- 1Faculty of Psychology, Tianjin Normal University, Tianjin, China
- 2National Key Laboratory of Human Factor & Ergonomics, China National Institute of Standardization, Beijing, China
Social exclusion stands as a source of social discord and holds substantial research value. Prior investigations on social exclusion have overlooked the interactive relationship between the excluded individuals and the observers. Hence, this study comparatively explores the neural mechanisms underlying the psychological responses of two distinct roles within the same social exclusion context. A total of 35 pairs (19 pairs of females) participated in the experiment. Within each pair, one individual assumed the role of a socially excluded participant (target), while the other acted as a social exclusion observer. Targets engaged in an online ball-passing game where controlled ball allocations to the participants created an exclusion scenario. Meanwhile, observers spectated the targets playing the game. Throughout the ball-passing activity, functional near-infrared spectroscopy (fNIRS) recorded the blood oxygen data in the prefrontal cortex (PFC) and temporoparietal junction (TPJ) of both participants. Our findings revealed varied levels of rejection sensitivity elicited by direct or observed social exclusion experiences. Additionally, distinct patterns of neural activation were observed: targets displayed conditional differences in the medial prefrontal cortex (mPFC), while male observers exhibited conditional activation differences in the mPFC, and female observers showed conditional activation differences in the right dorsolateral prefrontal cortex (dlPFC). This study juxtaposes the behavioral and neural activation variances between targets and observers within the same social context, offering a novel perspective on investigating the neural mechanisms of social exclusion.
1 Introduction
Social exclusion occurs when an individual’s need for belonging is obstructed by rejection from a social group (Williams, 2007, 2009). Humans naturally seek social connections, a trait essential for survival. Exclusion disrupts these connections, leading to isolation, impacting cognition, emotions, and behavior (Baumeister et al., 2002; Twenge and Campbell, 2003; Iffland et al., 2014). Excluded individuals are prone to depression, anger, and aggression, potentially causing broader societal issues. Researching the mechanisms and factors behind social exclusion is crucial for resolving conflicts and improving individual well-being.
Research on social exclusion has traditionally examined both excluded individuals and observers. Studies reveal that ostracism leads to social pain, sharing physiological foundations with physical pain (Eisenberger et al., 2003). Even brief exclusion triggers strong rejection feelings, leading to psychological responses like diminished self-esteem and reduced meaning (Williams, 2007). Exclusion can provoke aggressive behavior (Ren et al., 2018). Observers of exclusion scenarios may experience vicarious pain (Giesen and Echterhoff, 2018), with stronger guilt, anger, and sadness when witnessing marginalized groups’ exclusion compared to dominant groups (Petsnik and Vorauer, 2020). Empathy is the ability to share the emotions and sensations of others (Singer et al., 2004). There are many theoretical explanations for vicarious exclusion, but empathy theory is the only one that can simultaneously explain changes in the fundamental needs, emotions, behaviors, and neural networks of individuals experiencing vicarious exclusion (Wesselmann et al., 2009).
Research indicates that different sexes exhibit distinct responses when faced with social exclusion (Hawes et al., 2012; Seidel et al., 2013; Brown et al., 2019). In females, rejection affects various variables such as negative emotions, control, sadness, anger, disgust, and happiness, whereas rejected males tend to display more anger and a heightened need for belongingness, control, and meaningful existence (Iffland et al., 2014). Additionally, a study investigating social exclusion in observers revealed gender disparities, with boys showing increased demand for control in social settings when exposed to social exclusion, whereas no such variations were evident among girls (Marinović and Träuble, 2021). Nevertheless, there’s a dearth of research on gender differences, especially regarding systematic investigations into neural response disparities among individuals of different genders experiencing social exclusion.
Prior research on the psychological and neural mechanisms of social exclusion has typically focused on either the individuals directly experiencing exclusion (‘targets’) or those observing it (‘observers’), neglecting the interaction between both. However, social exclusion involves both targets and observers, and the presence of observers can impact the targets’ psychological well-being, intensifying their pain (Hales et al., 2021). Additionally, there is a lack of studies exploring gender differences in the neural mechanisms of social exclusion.
To address these gaps, this study aims to simultaneously measure the neural activation patterns of both targets and observers, incorporating gender as a variable. Using functional near-infrared spectroscopy (fNIRS), which offers high ecological validity and good temporal resolution for studying social interactions (Ferrari and Quaresima, 2012; Pinti et al., 2020), this study will capture brain activity signals. Previous research has shown that social exclusion increases activation in the prefrontal cortex (PFC) among adolescents (Will et al., 2016) and enhances activation in the temporoparietal junction (TPJ) of observers (Tousignant et al., 2018). Given the importance of the PFC and TPJ in social exclusion, they have been selected as regions of interest (ROI) for measurement (Derntl et al., 2010; Meyer et al., 2013; Wudarczyk et al., 2015; Will et al., 2016; Radke et al., 2018; Tousignant et al., 2018; Jie et al., 2019; Yanagi et al., 2020).
Based on these considerations, we propose the following hypotheses: (1) Targets undergoing direct exclusion may exhibit stronger emotional reactions and greater neural activation in the PFC and TPJ compared to observers; (2) Males and females show differences in brain activation regions when encountering social exclusion. This study has significant theoretical implications: (1) It provides a comprehensive view by measuring neural activation in both targets and observers, revealing their psychological and neural mechanisms; (2) By including gender as a variable, it explores different brain activation patterns in males and females, aiding in understanding gender-specific neural mechanisms and supporting personalized psychological interventions.
2 Method
2.1 Participants
Using G*Power 3 software, sample size estimation based on relevant research (Hartgerink et al., 2015) revealed an effect size (d) greater than |1.4|, suggesting 18 participants would achieve a statistical power of 0.95. However, 18 participants are too few for fNIRS-based research, so we expanded the sample size. Under controlled social exclusion conditions, this study included 35 pairs of participants (19 female pairs; M = 20.15 years, SD = 2.37). Each pair was same-gender, unacquainted, right-handed, with normal or corrected vision, and no psychiatric history. The study was ethically approved by Tianjin Normal University’s Ethics Committee. Participants will receive a monetary compensation after the completion of the experiment.
2.2 Experimental design
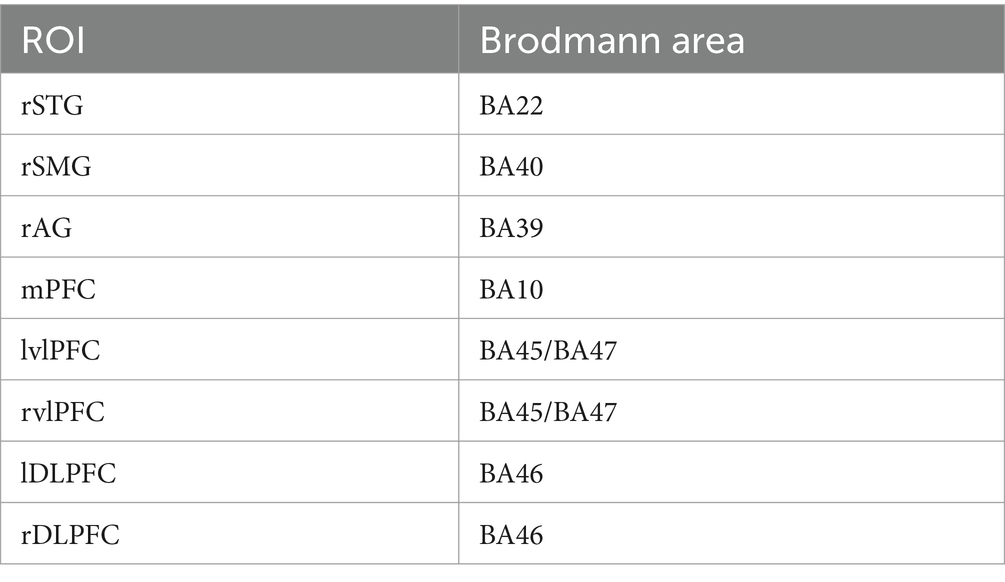
The experiment used the Cyberball paradigm to simulate exclusion scenarios (Williams et al., 2000; Tousignant et al., 2018). Participants were paired as ‘targets’ or ‘observers’. Targets played a ball-tossing game, while observers watched in the same lab setup. Targets were told they were playing with two others (actually computer confederates) and used keys to choose the recipients. Observers watched and considered thoughts during inclusion (equal tosses) and exclusion (one toss) conditions, arranged pseudo-randomly across eight blocks. A 20-s rest interval separated each block to ensure the participants’ blood oxygen levels returned to baseline. Furthermore, the time intervals between ball tosses by the two computer-programmed confederates were set to vary within a broad range of milliseconds. Please refer to Figure 1 for details of the experimental design.
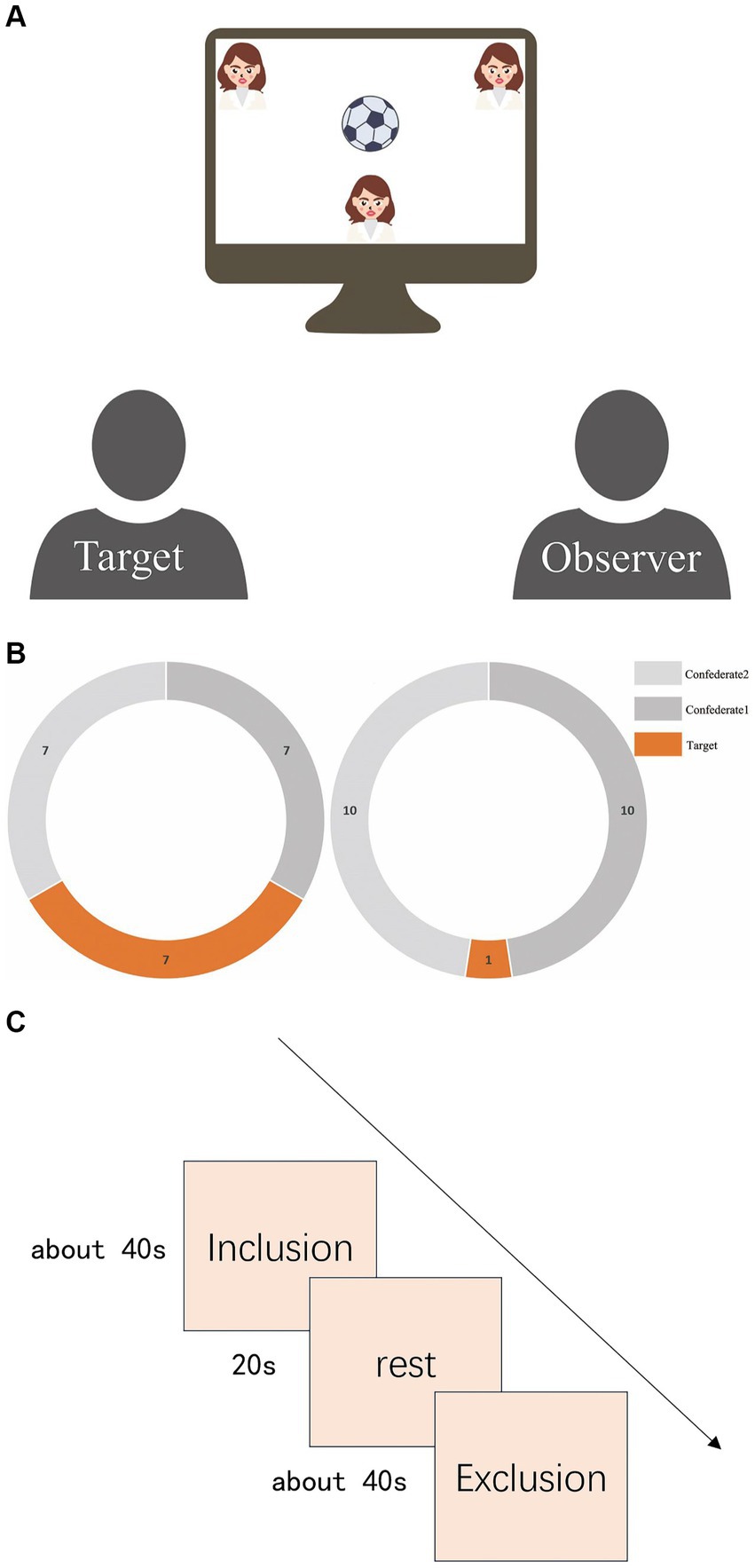
Figure 1. (A) Schematic of experimental scene. The Target is on the left, and the Observer is on the right. Both will be in the same room, watching a screen. At the top of the screen are computer-programmed confederates, but both participants will be told that these are real people participating in the experiment from another laboratory. The small figure at the bottom of the screen represents the Target. The Target will follow prompts to pass the ball by pressing keys, while the Observer will only watch this process and consider the intentions behind the ball passes. (B) Number of catches by targets. The left side represents the number of catches in the inclusion blocks, where the target and the other two confederates each caught the ball seven times. The right side represents the number of catches in the exclusion blocks, where the target could only catch the ball once. (C) Experimental procedure flowchart. There were a total of eight blocks of trials arranged in a pseudo-random distribution sequence: “inclusion, inclusion, exclusion, inclusion, exclusion, exclusion, inclusion, exclusion. “A 20-s rest interval separated each block to ensure the participants’ blood oxygen levels returned to baseline.
2.3 Data acquisition
2.3.1 Post-game assessment
After the completion of all eight blocks of the Cyberball game, the post-Cyberball assessment was used to evaluate the degree of social exclusion experienced by the targets. This scale, which ranges from 1 (indicating ‘completely absent’) to 5 (indicating ‘completely present’) on a five-point rating system, encompasses dimensions such as Belonging, Self-Esteem, Control, and Meaningful Existence. Each of these dimensions comprises five items (Williams, 2009; Cogoni et al., 2018; Ren et al., 2018). Additionally, a questionnaire on empathy was administered to measure observers’ empathy levels toward the targets. This questionnaire was based on previous studies (Fengfeng et al., 2010; Masten et al., 2011), and the relevant references have been included in the Supplementary materials for readers’ convenience.
2.3.2 Empathy and rejection sensitivity assessment
We employed the Chinese revised version of the Interpersonal Reactivity Index (IRI-C) (Fengfeng et al., 2010) to gather empathy data from both targets and observers (Cronbach’s α = 0.75). The IRI, developed by Davis (1980, 1983), evaluates empathy across four dimensions: Emotional Understanding, Empathy Concern, Personal Distress, and Perspective Taking, using 22 items on a 5-point Likert scale. We also used the Chinese revised version of the Rejection Sensitivity Questionnaire (RSQ) (Wei, 2012) to assess participants’ sensitivity to rejection (Downey and Feldman, 1996). Participants imagined themselves in specific scenarios and rated the likelihood of receiving assistance and their apprehension about the response on a 6-point scale. The RSQ includes 18 scenarios and 36 items.
2.3.3 Neural signal assessment
The LABNIRS functional near-infrared spectroscopy imaging system (LABNIRS/16, Shimadzu Corporation, Kyoto, Japan) was used. It employs three wavelengths (780, 805, and 830 nm) and the modified Beer–Lambert law (MBLL) to measure changes in oxyhemoglobin (Δ[HbO]), deoxyhemoglobin (Δ[HbR]), and total hemoglobin (Δ[HbT]) concentrations continuously. The sampling rate was 30.303 Hz. After the experiment, a 3D positioning system collected brain positional information. Each participant’s optodes were divided into two sections: the PFC with a 3 × 7 array of 10 emitters and 11 detectors forming 32 channels, and the temporo-parietal junction with a 3 × 3 array of four emitters and five detectors forming 12 channels (see Figure 2). The emitter-detector distance was 3 cm.
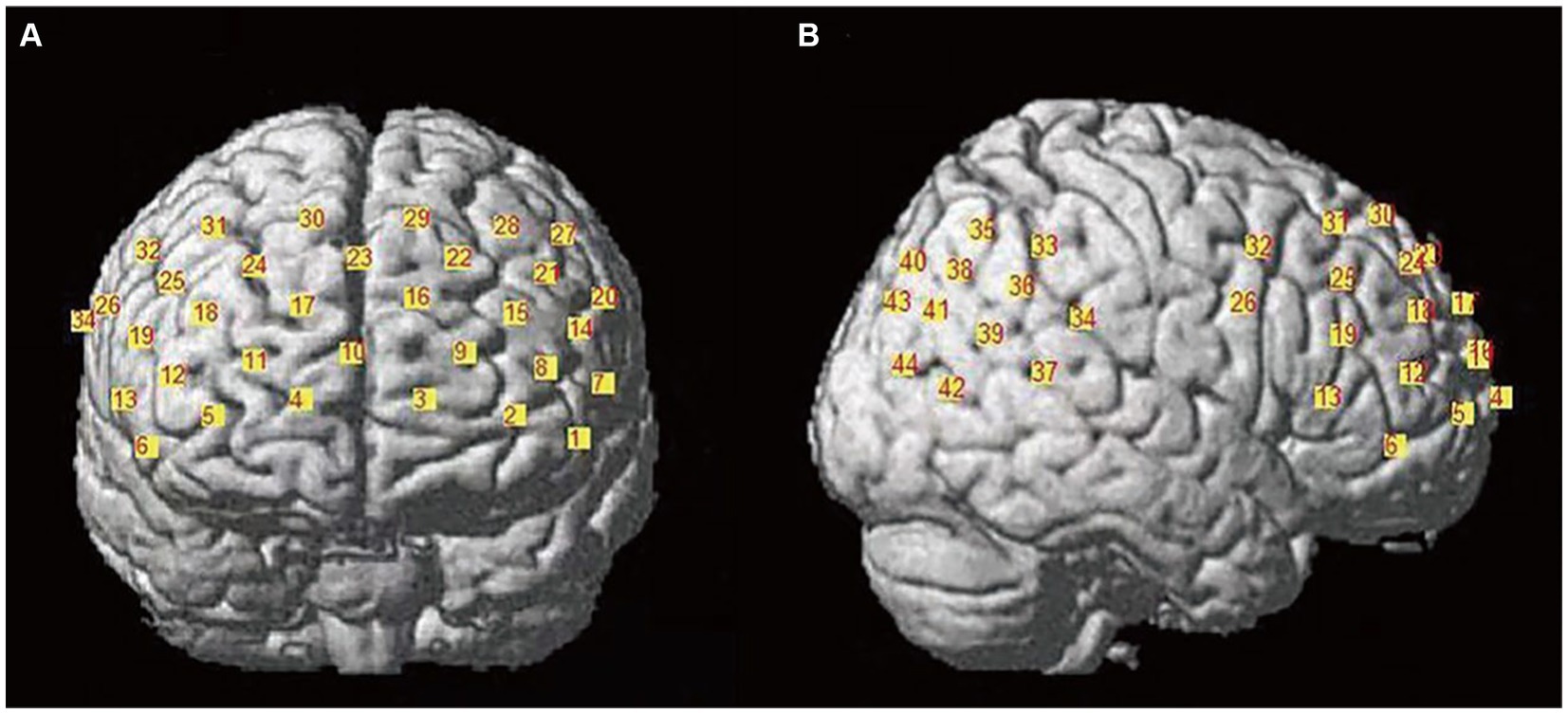
Figure 2. Channel layout diagram. (A) The left side shows the prefrontal cortex channel layout, comprising a total of 32 channels; (B) the right side shows the temporoparietal junction channel layout, consisting of 12 channels.
2.4 Data analysis
2.4.1 Behavioral data analysis
Firstly, descriptive statistics and reliability analyses were conducted on the post-questionnaire results to understand the extent of psychological changes elicited by the experimental manipulation and to establish a foundation for further data selection. Subsequently, a correlation analysis was performed on the post-questionnaires of both groups to examine whether there was a correlation between the psychological changes in the two groups. Finally, independent sample t-tests were employed to assess differences between the IRI scores and the RSQ scores for both groups. This aimed to investigate potential discrepancies in empathy and rejection sensitivity between the two groups.
2.4.2 Neural data analysis
Some studies have indicated a heightened sensitivity of oxyhemoglobin to task stimuli (Hoshi, 2003). Hence, this study exclusively focused on the analysis of Δ[HbO] indicators. Individual raw data underwent processing using NIRS_SPM, operated through Matlab2013a (Ye et al., 2009). The data were processed based on the Hemodynamic Response Functions (HRF) and Wavelet-Minimum Description Length (Wavelet-MDL) method to eliminate noise sources such as head movement and heartbeat, as well as drift. The HRF method was selected to conduct low-pass filtering for high-frequency noise removal. Subsequently, task-related β-values for each channel were assessed using the General Linear Model (GLM).
The study primarily focused on the PFC and TPJ. Therefore, The ROIs were delineated for these areas. The PFC encompassed bilateral dorsolateral prefrontal cortex (dlPFC), medial prefrontal cortex (mPFC), and bilateral ventrolateral prefrontal cortex (vlPFC), while the TPJ comprised the right superior temporal gyrus (STG), right angular gyrus (AG), and right supramarginal gyrus (SMG). These corresponded to Brodmann areas as outlined in Table 1. Considering the lower spatial resolution and signal-to-noise ratio of NIRS technology, this study employed individualized β-value weighted averaging of channels to transform them into ROIs. Previous research often used averaged channel ROIs, neglecting individualization when converting channel β-values to ROIs (Okamoto et al., 2009; Sun et al., 2019). However, this study collected spatial information for all participants’ channels, enabling individualized channel-to-ROI operations based on each individual’s spatial coordinates. Specifically, for example, participant A has channels 5, 7, and 10, with spatial coverage ratios in the medial prefrontal cortex of 60, 70, and 80%, respectively, (to avoid the influence of extreme values, only brain regions with coverage probabilities greater than 50% are taken as channels for merging into specific brain regions). Under the exclusion condition, the likelihood estimates for these three channels are β5, β7, and β10, respectively. Therefore, we calculate the likelihood estimate for participant A’s medial prefrontal cortex under the exclusion condition as:
The inclusion condition is similar. Each ROI for all participants is calculated using this method. Although this approach requires more work, it provides individualized localization information, theoretically offering greater credibility and validity. Then we will conduct a Fisher’s Z transformation on the β values based on the ROI to obtain standardized β values for the subsequent statistical tests.
Subsequently, a repeated measures analysis of variance (ANOVA) was conducted using SPSS software (IBM SPSS Statistics 26.0). The design included factors of 8 (ROIs: right STG, right SMG, right AG, mPFC, left vlPFC, right vlPFC, left dlPFC, right dlPFC) × 2 (conditions: inclusion, exclusion) × 2 (gender: male, female). Corrections for degrees of freedom were applied using the Greenhouse–Geisser method, and post-hoc multiple comparison corrections were conducted using the Bonferroni method.
2.4.3 Neuro-behavioral correlation analysis
We conducted a correlation analysis between brain activation during exclusion compared to inclusion and behavioral data. Specifically, we calculated the standardized β values difference (exclusion condition minus inclusion condition) for the eight ROIs in both Targets and Observers. We then performed Pearson correlation analysis between the exclusion–inclusion increment and IRI-C scores as well as RSQ scores. Finally, we applied FDR correction to the results based on the number of ROIs.
3 Results
3.1 Behavioral data results
The post-questionnaire for targets yielded a Cronbach’s α coefficient of 0.88. Targets self-assessed their average probability of receiving the ball at 23.51%. A one-sample t-test comparing the observed ball receptions by targets (33.3%) against an expected rate revealed significant awareness of receiving fewer passes, t(34) = −5.79, p < 0.0001, Cohen’s d = 0.98. Moreover, descriptive statistics were conducted on the Need Threat Scale, showing an overall mean score of 56.29, indicating a range between ‘rarely (60)’ and ‘somewhat true (40)’. This suggests that targets experienced exclusion during the experiment, indicating the effectiveness of our experimental manipulation.
The post-questionnaire for observers exhibited a Cronbach’s α coefficient of 0.76. Descriptive analysis of the questionnaire revealed that the average empathy score for observers was 25.83, which falls within the range of ‘somewhat appropriate (20)’ to ‘reasonably appropriate (30)’. This suggests that observers developed empathy toward the targets during the experimental process.
Correlation analysis was conducted between the post-questionnaire results of targets and observers, revealing a non-significant correlation (r = −0.14, p = 0.41). An independent sample t-test was performed on the IRI-C and RSQ scores of both groups. Observers’ rejection sensitivity (the mean score = 19.70) was notably higher than that of targets (the mean score = 16.66), t(78) = −2.67, p < 0.01, Cohen’s d = 0.64.
3.2 Neural data results
3.2.1 Targets results
The Greenhouse–Geisser correction did not yield significant results; therefore, we referred to the multivariate test results for a more robust assessment. There was a significant interaction between the condition and ROIs, F(7, 27) = 2.78, p = 0.026, ηp2 = 0.418. Simple effects analysis revealed that within the mPFC, activation was greater under the exclusion condition compared to the inclusion condition, F(1, 33) = 5.15, p = 0.03, ηp2 = 0.14.
3.2.2 Observers results
An 8 (ROIs: right STG, right SMG, right AG, mPFC, left vlPFC, right vlPFC, left dlPFC, right dlPFC) × 2 (condition: inclusion, exclusion) × 2 (gender: male, female) mixed-measures analysis of variance was conducted to analyze the activation across regions of interest. A significant three-way interaction was found, F(7, 27) = 2.77, p = 0.039, ηp2 = 0.078. Simple effects analyses revealed that in males, the mPFC exhibited higher activation under the exclusion condition compared to the inclusion condition, F(1, 33) = 7.19, p = 0.011, ηp2 = 0.18. Additionally, in females, the right dlPFC showed greater activation under the exclusion condition compared to the inclusion condition, F(1, 33) = 4.19, p = 0.049, ηp2 = 0.11. All significant results of neural activation differences are presented in Figure 3.
Figure 3. (A) Activation of target’s mPFC under different conditions. (B) Activation of mPFC in male observers under different conditions. (C) Activation of rdlPFC in female observers under different conditions.
3.3 Neuro-behavioral results
After FDR correction, we found a positive correlation between rSMG brain activation and RSQ scores in the target group (r = 0.48, p < 0.05). This means that the higher the rejection sensitivity of the participants, the stronger the rSMG activation during exclusion. Other neuro-behavioral correlation results were not significant; detailed results can be found in Figure 4.
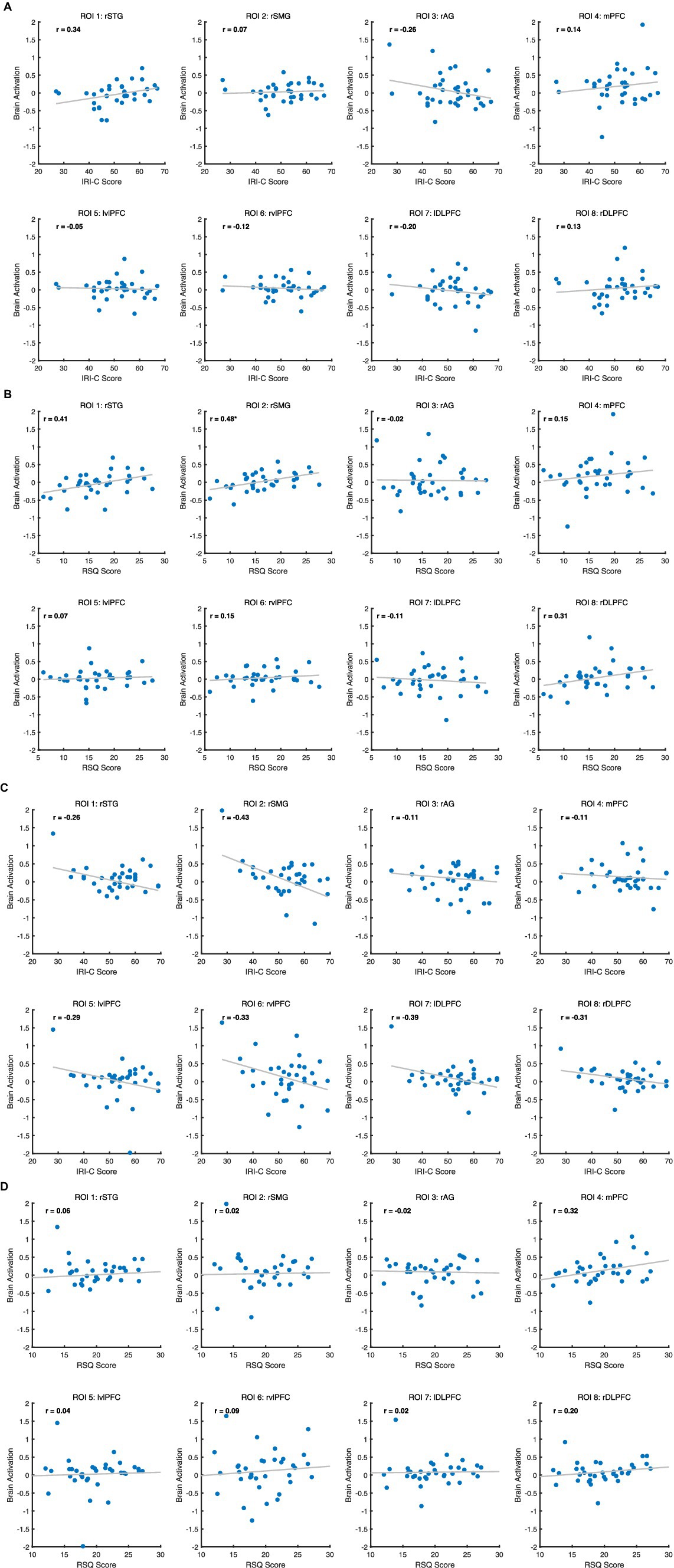
Figure 4. (A) Correlation between ROIs and IRI-C score for Target. (B) Correlation between ROIs and RSQ score for Target. (C) Correlation between eight ROIs and IRI-C score for Observer. (D) Correlation between eight ROIs and RSQ score for Observer.
4 Discussion
Social exclusion stands as a crucial area in the study of emotion generation and development. In order to simulate more authentic responses of individuals within scenarios of social exclusion and to compare the neural mechanisms between vicarious experience and vicarious exposure to social exclusion, this study employs fNIRS technology to concurrently measure the PFC and TPJ of both targets and observers. Additionally, we investigate whether gender influences psychological and neural response patterns. At the behavioral level, it was found that targets and observers exhibited differences in levels of rejection sensitivity following experiences of social exclusion. The extent of exclusion experienced by targets did not correlate with the empathy observed in observers. In terms of neural mechanisms, compared to inclusion blocks, targets exhibited significant activation in the mPFC when exposed to exclusion blocks. Moreover, observers showed gender-specific differences in neural activation patterns: males, similar to targets, displayed increased activity in the mPFC during exclusion blocks compared to inclusion blocks, whereas females exhibited heightened activation in the right dlPFC during exclusion blocks. Finally, we found that the increase in rSMG activation in Targets during exclusion was significantly positively correlated with their rejection sensitivity.
The finding that targets exhibit lower rejection sensitivity compared to observers is noteworthy. Rejection sensitivity reflects an individual’s anxiety about anticipating negative feedback, their tendency to perceive rejection, and their propensity to overreact (Downey and Feldman, 1996). It encompasses emotional and psychological responses to rejection, denial, or non-acceptance, and is influenced by factors such as gender, appearance, personality, early trauma, attachment styles, parenting, peer relationships, and cultural differences (Qinyi et al., 2019). In this study, both targets and observers underwent social exclusion, but targets experienced it directly while observers experienced it vicariously. We hypothesize that direct experience may lead to temporary emotional numbing. Baumeister et al. (2009) suggest that social exclusion can induce emotional and physiological numbing, making individuals less sensitive to emotional stimuli and leading to delayed reactions. This numbing helps in reallocating cognitive resources away from painful information, similar to how animals release opioid-like substances to mitigate pain (Baumeister et al., 2009). Thus, compared to vicarious observers, those who directly experience social exclusion show greater emotional numbness and lower rejection sensitivity.
The sense of exclusion in targets does not correlate with the empathy level of observers, potentially due to the social pain cues evoked by social exclusion, which are significantly influenced by individual experiences compared to cues from physical pain. In a study on social pain empathy, it was found that individuals who vicarious experienced social exclusion tend to underestimate the severity of social pain felt by targets, unless they genuinely comprehend the extent of social exclusion, such as through vicarious experience (Nordgren et al., 2011). Reviews on the effects of vicarious social exclusion suggest that it is impacted by the social relationship, familiarity, and similarity between observers and targets (Singer and Lamm, 2009). Consequently, unfamiliarity, lack of familiarity, and dissimilarity in social relationships diminish the extent to which basic needs of individuals experiencing vicarious exclusion are obstructed (Giesen and Echterhoff, 2018). The perception of rejection by targets is also influenced by individual personality factors and early experiences. Hence, each individual exhibits varying degrees of perceived threat when faced with exclusion triggered by the Cyberball paradigm. In summary, the empathetic resonance to social pain is not as straightforward as physical pain and is contingent upon the perceptions of both targets and observers.
Targets experiencing social exclusion show increased activation in the mPFC compared to experiencing social inclusion, akin to findings in previous related studies (Moor et al., 2012; Vijayakumar et al., 2017; Wasylyshyn et al., 2018). The mPFC, as part of the social monitoring system, is considered a crucial structure in dynamic exclusion processes (Kawamoto et al., 2015). Studies have revealed that individuals with strong secure attachment exhibit weaker mPFC activation when faced with exclusion, rendering them less susceptible to experiencing exclusion (Karremans et al., 2011). Additionally, poorer quality of adolescent community relationships is associated with increased mPFC activation in response to social exclusion (Gonzalez et al., 2015). Individuals with specific mental health conditions also demonstrate varied mPFC responses to social exclusion; for example, people with schizophrenia exhibit decreased activity in the medial prefrontal cortex (mPFC) during social exclusion, while individuals diagnosed with borderline personality disorder and those who engage in self-harm show increased mPFC activation during instances of social exclusion (Gradin et al., 2012; Groschwitz et al., 2016; Wrege et al., 2019). The close association between the mPFC and the recognition of social exclusion is evident. In this study, the mPFC of socially excluded targets exhibited significant activation after experiencing social exclusion, compared to the inclusion phase, reiterating the mPFC’s close association with firsthand experiences of social exclusion.
In the group of observers, gender differences in brain region activation were identified, suggesting that men and women exhibit distinct neural activities when observing others experiencing social exclusion. Specifically, significant conditional differences in the mPFC were observed in males, showing stronger activation when witnessing others being excluded compared to included. Conversely, females displayed significant enhancement in right dlPFC activation when observing others facing exclusion. As mentioned earlier, the mPFC is associated with theory of mind and self-processing (Singer et al., 2006), while the right dlPFC plays a significant role in emotional regulation (Eisenberger et al., 2003). These findings suggest that when witnessing others undergoing social exclusion, males engage the brain areas related to theory of mind, while females show more emotional arousal and regulation. Men might approach situations of social exclusion with a more rational perspective, engaging in more top-down processing of contextual information, contemplating the source and reasons behind social exclusion. Conversely, women tend to be more emotionally responsive, displaying greater empathy toward socially excluded targets and triggering emotional resonance (Derntl et al., 2010; Iffland et al., 2014).
Target’s rSMG activation correlates positively with their rejection sensitivity. The rSMG, part of the TPJ, crucially handles social cognition and empathy, affecting interaction with others and processing social cues (Tousignant et al., 2018). This region often activates during social information processing and emotional responses. Rejection sensitivity (RSQ scores) mirrors one’s anticipation and emotional reaction to social exclusion. Higher sensitivity suggests greater emotional vulnerability to rejection, intensifying negative emotions and stress during exclusion. This emotional response likely boosts rSMG activity, engaged in processing such complex social cues. Surprisingly, we found no brain region activation in observers correlating with their IRI-C levels. Observers may favor cognitive over emotional processing of exclusion, focusing on event context and causality, unlike Targets emotionally affected by exclusion. Therefore, their IRI-C levels may be influenced by non-neural factors. Future studies should refine paradigms for a more comprehensive investigation.
The study has some limitations. Firstly, the inability to measure the anterior cingulate cortex (ACC), an important brain region associated with social exclusion, was due to the constraints of fNIRS in detecting deep brain areas. Accommodating deep brain assessments was unfeasible to achieve multi-brain real-time interaction measurements. Secondly, the participant pool predominantly comprised university students, resulting in a narrow sample, raising questions about the generalizability of the findings to a broader population. Lastly, the ROI analysis in this study focused solely on condition differences within the regions of interest, leaving uncertainties about differences in other cortical brain regions. Future research could involve comprehensive measurements and comparisons across the entire cortical area within this paradigm.
In conclusion, the study yields the following conclusions: Experiencing social exclusion directly versus vicarious evokes different levels of rejection sensitivity. The experienced feelings after personal social exclusion are not directly related to the empathy level experienced following vicarious social exclusion. Direct and vicarious experiences of social exclusion result in distinct patterns of brain activation, and gender influences the expression of these patterns.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Tianjin Normal University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JS: Formal analysis, Data curation, Funding acquisition, Conceptualization, Writing – review & editing, Writing – original draft, Methodology. TL: Methodology, Formal analysis, Data curation, Writing – review & editing, Writing – original draft. YZ: Resources, Funding acquisition, Conceptualization, Methodology, Writing – review & editing, Writing – original draft. MC: Writing – review & editing, Writing – original draft. ZJ: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by Youth Fund for Humanities and Social Sciences Research, Ministry of Education (MOE) of China (19YJC190021); Philosophy and Social Science Foundation of China (22BSH102); Tianjin Research Innovation Project for Postgraduate Students (2019YJSS124); China National Institute of Standardization through the “special funds for the basic R&D undertakings by welfare research institutions” (292022Y-9455) and the Science &Technology Plan Project of State Administration for Market Regulation (2021MK158).
Acknowledgments
We would like to acknowledge the assistance of ChatGPT, developed by OpenAI, version GPT-4, for providing language editing and refinement for this manuscript. The use of this generative AI tool has helped improve the clarity and coherence of the text.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyg.2024.1368214/full#supplementary-material
References
Baumeister, R. F., DeWall, C. N., and Vohs, K. D. (2009). Social rejection, control, numbness, and emotion: how not to be fooled by Gerber and wheeler. Perspect. Psychol. Sci. 4, 489–493. doi: 10.1111/j.1745-6924.2009.01159.x
Baumeister, R. F., Twenge, J. M., and Nuss, C. K. (2002). Effects of social exclusion on cognitive processes: anticipated aloneness reduces intelligent thought. J. Pers. Soc. Psychol. 83, 817–827. doi: 10.1037/0022-3514.83.4.817
Brown, M., Sacco, D. F., and Medlin, M. M. (2019). Approaching extraverts: socially excluded men prefer extraverted faces. Personal. Individ. Differ. 137, 198–203. doi: 10.1016/j.paid.2018.09.007
Cogoni, C., Carnaghi, A., and Silani, G. (2018). Reduced empathic responses for sexually objectified women: an fMRI investigation. Cortex 99, 258–272. doi: 10.1016/j.cortex.2017.11.020
Davis, M. H. (1980). A multidimensional approach to individual differences in empathy. JSAS Catalog Selected Documents Psychol. 10:85.
Davis, M. H. (1983). Measuring individual differences in empathy: evidence for a multidimensional approach. J. Pers. Soc. Psychol. 44, 113–126. doi: 10.1037/0022-3514.44.1.113
Derntl, B., Finkelmeyer, A., Eickhoff, S., Kellermann, T., Falkenberg, D. I., Schneider, F., et al. (2010). Multidimensional assessment of empathic abilities: neural correlates and gender differences. Psychoneuroendocrinology 35, 67–82. doi: 10.1016/j.psyneuen.2009.10.006
Downey, G., and Feldman, S. (1996). Implications of rejection sensitivity for intimate relationships. J. Pers. Soc. Psychol. 70, 1327–1343. doi: 10.1037/0022-3514.70.6.1327
Eisenberger, N. I., Lieberman, M. D., and Williams, K. D. (2003). Does rejection hurt? An FMRI study of social exclusion. Science 302, 290–292. doi: 10.1126/science.1089134
Fengfeng, Z., Yi, D., Kai, W., Zhiyu, Z., and Lunfang, X. (2010). Reliability and validity of the Chinese version of the interpersonal reactivity index-C. Chin. J. Clin. Psych. 18, 155–157. doi: 10.16128/j.cnki.1005-3611.2010.02.019
Ferrari, M., and Quaresima, V. (2012). A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. NeuroImage 63, 921–935. doi: 10.1016/j.neuroimage.2012.03.049
Giesen, A., and Echterhoff, G. (2018). Do I really feel your pain? Comparing the effects of observed and personal ostracism. Personal. Soc. Psychol. Bull. 44, 550–561. doi: 10.1177/0146167217744524
Gonzalez, M. Z., Beckes, L., Chango, J., Allen, J. P., and Coan, J. A. (2015). Adolescent neighborhood quality predicts adult dACC response to social exclusion. Soc. Cogn. Affect. Neurosci. 10, 921–928. doi: 10.1093/scan/nsu137
Gradin, V. B., Waiter, G., Kumar, P., Stickle, C., Milders, M., Matthews, K., et al. (2012). Abnormal neural responses to social exclusion in schizophrenia. PLoS One 7:e42608. doi: 10.1371/journal.pone.0042608
Groschwitz, R. C., Plener, P. L., Groen, G., Bonenberger, M., and Abler, B. (2016). Differential neural processing of social exclusion in adolescents with non-suicidal self-injury: an fMRI study. Psychiatry Res. Neuroimaging 255, 43–49. doi: 10.1016/j.pscychresns.2016.08.001
Hales, A. H., McIntyre, M. M., Rudert, S. C., Williams, K. D., and Thomas, H. (2021). Ostracized and observed: the presence of an audience affects the experience of being excluded. Self Identity 20, 94–115. doi: 10.1080/15298868.2020.1807403
Hartgerink, C. H., van Beest, I., Wicherts, J. M., and Williams, K. D. (2015). The ordinal effects of ostracism: a meta-analysis of 120 Cyberball studies. PLoS One 10:e0127002. doi: 10.1371/journal.pone.0127002
Hawes, D. J., Zadro, L., Fink, E., Richardson, R., O'Moore, K., Griffiths, B., et al. (2012). The effects of peer ostracism on children's cognitive processes. Eur. J. Dev. Psychol. 9, 599–613. doi: 10.1080/17405629.2011.638815
Hoshi, Y. (2003). Functional near-infrared optical imaging: utility and limitations in human brain mapping. Psychophysiology 40, 511–520. doi: 10.1111/1469-8986.00053
Iffland, B., Sansen, L. M., Catani, C., and Neuner, F. (2014). Rapid heartbeat, but dry palms: reactions of heart rate and skin conductance levels to social rejection. Front. Psychol. 5:956. doi: 10.3389/fpsyg.2014.00956
Jie, J., Luo, P., Zhuang, M., Fan, M., Wang, Y., Yang, Y., et al. (2019). Gender differences in empathic responses to others’ economic payoffs: an event-related potentials study. Exp. Brain Res. 237, 1347–1359. doi: 10.1007/s00221-019-05518-x
Karremans, J. C., Heslenfeld, D. J., van Dillen, L. F., and Van Lange, P. A. (2011). Secure attachment partners attenuate neural responses to social exclusion: an fMRI investigation. Int. J. Psychophysiol. 81, 44–50. doi: 10.1016/j.ijpsycho.2011.04.003
Kawamoto, T., Ura, M., and Nittono, H. (2015). Intrapersonal and interpersonal processes of social exclusion. Front. Neurosci. 9:62. doi: 10.3389/fnins.2015.00062
Marinović, V., and Träuble, B. (2021). Vicarious ostracism and control in young children. Soc. Dev. 30, 225–238. doi: 10.1111/SODE.12465
Masten, C. L., Morelli, S. A., and Eisenberger, N. I. (2011). An fMRI investigation of empathy for ‘social pain’ and subsequent prosocial behavior. NeuroImage 55, 381–388. doi: 10.1016/j.neuroimage.2010.11.060
Meyer, M. L., Masten, C. L., Ma, Y., Wang, C., Shi, Z., Eisenberger, N. I., et al. (2013). Empathy for the social suffering of friends and strangers recruits distinct patterns of brain activation. Soc. Cogn. Affect. Neurosci. 8, 446–454. doi: 10.1093/scan/nss019
Moor, B. G., Guroglu, B., Op de Macks, Z. A., Rombouts, S. A., Van der Molen, M. W., and Crone, E. A. (2012). Social exclusion and punishment of excluders: neural correlates and developmental trajectories. NeuroImage 59, 708–717. doi: 10.1016/j.neuroimage.2011.07.028
Nordgren, L. F., Banas, K., and MacDonald, G. (2011). Empathy gaps for social pain: why people underestimate the pain of social suffering. J. Pers. Soc. Psychol. 100, 120–128. doi: 10.1037/a0020938
Okamoto, M., Tsuzuki, D., Clowney, L., Dan, H., Singh, A. K., and Dan, I. (2009). Structural atlas-based spatial registration for functional near-infrared spectroscopy enabling inter-study data integration. Clin. Neurophysiol. 120, 1320–1328. doi: 10.1016/j.clinph.2009.01.023
Petsnik, C., and Vorauer, J. D. (2020). Do dominant group members have different emotional responses to observing dominant-on-dominant versus dominant-on-disadvantaged ostracism? Some evidence for heightened reactivity to potentially discriminatory ingroup behavior. PLoS One 15:e0234540. doi: 10.1371/journal.pone.0234540
Pinti, P., Tachtsidis, I., Hamilton, A., Hirsch, J., Aichelburg, C., Gilbert, S., et al. (2020). The present and future use of functional near-infrared spectroscopy (fNIRS) for cognitive neuroscience. Ann. N. Y. Acad. Sci. 1464, 5–29. doi: 10.1111/nyas.13948
Qinyi, Z., Na, H., and Xuechen, D. (2019). Predictors of individual’s rejection sensitivity: base on child × environment model. Chinese J. Appl. Psychol. 26, 83–91. doi: 10.3969/j.issn.1006-6020.2020.01.009
Radke, S., Seidel, E. M., Boubela, R. N., Thaler, H., Metzler, H., Kryspin-Exner, I., et al. (2018). Immediate and delayed neuroendocrine responses to social exclusion in males and females. Psychoneuroendocrinology 93, 56–64. doi: 10.1016/j.psyneuen.2018.04.005
Ren, D., Wesselmann, E. D., and Williams, K. D. (2018). Hurt people hurt people: ostracism and aggression. Curr. Opin. Psychol. 19, 34–38. doi: 10.1016/j.copsyc.2017.03.026
Seidel, E. M., Silani, G., Metzler, H., Thaler, H., Lamm, C., Gur, R. C., et al. (2013). The impact of social exclusion vs. inclusion on subjective and hormonal reactions in females and males. Psychoneuroendocrinology 38, 2925–2932. doi: 10.1016/j.psyneuen.2013.07.021
Singer, T., and Lamm, C. (2009). The social neuroscience of empathy. Ann. N. Y. Acad. Sci. 1156, 81–96. doi: 10.1111/j.1749-6632.2009.04418.x
Singer, T., Seymour, B., O'Doherty, J., Kaube, H., Dolan, R. J., and Frith, C. D. (2004). Empathy for pain involves the affective but not sensory components of pain. Science 303, 1157–1162. doi: 10.1126/science.1093535
Singer, T., Seymour, B., O'Doherty, J. P., Stephan, K. E., Dolan, R. J., and Frith, C. D. (2006). Empathic neural responses are modulated by the perceived fairness of others. Nature 439, 466–469. doi: 10.1038/nature04271
Sun, P. P., Tan, F. L., Zhang, Z., Jiang, Y. H., Zhao, Y., and Zhu, C. Z. (2019). Corrigendum: feasibility of functional near-infrared spectroscopy (fNIRS) to investigate the Mirror neuron system: an experimental study in a real-life situation. Front. Hum. Neurosci. 13:148. doi: 10.3389/fnhum.2019.00148
Tousignant, B., Eugene, F., Sirois, K., and Jackson, P. L. (2018). Difference in neural response to social exclusion observation and subsequent altruism between adolescents and adults. Neuropsychologia 116, 15–25. doi: 10.1016/j.neuropsychologia.2017.04.017
Twenge, J. M., and Campbell, W. K. (2003). ‘Isn't it fun to get the respect that we're going to deserve?’ Narcissism, social rejection, and aggression. Personal. Soc. Psychol. Bull. 29, 261–272. doi: 10.1177/0146167202239051
Vijayakumar, N., Cheng, T. W., and Pfeifer, J. H. (2017). Neural correlates of social exclusion across ages: a coordinate-based meta-analysis of functional MRI studies. NeuroImage 153, 359–368. doi: 10.1016/j.neuroimage.2017.02.050
Wasylyshyn, N., Hemenway Falk, B., Garcia, J. O., Cascio, C. N., O'Donnell, M. B., Bingham, C. R., et al. (2018). Global brain dynamics during social exclusion predict subsequent behavioral conformity. Soc. Cogn. Affect. Neurosci. 13, 182–191. doi: 10.1093/scan/nsy007
Wei, Z. (2012). The reliability and validity of Chinese version of the rejection sensitivity questionnaire. Chinese J. Behav. Med. Brain Sci. 21, 757–759. doi: 10.3760/cma.j.issn.1674-6554.2012.08.027
Wesselmann, E. D., Bagg, D., and Williams, K. D. (2009). “I feel your pain”: the effects of observing ostracism on the ostracism detection system. J. Exp. Soc. Psychol. 45, 1308–1311. doi: 10.1016/j.jesp.2009.08.003
Will, G. J., van Lier, P. A., Crone, E. A., and Guroglu, B. (2016). Chronic childhood peer rejection is associated with heightened neural responses to social exclusion during adolescence. J. Abnorm. Child Psychol. 44, 43–55. doi: 10.1007/s10802-015-9983-0
Williams, K. D. (2007). Ostracism. Ann. Rev. Psychol. 58, 425–452. doi: 10.1146/annurev.psych.58.110405.085641
Williams, K. D. (2009). Ostracism: a temporal need-threat model. Adv. Exp. Soc. Psychol. 41, 275–314. doi: 10.1016/S0065-2601(08)00406-1
Williams, K. D., Cheung, C. K., and Choi, W. (2000). Cyberostracism effects of being ignored over the internet. J. Pers. Soc. Psychol. 79, 748–762. doi: 10.1037/0022-3514.79.5.748
Wrege, J. S., Ruocco, A. C., Euler, S., Preller, K. H., Busmann, M., Meya, L., et al. (2019). Negative affect moderates the effect of social rejection on frontal and anterior cingulate cortex activation in borderline personality disorder. Cogn. Affect. Behav. Neurosci. 19, 1273–1285. doi: 10.3758/s13415-019-00716-0
Wudarczyk, O. A., Kohn, N., Bergs, R., Gur, R. E., Turetsky, B., Schneider, F., et al. (2015). Chemosensory anxiety cues moderate the experience of social exclusion – an fMRI investigation with Cyberball. Front. Psychol. 6:1475. doi: 10.3389/fpsyg.2015.01475
Yanagi, M., Hosomi, F., Kawakubo, Y., Tsuchiya, A., Ozaki, S., and Shirakawa, O. (2020). A decrease in spontaneous activity in medial prefrontal cortex is associated with sustained hallucinations in chronic schizophrenia: an NIRS study. Sci. Rep. 10:9569. doi: 10.1038/s41598-020-66560-2
Keywords: social exclusion, observer, vicarious experience, empathy, functional near-infrared spectroscopy
Citation: Song J, Lian T, Zhang Y, Cao M and Jiao Z (2024) Social exclusion: differences in neural mechanisms underlying direct versus vicarious experience. Front. Psychol. 15:1368214. doi: 10.3389/fpsyg.2024.1368214
Edited by:
Zachary Hohman, Texas Tech University, United StatesReviewed by:
Joshua Kurtis Brown, University of Wisconsin-Madison, United StatesHanxuan Zhao, Shanghai International Studies University, China
Copyright © 2024 Song, Lian, Zhang, Cao and Jiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunhong Zhang, MTg5MTExNTA4OTZAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Juan Song1†
Juan Song1† Tao Lian
Tao Lian Yunhong Zhang
Yunhong Zhang Mingjing Cao
Mingjing Cao