- 1Laboratory for Innovation in Autism, University of Strathclyde, Glasgow, United Kingdom
- 2British Association of Play Therapists, London, United Kingdom
- 3Wooley Wood School, Sheffield, United Kingdom
- 4Concept Training Ltd., Lancashire, United Kingdom
- 5Strathclyde Institute of Education, University of Strathclyde, Glasgow, United Kingdom
We present a handbook for Rhythmic Relating, an approach developed to support play, learning and therapy with young autistic children, unconventional communicators, and autistic people who have additional learning needs. Rhythmic Relating is based on the Movement Sensing perspective, a growing body of research that recognizes that autistic social difficulties stem from more basic sensory and motor differences. These sensorimotor differences directly affect embodied experience and social timing in communication. The Rhythmic Relating approach acknowledges that autistic/non-autistic interactive mismatch goes both ways and offers bidirectional support for social timing and expressive action in play. This handbook is presented in an accessible fashion, allowing the reader to develop at their own pace through three skill-levels and encouraging time out to practice. We begin with the basics of building rapport (seeing, copying, and celebrating interactional behaviors), introduce the basic foundations of sensory stability, and then move on to developing reciprocal play (using mirroring, matching, looping, and “Yes…and” techniques), and further to understanding sensory impetus (using sensory contours, accents and flows) and its potential in support of social timing. Rhythmic Relating is offered in support of each practitioner’s creative practice and personal sense of fun and humor in play. The model is offered as a foundation for interaction and learning, as a base practice in schools, for Occupational Therapists, Speech Therapists and Physiotherapists, and can also provide a basis for tailoring creative arts therapies when working with autistic clients.
Introduction
Overview
Autistic people1 often find themselves literally out of sync with non-autistic people, and vice versa. Interaction between neurotypes often involves considerable challenges with the social timing2 that active engagement in interactions typically depends on (for an overview, see Wimpory, 2015). Non-autistic adults have difficulties picking up subtleties of gesture from autistic children (Keen et al., 2005; Faso et al., 2015; Sheppard et al., 2016; Casartelli et al., 2020b). While autistic children have difficulties picking up subtleties of interaction, gesture, and flow from typically developing children (Rochat et al., 2013; Di Cesare et al., 2017). The difficulties go both ways.3 And the result: it is difficult for us to play, communicate, and learn together (Trevarthen and Delafield-Butt, 2013; Cook, 2016; Delafield-Butt et al., 2019). Rhythmic Relating is designed to help autistic and non-autistic people feel more in sync together, so we can share meaningful experiences, play together, and support each other in therapeutic contexts toward wellbeing4 (see Table 1). Given average population demographics, most carers and practitioners will be non-autistic and our model and assumptions in this paper reflect this fact. If you are an autistic carer or practitioner, Rhythmic Relating will also support your playful interactions with autistic play partners—you will simply have the advantage of autistic sensitivities and insight. Rhythmic Relating is offered in support of each practitioner’s creative practice and personal sense of fun and humor in play. The model is offered as a foundation for interaction and learning, as a base practice in schools, for Occupational Therapists, Speech Therapists and Physiotherapists, and can also provide a basis for tailoring creative arts therapies when working with autistic clients.
The autism research community continues to investigate the nature of sensory and movement challenges in autism5 and the impact these challenges might have on social timing.6 Into this mix, recent studies have added an examination of the relationship between good-enough social timing and wider relational factors such as rapport.7 Although we have much to learn about the fundamentals of social interaction for autistic people, it seems clear that any approach aiming to improve social timing will benefit from finding ways to facilitate rapport and the sensory organization of movement (for an overview, see Daniel et al., 2022).
The Rhythmic Relating model described in this article combines previous work on Rhythmic Relating and social timing (Daniel et al., 2022) and an approach called Rapport-Based Communication (Laurie, 2021). Rhythmic Relating aims to give you foundational skills to support your own creativity, insights, and personal sense of fun and humor in play. Here, we are attempting a how-to-guide and we will try to keep the ideas and the language we use as simple as possible. On occasions, we give more detail in footnotes. You can read these if interested, or skip to stick with the flow of the article. This article will build your understanding in layers (from the basics, through skill levels 1, 2, and 3), with take-home ideas at each level. It will be helpful to take in little bits at a time, stop reading for a while and try things out in practice. Then come back to this article again when you are ready. It might be that you simply do not connect with some aspects of the model, no worries, please take and use whatever works for you. In support of your learning, the online article includes live links to video examples.8 You can click on the example heading, to activate the links.
Social timing
To give you a sense of what we mean by social timing, we can start with an exercise. This exercise reproduces the Still Face experiment (Tronick et al., 1978) that proved the importance of active social responses for affective connection between babies and mothers. However, even as intelligent and knowledgeable adults, the Still Face experience still works for us, because it engages our evolutionary ancient social pathways for communication and connection (see Table 2).
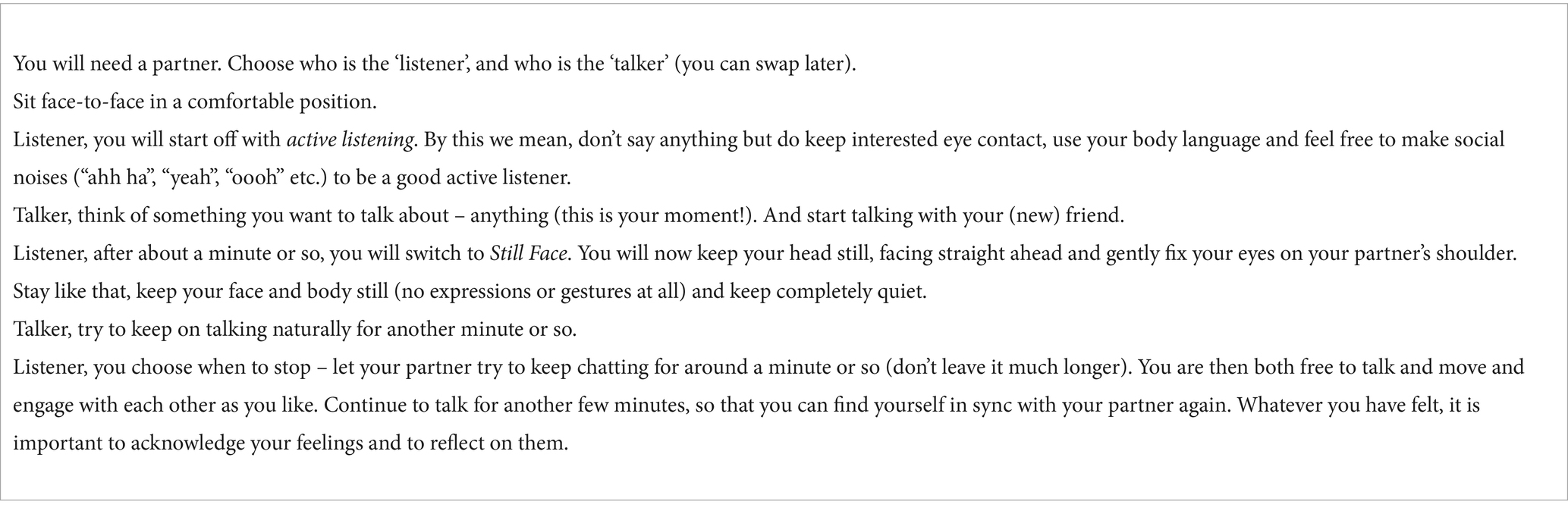
Table 2. The Still Face exercise. An optional exercise. Please feel free to skip if you prefer, it’s a helpful exercise, but not essential to your understanding of the article. Please also note, the exercise is designed to give neurotypical people an experience of a non-typical communication pattern. If you are neurodivergent or uncomfortable with it in any way, we recommend skipping this exercise.
When you were in the talker role, how did it feel initially when you were receiving active social prompts, and then how did it feel when your partner went Still Face? Most people feel awkward, many simply cannot continue talking. When we interact, we are continually feeding each other non-verbal social cues in a back-and-forth flow. This flow of non-verbal information is crucial to our feelings of togetherness and ease. Without it, we feel disturbed.
In a development of the Still Face experiment, known as the Double-Television experiment (Murray and Trevarthen, 1985), a baby and her mother interacted via screens. All was going great while they interacted in a natural and playfully attuned manner. The baby was happy and relaxed. Then the experimenters switched the live action to a loop of the mother’s responses from a minute earlier. What the baby was receiving was her mother’s real loving responses, but out of sync with the baby’s own live experience. The Double-Television experiment showed that an out-of-sync baby was just as disturbed as one experiencing her mother’s Still Face. Social timing is crucial to our feelings of ease and connectedness, and to our ability to share and make meaningful sense of things.
When babies experience Still Face, in the middle of active communication from their mothers, they quickly become distressed. In those initial experiments (Tronick et al., 1978), the mothers held Still Face just for a while, and quickly repaired the flowing connection with their babies. But imagine if your experience of other human beings, from when you were young onwards, was something like that Still Face experiment. Imagine if you were unable to pick-up on the social cues and flow which most of us take for granted. Imagine if you often, or always, felt out of sync with other people. And imagine how this might have affected your sense of who you are, your relationships with other people, and your ability to learn.
In using Rhythmic Relating, we support social timing in playful relationships with our autistic play partners. We use skills specifically developed to address the sensory needs of autistic people. Many of these skills are based on the science surrounding a concept we are calling sensory impetus (Daniel et al., 2022). Here, early on, we introduce a flavor of this idea, because this will help you to get a sense of where the article is heading. Sensory impetus will become much more tangible for you, as you work with the details and examples throughout the article at your own pace.
Sensory impetus
When we talk about sensory impetus, we are interested in sensory experiences that are directly experienced, before conscious reflection. These direct experiences are information-rich: they inherently suggest movement in a particular direction, toward a particular purpose or point in space and time; they communicate the intentions of our actions; and they compel us to move. Here are a few examples of sensory impetus (in these cases, not from human interaction) to give you more of a sense of what we mean:
Stu (one of the authors here) has a play therapy room up some stairs, round the corner, and down the corridor from the clinic’s front door. A few years ago, a five-year-old autistic boy came to the clinic. His mother was concerned he would not come in; he was very worried about transitions. But she did not know about the big blue and yellow floor tiles laid out chess-board style down the corridor. Out in the street, the boy used the pattern of the paving slabs as an impetus; up the stairs he counted; along the corridor he felt compelled to move from blue-to-blue square until (without really realizing it) he was outside the clinic door.
In the Oliver Sacks book and film Awakenings, Sacks described a patient who was stuck in a sort of physical stasis, looking, to an outside observer, like a statue. The patient never moved without manipulation. Yet when Oliver, the doctor, threw a baseball toward the patient, she immediately reached up and caught it (Sacks, 1991). The perceptual looming arc of the ball coming closer compelled an automatic and perfectly-timed response.
In Edinburgh, a group of researchers have improved the physical control of many people with cerebral palsy. How did they do this? Across various tasks involving reaching to grasp an object (a perceptual and motor control-arc in space), the researchers paired the arc of the grasp with a comparable arcing sound tone (the tone flowing up and then back down, in pitch and volume, to describe the arc of the grasping action) (Schögler et al., 2017).
The perceptual flow of the patterned floor tiles, the impetus of the looming baseball, and the compelling and information-rich sound tone are just a few examples of sensory impetus.
Throughout this article, we will be interested in how we can include such sensory experiences into our playful communication. As part of the Rhythmic Relating model, we will be using them to support the well-timed moments and flows of shared experience. We will develop the use of certain types of sound, movement, physical contact, rhythm, and patterned flows of actions which communicate timing, direction, and our intentions. In this way, we can help our play partner stay in sync and get a just-ahead-in-time sense of what we are about to do.
The basics
Rapport
Laurie (2021) has proposed that rapport is the central experience underpinning a number of systems for social support in autism, including Intensive Interaction (Nind and Hewett, 2012), Responsive Communication (Caldwell et al., 2019), Floortime (Greenspan and Wieder, 2006), Son-rise (Kaufman, 2011) etc., all of which stem from, and actively facilitate, principles of early infant-caregiver interaction. In 1990, a Harvard University study investigated the nature of high-quality rapport in human interaction (Tickle-Degnen and Rosenthal, 1990). This study suggests that rapport has three essential ingredients:
1. Mutual social attention.
2. Mutual positivity.
3. Mutual co-ordination (Tickle-Degnen and Rosenthal, 1990)
Remember that it is very likely that none of these three factors will come naturally to someone autistic. We will need to find ways to support mutual co-ordination—or social timing. When support is given for social timing, and play-partners begin to feel a little more in sync, then the stage is set for shared experiences of attention and positivity.
Here we introduce the three Cs. Laurie (2021) developed the three Cs as a highly accessible way of putting the above essential ingredients of rapport into practice. With the three Cs, you can start your journey into quality interaction even when, initially, connection is very hard to find. As we describe more about Rhythmic Relating throughout this paper, every step of the way we will build on the three Cs. And, if the three Cs is your take-home message from this article—fantastic!
Each of the three Cs relates to offers. Offers are the in-the-moment interests and behaviors of your play partner. Some typical offers are rocking, humming, tapping, grabbing, dancing, singing etc. The three Cs are:
“C” the offer—to see the in-the-moment behaviors of the person as a potential starting point.
Copy the offer—to join in with what the person is doing using 100% of your attention. It is important to note here, right at the start, that our conceptualization of copying is not limited to a direct mirroring of the person’s behaviors. We can join in, in many ways. Direct mirroring is one possibility with benefits in some circumstances, limitations in others. Here, copying will include possibilities of connection which deliberately bypass or go beyond these limitations: exaggerated or diminished mirroring; rhythm matching; and vitality matching.
Celebrate the offer—to use facial expressions, body language, and tone of voice to communicate acceptance and bring warmth.
Starting with sensory stability
Sensory stability is the essential ground-zero needed for autistic people to thrive and for connection to be possible. People on the autism spectrum often experience sensory disruption and feel, see, hear, and touch the world through hyper, hypo, or otherwise disorganized senses (Leekam et al., 2007; Tomchek and Dunn, 2007; Crane et al., 2009; for overviews see Ben-Sasson et al., 2019; Kojovic et al., 2019). When acute, experience like this is often frightening (Kojovic et al., 2019) and can be traumatizing (Tseng et al., 2011; Riquelme et al., 2023; Sung et al., 2024). When this is happening, interaction is impossible. Before attempting any interaction work, we suggest that you spend time observing the person you will be working with, and talking to them (if possible) and to those that know them. What is their sensory world like, and how can you design your play space to support them? There are some simple things you can look out for and do to help (see Table 3).
Skill level 1
C (see) the offer
The first aim of Rhythmic Relating is for the two of you to be able to share the space comfortably. If your play partner is uncomfortable in your presence and inclined to move away, then interaction is unlikely. Tickle-Degnen and Rosenthal (1990) found that postural mirroring is the most effective technique for establishing initial connection and rapport. So, we need to look for postures, gestures, body language and other offers that we can copy. We need to see the offer.
Almost anything can be an offer for interaction from your play partner. Here are a few possibilities: vocalizations (a sound, something spoken); vocal patterns (humming, singing, repetitive or patterned sounds or words); movements; movement patterns (dancing, reaching, tapping or otherwise contacting their own body/your body/objects/surfaces, other repeating movement patterns); shifts in body posture/positioning/proximity; object manipulation; patterned object-play (moving, tapping, or manipulating objects); a copying of something you are doing (or have done previously), eye contact, a break in eye contact,9 an expectant pause.
To see the offer means to view the things your play partner does as a gesture of communication. For example, Matt (one of the authors here) worked with the parent of an autistic child who spent a significant amount of time playing with wooden blocks while turned to face a wall in the family home. The mother thought, “my child does not want to play with me.” Matt helped her to turn the situation on its head and read the situation instead as, “my child would like to play blocks with me on his own terms.” This subtle, but significant shift in perspective allowed the mother to see the offer.
Celebrate the offer
It’s important that we value the person we are working with; value everything they do. Implicit in Rhythmic Relating is the notion that, through their offers, our play partner is making an intentional attempt to communicate. Even if we do not understand that intention, we can act as if each offer is rich with this potential. In doing so, we open the door to interaction and promote positive feedback for further possible engagement. So, part of celebrating the offer, is developing our ability to stay with, be patient with, and find genuine curiosity in what our play partner is doing. Our interest has to be real.
It is also important that we communicate this interest in the moment. In part, we do this through copying the offer. And, in general, we bring warmth, kindness and positivity shown in our eye contact, body language, expressions and vocalizations. Importantly, this does not mean being inauthentic, inappropriately loud, using meaningless praise, or bringing a shallow level of forced happiness into interactions. It’s okay not to be a children’s TV presenter! It’s more about real curiosity about the person in front of you, really caring for them and being interested in what they do.
Copy the offer 1: direct mirroring
We want to piggy-back our play on the natural behaviors of our play partner. We want to copy and flow with what they are doing spontaneously. Why is this a good idea? Four answers…
The short answer is because copying is the most direct and effective way of making an initial connection with another person (Tickle-Degnen and Rosenthal, 1990). As a technique, it is probably the most effective way of establishing quick rapport and is used either consciously or unconsciously by most care givers and therapists (Burns, 1984).
The second answer is that this simple instruction—to copy the offer directly—gives practitioners something immediate and effective to do. Practitioners new to these ideas often worry about what they should be doing. Sometimes thinking about too many options, too many nuances, can leave us feeling stuck. Perhaps this is where you are in your development? If so, direct mirroring can help you bypass all of this. You have something simple to work with right now. And when you become more confident, you can move on to assimilate some of the more nuanced ideas described later in this article.
The third answer is that copying and joining-in can be understood as the foundation of adjusting our social timing to that of our play partner. Copying offers will immediately result in a shared pace to an interaction with similarly timed actions and pauses; an initial alignment which is likely to help our play partner be comfortable with our presence.
The last, slightly longer answer is that tapping into natural behaviors is the best way to unlock more behavior, novelty and playfulness. A study (with the fabulously apt title: Give spontaneity and self-discovery a chance in ASD) by Torres et al. (2013) showed that when we engage with spontaneous behavior patterns (often seemingly variable, seemingly random) then these patterns can morph into more predictive and reliably intentional behaviors. Engaging with spontaneous behaviors holds much more interactive potential than if we introduce new stuff from the outside.
In general, we advise starting simply. Spend some time simply sitting in the space with your partner. Keep your own energy levels low and calm, just be with them. Allow time to acclimatize and get comfortable. When we start to copy, we focus in on one particular offer, one particular aspect of our partner’s movement, sound, or object play. Here, to start off, we try directly mirroring the offer, literally adopting the same posture or doing the same movement or making the same noise or picking up the same sort of object. In the example above, Matt advised the mother to sit at a sensitive distance from her child, copy his sitting posture, and have some wooden blocks of her own to hand. With real interest in what he was doing (celebrating her child’s actions) she played with her own blocks. After some time, the child turned to her mother to connect. This was a revelation moment for her.
It is helpful to play-down the other elements of your behavior, to allow your mirroring to stand out10. You can also try exaggerating or diminishing your mirroring to accentuate the expressive or emotional tone of your play-partner’s offer
(Tortora, 2006). You might try using big, distinct movements or clear, amplified sounds—but always check, does this exaggeration feel appropriate in the moment? Or you might use theatrical diminishing, taking the mirroring inwards, small, precious and special—but again, check, is this diminishing working for your play partner?
In the moment, it will be important to try out different possibilities and learn from your partner’s response. For instance, if you mirror your partner’s offer directly, this may be perceived as simplicity, clarity, and a welcome sense of recognition. Or, it risks being perceived as a patronizing over-simplification. It very much depends on the abilities and preferences of your play partner. Always stay open to their response, and to adapting in the moment. And remember this is play, and it’s about celebrating their offer. If direct mirroring is too obvious or felt as robotic, perhaps an exaggerated or diminished mirroring might work better, or perhaps you could play with rhythm matching or vitality matching (see below)? These techniques offer a way to copy and connect with more nuance. Or, of course, this approach may simply be inappropriate for your play partner on this occasion.
Skill level 2
Copy the offer 2: rhythm matching
Sometimes, your play partner’s offer might have a rhythmic pulse. Swaying, rocking, tapping, humming, vocalizing, singing, hand-clenching, beat-boxing, hand-flapping, shoulder shrugs, walking, running, jumping, all might have a fairly regular pulse (Bakan, 2014; Daniel, 2019; Daniel et al., 2022). We can join in and match their rhythm. There’s nothing expert here! We’re just talking basic humming, singing, tapping, creating sounds with an object, very simple beat-box, or sensitive physical strokes, squeezing, pressing, or tapping on your partner’s body. You will probably have a sense of your partners preferences and can go with those. Or you can explore different options to see what resonates. Mixing and matching sensory modalities is okay too. We can hear, see, and feel rhythm (Grahn and Brett, 2007). You can match a vocalization pattern by gently pulsing your partner’s little finger; sing their back-and-forth swaying; tap out their walking pattern on a cardboard box; beat-box their shoulder shrugs; eye-blink to their humming; click your fingers to their hand-flaps.
When we hum or sing or tap out a rhythm, we may well accent a beat at regular intervals. This is intuitive and natural for many people (Grahn and Brett, 2007). The simplest form of accenting is through a relative increase in volume and/or pitch.
Audio E.G.1 Simple Time Meters for Rhythm Matching
The intuition to accent is part of our human tendency to interpret a continuous pulse as having a rhythm with a time pattern or meter (Grahn and Brett, 2007). We can be aware of this and, when we rhythm match, we can choose to use a very simple,11 regular meter. In western music tradition, our simplest meters are: 2/4 (accented STRONG-weak-STRONG-weak; defined by a march; e.g., the Darth Vader theme in Star Wars); 3/4 (accented STRONG-weak-weak, STRONG-weak-weak; e.g., a waltz); or 4/4 (accented weak-weak- STRONG-weak, weak-weak- STRONG-weak; e.g., the straight “puh-te-Kuh-te, puh-te-Kuh-te” which opens Michael Jackson’s Billie Jean).
When we rhythm match, we can use our accents to add emphasis and vibrancy to a specific moment, action, or sound at regular intervals. This can bring regularity, flow and moments of shared focus into our shared rhythmic experience. Here are a few examples using rhythm matching with accents in play:
As our partner walks the edges of the room, we tap out a 2/4 march on the seat of a wooden chair. We accent every second beat with a louder tap.
Video E.G.1. Example of Rhythm Matching in Play

Our partner is humming a relatively repetitious phrase. We gently pulse their little finger, pressing it gently in a slow 4/4 rhythm. We gently accent the 1 (in, 1 and 2 and 1 and…) by squeezing with a little more pressure.
Video E.G.2. Example of Rhythm Matching in Play

A quick note on autism and sound
Timing delays in response to auditory information have been observed in autistic individuals across various studies (Lepisto et al., 2005, 2006; Oram Cardy et al., 2005; Russo et al., 2009; Kwakye et al., 2011; Torres et al., 2023). In the context of speech discrimination studies, Russo et al. (2009) found that the sensory experience of autistic children without background noise closely resembles that of typically developing children with background noise. Most autistic people find loudness intolerable (levels exceeding 80 dB, equivalent to shouting) (Khalfa et al., 2004), and some exhibit hypersensitivity to particular high-pitched sounds at volumes within the normal-to-mid-range (Rosenhall et al., 1999; Takahashi et al., 2014, 2016).
In support, we can use clarity and simplicity in our speech and choice of words; be patient and give time for response; avoid monotone and use a melodic “story-teller voice” or consider singing aspects of our communication; play with using rhythm matching (with simple meters and well-spaced timing); add further sensory impetus (see below); and maintain overall low-volume levels (we always suggest starting quietly, tailoring our ground-zero-volume to our partner’s needs, often below average conversational volume, i.e., <60 dB).
There’s something powerful in connecting with the rhythmic elements of autistic behavior and, also, in the possibility of adding rhythmic and melodic aspects to our communication. Rhythmic regularity helps people to predict what’s coming just-ahead-in-time12, and this holds true for autistic people (Knight et al., 2020). Knight et al. (ibid.) demonstrated that autistic individuals (6–21 years old), displayed no difference with encoding temporal patterns and predicting upcoming auditory events (in comparison with typically developing peers) with sounds presented in the context of simple or complex rhythms. The rhythmic context (ibid.) also nullified the delay effects demonstrated in relation to perceiving isolated sounds shown in previous studies, including, Lepisto et al., 2005, 2006; Oram Cardy et al., 2005; Russo et al., 2009; Kwakye et al., 2011; Torres et al., 2023.
Copy the offer 3: vitality matching
We copy the offer in order to connect with our play partner and to try to communicate that we care about their choices and interests. Yet we risk over-simplification, patronizing communication, or bringing a kind of dryness to our approach and losing our playfulness. Another option, is to try matching our play partner’s vitality rather than attempting a direct mirror.
Vitality is the energy, feel, style, flavor of any particular movement (Stern, 1999, 2010). It is the how in the way we do something. It communicates intention, feeling, force, and the direction of our actions. For instance, you could wave your hand vigorously with excitement in greeting a friend; or slowly with tentative sadness when your friend leaves; or in tick-tock staccato to a house music beat; or in chaotic desperation in fear and panic. In these examples, the action of hand-waving is basically the same, it’s the vitality you have changed.
We experience vitality with our whole being. A vocalized “wuuuUUUPP” can compel us to stand; make an energetic star-jump and you may well feel to vocalize with a “Huh!”; on seeing a dancer slide gracefully across the floor you may well match that slide with a vocalized “aahhhh.” Vitality can be experienced, communicated, and perceived via any sensory modality or combination (Malloch and Trevarthen, 2009).
We can copy an offer by matching and expressing the vitality communicated by our play partner.13 We can match our partner’s vitality in any modality (or combination of modalities) that feels right in the moment and appropriate for our partner—we are not limited to a direct mirror. This is brilliant for two reasons: 1. We can connect without risking patronizing over-simplification; 2. We can join in with the energy and intensity of our partner’s behavior without overinvesting in any particular emotional tone (allowing us authentic connection without adding fuel to negative emotions). As with direct mirroring, we can also play with exaggerating or diminishing our vitality matching. Here are a few examples of vitality matching (from Daniel et al., 2022).
Within the same sensory modality…
Video E.G.3. Example of Vitality Matching in Play

Violent jumping-stomps matched by star jumps (matching the energy release without turning up the anger).
Video E.G.4. Example of Vitality Matching in Play

Across different sensory modalities…
The arc of a repetitive arm movement matched by “WWWOOOhhhhhh” vocalized contours, falling in pitch and volume.
Video E.G.5. Example of Vitality Matching in Play

A withdrawing sad vocalization matched with a sensitive whole-body folding over and inwards.
Multimodal expressions…
A toy car is energetically pushed back and forth, matched by swaying vocalizations (rising then falling contours of sound) and accompanied by left-to-right body swaying from our sitting position.
Video E.G.6. Example of Vitality Matching in Play

As our partner hums repetitively, we rock our upper body back-and-forth in sync, while drumming on the floor in time to the repeating pattern.
When a game develops 1: 100% client-led
When we are patient and practicing deeply with the three Cs, we often find that two-way interactions simply emerge from the shared communicative space without us needing to contrive our actions. We trust the three Cs and do what we feel is natural. We copy our partner’s offers, wait, and celebrate. Often, at some point our partner will engage with either a slight change in their behavior or a new offer. We copy that new offer and what emerges can become a client-led back and forth interaction. It’s the beginnings of reciprocity, turn-taking and creativity in play. Here are a few examples:
Video E.G.7. Developing a Game through Mirroring

If our partner is stepping back and forth across a line in the floor boards and we copy this movement for some time, perhaps they pause, then we pause too, then they start again, but this time with a little smile. We do this many times, feeling more and more in sync.
Video E.G.8. Developing a Game through Vitality Matching

If two-way interactions are not emerging from the three Cs, we suggest thinking about the following. Firstly, consider the sensory environment and check if anything can be done to help (see Starting with Sensory Stability, above). Next, consider your physical proximity to your partner. Perhaps you are too far away for connection, or too close for comfort? Or perhaps your body and/or eye-line is uncomfortably above your partner? If so, adjust to match their eye-line. Or perhaps your body language is too complex, or physically or emotionally unavailable? If so, minimize, simplify, listen deeply, and be curious about your play partner. Or, as often is the case, perhaps you are failing to leave space for your partner to respond, going too fast? If we are a little impatient, we may well find ourselves out of sync with the other person and may well have missed an offer. Often, if we wait a little longer without adding anything, we find a response emerges. Being truly patient is probably the most powerful and challenging thing we need to learn.
When a game develops 2: “Yes and…”
Another way to conceive of how to support flowing interaction is through the process, “Yes and…,” a key activity in improvisational theater (Salinsky, 2017). Here, we welcome what our partner offers (“Yes”), expand on that action or theme just a little (“and…”) and then allow space for our partner’s response. Our play partner might pick up a yellow bucket and tip some sand out of it… we could “Yes and…” this offer by finding our own bucket and tipping sand slowly… our partner may “Yes and…” this offer by catching the sand with their bucket and tipping it again… we could “Yes and…” again and catch the sand once more. When offers are responded to by actions that say, “Yes and…,” then something remarkable happens: people start to want to add to the unfolding story, to build a story together, and it feels good!
In almost all contexts, it is crucially important that our “Yes and…” represents just a small variation on the theme of our partner’s offer. If our trusting relationship is very well established, big surprises and novelty can work (we do not rule this out 100%). But in almost all contexts if we over-step the mark, introduce too much novelty or too much of our own agenda, we risk shocking our partner out of connection and rapport. So, as a rule of thumb, “Yes and…” just a little.
But if our “Yes and…” does miss the mark, if our partner disconnects or if the flow becomes stilted or stuck, then we simply go back to listening carefully and trying to see the next offer. In this way, we repair the rapport. Tronick and Cohn (1989) found that typically developing infants regularly experience interactive miscoordination, yet mismatch is typically repaired close-on-instantaneously. “This constant oscillation between momentary miscoordination and interactive repair marks the essence of human dialogue, to which infants are sensitized in their earliest interactions” (Feldman, 2007b, p. 341). It can be very helpful to know that the flow of mismatch-and-repair is normal, and is itself very supportive of your developing relationship (Tronick and Cohn, 1989; Feldman, 2007a,b; Hughes, 2011).
Sometimes, as in the yellow bucket example above, your partner’s offer might be an action involving an object. But more often it will be an aspect of movement, expression, positioning, sound or rhythm. Here, it might feel less obvious how to “Yes and…” without changing things too much or adding something entirely new. We can find our way forwards by modifying our copying (our mirrored movement or sound or rhythm) just a little; one quality at a time. We see the offer, copy the offer, wait for response, and then copy the new offer but with a slight twist in the style, or the energy involved, the mood, the positioning, the physicality, the volume, the tempo etc. This is a big topic! Here, we offer a few pointers in some directions you might want to explore (see Table 4), followed by an example in play.
Video E.G.9. Using ‘Yes…and’ in a Developing Game

When a game develops 3: the common third
If things feel stuck, we can wait and be with that uncertainty. When we keep sensitively with the three Cs, staying open and giving more time, our partner usually finds a way. Play usually flows when we are led by our partners offers as to the nature of our “Yes…and.” Sometimes though, if we are experimenting when things feel stuck, we can try initiating a common third: “the common third is about creating a commonly shared situation that… brings the two of us together. It allows us to share an activity in a way that we can both be equal, two people connected by something we both enjoy doing.”14 Crucially, any common third needs to be communicated as an offer rather than as a demand. Our partner has to be genuinely free to choose how to respond to it, or indeed free to not respond at all.
There are two possibilities here. You could offer something that you know your play partner likes, something from their behavioral repertoire, something that you have seen them like or do before. This may be a movement, sound, object, game or activity, as long as it is something you know your partner likes. Or you could offer something neutral, a bridging gesture which hopes to extend an opportunity for connection. A simple offer of a common third may be to place our open hand halfway between ourself and our play partner. We leave no sense that our partner has to do anything with it, all the while extending an opportunity for connection that was not previously present. Keeping simple, neutral and low-arousal are good rules-of-thumb here. This gesture may be received as a demand, if the hand is extended too far into our partner’s personal space, if we touch our partner without consent, or if we say something like, “it’s your turn.”15 We know if our partner has received the gesture as a demand if they turn away, while an offer is much more likely to engender a turning-toward. And, as always, we need to be responsive to the need to repair our connection by returning to the three “C’s” if we miss the mark.
When a game develops 4: loops and pregnant pauses
A loop is a section of behavior or play which repeats itself, more or less, over time. Autistic people sometimes display looping behaviors: specific repetitive movements and/or sounds often referred to as “stimming” (Bakan, 2014; Kapp et al., 2019; Charlton et al., 2021; McCarty and Brumback, 2021); ritualized play patterns with hyper-focus on a particular sensation, sound, pattern or object; certain configurations of object or relational play (Daniel, 2019; Daniel et al., 2022).
As practitioners and carers, our response to loops will depend on the felt experience for our play partner in any one moment, including observable levels of dysregulation. A good example of the sensitivity needed here is in regards to stimming. Stimming is a commonly used term for “stereotypies”: semi-voluntary, stereotyped repetitive movements. Throughout the autistic community, stimming is widely reported as self-regulatory, self-calming and enjoyable with the only real stressful downside occurring if non-autistic people try to suppress, prevent or ridicule the actions (Bakan, 2014; Kapp et al., 2019; Charlton et al., 2021; McCarty and Brumback, 2021). In general, we should enable and support stimming. However, autistic adults report how stimming can, on occasion, become out of self-control and lead to intensity and dysregulation (Kapp et al., 2019; Charlton et al., 2021; McCarty and Brumback, 2021). We need to be sensitive to this possibility with all looping behaviors. If our partner is clearly dysregulated, if the intensity is cause for concern, or if they have expressed a prior wish for intervention, then we suggest direct (not directive) engagement with loops. Sometimes, when an autistic person is in a loop, they want to, but cannot stop. They may want your help in facilitating body-safety and control. In which case you can offer a behavior (remember that an offer is an action which the child can choose to engage with, or bypass easily otherwise). Here, our offer will have the potential to catalyze a change in the looping pattern, to unravel it or develop it in a different direction. Our play partner’s body and sensorimotor patterning knows what is best, yet might be stuck… For instance, if our partner is engaged in an intense pacing up-and-down the room we could try standing in front of them, or walking in front but slowing or changing the rhythm or style of our walk (this can shake-up the motor pattern), or standing or sitting close-by and engaging in something we know might grab our partner’s attention; or placing an object in the path of the looping behaviour (something which we know is of interest to our partner). If the loop changes, we can follow and flow into interaction, if there’s simply too much intensity, we can model self-calming behaviour, slowing down alongside our partner, breathing deeply, whole-body sighs, collapse gently to the floor if this feels right.
For the most part, when loops are positive or neutral experiences for our partner, we can engage in loops as wonderful way to connect and develop games. Loops have a type of rhythm (Malloch and Trevarthen, 2009; Bakan, 2014; Daniel, 2019; Daniel et al., 2022). Repetitive movements and/or sounds have their own regular beat-pattern. Looping behavioral patterns or object play have discrete behavioral steps which repeat in pattern. Our spontaneous response to feeling a pattern or beat, “is often to move at the time when the next beat is predicted” (Grahn and Brett, 2007, p. 902).16 In this way, loops have a kind of impetus and flow which helps us know what comes next, organize our actions just-ahead-in-time, and compel us into our next action.
Each loop is self-completing. By this we mean that each beat or behavioral step in the loop is essential to the feeling of rhythm, the impetus and flow, and the overall sense of completeness. If our actions become part of the pattern, replace a beat or behavioral step, then we can become essential in that rhythm and for that sense of completeness; a necessary link in our partner’s loop. From there, we can sensitively encourage the loop toward interaction and away from solo play. Working within loops in this way represents an autism-specific and powerful interpretation of the common third principle in action. Here are a few examples taken from Daniel et al., 2022:
Video E.G.10. Becoming Part of a Loop

Our partner is sitting and rocking back and forth. On every fourth rock forward, we offer both our hands out, close enough to be held, not too close as to be a demand. Perhaps, after sometime, our partner reaches out and we move into rocking together hand in hand. From here we can play with all kinds of qualities of shared movement and sound.
Video E.G.11. Becoming Part of a Loop

Our partner is pushing a toy train around a track in a loop. Each time the train passes the station, we start to run a toy car alongside (just behind) the train. Perhaps this becomes a race, or a crash, or our partner takes the car on as a passenger.
When our movements or sounds have become integral to our partner’s loop, occasionally and sensitively we can start to play with pregnant pauses. Sometimes we can deliberately interject a pregnant pause into the rhythmic flow of our partner’s behavior. A pregnant pause holds a silence longer than the natural on-beat demands. It has the energy of needing to be filled. A beat or behavioral step is expected, and movement is compelled.
Pregnant pauses are an unexpected novelty for our partner. As such, we only recommend experimenting with them when your rapport is strong and you share familiar loops or behavioral patterns. We can use a pregnant pause to replace an accent in rhythm or movement, or to replace any sound or movement which has become a link in our partner’s loop. When we leave a pregnant pause, with all the rhythmic build-up of impetus, flow and expectation, something is going to happen! And often it is fun and interactive and full of potential for us to build on together.
It is surprising how long we can hold a pregnant pause for, when waiting for our partner’s response. We can play with the held duration of a pregnant pause over a range up to around 6 s. If we push the duration much past this range, we lose the rhythmical impetus of the “here and now.” “The current consensus in music psychology and cognitive neuroscience is that the ability to associate beats, or perform them meaningfully as a pulse, stops at around 6 s or 0.16 Hz… It is at this point that the mind and body can no longer “lock on”—either actively through playing, or passively through listening—to the rhythm as a pulse” (Osborne, 2017, p. 18–19).17
Skill level 3
Here, the concepts we introduce begin to get a little more complex. If you feel you have enough to be getting on with, feel free to take a pause and spend some time trying out what you have already read about in practice. You can come back here any time.
We will now go on to talk in more depth about social timing, about the way neurotypical and autistic people organize their intentional movements, and then develop the use of activation contours and accents. These tools will help bring an extra level of sensory impetus and shared timing into our interactions.
Good-enough social timing
To be very clear here, when we talk of social timing we are not aiming for spot-on alignment or consistent synchrony in our shared experiences. In play with typically developing infants, partners flow in and out of levels of synchrony (Feldman, 2007b).18 This is natural and developmentally important (Feldman, 2007b) Sometimes partners do fall directly in sync (such as the co-occurrence of social gaze, vocalizing together, the matching of arousal level, or the coordination of parent affectionate touch with infant social gaze). More often partners experience a kind of complementarity; playing and teasing around a game within a shared time-frame (Feldman, 2007b). And indeed, partners are often out of sync, leading naturally to moments of interactive repair which constitute important practice steps in the development of robust social skills (Tronick and Cohn, 1989; Feldman, 2007a,b). We are looking for good-enough social timing.
We need to share moments where our intentional actions meet. Sharing our intentions, through timing and sequencing our actions together, is crucial for the development of togetherness and meaning (Fantasia and Delafield-Butt, 2023). For example, if your partner is reaching to touch a ball, then truly connecting means to share that intention, to move together toward it, to touch the ball together in space and time. Or if you are playfully high-fiving, then connecting means you share the intention and organized movement patterns which bring your hands together. We need also to share flows over time in which our intentional actions are complimentary, run alongside, connect with and develop each other. For example, if you are rocking together, then connecting means that both of you share the intention, the rhythm, and the movement flow which enables your bodies to be coupled in that movement. If your partner is walking slowly, then connecting means to share the arc of your steps together; arcs which culminate in your steps happening together.
The way we organize movements and social timing
Neurotypical individuals organize their intentional movements just-ahead-in-time. A motor image is generated in the brain just before action. It’s a neural image full of predictive information. In reaching out an arm to grasp an object, for example, the motor image pre-empts the movement needed and builds in an integrated sense of specific intention (i.e., to reach the object in a way advantageous of the type of object and the intended relationship to the object—is it heavy, delicate, difficult to hold, full of water; is it to be held gently, turned upside down, squashed?).
Often, these motor images are contours; contours of energy, direction, force, and intention (Lee, 1998; Stern, 1999; Rousanoglou and Boudolos, 2006; Delafield-Butt et al., 2018). In our reach-to-grasp example, the motor image is a contour of energetic enervation: rising on initiation, increasing to reach, falling in expectation to control the timing and nature of contact with the object. The contour gives the organization and sensory impetus for the action; an action with an intentional goal in space and time. When actually reaching for that object in real time, neurotypical individuals activate the motor image, start to move, and then use continuous sensory feedback to adjust and assess the image in action. It’s through combining our motor images (movement contours) with our real-time sense of an object in motion (perceptual contours), that we are able to sync-up with that object when, for instance, catching a ball (Lee, 1998). And, most importantly for us here, it’s this coupling of movement and perceptual contours which enables neurotypical individuals to couple their moving actions with those of another person (Lee, 1998; Rousanoglou and Boudolos, 2006; Delafield-Butt et al., 2018). It’s when our intentional actions are aligned in this way that we are able to feel and share the intentions inherent in another person’s actions. Good-enough social timing and our shared sense of play relies on this happening over and over in real-time.
Importantly, autistic individuals have challenges in integrating information for the generation and implementation of motor images for intentional actions19 (Mari et al., 2003; Rinehart et al., 2006; Cattaneo et al., 2007; Fabbri-Destro et al., 2009; Chua et al., 2022; Fourie et al., 2024; for an overview, see Daniel et al., 2022). In play with autistic partners, we need tools to magnify the clarity of our actions, the clarity of our own intentional movements. We also need tools to help our autistic partners organize, enact and compel their own intentional motor contours. And we need tools to bring our movement contours into alignment for good-enough social timing.
Activation contours
In the introduction, we mentioned an Edinburgh study in which sound tones were used to help people with cerebral palsy control their reach-to-grasp arm movements. How did this work? The sound used was a tonal flow, a “wooooOOOOOooooo” type sound, flowing up and then back down to stillness. This tonal flow was designed to imitate the neural flow of energy used by animals of all kinds when generating action (Delafield-Butt et al., 2010, 2012), including human babies (Delafield-Butt et al., 2018) and in creating music together (Schögler et al., 2008). When the tone was paired with the participant’s real-time attempts at reach-to-grasp., the tonal flow gave impetus and organization to the participant’s motor image (their motor contour). The results showed greatly improved movement control and synchrony (Schögler et al., 2017). The type of sound tone used in these studies is an example of an activation contour.
Activation contours contain, “…the felt experience of force… with a temporal contour and a sense of aliveness, of going somewhere” (Stern, 2010, p. 3).20 Importantly for us, in “going somewhere,” they point toward, and compel us toward a point in space and time and a focus for intention. They contain a sense of what’s coming next. Activation contours are often bell-shaped curves (like the sound tone described above, or the motor contour when we reach-to-grasp an object). They can also be upwards contours (starting low in energy and rising to a point in space, time, and intensity) or downwards (starting with higher energy and falling or fading to a point in space, time, and intensity). Activation contours can be expressed in any sensory modality or combination of modalities. Here are a few playful examples of the use of activation contours to facilitate shared moments, flows, and intentions:
If our partner is reaching to touch a ball, we can playfully exaggerate our own movement arc toward the ball and add a “wwoooOOOOOoooo” sound effect (a bell-shaped acoustic activation contour), all in real-time sync with their intentional movement. Our movement and sound contours match and guide our partners movement contour, with the climax of all contours landing exactly on contact with the ball.
Video E.G.12. Using Vocal Activation Contours

If our partner is walking slowly, we can match the movement contours of each step (and the overall rhythm) with theatrically diminished steps of our own. We can emphasize each movement contour, and the moment each step contacts the floor, with precise, careful, magical tip-toeing. And we can add further sensory impetus, clarity and mood through adding whispery “SSSSSSsssshhhhh” falling sound effects as acoustic activation contours guiding each step to contact with the floor.
We can also play with tactile contours—carefully, and always checking if physical contact seems appropriate in any one moment. We can generate supportive felt contours in our partner through touch or through supported movement.
Video E.G.13. Using Tactile Activation Contours

Next, we explore the types and qualities of sound which are particularly useful when using acoustic activation contours with autistic individuals. We will also build on our concept of accents, which will be particularly relevant when we go on to develop our practice of rhythm matching a little later on.
The neuroscience of sensory impetus in sound
Recently, a computational method for automatically categorizing music (X-System), has demonstrated success in predicting emotional, arousal, and mood responses to music (Sice et al., 2020). The X-System analyses distinct attributes of sounds that humans have evolved to be moved by. These sounds include the acoustic startle response (ASR) and acoustic activation contours. They elicit highly immediate and direct movement responses.
The human acoustic startle response (ASR) represents a neurophysiologically immediate reaction to sudden and unexpected auditory stimuli.21 Acoustic activation contours are evolutionarily significant sounds indicative of the positioning and movement of the human body in space and time (from sudden approach, to slowly moving away). These include separation cries (Panksepp, 2003), or the hissing of snakes (Erlich et al., 2013), and rapidly approaching sounds, glides, falling, fast crescendos, or bursts of sound (Osborne, 2009).22
Above, we mentioned various studies which have reported auditory timing delays in autism. If used sensitively, it is possible that we can use the activation and immediacy of acoustic activation contours and ASR-type sounds to bypass the neural pathways involved in these delays (see Daniel et al., 2022 for a detailed analysis). If so, we may be able to add clarity and impetus to our own movements and sounds, and augment our partner’s offers of movement and sound, in ways more immediate, more in sync, and more compelling than otherwise available.
In music, the sensitive use of such activating sounds involves modulation, context, timing, and expectation. With this sensitivity, music deliberately takes the “dangerous” edge off acoustic activation and startle (the fight/flight/freeze edge) and leverages that energy for joy (bursts, fast upwards contours, energetic building crescendos, sounds full of fun, randomized internal movement), wonderment (subtle downwards contours, quick fades, quiet hisses, whispers, jangles), and anticipation (contours in volume and pitch leading somewhere, pregnant pauses, shifts in tempo) (Osborne, 2020).
If we want to play with using activating sounds in support of social timing, we need to be conscious that these experiences are on the edge of edgy! They are neurologically reminiscent of fight/flight/freeze sounds. This is what makes them activating and exciting. But we need to be mindful of the parameters that make them fun as opposed to scary. For autistic people specifically, what takes the dangerous edge off these sounds, and makes them sounds of delight, intrigue, and anticipation is: a safe relationship; familiarity in context; familiarity of patterns of behavior and play (repetition23 and small-step changes); low-level ambient and spoken sound (<60 dB); and relatively low-level accent volumes (relative acoustic startle—just above 60 dB) (for further detail see, Daniel et al., 2022).
Accents and acoustic activation contours in play
Perfect for acoustic activation contours are any sounds with lots of inherent movement: glides (“sssssssooooo”); crescendos (including upwards “whhhHHOOOOP” effects, animals “wooOOF,” growl, squark); decrescendos (including downwards expressive sighs, “ZZZEEEEeeeooooo” effects); variations on playful hissing (“ssss,” “ssshhh,” blowing sounds); sounds with high levels of randomized internal movement (bubbling noises, raspberries, tongue wobbles); contoured spoken words; contoured sound effects (cars going past go “vrroOOOoom,” contoured “woooOOOOooo” effects; cartoon characters are literally designed for this, Scooby-doo’s “zzoiiEEKKS! for example; and if you can manage an elephant trumpet…!).
We can explore the playful “shock” factor of relative acoustic startle in our accents. We can choose our tones and relative volume levels to modulate ASR-type effects down to happy-startles—sonic moments full of wonder, surprise and delight. Initially, just over 60 dB, we can consider using drum-like bass tones; the quick hiss-factor of a high-hat-like-tone; the surprise of a higher-pitched machine-like pulse (e.g., a laser gun sound effect); “magical” pulses like bell and triangle tings; animal noises; cartoon character refrains (Homer Simpson’s “Doh!” as a perfect beat); machine and vehicle sounds; impressions; and focus words.
Accents and activation contours in rhythm matching
Developing our examples from, Copy the Offer 2: Rhythm Matching, we now include the sensitive use of accents with relative acoustic startle…
Video E.G.14. Using Accents in Rhythm Matching

In our example where our partner is walking, we keep tapping out the 2/4 march but replace every other beat with a relatively loud, comical “Oooh!.” We get, tap—“Oooh!”—tap—“Oooh!”—tap.
Video E.G.15. Using Accents in Rhythm Matching

Or we can turn the volume down low in gentleness and magical intrigue…
Our partner continues humming. We continue to gently pulse their little finger, pressing it gently in a slow 4/4 rhythm. Every fourth bar, we add extra accent to the already accented 1 by blowing on our partner’s hand (a tactile accent with a whispery sound).
We can also play with replacing a few beats or a full bar of our rhythm pattern with an activation contour. The contour could be in sound, or movement, or touch, or any combination. Such contours will guide us and our partner to land together on-beat with our accent. They compel us to move together.
Video E.G.16. Using Activation Contours in Rhythm Matching

Loops and activation contours
A powerful use of activation contours is in combination with our link role in our partner’s loops. We can use activation contours to compel our combined attention toward the link, and give energy and impetus to that shared moment. Developing the above examples from When a Game Develops 4: Loops and Pregnant Pauses, we add activation contours:
Video E.G.17. Using Activation Contours as part of a Loop

Our partner has, after some time, taken our hands and we sit facing each other and rocking back and forth in a shared rowing motion. We add upwards, then downwards vocal contours to accompany each forward and backward motion (as experienced by our partner). We use whispery, soft timbre sounds like waves as they move in and out on the shore.
Video E.G.18. Using Activation Contours as part of a Loop

Our partner has developed the moment of meeting between their train and our car into a regular crash point in their looping play. To add energy and impetus, as we move the car up toward the crash point, we add the cartoon sound effect of an object falling from the sky, “SSSHHHOOoooowwww”! The lowest point of the contour is synchronous with the crash point.
Loops, activation contours, and changing things up
Along with activation contours, we can start to play with replacing our link-moment in our partners loop, with a pregnant pause. Developing two of the above examples:
With the impetus of the car moving and the accompanying vocal contour the car crash becomes essential and expected, but this time we playfully stop just before the crash (our play partner looks surprised and engages in a brand-new way).
Video E.G.19. Using Activation Contours and Pregnant Pauses as part of a Loop

Or, instead of a pregnant pause, when we have a good level of rapport and a well-established loop, we could playfully, cheekily, do something completely unexpected! Developing two of the above examples:
Video E.G.20. Using Activation Contours and Novelty as part of a Loop

Our link has brought us and our play partner together in a hand-held rocking motion, but this time we just fall over to the side in the middle of it all (our partner laughs and interacts in a new way).
All these examples come with some risk of shocking or jarring our play partner out of an easy flowing connection with us. But, done sensitively the risk can have a huge pay off with new levels of play and relationship. And please remember, if that jarring does happen, we can simply return to the three C’s and repair our connection. No worries.
When a game develops 5: the story of a game
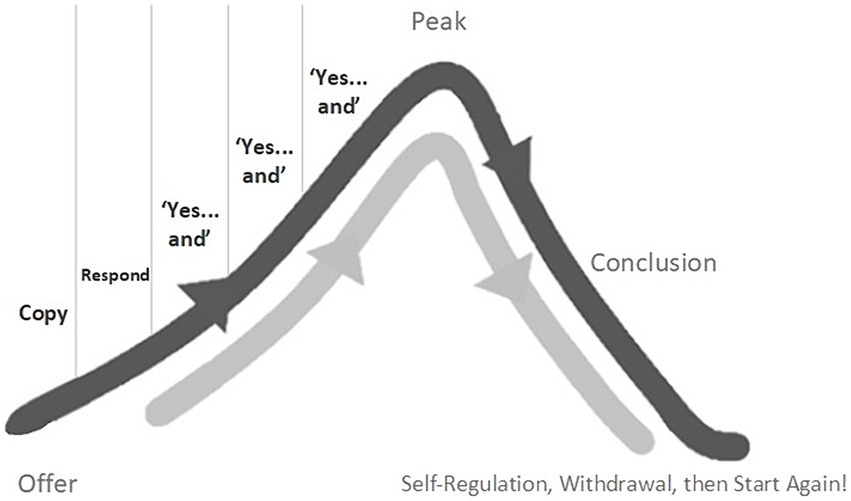
Stern (1974) described how, in the first 18 months or so of typical development, play between young children and their carers often takes the loose structure of a skewed bell-curve (see Figure 1).
After an initial orientation around an offer, Stern noticed that games tend to build with a phase of growing momentum, flowing up toward a “peak.” He also noted that, nested within this phase, play partners tend to share several “Yes…and” moments which provide the impetus of the game. Each “Yes…and” tends to build just a little on the previous, adding a little more intrigue, curiosity, energy, often developing the shared movement or sound just a little. This natural pattern of early game play supports social timing between play partners in several ways: impetus is added through each “Yes…and”; the expected structural flow helps partners feel and anticipate what is coming next; moods are built and shared, each with their own felt ambiance and expectations. If we play with these elements in our interactions, we will be helping our autistic partner to feel what’s about to come next, to anticipate it, to plan their actions, and to feel in sync with us.
Significantly, these “Yes…and” build-ups do not go on indefinitely. They form units or “stories” that start, build, reach a peak-moment of excitation, then draw to a natural conclusion (Stern, 1999; Malloch and Trevarthen, 2009; Delafield-Butt, 2018; McGowan and Delafield-Butt, 2022). Typically developing young children regulate these bell-curves of play by switching their behavior at the “peak.” If the game has been defined by increasing joy, perhaps the peak will be in laughter and physical release. If the game is one defined by magical curiosity and whispery wonderment, then perhaps the peak is in quiet shared eye contact or a whole-body contraction. If the game has been an exploration of anger, perhaps the peak is in a burst of physical power and release or a pushing out or through.
After the peak, typically developing young children will initiate a short period of conclusion and then withdraw from active interaction; a chill-out self-regulatory moment. But they are always ready for the next round, often literally with the words “again, again!” These moments of chill-out withdrawal are crucial practice-steps in the child’s journey toward self-regulation.
In our play with autistic play partners, it’s essential we get to know and feel the differences between these positive periods of self-regulation and moments in which our “Yes…and” offers have missed the mark. The former are usually short periods of active down-time. Your partner might turn away briefly, break eye-contact, and relax their anticipation. But throughout, there is a sense of healthy connection. Your partner will be immediately back to engage when they are ready. The latter (a moment in need of repair), will probably look more obvious and will lead to a more significant jolt in the interaction. Your partner is likely to disengage for longer, distancing themselves, the mood shifting; they might perhaps move away or engage in something entirely different.
It’s also important we understand that it’s likely our autistic play partner will find it difficult to instigate chill-out moments for themselves. We can model self-regulatory behaviors as part of our play.
Video E.G.21. Using ‘Yes…and’, and Modelling Self-Regulation

Discussion
In this article, we have presented Rhythmic Relating in support of playful interactions between autistic people and non-autistic carers or practitioners. Rhythmic Relating is based on a growing body of literature that recognizes that autistic social difficulties stem from more basic sensory and motor differences. These sensorimotor differences directly affect embodied experience and social timing in communication. The Rhythmic Relating model focuses on bidirectional support for the fundamentals of sensorimotor organization; addressing interactive mismatch and facilitating expressive action, social timing and play. When autistic people and their play partners are experiencing good-enough social timing—experiencing shared moments, flows and feelings—then the dynamics are right for rapport, co-regulation, shared meaning and learning.
Rhythmic Relating aims to give you skills to support your own creativity, insights, and personal sense of fun and humor in play. Please use what works for you, taking the model as a starting point and not a limiting prescription. The model is offered as a foundation for interaction and learning, as a base practice in schools, for Occupational Therapists, Speech Therapists and Physiotherapists, and can also provide a basis for tailoring creative arts therapies when working with autistic clients. To date, two pilot intervention studies have demonstrated significant positive effects on Emotion Regulation measures for young autistic children receiving a combination of Rhythmic Relating and Child-Centered Play Therapy (Daniel et al., 2023; Daniel et al., 2024b). Future work with case study designs and large-scale efficacy studies will support improved understanding of the role of timing and shared social rhythms in autism interaction, while also testing and developing the Rhythmic Relating model across a range of contexts.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SD: Conceptualization, Writing – original draft, Writing – review & editing. ML: Conceptualization, Writing – original draft, Writing – review & editing. JD-B: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
Immense gratitude for all 18 co-authors on the original Rhythmic Relating theory paper (Daniel et al., 2022) for their input in developing the ideas underlying the Rhythmic Relating model presented here. A huge thankyou to the following people for their insightful feedback in the development phase of Rhythmic Relating: Jess Lane and Sam Gilronan (Principal) at Glendinning Academy, Newton Abbot, UK; Dawn Mallon, Adrian Pierce, Debbie Hobson, and Jo Howarth (Headteacher) at Mossbrook School, Sheffield, UK; Suzi Tortora; Charles Gallagher; Pat Amos; and Lea Zaccari. And, thanks so much to Lea Zaccari in creation of the online video examples. All the examples featured in this manuscript (video and text) are generic. They are inspired by the themes and content of real play sessions, informed by clinical practice, but are non-specific and non-identifiable to any one person. Both video participants (Lea Zaccari and Stuart Daniel) have given their full permission to appear publicly (on YouTube) and via the platform of this article.
Conflict of interest
ML was employed by the Concept Training Ltd. SD and ML were self-employed as freelance therapy/education practitioners.
The remaining author declares that the research was conducted in the absence of any other commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1. ^The authors note the sensitively evolving nature of the discussion on societal, community, and individual preferences with regards to the choice between person-first and identity-first language when talking about autism. For an overview of this discussion, please see: https://www.nih.gov/about-nih/what-we-do/science-health-public-trust/perspectives/writing-respectfully-person-first-identity-first-language.We note that at all times, individual preferences should be prioritized in direct communication. When writing, it is necessary for authors to choose an option. Currently, many sources of information and opinion are focussing on a very positive community trend opposing deficit or disorder models, and drawing attention to autism as a highly positive factor of identity. For an overview of this position, please see: https://www.autism.org.uk/what-we-do/help-and-support/how-to-talk-about-autism.As such, we will be employing identity-first language throughout this article. We ask for your patience and understanding if this is not your personal preference. Thank you.
2. ^Social Timing encompasses the processes and information-sharing involved in the meaningful organisation and production of temporally-matched movements and sounds that occur during interaction.
3. ^The Double Empathy Problem (Milton et al., 2022) and the Interactional Heterogeneity Hypothesis (Georgescu et al., 2020), predict that social timing challenges in autistic/non-autistic pairings are due to interpersonal mismatch, as opposed to impairments isolated in the autistic partner—a prediction supported by evidence that autistic people prefer interactions with other autistic people, finding the quality of interaction considerably easier, from Sasson et al. (2017), Bolis and Schilbach (2018), Morrison et al. (2019), Crompton et al. (2020), Georgescu et al. (2020), and Wilson and Bishop (2021).
4. ^We encapsulate wellbeing as the movement towards happiness and good-enough self-regulation which, in turn, we define as, the ability to attain and maintain a good-enough state of arousal fit for task/environment/moment. Here we are suggesting co-regulatory play is the primary way human children learn to self-regulate. Through physical touch, voice and early play, typically developing infants and their attachment figures engage in co-regulation. Co-regulation is when one person’s nervous system supports another’s nervous system through vulnerability and back to okayness. During play, typically developing infants regularly push the boundaries of safety—think of the uncertainty inherent in the games “hide-n-seek” and “peek-a-boo.” In these games, the infant and adult travel together, through vulnerability (on the edge of dysregulation) and back down to good-enough regulation. In this way, the infant’s nervous system is trained to down-regulate to healthy working levels (Porges, 2021). Co-regulatory play relies on a degree of togetherness between play partners; good-enough social timing is essential. We also note there are other routes to learning to self-regulate, such as, time with a calming animal, sensitive physical pressure, taught techniques (such as breath work), rhythmical physicality etc. These can also be highly beneficial for autistic individuals.
5. ^Movement is fundamental to the human mind. Through movement we experience, interact, and learn. Importantly, neurotypical people generate movements expectant of their sensory contingencies, their sensory responses. For example, it is neurotypical to grasp a glass of water expecting that glass to be at the end of a 1-s-long reach, with its familiar tactile sensations and resistances at the fingertips, and weight of the glass as it is picked up to drink. This fundamental prospective sensorimotor organisation is impacted in autism. Evidence demonstrates that movements are organised with a different awareness of their anticipated effects in autism (for an overview, see Torres et al., 2013; Delafield-Butt and Trevarthen, 2017; + click here for a latest exploration of The Movement Perspective online), which challenges expectations and can lead to distress, social timing challenges, and withdrawal (for an overview, see Trevarthen and Delafield-Butt, 2013).
6. ^Social Motor Synchrony (SMS) challenges have been demonstrated in various studies involving mixed pairings (autistic/non-autistic) as opposed to non-autistic pairings (Fitzpatrick et al., 2016; Kaur et al., 2018; Su et al., 2020; Zampella et al., 2020; for an overview, see Glass and Yuill, 2023; McNaughton and Redcay, 2020). The degree of SMS disruption evidenced in autistic-non-autistic interactions correlates significantly with severity of autism diagnosis (Nebel et al., 2016; Kaur et al., 2018; Su et al., 2020; for an overview, see McNaughton and Redcay, 2020).
7. ^Synchrony is associated with rapport (Tickle-Degnen and Rosenthal, 1990; Au and Lo, 2020), and is quickly disrupted by its opposite: social anxiety (Asher et al., 2020). Many of the above reported SMS study designs (see footnote 6) did not allow for acclimatization, sensory stability, or relational rapport (for an overview, see Glass and Yuill, 2023). When these factors were facilitated via familiar settings, personalized tasks, and relationship building (ibid.), and in particular, via the Intensive Interaction model (Glass and Yuill, 2024), tentative findings suggest that observed synchrony differences in autistic/non-autistic dyads (as opposed to non-autistic dyads) are minimized. Also, note here, the fact that SMS study designs have consistently failed to include autistic-autistic dyads within their design—and the significance of this fact, given the strong evidence for the Interactional Heterogeneity Hypothesis (see footnote 3).
8. ^All the examples featured in this paper (video and text) are generic. They are inspired by the themes and content of real play sessions, informed by clinical practice, but are non-specific and non-identifiable to any one person. Both video participants (Lea Zaccari and Stuart Daniel) have given their full permission to appear publicly (on YouTube) and via the platform of this article.
9. ^A well understood aspect of autism is that eye-contact is difficult for many autistic individuals. It is very important to note that a lack of eye contact does not mean that an autistic person is not listening or paying attention.
10. ^Di Cesare et al. (2017) have shown that autistic children have difficulties in perceiving vitality-form differences between two contiguous stimuli (smallest change detected at >100 ms apart). This suggests that, “during action observation, children with ASD need greater stimuli variations than TD children to detect their differences in terms of vitality forms” (Di Cesare et al., 2017, p. 8).
11. ^Simple meter patterns, as opposed to complex ones, have been shown to improve synchronization dynamics (Patel et al., 2005).
12. ^Here, our concept of rhythm is information-rich with sensory impetus and flow (including temporal accents—e.g., the future-oriented emphasis of an iambic pentameter (for more details please see Daniel et al., 2022), and non-temporal intensity accents—use of changes in volume, pitch, timbre; and acoustic activation contours (all explained later in Skill Level 3). Dry metronomic time keeping is not enough to support the good-enough social timing needed for interactive play. It is the use of information-rich rhythms, as opposed to information-dry metronomic rhythms, that makes the difference between success and failure in synchronization tasks in autism studies (tapping studies, Tryfon et al., 2017; Yoo and Yoon, 2019; + for analysis see Daniel et al., 2022).
13. ^The authors are aware of the challenges of communicating vitality in autistic-non-autistic interactions. Autistic children, as compared with typically developing (TD) children, demonstrate significant differences in vitality-form expression (Casartelli et al., 2020a) and challenges in recognition of TD vitality-forms (imitation studies, Hobson and Lee, 1999; similarity judgments, Rochat et al., 2013; immediate evaluations, Di Cesare et al., 2017, 2020). Di Cesare et al. (2017) have shown that children with “high functioning” autism have difficulties in perceiving vitality-form differences between two contiguous stimuli (smallest change detected at >100 ms apart). This suggests that, “during action observation, children with ASD need greater stimuli variations than TD children to detect their differences in terms of vitality forms” (Di Cesare et al., 2017, p. 8). And, in a study of visual and auditory expression of vitality, Di Cesare et al. (2017) have concluded, “…it may be plausible that visual information is not sufficient for children with ASD to encode vitality forms correctly and that the use of alternative (additional) perceptual information may help vitality form perception” (p8, italics added). As such, Rhythmic Relating consciously supports understanding of vitality through: clarity and contiguity through isolation of copied movements; exaggerated and diminished copying where appropriate; multi and cross modal vitality matching; and the use of acoustic sensory impetus (through rhythm, accents, and acoustic activation contours) to give extra dimensionality and energy to vitality expressions (for further analysis see Daniel et al., 2022).
14. ^ https://www.thempra.org.uk/social-pedagogy/key-concepts-in-social-pedagogy/the-common-third/
15. ^Of course, if we know our partner well, there will be exceptions to this. There will be many circumstances where touch is playful and appropriate and flowing consent is clear. There will be circumstances where very direct language, like “it’s your turn’ will be helpful and dynamic.
16. ^As we are suggesting practical use of rhythmic experiences, and a reliance on a felt sense of a repetitious behavioural pattern, it is important that we address potential beat-perception in people on the autism spectrum. Across simple and complex meter conditions, pre-SMA, basal ganglia, and cerebellar dysfunction in autism appears to be functionally specific, leaving beat-perception largely intact (Rinehart et al., 2006; Qiu et al., 2010; DePape et al., 2012). The anatomically and functionally specific nature of basal ganglia dysfunction in particular (Rinehart et al., 2006; Qiu et al., 2010) may likely allow for a window of beat-based experience and support (for detailed analysis, see Daniel et al., 2022).
17. ^No studies have been identified that establish equivalence for individuals with autism. Consequently, we will move forward with the provisional working assumption that the six-second window is suitable for the upper range of a sense of rhythm. In practical terms, it is crucial for the practitioner to maintain their awareness of the ongoing rhythm, making the six-second estimate entirely applicable in this context.
18. ^Our concepts here, are closely akin to Feldman’s matching synchrony and sequential synchrony (Feldman, 2007a,b).
19. ^Such “autism motor signatures” can be computationally identified from 2½ years of age (Anzulewicz et al., 2016).
20. ^Stern (1999) described motor contours in action as, vitality contours. Our concept of activation contours, encompasses this movement concept but also integrates the inclusion of compelling contours of sound.
21. ^The ASR operates along a pathway leading directly from the cochlea, along cranial (auditory) nerve VIII by way of the lateral leminiscus, to the caudate reticular nucleus. From here, there are descending projections to spinal and limb motor neurons, provoking the “jump” or “blink” effect (Frankland et al., 1997; Osborne, 2009).
22. ^It is very likely that these sounds are recognized by innate systems early in auditory pathways (Erlich et al., 2013; re: the Inferior Colliculus, Jorris et al., 2004; Sivaramakrishnan et al., 2004; Osborne, 2009). There is clear evidence of these pathways ascending to emotional systems (Heldt and Falls, 2003), as well as a descending, emotional “feedback” pathway from the amygdala (Marsh et al., 2002).
23. ^Autistic individuals have demonstrated prolonged unmodulated ASR latency as compared with age-matched typically developing controls (though without controls for learning disability) (Ornitz et al., 1993). Understanding the conditions which modulate latency is important for us here as, if we can minimize relative autistic-non-autistic latency difference, we can improve alignment for synchrony. When startle stimuli were presented with pre-stimulation and/or with habituation, latency differences and auditory hypersensitivities (shown via ASR amplitude) became non-significant (Ornitz et al., 1993) Clearly, predictive information - through context and familiarity - matters. In addition, autistic participants were slower to gain the benefits of habituation, needing a longer period before ASR latency effects were minimized, as compared with age-matched typically developing controls (Ornitz et al., 1993). So, repetition and patience matter too.
References
Anzulewicz, A., Sobota, K., and Delafield-Butt, J. T. (2016). Toward the autism motor signature: gesture patterns during smart tablet gameplay identify children with autism. Sci. Rep. 6, 1–13. doi: 10.1038/srep31107
Asher, M., Kauffmann, A., and Aderka, I. M. (2020). Out of sync: nonverbal synchrony in social anxiety disorder. Clin. Psychol. Sci. 8, 280–294. doi: 10.1177/2167702619894566
Ashwin, C., Chapman, E., Howells, J., Rhydderch, D., Walker, I., and Baron-Cohen, S. (2014). Enhanced olfactory sensitivity in autism spectrum conditions. Mol. Autism. 5, 53–59. doi: 10.1186/2040-2392-5-53
Au, K. B., and Lo, L. Y. (2020). March for unity: a study on an effect of synchronized actions to perceived closeness. Curr. Psychol. 39, 1012–1019. doi: 10.1007/s12144-018-9820-z
Bakan, M. B. (2014). The musicality of stimming: promoting neurodiversity in the ethnomusicology of autism. Musicultures 41, 133–161.
Bartenieff, I., and Lewis, D. (1980). Body movements: coping with the Environment. New York, NY: Gordon and Breach Science Publishers.
Ben-Sasson, A., Gal, E., Fluss, R., Katz-Zetler, N., and Cermak, S. A. (2019). Update of a meta-analysis of sensory symptoms in ASD: a new decade of research. J. Autism Dev. Disord. 49, 4974–4996. doi: 10.1007/s10803-019-04180-0
Bolis, D., and Schilbach, L. (2018). Observing and participating in social interactions: action perception and action control across the autistic spectrum. Dev. Cogn. Neurosci. 29, 168–175. doi: 10.1016/j.dcn.2017.01.009
Caldwell, P., Bradley, E., Gurney, J., Heath, J., Lightowler, H., Richardson, K., et al. (2019). Responsive communication: combining attention to sensory issues with using body language (intensive interaction) to interact with autistic adults and children. West Sussex, UK: Pavilion.
Casartelli, L., Cesareo, A., Biffi, E., Campione, G. C., Villa, L., Molteni, M., et al. (2020a). Vitality form expression in autism. Sci. Rep. 10:17182. doi: 10.1038/s41598-020-73364-x
Casartelli, L., Federici, A., Fumagalli, L., Cesareo, A., Nicoli, M., Ronconi, L., et al. (2020b). Neurotypical individuals fail to understand action vitality form in children with autism spectrum disorder. Proc. Natl. Acad. Sci. USA 117, 27712–27718. doi: 10.1073/pnas.2011311117
Cattaneo, L., Fabbri-Destro, M., Boria, S., Pieraccini, C., Monti, A., Cossu, G., et al. (2007). Impairment of actions chains in autism and its possible role in intention understanding. Proc. Natl. Acad. Sci. USA 104, 17825–17830. doi: 10.1073/pnas.0706273104
Charlton, R. A., Entecott, T., Belova, E., and Nwaordu, G. (2021). “It feels like holding back something you need to say”: Autistic and non-autistic adults accounts of sensory experiences and stimming. Res. Autism Spectr. Disord. 89:101864. doi: 10.1016/j.rasd.2021.101864
Chu, V. W. T. (2017). Assessing proprioception in children: a review. J. Mot. Behav. 49, 458–466. doi: 10.1080/00222895.2016.1241744
Chua, Y. W., Lu, S.-C., Anzulewicz, A., Sobota, K., Tachtatzis, C., Andonovic, I., et al. (2022). Developmental differences in the prospective organisation of goal-directed movement between children with autism and typically developing children: a smart tablet serious game study. Dev. Sci. 25:e13195. doi: 10.1111/desc.13195
Cook, J. (2016). From movement kinematics to social cognition: the case of autism. Philos. Trans. R. Soc. Lond. B Biol. Sci. 371:20150372. doi: 10.1098/rstb.2015.0372
Coulter, R. A. (2009). Understanding the visual symptoms of individuals with Autism Spectrum Disorder (ASD). Optom. Vis. Dev. 40, 164–175.
Crane, L., Goddard, L., and Pring, L. (2009). Sensory processing in adults with autism spectrum disorders. Autism 13, 215–228. doi: 10.1177/1362361309103794
Crompton, C. J., Hallett, S., Ropar, D., Flynn, E., and Fletcher-Watson, S. (2020). ‘I never realised everybody felt as happy as I do when I am around autistic people’: a thematic analysis of autistic adults’ relationships with autistic and neurotypical friends and family. Autism 24, 1438–1448. doi: 10.1177/1362361320908976
Crow, A. J., Janssen, J. M., Vickers, K. L., Parish-Morris, J., Moberg, P. J., and Roalf, D. R. (2020). Olfactory dysfunction in neurodevelopmental disorders: a meta-analytic review of autism spectrum disorders, attention deficit/hyperactivity disorder and obsessive–compulsive disorder. J. Autism Dev. Disord. 50, 2685–2697. doi: 10.1007/s10803-020-04376-9
Daluwatte, C., Miles, J. H., Sun, J., and Yao, G. (2015). Association between pupillary light reflex and sensory behaviors in children with autism spectrum disorders. Res. Dev. Disabil. 37, 209–215. doi: 10.1016/j.ridd.2014.11.019
Daniel, S. (2019). Loops and jazz gaps: engaging the feedforward qualities of communicative musicality in play therapy with children with autism. Arts Psychother. 65:101595. doi: 10.1016/j.aip.2019.101595
Daniel, S., Berkovits, L., Eisenhower, A., and Blacher, J. (2023). Child-centred play therapy and rhythmic relating improves emotion regulation in autism: a single-N pilot intervention study. Couns. Psychother. Res. 1–10. doi: 10.1002/capr.12671
Daniel, S., Mahler, K., Clairy, K., Ray, D., Inderbitzen, S., Sharp, K., et al. (2024a). Sensory-processing informed autism practice for child-centered therapists. Front. Psychol. Psychology for Clinical Settings (In Preparation).
Daniel, S., Blacher, J., Eisenhower, A., and Berkovits, L. (2024b). Emotion regulation in autism is improved in a single-n pilot intervention study combining child-centered play therapy and rhythmic relating. Couns. Psychother. Res. (In Press).
Daniel, S., Wimpory, D., Delafield-Butt, J. T., Malloch, S., Holck, U., Geretsegger, M., et al. (2022). Rhythmic Relating: bidirectional support for social timing in autism therapies. Front. Psychol. 13:793258. doi: 10.3389/fpsyg.2022.793258
Delafield-Butt, J. (2018). “The emotional and embodied nature of human understanding: sharing narratives of meaning” in The child's curriculum: working with the natural voices of young children. eds. C. Trevarthen, J. Delafield-Butt, and A.-W. Dunlop (Oxford University Press), Available at: https://doi.org/10.1093/oso/9780198747109.003.0004 (Accessed 15 July 2024).
Delafield-Butt, J. T., Freer, Y., Perkins, J., Skulina, D., Schögler, B., and Lee, D. N. (2018). Prospective organization of neonatal arm movements: a motor foundation of embodied agency, disrupted in premature birth. Dev. Sci. 21:e12693. doi: 10.1111/desc.12693
Delafield-Butt, J. T., Galler, A., Schögler, B., and Lee, D. N. (2010). A perception-action strategy for hummingbirds. Perception 39, 1172–1174. doi: 10.1068/p6708
Delafield-Butt, J. T., Pepping, G.-J., McCaig, C. D., and Lee, D. N. (2012). Prospective guidance in a free-swimming cell. Biol. Cybern. 106, 283–293. doi: 10.1007/s00422-012-0495-5
Delafield-Butt, J., and Trevarthen, C. (2017). On the brainstem origin of autism: disruption to movements of the primary self. Boca Raton, FL: CRC Press.
Delafield-Butt, J., Trevarthen, C., Rowe, P., and Gillberg, C. (2019). Being misunderstood in autism: the role of motor disruption in expressive communication, implications for satisfying social relations. Behav. Brain Sci. 42:E86. doi: 10.1017/S0140525X1800242X
DePape, A.-M. R., Hall, G. B. C., Tillmann, B., and Trainor, L. J. (2012). Auditory processing in high-functioning adolescents with autism spectrum disorder. PLoS One 7:e44084. doi: 10.1371/journal.pone.0044084
Di Cesare, G., Gerbella, M., and Rizzolatti, G. (2020). The neural bases of vitality forms. Natl. Sci. Rev. 7, 202–213. doi: 10.1093/nsr/nwz187
Di Cesare, G., Sparaci, L., Pelosi, A., Mazzone, L., Giovagnoli, G., Menghini, D., et al. (2017). Differences in action style recognition in children with autism spectrum disorders. Front. Psychol. 8:1456. doi: 10.3389/fpsyg.2017.01456
Eberhard-Kaechele, M. (2012). “Memory, metaphor, and mirroring in movement therapy with trauma patients” in Body memory, metaphor and movement. eds. S. Koch, T. Fuchs, M. Summa, and C. Muller (John Benjamins Publishing Company), 267–287.
Erlich, N., Lipp, O. V., and Slaughter, V. (2013). Of hissing snakes and angry voices: human infants are differently responsive to evolutionary fear-relevant sounds. Dev. Sci. 16, 894–904. doi: 10.1111/desc.12091
Fabbri-Destro, M., Cattaneo, L., Boria, S., and Rizzolatti, G. (2009). Planning actions in autism. Exp. Brain Res. 192, 521–525. doi: 10.1007/s00221-008-1578-3
Fantasia, V., and Delafield-Butt, J. (2023). Time and sequence as key developmental dimensions of joint action. Develop. Rev. 69:101091. doi: 10.1016/j.dr.2023.101091
Faso, D. J., Sasson, N. J., and Pinkham, A. E. (2015). Evaluating posed and evoked facial expressions of emotion from adults with autism spectrum disorder. J. Autism Dev. Disord. 45, 75–89. doi: 10.1007/s10803-014-2194-7
Feldman, R. (2007a). Parent-infant synchrony and the construction of shared timing; physiological precursors, developmental outcomes, and risk conditions. J. Child Psychol. Psychiatry 48, 329–354. doi: 10.1111/j.1469-7610.2006.01701.x
Feldman, R. (2007b). Parent–infant synchrony: biological foundations and developmental outcomes. Curr. Dir. Psychol. Sci. 16, 340–345. doi: 10.1111/j.1467-8721.2007.00532.x
Fitzpatrick, P., Frazier, J. A., Cochran, D. M., Mitchell, T., Coleman, C., and Schmidt, E. R. (2016). Impairments of social motor synchrony evident in autism spectrum disorder. Front. Psychol. 7:1323. doi: 10.3389/fpsyg.2016.01323
Fourie, E., Lu, S.-C., Delafield-Butt, J., and Rivera, S. M. (2024). Motor control adherence to the two-thirds power law differs in autistic development. J. Autism Dev. Disord. doi: 10.1007/s10803-024-06240-6
Frankland, P. W., Josselyn, S. A., Bradwejn, J., Vaccarino, F. J., and Yeomans, J. S. (1997). Activation of amygdala cholecystokinin B receptors potentiates the acoustic startle response in rats. J. Neurosci. 17, 1838–1847. doi: 10.1523/JNEUROSCI.17-05-01838.1997
Franklin, A., Sowden, P., Notman, L., Gonzalez-Dixon, M., West, D., Alexander, I., et al. (2010). Reduced chromatic discrimination in children with autism spectrum disorders. Dev. Sci. 13, 188–200. doi: 10.1111/j.1467-7687.2009.00869.x
Georgescu, A. L., Koeroglu, S., Hamilton, D. C., Vogeley, K., Falter-Wagner, C. M., and Tschacher, W. (2020). Reduced nonverbal interpersonal synchrony in autism spectrum disorder independent of partner diagnosis: a motion energy study. Mol. Autism. 11, 1–14. doi: 10.1186/s13229-019-0305-1
Geretsegger, M., Holck, U., Carpente, J., Elefant, C., Kim, J., and Gold, C. (2015). Common characteristics of improvisational approaches in music therapy for children with autism spectrum disorder: developing treatment guidelines. J. Music. Ther. 52, 258–281. doi: 10.1093/jmt/thv005
Glass, D., and Yuill, N. (2023). Social motor synchrony in autism spectrum conditions: a systematic review. Autism. doi: 10.1177/13623613231213295
Glass, D., and Yuill, N. (2024). Evidence of mutual non-verbal synchrony in autistic learners and support workers: a motion energy analysis study. Front. Integr. Neurosci. doi: 10.3389/fnint.2024.1353966
Grahn, J. A., and Brett, M. (2007). Rhythm and beat perception in motor areas of the brain. J. Cogn. Neurosci. 19, 893–906. doi: 10.1162/jocn.2007.19.5.893
Greenspan, S. I., and Wieder, S. (2006). Engaging autism: using the floortime approach to help children relate, communicate, and think : Da Capo Lifelong Books.
Güçlü, B., Tanidir, C., Mukaddes, N. M., and Ünal, F. (2007). Tactile sensitivity of normal and autistic children. Somatosens. Mot. Res. 24, 21–33. doi: 10.1080/08990220601179418
Heldt, S. A., and Falls, W. A. (2003). Destruction of the inferior collicus disrupts the production and inhibition of fear conditioned to an acoustic stimulus. Behav. Brain Res. 144, 175–185. doi: 10.1016/s0166-4328(03)00092-5
Hobson, R. P., and Lee, A. (1999). Imitation and identification in autism. J. Child Psychol. Psychiatry 40, 649–659. doi: 10.1111/1469-7610.00481
Hughes, D. (2011). Attachment-focused family therapy workbook (Pap/DVD Wk edition). New York, NY: W.W. Norton.
Iacono, T., Trembath, D., and Erickson, S. (2016). The role of augmentative and alternative communication for children with autism: current status and future trends. Neuropsychiatr. Dis. Treat. 12, 2349–2361. doi: 10.2147/NDT.S95967
Ikuta, N., Iwanaga, R., Tokunaga, A., Nakane, H., Tanaka, K., and Tanaka, G. (2016). Effectiveness of earmuffs and noise-cancelling headphones for coping with hyper-reactivity to auditory stimuli in children with autism spectrum disorder: a preliminary study. Hong Kong J. Occup. Ther. 28, 24–32. doi: 10.1016/j.hkjot.2016.09.001
Jorris, P. X., Schreiner, C. E., and Rees, A. (2004). Neural processing of amplitude-modulated sounds. Physiol. Rev. 84, 541–577. doi: 10.1152/physrev.00029.2003
Kapp, S. K., Steward, R., Crane, L., Elliott, D., Elphick, C., Pellicano, E., et al. (2019). ‘People should be allowed to do what they like’: autistic adults’ views and experiences of stimming. Autism 23, 1782–1792. doi: 10.1177/1362361319829628
Kaur, M., Srinivasan, S. M., and Bhat, A. N. (2018). Comparing motor performance, praxis, coordination, and interpersonal synchrony between children with and without Autism Spectrum Disorder (ASD). Res. Dev. Disabil. 72, 79–95. doi: 10.1016/j.ridd.2017.10.025
Keen, D., Sigafoos, J., and Woodyatt, G. (2005). Teacher responses to the communicative attempts of children with autism. J. Dev. Phys. Disabil. 17, 19–33. doi: 10.1007/s10882-005-2198-5
Khalfa, S., Bruneau, N., Rogé, B., Georgieff, N., Veuillet, E., Adrien, J. L., et al. (2004). Increased perception of loudness in autism. Hear. Res. 198, 87–92. doi: 10.1016/j.heares.2004.07.006
Knight, E. J., Oakes, L., Hyman, S. L., Freedman, E. G., and Foxe, J. J. (2020). Individuals with autism have no detectable deficit in neural markers of prediction error when presented with auditory rhythms of varied temporal complexity. Autism Res. 13, 2058–2072. doi: 10.1002/aur.2362
Koh, H. C., Milne, E., and Dobkins, K. (2010). Spatial contrast sensitivity in adolescents with autism spectrum disorders. J. Autism Dev. Disord. 40, 978–987. doi: 10.1007/s10803-010-0953-7
Kojovic, N., Ben Hadid, L., Franchini, M., and Schaer, M. (2019). Sensory processing issues and their association with social difficulties in children with autism spectrum disorders. J. Clin. Med. 8:1508. doi: 10.3390/jcm8101508
Kwakye, L. D., Foss-Feig, J. H., Cascio, C. J., Stone, W. L., and Wallace, M. T. (2011). Altered auditory and multisensory temporal processing in autism spectrum disorders. Front. Integr. Neurosci. 4:129. doi: 10.3389/fnint.2010.00129
Laurie, C. (2022). Sensory and motor strategies: practical ways to help autistic children and young people learn and achieve. London, UK: Jessica Kingsley Publishers.
Lee, D. N. (1998). Guiding movement by coupling taus. Ecol. Psychol. 10, 221–250. doi: 10.1207/s15326969eco103&4_4
Leekam, S. R., Nieto, C., Libby, S. J., Wing, L., and Gould, J. (2007). Describing the sensory abnormalities of children and adults with autism. J. Autism Dev. Disord. 37, 894–910. doi: 10.1007/s10803-006-0218-7
Lepisto, T., Kujala, T., Vanhala, R., Alku, P., Huotilainen, M., and Naatanen, R. (2005). The discrimination of and orienting to speech and non-speech sounds in children with autism. Brain Res. 1066, 147–157. doi: 10.1016/j.brainres.2005.10.052
Lepisto, T., Silokallio, S., Nieminen-von Wendt, T., Alku, P., Naatanen, R., and Kujala, T. (2006). Auditory perception and attention as reflected by the brain event-related potentials in children with Asperger syndrome. Clin. Neurophysiol. 117, 2161–2171. doi: 10.1016/j.clinph.2006.06.709
Little, J. A. (2018). Vision in children with autism spectrum disorder: a critical review. Clin. Exp. Optom. 101, 504–513. doi: 10.1111/cxo.12651
Ludlow, A. K., Wilkins, A. J., and Heaton, P. (2006). The effect of coloured overlays on reading ability in children with autism. J. Autism Dev. Disord. 36, 507–516. doi: 10.1007/s10803-006-0090-5
Malloch, S., and Trevarthen, C. (2009). “Musicality: communicating the vitality and interests of life” in Communicative musicality: exploring the basis of human companionship. eds. S. Malloch and C. Trevarthen (Oxford: Oxford University Press).
Mari, M., Castiello, U., Marks, D., Marraffa, C., and Prior, M. (2003). The reach-to-grasp movement in children with autism spectrum disorder. Philos. Trans. R. Soc. B Biol. Sci. 358, 393–403. doi: 10.1098/rstb.2002.1205
Marsh, R. A., Fuzessery, Z. M., Grose, C. D., and Wenstrup, J. J. (2002). Projection to the inferior colliculus from the basal nucleus of the amygdala. J. Neurosci. 22, 10449–10460. doi: 10.1523/JNEUROSCI.22-23-10449.2002
McCarty, M. J., and Brumback, A. C. (2021). Rethinking stereotypies in autism. Semin. Pediatr. Neurol. 38:100897. doi: 10.1016/j.spen.2021.100897
McGowan, T., and Delafield-Butt, J. (2022). Narrative as co-regulation: A review of embodied narrative in infant development. Behav. Develop. 68:101747. doi: 10.1016/j.infbeh.2022.101747
McNaughton, K. A., and Redcay, E. (2020). Interpersonal synchrony in autism. Curr. Psychiatry Rep. 22:12. doi: 10.1007/s11920-020-1135-8
Milton, D., Emine, G., and López, B. (2022). The “Double Empathy Problem”: Ten Years On. Autism 26, 1901–1903. doi: 10.1177/13623613221129123
Morrison, K. E., DeBrabander, K. M., Faso, D. J., and Sasson, N. J. (2019). Variability in first impressions of autistic adults made by neurotypical raters is driven more by characteristics of the rater than by characteristics of autistic adults. Autism 23, 1817–1829. doi: 10.1177/1362361318824104
Murray, L., and Trevarthen, C. (1985). “Emotional regulation of interactions between two-month-olds and their mothers” in Social perception in Infants (Ablex), 177–197.
Nebel, M. B., Eloyan, A., Nettles, C. A., Sweeney, K. L., Ament, K., Ward, R. E., et al. (2016). Intrinsic visual-motor synchrony correlates with social deficits in autism. Biol. Psychiatry 79, 633–641. doi: 10.1016/j.biopsych.2015.08.029
Nind, M., and Hewett, D. (2012). Access to communication: developing the basics of communication with people with severe learning difficulties through intensive interaction. Oxford, UK: Routledge.
Oram Cardy, J., Flagg, E., Roberts, W., and Roberts, T. (2005). Delayed mismatch field for speech and non-speech sounds in children with autism. Neuroreport 16, 521–525. doi: 10.1097/00001756-200504040-00021
Ornitz, E. M., Lane, S. J., Sugiyama, T., and de Traversay, J. (1993). Startle modulation studies in autism. J. Autism Dev. Disord. 23, 619–637. doi: 10.1007/BF01046105
Osborne, N. (2009). “Towards a chronobiology of musical rhythm” in Communicative musicality: exploring the basis of human companionship. eds. S. Malloch and C. Trevarthen (Oxford: Oxford University Press).
Osborne, N. (2017). “Love, rhythm, and chronobiology”, Rhythms of Relating in Children’s Therapies: Connecting Creatively with Vulnerable Children. eds. S. Daniel and C. Trevarthen (London: Jessica Kingsley Publishers).
Osborne, N. (2020). “Imagination, intersubjectivity and a musical therapeutic process: a personal narrative” in The Cambridge handbook of the imagination. ed. A. Abraham (Cambridge: Cambridge University Press), 636–656.
Panksepp, J. (2003). Can anthropomorphic analyses of separation cries in other animals inform us about the emotional nature of social loss in humans? Comment on Blumberg and Sokoloff (2001). Psychol. Rev. 110, 376–388. doi: 10.1037/0033-295x.110.2.376
Patel, A. D., Iversen, J. R., Chen, Y., and Repp, B. H. (2005). The influence of metricality and modality on synchronization with a beat. Exp. Brain Res. 163, 226–238. doi: 10.1007/s00221-004-2159-8
Pfeiffer, B., Stein Duker, L., Murphy, A., and Shui, C. (2019). Effectiveness of noise-attenuating headphones on physiological responses for children with autism spectrum disorders. Front. Integr. Neurosci. 13:65. doi: 10.3389/fnint.2019.00065
Porges, S. W. (2021). “Play as a neural exercise: insights from the polyvagal theory” in Polyvagal safety: attachment, communication, self-regulation. ed. S. W. Porges (New York, US: WW Norton and Company), 61–65.
Proff, I., Williams, G. L., Quadt, L., and Garfinkel, S. N. (2022). Sensory processing in autism across exteroceptive and interoceptive domains. Psychol. Neurosci. 15, 105–130. doi: 10.1037/pne0000262
Qiu, A., Adler, M., Crocetti, D., Miller, M. I., and Mostofsky, S. H. (2010). Basal Ganglia shapes predict social, communication, and motor dysfunctions in boys with autism spectrum disorder. J. Am. Acad. Child Adolesc. Psychiatry 49, 539–551. doi: 10.1016/j.jaac.2010.02.012
Rinehart, N. J., Tonge, B. J., Iansek, R., McGinley, J., Brereton, A. V., Enticott, P. G., et al. (2006). Gait function in newly diagnosed children with autism: cerebellar and basal ganglia related motor disorder. Dev. Med. Child Neurol. 48, 819–824. doi: 10.1017/S0012162206001769
Riquelme, I., Hatem, S. M., Sabater-Gárriz, Á., and Montoya, P. (2023). A multidimensional investigation of the relationship between skin-mediated somatosensory signals, emotion regulation and behavior problems in autistic children. Front. Neurosci. 17:1227173. doi: 10.3389/fnins.2023.1227173
Rochat, M., Veroni, V., Bruschweiler-Stern, N., Pieraccini, C., Bonnet-Brilhault, F., Barthélémy, C., et al. (2013). Impaired vitality form recognition in autism. Neuropsychologia 51, 1918–1924. doi: 10.1016/j.neuropsychologia.2013.06.002
Rosenhall, U., Nordin, V., Sandström, M., Ahlsen, G., and Gillberg, C. (1999). Autism and hearing loss. J. Autism Dev. Disord. 29, 349–357. doi: 10.1023/a:1023022709710
Rousanoglou, E. N., and Boudolos, K. D. (2006). Rhythmic performance during a whole body movement: dynamic analysis of force–time curves. Hum. Move. Sci. 25, 393–408. doi: 10.1016/j.humov.2005.12.004
Russo, N., Zecker, S., Trommer, B., Chen, J., and Kraus, N. (2009). Effects of background noise on cortical encoding of speech in autism spectrum disorders. J. Autism Dev. Disord. 39, 1185–1196. doi: 10.1007/s10803-009-0737-0
Salinsky, T. (2017). The improv handbook: the ultimate guide to improvising in comedy, theatre, and beyond. London, UK: Bloomsbury Publishing PLC.
Sasson, N. J., Faso, D. J., Nugent, J., Lovell, S., Kennedy, D. P., and Grossman, R. B. (2017). Neurotypical peers are less willing to interact with those with autism based on thin slice judgments. Sci. Rep. 7, 1–10. doi: 10.1038/srep40700
Schögler, B., Pepping, G.-J., and Lee, D. N. (2008). TauG-guidance of transients in expressive musical performance. Exp. Brain Res. 189, 361–372. doi: 10.1007/s00221-008-1431-8
Schögler, B., Polokoff, R., Pepping, G. J., Perkins, J., and Lee, D. N. (2017). Improving tauG-control of action in PD. bioRxiv. doi: 10.1101/143313
Sheppard, E., Pillai, D., Wong, G. T. L., Ropar, D., and Mitchell, P. (2016). How easy is it to read the minds of people with autism spectrum disorder? J. Autism Dev. Disord. 46, 1247–1254. doi: 10.1007/s10803-015-2662-8
Sice, P., Elvin, G., Riachy, C., Shang, Y., Ogwu, S., and Zink, C. (2020). Online screening of x-system music playlists using an integrative wellbeing model informed by the theory of autopoiesis. IEEE Access 8, 182307–182319. doi: 10.1109/ACCESS.2020.3029142
Sivaramakrishnan, S., Sterbing-D’Angelo, S. J., Filipovic, B., D’Angelo, W. R., Oliver, D. L., and Kuwada, S. (2004). GABA (A) synapses shape neuronal responses to sound intensity in the Inferior Colliculus. J. Neurosci. 24, 5031–5043. doi: 10.1523/JNEUROSCI.0357-04.2004
Stern, D. N. (1974). “Mother and infant at play: the dyadic interaction involving facial, vocal, and gaze behaviors” in The effect of the infant on its caregiver. eds. M. Lewis and L. A. Rosenblum (Wiley-Interscience).
Stern, D. N. (1999). “Vitality contours: the temporal contour of feelings as a basic unit for constructing the infant's social experience” in Early social cognition: understanding others in the first months of life. ed. P. Rochat (Lawrence Erlbaum Associates Publishers), 67–80.
Stern, D. N. (2010). Forms of vitality: exploring dynamic experience in psychology, the arts, psychotherapy, and development. Oxford: Oxford University Press.
Su, W.-C., Culotta, M., Mueller, J., Tsuzuki, D., Pelphrey, K., and Bhat, A. (2020). Differences in cortical activation patterns during action observation, action execution, and interpersonal synchrony between children with or without autism spectrum disorder (ASD): an fNIRS pilot study. PLoS One 15:e0240301. doi: 10.1371/journal.pone.0240301
Sung, Y. S., Lin, C. Y., Chu, S. Y., and Lin, L. Y. (2024). Emotion dysregulation mediates the relationship between sensory processing and behavior problems in young children with autism spectrum disorder: a preliminary study. J. Autism Dev. Disord. 54, 738–748. doi: 10.1007/s10803-022-05839-x
Takahashi, H., Komatsu, S., Nakahachi, T., Ogino, K., and Kamio, Y. (2016). Relationship of the acoustic startle response and its modulation to emotional and behavioral problems in typical development children and those with autism spectrum disorders. J. Autism Dev. Disord. 46, 534–543. doi: 10.1007/s10803-015-2593-4
Takahashi, H., Nakahachi, T., Komatsu, S., Ogino, K., Iida, Y., and Kamio, Y. (2014). Hyperreactivity to weak acoustic stimuli and prolonged acoustic startle latency in children with autism spectrum disorders. Mol. Autism. 5, 23–28. doi: 10.1186/2040-2392-5-23
Tickle-Degnen, L., and Rosenthal, R. (1990). The nature of rapport and its nonverbal correlates. Psychol. Inq. 1, 285–293. doi: 10.1207/s15327965pli0104_1
Tomchek, S. D., and Dunn, W. (2007). Sensory processing in children with and without autism: a comparative study using the short sensory profile. Am. J. Occup. Ther. 61, 190–200. doi: 10.5014/ajot.61.2.190
Torres, E. B., Brincker, M., Isenhower, R. W., Yanovich, P., Stigler, K. A., Nurnberger, J. I., et al. (2013). Autism: the micro-movement perspective. Front. Integr. Neurosci. 7:32. doi: 10.3389/fnint.2013.00032
Torres, E. B., Varkey, H., Vero, J., London, E., Phan, H., Kittler, P., et al. (2023). Sensing echoes: temporal misalignment in auditory brainstem responses as the earliest marker of neurodevelopmental derailment. PNAS Nexus 2:pgac315. doi: 10.1093/pnasnexus/pgac315
Torres, E., Yanovich, P., and Metaxas, D. (2013). Give spontaneity and self-discovery a chance in ASD: spontaneous peripheral limb variability as a proxy to evoke centrally driven intentional acts. Front. Integr. Neurosci. 7:46. doi: 10.3389/fnint.2013.00046
Tortora, S. (2006). The dancing dialogue: using the communicative power of movement with young children. Baltimore, MD: Paul H. Brookes Publishing Company.
Trevarthen, C., and Delafield-Butt, J. T. (2013). Autism as a developmental disorder in intentional movement and affective engagement. Front. Integr. Neurosci. 7:49. doi: 10.3389/fnint.2013.00049
Tronick, E., Als, H., Adamson, L., Wise, S., and Brazelton, T. B. (1978). The infant's response to entrapment between contradictory messages in face-to-face interaction. J. Am. Acad. Child Psychiatry 17, 1–13. doi: 10.1016/S0002-7138(09)62273-1
Tronick, E. Z., and Cohn, J. F. (1989). Infant-mother face-to-face interaction: age and gender differences in coordination and the occurrence of miscoordination. Child Dev. 60, 85–92. doi: 10.2307/1131074
Tryfon, A., Foster, N. E., Ouimet, T., Doyle-Thomas, K., Anagnostou, E., Sharda, M., et al. (2017). Auditory-motor rhythm synchronization in children with autism spectrum disorder. Res. Autism Spectrum Disord. 35, 51–61. doi: 10.1016/J.RASD.2016.12.004
Tseng, M. H., Fu, C. P., Cermak, S. A., Lu, L., and Shieh, J. Y. (2011). Emotional and behavioral problems in preschool children with autism: relationship with sensory processing dysfunction. Res. Autism Spectr. Disord. 5, 1441–1450. doi: 10.1016/j.rasd.2011.02.004
Van Hecke, R., Danneels, M., Dhooge, I., Van Waelvelde, H., Wiersema, J. R., Deconinck, F. J., et al. (2019). Vestibular function in children with neurodevelopmental disorders: a systematic review. J. Autism Dev. Disord. 49, 3328–3350. doi: 10.1007/s10803-019-04059-0
Wigram, T. (2004). Improvisation: methods and techniques for music therapy clinicians, educators, and students. London, UK: Jessica Kingsley Publishers.
Wilson, A. C., and Bishop, D. V. (2021). “Second guessing yourself all the time about what they really mean…”: cognitive differences between autistic and non-autistic adults in understanding implied meaning. Autism Res. 14, 93–101. doi: 10.1002/aur.2345
Wimpory, D. (2015). “A social timing model of autism, informed by typical development” in Time distortions in mind: temporal processing in clinical populations. eds. A. Vatakis and M. Allman, Brill.com publishers. Ebook. 57–92.
Yoo, G. E., and Yoon, Y. E. (2019). Rhythmic tapping task performance in children with autism spectrum disorder: a meta-analysis. J. Music Hum. Behav. 16, 47–72. doi: 10.21187/JMHB.2019.16.1.047
Keywords: autism, Rhythmic Relating, education, therapy, early intervention (EI), movement, synchrony, coregulation
Citation: Daniel S, Laurie M and Delafield-Butt JT (2024) A handbook for Rhythmic Relating in autism: supporting social timing in play, learning and therapy. Front. Psychol. 15:1384068. doi: 10.3389/fpsyg.2024.1384068
Edited by:
Pamela Bryden, Wilfrid Laurier University, CanadaReviewed by:
Wan-Chun Su, National Institutes of Health (NIH), United StatesEdward S. Brodkin, University of Pennsylvania, United States
Copyright © 2024 Daniel, Laurie and Delafield-Butt. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stuart Daniel, c3R1ZGFuaWVsQGdtYWlsLmNvbQ==; Jonathan T. Delafield-Butt, am9uYXRoYW4uZGVsYWZpZWxkLWJ1dHRAc3RyYXRoLmFjLnVr
 Stuart Daniel
Stuart Daniel Matthew Laurie
Matthew Laurie Jonathan T. Delafield-Butt
Jonathan T. Delafield-Butt