
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
HYPOTHESIS AND THEORY article
Front. Psychol., 04 October 2023
Sec. Evolutionary Psychology
Volume 14 - 2023 | https://doi.org/10.3389/fpsyg.2023.1215481
This article is part of the Research TopicRising Stars In: Evolutionary Psychology 2022View all 5 articles
Human musicality exhibits the necessary hallmarks for biological adaptations. Evolutionary explanations focus on recurrent adaptive problems that human musicality possibly solved in ancestral environments, such as mate selection and competition, social bonding/cohesion and social grooming, perceptual and motor skill development, conflict reduction, safe time-passing, transgenerational communication, mood regulation and synchronization, and credible signaling of coalition and territorial/predator defense. Although not mutually exclusive, these different hypotheses are still not conceptually integrated nor clearly derived from independent principles. I propose The Nocturnal Evolution of Human Musicality and Performativity Theory in which the night-time is the missing piece of the adaptationist puzzle of human musicality and performing arts. The expansion of nocturnal activities throughout human evolution, which is tied to tree-to-ground sleep transition and habitual use of fire, might help (i) explain the evolution of musicality from independent principles, (ii) explain various seemingly unrelated music features and functions, and (iii) integrate many ancestral adaptive values proposed. The expansion into the nocturnal niche posed recurrent ancestral adaptive challenges/opportunities: lack of luminosity, regrouping to cook before sleep, imminent dangerousness, low temperatures, peak tiredness, and concealment of identity. These crucial night-time features might have selected evening-oriented individuals who were prone to acoustic communication, more alert and imaginative, gregarious, risk-taking and novelty-seeking, prone to anxiety modulation, hedonistic, promiscuous, and disinhibited. Those night-time selected dispositions may have converged and enhanced protomusicality into human musicality by facilitating it to assume many survival- and reproduction-enhancing roles (social cohesion and coordination, signaling of coalitions, territorial defense, antipredatorial defense, knowledge transference, safe passage of time, children lullabies, and sexual selection) that are correspondent to the co-occurring night-time adaptive challenges/opportunities. The nocturnal dynamic may help explain musical features (sound, loudness, repetitiveness, call and response, song, elaboration/virtuosity, and duetting/chorusing). Across vertebrates, acoustic communication mostly occurs in nocturnal species. The eveningness chronotype is common among musicians and composers. Adolescents, who are the most evening-oriented humans, enjoy more music. Contemporary tribal nocturnal activities around the campfire involve eating, singing/dancing, storytelling, and rituals. I discuss the nocturnal integration of musicality’s many roles and conclude that musicality is probably a multifunctional mental adaptation that evolved along with the night-time adaptive landscape.
For the last 30 years, evolutionary psychology (EP) has advanced the comprehension of the evolved nature of human cognition (Schmitt and Pilcher, 2004; Tooby, 2020; Nettle and Scott-Phillips, 2023). However, its evolved temporal dynamicity (i.e., the daily and seasonal timings of cognitive functioning) has received little attention. The same happens in the literature on animal ornaments and primate vocalization (Hau et al., 2017; Piel, 2018). Most temporal concern in EP focuses on life-history development (Webster et al., 2014; Figueredo et al., 2021) or the menstrual cycle (Gildersleeve et al., 2014). Nevertheless, a comprehensive ‘Evolutionary Chronopsychology’ is needed, integrating EP and chronopsychology (Putilov, 2021), in which the seasonal, monthly, and circadian rhythms are also considered as evolved temporal signatures of mental adaptations integrated into the underlying chronobiological patterns of neural and endocrine systems. Early ethologists knew that “Behavior must occur not only in the right place and with the right orientation but also at the right moment. Quite generally, releasing mechanisms, whether innate or acquired, are programmed so as to set off adequate behavior at the teleonomically correct time” (Lorenz, 1981, p. 231).
There is heuristic value in considering the evolved temporal dimension of cognition. Jonason et al. (2013) predicted and found that individuals with high dark-triad personality (Machiavellianism, psychopathy, and narcissism) are evening-oriented because the nocturnal context favors the expression of such self-centered/antisocial tendencies. Their cognitive niche hypothesis has been corroborated (Akram et al., 2019; Porfírio and Varella, 2022). Similarly, Varella et al. (2021) predicted that evening-oriented individuals would be less compliant with the COVID-19 pandemic safety guidelines based on evolutionary consideration of some recurrent nocturnal challenges/opportunities (dangerousness, peak tiredness, and concealment of identity) and how it might have amplified risk-taking and rule-breaking tendencies in evening-oriented individuals. Their eveningness epidemiological liability hypothesis has been corroborated (Li, 2022). In general, phenotypic correlations between psychological traits show significant and substantial genetic mediation and the individual variation of psychological traits that have been studied shows significant and substantial genetic influence (Polderman et al., 2015; Plomin et al., 2016); thus, the genetic basis necessary for those evolutionary propositions is plausible.
Here, I apply the evolutionary perspective to the temporal dimension of human musicality, connecting evolutionary musicology (Wallin, 1991; Brown et al., 2000; Fitch, 2015) with evolutionary chronopsychology. The heuristic value of this endeavor might help advance and integrate the evolutionary debate concerning musicality. First, I outline definitions and the confluence of evidence indicating the evolved and multifunctional nature of human musicality. Then, I present the ancestral adaptive explanations for musicality stressing that they are not mutually exclusive, conceptually integrated, or clearly derived from independent principles. I fill this gap by proposing that the night-time selective landscape is the missing piece of the adaptationist puzzle of human musicality.
I put forward the Nocturnal Evolution of Human Musicality and Performativity Theory (NEHMPT). I provide evidence for the evolved expansion of human nocturnal activities and argue that the recurrent night-time ancestral adaptive challenges/opportunities (i.e., lack of luminosity, low temperatures, regroup to cook before sleep, dangerousness, peak tiredness, and easy concealment of identity) might have molded human musicality from ape-like protomusicality, in relation to the concomitant nocturnal ecosocial contexts. I present and discuss the nocturnal signatures of musicality. I hope to (i) explain why humans are more musical than other great apes, (ii) explain the evolution of musicality from independent principles, (iii) explain and interrelate the various seemingly unconnected features/functions of musicality, and (iv) integrate different ancestral adaptive values proposed (knowledge transference, social cohesion, signaling of coalitions, territorial/antipredatorial defense, safe passage of time, children lullaby, and sexual selection). I expand on previous attempts to relate the night-time with the evolution of musicality (Jordania, 2011; Putilov, 2014; Nowell, 2018; Varella and Valentova, 2023) and on previous attempts to synthesize existing adaptive hypotheses into a comprehensive evolutionary framework for human musicality (Savage P. et al., 2021).
Musicality has many meanings, such as a potential/propensity/predisposition, a universal capacity/ability, or an individual difference aptitude/talent for music engagement (Hallam, 2006). It can be understood as the species-specific propensity (i.e., genotypic or “potential” musicality) to ontogenetically develop an integrated set of broad musically related psychological capacities (phenotypic or “operational” musicality), including general capacities (e.g., auditory scene analysis, creativity, play, emotional recognition, and language), and the narrow musically specific melodic/harmonic and rhythmic cognition, subdivided into cognitive capacities for musical production (creation and imitation), musical discrimination, appreciation and memorization, and musical motivations (cf. Peretz and Coltheart, 2003; Jackendoff and Lerdahl, 2006; Bispham, 2009; Honing et al., 2015a; Leongómez et al., 2022). Music, as the sociocultural product of musical behavior and evocated/enabled by musical capacities/propensities, can be defined as mostly acoustic communication that intentionally uses variations in pitch, contour, rhythm, tempo, timbre, and volume in a combined and organized way according to an internal generative grammar forming harmonized and rhythmic melodies exploring repetition, modifications and expectation through the use of voice, body, and/or instruments leading to intense and shared esthetic and emotional reactions (cf. Jackendoff and Lerdahl, 2006; Peretz, 2006; Mithen, 2009; Honing et al., 2015a; Mehr et al., 2021; Savage P. E. et al., 2021). Music is a costly activity of sound-focused elaboration that produces an extraordinary and esthetically crafted sonic temporal sequence and engaging performance that attracts attention and inspires engagement and esthetic evaluations aligned with emotional and interpersonal reactions (Dissanayake, 2008; Varella et al., 2011, 2012, 2017). Beyond sound, other multimodal factors comprise musical activities, such as the visual self-presentation and movements of those musically engaged, including dancing, and the related linguistic and socio-historic aspects of music (Miller, 2000; Cross and Morley, 2002, 2008; Dissanayake, 2008; Fink et al., 2021).
Figure 1 organizes the evolution and development and represents the relationship between musical propensity, capacity, behavior, and product.
Evolutionary musicology approaches human musicality phylogenetically and (non)adaptively (Wallin, 1991; Brown et al., 2000; Fitch, 2015). For evolutionary psychological purposes, the focus is on the operational cognitive traits and their corresponding genotypic propensity, not so much on manifested behavior or its products (Honing and Ploeger, 2012; Varella et al., 2013, 2017). Some authors consider musical behavior as a recent by-product of mostly preexisting general (not music-specific) cognitive capacities that evolved because of other-than-musical reasons (Spencer, 1875; Pinker, 1997; Patel, 2018). In fact, musical activities recruit many general capacities (e.g., attention, creativity), but there are also many music specificities (Peretz and Coltheart, 2003; Samson et al., 2009; Zatorre, 2015) that require their own conjoint evolutionary explanations.
Moreover, the individual variation in human musicality per se has a considerable genetic component: between 44 and 90% of the individual variation on musical ability is inherited (Coon and Carey, 1989), between 71 and 80% of the individual variation on pitch discrimination is inherited (Drayna et al., 2001), between 38 and 51% of the individual variation on musical aptitude, and between 9 and 57% of music achievement is inherited (Mosing et al., 2015). As for musical ability, the individual variance in musical practice also is substantially heritable (40–70%), and the association between both practice and ability is predominantly genetic (Mosing et al., 2014). Furthermore, a genome-wide association study has identified 69 genetic variants that influence variation in self-reported beat synchronization ability (Niarchou et al., 2022). Some musically related genetic variations are in chromosomes 4, 8, 12, and 17 (Tan et al., 2014). Importantly, the polygenic score for rhythmicity predicts melodic capacities and music-related outcomes but does not predict traits that are not directly related to music (Wesseldijk et al., 2022), suggesting that there is specificity and inherited individual variation which are necessary for there is selection to operate (Croston et al., 2015).
Furthermore, a nomological network of converging empirical evidence (Schmitt and Pilcher, 2004) has been assembled strongly indicating that human musicality exhibits the necessary hallmarks for biological adaptations (see Table 1). Human musicality has homologous precursors, adaptive convergence with songs of other species, antiquity, universality (across history, cultures, and individuals), heritability, precocity, standard ontogeny, and intrinsic motivation; it conveys pleasure and emotion, and exhibits complex cognitive modularity and neurological organization; it is beneficial to health, sexual, and psychological domains; and it is associated with social roles and cultural importance (Miller, 2000; Huron, 2001; Cross and Morley, 2002, 2008; Peretz, 2006; Dissanayake, 2008; Varella et al., 2010; Honing and Ploeger, 2012; Mehr et al., 2021). Overall, the full account of human musicality requires consideration of primordial acoustic/sonic capacities (precursors), ancient music-specific secondary adaptations, and recent cooptation of non-musical capacities within cultural dynamics (Varella et al., 2012; Trainor, 2021).
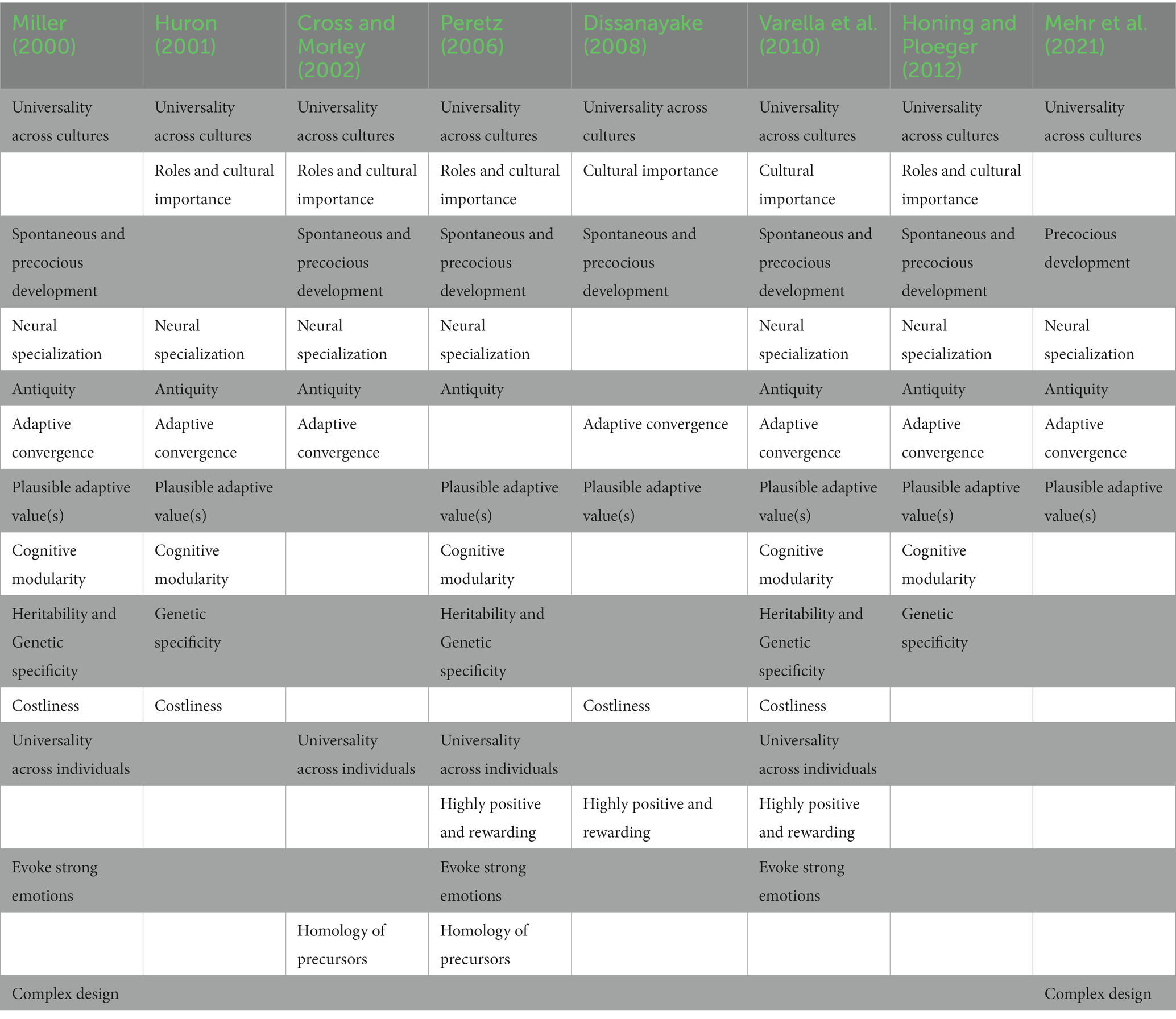
Table 1. Compilation of the 15 types of cross-disciplinary pieces of evidence pointing to the probable evolved status of music propensities and capacities.
The inquiry about the evolution of musicality is legitimate and remains open to debate and investigation (Kalinowski et al., 2021; Mehr et al., 2021; Savage P. et al., 2021; Savage P. E. et al., 2021). Since Darwin and Spencer initially discussed it (Kleinman, 2015), evolutionary theorizations about musicality have improved and diversified. Many empirical articles (e.g., Hagen and Bryant, 2003; Varella et al., 2010, 2022; Pearce et al., 2016), review articles (e.g., Hauser and McDermott, 2003; Fitch, 2006; Snowdon et al., 2015; Killin, 2018; Fink et al., 2021), books (e.g., Wallin et al., 2000; Mithen, 2005; Ball, 2010; Morley, 2013; Honing, 2018), and special issues (Peretz, 2006; Honing et al., 2015b; Nikolsky and Perlovsky, 2020) have been published, including guiding principles to improve/advance the field (Honing and Ploeger, 2012; Fitch, 2015; Merker et al., 2015; Currie and Killin, 2016).
The proposed evolutionary hypotheses are focused on different recurrent adaptive problems that human (proto)musicality possibly solved on ancestral environments: mate attraction/bonding and intrasexual competition (e.g., Darwin, 1871; Zahavi and Zahavi, 1999; Miller, 2000; Varella et al., 2017, 2022), group cohesion and social bonding (e.g., Brown, 2000; Dissanayake, 2008; Launay et al., 2016; Savage P. et al., 2021), mother–infant bonding (e.g., Trehub, 2003; Dissanayake, 2008; Mehr et al., 2021; Savage P. et al., 2021), mood/emotional induction/regulation and synchronization (Eibl-Eibesfeldt, 1989; Snowdon et al., 2015), coalitional signaling (Hagen and Bryant, 2003; Mehr et al., 2021), territorial/predator defense (Hagen and Hammerstein, 2009; Jordania, 2011; Hagen, 2022), group effort, perceptual and motor skill development, safe time-passing, transgenerational communication (Huron, 2001), conflict reduction, and social tolerance (Huron, 2001; Fukui and Toyoshima, 2023). Twenty years ago, there was a ‘lack of empirical evidence’ and ‘little basis’ on which to distinguish among the various evolutionary hypotheses about human musicality (Hauser and McDermott, 2003). Despite the empirical advances (e.g., Loersch and Arbuckle, 2013; Marin and Rathgeber, 2022), it is still ‘premature’ and ‘unjustified’ to rule out alternative hypotheses (Bowling et al., 2021). More empirical testing is required, and there is a still need for theoretical development.
Different accounts for the evolution of musicality rely on one or a few major factors influencing the hominin evolution (i.e., since the Pan and human lineage separated 6–8 Ma [Megaanni; million years] ago, Langergraber et al., 2012). Miller (2000) stressed the increase in brain size and monogamy; Merker (1999, 2021), the necessity of attracting exogamously migrating females; Dissanayake (2008), Mehr et al. (2021) and Leongómez et al. (2022), the increase in immaturity and dependence of human offspring; Launay et al. (2016), Fink et al. (2021), and Savage P. et al. (2021), the increase in group size and a ‘bonding gap’ in human sociality; Jordania (2011) and Hagen (2022) emphasized the increase in predation risk and intergroup conflict; and Benítez-Burraco and Nikolsky (2023) focused on self-domestication. Because those factors were recurrent and ancient enough during hominin evolution, their effects are likely complementary. This makes most of the evolutionary explanations for human musicality non-exclusive and mutually compatible (Menninghaus, 2019; De Tiège et al., 2021).
As human musicality is closely related to acoustic communications in other animals (Miller, 2000; Hauser and McDermott, 2003; Fitch, 2006; Snowdon et al., 2015; Honing, 2018), it might also serve the adaptive functions found in other animals’ signals, i.e., courtship displays and mate-bonding, ritualized combat, territorial defense/advertisement, coalitional displays, social bonding, and group-cohesion signaling (De Tiège et al., 2021). Furthermore, human musicality plays many important roles not restricted to signaling, indicating it might have accumulated concomitant adaptive values. The functioning of human musicality delivers (1) health, (2) psychological, (3) sexual, and (4) social benefits: (1) Music listening alleviates pain, diminishes stress and anxiety, reduces blood glucose, boosts immunity, and increases the quality of post-operatory, pregnancy, childbirth, and the health of the newborn baby (North and Hargreaves, 2008; Finn and Fancourt, 2018; McCaffrey et al., 2020); (2) listening to music makes people more positive, more alert, and focused on the present (Sloboda et al., 2001), and it also counteracts insomnia, improves sleep onset, latency, and quality (Feng et al., 2018). Despite that individuals who self-select musical training tend to have better cognitive and academic performance at baseline, musical training has a small but positive impact on cognitive skills and academic achievement (Román-Caballero et al., 2022). Musical training seems to enhance verbal intelligence and executive function (Schellenberg, 2004, 2011; Moreno et al., 2011), phonological awareness (Gordon et al., 2015), verbal working and long-term memories (Ho et al., 2003; Franklin et al., 2008), language development, literacy, numeracy, intelligence, general attainment, creativity, fine motor coordination, concentration, self-confidence, emotional sensitivity, social skills, teamwork, self-discipline, and relaxation (Hallam, 2010); (3) listening to music makes women find men more attractive and increases men’s and women’s desire to date other-sex target individuals (May and Hamilton, 1980; Marin et al., 2017; Chang et al., 2021; Marin and Rathgeber, 2022). The ability to sing, play a musical instrument, or dance is considered attractive in a mate (Weege et al., 2012; Charlton, 2014; Wade et al., 2015; Röder et al., 2016; Bongard et al., 2019; Valentova et al., 2019). Dancing with someone is perceived as an effective flirting tactic (Wade et al., 2021; Kennair et al., 2022). Music is sometimes present in individuals’ sexual fantasies (Lehmiller, 2018), it enhances feelings of sexual arousal when paired with a consensual sex story (Wan and Lalumière, 2017), and it has been effectively used in ‘sex playlists’ during sexual activities (Van Bohemen et al., 2018) and in couple/sexual therapies (Duba and Roseman, 2012; Micoogullari et al., 2021); (4) music functions as a way to express emotions, get esthetic enjoyment, entertain, communicate, represent symbolically, manage self-identity/self-awareness/mood/interpersonal relationships, coordinate action, enforce social norms, validate social institutions and rituals, contribute to continuity/stability of culture, and contribute to integration of society (Merriam, 1964; Hargreaves and North, 1999; Schäfer et al., 2013; Clayton, 2016). Thus, far from being useless, musicality offers many evolutionarily relevant benefits.
The different evolutionary hypotheses for musicality and its multifunctional nature are still not conceptually integrated. De Tiège et al. (2021) offer an animal signaling theory as a unifying framework for the multifunctional communicative nature of human musicality and artisticality (cf. Zahavi and Zahavi, 1999; Miller, 2000; Varella et al., 2011; Fink et al., 2021). More restricted to musicality and to mother–offspring and intergroup interactions, Mehr et al. (2021) have also employed signaling theory to propose an account that human musicality evolved as a credible signaling device indicating parental attention and coalitional quality. Savage P. et al. (2021) proposed an overarching social bonding function of musicality connecting mother–offspring bonding, romantic pair bonding, and in-group bonding. Others have explained the multiple functions of musicality as a fortuitous sequence of co-optations/exaptations to perform new functions followed by new selection (e.g., Trainor, 2015; Garfinkel, 2018; Dubourg et al., 2021).
I integrate these models by arguing that human musicality is multifunctional because the need and situation for accomplishing most of those functions were recurrently co-occurring during night-time.
The explanations for the design features and functions of musicality should rely on processes/regularities external to music cognition per se. The musical capacities and functions should be derived from independent principles (Pinker, 2005), the same way the designs and functions of the human eye and the human cochlea correspondent to the optics and acoustic principles, respectively (cf. Pinker, 1997, 2005; Trainor, 2015).
Pinker (2005) argued that the proposed adaptive functions for musicality (e.g., group cohesion) are circular and consequently not proper explanations because they do not rely on a set of principles independent of the part of our psychology being explained. According to him, such an explanation only begs the question of why harmonically and rhythmically organized sound sequences should in principle foster group cohesion. “Generating and sensing sequences of sounds is not an independently motivated solution to the problem of maintaining group solidarity, in the way that, say, the emotion of empathy, or a motive to punish free riders, is part of such a solution” (p. xv).
Here, I suggest that the night-time features (i.e., lack of luminosity, low temperatures, regrouping to cook before sleep, dangerousness, peak tiredness, and concealment of identity) are the independent principles necessary to a new comprehensive evolutionary understanding of human musicality.
The common ancestor of all primates was likely nocturnal, and apes are considered as cathemeral (i.e., activity not restricted to a particular time of day or night) (Wu et al., 2017). Male Orangutans in captivity perform premeditated nocturnal long calls (Samson et al., 2014). Wild Chimpanzees exhibit episodes of rain-dancing, pant-hoot chorusing, or “carnival displays” that can last from hours to the whole night (Dufour et al., 2015; Merker et al., 2015). During night-time, Chimpanzees can play (Tagg et al., 2018), feed, vocalize, travel, and mate (Anderson et al., 2019), taking 1.8% of their awake activity (Tagg et al., 2018). Interestingly, at night-time, Chimpanzees perform twice as many long-distance vocalizations as social proximity calls (Pruetz, 2018). Every night, and at a rate of almost twice an hour, Chimpanzees perform such long-distance communication maintaining contact with those traveling nearby (Zamma, 2014; Pruetz, 2018). This indicates a tendency for loud nocturnal vocalizations in Chimpanzees.
Importantly, there is a spontaneous inclination toward some level of rhythmic synchronization and entrainment in chimpanzees (Hattori et al., 2013) and bonobos (Large and Gray, 2015). Spontaneous drumming is also found in Chimpanzees (Arcadi et al., 1998; Dufour et al., 2015). Finally, Chimpanzees prefer consonant music (Sugimoto et al., 2009) and African and Indian traditional music over silence (Mingle et al., 2014); human instrumental music seems to increase affiliative behavior in captive Chimpanzees, while vocal music seems to decrease their agonistic behavior (Videan et al., 2007). Thus, the common ancestor between the pan and the hominin lineage (6–8 Ma ago, Langergraber et al., 2012) likely exhibited a tendency toward nocturnal vocalizations in this much older basic protomusicality, which was later ‘upgraded’ in hominin ancestors during Pleistocene.
Primate lineages rapidly adapt their circadian pattern to diurnal or nocturnal niches (Ankel-Simons and Rasmussen, 2008). During hot days and maize season, Chimpanzees expand their nocturnal activities after twilight feeding from the unguarded crops (Lacroux et al., 2022). This general ape-like flexible and occasional evening-oriented behavior could have enabled ancestral hominins’ adaptability (Tagg et al., 2018). Furthermore, ancient humans were selected for the nocturnal niche beyond the basic ape-like evening orientation (Coolidge and Wynn, 2006).
Humans have a uniquely evolved sleep pattern, suggesting an ancestral selective pressure to rapidly accomplish sleep necessities (Nunn and Samson, 2018). Human sleep is shorter, deeper, and without much movement and exhibits more occasions of REM sleep making it more efficient (improving memory consolidation and insightful problem solving while dreaming) and freeing the evening for socialization (Coolidge and Wynn, 2006; Samson and Nunn, 2015; Nunn et al., 2016; Nunn and Samson, 2018). Based on variation across primates, humans should sleep 9.55 h but actually sleep 7 h on average worldwide, making us a phylogenetic outlier (Nunn and Samson, 2018). Given that in Central Africa, the sun sets approximately at 18:00 and rises at 6:00, humans waking up at sunrise, even without a noon nap, could have had some 5 h awake after sunset (30% of the awake time). The tree-to-ground sleep transition (Coolidge and Wynn, 2006; Samson and Nunn, 2015; Fruth et al., 2018), predation risk (Samson, 2021), and use of fire (Coolidge and Wynn, 2006; Samson and Nunn, 2015; Fruth et al., 2018; Killin, 2018) represent significant evolutionary factors concerning the expansion of human evening activities setting the stage for unique subsequent evolutionary changes.
Early Homo erectus might have opportunistically and sporadically made use of fire as early as 1.6 Ma ago (Roebroeks and Villa, 2011; Gowlett, 2016). This is concomitant to the increased out-of-Africa migration toward colder locations (Gowlett, 2006; Brown et al., 2009), the appearance of the more developed Acheulean technology used in the butchery of fauna (Diez-Martín et al., 2015), and to the origin of digestive adaptations for cooked food, such as gracile teeth and jaw (Wrangham, 2017). During the early opportunistic phase of fire use, ancestral hominins were adapting to the progressively dry and fire-prone environments (Parker et al., 2016) and were already cooking fish 780 ka (i.e., kiloanni; thousand years) ago (Zohar et al., 2022). Wildfires tend to scare, confuse, and kill prey and predators, opening predator-free scavenging/hunting grounds (Herzog et al., 2020).
Despite the ongoing debate, this initial timeline of fire use is also considered to be aligned with the tree-to-ground nesting transition by the fully bipedal early Homo erectus (Coolidge and Wynn, 2006; Wrangham, 2009; Samson and Nunn, 2015; Fruth et al., 2018). Nesting on the ground eliminates the danger of eventually falling out of the tree; the ground makes for a more stable nest enabling a more uninterrupted and efficient sleep, so much so that nesting on the ground or in lower branches happens sometimes in Chimpanzees when there is less predation risk (Hernandez-Aguilar et al., 2013; Fruth et al., 2018; Pozzi et al., 2022). Thus, sleeping hidden in caves, probably protected by sentinels (Samson and Nunn, 2015; Samson et al., 2017) capable of stone throwing (which appeared 2 Ma ago, Lombardo and Deaner, 2018) and by sporadic use of fire arguably, could have favored the tree-to-ground nesting in Homo erectus speeding sleep efficiency freeing nocturnal awake time. The initial phase of sporadic use of fire was followed by a transitory phase that culminated more than a million years later in habitual fire use (Gowlett, 2016), dating 400–300 ka ago in Neanderthals and slightly later in early Homo sapiens (Rolland, 2004; Roebroeks and Villa, 2011; Scott and Hosfield, 2021). So far, the earliest fossil of Homo sapiens dates 315 ka (Hublin et al., 2017), this period is considered to be the boundary of a new phase in the evolution of Homo sapiens (Rolland, 2004; Bergström et al., 2021), and it is likely when the incremental evolution of protomusicality occurred (Killin, 2018).
The Homo sapiens is a pyrophilic primate (Parker et al., 2016). The human use of fire is very ancient and universal, and despite its dangerousness, children across cultures have an intrinsic curiosity about fire and achieve mastery of fire by middle childhood with little instruction (Fessler, 2006). This suggests that humans might have evolved learning mechanisms focused on controlling/maintaining and starting fire (Fessler, 2006). Paleolithic humans 170–150 ka ago optimally located the cave fireplace enabling maximum fire use and minimum exposure to smoke (Kedar et al., 2022). Fire provided our ancestors with heat, light, protection against predators and hostile conspecifics, smoke repellent of insects, aid in hunting and in preparing tasty, sterilized, preserved, and easily digestible cooked food, cremation of the dead, even improving flaking properties of stones, and aiding other tool manufacturing (Rolland, 2004; Coolidge and Wynn, 2006; Fessler, 2006; Brown et al., 2009; Wrangham, 2009; Dunbar and Gowlett, 2014). These benefits of habitual fire use set the stage for cognitive (Twomey, 2013), socioecological (Rolland, 2004; Dunbar and Gowlett, 2014), and cultural (Mithen, 2019) evolutionary change.
Therefore, the tree-to-ground nest transition, predation, and fire were recurrently enough to have converged and acted as selective pressures (Rolland, 2004; Coolidge and Wynn, 2006; Roebroeks and Villa, 2011; Twomey, 2013; Dunbar and Gowlett, 2014; Gowlett, 2016; Wrangham, 2017; Fruth et al., 2018; Samson, 2021; Hagen, 2022), expanding the ape-like flexible nocturnal niche in humans (Samson and Nunn, 2015; Tagg et al., 2018). The derived/efficient human sleep has allowed more nocturnal activities and higher exposure to nocturnal selective pressure (Samson and Nunn, 2015; Nunn et al., 2016; Nunn and Samson, 2018), which, I argue, could lead the ape-like protomusicality (including long-distance nocturnal vocalizations) to be refined into human musicality.
Adaptive problems can impose “must-solve” challenges to survival and reproduction or offer beneficial opportunities to increase inclusive fitness (Lewis et al., 2017). The strength of each adaptive problem depends on its frequency and the magnitude of its impact on fitness (Lewis et al., 2017), as well as on its duration and how enduring it was throughout human evolution. Night-time happens every day (i.e., frequently and consistently), lasts roughly 12 h per day (i.e., long-lasting), and humans have been expanding into the nocturnal niche at least since the habitual use of fire (400–300 ka ago) (i.e., ancient/long-standing). The night-time exhibits a predictable selective landscape consisting of a collection of adaptive problems of varying magnitudes (e.g., predation and thermoregulation) (Fruth et al., 2018; Pozzi et al., 2022). Expanding on Varella et al. (2021) and Varella and Valentova (2023) and others (e.g., Putilov, 2014; Killin, 2017; Nowell, 2018), I propose that the six crucial specific and recurrent ancient adaptive challenges/opportunities faced by early hominins during awakening night-time were lack of luminosity, low temperatures, regrouping to cook before sleep, dangerousness, peak tiredness, and concealment of identity.
Those nocturnal adaptive challenges/opportunities could have, in combination, generated adaptive responses reusing, modifying, and interconnecting the preexisting variation in psychological traits, including protomusicality, that were reasonably suitable to solve each task. As a probable signature of the nocturnal selection, those improved preexisting psychological capacities might have coevolved and be phenotypically associated with the eveningness chronotype. The circadian rhythm of an individual’s psychophysiological well-functioning and intrinsic timing preferences for awakening and sleeping is conceptualized as the chronotype continuum and governed by internal clocks open to environmental cues (Vink et al., 2001; Preckel et al., 2011; Jones et al., 2019). Morning-oriented individuals wake up and go to sleep early while evening-oriented individuals wake up and go to sleep late (Vink et al., 2001; Preckel et al., 2011; Jones et al., 2019).
Low luminosity impairs vision, creating a selection favoring hearing and night vision, leading to increased acoustic communication (Yoshida and Okanoya, 2005). Ontogenetically, initial auditory capacities could be improved following many night-time experiences. A comparable dynamic occurs in a compensatory way in early blind individuals, given that they have better spatial abilities (Collignon et al., 2006), auditory memory (Röder et al., 2001), and emotional activation to auditory stimuli (Klinge et al., 2010) and rely more on acoustic perception to evaluate a potential romantic/sexual partner (Sorokowska et al., 2018) than sighted counterparts. These auditory expansions throughout ontogeny were probably further intensified while living in dark caves. Interestingly, parietal paleoart is habitually found in places of caves, cliffs, and canyons with higher acoustic resonance, echoes, and reverberation (Waller, 2002; Reznikoff, 2008), indicating that ancient humans in the dark were very focused on sounds and gathering around places with exquisite acoustics.
Phylogenetically, a recent investigation including 1,800 tetrapod species showed that the origin of acoustic communication is strongly associated with a nocturnal lifestyle (Chen and Wiens, 2020). Living longer under low luminosity likely selects for higher acoustic communicative abilities, so that various nocturnal species independently evolved similar increased acoustic communication (Chen and Wiens, 2020). This adaptive convergence gives a strong indication that the improvement upon the ape-like protomusicality into the full human musicality (and even human language) likely occurred under nocturnal socioecological pressures.
Acoustic communication is flexible, fast, and long-ranged, allows sending complex and long messages, and overcomes obstacles. However, it is costly because it has a high degradation rate, is subject to interference, attenuation, and distortion with distance, and gives off the localization of the emitter allowing interception by natural enemies (Grier and Burk, 1992; Römer, 2001). The high diversity of species exhibiting nocturnal acoustic communication (Chen and Wiens, 2020) results in a noisier nocturnal environment (Römer, 2001). There are many strategies used across animal species to increase the signal-to-noise ratio (i.e., to avoid the background noise creating sound interference and distortion). The increases in signal amplitude and repetition are important and disseminated strategies across species to boost signal-to-noise ratio (Römer, 2001). Interestingly, humans are among the loudest terrestrial animals (Jakobsen et al., 2021). Compared to speaking, singing is louder and requires more lung capacity and more muscle activity (Leanderson et al., 1987; Åkerlund and Gramming, 1994; Lindblom and Sundberg, 2014; Savage et al., 2015), and music allows much more repetition of motifs/phrases, song sections, and entire songs/repertoire (Fitch, 2006; Savage et al., 2015). The consequent high accepting/liking of repetition in music (Madison and Schiölde, 2017) might have, along with other attributes (Levitin, 2021), made music a good mnemonic device (Schulkind, 2009; Reagh et al., 2017), explaining the cross-cultural existence of knowledge songs (Levitin, 2008, 2021). A similar mnemonic effect is found in the repeating patterns of whale song (Guinee and Payne, 1988).
After sunset, there is a period of temperature inversion when the ground gets cold faster than the air above trapping the sound energy in a sequence of refraction downward and reflection upwards creating a ‘sound duct’, a window of opportunity for optimal long-distance sound transmission (Larom et al., 1997; Römer, 2001; Piel, 2018). For instance, the calling range of savanna elephants increases up to 3-fold in the 2 h between late afternoon and early evening in dry and low wind conditions (Larom et al., 1997). Chimpanzees’ twilight calls are more frequent in dry seasons than in wet seasons (Piel, 2018), and at night, they perform long-distance vocalizations twice more often than proximity calls (Pruetz, 2018). The evening optimal transmission period for long-distance communication has probably been a selective pressure on many species in the African Savanna, including the lions, which mostly vocalize during the evening/night, and many others in savanna-like ecosystems such as coyotes and wolves which are also crepuscular vocalizers (Larom et al., 1997). Even diurnal animals, such as most birds and insects, tend to concentrate their acoustic communication during the times of temperature inversion near sunrise and sunset, generating the dawn and dusk choruses (Römer, 2001). Thus, due to the early evening cold ground creating favorable conditions for loud long-distance calls (Larom et al., 1997), early hominins could have been selected to make use of this acoustic opportunity to increase their nocturnal long-distance communication, expanding upon the ape-like correspondent precursors. Furthermore, the warm air/smoke from the campfire could, in principle, help maintain, for a longer time the temperature inversion favoring long-call communication.
Ancient hominins increased their nocturnal vocal activities, but night-time creates the challenge of avoiding hypothermia. The use of fire and the use of clothing are important strategies for thermoregulation. However, the habitual use of fire only started approximately 400–300 ka ago (Rolland, 2004; Roebroeks and Villa, 2011; Scott and Hosfield, 2021), and the earliest indication of clothing dates only 170–120 ka ago (Toups et al., 2011; Hallett et al., 2021). Thus, before habitual use of fire and clothing, during awake nocturnal activities, early hominins needed to rely on maintaining enduring physical activity and increase close contact with conspecifics to cope with the cold night-time, as a social thermoregulation (IJzerman et al., 2015). Arguably, like the penguins, early hominins could have performed social thermoregulation by maintaining group cohesion (IJzerman et al., 2015), doing useful nocturnal social activities in close proximity, including vocalizations and movements such as proto-dances.
In the dark, it is unpractical/difficult for humans to forage (cf. Nowell, 2018). Moreover, humans, as animals, need to rest and sleep. Thus, after fission into subgroups for daily foraging comes the evening multi-male multi-female fusion (Foley and Gamble, 2009). Around the sunset is the time for foraging subgroups to reunite to the safety of the collective sleeping site. This place of regrouping bears similarities with the great ape night-time land use: a well-chosen place near resources such as water or fruiting trees, and at a period of increased sociality and group cohesion (Anderson et al., 2019). The optimal long-distance sound transmission (Larom et al., 1997; Römer, 2001; Piel, 2018) could have been used to call group members to the nearest sleeping site, because, in Chimpanzees, long-distance calls facilitate fusion (Fedurek et al., 2014). This might be one reason for the existence of responsorial singing (i.e., call and response) in traditional music (Kubik, 2005; Brown, 2017).
Before the habitual use of fire, the place of regrouping of early homo (2 Ma ago) was probably a “core area”: A preferred, but not fixed, night-time place containing natural protective topographies (caves and hills) combined with artificially made protective barriers in an ecotone habitat (overlapping grasslands and woodlands) where a small extended family congregated to fulfill subsistence activities, socialize, and, in a more reserved part far from eating location, prepare the ground nests (Rolland, 2004; Carotenuto et al., 2016). The core area could have been protected by stone-throwing sentinels (Samson et al., 2017; Lombardo and Deaner, 2018). Homo erectus were skilled tool makers, gatherers, scavengers, and carnivorous, fully biped with increased mobility and dispersion pattern, so the core areas could have been distantly scattered along increased home ranges (Rolland, 2004; Foley and Gamble, 2009). Homo erectus were able to expand from Africa to Eurasia and Southeast Asia following large herbivores, courses of water, and avoiding predators (Carotenuto et al., 2016). The increased home ranges certainly augmented the difficulty of males to find and monopolize resources (which were dispersed, and prey are very mobile) and mates (who were dispersed, very mobile, and had kin in defense), which put them in a socioecological context with key elements (including the ‘sound duct’ of optimal long-distance transmission) of three-dimensional niches (water and air), favoring the evolution of elaborated acoustic displays via sexual selection (cf. Puts, 2010; Varella et al., 2017; Verpooten and Eens, 2021).
Moreover, given that Homo erectus were also less sexually dimorphic, and due to increased infant dependence, they were probably more (serial) monogamously oriented exhibiting biparental provisioning/care (Fisher, 1989; Eastwick, 2009) and grandmother provisioning/care (O’Connell et al., 1999; Bell et al., 2013). Indeed, social monogamy and small family-sized groups are consistent predictors of complex song in vocal communication across primates (Haimoff, 1986; Geissmann, 2000; Schruth et al., 2021), and the majority of duetting primates are nocturnal or live across great distances in visually unreachable environments (cf. Geissmann, 2000; Clink et al., 2020; Pozzi et al., 2022). Duetting songbirds also tend to be monogamous (Geissmann, 2000). The evolution of elaborated songs across species, such as baleen whales, seals, songbirds, and hummingbirds, is also related to small, less complex social groups interacting over large distances beyond visual reach (Verpooten, 2021; Verpooten and Eens, 2021; Vanderhoff and Bernal, 2022). Thus, it is possible that, before the habitual use of fire and its consequent increase in social complexity (Foley and Gamble, 2009), early Homo was already prone to singing (cf. Mithen, 2005; Savage P. E. et al., 2021) to defend/advertise the night-time territory and to attract/maintain mates (cf. Hagen and Bryant, 2003) as a result of the ongoing amplification of nocturnal long-distance elaborated vocalizations (cf. Geissmann, 2000).
After the habitual use of fire, ancient hominins most probably had a “home base,” which is a more fixed base for both day and night operations, near valuable resources and strategic ecotone settings, with marked multipurpose fire use, cooking and social eating, protection of juvenile, transmission of knowledge, and a more patterned spatial organization (Rolland, 2004; Foley and Gamble, 2009). In current hunter-gatherer societies, the overnight camp has 35–70 individuals (Gowlett et al., 2012). After day-time fission foraging in transient sites, ancient hominins needed to find the way back to their home base, to process, cook, and share food, which takes time. The easily digestible cooked food increased caloric intake diminishing foraging time and enabling more time for evening socialization in a bigger group (Wrangham, 2009, 2017; Dunbar and Gowlett, 2014). There was a need for safe time-passing awake activities (Huron, 2001). During this forage-free time, the kin-based bigger group could engage in many social interactions (Nowell, 2018) such as childcare and play, information exchange, gossip, flirt, treat their wounds, prepare the ground sleeping nest, and guard the area against predators/enemies. The need to sleep near each other, to share cooked food, and to coordinate firewood gathering and keeping fire alight are important factors increasing in-group cohesion (Gowlett et al., 2012; Dunbar and Gowlett, 2014). In addition to domestic necessities, humans gained longer leisure time after habitual fire use (Killin, 2018). At least in contemporary societies, most leisure time in modern humans happens during the evening (Glorieux et al., 2010) and at home (Cushman et al., 2005). Thus, since musical activities are intrinsically motivated (Wang et al., 2018; Varella, 2021) and very popular leisure activity (McManus and Furnham, 2006; Varella, 2022), it is possible that musicality would be mostly expressed during ancestral evenings.
Despite the communal and comforting home base atmosphere, the night-time was, and still is, dangerous. Even baboons are more vulnerable to leopard attacks at night (Isbell et al., 2018). Hominin ancestors were preyed by a diversity of large diurnal and nocturnal carnivores, such as hyaenids and felids (Lee-Thorp et al., 2000; Barrett, 2005; Treves and Palmqvist, 2007; Hagen, 2022). In current night-times, humans in small-scale African societies still suffer from lion attacks (Packer et al., 2011), and in industrial societies, there are more personal contact crimes (Averdijk and Bernasco, 2015). In the dark, it is difficult to detect danger from predators and enemies, and as time passes, there are fewer bystanders awake who could promptly help in case of an attack (Jonason et al., 2013). These select for higher imagination and alertness toward harmful agents, openness to experience, conspicuous preventive defenses, social agglomeration, risk-taking/sensation-seeking, and forms of anxiety alleviation (Varella et al., 2021; Varella and Valentova, 2023).
The absence of visual stimuli alone upturns tension, uncertainty, and apprehension predisposing to the acoustic startle reflex (Grillon et al., 1997); nevertheless, it is not darkness alone, but the night that is most related to fear responses (Levos and Zacchilli, 2015; Li et al., 2015). Anxiety keeps the body in alert mode; thus, it is linked to insomnia (Oh et al., 2019). Interestingly, some musical passages related to peak emotional experiences and violation of expectations tend to trigger ‘chill’ experiences comprising heart racing, pupil dilation, and specially piloerection (Grewe et al., 2005; Laeng et al., 2016), which are old phylogenetic reactions among primates toward threatening (Mobbs, 2018; Kret et al., 2020) and cold situations (Chaplin et al., 2014), both nocturnal challenges.
The emotion of fear reorganizes our mindset strategically lowering the thresholds for the detection of danger. When there is a greater cost of missing a real threat than seeing a misapprehension of a hazard, evolution selects the less costly error leading to a higher false alarm rate (Nesse, 2001; Haselton and Nettle, 2006). When we are frightened, less evidence is needed to trigger the threat response, protecting against the cost of not perceiving a real threat (Tooby and Cosmides, 2008). Because of the nocturnal dangerousness, we might have evolved an overexcited imagination; a tendency to overestimate threats, purposes, and agency, particularly stemming from opponents (Barrett, 2005; Varella, 2018; Needham et al., 2022). Interestingly, in many species, there is the phenomenon of predator inspection, which is a contra-intuitive curiosity toward dangerous agents probably enabling better assessment of the situation and learning about the habits and behavioral patterns of predators (cf. Fishman, 1999). Hence, individual personality features, such as risk-taking and openness to experience (i.e., curiosity, fantasy, and esthetics), would improve responses to night-time dangers and mysteries, particularly in those less vulnerable individuals (i.e., adolescents and adults) who would have more to lose in terms of social networking, sexual opportunities, knowledge gathered by not socializing facing the nocturnal risks. Indeed, the evidence suggests that risk-taking and openness to experience are related to evening orientation and aspects of musicality. Evening-oriented individuals are risk-takers (Ponzi et al., 2014; Gowen et al., 2019), low on fear and caution (Ottoni et al., 2012), high on openness to experience (Randler et al., 2017), high on originality, open to culture and novelties, curious and well-informed (Cavallera and Giampietro, 2007), and creative and intuitive (Diaz-Morales and Escribano, 2013). Individuals open to experience are more musically/artistically/esthetically oriented (Rawlings et al., 2000; Feist and Brady, 2004; Gjermunds et al., 2020). Risk-taking/sensation-seeking/impulsivity is high in individuals with increased emotionality toward music (Roberts et al., 1998), in male individuals exhibiting more attractive dance movements (Hugill et al., 2011), and in individuals who prefer rock/heavy music (Little and Zuckerman, 1986; Arnett, 1992). Finally, young pop musicians are twice as likely to die of violent death (Kenny and Asher, 2016).
Moreover, the necessity to solve new practical problems during the night and the increased rate of REM sleep (i.e., indicative of dreaming) also converged possibly leading to higher creativity (Coolidge and Wynn, 2006; Samson and Nunn, 2015). Creativity is positively related to musicality and visual arts (Ismail et al., 2020; Sotiropoulos and Anagnostouli, 2021). Evidence for a nocturnal overexcited imagination which corroborates the Nocturnal Evolution of Human Musicality and Performativity Theory ranges from contemporary industrial societies, traditional societies, and prehistory. In industrial societies, music composers (Gjermunds et al., 2019), art students (Wang and Chern, 2008), and visually creative individuals (Giampietro and Cavallera, 2007) are indeed more evening-oriented. In traditional society, there is the highest frequency of storytelling in the evening (81% vs. 6% of day conversations) with background music around the campfire (Wiessner, 2014). Even some recent prehistoric findings support the nocturnal theory, such as some esthetically engraved plaquettes from 23 to 14 ka ago, which were likely appreciated by early humans close to the night-time firelight (Needham et al., 2022), and possible ‘shadow play’ storytelling 6.8–5.2 ka ago (Ahola and Lassila, 2022).
Combined with fire, human sounds, particularly when collective, loud, and rhythmically coordinated, which maximizes the salience of the collective broadcast (Keller et al., 2017), might have frightened ancestral predators as a conspicuous audio-visual intimidating display for night-time defense (Jordania, 2011; Hagen, 2022). Large carnivores avoid places with human voices (Suraci et al., 2019). When vervet monkeys give ‘leopard’ alarm calls in their sleeping site, most often near dusk and dawn, nearby leopards indeed move away (Isbell and Bidner, 2016). There is a Scandinavian pastoral musical tradition sung by women over long distances that is suggested to have long been used to coordinate the grazing of livestock during day-time and to frighten predators, such as bears and wolves, with horns and lures when women returned home in the evening (Ivarsdotter, 2004). In addition to the predators, ambush tactics and night-time attacks have long been modes of warfare (Sheldon, 2012; Redmond, 2016). The optimal long-distance sound transmission during the evening (Larom et al., 1997; Römer, 2001; Piel, 2018) could increase the range and effect of acoustic displays for predator and enemy deterrence, by canalizing loud calls (cf. Hagen and Hammerstein, 2009). According to Hagen and Bryant (2003), under night’s poor visibility, synchronized musical displays might have distantly signaled coalition size and formidability, frightening enemies (cf. Jordania, 2011; Mehr et al., 2021; Hagen, 2022). Indeed, evening-oriented men are high on intrasexual competitiveness (Ponzi et al., 2015a). Furthermore, the ‘many eyes’/‘security in numbers’ principles (Quenette, 1990; Musharbash, 2013) could have been selected for higher social nocturnal agglomeration together with low temperatures and the necessity to regroup before sleep. The ‘better safe than sorry’ principle might have led to exaggerated and frequent defensive rituals (Boyer and Linard, 2006; Eilam et al., 2011). Interestingly, musicality’s role in maintaining and promoting prosociality (Kirschner and Tomasello, 2010) and interpersonal social bonds (Boer et al., 2011; Savage P. et al., 2021) might indicate that it was selected under nocturnal selective pressures when the group is already on fusion mode, not fission (cf. Nowell, 2018).
The dangerousness of night-time also selects for anxiolytic activities: At the end of the day, ancient hominins needed to feel safe and relaxed enabling sleep. Anxiety often results in rituals (Eilam et al., 2011). The patterned repetitions of rituals have tension-reducing and comforting effects necessary to cope with dangerous/unpredictable environments (Dissanayake, 2008; Tonna et al., 2020). The very predator and enemy deterrence effects of music could attest/explain its anxiolytic properties. Among other health benefits, music demonstrably alleviates pain and diminishes stress and anxiety (Finn and Fancourt, 2018), particularly in social contexts (Linnemann et al., 2016). Indeed, late at night individuals prefer to listen to relaxing music (Park et al., 2019; Heggli et al., 2021). Since children are most vulnerable to nocturnal predation and infanticide, they mostly need lullabies, rocking, and bedtime stories for mood regulation and relaxation. An important complement to Savage P. et al. (2021) and Mehr et al. (2021) is that the parent–offspring bond is particularly challenged during bedtime to the point that the children need to be put to sleep by requiring costly displays of all-night parental protection strengthening their bond in order to relax and fall asleep. In fact, music has an important sleep-promoting role for infants and adults (Feng et al., 2018; Akkermann et al., 2021), explaining the existence of lullaby songs (Mehr et al., 2018, 2019), and a nightly bedtime routine is beneficial to children’s healthy sleep and wellbeing leading to satisfactory development (Mindell and Williamson, 2018).
At night-time, individuals are most tired. Tired individuals self-regulate less, thus easily succumb to immediate pleasures (Millar et al., 2019). Cravings occur mostly at night (Piasecki et al., 2011; Sevincer et al., 2016). Moreover, during off-peak periods in alertness and energy reserves, humans increase emotional reactivity (Tucker et al., 2012). Evening-oriented individuals have lower self-control (Digdon and Howell, 2008), lower emotional control, coping, and volition, but more emotional sensitivity (Ottoni et al., 2012), are impulsive (Cross, 2010), and hedonistically present-oriented (Nowack and Van Der Meer, 2013). The lower self-control, increased emotionality, immediacy, and hedonism in tired individuals at night-time might have further enhanced the ancestral appeal of recreational musical activities (cf. Nowell, 2018) and manipulation (De Tiège et al., 2021), given that music activates cerebral pleasure centers (Peretz, 2006), is among the most pleasurable activities (Dubé and Le Bel, 2003), and stimulates emotional reactions (Peretz, 2006; Altenmüller et al., 2013; Nowell, 2018).
Darkness partially conceals one’s identity providing a sense of privacy and anonymity, disinhibiting behavior usually repressed due to social desirability concerns (Zhong et al., 2010; Hirsh et al., 2011). Darkness improves individual autonomy by allowing control of emitting flow of information and external interferences (Klopfer and Rubenstein, 1977), lowering the risk for social embarrassment and reputational damage. Thus, by enabling high disinhibition, social proximity, emotional sensitiveness, risk-taking, hedonism, entertainment, creativity, and low self-control, the expanded nocturnal niche has favored an intense peruse of sexuality goals. As humans are among the rare animal species exhibiting indirect/covert courtship (Gersick and Kurzban, 2014) and private sexual intercourse (Ben Mocha, 2020), possibly because of the third-party costs (a jealous partner/competitor intercepting) among other reasons, it makes sense that humans lose their virginity at night (Barak et al., 1997) and exhibit the major peak of sexual activity at night, after 21:00 (Refinetti, 2005; Jankowski et al., 2014). Moreover, evening-oriented individuals tend to be single (Maestripieri, 2014), be more flirtations later in the day and have more sexual partners (Gunawardane et al., 2011), lose virginity early in life and have a short-term relationship orientation (Kasaeian et al., 2019), be more inclined to casual sex (Cross, 2010; Piffer, 2010; Matchock, 2018; Díaz-Morales et al., 2019), and have a fast life-history strategy (Ponzi et al., 2015b; Marvel-Coen et al., 2018).
This elevated night-time mating action amplified sexual selection pressures acting on human sleep (Piffer, 2010), chronotype (Putilov, 2014), and, possibly, musicality (Putilov, 2014; Varella and Valentova, 2023) and other artistic propensities (Putilov, 2014). Importantly, sexual selection is not only about male-biased sex differences (Varella et al., 2014, 2017; Bowling et al., 2021; Merker, 2021; Verpooten and Eens, 2021). As human exhibit mutual mate choice (Stewart-Williams and Thomas, 2013a,b), parental influence over mate choice (Apostolou, 2007), multifunctional signals (Berglund et al., 1996; De Block and Dewitte, 2007; De Tiège et al., 2021), multiple/multimodal cues (Candolin, 2003), self-presentation modification (Valentova et al., 2021), and indirect/covert flirtation (Gersick and Kurzban, 2014), the boundaries between sexual and social selection tend to get blurred (cf. West-Eberhard, 2014; Winegard et al., 2018; Crespi et al., 2022). Socialization at the ancestral home base, containing self-promotion and prestige/ornamented competition (Varella et al., 2017), could also function as a nocturnal mixed-sex lekking ground, where adolescent and young adults exhibit their desirable qualities to attract, compete for, and defend/maintain romantic partners (Dunbar et al., 1997; Piffer, 2010; Putilov, 2014). Miller (2000) reasoned that youngsters dancing all night at rave parties paint a somewhat accurate picture of how our ancestors engaged with their music (cf. Jenkinson et al., 2014). Adolescents, in the prime of mating competition, are the most evening-oriented humans (peaking around age 19, Randler, 2016; Fischer et al., 2017). They listen to and enjoy music the most, give more importance to music, and listen to music in many contexts (Bonneville-Roussy et al., 2013). Adults mostly remember and self-identify with music heard during adolescence and young adulthood (Jakubowski et al., 2020; Loveday et al., 2020).
Many recognized locations/contexts for short-term mating are shifted to night-time and contain loud music, e.g., nightclubs, dance clubs, bars, (fraternity) parties, concerts/festivals, and weddings (Jonason et al., 2015), where seduction culminates into the high synchrony of couple’s body movements (Brak-Lamy, 2015). Compared to afternoon, music-listening preferences after sunset are focused on increasingly higher tempo and danceability (Heggli et al., 2021). Listening to music increases the desire to date someone (Marin and Rathgeber, 2022), dancing with someone is ranked as an effective flirting tactic for short-term mating (Wade et al., 2021; Kennair et al., 2022), and the music-induced coupling of body sway predicts long-term romantic interest (Chang et al., 2021). The effects of darkness in reducing social embarrassment (Hirsh et al., 2011) might have encouraged individuals to overcome shyness/face-saving tendencies (Garland and Brown, 1972) to overtly perform (sing, dance, and tell stories) in front of others increasing the odds of attracting/maintaining a partner. Indeed, compared to the entrance, there is a 50% increase in coupled individuals exiting the nightclub, which is dark and musical (Mannion et al., 2009). Thus, musicality and other performances could have entered the playful human mating games (De Block and Dewitte, 2007; Moraes et al., 2022).
The complexity of social interactions, the conflicts, and alignments of interests inherent to sexual reproduction and animal communication (senders and receivers) create arms races and runaway dynamics in which the need to impress and persuade in competitive ornamentation leads to an increase in complexity, elaboration, and embellishments of songs (Miller, 2000; De Block and Dewitte, 2007; Varella et al., 2011, 2017; Keller et al., 2017; Mehr et al., 2021; Verpooten and Eens, 2021; Crespi et al., 2022). Indeed, in birds, song complexity is positively related to reproductive success (Soma and Garamszegi, 2011). Women in their fertile phase prefer composers of complex music for short-term relationships (Charlton, 2014) and dance seductively (Miller et al., 2007). Mating motives increase creativity in both sexes (Griskevicius et al., 2006). Overall, the increased nocturnal sexual selection can help explain the existence of serenade, seduction, love, and dance songs (e.g., Levitin, 2008; Lehman et al., 2009; Mehr et al., 2018, 2019), the diversity and appeal of love songs (Werner and Todd, 1997; Knobloch and Zillmann, 2003), the prevalence of sexual/reproductive messages in popular songs across genres (Dukes et al., 2003; Hobbs and Gallup Jr., 2011), and the sexual/relationship benefits of musicality (Van Bohemen et al., 2018; Micoogullari et al., 2021; Marin and Rathgeber, 2022).
Sexual selection might have influenced musicality early in hominin evolution (Garfinkel, 2018; Verpooten and Eens, 2021). Before the habitual use of fire, the socioecological context of Homo erectus favoring the emergence of elaborated songs (i.e., difficulty in monopolizing resources/partners, high mobility, vast home ranges, small social groups, and optimal context for long-distance sound transmission) is fairly disseminated across singing species, and serving sexual/social selection: mate attraction/maintenance and territory advertising/defense (Searcy and Andersson, 1986; Yoshida and Okanoya, 2005; Mikula et al., 2021; Schruth et al., 2021; Verpooten and Eens, 2021), as Darwin first proposed Darwin (1871). Social monogamy is another socioecological context favoring the evolution of complex song across primates (Schruth et al., 2021) and mutual display by duetting (Geissmann, 2000; Yoshida and Okanoya, 2005). In birds, when biparental care, leading to social monogamy with low rates of extra-pair paternity, is combined with cooperative defense of territories/breeding sites, there is the evolution of ornamentation in both sexes (Tobias et al., 2012). Mutual ornaments also function as social armaments in both sexes of birds (Tobias et al., 2011) and humans (De Block and Dewitte, 2007; Varella et al., 2017; Mafra et al., 2020). Interestingly, the combination of grandmother alloparental care (O’Connell et al., 1999; Bell et al., 2013) and children and elderly individuals sleeping early (Randler, 2016; Fischer et al., 2017) can effectively free women from the parental burden of late evening, enabling them to allocate efforts into mating attraction and intrasexual competition. In cooperative breeding birds, female ornamentation is indeed increased, decreasing sexual dimorphism (Dale et al., 2015). Thus, sexual selection in humans under monogamy, mutual mate choice, and alloparenting might have favored musical displays in both sexes, with an increased role of attraction and ornamental competition in women (Fisher and Candea, 2012; Varella et al., 2017, 2022) in comparison with other non-monogamous species without alloparental care.
Indeed, both men and women tend to have basically the same musical capacities with some minor distinctions on how musicality is expressed (cf. Mosing et al., 2015; Bertolo et al., 2023). Women are slightly better at recognizing familiar melodies (Miles et al., 2016), at mistuning perception (Bertolo et al., 2023), at music achievement (Mosing et al., 2015), appreciating more music (Hagen and Bryant, 2003; Varella et al., 2010), liking to sing more than men (Varella et al., 2010; De Moor et al., 2013; Yan et al., 2021); even the complexity of songs across primates is female-biased (Schruth et al., 2021). Compared to women, men tend to enjoy playing musical instruments more (Varella et al., 2010), tend to be slightly better in rhythmic discrimination task (Mosing et al., 2015; Bertolo et al., 2023), and slightly better in melodic and pitch discrimination tasks (Mosing et al., 2015); cross-culturally, music performers tend to be male (Savage et al., 2015; Mehr et al., 2018, 2019). Given that lower sound frequencies travel longer distances (Larom et al., 1997) and that non-human primates use low frequency for long-distance loud calls (Mitani and Stuht, 1998), there is a possibility that ancestor male hominins sang together to attract exogamic females traveling nearby (Merker, 1999, 2021) using the evening ‘sound duct’ (Delgado, 2006). Interestingly, a majority of adult humans fantasize about sex in traveling contexts (Lehmiller, 2018).
Another factor that can lead to mutual ornamentation is assortative mating (Tobias et al., 2012). The exaggeration of costly signaling can evolve under perfect monogamy through mutual mate choice and assortative mating (Hooper and Miller, 2008). In fact, assortative mating occurs among individuals similar in vocational interests (Etzel et al., 2019; Banov et al., 2022), playing musical instruments and singing (Bongard et al., 2019), musical and artistic talents (De Moor et al., 2013), creative achievements (Kaufman et al., 2016), sexual strategies (Penke and Asendorpf, 2008), and chronotype (Randler and Kretz, 2011), but see Horwitz et al. (2023). In addition, couples with corresponding chronotypes are more sexually active (Jocz et al., 2018) and sexually satisfied than mismatched couples (Sprajcer et al., 2022). Furthermore, assortative mating, which leads to the evolution of trait covariation (De Moor et al., 2013; Conroy-Beam et al., 2019), may have created the phenotypic relationships between the eveningness chronotype and musicality. Indeed, musicians and composers tend to be evening-oriented (Gjermunds et al., 2019; Wright and Palmer, 2022), and there is a genetic correlation between the eveningness chronotype, more accurate capacity for musical beat synchronization, and exposure to loud music (Niarchou et al., 2022).
Why did musicality evolve? This “why” question has two aspects to be unpacked (cf. Dennett, 2017). The “how come that musicality evolved?” question requires a plausible step-by-step phylogenetic process narrative connecting ancestral ape-like protomusicality with human musicality. The “what reasons made musicality evolve?” question requires a probable combination of specific recurring selective pressures fitting most fitness-enhancing functions/roles of human musicality. The Nocturnal Evolution of Human Musicality and Performativity Theory (NEHMPT) tackles both aspects. It relies on interconnecting paleoanthropological phylogenetic patterns with a detailed evolutionary psychological analysis of recurrent nocturnal ancestral challenges/opportunities and correspondent outcomes, and with cross-species adaptive convergences, which offers a strong indication of adaptation (McGhee, 2011; Currie, 2012; Oikkonen et al., 2016). This newly proposed nocturnal theory is an extension and interconnection of previous related attempts that relate the evolution of musicality with night/eveningness (cf. Jordania, 2011; Dunbar and Gowlett, 2014; Putilov, 2014; Killin, 2017; Nowell, 2018; Varella et al., 2021; Varella and Valentova, 2023); it integrates many adaptive explanations/proposed proper functions for human musicality (e.g., parenting, group-cohesion, territorial/antipredator defense, and sexuality/romance) under the same umbrella theory. It also integrates ecological and social factors in shaping human musicality (cf. Schruth et al., 2021).
The possibility of a nocturnal evolution of human musicality does not imply that all humans should be evening-oriented nor that musical activities should only be found after sunset. Acoustic communication is often retained in lineages that evolved diurnality (Chen and Wiens, 2020), and mammals, in general, have retained many nocturnal features from the nocturnal bottleneck of the time of dinosaurs (Gerkema et al., 2013). Humans listen to music all day, but out of the five daily time blocks of music-listening preferences, three occur after sunset (evening, night, and late night; Heggli et al., 2021). I have provided evidence that musicality has a nocturnal bias. Even the music-listening effects on tennis players’ cognitive abilities are stronger during the evening (Jarraya and Jarraya, 2019). The evolutionary roots of this nocturnal bias are the focus of this proposal. I argue that the ancestral evening eco-social fusion context contained most of the key adaptive problems that musicality is suited to help solving. Throughout hominin evolution, there was an expansion of activities into a nocturnal niche, recurrently exposing humans (less vulnerable humans more than others) to enduring nocturnal challenges/opportunities impacting inclusive fitness: lack of luminosity, low temperatures, regrouping to cook before sleep, imminent dangerousness, peak tiredness, and concealment of identity. Despite being mostly active during the day, as humans became a more evening-inclined species in comparison with closely related apes, these high-frequency, long-lasting, and ancient night-time features might have selected together with the evening orientation, a propensity to acoustic communication, to be more alert and imaginative, gregarious, risk-taking and novelty-seeking, prone to anxiety modulation, hedonistic, and promiscuous and disinhibited, particularly in adolescents and adults, which in turn coevolved with protomusicality in different nocturnal contexts leading to the multifunctional human musicality. Those night-time features are independent from musical cognition and acted in combination as a coherent nocturnal selective landscape (i) directly promoting the enhancement of aspects of ape-like protomusicality into human musicality (e.g., darkness and temperature inversion promoting more long-distance acoustic communication, loudness, and repetition), (ii) selecting many psychological tendencies that coevolved with musicality (e.g., chronotype, openness to experience, and creativity), and (iii) creating the many co-occurring situational contexts to which musicality functionally adjusted (e.g., knowledge transference, recreation, parenting, group-cohesion, territorial/antipredator defense, infant-directed singing, and sexuality/romance).
This nocturnal theory does not invalidate/exclude other seriously proposed evolutionary theories of human musicality. It reorganizes and interconnects previous attempts into a parsimonious, overarching, and comprehensive framework. Instead of the final word, the current version of this nocturnal theory is the beginning of explorations of its heuristic value in generating new discoveries and connecting findings. For instance, it has been shown that music listening helps childbirth (Finn and Fancourt, 2018; McCaffrey et al., 2020), and given how evolutionarily problematic human delivery is, posing a survival problem to the mother and the newborn (Dunsworth and Eccleston, 2015), this might be considered another candidate adaptive effect of musicality. The nocturnal theory would predict that if there is any evolutionarily relevant activity recurrently happening during night-time, it is possible that musicality might have coevolved with it and be exapted to benefit the activity, in a process of cooptation by temporal juxtaposition. Indeed, it has been shown that non-human primates and humans tend to spontaneously give birth mostly during the night and early morning (Honnebier and Nathanielsz, 1994; Martin et al., 2018; Macfarlane et al., 2019; Declercq et al., 2023). The nocturnal onset of childbirth might be more temperature efficient (McFarland et al., 2022), free from insects and diurnal predators, and surrounded by more supporting conspecifics, despite vulnerability to nocturnal predators. Thus, activities that promote cohesion and deter predators decreasing pain, danger and anxiety would greatly benefit human childbirth; musical pain, activities seem to do so.
The Nocturnal Evolution of Human Musicality and Performativity Theory does not explain every single musical feature or underlying proximate mechanisms. Instead, it offers a plausible evidence-based scenario in which some important features (i.e., loudness, repetitiveness, call and response, chills, playfulness, complex ornamentation/virtuosity, and duets/chorus) and functions (i.e., long-distance communication, mnemonic device, dyadic and social bonds, leisure, territoriality, antipredator defense, emotional and mood regulation, sexuality, and childbirth) of musicality fit together. Furthermore, it does not suggest that only musicality was selected during the gradual expansion into the nocturnal niche. There are many non-exclusive strategic routes to reproductive success (De Block and Dewitte, 2007; Varella et al., 2017, 2022; Winegard et al., 2018). Similar to musicians, art students (Wang and Chern, 2008) and visually creative individuals (Giampietro and Cavallera, 2007) are also evening oriented. Thus, the nocturnal selective landscape is also relevant to the evolution of other forms of performing arts and performative entertainment using voice, such as humor, drama, and storytelling (Putilov, 2014; Wiessner, 2014; Lauer, 2022), including those forms focused on night-time horror/thriller stories (Clasen, 2012). Because of the firelight, visual arts by the fire (Needham et al., 2022) or in places with extraordinary acoustic properties (Waller, 2002; Reznikoff, 2008), including ‘shadow play’ storytelling (Ahola and Lassila, 2022) and fire-walking rituals (Xygalatas, 2015), are expected to be included in the scope of the Nocturnal Evolution of Human Musicality and Performativity Theory. This multimodal inclusive theoretical extension of the nocturnal theory might also help explain and integrate aspects of the proposed adaptive functions for visual arts (Power, 1999; Coe, 2003), literary arts and fiction (Gottschall, 2012; Smith et al., 2017; Dubourg and Baumard, 2022), language (Dunbar, 2004; Masataka, 2009; Dunbar and Gowlett, 2014), religiosity (Bulbulia, 2004; Sterelny, 2018), and popular culture (Salmon, 2018). Finally, even the cognitive niche hypothesis (Jonason et al., 2013) and eveningness epidemiological liability hypothesis (Varella et al., 2021) can be integrated into the Nocturnal Evolution of Human Musicality and Performativity Theory because the main nocturnal factors stressed by each hypothesis are inherent part among the night-time challenges/opportunities substantiating this nocturnal theory.
The relative lack of evidence connecting musicality with chronobiology/chronopsychology is a limitation. Some parts of the argument are still speculative in nature and need to be further developed and tested. The empirical support offered for this proposal still needs to be cross-culturally replicated. The current version of the nocturnal theory still needs further testing. Many questions remain unanswered. Evolutionary musicology should begin investigating the night following archeology (Gonlin and Nowell, 2017). How far can a singing group be heard or interact with other groups during the evening? Anthropologists studying traditional societies should try to determine using microphones how far a group sings can be heard under evening versus day-time conditions. Is there a nocturnal bias to sing loud, prefer repetitiveness, get the musical chills, compose, maintain a rhythm, perform, and appreciate music? Are the group cohesion effects and the anxiety-alleviating effects of music more pronounced at night? Are the effects of music on attractiveness evaluation and sexual excitement more pronounced in darker/nocturnal contexts? Music cognition researchers should try to compare the musical capacities and performances of the same individuals during day- and night-time conditions, controlling for other non-musical tasks or activities. Are nocturnal feline predators scared by group vocal music? Zoologists should try to investigate using playback whether big carnivores are attracted or repelled by human vocal group music.
The current theoretical proposition could be empirically refuted if the phenotypic and the genetic correlation between musicality and eveningness orientation is not replicated cross-culturally, or if the differences in musicality of adolescents and adults compared to youngsters and elderly are not replicated. It also could be refuted if future studies find no nocturnal bias on human musicality (production, appreciation, and motivation), particularly in adolescents and adults, and if future studies find no nocturnal bias for the effects and functions of musicality (i.e., long-distance communication, mnemonic device, dyadic and social bonds, leisure, territoriality, antipredator defense, emotional and mood regulation, sexuality, and childbirth). The current proposition offers precise and new ways of empirically testing human musicality and other performances.
The Nocturnal Evolution of Human Musicality and Performativity Theory (NEHMPT) argues that it is not a coincidence that humans are more nocturnal and musical than chimpanzees; that musicians, composers, and individuals with better beat synchronization capacities are evening-oriented; that most music-listening contexts are nocturnal; that adolescents, who are the most evening-oriented humans, enjoy more music; and that tribal nocturnal social activities around the campfire involve eating, singing, dancing, storytelling, and rituals. It presents convergent evidentiary support for the possibility that human musicality and performativity evolved mostly (but not only) under the influence of the nocturnal eco-social selective landscape. The nocturnal eco-social context is a common denominator among acoustic communication and the many functions, roles, and proposed adaptive functions of musicality. Musicality might function as a human’s night “long-distance vision”, night “sleeping bag”, night “social glue”, night entertainment and memory device, night “scarecrow” against predators/enemies, night anxiolytic and sleeping promoter, night “gala dress”, and night “midwife”. By integrating an evolutionary chronopsychology with evolutionary musicology, it is simultaneously possible to (i) explain why humans are more musical than the other great apes, (ii) explain the evolution of many aspects of musicality from independent principles, (iii) explain various features and functions of musicality, and (iv) integrate the different proposed ancestral adaptive values into an inclusive and coherent approach. Musicality is probably a multifunctional mental adaptation that evolved hand in hand with the many night-time adaptive problems. I hope to have shed some light on a missing piece of the adaptationist puzzle of human musicality.
The theoretical contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
MV searched the literature, conceptually analyzed, and organized the published evidence, conceived the main theoretical proposition, drafted, corrected, improved, and formatted the manuscript.
MV was supported by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), number PNPD 33002010037P0 – MEC/CAPES. The APC was jointly paid by PROEX-CAPES, IPEVSC - Instituto de Pesquisa de Variações Socioculturais (Institute for Research on Sociocultural Variations), and MV.
The author thanks the Dept. of Experimental Psychology, Institute of Psychology, University of São Paulo for the support and the PNPD/CAPES scholarship for the salary. The author also thanks the IPEVSC - Instituto de Pesquisa de Variações Socioculturais (Institute for Research on Sociocultural Variations) for the financial support. The author also gratefully thanks Tobias Kordsmeyer-Storp, Eduardo Ottoni, and Edward Hagen for constructive comments on previous versions of this manuscript. Any remaining mistake is my own. The author also thanks both reviewers for their constructive, helpful, and pertinent comments and suggestions.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor PJ declared a past co-authorship with the author.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ahola, M., and Lassila, K. (2022). Mesolithic shadow play? Exploring the performative attributes of a zoomorphic wild reindeer (Rangifer tarandus) antler artefact from Finland. Time Mind 15, 167–185. doi: 10.1080/1751696X.2022.2098047
Åkerlund, L., and Gramming, P. (1994). Average loudness level, mean fundamental frequency, and subglottal pressure: comparison between female singers and nonsingers. J. Voice 8, 263–270. doi: 10.1016/S0892-1997(05)80298-X
Akkermann, M., Akkaya, U. C., Dermiel, C., Pflüger, D., and Dresler, M. (2021). Sound sleep: lullabies as a test case for the neurobiological effects of music. Behav. Brain Sci. 44:E96. doi: 10.1017/S0140525X20001259
Akram, U., Stevenson, J. C., Gardani, M., Akram, A., and Allen, S. (2019). Psychopathy and chronotype disposition: the mediating role of depression. Heliyon 5:e02894. doi: 10.1016/j.heliyon.2019.e02894
Altenmüller, E., Kopiez, R., and Grewe, O. (2013). “Strong emotions in music: are they an evolutionary adaptation?” in Sound-perception-performance. ed. R. Bader, vol. 1 (Cham: Springer International Publishing), 131–156.
Anderson, J. R., Ang, M. Y., Lock, L. C., and Weiche, I. (2019). Nesting, sleeping, and nighttime behaviors in wild and captive great apes. Primates 60, 321–332. doi: 10.1007/s10329-019-00723-2
Ankel-Simons, F., and Rasmussen, D. T. (2008). Diurnality, nocturnality, and the evolution of primate visual systems. Yearb. Phys. Anthropol. 51, 100–117. doi: 10.1002/ajpa.20957
Apostolou, M. (2007). Sexual selection under parental choice: the role of parents in the evolution of human mating. Evol. Hum. Behav. 28, 403–409. doi: 10.1016/j.evolhumbehav.2007.05.007
Arcadi, A. C., Robert, D., and Boesch, C. (1998). Buttress drumming by wild chimpanzees: temporal patterning, phrase integration into loud calls, and preliminary evidence for individual distinctiveness. Primates 39, 505–518. doi: 10.1007/BF02557572
Arnett, J. (1992). Musical preferences and reckless behavior among adolescents. J. Adolesc. Res. 7, 313–331. doi: 10.1177/074355489273003
Averdijk, M., and Bernasco, W. (2015). Testing the situational explanation of victimization among adolescents. J. Res. Crime Delinq. 52, 151–180. doi: 10.1177/0022427814546197
Ball, P. (2010). The music instinct: How music works and why we can't do without it. New York: Oxford University Press.
Banov, K., Krapic, N., and Kardum, I. (2022). Do vocational interests matter for the selection of romantic partners? Evidence from variable-and couple-centered approaches. Appl. Psychol. 72, 697–717. doi: 10.1111/apps.12396
Barak, Y., Stein, D., Ring, A., Ticher, A., and Elizur, A. (1997). Patterns of first intercourse: a survey among Israeli women. Biol. Rhythm. Res. 28, 36–41. doi: 10.1076/brhm.28.1.36.12972
Barrett, H. (2005). “Adaptations to predators and prey” in The handbook of evolutionary psychology. ed. D. M. Buss (New York, NY: Wiley), 200–223.
Bell, A. V., Hinde, K., and Newson, L. (2013). Who was helping? The scope for female cooperative breeding in early homo. PLoS One 8:e83667. doi: 10.1371/journal.pone.0083667
Ben Mocha, Y. (2020). Why do human and non-human species conceal mating? The cooperation maintenance hypothesis. Proc. R. Soc. B. 287:1330. doi: 10.1098/rspb.2020.1330
Benítez-Burraco, A., and Nikolsky, A. (2023). The (co)evolution of language and music under human self-domestication. Hum. Nat. 34, 229–275. doi: 10.1007/s12110-023-09447-1
Berglund, A., Bisazza, A., and Pilastro, A. (1996). Armaments and ornaments: an evolutionary explanation of traits of dual utility. Biol. J. Linn. Soc. 58, 385–399. doi: 10.1111/j.1095-8312.1996.tb01442.x
Bergström, A., Stringer, C., Hajdinjak, M., Scerri, E. M., and Skoglund, P. (2021). Origins of modern human ancestry. Nature 590, 229–237. doi: 10.1038/s41586-021-03244-5
Bertolo, M., Müllensiefen, D., Peretz, I., Woolley, S. C., Sakata, J., and Mehr, S. (2023). Human music perception ability is not a sexually dimorphic trait. In M. Goldwater, F. K. Anggoro, B. K. Hayes, and D. C. Ong (Eds.), Proceedings of the Annual Meeting Cognition Science Society.
Bispham, J. C. (2009). Music's “design features”: musical motivation, musical pulse, and musical pitch. Music. Sci. 13, 41–61. doi: 10.1177/1029864909013002041
Boer, D., Fischer, R., Strack, M., Bond, M. H., Lo, E., and Lam, J. (2011). How shared preferences in music create bonds between people: values as the missing link. Personal. Soc. Psychol. Bull. 37, 1159–1171. doi: 10.1177/0146167211407521
Bongard, S., Schulz, I., Studenroth, K. U., and Frankenberg, E. (2019). Attractiveness ratings for musicians and non-musicians: an evolutionary-psychology perspective. Front. Psychol. 10:2627. doi: 10.3389/fpsyg.2019.02627
Bonneville-Roussy, A., Rentfrow, P. J., Xu, M. K., and Potter, J. (2013). Music through the ages: trends in musical engagement and preferences from adolescence through middle adulthood. J. Pers. Soc. Psychol. 105, 703–717. doi: 10.1037/a0033770
Bowling, D. L., Hoeschele, M., and Dunn, J. C. (2021). Progress without exclusion in the search for an evolutionary basis of music. Behav. Brain Sci. 44:E97. doi: 10.1017/S0140525X20001466
Boyer, P., and Linard, P. (2006). Precaution systems and ritualized behavior. Behav. Brain Sci. 29, 635–650. doi: 10.1017/S0140525X06009575
Brak-Lamy, G. (2015). Heterosexual seduction in the urban night context: behaviors and meanings. J. Sex Res. 52, 690–699. doi: 10.1080/00224499.2013.8
Brown, S. (2000). “Evolutionary models of music: from sexual selection to group selection” in Perspectives in ethology. eds. F. Tonneau and N. S. Thompson (New York: Plenum), 231–281.
Brown, S. (2017). A joint prosodic origin of language and music. Front. Psychol. 8:1894. doi: 10.3389/fpsyg.2017.01894
Brown, K. S., Marean, C. W., Herries, A. I., Jacobs, Z., Tribolo, C., Braun, D., et al. (2009). Fire as an engineering tool of early modern humans. Science 325, 859–862. doi: 10.1126/science.1175028
Brown, S., Merker, B., and Wallin, N. L. (2000). “An introduction to evolutionary musicology” in The origins of music. eds. N. L. Wallin, B. Merker, and S. Brown (Massachusetts: MIT Press), 3–24.
Bulbulia, J. (2004). The cognitive and evolutionary psychology of religion. Biol. Philos. 19, 655–686. doi: 10.1007/s10539-005-5568-6
Candolin, U. (2003). The use of multiple cues in mate choice. Biol. Rev. 78, 575–595. doi: 10.1017/S1464793103006158
Carotenuto, F., Tsikaridze, N., Rook, L., Lordkipanidze, D., Longo, L., Condemi, S., et al. (2016). Venturing out safely: the biogeography of Homo erectus dispersal out of Africa. J. Hum. Evol. 95, 1–12. doi: 10.1016/j.jhevol.2016.02.005
Cavallera, G. M., and Giampietro, M. (2007). Morning and evening personality characteristics in a sample of young Italians. Percept. Mot. Skills 104, 277–286. doi: 10.2466/pms.104.1.277-286
Chang, A., Kragness, H. E., Tsou, W., Bosnyak, D. J., Thiede, A., and Trainor, L. J. (2021). Body sway predicts romantic interest in speed dating. Soc. Cogn. Affect. Neurosci. 16, 185–192. doi: 10.1093/scan/nsaa093
Chaplin, G., Jablonski, N. G., Sussman, R. W., and Kelley, E. A. (2014). The role of piloerection in primate thermoregulation. Folia Primatol. 85, 1–17. doi: 10.1159/000355007
Charlton, B. D. (2014). Menstrual cycle phase alters women's sexual preferences for composers of more complex music. Proc. R. Soc. B Biol. Sci. 281:20140403. doi: 10.1098/rspb.2014.0403
Chen, Z., and Wiens, J. J. (2020). The origins of acoustic communication in vertebrates. Nat. Commun. 11:369. doi: 10.1038/s41467-020-14356-3
Clasen, M. (2012). Monsters evolve: a biocultural approach to horror stories. Rev. Gen. Psychol. 16, 222–229. doi: 10.1037/a0027918
Clayton, M. (2016). “The social and personal functions of music in cross-cultural perspective” in Oxford handbook of music psychology. eds. S. Hallam, I. Cross, and M. Thaut. 2nd ed (Oxford: Oxford University Press)
Clink, D. J., Tasirin, J. S., and Klinck, H. (2020). Vocal individuality and rhythm in male and female duet contributions of a nonhuman primate. Curr. Zool. 66, 173–186. doi: 10.1093/cz/zoz035
Coe, K. (2003). The ancestress hypothesis: Visual art as adaptation. New Brunswick, NJ: Rutgers University Press.
Collignon, O., Renier, L., Bruyer, R., Tranduy, D., and Veraart, C. (2006). Improved selective and divided spatial attention in early blind subjects. Brain Res. 1075, 175–182. doi: 10.1016/j.brainres.2005.12.079
Conroy-Beam, D., Roney, J. R., Lukaszewski, A. W., Buss, D. M., Asao, K., Sorokowska, A., et al. (2019). Assortative mating and the evolution of desirability covariation. Evol. Hum. Behav. 40, 479–491. doi: 10.1016/j.evolhumbehav.2019.06.003
Coolidge, F., and Wynn, T. (2006). The effects of the tree-to-ground sleep transition in the evolution of cognition in early Homo. Before Farm. 4, 1–18. doi: 10.3828/bfarm.2006.4.11
Coon, H., and Carey, G. (1989). Genetic and environmental determinants of musical ability in twins. Behav. Genet. 19, 183–193. doi: 10.1007/BF01065903
Crespi, B. J., Flinn, M. V., and Summers, K. (2022). Runaway social selection in human evolution. Front. Ecol. Evol. 10:894506. doi: 10.3389/fevo.2022.894506
Cross, C. P. (2010). Sex differences in same-sex direct aggression and sociosexuality: the role of risky impulsivity. Evol. Psychol. 8, 779–792. doi: 10.1177/147470491000800418
Cross, I., and Morley, I. (2002). “Music and evolution: the nature of the evidence,” In Proceedings of the 7th International Conference on Music Perception and Cognition, eds. C. Stevens, D. Burnham, G. McPherson, E. Schubert, and J. Renwick (Adelaide: Causal Productions).
Cross, I., and Morley, I. (2008). “The evolution of music: theories, definitions and the nature of the evidence” in Communicative musicality. eds. S. Malloch and C. Trevarthen (Oxford: Oxford University Press), 61–82.
Croston, R., Branch, C. L., Kozlovsky, D. Y., Dukas, R., and Pravosudov, V. V. (2015). Heritability and the evolution of cognitive traits. Behav. Ecol. 26, 1447–1459. doi: 10.1093/beheco/arv088
Currie, A. (2012). Convergence as evidence. Brit. J. Phil. Sci. 64, 763–786. doi: 10.1093/bjps/axs027
Currie, A., and Killin, A. (2016). Musical pluralism and the science of music. Eur. J. Philos. Sci. 6, 9–30. doi: 10.1007/s13194-015-0123-z
Cushman, G., Veal, A. J., and Zuzamek, J. (2005). Free time and leisure participation international perspectives. Wallingford, UK: CABI Publishing.
Dale, J., Dey, C. J., Delhey, K., Kempenaers, B., and Valcu, M. (2015). The effects of life history and sexual selection on male and female plumage colouration. Nature 527, 367–370. doi: 10.1038/nature15509
De Block, A., and Dewitte, S. (2007). Mating games: cultural evolution and sexual selection. Biol. Philos. 22, 475–491. doi: 10.1007/s10539-006-9041-y
De Moor, M. H. M., Roeling, M. P., and Boomsma, D. I. (2013). “Creativity and talent: etiology of familial clustering” in Neuroscience of creativity. eds. A. S. Bristol, O. Vartanian, and A. B. Kaufman (Cambridge, Mass: MIT Press), 95–112.
De Tiège, A., Verpooten, J., and Braeckman, J. (2021). From animal signals to art: manipulative animal signaling and the evolutionary foundations of aesthetic behavior and art production. Q. Rev. Biol. 96, 1–27. doi: 10.1086/713210
Declercq, E., Wolterink, A., Rowe, R., de Jonge, A., De Vries, R., Nieuwenhuijze, M., et al. (2023). The natural pattern of birth timing and gestational age in the US compared to England, and the Netherlands. PLoS One 18:E0278856. doi: 10.1371/journal.pone.0278856
Delgado, R. A. (2006). Sexual selection in the loud calls of male primates: signal content and function. Int. J. Primatol. 27, 5–25. doi: 10.1007/s10764-005-9001-4
Dennett, D. C. (2017). From bacteria to Bach and back: The evolution of minds. New York: W.W. Norton & Company.
Diaz-Morales, J. F., and Escribano, C. (2013). Circadian preference and thinking styles: implications for school achievement. Chronobiol. Int. 30, 1231–1239. doi: 10.3109/07420528.2013.813854
Díaz-Morales, J. F., Jankowski, K. S., Prokop, P., and Rahafar, A. (2019). Sleep timing is linked to sociosexuality: evidence from German, polish, Slovak, and Spanish females. Time Soc. 28, 1272–1287. doi: 10.1177/0961463X18757390
Diez-Martín, F., Sánchez Yustos, P., Uribelarrea, D., Baquedano, E., Mark, D. F., Mabulla, A., et al. (2015). The origin of the Acheulean: the 1.7 million-year-old site of FLK West, Olduvai Gorge (Tanzania). Sci. Rep. 5:17839. doi: 10.1038/srep17839
Digdon, N. L., and Howell, A. J. (2008). College students who have an eveningness preference report lower self-control and greater procrastination. Chronobiol. Int. 25, 1029–1046. doi: 10.1080/07420520802553671
Dissanayake, E. (2008). If music is the food of love, what about survival and reproductive success? Mus. Sci. 12, 169–195. doi: 10.1177/1029864908012001081
Drayna, D., Manichaikul, A., de Lange, M., Snieder, H., and Spector, T. (2001). Genetic correlates of musical pitch recognition in humans. Science 291, 1969–1972. doi: 10.1126/science.291.5510.1969
Duba, J. D., and Roseman, C. (2012). Musical “tune-ups” for couples: brief treatment interventions. Fam. J. 20, 322–326. doi: 10.1177/1066480712449604
Dubé, L., and le bel, J. (2003). The content and structure of laypeople's concept of pleasure. Cogn. Emot. 17, 263–295. doi: 10.1080/02699930302295
Dubourg, E., André, J. B., and Baumard, N. (2021). The evolution of music: one trait, many ultimate-level explanations. Behav. Brain Sci. 44:E98. doi: 10.1017/S0140525X20001156
Dubourg, E., and Baumard, N. (2022). Why and how did narrative fictions evolve? Fictions as entertainment technologies. Front. Psychol. 13:786770. doi: 10.3389/fpsyg.2022.786770
Dufour, V., Poulin, N., Curé, C., and Sterck, E. H. (2015). Chimpanzee drumming: a spontaneous performance with characteristics of human musical drumming. Sci. Rep. 5:11320. doi: 10.1038/srep11320
Dukes, R. L., Bisel, T. M., Borega, K. N., Lobato, E. A., and Owens, M. D. (2003). Expressions of love, sex, and hurt in popular songs: a content analysis of all-time greatest hits. Soc. Sci. J. 40, 643–650. doi: 10.1016/S0362-3319(03)00075-2
Dunbar, R. I. M. (2004). “Language, music, and laughter in evolutionary perspective” in Evolution of communication systems: A comparative approach. eds. D. K. Oller and U. Griebel (Cambridge, MA: MIT Press), 257–274.
Dunbar, R., and Gowlett, J. (2014). “Fireside chat: the impact of fire on hominin socioecology” in Lucy to language: The benchmark papers. eds. R. Dunbar, C. Gamble, and J. Gowlett (Oxford: Oxford University Press), 277–296.
Dunbar, M., Marriott, A., and Duncan, C. (1997). Human conversational behavior. Hum. Nat. 8, 231–246. doi: 10.1007/BF02912493
Dunsworth, H., and Eccleston, L. (2015). The evolution of difficult childbirth and helpless hominin infants. Annu. Rev. Anthropol. 44, 55–69. doi: 10.1146/annurev-anthro-102214-013918
Eastwick, P. W. (2009). Beyond the pleistocene: using phylogeny and constraint to inform the evolutionary psychology of human mating. Psychol. Bull. 135, 794–821. doi: 10.1037/a0016845
Eilam, D., Izhar, R., and Mort, J. (2011). Threat detection: behavioral practices in animals and humans. Neurosci. Biobehav. Rev. 35, 999–1006. doi: 10.1016/j.neubiorev.2010.08.002
Etzel, J. M., Lüdtke, O., Wagner, J., and Nagy, G. (2019). Similarity of vocational interest profiles within families: a person-centered approach for examining associations between circumplex profiles. J. Pers. 87, 593–606. doi: 10.1111/jopy.12418
Fedurek, P., Donnellan, E., and Slocombe, K. E. (2014). Social and ecological correlates of long-distance pant hoot calls in male chimpanzees. Behav. Ecol. Sociobiol. 68, 1345–1355. doi: 10.1007/s00265-014-1745-4
Feist, G. J., and Brady, T. R. (2004). Openness to experience, non-conformity, and the preference for abstract art. Empir. Stud. Arts 22, 77–89. doi: 10.2190/Y7CA-TBY6-V7LR-76GK
Feng, F., Zhang, Y., Hou, J., Cai, J., Jiang, Q., Li, X., et al. (2018). Can music improve sleep quality in adults with primary insomnia? A systematic review and network meta-analysis. Int. J. Nurs. Stud. 77, 189–196. doi: 10.1016/j.ijnurstu.2017.10.011
Fessler, D. (2006). A burning desire: steps toward an evolutionary psychology of fire learning. J. Cogn. Cult. 6, 429–451. doi: 10.1163/156853706778554986
Figueredo, A. J., Black, C. J., Patch, E. A., Heym, N., Ferreira, J. H. B. P., Varella, M. A. C., et al. (2021). The cascade of chaos: from early adversity to interpersonal aggression. Evol. Behav. Sci. 15, 231–250. doi: 10.1037/ebs0000241
Fink, B., Bläsing, B., Ravignani, A., and Shackelford, T. K. (2021). Evolution and functions of human dance. Evol. Hum. Behav. 42, 351–360. doi: 10.1016/j.evolhumbehav.2021.01.003
Finn, S., and Fancourt, D. (2018). “The biological impact of listening to music in clinical and nonclinical settings: a systematic review” in The arts and the brain: Psychology and physiology beyond pleasure. eds. J. F. Christensen and A. Gomila (Amsterdam: Elsevier B.V), 173–200.
Fischer, D., Lombardi, D. A., Marucci-Wellman, H., and Roenneberg, T. (2017). Chronotypes in the US–influence of age and sex. PLoS One 12:E0178782. doi: 10.1371/journal.pone.0178782
Fisher, H. E. (1989). Evolution of human serial pairbonding. Am. J. Phys. Anthropol. 78, 331–354. doi: 10.1002/ajpa.1330780303
Fisher, M. L., and Candea, C. (2012). You ain’t woman enough to take my man: female intrasexual competition as portrayed in songs. J. Soc. Evol. Cult. Psychol. 6:480. doi: 10.1037/h0099238
Fishman, M. A. (1999). Predator inspection: closer approach as a way to improve assessment of potential threats. J. Theor. Biol. 196, 225–235. doi: 10.1006/jtbi.1998.0834
Fitch, W. T. (2006). The biology and evolution of music: a comparative perspective. Cognition 100, 173–215. doi: 10.1016/j.cognition.2005.11.009
Fitch, W. T. (2015). Four principles of bio-musicology. Philos. Trans. R. Soc. B 370:20140091. doi: 10.1098/rstb.2014.0091
Foley, R., and Gamble, C. (2009). The ecology of social transitions in human evolution. Philos. Trans. R. Soc. B 364, 3267–3279. doi: 10.1098/rstb.2009.0136
Franklin, M. S., Rattray, K., Moore, K. S., Moher, J., Yip, C., and Jonides, J. (2008). The effects of musical training on verbal memory. Psychol. Music 36, 353–365. doi: 10.1177/0305735607086044
Fruth, B., Tagg, N., and Stewart, F. (2018). Sleep and nesting behavior in primates: a review. Am. J. Phys. Anthropol. 166, 499–509. doi: 10.1002/ajpa.23373
Fukui, H., and Toyoshima, K. (2023). Testosterone, oxytocin and co-operation: a hypothesis for the origin and function of music. Front. Psychol. 14:1055827. doi: 10.3389/fpsyg.2023.1055827
Garfinkel, Y. (2018). The evolution of human dance: courtship, rites of passage, trance, calendrical ceremonies and the professional dancer. Camb. Archaeol. J. 28, 283–298. doi: 10.1017/S0959774317000865
Garland, H., and Brown, B. R. (1972). Face-saving as affected by subjects’ sex, audiences’ sex and audience expertise. Sociometry 35, 280–289. doi: 10.2307/2786623
Geissmann, T. (2000). “Gibbon songs and human music from an evolutionary perspective” in The origins of music. eds. N. L. Wallin, B. Merker, and S. Brown (Cambridge, MA: MIT Press), 103–123.
Gerkema, M. P., Davies, W. I. L., Foster, R. G., Menaker, M., and Hut, R. A. (2013). The nocturnal bottleneck and the evolution of activity patterns in mammals. Proc. R. Soc. B 280:20130508. doi: 10.1098/rspb.2013.0508
Gersick, A., and Kurzban, R. (2014). Covert sexual signaling: human flirtation and implications for other social species. Evol. Psychol. 12:305. doi: 10.1177/147470491401200305
Giampietro, M., and Cavallera, G. M. (2007). Morning and evening types and creative thinking. Pers. Individ. Differ. 42, 453–463. doi: 10.1016/j.paid.2006.06.027
Gildersleeve, K., Haselton, M. G., and Fales, M. R. (2014). Do women’s mate preferences change across the ovulatory cycle? A meta-analytic review. Psychol. Bull. 140:1205. doi: 10.1037/a0035438
Gjermunds, N., Brechan, I., Johnsen, S. Å. K., and Watten, R. G. (2019). Musicians: larks, owls or hummingbirds? J. Circadian Rhythms 17:4. doi: 10.5334/jcr.173
Gjermunds, N., Brechan, I., Johnsen, S. Å. K., and Watten, R. G. (2020). Personality traits in musicians. Curr. Issues Personal. Psychol. 8, 100–107. doi: 10.5114/cipp.2020.97314
Glorieux, I., Laurijssen, I., Minnen, J., and van Tienoven, T. P. (2010). In search of the harried leisure class in contemporary society: time-use surveys and patterns of leisure time consumption. J. Consum. Policy 33, 163–181. doi: 10.1007/s10603-010-9124-7
Gonlin, N., and Nowell, A. (2017). Archaeology of the night: life after dark in the ancient world. Boulder: University Press of Colorado.
Gordon, R. L., Fehd, H. M., and McCandliss, B. D. (2015). Does music training enhance literacy skills? A meta-analysis. Front. Psychol. 6:1777. doi: 10.3389/fpsyg.2015.01777
Gottschall, J. (2012). The storytelling animal: How stories make us human. New York: Houghton Mifflin Harcourt.
Gowen, R., Filipowicz, A., and Ingram, K. K. (2019). Chronotype mediates gender differences in risk propensity and risk-taking. PLoS One 14:E0216619. doi: 10.1371/journal.pone.0216619
Gowlett, J. A. J. (2006). The early settlement of northern Europe: fire history in the context of climate change and the social brain. C. R. Palevol. 5, 299–310. doi: 10.1016/j.crpv.2005.10.008
Gowlett, J. A. J. (2016). The discovery of fire by humans: a long and convoluted process. Philos. T. R. Soc. B. 371:20150164. doi: 10.1098/rstb.2015.0164
Gowlett, J., Gamble, C., and Dunbar, R. (2012). Human evolution and the archaeology of the social brain. Curr. Anthropol. 53, 693–722. doi: 10.1086/667994
Grewe, O., Nagel, F., Kopiez, R., and Altenmüller, E. (2005). How does music arouse “chills”? Investigating strong emotions, combining psychological, physiological, and psychoacoustical methods. Ann. N. Y. Acad. Sci. 1060, 446–449. doi: 10.1196/annals.1360.041
Grillon, C., Pellowski, M., Merikangas, K. R., and Davis, M. (1997). Darkness facilitates the acoustic startle reflex in humans. Biol. Psychiatry 42, 453–460. doi: 10.1016/S0006-3223(96)00466-0
Griskevicius, V., Cialdini, R. B., and Kenrick, D. T. (2006). Peacocks, Picasso, and parental investment: the effects of romantic motives on creativity. J. Pers. Soc. Psychol. 91:63. doi: 10.1037/0022-3514.91.1.63
Guinee, L. N., and Payne, K. B. (1988). Rhyme-like repetitions in songs of humpback whales. Ethology 79, 295–306. doi: 10.1111/j.1439-0310.1988.tb00718.x
Gunawardane, K. G., Custance, D. M., and Piffer, D. (2011). Evidence of sexual selection for evening orientation in human males: a cross cultural study in Italy and Sri Lanka. Interdiscip. Biol. Central 3, 13–17. doi: 10.4051/ibc.2011.3.4.0013
Hagen, E. H. (2022). The biological roots of music and dance: extending the credible signaling hypothesis to predator deterrence. Hum. Nat., 33, 261–279. doi: 10.1007/s12110-022-09429-9
Hagen, E. H., and Bryant, G. A. (2003). Music and dance as a coalition signalling system. Hum. Nat. 14, 21–51. doi: 10.1007/s12110-003-1015-z
Hagen, E. H., and Hammerstein, P. (2009). Did Neanderthals and other early humans sing? Seeking the biological roots of music in the territorial advertisements of primates, lions, hyenas, and wolves. Mus. Sci. 13, 291–320. doi: 10.1177/1029864909013002131
Haimoff, E. H. (1986). Convergence in the duetting of monogamous Old World primates. J. Hum. Evol. 15, 51–59. doi: 10.1016/S0047-2484(86)80065-3
Hallam, S. (2010). The power of music: its impact on the intellectual, social and personal development of children and young people. Int. J. Music. Educ. 28, 269–289. doi: 10.1177/0255761410370658
Hallett, E. Y., Marean, C. W., Steele, T. E., Álvarez-Fernández, E., Jacobs, Z., Cerasoni, J. N., et al. (2021). A worked bone assemblage from 120,000-90,000 year old deposits at Contrebandiers cave, Atlantic Coast, Morocco. iScience 24:102988. doi: 10.1016/j.isci.2021.102988
Hargreaves, D. J., and North, A. C. (1999). The functions of music in everyday life: redefining the social in music psychology. Psychol. Music 27, 71–83. doi: 10.1177/0305735699271007
Haselton, M. G., and Nettle, D. (2006). The paranoid optimist: an integrative evolutionary model of cognitive biases. Personal. Soc. Psychol. Rev. 10, 47–66. doi: 10.1207/s15327957pspr1001_3
Hattori, Y., Tomonaga, M., and Matsuzawa, T. (2013). Spontaneous synchronized tapping to an auditory rhythm in a chimpanzee. Sci. Rep. 3:1566. doi: 10.1038/srep01566
Hau, M., Dominoni, D., Casagrande, S., Buck, C. L., Wagner, G., Hazlerigg, D., et al. (2017). Timing as a sexually selected trait: the right mate at the right moment. Philos. Trans. R. Soc. B 372:20160249. doi: 10.1098/rstb.2016.0249
Hauser, M. D., and McDermott, J. (2003). The evolution of the music faculty: a comparative perspective. Nat. Neurosci. 6, 663–668. doi: 10.1038/nn1080
Heggli, O. A., Stupacher, J., and Vuust, P. (2021). Diurnal fluctuations in musical preference. R. Soc. Open Sci. 8:210885. doi: 10.1098/rsos.210885
Hernandez-Aguilar, R. A., Moore, J. I. M., and Stanford, C. B. (2013). Chimpanzee nesting patterns in savanna habitat: environmental influences and preferences. Am. J. Primatol. 75, 979–994. doi: 10.1002/ajp.22163
Herzog, N. M., Parker, C., Keefe, E., and Hawkes, K. (2020). Fire’s impact on threat detection and risk perception among vervet monkeys: implications for hominin evolution. J. Hum. Evol. 145:102836. doi: 10.1016/j.jhevol.2020.102836
Hirsh, J. B., Galinsky, A. D., and Zhong, C. B. (2011). Drunk, powerful, and in the dark: how general processes of disinhibition produce both prosocial and antisocial behavior. Pers. Psychol. Sci. 6, 415–427. doi: 10.1177/1745691611416992
Ho, Y., Cheung, M., and Chan, A. S. (2003). Music training improves verbal but not visual memory: crosssectional and longitudinal explorations in children. Neuropsychology 17, 439–450. doi: 10.1037/0894-4105.17.3.439
Hobbs, D. R., and Gallup, G. G. Jr. (2011). Songs as a medium for embedded reproductive messages. Evol. Psychol. 9, 390–416. doi: 10.1177/147470491100900309
Honing, H., and Ploeger, A. (2012). Cognition and the evolution of music: pitfalls and prospects. Top. Cogn. Sci. 4, 513–524. doi: 10.1111/j.1756-8765.2012.01210.x
Honing, H., ten Cate, C., Peretz, I., and Trehub, S. E. (2015a). Without it no music: cognition, biology and evolution of musicality. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 370:88. doi: 10.1098/rstb.2014.0088
Honing, H., ten Cate, C., Peretz, I., and Trehub, S. E. (2015b). Biology, cognition and origins of musicality [special issue]. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 370:88
Honnebier, M. B. O. M., and Nathanielsz, P. W. (1994). Primate parturition and the role of the maternal circadian system. Eur. J. Obstet. Gynecol. Reprod. Biol. 55, 193–203. doi: 10.1016/0028-2243(94)90038-8
Hooper, P. L., and Miller, G. F. (2008). Mutual mate choice can drive costly signaling even under perfect monogamy. Adapt. Behav. 16, 53–70. doi: 10.1177/1059712307087283
Horwitz, T. B., Balbona, J. V., Paulich, K. N., and Keller, M. C. (2023). Evidence of correlations between human partners based on systematic reviews and meta-analyses of 22 traits and UK Biobank analysis of 133 traits. Nat. Hum. Behav, 7, 1568–1583. doi: 10.1038/s41562-023-01672-z
Hublin, J. J., Ben-Ncer, A., Bailey, S. E., Freidline, S. E., Neubauer, S., Skinner, M. M., et al. (2017). New fossils from Jebel Irhoud, Morocco and the pan-African origin of Homo sapiens. Nature 546, 289–292. doi: 10.1038/nature22336
Hugill, N., Fink, B., Neave, N., Besson, A., and Bunse, L. (2011). Women’s perception of men’s sensation seeking propensity from their dance movements. Personal. Individ. Differ. 51, 483–487. doi: 10.1016/j.paid.2011.05.002
Huron, D. (2001). Is music an evolutionary adaptation? Ann. N. Y. Acad. Sci. 930, 43–61. doi: 10.1111/j.1749-6632.2001.tb05724.x
IJzerman, H., Coan, J. A., Wagemans, F. M. A., Missler, M. A., Beest, I. v., Lindenberg, S., et al. (2015). A theory of social thermoregulation in human primates. Front. Psychol. 6:464. doi: 10.3389/fpsyg.2015.00464
Isbell, L. A., and Bidner, L. R. (2016). Vervet monkeys (Chlorocebus pygerythrus) alarm calls to leopards (Panthera pardus) function as a predator deterrent. Behaviour 153, 591–606. doi: 10.1163/1568539X-00003365
Isbell, L. A., Bidner, L. R., Van Cleave, E. K., Matsumoto-Oda, A., and Crofoot, M. C. (2018). GPS-identified vulnerabilities of savannah-woodland primates to leopard predation and their implications for early hominins. J. Hum. Evol. 118, 1–13. doi: 10.1016/j.jhevol.2018.02.003
Ismail, M. J., Anuar, A. F., and Kamis, M. S. (2020). Divergent thinking in musically gifted practices: a review. Quan. J. Soc. Sci. Hum. 1, 13–26. doi: 10.55197/qjssh.v1i5.22
Ivarsdotter, A. (2004). “And the cattle follow her, for they know her voice …on communication between women and cattle in Scandinavian pastures” in Pecus. Man and animal in antiquity. ed. B. S. Frizell (Rome: The Swedish Institute in Rome), 146–149.
Jackendoff, R., and Lerdahl, F. (2006). The capacity for music: what is it, and what's special about it? Cognition 100, 33–72. doi: 10.1016/j.cognition.2005.11.005
Jakobsen, L., Christensen-Dalsgaard, J., Juhl, P. M., and Elemans, C. P. H. (2021). How loud can you go? Physical and physiological constraints to producing high sound pressures in animal vocalizations. Front. Ecol. Evol. 9:657254. doi: 10.3389/fevo.2021.657254
Jakubowski, K., Eerola, T., Tillmann, B., Perrin, F., and Heine, L. (2020). A cross-sectional study of reminiscence bumps for music-related memories in adulthood. Music. Sci. 3:2059204320965058. doi: 10.1177/2059204320965058
Jankowski, K. S., Díaz-Morales, J. F., and Randler, C. (2014). Chronotype, gender and time for sex. Chronobiol. Int. 31, 911–916. doi: 10.3109/07420528.2014.925470
Jarraya, S., and Jarraya, M. (2019). The effects of music and the time-of-day on cognitive abilities of tennis player. J. Sport Exerc. Psychol. 17, 185–196. doi: 10.1080/1612197X.2017.1292299
Jenkinson, R., Bowring, A., Dietze, P., Hellard, M., and Lim, M. S. (2014). Young risk takers: alcohol, illicit drugs and sexual practices among a sample of music festival attendees. J. Sex. Transm. Dis. 2014:357239. doi: 10.1155/2014/357239
Jocz, P., Stolarski, M., and Jankowski, K. S. (2018). Similarity in chronotype and preferred time for sex and its role in relationship quality and sexual satisfaction. Front. Psychol. 9:443. doi: 10.3389/fpsyg.2018.00443
Jonason, P. K., Foster, J. D., McCain, J., and Campbell, W. K. (2015). Where birds flock to get together: the who, what, where, and why of mate searching. Pers. Individ. Dif. 80, 76–84. doi: 10.1016/j.paid.2015.02.018
Jonason, P. K., Jones, A., and Lyons, M. (2013). Creatures of the night: chronotypes and the dark triad traits. Personal. Individ. Differ. 55, 538–541. doi: 10.1016/j.paid.2013.05.001
Jones, S. E., Lane, J. M., Wood, A. R., and Weedon, M. N. (2019). Genome-wide association analyses of chronotype in 697,828 individuals provides insights into circadian rhythms. Nat. Commun. 10:343. doi: 10.1038/s41467-018-08259-7
Jordania, J. (2011). Sexual selection or natural selection? New look on the evolution of human morphology, behavior and art. Kadmos 3, 400–411.
Kalinowski, K., Kozłowska, A., Malesza, M., and Danel, D. P. (2021). Evolutionary origins of music. Classical and recent hypotheses. Przegląd Antropologiczny 84, 213–231. doi: 10.2478/anre-2021-0011
Kasaeian, A., Weidenauer, C., Hautzinger, M., and Randler, C. (2019). Reproductive success, relationship orientation and sexual behaviour in heterosexuals: relationship with chronotype, sleep and sex. Evol. Psychol. 17:1474704919859760. doi: 10.1177/1474704919859760
Kaufman, S. B., Kozbelt, A., Silvia, P., Kaufman, J. C., Ramesh, S., and Feist, G. J. (2016). Who finds bill gates sexy? Creative mate preferences as a function of cognitive ability, personality, and creative achievement. J. Creat. Behav. 50, 294–307. doi: 10.1002/jocb.78
Kedar, Y., Kedar, G., and Barkai, R. (2022). The influence of smoke density on hearth location and activity areas at lower Paleolithic lazaret cave, France. Sci. Rep. 12, 1–14. doi: 10.1038/s41598-022-05517-z
Keller, P. E., König, R., and Novembre, G. (2017). Simultaneous cooperation and competition in the evolution of musical behavior: sex-related modulations of the singer's formant in human chorusing. Front. Psychol. 8:1559. doi: 10.3389/fpsyg.2017.01559
Kennair, L. E. O., Wade, T. J., Tallaksen, M. T., Grøntvedt, T. V., Kessler, A. M., Burch, R. L., et al. (2022). Perceived effectiveness of flirtation tactics: the effects of sex, mating context and individual differences in US and Norwegian samples. Evol. Psychol. 20:1088011. doi: 10.1177/14747049221088011
Kenny, D. T., and Asher, A. (2016). Life expectancy and cause of death in popular musicians: is the popular musician lifestyle the road to ruin? Med. Probl. Perform. Art. 31, 37–44. doi: 10.21091/mppa.2016.1007
Killin, A. (2017). Plio-pleistocene foundations of hominin musicality: coevolution of cogition, sociality, and music. Biol. Theor. 12, 222–235. doi: 10.1007/s13752-017-0274-6
Killin, A. (2018). The origins of music: evidence, theory, and prospects. Music. Sci. 1, 1–23. doi: 10.1177/2059204317751971
Kirschner, S., and Tomasello, M. (2010). Joint music making promotes prosocial behaviour in 4-year-old children. Evol. Hum. Behav. 31, 354–364. doi: 10.1016/j.evolhumbehav.2010.04.004
Kleinman, K. (2015). Darwin and Spencer on the origin of music: is music the food of love? Prog. Brain Res. 217, 3–15. doi: 10.1016/bs.pbr.2014.11.018
Klinge, C., Röder, B., and Büchel, C. (2010). Increased amygdala activation to emotional auditory stimuli in the blind. Brain 133, 1729–1736. doi: 10.1093/brain/awq102
Klopfer, P. H., and Rubenstein, D. I. (1977). The concept privacy and its biological basis. J. Soc. Issues 33, 52–65. doi: 10.1111/j.1540-4560.1977.tb01882.x
Knobloch, S., and Zillmann, D. (2003). Appeal of love themes in popular music. Psychol. Rep. 93, 653–658. doi: 10.2466/pr0.2003.93.3.653
Kret, M. E., Prochazkova, E., Sterck, E. H., and Clay, Z. (2020). Emotional expressions in human and non-human great apes. Neurosci. Biobehav. Rev. 115, 378–395. doi: 10.1016/j.neubiorev.2020.01.027
Lacroux, C., Robira, B., Kane-Maguire, N., Guma, N., and Krief, S. (2022). Between forest and croplands: nocturnal behavior in wild chimpanzees of Sebitoli, Kibale National Park Uganda. PloS One 17:E0268132. doi: 10.1371/journal.pone.0268132
Laeng, B., Eidet, L. M., Sulutvedt, U., and Panksepp, J. (2016). Music chills: the eye pupil as a mirror to music’s soul. Conscious. Cogn. 44, 161–178. doi: 10.1016/j.concog.2016.07.009
Langergraber, K. E., Prüfer, K., Rowney, C., Boesch, C., Crockford, C., Fawcett, K., et al. (2012). Generation times in wild chimpanzees and gorillas suggest earlier divergence times in great ape and human evolution. Proc. Natl. Acad. Sci. U. S. A. 109, 15716–15721. doi: 10.1073/pnas.1211740109
Large, E. W., and Gray, P. (2015). Spontaneous tempo and entrainment in a bonobo (Pan paniscus). J. Comp. Psychol. 129, 317–328. doi: 10.1037/com0000011
Larom, D., Garstang, M., Payne, K., Raspet, R., and Lindeque, M. (1997). The influence of surface atmospheric conditions on the range and area reached by animal vocalizations. J. Exp. Biol. 200, 421–431. doi: 10.1242/jeb.200.3.421
Lauer, G. (2022). Language, childhood, and fire: how we learned to love sharing stories. Front. Psychol. 12:787203. doi: 10.3389/fpsyg.2021.787203
Launay, J., Tarr, B., and Dunbar, R. (2016). Synchrony as an adaptive mechanism for large-scale human social bonding. Ethology 122, 779–789. doi: 10.1111/eth.12528
Leanderson, R., Sundberg, J., and Von Euler, C. (1987). Breathing muscle activity and subglottal pressure dynamics in singing and speech. J. Voice 1, 258–261. doi: 10.1016/S0892-1997(87)80009-7
Lee-Thorp, J., Thackeray, J. F., and van der Merwe, N. (2000). The hunters and the hunted revisited. J. Hum. Evol. 39, 565–576. doi: 10.1006/jhev.2000.0436
Lehman, C., Welker, L., and Schiefenhövel, W. (2009). Towards an ethology of song: a categorization of musical behaviour. Music. Sci. 13, 321–338. doi: 10.1177/1029864909013002141
Lehmiller, J. J. (2018). Tell me what you want: The science of sexual desire and how it can help you improve your sex life. New York, NY: Da Capo Press.
Leongómez, J. D., Havlíček, J., and Roberts, S. C. (2022). Musicality in human vocal communication: an evolutionary perspective. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 377:391. doi: 10.1098/rstb.2020.0391
Levitin, D. J. (2008). The world in six songs: How the musical brain created human nature. London, UK: Atlantic.
Levitin, D. J. (2021). Knowledge songs as an evolutionary adaptation to facilitate information transmission through music. Behav. Brain Sci. 44:E105. doi: 10.1017/S0140525X20001090
Levos, J., and Zacchilli, T. L. (2015). Nyctophobia: from imagined to realistic fears of the dark. Psi Chi J. Psychol. Res. 20, 102–110. doi: 10.24839/2164-8204.JN20.2.102
Lewis, D. M. G., Conroy-Beam, D., Asao, K., and Buss, D. M. (2017). Evolutionary psychology: a how-to guide. Am. Psychol. 72, 353–373. doi: 10.1037/a0040409
Li, H. (2022). Normal lark, deviant owl: the relationship between chronotype and compliance with COVID-19 mitigation measures. Chronobiol. Int. 39, 1524–1532. doi: 10.1080/07420528.2022.2123276
Li, Y., Ma, W., Kang, Q., Qiao, L., Tang, D., Qiu, J., et al. (2015). Night or darkness, which intensifies the feeling of fear? J. Psychophysiol. 97, 46–57. doi: 10.1016/j.ijpsycho.2015.04.021
Lindblom, B., and Sundberg, J. (2014). “The human voice in speech and singing” in Springer handbook of acoustics. ed. T. D. Rossing (Berlin/Heidelberg: Springer), 703–746.
Linnemann, A., Strahler, J., and Nater, U. M. (2016). The stress-reducing effect of music listening varies depending on the social context. Psychoneuroendocrinology 72, 97–105. doi: 10.1016/j.psyneuen.2016.06.003
Little, P., and Zuckerman, M. (1986). Sensation seeking and music preferences. Pers. Individ. Differ. 7, 575–577. doi: 10.1016/0191-8869(86)90136-4
Loersch, C., and Arbuckle, N. L. (2013). Unraveling the mystery of music: music as an evolved group process. J. Pers. Soc. Psychol. 105, 777–798. doi: 10.1037/a0033691
Lombardo, M. P., and Deaner, R. O. (2018). Born to throw: the ecological causes that shaped the evolution of throwing in humans. Q. Rev. Biol. 93, 1–16. doi: 10.1086/696721
Loveday, C., Woy, A., and Conway, M. A. (2020). The self-defining period in autobiographical memory: evidence from a long-running radio show. Q. J. Exp. Physiol. 73, 1969–1976. doi: 10.1177/1747021820940300
Macfarlane, A., Dattani, N., Gibson, R., Harper, G., Martin, P., Scanlon, M., et al. (2019). Births and their outcomes by time, day and year: a retrospective birth cohort data linkage study. Health Serv. Deliv. Res. 7:hsdr07180. doi: 10.3310/hsdr07180
Madison, G., and Schiölde, G. (2017). Repeated listening increases the liking for music regardless of its complexity: implications for the appreciation and aesthetics of music. Front. Neurosci. 11:147. doi: 10.3389/fnins.2017.00147
Maestripieri, D. (2014). Night owl women are similar to men in their relationship orientation, risk-taking propensities, and cortisol levels: implications for the adaptive significance and evolution of eveningness. Evol. Psychol. 12, 130–147. doi: 10.1177/147470491401200111
Mafra, A. L., Varella, M. A. C., Defelipe, R. P., Anchieta, N. M., de Almeida, C. A. G., and Valentova, J. V. (2020). Makeup usage in women as a tactic to attract mates and compete with rivals. Pers. Individ. Differ. 163:110042. doi: 10.1016/j.paid.2020.110042
Mannion, H., Hendrie, C., and Godfrey, G. (2009). Evidence to suggest that nightclubs function as human sexual display grounds. Behaviour 146, 1331–1348. doi: 10.1163/156853909X427704
Marin, M. M., and Rathgeber, I. (2022). Darwin’s sexual selection hypothesis revisited: musicality increases sexual attraction in both sexes. Front. Psychol. 13:971988. doi: 10.3389/fpsyg.2022.971988
Marin, M. M., Schober, R., Gingras, B., and Leder, H. (2017). Misattribution of musical arousal increases sexual attraction towards opposite-sex faces in females. PLoS One 12:e0183531. doi: 10.1371/journal.pone.0183531
Martin, P., Cortina-Borja, M., Newburn, M., Harper, G., Gibson, R., Dodwell, M., et al. (2018). Timing of singleton births by onset of labour and mode of birth in NHS maternity units in England, 2005–2014: a study of linked birth registration, birth notification, and hospital episode data. PLoS One 13:E0198183. doi: 10.1371/journal.pone.0198183
Marvel-Coen, J., Scrivner, C., and Maestripieri, D. (2018). “Morningness–eveningness and sociosexuality from a life history perspective”, in The SAGE Handbook of Personality and Individual Differences. Eds. V. Zeigler-Hill and T. Shackelford (Thousand Oaks, CA: Sage).
Masataka, N. (2009). The evolution of music: theories, definitions and the nature of the evidence. The origins of language and the evolution of music: a comparative perspective. Phys Life Rev 6, 11–22. doi: 10.1016/j.plrev.2008.08.003
Matchock, R. L. (2018). Evening chronotype is associated with a more unrestricted sociosexuality in men and women. Pers. Indiv. Dif. 135, 56–59. doi: 10.1016/j.paid.2018.06.054
May, J. L., and Hamilton, P. A. (1980). Effects of musically evoked affect on women's interpersonal attraction toward and perceptual judgments of physical attractiveness of men. Motiv. Emot. 4, 217–228. doi: 10.1007/BF00995420
McCaffrey, T., Cheung, P. S., Barry, M., Punch, P., and Dore, L. (2020). The role and outcomes of music listening for women in childbirth: an integrative review. Midwifery 83:102627. doi: 10.1016/j.midw.2020.102627
McFarland, R., Henzi, S. P., Fuller, A., Hetem, R. S., Young, C., and Barrett, L. (2022). The thermal consequences of primate birth hour and its evolutionary implications. Biol. Lett. 18:20210574. doi: 10.1098/rsbl.2021.0574
McManus, I. C., and Furnham, A. (2006). Aesthetic activities and aesthetic attitudes: influences of education, background and personality on interest and involvement in the arts. Br. J. Psychol. 97, 555–587. doi: 10.1348/000712606X101088
Mehr, S. A., Krasnow, M. M., Bryant, G. A., and Hagen, E. H. (2021). Origins of music in credible signaling. Behav. Brain Sci. 44:E60. doi: 10.1017/S0140525X20000345
Mehr, S. A., Singh, M., Knox, D., Ketter, D. M., Pickens-Jones, D., Atwood, S., et al. (2019). Universality and diversity in human song. Science 366:17. doi: 10.1126/science.aax0868
Mehr, S. A., Singh, M., York, H., Glowacki, L., and Krasnow, M. M. (2018). Form and function in human song. Curr. Biol. 28, 356.e5–368.e5. doi: 10.1016/j.cub.2017.12.042
Menninghaus, W. (2019). Aesthetics after Darwin: the multiple origins and functions of art. Boston: Academic Studies Press.
Merker, B. (1999). Synchronous chorusing and the origins of music. Music. Sci. 3, 59–73. doi: 10.1177/10298649000030S105
Merker, B. (2021). Music, bonding, and human evolution: a critique. Behav. Brain Sci. 44:E83. doi: 10.1017/S0140525X20001429
Merker, B., Morley, I., and Zuidema, W. (2015). Five fundamental constraints on theories of the origin of music. Philos. Trans. R. Soc. B 370:20140095. doi: 10.1098/rstb.2014.0095
Micoogullari, U., Kisa, E., Celik, O., Erbay, O. F., Koc, E., and Gok, B. (2021). New behavior therapy in the treatment of acquired premature ejaculation: a comparative study of listening to music. Arch. Esp. Urol. 74, 519–525.
Mikula, P., Valcu, M., Brumm, H., Bulla, M., Forstmeier, W., Petrusková, T., et al. (2021). A global analysis of song frequency in passerines provides no support for the acoustic adaptation hypothesis but suggests a role for sexual selection. Ecol. Lett. 24, 477–486. doi: 10.1111/ele.13662
Miles, S. A., Miranda, R. A., and Ullman, M. T. (2016). Sex differences in music: a female advantage at recognizing familiar melodies. Front. Psychol. 7:278. doi: 10.3389/fpsyg.2016.00278
Millar, B. M., Rendina, H. J., Starks, T. J., Grov, C., and Parsons, J. T. (2019). The role of chronotype, circadian misalignment, and tiredness in the substance use behaviors of gay and bisexual men. Psychol. Sex. Orientat. Gend. Divers. 6, 96–106. doi: 10.1037/sgd0000311
Miller, G. (2000). “Evolution of human music through sexual selection” in The origins of music. eds. N. L. Wallin, B. Merker, and S. Brown (Cambridge: The MIT Press), 329–360.
Miller, G., Tybur, J. M., and Jordan, B. D. (2007). Ovulatory cycle effects on tip earnings by lap dancers: economic evidence for human estrus? Evol. Hum. Behav. 28, 375–381. doi: 10.1016/j.evolhumbehav.2007.06.002
Mindell, J. A., and Williamson, A. A. (2018). Benefits of a bedtime routine in young children: sleep, development, and beyond. Sleep Med. Rev. 40, 93–108. doi: 10.1016/j.smrv.2017.10.007
Mingle, M. E., Eppley, T. M., Campbell, M. W., Hall, K., Horner, V., and De Waal, F. (2014). Chimpanzees prefer African and Indian music over silence. J. Exp. Psychol. Anim. Learn. Cogn. 40, 502–505. doi: 10.1037/xan0000032
Mitani, J. C., and Stuht, J. (1998). The evolution of nonhuman primate loud calls: acoustic adaptation for long-distance transmission. Primates 39, 171–182. doi: 10.1007/BF02557729
Mithen, S. (2005). The singing Neanderthals: The origins of music, language, mind and body. London: Weidenfeld and Nicolson.
Mithen, S. (2009). The music instinct: the evolutionary basis of musicality. Ann. N. Y. Acad. Sci. 1169, 3–12. doi: 10.1111/j.1749-6632.2009.04590.x
Mithen, S. (2019). Mesolithic fireplaces and the enculturation of early holocene landscapes in Britain, with a case study from Western Scotland. P. Prehist. Soc. 85, 131–159. doi: 10.1017/ppr.2019.6
Mobbs, D. (2018). The ethological deconstruction of fear(s). Curr. Opin. Behav. Sci. 24, 32–37. doi: 10.1016/j.cobeha.2018.02.008
Moraes, Y. L., Valentova, J. V., and Varella, M. A. C. (2022). The evolution of playfulness, play and play-like phenomena in relation to sexual selection. Front. Psychol. 13:925842. doi: 10.3389/fpsyg.2022.925842
Moreno, S., Bialystok, E., Barac, R., Schellenberg, E. G., Cepeda, N. J., and Chau, T. (2011). Short-term music training enhances verbal intelligence and executive function. Psychol. Sci. 22, 1425–1433. doi: 10.1177/0956797611416999
Morley, I. (2013). The prehistory of music: Human evolution, archaeology, and the origins of musicality. Oxford: Oxford University Press.
Mosing, M. A., Madison, G., Pedersen, N. L., Kuja-Halkola, R., and Ullén, F. (2014). Practice does not make perfect. Psychol. Sci. 25, 1795–1803. doi: 10.1177/0956797614541990
Mosing, M. A., Verweij, K. J., Madison, G., Pedersen, N. L., Zietsch, B. P., and Ullén, F. (2015). Did sexual selection shape human music? Testing predictions from the sexual selection hypothesis of music evolution using a large genetically informative sample of over 10,000 twins. Evol. Hum. Behav. 36, 359–366. doi: 10.1016/j.evolhumbehav.2015.02.004
Musharbash, Y. (2013). Night, sight, and feeling safe: an exploration of aspects of Warlpiri and Western sleep. Aust. J. Anthropol. 24, 48–63. doi: 10.1111/taja.12021
Needham, A., Wisher, I., Langley, A., Amy, M., and Little, A. (2022). Art by firelight? Using experimental and digital techniques to explore Magdalenian engraved plaquette use at Montastruc (France). PLoS One 17:E0266146. doi: 10.1371/journal.pone.0266146
Nesse, R. M. (2001). The smoke detector principle: natural selection and the regulation of defensive responses. Ann. N. Y. Acad. Sci. 935, 75–85. doi: 10.1111/j.1749-6632.2001.tb03472.x
Nettle, D., and Scott-Phillips, T. (2023). “Is a non-evolutionary psychology possible?” in Evolutionary thinking across disciplines: problems and perspectives in generalized Darwinism. eds. A. Crest, M. Valkovic, A. Ariew, H. Desmond, P. Huneman, and T. Reydon (New York, NY: Springer), 27–55.
Niarchou, M., Gustavson, D. E., Sathirapongsasuti, J. F., Anglada-Tort, M., Eising, E., Bell, E., et al. (2022). Genome-wide association study of musical beat synchronization demonstrates high polygenicity. Nat. Hum. Behav. 6, 1292–1309. doi: 10.1038/s41562-022-01359-x
Nikolsky, A., and Perlovsky, L. (2020). The evolution of music [special issue]. Front. Psychol. 11:595517. doi: 10.3389/fpsyg.2020.595517
North, A., and Hargreaves, D. (2008). The social and applied psychology of music. New York: Oxford University Press.
Nowack, K., and Van Der Meer, E. (2013). Are larks future-oriented and owls present-oriented? Age-and sex-related shifts in chronotype–time perspective associations. Chronobiol. Int. 30, 1240–1250. doi: 10.3109/07420528.2013.815197
Nowell, A. (2018). “Upper Paleolithic soundscapes and the emotional resonance of nighttime” in Archaeology of the night: Life after dark in the ancient world. eds. N. Gonlin and A. Nowell (Boulder, CO: University Press of Colorado), 27–44.
Nunn, C. L., and Samson, D. R. (2018). Sleep in a comparative context: investigating how human sleep differs from sleep in other primates. Am. J. Phys. Anthropol. 166, 601–612. doi: 10.1002/ajpa.23427
Nunn, C. L., Samson, D. R., and Krystal, A. D. (2016). Shining evolutionary light on human sleep and sleep disorders. Evol. Med. Public Health 2016, 227–243. doi: 10.1093/emph/eow018
O’Connell, J. F., Hawkes, K., and Jones, N. B. (1999). Grandmothering and the evolution of Homo erectus. J. Hum. Evol. 36, 461–485. doi: 10.1006/jhev.1998.0285
Oh, C. M., Kim, H. Y., Na, H., Cho, K. H., and Chu, M. K. (2019). The effect of anxiety and depression on sleep quality of individuals with high risk for insomnia: a population-based study. Front. Neurol. 10:849. doi: 10.3389/fneur.2019.00849
Oikkonen, J., Onkamo, P., Järvelä, I., and Kanduri, C. (2016). Convergent evidence for the molecular basis of musical traits. Sci. Rep. 6:39707. doi: 10.1038/srep39707
Ottoni, G. L., Antoniolli, E., and Lara, D. R. (2012). Circadian preference is associated with emotional and affective temperaments. Chronobiol. Int. 29, 786–793. doi: 10.3109/07420528.2012.679329
Packer, C., Swanson, A., Ikanda, D., and Kushnir, H. (2011). Fear of darkness, the full moon and the nocturnal ecology of African lions. PLoS One 6:E22285. doi: 10.1371/journal.pone.0022285
Park, M., Thom, J., Mennicken, S., Cramer, H., and Macy, M. (2019). Global music streaming data reveal diurnal and seasonal patterns of affective preference. Nat. Hum. Behav. 3, 230–236. doi: 10.1038/s41562-018-0508-z
Parker, C. H., Keefe, E. R., Herzog, N. M., O’connell, J. F., and Hawkes, K. (2016). The pyrophilic primate hypothesis. Evol. Anthropol. 25, 54–63. doi: 10.1002/evan.21475
Patel, A. D. (2018). “Music as a transformative technology of the mind: an update” in The origins of musicality. ed. H. Honing (Cambridge, MA: MIT Press), 113–126.
Pearce, E., Launay, J., van Duijn, M., Rotkirch, A., David-Barrett, T., and Dunbar, R. I. M. (2016). Singing together or apart: the effect of competitive and cooperative singing on social bonding within and between sub-groups of a university fraternity. Psychol. Music 44, 1255–1273. doi: 10.1177/0305735616636208
Penke, L., and Asendorpf, J. B. (2008). Beyond global sociosexual orientations: a more differentiated look at sociosexuality and its effects on courtship and romantic relationships. J. Pers. Soc. Psychol. 95:1113. doi: 10.1037/0022-3514.95.5.1113
Peretz, I. (2006). The nature of music from a biological perspective. Cognition 100, 1–32. doi: 10.1016/j.cognition.2005.11.004
Peretz, I., and Coltheart, M. (2003). Modularity of music processing. Nat. Neurosci. 6, 688–691. doi: 10.1038/nn1083
Piasecki, T. M., Jahng, S., Wood, P. K., Robertson, B. M., Epler, A. J., Cronk, N. J., et al. (2011). The subjective effects of alcohol–tobacco co-use: an ecological momentary assessment investigation. J. Abnorm. Psychol. 120, 557–571. doi: 10.1037/a0023033
Piel, A. K. (2018). Temporal patterns of chimpanzee loud calls in the Issa Valley, Tanzania: evidence of nocturnal acoustic behavior in wild chimpanzees. Am. J. Phys. Anthropol. 166, 530–540. doi: 10.1002/ajpa.23609
Piffer, D. (2010). Sleep patterns and sexual selection: an evolutionary approach. Mank. Q. 50:361. doi: 10.46469/mq.2010.50.4.6
Pinker, S. (2005). “Foreword” in The handbook of evolutionary psychology. ed. D. M. Buss (New Jersey: John Wiley & Sons), xi–xvi.
Plomin, R., DeFries, J. C., Knopik, V. S., and Neiderhiser, J. M. (2016). Top 10 replicated findings from behavioral genetics. Perspect. Psychol. Sci. 11, 3–23. doi: 10.1177/1745691615617439
Polderman, T. J., Benyamin, B., de Leeuw, C. A., Sullivan, P. F., van Bochoven, A., Visscher, P. M., et al. (2015). Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nat. Genet. 47, 702–709. doi: 10.1038/ng.3285
Ponzi, D., Henry, A., Kubicki, K., Nickels, N., Wilson, M. C., and Maestripieri, D. (2015a). Morningness–eveningness and intrasexual competition in men. Pers. Individ. Dif. 76, 228–231. doi: 10.1016/j.paid.2014.12.023
Ponzi, D., Henry, A., Kubicki, K., Nickels, N., Wilson, M. C., and Maestripieri, D. (2015b). The slow and fast life histories of early birds and night owls: their future-or present-orientation accounts for their sexually monogamous or promiscuous tendencies. Evol. Hum. Behav. 36, 117–122. doi: 10.1016/j.evolhumbehav.2014.09.008
Ponzi, D., Wilson, M. C., and Maestripieri, D. (2014). Eveningness is associated with higher risk-taking, independent of sex and personality. Psychol. Rep. 115, 932–947. doi: 10.2466/19.12.PR0.115c28z5
Porfírio, J. C. C., and Varella, M. A. C. (2022). Testing the cognitive niche hypothesis with structural equation modeling: different dark traits predict an evening-chronotype in males and females. Curr. Psychol. :s12144-022-04111-w. doi: 10.1007/s12144-022-04111-w
Power, C. (1999). “‘Beauty magic’: the origins of art” in The evolution of culture. eds. R. Dunbar, C. Knight, and C. Power (New Brunswick, NJ: Rutgers University Press), 92–112.
Pozzi, L., Voskamp, M., and Kappeler, P. M. (2022). The effects of body size, activity, and phylogeny on primate sleeping ecology. Am. J. Biol. Anthropol. 179, 598–608. doi: 10.1002/ajpa.24640
Preckel, F., Lipnevich, A. A., Schneider, S., and Roberts, R. D. (2011). Chronotype, cognitive abilities, and academic achievement: a meta-analytic investigation. Learn. Individ. Diff. 21, 483–492. doi: 10.1016/j.lindif.2011.07.003
Pruetz, J. D. (2018). Nocturnal behavior by a diurnal ape, the West African chimpanzee (Pan troglodytes verus), in a savanna environment at Fongoli. Senegal. Am. J. Phys. Anthropol. 166, 541–548. doi: 10.1002/ajpa.23434
Putilov, A. A. (2014). What were “owls” doing in our ancestral photoperiodic environment? Chronobiological account for the evolutionary advantage of nocturnal lifestyle. Biol. Rhythm. Res. 45, 759–787. doi: 10.1080/09291016.2014.913950
Putilov, A. A. (2021). Quo Vadis, Chronopsychology? Neurosci. Behav. Physiol. 51, 1244–1261. doi: 10.1007/s11055-021-01187-y
Puts, D. A. (2010). Beauty and the beast: mechanisms of sexual selection in humans. Evol. Hum. Behav. 31, 157–175. doi: 10.1016/j.evolhumbehav.2010.02.005
Quenette, P. Y. (1990). Functions of vigilance behaviour in mammals: a review. Acta Oecol. 11, 801–818.
Randler, C. (2016). Chronotype in children and adolescents. Somnologie 20, 166–171. doi: 10.1007/s11818-016-0073-5
Randler, C., and Kretz, S. (2011). Assortative mating in morningness–eveningness. Int. J. Psychol. 46, 91–96. doi: 10.1080/00207594.2010.518237
Randler, C., Schredl, M., and Göritz, A. S. (2017). Chronotype, sleep behavior, and the big five personality factors. SAGE Open 7:728321. doi: 10.1177/2158244017728321
Rawlings, D., Barrantes i Vidal, N., and Furnham, A. (2000). Personality and aesthetic preference in Spain and England: two studies relating sensation seeking and openness to experience to liking for paintings and music. Eur. J. Personal. 14, 553–576. doi: 10.1002/1099-0984(200011/12)14:6<553::AID-PER384>3.0.CO;2-H
Reagh, Z. M., Murray, E. A., and Yassa, M. A. (2017). Repetition reveals ups and downs of hippocampal, thalamic, and neocortical engagement during mnemonic decisions. Hippocampus 27, 169–183. doi: 10.1002/hipo.22681
Redmond, E. M. (2016). Meeting with resistance: early Spanish encounters in the Americas, 1492–1524. Ethnohistory 63, 671–695. doi: 10.1215/00141801-3633264
Refinetti, R. (2005). Time for sex: nycthemeral distribution of human sexual behavior. J. Circadian Rhythms 3:4. doi: 10.1186/1740-3391-3-4
Reznikoff, I. (2008). Sound resonance in prehistoric times: a study of Paleolithic painted caves and rocks. J. Acoust. Soc. Am. 123:3603. doi: 10.1121/1.2934773
Roberts, K. R., Dimsdale, J., East, P., and Friedman, L. (1998). Adolescent emotional response to music and its relationship to risk-taking behaviors. J. Adolesc. Health 23, 49–54. doi: 10.1016/S1054-139X(97)00267-X
Röder, S., Carbon, C.-C., Shackelford, T. K., Pisanski, K., Weege, B., and Fink, B. (2016). Men’s visual attention and perceptions of women’s dance movements. Pers. Individ. Differ. 101, 1–3. doi: 10.1016/j.paid.2016.05.025
Röder, B., Rösler, F., and Neville, H. J. (2001). Auditory memory in congenitally blind adults: a behavioral-electrophysiological investigation. Cogn. Brain Res. 11, 289–303. doi: 10.1016/S0926-6410(01)00002-7
Roebroeks, W., and Villa, P. (2011). On the earliest evidence for habitual use of fire in Europe. Proc. Natl. Acad. Sci. U. S. A. 108, 5209–5214. doi: 10.1073/pnas.1018116108
Rolland, N. (2004). Was the emergence of home bases and domestic fire a punctuated event? A review of the middle Pleistocene record in Eurasia. Asian Perspect. 43, 248–280. doi: 10.1353/asi.2004.0027
Román-Caballero, R., Vadillo, M. A., Trainor, L. J., and Lupiánez, J. (2022). Please don't stop the music: a meta-analysis of the cognitive and academic benefits of instrumental musical training in childhood and adolescence. Educ. Res. Rev. 35:100436. doi: 10.1016/j.edurev.2022.100436
Römer, H. (2001). “Ecological constraints for sound communication: from grasshoppers to elephants” in Ecology of sensing. eds. F. G. Barth and A. Schmid (Berlin: Springer), 59–78.
Salmon, C. (2018). Evolutionary perspectives on popular culture: state of the art. Evol. Stud. Imaginative Cult. 2, 47–66. doi: 10.26613/esic.2.2.92
Samson, D. R. (2021). The human sleep paradox: the unexpected sleeping habits of Homo sapiens. Annu. Rev. Anthropol. 50, 259–274. doi: 10.1146/annurev-anthro-010220-075523
Samson, D. R., Crittenden, A. N., Mabulla, I. A., Mabulla, A. Z., and Nunn, C. L. (2017). Chronotype variation drives night-time sentinel-like behaviour in hunter–gatherers. Proc. R. Soc. B Biol. Sci. 284:967. doi: 10.1098/rspb.2017.0967
Samson, S., Dellacherie, D., and Platel, H. (2009). Emotional power of music in patients with memory disorders. Ann. N. Y. Acad. Sci. 1169, 245–255. doi: 10.1111/j.1749-6632.2009.04555.x
Samson, D. R., Hurst, D., and Shumaker, R. W. (2014). Orangutan night-time long call behavior: sleep quality costs associated with vocalizations in captive Pongo. Adv. Zoo. 2014:101763. doi: 10.1155/2014/101763
Samson, D. R., and Nunn, C. L. (2015). Sleep intensity and the evolution of human cognition. Evol. Anthropol. 24, 225–237. doi: 10.1002/evan.21464
Savage, P. E., Brown, S., Sakai, E., and Currie, T. E. (2015). Statistical universals reveal the structures and functions of human music. Proc. Natl. Acad. Sci. U. S. A. 112, 8987–8992. doi: 10.1073/pnas.1414495112
Savage, P., Loui, P., Tarr, B., Schachner, A., Glowacki, L., Mithen, S., et al. (2021). Music as a coevolved system for social bonding. Behav. Brain Sci. 44:E59. doi: 10.1017/S0140525X20000333
Savage, P. E., Loui, P., Tarr, B., Schachner, A., Glowacki, L., Mithen, S., et al. (2021). Toward inclusive theories of the evolution of musicality. Behav. Brain Sci. 44:E121. doi: 10.1017/S0140525X21000042
Schäfer, T., Sedlmeier, P., Städtler, C., and Huron, D. (2013). The psychological functions of music listening. Front. Psychol. 4:511. doi: 10.3389/fpsyg.2013.00511
Schellenberg, E. G. (2004). Music lessons enhance IQ. Psychol. Sci. 15, 511–514. doi: 10.1111/j.0956-7976.2004.00711.x
Schellenberg, E. G. (2011). Music lessons, emotional intelligence, and IQ. Music. Percept. 29, 185–194. doi: 10.1525/mp.2011.29.2.185
Schmitt, D. P., and Pilcher, J. J. (2004). Evaluating evidence of psychological adaptation: how do we know one when we see one? Psychol. Sci. 15, 643–649. doi: 10.1111/j.0956-7976.2004.00734.x
Schruth, D. M., Templeton, C. N., and Holman, D. J. (2021). On reappearance and complexity in musical calling. PLoS One 16:E0218006. doi: 10.1371/journal.pone.0218006
Schulkind, M. D. (2009). Is memory for music special? Ann. N. Y. Acad. Sci. 1169, 216–224. doi: 10.1111/j.1749-6632.2009.04546.x
Scott, R. V., and Hosfield, R. (2021). Fire in the round: a holistic approach to the lower Palaeolithic record. J. Archaeolog. Sci. Rep. 37:102938. doi: 10.1016/j.jasrep.2021.102938
Searcy, W. A., and Andersson, M. (1986). Sexual selection and the evolution of song. Annu. Rev. Ecol. Evol. Syst. 17, 507–533. doi: 10.1146/annurev.es.17.110186.002451
Sevincer, G. M., Ince, E., Taymur, I., and Konuk, N. (2016). Night eating syndrome frequency in university students: association with impulsivity, depression, and anxiety. J. Clin. Psychopharmacol. 26, 238–247. doi: 10.5455/bcp.20160322093750
Sheldon, R. M. (2012). Ambush: Surprise attack in ancient Greek warfare. Philadelphia: Casemate Publishers.
Sloboda, J. A., O'Neill, S. A., and Ivaldi, A. (2001). Functions of music in everyday life: an exploratory study using the experience sampling method. Music. Sci. 5, 9–32. doi: 10.1177/102986490100500102
Smith, D., Schlaepfer, P., Major, K., Dyble, M., Page, A. E., Thompson, J., et al. (2017). Cooperation and the evolution of hunter-gatherer storytelling. Nat. Commun. 8:1853. doi: 10.1038/s41467-017-02036-8
Snowdon, C. T., Zimmermann, E., and Altenmüller, E. (2015). “Music evolution and neuroscience” in Progress in brain research. eds. E. Altenmüller, S. Finger, and F. Boller, vol. 217 (Amsterdam: Elsevier), 17–34.
Soma, M., and Garamszegi, L. Z. (2011). Rethinking birdsong evolution: meta-analysis of the relationship between song complexity and reproductive success. Behav. Ecol. 22, 363–371. doi: 10.1093/beheco/arq219
Sorokowska, A., Oleszkiewicz, A., and Sorokowski, P. (2018). A compensatory effect on mate selection? Importance of auditory, olfactory and tactile cues in partner choice among blind and sighted individuals. Arch. Sex. Behav. 47, 597–603. doi: 10.1007/s10508-018-1156-0
Sotiropoulos, M. G., and Anagnostouli, M. (2021). Genes, brain dynamics and art: the genetic underpinnings of creativity in dancing, musicality and visual arts. J. Integr. Neurosci. 20, 1095–1104. doi: 10.31083/j.jin2004110
Spencer, H. (1875). “The origin and function of music” in Illustrations of universal progress: a series of discussions. ed. H. Spencer (New York, NY: D Appleton & Company), 210–238.
Sprajcer, M., Stewart, D., Miller, D., and Lastella, M. (2022). Sleep and sexual satisfaction in couples with matched and mismatched chronotypes: a dyadic cross-sectional study. Chronobiol. Int. 39, 1249–1255. doi: 10.1080/07420528.2022.2093213
Sterelny, K. (2018). Religion re-explained. Relig. Brain Behav. 8, 406–425. doi: 10.1080/2153599X.2017.1323779
Stewart-Williams, S., and Thomas, A. G. (2013a). The ape that thought it was a peacock: does evolutionary psychology exaggerate human sex differences? Psychol. Inq. 24, 137–168. doi: 10.1080/1047840X.2013.804899
Stewart-Williams, S., and Thomas, A. G. (2013b). The ape that kicked the hornet's nest: response to commentaries on “the ape that thought it was a peacock”. Psychol. Inq. 24, 248–271. doi: 10.1080/1047840X.2013.823831
Sugimoto, T., Kobayashi, H., Nobuyoshi, N., Kiriyama, Y., Takeshita, H., Nakamura, T., et al. (2009). Preference for consonant music over dissonant music by an infant chimpanzee. Primates 51, 7–12. doi: 10.1007/s10329-009-0160-3
Suraci, J. P., Clinchy, M., Zanette, L. Y., and Wilmers, C. C. (2019). Fear of humans as apex predators has landscape-scale impacts from mountain lions to mice. Ecol. Lett. 22, 1578–1586. doi: 10.1111/ele.13344
Tagg, N., McCarthy, M., Dieguez, P., and Agbor, A. (2018). Nocturnal activity in wild chimpanzees (Pan troglodytes): evidence for flexible sleeping patterns and insights into human evolution. Am. J. Phys. Anthropol. 166, 510–529. doi: 10.1002/ajpa.23478
Tan, Y. T., McPherson, G. E., Peretz, I., Berkovic, S. F., and Wilson, S. J. (2014). The genetic basis of music ability. Front. Psychol. 5:658. doi: 10.3389/fpsyg.2014.00658
Tobias, J. A., Gamarra-Toledo, V., García-Olaechea, D., Pulgarin, P. C., and Seddon, N. (2011). Year-round resource defence and the evolution of male and female song in suboscine birds: social armaments are mutual ornaments. J. Evol. Biol. 24, 2118–2138. doi: 10.1111/j.1420-9101.2011.02345.x
Tobias, J. A., Montgomerie, R., and Lyon, B. E. (2012). The evolution of female ornaments and weaponry: social selection, sexual selection and ecological competition. Philos. Trans. R. Soc. B: Biol. Sci. 367, 2274–2293. doi: 10.1098/rstb.2011.0280
Tonna, M., Ponzi, D., Palanza, P., Marchesi, C., and Parmigiani, S. (2020). Proximate and ultimate causes of ritual behaviour. Behav. Brain Res. 393:112772. doi: 10.1016/j.bbr.2020.112772
Tooby, J. (2020). Evolutionary psychology as the crystalizing core of a unified modern social science. Evol. Behav. Sci. 14, 390–403. doi: 10.1037/ebs0000250
Tooby, J., and Cosmides, L. (2008). “The evolutionary psychology of the emotions and their relationship to internal regulatory variables” in Handbook of emotions. eds. M. Lewis, J. M. Haviland-Jones, and L. F. Barrett (New York: Guilford Press), 114–137.
Toups, M. A., Kitchen, A., Light, J. E., and Reed, D. L. (2011). Origin of clothing lice indicates early clothing use by anatomically modern humans in Africa. Mol. Biol. Evol. 28, 29–32. doi: 10.1093/molbev/msq234
Trainor, L. J. (2015). The origins of music in auditory scene analysis and the roles of evolution and culture in musical creation. Philos. Trans. R. Soc. B 370:0089. doi: 10.1098/rstb.2014.0089
Trainor, L. J. (2021). Understanding the origins of musicality requires reconstructing the interactive dance between music-specific adaptations, exaptations, and cultural creations. Behav. Brain Sci. 44:E116. doi: 10.1017/S0140525X20001363
Trehub, S. E. (2003). The developmental origins of musicality. Nat. Neurosci. 6, 669–673. doi: 10.1038/nn1084
Treves, A., and Palmqvist, P. (2007). “Reconstructing hominin interactions with mammalian carnivores (6.0–1.8 Ma)” in Primate Anti-Predator Strategies. eds. S. Gurski and K. A. I. Nekaris (New York: Springer), 355–381.
Tucker, A. M., Feuerstein, R., Mende-Siedlecki, P., Ochsner, K. N., and Stern, Y. (2012). Double dissociation: circadian off-peak times increase emotional reactivity; aging impairs emotion regulation via reappraisal. Emotion 12, 869–874. doi: 10.1037/a0028207
Twomey, T. (2013). The cognitive implications of controlled fire use by early humans. Camb. Archaeol. J. 23, 113–128. doi: 10.1017/S0959774313000085
Valentova, J. V., Mafra, A. L., and Varella, M. A. C. (2021). Enhancing the evolutionary science of self-presentation modification. Arch. Sex. Behav. 51, 79–84. doi: 10.1007/s10508-021-01975-0
Valentova, J. V., Tureček, P., Varella, M. A. C., Šebesta, P., Mendes, F. D. C., Pereira, K. J., et al. (2019). Vocal parameters of speech and singing covary and are related to vocal attractiveness, body measures, and sociosexuality: a cross-cultural study. Front. Psychol. 10:2029. doi: 10.3389/fpsyg.2019.02029
Van Bohemen, S., Den Hertog, L., and van Zoonen, L. (2018). Music as a resource for the sexual self: an exploration of how young people in the Netherlands use music for good sex. Poetics, 66, 19–29. doi: 10.1016/j.poetic.2017.12.001
Vanderhoff, E. N., and Bernal, H. N. (2022). Perspectives on antiphonal calling, duetting and counter-singing in non-primate mammals: an overview with notes on the coordinated vocalizations of bamboo rats (Dactylomys spp., Rodentia: Echimyidae). Front. Ecol. Evol. 10:906546. doi: 10.3389/fevo.2022.906546
Varella, M. A. C. (2018). The biology and evolution of the three psychological tendencies to anthropomorphize biology and evolution. Front. Psychol. 9:1839. doi: 10.3389/fpsyg.2018.01839
Varella, M. A. C. (2021). Evolved features of artistic motivation: analyzing a Brazilian database spanning three decades. Front. Psychol. 12:769915. doi: 10.3389/fpsyg.2021.769915
Varella, M. A. C. (2022). Artistic motivations are intrinsic, specific, and temporally stable by nature: evidence from large real-life Brazilian public data between 1987–2004. Cult. Evol. 19, 68–80. doi: 10.1556/2055.2022.00012
Varella, M. A. C., Ferreira, J. H. B. P., Cosentino, L. A. M., Ottoni, E., and Bussab, V. S. R. (2010). Sex differences in aspects of musicality in a Brazilian sample: adaptative hypotheses. Cogn. Music. Arts 4, 10–16.
Varella, M. A. C., Ferreira, J. H. B. P., and Souza, A. A. L. (2014). The role of male and female in reproduction, and understanding of sexual selection when applied to human artistic propensities. Rock Art Res. 31, 239–240.
Varella, M. A. C., Luoto, S., Soares, R. B. S., and Valentova, J. V. (2021). COVID-19 pandemic on fire: evolved propensities for nocturnal activities as a liability against epidemiological control. Front. Psychol. 12:646711. doi: 10.3389/fpsyg.2021.646711
Varella, M. A. C., Santos, I. B. C., Ferreira, J. H. B. P., and Bussab, V. S. R. (2013). Misunderstandings in applying evolution to human mind and behavior and its causes: a systematic review. EvoS J. 5, 81–107.
Varella, M. A. C., Souza, A. A. L., and Ferreira, J. H. B. P. (2011). Evolutionary aesthetics and sexual selection in the evolution of rock art aesthetics. Rock Art Res. 28, 153–163.
Varella, M. A. C., Souza, A. A. L., and Ferreira, J. H. B. P. (2012). Considering both proximal and distal explanations for (rock) art production and appreciation as fruitful. Rock Art Res. 29, 227–229.
Varella, M. A. C., Štěrbová, Z., Bártová, K., Fisher, M., and Valentova, J. V. (2022). Evolution of artistic and athletic propensities: testing of intersexual selection and intrasexual competition. Front. Psychol. 13:925862. doi: 10.3389/fpsyg.2022.925862
Varella, M. A. C., and Valentova, J. V. (2023). “Because the night belongs to sex, ‘drugs’ and rock ‘n’ roll: the nocturnal integration of promiscuity, psychoactive substance-seeking and musicality in human evolution” in Musical psychedelia research at the intersection of music and psychedelic experience. Ed. G. L. Farrell (London: Taylor and Francis Press), 192–210.
Varella, M. A. C., Valentova, J. V., and Fernández, A. M. (2017). “Evolution of artistic and aesthetic propensities through female competitive ornamentation” in The Oxford handbook of female competition. ed. M. L. Fisher (New York, NY: Oxford University Press), 757–783.
Verpooten, J. (2021). Complex vocal learning and three-dimensional mating environments. Biol. Philos. 36, 78–86. doi: 10.1007/s10539-021-09786-2
Verpooten, J., and Eens, M. (2021). Singing is not associated with social complexity across species. Behav. Brain Sci. 44:E92. doi: 10.1017/S0140525X20001120
Videan, E. N., Fritz, J., Howell, S., and Murphy, J. (2007). Effects of two types and two genre of music on social behavior in captive chimpanzees (Pan troglodytes). J. Am. Assoc. Lab. Anim. Sci. 46, 66–70.
Vink, J. M., Groot, A. S., Kerkhof, G. A., and Boomsma, D. I. (2001). Genetic analysis of morningness and eveningness. Chronobiol. Int. 18, 809–822. doi: 10.1081/cbi-100107516
Wade, T. J., Fisher, M. L., Salmon, C., and Downs, C. (2021). Want to hookup?: sex differences in short-term mate attraction tactics. Evol. Psychol. Sci. 7, 430–438. doi: 10.1007/s40806-021-00282-0
Wade, T. J., Weinstein, E., Dalal, N., and Salerno, K. J. (2015). I can dance: further investigations of the effect of dancing ability on mate value. Hum. Ethol. Bull. 30, 10–20.
Waller, S. J. (2002). Psychoacoustic influences of the echoing environments of prehistoric art. J. Acoust. Soc. Am. 112:2284. doi: 10.1121/1.4779166
Wallin, N. L. (1991). Biomusicology. Neurophysiological, neuropsychological, and evolutionary perspectives on the origins and purposes of music. Stuyvesant, NY: Pendragon Press.
Wan, C., and Lalumière, M. L. (2017). Can music cue sexual arousal? Can. J. Hum. Sex. 26, 238–248. doi: 10.3138/cjhs.2017-0011
Wang, S. C., and Chern, J. Y. (2008). The ‘night-owl’ learning style of art students: creativity and daily rhythm. Int. J. Art Des. Educ. 27, 202–209. doi: 10.1111/j.1476-8070.2008.00575.x
Wang, C. K. J., Tan, L., and Dairianathan, E. I. (2018). Achievement goals, implicit theories, and intrinsic motivation: a test of domain specificity across music, visual art, and sports. J. Res. Music. Educ. 66, 320–337. doi: 10.1177/0022429418784563
Webster, G. D., Graber, J. A., Gesselman, A. N., Crosier, B. S., and Schember, T. O. (2014). A life history theory of father absence and menarche: a meta-analysis. Evol. Psychol. 12:147470491401200202. doi: 10.1177/147470491401200202
Weege, B., Lange, B. P., and Fink, B. (2012). Women’s visual attention to variation in men’s dance quality. Pers. Individ. Differ. 53, 236–240. doi: 10.1016/j.paid.2012.03.011
Werner, G. M., and Todd, P. M. (1997). “Too many love songs: sexual selection and the evolution of communication,” in Fourth European Conference on Artificial Life (Cambridge, MA: MIT Press, Bradford Books), 434–443.
Wesseldijk, L. W., Abdellaoui, A., Gordon, R. L., 23andMe Research Team, Aslibekyan, S., Auton, A., et al. (2022). Using a polygenic score in a family design to understand genetic influences on musicality. Sci. Rep. 12:14658. doi: 10.1038/s41598-022-18703-w
West-Eberhard, M. J. (2014). Darwin's forgotten idea: the social essence of sexual selection. Neurosci. Biobehav. Rev. 46, 501–508. doi: 10.1016/j.neubiorev.2014.06.015
Wiessner, P. W. (2014). Embers of society: firelight talk among the Ju/'hoansi bushmen. Proc. Natl. Acad. Sci. U. S. A. 111, 14027–14035. doi: 10.1073/pnas.1404212111
Winegard, B., Winegard, B., and Geary, D. C. (2018). The status competition model of cultural production. Evol. Psychol. Sci. 4, 351–371. doi: 10.1007/s40806-018-0147-7
Wrangham, R. (2017). Control of fire in the paleolithic: evaluating the cooking hypothesis. Curr. Anthropol. 58, S303–S313. doi: 10.1086/692113
Wright, S. E., and Palmer, C. (2022). Does chronotype explain daily timing of music behaviors? Chronobiol. Int. 39, 186–197. doi: 10.1080/07420528.2021.1989449
Wu, Y., Wang, H., Wang, H., and Hadly, E. A. (2017). Rethinking the origin of primates by reconstructing their diel activity patterns using genetics and morphology. Sci. Rep. 7, 1–12. doi: 10.1038/s41598-017-12090-3
Xygalatas, D. (2015). The biosocial basis of collective effervescence: an experimental anthropological study of a fire-walking ritual. Fieldw. Rel. 9, 53–67. doi: 10.1558/fiel.v9i1.53
Yan, R., Jessani, G., Spelke, E. S., de Villiers, P., de Villiers, J., and Mehr, S. A. (2021). Across demographics and recent history, most parents sing to their infants and toddlers daily. Philos. Trans. R. Soc. B 376:20210089. doi: 10.1098/rstb.2021.0089
Yoshida, S., and Okanoya, K. (2005). Evolution of turn-taking: a bio-cognitive perspective. Cogn. Stud. 12, 153–165. doi: 10.11225/jcss.12.153
Zahavi, A., and Zahavi, A. (1999). The handicap principle: A missing piece of Darwin's puzzle. New York, NY, Oxford: Oxford University Press.
Zamma, K. (2014). What makes wild chimpanzees wake up at night? Primates 55, 51–57. doi: 10.1007/s10329-013-0367-1
Zatorre, R. J. (2015). Musical pleasure and reward: mechanisms and dysfunction. Ann. N. Y. Acad. Sci. 1337, 202–211. doi: 10.1111/nyas.12677
Zhong, C. B., Bohns, V. K., and Gino, F. (2010). Good lamps are the best police: darkness increases dishonesty and self-interested behavior. Psychol. Sci. 21, 311–314. doi: 10.1177/0956797609360754
Keywords: music, song, night, esthetic behavior, evolutionary psychology, evolutionary musicology, chronotype, vocal communication
Citation: Varella MAC (2023) Nocturnal selective pressures on the evolution of human musicality as a missing piece of the adaptationist puzzle. Front. Psychol. 14:1215481. doi: 10.3389/fpsyg.2023.1215481
Received: 02 May 2023; Accepted: 11 September 2023;
Published: 04 October 2023.
Edited by:
Peter Karl Jonason, University of Padua, ItalyReviewed by:
Bruno Gingras, University of Vienna, AustriaCopyright © 2023 Varella. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marco Antonio Correa Varella, bWFjdmFyZWxsYUBhbHVtbmkudXNwLmJy
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.