- 1Institute for Biodiversity and Ecosystem Dynamics (IBED), University of Amsterdam, Amsterdam, Netherlands
- 2Department of Evolutionary Anthropology, University of Zurich, Zurich, Switzerland
- 3Department of Human Behavior, Ecology and Culture, Max Planck Institute for Evolutionary Anthropology, Leipzig, Germany
- 4Department of Human Evolutionary Biology, Harvard University, Cambridge, MA, United States
- 5Cooperative Evolution Lab, German Primate Center, Göttingen, Germany
- 6Department of Anthropology, University College London, London, United Kingdom
- 7Music Cognition Group, Institute for Logic, Language and Computation, University of Amsterdam, Amsterdam, Netherlands
- 8Department of Cognitive Psychology, Leiden University, Leiden, Netherlands
- 9ARTIS Amsterdam Royal Zoo, Amsterdam, Netherlands
Music is a cultural activity universally present in all human societies. Several hypotheses have been formulated to understand the possible origins of music and the reasons for its emergence. Here, we test two hypotheses: (1) the coalition signaling hypothesis which posits that music could have emerged as a tool to signal cooperative intent and signal strength of alliances and (2) music as a strategy to deter potential predators. In addition, we further explore the link between tactile cues and the propensity of mothers to sing toward infants. For this, we investigated the singing behaviors of hunter-gatherer mothers during daily foraging trips among the Mbendjele BaYaka in the Republic of the Congo. Although singing is a significant component of their daily activities, such as when walking in the forest or collecting food sources, studies on human music production in hunter-gatherer societies are mostly conducted during their ritual ceremonies. In this study, we collected foraging and singing behavioral data of mothers by using focal follows of five BaYaka women during their foraging trips in the forest. In accordance with our predictions for the coalition signaling hypothesis, women were more likely to sing when present in large groups, especially when group members were less familiar. However, predictions of the predation deterrence hypothesis were not supported as the interaction between group size and distance from the village did not have a significant effect on the likelihood of singing. The latter may be due to limited variation in predation risk in the foraging areas, because of the intense bush meat trade, and hence, future studies should include foraging areas with higher densities of wild animals. Lastly, we found that mothers were more likely to sing when they were carrying infants compared to when infants were close, but carried by others, supporting the prediction that touch plays an important prerequisite role in musical interaction between the mother and child. Our study provides important insight into the role of music as a tool in displaying the intent between or within groups to strengthen potentially conflict-free alliances during joint foraging activities.
Introduction
Music is a ubiquitous cultural feature that is present wherever human beings are found (Brown, 1991; Savage et al., 2015; Trehub et al., 2015; Mehr et al., 2019) and has been actively studied in an attempt to deconstruct its physiological, neurological, psychological, genetic, developmental, and cultural impacts on humans (Wallin et al., 2000; Honing, 2018). Theories on the origins and evolution of music have been diverse and plenty (Huron, 2001), although they are regularly debated (Bloom and Finlay, 2021; Honing, 2021, 2023). One of the earliest approaches to explain the evolution of music was taken by Darwin (1871) who compared music to mating calls and suggested that music could have arisen as an adaptation through sexual selection allowing early men to compete with each other to court and win over women. However, the sexual selection hypothesis fails to explain the variety of contexts in which music takes place such as war rituals, work, mourning, and social gatherings, as well as the presence of music in children and the listening by people of all ages (Hagen and Bryant, 2003; Dissanayake, 2006; Clayton, 2009; Kirschner and Tomasello, 2010; Mehr et al., 2019, 2021).
As a result, another new hypothesis was put forward to explain the occurrence of music in social groups, stating that music could have originated to initiate and enhance social bonding within and between groups: the social bonding hypothesis (Roederer, 1984; Savage et al., 2021). Novel social bonds and bond strength are crucial for humans and non-human primates as they are suggested to positively affect longevity, mental wellbeing, access to nutritionally rich but difficult-to-acquire food, dominance rank, territorial defense and expansion, predator protection, and reproductive success (Kaplan et al., 2000; Silk, 2007; Dunbar and Shultz, 2010; Holt-Lunstad et al., 2010; Silk et al., 2010; Dunbar, 2012; Gilby et al., 2013; Samuni et al., 2018). According to the social bonding hypothesis, music is thought to achieve increased social bonding by facilitating synchronization between and among individuals in groups (Demos et al., 2012; Weinstein et al., 2016; Savage et al., 2021).
However, as music is largely uninformative concerning personal goals, skills, and fitness benefits of individuals (an important outcome of social bonding), it may hinder reciprocal exchange (Hagen and Bryant, 2003). In addition, Mehr et al. (2021) suggested that music production is costly due to its energy and time-intensive nature. Hence, an alternative to the social bonding hypothesis has been raised where music does not cause bonding and boost cohesion directly but can reliably signal coalition strength or quality and cooperative intent or the intention to signal information on the willingness to cooperate within and between groups (Hagen and Bryant, 2003; Mehr et al., 2021). According to Mehr et al. (2021), the coalition signaling hypothesis is part of a larger hypothesis (the credible signaling hypothesis), where music could act as a reliable signal to deter predation, signal attention toward altricial infants, and signal coalition strength or intent. Therefore, music in group contexts could signal group identity, coalition strength, or quality through showcasing complex coordinated or synchronized musical performances for spectating groups and, hence, also displaying a group identity through celebration or remembrance of ancestors (McCall, 1993; Feld, 2012; Mehr et al., 2021). Furthermore, music could be used as an intimidation strategy in inter-group encounters (Zavos, 1998; Jordania, 2015). At the same time, music under certain contexts has been argued to signal information regarding the intention to form and strengthen coalitions by “breaking the ice” between individuals from different groups or within groups and in turn indirectly benefitting group members by giving potential future access to valuable information regarding resources. Feasts, rituals, and processions are major social contexts that are largely accompanied by music to encourage new alliances and exhibit group identities and cooperation across several cultures (Zvelebil, 1988; Hagen and Bryant, 2003; Levitin, 2008; Ng and Verkuyten, 2010; Boer and Abubakar, 2014; Hayden, 2014; Lewis, 2015; Mehr et al., 2021). For example, among the Columbian Barasana, food is donated by one of the groups to other neighboring local groups as an act of gratitude and as an incentive to build trust. The period of food exchange is accompanied by music and dance throughout the entire day and night (Hugh-Jones, 1979). Furthermore, the number of individuals singing and dancing, the extent of coordination, or the amount of time invested in practicing could signify the eagerness to achieve a particular group-benefitting goal (Rouget, 1980; Hagen and Bryant, 2003; Mehr et al., 2021). Although the role of music in signaling coalitions has been thoroughly formulated (Mehr et al., 2021), to our knowledge, it has not been empirically tested yet on a non-WEIRD (Western, Educated, Industrialized, Rich, and Democratic; Henrich et al., 2010) population. This current article is one of the first attempts to do so.
Another argument under the credible signaling hypothesis posits that music originated as a strategy along with the use of masks, body painting, and clothes to enable a group to intimidate and deter threats, such as predators (Jordania, 2011; Hagen, 2022). Singing in large numbers is shown to have a positive effect on singing amplitude, although conditional on the intensity range of individual members (Titze and Maxfield, 2017), thereby making it potentially effective in deterring wild animals. We include this strategy under the predation deterrence hypothesis and suggest that beyond the deterrence of predators, it can be also used to describe the function of music to deter potentially dangerous animals in general, such as elephants, buffaloes, and gorillas. Although it is a part of the credible signaling hypothesis (which includes coalition signaling), we call our hypothesis predation deterrence to make a distinction between signaling among humans and signaling by humans toward non-human animals. According to our hypothesis, the strategy to repel any potential non-human threat is ornate and loud, especially when implemented in large numbers instead of being quiet and inconspicuous (Knight and Lewis, 2017).
While discussing the possible reasons for the origin of music, the occurrence of music in the context of a parent and infant cannot be ignored. The use of lullabies by the mother is shown to be a human universal and is the second most identifiable type of music after dance songs (Trehub, 2001; Mehr et al., 2019). A probable explanation for these observations is that music is a result of kin selection where music enhances the bonding between the mother and the infant. Fancourt and Perkins (2018) showed that infant-directed singing by mothers can induce more closeness compared to talking or playing. Furthermore, music is suggested to be a part of a unique well-coordinated multimodal exchange involving touch, eye contact, body movement, and infant-directed speech (Feldman, 2012; Pérez and Español, 2016; Dissanayake, 2021; Trehub, 2021; Hilton et al., 2022). This multimodal exchange could represent a primordial communicative system of information since prosodic changes and intonation of the mother toward the infant (infant-directed speech) along with touch and eye contact create a foundation for stable relationships later in life (Dissanayake, 2000; Koulomzin et al., 2002; Falk, 2004). Within this communication system, touch is specifically shown to play a crucial role in displaying parental attention. Specifically, affectionate touch by the parent which consists of gentle stroking and holding has been shown to play an important role in facilitating parent–infant bonds and social bonds along with impacting infant somatosensory development (Hertenstein, 2002; Bigelow and Williams, 2020; Carozza and Leong, 2021). The importance of the role of touch has also been studied in non-WEIRD societies such as the Aka, where mothers regularly carry and breastfeed their infants (Hewlett et al., 1998; Hewlett and Lamb, 2002; Konner, 2010, 2016). Importantly, Velandia et al. (2010) showed that the increase in vocal interactions between a mother and infant is dependent on skin-to-skin contact. However, to date, there is no evidence for a direct link between tactile cues and the propensity to sing to infants, especially in non-WEIRD societies. If music originated to signal attention (Mehr et al., 2021) instead of directly increasing social bonding between mother and infant, we propose that touch could be a prerequisite for singing behavior. In other words, the prevalence of touch could be crucial in initiating parental singing during parent–infant interactions. Therefore, in this study, we also investigate whether touch (through carrying) influences the likelihood of parental singing by studying the unique context of children being carried by the mother as well as alloparents in hunter-gatherer societies.
Here, we test the coalition signaling and predation deterrence hypotheses, both within the overarching credible signaling hypothesis. In addition, we study the proposed link between touch and singing in a parent–infant context. We conducted our study with a hunter-gatherer population: Mbendjele BaYaka (hereafter: “BaYaka”) in the Republic of Congo. The BaYaka are an egalitarian community with semi-nomadic (move from one seasonal camp to another every few months) lifestyles (Woodburn, 1982; Lewis, 2002; Salali and Migliano, 2015; Thompson, 2018). The BaYaka forage for wild food sources daily, such as fish, fruits, nuts, tubers, mushrooms, leaves, caterpillars, and meat, but they also engage in some crop cultivation and trade (Kitanishi, 1995; Lewis, 2002; Veen et al., 2023). Foraging activities are gendered among the BaYaka, where women generally focus on the gathering of fruits, nuts, and tubers while men preferably target meat, high-hanging fruits, and honey (Thompson, 2018; Veen et al., 2023). Music and dance are an integral part of the BaYaka society which dictates their day-to-day lives (Lewis, 2002, 2013; Knight and Lewis, 2017; Oloa-Biloa, 2017). The BaYaka exhibit music in both the individual and group levels, especially, BaYaka women, who often sing during foraging (Lewis, 2013) as well as in larger gatherings such as spirit plays (Oloa-Biloa, 2017). The BaYaka song structure consists of an ever-present gradient in terms of the complexity of the song. When they are alone in the forest, the song can either consist of a single note or display an alternation between the chest voice and to head voice, hence giving the impression of multiple singers instead of one (pers. observation by J.L). In addition, the BaYaka often showcase a complex polyphonic song structure intertwined with polyrhythms during social contexts such as spirit plays (Mokondi Massana) (Lewis, 2009; Knight and Lewis, 2017; Oloa-Biloa, 2017).
For our study, we observed the singing behaviors of BaYaka women during foraging activities in the forest, especially, when the women were searching and digging for wild tubers which are an essential part of the BaYaka diet (Salali, 2017; Veen et al., 2023). Previous studies on hunter-gatherers have largely focused on musical performances during stand-alone large-scale events such as rituals and ceremonies but rarely consider musical activities well-integrated with daily activities such as foraging contexts. Importantly, BaYaka women often sing while walking in the forest and during subsistence activities. Hence, studying their singing behaviors during foraging trips offers an ideal context to test the aforementioned hypotheses. The BaYaka women often travel long distances in the forest (median travel distance = 3.93 km on human-made trails and 0.42 km off-trail; see Jang 2019a for more information) for foraging trips, allowing us to examine the predation deterrence hypothesis as the prevalence of wild animals increases deep in the forest. Tracks of leopards and gorillas have been regularly spotted during the foraging trips with occasional narration or accounts of encounters with wild animals by the women (pers. observation by K.R.L.J). Moreover, the women are often accompanied by infants on foraging trips, which allows us to simultaneously study the effect of carrying an infant (a cue for touch) on singing behavior. Importantly, childcare in BaYaka societies is not restricted to mothers but extended to non-maternal caregivers, including genetically unrelated individuals (Meehan, 2009; Bogin et al., 2014; Meehan et al., 2014; Jang et al., 2022).
To test our hypotheses on the evolutionary function of singing, we used the following measures. The probability of singing was measured as the probability (yes or no), with which BaYaka women sang during a tuber searching and digging bout in the forest. First, we predicted that BaYaka women would sing more during foraging with an increase in the number of individuals in the foraging group, especially in the presence of less familiar individuals, who are socially less close to the focal women (coalition signaling hypothesis). When socially closer individuals are present in larger groups, we expect the individuals to use more efficient ways of communication such as talking as singing requires more effort (Hagen and Bryant, 2003; Mehr et al., 2021). By “more efficient”, we mean that language is an explicit and precise communication tool to immediately acquire information regarding resources (Lewis, 2013). In addition, we investigated the predation deterrence hypothesis. Based on prior observations, BaYaka women were reported to sing loudly in a synchronized choral manner, while walking in the forest which they reported would deter and prevent the startling of wild animals such as buffalos, elephants, gorillas, and leopards (Lewis, 2009; Knight and Lewis, 2017; Salali, 2017). As animal densities would be higher in the deep forest far away from villages and decrease in an area close to human settlements due to hunting pressure for bushmeat (Wilkie and Carpenter, 1999; Hennessey and Rogers, 2008; Tweh et al., 2015), we expected the women to be more vulnerable to the potential threat from wild animals once they moved further away from the village. Furthermore, the foraging women are thought to increase their singing frequencies when present deep in the forest and in larger numbers during menstruation. It is suggested that singing repels predators and animals that could be potentially attracted to the smell of the blood of these foraging women (Lewis, 2009, 2014). Therefore, being in large groups could enable them to incorporate loud synchronized singing behavior along with conspicuous body movements that could be influential in warding off potential non-human threats (Jordania, 2011, 2015; Lewis, 2014; Salali, 2017). In addition, singing loudly in large groups could reduce the risk of startling a distant potentially dangerous animal, such as elephants or gorillas, decreasing the risk that foragers would be attacked.
Hence, we predicted that BaYaka women would sing more especially in large groups when they were distant from the village (where the risk of encountering dangerous animals was presumed to be higher). Lastly, we also predicted that BaYaka women would sing more frequently the longer they carried infants in a foraging bout (see methods for more information), as a measure of touch.
Methods
Participants and data description
The data used for this study were collected in a BaYaka community near the village of Djoube, along the Motaba River in the Likoula department of the north-western Republic of the Congo by H.J. and K.R.L.J. The BaYaka community is often referred to as “Aka”, “Baaka”, “Baka”, and “Mbendjele” (Kitanishi, 1995; Bombjaková, 2018; Jang et al., 2019a). The data were collected from March to August 2015 and 2016 (Jang et al., 2019a,b) as part of a larger project investigating the navigation strategies of BaYaka women. During the 230 total observation days, H.J. and K.R.L.J. conducted continuous focal sampling of five focal women (estimated mean age = 31 years, range = 26–41; Supplementary Table 1 for a detailed summary) from the beginning (the moment the focal woman left the camp) to the end (when she returned to the camp) of the women foraging expeditions (Martin and Bateson, 2009). The same focal women were followed twice for consecutive days each in 2015 and 2016. In addition to foraging expeditions, data were also collected when the focal women traveled to nearby villages or went gardening for crop cultivation. Focal data included the behaviors, gestures, and vocalization of the focal woman, as well as her expedition group composition and whether she carried an infant (not necessarily her infant) at the point of observation. We recorded the focal women's diverse vocalizing actions using a voice recorder (Supplementary Table 4 for codes). We categorized these vocalizations based on the nature of communication, singing (roughly categorized into loud and soft), silence or non-vocalization, discussion (mentioning tuber species names or their locations), and conversation (talking about anything else other than food or the food locations). In this study, our main interest was the singing (both loud and soft) vocalization (Supplementary Figure 1 for the duration of all vocalizations). Additional metrics such as the species of the tuber collected (Supplementary Table 2) and Global Positioning System (GPS; Garmin-62)-based space-time stamps were accounted for in the dataset. See Supplementary Tables 3, 5 for full details of the data description.
Definition and calculation of tuber searching and digging bout
The unit of our analyses is a tuber searching and digging bout, which consists of a starting point where a focal woman first exhibited tuber digging behavior and an end point where she exhibited a non-digging behavior before the next digging behavior (that would indicate the start of the next searching and digging bout). When the focal woman exhibited walking or inspection behaviors between two digging behaviors, we considered the duration of those activities also as a tuber searching duration. It is difficult to define a tuber patch by observation. Hence, to identify whether walking occurred between two different tuber patches or within the same tuber patch, we defined a tuber patch based on the BaYaka women's digging and traveling behavior and calculated the 75th percentile of the duration of all the tuber searching and digging bouts. If any of the bout durations exceeded the 75th percentile, we considered those as bouts that included focal women's movement between two different tuber patches. We then excluded those bouts from our analyses as we did not consider this type of long-distance movement as part of the foraging activity (Supplementary Figure 2). To calculate the duration of the tuber searching and digging bouts, we used a customized function along with aggregate and merge in RStudio (R Core Team, 2020).
Calculation of relevant variables
The response variable in the model was singing probability. This was a binary response as an indication of whether the focal woman sang (1) or not (0) during a tuber searching and digging bout. As predictor variables, we included group size, dyadic association index, distance from the village, and duration of carrying a baby. The group size was determined by counting the number of individuals present along with the focal individual during each behavior carried out by the focal woman and averaged per tuber searching and digging bout.
The Dyadic Association Index (hereafter “DAI”) was calculated as a proxy for bond strength (or social closeness) and the relationship between the focal individual and all the other individuals. The other individuals observed along with the focal women consisted of individuals (both men and women) from the same camp (either genetically related or not and including children) and BaYaka individuals from other villages. The DAI is the proportion of time that two individuals were observed together during foraging activities out of the amount of time that either of them was observed foraging in total, representing how likely are two individuals to forage together (Nishida, 1968; Cairns and Schwager, 1987). For our study, we calculated a yearly DAI value for each dyad (the proportion of time two individuals spent together in a year). The value was calculated for two separate years, 2015 and 2016. The DAI ranged from 0 to 1, with 1 indicating that individuals were always observed together.
where A (Focal Individual) = the total time individual A was observed; B (Individual present in the foraging group) = the total time individual B was observed; AB = the time A and B were observed together.
To determine social closeness between focal women and other individuals in a foraging group during the observed tuber searching and digging bouts, we averaged the DAI values across all focal-partner dyads per tuber searching and digging bout and included the average values in the statistical model.
Using the GPS coordinates, the distances between the location at each observation point and the village were calculated (km) with a formula based on the spherical law of cosines (Supplementary Figure 1). The distances from the village were then further averaged for all locations where the women had been during a tuber searching and digging bout. We observed that children aged between 1 month and 4 years were carried while walking (Lancy and Grove, 2011; Salali et al., 2019). As a result, a total of 13 individuals (Nboys=5, Ngirls=8) were identified as children who were carried, with a mean age of 1.546 in 2015 (refer to Supplementary Figure 3 for more information on how the ages were calculated). We calculated the total duration of carrying an infant per tuber searching and digging bout to use it as a cue for touch. We observed that the focal women often carried or spent time with their infants during foraging trips or with the infants of other women in the foraging group when they were not digging.
Statistical analysis
The dataset used for statistical analyses consisted of a total of 1,704 tuber searching and digging bouts (with mean ± SD: 1,797.795 ± 6,823.564 s for the duration of the bouts) from five focal individuals with similar observation efforts per woman (Supplementary Table 1). The foraging parties existed mainly of groups of females. Only one male was present in the foraging group in 53 of the total 1,704 bouts (3.1% of the bouts) with a mean ratio of 0.003 for all bouts (number of males to the total number of individuals in a foraging group). We fitted a Generalized Linear Mixed Model (GLMM) with a Binomial error structure and logit link function (Baayen, 2008). The model simultaneously tested the coalition signaling and predation deterrence hypothesis. We used singing probability as a binary response variable (18.54% of the bouts, N = 316 included singing; see Supplementary Table 1 for a detailed summary). The model consisted of the interaction between group size and DAI, the interaction between group size and distance from the village, and the duration of carrying a baby (19.31% of the bouts included the carrying of a baby, N = 329; with mean ± SD: 493.508± 3,103.675 s for the baby carrying duration; Supplementary Table 1 for a detailed summary) as predictors (Table 1, Supplementary Figure 2 for the frequency distribution of model variables). We included the focal individual as a random effect, with the DAI, duration of carrying a baby, and interaction between group size and distance from the village as random slopes (Supplementary Table 7). The remaining random slopes were deemed to be unidentifiable and hence discarded. Including random slopes was done to keep the Type I error probability at the desired level of 0.05 (Schielzeth and Forstmeier, 2009; Barr et al., 2013). The correlations between the random slopes and intercepts were not included in the model. The distributions of the predictor and response variables were monitored accordingly before model construction (Supplementary Figure 2). All the predictors were then z-transformed to a mean of zero and standard deviation of one to ensure easier interpretation of model coefficients (Schielzeth, 2010). We used the glmer function of the package lme4 (Bates et al., 2015). The model converged and the full-null model comparison was carried out using a likelihood ratio test (Dobson, 2002) to avoid multiple testing where the null model consisted of the intercept and the random structure of the full model (Forstmeier and Schielzeth, 2011). By omitting the levels of the random effects one at a time, the model's stability was evaluated based on the calculated coefficients and standard deviations (Nieuwenhuis et al., 2012). The stability was estimated through customized scripts. Overall, the model was found to be of acceptable stability except for the intercept (Supplementary Figures 10, 13). Potential problems of collinear predictable variables were checked by analyzing the variance inflation factors (VIFs) using the vif function of the car package (Field 2005, Fox and Weisberg, 2019). None of the predictor variables were found to be collinear (Highest VIF = 1.3895 and 1.2064 for DAI and baby carrying duration, respectively). Furthermore, we used a parametric bootstrap to obtain confidence intervals of model estimates (function bootMer of the package lme4; N = 1,000 bootstraps). The confidence intervals were the widest for the intercept (C95% = [−2.239, −1.205]) and DAI (C95% = [−0.894, −0.121] (Supplementary Figures 8, 9). The explained variance was calculated using the r.squaredGLMM function of the MuMIn package (Barton, 2020). The marginal (only fixed effects) and conditional (fixed and random effects) R-squared values were found to be 0.2216 and 0.2954, respectively (Nakagawa et al., 2017). The graphs for the model were plotted in R (R Core Team, 2020) using the sjPlot (Lüdecke, 2020) and visreg packages (Breheny and Burchett, 2017; see Supplementary Figures 5, 6, 8 for alternative plots). All the analysis was done in R Studio (version 4.0.2, R Core Team, 2020). In addition to the main model, we ran a post-hoc model to test whether the focal women were more likely to sing specifically when they carried a baby for longer durations in bouts. To do so, we only used tuber and searching bouts where the baby was present in the foraging group.
Results
The full model showed significance when compared with the null model (full-null model comparison: χ2 = 30.7812, df = 6, P < 0.0001). In general, the singing probability of the women increased with a decrease in average DAI, with a more prominent increase when the women foraged in large groups compared to small ones. However, when in the presence of familiar individuals (high average DAI), the focal women were more likely to sing when they foraged in small groups compared to large groups (Estimate = −0.3782, SE = 0.1150, P = 0.0008; Table 2, Figure 1). In contrast, the interaction between group size and distance from village did not have a significant effect on the response (Estimate = −0.1272, SE = 0.0940, P = 0.1762), nor did the single term of distance from the village (after reducing the non-significant interaction). The duration of carrying a baby had a strong positive impact on singing probability within the foraging bouts (Estimate = 1.2554, SE = 0.1295, P < 0.0001; Table 2, Figure 2). In addition, the effect of duration of carrying a baby on singing probability remained strongly significant (Estimate = 1.4497, SE = 0.1694, P < 0.0001; Refer to Supplementary Tables 6, 7 for a detailed summary) in the post-hoc model (only consisting of bouts in which the baby was present in the foraging group). Therefore, the more time the focal woman under observation carried the baby herself (instead of having it carried by others), the higher the probability that she would sing.
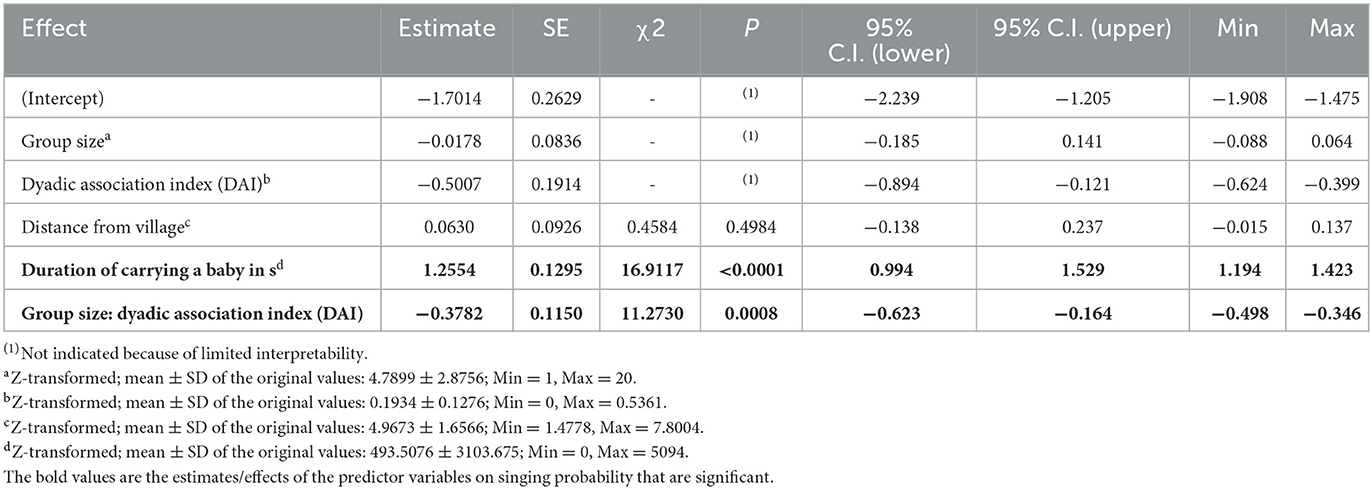
Table 2. Results of the singing probability model (together with estimates, standard errors, significance tests, confidence intervals, as well as minimum and maximum of model estimates when excluding focal individuals one at a time to test model stability).

Figure 1. Effect of interaction between mean dyadic association index (DAI) and average party size on singing probability. The fitted model (surface; conditional on the distance from the village and duration of carrying a baby being at their average) and singing probability per level of the cell. The filled points indicate values above the fitted model while the open points depict values below the fitted model. The size or volume of the points indicates the number of individual data points per cell (Range = 1 to 244 points per cell. Mean=34.08 ± 44.7578). Mean group size has a strong positive effect on singing probability for low values of DAI, but the singing probability decreases steeply with an increase in the DAI for large party sizes. The focal women were more likely to sing more when present in large groups of less familiar individuals but were less likely to sing when present in large groups of familiar individuals (Estimate = −0.3782, SE = 0.1150, p = 0.0008). An alternative 2D version of this relationship (showing the effect of interaction between mean dyadic association index (DAI) and average party size on singing probability) is given in Supplementary Figures 3–7.
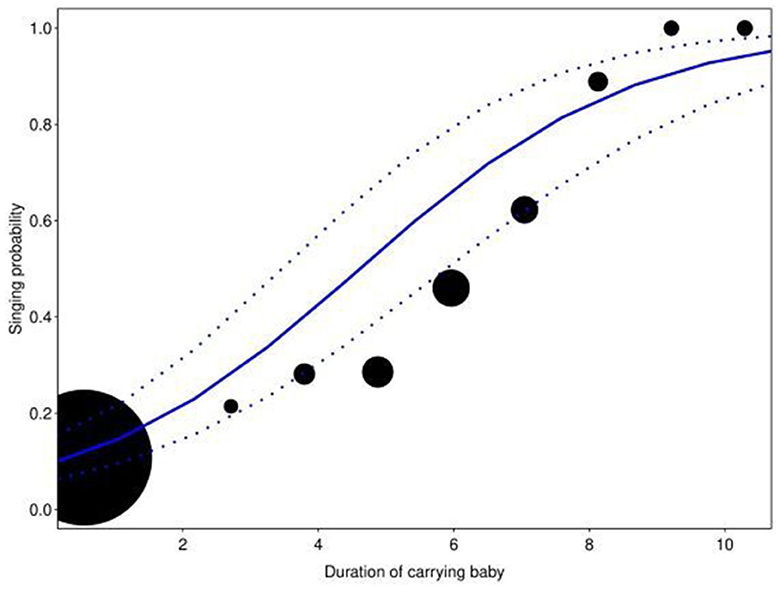
Figure 2. Effect of log-transformed duration of carrying baby (in s) on singing probability. The circles represent the amount of time (in s) the focal woman carried a baby. The size of the circles indicates the number of data points for particular ranges of duration (total N=14 to 1,375). The blue line represents the fitted model with the dashed lines representing the upper and lower confidence intervals, respectively. Singing probability increases with an increase in the duration of carrying a baby (Estimate = 1.2554, SE = 0.1295, p < 0.0001).
Discussion
The singing probability model contrasted two key hypotheses on the origins of singing behavior, the coalition signaling and the predation hypotheses. Our results were consistent with the coalition signaling hypothesis as the focal women sang more frequently in larger groups of less familiar individuals and less frequently in larger groups consisting of more closely bonded individuals. In the presence of closely bonded individuals, singing probability decreased with an increase in group size. However, our results were not in line with the predation deterrence hypothesis.
Forming highly cooperative relationships with less familiar individuals between groups is an important component of human societies (Hagen and Bryant, 2003). Cooperation is important not only during the process of joint food acquisition, such as dam-fishing, nut-cracking, and tuber digging, but can also result in the shared knowledge of food locations and handling skills (Hagen and Bryant, 2003; Oloa-Biloa, 2017; Bombjaková, 2018; pers. observations by K.R.L.J and H.J). The effects of social connections with less familiar partners could, therefore, be beneficial over the long term as these members may possess skills and knowledge that closely bonded individuals may not have and can contribute to the labor needed for certain food-finding and handling techniques in future (Laden, 1992; Lewis, 2013). Bahuchet (1992) reported that several BaYaka groups unite for cooperative activities such as hunting and singing in large groups for festivals.
However, joint foraging can also result in conflicts if acquired food or information about food locations is not equally shared or is monopolized (pers. observation by H.J and K.R.L.J, Lewis, 2002; Oloa-Biloa, 2017). An increased proportion of less familiar and less related individuals during temporary foraging alliances will likely result in an increased probability of conflicts regarding equal sharing. In our study, although some of the individuals from the same camp or a different village are relatively less socially close with the focal women, they are united through crucial shared values such as egalitarianism, spreading positive emotions (such as “joy” or bisengo), and monogamy (Lewis, 2002, 2015, 2019; Knight and Lewis, 2017; Oloa-Biloa, 2017). Violations of these values can involve discussion and reinforcement of these values through songs and stories (Oloa-Biloa, 2017). Therefore, we also propose the possibility that song production in the BaYaka could have played an important role in minimizing the likelihood of potential conflict or mitigating existing conflicts during foraging. There are several possibilities for potential conflicts during foraging that have to be considered by the foraging women such as the locations on where to dig, who gets to use the iron tools which are essential for digging (pers. observation by K.R.L.J and H.J), and through stealing (Bombjaková, 2018, pers. observations by K.R.L.J and H.J). Through reinforcement of values such as spreading positive emotions within the continuously changing foraging alliances, cooperation and foraging success is expected to maintain and improve. Apart from singing, other strategies can be also incorporated to resolve conflicts during foraging such as avoidance (not foraging together with people with past disputes; pers. observation by K.R.L.J and J.L), announcing or warning other women publicly to not steal from the house gardens, hiding of tubers by placing them under leaves in baskets or on roofs of empty houses (theft occurred in 7 out of 1,704 bouts and hiding in 37 out of 1,704 bouts during our study; the percentage is 0.4% and 2.2%, respectively), and oral concealment of the location of a new tuber patch from the rest of the camp by foraging women (Bombjaková, 2018, pers. observation by K.R.L.J).
Our results are best explained by the coalition signaling hypothesis, supporting the idea that music could have originated as a tool to signal the intent to establish and strengthen temporary potentially conflict-free alliances with less familiar individuals, especially when they are large in number. In addition, song production could have been used as a mode to gain trust by acting as an “icebreaker” and assessing the intentions of less familiar members, thereby making music an inclusive activity that does not discriminate against individuals (Pearce et al., 2016). Among the BaYaka, the charisma and initiative of individuals are highly valued as such characteristics can positively contribute to cooperation and enhance trust among individuals (Oloa-Biloa, 2017). We suspect that song production also allows the focal women to gauge these valued characteristics in less familiar individuals.
Large groups usually act as an incentive to fuel more elaborate musical behavior with body movements. The BaYaka use music in a variety of different contexts (Lewis, 2013). One of the main occurrences of elaborate music behavior is during spirit plays (also known as Mokondi Massana). Spirit plays are mostly conducted in villages that also house a large number of genetically unrelated or less socially close individuals (Hill et al., 2011; Lewis, 2013, 2015). During these plays, there is no hierarchy dictating the rules of music performance regarding what can or cannot be included. However, the vocal contribution of each individual is still dependent on the melodies of other group members. In addition, the underlying principles dictating the musical principles of BaYaka could be also transferred to certain daily activities such as hunting (Widdess, 2012; Lewis, 2013). Personal observations by K.R.L.J have indicated that certain singing events also occur outside the context of spirit plays such as when the BaYaka go out or return from tuber foraging trips. These events could involve elaborate body movements such as stomping, clapping, and swaying during foraging expeditions. Among adults, practicing for big musical events seems quite universal in the ethnographic record (Merriam, 1964). Therefore, there is a strong possibility that singing during foraging excursions acts as rehearsals for large gatherings and events (Oloa-Biloa, 2017).
Furthermore, we suspect that singing during foraging excursions could enable the focal individuals to identify the less familiar individuals in a foraging group and assess their unique musical approaches or contributions. The less familiar individuals could introduce new melodies which can make the forest “happy” and bring joy to the focal individuals (Lewis, 2015). In BaYaka society, new melodies are often encouraged and highly valued. Hence, our findings are also in line with the role of music acting as an “icebreaker” among individuals (Pearce et al., 2016). In contrast, the reasons for the decrease in singing probability with increasing group size for high DAI values could be that singing invariably requires more effort and control over the voice than talking (Natke et al., 2003). Therefore, a group of related or familiar individuals could incorporate less costly vocalizations such as talking. We suspect that it could be easier for the focal women to use language to instruct familiar instead of less familiar individuals for the procurement of immediate knowledge regarding resources (e.g., where a tuber patch is). Language is more precise and explicit in facilitating communication of short-term intentions, goals, and knowledge sharing (Lewis, 2013). Since the focal women are socially close to these individuals already, trust could have already been achieved. Furthermore, we suspect the foraging women who are more familiar with each other will be less motivated to sing as they already know the melodies the socially close individuals could sing. Alternatively, we are open to the possibility of music being used to communicate with large groups of less familiar individuals to keep the group coordinated, as the group spread is expected to be larger, akin to the instances of “drumming” and call combination in chimpanzees and bonobos (Schamberg et al., 2016; Eleuteri et al., 2022; Fitzgerald et al., 2022). Music and dance could be an indicator of coalition quality, and stable coalitions tend to have more elaborate, complex, well-choreographed musical performances and are more synchronized (Hagen and Bryant, 2003; Mehr et al., 2021). However, our study unfortunately did not directly measure coalition quality which is a variable that determines how stable a coalition is or how collectively a group of people act to perform a task (Hagen and Bryant, 2003; Mehr et al., 2021).
In addition, we continued testing the credible signaling hypothesis by investigating the role of music in the protection against predators (Hagen, 2022). To test our predation deterrence hypothesis, the interaction between group size and distance from the village was selected as the proxy for predation risk. We predicted that singing probability would increase with an increase in distance from the village, especially when the women foraged in large groups. However, we found limited support for the predation deterrence hypothesis, as the interaction between group size and distance from the village had no significant effect on singing probability. A potential reason for the observed result is that the density of animals was probably not high in the areas where the women foraged for tubers, and hence singing may not have been necessary for these parts of the forest. Although tracks of animals such as leopards and gorillas were encountered along with repeated discussions in the camp regarding animal sightings, the density of these animals has not been regularly monitored (through camera traps) or verified in the area. Another possibility is that the animals were scared of humans in the area due to intense hunting pressure (Wilkie and Carpenter, 1999; Tweh et al., 2015). Due to the bushmeat trade, the animal density near the camp has decreased (pers. observation by K.R.L.J and M. Dzabatou). However, Knight and Lewis (2017) report instances of continuous, loud, and all-night singing by BaYaka women in the proximity of a predator. Therefore, future testing of the predation deterrence hypothesis should incorporate analyses of the singing behavior of women who reside in camps that are deeper in the forest—more than 15 km from villages—where there is sufficient evidence of the presence of predators or dangerous animals (through sightings, camera footage, and vocalizations).
In parallel, the total duration of carrying a baby had a significant positive effect on singing probability. The results were in line with our predictions and showed a possible link between singing and tactile cues which could have an impact on parent–infant bonds (Pérez and Español, 2016; Dissanayake, 2021). Furthermore, the results from the post-hoc model indicated that the focal women were more likely to sing when they carried a baby compared to when the baby was in the foraging party, but was carried or just accompanied by alloparents. Among the BaYaka, the presence of alloparents in foraging trips affect mother's foraging productivity (Jang et al., 2022). Our results further suggest that the presence of infants and alloparents during mothers' foraging activities affect also mothers' singing behaviors during foraging and that touch plays a vital supplementary role in parent-infant singing behavior. BaYaka women yodel loudly or rhythmically pat their infants' backs whenever they start crying profusely to coax and console the unsettled baby (Lewis, 2013). Lewis (2013) suggested that this behavior could be critical in developing musical skills and important tools to learn to create music. Furthermore, children are encouraged by adults to sing at an early age, especially during singing events, and are frequently exposed to sounds produced by musical instruments such as drums (Lewis, 2002, 2013; Oloa-Biloa, 2017). Therefore, our results were expected and unsurprising. As yet, we did not directly assess whether the singing was associated with the demeanor of the infants (i.e., relaxed or not) which could be measured in future studies by expanding on the previous work done by Hewlett et al. (1998). Importantly, our result can act as a primer to carry out future studies where it is tested whether an interaction between touch and parental singing affects affiliative behavioral cues such as happiness or laughing behavior in the infant. In other words, we can investigate if touch in tandem with singing increases bonding and trust between the parent and infant by regulating the mood and emotions of the infant. Maternal body movements during touch have been shown to drastically reduce restlessness and crying behavior in infants (Esposito et al., 2013). We further suggest that such reduction of restlessness and keeping the infant quiet could prevent potential predation, especially from leopards, which are an important cause of adult and especially infant casualties in foragers (Athreya et al., 2011; Janmaat et al., 2014; Kandel et al., 2023). Future studies that compare our data to those collected in areas with higher predation risks will therefore be crucial to disentangle the variables that explain singing behavior in BaYaka women who carry babies. We stress the urgency of conducting studies in areas with high predation risk as wildlife is on an alarming decline due to the increasing bushmeat trade (Hennessey and Rogers, 2008; Van Vliet et al., 2019). Lastly, we suspect that singing could be a way to increase endorphins to lessen the burden of carrying a baby in a similar way to the use of sea shanties sung by sailors during hard labor tasks such as rope hauling (Milne, 2017). This could be investigated by considering an interaction between carrying a baby and energy expenditure in future studies.
In conclusion, our study sheds light on the possibility that music could have originated to signal coalition, particularly cooperative intent or intention to form coalitions within and between groups. In addition, music could have a potential impact on parent–infant bonds by working in tandem with parental touch with a hunter-gatherer community as the sole focus. However, the signaling role of music is likely dependent on the societal structure as not all communities exhibit communal or collective music-making (Rudge, 2019; Patel and Von Rueden, 2021; e.g., the Tsimane of Bolivia and Batek of Malaysia). According to Moser et al. (2021), the diversity in musical forms is potentially an outcome of the complex and variable nature of human social structures and cultural niches. Future research assessing coalition signaling in different societies should account for the role of music in signaling their particular structure or identity, and hence, its contribution to the preservation or cohesion of that community.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: personal information regarding the identities of the individuals. Requests to access these datasets should be directed to Y2hpcmFnLmNoaXR0YXJAZ21haWwuY29t.
Ethics statement
The studies involving humans were approved by Comité d'Ethique de la Recherche en Sciences de la Santé (N°095/MRSIT/IRSA/CERSSA). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
CC and KJ: conceptualization and writing—original draft preparation. HJ and KJ: data collection. CC, LS, KJ, and EL: formal analysis and investigation. CC, HJ, HH, JL, LS, EL, and KJ: writing—reviewing and editing. KJ: funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
The Max Planck Society and the Leakey Foundation provided funding to HJ and KJ for the field research and received funding from the Max Planck Institute for Evolutionary Anthropology, Leipzig. HH was supported by an Open Competition grant from the Dutch Research Council NWO (406.20.CW.002). The study's design, data collection, analysis, publication decision, and manuscript preparation were all done independently from the sponsors.
Acknowledgments
We thank the Ministère de la Recherche Scientifique et de l'Innovation Technologique, the Comité d'Ethique de la Recherche en Sciences de la Santé and Institut de Recherche en Sciences Exactes et Naturelles (IRSEN) for their permission to execute our studies. We gratefully acknowledge Prof. C. Bouka-Biona and Prof. J.M. Moutsambote at IRSEN and M.J. Dzabatou in Makao for the logistic support of our studies. We thank D. Bombjaková for introducing us to the BaYaka family in Djoube. We especially thank R. Mundry and S. Pulla for their help with the statistical analysis. We would also like to thank J. Veen for his help in clarifying doubts related to the tuber species list. Lastly, our results supporting the coalition signaling hypothesis and rejecting the predation deterrence hypothesis have been featured earlier in an MSc thesis by Chittar (2020).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyg.2023.1218394/full#supplementary-material
References
Athreya, V., Odden, M., Linnell, J. D. C., and Karanth, K. U. (2011). Translocation as a tool for mitigating conflict with leopards in human-dominated landscapes of India. Conservat. Biol. 25, 133–141. doi: 10.1111/j.1523-1739.2010.01599.x
Bahuchet, S. (1992). “Spatial mobility and access to resources among the African Pygmies,” in Mobility and Territoriality. Social and Spatial Boundaries among Foragers, Fishers, Pastoralists and Peripatetics, eds. M. J. Casimir and A. Rao. Oxford: Berg Publishers, 205–257.
Barr, D. J., Levy, R., Scheepers, C., and Tily, H. J. (2013). Random effects structure for confirmatory hypothesis testing: keep it maximal. J. Mem. Lang. 68, 255–278. doi: 10.1016/j.jml.2012.11.001
Bartoń, K. (2020). MuMIn: Multi-Model Inference. Available online at: https://CRAN.R-project.org/package=MuMIn
Bates, D., Machler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Bigelow, A. E., and Williams, L. R. (2020). To have and to hold: Effects of physical contact on infants and their caregivers. Infant Behav. Dev. 61, 101494. doi: 10.1016/j.infbeh.2020.101494
Boer, D., and Abubakar, A. (2014). Music listening in families and peer groups: benefits for young people's social cohesion and emotional well-being across four cultures. Front. Psychol. 5, 392. doi: 10.3389/fpsyg.2014.00392
Bogin, B., Bragg, J., and Kuzawa, C. (2014). Humans are not cooperative breeders but practice biocultural reproduction. Ann. Hum. Biol. 41, 368–380. doi: 10.3109/03014460.2014.923938
Bombjaková, D. (2018). The Role of Public Speaking, Ridicule, and Play in Cultural Transmission among Mbendjele BaYaka Forest Hunter-gatherers (dissertation/doctoral thesis). London: University College London.
Breheny, P., and Burchett, W. (2017). Visualization of regression models. Using visreg. R J. 9, 56–71. doi: 10.32614/RJ-2017-046
Cairns, S. J., and Schwager, S. J. (1987). A comparison of association indices. Anim. Behav. 35, 1454–1469. doi: 10.1016/S0003-3472(87)80018-0
Carozza, S., and Leong, V. (2021). The role of affectionate caregiver touch in early neurodevelopment and parent-infant interactional synchrony. Front. Neurosci. 14, 613378. doi: 10.3389/fnins.2020.613378
Chittar, C. (2020). Deciphering the Various Integral Roles of Music in a Hunter-Gatherer Society and Quest for an Explanation for the Origins of Music (Dissertation/Master's Thesis). Amsterdam: University of Amsterdam. Available online at: https://scripties.uba.uva.nl/search?id=725037
Clayton, M. (2009). “The social and personal functions of music in cross-cultural perspective,” in Oxford Handbook of Music Psychology, eds. S. Hallam, I. Cross, and M. Thaut. Oxford: Oxford University Press, pp. 35–44.
Demos, A. P., Chaffin, R., Begosh, K. T., Daniels, J. R., and Marsh, K. L. (2012). Rocking to the beat: effects of music and partner's movements on spontaneous interpersonal coordination. J. Exp. Psychol.: General 141, 49–53. doi: 10.1037/a0023843
Dissanayake, E. (2000). “Antecedents of the temporal arts in early mother–infant interaction,” in The Origins of Music, N. L. Wallin, B. Merker, and S. Brown. Cambridge, MA: The MIT Press, 389–410.
Dissanayake, E. (2006). “Ritual and ritualization: Musical means of conveying and shaping emotion in humans and other animals,” in Music and Manipulation: On the Social Uses and Social Control of Music, eds. S. Brown, and U. Voglsten. New York: Berghahn Books, 31–56.
Dissanayake, E. (2021). Ancestral human mother–infant interaction was an adaptation that gave rise to music and dance. Behav. Brain Sci. 44, E68. doi: 10.1017/S0140525X20001144
Dunbar, R. I. M. (2012). Bridging the bonding gap: the transition from primates to humans. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 367, 1837–1846. doi: 10.1098/rstb.2011.0217
Dunbar, R. I. M., and Shultz, S. (2010). Bondedness and sociality. Behavior 147, 775–803. doi: 10.1163/000579510X501151
Eleuteri, V., Henderson, M., Soldati, A., Badihi, G., Zuberbühler, K., and Hobaiter, C. (2022). The form and function of chimpanzee buttress drumming. Anim. Behav. 192, 189–205. doi: 10.1016/j.anbehav.2022.07.013
Esposito, G., Yoshida, S., Ohnishi, R., Tsuneoka, Y., Rostagno, M., del, C., et al. (2013). Infant calming responses during maternal carrying in humans and mice. Curr. Biol CB. 23, 739–745. doi: 10.1016/j.cub.2013.03.041
Falk, D. (2004). Prelinguistic evolution in early hominins: whence motherese? Behav. Brain Sci. 27, 491–503. doi: 10.1017/S0140525X04000111
Fancourt, D., and Perkins, R. (2018). The effects of mother-infant singing on emotional closeness, affect, anxiety, and stress hormones. Music Sci. 1. doi: 10.1177/2059204317745746
Feld, S. (2012). Sound and Sentiment: Birds, Weeping, Poetics, and Song in Kaluli Expression. Durham, NC: Duke University Press.
Feldman, R. (2012). Oxytocin and social affiliation in humans. Horm. Behav. 61, 380–391. doi: 10.1016/j.yhbeh.2012.01.008
Fitzgerald, M., Willems, E. P., Soumah, A. G., Matsuzawa, T., and Koops, K. (2022). To drum or not to drum: selectivity in tree buttress drumming by chimpanzees (Pan troglodytes verus) in the Nimba Mountains, Guinea. Am. J. Primatol. 84, e23382. doi: 10.1002/ajp.23382
Forstmeier, W., and Schielzeth, H. (2011). Cryptic multiple hypotheses testing in linear models: overestimated effect sizes and the winners curse. Behav. Ecol. Sociobiol. 65, 47–55. doi: 10.1007/s00265-010-1038-5
Fox, J., and Weisberg, S. (2019). An R Companion to Applied Regression, 2nd Edn. Thousand Oaks CA: Sage. Available online at: https://socialsciences.mcmaster.ca/jfox/Books/Companion/
Gilby, I. C., Brent, L. J., Wroblewski, E. E., Rudicell, R. S., Hahn, B. H., Goodall, J., et al. (2013). Fitness benefits of coalitionary aggression in male chimpanzees. Behav. Ecol. Sociobiol. 67, 373–381. doi: 10.1007/s00265-012-1457-6
Hagen, E. H. (2022). The biological roots of music and dance. Hum. Nat. 33, 261–279. doi: 10.1007/s12110-022-09429-9
Hagen, H., and Bryant, G. A. (2003). Music and dance as a coalition signaling system. Hum. Nat. 14, 21–51. doi: 10.1007/s12110-003-1015-z
Hennessey, A. B., and Rogers, J. (2008). A study of the bushmeat trade in Ouesso, Republic of Congo. Conservat. Soc. 6, 179–184. doi: 10.4103/0972-4923.49211
Henrich, J., Heine, S., and Norenzayan, A. (2010). The weirdest people in the world? Behav. Brain Sci. 33, 61–83. doi: 10.1017/S0140525X0999152X
Hertenstein, M. J. (2002). Touch: Its Communicative Functions in Infancy. Berlin: Karger Publishers. doi: 10.1159/000048154
Hewlett, B. S., and Lamb, M. E. (2002). “Integrating evolution, culture and developmental psychology: explaining caregiver-infant proximity and responsiveness in central Africa and the USA,” in Between Culture and Biology: Perspectives on Ontogenetic Development, eds. H. Keller, Y. H. Poortinga, and A. Schölmerich. Cambridge: Cambridge University Press, 241–269.
Hewlett, B. S., Lamb, M. E., Shannon, D., Leyendecker, B., and Schölmerich, A. (1998). Culture and early infancy among central African foragers and farmers. Dev. Psychol. 34, 653–661. doi: 10.1037/0012-1649.34.4.653
Hill, K., Walker, R. S., Bozicević, M., Eder, J., Headland, T., Hewlett, B. S., and Wood, B. (2011). Co-residence patterns in hunter-gatherer societies show unique human social structure. Science 331, 1286–89. doi: 10.1126/science.1199071
Hilton, C. B., Moser, C. J., Bertolo, M., Lee-Rubin, H., Amir, D., Bainbridge, C. M., et al. (2022). Acoustic regularities in infant-directed speech and song across cultures. Nat. Hum. Behav. 6, 1545–1556. doi: 10.1038/s41562-022-01410-x
Holt-Lunstad, J., Smith, T. B., and Bradley Layton, J. (2010). Social relationships and mortality risk: a meta-analytic review. PLoS Med. 7, 859. doi: 10.1371/journal.pmed.1000316
Honing, H. (2018). The Origins of Musicality. Cambridge, MA: The MIT Press. Available online at: https://mitpress.mit.edu/books/origins-musicality
Honing, H. (2021). Unraveling the origins of musicality: beyond music as an epiphenomenon of language. Behav. Brain Sci. 44, 66–68. doi: 10.1017/S0140525X20001211
Honing, H. (2023). “What is your position in the BBS discussion on the origins of music/ality?,” in Music Matters. A Blog on Music Cognition. Available online at: http://musiccognition.blogspot.com/2021/10/position-in-bbs-discussion-on-origins.html
Hugh-Jones, S. (1979). The Palm and the Pleiades: Initiation and Cosmology in Northwest Amazonia. Cambridge: Cambridge University Press.
Huron, D. (2001). Is music an evolutionary adaptation?. Ann. N. Y. Acad. Sci. 930, 43–61. doi: 10.1111/j.1749-6632.2001.tb05724.x
Jang, H., Boesch, C., Mundry, R., Ban, S. D., and Janmaat, K. R. L. (2019a). Travel linearity and speed of human foragers and chimpanzees during their daily search for food in tropical rainforests. Sci. Rep. 9, 11066. doi: 10.1038/s41598-019-47247-9
Jang, H., Boesch, C., Mundry, R., Kandza, V., and Janmaat, K. R. L. (2019b). Sun, age and test location affect spatial orientation in human foragers in rainforests. Proc. R. Soc. B Biol. Sci. 286, 20190934. doi: 10.1098/rspb.2019.0934
Jang, H., Janmaat, K. R. L., Kandza, V., and Boyette, A. H. (2022). Girls in early childhood increase food returns of nursing women during subsistence activities of the Bayaka in the Republic of Congo. Proc. R. Soc. 2022, 1407. doi: 10.1098/rspb.2022.1407
Janmaat, K. R., Polansky, L., Ban, S. D., and Boesch, C. (2014). Wild chimpanzees plan their breakfast time, type, and location. Proc. National Acad. Sci. 111, 16343–16348. doi: 10.1073/pnas.1407524111
Jordania, J. (2015). New interdisciplinary approach to the study of the origins of traditional polyphony. Musicology 18, 77–79. doi: 10.2298/MUZ1518077J
Kandel, S. R., Neupane, B., Miya, M. S., Sadadev, B. M., Khatri, N. D., and Dhami, B. (2023). An emerging issue of human-leopard conflict in the human-dominated landscape of mid-hills: a case study from Tanahun District of Nepal. Int. J. Zool. 2023, 12. doi: 10.1155/2023/5690289
Kaplan, H., Hill, K., Lancaster, J., and Hurtado, A. M. (2000). A theory of human life history evolution: diet, intelligence, and longevity. Evol. Anthropo. 9, 156–185. doi: 10.1002/1520-6505(2000)9:4<156::AID-EVAN5>3.0.CO;2-7
Kirschner, S., and Tomasello, M. (2010). Joint music making promotes prosocial behavior in 4-year-old children. Evol. Hum. Behav. 31, 354–364. doi: 10.1016/j.evolhumbehav.2010.04.004
Kitanishi, K. (1995). Seasonal changes in the subsistence activities and food intake of the aka hunter-gatherers in Northeastern Congo. Afr. Study Monogr. 16, 73–118.
Knight, C., and Lewis, J. (2017). Wild voices. Mimicry, reversal, metaphor and the emergence of language. Curr. Anthrop. 58, 435–453 doi: 10.1086/692905
Konner, M. (2016). “Hunter-gatherer infancy and childhood in the context of human evolution,” in Childhood: Origins, Evolution, and Implications, eds. C. L. Meehan and A. N. Crittenden. Santa Fe, Albuquerque: University of New Mexico Press, School for Advanced Research Press, 123–154.
Koulomzin, M., Beebe, B., Anderson, S., Jaffe, J., Feldstein, S., and Crown, C. (2002). Infant gaze, head, face and self-touch at 4 months differentiate secure vs. avoidant attachment at 1 year: A microanalytic approach. Attach. Human Dev. 4, 3–24. doi: 10.1080/14616730210123120
Laden, G. T. (1992). Ethnoarchaeology and Land Use Ecology of the Efe (Pygmies) of the Ituri Rain Forest, Zaire (PhD thesis). Cambridge: Harvard University.
Lancy, D. F., and Grove, M. A. (2011). Getting noticed: middle childhood in cross-cultural perspective. Hum. Nat. 22, 281–302. doi: 10.1007/s12110-011-9117-5
Levitin, D. J. (2008). The World in Six Songs: How the Musical Brain Created Human Nature. New York, NY: Penguin Random House.
Lewis, J. (2002). Forest Hunter-Gatherers and Their World: a Study of the Mbendjele Yaka Pygmies of Congo-Brazzaville and Their Secular and Religious Activities and Representations. (Dissertation/doctoral thesis). London: University College London.
Lewis, J. (2009). “As well as words: Congo Pygmy hunting, mimicry, and play,” in The Cradle of Language, eds. R. Botha and C. Knight. Oxford: Oxford University Press, 236–256.
Lewis, J. (2013). “A cross-cultural perspective on the significance of music and dance to culture and society,” in Insight from BaYaka Pygmies, ed. M. A. Arbib. Cambridge MA: MIT Press, 45–65.
Lewis, J. (2014). “Pygmy hunter-gatherer egalitarian social organization: the case of the Mbendjele BaYaka,” in Congo Basin Hunter-Gatherers, ed. B. Hewlett, 219–244.
Lewis, J. (2015). Where goods are free but knowledge costs. Hunter Gatherer Res. 1, 1–27. doi: 10.3828/hgr.2015.2
Lewis, J. (2019). “Sharing pleasures to share rare things: hunter-gatherers' dual distribution systems in Africa,” in Lavi, Noa [Book chapter].
Lüdecke, D. (2020). “sjPlot: data visualization for statistics in social science,” in R Package Version 2.8.4. Available online at: https://CRAN.R-project.org/package=sjPlot
Martin, P., and Bateson, P. P. G. (2009). Measuring Behavior: An Introductory Guide. Cambridge: Cambridge University Press.
McCall, J. (1993). Dancing the past: experiencing historical knowledge in Ohafia, Nigeria. Evanston, IL: Program of African Studies, Northwestern University, 8-9.
Meehan, C. L. (2009). Maternal time allocation in two cooperative childrearing societies. Hum. Nat. 20, 375. doi: 10.1007/s12110-009-9076-2
Meehan, C. L., Helfrecht, C., and Quinlan, R. J. (2014). Cooperative breeding and Aka children's nutritional status: is flexibility key? Am. J. Phys. Anthropol. 153:513–525 doi: 10.1002/ajpa.22415
Mehr, S., Krasnow, M., Bryant, G., and Hagen, E. (2021). Toward a productive evolutionary understanding of music. Behav. Brain Sci. 44, E122. doi: 10.1017/S0140525X21000030
Mehr, S. A., Singh, M., Knox, D., Lucas, C., Ketter, D. M., Pickens-Jones, D., et al. (2019). Universality and diversity in human song. Science 366, eaax0868. doi: 10.1126/science.aax0868
Merriam, A. P. (1964). The Anthropology of Music. Evanston, Illinois: Northwestern University Press.
Milne, G. J. (2017). Collecting the sea shanty: British maritime identity and Atlantic musical cultures in the early twentieth century. Int. J. Marit. Hist. 29, 370–386. doi: 10.1177/0843871417693997
Moser, C., Ackerman, J., Dayer, A., Proksch, S., and Smaldino, P. (2021). Why don't cockatoos have war songs? Behav. Brain Sci. 44, E108. doi: 10.1017/S0140525X20001223
Nakagawa, S., Johnson, P. C. D., and Schielzeth, H. (2017). The coefficient of determination r and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J. R. Soc. Interface14:20170213. doi: 10.1098/rsif.2017.0213
Natke, U., Donath, T., and Kalveram, K. (2003). Control of voice fundamental frequency in speaking versus singing. J. Acoust. Soc. Am. 113, 1587–1593. doi: 10.1121/1.1543928
Ng, T.-W. C., and Verkuyten, M. (2010). Intergroup evaluations, group indispensability and prototypicality judgments: a study in Mauritius. Group Process. Intergr. Relat. 13, 621–638 doi: 10.1177/1368430210369345
Nieuwenhuis, R., Grotenhuis, M., and Pelzer, B. (2012). influence.me: tools for detecting influential data in mixed effects models. R J. 4, 38–47. doi: 10.32614/RJ-2012-011
Nishida, T. (1968). The social group of wild chimpanzees in the Mahali Mountains. Primates 9, 167–224. doi: 10.1007/BF01730971
Oloa-Biloa, C. (2017). The Egalitarian Body. A Study of Aesthetic and Emotional Processes in Massana Performances Among the Mbendjele of the Likouala region (Republic of Congo) (Dissertation/doctoral thesis). London: University College London.
Patel, A., and Von Rueden, C. (2021). Where they sing solo: accounting for cross-cultural variation in collective music-making in theories of music evolution. Behav. Brain Sci. 44, E85. doi: 10.1017/S0140525X20001089
Pearce, E., Launay, J., Machin, A., and Dunbar, R. I. (2016). Is group singing special? Health, well-being and social bonds in community-based adult education classes. J. Commu. Appl. Social Psychol. 26, 518–533. doi: 10.1002/casp.2278
Pérez, S. C., and Español, S. A. (2016). Multimodal study of adult-infant interaction: a review of its origins and its current status. Paidéia 26, 377–385. doi: 10.1590/1982-43272665201613
R Core Team (2020). R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing.
Roederer, J. (1984). The search for a survival value of music. Music Percept. 1:350–356. doi: 10.2307/40285265
Rudge, A. (2019). Flexibility and egalitarianism: musical insights from hunter-gatherers. Ethnomusicol. Forum. 28, 163–183. doi: 10.1080/17411912.2019.1683875
Salali, G. D. (2017). “Social structure and knowledge sharing networks in hunter-gatherers,” in A Case Study on the Plant Knowledge of the Mbendjele BaYaka pygmies (Dissertation/doctoral thesis). London: University College London.
Salali, G. D., Chaudhary, N., Bouer, J., Thompson, J., Vinicius, L., and Migliano, A. B. (2019). Development of social learning and play in BaYaka hunter-gatherers of Congo. Sci. Rep. 9, 11080. doi: 10.1038/s41598-019-47515-8
Salali, G. D., and Migliano, A. B. (2015). Future discounting in congo basin hunter-gatherers declines with socio-economic transitions. PLoS ONE 10, e0137806. doi: 10.1371/journal.pone.0137806
Samuni, L., Preis, A., Mielke, A., Deschner, T., Wittig, R. M., and Crockford, C. (2018). Social bonds facilitate cooperative resource sharing in wild chimpanzees. Proc. Biol. Sci. 285, 20181643. doi: 10.1098/rspb.2018.1643
Savage, P., Loui, P., Tarr, B., Schachner, A., Glowacki, L., Mithen, S., et al. (2021). Toward inclusive theories of the evolution of musicality. Behav. Brain Sci. 44, E121. doi: 10.1017/S0140525X21000042
Savage, P. E., Brown, S., Sakai, E., and Currie, T. E. (2015). Statistical universals reveal the structures and functions of human music. Proc. National Acad. Sci. USA 112, 8987–8992. doi: 10.1073/pnas.1414495112
Schamberg, I., Cheney, D. L., Clay, Z., Hohmann, G., and Seyfarth, R. M. (2016). Call combinations, vocal exchanges and interparty movement in wild bonobos. Anim. Behav. 122, 109–116. doi: 10.1016/j.anbehav.2016.10.003
Schielzeth, H. (2010). Simple means to improve the interpretability of regression coefficients. Methods Ecol. Evolut. 1, 103–113. doi: 10.1111/j.2041-210X.2010.00012.x
Schielzeth, H., and Forstmeier, W. (2009). Conclusions beyond support: overconfident estimates in mixed models. Behav. Ecol. 20:416–420. doi: 10.1093/beheco/arn145
Silk, J.B., Beehner, J.C., Bergman, T.J., Crockford, C., Engh, A.L., Moscovice, L.R., et al. (2010). Strong and consistent social bonds enhance the longevity of female baboons. Curr. Biol. 20, 1359–1361. doi: 10.1016/j.cub.2010.05.067
Silk, J. B. (2007). Social components of fitness in primate groups. Science 317, 1347–1351. doi: 10.1126/science.1140734
Thompson, J. (2018). The Social Foraging Niche of the Mbendjele Bayaka (PhD thesis). London: University College London.
Titze, I., and Maxfield, L. (2017). Acoustic factors affecting the dynamic range of a choir. The J. Acoustical Soc. Am. 142, 2464–2468. doi: 10.1121/1.5004569
Trehub, S. (2021). Challenging infant-directed singing as a credible signal of maternal attention. Behav. Brain Sci. 44, E117. doi: 10.1017/S0140525X20001442
Trehub, S. E. (2001). Musical predispositions in infancy. Ann. N. Y. Acad. Sci. 930, 1–16. doi: 10.1111/j.1749-6632.2001.tb05721.x
Trehub, S. E., Becker, J., and Morley, I. (2015). Cross-cultural perspectives on music and musicality. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 370, 20140096. doi: 10.1098/rstb.2014.0096
Tweh, C., Lormie, M., Kouakou, C., Hillers, A., Kühl, H., and Junker, J. (2015). Conservation status of chimpanzees Pan troglodytes verus and other large mammals in Liberia: a nationwide survey. Oryx 49, 710–718. doi: 10.1017/S0030605313001191
Van Vliet, N., Muhindo, J., Nyumu, J. K., and Nasi, R. (2019). From the forest to the dish: a comprehensive study of the wildmeat value chain in yangambi, democratic Republic of Congo. Front. Ecol. Evol. 7, 132. doi: 10.3389/fevo.2019.00132
Veen, J., Jang, H., Raubenheimer, D., van Pinxteren, B., Kandza, V., Meirmans, P., et al. (2023). Development of embodied capital: Diet composition, foraging skills, and botanical knowledge of forager children in the Congo Basin. Front. Ecol. Evolut. 11. 935987. doi: 10.3389/fevo.2023.935987
Velandia, M., Matthisen, A.-S., Uvnäs-Moberg, K., and Nissen, E. (2010). Onset of vocal interaction between parents and newborns in skin-to-skin contact immediately after elective cesarean section. Birth 37, 192–201. doi: 10.1111/j.1523-536X.2010.00406.x
Weinstein, D., Launay, J., Pearce, E., Dunbar, R. I. M., and Stewart, L. (2016). Group music performance causes elevated pain thresholds and social bonding in small and large groups of singers. Evol. Hum. Behav. 37, 152–158. doi: 10.1016/j.evolhumbehav.2015.10.002
Widdess, R. (2012). Music, meaning and culture. Emp. Musicol. Rev. 7. 10.18061/1811/52985. doi: 10.18061/1811/52985
Wilkie, D., and Carpenter, J. (1999). Bushmeat hunting in the Congo Basin: an assessment of impacts and options for mitigation. Biodiv. Conservat. 8, 927–955. doi: 10.1023/A:1008877309871
Zavos, S. (1998). Ka Mate! Ka Mate! New Zealand's Conquest of British Rugby. Auckland, New Zealand: Viking.
Keywords: music, foraging, coalition signaling, less familiar, predation, hunter-gatherer
Citation: Chittar CR, Jang H, Samuni L, Lewis J, Honing H, Loon EEv and Janmaat KRL (2023) Music production and its role in coalition signaling during foraging contexts in a hunter-gatherer society. Front. Psychol. 14:1218394. doi: 10.3389/fpsyg.2023.1218394
Received: 07 May 2023; Accepted: 06 September 2023;
Published: 01 November 2023.
Edited by:
Prof Alan Harvey, University of Western Australia, AustraliaReviewed by:
Robin Dunbar, University of Oxford, United KingdomIan Cross, University of Cambridge, United Kingdom
Copyright © 2023 Chittar, Jang, Samuni, Lewis, Honing, Loon and Janmaat. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chirag Rajendra Chittar, Y2hpcmFnLmNoaXR0YXJAZ21haWwuY29t
†ORCID: Chirag Rajendra Chittar orcid.org/0009-0004-2292-3356
Haneul Jang orcid.org/0000-0001-5994-6413
Liran Samuni orcid.org/0000-0001-7957-6050
Jerome Lewis orcid.org/0000-0001-8629-6321
Henkjan Honing orcid.org/0000-0001-7046-7923
E. Emiel van Loon orcid.org/0000-0002-8895-0427
Karline R. L. Janmaat orcid.org/0000-0003-2533-6881
 Chirag Rajendra Chittar
Chirag Rajendra Chittar Haneul Jang
Haneul Jang Liran Samuni4,5†
Liran Samuni4,5† Jerome Lewis
Jerome Lewis Henkjan Honing
Henkjan Honing E. Emiel van Loon
E. Emiel van Loon Karline R. L. Janmaat
Karline R. L. Janmaat