
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychol. , 30 March 2023
Sec. Neuropsychology
Volume 14 - 2023 | https://doi.org/10.3389/fpsyg.2023.1089856
 Qian-Qian Wei1†
Qian-Qian Wei1† Yuan Guo2†
Yuan Guo2† Shirong Li1,3
Shirong Li1,3 Tianmi Yang1
Tianmi Yang1 Yanbing Hou1
Yanbing Hou1 Ruwei Ou1
Ruwei Ou1 Junyu Lin1
Junyu Lin1 Qirui Jiang1
Qirui Jiang1 Huifang Shang1*
Huifang Shang1*Objectivve: This study aimed to explore the prevalence and clinical correlates of apathy in amyotrophic lateral sclerosis (ALS) in a cohort of Chinese patients.
Methods: A total of 1,013 ALS patients were enrolled in this study. Apathy was recorded during face-to-face interviews using Frontal Behavioral Inventory, and other patient characteristics, including depression, anxiety, and cognitive function, were collected using Hamilton Depression Rating Scale (HDRS), Hamilton Anxiety Rating Scale (HARS), and Chinese version of Addenbrooke’s Cognitive Examination-revised. Health-related quality of life of ALS patients and their caregivers was also evaluated, and the potential factors associated with apathy were explored using forward binary regression analysis. Survival was analyzed using the Cox proportional hazards model.
Results: The prevalence of apathy in all patients was 28.9%. Patients in the late disease stage had a higher prevalence of apathy than those in the early disease stage. Furthermore, patients with apathy had a lower ALS Functional Rating Scale revised (ALSFRS-R) score, higher HDRS score, HARS score and higher proportion of reported problems in the anxiety/depression. Additionally, their caregivers had higher score of depression and higher Zarit-Burden Interview scores. Multivariate regression analysis revealed that apathy in ALS was associated with the onset region (p = 0.027), ALSFRS-R score (p = 0.007), depression (p = 0.001) and anxiety (p < 0.001). Apathy had a significant negative effect on survival in ALS patients (p = 0.032).
Conclusion: Apathy is relatively common (28.9%) in Chinese patients with ALS. Apathy is related to both the severity of the disease, and the presentation of non-motor symptoms in ALS, such as depression and anxiety disorders. Apathy is an independent prognostic factor for survival and requires early intervention and management.
Amyotrophic lateral sclerosis (ALS) is an irreversible progressive motor neurodegenerative disease featured with a deterioration of the upper and/or lower motor neurons (Brown and Al-Chalabi, 2017). In addition to the typical clinical symptoms of limb weakness, dysarthria, dysphagia, and muscle atrophy, ALS patients also experience a profile of cognitive impairments and behavioral changes in different degree. Cognitive changes are characterized by executive and memory dysfunction, broadening to impairments in social cognition and language (Goldstein and Abrahams, 2013). Behavioral changes in ALS manifests most commonly as apathy, loss of sympathy, disinhibition and changes in eating habits (Pender et al., 2020).
Among the many neuropsychiatric changes associated with ALS; apathy has received the most scrutiny. Apathy is characterized by decreased motivation toward goal-directed behaviors, which are variably featured by reduced emotions or interests (Robert et al., 2018). It is one of the most prevalent neuropsychiatric symptoms in neurodegenerative diseases, such as behavioral variant frontotemporal dementia (FTD), Alzheimer’s disease (Leung et al., 2021), and Parkinson’s disease (Liu et al., 2017). The prevalence of apathy ranged from to 8.6 to 80% in ALS (Santangelo et al., 2017), depending on the use of variable assessment instruments and patients selected methods. The prevalence rate of apathy assessed by ALS patient informants or caregivers was significantly higher than that self-rated by the patients. One review showed that the frequency of apathy was 25% in studies that using self-rated tools and 34% in studies using informant-rated tools in ALS (Kutlubaev et al., 2022). This review study also found the lowest frequency of apathy in ALS was registered in the studies from Asia (Kutlubaev et al., 2022). Only a few studies were concerned about apathy in Chinese ALS. In our previous observational studies, apathy was a common impaired neurobehavioral domain with a prevalence of 16.5–19% in ALS patients, which is a lower rate than that reported in previous literature (Wei et al., 2014, 2016). Another study reported that 14% of patients displayed abnormal apathy behavior (Cui et al., 2015). Compared with German ALS patients, the frequency of apathy was lower in Chinese ALS patients, but the difference was not statistically significant (Ye et al., 2019). The occurrence and clinical correlates of apathy require further investigation.
In ALS, there is limited evidence to suggest associations between disease and non-motor features with apathy. Apathy is associated with bulbar involvement in ALS, but whether the bulbar onset or severity of bulbar symptoms contributes to the development of apathy remains unknown (Chiò et al., 2010). Previous studies reported that there was no association between the apathy score and ALS Functional Rating Scale revised (ALSFRS-R) score (Chiò et al., 2010; Salas et al., 2020). Further, there was no consistent correlation between apathy and depression severity or quality of life (QoL). An observational study with a small simple size found that patients with apathy showed higher levels of depression and lower QoL than non-apathetic patients (Caga et al., 2018b); however, no association was found in other studies (Bock et al., 2016; Caga et al., 2016; Radakovic et al., 2017). The development of apathy has been related to cognitive decline in one study (Unglik et al., 2018), while another showed that patients with cognitively impaired ALS have worse apathy scores (Witgert et al., 2010). Apathy may be a critical prognostic factor in ALS. A longitudinal study of 76 patients with ALS indicated that apathy was a significant predictor of survival after adjusting for cognitive status and other clinical parameters (Caga et al., 2016). A study of 152 patients indicated that apathy negatively correlated with survival time (Unglik et al., 2018). Some studies have found no association between apathy scores and survival (Mioshi et al., 2014, 2015). Another observational study of 51 patients found that apathy was related to a higher care burden in ALS caregivers (Caga et al., 2018a). Apathy may even affect the sufferer’s ability to engage competently in end-of-life decisions. Therefore, a better understanding of apathy would be helpful in personalized care and early intervention.
Therefore, the study was designed to examine the prevalence and clinical correlates (emotional state and cognitive function) of apathy in ALS patients. We further investigated the associations between apathy and QOL in ALS and care burden in their caregivers. Finally, we established the influence of apathy on ALS prognosis.
The study was conducted in our motor neuron disease center from southwest China from August 2012 to July 2021. According to the revised El Escorial criteria, definite or probable ALS were enrolled. Written informed consent was obtained from all participants. The study was approved by the Ethics Committee of the West China Hospital of Sichuan University [approval No.2015 (236)].
Demographic data and disease-related data were collected. The ALS Functional Rating Scale revised (ALSFRS-R) scale was used to evaluate functional impairment. The rate of disease progression was evaluated as the changes in ALSFRS-R per month (Formula: (48-ALSFRS-R score at the baseline)/month intervals between first symptom onset and baseline). The disease stage was identified using the King’s College Staging System. Early stage subgroup included patients from stage 1 or stage 2 and late-stage subgroup included patients from stage 3 or stage 4.
The Frontal Assessment Battery (FAB) to assess frontal lobe executive function in a face-to-face interview. A Hamilton Depression Rating Scale-24 (HDRS) score > 20 indicates depression and a Hamilton Anxiety Rating Scale (HARS) score > 14 indicates anxiety. Cognitive dysfunction was defined as score of less than 75 in our previous study using the Chinese version of Addenbrooke’s Cognitive Examination-revised (ACE-R) (Wei et al., 2015). Frontal Behavioral Inventory (FBI) was used to frontal behavioural symptoms. Lower scores indicate better frontal behavioral function. Item number one of the FBI was used to evaluate apathy in ALS patients. Patients with scores ≥1 were considered to be “with the presence of apathy,” <1 as “without the presence of apathy.” Health-related QoL was assessed using the five-level EuroQol five-dimension (EQ-5D-5L) scale, which is a standardized QoL scale. The basic activities of daily living (BADL) and instrumental activities of daily living (IADL) were used to evaluate QoL and ALS ability. Higher scores indicate a better QoL. Caregivers’ depressive symptoms and burden were investigated by Beck Depression Inventory (BDI) and Zarit-Burden Interview (ZBI). All clinical and treatment data, including ALSFRS-R score, medication, survival and other treatment, were collected in the followed-up.
All analyses were performed using SPSS 26.0 (SPSS, Inc., Chicago, IL, USA). Continuous parameters that were normally distributed are described as mean ± standard deviation (SD), and those with a non-normal distribution are presented as median values. Categorical variables are presented as percentages. Comparisons between the groups were performed using Student’s t-test or Chi-square test. The Bonferroni correction were applied in multiple comparisons. Stepwise backward binary logistic regression analysis was used to assess the potential factors associated with the presence of apathy. Kaplan–Meier (KM) curves and log-rank tests were used to assess survival in univariate analysis. The censoring time for follow-up was the end of April 2022. Multivariate analysis was performed using the Cox proportional hazards regression model to assess the effect of apathy on survival (stepwise forward). Hazard ratios (HRs) and 95% confidence interval (CI) were also calculated. A level of p < 0.05 was statistically significant.
The demographic and clinical features of the ALS patients are shown in Table 1. The prevalence of apathy among all registered patients was 28.9%. Intergroup comparisons showed that patients with apathy had significantly lower ALSFRS-R score than patients without apathy. While, the mean age, sex distribution, marital status, education level, body mass index (BMI), family history, disease duration, disease delay, onset region, and classical phenotype distribution did not differ in apathy subgroups.
The prevalence of apathy is related to ALS stage. Patients in the late-stage subgroup (Stage 3,34.3%; Stage 4,34.8%) had a higher percentage of apathy than patients in the early stage subgroup (Stage 1:21.9% and Stage 4:29.2%) (p = 0.009). The assessment of emotional state and cognitive function in ALS are listed in Table 2. Patients with apathy had significantly higher HDRS and HARS scores. Of the 293 patients with apathy, 201 (68.6%) reported depression and 157 (53.6%) reported anxiety. No differences in the FAB, ACE-R, and five domains of the ACE-R scores were found between apathy subgroups.
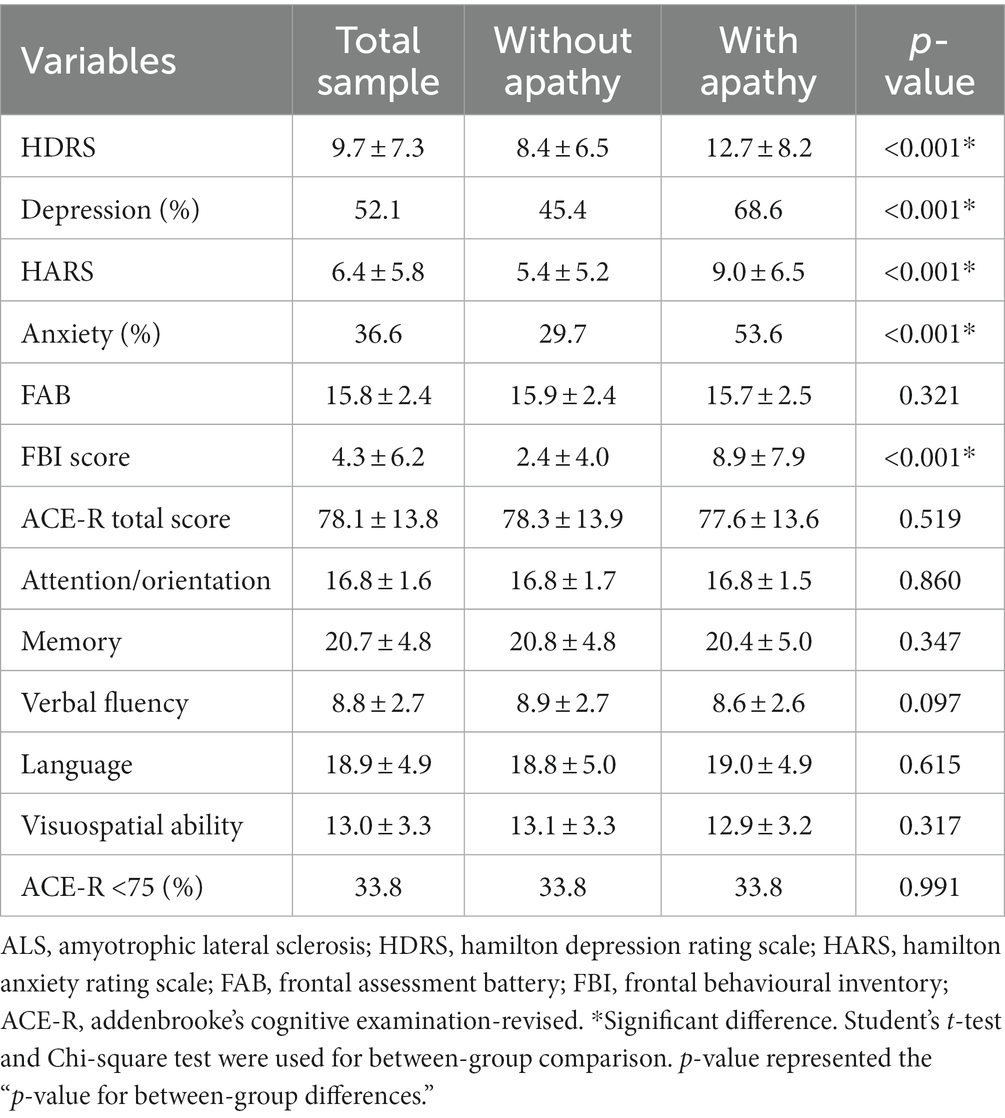
Table 2. Cognitive function and health-related quality of life of ALS patients in different subgroups.
For the health related QoL analysis, 642 patients and caregivers with complete available data were included (Table 3). Patients with apathy had higher proportion of reported problems in the anxiety/depression (68.5% vs. 51.8%, p < 0.001) dimensions. Furthermore, higher BDI and ZBI scores were found in caregivers in the apathy subgroup.
Logistic regression model showed that the onset region (OR = 0.789, 95%CI = 0.639–0.974, p = 0.027), ALSFRS-R score (OR = 0.0.965, 95%CI = 0.940–0.990, p = 0.007), depression (OR = 1.824, 95%CI = 1.289–2.580, p = 0.001), and anxiety (OR = 1.903, 95%CI = 1.359–2.664, p < 0.001) were associated with apathy (Table 4). Other parameters, including age, sex, marital status, BMI, family history, disease duration, diagnostic delay, stages, classical phenotype, disease progression rate, or ACE-R score showed no significant associations with apathy.
In the end of the follow-up (April 2022), 641 patients (63.3%) had died, 346 patients (34.2%) were alive, and 26 patients (2.6) were lost to follow-up. The median survival time of all the patients was 42.8 months. 54.9% of the patients took riluzole. Kaplan–Meier survival analysis of all patients showed that patients with apathy had a shorter survival time than patients without survival (log-rank p = 0.006) (estimated median survival time:39.6 months vs. 44.4 months, Figure 1). Apathy had a significant effect on survival (HR = 1.210, 95%CI:1.016–1.440, p = 0.032) after adjusting for age, sex, marital status, BMI, family history, disease duration, onset region, classical phenotype, disease stage, ALSFRS-R score, depression, anxiety, ACER, and treatment. Using the apathy score instead of apathy groups, we generated similar findings for the apathy score (HR = 1.164. 95%CI:1.037–1.308, p = 0.010).

Figure 1. Survival curves according to apathy in ALS patients (blue, without apathy; red, with apathy).
Overall, apathy (28.9%) is relatively frequent in Chinese ALS patients. Late stages of ALS patients had A higher prevalence of apathy. Furthermore, we showed that patient with apathy had lower health-related QoL and their caregivers had a higher care burden. We also highlighted that emotional state had a possible relationship with apathy in ALS. Apathy is strongly and independently associated with survival in ALS patients.
Apathy is a common neuropsychiatric symptom that has been observed in many neurodegenerative diseases. According to previous studies, the prevalence of apathy in ALS varies greatly, ranged from 8.6 to 80% (Santangelo et al., 2017). This large heterogeneity is likely associated with the array of methodological issues and assessment scales utilized to date. For example, in some, apathy was measured using non-ALS-specific tools, which might have led to bias in the results caused by muscle weakness or dysarthria, potentially contribute to an overestimation of apathy. To avoid the confounding influences of motor impairments, some studies have used the Dimensional Apathy Scale to evaluate apathy and apathetic subtypes in ALS patients. This scale provides scores both for patient-related and informant/caregiver-related apathy, and further comment on methodological bias for apathy in ALS when only caregivers are assessed. In a previous review, the prevalence of apathy was higher in studies that assessed by informant-rated tools (34%) than self-rated tools (25%) (Kutlubaev et al., 2022). In our study, based on information gathered from their caregivers, we found that apathy occurred in 28.9% of our patients. This finding is consistent with that of the prior review (Kutlubaev et al., 2022). Despite the methodological differences and bias on the prevalence of apathy, it appeared that specific neuroanatomical regions contributed to the observed apathy and apathy subtypes. Neuroimaging studies provided evidence of the involvement of brain circuits in apathy in ALS. A previous study observed that the severity of apathy was related to fractional anisotropy values in more widespread white matter areas, including frontal, parietal, and temporal lobes (Femiano et al., 2018). Another study found that increased initiation apathy correlated with reduced gray matter within the bilateral superior frontal gyrus and increased emotional apathy correlated with reduced gray matter in the prefrontal cortices and right anterior cingulate (Caga et al., 2021). Neuroimaging findings complement and extend the pathophysiological mechanisms of apathy in ALS.
The correlations between disease stage or severity and the frequency of apathy have inconsistent in prior reports. In one study, no significant difference was revealed in apathy in different stages according to King’s college staging system (Devenney et al., 2021). In addition, the severity of apathy was negative according to the ALSFRS-R score (Devenney et al., 2021). Other studies found that comparison of ALSFRS-R scores between apathetic and non-apathetic patients is controversial (Chiò et al., 2010; Caga et al., 2016; Santangelo et al., 2017; Salas et al., 2020). Another study indicated that behavioral changes were common and severe in advanced disease stages (Crockford et al., 2018). We found a higher prevalence of apathy in the late stages of ALS. In the multiple logistic regression model, ALSFRS-R scores other than disease stage were associated with apathy. Further longitudinal studies are required to explore the complex relationship in apathy and other ALS disease factors. For example, we found that the emergence of apathy in ALS was related to bulbar onset, which is reported in previous studies (Grossman et al., 2007; Chiò et al., 2010; Santangelo et al., 2017). This further supports the concept that bulbar onset is a risk factor for cognitive and behavioral involvement in ALS (Consonni et al., 2021; Yang et al., 2021).
For the effect of apathy on QoL, some studies have identified that increased severity of apathy was associated with lower patient QoL (Caga et al., 2018a), while other studies have found no such relationships (Chiò et al., 2010; Bock et al., 2016). In our study, patients with apathy had lower health-related QoL according to the EQ-5D-5L, which was most pronounced in anxiety/depression domains. Apathy may be a correlated factor for poor QoL in ALS, leading to issues with treatment compliance. Apathy also may impact different aspects of QoL, further highlighting the importance of assessing QoL domains other than those related to physical function in ALS with apathy (Pagnini, 2013). Early diagnosis and management of apathy would improve QoL in patients with ALS. We found that apathy in ALS was related to higher caregiver burden and depression. Previous study found that caregiver depression was correlated with patients’ apathy scores (Chiò et al., 2010). Another observational study also found that apathy aggravated the burden on caregivers (Caga et al., 2018a). Thus, the management of apathy and provision of tailored therapeutic interventions could help improve caregivers’ QoL.
In line with the previous study, we observed no significant difference for cognitive function in the subgroups with and without apathy (Caga et al., 2018b). They found that the difference in cognitive functioning using Mini-Addenbrooke’s Cognitive Examination between the groups was not statistically significant (Caga et al., 2018b). Another study found that patients with moderate to severe apathy had a higher percentage of cognitive impairment, as evaluated by the ACE-R (Caga et al., 2016). Other studies have also reported that patients with apathy perform worse in cognitive tests, such as verbal fluency, block design, and animal fluency (Grossman et al., 2007; Witgert et al., 2010). Furthermore, the association between the apathetic subtypes and cognitive function was explored. Initiation apathy was associated with verbal fluency deficits, while emotional apathy was associated with emotional recognition deficits, indicating the possible underlying pathological mechanisms of these cognitive and behavioral symptoms (Radakovic et al., 2017). The complex association between apathy and cognitive function is most likely due to methodological variability in assessing cognitive impartments. Therefore, future studies using ALS-specific instruments need to be performed to further clarify the association between apathy and cognitive function.
Few studies have so far reported on the relationship between apathy and emotional states. Patients with severe apathy have anxiety or depression than those with mild or moderate apathy (Witgert et al., 2010; Caga et al., 2018b; Siciliano et al., 2019). Previous study detected higher depression scores in ALS with apathy (Crockford et al., 2018). This significant correlation reflected an overlap between apathy and affective symptoms. In addition, a history of mood disorders increases the possibility of developing apathy in ALS patients (McHutchison et al., 2020). We also highlighted the possible relationship between emotional state and apathy in ALS using multivariate regression analysis. Patients with apathy had significantly higher HDRS and HARS scores than those without apathy. However, no association was found in other studies. There was no significant association between the level of apathy and depression and demoralization, suggesting that apathy and specific symptoms of depression may occur independently of each other in ALS. The association between apathy and emotional state is controversial in ALS, and further reports with stratification analysis are required to explore the possible relationships between these phenomena in ALS.
Apathy patients had shorter survival time in the Kaplan–Meier survival analysis compared to those without (estimated median survival time: 39.6 months vs. 44.4 months). Furthermore, apathy was found to have a negative effect on survival after adjusting for other parameters in our study, which is consistent with previous studies (Unglik et al., 2018). Higher level of apathy was significantly associated with mortality after controlling for clinical factors; for each one-unit change in the level of apathy, the risk of death more than tripled (Caga et al., 2016). Other studies have also indicated that apathy is a significant predictor of survival after when adjusted cognitive status and other clinical factors (Unglik et al., 2018). The presence of apathy preceded motor symptoms and worsen ALS prognosis. This might be due to poorer management with multidisciplinary care, that was associated with prolonged survival and better QoL. Early diagnosis and personalised intervention for apathy were helpful at reducing the involvement in worse ALS outcomes.
The inclusion of non-specific apathy scale is a major limitation of the study. Apathy was evaluated based on one item from FBI questionnaire. In addition, using a single item to categorize participants into two subgroups may result in further methodological bias. Another limitation of the study is the use of a less sensitive and specific scale for cognitive impairment in ALS. Previous studies have shown that ACE may not captured mild cognitive impairment in ALS and has higher ceiling effects compared to ALS-specific scales (De Icaza Valenzuela et al., 2018; Kourtesis et al., 2020). ALS-specific and apathy-specific scales were needed to evaluate cognition and apathy in ALS. Finally, other factors, such as the C9orf72 gene mutation, which interferes with cognitive function, should been considered in the Cox analysis (Table 5).
Apathy is common in Chinese ALS patients and has a negative effect on survival. Apathy in ALS is related not only to the severity of the disease, but also to some non-motor symptoms, such as depression and anxiety disorders. Apathy is an independent prognostic factor for ALS. Early intervention and management of apathy could prolong survival and improve QoL in patients with ALS.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of West China Hospital of Sichuan University. The patients/participants provided their written informed consent to participate in this study.
Q-QW research project (conception, organization, execution), statistical analysis (design), and manuscript (writing of the first draft). YG statistical analysis (review and critique) and patients enrollment. SL and TY patients enrollment and follow up. YH, RO, JL, and QJ patients enrollment. HS research project (conception), statistical analysis (review and critique), and manuscript (review and critique). All authors read and approved the final manuscript.
This study was supported by funding from the National Natural Science Foundation of China (Grant No. 82101485), Sichuan Science and Technology Program (Grant No. 2022ZDZX0023), Science and Technology Commission Foundation of Chengdu City (Grant No. 2021-YF05-00242-SN), and Postdoctoral Science Foundation of Sichuan University (Grant No. 2021SCU12019).
The authors thank the patients for their participation in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Bock, M., Duong, Y. N., Kim, A., Allen, I., Murphy, J., and Lomen-Hoerth, C. (2016). Cognitive-behavioral changes in amyotrophic lateral sclerosis: screening prevalence and impact on patients and caregivers. Amyotroph. Lateral Scler. Frontotemporal Degener. 17, 366–373. doi: 10.3109/21678421.2016.1165257
Brown, R. H. Jr., and Al-Chalabi, A. (2017). Amyotrophic lateral sclerosis. N. Engl. J. Med. 377:1602. doi: 10.1056/NEJMc1710379
Caga, J., Hsieh, S., Highton-Williamson, E., Zoing, M. C., Ramsey, E., Devenney, E., et al. (2018a). The burden of apathy for caregivers of patients with amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Frontotemporal Degener. 19, 599–605. doi: 10.1080/21678421.2018.1497659
Caga, J., Hsieh, S., Highton-Williamson, E., Zoing, M. C., Ramsey, E., Devenney, E., et al. (2018b). Apathy and its impact on patient outcome in amyotrophic lateral sclerosis. J. Neurol. 265, 187–193. doi: 10.1007/s00415-017-8688-4
Caga, J., Tu, S., Dharmadasa, T., Tse, N. Y., Zoing, M. C., Huynh, W., et al. (2021). Apathy is associated with parietal cortical-subcortical dysfunction in ALS. Cortex 145, 341–349. doi: 10.1016/j.cortex.2021.02.029
Caga, J., Turner, M. R., Hsieh, S., Ahmed, R. M., Devenney, E., Ramsey, E., et al. (2016). Apathy is associated with poor prognosis in amyotrophic lateral sclerosis. Eur. J. Neurol. 23, 891–897. doi: 10.1111/ene.12959
Chiò, A., Vignola, A., Mastro, E., Giudici, A. D., Iazzolino, B., Calvo, A., et al. (2010). Neurobehavioral symptoms in ALS are negatively related to caregivers' burden and quality of life. Eur. J. Neurol. 17, 1298–1303. doi: 10.1111/j.1468-1331.2010.03016.x
Consonni, M., Dalla Bella, E., Bersano, E., and Lauria, G. (2021). Cognitive and behavioural impairment in amyotrophic lateral sclerosis: a landmark of the disease? A mini review of longitudinal studies. Neurosci. Lett. 754:135898. doi: 10.1016/j.neulet.2021.135898
Crockford, C., Newton, J., Lonergan, K., Chiwera, T., Booth, T., Chandran, S., et al. (2018). ALS-specific cognitive and behavior changes associated with advancing disease stage in ALS. Neurology 91, e1370–e1380. doi: 10.1212/wnl.0000000000006317
Cui, B., Cui, L. Y., Liu, M. S., Li, X. G., Ma, J. F., Fang, J., et al. (2015). Behavioral symptoms in motor neuron disease and their negative impact on caregiver burden. Chin. Med. J. 128, 2295–2300. doi: 10.4103/0366-6999.163393
De Icaza Valenzuela, M. M., Bak, T. H., Pal, S., and Abrahams, S. (2018). The Edinburgh cognitive and behavioral ALS screen: relationship to age, education, IQ and the Addenbrooke's cognitive examination-III. Amyotroph. Lateral Scler. Frontotemporal Degener. 19, 585–590. doi: 10.1080/21678421.2018.1491601
Devenney, E. M., McErlean, K., Tse, N. Y., Caga, J., Dharmadasa, T., Huynh, W., et al. (2021). Factors that influence non-motor impairment across the ALS-FTD spectrum: impact of phenotype, sex, age, onset and disease stage. Front. Neurol. 12:743688. doi: 10.3389/fneur.2021.743688
Femiano, C., Trojsi, F., Caiazzo, G., Siciliano, M., Passaniti, C., Russo, A., et al. (2018). Apathy is correlated with widespread diffusion tensor imaging (DTI) impairment in amyotrophic lateral sclerosis. Behav. Neurol. 2018, 2635202–2635210. doi: 10.1155/2018/2635202
Goldstein, L. H., and Abrahams, S. (2013). Changes in cognition and behaviour in amyotrophic lateral sclerosis: nature of impairment and implications for assessment. Lancet Neurol. 12, 368–380. doi: 10.1016/s1474-4422(13)70026-7
Grossman, A. B., Woolley-Levine, S., Bradley, W. G., and Miller, R. G. (2007). Detecting neurobehavioral changes in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 8, 56–61. doi: 10.1080/17482960601044106
Kourtesis, P., Christidi, F., Margioti, E., Demenega, C., Rentzos, M., Evdokimidis, I., et al. (2020). The Edinburgh cognitive and behavioral amyotrophic lateral sclerosis screen (ECAS): sensitivity in differentiating between ALS and Alzheimer's disease in a Greek population. Amyotroph. Lateral Scler. Frontotemporal Degener. 21, 78–85. doi: 10.1080/21678421.2019.1655059
Kutlubaev, M. A., Caga, J., Xu, Y., Areprintseva, D. K., Pervushina, E. V., and Kiernan, M. C. (2022). Apathy in amyotrophic lateral sclerosis: systematic review and meta-analysis of frequency, correlates, and outcomes. Amyotroph. Lateral Scler. Frontotemporal Degener. 24, 14–23. doi: 10.1080/21678421.2022.2053721
Leung, D. K. Y., Chan, W. C., Spector, A., and Wong, G. H. Y. (2021). Prevalence of depression, anxiety, and apathy symptoms across dementia stages: a systematic review and meta-analysis. Int. J. Geriatr. Psychiatry 36, 1330–1344. doi: 10.1002/gps.5556
Liu, H., Ou, R., Wei, Q., Hou, Y., Zhang, L., Cao, B., et al. (2017). Apathy in drug-naïve patients with Parkinson's disease. Parkinsonism Relat. Disord. 44, 28–32. doi: 10.1016/j.parkreldis.2017.08.008
McHutchison, C. A., Leighton, D. J., McIntosh, A., Cleary, E., Warner, J., Porteous, M., et al. (2020). Relationship between neuropsychiatric disorders and cognitive and behavioural change in MND. J. Neurol. Neurosurg. Psychiatry 91, 245–253. doi: 10.1136/jnnp-2019-321737
Mioshi, E., Caga, J., Lillo, P., Hsieh, S., Ramsey, E., Devenney, E., et al. (2014). Neuropsychiatric changes precede classic motor symptoms in ALS and do not affect survival. Neurology 82, 149–155. doi: 10.1212/wnl.0000000000000023
Mioshi, E., Roberts, R., and Hornberger, M. (2015). Neuropsychiatric symptoms and survival in amyotrophic lateral sclerosis: a missing link? Neurodegener. Dis. Manag. 5, 89–91. doi: 10.2217/nmt.15.1
Pagnini, F. (2013). Psychological wellbeing and quality of life in amyotrophic lateral sclerosis: a review. Int. J. Psychol. 48, 194–205. doi: 10.1080/00207594.2012.691977
Pender, N., Pinto-Grau, M., and Hardiman, O. (2020). Cognitive and behavioural impairment in amyotrophic lateral sclerosis. Curr. Opin. Neurol. 33, 649–654. doi: 10.1097/wco.0000000000000862
Radakovic, R., Stephenson, L., Newton, J., Crockford, C., Swingler, R., Chandran, S., et al. (2017). Multidimensional apathy and executive dysfunction in amyotrophic lateral sclerosis. Cortex 94, 142–151. doi: 10.1016/j.cortex.2017.06.023
Robert, P., Lanctôt, K. L., Agüera-Ortiz, L., Aalten, P., Bremond, F., Defrancesco, M., et al. (2018). Is it time to revise the diagnostic criteria for apathy in brain disorders? The 2018 international consensus group. Eur. Psychiatry 54, 71–76. doi: 10.1016/j.eurpsy.2018.07.008
Salas, T., Radakovic, R., Rodriguez-Castillo, V., Marín, S., Chaverri, D., and Rodriguez-Santos, F. (2020). Spanish adaptation of the dimensional apathy scale (DAS) in amyotrophic lateral sclerosis. Front. Neurol. 11:562837. doi: 10.3389/fneur.2020.562837
Santangelo, G., Siciliano, M., Trojano, L., Femiano, C., Monsurrò, M. R., Tedeschi, G., et al. (2017). Apathy in amyotrophic lateral sclerosis: insights from dimensional apathy scale. Amyotroph. Lateral Scler. Frontotemporal Degener. 18, 434–442. doi: 10.1080/21678421.2017.1313865
Siciliano, M., Trojano, L., Trojsi, F., Monsurrò, M. R., Tedeschi, G., and Santangelo, G. (2019). Assessing anxiety and its correlates in amyotrophic lateral sclerosis: the state-trait anxiety inventory. Muscle Nerve 60, 47–55. doi: 10.1002/mus.26475
Unglik, J., Bungener, C., Delgadillo, D., Salachas, F., Pradat, P. F., Bruneteau, G., et al. (2018). Emotional feeling in patients suffering from amyotrophic lateral sclerosis. Geriatr. Psychol. Neuropsychiatr. Vieil. 16, 414–422. doi: 10.1684/pnv.2018.0762
Wei, Q., Chen, X., Cao, B., Ou, R., Zhao, B., Wu, Y., et al. (2016). Associations between neuropsychiatric symptoms and cognition in Chinese patients with amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Frontotemporal Degener. 17, 358–365. doi: 10.3109/21678421.2016.1154574
Wei, Q., Chen, X., Zheng, Z., Huang, R., Guo, X., Cao, B., et al. (2015). Screening for cognitive impairment in a Chinese ALS population. Amyotroph. Lateral Scler. Frontotemporal Degener. 16, 40–45. doi: 10.3109/21678421.2014.966311
Wei, Q., Chen, X., Zheng, Z., Huang, R., Guo, X., Cao, B., et al. (2014). Frontal lobe function and behavioral changes in amyotrophic lateral sclerosis: a study from Southwest China. J. Neurol. 261, 2393–2400. doi: 10.1007/s00415-014-7508-3
Witgert, M., Salamone, A. R., Strutt, A. M., Jawaid, A., Massman, P. J., Bradshaw, M., et al. (2010). Frontal-lobe mediated behavioral dysfunction in amyotrophic lateral sclerosis. Eur. J. Neurol. 17, 103–110. doi: 10.1111/j.1468-1331.2009.02801.x
Yang, T., Hou, Y., Li, C., Cao, B., Cheng, Y., Wei, Q., et al. (2021). Risk factors for cognitive impairment in amyotrophic lateral sclerosis: a systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 92, 688–693. doi: 10.1136/jnnp-2020-325701
Ye, S., Rosenbohm, A., Böhm, S., Uttner, I., Ji, Y., Ludolph, A. C., et al. (2019). Cognitive and behavioral impairments in German and Chinese ALS populations – a post-hoc comparison of national study data. Amyotroph. Lateral Scler. Frontotemporal Degener. 20, 28–36. doi: 10.1080/21678421.2018.1542535
Keywords: amyotrophic lateral sclerosis, apathy, depression, anxiety, health-related quality of life, survival
Citation: Wei Q-Q, Guo Y, Li S, Yang T, Hou Y, Ou R, Lin J, Jiang Q and Shang H (2023) Prevalence and associated factors of apathy in Chinese ALS patients. Front. Psychol. 14:1089856. doi: 10.3389/fpsyg.2023.1089856
Received: 01 December 2022; Accepted: 06 March 2023;
Published: 30 March 2023.
Edited by:
Grigorios Nasios, University of Ioannina, GreeceReviewed by:
Foteini Christidi, National and Kapodistrian University of Athens, GreeceCopyright © 2023 Wei, Guo, Li, Yang, Hou, Ou, Lin, Jiang and Shang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huifang Shang, aGZzaGFuZzIwMDJAMTI2LmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.