- 1Mood Disorders Psychopharmacology Unit, University Health Network, Toronto, ON, Canada
- 2Department of Psychological Medicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore
- 3Department of Affective Disorder, the Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou Medical University, Guangzhou, China
- 4Laboratory of Emotion and Cognition, the Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou Medical University, Guangzhou, China
- 5Key Laboratory of Cognition and Personality, Faculty of Psychology, Ministry of Education, Southwest University, Chongqing, China
- 6Department of Psychiatry, University of Toronto, Toronto, ON, Canada
- 7Department of Pharmacology, University of Toronto, Toronto, ON, Canada
- 8Brain and Cognition Discovery Foundation, Toronto, ON, Canada
The prevalence and etiology of COVID-19's impact on brain health and cognitive function is poorly characterized. With mounting reports of delirium, systemic inflammation, and evidence of neurotropism, a statement on cognitive impairment among COVID-19 cases is needed. A substantial literature has demonstrated that inflammation can severely disrupt brain function, suggesting an immune response, a cytokine storm, as a possible cause of neurocognitive impairments. In this light, the aim of the present study was to summarize the available knowledge of the impact of COVID-19 on cognition (i.e., herein, we broadly define cognition reflecting the reporting on this topic in the literature) during the acute and recovery phases of the disease, in hospitalized patients and outpatients with confirmed COVID-19 status. A systematic review of the literature identified six studies which document the prevalence of cognitive impairment, and one which quantifies deficits after recovery. Pooling the samples of the included studies (total sample n = 644) at three standards of quality produced conservative estimates of cognitive impairment ranging from 43.0 to 66.8% prevalence in hospitalized COVID-19 patients only, as no studies which report on outpatients met criteria for inclusion in the main synthesis. The most common impairment reported was delirium and frequent reports of elevated inflammatory markers suggest etiology. Other studies have demonstrated that the disease involves marked increases in IL-6, TNFα, and IL-1β; cytokines known to have a profound impact on working memory and attention. Impairment of these cognitive functions is a characteristic aspect of delirium, which suggests these cytokines as key mediators in the etiology of COVID-19 induced cognitive impairments. Researchers are encouraged to assay inflammatory markers to determine the potential role of inflammation in mediating the disturbance of cognitive function in individuals affected by COVID-19.
Introduction
The coronavirus disease 2019 (COVID-19) is a respiratory condition caused by the RNA virus known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The disease can result in several complex syndromes due to far reaching and variable effects on the human body. The virus binds the angiotensin-converting enzyme 2 (ACE2) receptor (1) which induces its internalization (2) and begins its replication cycle (3). In many viral infections, immune cells detect pathogenic RNAs and activate the inflammatory response, which triggers wide-ranging effects that contain the spread of the pathogen (4). However, SARS-CoV-2 can overcome this containment, which results in a positive feedback loop between viral propagation and the release of cytokines/chemokines (5); the molecular signals that regulate inflammation. This mutual amplification causes the disease's characteristic cytokine storm; a destabilizing increase in circulating inflammatory cytokines. The inflammation storm caused by SARS-CoV-2 is the main reason the disease has far reaching physiological effects.
The disease course of COVID-19 involves the elevation of key cytokines such as interleukin-6 (IL-6), tumor necrosis factor-α (TNFα) (3), and interleukin-1β (IL-1β), among others (5). Convergent evidence from laboratory, clinical, and epidemiological studies suggest that the foregoing key cytokines, among several others, are produced in greater quantities when the active hormonal form of Vitamin D3 is low (6). Indeed, these findings have shown that Vitamin D3 deficiency is common among COVID-19 patients, and it has been known for decades that the biosynthesis of TNFα and IL-1β are reduced by calcitriol in a dose dependent manner (7). Furthermore, some of these cytokines can cross the blood brain barrier and prompt their own release from microglia (8). This amplification of the inflammatory signal in the CNS can bias the excitation-inhibition ratio toward excitation (9). The foregoing excitation may explain the disproportionate number of seizures in COVID-19 cases as compared to the typical incidence of seizures observed in intensive care units (ICUs) (10). Due to substantial sequence homology with better characterized coronaviruses, some have speculated that the virus might be neurotropic like many of its predecessors (11). Angiotensin-converting enzyme 2 (ACE2) receptors are expressed in both the nasal cavity and the CNS. Consequently, researchers have proposed that the virus traverses the cribriform plate and infects the brain (10).
The foregoing observations have prompted a recent wave of publications characterizing the neurological and mental health ramifications of SARS-CoV-2 infection (10–15). Although this literature adequately characterized the variety of COVID-19 related neuropsychological conditions, the data on the cognitive effects of the disease are insufficient, and these data are often reported ambiguously. For instance, one of the most widely cited studies on the neurological manifestations of COVID-19, Mao et al. (12), conflated the prevalence of somnolence with that of delirium, by reporting them jointly as “impaired consciousness.” This kind of nebulosity regarding cognitive outcomes is evident throughout the current COVID-19 literature and results are often confounded by pre-existing cognitive impairment. Nevertheless, several lines of research indicate that even peripheral viral infections or inflammatory signaling may affect cognitive function (16–19). Accordingly, the recognition of SARS-CoV-2 neurotropism (1, 10, 11, 20) as well as significant immune system activation (3, 5, 8) provides the basis for hypothesizing that COVID-19 patients may be susceptible to multi-dimensional cognitive impairments across the domains of the Research Domain Criteria (RDoC) framework (18).
In light of the aforementioned shortcomings of the extant literature, this review aimed to summarize the available knowledge of the impact of COVID-19 on cognition (i.e., herein, we broadly define cognition reflecting the reporting on this topic in the literature) during the acute and recovery phases of the disease, in hospitalized patients and outpatients with confirmed COVID-19 status. The prevalence of cognitive impairments among hospitalized COVID-19 adult cases has been quantified, and the most prevalent types of cognitive conditions have been reported. No studies which report on outpatients met criteria for inclusion in the main synthesis of the present study. Non-primary sources and publications with conspicuous signs of selective reporting (e.g., selected cases of cognitive impairment) have been excluded from the main synthesis and are referenced, either directly or indirectly, only as sources of etiological insight.
Materials and Methods
This review has been registered on PROSPERO (ID: CRD42020201232) prior to its commencement and was conducted in accordance with the recommendations of the PRISMA statement (21). Much of the relevant methodological details were described and updated on PROSPERO throughout the review process.
Search Strategy
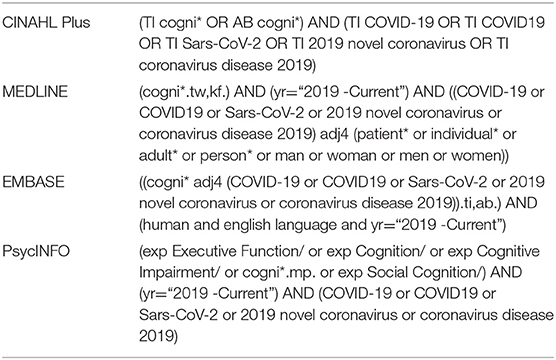
A systematic search of the literature was conducted on CINAHL Plus, MEDLINE, EMBASE, and APA PsycINFO. A manual citation search was conducted in the reference lists of articles included in full-text screening. As shown in Table 1, the searches involved both the “cognition” and the “COVID-19” concepts on all databases. Functional synonyms were used for COVID-19, and the word “cognition” was truncated to include all variations of the term. Time of publication was restricted to the interval between 2019 and 26/08/2020. EMBASE search yielded numerous generic and irrelevant documents. To exclude these results, the EMBASE search was restricted to papers with the two concepts appearing within four words of each other. MEDLINE search yielded numerous generic results that did not report patient data. To exclude these results, the MEDLINE search was restricted to papers with the “COVID-19” and “patient” concepts appearing within four words of each other. All searches on all databases were only applied to the title, abstract, and related keyword fields. The OVID platform was used to search all databases, with the exception of CINAHL Plus, for which the EBSCOhost platform was used. Database-specific restrictions and keywords are shown in Table 1.
Inclusion and Exclusion Criteria
To be included, studies were required to report either primary or secondary cognitive outcomes of SARS-CoV-2 infections confirmed by the presence of biological markers, as indicated by Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) or antibody assays, of blood, cerebrospinal fluid (CSF), or oronasopharyngeal swabs. Studies that only reported on suspected COVID-19 cases or on patients under the age of 18 were excluded, along with publications that did not report explicitly on cognitive function as characterized by reliable medical tests (e.g., CAM) or DSM-IV/V criteria. Papers in languages other than English, and papers which reported the cognitive outcomes of the socioeconomic or cultural circumstances of the COVID-19 pandemic, were also excluded. Within the included samples, data from those with cognitive impairments known or suspected to have existed prior to infection, were omitted from data analysis wherever possible. Peer-reviewed letters, case series, case-control studies, retrospective chart reviews, cohort studies, and point prevalence studies were included for analysis. Reviews, perspective/position papers, protocols/study designs, editorials, individual cases, or any non-primary sources were excluded to minimize the risks of redundant data collection and publication bias.
In compliance with the PRISMA statement (21), this review has been conducted in accordance with the PI(E)COS outline below:
• Participants: Patients aged ≥18 years with no known pre-exisiting cognitive impairments.
• Intervention: No intervention was evaluated in the present review.
• Exposure: SARS-CoV-2 infection confirmed by the presence of biological markers, as indicated by Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) or antibody assays, of blood, cerebrospinal fluid (CSF), or oronasopharyngeal swabs.
• Comparator: No overarching comparator applied to the present study, as assessments of cognitive function were categorical.
• Outcome: Prevalence of cognitive impairment during acute and recovery phases of COVID-19, as identified by the Confusion Assessment Method (CAM), 4 A's Test (4AT), DSM-IV/V criteria, or clinical diagnosis.
• Studies: Peer-reviewed case series, case-control studies, retrospective chart reviews, cohort studies, and point prevalence studies, which do not restrict selection to cognitively impaired patients.
Data Extraction Protocol
In compliance with the PRISMA statement (21), articles were assessed for relevance by title and abstract screening conducted by three independent reviewers. Full texts were examined for relevance when titles and abstracts were uninformative. Deduplication, screening, and quality assessments were conducted on the Covidence platform for systematic review management (https://www.covidence.org/). Conflicts in judgement were either resolved by discussion or by the judgement of the third reviewer. Throughout the review process, publications were only advanced to the next phase of examination upon the agreement of at least two reviewers. One reviewer extracted the data, and the results of these extractions were closely inspected by the co-authors.
The extracted data included: first author, year of publication, study design, sample size, sex ratio, average age, location, diagnostic test or criteria, and the prevalence of cognitive impairments. The percent prevalence of impairments and mean age of the total sample were calculated as weighted averages of the corresponding values (i.e., percent prevalence values and average age of the constituent samples, with sample sizes as weights). “Impairment” was used as a broad umbrella term that included the following conditions: altered mental status (AMS), confusion, delirium, encephalitis, encephalopathy, psychosis, dysexecutive syndrome, or any other condition explicitly reported as entailing cognitive deficits.
Methodological Quality Assessment
The quality assessment tool for case studies proposed by Murad et al. (22) was adapted to the final collection of articles of the present study. The adapted form used in the present study is presented in Table 2. The original tool assesses risks of bias with eight items across four domains: selection, ascertainment, causality, and reporting. Three items in the causality domain were omitted due to irrelevance; namely, the items for dose-response, challenge-rechallenge, and adequacy of time period from exposure/treatment till follow-up. Each included study was assessed by two reviewers, and conflicts were resolved by discussion. For domains in which judgments were necessarily made for separate participant subgroups, the weight of the associated domain was divided by the number of subgroups, and the sum of weights associated with items demonstrating low risk of bias was divided by the total number of items for a final quality score. Studies with scores ≤ 0.6 were considered to be at risk of being biased, and studies were ranked in accordance with this standard of quality.
Results
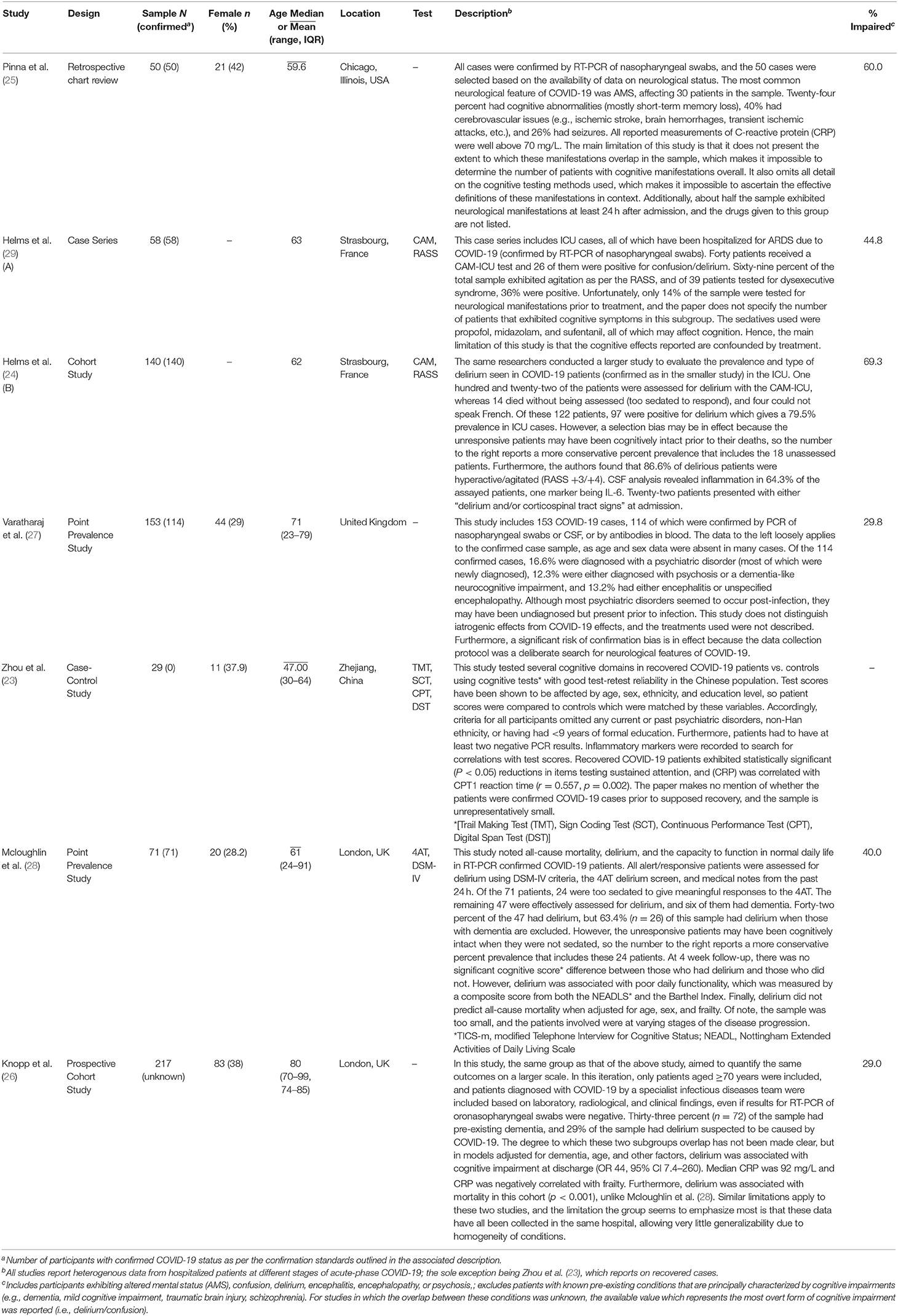
Seven studies which report on the prevalence of cognitive impairments associated with SARS-CoV-2 infection were included in this systematic review. The overall prevalence estimates from pooled and nested samples ranged from 43.0 to 66.8%, and one study demonstrated a correlation (r = 0.557, p = 0.002) between C-reactive protein (CRP) and reaction time in recovered COVID-19 patients (23). It is noteworthy that delirium was the most represented type of cognitive impairment in the prevalence estimates included. These conservative estimates along with the main findings of their associated studies, are summarized in Table 4.
Systematic Search Results
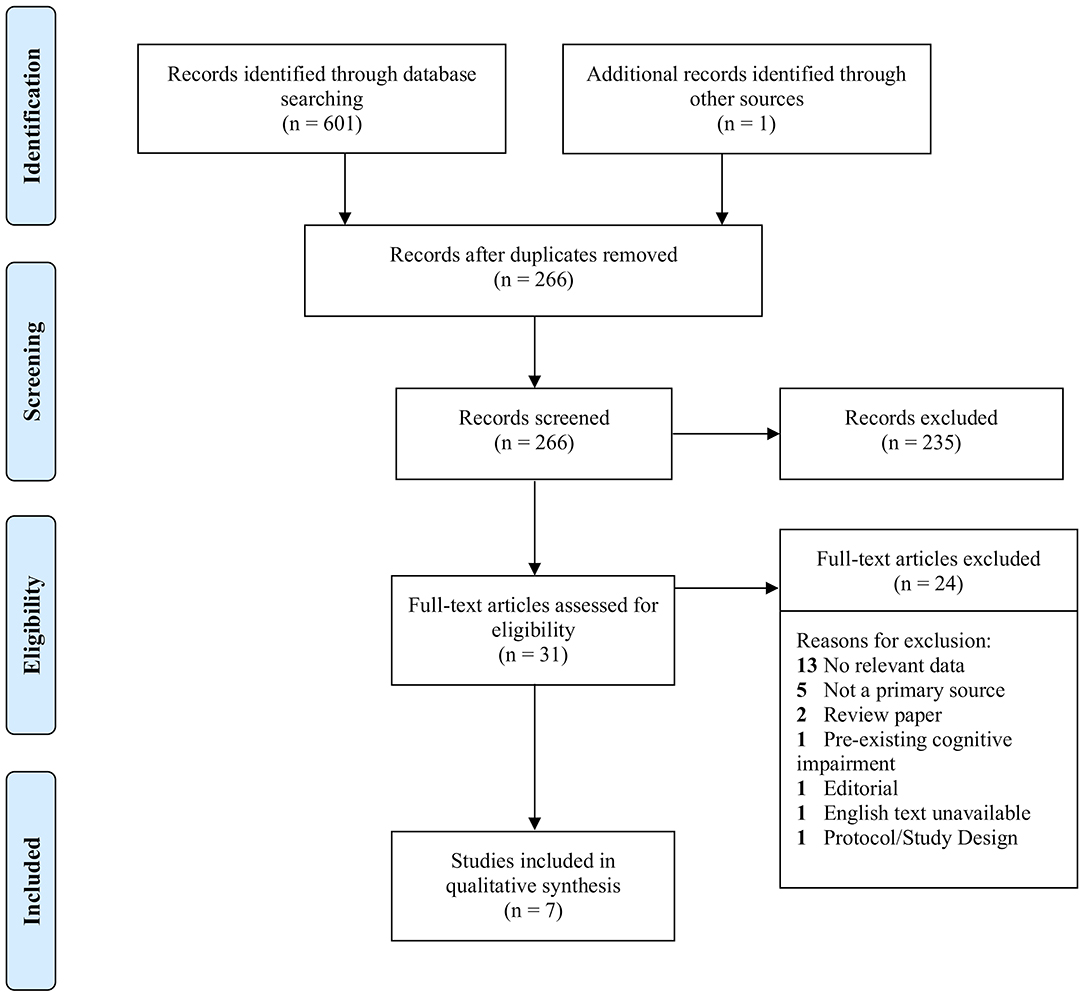
Due to the continuing publication of studies on COVID-19 and the scarcity of studies on cognition, databases were systematically searched at three time points: 19/07/2020, 09/08/2020, and 26/08/2020. Of 601 studies found in databases, 336 were identified as duplicates. After title and abstract screening of 266 studies, a total of 31 studies met criteria for full text assessment, which included one study found in a reference list. Of the 31, only seven met criteria for inclusion, and 24 studies were excluded for reasons listed in Figure 1. Six of the included studies reported the prevalence of cognitive impairment in COVID-19 patients during hospitalization, and one study (23) reported on cognitive function after recovery. The foregoing study was omitted from the total sample (n = 644) because the cases may not have been confirmed, and the impairments reported therein were not comparable to those in the other six studies. Nevertheless, the paper was included for its relevant findings, and because such few studies met requirements for inclusion. Notwithstanding, there was a significant lack of studies investigating the cognitive effects of SARS-CoV-2 infection. An informal search of the literature on October 11th, 2020 demonstrated that newer publications which discuss the COVID-19-cognition relationship mostly relied on the same studies found in the three formal searches of this review. Notably, Mao et al. (12) was cited often when relating infection to cognitive outcomes, but its methodological limitations necessitated its exclusion.
Quality Assessment Results
Five of the included studies had satisfactory scores above the 0.6 threshold, and two were considered unsatisfactory. The quality assessment naturally resulted in three tiers of quality: two studies had scores above 0.8, three studies had identical scores of 0.8, and two studies had identical scores of 0.6. Table 3 lists the three tiers in order of decreasing quality, along with the domains in which each study was deemed to be methodologically lacking.
Prevalence of Neurocognitive Impairments
All data gathered from the included articles are presented in Table 4, which also includes summaries of study methods, major findings, and limitations. The total sample was n = 644 and the weighted mean of the reported average ages from its constituent samples was 69 years (SD = 7.90). Of this sample, at least 43.0% (SD = 16.2) exhibited one of the following neurocognitive impairments: delirium, confusion, AMS, encephalopathy, encephalitis, or psychosis. However, this percentage involves one study (26) which included 72 patients with premorbid dementia. Nevertheless, this study produced relevant results from models corrected for dementia in a separate analysis (reported in Table 4). Upon omission of this study's sample (n = 217), at least 49.9% (SD = 15.8) of the remaining pooled sample (n = 427) were cognitively impaired (the same neurocognitive impairments reported in the overall sample), and the weighted mean age for this sample was 64 years (SD = 4.50). Upon exclusion of Zhou et al. (23) and Knopp et al. (26), the percent prevalence for the combined samples of the top two tiers of quality (n = 304) is 53.0% (SD = 20.6), and the weighted mean age was 65 years (SD = 6.01). The types of impairment remained the same after these exclusions, with the exception of confusion; however, the more severe form of this impairment (i.e., delirium) retained its status as the most represented. The percent prevalence for the sample of the top tier alone (n = 190) is 66.8% (SD = 6.57), with weighted mean age of 61 years (SD = 1.70); only delirium and AMS were reported in this sample. There are additional cases of cognitive impairment that have not been incorporated into these percentages. The prevalence of some such cases along with the types of impairments as well as reports on inflammatory markers are mentioned under “Descriptions” in Table 4. Overall, four studies reported on inflammatory markers [C-reactive protein (CRP) or IL-6] and all four publications reported elevations in at least one of these inflammatory markers which were concomitant with cognitive impairment. Helms et al. (24) and Knopp et al. (26) reported respective elevations of IL-6 and CRP in delirious patients. Pinna et al. (25) found elevations of CRP in cases of AMS, and Zhou et al. (23) found a positive correlation between reaction time and serum (CRP) (r = 0.557, p = 0.002).
Discussion
Quality of Information
One of the limitations with respect to the interpretation of the available studies was that the medications prescribed to treat COVID-19 may have significantly confounded results. As suggested in Table 3, four of the included studies did not exclude confounds, the most significant of which was the dyscognitive effect of medications which may have been used to treat COVID-19 (e.g., steroids). Furthermore, much of the literature does not adequately separate cases with pre-existing neurocognitive impairments from cases of cognitive impairment associated with COVID-19. Stringent as the inclusion criteria were, these problems still presented themselves in the included studies to varying degrees. For instance, Knopp et al. (26) did not clarify whether some of the 72 participants with pre-existing dementia were included in the delirious subgroup. Dementia and delirium are often confused and misdiagnosed in clinical practice (30), and some evidence has suggested that patients with dementia are especially at risk of developing persistent delirium (31). This suggests that there may have been an overestimation of COVID-19 related delirium due to the inclusion of patients with dementia. There was also ambiguity in Knopp et al. (26) regarding the methods used to confirm SARS-CoV-2 infection. The article reports that these assessments were conducted by infectious disease experts but does not mention the exact methods used, or whether they were contested in the scientific literature. Nevertheless, this article was an exemplar of the fundamental challenges involved in gathering large datasets from the busy hospital environment. It is a testament to the difficulty of the situation that Knopp et al. (26) was one of the best studies available. The other included studies did allow for the exclusion of patients with known pre-existing cognitive impairments but had other significant limitations, as indicated in Tables 3, 4. It is likely due to such challenges that most studies did not quantify the extent of overlap between subgroups with different types of COVID-19-related cognitive impairments. In those cases, conservative prevalence statistics were produced, involving only the most severe and overt cognitive conditions (i.e., delirium).
Implicit Reporting Bias in Prevalence Results
As mentioned in “Prevalence of Neurocognitive Impairments,” the prevalence statistics produced for various combinations of the included samples ranged from 43.0 to 66.8%. Although these numbers were calculated conservatively on the study level, a reporting bias may have been amplified by pooling the results. One of the limitations of extant literature is the non-publication of negative study results. In an analysis of 64 randomly selected scientific articles, out of 145 empirically supported potential determinants of selective reporting, it was found that the leading determinant was a “focus on preferred findings,” accounting for 36% of cases (32). Despite best efforts, this review may have implicitly amplified this type of bias. It is certainly possible that some of the excluded attempts to characterize the presentations of COVID-19 involved cognitive assessments that produced negative results. Aside from bias toward preferred findings, these results may not have been reported simply for the sake of brevity. Many of the foregoing studies considered throughout this review were very broad in scope, attempting to provide a complete impression of the COVID-19 syndrome. In such cases, the omission of negative results on cognitive assessments may have seemed prudent. This implicit risk of selective reporting is difficult to rectify and is a fundamental problem in the systematic review methodology. Furthermore, the unspecified diagnostic criteria in three of the included studies may have masked loose definitions of cognitive impairment, which may have resulted in the overestimation of the associated prevalence statistics. Taking these considerations in isolation, the 43.0–66.8% prevalence range may be viewed as non-representative of the real-world prevalence of COVID-19 induced cognitive impairments. However, considering the parsimonious neurobiological models which predict these impairments, the results included herein cannot be dismissed.
Neurobiological Model for COVID-19 Related Cognitive Impairments
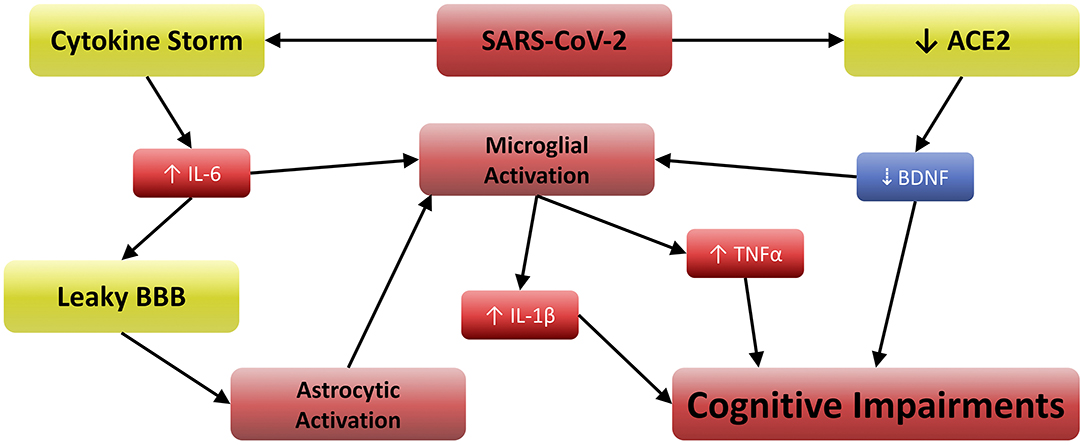
As mentioned in the introduction, COVID-19 involves elevations in IL-6, TNFα (3), and IL-1β (5), which are often exacerbated by Vitamin D3 deficiency (6). Furthermore, IL-6 and TNFα can cross the blood brain barrier and activate microglia (8). These activated cells release IL-1β, the receptors for which are especially concentrated in the postsynaptic compartments of hippocampal neurons (33). This renders the hippocampus especially vulnerable to IL-1β, which has been shown to disrupt long term potentiation (LTP) and memory (34). Other work has also suggested that attentional processes are subserved by hippocampal activity, demonstrating the importance of working memory in determining how attention is directed and sustained (35). Attention and working memory are among the principle cognitive functions impaired in delirium (30), and clinically manifested neurotropism may exacerbate this through additional pathways.
ACE2 acts as the functional and host receptor for coronaviruses (1) and regulates normal brain function by stimulating brain-derived neurotrophic factor (BDNF) activity (36). BDNF plays a critical role in attenuating microglial activation (37) and neuronal inflammation (38), and low BDNF levels are associated with cognitive impairment in both human and animal studies (37, 39, 40). SARS-CoV-2 is now known to decrease ACE2-mediated BDNF activity (20), possibly by acting as a competitive angiotensin-II-antagonist via spike protein-ACE2 binding. Regardless of the mechanism by which SARS-CoV-2 inhibits ACE2, the resulting reduction of BDNF is likely to cause cognitive impairment (20). Furthermore, the permeability of the blood-brain barrier (BBB) can be increased by IL-6 (41), which can further microglial activation by enhancing the CNS effects of serum cytokines. Astrocytic activation also contributes to the inflammatory signal in the CNS, which is especially pronounced when BBB integrity is compromised (41). Indeed, increased BBB permeability has been observed in COVID-19 patients (26), and high CRP/IL-6 concentrations are reported by several studies (24–26).
Taken together in the context of the present study, these findings suggest that impairment of working memory and attention can both be affected by TNFα (42) and IL-1β, because both can disrupt normal firing in the neurons involved. Furthermore, these same effects would be greater in the case of clinically manifested neurotropism. In such scenarios, it is reasonable to assume that greater proportions of the microglial and astrocytic populations would be activated due to direct toll-like receptor 3/7/8 stimulation (4), and this inflammation would be furthered by reductions in BDNF (20, 36, 39). Figure 2 depicts the relationships between these variables, suggesting a well-supported neurobiological model for the etiology of COVID-19 related cognitive impairments. It is noteworthy that tests for delirium and other conditions provide categorical measures of cognitive outcomes, but a quantitative assessment such as that conducted in Zhou et al. (23) may aid future researchers in revealing the continuous cognitive effects of the neurobiological mechanism described herein. Furthermore, clinicians are urged to consider Vitamin D3 supplementation, as its active metabolite may attenuate such effects via reductions in TNFα and IL-1β production (7).
Limitations
The main limitation of the analysis herein is a function of the limitations of the included studies (e.g., reported outcomes may have been confounded by iatrogenic effects). Sedatives are often used to treat COVID-19 patients, and other drugs may also have effects on measures of cognition. For example, research has linked chloroquine and hydroxychloroquine to psychotic symptoms and irritability (43). Other studies have linked tocilizumab to headaches, dizziness, and in some cases, strokes (44). It is also important to note that the patients included for synthesis were all hospitalized, presumably due to the severity of symptoms. Accordingly, less severe COVID-19 cases may have escaped inclusion merely due to lack of adequate reporting; a possibility which restricts the generalizability of the results reported herein. Furthermore, the diagnostic tools applied to classify cognitive impairments were nebulous in three of the included studies (as suggested in Table 4). Theoretically, this raises concerns regarding misdiagnoses which may have exaggerated the prevalence of cognitive impairments. Aside from the risk of selective reporting explained in “Implicit Reporting Bias in Prevalence Results,” this review may also be limited by the exclusivity of the search strategy. The use of the adjacency operator on EMBASE was necessary to exclude an unmanageable number of irrelevant publications, but by applying this restriction, some relevant studies may not have been identified. Nevertheless, this review provided a quantitative assessment of cognitive dysfunction associated with COVID-19 as well as a call, for both clinical and research purposes, to apply measures of cognitive function and inflammatory markers in COVID-19 patients at presentation.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
Author Contributions
RM conceived of and supervised the project, among other contributions. RM and FN were responsible for managing the project and advising the team, among other contributions. YA, AS, and LL jointly determined the inclusion and exclusion criteria, screened the literature, formally assessed the quality of the included literature, and contributed to team discussions regarding data analysis. YA conducted the systematic searches, summarized the included literature, and wrote the first draft of the article. All authors contributed to team discussions regarding data analysis, synthesis, and interpretation and fact checked, edited, contributed to, and approved the submitted draft.
Conflict of Interest
YL received salary support from the Global Alliance for Chronic Diseases/Canadian Institutes of Health Research (CIHR)/National Natural Science Foundation of China's Mental Health Team Grant and the CIHR Frederick Banting and Charles Best Canada Graduate Scholarship; personal fees from Champignon Brands. RM has received research grant support from CIHR/GACD/Chinese National Natural Research Foundation; speaker/consultation fees from Lundbeck, Janssen, Purdue, Pfizer, Otsuka, Allergan, Takeda, Neurocrine, Sunovion, Minerva, Intra-Cellular, Abbvie, and Eisai. RM is a shareholder and CEO of Champignon. JR has received research grant support from the Canadian Cancer Society, Canadian Psychiatric Association, American Psychiatric Association, American Society of Psychopharmacology, University of Toronto, University Health Network Centre for Mental Health, Joseph M. West Family Memorial Fund and Timeposters Fellowship and industry funding for speaker/consultation/research fees from Allergan, Lundbeck and COMPASS. JR is the medical director of a private clinic providing off-label ketamine infusions for depression.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Earlier drafts of this article were posted on https://www.researchgate.net through the first author's account. Some of these drafts did not list all authors as they were not edited and approved by the review team. Drafts were only posted for additional feedback from the online community. All drafts of this article can be viewed at: https://www.researchgate.net/publication/344622934_Impact_of_SARS-CoV-2_Infection_on_Cognitive_Functions_A_Systematic_Review.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2020.621773/full#supplementary-material
References
1. Kuhn JH, Radoshitzky SR, Li W, Wong SK, Choe H, Farzan M. The SARS Coronavirus receptor ACE 2 A potential target for antiviral therapy. In: Holzenburg A, Bogner E, editors. New Concepts of Antiviral Therapy. Boston, MA: Springer, U. S. (2006). p. 397–418.
2. Walls AC, Park Y-J, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. (2020) 181:281–92.e6. doi: 10.1016/j.cell.2020.02.058
3. Poduri R, Joshi G, Jagadeesh G. Drugs targeting various stages of the SARS-CoV-2 life cycle: exploring promising drugs for the treatment of Covid-19. Cell Signal. (2020) 74:109721. doi: 10.1016/j.cellsig.2020.109721
4. Sariol CA, Martínez MI, Rivera F, Rodríguez IV, Pantoja P, Abel K, et al. Decreased dengue replication and an increased anti-viral humoral response with the use of combined toll-like receptor 3 and 7/8 agonists in Macaques. PLoS ONE. (2011) 6:e19323. doi: 10.1371/journal.pone.0019323
5. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J Infect. (2020) 80:607–13. doi: 10.1016/j.jinf.2020.03.037
6. Daneshkhah A, Agrawal V, Eshein A, Subramanian H, Roy HK, Backman V. Evidence for possible association of vitamin D status with cytokine storm and unregulated inflammation in COVID-19 patients. Aging Clin Exp Res. (2020) 32:2141–58. doi: 10.1007/s40520-020-01677-y
7. Panichi V, De Pietro S, Andreini B, Bianchi AM, Migliori M, Taccola D, et al. Calcitriol modulates in vivo and in vitro cytokine production: a role for intracellular calcium. Kidney Int. (1998) 54:1463–9. doi: 10.1046/j.1523-1755.1998.00152.x
8. Akrout N, Sharshar T, Annane D. Mechanisms of brain signaling during sepsis. Curr Neuropharmacol. (2009) 7:296–301. doi: 10.2174/157015909790031175
9. Chiavegato A, Zurolo E, Losi G, Aronica E, Carmignoto G. The inflammatory molecules IL-1β and HMGB1 can rapidly enhance focal seizure generation in a brain slice model of temporal lobe epilepsy. Front Cell Neurosci. (2014) 8:155. doi: 10.3389/fncel.2014.00155
10. Fotuhi M, Mian A, Meysami S, Raji CA. Neurobiology of COVID-19. J Alzheimer's Dis. (2020) 76:3–19. doi: 10.3233/JAD-200581
11. Holmes EA, O'Connor RC, Perry VH, Tracey I, Wessely S, Arseneault L, et al. Multidisciplinary research priorities for the COVID-19 pandemic: a call for action for mental health science. Lancet Psychiatry. (2020) 7:547–60. doi: 10.1016/S2215-0366(20)30168-1
12. Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. (2020) 77:683. doi: 10.1001/jamaneurol.2020.1127
13. McIntyre RS, Lee Y. Preventing suicide in the context of the COVID-19 pandemic. World Psychiatry. (2020) 19:250–1. doi: 10.1002/wps.20767
14. Xiong J, Lipsitz O, Nasri F, Lui LMW, Gill H, Phan L, et al. Impact of COVID-19 pandemic on mental health in the general population: A systematic review. J Affect Disord. (2020) 277:55–64. doi: 10.1016/j.jad.2020.08.001
15. Wang C, Pan R, Wan X, Tan Y, Xu L, McIntyre RS, et al. A longitudinal study on the mental health of general population during the COVID-19 epidemic in China. Brain Behav Immun. (2020) 87:40–8. doi: 10.1016/j.bbi.2020.04.028
16. Rosenblat JD, Brietzke E, Mansur RB, Maruschak NA, Lee Y, McIntyre RS. Inflammation as a neurobiological substrate of cognitive impairment in bipolar disorder: evidence, pathophysiology and treatment implications. J Affect Disord. (2015) 188:149–59. doi: 10.1016/j.jad.2015.08.058
17. Hung T-H, Chen VC-H, Yang Y-H, Tsai C-S, Lu M-L, McIntyre RS, et al. Association between enterovirus infection and speech and language impairments: a nationwide population-based study. Res Dev Disabil. (2018) 77:76–86. doi: 10.1016/j.ridd.2018.04.017
18. Lee Y, Subramaniapillai M, Brietzke E, Mansur RB, Ho RC, Yim SJ, et al. Anti-cytokine agents for anhedonia: targeting inflammation and the immune system to treat dimensional disturbances in depression. Ther Adv Psychopharmacol. (2018) 8:337–48. doi: 10.1177/2045125318791944
19. Dalkner N, Reininghaus E, Schwalsberger K, Rieger A, Hamm C, Pilz R, et al. C-Reactive protein as a possible predictor of trail-making performance in individuals with psychiatric disorders. Nutrients. (2020) 12:3019. doi: 10.3390/nu12103019
20. Motaghinejad M, Gholami M. Possible neurological and mental outcomes of COVID-19 infection: a hypothetical role of ACE-2\Mas\BDNF signaling pathway. Int J Prev Med. (2020) 11:84. doi: 10.4103/ijpvm.IJPVM_114_20
21. Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
22. Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. (2018) 23:60–3. doi: 10.1136/bmjebm-2017-110853
23. Zhou H, Lu S, Chen J, Wei N, Wang D, Lyu H, et al. The landscape of cognitive function in recovered COVID-19 patients. J Psychiatr Res. (2020) 129:98–102. doi: 10.1016/j.jpsychires.2020.06.022
24. Helms J, Kremer S, Merdji H, Schenck M, Severac F, Clere-Jehl R, et al. Delirium and encephalopathy in severe COVID-19: a cohort analysis of ICU patients. Crit Care. (2020) 24:491. doi: 10.1186/s13054-020-03200-1
25. Pinna P, Grewal P, Hall JP, Tavarez T, Dafer RM, Garg R, et al. Neurological manifestations and COVID-19: experiences from a tertiary care center at the Frontline. J Neurol Sci. (2020) 415:116969. doi: 10.1016/j.jns.2020.116969
26. Knopp P, Miles A, Webb TE, Mcloughlin BC, Mannan I, Raja N, et al. Presenting features of COVID-19 in older people: relationships with frailty, inflammation and mortality. Eur Geriatr Med. (2020) 11:1089–94. doi: 10.1007/s41999-020-00373-4
27. Varatharaj A, Thomas N, Ellul MA, Davies NWS, Pollak TA, Tenorio EL, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. (2020) 7:875–82. doi: 10.1016/S2215-0366(20)30287-X
28. Mcloughlin BC, Miles A, Webb TE, Knopp P, Eyres C, Fabbri A, et al. Functional and cognitive outcomes after COVID-19 delirium. European Geriatric Medicine. (2020) 11:857–62. doi: 10.1007/s41999-020-00353-8
29. Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, et al. Neurologic features in severe SARS-CoV-2 infection. New England J Med. (2020) 382:2268–70. doi: 10.1056/NEJMc2008597
30. Leonard M, McInerney S, McFarland J, Condon C, Awan F, O'Connor M, et al. Comparison of cognitive and neuropsychiatric profiles in hospitalised elderly medical patients with delirium, dementia and comorbid delirium–dementia. BMJ Open. (2016) 6:e009212. doi: 10.1136/bmjopen-2015-009212
31. Boettger S, Jenewein J, Breitbart W. Delirium in advanced age and dementia: a prolonged refractory course of delirium and lower functional status. Pall Supp Care. (2015) 13:1113–21. doi: 10.1017/S1478951514000972
32. Steen JT, van der Bogert CA, van den Soest-Poortvliet MC, van Farsani SF, Otten RHJ, Riet G, et al. Determinants of selective reporting: a taxonomy based on content analysis of a random selection of the literature. PLoS ONE. (2018) 13:e0188247. doi: 10.1371/journal.pone.0188247
33. Gardoni F, Boraso M, Zianni E, Corsini E, Galli CL, Cattabeni F, et al. Distribution of interleukin-1 receptor complex at the synaptic membrane driven by interleukin-1β and NMDA stimulation. J Neuroinflamm. (2011) 8:14. doi: 10.1186/1742-2094-8-14
34. Prieto GA, Tong L, Smith ED, Cotman CW. TNFα and IL-1β but not IL-18 suppresses hippocampal long-term potentiation directly at the synapse. Neurochem Res. (2019) 44:49–60. doi: 10.1007/s11064-018-2517-8
35. Goldfarb EV, Chun MM, Phelps EA. Memory-guided attention: independent contributions of the hippocampus and striatum. Neuron. (2016) 89:317–24. doi: 10.1016/j.neuron.2015.12.014
36. Zheng J, Li G, Chen S, Wang J, Olson JE, Xia H, et al. Angiotensin converting enzyme 2/Ang-(1–7)/Mas axis protects brain from ischemic injury with a tendency of age-dependence. CNS Neurosci Ther. (2014) 20:452–9. doi: 10.1111/cns.12233
37. Wu S-Y, Pan B-S, Tsai S-F, Chiang Y-T, Huang B-M, Mo F-E, et al. BDNF reverses aging-related microglial activation. J Neuroinflamm. (2020) 17:210. doi: 10.1186/s12974-020-01887-1
38. Joosten EAJ, Houweling DA. Local acute application of BDNF in the lesioned spinal cord anti-in£ammatory and anti-oxidant effects. Neuroreport. (2004) 1163–6. doi: 10.1097/00001756-200405190-00016
39. Ng TKS, Ho CSH, Tam WWS, Kua EH, Ho RC-M. Decreased serum brain-derived neurotrophic factor (BDNF) levels in patients with Alzheimer's Disease (AD): a systematic review and meta-analysis. Int J Mol Sci. (2019) 20:257. doi: 10.3390/ijms20020257
40. de Pins B, Cifuentes-Díaz C, Thamila Farah A, López-Molina L, Montalban E, Sancho-Balsells A, et al. Conditional BDNF delivery from astrocytes rescues memory deficits, spine density and synaptic properties in the 5xFAD mouse model of Alzheimer disease. J Neurosci. (2019) 39:2121–18. doi: 10.1523/JNEUROSCI.2121-18.2019
41. Varatharaj A, Galea I. The blood-brain barrier in systemic inflammation. Brain Behav Immunity. (2017) 60:1–12. doi: 10.1016/j.bbi.2016.03.010
42. Tancredi V, D'Arcangelo G, Grassi F, Tarroni P, Palmieri G, Santoni A, et al. Tumor necrosis factor alters synaptic transmission in rat hippocampal slices. Neurosci Lett. (1992) 146:176–8. doi: 10.1016/0304-3940(92)90071-E
43. Manzo C, Gareri P, Castagna A. Psychomotor agitation following treatment with hydroxychloroquine. Drug Saf - Case Rep. (2017) 4:6. doi: 10.1007/s40800-017-0048-x
Keywords: neurotropism, cognitive function, delirium, depression, neuroinflammation, cytokines, COVID-19, brain health
Citation: Alnefeesi Y, Siegel A, Lui LMW, Teopiz KM, Ho RCM, Lee Y, Nasri F, Gill H, Lin K, Cao B, Rosenblat JD and McIntyre RS (2021) Impact of SARS-CoV-2 Infection on Cognitive Function: A Systematic Review. Front. Psychiatry 11:621773. doi: 10.3389/fpsyt.2020.621773
Received: 27 October 2020; Accepted: 22 December 2020;
Published: 10 February 2021.
Edited by:
Alessandro Colasanti, Brighton and Sussex Medical School, United KingdomReviewed by:
Kamilla Miskowiak, University of Copenhagen, DenmarkMaurice Preter, Columbia University, United States
Copyright © 2021 Alnefeesi, Siegel, Lui, Teopiz, Ho, Lee, Nasri, Gill, Lin, Cao, Rosenblat and McIntyre. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roger S. McIntyre, cm9nZXIubWNpbnR5cmVAdWhuLmNh
 Yazen Alnefeesi
Yazen Alnefeesi Ashley Siegel
Ashley Siegel Leanna M. W. Lui1
Leanna M. W. Lui1 Kayla M. Teopiz
Kayla M. Teopiz Roger C. M. Ho
Roger C. M. Ho Kangguang Lin
Kangguang Lin Bing Cao
Bing Cao Roger S. McIntyre
Roger S. McIntyre