- 1EngageMinds HUB – Consumer, Food and Health Engagement Research Center, Università Cattolica del Sacro Cuore, Milan, Italy
- 2Department of Psychology, Università Cattolica del Sacro Cuore, Milan, Italy
- 3Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy
- 4Department of General Psychology, University of Padua, Padua, Italy
- 5Liverpool Centre for Cardiovascular Science and Department of Cardiovascular and Metabolic Medicine, Faculty of Health and Life Sciences, University of Liverpool, Liverpool, United Kingdom
- 6Department of Clinical Medicine, Aalborg University, Aalborg, Denmark
- 7Arrhythmia Alliance, Stratford-upon-Avon, United Kingdom
- 8Department of Medical Sciences, University of Ferrara, Ferrara, Italy
- 9Faculty of Agriculture, Food and Environmental Sciences, Università Cattolica del Sacro Cuore, Cremona, Italy
Background: People with multimorbidity are increasingly engaged, enabled, and empowered to take responsibility for managing their health status. The purpose of the study was to systematically review and appraise the psychometric properties of tools measuring patient engagement in adults with multimorbidity and their applicability for use within engagement programs.
Methods: PubMed, Scopus, Web of Science, and PsycInfo were searched from inception to 1 July 2021. Gray literature was searched using EBSCO host-database “Open dissertation”. The reference lists of studies meeting the inclusion criteria were searched to identify additional eligible studies. The screening of the search results and the data extraction were performed independently by two reviewers. The methodological quality of the included studies was evaluated with the COSMIN checklist. Relevant data from all included articles were extracted and summarized in evidence synthesis tables.
Results: Twenty articles on eight tools were included. We included tools that measure all four dimensions of patient engagement (i.e., engagement, empowerment, activation, and participation). Their psychometric properties were analyzed separately. Most tools were developed in the last 10 years in Europe or the USA. The comparison of the estimated psychometric properties of the retrieved tools highlighted a significant lack of reliable patient engagement measures for people with multimorbidity. Available measures capture a diversity of constructs and have very limited evidence of psychometric properties that are vital for patient-reported measures, such as invariance, reliability, and responsiveness.
Conclusion: This review clarifies how patient engagement, as operationalized in measures purporting to capture this concept, overlaps with, and differs from other related constructs in adults with multimorbidity. The methodological quality of psychometric tools measuring patient engagement in adults with multimorbidity could be improved.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=259968, identifier CRD42021259968.
1 Background
In recent years the population aging has led to increase the proportion of people with multiple chronic conditions (i.e., multimorbidity) (World Health Organization, 2016). Risky habits and lifestyles, longer life expectancy, and improved health care have led one in three adults to suffer from multimorbidity (Divo et al., 2014). People with multimorbidity are individuals who live with two or more long-term conditions, one of which is either physical non-communicable disease or a mental health condition, or an infectious disease of long duration (World Health Organization, 2016). People with multiple long-term conditions are challenging to treat, are prone to experience complications such as readmissions, adverse drug interactions or death, and often require a great deal of social and psychological support (Divo et al., 2014; World Health Organization, 2016). Moreover, the risk of being diagnosed with multiple long-term conditions rises with age, is more common among women and in people of lower socio-economic status (Divo et al., 2014; World Health Organization, 2016). People with multimorbidity often report difficulties in managing their care pathways that are often designed to control and treat single health conditions (Dhere, 2016). Collectively this makes caring for these people, particularly challenging. Clinicians often struggle to find, personalize, and provide the best therapeutic pathways, interventions, and protocols for people with multiple long-term conditions (Smoth et al., 2013).
Simultaneously, Western culture has gradually shifted from a paternalistic care approach toward patient-centered care and participatory medicine (Weil, 2016; deBronkart, 2018). People with multimorbidity are increasingly engaged, enabled, and empowered to take responsibility for managing their health (Pushparajah, 2018). Health researchers and stakeholders have started to design, test, and implement engagement interventions for people with multiple long-term conditions, showing their positive effects on health outcomes, user satisfaction, communication between patients and health professionals, adherence to treatment regimes, and healthcare resources usage (Barello et al., 2016; Bombard et al., 2018). This has led to the increased relevance of the concept of patient engagement and its synonyms (e.g., patient empowerment, activation, participation) in the literature (Castro et al., 2016; Náfrádi et al., 2017). In the last ten years, several studies have attempted to clarify the concept of patient engagement (Barello et al., 2012; Fumagalli et al., 2015; Higgins et al., 2017). Menichetti et al. (2016) highlighted that many concepts in the current literature overlap with patient engagement, such as patient enablement, empowerment, activation, and participation, since all these concepts refer to people’ proactive role in the management of their own healthcare.
ln this context, the use of tools designed and tested to engage people with multiple long-term diseases should be promoted among clinicians. Despite longstanding calls for greater engagement of older adults with multiple long-term conditions in healthcare, current evidence suggests that this population can be successfully engaged (Dambha-Miller et al., 2021; Markle-Reid et al., 2021). People with multiple long-term diseases are a diverse group, ranging from relatively healthy, independent living individuals to very frail individuals with poor physical functioning and cognitive problems, which often can make patient engagement in healthcare a challenging goal.
Therefore, a systematic review of the available engagement measurement tools to evaluate and monitor the benefits of engagement programs for people with multiple long-term conditions may help clinicians improve their care pathways. In particular, the examination of reliability, validity, feasibility, and clinical utility of engagement tools is required to inform the selection of appropriate instruments and address how to effectively enhance engagement in individuals and groups. Thus, the main object of the study was to systematically review and appraise the psychometric properties of tools measuring patient engagement in adults with multimorbidity and their applicability for use within empowerment programs, with a distinct focus on tools which have been validated in people with cardiovascular diseases.
This systematic review has been guided by the following research questions:
• What tools have been developed and validated in the literature to measure patient engagement in adults with multiple long-term conditions?
• What are the best tools, in terms of methodological quality and goodness-of-fit, to measure patient engagement in adults with multiple long-term conditions?
• What are the main conceptual components of engagement tools to shape future engagement interventions in this population?
2 Methods
2.1 Design
This study was performed in two steps: (i) a systematic review of the psychometric properties of engagement scales and tools was performed; then (ii) the psychometric properties were assessed by following the COnsensus-based Standards for the selection of health Measurement Instruments (COSMIN) guideline for systematic reviews of patient-reported outcome measures (Mokkink et al., 2016; Prinsen et al., 2018). The study protocol was registered on PROSPERO (registration number: CRD42021259968).
2.2 Search methods
A search strategy was designed to retrieve published and unpublished studies measuring patient engagement in adults with long-term conditions (Supplementary Material 1). The search filters developed by the Oxford PROM group and Terwee et al. (2007) were then used to refine the search strategy. Pubmed, Scopus, Web of Science, and PsycInfo were searched from their inception to April 2024. Gray literature was checked on EBSCOhost-database “Open dissertation” to identify any other significant publications. A forward and backward snowball search was performed to identify additional relevant publications.
The following eligibility criteria were used to select studies: (a) concerned with the development and/or evaluation of measurement properties of instruments that measure engagement and all the related concept such as empowerment, patient participation and patient involvement; (b) including adults with long-term conditions, including either instruments validated on people with multiple long term conditions or validated on people with at least three different long-term conditions; (c) published or unpublished up to April 2024; and (d) available in a language accessible to the authors (English and Italian). Tools were excluded if they: (a) were based on a single item. The literature search was performed by one researcher and then two researchers independently screened the records based on the title and abstract against the inclusion criteria. For eligible studies, the full texts were retrieved, and the same two researchers independently evaluated the eligibility of each study, and decisions on study inclusion were based on joint agreement.
Data extraction was performed by two researchers and the following data was recorded: (i) author, year and country; (ii) language and setting; (iii) study design; (iv) key characteristics of study subjects; (v) name of measurement instruments and domains measured; (vi) number of items and (sub)scales and number and type of response categories; (vii) recall period and time needed for administration; (viii) scoring algorithm; (ix) mode of administration; (x) instructions given to those who complete the questionnaire; and (xi) licensing information and costs. The psychometric properties reported in the studies were independently extracted by four authors. Then, another researcher independently revised the data extracted for accuracy. Any changes were discussed, and a full agreement was reached among the researchers.
2.3 Quality appraisal
The COSMIN checklist (Mokkink et al., 2018) was used to evaluate the methodological quality of studies on measurement properties. The checklist uses a standardized descriptive framework to assess the measurement properties against quality markers in ten boxes (Mokkink et al., 2018). Each box includes a pool of items (from five to 18) scored on a four-point scale (from 1 ‘poor’ to 4 ‘excellent’). The overall score is obtained by taking the lowest score indicated by the items in the box: therefore, a final score is given for each psychometric property, ranging from ‘poor’ to ‘excellent’. The measurement property ‘criterion validity’ was not considered in this systematic review since no “gold standard” exists for measuring engagement; therefore, eight boxes were rated. One researcher underwent training in the use of the COSMIN guidelines while the second reviewer had previous experience in the field. The inter-rater agreement between the two reviewers for the quality appraisal was 86.36% (k = 0.79).
2.4 Synthesis
Included validation studies have been summarized according to the data extracted. The values of the psychometric properties evaluated, and the quality of the methodologies used in assessing these psychometric properties have been also summarized using a descriptive approach. The conceptual components for future engagement interventions were synthesized based on the conceptual framework underlying the single engagement tools.
3 Results
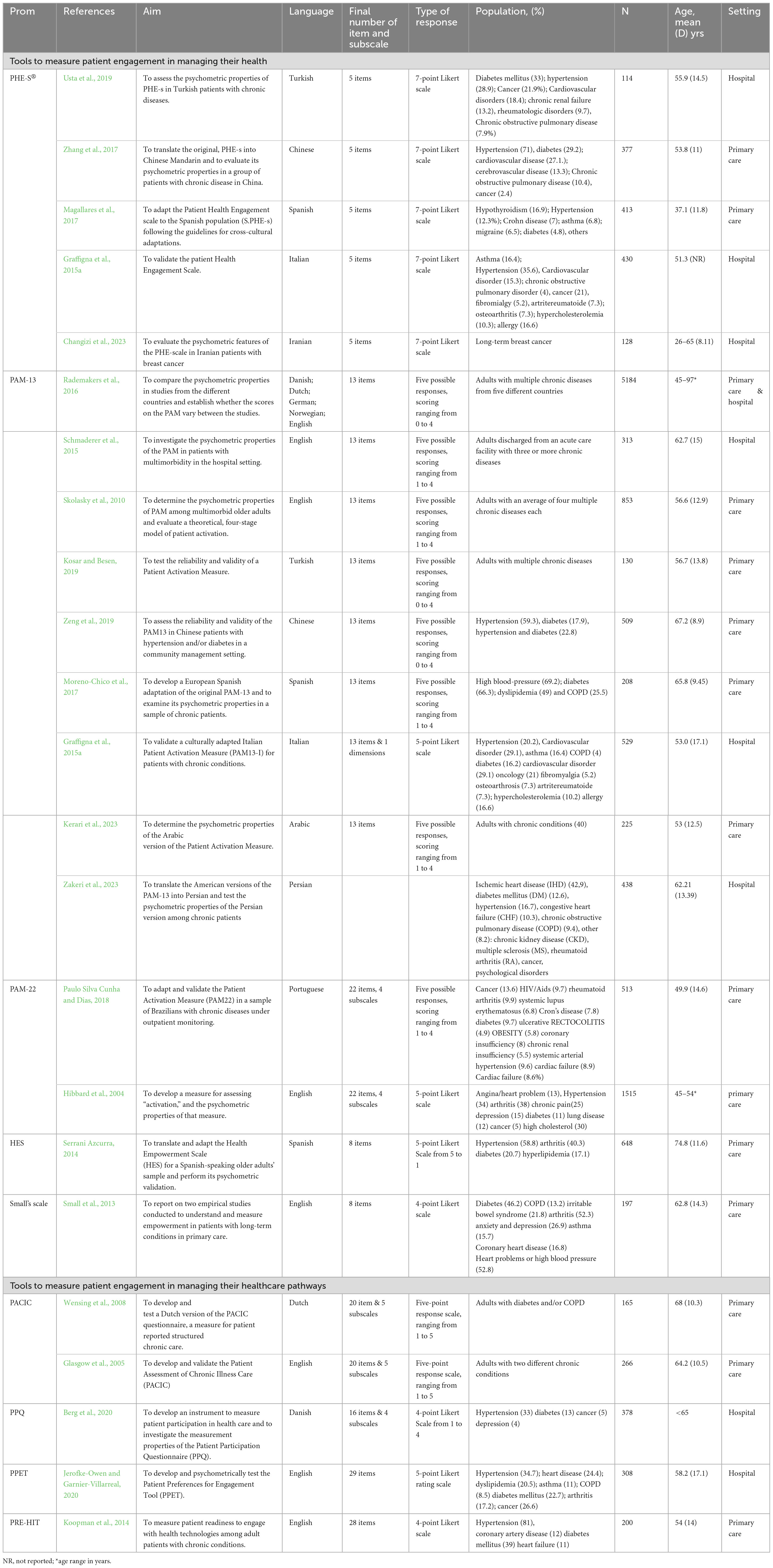
The literature search produced 6,561 results, of which 942 duplicates were excluded. A total of 5,473 articles were excluded at the title and abstract screening stage, while other 123 articles were excluded at the full-text stage. Twenty-three articles (Hibbard et al., 2004; Glasgow et al., 2005; Wensing et al., 2008; Skolasky et al., 2011; Small et al., 2013; Koopman et al., 2014; Serrani Azcurra, 2014; Graffigna et al., 2015a,b; Schmaderer et al., 2015; Rademakers et al., 2016; Magallares et al., 2017; Moreno-Chico et al., 2017; Zhang et al., 2017; Cunha et al., 2019; Kosar and Besen, 2019; Usta et al., 2019; Zeng et al., 2019; Berg et al., 2020; Jerofke-Owen and Garnier-Villarreal, 2020) met the inclusion criteria describing eight families of tools as reported in Figure 1.
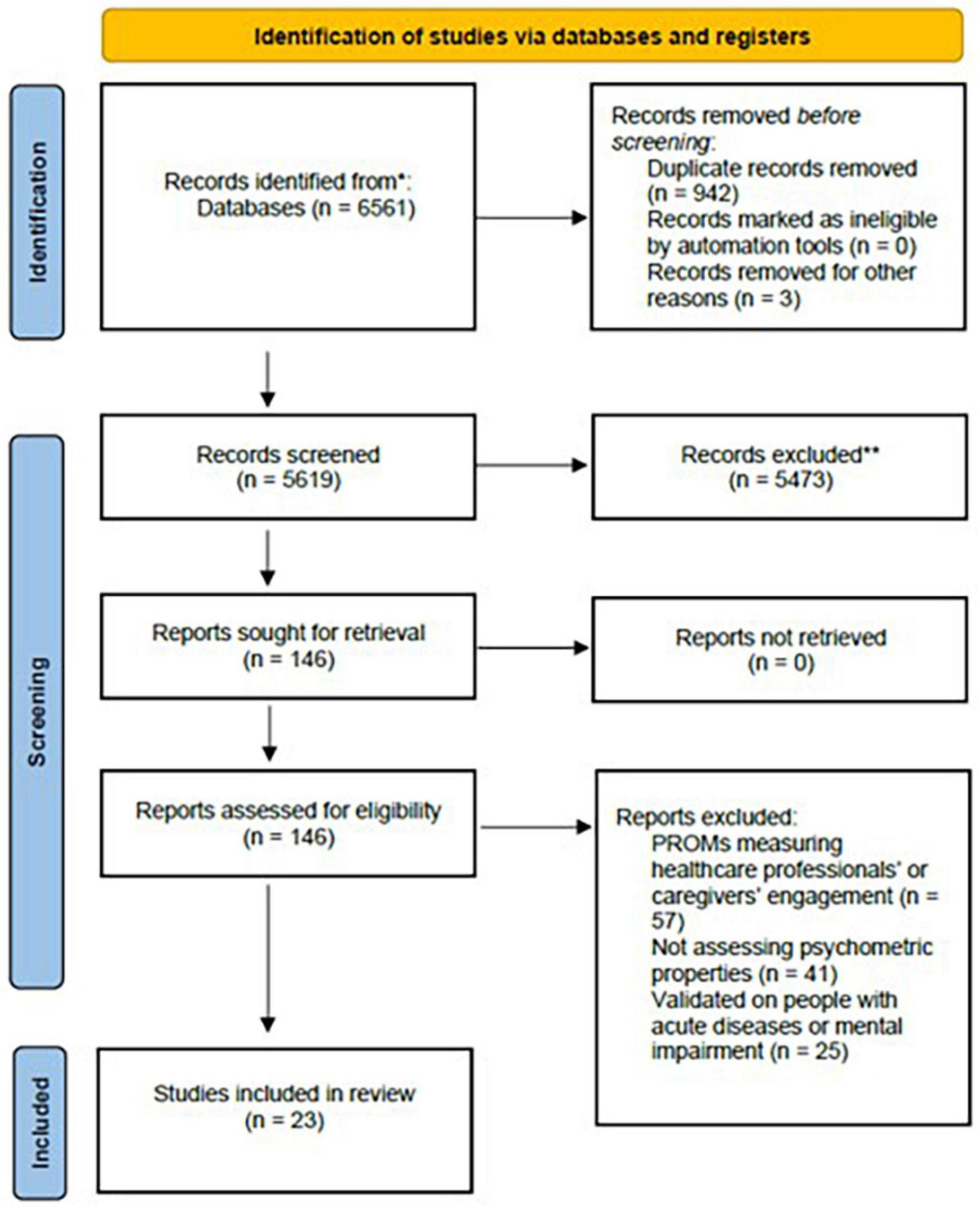
Figure 1. PRISMA flow diagram of the studies’ selection. *Consider, if feasible to do so, reporting the number of records identified from each database or register searched (rather than the total number across all databases/registers). **If automation tools were used, indicate how many records were excluded by a human and how many were excluded by automation tools.
3.1 Study features
The main characteristics of the 23 articles (Hibbard et al., 2004; Glasgow et al., 2005; Wensing et al., 2008; Skolasky et al., 2011; Small et al., 2013; Koopman et al., 2014; Serrani Azcurra, 2014; Graffigna et al., 2015a,b; Schmaderer et al., 2015; Rademakers et al., 2016; Magallares et al., 2017; Moreno-Chico et al., 2017; Zhang et al., 2017; Cunha et al., 2019; Kosar and Besen, 2019; Usta et al., 2019; Zeng et al., 2019; Berg et al., 2020; Jerofke-Owen and Garnier-Villarreal, 2020) are reported in Table 1. The eight families of tools were categorized as those used to measure patient engagement in managing their own health and those used to measure patient engagement in managing their healthcare pathways (Table 1). Most studies validated or investigated the psychometric properties of the following tools: (i) the Patient Activation Measurement (PAM) (n = 10) (Hibbard et al., 2004; Skolasky et al., 2011; Graffigna et al., 2015b; Schmaderer et al., 2015; Rademakers et al., 2016; Moreno-Chico et al., 2017; Cunha et al., 2019; Kosar and Besen, 2019; Zeng et al., 2019); (ii) The Patient Assessment Care for Chronic Conditions (PACIC) (n = 3) (Glasgow et al., 2005; Wensing et al., 2008; Berg et al., 2020); and (iii) The Patient Health Engagement Scale (PHE-S®) (n = 5) (Graffigna et al., 2015a; Magallares et al., 2017; Zhang et al., 2017; Usta et al., 2019).
The majority (78%) of the included studies were published in the last 10 years and included patients from 15 different countries, mainly North America (e.g., USA, Canada) and Europe (e.g., Denmark, Netherlands, UK, Italy) (Table 1). Six studies focused on the development and validation of these tools, while the others were adaptation, translation, and evaluation of their psychometric properties (Table 1). Among primary studies, the first data collection was performed in 2003 (Hibbard et al., 2004).
Overall, the number of participants involved ranged from 114 (Usta et al., 2019) to 5,184 patients (Skolasky et al., 2011). The response rate was only reported in ten studies and ranged from 48% (Hibbard et al., 2004) to 96.2% (Zhang et al., 2017). As shown in Table 1, tools were mainly validated among patients with diabetes (66%), hypertension and other cardiovascular morbidities (52%), or on people with multiple long-term conditions (23%). Most participants were female, and the mean age of participants varied from 37 (Magallares et al., 2017) to 74 years old (Small et al., 2013). The ethnicity of participants was only reported in eleven studies, and most participants were Caucasian. Most of the scales required patients to have a basic level of health literacy. Patients with cognitive or mental health problems were often excluded from the validation studies.
Almost all tools were validated either in hospitalized (35%) or in primary care populations (65%), except Skolasky et al. (2011) which employed data from both settings. All the included tools were self-report questionnaires. Few studies reported the completion time and ranged from less 7 min (Glasgow et al., 2005) to 12 min (Usta et al., 2019).
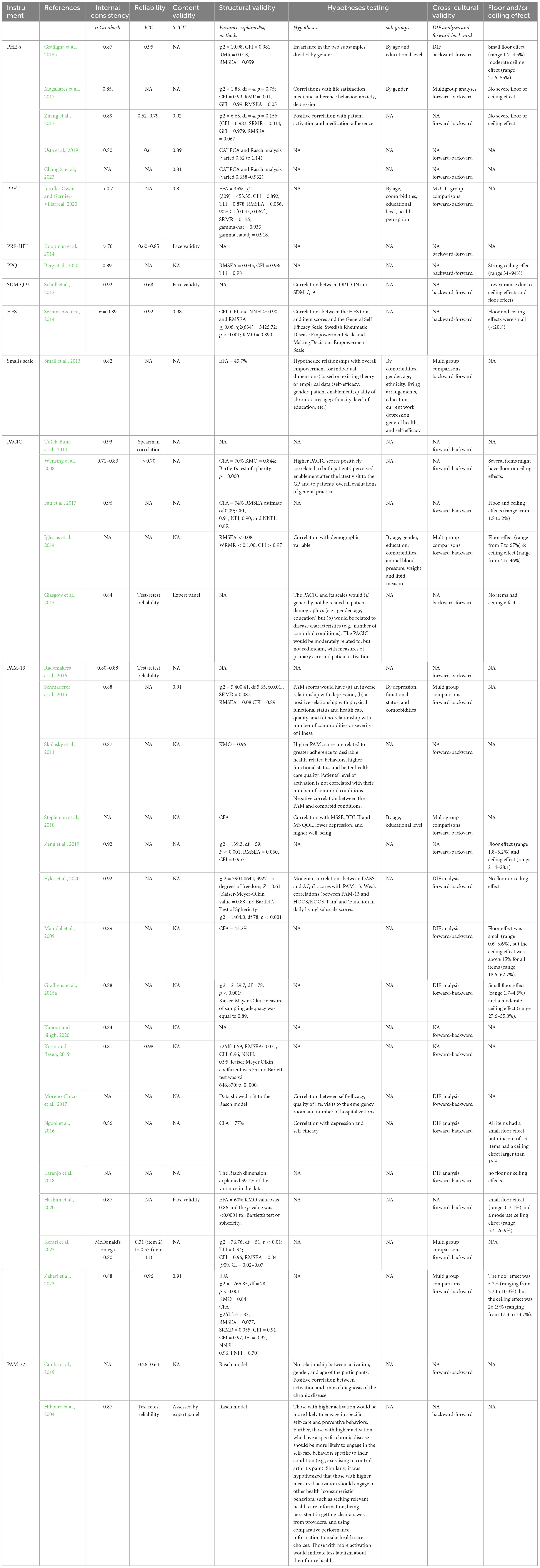
The number of evaluated psychometric properties ranged from two to six (Table 2). The most commonly assessed properties were structural validity and internal consistency. Only two studies evaluated measurement error (Hibbard et al., 2004; Graffigna et al., 2015a). None of the included studies evaluated measurement variance. However, given that the items included are a manifestation of different underlying constructs, these properties were evaluated individually for each group of tools (Table 2).
3.1.1 Tools to measure patient engagement in managing their health
Five tools to measure patient engagement in managing their health were retrieved (Table 1).
The Patient Health Engagement Scale (PHE-S®) is a patient self-administered short psychometric questionnaire developed to measure the level of patient engagement in their healthcare function (Graffigna et al., 2015a). It consists of five items measured on a 7-point Likert scale, that allows patients to easily mirror their current emotional states and illnesses experience. The PHE-S® has a robust theoretical foundation since it was developed from the Patient Health Engagement model (Graffigna et al., 2015a). Currently, six versions of this scale are available: Italian (Graffigna et al., 2015a); English (Graffigna et al., 2015a); Turkish (Usta et al., 2019); Spanish (Magallares et al., 2017); Chinese (Zhang et al., 2017); Persian [XXX]. Across these tools, the psychometric properties remain the same as the original version (Table 2), demonstrating the consistency of PHE-S®. All the validation studies tested the internal consistency of the tool. Structural validity was evaluated using the Categorical Principal Component Analysis (CATPCA), a confirmatory factor analysis (CFA) and a RASCH model (Table 2). Reliability was evaluated in three studies (from acceptable to very good), while cross-cultural validity was assessed in two (Table 2). All the PHE-S psychometric properties were judged as good or adequate. The only exception was the reliability of the Turkish version which was judged as doubtful (Table 2).
The Patient Activation Measure (PAM) (Hibbard et al., 2004) is a well-known tool to assess patients’ knowledge, skills, and confidence for managing their health. There are currently two versions of the PAM, the original 22-item (PAM-22) and the 13-item short form (PAM-13). The PAM measures patient activation on a 0–100 scale, and the patients’ responses are measured on a 5-point Likert scale. Several translations and validations of the PAM are available (Table 1), as well as the original version developed by Hibbard et al. (2004). The PAM shows different judgments of its psychometric properties among its validations: in some of the studies, the PAM demonstrated good construct validity, reliability, and internal consistency overall, in others the judgment is doubtful or inadequate (Table 2). However, the PAM is the only patient activation measures retrieved that has been validated in a wide range of chronic or multimorbid populations (Table 1).
The Health Empowerment Scale (HES) is a survey that measures patients’ self-management skills and decision-making abilities (Serrani Azcurra, 2014). The HES was adapted from the Diabetes Empowerment Short Form Scale (DES-SSF) and has 8 items measured on a 5-point Likert scale. The HES shows good internal consistency, construct validity and adequate reliability (Table 2). Small floor and ceiling effects were reported (Table 2). Its content validity and theoretical conceptualization were judged as doubtful since the HES has no real underlying conceptual model. Other studies are needed to evaluate the consistency of the HES psychometric properties.
Small et al. (2013) developed a short questionnaire to measure empowerment in patients with long-term conditions (primarily diabetes, irritable bowel syndrome, coronary heart disease, or chronic obstructive pulmonary disease). It has 8 items measured on a 4-point Likert scale. Its structural validity appears to be doubtful, and no content validity was provided (Table 2).
3.1.2 Tools to measure patient engagement in managing their healthcare pathways
Four tools measuring patient engagement in healthcare were identified.
The Patient Assessment of Care for Chronic Conditions (PACIC) is a survey that measures specific actions that chronic patients report they have experienced in the healthcare system (Glasgow et al., 2005). The PACIC was developed from the Patient Centered model and has five subscales, measuring patients’ activation, delivery system experience, goal setting, problem-solving, and coordination involvement. Five studies utilizing the PACIC were retrieved (Table 1). The PACIC is a 20-item questionnaire, and it uses a 5-point response scale, with higher scores indicating better quality of care. Similar to the PAM, the various PACIC validation studies report different judgments of its psychometric properties (Table 2). The PACIC content validity has been assessed by Glasgow et al. (2005) and was rated as inadequate. Its’ structural validity was judged as very good only by two studies (Table 2). PACIC reliability was only assessed by three studies with two deeming its reliability as inadequate or doubtful.
The Patient Participation Questionnaire (PPQ) is an instrument developed to measure patient participation in their treatment and care (Berg et al., 2020). It has been validated in patients with multi-morbidity, where one-third of the sample were patients with hypertension (Berg et al., 2020). The PPQ is a short questionnaire with 16 items and four subscales, measured on a 4-point Likert scale. The PPQ has a good internal consistency, but its structural validity has been judged as doubtful, and no measures of its reliability have been provided yet (Table 2).
The Patient Readiness to Engage in Health Internet Technology (PRE-HIT) is a tool developed to measure the likelihood of using health information technology among patients with chronic conditions (Koopman et al., 2014). The PRE-HIT focuses on the measurement of patients’ engagement in specific conditions and 28 items measured on a 4-point Likert scale. Only its content validity, internal consistency and reliability were reported (Table 2).
The Patient Preferences for Engagement (PPET) tool was developed to assess patients’ preferences for engaging in healthcare (Jerofke-Owen and Garnier-Villarreal, 2020). The PPET was designed to inform the planning and delivery of individualized healthcare. The PPET consists of 29 items weighted with a 5-point Likert scale. No PPET composite score has been computed yet. The content validity was judged doubtful, while its reliability, structural validity, and internal consistency were rated as adequate or very good (Table 2). Other studies are needed to further evaluate the consistency of the PPET psychometric properties.
3.1.3 Conceptual components for future engagement interventions
According to the synthesis of the conceptual models or frameworks behind the tools included in this review, we extracted eight main conceptual components to be considered for future patient engagement interventions. The conceptual components are emotional adjustment, self-efficacy, self-management, health literacy, shared decision making, collaborative goal setting, proactive communication with the care teams, and problem solving (Table 3).
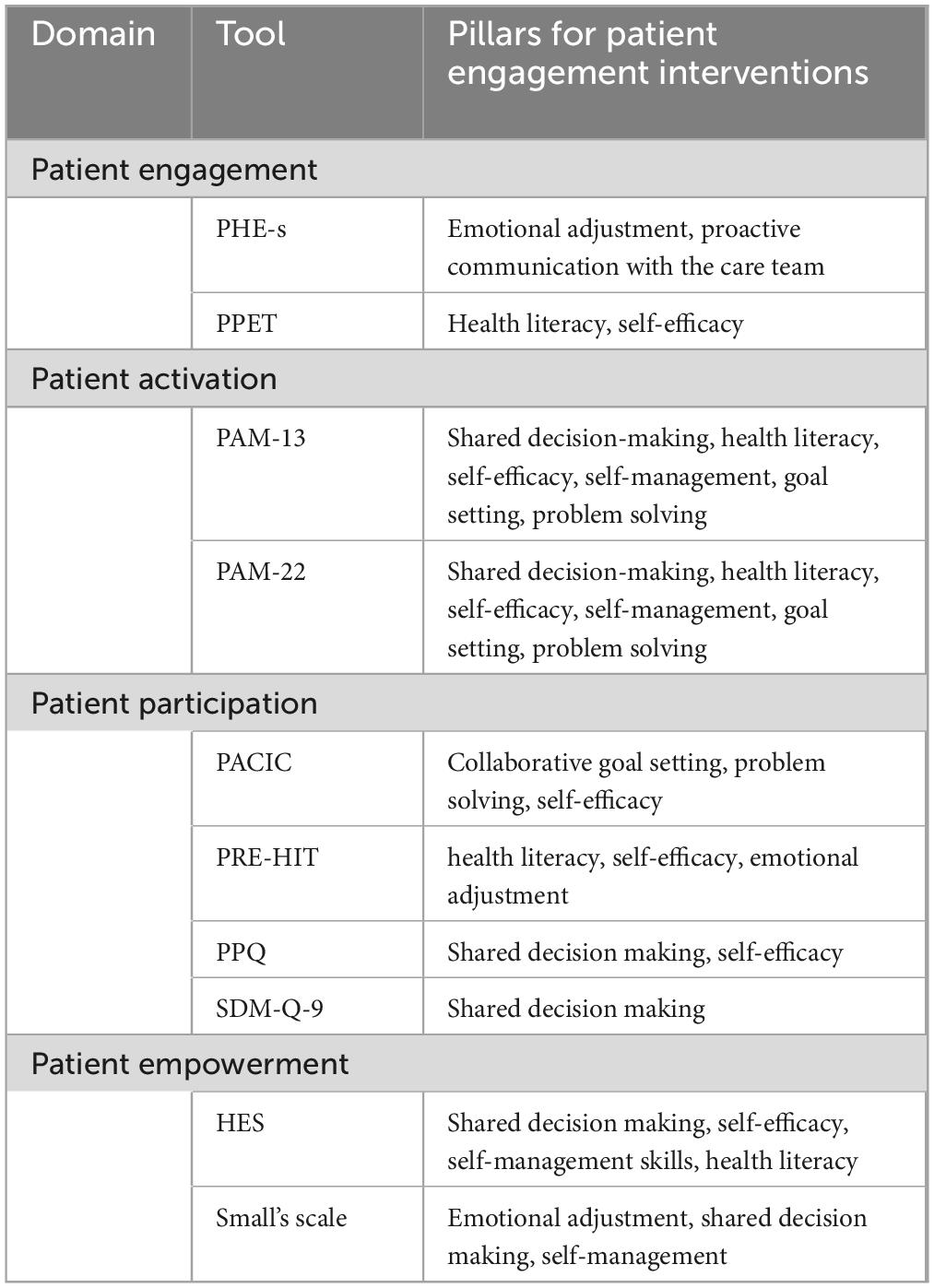
Table 3. Components of engagement interventions for patients diagnosed with multiple chronic diseases.
Emotional adjustment, mainly related to the “patient engagement” domain, - refers to the patients’ ability to cope with the diagnosis and to elaborate their own role in the disease management. Self-management and self-efficacy – mainly related to the “patient activation domain” - are two well-known components of engagement interventions and refer to patients’ ability to effectively recognize their needs and act proactively to fulfill them. Health literacy, mainly linked to the “patient empowerment” domain, refers to patients’ knowledge and ability to understand information provided by the healthcare providers or caregivers about the disease and treatment journey. Also shared decision making and proactive communication are common conceptual components of engagement measurement tools. Indeed, shared decision making – which is mainly related to the “patient participation” domain - is essential in making them able to proactively manage their disease by enabling an open dialogue with the healthcare team about therapeutic choices and strategies. Collaborative goal setting and problem-solving, mainly related to the patient are crucial skills that make patients able to effectively plan self-care activities and to engage in proactive behaviors toward their disease management.
4 Discussion
This systematic review retrieved eight different tools that measure patient engagement in people with multiple long-term diseases. The tools were analyzed separately, based on the construct they measured. Half of the tools retrieved focused on measuring patient engagement as the process of emotional adjustment and the acquisition of motivation to manage their disease or as a general process of acquisition of a higher level of power. The other half measured people’s ability to take an active part in their consultations with healthcare professionals. Overall, the structure of the instruments was heterogeneous, as were their psychometric properties. Many tools only partially described their psychometric properties, with few outlining their theoretical foundation. The best psychometric properties were reported by the PAM® (Hibbard et al., 2004) and the PHE-S® (Graffigna et al., 2015a), which are the most tested and cross-culturally validated measures of patient engagement in managing their health to date.
Most of the tools retrieved were developed and/or adapted in the last 10 years, highlighting the growing importance of the concept of patient engagement in healthcare. The tools were tested mainly in populations with diabetes or hypertension. This is not surprising given the mean age of people with long-term conditions (Busse et al., 2010) and the importance of engaging with these people to help them achieve a suitable quality of life (Yen and Lin, 2018; Søgaard et al., 2021). Most instruments were short (<15 items) and had a short completion time (less than 10 min). The psychometric properties most often measured and reported were internal validity, content validity and construct validity. Many tools which showed a good theoretical foundation and reliability (Table 2), lacked a formal assessment of their structural validity. It is important that future studies further clarify the construct validity of these tools. Floor and ceiling effects were reported with some tools, and this may be problematic as the response scale of these instruments was all measured using Likert scales. Only three tools (PAM, PACIC, and PHE-S®) were tested in more than two different populations. This highlights the importance of increasing the dissemination of the concept of engagement and its measurement tools across healthcare conditions and especially in developing countries.
None of the identified tools measured both patient engagement in managing their own health and the healthcare pathways. This may be due to the lack of consensus on a unique definition of patient engagement (Barello et al., 2012; Fumagalli et al., 2015; Higgins et al., 2017). Patient engagement is a construct that in the literature overlaps with other psychological constructs such as activation, participation, and empowerment. However, even if many of these concepts are strongly intersecting (e.g., patient engagement and patient empowerment), others clearly measure different aspects of the process of engagement (e.g., patient participation). This problem was originally highlighted by Fumagalli et al. (2015) and almost 7 years later remains unresolved. The development of a single tool that measures all the different constructs underlying the concept of patient engagement may be an effective way to ease the process of measuring engagement.
To our knowledge, only one previous review has focused on measuring the concept of patient engagement in healthcare. Jerofke-Owen et al. (2020) limited their review on tools measuring patients’ preferences for engagement in healthcare; however, they did not systematically retrieve and evaluated also the tools measuring patients’ engagement in managing their own health. While this approach may increase accuracy in the analysis of the finding, given the lack of clarity on the concept of engagement it could also limit the ability to synthesize the concept’s use in the literature and lead to the loss of many valuable tools. Instead, we choose to use an inclusive approach to gain a deeper understanding of all the tools available to measure the concept of patient engagement.
This review allowed us to reflect on the components that should characterize engagement interventions in the future. The conceptual models and frameworks of the engagement tools are characterized by components such as emotional adjustment, self-efficacy, self-management, health literacy, shared decision making, collaborative goal setting, proactive communication with the care teams, and problem-solving. Some of these components (e.g., shared decision making, and proactive communication with the care team) are particularly important to identify the best care pathways for people with multiple chronic conditions. Others instead (e.g., emotional adjustment, self-efficacy, self-management) are necessary to guarantee that people with multiple chronic conditions are confident and able to partake in complex decisions on prognosis, treatment options and prioritizing care driven by their own perspective on what is acceptable, feasible or meaningful. These findings suggest that future engagement interventions should consider all these components to be effective. Current literature on patient engagement intervention for people with multiple long-term conditions is very heterogeneous (Søgaard et al., 2021). This diversity in the evidence base challenges the ability to draw robust conclusions and the increasing interest in patient engagement in the last 10 years in Europe and America sets the stage for reflection.
This review has some limitations. Firstly, while there are many different related concepts of engagement, some central terms might be lacking. Therefore, we excluded some concepts, for instance, self-care, patient adherence, or patient compliance although they have been used as related concepts of engagement. From our perspective, these concepts are outcomes of engagement. We chose the concepts which have in recent years been used as describing the active role of patients in healthcare (Fumagalli et al., 2015; Magallares et al., 2017), assuming they had an up-to-date view of related concepts. Secondly, some measures were rather new, and their validation process may be still ongoing. Lastly, it is possible that some relevant articles written in languages other than English or Italian may have been missed.
5 Conclusion
This systematic review highlights the need for a more comprehensive measure of patient engagement which includes all its related concepts (i.e., patient empowerment, patient activation, patient participation) and addresses all the possible components of patient engagement (i.e., emotional adjustment, self-efficacy, self-management, health literacy, shared decision making, collaborative goal setting, proactive communication with the care teams, problem-solving). Despite policy interest and initiatives relating to patient engagement, there is limited evidence to support the reliability and validity of existing tools and for the specific application to people with multiple long-term conditions. Moreover, retrieved studies often lack cross-cultural validation of the measures. This is particularly relevant as research suggests that there are ethnic differences in illness perception and management (Hillier, 1991; Lip et al., 2002). Future research could usefully develop a definitive more comprehensive measure of patient engagement.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
SB: Writing – review and editing, Writing – original draft, Methodology, Investigation, Data curation. GA: Writing – review and editing, Writing – original draft, Methodology, Investigation, Data curation. CB: Writing – review and editing, Writing – original draft, Project administration, Investigation. DAL: Writing – review and editing. DGL: Writing – review and editing. TL: Writing – review and editing, Supervision, Conceptualization. CT: Writing – review and editing. GG: Writing – review and editing, Writing – original draft, Supervision, Methodology, Conceptualization.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This project has received funding from the European Union’s Horizon 2020 Research and Innovation Programme under grant agreement no. 899871.
Acknowledgments
The authors would like to thank the AFFIRMO project consortium.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyg.2024.1345117/full#supplementary-material
References
Barello, S., Graffigna, G., and Vegni, E. (2012). Patient engagement as an emerging challenge for healthcare services: Mapping the literature. Nurs. Res. Pract. 2012:905934. doi: 10.1155/2012/905934
Barello, S., Triberti, S., Graffigna, G., Libreri, C., Serino, S., Hibbard, J., et al. (2016). eHealth for patient engagement: A systematic review. Front. Psychol. 6:2013. doi: 10.3389/fpsyg.2015.02013
Berg, S. K., Færch, J., Cromhout, P. F., Tewes, M., Pedersen, P. U., Rasmussen, T. B., et al. (2020). Questionnaire measuring patient participation in health care: Scale development and psychometric evaluation. Eur. J. Cardiovasc. Nurs. 19, 600–608. doi: 10.1177/1474515120913809
Bombard, Y., Baker, G. R., Orlando, E., Fancott, C., Bhatia, P., Casalino, S., et al. (2018). Engaging patients to improve quality of care: A systematic review. Implement Sci. 13:98. doi: 10.1186/s13012-018-0784-z
Busse, R., Blumel, M., Scheller-Kreinsen, D., and Zentner, A. (2010). Tackling chronic diseases in Europe. Strategies, interventions and challenges. Copenhagen: WHO Regional Office for Europe.
Castro, E. M., Van Regenmortel, T., Vanhaecht, K., Sermeus, W., and Van Hecke, A. (2016). Patient empowerment, patient participation and patient-centeredness in hospital care: A concept analysis based on a literature review. Patient Educ. Couns. 99, 1923–1939. doi: 10.1016/j.pec.2016.07.026
Changizi, M., Cheraghiyan, B., Mohamadian, H., Ghorbani Kalkhajeh, S., Salmanzadeh, S., and Maghsoudi, F. (2023). The association between covid-19 information channels and preventive behaviors in Southwest of Iran: Application of Protection Motivation Theory (PMT). Health Educ. Health Promot. 11, 1001–1008.
Cunha, C. M., da Cunha, D., Manzato, R. O., Nepomuceno, E., da Silva, D., and Dantas, R. (2019). Validation of the Brazilian version of the patient activation measure 13. J. Nurs. Meas. 27, 97–113. doi: 10.1891/1061-3749.27.1.97
Dambha-Miller, H., Simpson, G., Hobson, L., Roderick, P., Little, P., Everitt, H., et al. (2021). Integrated primary care and social services for older adults with multimorbidity in England: A scoping review. BMC Geriatr. 21:674. doi: 10.1186/s12877-021-02618-8
deBronkart, D. (2018). The patient’s voice in the emerging era of participatory medicine. Int. J. Psychiatry Med. 53, 350–360. doi: 10.1177/0091217418791461
Dhere, A. (2016). Managing complex long-term conditions and multimorbidity. Clin. Med. (Lond). 16, 545–547. doi: 10.7861/clinmedicine.16-6-545
Divo, M. J., Martinez, C. H., and Mannino, D. M. (2014). Ageing and the epidemiology of multimorbidity. Eur. Respir. J. 44, 1055–1068. doi: 10.1183/09031936.00059814
Eyles, A., Gibbons, S., and Montebruno, P. (2020). Covid-19 school shutdowns: What will they do to our children’s education? London: London School of Economics and Political Science.
Fan, L. H., Gao, L., Liu, X., Zhao, S. H., Mu, H. T., Li, Z., et al. (2017). Patients’ perceptions of service quality in China: An investigation using the SERVQUAL model. PloS one 12:e0190123.
Fumagalli, L. P., Radaelli, G., Lettieri, E., Bertele’, P., and Masella, C. (2015). Patient Empowerment and its neighbours: Clarifying the boundaries and their mutual relationships. Health Policy 119, 384–394. doi: 10.1016/j.healthpol.2014.10.017
Glasgow, R. E., Neta, G., Carpenter, C. R., Grimshaw, J. M., Rabin, B. A., Fernandez, M. E., et al. (2015). A framework for enhancing the value of research for dissemination and implementation. Am. J. Public Health 105, 49–57.
Glasgow, R. E., Wagner, E. H., Schaefer, J., Mahoney, L. D., Reid, R. J., and Greene, S. M. (2005). Development and validation of the Patient Assessment of Chronic Illness Care (PACIC). Med. Care 43, 436–444. doi: 10.1097/01.mlr.0000160375.47920.8c
Graffigna, G., Barello, S., Bonanomi, A., and Lozza, E. (2015a). Measuring patient engagement: Development and psychometric properties of the Patient Health Engagement (PHE) Scale. Front. Psychol. 6:274. doi: 10.3389/fpsyg.2015.00274
Graffigna, G., Barello, S., Bonanomi, A., Lozza, E., and Hibbard, J. (2015b). Measuring patient activation in Italy: Translation, adaptation and validation of the Italian version of the patient activation measure 13 (PAM13-I). BMC Med. Inform. Decis. Mak. 15:109. doi: 10.1186/s12911-015-0232-9
Hashim, H. A., Maulood, M. F., Rasheed, A. M., Fatak, D. F., Kabah, K. K., and Abdulamir, A. S. (2020). Controlled randomized clinical trial on using Ivermectin with Doxycycline for treating COVID-19 patients in Baghdad, Iraq. MedRxiv. [Preprint] doi: 10.1101/2020.10.26.20219345
Hibbard, J. H., Stockard, J., Mahoney, E. R., and Tusler, M. (2004). Development of the Patient Activation Measure (PAM): Conceptualizing and measuring activation in patients and consumers. Health Serv. Res. 39(4 Pt 1), 1005–1026. doi: 10.1111/j.1475-6773.2004.00269.x
Higgins, T., Larson, E., and Schnall, R. (2017). Unraveling the meaning of patient engagement: A concept analysis. Patient Educ. Couns. 100, 30–36. doi: 10.1016/j.pec.2016.09.002
Hillier, S. (1991). The health and health care of ethnic minority groups. A: SCRAM-BLER, G. Sociology as applied to medicine. Londres: Baillière Tindall, 146–159.
Iglesias, E. B., del Río, E. F., Calafat, A., and Hermida, J. R. F. (2014). Attachment and substance use in adolescence: A review of conceptual and methodological aspects. Adicciones 26, 77–86.
Jerofke-Owen, T. A., and Garnier-Villarreal, M. (2020). Development and psychometric analysis of the patient preferences for engagement tool. Nurs. Res. 69, 289–298. doi: 10.1097/NNR.0000000000000423
Jerofke-Owen, T., Garnier-Villarreal, M., Fial, A., and Tobiano, G. (2020). Systematic review of psychometric properties of instruments measuring patient preferences for engagement in health care. J. Adv. Nurs. 76, 1988–2004. doi: 10.1111/jan.14402
Kapoor, A., and Singh, E. (2020). Empowering smart cities though community participation a literature review. Smart Cities—Opportun. Chall. 2019, 117–125.
Kerari, A., Almalki, M., Bahari, G., and Alharbi, M. F. (2023). Validation of the Arabic version of the Patient Activation Measure (PAM-13) for Application within the primary healthcare context in Saudi Arabia. Healthcare 11:3090).
Koopman, R. J., Petroski, G. F., Canfield, S. M., Stuppy, J. A., and Mehr, D. R. (2014). Development of the PRE-HIT instrument: Patient readiness to engage in health information technology. BMC Fam. Pract. 15:18. doi: 10.1186/1471-2296-15-18
Kosar, C., and Besen, D. B. (2019). Adaptation of a patient activation measure (PAM) into Turkish: Reliability and validity test. Afr. Health Sci. 19, 1811–1820. doi: 10.4314/ahs.v19i1.58
Laranjo, L., Dunn, A. G., Tong, H. L., Kocaballi, A. B., Chen, J., Bashir, R., et al. (2018). Conversational agents in healthcare: A systematic review. J. Am. Med. Inform. Assoc. 25, 1248–1258.
Lip, G. Y., Kamath, S., Jafri, M., Mohammed, A., and Bareford, D. (2002). Ethnic differences in patient perceptions of atrial fibrillation and anticoagulation therapy: The West Birmingham Atrial Fibrillation Project. Stroke 33, 238–242.
Magallares, A., Graffigna, G., Barello, S., Bonanomi, A., and Lozza, E. (2017). Spanish adaptation of the patient health engagement scale (S.PHE-s)in patients with chronic diseases. Psicothema 29, 408–413. doi: 10.7334/psicothema2017.75
Maindal, H. T., Sokolowski, I., and Vedsted, P. (2009). Translation, adaptation and validation of the American short form Patient Activation Measure (PAM13) in a Danish version. BMC Public Health 9:209. doi: 10.1186/1471-2458-9-209
Markle-Reid, M., Ganann, R., Ploeg, J., Heald-Taylor, G., Kennedy, L., McAiney, C., et al. (2021). Engagement of older adults with multimorbidity as patient research partners: Lessons from a patient-oriented research program. J. Comorb. 11:2633556521999508. doi: 10.1177/2633556521999508
Menichetti, J., Libreri, C., Lozza, E., and Graffigna, G. (2016). Giving patients a starring role in their own care: A bibliometric analysis of the on-going literature debate. Health Expect. 19, 516–526. doi: 10.1111/hex.12299
Mokkink, L. B., de Vet, H. C. W., Prinsen, C. A. C., Patrick, D. L., Alonso, J., Bouter, L. M., et al. (2018). Risk of bias checklist for systematic reviews of patient-reported outcome measures. Qual. Life Res. 27, 1171–1179. doi: 10.1007/s11136-017-1765-4
Mokkink, L. B., Prinsen, C. A., Bouter, L. M., Vet, H. C., and Terwee, C. B. (2016). The COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) and how to select an outcome measurement instrument. Braz. J. Phys. Ther. 20, 105–113. doi: 10.1590/bjpt-rbf.2014.0143
Moreno-Chico, C., González-de Paz, L., Monforte-Royo, C., Arrighi, E., Navarro-Rubio, M. D., and Gallart Fernández-Puebla, A. (2017). Adaptation to European Spanish and psychometric properties of the patient activation measure 13 in patients with chronic diseases. Fam. Pract. 34, 627–634. doi: 10.1093/fampra/cmx022
Náfrádi, L., Nakamoto, K., and Schulz, P. J. (2017). Is patient empowerment the key to promote adherence? A systematic review of the relationship between self-efficacy, health locus of control and medication adherence. PLoS One 12:e0186458. doi: 10.1371/journal.pone.0186458
Ngooi, S. S., Lee, W. W., Alkureishi, M. A., Ukabiala, O., Venable, L. R., Staisiunas, D. D., et al. (2016). Patient perceptions of electronic medical record use by faculty and resident physicians: a mixed methods study. J. Gen. Intern. Med. 31, 1315–1322.
Paulo Silva Cunha, J., and Dias, D. (2018). Wearable health devices—vital sign monitoring, systems and technologies. Sensors 18:2414.
Prinsen, C. A. C., Mokkink, L. B., Bouter, L. M., Alonso, J., Patrick, D. L., de Vet, H. C. W., et al. (2018). COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual. Life Res. 27, 1147–1157. doi: 10.1007/s11136-018-1798-3
Pushparajah, D. S. (2018). Making patient engagement a reality. Patient 11, 1–8. doi: 10.1007/s40271-017-0264-6
Rademakers, J., Maindal, H. T., Steinsbekk, A., Gensichen, J., Brenk-Franz, K., and Hendriks, M. (2016). Patient activation in Europe: An international comparison of psychometric properties and patients’ scores on the short form Patient Activation Measure (PAM-13). BMC Health Serv. Res. 16:570. doi: 10.1186/s12913-016-1828-1
Schmaderer, M., Pozehl, B., Hertzog, M., and Zimmerman, L. (2015). Psychometric properties of the patient activation measure in multimorbid hospitalized patients. J. Nurs. Meas. 23, 128–141. doi: 10.1891/1061-3749.23.3.E128
Scholl, I., Kriston, L., Dirmaier, J., Buchholz, A., and Härter, M. (2012). Development and psychometric properties of the Shared Decision Making Questionnaire-physician version (SDM-Q-Doc). Patient Educ. Couns. 88, 284–290.
Serrani Azcurra, D. J. (2014). Elders Health Empowerment Scale: Spanish adaptation and psychometric analysis. Colombia Med. (Cali, Colombia) 45, 179–185.
Skolasky, R. L., Green, A. F., Scharfstein, D., Boult, C., Reider, L., and Wegener, S. T. (2011). Psychometric properties of the patient activation measure among multimorbid older adults. Health Serv. Res. 46, 457–478. doi: 10.1111/j.1475-6773.2010.01210.x
Skolasky, R. L., Ter Gunne, A. F. P., Mohamed, A. S., Van Laarhoven, C. J., and Cohen, D. B. (2010). The presentation, incidence, etiology, and treatment of surgical site infections after spinal surgery. Spine 35, 1323–1328.
Small, N., Bower, P., Chew-Graham, C. A., Whalley, D., and Protheroe, J. (2013). Patient empowerment in long-term conditions: Development and preliminary testing of a new measure. BMC Health Serv. Res. 13:263. doi: 10.1186/1472-6963-13-263
Smoth, M. D., Bagian, J. P., Bryk, A. S., Cassell, G. H., Conway, J. B., Darling, H. B., et al. (2013). “Best care at lower cost: The path to continuously learning health care in America,” in Committee on the learning health care system in America; Institute of Medicine, eds M. Smith, R. Saunders, L. Stuckhardt, and J. M. McGinnis (Washington, DC: National Academies Press (US).
Søgaard, M. B., Andresen, K., and Kristiansen, M. (2021). Systematic review of patient-engagement interventions: Potentials for enhancing person-centred care for older patients with multimorbidity. BMJ Open 11:e048558. doi: 10.1136/bmjopen-2020-048558
Stepleman, L., Rutter, M-C., Hibbard, J., Johns, L., Wright, D., and Hughes, M. (2010). Validation of the patient activation measure in a multiple sclerosis clinic sample and implications for care. Disabil. Rehabil. 32, 1558–1567.
Terwee, C. B., Bot, S. D., de Boer, M. R., van der Windt, D. A., Knol, D. L., Dekker, J., et al. (2007). Quality criteria were proposed for measurement properties of health status questionnaires. J. Clin. Epidemiol. 60, 34–42. doi: 10.1016/j.jclinepi.2006.03.012
Tušek-Bunc, K., Petek-Šter, M., Šter, B., Petek, D., and Kersnik, J. (2014). Validation of the Slovenian version of patient assessment of chronic illness care (PACIC) in patients with coronary heart disease. Coll. Antropol. 38, 437–444.
Usta, D., Korkmaz, F., Akyar, I., and Bonanomi, A. (2019). Patient health engagement scale (PHE-s): Validity and reliability for Turkish patients with chronic diseases. Cukurova Universitesi Tip Fakultesi Dergisi 44, 1055–1063. doi: 10.17826/cumj.482420
Weil, A. R. (2016). The patient engagement imperative. Health Aff. (Millwood). 35:563. doi: 10.1377/hlthaff.2016.0337
Wensing, M., van Lieshout, J., Jung, H. P., Hermsen, J., and Rosemann, T. (2008). The Patients Assessment Chronic Illness Care (PACIC) questionnaire in The Netherlands: A validation study in rural general practice. BMC Health Serv. Res. 8:182. doi: 10.1186/1472-6963-8-182
World Health Organization (2016). Multimorbidity. Available online at: https://apps.who.int/iris/bitstream/handle/10665/252275/9789241511650-eng.pdf
Yen, H. Y., and Lin, L. J. (2018). Quality of life in older adults: Benefits from the productive engagement in physical activity. J. Exerc. Sci. Fit. 16, 49–54. doi: 10.1016/j.jesf.2018.06.001
Zakeri, S., Chatterjee, P., Konstantas, D., and Ecer, F. (2023). A decision analysis model for material selection using simple ranking process. Sci. Rep. 13:8631.
Zeng, H., Jiang, R., Zhou, M., Wu, L., Tian, B., Zhang, Y., et al. (2019). Measuring patient activation in Chinese patients with hypertension and/or diabetes: Reliability and validity of the PAM13. J. Int. Med. Res. 47, 5967–5976. doi: 10.1177/0300060519868327
Keywords: patient engagement, patient empowerment, assessment, measures, multimorbidity
Citation: Barello S, Anderson G, Bosio C, Lane DA, Leo DG, Lobban TCA, Trevisan C and Graffigna G (2024) Patient engagement in multimorbidity: a systematic review of patient-reported outcome measures. Front. Psychol. 15:1345117. doi: 10.3389/fpsyg.2024.1345117
Received: 27 November 2023; Accepted: 06 May 2024;
Published: 17 July 2024.
Edited by:
Khaled Trabelsi, University of Sfax, TunisiaReviewed by:
Hajer Sahli, University of Jendouba, TunisiaAshten Duncan, University of New Mexico Health Sciences Center, United States
Copyright © 2024 Barello, Anderson, Bosio, Lane, Leo, Lobban, Trevisan and Graffigna. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Caterina Bosio, Y2F0ZXJpbmEuYm9zaW9AdW5pY2F0dC5pdA==
 Serena Barello
Serena Barello Gloria Anderson
Gloria Anderson Caterina Bosio1*
Caterina Bosio1* Deirdre A. Lane
Deirdre A. Lane Donato G. Leo
Donato G. Leo Trudie C. A. Lobban
Trudie C. A. Lobban Caterina Trevisan
Caterina Trevisan Guendalina Graffigna
Guendalina Graffigna