- 1Department of Psychology, University of Alabama at Birmingham, Birmingham, AL, United States
- 2Department of Psychological Sciences, University of Connecticut, Storrs, CT, United States
- 3Department of Psychology, University of California, Berkeley, Berkeley, CA, United States
- 4Department of Brain and Cognitive Sciences, University of Rochester, Rochester, NY, United States
- 5CogT Lab, Department of Psychiatry and Behavioral Sciences, Stanford University, Stanford, CA, United States
- 6Intensive Care Pediatrician, Pediatric Intensive Care Unit, Hospital Moinhos de Vento, Porto Alegre, Brazil
- 7Center for Healthy Minds, University of Wisconsin, Madison, WI, United States
- 8Department of Psychology, University of Wisconsin, Madison, WI, United States
- 9Waisman Laboratory for Brain Imaging and Behavior, University of Wisconsin, Madison, WI, United States
- 10Department of Psychiatry, University of Wisconsin, Madison, WI, United States
- 11Haskins Laboratories, New Haven, CT, United States
- 12Brain Imaging Research Center (BIRC), University of Connecticut, Storrs, CT, United States
- 13Department of Psychiatry and Behavioral Sciences, and Weill Institute for Neurosciences, University of California San Francisco, San Francisco, CA, United States
- 14Department of Neuropsychiatry, Keio University School of Medicine, Shinanomachi Shinjuku Tokyo, Tokyo, Japan
This scoping review provides an overview of previous empirical studies that used brain imaging techniques to investigate the neural correlates of emotional well-being (EWB). We compiled evidence on this topic into one accessible and usable document as a foundation for future research into the relationship between EWB and the brain. PRISMA 2020 guidelines were followed. We located relevant articles by searching five electronic databases with 95 studies meeting our inclusion criteria. We explored EWB measures, brain imaging modalities, research designs, populations studied, and approaches that are currently in use to characterize and understand EWB across the literature. Of the key concepts related to EWB, the vast majority of studies investigated positive affect and life satisfaction, followed by sense of meaning, goal pursuit, and quality of life. The majority of studies used functional MRI, followed by EEG and event-related potential-based EEG to study the neural basis of EWB (predominantly experienced affect, affective perception, reward, and emotion regulation). It is notable that positive affect and life satisfaction have been studied significantly more often than the other three aspects of EWB (i.e., sense of meaning, goal pursuit, and quality of life). Our findings suggest that future studies should investigate EWB in more diverse samples, especially in children, individuals with clinical disorders, and individuals from various geographic locations. Future directions and theoretical implications are discussed, including the need for more longitudinal studies with ecologically valid measures that incorporate multi-level approaches allowing researchers to better investigate and evaluate the relationships among behavioral, environmental, and neural factors.
Systematic review registration: https://osf.io/t9cf6/.
1 Introduction
According to the Centers for Disease Control and Prevention ([CDC], 2014), well-being generally refers to “judging life positively and feeling good,” yet there is no consensus around a single definition of well-being, and research indicates that well-being is a multifaceted construct. Many factors contribute to perceived well-being, including mental and physical health, social relationships, and quality of life (Diener, 1984; Organisation for Economic Co-operation and Development, 2020). Out of all the aspects of well-being, the focus of this review is on emotional well-being (EWB). EWB is not synonymous with the absence of negative states such as depressed or anxious thoughts or feelings, but instead comprises an important independent domain of positive functioning (Feller et al., 2018; Organisation for Economic Co-operation and Development, 2020).
Definitions of EWB differ widely across the literature, and the diversity of methods and measures used to study EWB reflect a lack of consensus of this concept. In an effort to build consensus around the construct and advance research on EWB, NIH funded six EWB High Priority Research Networks (RFA-AT-20-003) from across the country in early 2021, whose main goals included the development of a working definition of EWB (National Institute of Health [NIH], 2018). The work of this NIH-funded consortium led to the following working definition for EWB: “EWB is a multi-dimensional composite that encompasses how positive an individual feels generally and about life overall. It includes both experiential features (emotional quality of momentary and everyday experiences) and reflective features (judgments about life satisfaction, sense of meaning, and ability to pursue goals that can include and extend beyond the self). These features occur in the context of culture, life circumstances, resources, and life course.” (Park et al., 2023a, p. 16). Importantly, the working definition of EWB includes both evaluative (e.g., life satisfaction, sense of meaning) and experienced (e.g., positive mood, day-to-day feelings of happiness) aspects of emotion, as well as hedonic (i.e., positive affect and life satisfaction) and eudaimonic aspects (i.e., sense of meaning in life and goal pursuit) of positive affect. As emphasized by Park et al. (2023a,b) this definition of EWB is still evolving, and aspects of the definition can be mapped onto the well-established constructs of subjective well-being (Diener, 1984) and psychological well-being (Ryff, 1989). Furthermore, Park et al. (2023b) subsequent commentary clarifies that although this definition emphasizes the positive aspects of EWB, it is designed to encompass varying degrees of positive experience and affect. Moreover, it is designed to capture the importance of negative emotional experiences in shaping the emotional quality of quotidian and moment-to-moment experiences.
Previous studies have demonstrated that elevated EWB is associated with decreased mortality risk and increased physical and psychological health (Feller et al., 2018; Trudel-Fitzgerald et al., 2021). Research has consistently linked higher levels of happiness to an increased likelihood of maintaining healthy lifestyles over extended periods, with one longitudinal study reporting evidence of this relationship over 12 years (Trudel-Fitzgerald et al., 2019). Furthermore, elevated EWB is correlated with a lower risk of chronic illnesses such as cardiovascular disease and diabetes. It also appears to contribute to healthier aging and increased longevity (Boehm and Kubzansky, 2012; Pressman et al., 2019; Steptoe, 2019). Meta-analyzes indicate that individuals with higher EWB, compared to those with lower levels, have a 17% reduced risk of mortality (Cohen et al., 2016), a 14% decrease in all-cause mortality risk (Rozanski et al., 2019), and a notable 25% reduction in mortality risk among older adults (Zhang and Han, 2016).
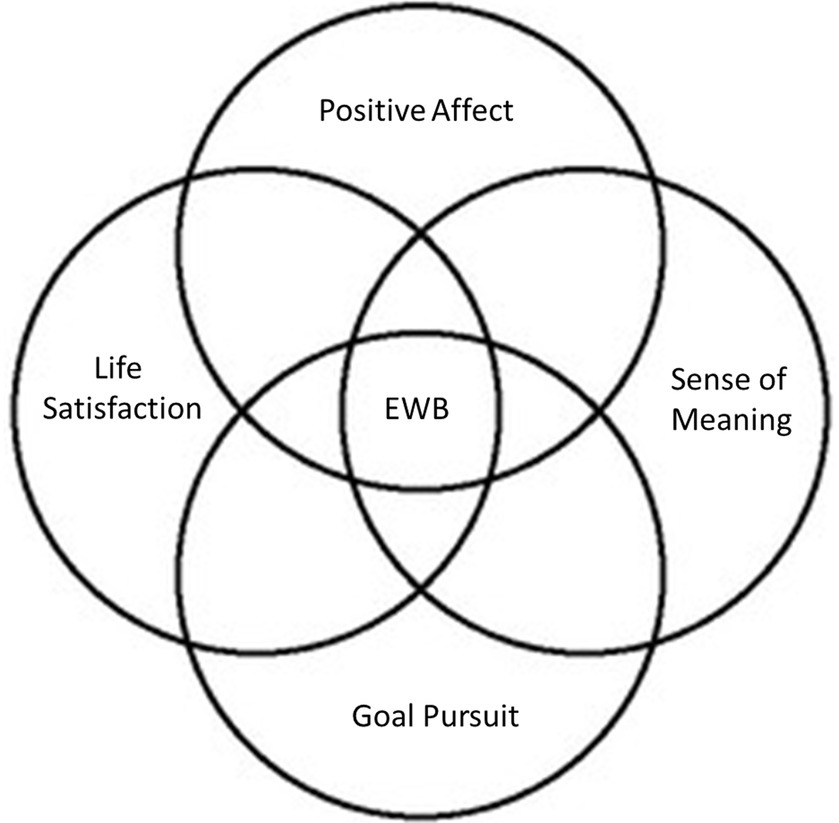
In the present scoping systematic review, we seek to contribute to the scientific understanding of EWB by organizing existing knowledge in the field into a coherent and accessible framework and by making neuroimaging recommendations for future research into the neural processes related to EWB. Our scoping review is organized following this working definition of EWB (see Figure 1).
1.1 Overview of EWB neuroimaging research
EWB broadly relates to the subjective experience of the goodness of one’s life and, thus, has traditionally been evaluated using self-report instruments. Neuroimaging research, by contrast, has contributed to our understanding of the brain-based mechanisms underlying EWB and may help researchers to identify new avenues for interventions to increase EWB. It may also someday provide a measure of EWB in populations with cognitive or communication differences that can make self-report measures unreliable, such as those with severe or profound intellectual disabilities (Flynn et al., 2017). Non-invasive brain imaging is widely used to understand neural mechanisms in humans and is particularly relevant for the study of EWB, given the limits of examining EWB in animal models (but see Weiss et al., 2011; Ben-Shaanan et al., 2016). Neuroimaging has enhanced our understanding of emotional processing and paved the way for innovative treatments for emotional challenges. As an example, studies of the brain regions and networks most affected by depression have informed the development of brain stimulation techniques that demonstrate promising outcomes in treating depressive symptoms (Cash et al., 2021). An improved understanding of the neural correlates of EWB may allow for the development of similar interventions to improve EWB in groups at-risk for low EWB and the broader population and identification of potential biomarkers for those interventions.
Research indicates that EWB is linked to variations in network connectivity, both within and between networks, as well as the dynamic interactions therein (Shi et al., 2018). A number of large-scale brain networks, such as the default mode, salience, and frontoparietal networks, have been associated with EWB (Lindquist et al., 2012; Kragel and LaBar, 2016; Riedel et al., 2018). These networks have also been linked with self-reflection (van der Meer et al., 2010), interoception (Kleckner et al., 2017), theory of mind (Schurz et al., 2014), emotion regulation (Morawetz et al., 2020), and cognitive control (Cocchi et al., 2013).
Both regional and network-level brain variations appear to correlate to aspects of EWB. However, there is limited agreement amongst study findings and a dearth of well-defined theories that add explanatory value and predictive power to these results. Moreover, consistent with trends in neuroimaging more broadly (Siddiqi et al., 2022), few studies provide causal insight into the neural foundation of EWB. This is largely due to a lack of temporal clarity (i.e., whether brain changes precede behavioral ones), experimental manipulation (i.e., if altering the brain influences behavior), and convergence amongst study findings (i.e., whether research consistently highlights a specific brain region or network).
The variety and complexity of literature investigating the neural basis of EWB might stem from the methodological variability inherent in neuroimaging studies (Carp, 2012), including differences in MRI techniques (e.g., structural MRI, resting-state fMRI, task-based fMRI), fMRI tasks, and preprocessing strategies. Additionally, the low reproducibility of this research (Poldrack et al., 2020) complicates drawing definitive conclusions from individual studies. A systematic investigation of neuroimaging research on EWB is necessary to lay the groundwork for future studies and to evaluate the readiness of specific sub-domains of EWB and its neural correlates for eventual meta-analysis. Such meta-analyzes should ideally encompass studies with consistent methodologies (Müller et al., 2018). While systematic reviews are known to have biases, such as publication bias, inclusion of low-quality articles, and lack of homogeneity on the data summarized (e.g., Murad et al., 2018; Uttley et al., 2023), they nevertheless provide clear advantages over individual studies or narrative reviews, by offering a more impartial assessment of the existing evidence (e.g., Finckh and Tramèr, 2008; Haddaway et al., 2020).
Motivated by the aforementioned identified need and formal efforts to better operationalize EWB, we undertook a systematic review of neuroimaging studies that include behavioral measures of constructs related to EWB. Additionally, we sought to capture various definitions and conceptualizations of EWB and how they have evolved over time in relation to neuroimaging, as well as identify gaps in the extant literature. It’s important to emphasize that our review does not seek to identify or establish the neural correlates of EWB directly; the diversity in design, imaging modality, and behavioral measures across included studies renders comparison and conclusion difficult, if not impossible. Our aim, rather, is to present a current overview of the literature, paving the way for more focused future research on the neural underpinnings of EWB.
1.2 Previous reviews of EWB neuroimaging research
Our team conducted a systematic search of previously published reviews—including chapters, narrative reviews, systematic reviews, and meta-analyzes—that explored concepts related to EWB and incorporated any form of brain imaging. Out of the 17 reviews we identified: Four examined the neural correlates of well-being, encompassing concepts including overall well-being, positive affect, happiness, and psychological well-being (Keverne, 2004; Huppert, 2009; King, 2019; Alexander et al., 2021); four focused on positive and negative emotions (Critchley, 2003; Murphy et al., 2003; Vytal and Hamann, 2010; Machado and Cantilino, 2017); three examined well-being in specific scenarios or amongst specific populations, such as the relation between aesthetic emotion and psychological well-being and aging (Kryla-Lighthall and Mather, 2009; St. Jacques et al., 2013; Mastandrea et al., 2019); three specifically targeted happiness (Subramaniam and Vinogradov, 2013; Suardi et al., 2016; Tanzer and Weyandt, 2020); two evaluated neural processes linked to mindfulness practices by using mindfulness as their EWB-related measure probed with neuroimaging (Marchand, 2014; Kaur and Singh, 2015); and one focused on the neural foundation of anomalies in emotional stimuli processing in individuals with mood disorders (Leppänen, 2006).
With respect to previously published systematic reviews of constructs related to EWB (e.g., Keverne, 2004; Huppert, 2009; Subramaniam and Vinogradov, 2013; Suardi, 2016; King, 2019; Tanzer and Weyandt, 2020; Alexander et al., 2021), our current scoping review distinguishes itself in several important ways: (1) We focused specifically on EWB, aligning our search with the working definition proposed by Park et al. (2023a). (2) Our inclusion criteria required that the brain imaging studies also include at least one subjective measure of constructs related to EWB identified by the scoping review of reviews conducted by Koslouski et al. (2022) that compiled a list of EWB measures extracted from previous reviews. (3) Instead of limiting our focus to a specific imaging method, we included a broad spectrum of imaging modalities. (4) We sought to identify broad trends in existing research rather than focus narrowly on the neural correlates of EWB, a task better suited for future meta-analyzes within brain imaging modalities.
1.3 The present review
The objective for the present study was to undertake a scoping review of past empirical studies employing brain imaging techniques and self-report measures to explore the neural and behavioral underpinnings of EWB. Identifying, describing, and synthesizing prior works on this subject is of considerable value to the scientific community as it offers insights into the progression of EWB research, refines our understanding of the construct, and paves the way for subsequent inquiries in this domain. Specifically, this review investigates imaging modalities and measures used to capture and comprehend EWB. Furthermore, it describes and analyzes trends in extant research, encompassing research design, target population, imaging methodologies, and findings, as well as how these have evolved over time.
2 Methods
2.1 Search strategy
This is a scoping review of the literature on brain imaging studies including measures of EWB. We followed the PRISMA 2020 guidelines (Page et al., 2021) in conducting and reporting our search and preregistered this study on Open Science Framework on July 15, 2021 (doi: 10.17605/OSF.IO/T9CF6). We located relevant articles by searching five electronic databases: PubMed, PsycInfo, Web of Science, ERIC (EBSCO), Embase, with the latest search conducted on July 09, 2021. Complete and detailed searches per database can be found in Supplemental materials. To obtain the maximum number of articles, we elected not to restrict our search by date. Articles originating in any nation were included, but searches were limited to articles published in peer reviewed journals and in English. Following each database’s guidelines, we entered keywords that combined terms related to EWB and terms related to brain imaging modalities. Articles that included at least one search term from each Group (A: EWB and its components, B: brain imaging modalities, and C: brain or neuro-related terms) were captured (see Supplementary Table S1) for screening and eventual review. Based on this search, we identified a total of 4,243 articles after duplicates were removed (see Figure 2 for PRISMA 2020 flow diagram).
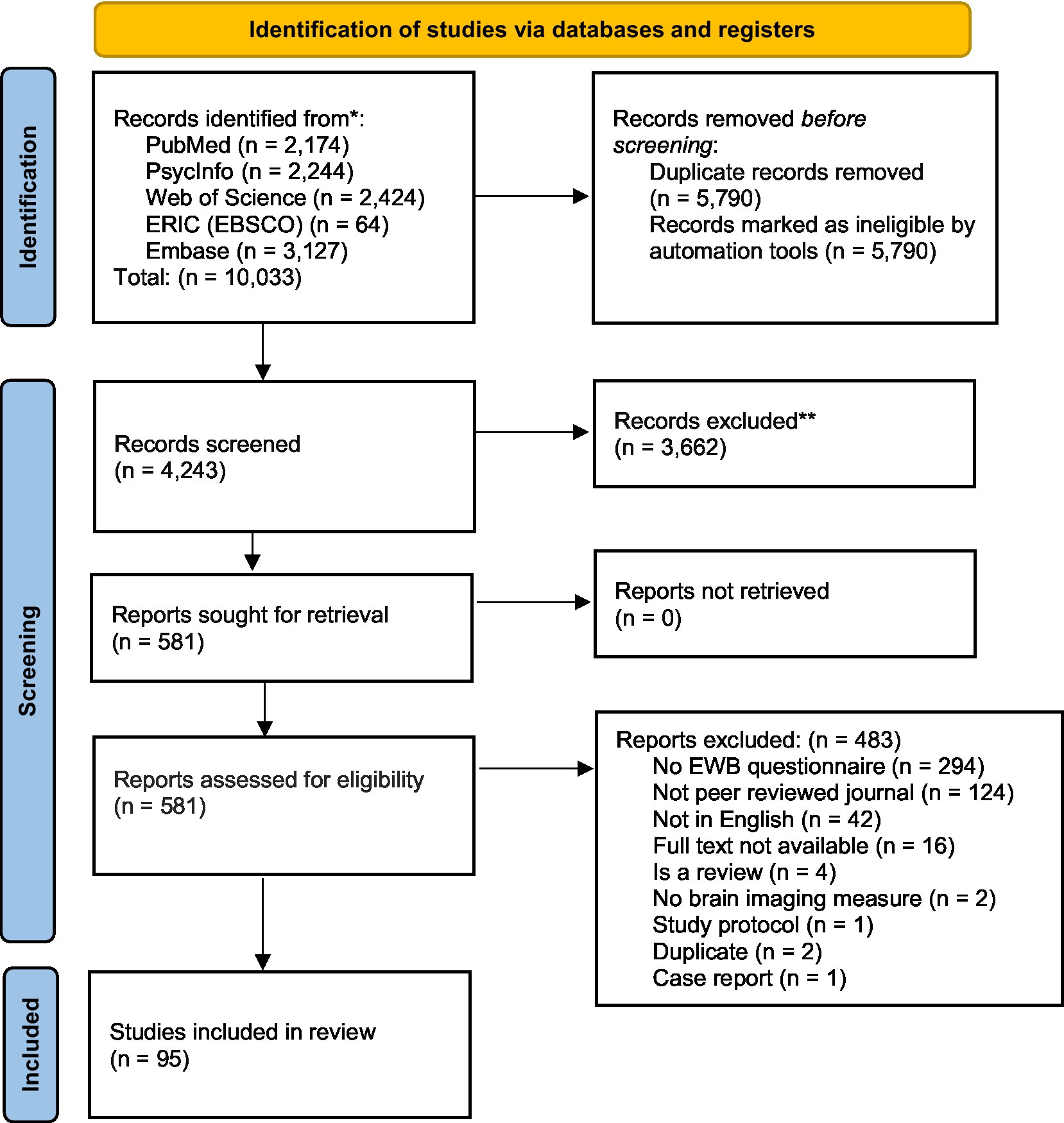
Figure 2. PRISMA 2020 flow diagram for the systematic search on brain imaging studies of emotional well-being. Page et al. (2021) for more information, visit: http://www.prisma-statement.org/.
2.2 Inclusion and exclusion criteria
We reviewed the articles for inclusion based on the following inclusion criteria: (1) The use of at least one brain imaging modality; (2) At least one measure of EWB or its components (see list in Supplementary Table S2 andrationale below); (3) Articles published in peer reviewed journals; (4) Studies published in English. We excluded articles based on the following criteria: (1) Book chapters, reviews, case studies, qualitative studies, meta-analysis, systematic reviews; (2) Unrelated articles, duplicates, unavailable full texts, or abstract-only papers; (3) Articles published on Google Scholar only, dissertations, theses, conference papers, opinion papers; and (4) Animal research. We did not impose restrictions on publication date, methodological rigor, characteristics of participants, or age of participants included in the study. As the current study is a scoping review, study quality was not investigated (Munn et al., 2018).
2.3 Search procedures
Search procedures comprised three separate stages: (1) Title and abstract screening, (2) Full-text screening, and (3) Data extraction. At each stage, relevant procedures were conducted independently by at least two trained research assistants using Covidence (systematic review software; Veritas Health Innovation, 2021). Discrepancies at each stage were resolved by a third rater (authors of this current article) also using Covidence. We followed the procedures outlined by Polanin et al. (2019), including the creation of a checklist file with specific screening questions that guided raters throughout the screening process (see Supplemental materials).
To pass to the full-text screening phase, each study must have included at least one of the 135 EWB measures outlined in Supplementary Table S2 (a comprehensive list of these measures, with citations, can be found in Supplementary Table S2). These measures originated from a scoping review of reviews that gathered questionnaires designed to capture individual experiences of EWB (Koslouski et al., 2022). Consistent with our operational definition of EWB, instruments focusing solely on depression, anxiety, or other negative emotions were omitted from their assembled list. Our scoping review was conducted concurrently with that of Koslouski et al. (2022), employing analogous search terms, with the intention of yielding a cohesive set of outcomes to further enrich the existing literature.
2.4 EWB constructs
As previously noted, our scoping review follows the theoretical framework proposed by Park et al. (2023a). Accordingly, we classified the included studies into five core EWB domains as conceptualized by Park et al. (2023a): (1) positive affect, (2) life satisfaction, (3) goal pursuit, (4) quality of life, and (5) sense of meaning. These classifications were based on the EWB measures employed by the studies included in this review.
2.5 Brain imaging modalities
Studies were classified into three main categories based on their design and purpose. Studies were characterized as task-based functional imaging studies if they examined the function of the brain using a task-based paradigm. Task-based imaging methods included fMRI, EEG, ERP, PET, SPECT, TMS, and tDCS. For the task-based functional imaging studies, we provided a brief description of the task or paradigm used in the study, along with the main EWB domain investigated in the study (e.g., experienced affect, affective perception, reward, and emotion regulation). Studies were characterized as resting-state if functional images were acquired under resting-state conditions, using methods such as fMRI, EEG, PET, MRS, rTMS, and TBS. Finally, those studies examining structural properties of the brain such as grey and white matter properties, were categorized as structural MRI studies and included methods such as MRI and resting DTI. For studies in all three categories, main regions of interest (ROI) were documented, and main brain outcome measures were also reported.
3 Results
3.1 Study and sample characteristics
The distribution of papers by year can be found in Figure 3. There was a clear increase in the number of articles published after 2010, with a peak in 2015 (n = 16), then a slight decline. Of the 95 articles in this review, 69 were published between 2013 and 2021. This trend corresponds with the advancements in brain imaging techniques and their growing use in EWB studies.
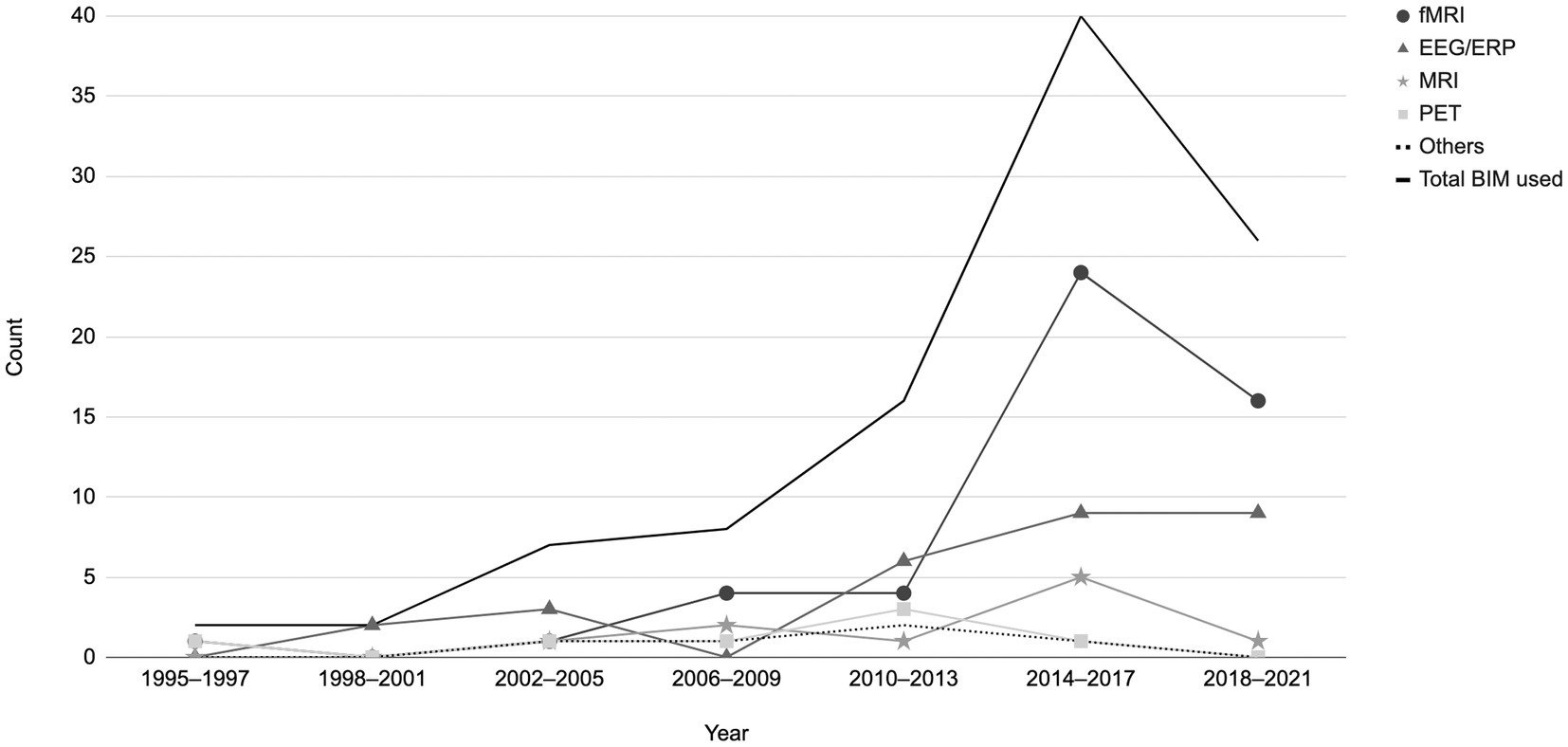
Figure 3. Distribution of papers by year based on the brain imaging modalities utilized (fMRI, EEG/ERP, MRI, PET). Others = TMS, rTMS/TBS, tDCS, SPECT and MRS. A total of 101 brain imaging modalities used across the 95 studies included in the current review.
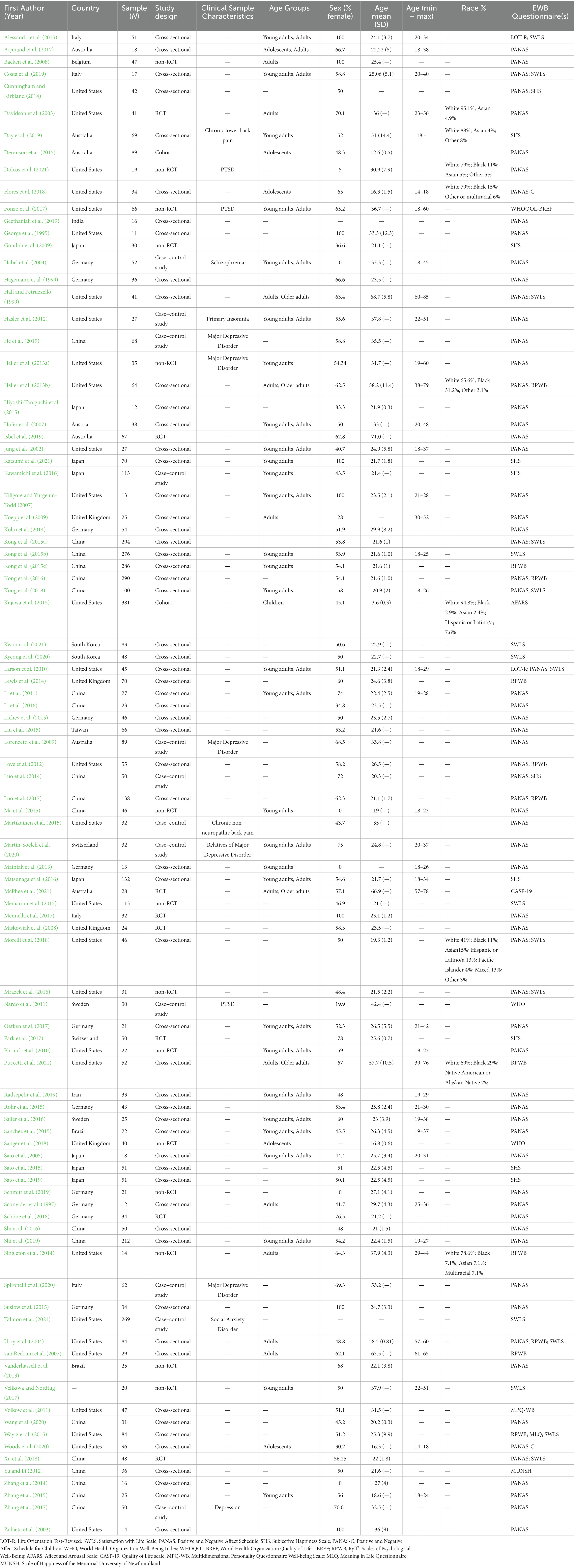
Table 1 presents the sample characteristics of the 95 studies included in this review and Supplementary Table S3 presents the studies’ aims and main findings. A minority (n = 14) was conducted with clinical populations. This includes one study with a subclinical group and another with an at-risk group. The specific disorders investigated were: post-traumatic stress disorder (n = 3), schizophrenia (n = 1), major depressive disorder or depression (n = 6), social anxiety disorder (n = 1), chronic lower back pain (n = 2), and primary insomnia (n = 1). The large majority of the studies (n = 81) involved samples reported to be non-clinical, not at risk, or healthy.
With respect to developmental stages, we classified studies based on the mean age reported in the articles. Seven studies did not provide this information. Of the remaining 88 studies, the preponderance (n = 82) focused on adults (age ≥ 18). Only four of these included older adults in their sample (age > 65; Hall and Petruzzello, 1999; Heller et al., 2013b; McPhee et al., 2021; Puccetti et al., 2021). Five studies included adolescents (age 12–18) in their sample (Dennison et al., 2015; Arjmand et al., 2017; Flores et al., 2018; Sanger et al., 2018; Woods et al., 2020), and one study, by Kujawa et al. (2015), included children aged 12 or younger.
Only nine of the included articles reported the racial or ethnic composition of their samples, and all but one (Day et al., 2019; conducted in Australia) of these were conducted in the United States (Davidson et al., 2003; Heller et al., 2013b; Singleton et al., 2014; Kujawa et al., 2015; Flores et al., 2018; Morelli et al., 2018; Dolcos et al., 2021; Puccetti et al., 2021). Amongst the nine studies reporting racial or ethnic demographic data, the majority of the participants were White (ranging from 65.6 to 95.1%). Black/African American participants ranged from 2.9 to 31.3% of the total sample, whilst Asian participants varied between 2.4 and 15% of the total sample. Three studies (Singleton et al., 2014; Flores et al., 2018; Morelli et al., 2018) included a multiracial category. Only two studies (Kujawa et al., 2015; Morelli et al., 2018) mentioned the inclusion of Hispanic or Latino/a participants (see Table 1 for details).
With respect to socio-economic status (SES), 16 studies explicitly reported information on education, and only two reported income of participants. Thirty-six studies reported university student participants, suggesting their samples had some level of college education; however, factors explicitly related to SES were not reported.
3.2 Journals
Studies included in this review were published in a variety of peer reviewed journals (n = 52) varying widely in impact factor (see Supplementary Table S4). Specifically, nine of the studies were published in NeuroImage and another nine in Social Cognitive and Affective Neuroscience, followed by 6 in Frontiers in Human Neuroscience and 4 in the American Journal of Psychiatry. To evaluate the impact of journals, we used the five-year impact factor (2017–2021) from Journal Citation Reports (JCR) - Clarivate. This impact factor is calculated by dividing the total citations in 2021 from articles published in 2016 to 2020 by the total number citable articles in 2016–2020. The JCR impact factor of the journals ranged from 2.826 (i.e., Social Neuroscience) to 19.59 (i.e., American Journal of Psychiatry) for the 18 journals that have two or more studies included in this current review.
3.3 Countries
Most of the articles included in the current review were conducted in the United States (n = 29) and China (n = 19), followed by Germany (n = 11). It is noteworthy that the studies published in North America, Europe, and Australia/Oceania are shown to be overrepresented in proportion to the world population share; on the other hand, articles published in Asia, Africa, and South America appear to be underrepresented to the world population share (see Supplementary Figure S1).
3.4 EWB measures used and EWB constructs investigated
Supplementary Table S5 reports EWB measures and constructs across included studies. Thirteen of the 135 EWB measures identified by Koslouski et al. (2022), were employed in the 95 studies, with a reported total of 115 instances of use. This indicates multiple EWB measures were used in some studies. The Positive and Negative Affect Schedule (PANAS; Watson et al., 1988) was most commonly used at 68.4% of the studies (excluding 2.1% using the children’s version). This was followed by the Satisfaction with Life Scale (SWLS; Diener, 1984) at 17.9%. The Subjective Happiness Scale (SHS; Lyubomirsky and Lepper, 1999) and Ryff’s Psychological Well-being Scale (PWB; Ryff, 1989) had similar frequencies of use, at 10.5 and 11.6%, respectively. Another nine measures were employed in 12 studies.
Of the studies examined, all 95 incorporated at least one measure designed to evaluate the constructs of positive affect, mood, or emotion. All but one examined the construct of life satisfaction. This overlap in constructs stems from the fact that many EWB measures are designed to probe both areas. For example, PANAS is a widely-used instrument measuring both life satisfaction and positive affect. Far fewer studies measured more evaluative aspects of EWB, including sense of meaning (14 studies), goal pursuit (13 studies), and quality of life (2 studies).
3.5 Study design
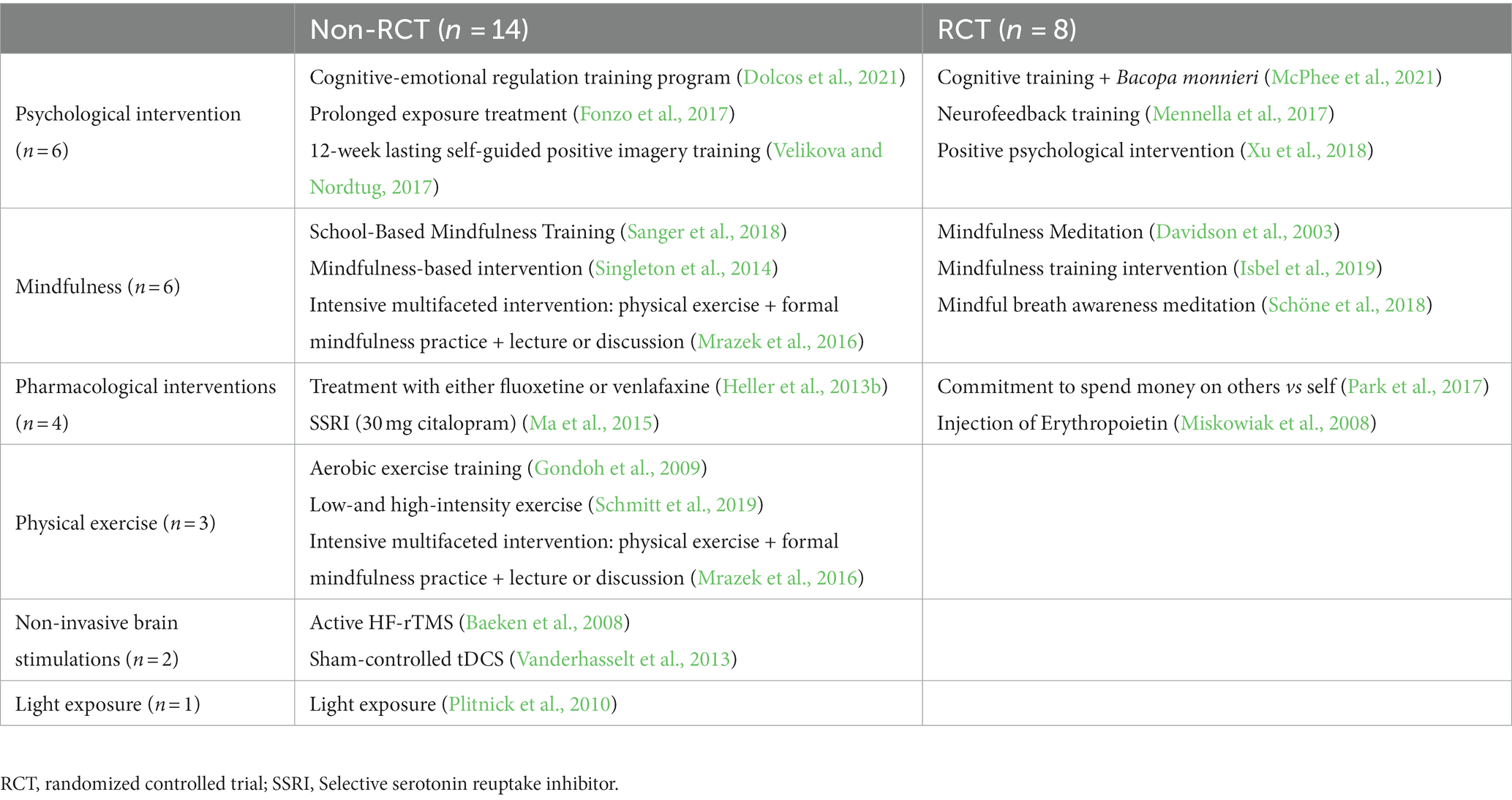
As reported in Table 1, a preponderance of studies were cross-sectional (n = 59), followed by 22 intervention studies, 12 case–control studies, and only two cohort studies. A description of the intervention studies can be found in Table 2. Of these,13 used a quasi-experimental (non-randomized) design, and eight constituted randomized controlled trials (RCTs). These intervention studies can be further classified into six distinct categories: (1) psychological interventions (n = 6), (2) mindfulness-based interventions (n = 6), (3) pharmacological interventions (n = 4), (4) physical exercise interventions (n = 3), (5) non-invasive brain stimulation techniques (n = 2), and (6) light-exposure based interventions (n = 1). It is important to note that few of these studies aimed at drawing causal inferences about the neural basis of EWB: Instead, most sought to identify and describe behavioral and neural correlates of their respective interventions. For example, Dolcos et al. (2021) examined changes in EWB measures and brain measures following cognitive-emotional training but did not establish a causal link between neural and behavioral changes.
3.6 Brain imaging modalities
Among the 95 studies included in this review, neuroimaging modalities were used 101 times, indicating that some studies used more than one modality. Functional magnetic resonance imaging (fMRI) was the predominant modality, used in 50 studies. Continuous electroencephalogram (EEG) and event-related potentials (ERPs) were employed in 24 and five studies, respectively. A limited number of studies (n = 9) used structural MRI. Both magnetic resonance spectroscopy (MRS) and diffusion-weighted imaging (DWI) were used in a single study. Positron emission tomography (PET) imaging was employed in 7 of the studies, whereas single-photon emission computerized tomography (SPECT) was used in only single study. This distribution underscores a pronounced pattern of functional over structural investigations.
Complementary to these imaging methods, certain studies incorporated neuromodulatory techniques. Notably, transcranial magnetic stimulation (TMS), transcranial direct current stimulation (tDCS), and repetitive TMS/theta-burst stimulation (TBS) each appeared in a singular study (refer to Tables 3–5 for details).
Figure 4 details the use of various brain imaging modalities across five distinct EWB constructs. Amongst the 94 studies examining the construct of life satisfaction, fMRI emerged as the predominant modality, used in 50 studies. This was followed by EEG/ERP in 27 studies, MRI in nine studies, PET in seven studies, and other modalities in six studies. Of the 86 studies examining positive affect, fMRI was again used in a preponderance of studies (44 studies). EEG/ERP was used in 27 studies, MRI in nine studies, PET in seven studies, and other techniques were used in four studies. Of those examining goal pursuit, fMRI was employed in seven of 13 studies, and EEG/ERP and MRI were used in two studies each. PET and other techniques were each used in a single study. Amongst the 14 studies evaluating sense of meaning, fMRI was the modality of choice in eight. EEG/ERP and MRI were each employed in two studies, with PET used in one study, and other modalities in two studies. Collectively, these findings underscore a consistent preference for the use of fMRI across EWB constructs, highlighting its widespread use in the field.
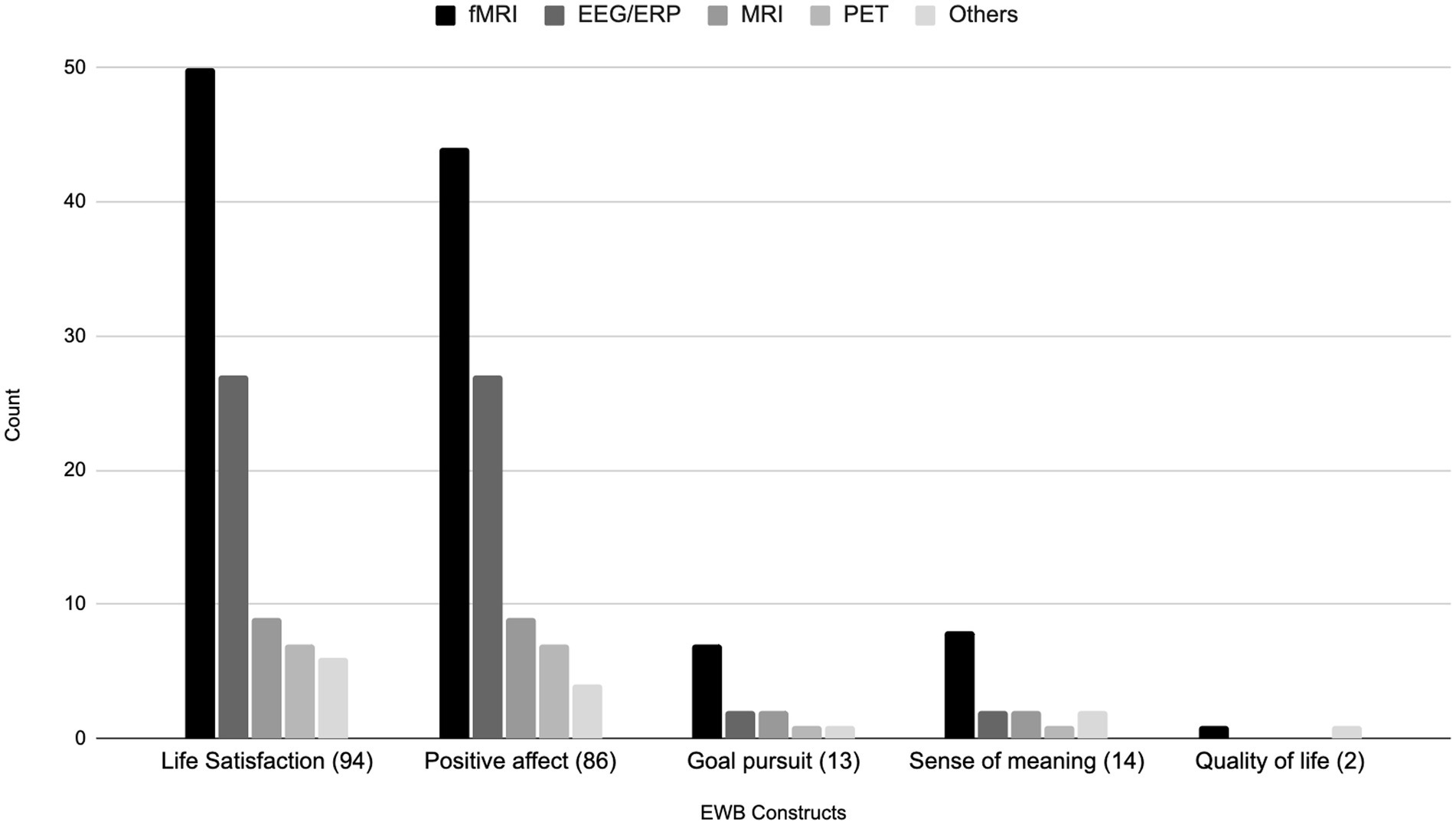
Figure 4. Bar chart of EWB constructs being investigated by brain imaging modalities. Others = TMS, rTMS/TBS, tDCS, SPECT and MRS.
3.7 Task-based functional imaging studies
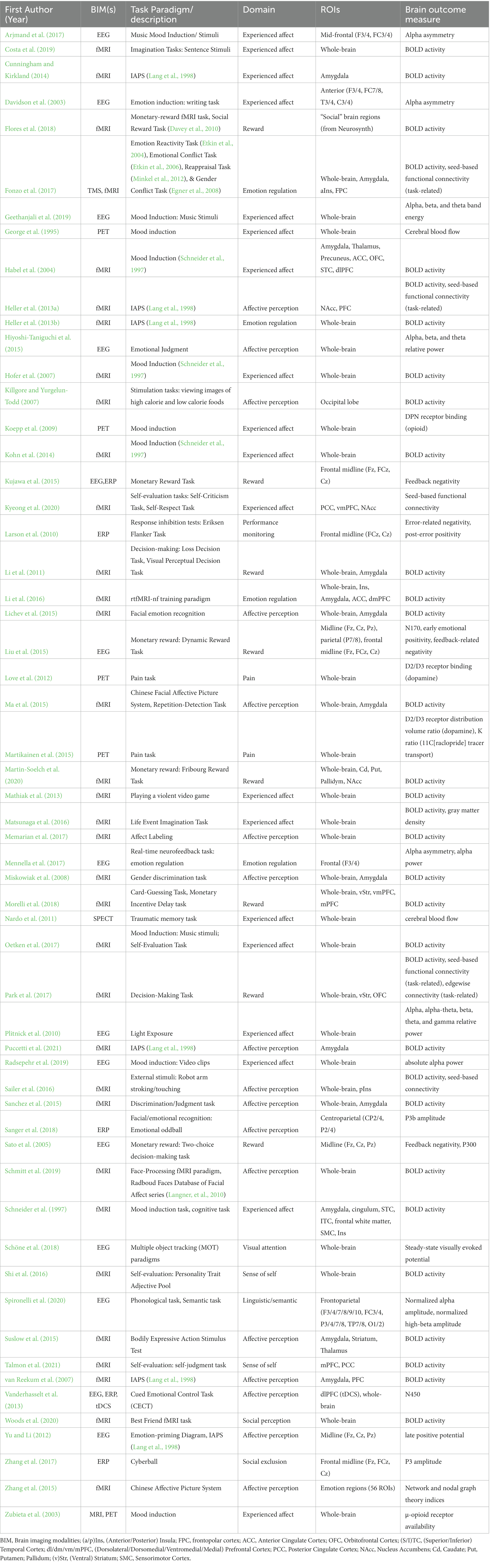
Table 3 depicts the task-based functional imaging studies (n = 57). Among the 57 studies measuring brain activity during task performance, the most commonly used (n = 10) instrument was the International Affective Picture System (IAPS; Lang et al., 1998). Various mood induction tasks were employed in 18 studies (e.g., Schneider et al., 1997), and a range of other paradigms were also used, with most used only in one or two studies.
We classified study paradigms into domains corresponding to the cognitive or emotional processes they were designed to evaluate. These domains comprised: experienced affect, affective perception, emotion regulation, reward, linguistic/semantic processing, pain induction, performance monitoring, sense of self, social exclusion, social perception, and visual attention. As an example, paradigms designed to explore brain activity during distinct affective states, such as positive/negative moods or self-judgment, were categorized under the domain of experienced affect. Paradigms that required participants to modulate their emotional responses were classified as emotion regulation tasks. By contrast, those assessing brain activity in response to emotionally-valenced stimuli were classed as affective perception. Paradigms designed to probe affective perception included both passive viewing of stimuli (e.g., IAPS), as well as active tasks that asked participants to judge, recognize, label, and discriminate emotionally-valenced stimuli. Notably, the domains of experienced affect and affective perception predominated in our dataset, comprising 19 and 17 of the 57 task-based studies, respectively. These were followed by studies focused on emotion regulation (n = 4). This emphasis on the evaluation of neuroactivity underpinning emotion processing aligns with the EWB used measures in these studies, specifically those examining experiential facets of EWB, such as positive affect.
The next most commonly studied domain was reward, with eight studies measuring brain function during processes related to reward. Two studies measured brain activity during pain induction, and two during paradigms designed to explore sense of self. Several studies used paradigms aimed at understanding cognitive processes, including one performance monitoring task, one visual attention task, and one study using linguistic/semantic tasks. Finally, two studies used paradigms designed to measure brain activity during social processing, one using a social perception task (video clips of best friends vs. strangers) and one using a social exclusion task (Cyberball game, in which participants are excluded from a virtual game).
With respect to brain regions examined, 21 of task-based functional imaging studies used exploratory whole-brain approaches (looking for any relationships to either connectivity or activity across every brain region) with no a priori defined ROI. After taking this exploratory whole brain approach, most of these studies (n = 12) identified the regions that were most active during the chosen task, and then within those specific regions (ROI) investigated the relations with a particular EWB measure (see Table 3 for details). In studies with a priori ROIs, a number of EEG studies targeted frontal/mid-frontal electrodes as a means of calculating frontal asymmetry, a measure commonly linked to positive affect. In general, EEG studies used various approaches to quantify neural activity, including calculating energy/power within different frequency bands (mostly alpha, beta, and theta), as well as ERPs such as the feedback negativity response, N170, and P3. Across fMRI studies, a range of target regions were explored across both the cortex and subcortex. Frontal regions (e.g., dorsolateral prefrontal cortex, orbitofrontal cortex, anterior cingulate cortex) were more commonly targeted than others, although several studies examined the posterior cingulate cortex as a ROI. The insula and amygdala, two regions commonly linked to emotional processing, were also well represented. Other subcortical regions, including the striatum and thalamus, were also targeted in multiple studies. fMRI studies predominantly looked at brain activity measured using BOLD signal response, although several studies also looked at functional connectivity during task performance.
3.8 Resting-state studies
Table 4 presents those studies that used resting state methodologies to examine brain function, accounting for 30 studies. Of these, fMRI was used in 16 studies. EEG was employed in 10 studies, and PET in two, while rTMS or TBS and MRS were each used in a single study. Of note, almost half of these studies (n = 15) explored connectivity or activity across the entire brain, indicating an absence of a priori hypotheses focused on specific brain regions or networks. As with the task-based studies, several of the EEG studies targeted frontal/mid-frontal electrodes to calculate measures of alpha asymmetry. The fMRI resting state studies that did define ROIs a priori targeted a range of regions across all four cortical lobes, as well as the subcortex. Studies used a range of resting state analytical approaches, including seed-based functional connectivity, fractional amplitude of low-frequency fluctuations (fALFF), and regional homogeneity (ReHo) to measure brain function at rest. In line with the EEG studies, areas the frontal lobe were identified as ROIs more often than those in the occipital, parietal, or temporal lobes, with several studies targeting the orbitofrontal cortex, medial prefrontal cortex, dorsolateral prefrontal cortex, and anterior cingulate cortex (as was found for the task-based fMRI studies). Similar to task-based studies, the insula and amygdala were also commonly identified as ROIs. Two studies also used the default mode network as a network-of-interest, the only network to be targeted in this way.
3.9 Structural MRI studies
Table 5 includes studies that used structural MRI approaches (n = 8). Of these studies, one used DTI while the remainder used MRI. Three studies used whole brain approaches, while the others defined ROI a priori. In contrast with the task-based and resting state studies, the majority of regions targeted by structural MRI studies were subcortical regions, including the amygdala, striatum, and hippocampus, and one study targeting the pituitary gland. All studies (except the DTI study) in this category assessed gray matter structure, measuring total MRI volume, gray matter volume, gray matter density, and/or gray matter concentration. The single DTI study measured white matter mean diffusivity/fractional anisotropy and gray matter dispersion/neurite density.
4 Discussion and future directions
We conducted a scoping review of neuroimaging studies designed to explore and evaluate the relationship between EWB and the brain. By compiling, organizing, and assessing included articles, we sought to establish a foundation for future modality-specific meta-analyzes and provide insight into the current state of the evidence, as well as identify gaps in the literature and highlight directions for future research.
With respect to overall trends, few studies evaluated the neural correlates of EWB in populations other than White healthy young adults; as such, there is a notable gap in the research involving children, older adults, clinical populations, and more diverse, underrepresented groups and populations. It’s likewise evident that the neural basis of experienced affect, especially positive mood, has been better researched than the evaluative components of EWB. The majority of these studies used exploratory (whole-brain) imaging techniques, and among those with pre-determined ROIs, there was little consistency among targeted regions. This suggests a lack of consensus around the neural correlates of aspects of EWB. However, the existence of whole-brain maps of EWB associations suggests that future meta-analyzes, particularly leveraging novel cross-modality analyzes such as standard permutation of subject images (Albajes-Eizagirre et al., 2019), may be able to better summarize this research and provide greater clarity on which brain regions and networks are most strongly implicated in EWB to guide future research and intervention development.
While trait associations can be summarized—for example, those derived from resting state and structural MRI—a significant challenge arises due to the considerable variability in tasks employed to probe EWB. Aggregating data from varied tasks in meta-analyzes is rare due to the complexities associated with interpreting analogous activation patterns within diverse contexts. We must reiterate that our inclusive search criteria, encompassing diverse studies that included any form of neuroimaging and a wide variety of EWB measures, resulted in the inclusion of a large number of studies whose primary aims did not include the establishment of causal links between EWB and brain functioning. This diversity, coupled with a dearth of causal design, complicates summarization and renders generalization impossible. Nevertheless, we contend that our review offers valuable insights into the current state of the EWB neuroimaging literature, potentially informing subsequent focused research. Specifically, the findings from this review might guide the development of a taxonomy of tasks used in EWB research, categorizing them based on the aspects of EWB they measure and the brain regions they activate. In the remainder of the Discussion, we discuss the main findings of this study, limitations, and directions for future research.
4.1 EWB measures
The majority of included studies used PANAS; although previous research has demonstrated that this scale is a valid and reliable measure of certain aspects of EWB (e.g., Pavot et al., 1991; Crawford and Henry, 2004), the manner in which the instrument is used and interpreted can vary widely. For example, the PANAS questionnaire can be used as a tool to measure momentary affect (e.g., Indicate to what extent you feel this way right now, that is, at the present moment.) or trait affect (e.g., Indicate to what extent you generally feel this way, that is how you feel on average.). Yet, the majority of studies included in our sample did not describe how PANAS was used in their protocol (i.e., whether it was used as a measure of momentary affect or trait affect). In addition, several studies reported using the PANAS to evaluate changes in affect during tasks (e.g., emotion regulation and affect perception tasks), which suggests that the instrument was used to measure affect in response to task-specific stimuli, rather than to evaluate momentary or trait affect in daily life. Future studies should be more transparent while reporting on the use of questionnaires.
In light of PANAS’s widespread adoption in EWB assessment, it’s important to acknowledge its usefulness in efficiently measuring positive and negative affect. However, a potential weakness of the PANAS is its emphasis on high-arousal positive affect dimensions (e.g., alertness, excitation), to the exclusion of low-arousal positive emotions like calmness (cf. Pressman et al., 2019). The utility of the PANAS is tempered by its lack of scope, specifically its limitation in capturing a diverse range of discrete positive emotions. Future research should address this limitation by selecting or designing instruments designed to evaluate a broader range of EWB constructs in greater granularity. In this context, the circumplex model of affect presents a potential alternative (Russell, 1980).
The majority of the studies included in this review were task-based functional imaging studies. Of these studies, one third measured brain activity during an experienced affect task and 28 measured brain activity during affective perception (e.g., viewing affective images). Notably, a limited number of studies endeavored to discern associations between brain activity and responses on EWB questionnaires, potentially shedding light on the influence of trait EWB on neural functioning in specific contexts. Overall, the literature appears to exhibit a paucity of evidence concerning neural underpinnings of evaluative EWB as a trait.
While certain resting state and structural studies—which reflect trait aspects of brain function and structure— did examine the relationships between brain activity and evaluative EWB questionnaire outcomes (e.g., Urry et al., 2004; Kong et al., 2015a,b,c; Kwon et al., 2021), others analyzed these measures separately without seeking to establish correlational or causal links (e.g., Mrazek et al., 2016; Dolcos et al., 2021). In some cases, brain function was evaluated in relation to trait measures of experienced affect over extended periods, as observed in studies addressing the trait neural markers of subjective well-being (e.g., Luo et al., 2014; Sato et al., 2015, 2019; Katsumi et al., 2021).
4.2 Brain imaging modalities
Our findings indicate substantial variability not only in the use of instruments (e.g., the PANAS), but also in the use of stimuli across studies. For example, while the IAPS was used in six of the included studies, its application varied: Some studies used IAPS as a tool for mood induction (e.g., asking participants to describe images with the intent of affecting change in their mood, as measured by the PANAS; Cunningham and Kirkland, 2014), while others (e.g., van Reekum et al., 2007; Heller et al., 2013b) used IAPS to stimulate emotion regulation (e.g., suppression) or promote affective perception. As such, it is difficult to generalize findings, even amongst studies using the same stimuli.
This lack of uniformity in study design and use of measures and stimuli hampers our capacity to establish an integrated knowledge base on EWB. Given the multifaceted nature of EWB, which encompasses elements such as positive affect, life satisfaction, sense of meaning, and goal pursuit, a number of measures would likely be required to measure the construct holistically. Future research, centered on well-established facets of experienced EWB—such as mood induction or affective image viewing—adopting standardized measures and validated tasks, could guide the field towards a more unified understanding of the construct and its neural underpinnings.
Alternatively, research into evaluative aspects of EWB may benefit from careful theory-driven task design (e.g., those aimed at understanding the dynamic processes involved in cognitive appraisal of experienced EWB) given the current lack of findings in these areas. Brain imaging measures that allow us to capture these EWB domains dynamically, accounting for their relationship with the environment, could provide crucial insights into the construct and its neural and environmental correlates. Moreover, imaging modalities that allow real-world assessments (i.e., assessments in naturalistic environments) and that are used along with ecological momentary assessments (EMA; Shiffman et al., 2008) would provide the basis for further advancement in the field.
A promising avenue for future research is the use of functional near-infrared spectroscopy (fNIRS). In contrast to the fMRI and EEG modalities used in the included studies, fNIRS offers distinct advantages. Specifically, it demonstrates greater tolerance to bodily movements and boasts heightened portability, compared to MRI, making it well-suited to studies in naturalistic settings (e.g., Hu et al., 2019; Pinti et al., 2020). Researchers can further enhance ecological validity by adopting naturalistic paradigms, such as contrasting neural responses during the viewing of neutral versus emotional films.
Finally, future studies can focus on investigating structural connections in addition to functional connections and interventions to promote EWB or to induce neuroplasticity at grey matter and white matter levels. Innovative approaches that can be incorporated in future studies include the use of multimodal imaging techniques such as fMRI and DTI, or a combination of EEG, fMRI, and DTI. Finally designs that allow investigation of neuromodulation such as TMS-fMRI studies and focused ultrasound (Thaler et al., 2023) are promising.
4.3 Brain regions investigated
Although the goal of this review was not to establish neural correlates of EWB, and the search strategy provided a large range of methodologies that make comparison difficult, there were some broad trends in the findings. In general, frontal brain regions are more commonly implicated by studies of positive affect (e.g., van Reekum et al., 2007; Vanderhasselt et al., 2013; Kyeong et al., 2020), while subcortical regions appear more often in studies looking at structural neural correlates of EWB (e.g., Habel et al., 2004; Cunningham and Kirkland, 2014; Lichev et al., 2015; Li et al., 2016). It is difficult to know whether these reflect consistent findings or researchers with different expertise and interest. Similarly, in the temporal domain EEG studies of positive affect mostly targeted alpha frequency but findings related to beta and theta were also reported. Future studies guided by this review to systematically evaluate the neural correlates of EWB should aim to use consistent and well-described neuroimaging methods (Carp, 2012) and share the results (Poldrack et al., 2017) from both whole-brain and ROI analyzes to allow for much needed meta-analyzes (Müller et al., 2018).
5 Limitations
5.1 Limitations of the studies included in the review
5.1.1 Small sample size
This review identifies several noteworthy limitations within the current body of EWB neuroimaging research. Notably, our analysis revealed that the majority of the studies included relatively small sample sizes. Only 11 of the 95 examined studies included a sample size of 100 or more (e.g., Kujawa et al., 2015; Kong et al., 2015a; Kawamichi et al., 2016). While smaller sample sizes may not compromise interpretation when the observed effect sizes are large, they invariably constrain the power to discern small to medium effects. It is imperative for researchers to judiciously weigh the feasibility and benefits of expanding sample sizes against anticipated effect sizes. It’s also important to acknowledge that advocating for larger sample sizes might serve to exclude researchers from less affluent laboratories globally, potentially perpetuating Western-centric biases in this domain. Furthermore, given the documented volatility of effect sizes within smaller samples (Schönbrodt and Perugini, 2013) and the small to medium effects characteristic of neuroimaging-behavior correlations (Marek et al., 2022; but see Makowski et al., 2023), results from such studies warrant cautious interpretation. Future research would benefit from larger-scale studies and meta-analyzes to bolster confidence in the reported findings.
5.1.2 Lack of studies in diverse samples
As mentioned in previous sections, more than a third of the included studies were conducted in a university setting within healthy individuals. This suggests a probable homogeneity in participants, primarily comprising college students with similar educational backgrounds. While 15.9% of studies delved into EWB within clinical cohorts (e.g., individuals with schizophrenia, those experiencing chronic non-neuropathic back pain, and others), only one study (Martin-Soelch et al., 2020) specifically targeted university students who were kin to a clinical population. Notably absent were studies exploring university students diagnosed with disorders or illnesses; if such participants were included in the studies retained for review, they were not explicitly referenced.
Moreover, our review reveals a conspicuous gap in the literature pertaining to EWB research in children, adolescents, and older adults. This underscores the paucity of dedicated research in these demographics and points to a probable deficiency in the development of validated methodologies and tools tailored for these groups. It is imperative that future endeavors in the field prioritize the selection or development of validated instruments to measure aspects of EWB within these populations. Future studies that consider the impact of life-transition events on emotional well-being are also needed (e.g., middle-aged women hormonal transition and EWB).
Remarkably, none of the studies included in our review presented data on participants’ multifaceted social identities that may bear significance to EWB, such as gender identity, sexual identity, and ability, as highlighted by Villanueva-Moya and Expósito (2022). An intersectional approach to the collection, analysis and reporting of these data would contribute to the collective understand of the diverse elements of identity that contribute to the lived experience of EWB.
5.1.3 Lack of information on race and ethnicity
Another significant limitation observed within the current body of EWB neuroimaging research is the inadequate documentation of participants’ racial and ethnic backgrounds. A mere 9.5% of the articles under review provided details regarding the racial-ethnic demographics of their participants, and this information was exclusively reported by studies based in the United States or Australia.1 This underscores a broader oversight in the literature regarding the potential role of racial and ethnic factors on the lived experience of EWB, even in light of considerable research into race-associated stress (refer to Paradies et al. (2015) for an encompassing meta-analysis). We advocate for EWB researchers to exercise heightened diligence in gathering and disclosing race/ethnicity data, while also considering the role these factors play in shaping EWB outcomes. Future research should be committed to inclusivity, ensuring the representation and equitable examination of diverse populations (see National Academies of Sciences, Engineering, and Medicine, 2022 for guidelines). To streamline and elevate global research efforts, there is a need for a consensus on the standardization of racial and ethnic data reporting, recognizing that reporting norms may vary considerably across nations.
5.1.4 Lack of socioeconomic status (SES) information
In addition to the notable absence of racial and ethnic data in the literature, we observed that SES is another demographic characteristic frequently either omitted or incompletely documented. Of the 95 studies included in this review, fewer than 20% provided detailed information on SES (n = 17 studies), and among those that did, reporting was typically scant: 16 detailed education, whilst two reported participants’ income. Though several studies implicitly document education level and occupation (e.g., those focusing solely on university student participants), other essential SES metrics, such as family income, remained unreported. The relationship between EWB and SES has been robustly established in prior research (refer to Tan et al. (2020) for a comprehensive meta-analysis). Thus, the importance of SES in the context of EWB studies cannot be overstated, and researchers should ensure it is consistently considered and documented within the context of neural correlates of EWB research.
5.1.5 Lack of causal inference regarding the neural basis of EWB
A significant limitation of the current literature is a paucity of studies designed to elucidate neural basis of EWB. The majority of studies included in this review used a single neuroimaging modality in a cross-sectional design, making causal inferences difficult if not impossible. Many of the longitudinal/intervention studies did not examine links between EWB and neuroimaging measures over time, a missed opportunity to gain improved causal insight into these relationships. Recently, there has been an increased focus on the lack of causal insights in neuroimaging research in general (Siddiqi et al., 2022). We believe that the EWB neuroimaging literature would benefit from Siddiqi and coauthors’ (Ibid) recommendations to address this limitation, particularly with respect to the increased use of brain imaging studies that experimentally manipulate the brain (e.g., targeted lesions and stimulation, neurofeedback) and the investigation of temporality (i.e., do brain changes precede changes in EWB). Finally, the literature would benefit from multi-modal studies that can help establish convergence across methodologies to increase confidence of the causal role of certain brain regions in specific aspects of EWB.
5.2 Limitations of the present review and future directions
The current findings should be interpreted in light of some limitations specific to this review. First, while we included some studies conducted in Asian countries (e.g., Japan, China), we only included articles that were published in English and mostly in English-speaking countries. As conceptions of EWB differ across cultures (which is highlighted by the working definition used in this study), a more diverse and global approach to this topic is warranted. The growing influence of cultural neuroscience will help to rectify this deficiency in the current literature.
Second, although the range of the included EWB measures provides a wealth of valuable data, the use of self-report scales comes with significant methodological limitations, such as the tendency for self-deception, inability to internalize the relevant features of EWB, and also the subsequent inaccuracy when reporting (e.g., Paulhus and Vazire, 2007; Chan, 2010). Third, we reviewed used studies that included EWB self-report measures that were previously selected by the Koslouski et al. (2022) scoping review of reviews.
Finally, the cultural understanding and interpretation of EWB and its relevant features may differ across cultures and languages. Our inclusion of studies published in English is therefore a limitation. For example, questionnaires in languages other than English might contain content that is difficult to translate into English with fidelity. Moreover, researchers and participants across the globe may have culturally-specific understandings of the concept and essence of EWB [see for example Lomas et al. (2022) and Ruggeri et al., 2020 for global initiatives aiming to understand EWB]. Despite its acknowledged limitations, this scoping review represents an important step towards a better understanding of the neural correlates of EWB, providing an important compilation of the previous studies in this area into one accessible and usable document. The growing influence of cultural neuroscience will be important to address the cultural aspects related to EWB [see Kim and Sasaki (2014) for a review].
Future studies should take a longitudinal approach and consider the complexity of EWB and its relation to a variety of contextual factors (e.g., social, environmental) that could impact an individual’s EWB and their response to interventions. Given its complexity, a team science approach (Hall et al., 2018) is required to allow advancement in the field. The use of multi-level approaches that incorporate multiple imaging modalities in the same study along with multiple behavioral, physiological, and environmental measures would allow us a whole-person integrative approach to investigating EW.B. Finally, a whole person approach (Thomas et al., 2018; Langevin, 2021; Pitcher et al., 2023) is needed to advance the field of EWB.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
CR: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing. CL: Data curation, Formal analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing. AT: Conceptualization, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. SH: Formal analysis, Investigation, Writing – review & editing. DS: Data curation, Formal analysis, Validation, Writing – review & editing. JL: Formal analysis, Writing – review & editing. DL: Formal analysis, Writing – review & editing. FL: Conceptualization, Funding acquisition, Writing – review & editing. RD: Conceptualization, Funding acquisition, Writing – review & editing. FH: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD), the National Center for Complementary & Integrative Health (NCCIH), and the Office of the Director, National Institutes of Health (NIH) Award Number U24AT011281 “Network to Advance the Study of Mechanisms Underlying Mind–Body Interventions and Measurement of Emotional Wellbeing.” This work was also supported by the NIH/National Institute on Aging U24 AG072701 “Network for Emotional Wellbeing and Brain Aging” (NEW Brain Aging). Finally, this work was supported by the NCCIH Award Number U24AT011289 “The plasticity of well-being: A research network to define, measure and promote human flourishing.” The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Acknowledgments
We would like to thank all the trained research assistants that helped in the screening and data extraction process (i.e., Ashley Williamson, Amy O’Rourke, Bryanna D’Souza-Bohannon, Kora Makarska, Shreya Sreenivas, Yasmin Andalib, Kelly Lee). We also thank the University of Connecticut librarian Hilary Kraus who provided us with great support as we designed the search process for this review. Finally, we thank Erin Burke Quinlan from NCCIH for her feedback on the conceptualization and writing of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyg.2023.1328523/full#supplementary-material
Footnotes
1. ^It is plausible that research conducted in nations with more racially homogeneous populations, such as Japan and China, may not prioritize or even collect demographic information, perhaps perceiving it as redundant.
References
Albajes-Eizagirre, A., Solanes, A., Vieta, E., and Radua, J. (2019). Voxel-based meta-analysis via permutation of subject images (PSI): theory and implementation for SDM. NeuroImage 186, 174–184. doi: 10.1016/j.neuroimage.2018.10.077
Alessandri, G., Caprara, G. V., and De Pascalis, V. (2015). Relations among EEG-alpha asymmetry and positivity personality trait. Brain Cogn. 97, 10–21. doi: 10.1016/j.bandc.2015.04.003
Alexander, R., Aragón, O. R., Bookwala, J., Cherbuin, N., Gatt, J. M., Kahrilas, I. J., et al. (2021). The neuroscience of positive emotions and affect: implications for cultivating happiness and wellbeing. Neurosci. Biobehav. Rev. 121, 220–249. doi: 10.1016/j.neubiorev.2020.12.002
Arjmand, H.-A., Hohagen, J., Paton, B., and Rickard, N. S. (2017). Emotional responses to music: shifts in frontal brain asymmetry mark periods of musical change. Front. Psychol. 8:2044. doi: 10.3389/fpsyg.2017.02044
Baeken, C., Leyman, L., De Raedt, R., Vanderhasselt, M. A., and D’haenen, H. (2008). Left and right high frequency repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex does not affect mood in female volunteers. Clin. Neurophysiol. 119, 568–575. doi: 10.1016/j.clinph.2007.11.044
Ben-Shaanan, T. L., Azulay-Debby, H., Dubovik, T., Starosvetsky, E., Korin, B., Schiller, M., et al. (2016). Activation of the reward system boosts innate and adaptive immunity. Nat. Med. 22, 940–944. doi: 10.1038/nm.4133
Boehm, J. K., and Kubzansky, L. D. (2012). The heart’s content: the association between positive psychological well-being and cardiovascular health. Psychol. Bull. 138, 655–691. doi: 10.1037/a0027448
Carp, J. (2012). The secret lives of experiments: methods reporting in the fMRI literature. NeuroImage 63, 289–300. doi: 10.1016/j.neuroimage.2012.07.004
Cash, R. F., Weigand, A., Zalesky, A., Siddiqi, S. H., Downar, J., Fitzgerald, P. B., et al. (2021). Using brain imaging to improve spatial targeting of transcranial magnetic stimulation for depression. Biol. Psychiatry 90, 689–700. doi: 10.1016/j.biopsych.2020.05.033
Chan, D. (2010). “So why ask me? Are self-report data really that bad?” in Statistical and methodological myths and urban legends. eds. C. E. Lance and R. J. Vandenberg (London: Routledge), 329–356.
Cocchi, L., Zalesky, A., Fornito, A., and Mattingley, J. B. (2013). Dynamic cooperation and competition between brain systems during cognitive control. Trends Cogn. Sci. 17, 493–501. doi: 10.1016/j.tics.2013.08.006
Cohen, R., Bavishi, C., and Rozanski, A. (2016). Purpose in life and its relationship to all-cause mortality and cardiovascular events: a meta-analysis. Psychosom. Med. 78, 122–133. doi: 10.1097/PSY.0000000000000274
Costa, T., Suardi, A. C., Diano, M., Cauda, F., Duca, S., Rusconi, M. L., et al. (2019). The neural correlates of hedonic and eudaimonic happiness: an fMRI study. Neurosci. Lett. 712:134491. doi: 10.1016/j.neulet.2019.134491
Crawford, J. R., and Henry, J. D. (2004). The positive and negative affect schedule (PANAS): construct validity, measurement properties and normative data in a large non-clinical sample. Br. J. Clin. Psychol. 43, 245–265. doi: 10.1348/0144665031752934
Cunningham, W. A., and Kirkland, T. (2014). The joyful, yet balanced, amygdala: moderated responses to positive but not negative stimuli in trait happiness. Soc. Cogn. Affect. Neurosci. 9, 760–766. doi: 10.1093/scan/nst045
Davey, C. G., Allen, N. B., Harrison, B. J., Dwyer, D. B., and Yücel, M. (2010). Being liked activates primary reward and midline self‐related brain regions. Hum. Brain Mapp. 31, 660–668. doi: 10.1002/hbm.20895
Davidson, R. J., Kabat-Zinn, J., Schumacher, J., Rosenkranz, M., Muller, D., Santorelli, S. F., et al. (2003). Alterations in brain and immune function produced by mindfulness meditation. Psychosom. Med. 65, 564–570. doi: 10.1097/01.PSY.0000077505.67574.E3
Day, M. A., Matthews, N., Newman, A., Mattingley, J. B., and Jensen, M. P. (2019). An evaluation of the behavioral inhibition and behavioral activation system (BIS-BAS) model of pain. Rehabil. Psychol. 64, 279–287. doi: 10.1037/rep0000274
Dennison, M., Whittle, S., Yücel, M., Byrne, M. L., Schwartz, O., Simmons, J. G., et al. (2015). Trait positive affect is associated with hippocampal volume and change in caudate volume across adolescence. Cogn. Affect. Behav. Neurosci. 15, 80–94. doi: 10.3758/s13415-014-0319-2
Diener, E. (1984). Subjective well-being. Psychol. Bull. 95, 542–575. doi: 10.1037/0033-2909.95.3.542
Dolcos, S., Hu, Y., Williams, C., Bogdan, P. C., Hohl, K., Berenbaum, H., et al. (2021). Cultivating affective resilience: proof-of-principle evidence of translational benefits from a novel cognitive-emotional training intervention. Front. Psychol. 12:585536. doi: 10.3389/fpsyg.2021.585536
Egner, T., Etkin, A., Gale, S., and Hirsch, J. (2008). Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cereb. Cortex. 18, 1475–1484. doi: 10.1093/cercor/bhm179
Etkin, A., Egner, T., Peraza, D. M., Kandel, E. R., and Hirsch, J. (2006). Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron, 51, 871–882. doi: 10.1016/j.neuron.2006.07.029
Etkin, A., Klemenhagen, K. C., Dudman, J. T., Rogan, M. T., Hen, R., Kandel, E. R., et al. (2004). Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron, 44, 1043–1055. doi: 10.1016/j.neuron.2004.12.006
Feller, S. C., Castillo, E. G., Greenberg, J. M., Abascal, P., Van Horn, R., Wells, K. B., et al. (2018). Emotional well-being and public health: Proposal for a model national initiative. Public Health Rep. 133, 136–141. doi: 10.1177/0033354918754540
Finckh, A., and Tramèr, M. R. (2008). Primer: strengths and weaknesses of meta-analysis. Nat. Clin. Pract. Rheumatol. 4, 146–152. doi: 10.1038/ncprheum0732
Flores, L. E., Eckstrand, K. L., Silk, J. S., Allen, N. B., Ambrosia, M., Healey, K. L., et al. (2018). Adolescents’ neural response to social reward and real-world emotional closeness and positive affect. Cogn. Affect. Behav. Neurosci. 18, 705–717. doi: 10.3758/s13415-018-0598-0
Flynn, S., Vereenooghe, L., Hastings, R. P., Adams, D., Cooper, S. A., Gore, N., et al. (2017). Measurement tools for mental health problems and mental well-being in people with severe or profound intellectual disabilities: a systematic review. Clin. Psychol. Rev. 57, 32–44. doi: 10.1016/j.cpr.2017.08.006
Fonzo, G. A., Goodkind, M. S., Oathes, D. J., Zaiko, Y. V., Harvey, M., Peng, K. K., et al. (2017). Selective effects of psychotherapy on frontopolar cortical function in PTSD. Am. J. Psychiatr. 174, 1175–1184. doi: 10.1176/appi.ajp.2017.16091073
Geethanjali, B., Adalarasu, K., Jagannath, M., and Scshadri, N. P. G. (2019). Music-induced brain functional connectivity using EEG sensors: a study on Indian music. IEEE Sensors J. 19, 1499–1507. doi: 10.1109/jsen.2018.2873402
George, M. S., Ketter, T. A., Parekh, P. I., Horwitz, B., Herscovitch, P., and Post, R. M. (1995). Brain activity during transient sadness and happiness in healthy women. Am. J. Psychiatr. 152, 341–351. doi: 10.1176/ajp.152.3.341
Gondoh, Y., Sensui, H., Kinomura, S., Fukuda, H., Fujimoto, T., Masud, M., et al. (2009). Effects of aerobic exercise training on brain structure and psychological well-being in young adults. J. Sports Med. Phys. Fitness 49, 129–135.
Habel, U., Klein, M., Shah, N. J., Toni, I., Zilles, K., Falkai, P., et al. (2004). Genetic load on amygdala hypofunction during sadness in nonaffected brothers of schizophrenia patients. Am. J. Psychiatr. 161, 1806–1813. doi: 10.1176/appi.ajp.161.10.1806
Haddaway, N. R., Bethel, A., Dicks, L. V., Koricheva, J., Macura, B., Petrokofsky, G., et al. (2020). Eight problems with literature reviews and how to fix them. Nat. Ecol. Evol. 4, 1582–1589. doi: 10.1038/s41559-020-01295-x
Hagemann, D., Naumann, E., Lürken, A., Becker, G., Maier, S., and Bartussek, D. (1999). EEG asymmetry, dispositional mood and personality. Personal. Individ. Differ. 27, 541–568. doi: 10.1016/S0191-8869(98)00263-3
Hall, E. E., and Petruzzello, S. J. (1999). Frontal asymmetry, dispositional affect, and physical activity in older adults. J. Aging Phys. Act. 7, 76–90. doi: 10.1123/japa.7.1.76
Hall, K. L., Vogel, A. L., Huang, G. C., Serrano, K. J., Rice, E. L., Tsakraklides, S. P., et al. (2018). The science of team science: a review of the empirical evidence and research gaps on collaboration in science. Am. Psychol. 73, 532–548. doi: 10.1037/amp0000319
Hasler, B. P., Germain, A., Nofzinger, E., Kupfer, D. J., Krafty, R., Rothenberger, S., et al. (2012). Chronotype and diurnal patterns of positive affect and affective neural circuitry in primary insomnia. J. Sleep Res. 21, 515–526. doi: 10.1111/j.1365-2869.2012.01002.x
He, Z., Lu, F., Sheng, W., Han, S., Long, Z., Chen, Y., et al. (2019). Functional dysconnectivity within the emotion-regulating system is associated with affective symptoms in major depressive disorder: a resting-state fMRI study. Aust. N. Z. J. Psychiatry 53, 528–539. doi: 10.1177/0004867419832106
Heller, A. S., Johnstone, T., Light, S. N., Peterson, M. J., Kolden, G. G., Kalin, N. H., et al. (2013a). Relationships between changes in sustained fronto-striatal connectivity and positive affect in major depression resulting from antidepressant treatment. Am. J. Psychiatr. 170, 197–206. doi: 10.1176/appi.ajp.2012.12010014
Heller, A. S., van Reekum, C. M., Schaefer, S. M., Lapate, R. C., Radler, B. T., Ryff, C. D., et al. (2013b). Sustained striatal activity predicts Eudaimonic well-being and cortisol output. Psychol. Sci. 24, 2191–2200. doi: 10.1177/0956797613490744
Hiyoshi-Taniguchi, K., Kawasaki, M., Yokota, T., Bakardjian, H., Fukuyama, H., Cichocki, A., et al. (2015). EEG correlates of voice and face emotional judgments in the human brain. Cogn. Comput. 7, 11–19. doi: 10.1007/s12559-013-9225-0
Hofer, A., Siedentopf, C. M., Ischebeck, A., Rettenbacher, M. A., Verius, M., Felber, S., et al. (2007). Sex differences in brain activation patterns during processing of positively and negatively valenced emotional words. Psychol. Med. 37, 109–119. doi: 10.1017/S0033291706008919
Hu, X., Zhuang, C., Wang, F., Liu, Y. J., Im, C. H., and Zhang, D. (2019). fNIRS evidence for recognizably different positive emotions. Front. Hum. Neurosci. 13:120. doi: 10.3389/fnhum.2019.00120
Huppert, F. A. (2009). Psychological well-being: evidence regarding its causes and consequences. Appl. Psychol. Health Well-Being 1, 137–164. doi: 10.1111/j.1758-0854.2009.01008.x
Isbel, B., Lagopoulos, J., Hermens, D. F., and Summers, M. J. (2019). Mindfulness induces changes in anterior alpha asymmetry in healthy older adults. Mindfulness 10, 1381–1394. doi: 10.1007/s12671-019-01106-w
Jung, R. E., Yeo, R. A., Love, T. M., Petropoulos, H., Sibbitt, W. L. Jr., and Brooks, W. M. (2002). Biochemical markers of mood: a proton magnetic resonance spectroscopy study of normal human brain. Biol. Psychiatry 51, 224–229. doi: 10.1016/S0006-3223(01)01224-0
Katsumi, Y., Kondo, N., Dolcos, S., Dolcos, F., and Tsukiura, T. (2021). Intrinsic functional network contributions to the relationship between trait empathy and subjective happiness. Neuro Image 227:117650. doi: 10.1016/j.neuroimage.2020.117650
Kaur, C., and Singh, P. (2015). EEG derived neuronal dynamics during meditation: Progress and challenges. Adv. Prev. Med. 2015:614723, 1–10. doi: 10.1155/2015/614723
Kawamichi, H., Sugawara, S. K., Hamano, Y. H., Makita, K., Matsunaga, M., Tanabe, H. C., et al. (2016). Being in a romantic relationship is associated with reduced gray matter density in striatum and increased subjective happiness. Front. Psychol. 7:1763. doi: 10.3389/fpsyg.2016.01763
Keverne, E. B. (2004). Understanding well-being in the evolutionary context of brain development. Philosophical transactions of the Royal Society of London. Proc. Royal Soc. 359, 1349–1358. doi: 10.1098/rstb.2004.1517
Killgore, W. D. S., and Yurgelun-Todd, D. A. (2007). Positive affect modulates activity in the visual cortex to images of high calorie foods. Int. J. Neurosci. 117, 643–653. doi: 10.1080/00207450600773848
Kim, H. S., and Sasaki, J. Y. (2014). Cultural neuroscience: biology of the mind in cultural contexts. Annu. Rev. Psychol. 65, 487–514. doi: 10.1146/annurev-psych-010213-115040
King, M. L. (2019). The neural correlates of well-being: a systematic review of the human neuroimaging and neuropsychological literature. Cogn. Affect. Behav. Neurosci. 19, 779–796. doi: 10.3758/s13415-019-00720-4
Kleckner, I. R., Zhang, J., Touroutoglou, A., Chanes, L., Xia, C., Simmons, W. K., et al. (2017). Evidence for a large-scale brain system supporting allostasis and interoception in humans. Nat. Hum. Behav. 1, 1–14. doi: 10.1038/s41562-017-0069
Koepp, M. J., Hammers, A., Lawrence, A. D., Asselin, M. C., Grasby, P. M., and Bench, C. J. (2009). Evidence for endogenous opioid release in the amygdala during positive emotion. Neuro Image 44, 252–256. doi: 10.1016/j.neuroimage.2008.08.032
Kohn, N., Falkenberg, I., Kellermann, T., Eickhoff, S. B., Gur, R. C., and Habel, U. (2014). Neural correlates of effective and ineffective mood induction. Soc. Cogn. Affect. Neurosci. 9, 864–872. doi: 10.1093/scan/nst055
Kong, F., Hu, S., Wang, X., Song, Y., and Liu, J. (2015a). Neural correlates of the happy life: the amplitude of spontaneous low frequency fluctuations predicts subjective well-being. Neuro Image 107, 136–145. doi: 10.1016/j.neuroimage.2014.11.033
Kong, F., Liu, L., Wang, X., Hu, S., Song, Y., and Liu, J. (2015c). Different neural pathways linking personality traits and eudaimonic well-being: a resting-state functional magnetic resonance imaging study. Cogn. Affect. Behav. Neurosci. 15, 299–309. doi: 10.3758/s13415-014-0328-1
Kong, F., Ma, X., You, X., and Xiang, Y. (2018). The resilient brain: psychological resilience mediates the effect of amplitude of low-frequency fluctuations in orbitofrontal cortex on subjective well-being in young healthy adults. Soc. Cogn. Affect. Neurosci. 13, 755–763. doi: 10.1093/scan/nsy045
Kong, F., Wang, X., Hu, S., and Liu, J. (2015b). Neural correlates of psychological resilience and their relation to life satisfaction in a sample of healthy young adults. Neuro Image 123, 165–172. doi: 10.1016/j.neuroimage.2015.08.020
Kong, F., Wang, X., Song, Y., and Liu, J. (2016). Brain regions involved in dispositional mindfulness during resting state and their relation with well-being. Soc. Neurosci. 11, 331–343. doi: 10.1080/17470919.2015.1092469
Koslouski, J. B., Wilson-Mendenhall, C. D., Parsafar, P., Goldberg, S. B., Martin, M. Y., and Chafouleas, S. M. (2022). Measuring emotional well-being through subjective report: A Scoping Review of Reviews. BMJ Open 12:e062120. doi: 10.1136/bmjopen-2022-062120
Kragel, P. A., and LaBar, K. S. (2016). Decoding the nature of emotion in the brain. Trends Cogn. Sci. 20, 444–455. doi: 10.1016/j.tics.2016.03.011
Kryla-Lighthall, N., and Mather, M. (2009). “The role of cognitive control in older adults’ emotional well-being” in Handbook of theories of aging. eds. V. L. Bengston, D. Gans, N. M. Pulney, and M. Silverstein. 2nd ed (Berlin: Springer Publishing Company), 323–344.
Kujawa, A., Proudfit, G. H., Kessel, E. M., Dyson, M., Olino, T., and Klein, D. N. (2015). Neural reactivity to monetary rewards and losses in childhood: longitudinal and concurrent associations with observed and self-reported positive emotionality. Biol. Psychol. 104, 41–47. doi: 10.1016/j.biopsycho.2014.11.008
Kwon, J. H., Kim, H. E., Kim, J., Kim, E. J., and Kim, J.-J. (2021). Differences in basic psychological needs-related resting-state functional connectivity between individuals with high and low life satisfaction. Neurosci. Lett. 750:135798. doi: 10.1016/j.neulet.2021.135798
Kyeong, S., Kim, J., Kim, J., Kim, E. J., Kim, H. E., and Kim, J.-J. (2020). Differences in the modulation of functional connectivity by self-talk tasks between people with low and high life satisfaction. Neuro Image 217:116929. doi: 10.1016/j.neuroimage.2020.116929
Lang, P. J., Bradley, M. M., and Cuthbert, B. N. (1998). International affective picture system (IAPS): Technical manual and affective ratings. Gainesville, FL. The Center for Research in Psychophysiology, University of Florida.
Langevin, H. M. (2021). Moving the complementary and integrative health research field toward whole person health. J. Altern. Complement. Med. 27, 623–626. doi: 10.1089/acm.2021.0255
Langner, O., Dotsch, R., Bijlstra, G., Wigboldus, D. H., Hawk, S. T., and Van Knippenberg, A. D. (2010). Presentation and validation of the Radboud Faces Database. Cogn. Emot. 24, 1377–1388. doi: 10.1080/02699930903485076
Larson, M. J., Good, D. A., and Fair, J. E. (2010). The relationship between performance monitoring, satisfaction with life, and positive personality traits. Biol. Psychol. 83, 222–228. doi: 10.1016/j.biopsycho.2010.01.003
Leppänen, J. M. (2006). Emotional information processing in mood disorders: a review of behavioral and neuroimaging findings. Curr. Opin. Psychiatry 19, 34–39. doi: 10.1097/01.yco.0000191500.46411.00
Lewis, G. J., Kanai, R., Rees, G., and Bates, T. C. (2014). Neural correlates of the “good life”: Eudaimonic well-being is associated with insular cortex volume. Soc. Cogn. Affect. Neurosci. 9, 615–618. doi: 10.1093/scan/nst032
Li, Q., Qin, S., Rao, L.-L., Zhang, W., Ying, X., Guo, X., et al. (2011). Can Sophie’s choice be adequately captured by cold computation of minimizing losses? An fMRI study of vital loss decisions. PLoS One 6:e17544. doi: 10.1371/journal.pone.0017544
Li, Z., Tong, L., Wang, L., Li, Y., He, W., Guan, M., et al. (2016). Self-regulating positive emotion networks by feedback of multiple emotional brain states using real-time fMRI. Exp. Brain Res. 234, 3575–3586. doi: 10.1007/s00221-016-4744-z
Lichev, V., Sacher, J., Ihme, K., Rosenberg, N., Quirin, M., Lepsien, J., et al. (2015). Automatic emotion processing as a function of trait emotional awareness: an fMRI study. Soc. Cogn. Affect. Neurosci. 10, 680–689. doi: 10.1093/scan/nsu104
Lindquist, K. A., Wager, T. D., Kober, H., Bliss-Moreau, E., and Barrett, L. F. (2012). The brain basis of emotion: a meta-analytic review. Behav. Brain Sci. 35, 121–143. doi: 10.1017/S0140525X11000446
Liu, H.-H., Hsieh, M. H., Hsu, Y.-F., and Lai, W.-S. (2015). Effects of affective arousal on choice behavior, reward prediction errors, and feedback-related negativities in human reward-based decision making. Front. Psychol. 6:592. doi: 10.3389/fpsyg.2015.00592
Lomas, T., Ishikawa, Y., Diego-Rosell, P., Daly, J., English, C., Harter, J., et al. (2022). Balance and harmony in the Gallup world poll: the development of the global wellbeing initiative module. Int. J. Health Sci. 12, 1–19. doi: 10.5502/ijw.v12i4.2655
Lorenzetti, V., Allen, N. B., Fornito, A., Pantelis, C., De Plato, G., Ang, A., et al. (2009). Pituitary gland volume in currently depressed and remitted depressed patients. Psychiatry Res. Neuroimaging 172, 55–60. doi: 10.1016/j.pscychresns.2008.06.006
Love, T. M., Enoch, M.-A., Hodgkinson, C. A., Peciña, M., Mickey, B., Koeppe, R. A., et al. (2012). Oxytocin gene polymorphisms influence human dopaminergic function in a sex-dependent manner. Biol. Psychiatry 72, 198–206. doi: 10.1016/j.biopsych.2012.01.033
Luo, Y., Huang, X., Yang, Z., Li, B., Liu, J., and Wei, D. (2014). Regional homogeneity of intrinsic brain activity in happy and unhappy individuals. PLoS One 9:e85181. doi: 10.1371/journal.pone.0085181
Luo, Y., Qi, S., Chen, X., You, X., Huang, X., and Yang, Z. (2017). Pleasure attainment or self-realization: the balance between two forms of well-beings are encoded in default mode network. Soc. Cogn. Affect. Neurosci. 12, 1678–1686. doi: 10.1093/scan/nsx078
Lyubomirsky, S., and Lepper, H. S. (1999). A measure of subjective happiness: Preliminary reliability and construct validation. Soc. Indic. Res. 46, 137–155. doi: 10.1023/A:1006824100041
Ma, Y., Li, B., Wang, C., Zhang, W., Rao, Y., and Han, S. (2015). Allelic variation in 5-HTTLPR and the effects of citalopram on the emotional neural network. Br. J. Psychiatry 206, 385–392. doi: 10.1192/bjp.bp.114.150128
Machado, L., and Cantilino, A. (2017). A systematic review of the neural correlates of positive emotions. Rev. Bras. Psiquiatr. 39, 172–179. doi: 10.1590/1516-4446-2016-1988
Makowski, C., Brown, T. T., Zhao, W., Hagler, D. J., Parekh, P., Garavan, H., et al. (2023). Leveraging the adolescent brain cognitive development study to improve behavioral prediction from neuroimaging in smaller replication samples. bioRxiv 1:2023.06.16.545340. doi: 10.1101/2023.06.16.545340
Marchand, W. R. (2014). Neural mechanisms of mindfulness and meditation: evidence from neuroimaging studies. World J. Radiol. 6, 471–479. doi: 10.4329/wjr.v6.i7.471
Marek, S., Tervo-Clemmens, B., Calabro, F. J., Montez, D. F., Kay, B. P., Hatoum, A. S., et al. (2022). Reproducible brain-wide association studies require thousands of individuals. Nature 603, 654–660. doi: 10.1038/s41586-022-04492-9
Martikainen, I. K., Nuechterlein, E. B., Peciña, M., Love, T. M., Cummiford, C. M., Green, C. R., et al. (2015). Chronic back pain is associated with alterations in dopamine neurotransmission in the ventral striatum. J. Neurosci. 35, 9957–9965. doi: 10.1523/JNEUROSCI.4605-14.2015
Martin-Soelch, C., Guillod, M., Gaillard, C., Recabarren, R. E., Federspiel, A., Mueller-Pfeiffer, C., et al. (2020). Increased reward-related activation in the ventral striatum during stress exposure associated with positive affect in the daily life of Young adults with a family history of depression. Preliminary findings. Front. Psychiatry 11:563475. doi: 10.3389/fpsyt.2020.563475
Mastandrea, S., Fagioli, S., and Biasi, V. (2019). Art and psychological well-being: linking the brain to the aesthetic emotion. Front. Psychol. 10:739. doi: 10.3389/fpsyg.2019.00739
Mathiak, K. A., Klasen, M., Zvyagintsev, M., Weber, R., and Mathiak, K. (2013). Neural networks underlying affective states in a multimodal virtual environment: contributions to boredom. Front. Hum. Neurosci. 7:820. doi: 10.3389/fnhum.2013.00820
Matsunaga, M., Kawamichi, H., Koike, T., Yoshihara, K., Yoshida, Y., Takahashi, H. K., et al. (2016). Structural and functional associations of the rostral anterior cingulate cortex with subjective happiness. Neuro Image 134, 132–141. doi: 10.1016/j.neuroimage.2016.04.020
McPhee, G. M., Downey, L. A., Wesnes, K. A., and Stough, C. (2021). The neurocognitive effects of Bacopa monnieri and cognitive training on markers of brain microstructure in healthy older adults. Front. Aging Neurosci. 13:638109. doi: 10.3389/fnagi.2021.638109
Memarian, N., Torre, J. B., Haltom, K. E., Stanton, A. L., and Lieberman, M. D. (2017). Neural activity during affect labeling predicts expressive writing effects on well-being: GLM and SVM approaches. Soc. Cogn. Affect. Neurosci. 12, 1437–1447. doi: 10.1093/scan/nsx084
Mennella, R., Patron, E., and Palomba, D. (2017). Frontal alpha asymmetry neurofeedback for the reduction of negative affect and anxiety. Behav. Res. Ther. 92, 32–40. doi: 10.1016/j.brat.2017.02.002
Minkel, J. D., McNealy, K., Gianaros, P. J., Drabant, E. M., Gross, J. J., and Manuck, S. B. (2012). Sleep quality and neural circuit function supporting emotion regulation. Biol. Mood Anxiety Disord. 2. doi: 10.1186/2045-5380-2-22
Miskowiak, K., Inkster, B., Selvaraj, S., Wise, R., Goodwin, G. M., and Harmer, C. J. (2008). Erythropoietin improves mood and modulates the cognitive and neural processing of emotion 3 days post administration. Neuropsychopharmacology 33, 611–618. doi: 10.1038/sj.npp.1301439
Morawetz, C., Riedel, M. C., Salo, T., Berboth, S., Eickhoff, S. B., Laird, A. R., et al. (2020). Multiple large-scale neural networks underlying emotion regulation. Neurosci. Biobehav. Rev. 116, 382–395. doi: 10.1016/j.neubiorev.2020.07.001
Morelli, S. A., Knutson, B., and Zaki, J. (2018). Neural sensitivity to personal and vicarious reward differentially relate to prosociality and well-being. Soc. Cogn. Affect. Neurosci. 13, 831–839. doi: 10.1093/scan/nsy056
Mrazek, M. D., Mooneyham, B. W., Mrazek, K. L., and Schooler, J. W. (2016). Pushing the limits: cognitive, affective, and neural plasticity revealed by an intensive multifaceted intervention. Front. Hum. Neurosci. 10:117. doi: 10.3389/fnhum.2016.00117
Müller, V. I., Cieslik, E. C., Laird, A. R., Fox, P. T., Radua, J., Mataix-Cols, D., et al. (2018). Ten simple rules for neuroimaging meta-analysis. Neurosci. Biobehav. Rev. 84, 151–161. doi: 10.1016/j.neubiorev.2017.11.012
Munn, Z., Peters, M. D. J., Stern, C., Tufanaru, C., McArthur, A., and Aromataris, E. (2018). Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 18:143. doi: 10.1186/s12874-018-0611-x
Murad, M. H., Chu, H., Lin, L., and Wang, Z. (2018). The effect of publication bias magnitude and direction on the certainty in evidence. BMJ Evid. Based Med. 23, 84–86. doi: 10.1136/bmjebm-2018-110891
Murphy, F. C., Nimmo-Smith, I., and Lawrence, A. D. (2003). Functional neuroanatomy of emotions: a meta-analysis. Cogn. Affect. Behav. Neurosci. 3, 207–233. doi: 10.3758/cabn.3.3.207
Nardo, D., Högberg, G., Flumeri, F., Jacobsson, H., Larsson, S. A., Hällström, T., et al. (2011). Self-rating scales assessing subjective well-being and distress correlate with rCBF in PTSD-sensitive regions. Psychol. Med. 41, 2549–2561. doi: 10.1017/S0033291711000912
National Academies of Sciences, Engineering, and Medicine. (2022). Improving representation in clinical trials and research: Building research equity for women and underrepresented groups. Washington, DC: The National Academies Press
National Institute of Health [NIH] (2018). Emotional well-being: High priority research networks (U24, clinical trial optional). Available at: https://grants.nih.gov/grants/guide/rfa-files/rfa-at-20-003.html
Oetken, S., Pauly, K. D., Gur, R. C., Schneider, F., Habel, U., and Pohl, A. (2017). Don’t worry, be happy—neural correlates of the influence of musically induced mood on self-evaluation. Neuropsychologia 100, 26–34. doi: 10.1016/j.neuropsychologia.2017.04.010
Organisation for Economic Co-operation and Development (2020). Measuring Well-being and Progress. Available at: https://www.oecd.org/wise/measuring-well-being-and-progress.htm
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. doi: 10.1136/bmj.n71
Paradies, Y., Ben, J., Denson, N., Elias, A., Priest, N., Pieterse, A., et al. (2015). Racism as a determinant of health: a systematic review and Meta-analysis. PLoS One 10:e0138511. doi: 10.1371/journal.pone.0138511
Park, S. Q., Kahnt, T., Dogan, A., Strang, S., Fehr, E., and Tobler, P. N. (2017). A neural link between generosity and happiness. Nat. Commun. 8:15964. doi: 10.1038/ncomms15964
Park, C., Kubzansky, L., Chafouleas, S. M., Davidson, R., Keltner, D., Parsafar, P., et al. (2023a). Emotional well-being: what it is and why it matters. Affect. Sci. 4, 10–20. doi: 10.1007/s42761-022-00163-0
Park, C. L., Kubzansky, L. D., Chafouleas, S. M., Davidson, R. J., Keltner, D., Parsafar, P., et al. (2023b). A perfect storm to set the stage for ontological exploration: response to commentaries on “emotional well-being: what it is and why it matters”. Affect. Sci. 4, 52–58. doi: 10.1007/s42761-022-00169-8
Paulhus, D. L., and Vazire, S. (2007). “The self-report method” in Handbook of research methods in personality psychology. eds. R. W. Robins, R. C. Fraley, and R. F. Krueger (New York: The Guilford Press), 224–239.
Pavot, W., Diener, E., Colvin, C. R., and Sandvik, E. (1991). Further validation of the satisfaction with life scale: evidence for the cross-method convergence of well-being measures. J. Pers. Assess. 57, 149–161. doi: 10.1207/s15327752jpa5701_17
Pinti, P., Tachtsidis, I., Hamilton, A., Hirsch, J., Aichelburg, C., Gilbert, S., et al. (2020). The present and future use of functional near-infrared spectroscopy (fNIRS) for cognitive neuroscience. Ann. N. Y. Acad. Sci. 1464, 5–29. doi: 10.1111/nyas.13948
Pitcher, M. H., Edwards, E., Langevin, H. M., Rusch, H. L., and Shurtleff, D. (2023). Complementary and integrative health therapies in whole person resilience research. Stress Health 39, 55–61. doi: 10.1002/smi.3276
Plitnick, B., Figueiro, M. G., Wood, B., and Rea, M. S. (2010). The effects of red and blue light on alertness and mood at night. Light. Res. Technol. 42, 449–458. doi: 10.1177/1477153509360887
Polanin, J. R., Pigott, T. D., Espelage, D. L., and Grotpeter, J. K. (2019). Best practice guidelines for abstract screening large-evidence systematic reviews and meta-analyzes. Res. Synth. Methods 10, 330–342. doi: 10.1002/jrsm.1354
Poldrack, R. A., Baker, C. I., Durnez, J., Gorgolewski, K. J., Matthews, P. M., Munafò, M. R., et al. (2017). Scanning the horizon: towards transparent and reproducible neuroimaging research. Nat. Rev. Neurosci. 18, 115–126. doi: 10.1038/nrn.2016.167
Poldrack, R. A., Whitaker, K., and Kennedy, D. N. (2020). Introduction to the special issue on reproducibility in neuroimaging. Neuro Image 218:116357. doi: 10.1016/j.neuroimage.2019.116357
Pressman, S. D., Jenkins, B. N., and Moskowitz, J. T. (2019). Positive affect and health: what do we know and where next should we go? Annu. Rev. Psychol. 70, 627–650. doi: 10.1146/annurev-psych-010418-102955
Puccetti, N. A., Schaefer, S. M., van Reekum, C. M., Ong, A. D., Almeida, D. M., Ryff, C. D., et al. (2021). Linking amygdala persistence to real-world emotional experience and psychological well-being. J. Neurosci. 41, 3721–3730. doi: 10.1523/JNEUROSCI.1637-20.2021
Radsepehr, H., Shareh, H., and Dehnabi, A. (2019). Evaluation of positive and negative affect induction on the regional brain activity and personality traits. Pract. Clin. Psychol. 7, 95–106. doi: 10.32598/jpcp.7.2.95
Riedel, M. C., Yanes, J. A., Ray, K. L., Eickhoff, S. B., Fox, P. T., Sutherland, M. T., et al. (2018). Dissociable meta-analytic brain networks contribute to coordinated emotional processing. Hum. Brain Mapp. 39, 2514–2531. doi: 10.1002/hbm.24018
Rohr, C. S., Dreyer, F. R., Aderka, I. M., Margulies, D. S., Frisch, S., Villringer, A., et al. (2015). Individual differences in common factors of emotional traits and executive functions predict functional connectivity of the amygdala. Neuro Image 120, 154–163. doi: 10.1016/j.neuroimage.2015.06.049
Rozanski, A., Bavishi, C., Kubzansky, L. D., and Cohen, R. (2019). Association of optimism with cardiovascular events and all-cause mortality: a systematic review and meta-analysis. JAMA Netw. Open 2:e1912200. doi: 10.1001/jamanetworkopen.2019.12200
Ruggeri, K., Garcia-Garzon, E., Maguire, Á., Matz, S., and Huppert, F. A. (2020). Well-being is more than happiness and life satisfaction: a multidimensional analysis of 21 countries. Health Qual. Life Outcomes 18, 192–116. doi: 10.1186/s12955-020-01423-y
Russell, J. A. (1980). A circumplex model of affect. J. Pers. Soc. Psychol. 39, 1161–1178. doi: 10.1037/h0077714
Ryff, C. D. (1989). Happiness is everything, or is it? Explorations on the meaning of psychological well-being. J. Pers. Soc. Psychol. 57, 1069–1081. doi: 10.1037/0022-3514.57.6.1069
Sailer, U., Triscoli, C., Häggblad, G., Hamilton, P., Olausson, H., and Croy, I. (2016). Temporal dynamics of brain activation during 40 minutes of pleasant touch. Neuro Image 139, 360–367. doi: 10.1016/j.neuroimage.2016.06.031
Sanchez, T. A., Mocaiber, I., Erthal, F. S., Joffily, M., Volchan, E., Pereira, M. G., et al. (2015). Amygdala responses to unpleasant pictures are influenced by task demands and positive affect trait. Front. Hum. Neurosci. 9:107. doi: 10.3389/fnhum.2015.00107
Sanger, K. L., Thierry, G., and Dorjee, D. (2018). Effects of school-based mindfulness training on emotion processing and well-being in adolescents: evidence from event-related potentials. Dev. Sci. 21:e12646. doi: 10.1111/desc.12646
Sato, W., Kochiyama, T., Uono, S., Kubota, Y., Sawada, R., Yoshimura, S., et al. (2015). The structural neural substrate of subjective happiness. Sci. Rep. 5:16891. doi: 10.1038/srep16891
Sato, W., Kochiyama, T., Uono, S., Sawada, R., Kubota, Y., Yoshimura, S., et al. (2019). Resting-state neural activity and connectivity associated with subjective happiness. Sci. Rep. 9:12098. doi: 10.1038/s41598-019-48510-9
Sato, A., Yasuda, A., Ohira, H., Miyawaki, K., Nishikawa, M., Kumano, H., et al. (2005). Effects of value and reward magnitude on feedback negativity and P300. Neuroreport 16, 407–411. doi: 10.1097/00001756-200503150-00020
Schmitt, A., Martin, J. A., Rojas, S., Vafa, R., Scheef, L., Strüder, H. K., et al. (2019). Effects of low-and high-intensity exercise on emotional face processing: an fMRI face-matching study. Soc. Cogn. Affect. Neurosci. 14, 657–665. doi: 10.1093/scan/nsz042
Schneider, F., Grodd, W., Weiss, U., Klose, U., Mayer, K. R., Nägele, T., et al. (1997). Functional MRI reveals left amygdala activation during emotion. Psychiatry Res. Neuroimaging 76, 75–82. doi: 10.1016/S0925-4927(97)00063-2
Schönbrodt, F. D., and Perugini, M. (2013). At what sample size do correlations stabilize? J. Res. Pers. 47, 609–612. doi: 10.1016/j.jrp.2013.05.009
Schöne, B., Gruber, T., Graetz, S., Bernhof, M., and Malinowski, P. (2018). Mindful breath awareness meditation facilitates efficiency gains in brain networks: a steady-state visually evoked potentials study. Sci. Rep. 8:13687. doi: 10.1038/s41598-018-32046-5
Schurz, M., Radua, J., Aichhorn, M., Richlan, F., and Perner, J. (2014). Fractionating theory of mind: a meta-analysis of functional brain imaging studies. Neurosci. Biobehav. Rev. 42, 9–34. doi: 10.1016/j.neubiorev.2014.01.009
Shi, Z., Ma, Y., Wu, B., Wu, X., Wang, Y., and Han, S. (2016). Neural correlates of reflection on actual versus ideal self-discrepancy. Neuro Image 124, 573–580. doi: 10.1016/j.neuroimage.2015.08.077
Shi, L., Sun, J., Wei, D., and Qiu, J. (2019). Recover from the adversity: functional connectivity basis of psychological resilience. Neuropsychologia 122, 20–27. doi: 10.1016/j.neuropsychologia.2018.12.002
Shi, L., Sun, J., Wu, X., Wei, D., Chen, Q., Yang, W., et al. (2018). Brain networks of happiness: dynamic functional connectivity among the default, cognitive and salience networks relates to subjective well-being. Soc. Cogn. Affect. Neurosci. 13, 851–862. doi: 10.1093/scan/nsy059
Shiffman, S., Stone, A. A., and Hufford, M. R. (2008). Ecological momentary assessment. Annu. Rev. Clin. Psychol. 4, 1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415
Siddiqi, S. H., Kording, K. P., Parvizi, J., and Fox, M. D. (2022). Causal mapping of human brain function. Nat. Rev. Neurosci. 23, 361–375. doi: 10.1038/s41583-022-00583-8
Singleton, O., Hölzel, B. K., Vangel, M., Brach, N., Carmody, J., and Lazar, S. W. (2014). Change in brainstem gray matter concentration following a mindfulness-based intervention is correlated with improvement in psychological well-being. Front. Hum. Neurosci. 8:33. doi: 10.3389/fnhum.2014.00033
Spironelli, C., Maffei, A., Romeo, Z., Piazzon, G., Padovan, G., Magnolfi, G., et al. (2020). Evidence of language-related left hypofrontality in major depression: An EEG beta band study. Sci. Rep. 10:8166. doi: 10.1038/s41598-020-65168-w
St. Jacques, P. L., Winecoff, A., and Cabeza, R. (2013). “Emotion and aging: linking neural mechanisms to psychological theory” in The Cambridge handbook of human affective neuroscience. eds. J. Armony and P. Vuilleumier (Cambridge: Cambridge University Press), 635–661.
Steptoe, A. (2019). Happiness and health. Annu. Rev. Public Health 40, 339–359. doi: 10.1146/annurev-publhealth-040218-044150
Suardi, A., Sotgiu, I., Costa, T., Cauda, F., and Rusconi, M. (2016). The neural correlates of happiness: a review of PET and fMRI studies using autobiographical recall methods. Cogn. Affect. Behav. Neurosci. 16, 383–392. doi: 10.3758/s13415-016-0414-7
Subramaniam, K., and Vinogradov, S. (2013). Improving the neural mechanisms of cognition through the pursuit of happiness. Front. Hum. Neurosci. 7:452. doi: 10.3389/fnhum.2013.00452
Suslow, T., Ihme, K., Quirin, M., Lichev, V., Rosenberg, N., Bauer, J., et al. (2015). Implicit affectivity and rapid processing of affective body language: an fMRI study. Scand. J. Psychol. 56, 545–552. doi: 10.1111/sjop.12227
Talmon, A., Dixon, M. L., Goldin, P. R., Heimberg, R. G., and Gross, J. J. (2021). Neurocognitive heterogeneity in social anxiety disorder: the role of self-referential processing and childhood maltreatment. Clin. Psychol. Sci. 9, 1045–1058. doi: 10.1177/21677026211004452
Tan, J. J. X., Kraus, M. W., Carpenter, N. C., and Adler, N. E. (2020). The association between objective and subjective socioeconomic status and subjective well-being: a meta-analytic review. Psychol. Bull. 146, 970–1020. doi: 10.1037/bul0000258
Tanzer, J. R., and Weyandt, L. (2020). Imaging happiness: Meta analysis and review. J. Happiness Stud. 21, 2693–2734. doi: 10.1007/s10902-019-00195-7
Thaler, C., Tian, Q., Wintermark, M., Ghanouni, P., Halpern, C. H., Henderson, J. M., et al. (2023). Changes in the Cerebello-Thalamo-cortical network after magnetic resonance-guided focused ultrasound Thalamotomy. Brain Connect. 13, 28–38. doi: 10.1089/brain.2021.0157
Thomas, H., Mitchell, G., Rich, J., and Best, M. (2018). Definition of whole person care in general practice in the English language literature: a systematic review. BMJ Open 8:e023758. doi: 10.1136/bmjopen-2018-023758
Trudel-Fitzgerald, C., James, P., Kim, E. S., Zevon, E. S., Grodstein, F., and Kubzansky, L. D. (2019). Prospective associations of happiness and optimism with lifestyle over up to two decades. Prev. Med. 126:105754. doi: 10.1016/j.ypmed.2019.105754
Trudel-Fitzgerald, C., Kubzansky, L. D., and Vander Weele, T. J. (2021). “A review of psychological well-being and mortality risk: are all dimensions of psychological well-being equal?” in Measuring well-being: Interdisciplinary perspectives from the social sciences and the humanities. eds. M. T. Lee, L. D. Kubzansky, and T. J. VanderWeele (Oxford: Oxford University Press), 136–188.
Urry, H. L., Nitschke, J. B., Dolski, I., Jackson, D. C., Dalton, K. M., Mueller, C. J., et al. (2004). Making a life worth living: neural correlates of well-being. Psychol. Sci. 15, 367–372. doi: 10.1111/j.0956-7976.2004.00686.x
Uttley, L., Quintana, D. S., Montgomery, P., Carroll, C., Page, M. J., Falzon, L., et al. (2023). The problems with systematic reviews: a living systematic review. J. Clin. Epidemiol. 156, 30–41. doi: 10.1016/j.jclinepi.2023.01.011
van der Meer, L., Costafreda, S., Aleman, A., and David, A. S. (2010). Self-reflection and the brain: a theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neurosci. Biobehav. Rev. 34, 935–946. doi: 10.1016/j.neubiorev.2009.12.004
van Reekum, C. M., Urry, H. L., Johnstone, T., Thurow, M. E., Frye, C. J., Jackson, C. A., et al. (2007). Individual differences in amygdala and ventromedial prefrontal cortex activity are associated with evaluation speed and psychological well-being. J. Cogn. Neurosci. 19, 237–248. doi: 10.1162/jocn.2007.19.2.237
Vanderhasselt, M.-A., De Raedt, R., Brunoni, A. R., Campanhã, C., Baeken, C., Remue, J., et al. (2013). TDCS over the left prefrontal cortex enhances cognitive control for positive affective stimuli. PLoS One 8:e62219. doi: 10.1371/journal.pone.0062219
Velikova, S., and Nordtug, B. (2017). Self-guided positive imagery training: effects beyond the emotions-a Loreta study. Front. Hum. Neurosci. 11:644. doi: 10.3389/fnhum.2017.00644
Veritas Health Innovation (2021). Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. Available at: https://www.covidence.org
Villanueva-Moya, L., and Expósito, F. (2022). Are gender roles associated with well-being indicators? The role of femininity, fear of negative evaluation, and regret in decision-making in a Spanish sample. Curr. Psychol. 42, 20790–20803. doi: 10.1007/s12144-022-03142-7
Volkow, N. D., Tomasi, D., Wang, G.-J., Fowler, J. S., Telang, F., Goldstein, R. Z., et al. (2011). Positive emotionality is associated with baseline metabolism in orbitofrontal cortex and in regions of the default network. Mol. Psychiatry 16, 818–825. doi: 10.1038/mp.2011.30
Vytal, K., and Hamann, S. (2010). Neuroimaging support for discrete neural correlates of basic emotions: a voxel-based meta-analysis. J. Cogn. Neurosci. 22, 2864–2885. doi: 10.1162/jocn.2009.21366
Wang, Y.-J., Duan, W., and Lei, X. (2020). Impaired coupling of the Brain’s default network during sleep deprivation: a resting-state EEG study. Nat. Sci. Sleep 12, 937–947. doi: 10.2147/nss.S277655
Watson, D., Clark, L. A., and Tellegen, A. (1988). Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Soc. Psychol. 54:1063. doi: 10.1037//0022-3514.54.6.1063
Waytz, A., Hershfield, H. E., and Tamir, D. I. (2015). Mental simulation and meaning in life. J. Pers. Soc. Psychol. 108, 336–355. doi: 10.1037/a0038322
Weiss, A., Adams, M. J., and King, J. E. (2011). Happy orangutans live longer lives. Biol. Lett. 7, 872–874. doi: 10.1098/rsbl.2011.0543
Woods, B. K., Forbes, E. E., Sheeber, L. B., Allen, N. B., Silk, J. S., Jones, N. P., et al. (2020). Positive affect between close friends: brain-behavior associations during adolescence. Soc. Neurosci. 15, 128–139. doi: 10.1080/17470919.2019.1662840
Xu, Y.-Y., Feng, Z.-Q., Xie, Y.-J., Zhang, J., Peng, S.-H., Yu, Y.-J., et al. (2018). Frontal alpha EEG asymmetry before and after positive psychological interventions for medical students. Front. Psych. 9:432. doi: 10.3389/fpsyt.2018.00432
Yu, G.-M., and Li, B. (2012). How subjective well-being affects emotional processing: the role of event-related potentials. Soc. Behav. Personal. Int. J. 40, 1285–1292. doi: 10.2224/sbp.2012.40.8.1285
Zhang, Y., and Han, B. (2016). Positive affect and mortality risk in older adults: a meta-analysis. Psy. Ch J. 5, 125–138. doi: 10.1002/pchj.129
Zhang, J., Hu, B., Chen, W., Moore, P., Xu, T., Dong, Q., et al. (2014). Quantitative EEG and its correlation with cardiovascular, cognition and mood state: an integrated study in simulated microgravity. Microgravity Sci. Technol. 26, 401–418. doi: 10.1007/s12217-014-9388-7
Zhang, W., Li, H., and Pan, X. (2015). Positive and negative affective processing exhibit dissociable functional hubs during the viewing of affective pictures. Hum. Brain Mapp. 36, 415–426. doi: 10.1002/hbm.22636
Zhang, Q., Li, X., Wang, K., Zhou, X., Dong, Y., Zhang, L., et al. (2017). Dull to social acceptance rather than sensitivity to social ostracism in interpersonal interaction for depression: behavioral and electrophysiological evidence from cyberball tasks. Front. Hum. Neurosci. 11:162. doi: 10.3389/fnhum.2017.00162
Keywords: emotional well-being, neuroimaging, scoping review, positive affect, life satisfaction, sense of meaning, goal pursuit
Citation: Richter CG, Li CM, Turnbull A, Haft SL, Schneider D, Luo J, Lima DP, Lin FV, Davidson RJ and Hoeft F (2024) Brain imaging studies of emotional well-being: a scoping review. Front. Psychol. 14:1328523. doi: 10.3389/fpsyg.2023.1328523
Edited by:
Hans Henrik Knoop, Aarhus University, DenmarkReviewed by:
Gewnhi Park, Westmont College, United StatesKorrina Duffy, University of Colorado Anschutz Medical Campus, United States
Dmitriy Bondarev, University of Jyväskylä, Finland
Maria Blasa Sánchez Barrera, University of Granada, Spain
Copyright © 2024 Richter, Li, Turnbull, Haft, Schneider, Luo, Lima, Lin, Davidson and Hoeft. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fumiko Hoeft, ZnVtaWtvLmhvZWZ0QHVjb25uLmVkdQ==
†These authors have contributed equally to this work
†ORCID: Caroline G. Richter, https://orcid.org/0000-0002-4810-1770
Celine Mylx Li, https://orcid.org/0000-0002-0622-6292
Adam Turnbull, https://orcid.org/0000-0002-1629-562X
Stephanie L. Haft, https://orcid.org/0000-0002-5486-1581
Deborah Schneider, https://orcid.org/0000-0002-1868-1989
Jie Luo, https://orcid.org/0000-0002-0484-4441
Denise Pinheiro Lima, https://orcid.org/0000-0001-9350-1100
Feng Vankee Lin, https://orcid.org/0000-0003-1945-0362
Richard J. Davidson, https://orcid.org/0000-0002-8506-4964
Fumiko Hoeft, https://orcid.org/0000-0003-3932-9201
 Caroline G. Richter
Caroline G. Richter Celine Mylx Li
Celine Mylx Li Adam Turnbull
Adam Turnbull Stephanie L. Haft
Stephanie L. Haft Deborah Schneider
Deborah Schneider Jie Luo
Jie Luo Denise Pinheiro Lima6
Denise Pinheiro Lima6 Feng Vankee Lin
Feng Vankee Lin Richard J. Davidson
Richard J. Davidson Fumiko Hoeft
Fumiko Hoeft