- 1Banner Alzheimer’s Institute, Phoenix, AZ, United States
- 2College of Medicine, University of Arizona, Phoenix, AZ, United States
Background: The Montreal Cognitive Assessment (MoCA) is one of the most widely-used cognitive screening instruments and has been translated into several different languages and dialects. Although the original validation study suggested to use a cutoff of ≤26, subsequent studies have shown that lower cutoff values may yield fewer false-positive indications of cognitive impairment. The aim of this study was to summarize the diagnostic accuracy and mean difference of the MoCA when comparing cognitively unimpaired (CU) older adults to those with amnestic mild cognitive impairment (aMCI).
Methods: PubMed and EMBASE databases were searched from inception to 22 February 2022. Meta-analyses for area under the curve (AUC) and standardized mean difference (SMD) values were performed.
Results: Fifty-five observational studies that included 17,343 CU and 8,413 aMCI subjects were selected for inclusion. Thirty-nine studies were used in the AUC analysis while 44 were used in the SMD analysis. The overall AUC value was 0.84 (95% CI: 0.81, 0.87) indicating good diagnostic accuracy and a large effect size was noted for the SMD analysis (Hedge’s g = 1.49, 95% CI: 1.33, 1.64). Both analyses had high levels of between-study heterogeneity. The median cutoff score for identifying aMCI was <24.
Discussion and conclusion: The MoCA has good diagnostic accuracy for detecting aMCI across several different languages. The findings of this meta-analysis also support the use of 24 as the optimal cutoff when the MoCA is used to screen for suspected cognitive impairment.
Introduction
Amnestic mild cognitive impairment (aMCI) due to Alzheimer’s disease (AD) is a syndrome that is associated with future progression to clinical AD. While not all individuals with aMCI progress to AD, they are thought to be at the highest risk of progression and this classification is often referred to as “MCI due to AD” (Albert et al., 2011; Sperling et al., 2011). The diagnostic criteria for aMCI have remained largely the same since their initial publication (Petersen et al., 1999) and require that an individual’s episodic memory performance fall at least 1.5 standard deviations below what would be expected for their age and education level and is accompanied by a self-reported or collateral-reported complaint of cognitive decline. However, the aMCI diagnosis is made only after an extensive neuropsychological examination which prevents the diagnosis from being made in general practice settings where cognitive screening measures are often used to determine if an individual requires a more comprehensive cognitive assessment (Townley et al., 2019). Further refinements to the aMCI diagnostic criteria include the differentiation of those whose impairments are only in the memory domain (single domain) versus those who are impaired in memory and another cognitive domain (multiple domain) (Petersen and Negash, 2008). These classifications also apply for cases where the memory domain is not impaired (non-amnestic MCI), but other domains are (Petersen and Negash, 2008).
For several decades the Mini-Mental State Exam (MMSE) (Folstein et al., 1975) has been the most ubiquitous cognitive screening instrument, however the Montreal Cognitive Assessment (MoCA) (Nasreddine et al., 2005) is now among the most widely-used assessments for cognitive screening in general practice settings. Recent evidence indicates that the MoCA is superior to the MMSE in its ability to differentiate aMCI from normal cognition (Pinto et al., 2019a) as many individuals with aMCI often obtain normal scores on the MMSE (26–30) despite collateral reports of significant cognitive decline. The initial validation study of the MoCA recommended the same cutoff score as the MMSE (<26), however subsequent studies have indicated this cutoff may be too stringent and result in false positive indications of possible cognitive impairment (Wong et al., 2015; Carson et al., 2018; Ilardi et al., 2023).
To date, there has not been an extensive review and quantitative analysis of the MoCA’s diagnostic accuracy for aMCI. Given that the MoCA has been translated into many different languages and dialects it is important to understand how consistent its diagnostic accuracy is across its various translations. The aims of this meta-analysis are to characterize the MoCA’s diagnostic accuracy for aMCI and to characterize its relative effect size for mean differences between cognitively unimpaired (CU) older adults and those with aMCI using a large sample of published observational studies that cover a wide array of the languages that the MoCA has been translated in.
Methods
Inclusion criteria
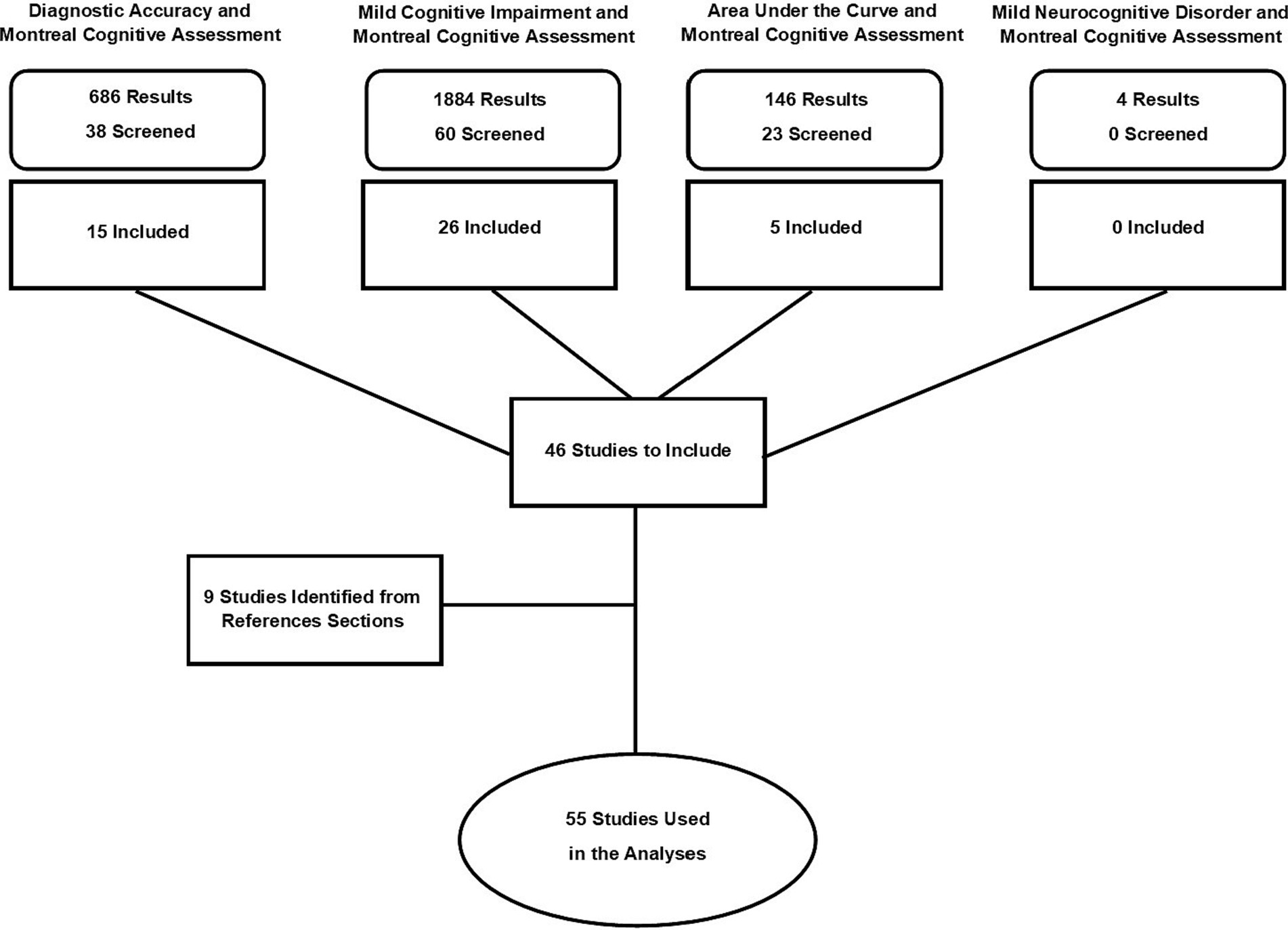
Prior to conducting the literature searches, the following criteria for study selection and inclusion were established: (1) The data could not come from a treatment or intervention trial, (2) The study should report either raw means and standard deviations for MoCA performance in both the CU and aMCI groups OR the study should report should report area under the curve (AUC) values with standard error (SE) or 95% confidence intervals (CI), (3) The study should use either Petersen criteria (Petersen and Negash, 2008) to classify its aMCI subjects or DSM-V criteria for mild neurocognitive disorder (MND). Although in most circumstances using only one set of diagnostic criteria is preferred, we felt that including studies that used either the Petersen or DSM-V MND criteria would provide greater ecological validity for the study results since the MoCA is used primarily as a screening instrument in general practice settings where formal diagnostic criteria for cognitive impairment are not usually applied. PRISMA guidelines were followed for the analysis and a flow chart depicting study screening and selection is shown in Figure 1.
Literature search terms
Using the PubMed database, four different search terms were used. The first search term, “diagnostic accuracy and Montreal Cognitive Assessment” yielded 686 results from which 38 were screened and 15 were selected for inclusion. A second search using “mild cognitive impairment and Montreal Cognitive Assessment” yielded 1,884 results from which 60 were screened with 26 that were selected for inclusion. The third search using “area under the curve and Montreal Cognitive Assessment” yielded 146 results with 23 that were selected for screening from which five were included. A fourth search using the term “mild neurocognitive disorder and Montreal Cognitive Assessment” did not yield any additional studies beyond those already identified in the previous searches. All four searches were also carried out in the EMBASE database which yielded no additional articles. Nine additional articles were identified through reviews of references sections of the selected papers which brought the final total of included studies to 55 (Figure 1). The search approach taken for this study is consistent with “a multi-faceted approach that uses a series of searches” as described in the Cochrane Handbook of Systematic Reviews of Interventions (Lefebvre et al., 2023).
Data quality and extraction
From each of the included studies the following data were extracted: sample sizes for the CU and MCI groups, means and standard deviations of MoCA scores for the CU and MCI groups, AUC values with standard errors (SE). When 95% CIs were reported, SE was derived by taking the difference between the AUC estimate and the upper bound of the 95% CI and dividing by 3.92 (Higgins et al., 2022). The cutpoint associated with the AUC estimate, means and standard deviations for age and education levels (when education was reported in years), and the geographic region in which the study was conducted (Asia, Europe, North America) were also extracted from each study. The quality of each study was assessed using the National Heart, Lung, and Blood Institute (NHLBI) Study Quality Assessment of Case Control Studies1 which was used to grade each study as Good, Fair, or Poor.
Statistical analysis
The first analytic approach was a meta-analysis of AUC values derived from the receiver operator characteristic (ROC) analyses that differentiated aMCI from CU individuals. The second analytic approach included analyses of the standardized mean difference (SMD) (Hedge’s g) and the raw mean difference (RMD) for MoCA scores between CU and aMCI. For both analytic approaches, results from random effects analyses were reported and the I2 statistic was used to quantify between-study heterogeneity which was classified as low, moderate, or high based on proposed guidelines (Higgins et al., 2003). Additional AUC and SMD analyses were carried out for subgroups based on geographic region (Asia, Europe, North America). The Egger’s test was used to determine the presence of publication bias among the included studies. In addition, the median of the reported MoCA cutoff score was used to summarize the reported cutoff values for studies in the AUC analysis. Since the included studies came from a number of different geographic regions we anticipated a wide range of reported MoCA cutoff scores so using the median as a summary measure provides an overall estimate of the MoCA’s cutoff that is relatively robust to the variability of reported cutoff values among the studies. All analyses were carried out using MedCalc Statistical Software version 20.109 (MedCalc Software Ltd., Ostend, Belgium,2 2022).
Results
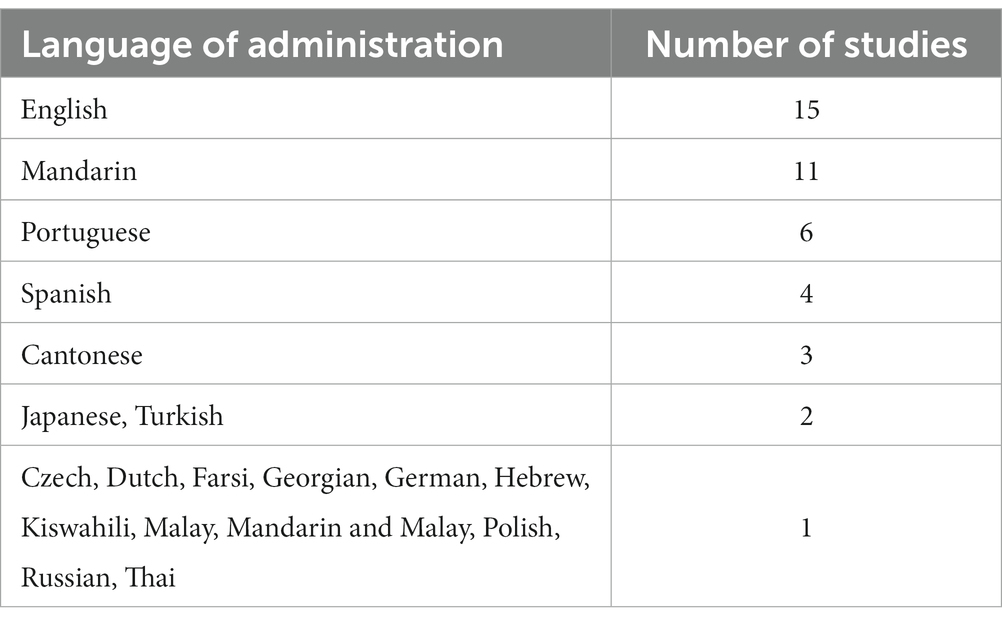
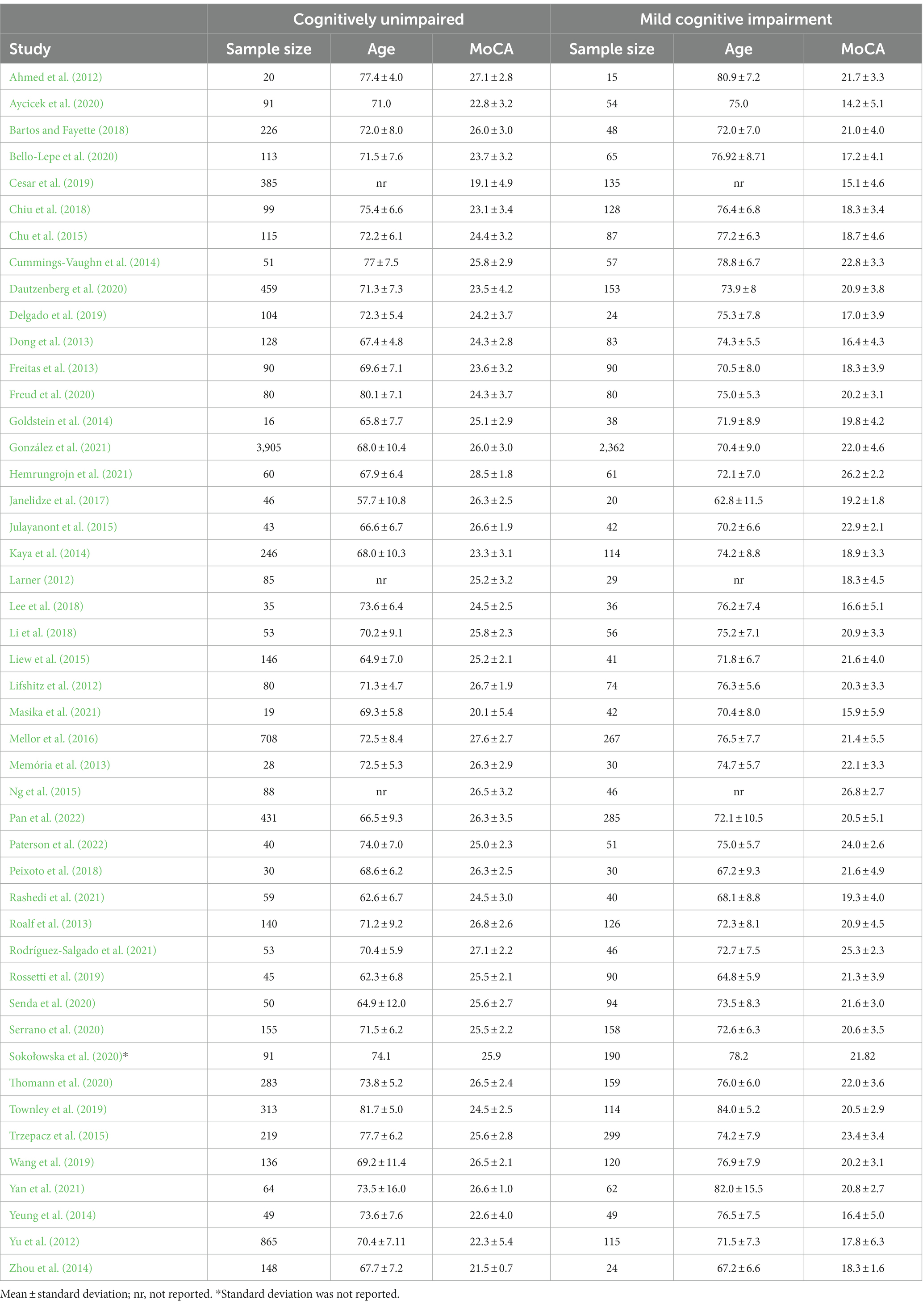
A total of 55 studies (Fujiwara et al., 2010; Lu et al., 2011; Ahmed et al., 2012; Larner, 2012; Yu et al., 2012; Dong et al., 2013; Freitas et al., 2013; Memória et al., 2013; Roalf et al., 2013; Cummings-Vaughn et al., 2014; Goldstein et al., 2014; Kaya et al., 2014; Malek-Ahmadi et al., 2014; Yeung et al., 2014; Zhou et al., 2014; Chu et al., 2015; Julayanont et al., 2015; Ng et al., 2015; Trzepacz et al., 2015; Mellor et al., 2016; O’Caoimh et al., 2016; Tsai et al., 2016; Cecato et al., 2017; Clarnette et al., 2017; Janelidze et al., 2017; Bartos and Fayette, 2018; Chiu et al., 2018; Lee et al., 2018; Li et al., 2018; Cesar et al., 2019; Delgado et al., 2019; Rossetti et al., 2019; Townley et al., 2019; Wang et al., 2019; Pinto et al., 2019b; Aycicek et al., 2020; Bello-Lepe et al., 2020; Dautzenberg et al., 2020; Freud et al., 2020; Senda et al., 2020; Serrano et al., 2020; Sokołowska et al., 2020; Thomann et al., 2020; González et al., 2021; Hemrungrojn et al., 2021; Masika et al., 2021; Rashedi et al., 2021; Rodríguez-Salgado et al., 2021; Yan et al., 2021; Pan et al., 2022; Paterson et al., 2022) were included in this meta-analysis from which 40 were used in the AUC analysis and 45 were used in the analysis of mean differences. Thirty-one of the included studies were used in both the AUC and mean difference analyses. An AUC-derived MoCA cutoff score for aMCI was reported by 45 studies. There was a great deal of diversity in the language of administration among the included studies which is shown in Table 1. English was the most prevalent among the studies (n = 15) followed by Mandarin (n = 11), Portuguese (n = 6), and Spanish (n = 4). 41% of the included studies were judged to be of good quality while 59% were judged to be of fair quality.
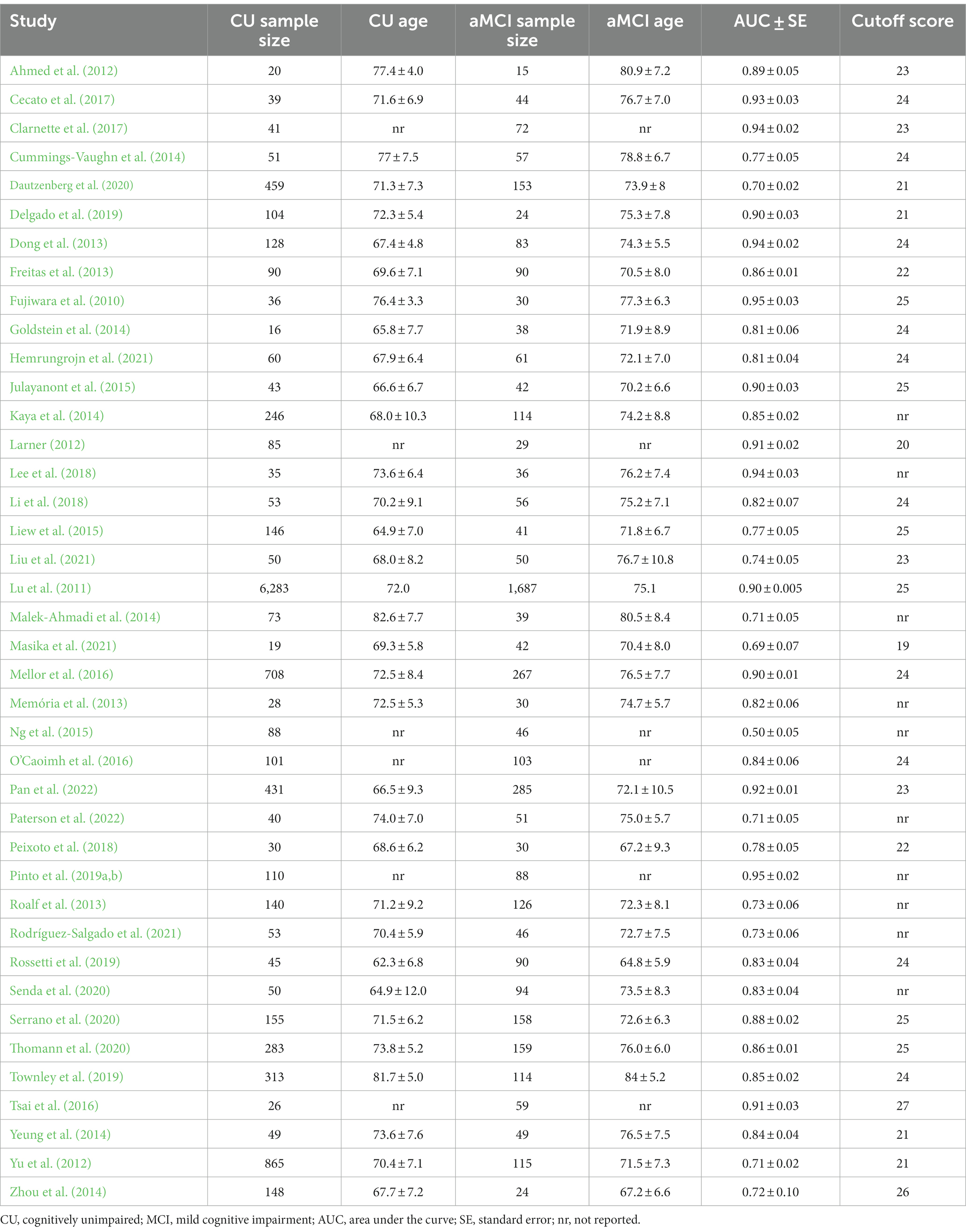
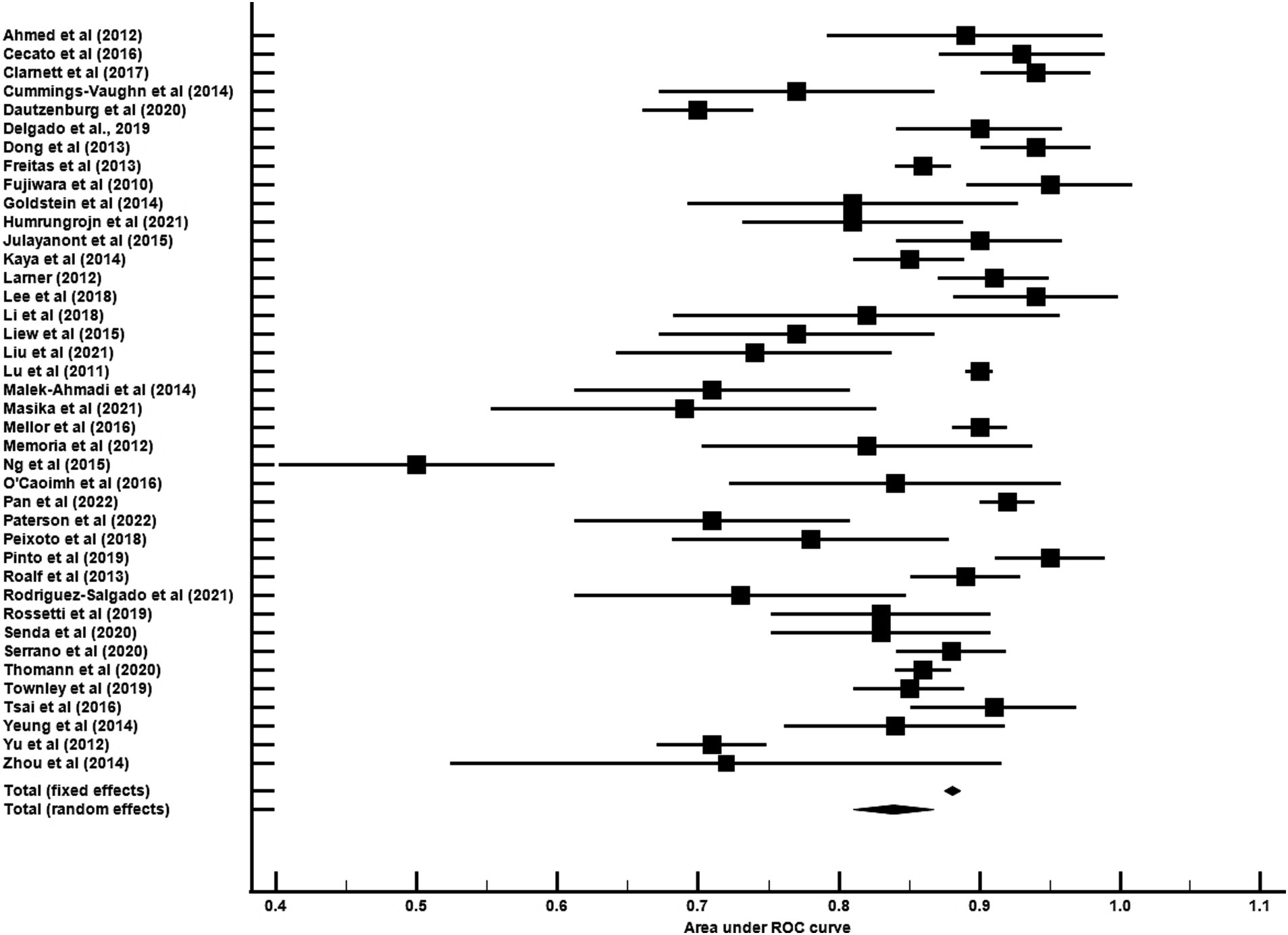
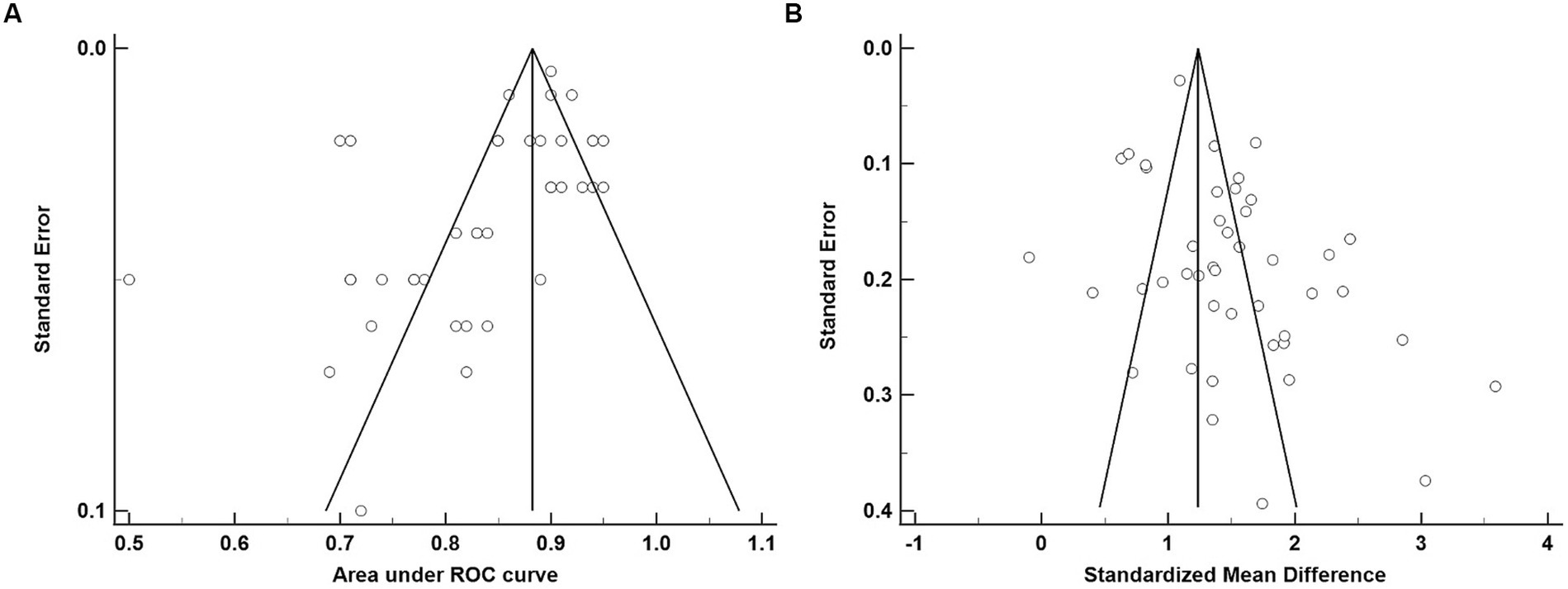
The average age for CU groups was 71.06 ± 7.37 years with an average of 11.44 ± 3.27 years of education. For aMCI groups, the average age was 73.99 ± 7.65 years with an average of 9.89 ± 3.47 years of education. Mean MoCA scores for the CU groups was 24.98 ± 2.88 and 20.11 ± 3.76 for the aMCI groups. Among studies that reported optimal cutoff values (n = 44), the median was 24 (range = 17–27). Characteristics of each study included in the AUC meta-analysis are shown in Table 2. The overall AUC value was 0.84, 95% CI (0.81, 0.87), p < 0.001 with very high heterogeneity [I2 = 90, 95% CI (87, 92%)] (Figure 2). The Egger’s test indicated the presence of publication bias in the analysis (p = 0.002). The meta-analysis for differences in means demonstrated a large effect size [Hedge’s g = 1.49, 95% CI (1.33, 1.64), p < 0.001; Table 3] with very high heterogeneity [I2 = 93, 95% CI (91, 94%)] and an Egger’s test that indicated the presence of publication bias (p = 0.003). The large effect size reported here equates to a 4.73 (95% CI: 4.20, 5.27) point difference on the MoCA between CU and aMCI groups (Figure 3). Characteristics of each study included in the SMD meta-analysis are shown in Table 2. Funnel plots depicting the publication bias in the AUC and SMD analyses are shown in Figure 4.
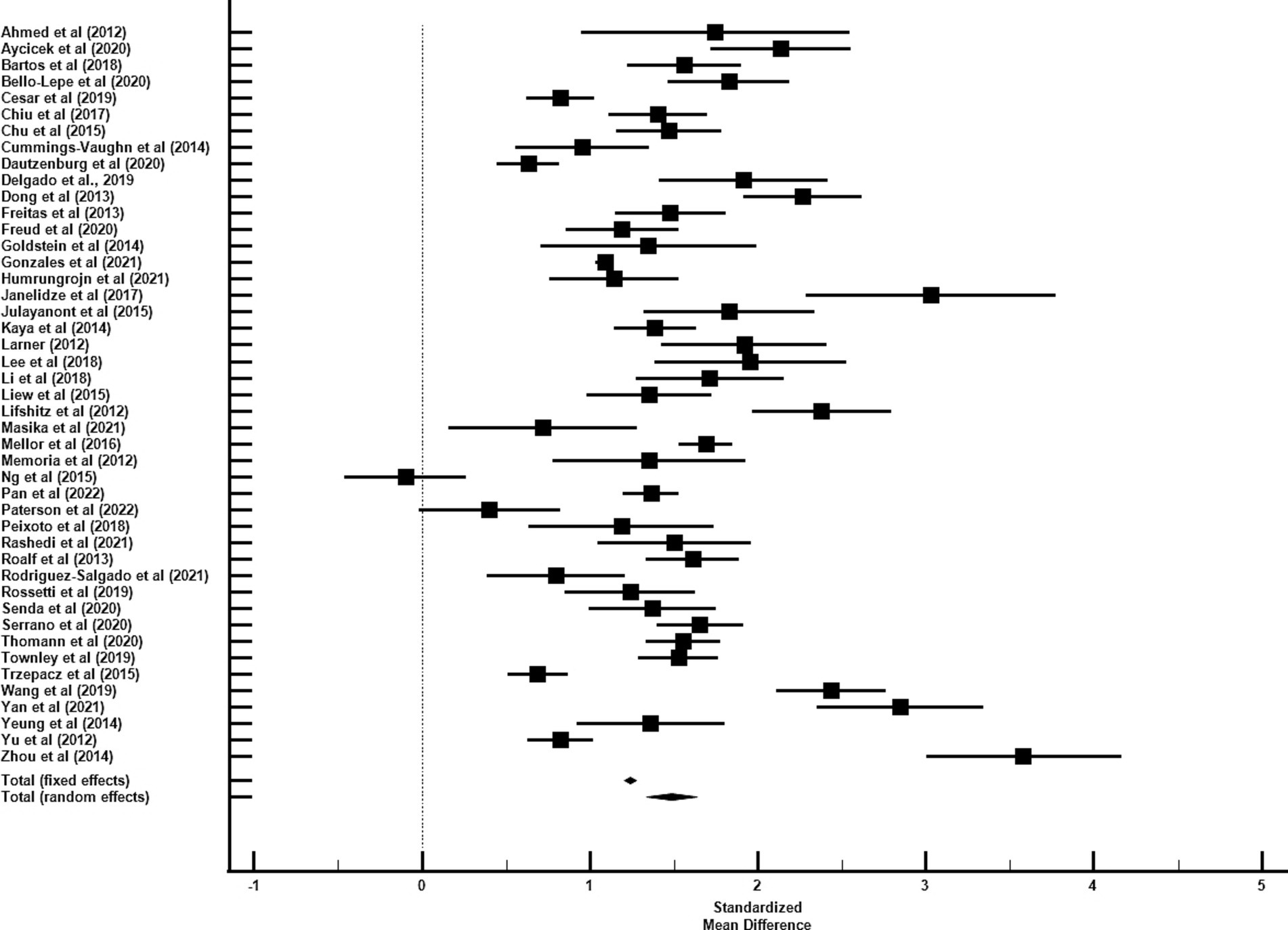
Figure 3. Forest plot for standardized mean difference of MoCA performance between CU and aMCI groups.
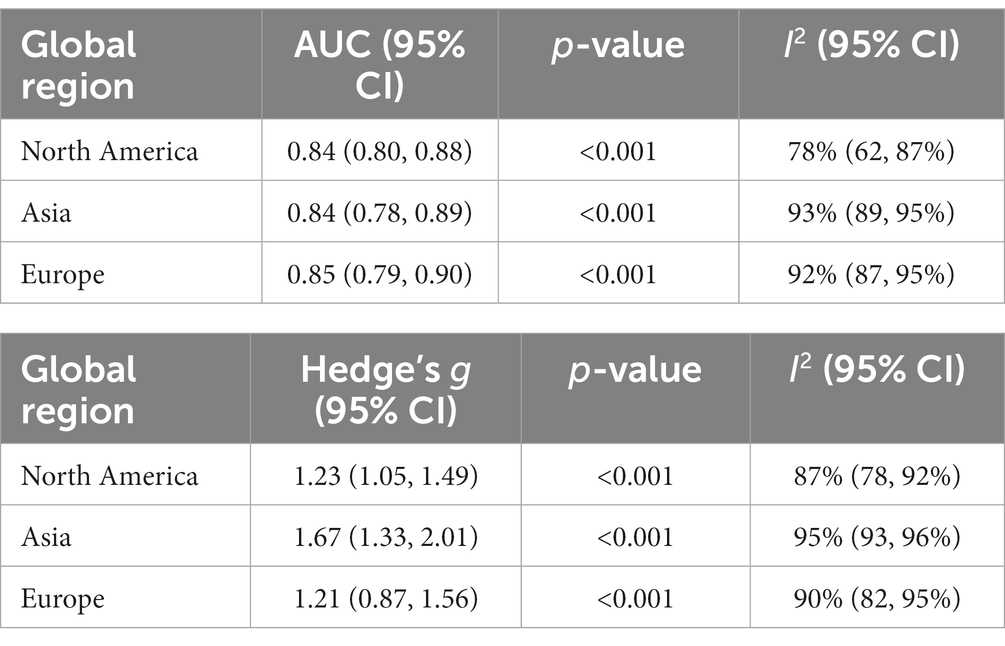
Analyses of ROC values by geographic region (Table 4) found that North American and Asian studies both yielded AUC values of 0.84 with European studies having a slightly higher AUC of 0.85. For the region-wise SMD analysis (Table 4), Asian studies had the largest effect size [Hedge’s g = 1.67, 95% CI (1.33, 2.01)], followed by North America [Hedge’s g = 1.23, 95% CI (1.05, 1.49)] and Europe [Hedge’s g = 1.21, 95% CI (0.87, 1.56)].
Discussion
This meta-analysis assessed the diagnostic accuracy and the mean difference of the MoCA when comparing aMCI older adults to those who are CU across several global regions. The overall AUC value of 0.84 indicates that the MoCA has good diagnostic accuracy for aMCI, however a very high degree of between-study heterogeneity was noted for this finding. The analysis of MoCA mean differences yielded a large effect size (Hedge’s g = 1.49) and a high degree of between-study heterogeneity was also noted for this analysis. English and Mandarin studies (n = 26) made up approximately half of the studies included in the meta-analysis and among the studies that assessed diagnostic accuracy a score of 24 was the most commonly-used cutoff for differentiating aMCI from CU individuals. However, it was noted that the range of reported cutoff values was 17 to 27 which suggests that optimal MoCA cutpoints may be population- and context-specific in order to avoid misclassification errors. A recent systematic review highlights this point by noting that cross-cultural differences necessitate the use of varying cutoff values as well as corrections for educational levels in different populations (O’Driscoll and Shaikh, 2017).
Others have also noted significant problems with misclassification on the MoCA when a single cutoff is used as higher rates of false positive indications of impairment were noted with increased age and decreased educational levels (Wong et al., 2015). Based on these previous reports the high levels of between-study heterogeneity in this meta-analysis may reflect the cultural, linguistic, and educational diversity among the included studies rather than any particular methodological weakness among them. These findings also emphasize the need to frame the MoCA’s utilization in a screening rather than a diagnostic context. Here it is also important to consider the sensitivity and specificity of a cognitive screening measure and how this impacts the utilization of full neuropsychological evaluations. The high false-positive rates of impairment on the MoCA using 26 as the cutoff could lead to many CU individuals being referred for unnecessary neuropsychological evaluations (Ilardi et al., 2023). In contrast, lowering the cutoff score for impairment also has the effect reducing the MoCA’s sensitivity in correctly detecting aMCI which further underscores the notion that the MoCA’s cutoff score can be adjusted for a given population in order to optimize its diagnostic accuracy. Additionally, adjustments to the cutoff score can be made when physical limitations (e.g., hearing loss) substantially impact MoCA performance (Utoomprurkporn et al., 2020).
A previous meta-analysis of nine studies investigating the MoCA’s diagnostic accuracy showed that the optimal MoCA cutoff for detecting aMCI was 23 (Carson et al., 2018) and a recent systematic review found that the AUC value for the MoCA in differentiating aMCI from CU individuals ranged from 0.71 to 0.99 across 34 studies (Pinto et al., 2019b) putting the AUC value of this meta-analysis (0.84) near the midpoint of this range. The three global regions examined in this meta-analysis (North America, Asia, Europe) all had comparable AUC and effect size values despite each region having a high degree of between-study heterogeneity.
There are some limitations to this meta-analysis. Despite the very large number of studies included for both the AUC and SMD analyses, a high degree of between-study heterogeneity was noted for all analyses which decreases the level of confidence one may have in the findings that are reported. A number of different factors may account for the high heterogeneity such as study setting (clinic vs. community-based), varying educational attainment of the populations among the different geographic regions, and cultural norms and values that may impact test performance. While the inconsistencies of the reported AUC and SMD values across studies warrant some degree of skepticism for the final results, there is also significant value in findings that are derived from such a large number of studies across different geographic regions and this aspect of the meta-analysis will likely appeal to clinicians who use the MoCA.
The findings of this meta-analysis provide further support for the use of the MoCA as an accurate cognitive screening tool for use in general practice settings. In line with other studies of the MoCA in aMCI and CU samples, a score of 24 appears to be the optimal cutoff to use for identifying cognitive impairment.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
MM-A: Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing. NN: Data curation, Formal analysis, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by National Institute on Aging P30AG072980 and P01AG014449.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyg.2024.1369766/full#supplementary-material
Footnotes
References
Ahmed, S., de Jager, C., and Wilcock, G. (2012). A comparison of screening tools for the assessment of mild cognitive impairment: preliminary findings. Neurocase 18, 336–351. doi: 10.1080/13554794.2011.608365
Albert, M. S., DeKosky, S. T., Dickson, D., Dubois, B., Feldman, H. H., Fox, N. C., et al. (2011). The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 7, 270–279. doi: 10.1016/j.jalz.2011.03.008
Aycicek, G. S., Çalıskan, H., Ozsurekci, C., Unsal, P., Kessler, J., Kalbe, E., et al. (2020). A reliable tool for assessing MCI and dementia: validation study of Dem Tect for Turkish population. Am. J. Alzheimers Dis. Other Dement. 35:1533317520949805. doi: 10.1177/1533317520949805
Bartos, A., and Fayette, D. (2018). Validation of the Czech Montreal cognitive assessment for mild cognitive impairment due to Alzheimer disease and Czech norms in 1, 552 elderly persons. Dement. Geriatr. Cogn. Disord. 46, 335–345. doi: 10.1159/000494489
Bello-Lepe, S., Alonso-Sánchez, M. F., Ortega, A., Gaete, M., Veliz, M., Lira, J., et al. (2020). Montreal cognitive assessment as screening measure for mild and major neurocognitive disorder in a Chilean population. Dement Geriatr Cogn Dis Extra. 10, 105–114. doi: 10.1159/000506280
Carson, N., Leach, L., and Murphy, K. J. (2018). A re-examination of Montreal cognitive assessment (MoCA) cutoff scores. Int. J. Geriatr. Psychiatry 33, 379–388. doi: 10.1002/gps.4756
Cecato, J. F., Martinelli, J. E., Izbicki, R., Yassuda, M. S., and Aprahamian, I. (2017). A subtest analysis of the Montreal cognitive assessment (MoCA): which subtests can best discriminate between healthy controls, mild cognitive impairment and Alzheimer's disease? Int. Psychogeriatr. 29:701. doi: 10.1017/S104161021600212X
Cesar, K. G., Yassuda, M. S., Porto, F. H. G., Brucki, S. M. D., and Nitrini, R. (2019). MoCA test: normative and diagnostic accuracy data for seniors with heterogeneous educational levels in Brazil. Arq. Neuropsiquiatr. 77, 775–781. doi: 10.1590/0004-282x20190130
Chiu, H. F. K., Zhong, B. L., Leung, T., Li, S. W., Chow, P., Tsoh, J., et al. (2018). Development and validation of a new cognitive screening test: the Hong Kong brief cognitive test (HKBC). Int. J. Geriatr. Psychiatry 33, 994–999. doi: 10.1002/gps.4883
Chu, L. W., Ng, K. H., Law, A. C., Lee, A. M., and Kwan, F. (2015). Validity of the Cantonese Chinese Montreal cognitive assessment in southern Chinese. Geriatr Gerontol Int 15, 96–103. doi: 10.1111/ggi.12237
Clarnette, R., O'Caoimh, R., Antony, D. N., Svendrovski, A., and Molloy, D. W. (2017). (2017). Comparison of the quick mild cognitive impairment (Qmci) screen to the Montreal cognitive assessment (MoCA) in an Australian geriatrics clinic. Int. J. Geriatr. Psychiatry 32, 643–649. doi: 10.1002/gps.4505
Cummings-Vaughn, L. A., Chavakula, N. N., Malmstrom, T. K., Tumosa, N., Morley, J. E., and Cruz-Oliver, D. M. (2014). Veterans affairs Saint Louis university mental status examination compared with the Montreal cognitive assessment and the short test of mental status. J. Am. Geriatr. Soc. 62, 1341–1346. doi: 10.1111/jgs.12874
Dautzenberg, G., Lijmer, J., and Beekman, A. (2020). Diagnostic accuracy of the Montreal cognitive assessment (MoCA) for cognitive screening in old age psychiatry: determining cutoff scores in clinical practice. Avoiding spectrum bias caused by healthy controls. Int. J. Geriatr. Psychiatry 35, 261–269. doi: 10.1002/gps.5227
Delgado, C., Araneda, A., and Behrens, M. I. (2019). Validation of the Spanish-language version of the Montreal cognitive assessment test in adults older than 60 years. Validación del instrumento Montreal cognitive assessment en español en adultos mayores de 60 años. Neurologia (Engl Ed). 34, 376–385. doi: 10.1016/j.nrl.2017.01.013
Dong, Y., Yean Lee, W., Hilal, S., Saini, M., Wong, T. Y., Chen, C. L.-H., et al. (2013). Comparison of the Montreal cognitive assessment and the Mini-mental state examination in detecting multi-domain mild cognitive impairment in a Chinese sub-sample drawn from a population-based study. Int. Psychogeriatr. 25, 1831–1838. doi: 10.1017/S1041610213001129
Folstein, M. F., Folstein, S. E., and McHugh, P. R. (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198. doi: 10.1016/0022-3956(75)90026-6
Freitas, S., Simões, M. R., Alves, L., and Santana, I. (2013). Montreal cognitive assessment: validation study for mild cognitive impairment and Alzheimer disease. Alzheimer Dis. Assoc. Disord. 27, 37–43. doi: 10.1097/WAD.0b013e3182420bfe
Freud, T., Vostrikov, A., Dwolatzky, T., Punchik, B., and Press, Y. (2020). Validation of the Russian version of the MoCA test as a cognitive screening instrument in cognitively asymptomatic older individuals and those with mild cognitive impairment. Front Med (Lausanne). 7:447. doi: 10.3389/fmed.2020.00447
Fujiwara, Y., Suzuki, H., Yasunaga, M., Sugiyama, M., Ijuin, M., Sakuma, N., et al. (2010). Brief screening tool for mild cognitive impairment in older Japanese: validation of the Japanese version of the Montreal cognitive assessment. Geriatr Gerontol Int 10, 225–232. doi: 10.1111/j.1447-0594.2010.00585.x
Goldstein, F. C., Ashley, A. V., Miller, E., Alexeeva, O., Zanders, L., and King, V. (2014). Validity of the Montreal cognitive assessment as a screen for mild cognitive impairment and dementia in African Americans. J. Geriatr. Psychiatry Neurol. 27, 199–203. doi: 10.1177/0891988714524630
González, D. A., Gonzales, M. M., Jennette, K. J., Soble, J. R., and Fongang, B. (2021). Cognitive screening with functional assessment improves diagnostic accuracy and attenuates bias. Alzheimers Dement (Amst). 13:e12250. doi: 10.1002/dad2.12250
Hemrungrojn, S., Tangwongchai, S., Charoenboon, T., Panasawat, M., Supasitthumrong, T., Chaipreserstud, P., et al. (2021). Use of the Montreal cognitive assessment Thai version to discriminate amnestic mild cognitive impairment from Alzheimer's disease and healthy controls: machine learning results. Dement. Geriatr. Cogn. Disord. 50, 183–194. doi: 10.1159/000517822
Higgins, JPT, Thomas, J, Chandler, J, Cumpston, M, Li, T, Page, MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022). (2022). Available at: www.training.cochrane.org/handbook.
Higgins, J. P. T., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ 327, 557–560. doi: 10.1136/bmj.327.7414.557
Ilardi, C. R., Menichelli, A., Michelutti, M., Cattaruzza, T., and Manganotti, P. (2023). Optimal MoCA cutoffs for detecting biologically-defined patients with MCI and early dementia. Neurol. Sci. 44, 159–170. doi: 10.1007/s10072-022-06422-z
Janelidze, M., Mikeladze, N., Bochorishvili, N., Dzagnidze, A., Kapianidze, M., Mikava, N., et al. (2017). Validity of the Georgian Montreal cognitive assessment for the screening of mild cognitive impairment and dementia. Am. J. Alzheimers Dis. Other Dement. 32, 36–40. doi: 10.1177/1533317516679304
Julayanont, P., Tangwongchai, S., Hemrungrojn, S., Tunvirachaisakul, C., Phanthumchinda, K., Hongsawat, J., et al. (2015). The Montreal cognitive assessment-basic: a screening tool for mild cognitive impairment in illiterate and low-educated elderly adults. J. Am. Geriatr. Soc. 63, 2550–2554. doi: 10.1111/jgs.13820
Kaya, Y., Aki, O. E., Can, U. A., Derle, E., Kibaroğlu, S., and Barak, A. (2014). Validation of Montreal cognitive assessment and discriminant power of Montreal cognitive assessment subtests in patients with mild cognitive impairment and Alzheimer dementia in Turkish population. J. Geriatr. Psychiatry Neurol. 27, 103–109. doi: 10.1177/0891988714522701
Larner, A. J. (2012). Screening utility of the Montreal cognitive assessment (MoCA): in place of--or as well as--the MMSE? Int. Psychogeriatr. 24, 391–396. doi: 10.1017/S1041610211001839
Lee, M. T., Chang, W. Y., and Jang, Y. (2018). Psychometric and diagnostic properties of the Taiwan version of the quick mild cognitive impairment screen. PLoS One 13:e0207851. doi: 10.1371/journal.pone.0207851
Lefebvre, C., Glanville, J., Briscoe, S., Featherstone, R., Littlewood, A., Metzendorf, M.-I., et al. (2023). “Chapter 4: searching for and selecting studies” in Cochrane handbook for systematic reviews of interventions version 6.4. eds. H. JPT, J. Thomas, J. Chandler, M. Cumpston, T. Li, and M. J. Page, et al. (Cochrane).
Li, X., Jia, S., Zhou, Z., Zhang, X., Zheng, W., Rong, P., et al. (2018). The role of the Montreal cognitive assessment (MoCA) and its memory tasks for detecting mild cognitive impairment. Neurol. Sci. 39, 1029–1034. doi: 10.1007/s10072-018-3319-0
Liew, T. M., Feng, L., Gao, Q., Ng, T. P., and Yap, P. (2015). Diagnostic utility of Montreal cognitive assessment in the fifth edition of diagnostic and statistical manual of mental disorders: major and mild neurocognitive disorders. J. Am. Med. Dir. Assoc. 16, 144–148. doi: 10.1016/j.jamda.2014.07.021
Lifshitz, M., Dwolatzky, T., and Press, Y. (2012). Validation of the Hebrew version of the MoCA test as a screening instrument for the early detection of mild cognitive impairment in elderly individuals. J. Geriatr. Psychiatry Neurol. 25, 155–161. doi: 10.1177/0891988712457047
Liu, X., Chen, X., Zhou, X., Shang, Y., Xu, F., Zhang, J., et al. (2021). Validity of the mem Trax memory test compared to the Montreal cognitive assessment in the detection of mild cognitive impairment and dementia due to Alzheimer's disease in a Chinese cohort. J. Alzheimers Dis. 80, 1257–1267. doi: 10.3233/JAD-200936
Lu, J., Li, D., Li, F., Zhou, A., Wang, F., Zuo, X., et al. (2011). Montreal cognitive assessment in detecting cognitive impairment in Chinese elderly individuals: a population-based study. J. Geriatr. Psychiatry Neurol. 24, 184–190. doi: 10.1177/0891988711422528
Malek-Ahmadi, M., Davis, K., Belden, C. M., and Sabbagh, M. N. (2014). Comparative analysis of the Alzheimer questionnaire (AQ) with the CDR sum of boxes, MoCA, and MMSE. Alzheimer Dis. Assoc. Disord. 28, 296–298. doi: 10.1097/WAD.0b013e3182769731
Masika, G. M., Yu, D. S. F., and Li, P. W. C. (2021). Accuracy of the Montreal cognitive assessment in detecting mild cognitive impairment and dementia in the rural African population. Arch. Clin. Neuropsychol. 36, 371–380. doi: 10.1093/arclin/acz086
Mellor, D., Lewis, M., McCabe, M., Byrne, L., Wang, T., Wang, J., et al. (2016). Determining appropriate screening tools and cut-points for cognitive impairment in an elderly Chinese sample. Psychol. Assess. 28, 1345–1353. doi: 10.1037/pas0000271
Memória, C. M., Yassuda, M. S., Nakano, E. Y., and Forlenza, O. V. (2013). Brief screening for mild cognitive impairment: validation of the Brazilian version of the Montreal cognitive assessment. Int. J. Geriatr. Psychiatry 28, 34–40. doi: 10.1002/gps.3787
Nasreddine, Z. S., Phillips, N. A., Bédirian, V., Charbonneau, S., Whitehead, V., Collin, I., et al. (2005). The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 53, 695–699. doi: 10.1111/j.1532-5415.2005.53221.x
Ng, T. P., Feng, L., Lim, W. S., Chong, M. S., Lee, T. S., Yap, K. B., et al. (2015). Montreal cognitive assessment for screening mild cognitive impairment: variations in test performance and scores by education in Singapore. Dement. Geriatr. Cogn. Disord. 39, 176–185. doi: 10.1159/000368827
O’Caoimh, R., Timmons, S., and Molloy, D. W. (2016). Screening for mild cognitive impairment: comparison of "MCI specific" screening instruments. J. Alzheimers Dis. 51, 619–629. doi: 10.3233/JAD-150881
O’Driscoll, C., and Shaikh, M. (2017). Cross-cultural applicability of the Montreal cognitive assessment (MoCA): a systematic review. J. Alzheimers Dis. 58, 789–801. doi: 10.3233/JAD-161042
Pan, F. F., Cui, L., Li, Q. J., and Guo, Q. H. (2022). Validation of a modified Chinese version of Mini-Addenbrooke's cognitive examination for detecting mild cognitive impairment. Brain Behav. 12:e2418. doi: 10.1002/brb3.2418
Paterson, T. S. E., Sivajohan, B., Gardner, S., Binns, M. A., Stokes, K. A., Freedman, M., et al. (2022). Accuracy of a self-administered online cognitive assessment in detecting amnestic mild cognitive impairment. J. Gerontol. B Psychol. Sci. Soc. Sci. 77, 341–350. doi: 10.1093/geronb/gbab097
Peixoto, B., Machado, M., Rocha, P., Macedo, C., Machado, A., Baeta, E., et al. (2018). Validation of the Portuguese version of Addenbrooke's cognitive examination III in mild cognitive impairment and dementia. Adv. Clin. Exp. Med. 27, 781–786. doi: 10.17219/acem/68975
Petersen, R. C., and Negash, S. (2008). Mild cognitive impairment. CNS Spectr. 13, 46–53. doi: 10.1017/s1092852900016151
Petersen, R. C., Smith, G. E., Waring, S. C., Ivnik, R. J., Tangalos, E. G., and Kokmen, E. (1999). Mild cognitive impairment: clinical characterization and outcome. Arch. Neurol. 56, 303–308. doi: 10.1001/archneur.56.3.303
Pinto, T. C. C., Machado, L., Bulgacov, T. M., Rodrigues-Júnior, A. L., Costa, M. L. G., Ximenes, R. C. C., et al. (2019a). Is the Montreal cognitive assessment (MoCA) screening superior to the Mini-mental state examination (MMSE) in the detection of mild cognitive impairment (MCI) and Alzheimer's disease (AD) in the elderly? Int. Psychogeriatr. 31, 491–504. doi: 10.1017/S1041610218001370
Pinto, T. C. C., Machado, L., Costa, M. L. G., Santos, M. S. P., Bulgacov, T. M., Rolim, A. P. P., et al. (2019b). Accuracy and psychometric properties of the Brazilian version of the Montreal cognitive assessment as a brief screening tool for mild cognitive impairment and Alzheimer's disease in the initial stages in the elderly. Dement. Geriatr. Cogn. Disord. 47, 366–374. doi: 10.1159/000501308
Rashedi, V., Foroughan, M., and Chehrehnegar, N. (2021). Psychometric properties of the Persian Montreal cognitive assessment in mild cognitive impairment and Alzheimer disease. Dement. Geriatr. Cogn. Dis. Extra. 11, 51–57. doi: 10.1159/000514673
Roalf, D. R., Moberg, P. J., Xie, S. X., Wolk, D. A., Moelter, S. T., and Arnold, S. E. (2013). Comparative accuracies of two common screening instruments for classification of Alzheimer's disease, mild cognitive impairment, and healthy aging. Alzheimers Dement. 9, 529–537. doi: 10.1016/j.jalz.2012.10.001
Rodríguez-Salgado, A. M., Llibre-Guerra, J. J., Tsoy, E., Penalver-Guia, A. I., Bringas, G., Erlhoff, S. J., et al. (2021). A brief digital cognitive assessment for detection of cognitive impairment in Cuban older adults. J. Alzheimers Dis. 79, 85–94. doi: 10.3233/JAD-200985
Rossetti, H. C., Smith, E. E., Hynan, L. S., Lacritz, L. H., Cullum, C. M., Van Wright, A., et al. (2019). Detection of mild cognitive impairment among community-dwelling African Americans using the Montreal cognitive assessment. Arch. Clin. Neuropsychol. 34, 809–813. doi: 10.1093/arclin/acy091
Senda, M., Terada, S., Takenoshita, S., Hayashi, S., Yabe, M., Imai, N., et al. (2020). Diagnostic utility of the Addenbrooke's cognitive examination-III (ACE-III), Mini-ACE, Mini-mental state examination, Montreal cognitive assessment, and Hasegawa dementia scale-revised for detecting mild cognitive impairment and dementia. Psychogeriatrics 20, 156–162. doi: 10.1111/psyg.12480
Serrano, C. M., Sorbara, M., Minond, A., Finlay, J. B., Arizaga, R. L., Iturry, M., et al. (2020). Validation of the argentine version of the Montreal cognitive assessment test (MOCA): a screening tool for mild cognitive impairment and mild dementia in elderly. Dement Neuropsychol. 14, 145–152. doi: 10.1590/1980-57642020dn14-020007
Sokołowska, N., Sokołowski, R., Oleksy, E., Kasperska, P., Klimkiewicz-Wszelaki, K., Polak-Szabela, A., et al. (2020). Usefulness of the polish versions of the Montreal cognitive assessment 7.2 and the Mini-mental state examination as screening instruments for the detection of mild neurocognitive disorder. Neurol. Neurochir. Pol. 54, 440–448. doi: 10.5603/PJNNS.a2020.0064
Sperling, R. A., Aisen, P. S., Beckett, L. A., Bennett, D. A., Craft, S., Fagan, A. M., et al. (2011). Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the national institute on aging Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 280–292. doi: 10.1016/j.jalz.2011.03.003
Thomann, A. E., Berres, M., Goettel, N., Steiner, L. A., and Monsch, A. U. (2020). Enhanced diagnostic accuracy for neurocognitive disorders: a revised cut-off approach for the Montreal cognitive assessment. Alzheimers Res. Ther. 12:39. doi: 10.1186/s13195-020-00603-8
Townley, R. A., Syrjanen, J. A., Botha, H., Kremers, W. K., Aakre, J. A., Fields, J. A., et al. (2019). Comparison of the short test of mental status and the Montreal cognitive assessment across the cognitive Spectrum. Mayo Clin. Proc. 94, 1516–1523. doi: 10.1016/j.mayocp.2019.01.043
Trzepacz, P. T., Hochstetler, H., Wang, S., Walker, B., and Saykin, A. J.Alzheimer’s Disease Neuroimaging Initiative (2015). Relationship between the Montreal cognitive assessment and Mini-mental state examination for assessment of mild cognitive impairment in older adults. BMC Geriatr. 15:107. doi: 10.1186/s12877-015-0103-3
Tsai, J. C., Chen, C. W., Chu, H., Yang, H.-L., Chung, M.-H., Liao, Y.-M., et al. (2016). Comparing the sensitivity, specificity, and predictive values of the Montreal cognitive assessment and Mini-mental state examination when screening people for mild cognitive impairment and dementia in Chinese population. Arch. Psychiatr. Nurs. 30, 486–491. doi: 10.1016/j.apnu.2016.01.015
Utoomprurkporn, N., Woodall, K., Stott, J., Costafreda, S. G., and Bamiou, D. E. (2020). Hearing-impaired population performance and the effect of hearing interventions on Montreal cognitive assessment (MoCA): systematic review and meta-analysis. Int. J. Geriatr. Psychiatry 35, 962–971. doi: 10.1002/gps.5354
Wang, B. R., Zheng, H. F., Xu, C., Sun, Y., Zhang, Y. D., and Shi, J. Q. (2019). Comparative diagnostic accuracy of ACE-III and MoCA for detecting mild cognitive impairment. Neuropsychiatr. Dis. Treat. 15, 2647–2653. doi: 10.2147/NDT.S212328
Wong, A., Law, L. S., Liu, W., Wang, Z., Lo, E. S. K., Lau, A., et al. (2015). Montreal cognitive assessment: one cutoff never fits all. Stroke 46, 3547–3550. doi: 10.1161/STROKEAHA.115.011226
Yan, M., Yin, H., Meng, Q., Wang, S., Ding, Y., Li, G., et al. (2021). A virtual supermarket program for the screening of mild cognitive impairment in older adults: diagnostic accuracy study. JMIR Serious Games. 9:e30919. doi: 10.2196/30919
Yeung, P. Y., Wong, L. L., Chan, C. C., Leung, J. L., and Yung, C. Y. (2014). A validation study of the Hong Kong version of Montreal cognitive assessment (HK-MoCA) in Chinese older adults in Hong Kong. Hong Kong Med. J. 20, 504–510. doi: 10.12809/hkmj144219
Yu, J., Li, J., and Huang, X. (2012). The Beijing version of the Montreal cognitive assessment as a brief screening tool for mild cognitive impairment: a community-based study. BMC Psychiatry 12:156. doi: 10.1186/1471-244X-12-156
Keywords: cognitive screen, mild cognitive impairment, cognitively unimpaired, diagnostic accuracy, cutoff score
Citation: Malek-Ahmadi M and Nikkhahmanesh N (2024) Meta-analysis of Montreal cognitive assessment diagnostic accuracy in amnestic mild cognitive impairment. Front. Psychol. 15:1369766. doi: 10.3389/fpsyg.2024.1369766
Edited by:
Alessio Facchin, University of Milano-Bicocca, ItalyReviewed by:
Ciro Rosario Ilardi, IRCCS SYNLAB SDN, ItalyNattawan Utoomprurkporn, Chulalongkorn University, Thailand
Copyright © 2024 Malek-Ahmadi and Nikkhahmanesh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael Malek-Ahmadi, bWljaGFlbC5tYWxla2FobWFkaUBiYW5uZXJoZWFsdGguY29t
†ORCID: Michael Malek-Ahmadi, http://orcid.org/0000-0001-9901-3650
 Michael Malek-Ahmadi
Michael Malek-Ahmadi Nia Nikkhahmanesh
Nia Nikkhahmanesh