- 1Department of General Psychology, University of Padua, Padua, Italy
- 2Padova Neuroscience Center (PNC), University of Padua, Padua, Italy
- 3Hospital Psychology Unit, Padua University Hospital, Padua, Italy
Considering that the classical categorical approach to mental disorders does not allow a clear identification of at-risk conditions, the dimensional approach provided by the Research Domain Criteria (RDoC) is useful in the exploration of vulnerability to psychopathology. In the RDoC era, psychophysiological models have an important role in the reconceptualization of mental disorders. Indeed, progress in the study of depression vulnerability has increasingly been informed by psychophysiological models. By adopting an RDoC lens, this narrative review focuses on how psychophysiological models can be used to advance our knowledge of the pathophysiological mechanisms underlying depression vulnerability. Findings from psychophysiological research that explored multiple RDoC domains in populations at-risk for depression are reviewed and discussed. Future directions for the application of psychophysiological research in reaching a more complete understanding of depression vulnerability and, ultimately, improving clinical utility, are presented.
1. Introduction
Major depressive disorder (MDD) is a mood disorder that affects psychological and physiological functioning causing an elevated functional impairment and represents a leading cause of disease burden worldwide (World Health Organization, 2017). Symptoms of MDD include depressive mood, anhedonia, appetite changes, sleep disturbances, apathy, psychomotor retardation or agitation, lack of energy, excessive guilt and worthlessness, poor concentration, and suicidal thoughts. According to the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5), MDD is defined by the presence of five or more of these symptoms, one of which must be depressed mood or anhedonia causing social and/or occupational impairment. With these criteria, there are 227 possible combinations of symptoms for an MDD diagnosis (Zimmerman et al., 2015). Hence, a few underlying factors may give rise to very different sets of symptoms.
Given the pervasive nature of MDD, improving the early identification of depression risk, and developing strategies to prevent the onset of full-blown depression is a core priority (Wahlbeck and Mäkinen, 2008). For prevention efforts to succeed, it is necessary to identify people at risk early and, ideally, before they become ill. Studying individuals who currently have depression prevents assumptions about whether the observed conditions represent mere correlates of depressive states or reliable markers of its risk. Hence, in the field of clinical psychobiology, researchers are shifting their focus to the study of biomarkers that characterize individuals that have a greater risk to develop a full-blown depressive episode. One reliable risk condition is a parental history of MDD: indeed, adolescents with a parental history of depression are 3–5 times more likely to develop depression themselves (Gottesman and Gould, 2003; Goodman et al., 2011). Other at-risk conditions include individuals with dysphoria, a condition characterized by subclinical depressive symptoms. Last, individuals with past depression but currently free from clinical symptoms represent a risk condition of having a recurrence of the disorder (Michelini et al., 2021). These three conditions (i.e., parental history of MDD, dysphoria, and past depression) are more vulnerable to the development or recurrence of a full-blown depressive episode than the general population, thus representing target conditions to the study of psychobiological markers of MDD (Hardeveld et al., 2010; Laborde-Lahoz et al., 2015). Another way to identify psychobiological markers of a disorder is to conduct longitudinal studies predicting future psychopathology (Raulin and Lilienfeld, 2009). Some researchers have focused on the prevention of MDD by targeting these at-risk conditions with universal psychological treatments and findings have been promising but rather mixed (e.g., Horowitz and Garber, 2006; Brunwasser and Garber, 2016). Efforts to advance effective prevention and treatment strategies might be hindered by our relatively limited understanding of mechanisms implicated in the development and maintenance of depression.
Considering that the “categorical-polythetic” approach provided by the DSM-5 may not allow a clear identification of all at-risk conditions, a viable way to improve our knowledge of the pathophysiological mechanisms linked to depression is to move beyond this approach and, instead, adopt a dimensional approach (Cuthbert and Insel, 2013; Weinberg, 2023). In this context, the National Institute of Mental Health (NIMH) launched the Research Domain Criteria (RDoC) project, which aims at linking biological and physiological mechanisms to clinical phenomena to generate empirically derived, psychobiological markers of psychopathology (Insel et al., 2010; Cuthbert and Insel, 2013). The RDoC assumes that mental disorders are multidimensional disorders observable at different levels of analysis (e.g., from genetics to behavior). The RDoC matrix is rooted in a dimensional approach to mental health and includes six domains: Positive Valence Systems, Negative Valence Systems, Arousal/Regulatory Systems, Cognitive Systems, Sensorimotor Systems, and Systems for Social Processes. The columns of the matrix include the different units of analysis: genes, molecules, cells, circuits, physiology, behavior, and self-report along dimensional neuro-environmental trajectories. The underlying principle is that by integrating different levels of analysis along these dimensions, the RDoC approach will also contribute to the advancement of our understanding of vulnerability to psychopathology (Dillon et al., 2014). Therefore, RDoC dimensions and constructs should not only be considered as a correlate of psychopathology but also of increased vulnerability. To determine whether dysfunctions within RDoC components relate to future psychopathology, conducting studies based on at-risk categories is warranted.
In the “RDoC era,” psychophysiological models have an important role in the reconceptualization of mental disorders and their vulnerability (Shankman and Gorka, 2015). Indeed, psychophysiological studies have highly contributed to the development and refinement of each RDoC dimension for numerous psychopathological conditions. Psychophysiological models cover multiple levels of analysis of constructs of the RDoC (e.g., neural, autonomic, and psychological; Kujawa and Burkhouse, 2017). In addition, psychophysiological measures have several methodological advantages, such as they are non-invasive, well-tolerated, relatively economical biological measures, and can be used in early infancy through old age. In the present narrative review, studies that have employed a wide array of psychophysiological measures for the investigation of RDoC dimensions in at-risk samples will be described. Ultimately, this review emphasizes the relevance that psychophysiology is playing in the refinement of the RDoC matrix in the context of depression risk.
Regarding the methods for studies selection employed in the current narrative review, the focus was on central (event-related potentials, spectral and time-frequency measures, and the startle eyeblink reflex), peripheral (cardiovascular activity and skin conductance) psychophysiological level of analysis as well as other measures related to specific RDoC domains (e.g., pupillometry, cortisol levels, actigraphy). This narrative review includes studies spanning all age groups, but significant attention is posed to studies on childhood and adolescence as they represent vulnerable windows to the development of psychopathology (Jaffee et al., 2002).
2. The positive valence systems
The Positive Valence Systems (PVS) are a set of systems involved in anticipating, obtaining, and responding to pleasant or rewarding stimuli (Olino, 2016). Reduced PVS functioning in depression has been evidenced by multiple units of analysis (e.g., self-report, behavioral, and psychophysiological units; McFarland and Klein, 2009; Treadway and Zald, 2011; Treadway et al., 2012; Liu et al., 2014; Hajcak Proudfit, 2015; Nusslock and Alloy, 2017). Indeed, depression is characterized by reduced positive affect, anhedonia, impaired motivational disposition, and reward insensitivity, which could also represent risk factors for the disorder. Indeed, interest has increasingly turned to insensitivity to pleasant or rewarding content as a putative risk factor that precedes depression and may represent a mechanism for the disorder (Kujawa and Burkhouse, 2017; Weinberg, 2023).
2.1. Event-related potentials
At the psychophysiological level, deficits in the approach-related brain system can be assessed by measuring neural responses to pleasant or rewarding stimuli through the computation of electroencephalographic (EEG) event-related potentials (ERPs) during specific tasks. For example, the Late Positive Potential (LPP), a positive sustained centroparietal component that begins around 300 ms after stimulus onset, has been found to be particularly important for the study of affective processing. The LPP is often investigated through affective picture viewing paradigms, and it is larger to emotionally arousing (pleasant and unpleasant) relative to neutral stimuli (images of scenes, faces, or words, e.g., Palomba et al., 1997; Cuthbert et al., 2000; Schupp et al., 2007; Dell'Acqua et al., 2022c). Reduced LPP to pleasant stimuli is considered a marker of clinical depression in adults (Grunewald et al., 2019; Klawohn et al., 2021a; for a review see Hajcak Proudfit, 2015) and children (Whalen et al., 2020). Moreover, there is some evidence that the altered LPP may precede the development of the disorder. For instance, reduced LPP amplitude to pleasant pictures has been observed in adults with dysphoria (Benning and Ait Oumeziane, 2017; Moretta et al., 2021), in adults and children with a parental history of depression (Kujawa et al., 2012; Nelson et al., 2015; Moretta and Messerotti Benvenuti, under review1), in adults with remitted depression (Allison et al., 2021), and to prospectively predict depression onset (Levinson et al., 2018; Sandre et al., 2019). Specifically, regarding the parental risk for depression, Kujawa et al. (2012) found that even preschool children with a maternal history of depression, but no depressive symptoms, had a reduced LPP to pleasant images relative to controls.
Another ERP component that has been robustly observed to be reduced in depression is reward positivity (RewP, previously referred to as feedback negativity, see Hajcak Proudfit, 2015), a positive feedback-locked frontocentral deflection occurring ~250 ms following the receipt of a reward relative to loss feedback in simple gambling tasks. The RewP represents a valuable and reliable index of reward responsiveness (Hajcak Proudfit, 2015) and there is strong evidence for considering blunted RewP as a vulnerability marker of depression (Kujawa and Burkhouse, 2017). A blunted RewP has been observed in adults and children with a parental history of depression (Foti et al., 2011; Kujawa et al., 2014a,b; Kujawa et al., 2019), siblings with depressive symptoms (Weinberg et al., 2015b), remitted depression (Whitton et al., 2016; Weinberg and Shankman, 2017), and to prospectively predict the first onset of a depressive disorder in adolescent girls with no lifetime depression (Bress et al., 2013; Nelson et al., 2016; Michelini et al., 2021; Burani et al., 2021c). A study that included a large sample of never-depressed adolescent girls reported that reduced RewP amplitude was cross-sectionally related to baseline subclinical depressive symptoms and parental depression history and longitudinally predicted first-onset depressive disorder (Nelson et al., 2016). Moreover, Burani et al. (2021c) showed how a blunted RewP and maternal suicidal thoughts and/or behaviors predicted the onset of depressive symptoms at a 1-year follow-up in a sample of adolescent girls. Notably, within a group of adults with clinical depression, the LPP to pleasant images and RewP predicted a remission status 9 months following the assessment, whereby larger potentials were associated with a higher probability of remission, relative to those that had lower ERPs values (Klawohn et al., 2021b). The reviewed literature suggests that the LPP and the RewP, two distinct measures of neural sensitivity to appetitive cues, might represent early emerging markers of depression vulnerability.
2.2. Alpha asymmetry and EEG time-frequency measures
Other studies have examined EEG frequency bands related to approach motivation. A well-established index of affective disposition is frontal asymmetric alpha activity (Davidson, 1998), considered to be inversely related to the level of cortical activation, and an EEG marker of positive affect (Gable et al., 2021). Depression has been associated with an asymmetric pattern of resting-state alpha activity characterized by increased alpha in the left frontal cortex compared to the right, possibly reflecting the hypoactivation of approach-related motivation (Allen et al., 2004; but see also Van Der Vinne et al., 2017). Reduced resting-state left relative to right frontal EEG activity has been observed in unaffected offspring of individuals with MDD (Dawson et al., 1997), and prospectively predicted the onset of depression (Pössel et al., 2008; Nusslock et al., 2011). To date, only a few studies have examined alpha asymmetry during emotional processing in at-risk samples. A study observed reduced left frontal EEG activation (i.e., greater left alpha) to both happy and sad clips in children with a parental history of depression, suggesting that at-risk individuals might have reduced approach motivation during the viewing of all affective cues (Feng et al., 2012; Lopez-Duran et al., 2012). Similarly, individuals with a parental history of MDD showed greater left relative to right frontal EEG alpha activity during a reward-based laboratory task (Nelson et al., 2013). Furthermore, Mennella et al. (2015) found that young adults with, but not without, dysphoria showed reduced alpha desynchronization in the left relative to right anterior sites during an emotional imagery task of pleasant, neutral, and unpleasant narratives, indicating an overall blunted motivation in this at-risk condition. Most of the studies have analyzed alpha activity only at anterior scalp sites, but a smaller alpha desynchronization (i.e., greater alpha) in frontal and right centro-parietal regions to pleasant images was recently found in dysphoria (Messerotti Benvenuti et al., 2019). Given that right parietal activity reflects arousal (Bruder et al., 2005; Stewart et al., 2011), these results were interpreted as an under-engagement of the approach-related motivational system in individuals with dysphoria. Alpha asymmetry during the engagement in emotional tasks remains to be fully explored in depression vulnerability.
Considering the limited and mixed evidence regarding alpha asymmetry in the study of approach motivation in at-risk samples, research has explored other time-frequency correlates of affective processing of pleasant/rewarding stimuli. Delta oscillations are of particular interest as they appear to have a functional role in monitoring the motivational relevance of affective cues and in the identification of pleasant/rewarding stimuli that are generated by subcortical regions involved in the reward system (Knyazev, 2007, 2012; Foti et al., 2015). Blunted delta power to pleasant or rewarding stimuli, which was associated with clinical depression (Foti et al., 2015; Dell’Acqua et al., 2022d), has been observed in individuals with dysphoria (Dell’Acqua et al., 2022a) and to prospectively predict first-onset depressive disorder in a sample of never-depressed adolescent girls at an 18-month follow-up (Nelson et al., 2018). Moreover, Ethridge et al. (2021) explored the intergenerational concordance of delta power to rewards in women with a history of depression and their daughters and found that there was a positive relationship between offspring and mothers’ delta power to rewards. Additionally, they found that having a mother with depression altered the typical increase in reward sensitivity seen during pubertal development, thereby interfering with neural development during this critical period (Ethridge et al., 2021).
2.3. Startle eyeblink reflex
Although most psychophysiological contributions to the study of the PVS in depression vulnerability come from EEG studies, other psychophysiological indices have also been useful in exploring this relation. For example, the startle eyeblink reflex consisting of the rapid evoked contraction of the orbicularis oculi muscle represents a measure of affective modulation when the startle probe is presented during affective processing mostly 500 ms after the beginning of the presentation of an emotional stimulus. Specifically, the reflex is potentiated during unpleasant affective states and inhibited during pleasant affective states (e.g., Bradley et al., 1999). The absence of startle attenuation to pleasant images, documented in depression (e.g., Dichter and Tomarken, 2008; see Boecker and Pauli, 2019), indicates reduced approach motivation and has also been documented in dysphoria (Mneimne et al., 2008) and in individuals with past but recurrent depression (Vaidyanathan et al., 2014).
2.4. Peripheral psychophysiology: Cardiovascular activity and skin conductance
Autonomic nervous system (ANS) changes are measures associated with both affective processing and arousal/metabolic requirements of emotional responding. Cardiac autonomic modulation, as the heart is dually innervated by the two branches of the autonomic nervous systems, can be detected by measuring heart rate that mirrors the sympathetic (acceleration) and parasympathetic (deceleration) nervous systems (Berntson et al., 1993). During active emotional tasks (e.g., imagery, public speaking) vagal withdrawal (i.e., cardiac acceleration) is considered a pattern of autonomic flexibility to respond to stimuli in the environment (Porges, 1997). Cardiac autonomic balance can also, and mostly, be measured through heart rate variability (HRV), a measure of beat-to-beat variation in the heart over time that reflects the balance between the two ANS branches on the heart (Task Force of the European Society of Cardiology and the North American Society for Pacing and Electrophysiology, 1996). Reduced HRV reflects reduced cardiac vagal inhibitory control and has been observed in response to pleasant and unpleasant emotions during active tasks (i.e., imagery, recall of events) in healthy individuals (e.g., Marci et al., 2007; Kreibig, 2010). Individuals with dysphoria showed reduced heart rate increases and less HRV reductions, which reflect inadequate cardiac vagal control, during the imagery of pleasant, but not unpleasant or neutral, scripts relative to controls (Messerotti Benvenuti et al., 2015). In addition, reduced skin conductance response, a measure of the activity of sympathetic cholinergic neurons at the level of eccrine dermal sweat glands (Venables and Christie, 1980), to pleasant stimuli (but also unpleasant) has been shown in individuals with dysphoria relative to a control group (Benning and Ait Oumeziane, 2017; De Zorzi et al., 2021).
2.5. Interim conclusion
Taken together, the reviewed psychophysiological findings provide consistent support for a lack of sensitivity to pleasant and rewarding stimuli in vulnerability to depression. More integrative research is needed to clarify which of these measures might be more useful in the early identification of MDD as well as whether they could be leveraged together to improve clinical utility in at-risk samples.
3. The negative valence systems
The Negative Valence Systems (NVS) encompass five constructs related to responses to aversive stimuli or events. These constructs include responses to acute threat, potential threat, sustained threat, loss, and frustrative non-reward. Compared to the PVS, data on the reactivity to unpleasant stimuli in depression and vulnerability to depression have been extensively produced in several (and different) research areas and therefore the findings are rather mixed and sometimes even unable to show any significant effect (for a meta-analysis and a review on psychophysiological studies on emotional reactivity see Bylsma et al., 2008 and Bylsma, 2021). Initial theories suggested that depression would be characterized by an increased reactivity to unpleasant emotional stimuli based on the idea that individuals’ background affective state would prime reactivity to a stimulus of matching valence (Rosenberg, 1998; Rottenberg, 2017). Cognitive theories of depression (Beck and Bredemeier, 2016) seem to have a similar hypothesis: negative cognitive schemas guide preferential processing of negative stimuli which, in turn, lead to enhanced attention and intake of these cues. For instance, in support of this claim, individuals with dysphoria, but not controls, repeatedly showed a prolonged cardiac deceleration during passive viewing of unpleasant stimuli as compared with neutral ones, suggesting a sustained intake of unpleasant cues and a mood-related bias in this at-risk group (Messerotti Benvenuti et al., 2020; Moretta et al., 2021). Additionally, children of mothers with MDD showed greater physiological reactivity, indexed by pupil dilation, to sad, but not happy or neutral faces compared to children of non-depressed mothers (Burkhouse et al., 2014). However, the greater processing of unpleasant images observed in dysphoria does not seem to lead to greater action preparation and reactivity. Indeed, from most research using both passive and active tasks and different psychophysiological measures, depression appears to be mostly characterized by a reduced emotional reactivity to unpleasant stimuli (Foti et al., 2010; MacNamara et al., 2016; Hill et al., 2019; for a review see Bylsma, 2021). The lack of reactivity to unpleasant contents is in line with the emotion context insensitivity hypothesis (ECI; Rottenberg et al., 2005; Bylsma et al., 2008; Bylsma, 2021), which suggests that depression might be characterized by an overall blunted emotional reactivity, with reduced psychophysiological responses to all affective cues.
3.1. Event-related potentials
In support of the ECI model in depression risk, studies on the LPP during affective picture processing have also observed reduced LPP to unpleasant images in dysphoria relative to a control group (Benning and Ait Oumeziane, 2017; Grunewald et al., 2019), although some studies failed to find this effect (Moretta et al., 2021). Besides, the offspring of parents with a history of MDD had a reduced LPP to unpleasant faces and scenes compared to a control group (Kujawa et al., 2012; Nelson et al., 2015; Moretta and Messerotti Benvenuti, under review1). Importantly, in a large longitudinal study (Michelini et al., 2021), blunted LPP to unpleasant stimuli was one of the main predictors of fist-onset depressive disorder over a period of 3 years. However, findings are rather mixed as other studies linked maternal risk for MDD with enhanced LPP to unpleasant images (Speed et al., 2016).
Another way to examine the Negative Valence System is to assess EEG responses to the commission of an error (i.e., error monitoring). Indeed, making a mistake is generally perceived as subjectively unpleasant and, at times, it can be perilous and threatening to one’s life (Weinberg et al., 2016). A specific physiological measure of error monitoring is EEG error-related negativity (ERN), which arises as a negative electrocortical deflection in the ERP at frontocentral scalp sites within 100 ms following the commission of an error vs. a correct response (Gehring et al., 1995). The ERN has been mostly employed in the study of anxiety disorders (e.g., Meyer et al., 2018a), but there is some evidence that the ERN is blunted in adults and children with clinical depression (Ruchsow et al., 2004, 2006; Schrijvers et al., 2008; Weinberg et al., 2015c; Dell'Acqua et al., 2023) and depression risk (Meyer et al., 2018b; Tabachnick et al., 2018). For instance, Meyer and colleagues reported that the offspring of women with recurrent MDD had a reduced ERN relative to a control group, even when accounting for maternal anxiety (Meyer et al., 2018b). Another study showed that subclinical depressive symptoms were linked to blunted ERN in children involved with Child Protective Services (Tabachnick et al., 2018). However, other studies reported greater ERN in clinical depression (Chiu and Deldin, 2007; Holmes and Pizzagalli, 2010). Although these results are promising, more research on multiple at-risk populations is needed to clarify whether a blunted ERN can be considered a psychobiological marker of depression.
3.2. Startle eyeblink reflex
Other evidence comes from studies on the startle reflex measured at the orbicularis oculi muscle during exposure to emotional cues, which have reported reduced startle potentiation to unpleasant stimuli in individuals with dysphoria (Messerotti Benvenuti et al., 2020) but also enhance startle potentiation in individuals with past but recurrent depression (Vaidyanathan et al., 2014), suggesting that risk may not be equivalent in remitted individuals.
3.3. Skin conductance
As noted above in the PVS section, reduced skin conductance during the viewing of all emotional stimuli in individuals with dysphoria relative to a control group was reported (Benning and Ait Oumeziane, 2017). However, while reduced reactivity to unpleasant cues in affective processing tasks may represent a psychobiological marker of depression, greater skin conductance during sad mood induction and recovery were observed in the offspring of mothers with depression relative to a control group (Daches et al., 2020).
3.4. Interim conclusion
Taken together, the literature examining the NVS functioning in depression risk is mostly inconsistent and this might be due to several reasons, including cross-study differences in tasks and types of stimuli used, and/or the presence of comorbid anxiety symptoms (for reviews, see Weinberg et al., 2015a; Dickey et al., 2021). Although the role of NVS functioning in vulnerability to depression is not definite, many reviewed psychophysiological studies on emotional reactivity in at-risk samples suggest that vulnerability might be related to blunted responses to unpleasant stimuli, indicating a general pattern of blunted motivation in accordance with the ECI model.
4. The arousal and regulatory systems
The DSM-5 criteria for MDD include physical alterations, such as fatigue, sleep disturbances, and appetite changes. Beyond these three bodily symptoms, no other physical symptom is mentioned. However, other somatic symptoms are prevalent in individuals with depression, including headaches, musculoskeletal symptoms, palpitations, and upset stomach (Breslau et al., 2000; Vaccarino A. L. et al., 2008). Arousal might have a primary role in the somatic and neurovegetative symptoms experienced by individuals with depression and they can be ascribable to the Arousal and Regulatory Systems (ARS) of the RDoC (Gunzler et al., 2020). Somatic symptoms of depression are associated with longer disease duration, greater disability, poorer clinical outcomes, and elevated healthcare costs (Vaccarino A. L. et al., 2008; Vaccarino V. et al., 2008). These somatic consequences could partly be due to metabolic, immuno-inflammatory, autonomic, and hypothalamic–pituitary–adrenal axis (HPA) imbalances which can also reflect an altered psychoneurimmumological interaction. These imbalances are often present among MDD patients (Penninx et al., 2013) and they can increase the risk of developing cardiovascular diseases, metabolic syndromes, and overall immune system deterioration (Wolkowitz et al., 2011).
4.1. Sleep quality: EEG and actigraphy
Circadian rhythm alterations, such as sleep problems and insomnia, are not only a correlate of MDD but accumulating evidence suggests that they may represent a biomarker of the disorder (Modell and Lauer, 2007; Wiebe et al., 2012). Sleep disturbances are also a typical residual symptom following remission from depression (Carney et al., 2007). For instance, fragmented REM sleep assessed with the EEG (e.g., reduced sleep spindles, shorter latencies to REM, longer REM), was related to subclinical depressive symptoms (Pesonen et al., 2019), was observed in remitted individuals (Jindal et al., 2002), in adolescents and adults with a parental history of MDD (Lopez et al., 2010; Bat-Pitault et al., 2013), and was predictive of depression onset in at-risk adolescents (Rao et al., 2009). Moreover, reduced sleep quality, as assessed by self-report and actigraphy measures, was reported in adolescents with a parental history of MDD (Chen et al., 2012; Wescott et al., 2019), and to prospectively predict depressive symptoms in adolescents (Bei et al., 2015). Notably, altered sleep structure, as assessed with actigraphy and EEG, was observed even in infants born from depressed mothers, suggesting that even the prenatal environment could promote depression vulnerability of the child (Armitage et al., 2009; Bat-Pitault et al., 2017). Other researchers have looked at EEG vigilance and arousal and reported reduced arousal, as indexed by greater posterior resting alpha power in individuals with a parental history of MDD (Bruder et al., 2012).
4.2. Peripheral psychophysiology: Cardiovascular activity and skin conductance
Vulnerability to depression has also been related to autonomic unbalances, such as increased heart rate and reduced HRV in resting conditions (in depression: Kemp et al., 2010; Koch et al., 2019; with dysphoria, familial risk, and remitted; Vaccarino V. et al., 2008; Dell’Acqua et al., 2020; Moretta and Messerotti Benvenuti, 2022). Reduced resting HRV in a wide array of at-risk samples suggests that decreased cardiac autonomic balance might serve as an early marker of depression vulnerability. Moreover, a multi-wave study on a large sample of university students showed that a smaller decrease in respiratory sinus arrhythmia (RSA, a measure of vagal activity) and greater increases in heart rate in response to sad clips predicted greater depressive symptoms when individuals encountered negative life events, perhaps due to attenuated self-regulatory abilities (Stange et al., 2017). A reduced cardiac autonomic balance, as indexed by greater parasympathetic activation (reduced decreases in HRV) and sympathetic withdrawal (reduced pre-ejection period), during psychological (e.g., unsolvable puzzle) and physical challenges (e.g., handgrip), was also observed in youths with past depression relative to a control group (Bylsma et al., 2015). Conversely, while individuals with MDD showed blunted RSA and reduced heart rate increase to stress tasks (i.e., cold pressor and speech task), those in remission did not show the same pattern (Salomon et al., 2013; Bylsma et al., 2014), suggesting that the lack of withdrawal of parasympathetic control during stress might be state-dependent and not a putative risk factor of MDD in remitted individuals.
Another psychophysiological measure related to autonomic activity is skin conductance. As previously described, skin conductance mirrors exclusively the sympathetic nervous system activity. Accordingly, during the viewing of pleasant and unpleasant pictures, healthy individuals showed comparable skin conductance responses to similarly arousing stimuli, both pleasant and unpleasant, relative to neutral ones. Instead, individuals with subclinical depression showed reduced skin conductance to all emotional stimuli, supporting both the hypothesis of reduced functioning of the Arousal and Regulatory Systems, as well as the PVS and NVS domains (Benning and Ait Oumeziane, 2017). Similarly, reduced skin conductance response was reported in individuals with depression during a mental arithmetic task (Kim et al., 2019) and in individuals with dysphoria during a public speaking task (Schwerdtfeger and Rosenkaimer, 2011). Additionally, even unaffected offspring of chronically depressed mothers showed reduced skin conductance to stressful situations (i.e., arguments between adults; Cummings et al., 2007).
4.3. Cortisol levels
Another measure related to the Arousal and Regulatory domain is cortisol, the main stress hormone that reflects HPA functioning and that has been widely used in the study of neuroendocrine and dysfunctions in MDD (Lopez-Duran et al., 2009; Herbert, 2013). Individuals with depression have been shown to have elevated morning cortisol (e.g., Michael et al., 2000) and a greater cortisol awakening response (CAR; e.g., Bhagwagar et al., 2005; Vreeburg et al., 2009; but see also Huber et al., 2006). Interestingly, increased morning cortisol (e.g., Young et al., 2006; Dougherty et al., 2009) and CAR (e.g., Vreeburg et al., 2010; Nederhof et al., 2015) have been found in never-depressed offspring of parents with a depressive disorder. Moreover, higher CAR cortisol levels were reported in adolescents who subsequently developed a major depressive episode in the following year (Goodyer et al., 2000; Adam et al., 2010; Vrshek-Schallhorn et al., 2013). Collectively, these findings suggest that vulnerability to depression may be related to a hyperactive HPA, mostly in relation to its circadian rhythm (morning cortisol and CAR). The increased activity of the HPA axis is thought to be mostly related to reduced inhibition by endogenous glucocorticoids in the synthesis and release of the adrenocorticotrophic hormone-releasing factor in the paraventricular nucleus and adrenocorticotrophic hormone in the pituitary (Pariante and Lightman, 2008). Regarding cortisol reactivity to a stressor, a relatively blunted cortisol stress reactivity even when controlling for baseline measures was repeatedly observed in MDD (Burke et al., 2005; Harkness et al., 2011). However, whether a blunted cortisol stress reactivity represents a psychobiological marker of depression is rather unclear, but some studies reported reduced cortisol reactivity to stressors in adults and children with dysphoria (de Rooij et al., 2010; Hankin et al., 2010; Suzuki et al., 2013), those with a familial risk for depression (Morris et al., 2017), and in individuals in remission (Morris et al., 2014; but see also Morris et al., 2012; Höhne et al., 2014). However, to our knowledge, cortisol stress reactivity has not been examined in individuals with a parental risk for depression, and whether depression risk relates to blunted cortisol reactivity should be further explored.
4.4. Interim conclusion
Collectively, vulnerability to depression seems to be characterized by alterations of the Arousal and Regulatory Systems, such as sleep disturbances and autonomic unbalances in resting and stress-related conditions. As supported by studies on heart rate and cortisol, individuals at-risk for depression seem to be characterized by somatic heightened activation in resting conditions. Studies that assessed cardiovascular reactivity, skin conductance, and cortisol changes to pleasant and unpleasant stimuli (i.e., images, stressors) suggest that at-risk samples might have reduced physiological arousal when mobilization is required. These results emphasize that the Arousal and Regulatory Systems support the affective systems in the PVS and NVS domains and are consistent with the ECI hypothesis of a blunted emotional reactivity (Bylsma et al., 2008; Bylsma, 2021).
5. The cognitive systems
In addition to affective and somatic symptoms, cognitive symptoms have been widely reported in individuals with depression. One of the DSM-5 criteria for depression is, indeed, a diminished ability to think, concentrate, or make decisions (American Psychiatric Association, 2013). Cognitive dysfunctions in depression include impairments in cognitive control. Studies have reported that individuals with depressive symptoms show reduced sustained and divided attention (McClintock et al., 2010), overgeneralized declarative memory (Zhou et al., 2017), reduced cognitive flexibility, set-shifting, planning, and updating (Dotson et al., 2020; Dell’Acqua et al., 2022b). These deficits align with the Cognitive Systems domain of the RDoC, which includes constructs of Attention, Perception, Declarative Memory, Language, Cognitive Control, and Working Memory (Insel et al., 2010; Cuthbert and Insel, 2013). Cognitive control deficits have emerged as one of the potential behavioral endophenotypes of depression (Webb et al., 2016). Indeed, cognitive control deficits often persist in remitted individuals (Snyder, 2013), are a stable and reliable characteristic (Sarapas et al., 2012), and showed moderate-to-high heritability (Friedman et al., 2008). In addition, there is some evidence of cognitive control impairments in healthy, unaffected twins at risk for MDD (Christensen et al., 2006).
5.1. Event-related potentials
Besides, impairments in the Cognitive Systems are strictly related to the PVS and NVS domains. For instance, numerous studies have investigated cognitive control in affective contexts in relation to depressive symptoms (e.g., Koster et al., 2011; Joormann and Vanderlind, 2014). Although most research on cognitive control typically focuses on behavioral measures, such as reaction times in various tasks (e.g., Go/No-Go, Stroop; Kertz et al., 2019), some studies have explored the electrocortical correlates of cognitive processing in emotional contexts in depression and individuals vulnerable to depression. A task that has been broadly used is the Emotional Go/No-Go. For example, an enhanced No-Go P300 to negative relative to positive faces was positively correlated with depressive symptoms (Zhang et al., 2016) and was observed in individuals with dysphoria but not in controls (Krompinger and Simons, 2009), suggesting that greater processing resources were needed to inhibit the motor response during the presentation of unpleasant stimuli. Indeed, the P300 is an ERP related to attentional processing and resource allocation (Gray et al., 2004). Similarly, in an oddball paradigm, individuals with dysphoria and remitted depression, but not controls, showed greater P300 following sad relative to happy target faces, suggesting that these samples showed more attentional bias to these stimuli (Bistricky et al., 2014). These initial ERPs findings suggest that a mood-related bias may characterize vulnerability to depression when a cognitive effort is required. Contrariwise, Messerotti Benvenuti et al. (2017) showed a reduced Go/No-Go effect for P3 and delta power in response to pleasant and neutral, but not unpleasant, stimuli in individuals with dysphoria relative to non-dysphoric individuals. These findings suggest that individuals with dysphoria need a reduced and/or less effortful response inhibition to pleasant stimuli, supporting the hypothesis of reduced PVS activity. Evidence for reduced attention to pleasant stimuli comes also from eye-tracking studies, which have shown that individuals with past depression and those with dysphoria spent less time attending to pleasant, but not unpleasant images relative to controls (Sears et al., 2010, 2011).
Another electrocortical measure of cognitive control is the ERN, an event-related potential discussed within the NVS domain. Indeed, the ERN does not only reflect sensitivity to an endogenous threat (i.e., commission of an error) but it is also implicated in cognitive control abilities, namely the ability to rapidly detect errors and adaptively regulate actions in a dynamic environment (Weinberg et al., 2016). Some models on the ERN suggest that this measure acts as an early warning signal following the commission of an error evaluating the need to raise cognitive control resources allocated to the task (Weinberg et al., 2016). As previously described, the literature on the ERN in depression and its risk is still conflicting, with studies evidencing reduced (Ruchsow et al., 2004, 2006; Schrijvers et al., 2008; Weinberg et al., 2015a; Meyer et al., 2018b; Tabachnick et al., 2018;Dell'Acqua et al., 2023) or greater ERN (Chiu and Deldin, 2007; Holmes and Pizzagalli, 2010) in these groups.
5.2. Interim conclusion
Collectively, from the reviewed studies, the interaction between cognition and the PVS and NVS in determining depression vulnerability becomes evident. Particularly, individuals at-risk for depression seem to have inhibition difficulties of unpleasant stimuli and facilitation of pleasant ones. Taken together, psychophysiological research on cognitive dysfunctions and the interference of emotion on cognitive processing in at-risk populations is still in its infancy and more studies with more heterogeneous paradigms are needed to further identify psychophysiological markers related to the Cognitive Systems of depression vulnerability.
6. The sensorimotor systems
Psychomotor disturbances (retardation or agitation) are core features of depression and are included as a diagnostic criterion in the DSM-5. Considering that motor activity (e.g., walking) is needed to increase the chances of rewarding and pleasant events (e.g., meeting some friends or a partner), it is not surprising that psychomotor retardation and reduced gross motor activity are core features of depression (Razavi et al., 2011; Bewernick et al., 2017; Walther et al., 2019; Shankman et al., 2020; Wüthrich et al., 2022). Indeed, motor processes are strictly related to motivational drive and positive emotionality that support approach actions (Walther et al., 2019). These motor disturbances align with the Sensorimotor Systems of the RDoC, a domain that was recently added to the matrix (Garvey and Cuthbert, 2017). The Sensorimotor domain includes four constructs, namely Motor Actions, Agency and Ownership, Habit, and Innate Motor Patterns. Psychomotor retardation can be ascribed to the Motor Actions Construct.
6.1. Actigraphy
The assessment of motor disturbances in depression has long been confined to self-report measures and only recently research is shifting toward more objective and ecological measures, such as actigraphy (Walther et al., 2019). Low levels of motor activity, as assessed by a wrist-worn actigraphy, were documented in older adults with remitted depression relative to an age-matched control group (Pye et al., 2021) and were related to subclinical depressive symptoms (Mendlowicz et al., 1999).
6.2. EEG spectral features of motor activity
The EEG correlates of motor activity disturbances have only been investigated in clinical depression and have focused on the examination of resting spectral characteristics in relation to psychomotor retardation levels (Nieber and Schlegel, 1992; Cantisani et al., 2015). For example, a left-lateralized pattern of frontal alpha activity was negatively associated with activity levels (assessed with an actigraphy) in individuals with MDD, suggesting that psychomotor retardation may be related to impaired motivational drive (Cantisani et al., 2015). A negative covariance between resting alpha power over motor areas and activity levels was also reported (Nieber and Schlegel, 1992; Cantisani et al., 2015). Considering that alpha power mirrors inhibition of a cortical region, these results might indicate that psychomotor retardation is reflected in reduced motor cortex activity even in conditions of rest, potentially representing a trait feature or these alternations (Cantisani et al., 2015). Overall, it would be valuable to further explore the link between psychomotor retardation and motivation dispositions by means of fine psychophysiological measures (e.g., startle reflex) in depression and its risk.
6.3. Interim conclusion
The lack of systematic research on psychomotor disturbances in at-risk samples does not allow for determining whether these disturbances represent a core underlying etiological mechanism of MDD. Studies on risk samples but also longitudinal designs are warranted to better identify whether motor disturbances may represent a viable target for MDD prevention. This would have several advantages, considering that targeting motor functions could be accomplished in different ways (i.e., physical activity, and brain stimulation; Walther et al., 2017).
7. The systems for social processes
Depressive symptoms have long been associated with social impairments and poor social functioning (Gotlib and Hammen, 1992). Social impairments are included within the Systems for Social Processes of the RDoC, which include the following domains: Affiliation and Attachment, Social Communication, Perception and Understanding of Self, and Perception and Understanding of Others. Depression is associated with social anhedonia, namely reduced drive for social affiliation, but also with increased sensitivity to social rejection. As might already be evident, this dimension is closely related to the PVS, particularly in the study of depression.
Sensitivity to social rewards has been included in the Affiliation and Attachment domain and can be assessed with the reward positivity component (RewP, see Section 2) during a social feedback task. For example, in the island gateway task, participants play a game in which they are traveling along the Hawaiian Islands and trying to avoid being voted off the island by other (computerized) players whom they are told are age-matched peers (Kujawa et al., 2014a,b). Participants create online profiles, and, in a series of rounds, vote other players on or off the island, while receiving feedback on which players voted them on or off. Participants receive approximately the same number of acceptances and rejections over the course of the task. It has been recently observed that reduced RewP to social rejection during this task significantly predicted the onset of depressive symptoms in a sample of adolescents (Pegg et al., 2019, 2021), suggesting that blunted neural sensitivity to being socially excluded might represent a psychobiological marker of MDD. Similarly, subclinical depressive symptoms were linked to reduced time-frequency delta power to social rewards (Jin et al., 2019). Additionally, other studies indicated a smaller RewP to social acceptance in individuals with depression (Kujawa et al., 2017; Distefano et al., 2018) and those at risk for the disorder (Freeman et al., 2022a,b).
Related to the Cognitive Systems, the offspring of parents with depression recruited more resources to have an optimal performance during a cognitive task (i.e., larger P300) to prevent making a speech in front of an audience (in case of suboptimal performance), thus avoiding social evaluation (Pérez-Edgar et al., 2006). These latter findings are at odds with the hypothesis of reduced sensitivity to social rejection in depression vulnerability. Further research is needed to better parse social functioning and its psychophysiological correlates in depression vulnerability. Further support for reduced sensitivity to social affiliation, individuals with dysphoria showed a reduced increase in heart rate to social rewards relative to a control group (Brinkmann et al., 2014).
8. Discussion
The present review integrated findings from psychophysiological research in individuals at elevated risk for depression development or maintenance, owing to a familial history, dysphoria, or past depression, as well as longitudinal studies that examined predictors of future depression, adopting an RDoC lens. Hence, each of the described psychophysiological underpinnings could confer a higher risk to develop or maintain full-blown depression and, notably, are apparent before the onset of the disorder (see Table 1; Figure 1 for a summary of the reviewed literature).
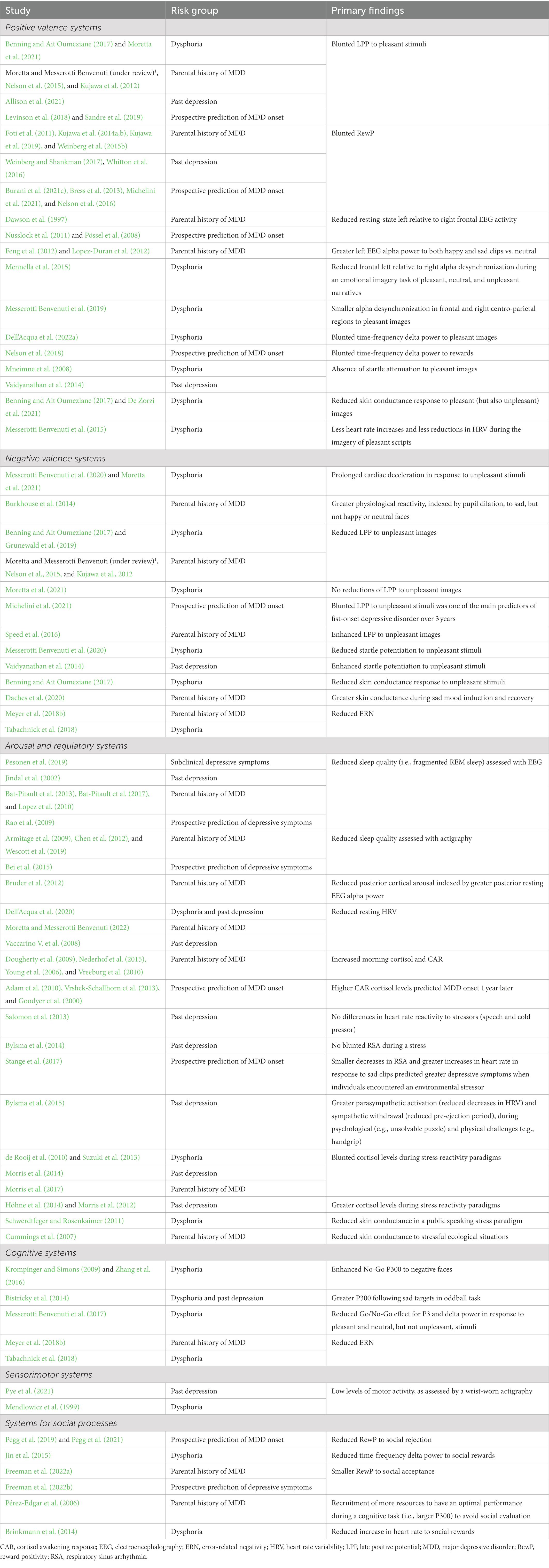
Table 1. Summary of the studies within each RDoC domain in relation to risk for depression development and maintenance included in the review.
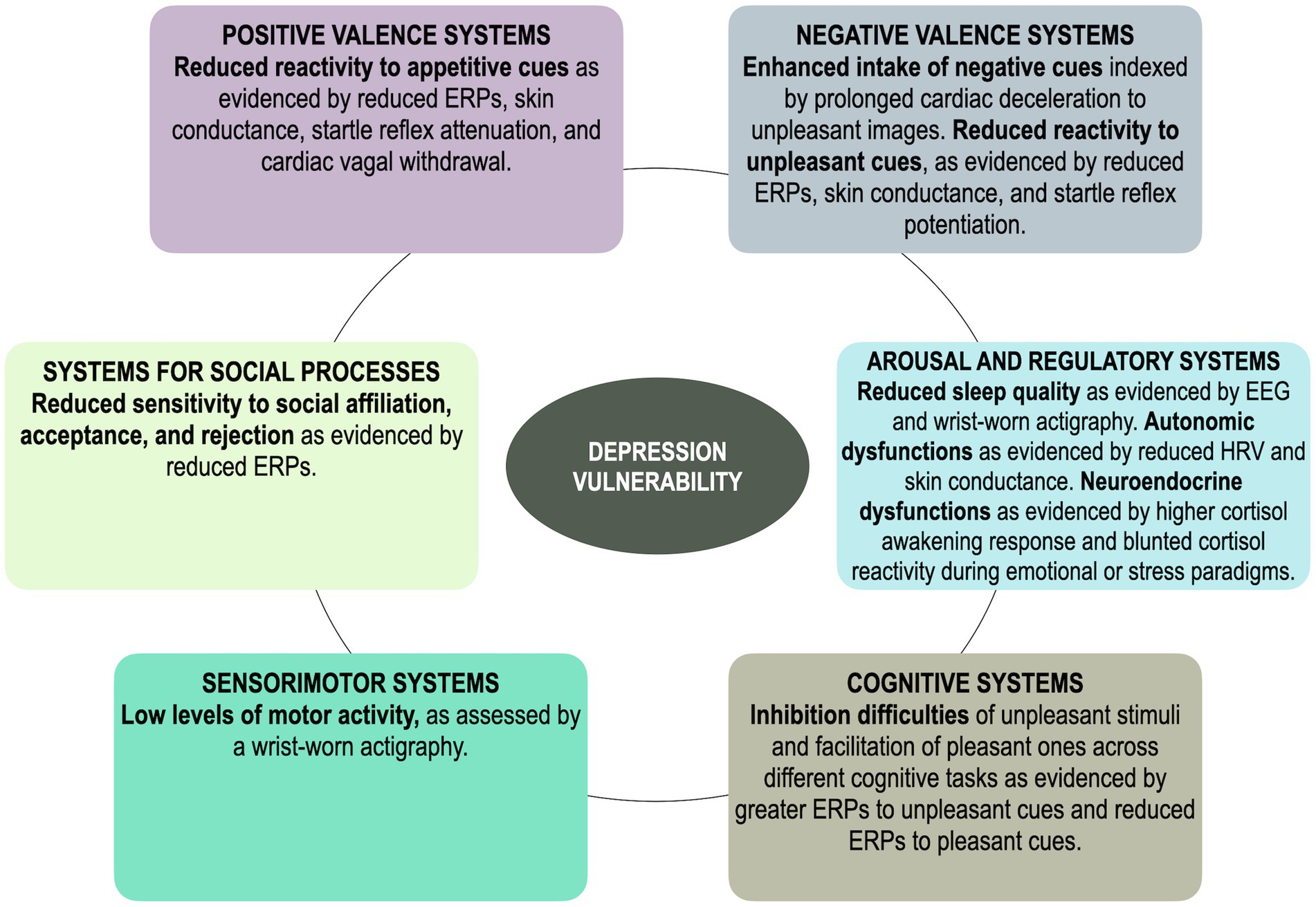
Figure 1. A simplified and schematic illustration of the main findings regarding all RDoC systems in vulnerability to depression. ERPs, event-related potentials.
In sum, consistent evidence across multiple psychophysiological levels indicates that reduced responses to appetitive/rewarding cues, belonging within the Positive Valence domain, characterize individuals that are more vulnerable to depression onset. Indeed, based on the reviewed literature, reduced approach motivation seems to represent the most consolidated and robust vulnerability marker of depression. Evidence on the Negative Valence is more mixed, but most studies suggest that at-risk samples may be characterized by a greater intake of unpleasant information that, however, does not lead to greater reactivity but to a blunted reactivity to unpleasant cues. These findings suggest that, in line with the ECI hypothesis (Bylsma et al., 2008), blunted positive and negative emotional reactivity might represent a risk factor for MDD. Depression risk appears to be also characterized by alterations in the Arousal and Regulatory domain, whereby at-risk samples are characterized by sleep alterations and autonomic unbalances in resting conditions and during the viewing of emotional images or stress induction paradigms. Within the Cognitive domain, EEG studies have looked at how emotional cues modulate cognitive resources in depression vulnerability and found that at-risk samples experience inhibition difficulties during the presentation of unpleasant content and facilitated inhibition to pleasant content, suggesting that – at the cognitive level – enhanced attention and processing of mood-related content might be a risk factor of MDD. Moreover, whether motor disturbances that characterize depression, ascribable to the Sensorimotor domain, are viable vulnerability factors of depression still needs to be properly explored, although some studies suggest that low levels of motor activity might characterize at-risk samples. Last, psychophysiological research has recently begun to examine correlates of social processes, but this work is still in its early stages and needs to be extended to other levels of analysis.
From the reviewed literature, what emerges is a strong interrelation among each of the RDoC domains in depression vulnerability. For example, by studying autonomic reactivity (Arousal and Regulatory domain) to unpleasant laboratory stressors (Negative Valence domain), cognitive processes (Cognitive domain) to affective stimuli (Positive and Negative Valence domain), and the relation between psychomotor retardation (Sensorimotor domain) and approach motivation (Positive valence domain) or baseline cortical arousal (i.e., posterior alpha; Arousal and Regulatory domain), researchers are concurrently tackling several dimensions related to depression vulnerability. Indeed, it becomes clear that vulnerability may not be conferred by a single process but by the interrelation of many processes. This highlights how the development of a single condition is truly a product of the interplay among multiple factors that can be potentially targeted for prevention and early intervention.
Nevertheless, another important aspect that, according to the RDoC framework, has a transversal impact on all domains is environmental influences. For example, exposure to negative stressful life events is a well-established risk factor for psychopathology and seems to have an impact on multiple domains. Chronic stress has significant adverse effects on brain regions implicated in reward processing (Pizzagalli, 2014; Ethridge et al., 2018; Burani et al., 2021b) and endocrine and autonomic regulation (Sheth et al., 2017), processes that have been described throughout this review. Of note, there is evidence of how stressful life events interact with neural activity to rewards to prospectively predict the development of depression (Burani et al., 2021a), further supporting the role of an environmental influence on the functioning of an RDoC domain in determining vulnerability for psychopathology. Although this was not the focus of this review, many other environmental factors may act as catalysts for vulnerability factors in determining the development of depression (Bronfenbrenner and Morris, 2007).
Future work should aim at incorporating multiple dimensions to identify narrower and specific vulnerability profiles to ultimately improve the ability of clinicians to recognize people early and implement ad-hoc strategies (e.g., Craske et al., 2016). However, to do this, some issues in the pursuit of psychophysiological vulnerabilities of depression will have to be addressed. Firstly, for the assessment of each RDoC domain, it is important to unify paradigms and methods to promote the replicability of results and build robust evidence across investigations. Then, it is also important to account for sociodemographic variables that may drive some of the mixed findings, such as gender, race, and socioeconomic status. Further, to precisely identify vulnerability profiles, more longitudinal investigations examining trajectories of risk are warranted. Another important point that should be further expanded is the role of development, emphasized in the RDoC model. In particular, the RDoC framework advises posing attention to the importance of improving the knowledge of typical and atypical developmental trajectories as well as enhancing prevention and intervention efforts by identifying reliable and valid biomarkers of risk for psychopathology in early in life. The current narrative review focused on the available studies that included a broad age range, including studies on children, but future efforts should be made to conduct more research on depression risk during early life. Additionally, the present review focused on studies that employed psychophysiological models as these represent a useful framework in redefining dimensions involved in psychopathology and present numerous methodological advantages (e.g., non-invasive, well-tolerated, and economic). However, there are still some barriers to these methods in improving the understanding of psychopathology among minoritized races and ethnicities (e.g., Kredlow et al., 2017; Choy et al., 2022).
In conclusion, the present work described, for each RDoC domain, studies aimed at identifying psychobiological markers of depression risk. Insights into some viable mechanisms that contribute to the development of depression in at-risk samples were provided and the effectiveness and potential of psychophysiological models within the RDoC framework for exploring and understanding depression pathophysiology were emphasized. Nonetheless, despite the significant progress that has been made, additional effort is required to better identify vulnerability profiles that can precisely predict the disorder.
Author contributions
CDA, EP, DP, and SMB: conceptualization. CDA: writing the original draft, editing, and reviewing. EP, DP, and SMB: supervision, review, and editing. DP and SMB: funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by a grant from MIUR [Dipartimenti di Eccellenza DM 11/05/2017 n. 262] to the Department of General Psychology, University of Padua, and by a grant from MIUR [PRIN n. 2017BC4MST] to DP. SMB’s work was supported by the University of Padua under the 2019 STARS Grants programme [Acronym and title of the project: A-CAOS-BIRD – Asymmetries and Connectivity in Alpha OScillations: toward Biomarkers of Intergenerational Risk for Depression].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1. ^Moretta, T., and Messerotti Benvenuti, S. (under review). Emotional processing in individuals with familial risk for depression: an ERP and cardiac deceleration study.
References
Adam, E. K., Doane, L. D., Zinbarg, R. E., Mineka, S., Craske, M. G., and Griffith, J. W. (2010). Prospective prediction of major depressive disorder from cortisol awakening responses in adolescence. Psychoneuroendocrinology 35, 921–931. doi: 10.1016/j.psyneuen.2009.12.007
Allen, J. J., Urry, H. L., Hitt, S. K., and Coan, J. A. (2004). The stability of resting frontal electroencephalographic asymmetry in depression. Psychophysiology 41, 269–280. doi: 10.1111/j.1469-8986.2003.00149.x
Allison, G. O., Kamath, R. A., Carrillo, V., Alqueza, K. L., Pagliaccio, D., Slavich, G. M., et al. (2021). Self-referential processing in remitted depression: an event-related potential study. Biol. Psychiatry Global Open Sci. 3, 119–129. doi: 10.1016/j.bpsgos.2021.12.005
American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders. 5th edn, American Psychiatric Publishing, Inc.
Armitage, R., Flynn, H., Hoffmann, R., Vazquez, D., Lopez, J., and Marcus, S. (2009). Early developmental changes in sleep in infants: the impact of maternal depression. Sleep 32, 693–696. doi: 10.1093/sleep/32.5.693
Bat-Pitault, F., Da Fonseca, D., Cortese, S., Le Strat, Y., Kocher, L., Rey, M., et al. (2013). The sleep macroarchitecture of children at risk for depression recruited in sleep centers. Eur. Psychiatry 28, 168–173. doi: 10.1016/j.eurpsy.2012.02.007
Bat-Pitault, F., Sesso, G., Deruelle, C., Flori, S., Porcher-Guinet, V., Stagnara, C., et al. (2017). Altered sleep architecture during the first months of life in infants born to depressed mothers. Sleep Med. 30, 195–203. doi: 10.1016/j.sleep.2016.11.018
Beck, A. T., and Bredemeier, K. (2016). A unified model of depression. Clin. Psychol. Sci. 4, 596–619. doi: 10.1177/2167702616628523
Bei, B., Coo, S., and Trinder, J. (2015). Sleep and mood during pregnancy and the postpartum period. Sleep Med. Clin. 10, 25–33. doi: 10.1016/j.jsmc.2014.11.011
Benning, S. D., and Ait Oumeziane, B. (2017). Reduced positive emotion and underarousal are uniquely associated with subclinical depression symptoms: evidence from psychophysiology, self-report, and symptom clusters. Psychophysiology 54, 1010–1030. doi: 10.1111/psyp.12853
Berntson, G. G., Cacioppo, J. T., and Quigley, K. S. (1993). Cardiac psychophysiology and autonomic space in humans: empirical perspectives and conceptual implications. Psychol. Bull. 114, 296–322. doi: 10.1037/0033-2909.114.2.296
Bewernick, B. H., Urbach, A. S., Bröder, A., Kayser, S., and Schlaepfer, T. E. (2017). Walking away from depression-motor activity increases ratings of mood and incentive drive in patients with major depression. Psychiatry Res. 247, 68–72. doi: 10.1016/j.psychres.2016.09.009
Bhagwagar, Z., Hafizi, S., and Cowen, P. J. (2005). Increased salivary cortisol after waking in depression. Psychopharmacology 182, 54–57. doi: 10.1007/s00213-005-0062-z
Bistricky, S. L., Atchley, R. A., Ingram, R., and O'Hare, A. (2014). Biased processing of sad faces: an ERP marker candidate for depression susceptibility. Cogn. Emot. 28, 470–492. doi: 10.1080/02699931.2013.837815
Boecker, L., and Pauli, P. (2019). Affective startle modulation and psychopathology: implications for appetitive and defensive brain systems. Neurosci. Biobehav. Rev. 103, 230–266. doi: 10.1016/j.neubiorev.2019.05.019
Bradley, M. M., Cuthbert, B. N., and Lang, P. J. (1999). “Affect and the startle reflex,” in Startle modification: Implications for neuroscience, cognitive science, and clinical science. eds. M. E. Dawson, A. M. Schell and A. H. Böhmelt (Cambridge University Press), 157–183.
Breslau, N., Schultz, L. R., Stewart, W. F., Lipton, R. B., Lucia, V. C., and Welch, K. M. A. (2000). Headache and major depression: is the association specific to migraine? Neurology 54:308. doi: 10.1212/WNL.54.2.308
Bress, J. N., Foti, D., Kotov, R., Klein, D. N., and Hajcak, G. (2013). Blunted neural response to rewards prospectively predicts depression in adolescent girls. Psychophysiology 50, 74–81. doi: 10.1111/j.1469-8986.2012.01485.x
Brinkmann, K., Franzen, J., Rossier, C., and Gendolla, G. H. (2014). I don’t care about others’ approval: dysphoric individuals show reduced effort mobilization for obtaining a social reward. Motiv. Emot. 38, 790–801. doi: 10.1007/s11031-014-9437-y
Bronfenbrenner, U., and Morris, P. A. (2007). “The bioecological model of human development,” in Handbook of child psychology. Vol. 1. eds. W. Damon, R. M. Lerner, K. A Renninger and I. E. Sigel (Hoboken, NJ: John Wiley and Sons).
Bruder, G. E., Bansal, R., Tenke, C. E., Liu, J., Hao, X., Warner, V., et al. (2012). Relationship of resting EEG with anatomical MRI measures in individuals at high and low risk for depression. Hum. Brain Map. 33, 1325–1333. doi: 10.1002/hbm.21284
Bruder, G. E., Tenke, C. E., Warner, V., Nomura, Y., Grillon, C., Hille, J., et al. (2005). Electroencephalographic measures of regional hemispheric activity in offspring at risk for depressive disorders. Biol. Psychiatry 57, 328–335. doi: 10.1016/j.biopsych.2004.11.015
Brunwasser, S. M., and Garber, J. (2016). Programs for the prevention of youth depression: evaluation of efficacy, effectiveness, and readiness for dissemination. J. Clin. Child Adolesc. Psychol. 45, 763–783. doi: 10.1080/15374416.2015.1020541
Burani, K., Brush, C. J., Gallyer, A., Joiner, T., Nelson, B., and Hajcak, G. (2021c). Maternal suicidality interacts with blunted reward processing to prospectively predict increases in depressive symptoms in 8-to-14-year-old girls. Int. J. Psychophysiol. 170, 67–74. doi: 10.1016/j.ijpsycho.2021.10.002
Burani, K., Gallyer, A., Ryan, J., Jordan, C., Joiner, T., and Hajcak, G. (2021b). Acute stress reduces reward-related neural activity: evidence from the reward positivity. Stress 24, 833–839. doi: 10.1080/10253890.2021.1929164
Burani, K., Klawohn, J., Levinson, A. R., Klein, D. N., Nelson, B. D., and Hajcak, G. (2021a). Neural response to rewards, stress and sleep interact to prospectively predict depressive symptoms in adolescent girls. J. Clin. Child Adolesc. Psychol. 50, 131–140. doi: 10.1080/15374416.2019.1630834
Burke, H. M., Davis, M. C., Otte, C., and Mohr, D. C. (2005). Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology 30, 846–856. doi: 10.1016/j.psyneuen.2005.02.010
Burkhouse, K. L., Siegle, G. J., and Gibb, B. E. (2014). Pupillary reactivity to emotional stimuli in children of depressed and anxious mothers. J. Child Psychol. Psychiatry 55, 1009–1016. doi: 10.1111/jcpp.12225
Bylsma, L. M. (2021). Emotion context insensitivity in depression: toward an integrated and contextualized approach. Psychophysiology 58:e13715. doi: 10.1111/psyp.13715
Bylsma, L. M., Morris, B. H., and Rottenberg, J. (2008). A meta-analysis of emotional reactivity in major depressive disorder. Clin. Psychol. Rev. 28, 676–691. doi: 10.1016/j.cpr.2007.10.001
Bylsma, L. M., Salomon, K., Taylor-Clift, A., Morris, B. H., and Rottenberg, J. (2014). RSA reactivity in current and remitted major depressive disorder. Psychosom. Med. 76:66. doi: 10.1097/PSY.0000000000000019
Bylsma, L. M., Yaroslavsky, I., Rottenberg, J., Jennings, J. R., George, C. J., Kiss, E., et al. (2015). Juvenile onset depression alters cardiac autonomic balance in response to psychological and physical challenges. Biol. Psychol. 110, 167–174. doi: 10.1016/j.biopsycho.2015.07.003
Cantisani, A., Koenig, T., Horn, H., Müller, T., Strik, W., and Walther, S. (2015). Psychomotor retardation is linked to frontal alpha asymmetry in major depression. J. Affect. Disord. 188, 167–172. doi: 10.1016/j.jad.2015.08.018
Carney, C. E., Segal, Z. V., Edinger, J. D., and Krystal, A. D. (2007). A comparison of rates of residual insomnia symptoms following pharmacotherapy or cognitive-behavioral therapy for major depressive disorder. J. Clin. Psych. 68, 254–260. doi: 10.4088/JCP.v68n0211
Chen, M. C., Burley, H. W., and Gotlib, I. H. (2012). Reduced sleep quality in healthy girls at risk for depression. J. Sleep Res. 21, 68–72. doi: 10.1111/j.1365-2869.2011.00934.x
Chiu, P. H., and Deldin, P. J. (2007). Neural evidence for enhanced error detection in major depressive disorder. Am. J. Psychiatry 164, 608–616. doi: 10.1007/s42761-021-00050-0
Choy, T., Baker, E., and Stavropoulos, K. (2022). Systemic racism in EEG research: considerations and potential solutions. Affec. Sci. 3, 14–20. doi: 10.1007/s42761-021-00050-0
Christensen, M. V., Kyvik, K. O., and Kessing, L. V. (2006). Cognitive function in unaffected twins discordant for affective disorder. Psychol. Med. 36, 1119–1129. doi: 10.1017/S0033291706007896
Craske, M. G., Meuret, A. E., Ritz, T., Treanor, M., and Dour, H. J. (2016). Treatment for anhedonia: a neuroscience driven approach. Dep. Anxiety 33, 927–938. doi: 10.1002/da.22490
Cummings, E. M., El-Sheikh, M., Kouros, C. D., and Keller, P. S. (2007). Children's skin conductance reactivity as a mechanism of risk in the context of parental depressive symptoms. J. Child Psychol. Psychiatry 48, 436–445. doi: 10.1111/j.1469-7610.2006.01713.x
Cuthbert, B. N., and Insel, T. R. (2013). Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 11, 1–8. doi: 10.1186/1741-7015-11-126
Cuthbert, B. N., Schupp, H. T., Bradley, M. M., Birbaumer, N., and Lang, P. J. (2000). Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biol. Psychol. 52, 95–111. doi: 10.1016/S0301-0511(99)00044-7
Daches, S., Vine, V., George, C. J., Jennings, J. R., and Kovacs, M. (2020). Sympathetic arousal during the processing of dysphoric affect by youths at high and low familial risk for depression. Psychophysiology 57:e13664. doi: 10.1111/psyp.13664
Davidson, R. J. (1998). Affective style and affective disorders: perspectives from affective neuroscience. Cogn. Emot. 12, 307–330. doi: 10.1080/026999398379628
Dawson, G., Frey, K., Panagiotides, H., Osterling, J., and Hess, I. D. (1997). Infants of depressed mothers exhibit atypical frontal brain activity a replication and extension of previous findings. J. Child Psychol. Psychiatry 38, 179–186. doi: 10.1111/j.1469-7610.1997.tb01852.x
de Rooij, S. R., Schene, A. H., Phillips, D. I., and Roseboom, T. J. (2010). Depression and anxiety: associations with biological and perceived stress reactivity to a psychological stress protocol in a middle-aged population. Psychoneuroendocrinology 35, 866–877. doi: 10.1016/j.psyneuen.2009.11.011
De Zorzi, L., Ranfaing, S., Honoré, J., and Sequeira, H. (2021). Autonomic reactivity to emotion: a marker of sub-clinical anxiety and depression symptoms? Psychophysiology 58:e13774. doi: 10.1111/psyp.13774
Dell’Acqua, C., Brush, C. J., Burani, K., Santopetro, N. J., Klawohn, J., Messerotti Benvenuti, S., et al. (2022d). Reduced electrocortical responses to pleasant pictures in depression: a brief report on time-domain and time-frequency delta analyses. Biol. Psychol. 170:108302. doi: 10.1016/j.biopsycho.2022.108302
Dell’Acqua, C., Dal Bò, E., Messerotti Benvenuti, S., and Palomba, D. (2020). Reduced heart rate variability is associated with vulnerability to depression. J. Affect. Disord. Reports 1:100006. doi: 10.1016/j.jadr.2020.100006
Dell’Acqua, C., Dal Bò, E., Moretta, T., Palomba, D., and Messerotti Benvenuti, S. (2022a). EEG time–frequency analysis reveals blunted tendency to approach and increased processing of unpleasant stimuli in dysphoria. Sci. Rep. 12, 8161–8113. doi: 10.1038/s41598-022-12263-9
Dell’Acqua, C., Messerotti Benvenuti, S., Vallesi, A., Palomba, D., and Ambrosini, E. (2022b). Depressive symptoms and cognitive control: the role of affective interference. Cogn. Emot. 36, 1389–1403. doi: 10.1080/02699931.2022.2128065
Dell'Acqua, C., Hajcak, G., Amir, N., Santopetro, N. J., Brush, C. J., and Meyer, A. (2023). Error-related brain activity in pediatric major depressive disorder: an ERP and time-frequency investigation. Int. J. Psychophysiol 184, 100–109. doi: 10.1016/j.ijpsycho.2023.01.005
Dell'Acqua, C., Moretta, T., Dal Bò, E., Messerotti Benvenuti, S., and Palomba, D. (2022c). Emotional processing prospectively modulates the impact of anxiety on COVID-19 pandemic-related post-traumatic stress symptoms: an ERP study. J. Affect. Disord. 303, 245–254. doi: 10.1016/j.jad.2022.02.027
Dichter, G. S., and Tomarken, A. J. (2008). The chronometry of affective startle modulation in unipolar depression. J. Abnorm. Psychol. 117:1. doi: 10.1037/0021-843X.117.1.1
Dickey, L., Politte-Corn, M., and Kujawa, A. (2021). Development of emotion processing and regulation: insights from event-related potentials and implications for internalizing disorders. Int. J. Psychophysiol. 170, 121–132. doi: 10.1016/j.ijpsycho.2021.10.003
Dillon, D. G., Rosso, I. M., Pechtel, P., Killgore, W. D., Rauch, S. L., and Pizzagalli, D. A. (2014). Peril and pleasure: an RDOC-inspired examination of threat responses and reward processing in anxiety and depression. Dep. Anxiety 31, 233–249. doi: 10.1002/da.22202
Distefano, A., Jackson, F., Levinson, A. R., Infantolino, Z. P., Jarcho, J. M., and Nelson, B. D. (2018). A comparison of the electrocortical response to monetary and social reward. Soc. Cogn. Affect. Neurosci. 13, 247–255. doi: 10.1093/scan/nsy006
Dotson, V. M., McClintock, S. M., Verhaeghen, P., Kim, J. U., Draheim, A. A., Syzmkowicz, S. M., et al. (2020). Depression and cognitive control across the lifespan: a systematic review and meta-analysis. Neuropsychol. Rev. 30, 461–476. doi: 10.1007/s11065-020-09436-6
Dougherty, L. R., Klein, D. N., Olino, T. M., Dyson, M., and Rose, S. (2009). Increased waking salivary cortisol and depression risk in preschoolers: the role of maternal history of melancholic depression and early child temperament. J. Child Psychol. Psychiatry 50, 1495–1503. doi: 10.1111/j.1469-7610.2009.02116.x
Ethridge, P., Freeman, C., Sandre, A., Banica, I., Dirks, M. A., and Weinberg, A. (2021). Intergenerational transmission of depression risk: mothers’ neural response to reward and history of depression are associated with daughters’ neural response to reward across adolescence. J. Abnorm. Psychol. 131, 598–610. doi: 10.1037/abn0000662
Ethridge, P., Sandre, A., Dirks, M. A., and Weinberg, A. (2018). Past-year relational victimization is associated with a blunted neural response to rewards in emerging adults. Soc. Cogn. Affect. Neurosci. 13, 1259–1267. doi: 10.1093/scan/nsy091
Feng, X., Forbes, E. E., Kovacs, M., George, C. J., Lopez-Duran, N. L., Fox, N. A., et al. (2012). Children’s depressive symptoms in relation to EEG frontal asymmetry and maternal depression. J. Abnorm. Child Psychol. 40, 265–276. doi: 10.1007/s10802-011-9564-9
Foti, D., Kotov, R., Klein, D. N., and Hajcak, G. (2011). Abnormal neural sensitivity to monetary gains versus losses among adolescents at risk for depression. J. Abnorm. Child Psychol. 39, 913–924. doi: 10.1007/s10802-011-9503-9
Foti, D., Olvet, D. M., Klein, D. N., and Hajcak, G. (2010). Reduced electrocortical response to threatening faces in major depressive disorder. Dep. Anxiety 27, 813–820. doi: 10.1002/da.20712
Foti, D., Weinberg, A., Bernat, E. M., and Proudfit, G. H. (2015). Anterior cingulate activity to monetary loss and basal ganglia activity to monetary gain uniquely contribute to the feedback negativity. Clin. Neurophysiol. 126, 1338–1347. doi: 10.1016/j.clinph.2014.08.025
Freeman, C., Ethridge, P., Banica, I., Sandre, A., Dirks, M. A., Kujawa, A., et al. (2022a). Neural response to rewarding social feedback in never-depressed adolescent girls and their mothers with remitted depression: associations with multiple risk indices. J. Psychopat. Clin. Sci. 131:141. doi: 10.1037/abn0000728
Freeman, C., Panier, L., Schaffer, J., and Weinberg, A. (2022b). Neural response to social but not monetary reward predicts increases in depressive symptoms during the COVID-19 pandemic. Psychophysiology, e14206. doi: 10.1111/psyp.14206
Friedman, N. P., Miyake, A., Young, S. E., DeFries, J. C., Corley, R. P., and Hewitt, J. K. (2008). Individual differences in executive functions are almost entirely genetic in origin. J. Exp. Psychol. Gen. 137, 201–225. doi: 10.1037/0096-3445.137.2.201
Gable, P. A., Paul, K., Pourtois, G., and Burgdorf, J. (2021). Utilizing electroencephalography (EEG) to investigate positive affect. Curr. Opin. Behav. Sci. 39, 190–195. doi: 10.1016/j.cobeha.2021.03.018
Garvey, M. A., and Cuthbert, B. N. (2017). Developing a motor systems domain for the NIMH RDoC program. Schizophr. Bull. 43, 935–936. doi: 10.1093/schbul/sbx095
Gehring, W. J., Coles, M. G., Meyer, D. E., and Donchin, E. (1995). A brain potential manifestation of error-related processing. Electroencephalogr. Clin. Neurophysiol. 44, 261–272.
Goodman, S. H., Rouse, M. H., Connell, A. M., Broth, M. R., Hall, C. M., and Heyward, D. (2011). Maternal depression and child psychopathology: a meta-analytic review. Clin. Child. Fam. Psychol. Rev. 14, 1–27. doi: 10.1007/s10567-010-0080-1
Goodyer, I. M., Herbert, J., Tamplin, A., and Altham, P. M. E. (2000). Recent life events, cortisol, dehydroepiandrosterone and the onset of major depression in high-risk adolescents. BJ Psych. 177, 499–504. doi: 10.1192/bjp.177.6.499
Gotlib, I. H., and Hammen, C. L. (1992). Psychological aspects of depression: toward a cognitive-interpersonal integration. Hoboken, NJ: John Wiley & Sons.
Gottesman, I. I., and Gould, T. D. (2003). The endophenotype concept in psychiatry: etymology and strategic intentions. Am. J. Psychiatry 160, 636–645. doi: 10.1176/appi.ajp.160.4.636
Gray, H. M., Ambady, N., Lowenthal, W. T., and Deldin, P. (2004). P 300 as an index of attention to self-relevant stimuli. J. Exper. Soc. Psychol. 40, 216–224. doi: 10.1016/S0022-1031(03)00092-1
Grunewald, M., Döhnert, M., Brandeis, D., Klein, A. M., Von Klitzing, K., Matuschek, T., et al. (2019). Attenuated LPP to emotional face stimuli associated with parent-and self-reported depression in children and adolescents. J. Abnormal Child Psychol. 47, 109–118. doi: 10.1007/s10802-018-0429-3
Gunzler, D., Sehgal, A. R., Kauffman, K., Davey, C. H., Dolata, J., Figueroa, M., et al. (2020). Identify depressive phenotypes by applying RDOC domains to the PHQ-9. Psychiatry Res. 286:112872. doi: 10.1016/j.psychres.2020.112872
Hajcak Proudfit, G. (2015). The reward positivity: from basic research on reward to a biomarker for depression. Psychophysiology 52, 449–459. doi: 10.1111/psyp.12370
Hankin, B. L., Badanes, L. S., Abela, J. R., and Watamura, S. E. (2010). Hypothalamic–pituitary–adrenal axis dysregulation in dysphoric children and adolescents: cortisol reactivity to psychosocial stress from preschool through middle adolescence. Biol. Psychiatry 68, 484–490. doi: 10.1016/j.biopsych.2010.04.004
Hardeveld, F., Spijker, J., De Graaf, R., Nolen, W. A., and Beekman, A. T. F. (2010). Prevalence and predictors of recurrence of major depressive disorder in the adult population. Acta Psychiatr. Scand. 122, 184–191. doi: 10.1111/j.1600-0447.2009.01519.x
Harkness, K. L., Stewart, J. G., and Wynne-Edwards, K. E. (2011). Cortisol reactivity to social stress in adolescents: role of depression severity and child maltreatment. Psychoneuroendocrinology 36, 173–181. doi: 10.1016/j.psyneuen.2010.07.006
Herbert, J. (2013). Cortisol and depression: three questions for psychiatry. Psychol. Med. 43, 449–469. doi: 10.1017/S0033291712000955
Hill, K. E., South, S. C., Egan, R. P., and Foti, D. (2019). Abnormal emotional reactivity in depression: contrasting theoretical models using neurophysiological data. Biol. Psychol. 141, 35–43. doi: 10.1016/j.biopsycho.2018.12.011
Höhne, N., Poidinger, M., Merz, F., Pfister, H., Brückl, T., Zimmermann, P., et al. (2014). Increased HPA axis response to psychosocial stress in remitted depression: the influence of coping style. Biol. Psychol. 103, 267–275. doi: 10.1016/j.biopsycho.2014.09.008
Holmes, A. J., and Pizzagalli, D. A. (2010). Effects of task-relevant incentives on the electrophysiological correlates of error processing in major depressive disorder. Cogn. Affect. Behav. Neurosci. 10, 119–128. doi: 10.3758/CABN.10.1.119
Horowitz, J. L., and Garber, J. (2006). The prevention of depressive symptoms in children and adolescents: a meta-analytic review. J. Consult. Clin. Psychol. 74:401. doi: 10.1037/0022-006X.74.3.401
Huber, T. J., Issa, K., Schik, G., and Wolf, O. T. (2006). The cortisol awakening response is blunted in psychotherapy inpatients suffering from depression. Psychoneuroendocrinology 31, 900–904. doi: 10.1016/j.psyneuen.2006.03.005
Insel, T., Cuthbert, B., Garvey, M., Heinssen, R., Pine, D. S., Quinn, K., et al. (2010). Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatry 167, 748–751. doi: 10.1176/appi.ajp.2010.09091379
Jaffee, S. R., Moffitt, T. E., Caspi, A., Fombonne, E., Poulton, R., and Martin, J. (2002). Differences in early childhood risk factors for juvenile-onset and adult-onset depression. Arch. Gen. Psychiatry 59, 215–222. doi: 10.1001/archpsyc.59.3.215
Jin, J., Sabharwal, A., Infantolino, Z. P., Jarcho, J. M., and Nelson, B. D. (2019). Time-frequency delta activity to social feedback demonstrates differential associations with depression and social anxiety symptoms. Front. Behav. Neurosci. 13:189. doi: 10.3389/fnbeh.2019.00189
Jin, A. B., Steding, L. H., and Webb, A. K. (2015). Reduced emotional and cardiovascular reactivity to emotionally evocative stimuli in major depressive disorder. Int. J. Psychophysiol. 97, 66–74. doi: 10.1016/j.ijpsycho.2015.04.014
Jindal, R. D., Thase, M. E., Fasiczka, A. L., Friedman, E. S., Buysse, D. J., Frank, E., et al. (2002). Electroencephalographic sleep profiles in single-episode and recurrent unipolar forms of major depression: II. Comparison during remission. Biol. Psychiatry 51, 230–236. doi: 10.1016/S0006-3223(01)01226-4
Joormann, J., and Vanderlind, W. M. (2014). Emotion regulation in depression: the role of biased cognition and reduced cognitive control. Clin. Psychol. Sci. 2, 402–421. doi: 10.1177/2167702614536163
Kemp, A. H., Quintana, D. S., Gray, M. A., Felmingham, K. L., Brown, K., and Gatt, J. M. (2010). Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol. Psychiatry 67, 1067–1074. doi: 10.1016/j.biopsych.2009.12.012
Kertz, S. J., Petersen, D. R., and Stevens, K. T. (2019). Cognitive and attentional vulnerability to depression in youth: a review. Clin. Psychol. Rev. 71, 63–77. doi: 10.1016/j.cpr.2019.01.004
Kim, A. Y., Jang, E. H., Choi, K. W., Jeon, H. J., Byun, S., Sim, J. Y., et al. (2019). Skin conductance responses in major depressive disorder (MDD) under mental arithmetic stress. PLoS One 14:e0213140. doi: 10.1371/journal.pone.0213140
Klawohn, J., Brush, C. J., and Hajcak, G. (2021a). Neural responses to reward and pleasant pictures prospectively predict remission from depression. J. Abnormal Psychol. 130, 702–712. doi: 10.1037/abn0000696
Klawohn, J., Burani, K., Bruchnak, A., Santopetro, N., and Hajcak, G. (2021b). Reduced neural response to reward and pleasant pictures independently relate to depression. Psychol. Med. 51, 741–749. doi: 10.1017/S0033291719003659
Knyazev, G. G. (2007). Motivation, emotion, and their inhibitory control mirrored in brain oscillations. Neurosci. Biobehav. Rev. 31, 377–395. doi: 10.1016/j.neubiorev.2006.10.004
Knyazev, G. G. (2012). EEG delta oscillations as a correlate of basic homeostatic and motivational processes. Neurosci. Biobehav. Rev. 36, 677–695. doi: 10.1016/j.neubiorev.2011.10.002
Koch, C., Wilhelm, M., Salzmann, S., Rief, W., and Euteneuer, F. (2019). A meta-analysis of heart rate variability in major depression. Psychol. Med. 49, 1948–1957. doi: 10.1017/S0033291719001351
Koster, E. H., De Lissnyder, E., Derakshan, N., and De Raedt, R. (2011). Understanding depressive rumination from a cognitive science perspective: the impaired disengagement hypothesis. Clin. Psychol. Rev. 31, 138–145. doi: 10.1016/j.cpr.2010.08.005
Kredlow, A. M., Pineles, S. L., Inslicht, S. S., Marin, M. F., Milad, M. R., Otto, M. W., et al. (2017). Assessment of skin conductance in African American and non–African American participants in studies of conditioned fear. Psychophysiology 54, 1741–1754. doi: 10.1111/psyp.12909
Kreibig, S. D. (2010). Autonomic nervous system activity in emotion: a review. Biol. Psychol. 84, 394–421. doi: 10.1016/j.biopsycho.2010.03.010
Krompinger, J. W., and Simons, R. F. (2009). Electrophysiological indicators of emotion processing biases in depressed undergraduates. Biol. Psychol. 81, 153–163. doi: 10.1016/j.biopsycho.2009.03.007
Kujawa, A., Arfer, K. B., Klein, D. N., and Proudfit, G. H. (2014a). Electrocortical reactivity to social feedback in youth: a pilot study of the island getaway task. Dev. Cogn. Neurosci. 10, 140–147. doi: 10.1016/j.dcn.2014.08.008
Kujawa, A., and Burkhouse, K. L. (2017). Vulnerability to depression in youth: advances from affective neuroscience. Biol. Psychiatry: Cogn. Neurosci. Neuroimaging 2, 28–37. doi: 10.1016/j.bpsc.2016.09.006
Kujawa, A., Hajcak, G., and Klein, D. N. (2019). Reduced reward responsiveness moderates the effect of maternal depression on depressive symptoms in offspring: evidence across levels of analysis. J. Child Psychol. Psychiatry 60, 82–90. doi: 10.1111/jcpp.12944
Kujawa, A., Hajcak, G., Torpey, D., Kim, J., and Klein, D. N. (2012). Electrocortical reactivity to emotional faces in young children and associations with maternal and paternal depression. J. Child Psychol. Psychiatry 53, 207–215. doi: 10.1111/j.1469-7610.2011.02461.x
Kujawa, A., Kessel, E. M., Carroll, A., Arfer, K. B., and Klein, D. N. (2017). Social processing in early adolescence: associations between neurophysiological, self-report, and behavioral measures. Biol. Psychol. 128, 55–62. doi: 10.1016/j.biopsycho.2017.07.001
Kujawa, A., Proudfit, G. H., and Klein, D. N. (2014b). Neural reactivity to rewards and losses in offspring of mothers and fathers with histories of depressive and anxiety disorders. J. Abnorm. Psychol. 123, 287–297. doi: 10.1037/a0036285
Laborde-Lahoz, P., El-Gabalawy, R., Kinley, J., Kirwin, P. D., Sareen, J., and Pietrzak, R. H. (2015). Subsyndromal depression among older adults in the USA: prevalence, comorbidity, and risk for new-onset psychiatric disorders in late life. Int. J. Geriatr. Psychiatry 30, 677–685. doi: 10.1002/gps.4204
Levinson, A. R., Speed, B. C., and Hajcak, G. (2018). Neural response to pleasant pictures moderates prospective relationship between stress and depressive symptoms in adolescent girls. J. Clin. Child Adolesc. Psychol. 48, 643–655. doi: 10.1080/15374416.2018.1426004
Liu, W. H., Wang, L. Z., Shang, H. R., Shen, Y., Li, Z., Cheung, E. F., et al. (2014). The influence of anhedonia on feedback negativity in major depressive disorder. Neuropsychologia 53, 213–220. doi: 10.1016/j.neuropsychologia.2013.11.023
Lopez, J., Hoffmann, R., and Armitage, R. (2010). Reduced sleep spindle activity in early-onset and elevated risk for depression. J. Am. Acad. Child Adolesc. Psychiatry 49, 934–943. doi: 10.1016/j.jaac.2010.05.014
Lopez-Duran, N. L., Kovacs, M., and George, C. J. (2009). Hypothalamic–pituitary–adrenal axis dysregulation in depressed children and adolescents: a meta-analysis. Psychoneuroendocrinology 34, 1272–1283. doi: 10.1016/j.psyneuen.2009.03.016
Lopez-Duran, N. L., Nusslock, R., George, C., and Kovacs, M. (2012). Frontal EEG asymmetry moderates the effects of stressful life events on internalizing symptoms in children at familial risk for depression. Psychophysiology 49, 510–521. doi: 10.1111/j.1469-8986.2011.01332.x
MacNamara, A., Kotov, R., and Hajcak, G. (2016). Diagnostic and symptom-based predictors of emotional processing in generalized anxiety disorder and major depressive disorder: an event-related potential study. Cogn. Ther. Res. 40, 275–289. doi: 10.1007/s10608-015-9717-1
Marci, C. D., Glick, D. M., Loh, R., and Dougherty, D. D. (2007). Autonomic and prefrontal cortex responses to autobiographical recall of emotions. Cogn. Affect. Behav. Neurosci. 7, 243–250. doi: 10.3758/CABN.7.3.243
McClintock, S. M., Husain, M. M., Greer, T. L., and Cullum, C. M. (2010). Association between depression severity and neurocognitive function in major depressive disorder: a review and synthesis. Neuropsychology 24, 9–34. doi: 10.1037/a0017336
McFarland, B. R., and Klein, D. N. (2009). Emotional reactivity in depression: diminished responsiveness to anticipated reward but not to anticipated punishment or to nonreward or avoidance. Dep. Anxiety 26, 117–122. doi: 10.1002/da.20513
Mendlowicz, M. V., Jean-Louis, G., von Gizycki, H., Zizi, F., and Nunes, J. (1999). Actigraphic predictors of depressed mood in a cohort of non-psychiatric adults. Aust. NZ J. Psychiatry 33, 553–558. doi: 10.1080/j.1440-1614.1999.00585.x
Mennella, R., Messerotti Benvenuti, S., Buodo, G., and Palomba, D. (2015). Emotional modulation of alpha asymmetry in dysphoria: results from an emotional imagery task. Int. J. Psychophysiol. 97, 113–119. doi: 10.1016/j.ijpsycho.2015.05.013
Messerotti Benvenuti, S., Buodo, G., Dal Bò, E., and Palomba, D. (2020). Attention and affect in dysphoria: insights from startle reflex modulation and cardiac deceleration. Behav. Res. Ther. 131:103626. doi: 10.1016/j.brat.2020.103626
Messerotti Benvenuti, S., Buodo, G., Mennella, R., Dal Bò, E., and Palomba, D. (2019). Appetitive and aversive motivation in depression: the temporal dynamics of task-elicited asymmetries in alpha oscillations. Sci. Rep. 9, 1–11. doi: 10.1038/s41598-019-53639-8
Messerotti Benvenuti, S., Buodo, G., and Palomba, D. (2017). Appetitive and aversive motivation in dysphoria: a time-domain and time-frequency study of response inhibition. Biol. Psychol. 125, 12–27. doi: 10.1016/j.biopsycho.2017.02.007
Messerotti Benvenuti, S., Mennella, R., Buodo, G., and Palomba, D. (2015). Dysphoria is associated with reduced cardiac vagal withdrawal during the imagery of pleasant scripts: evidence for the positive attenuation hypothesis. Biol. Psychol. 106, 28–38. doi: 10.1016/j.biopsycho.2014.11.017
Meyer, A., Bress, J. N., Hajcak, G., and Gibb, B. E. (2018b). Maternal depression is related to reduced error-related brain activity in child and adolescent offspring. J. Clin. Child Adol. Psychol. 47, 324–335. doi: 10.1080/15374416.2016.1138405
Meyer, A., Nelson, B., Perlman, G., Klein, D. N., and Kotov, R. (2018a). A neural biomarker, the error-related negativity, predicts the first onset of generalized anxiety disorder in a large sample of adolescent females. J. Child Psychol. Psychiatry 59, 1162–1170. doi: 10.1111/jcpp.12922
Michael, A., Jenaway, A., Paykel, E. S., and Herbert, J. (2000). Altered salivary dehydroepiandrosterone levels in major depression in adults. Biol. Psychiatry 48, 989–995. doi: 10.1016/S0006-3223(00)00955-0
Michelini, G., Perlman, G., Tian, Y., Mackin, D. M., Nelson, B. D., Klein, D. N., et al. (2021). Multiple domains of risk factors for first onset of depression in adolescent girls. J. Affect. Disord. 283, 20–29. doi: 10.1016/j.jad.2021.01.036
Mneimne, M., McDermut, W., and Powers, A. S. (2008). Affective ratings and startle modulation in people with nonclinical depression. Emotion 8, 552–559. doi: 10.1037/a0012827
Modell, S., and Lauer, C. J. (2007). Rapid eye movement (REM) sleep: an endophenotype for depression. Curr. Psychiatry Rep. 9, 480–485. doi: 10.1007/s11920-007-0065-z
Moretta, T., Dal Bò, E., Dell’Acqua, C., Messerotti Benvenuti, S., and Palomba, D. (2021). Disentangling emotional processing in dysphoria: an ERP and cardiac deceleration study. Behav. Res. Ther. 147:103985. doi: 10.1016/j.brat.2021.103985
Moretta, T., and Messerotti Benvenuti, S. (2022). Early indicators of vulnerability to depression: the role of rumination and heart rate variability. J. Affect. Disord. 312, 217–224. doi: 10.1016/j.jad.2022.06.049
Morris, M. C., Kouros, C. D., Mielock, A. S., and Rao, U. (2017). Depressive symptom composites associated with cortisol stress reactivity in adolescents. J. Affect. Disord. 210, 181–188. doi: 10.1016/j.jad.2016.12.023
Morris, M. C., Rao, U., and Garber, J. (2012). Cortisol responses to psychosocial stress predict depression trajectories: social-evaluative threat and prior depressive episodes as moderators. J. Affect. Disord. 143, 223–230. doi: 10.1016/j.jad.2012.05.059
Morris, M. C., Rao, U., Wang, L., and Garber, J. (2014). Cortisol reactivity to experimentally manipulated psychosocial stress in young adults at varied risk for depression. Dep. Anxiety 31, 44–52. doi: 10.1002/da.22125
Nederhof, E., Van Oort, F. V. A., Bouma, E. M. C., Laceulle, O. M., Oldehinkel, A. J., and Ormel, J. (2015). Predicting mental disorders from hypothalamic-pituitary-adrenal axis functioning: a 3-year follow-up in the TRAILS study. Psychol. Med. 45, 2403–2412. doi: 10.1017/S0033291715000392
Nelson, B. D., Infantolino, Z. P., Klein, D. N., Perlman, G., Kotov, R., and Hajcak, G. (2018). Time-frequency reward-related delta prospectively predicts the development of adolescent-onset depression. Biol. Psychiatry: Cogn. Neurosci. Neuroimaging 3, 41–49. doi: 10.1016/j.bpsc.2017.07.005
Nelson, B. D., McGowan, S. K., Sarapas, C., Robison-Andrew, E. J., Altman, S. E., Campbell, M. L., et al. (2013). Biomarkers of threat and reward sensitivity demonstrate unique associations with risk for psychopathology. J. Abnorm. Psychol. 122, 662–671. doi: 10.1037/a0033982
Nelson, B. D., Perlman, G., Hajcak, G., Klein, D. N., and Kotov, R. (2015). Familial risk for distress and fear disorders and emotional reactivity in adolescence: an event-related potential investigation. Psychol. Med. 45, 2545–2556. doi: 10.1017/S0033291715000471
Nelson, B. D., Perlman, G., Klein, D. N., Kotov, R., and Hajcak, G. (2016). Blunted neural response to rewards as a prospective predictor of the development of depression in adolescent girls. Am. J. Psychiatry 173, 1223–1230. doi: 10.1176/appi.ajp.2016.15121524
Nieber, D., and Schlegel, S. (1992). Relationships between psychomotor retardation and EEG power spectrum in major depression. Neuropsychobiology 25, 20–23. doi: 10.1159/000118804
Nusslock, R., and Alloy, L. B. (2017). Reward processing and mood-related symptoms: an RDoC and translational neuroscience perspective. J. Affect. Disord. 216, 3–16. doi: 10.1016/j.jad.2017.02.001
Nusslock, R., Shackman, A. J., Harmon-Jones, E., Alloy, L. B., Coan, J. A., and Abramson, L. Y. (2011). Cognitive vulnerability and frontal brain asymmetry: common predictors of first prospective depressive episode. J. Abnorm. Psychol. 120, 497–503. doi: 10.1037/a0022940
Olino, T. M. (2016). Future research directions in the positive valence systems: measurement, development, and implications for youth unipolar depression. J. Clin. Child Adole. Psychol. 45, 681–705. doi: 10.1080/15374416.2015.1118694
Palomba, D., Angrilli, A., and Mini, A. (1997). Visual evoked potentials, heart rate responses and memory to emotional pictorial stimuli. Int. J. Psychophysiol. 27, 55–67. doi: 10.1016/S0167-8760(97)00751-4
Pariante, C. M., and Lightman, S. L. (2008). The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 31, 464–468. doi: 10.1016/j.tins.2008.06.006
Pegg, S., Arfer, K. B., and Kujawa, A. (2021). Altered reward responsiveness and depressive symptoms: an examination of social and monetary reward domains and interactions with rejection sensitivity. J. Affect. Disord. 282, 717–725. doi: 10.1016/j.jad.2020.12.093
Pegg, S., Ethridge, P., Shields, G. S., Slavich, G. M., Weinberg, A., and Kujawa, A. (2019). Blunted social reward responsiveness moderates the effect of lifetime social stress exposure on depressive symptoms. Front. Behav. Neurosci. 13:178. doi: 10.3389/fnbeh.2019.00178
Penninx, B. W., Milaneschi, Y., Lamers, F., and Vogelzangs, N. (2013). Understanding the somatic consequences of depression: biological mechanisms and the role of depression symptom profile. BMC Med. 11, 1–14. doi: 10.1186/1741-7015-11-129
Pérez-Edgar, K., Fox, N. A., Cohn, J. F., and Kovacs, M. (2006). Behavioral and electrophysiological markers of selective attention in children of parents with a history of depression. Biol. Psychiatry 60, 1131–1138. doi: 10.1016/j.biopsych.2006.02.036
Pesonen, A. K., Gradisar, M., Kuula, L., Short, M., Merikanto, I., Tark, R., et al. (2019). REM sleep fragmentation associated with depressive symptoms and genetic risk for depression in a community-based sample of adolescents. J. Affect. Disord. 245, 757–763. doi: 10.1016/j.jad.2018.11.077
Pizzagalli, D. A. (2014). Depression, stress, and anhedonia: toward a synthesis and integrated model. Ann. Rev. Clin. Psychol. 10, 393–423. doi: 10.1146/annurev-clinpsy-050212-185606
Porges, S. W. (1997). Emotion: an evolutionary by-product of the neural regulation of the autonomic nervous system. Ann. N. Y. Acad. Sci. 807, 62–77. doi: 10.1111/j.1749-6632.1997.tb51913.x
Pössel, P., Lo, H., Fritz, A., and Seemann, S. (2008). A longitudinal study of cortical EEG activity in adolescents. Biol. Psychol. 78, 173–178. doi: 10.1016/j.biopsycho.2008.02.004
Pye, J., Phillips, A. J., Cain, S. W., Montazerolghaem, M., Mowszowski, L., Duffy, S., et al. (2021). Irregular sleep-wake patterns in older adults with current or remitted depression. J. Affect. Disord. 281, 431–437. doi: 10.1016/j.jad.2020.12.034
Rao, U., Hammen, C. L., and Poland, R. E. (2009). Risk markers for depression in adolescents: sleep and HPA measures. Neuropsychopharmacology 34, 1936–1945. doi: 10.1038/npp.2009.27
Razavi, N., Horn, H., Koschorke, P., Hügli, S., Höfle, O., Müller, T., et al. (2011). Measuring motor activity in major depression: the association between the Hamilton depression rating scale and actigraphy. Psychiatry Res. 190, 212–216. doi: 10.1016/j.psychres.2011.05.028
Rosenberg, E. L. (1998). Levels of analysis and the organization of affect. Rev. Gen. Psychol. 2, 247–270. doi: 10.1037/1089-2680.2.3.247
Rottenberg, J. (2017). Emotions in depression: what do we really know. Ann. Rev. Clin. Psychol. 13, 241–263. doi: 10.1146/annurev-clinpsy-032816-045252
Rottenberg, J., Gross, J. J., and Gotlib, I. H. (2005). Emotion context insensitivity in major depressive disorder. J. Abnorm. Psychol. 114, 627–639. doi: 10.1037/0021-843X.114.4.627
Ruchsow, M., Herrnberger, B., Beschoner, P., Grön, G., Spitzer, M., and Kiefer, M. (2006). Error processing in major depressive disorder: evidence from event-related potentials. J. Psychiatr. Res. 40, 37–46. doi: 10.1016/j.jpsychires.2005.02.002
Ruchsow, M., Herrnberger, B., Wiesend, C., Grön, G., Spitzer, M., and Kiefer, M. (2004). The effect of erroneous responses on response monitoring in patients with major depressive disorder: a study with event-related potentials. Psychophysiology 41, 833–840. doi: 10.1111/j.1469-8986.2004.00237.x
Salomon, K., Bylsma, L. M., White, K. E., Panaite, V., and Rottenberg, J. (2013). Is blunted cardiovascular reactivity in depression mood-state dependent? A comparison of major depressive disorder remitted depression and healthy controls. Int. J. Psychophysiol. 90, 50–57. doi: 10.1016/j.ijpsycho.2013.05.018
Sandre, A., Bagot, R. C., and Weinberg, A. (2019). Blunted neural response to appetitive images prospectively predicts symptoms of depression, and not anxiety, during the transition to university. Biol. Psychol. 145, 31–41. doi: 10.1016/j.biopsycho.2019.04.001
Sarapas, C., Shankman, S. A., Harrow, M., and Goldberg, J. F. (2012). Parsing trait and state effects of depression severity on neurocognition: evidence from a 26-year longitudinal study. J. Abnorm. Psychol. 121, 830–837. doi: 10.1037/a0028141
Schrijvers, D., De Bruijn, E. R., Maas, Y., De Grave, C., Sabbe, B. G., and Hulstijn, W. (2008). Action monitoring in major depressive disorder with psychomotor retardation. Cortex 44, 569–579. doi: 10.1016/j.cortex.2007.08.014
Schupp, H. T., Stockburger, J., Codispoti, M., Junghofer, M., Weike, A. I., and Hamm, A. O. (2007). Selective visual attention to emotion. J. Neurosci. 27, 1082–1089. doi: 10.1523/JNEUROSCI.3223-06.2007
Schwerdtfeger, A., and Rosenkaimer, A. K. (2011). Depressive symptoms and attenuated physiological reactivity to laboratory stressors. Biol. Psychol. 87, 430–438. doi: 10.1016/j.biopsycho.2011.05.009
Sears, C. R., Newman, K. R., Ference, J. D., and Thomas, C. L. (2011). Attention to emotional images in previously depressed individuals: an eye-tracking study. Cogn. Ther. Res. 35, 517–528. doi: 10.1007/s10608-011-9396-5
Sears, C. R., Thomas, C. L., LeHuquet, J. M., and Johnson, J. C. (2010). Attentional biases in dysphoria: an eye-tracking study of the allocation and disengagement of attention. Cogn. Emot. 24, 1349–1368. doi: 10.1080/02699930903399319
Shankman, S. A., and Gorka, S. M. (2015). Psychopathology research in the RDoC era: unanswered questions and the importance of the psychophysiological unit of analysis. Int. J. Psychophysiol. 98, 330–337. doi: 10.1016/j.ijpsycho.2015.01.001
Shankman, S. A., Mittal, V. A., and Walther, S. (2020). An examination of psychomotor disturbance in current and remitted MDD: an RDoC study. J. Psychiatry Brain Sci. 5:e200007. doi: 10.20900/jpbs.20200007
Sheth, C., McGlade, E., and Yurgelun-Todd, D. (2017). Chronic stress in adolescents and its neurobiological and psychopathological consequences: an RDoC perspective. Chronic Stress 1:2470547017715645. doi: 10.1177/2470547017715645
Snyder, H. R. (2013). Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychol. Bull. 139, 81–132. doi: 10.1037/a0028727
Speed, B. C., Nelson, B. D., Auerbach, R. P., Klein, D. N., and Hajcak, G. (2016). Depression risk and electrocortical reactivity during self-referential emotional processing in 8 to 14 year-old girls. J. Abnorm. Psychol. 125, 607–619. doi: 10.1037/abn0000173
Stange, J. P., Hamilton, J. L., Olino, T. M., Fresco, D. M., and Alloy, L. B. (2017). Autonomic reactivity and vulnerability to depression: a multi-wave study. Emotion 17, 602–615. doi: 10.1037/emo0000254
Stewart, J. L., Coan, J. A., Towers, D. N., and Allen, J. J. (2011). Frontal EEG asymmetry during emotional challenge differentiates individuals with and without lifetime major depressive disorder. J. Affect. Disord. 129, 167–174. doi: 10.1016/j.jad.2010.08.029
Suzuki, H., Belden, A. C., Spitznagel, E., Dietrich, R., and Luby, J. L. (2013). Blunted stress cortisol reactivity and failure to acclimate to familiar stress in depressed and sub-syndromal children. Psychiatry Res. 210, 575–583. doi: 10.1016/j.psychres.2013.06.038
Tabachnick, A. R., Valadez, E. A., Palmwood, E. N., Zajac, L., Simons, R. F., and Dozier, M. (2018). Depressive symptoms and error-related brain activity in CPS-referred children. Psychophysiology 55:e13211. doi: 10.1111/psyp.13211
Task Force of the European Society of Cardiology and the North American Society for Pacing and Electrophysiology (1996). Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 93, 1043–1065.
Treadway, M. T., Bossaller, N. A., Shelton, R. C., and Zald, D. H. (2012). Effort-based decision-making in major depressive disorder: a translational model of motivational anhedonia. J. Abnorm. Psychol. 121, 553–558. doi: 10.1037/a0028813
Treadway, M. T., and Zald, D. H. (2011). Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci. Biobehav. Rev. 35, 537–555. doi: 10.1016/j.neubiorev.2010.06.006
Vaccarino, V., Lampert, R., Bremner, J. D., Lee, F., Su, S., Maisano, C., et al. (2008). Depressive symptoms and heart rate variability: evidence for a shared genetic substrate in a study of twins. Psychosom. Med. 70, 628–636. doi: 10.1097/PSY.0b013e31817bcc9e
Vaccarino, A. L., Sills, T. L., Evans, K. R., and Kalali, A. H. (2008). Prevalence and association of somatic symptoms in patients with major depressive disorder. J. Affect. Disord. 110, 270–276. doi: 10.1016/j.jad.2008.01.009
Vaidyanathan, U., Welo, E. J., Malone, S. M., Burwell, S. J., and Iacono, W. G. (2014). The effects of recurrent episodes of depression on startle responses. Psychophysiology 51, 103–109. doi: 10.1111/psyp.12152
Van Der Vinne, N., Vollebregt, M. A., Van Putten, M. J., and Arns, M. (2017). Frontal alpha asymmetry as a diagnostic marker in depression: fact or fiction? A meta-analysis. Neuroimage: Clinical 16, 79–87. doi: 10.1016/j.nicl.2017.07.006
Venables, P. H., and Christie, M. J. (1980). “Electrodermal activity” in Techniques in Psychophysiology. eds. I. Martin and P. H Venables (New York, NY: John Wiley), 2–67.
Vreeburg, S. A., Hartman, C. A., Hoogendijk, W. J., van Dyck, R., Zitman, F. G., Ormel, J., et al. (2010). Parental history of depression or anxiety and the cortisol awakening response. B. J. Psychiatry 197, 180–185. doi: 10.1192/bjp.bp.109.076869
Vreeburg, S. A., Hoogendijk, W. J., van Pelt, J., DeRijk, R. H., Verhagen, J. C., van Dyck, R., et al. (2009). Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Arch. Gen. Psychiatry 66, 617–626. doi: 10.1001/archgenpsychiatry.2009.50
Vrshek-Schallhorn, S., Doane, L. D., Mineka, S., Zinbarg, R. E., Craske, M. G., and Adam, E. K. (2013). The cortisol awakening response predicts major depression: predictive stability over a 4-year follow-up and effect of depression history. Psychol. Med. 43, 483–493. doi: 10.1017/S0033291712001213
Walther, S., Bernard, J. A., Mittal, V. A., and Shankman, S. A. (2019). The utility of an RDoC motor domain to understand psychomotor symptoms in depression. Psychol. Med. 49, 212–216. doi: 10.1017/S0033291718003033
Walther, S., Stegmayer, K., Federspiel, A., Bohlhalter, S., Wiest, R., and Viher, P. V. (2017). Aberrant hyperconnectivity in the motor system at rest is linked to motor abnormalities in schizophrenia spectrum disorders. Schizophr. Bull. 43, 982–992. doi: 10.1093/schbul/sbx091
Webb, C. A., Dillon, D. G., Pechtel, P., Goer, F. K., Murray, L., Huys, Q. J., et al. (2016). Neural correlates of three promising endophenotypes of depression: evidence from the EMBARC study. Neuropsychopharmacology 41, 454–463. doi: 10.1038/npp.2015.165
Weinberg, A. (2023). Pathways to depression: dynamic associations between neural responses to appetitive cues in the environment, stress, and the development of illness. Psychophysiology 60:e14193. doi: 10.1111/psyp.14193
Weinberg, A., Dieterich, R., and Riesel, A. (2015a). Error-related brain activity in the age of RDoC: a review of the literature. Int. J. Psychophysiol. 98, 276–299. doi: 10.1016/j.ijpsycho.2015.02.029
Weinberg, A., Liu, H., Hajcak, G., and Shankman, S. A. (2015b). Blunted neural response to rewards as a vulnerability factor for depression: results from a family study. J. Abnorm. Psychol. 124, 878–889. doi: 10.1037/abn0000081
Weinberg, A., Kotov, R., and Hajcak Proudfit, G. (2015c). Neural indicators of error processing in generalized anxiety disorder, obsessive-compulsive disorder, and major depressive disorder. J. Abnorm. Psychol. 124, 172–185. doi: 10.1037/abn0000019
Weinberg, A., Meyer, A., Hale-Rude, E., Perlman, G., Kotov, R., Klein, D. N., et al. (2016). Error-related negativity (ERN) and sustained threat: conceptual framework and empirical evaluation in an adolescent sample. Psychophysiology 53, 372–385. doi: 10.1111/psyp.12538
Weinberg, A., and Shankman, S. A. (2017). Blunted reward processing in remitted melancholic depression. Clin. Psychol. Sci. 5, 14–25. doi: 10.1177/2167702616633158
Wescott, D. L., Morash-Conway, J., Zwicker, A., Cumby, J., Uher, R., and Rusak, B. (2019). Sleep in offspring of parents with mood disorders. Front. Psychiatry 10:225. doi: 10.3389/fpsyt.2019.00225
Whalen, D. J., Gilbert, K. E., Kelly, D., Hajcak, G., Kappenman, E. S., Luby, J. L., et al. (2020). Preschool-onset major depressive disorder is characterized by electrocortical deficits in processing pleasant emotional pictures. Res. Child Adolesc. Psychopathol. 48, 91–108. doi: 10.1007/s10802-019-00585-8
Whitton, A. E., Kakani, P., Foti, D., Vant Veer, A., Haile, A., Crowley, D. J., et al. (2016). Blunted neural responses to reward in remitted major depression: a high-density event-related potential study. Biol. Psychiatry: Cogn. Neurosci. Neuroimaging 1, 87–95. doi: 10.1016/j.bpsc.2015.09.007
Wiebe, S. T., Cassoff, J., and Gruber, R. (2012). Sleep patterns and the risk for unipolar depression: a review. Nat. Sci. Sleep 4, 63–71. doi: 10.2147/2FNSS.S23490
Wolkowitz, O. M., Mellon, S. H., Epel, E. S., Lin, J., Dhabhar, F. S., Su, Y., et al. (2011). Leukocyte telomere length in major depression: correlations with chronicity, inflammation and oxidative stress-preliminary findings. PLoS One 6:e17837. doi: 10.1371/journal.pone.0017837
World Health Organization (2017). Depression and other common mental disorders: global health estimates (No. WHO/MSD/MER/2017.2). Geneva: World Health Organization.
Wüthrich, F., Nabb, C. B., Mittal, V. A., Shankman, S. A., and Walther, S. (2022). Actigraphically measured psychomotor slowing in depression: systematic review and meta-analysis. Psychol. Med. 52, 1208–1221. doi: 10.1017/S0033291722000903
Young, E. A., Vazquez, D., Jiang, H., and Pfeffer, C. R. (2006). Saliva cortisol and response to dexamethasone in children of depressed parents. Biol. Psychiatry 60, 831–836. doi: 10.1016/j.biopsych.2006.03.077
Zhang, W., Ding, Q., Chen, N., Wei, Q., Zhao, C., Zhang, P., et al. (2016). The development of automatic emotion regulation in an implicit emotional go/NoGo paradigm and the association with depressive symptoms and anhedonia during adolescence. Neuro Image: Clinical 11, 116–123. doi: 10.1016/j.nicl.2016.01.018
Zhou, F. C., Wang, Y. Y., Zheng, W., Zhang, Q., Ungvari, G. S., Ng, C. H., et al. (2017). Prospective memory deficits in patients with depression: a meta-analysis. J. Affect. Disord. 220, 79–85. doi: 10.1016/j.jad.2017.05.042
Keywords: depression vulnerability, psychophysiology, research domain criteria, emotion, risk for psychiatric disorder
Citation: Dell’Acqua C, Palomba D, Patron E and Messerotti Benvenuti S (2023) Rethinking the risk for depression using the RDoC: A psychophysiological perspective. Front. Psychol. 14:1108275. doi: 10.3389/fpsyg.2023.1108275
Edited by:
Gianluca Castelnuovo, Catholic University of the Sacred Heart, ItalyReviewed by:
Aislinn Sandre, Columbia University, United StatesBarbara Penolazzi, University of Trieste, Italy
Copyright © 2023 Dell’Acqua, Palomba, Patron and Messerotti Benvenuti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carola Dell’Acqua, ✉ Y2Fyb2xhLmRlbGxhY3F1YUBwaGQudW5pcGQuaXQ=
 Carola Dell’Acqua
Carola Dell’Acqua Daniela Palomba
Daniela Palomba Elisabetta Patron
Elisabetta Patron Simone Messerotti Benvenuti
Simone Messerotti Benvenuti