- 1School of Nursing, The George Washington University, Washington, DC, United States
- 2Department of Biology, Morgan State University, Baltimore, MD, United States
- 3Department of Rheumatology, Ajou University School of Medicine, Suwon-si, Republic of Korea
Introduction: Pain is a prominent contributor to negative personal and social outcomes, including increased disability and mortality, in many rheumatic diseases. In the Biopsychosocial model of chronic pain, psychological and social factors share roles with the biology of the injury in determining each patient’s pain and suffering. The current study explored factors associated with clinical pain intensity and interference among patients with chronic secondary musculoskeletal pain in rheumatic diseases.
Methods: In total, 220 patients experiencing chronic secondary musculoskeletal pain participated. Biological factors (age, biological sex, pain condition, pain duration, pain sensitivity, and comorbidity), socio-economic factors, psychological factors (pain catastrophizing and depressive symptoms), and pain intensity and interference were measured. Descriptive, multivariable linear regression and partial correlation analyses were conducted. Subgroup analysis by sex was conducted to examine differences in how different factors affect the pain experience.
Results: The mean age of the participants was 52.3 years (SD = 12.07) and ranged from 22 to 78. Average pain intensity was 3.01 (0–10 scale) and average total pain interference score was 21.07 (0–70 scale). Partial correlation found positive correlations between pain intensity and interference with depression (intensity: R = 0.224; p = 0.0011; interference: R = 0.351; p < 0.001) and pain catastrophizing (intensity: R = 0.520; p < 0.001; interference: R = 0.464; p < 0.001). In males, pain condition (β = −0.249, p = 0.032) and pain catastrophizing (R = 0.480, p < 0.001) were associated with pain intensity. In males, the simple correlation between pain intensity and depression (R = 0.519; p < 0.001) was driven by pain catastrophizing. In females, pain catastrophizing (R = 0.536, p < 0.001) and depressive symptoms (R = 0.228, p = 0.0077) were independently associated with pain intensity. Age (β = −0.251, p = 0.042) and pain catastrophizing (R = 0.609, p < 0.001) were associated with pain interference in males, while depressive symptoms (R = 0.439, p < 0.001) and pain catastrophizing (R = 0.403, p < 0.001) were associated with pain interference in females. Again, in males, the simple correlation between pain interference and depression (R = 0.455; p < 0.001) was driven by pain catastrophizing.
Discussion: In this study, females were more directly affected by depressive symptoms than males, regarding pain intensity and interference. Pain catastrophizing was a significant factor influencing chronic pain for both males and females. Based on these findings, a sex-specific approach to the Biopsychosocial model should be considered in understanding and managing pain among Asians with chronic secondary musculoskeletal pain.
1. Introduction
Chronic pain affects a significant number of patients worldwide. Estimated prevalence of chronic pain ranged from 35.0 to 51.3% in the United Kingdom (Fayaz et al., 2016), and 11 to 40% in the US (Dahlhamer et al., 2018). In South Korea, nationwide prevalence of chronic pain in adults has been rarely reported. However, one study reported prevalence in older adults of 87.8% for females and 63.8% for males (Jung-Choi et al., 2014). Chronic pain, defined as pain lasting more than 3 months, is frequently refractory to treatment (Jensen, 2016); meta-analyses indicate that only about half of patients experience clinically meaningful pain relief from pharmacological therapies (Bjordal et al., 2007; Machado et al., 2015). The new International Classification of Diseases (ICD-11) introduced the concept of chronic primary and secondary pain, stating that chronic pain should be regarded as a condition in its own right, rather than being assigned by the underlying diagnosis (Perrot et al., 2019). This new definition integrates the biomedical, psychological, and social axes that comprise the complex experience of chronic pain, recognizing its independent impact on functioning. Treatments are frequently undermined by various psychological and social factors which influence the pain experience. Comprehensive and accurate assessment of chronic pain and long-term evidence-based treatment plans aimed at factors influencing pain outcomes are necessary to better care for patients with chronic pain.
Chronic pain is a complex, subjective experience influenced by multiple factors. The Biopsychosocial model of pain experience is the dominant framework for understanding the complexity of chronic pain (Fifield et al., 1991; Fordyce, 1994; Sullivan et al., 2022). The Biopsychosocial model seeks to acknowledge and measure the combined influence of sociological, psychological, and biological factors influencing the pain experience. Previous studies on the role of age and biological sex have shown that older patients and women experience chronic pain with greater intensity (Mills et al., 2019). An individual’s degree of sensitivity to painful stimuli has also been associated with chronic pain. Differences in experimental pain sensitivity were reported in patients with chronic pain (Cardoso et al., 2016), and higher experimental pain sensitivity was associated with greater clinical pain intensity among elderly patients with knee osteoarthritis (Cruz-Almeida et al., 2014). Furthermore, greater mechanical cutaneous pain sensitivity was associated with clinical pain intensity in patients with chronic knee osteoarthritis (Alabas et al., 2013). Including quantitative sensory testing (QST) assessment in chronic musculoskeletal pain may allow the patient to be categorized into phenotypic subgroups (Uddin and MacDermid, 2016).
In terms of psychosocial factors, depression, anxiety, sleep quality, quality of life, and pain catastrophizing are all reported to be associated with the chronic pain experience, impacting on both pain intensity and pain interference (Kim et al., 2020). In a racial/ethnic comparison study, depression and pain catastrophizing were significant factors explaining higher pain intensity among Asians compared to non-Hispanic Whites (Kim et al., 2019). Pain catastrophizing was also found to have a significant mediating effect on the relationship between depression and pain intensity in Asians with chronic pain (Kim et al., 2021). Socio-economic background was reported to be inversely related to chronic pain; severe pain intensity and greater level of pain-related disability were associated with socio-economic deprivation; those who have lower levels of education and perceived economic status were more likely to experience chronic pain than those with higher levels of education and economic status (Mills et al., 2019).
Asians have been under-represented in pain research in the US; evidence is lacking on chronic pain experiences of Asians due to their minimal sampling in chronic pain studies (Kawi et al., 2019). Without considering social and cultural aspects, chronic pain cannot be understood nor effectively managed (Bostick et al., 2021). Racial/ethnic and cultural impact on biopsychosocial aspects of pain experience in patients with chronic secondary musculoskeletal pain has been reported in general populations (Orhan et al., 2018), however, evidence on Asians on this matter is still limited. The purpose of this study was to examine concurrent influences of multidimensional correlates of chronic secondary musculoskeletal pain among Korean patients with various rheumatic disease, as an example of an Asian subpopulation. We also aimed to evaluate biological sex differences in how biopsychosocial factors affect chronic secondary musculoskeletal pain in Korean culture. We hypothesized that psychological factors will be the most significant factors influencing pain experiences (pain intensity/interference) compared to other social/biological factors, and there will be significant differences in those associations between pain experiences and biopsychosocial factors by biological sex.
2. Materials and methods
2.1. Design
An exploratory, cross-sectional design was used for this study.
2.2. Participants
The participants recruited for this study were patients with chronic musculoskeletal pain. The inclusion criteria were as follows: (1) age > 18 years, (2) able to communicate verbally and in written form and to provide written informed consent, and 3) chronic musculoskeletal pain (≥3 months). Exclusion criteria were: (1) serious medical conditions (e.g., uncontrolled hypertension, cardiac diseases), (2) peripheral neuropathy, (3) diagnosed with fibromyalgia or Systemic Lupus Erythematosus, (4) cognitive impairment, (5) daily use of opioids, or (6) hospitalization within the preceding year for psychiatric illness.
2.3. Procedure/ethical consideration
Participant recruitment was conducted in a rheumatic outpatient clinic at a hospital in a province in South Korea. Patients who met the inclusion/exclusion criteria were informed about the study by clinicians. If patients were interested in participating in the study, a research assistant approached the potential participants and explained the study process and purposes. Once they agreed to participate, written informed consent was obtained before data collection. Participants were assured that they could withdraw from the study if they wished at any time. The data collection procedure started with pain sensitivity measurement, conducted by a trained research assistant, and then participants completed self-reported questionnaires. To reduce missing data, the research assistant reviewed the written questionnaires after they were completed and answered any questions raised by the participants. Participants who completed the study were reimbursed with an $18 online gift-card. The study protocol and questionnaires were reviewed and approved by the Ajou University Hospital IRB (AJIRB-MED-SUR-21-319) prior to the data collection. Data was collected from September to October 2021.
2.4. Measures
We measured biological factors, including age, biological sex, diseases associated with pain, pain duration, comorbidity, and experimental pain sensitivity. Psychological factors include depressive symptoms and pain catastrophizing that was previously reported associated with chronic pain, especially among Asians (Kim et al., 2019, 2021). For social factors, we included level of education, perceived economic status, religion, type of residency, and marital status.
2.4.1. Quantitative sensory testing
Experimental pain sensitivity was measured using a QST procedure (Rolke et al., 2006). Pressure pain threshold, tactile threshold, mechanical cutaneous pain threshold, and temporal summation of painful cutaneous mechanical stimuli were included. Pressure pain threshold was measured using a digital pressure algometer (FPX25, Wagner Instrument, Greenwich, CT, United States); patients were asked to say “now” when they felt “pain or discomfort” when the examiner applied a pressure with a 30 kPa/s speed (increments of 30–50 N/m2 per second). We tested the forearm area, avoiding any painful areas. In total, five trials were conducted using adjacent skin areas. First trial data were excluded in data analyses. Mechanical cutaneous pain sensitivity was measured using monofilaments (Von Frey Filament, North Coast Medical Inc. Touch-Test Sensory Evaluator, United States). Participants were asked to say “now” when they first felt touch (tactile threshold) change to “pain or discomfort” by increasing the force exerted by the monofilament (from 0.008 to 300 g) to assess mechanical cutaneous pain thresholds. Temporal summation of painful cutaneous mechanical stimuli was assessed using the 300 g filament. One stimulus was applied, and the patient was asked to rate the pain from 0 to 100 scale. Subsequently 10 stimuli, delivered every 2 s, were applied and patients were asked to rate their pain every trial. The difference between the first and the highest pain rating was calculated and taken as the summation rating (Greenspan et al., 2011; Goodin et al., 2014).
2.4.2. Psychological factors
Pain catastrophizing was measured using the Pain Catastrophizing Scale (PCS) (Sullivan et al., 1995). The tool is a 13-item scale that assesses catastrophic thinking, including subscales of helplessness, rumination, and magnification, in response to pain. The PCS total score was calculated by summing the total item responses; higher scores on the PCS are indicative of greater pain-related catastrophizing. The Korean version of the PCS (K-PCS) used for this study has been previously validated in patients with chronic non-cancer pain (Cho et al., 2013). Cronbach’s alpha was 0.92 in this study.
The previously validated Patient Health Questionniaire-9 Korean version (PHQ-9 K) was used to measure depressive symptoms (Donnelly and Kim, 2008). The PHQ-9 is a self-administered questionnaire and includes nine items based on the nine diagnostic criteria for a major depressive episode in Diagnostic and Statistical Manual of Mental Disorders (DSM-5) (American Psychiatric Association, 2013). The tool has been validated in the Korean population previously (Shin et al., 2010), and the Cronbach’s alpha was 0.84 in this study.
2.4.3. Pain intensity/interference
The Korean version of Brief Pain Inventory (BPI) was used to measure the level of chronic pain of the participants (Yun et al., 2004). This instrument examines both pain intensity and the level of pain interference due to chronic pain (Cleeland, 1991). The BPI uses an 11-point numeric rating scale (0 = “no pain” and 10 = “pain as bad as you can imagine”) to evaluate pain at its worst, least, average, and current severity over the past week. The BPI also evaluates the degree of interference with general activity, mood, walking, work, sleep, relations with others, and enjoyment of life due to pain, using a numeric rating scale (0 = “no interference” and 10 = “interferes completely”). Cronbach’s alpha for pain severity was 0.84, and pain interference was 0.93.
2.4.4. Demographic and social factors
Age, biological sex, levels of education, perceived economic status, religion, marital status, and type of residency (e.g., living alone, living with children, etc.) were included. Chronic pain condition, duration, and comorbidities were also assessed.
2.5. Statistical analysis
Descriptive analyses were conducted to provide background information of the participants. Normality of the data was verified using histograms, normal probability plots, and skewness/kurtosis measures. Bivariate analyses were conducted to examine relationships between study variables (biological, social, and psychological factors) and the dependent variables (pain intensity and pain interference) using Pearson’s correlations. Subgroup analysis of multivariable linear regression was conducted to examine significant factors associated with pain intensity and pain interference by biological sex. Assumptions for regression analysis were checked and interdependence and linearity were evaluated through bivariate scatter plots. Scatter plots and the Levene’s test was used to determine homoscedasticity. The multivariable model included biological factors (age, sex, chronic pain condition, pain duration, comorbidity), social factors including education level, perceived economic status, religion, marital status, and type of residency, and psychological factors, including depressive symptoms and pain catastrophizing; these were entered into the regression models. All analyses were performed using Stata version 17.1.
For continuous and pseudo-continuous predictors without significant collinearity including age, depressive symptoms, pain catastrophizing, tactile threshold, mechanical pain threshold, area under the curve for temporal summation of mechanical pain, fold change in mechanical pain during temporal summation, pressure pain threshold at the forearm, and pain duration in a partial correlation model with pain interference, pain intensity or number of pain areas were used as the outcomes of interest. Partial correlation models were solved using the ppcor package in R 4.2.1 (Kim, 2015). This package solves the multivariable correlation problem by inverting the covariance matrix, solving all partial correlations simultaneously. This avoids the necessity of specifying a hierarchical model. For figures, we used the R package ggscatter to create bivariate scatterplots with corresponding 95% confidence curves for relationships of interest (Meeker et al., 2021). To control for testing multiple statistical hypotheses within each partial correlation model we used a Bonferroni correction for 45 possible tests (p < 0.0011).
3. Results
3.1. Participants characteristics
In total, 220 participants signed their informed consent and completed all study procedures. Descriptive statistics of the sample are presented in Table 1. The mean age of the participants was 52.3 years (SD = 12.07). The sample was 65.5% female and married (70%). A majority (82%) of the participants had at least a high school education. Sixty-six percent of participants indicated their perceived economic status as middle socioeconomic status (SES), while 25.6% indicated self-endorsed low SES. About half of the participants were living with children. In terms of chronic pain condition, 55.5% of the participants reported being diagnosed with rheumatoid arthritis, 23.6% with ankylosing spondylitis, and 14.1% with osteoarthritis. Thirty-seven percent of the participants reported having pain for more than 10 years, while 29.7% indicated that they had pain for 5–10 years. Twenty-eight percent of participants indicated they had at least one comorbid condition, whereas 60% of the participants reported that they did not have other diseases. The most common comorbid condition was hypertension (22.3%).
3.2. Depressive symptoms, pain catastrophizing, pain intensity, and pain interference
Table 2 presents the descriptive statistics of depressive symptoms, pain catastrophizing, pain intensity, and pain interference reported by the participants. The mean score of depressive symptoms was 5.62 (SD = 4.8). Fifty-one percent of participants reported no depression, while others were categorized as having mild (29.8%), moderate (11.0%), moderate–severe (6.4%), or severe depression (0.9%). The average pain catastrophizing score was 14.99 (SD = 11.05). Means of each subscale of the PCS were 7.2 (SD = 4.54) for rumination, 4.8 (SD = 5.07) for helplessness, and 3.0 (SD = 2.87) for magnification.
The average intensity of pain over the past week was 4.9 (SD = 2.69) for worst pain, 1.6 (SD = 1.71) for least pain, 3.2 (SD = 1.85) for average pain, and 2.2 (SD = 2.11) for current pain. The mean of the average scores of the four pain intensities was 3.00 (SD = 1.74). The average pain interference reported by the participants was 21.06 (SD = 16.75).
3.3. Pressure pain thresholds, mechanical cutaneous sensation thresholds, mechanical cutaneous pain thresholds, and temporal summation of mechanical cutaneous pain
The average of PPT was 8.12 Nm (SD = 2.11), 0.011 g (SD = 0.002) for mechanical cutaneous sensation threshold, and 0.021 g (SD = 0.002) for mechanical cutaneous pain threshold. The average of temporal summation of the mechanical cutaneous pain was 20.1 (SD = 14.5).
3.4. Depression, pain condition, and pain catastrophizing are associated with pain intensity and sex differences
We explored bivariate associations among the study variables; these results are presented in Supplementary Appendix 1. The first multivariable analysis included biological measures (age, pain-related disease type, pain duration, and comorbidity), socio-economic factors (level of education, perceived economic status, religion, marital status, and type of residency), and psychological factors, including depressive symptoms and pain catastrophizing, for both men and women (Table 3). The regression model for examining factors associated with pain intensity was significant, n = 216, F(21, 194) =8.79, p < 0.001, accounting for 43.2% of the variance in the pain intensity. The results indicated that pain-related disease type (β = −0.831, p = 0.030), pain catastrophizing (β = 0.071, p < 0.001), and depressive symptoms (β = 0.080, p < 0.001) were significantly associated with pain intensity.
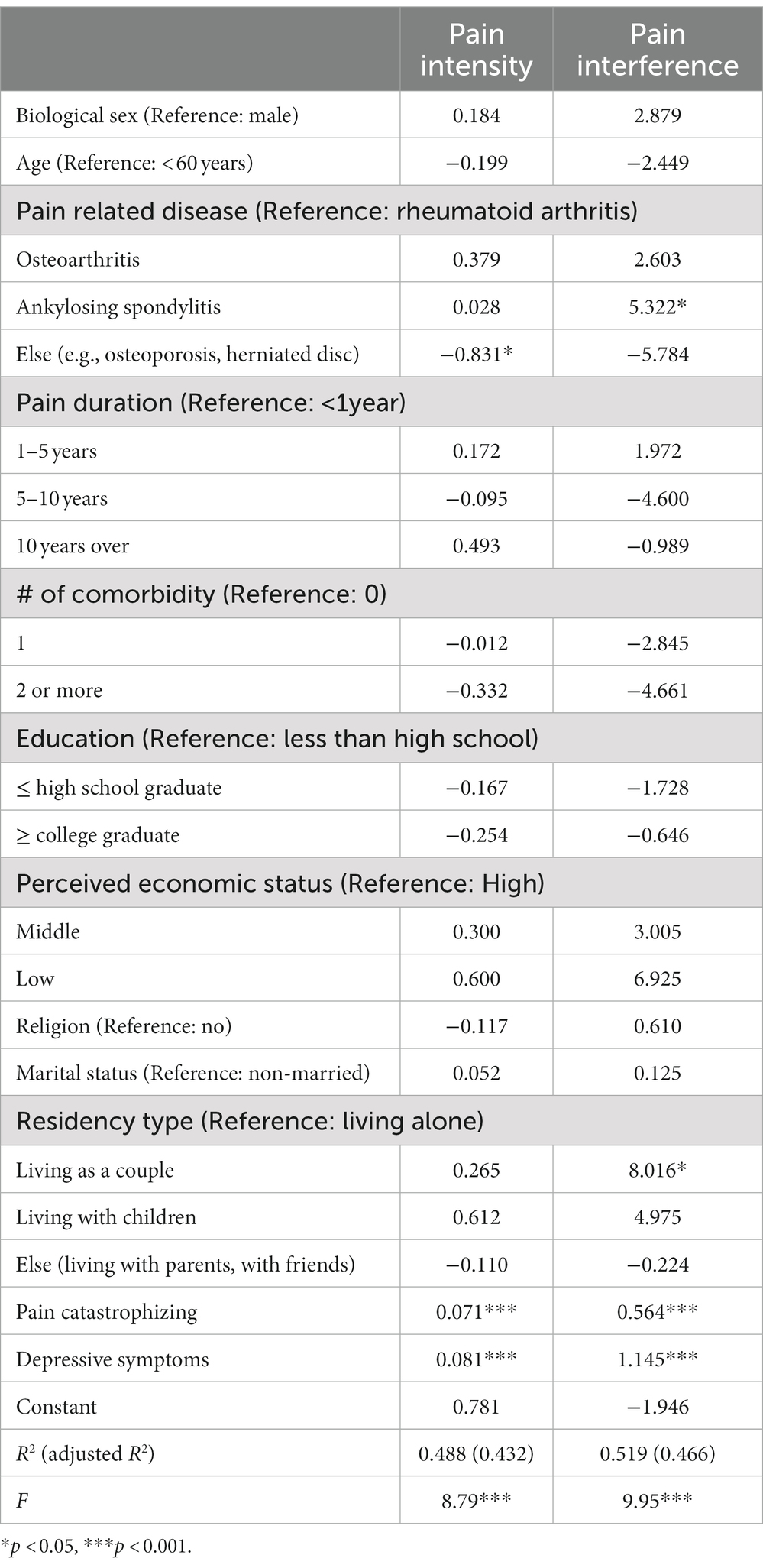
Table 3. Multivariate analysis of biopsychosocial factors influencing pain intensity/interference (N = 220).
The multivariable subgroup regression models separated by biological sex were tested (Table 4). The regression model for examining factors associated with pain intensity for males was significant, n = 74, F(20, 53) =2.99, p = 0.0076, accounting for 35.3% of the variance in the pain intensity. In this model, being diagnosed with other rheumatic diseases (e.g., herniated disc) compared to rheumatoid arthritis (β = −2.374, p = 0.032) and higher pain catastrophizing (β = 0.053, p = 0.020) was significantly associated with higher pain intensity. For female participants, both psychological factors were significantly associated with pain intensity, indicating higher depressive symptoms (β = 0.073, p = 0.012), and pain catastrophizing (β = 0.077, p < 0.001) was associated with higher pain intensity. The model was significant, n = 142, F(20, 121) = 5.59, p < 0.001, accounting for 39.4% of the variance in the pain intensity.
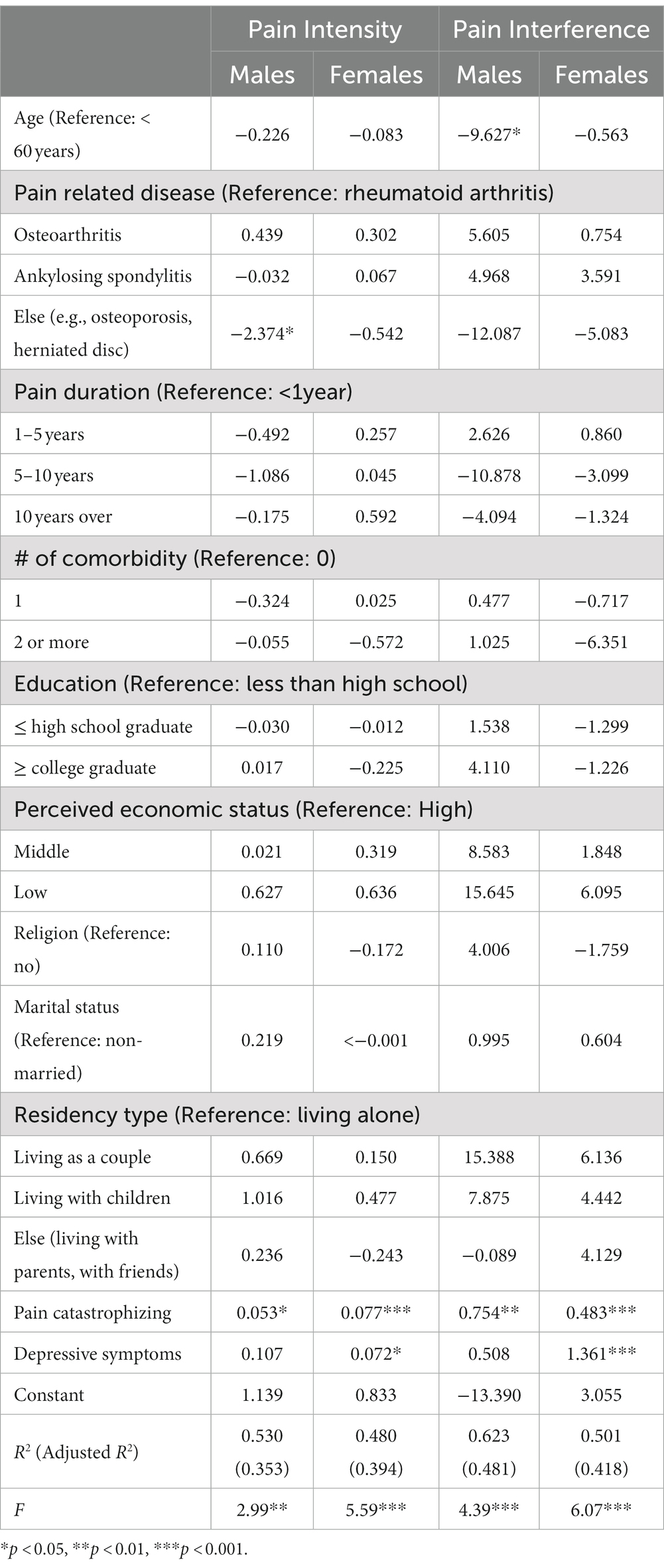
Table 4. Subgroup analysis of biopsychosocial factors influencing pain intensity/interference by gender.
To derive Pearson’s correlation coefficients for covariates associated with pain intensity, we included all continuous and pseudo-continuous covariates of interest without significant collinearity including age, depression (PHQ), pain catastrophizing (PCS), tactile threshold, mechanical pain threshold, area under the curve for temporal summation of mechanical pain, fold change in mechanical pain during temporal summation, pressure pain threshold at the forearm, and pain duration in a partial correlation model. The relationship between pain intensity and depression (n = 219; R = 0.224; t-stat = 3.32; p < 0.001) as well as pain catastrophizing (R = 0.520; t-stat = 8.80; p < 0.001) both passed BF correction for 45 pairs (p ≤ 0.0011). Simple bivariate relationships between pain intensity and depression (R = 0.449; t-stat = 7.40; p < 0.001) as well as pain catastrophizing (R = 0.627; t-stat = 11.9; p < 0.001) were also significant (Figures 1A,B).
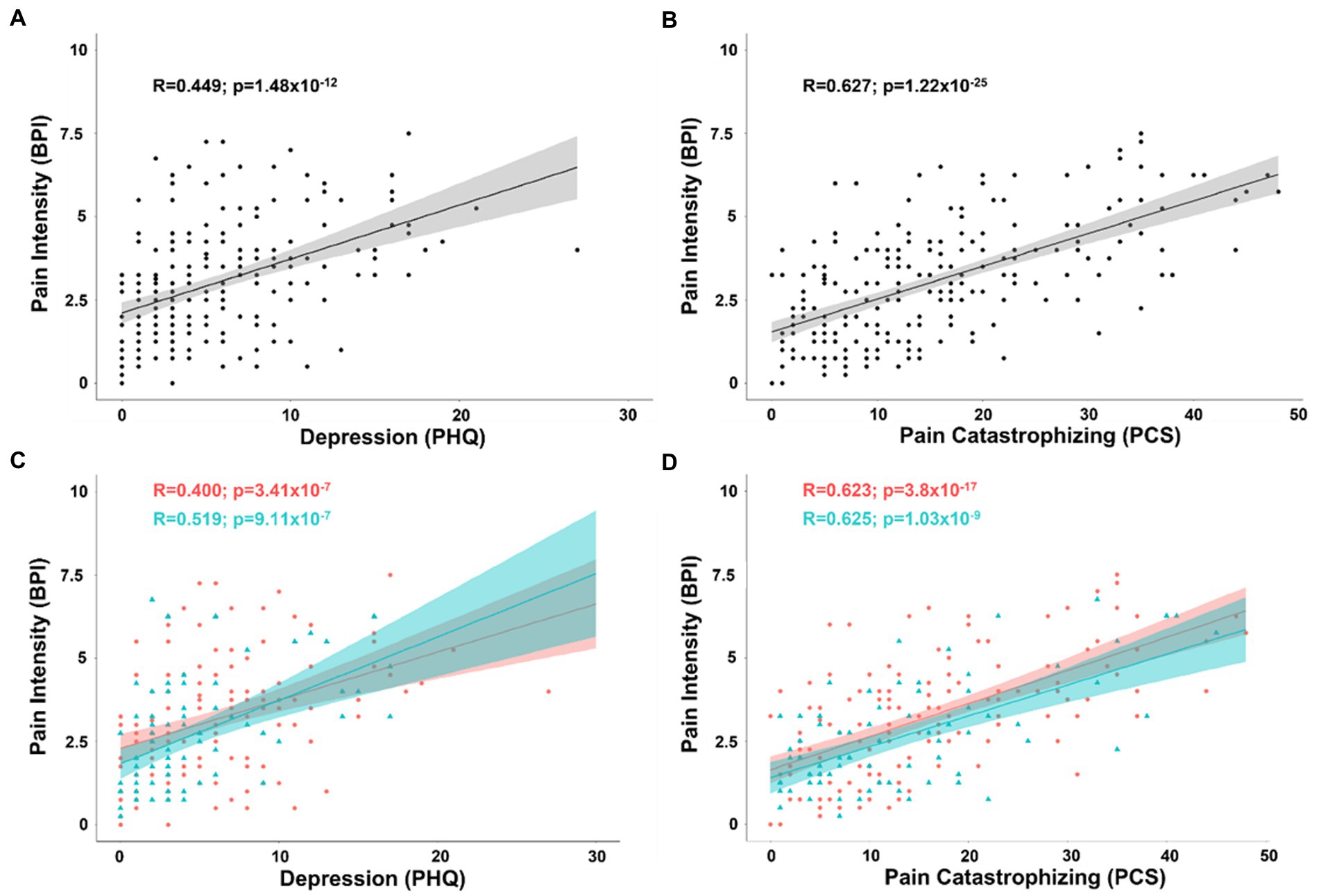
Figure 1. (A) Scatterplot displaying the correlation between intensity of chronic pain and depression as measured by the PHQ. (B) Scatterplot displaying the correlation between intensity of chronic pain and pain catastrophizing as measured by the PCS. (C) Scatterplot displaying the correlation between intensity of chronic pain and depression separated into female (coral/red) and male groups (arctic green/cyan). (D) Scatterplot displaying the correlation between intensity of chronic pain and pain catastrophizing separated into female (coral/red) and male groups (arctic green/cyan). Gray, coral, and arctic green shaded areas correspond to 95% confidence curves.
By separating patients into groups by biological sex, the same model revealed that while pain catastrophizing was significantly associated with pain intensity in both men (n = 75; R = 0.480; t-stat = 4.41; p < 0.001) and women (n = 144; R = 0.536; t-stat = 7.35; p < 0.001), depression showed a similar association in women (R = 0.228; t-stat = 2.71; p < 0.001), but was no longer a significant covariate with pain intensity in men (R = 0.189; t-stat = 1.56; p = 0.125). There was no significant difference between men and women in the effect of pain catastrophizing or depression with pain intensity (all tests p > 0.05). Simple bivariate relationships between pain intensity and depression (female: R = 0.400; t-stat = 5.2; p < 0.001; male: R = 0.519; t-stat = 5.19; p < 0.001) as well as pain catastrophizing (female: R = 0.623; t-stat = 9.49; p < 0.001; male: R = 0.625; t-stat = 6.84; p < 0.001) were significant for both women and men (Figures 1C,D).
Exploratory analyses of biological sex differences in correlation of pain intensity with covariates included in the partial correlation model found tactile threshold was negatively associated with pain intensity in men (R = −0.316; t-stat = −2.68; p = 0.00928), but not women (R = 0.025; t-stat = 0.285; p = 0.776). The difference between the correlations between pain intensity and tactile threshold was significant after controlling for factors included in the partial correlation model (pooled n = 109; ΔR = 0.341; t-stat = 3.75; p < 0.001) and in bivariate correlations (ΔR = 0.392; t-stat = 4.41; p < 0.001) (Figure 2).
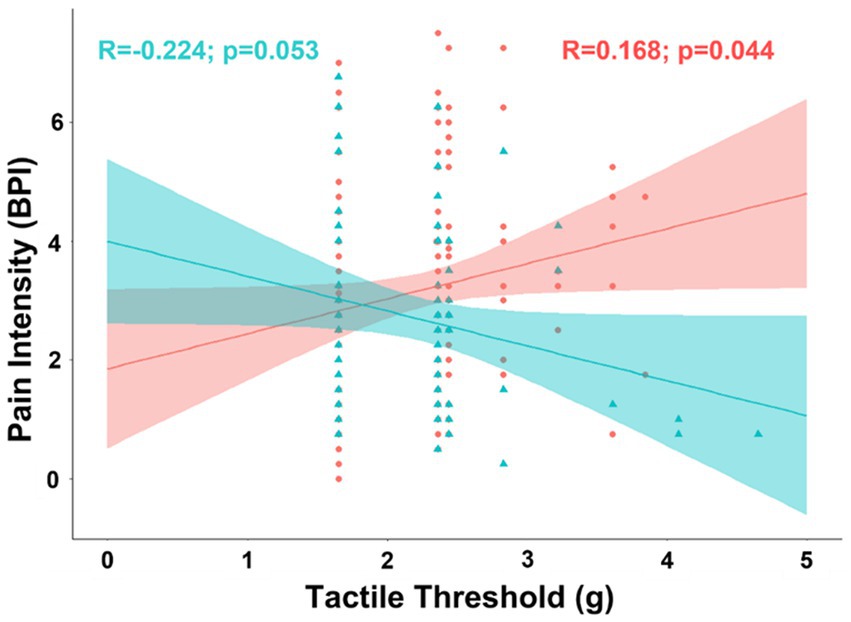
Figure 2. Scatterplot displaying the correlation between intensity of chronic pain and tactile sensitivity separated into female (coral/red) and male groups (arctic green/cyan). Coral and arctic green shaded areas correspond to 95% confidence curves.
3.5. Depression, pain condition, and pain catastrophizing are associated with pain interference and sex differences
For pain interference, being diagnosed with ankylosing spondylitis compared to rheumatoid arthritis (β = 5.322, p = 0.035), living as a couple compared to living alone (β = 8.016, p = 0.031), pain catastrophizing (β = 0.564, p < 0.001), and depressive symptoms (β = 1.145, p < 0.001) were significantly positively associated factors. The regression model for examining factors associated with pain interference was significant, n = 216, F(21, 194) = 9.95, p < 0.001, accounting for 46.6% of the variance in the pain interference (Table 3).
The regression model for examining factors associated with pain interference for males was significant, n = 74, F(20, 53) = 4.39, p < 0.001, accounting for 48.2% of the variance in the pain interference. In this model, being younger (β = −9.627, p = 0.042) and higher pain catastrophizing (β = 0.754, p = 0.001) was significantly associated with higher pain interference. For female participants, both psychological factors were significantly associated with pain interference, indicating higher depressive symptoms (β = 1.361, p < 0.001) and pain catastrophizing (β = 0.482, p < 0.001), which were associated with higher pain interference. The model was significant, n = 142, F(20, 121) = 6.07, p < 0.001, accounting for 41.8% of the variance in the pain interference (Table 4).
To derive Pearson’s correlation coefficients for covariates associated with pain inference, we included all continuous and pseudo-continuous covariates of interest without significant collinearity including age, depression (PHQ), pain catastrophizing (PCS), tactile threshold, mechanical pain threshold, area under the curve for temporal summation of mechanical pain, fold change in mechanical pain during temporal summation, pressure pain threshold at the forearm, and pain duration in a partial correlation model. The positive correlations between pain interference and depression (n = 219; R = 0.351; t-stat = 5.41; p < 0.001) as well as pain catastrophizing (R = 0.464; t-stat = 7.58; p < 0.001) both passed BF correction within the partial correlation model and in bivariate correlations of pain interference and depression (R = 0.540; t-stat = 9.47; p < 0.001) and pain catastrophizing (R = 0.604; t-stat = 11.2; p < 0.001) (Figures 3A,B).
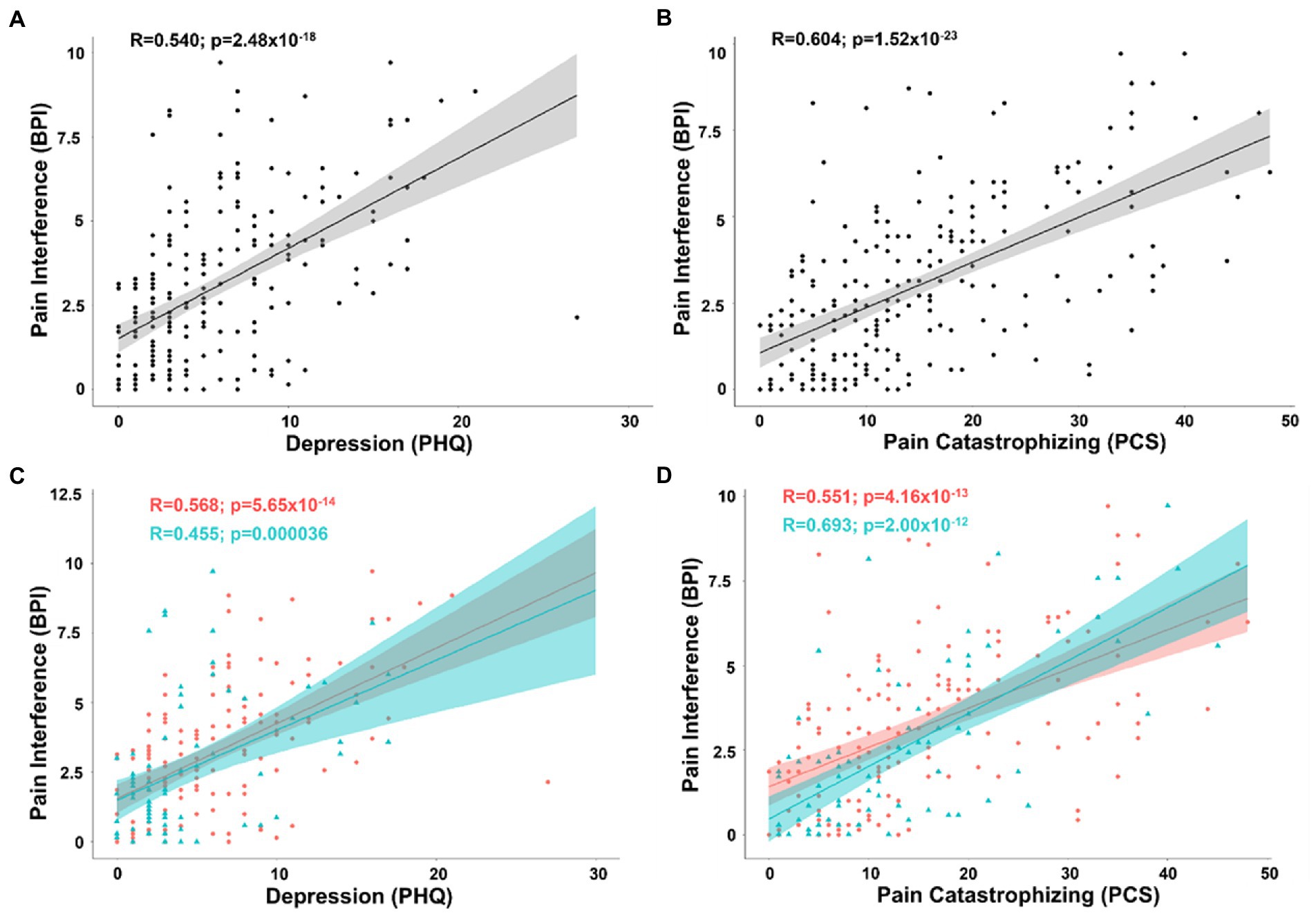
Figure 3. (A) Scatterplot displaying the correlation between pain interference and depression as measured by the PHQ. (B) Scatterplot displaying the correlation between pain interference and pain catastrophizing as measured by the PCS. (C) Scatterplot displaying the correlation between pain interference and depression separated into female (coral/red) and male groups (arctic green/cyan). (D) Scatterplot displaying the correlation between pain interference and pain catastrophizing separated into female (coral/red) and male groups (arctic green/cyan). Gray, coral, and arctic green shaded areas correspond to 95% confidence curves.
Separating patients into groups by biological sex, the same model revealed that while pain catastrophizing was significantly associated with pain interference in both men (n = 75; R = 0.609; t-stat = 6.20; p < 0.001) and women (n = 144; R = 0.403; t-stat = 5.10; p < 0.001), depression showed a similar association in women (R = 0.439; t-stat = 5.66; p < 0.001), but was no longer a significant covariate with pain interference in men (R = 0.022; t-stat = 0.179; p = 0.859). There was a significant difference between males and females in the association of depression on pain interference (pooled n = 109 ΔR = 0.417; t-stat = 4.75; p < 0.001). In contrast, in bivariate analysis, while not controlling for confounding variables, pain interference was positively correlated with depression in both men (R = 0.455; t-stat = 4.37; p < 0.001) and women (R = 0.568; t-stat = 8.22; p < 0.001) (Figure 3C). To determine the reason for this loss of correlation in the three-variable model between depressive symptoms and pain interference in men, we analyzed the partial correlation after removing pain catastrophizing from the covariance matrix. Removing catastrophizing from the partial correlation model resulted in a positive correlation between pain interference and depression (R = 0.439; t-stat = 3.97; p < 0.001). This demonstrates that, in this sample of men, the bivariate relationship between pain interference and depression is driven by pain catastrophizing as far as the cross-sectional study design allows us to determine drivers of effects. In women and men, bivariate positive correlations remained between pain interference and catastrophizing (female: R = 0.551; t-stat = 7.87; p < 0.001; male: R = 0.693; t-stat = 8.27; p < 0.001) (Figure 3D).
Exploratory analyses of biological sex differences in correlation of pain interference with covariates included in the partial correlation model found tactile threshold was negatively associated with pain interference in men (R = −0.253; t-stat = −2.11; p = 0.039), but positively correlated in women (R = 0.207; t-stat = 2.45; p = 0.016). The difference between the correlations between pain interference and tactile threshold was also significant in the partial correlation model (ΔR = 0.460; t-stat = 5.36; p < 0.001) and in simple bivariate correlations (ΔR = 0.462; t-stat = 5.39; p < 0.001) (Figure 4).
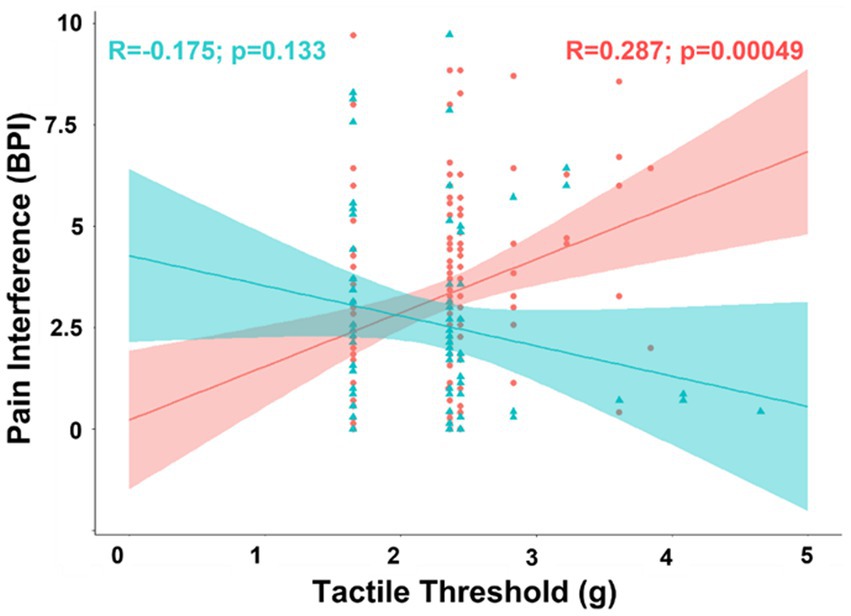
Figure 4. Scatterplot displaying the correlation between pain interference and tactile sensitivity separated into female (coral/red) and male groups (arctic green/cyan). Coral and arctic green shaded areas correspond to 95% confidence curves.
3.6. Depression is associated with number of pain areas reported
In an exploratory analysis of number of pain areas reported, we included all continuous and pseudo-continuous covariates of interest without significant collinearity including age, depression (PHQ), pain catastrophizing (PCS), tactile threshold, mechanical pain threshold, area under the curve for temporal summation of mechanical pain, fold change in mechanical pain during temporal summation, pressure pain threshold at the forearm, and pain duration in a partial correlation model. The relationship between number of pain areas and depression (n = 219; R = 0.215; t-stat = 3.19; p = 0.00165) as well as pain catastrophizing (R = 0.136; t-stat = 1.99; p = 0.048) were significant in exploratory analysis. In simple bivariate analyses, the relationship between number of pain areas and depression (R = 0.315; t-stat = 4.89; p < 0.001) as well as pain catastrophizing (R = 0.285; t-stat = 4.38; p < 0.001) were significant in exploratory analysis (Figures 5A,B). By separating patients into groups by biological sex, the partial correlation model revealed depression was more strongly related to number of pain areas reported in men (n = 75; R = 0.332; t-stat = 2.74; p = 0.0080) than women (n = 144; R = 0.148; t-stat = 1.73; p = 0.085), but this difference was not significant (Figure 5C).
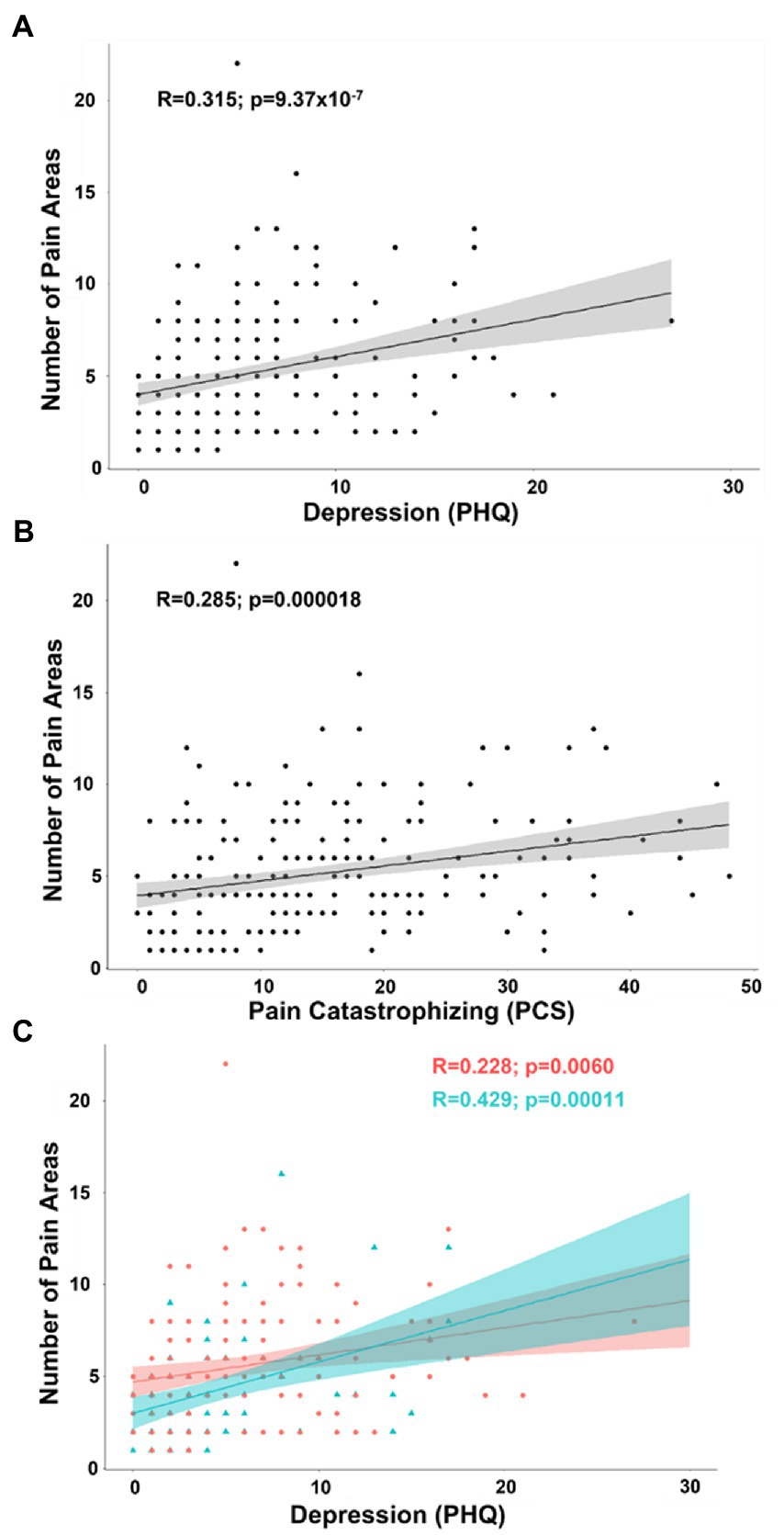
Figure 5. (A) Scatterplot displaying the correlation between number of pain areas reported and depression as measured by the PHQ. (B) Scatterplot displaying the correlation between number of pain areas reported and pain catastrophizing as measured by the PCS. (C) Scatterplot displaying the correlation between number of pain areas reported and depression separated into female (coral/red) and male groups (arctic green/cyan). Gray, coral, and arctic green shaded areas correspond to 95% confidence curves.
4. Discussion and conclusions
This study explored levels of pain intensity and pain interference among Korean patients with chronic secondary musculoskeletal pain in various rheumatic diseases, and its associations with multidimensional factors based on the Biopsychosocial model of pain. The average pain intensity of the current study was 3 on a scale from 0 to 10. This is similar to previous results in an elderly Korean population with chronic pain (Kim et al., 2020). However, this is somewhat lower compared to previous studies among Western populations which ranged from 6 to 8 in the US (Farrar et al., 2001; Smit et al., 2020; Zvolensky et al., 2020), in Spain (Bodes Pardo et al., 2018), and Sweden (Grimby-Ekman et al., 2020) in patients with chronic pain. This is supported by previous reports of underreporting and underdiagnosis of pain in Asians (Kawi et al., 2019). Cultural norms of Asian patients may discourage reporting their pain to avoid burdening others or being seen as weak (Tung and Li, 2015). Rather than seeking medical assistance, accepting the pain as a natural thing (Im et al., 2008) or suffering to maintain one’s independence (Dubus, 2014; Nguyen and Seal, 2014) has been reported in Asian immigrants in the US. The participants of the current study were recruited from a clinical setting in an outpatient clinic in a hospital, and most had experienced pain for more than 5 years. Previously, higher pain sensitivity has been reported in Asians compared to non-Hispanic Whites (Kim et al., 2017). The discrepancy of these results in pain intensity reports may be associated with the cultural background of the Korean population in the way they express their pain. Cultural aspects (e.g., stereotyped stoicism; Buddhism) and social norms embedded in Asian cultures may influence patients’ pain perception and their expression of pain intensity (Good and Ahn, 2008; Ahn et al., 2021). Therefore, careful attention may be required when assessing pain experience, and multidimensional assessment may mitigate this potential predisposition for under-reporting pain in this specific ethnic group when dealing with chronic secondary musculoskeletal pain.
Among the multidimensional factors associated with pain intensity and pain interference, we found several significant factors for each biological, psychological, and social measures. Among these three groups of factors, psychological factors (depressive symptoms and pain catastrophizing) were the most influential in both pain intensity and pain interference when compared to biological and social factors. Depressive symptoms (Zvolensky et al., 2020; Kim et al., 2021) and pain catastrophizing (Bodes Pardo et al., 2018; Kamonseki et al., 2021; Kim et al., 2021) are well known factors associated with chronic pain, and this study adds to the literature by providing additional evidence of the significance of these factors amongst other factors, specifically in this cultural group. About half of the participants in this study had some level of depressive symptoms, and this is comparable with a previous study in Koreans (Kim et al., 2020). We also found the significant mediating role of pain catastrophizing on the association between depression and pain interference among male participants. This result is in line with previous study on elderly Korean Americans with chronic pain (Kim et al., 2021). We suggest including these psychological factors in pain assessment in a Korean population to capture a more complete picture of their pain experience and to improve outcomes of pain management.
Biological sex is a well-known factor influencing chronic pain (Mogil and Bailey, 2010); we add to the literature by reporting sex differences on the impact of pain catastrophizing and depression on pain. For example, depressive symptoms were significantly associated with pain intensity and pain interference only for women, while there were no significant associations in men, including all other biopsychosocial factors in the model. Also, for men, being young (compared to those higher than 60 years old) was significantly associated with higher pain interference. These differences should inform healthcare providers to consider these factors for developing not only culturally adapted, but also sex-specific, programs targeted to reduce levels of pain intensity and pain interference.
Interestingly, measures of QST did not specifically contribute to explaining pain intensity or pain interference in the multivariable analyses. However, we found some sex differences in the correlational analyses, where tactile threshold was negatively associated with pain interference in men but positively correlated in women. This is a novel sex difference in the relationship between tactile sensitivity and pain intensity and interference and needs to be followed up and replicated. The source of the parallel finding that pain intensity and pain interference are both related to tactile sensitivity in a consistent manner in men and women is that pain intensity and pain interference are highly correlated (Cano et al., 2006; Fullwood et al., 2021; Kim et al., 2021). Several studies have demonstrated that both acute and chronic pain reduces tactile sensitivity and tactile acuity (Seltzer and Seltzer, 1986; Bolanowski et al., 2001; Magerl and Treede, 2004; Drummond and Blockey, 2009; Adamczyk W. et al., 2018; Adamczyk W.M. et al., 2018). Tactile sensitivity is directly related to tactile acuity (Craig and Kisner, 1998; Gibson and Craig, 2002; Zingaretti et al., 2019). Studies have shown that reductions in tactile acuity, measured as two-point discrimination, are related to the intensity of chronic pain. These behavioral changes have been linked to evidence of neuroplastic changes in the somatosensory cortex and somatosensory thalamus associated with chronic pain (Kong et al., 2013; Kim et al., 2020). On a group level, changes in tactile acuity are largely consistent across studies, however, efforts to train individuals with chronic pain in tactile discrimination as an analgesic intervention have not achieved consistent analgesia (Moseley et al., 2008; Ryan et al., 2014; Adamczyk W. et al., 2018; van Baal et al., 2020). Our findings suggest that there may be an important sex difference in how tactile acuity is related to chronic pain intensity and interference that has previously been ignored in these interventional studies.
While previously it has been reported that experimental pain sensitivity was associated with pain intensity (Bagnato et al., 2015; Amiri et al., 2021), besides the tactile sensitivity, we did not find any significant relationships between experimental pain sensitivity and clinical pain intensity or interference. The average PPT was 8.12 Nm, which is somewhat higher compared to previous studies in chronic pain patients (Bodes Pardo et al., 2018; Amiri et al., 2021). The results imply low levels of sensitivity in the current study participants, and this is in contrast with previous reports (Kim et al., 2017). It is difficult to explain these results, but we did not include the full modality (e.g., heat pain or cold pain) of experimental pain sensitivity in our study. An additional possible explanation is that participants were new to the QST procedure and showed difficulty in reporting their pain perception. We would suggest more studies with more extensive and rigorous QST measurement in this population. However, it must be cautioned that while QST has been shown to correlate with pain symptomatology and intensity, large studies have not demonstrated that QST predicts development of chronic pain (Slade et al., 2014). The usefulness of QST may be to diagnose subtypes of chronic pain, but not to usefully anticipate overall pain intensity or interference (Shraim et al., 2022).
This study has several limitations. First, we included multiple measures to build a comprehensive model explaining chronic pain, however, there are still other important factors that we did not include (e.g., anxiety, social support, quality of life, kinesiophobia, pain acceptance, and coping strategies) that have been reported previously (Esteve et al., 2007; Varallo et al., 2022). Also, participants were recruited from an outpatient center at a hospital which implies the participants were receiving medical care for their diseases. Therefore, the results of the current study may not be generalized to those in the community without appropriate chronic pain care. Finally, because of the nature of study design, associations reported in this study cannot be interpreted as causal relationships.
The current study explored biopsychosocial correlates associated with pain intensity and pain interference among patients with chronic secondary musculoskeletal pain. Pain catastrophizing had the greatest influence on pain experience for both male and female patients, and depressive symptoms were associated with pain intensity and pain interference among female patients. These identified factors should be included in pain assessment and pain management for patients with chronic pain. A sex-specific approach would be warranted to better manage patients with chronic secondary musculoskeletal pain in various rheumatic diseases. Clinicians and researchers who serve diverse racial/ethnic groups should consider the significant role of cultural and psychological factors in understanding chronic pain experiences, specifically for Asian populations.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ajou University Hospital IRB (AJIRB-MED-SUR-21-319). The patients/participants provided their written informed consent to participate in this study.
Author contributions
HK and TM designed and conceptualized the study, analyzed the data, and drew all the charts. HK, J-YJ, J-WK, and HK collected the data. HK, TM, and H-AK organized the data. HK organized the whole data collection process. H-AK supervised the quality of the data collection. HK wrote the first draft of the article. All authors participated in the revision of the manuscript and approved the final manuscript for publication.
Funding
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (no. 2021R1F1A1059877) and the National Institute of General Medical Sciences (RL5GM118972).
Acknowledgments
We thank all the patients who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyg.2023.1063164/full#supplementary-material
References
Adamczyk, W., Luedtke, K., and Saulicz, E. (2018). Lumbar tactile acuity in patients with low back pain and healthy controls: Systematic review and meta-analysis. Clin. J. Pain 34, 82–94. doi: 10.1097/AJP.0000000000000499
Adamczyk, W. M., Saulicz, O., Saulicz, E., and Luedtke, K. (2018). Tactile acuity (dys)function in acute nociceptive low back pain: A double-blind experiment. Pain 159, 427–436. doi: 10.1097/j.pain.0000000000001110
Ahn, H., Jackson, N., An, K., Fillingim, R. B., Miao, H., Lee, M., et al. (2021). Relationship between acculturative stress and pain catastrophizing in Korean Americans. J. Immigr. Minor. Health 23, 741–746. doi: 10.1007/s10903-020-01083-6
Alabas, O. A., Tashani, O. A., and Johnson, M. I. (2013). Effects of ethnicity and gender role expectations of pain on experimental pain: A cross-cultural study. Eur. J. Pain 17, 776–786. doi: 10.1002/j.1532-2149.2012.00229.x
American Psychiatric Association . Diagnostic and statistical manual of mental disorders (5th ed.) Washington DC: American Psychiatric Association (2013).
Amiri, M., Alavinia, M., Singh, M., and Kumbhare, D. (2021). Pressure pain threshold in patients with chronic pain: A systematic review and meta-analysis. Am. J. Phys. Med. Rehabil. 100, 656–674. doi: 10.1097/PHM.0000000000001603
Bagnato, G., De Andres, I., Sorbara, S., Verduci, E., Corallo, G., Ferrera, A., et al. (2015). Pain threshold and intensity in rheumatic patients: Correlations with the Hamilton Depression Rating scale. Clin. Rheumatol. 34, 555–561. doi: 10.1007/s10067-013-2477-y
Bjordal, J. M., Klovning, A., Ljunggren, A. E., and Slørdal, L. (2007). Short-term efficacy of pharmacotherapeutic interventions in osteoarthritic knee pain: A meta-analysis of randomised placebo-controlled trials. Eur. J. Pain 11, 125–138. doi: 10.1016/j.ejpain.2006.02.013
Bodes Pardo, G., Lluch Girbés, E., Roussel, N. A., Gallego Izquierdo, T., Jiménez Penick, V., and Pecos, M. D. (2018). Pain neurophysiology education and therapeutic exercise for patients with chronic low back pain: A single-blind randomized controlled trial. Arch. Phys. Med. Rehabil. 99, 338–347. doi: 10.1016/j.apmr.2017.10.016
Bolanowski, S. J., Gescheider, G. A., Fontana, A. M., Niemiec, J. L., and Tromblay, J. L. (2001). The effects of heat-induced pain on the detectability, discriminability, and sensation magnitude of vibrotactile stimuli. Somatosens. Mot. Res. 18, 5–9. doi: 10.1080/08990220020002015
Bostick, G. P., Norman, K. E., Sharma, A., Toxopeus, R., Irwin, G., and Dhillon, R. (2021). Improving cultural knowledge to facilitate cultural adaptation of pain management in a culturally and linguistically diverse community. Physiother. Can. 73, 19–25. doi: 10.3138/ptc-2019-0027
Cano, A., Mayo, A., and Ventimiglia, M. (2006). Coping, pain severity, interference, and disability: The potential mediating and moderating roles of race and education. J. Pain 7, 459–468. doi: 10.1016/j.jpain.2006.01.445
Cardoso, J. S., Riley, J. L. 3rd, Glover, T., Sibille, K. T., Bartley, E. J., Goodin, B. R., et al. (2016). Experimental pain phenotyping in community-dwelling individuals with knee osteoarthritis. Pain 157, 2104–2114. doi: 10.1097/j.pain.0000000000000625
Cho, S., Kim, H. Y., and Lee, J. H. (2013). Validation of the Korean version of the pain catastrophizing scale in patients with chronic non-cancer pain. Qual. Life Res. 22, 1767–1772. doi: 10.1007/s11136-012-0308-2
Cleeland, CS. The brief pain inventory, user guide. Houston, TX (1991). Available at: https://www.mdanderson.org/documents/Departments-and-Divisions/Symptom-Research/BPI_UserGuide.pdf
Craig, J. C., and Kisner, J. M. (1998). Factors affecting tactile spatial acuity. Somatosens. Mot. Res. 15, 29–45. doi: 10.1080/08990229870934
Cruz-Almeida, Y., Sibille, K. T., Goodin, B. R., Petrov, M. E., Bartley, E. J., Riley, J. L. 3rd, et al. (2014). Racial and ethnic differences in older adults with knee osteoarthritis. Arthritis Rheumatol. 66, 1800–1810. doi: 10.1002/art.38620
Dahlhamer, J., Lucas, J., Zelaya, C., Nahin, R., Mackey, S., DeBar, L., et al. (2018). Prevalence of chronic pain and high-impact chronic pain among adults - United States, 2016. MMWR Morb. Mortal. Wkly Rep. 67, 1001–1006. doi: 10.15585/mmwr.mm6736a2
Donnelly, P. L., and Kim, K. S. (2008). The patient health questionnaire (PHQ-9K) to screen for depressive disorders among immigrant Korean American elderly. J. Cult. Divers. 15, 24–29.
Drummond, P. D., and Blockey, P. (2009). Topically applied capsaicin inhibits sensitivity to touch but not to warmth or heat-pain in the region of secondary mechanical hyperalgesia. Somatosens. Mot. Res. 26, 75–81. doi: 10.3109/08990220903296761
Dubus, N. M. (2014). Self-perception of when old age begins for Cambodian elders living in the United States. J. Cross Cult. Gerontol. 29, 185–199. doi: 10.1007/s10823-014-9230-0
Esteve, R., Ramírez-Maestre, C., and López-Marínez, A. E. (2007). Adjustment to chronic pain: The role of pain acceptance, coping strategies, and pain-related cognitions. Ann. Behav. Med. 33, 179–188. doi: 10.1007/BF02879899
Farrar, J. T., Young, J. P. Jr., LaMoreaux, L., Werth, J. L., and Poole, M. R. (2001). Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 94, 149–158. doi: 10.1016/S0304-3959(01)00349-9
Fayaz, A., Croft, P., Langford, R. M., Donaldson, L. J., and Jones, G. T. (2016). Prevalence of chronic pain in the UK: A systematic review and meta-analysis of population studies. BMJ Open 6:e010364. doi: 10.1136/bmjopen-2015-010364
Fifield, J., Reisine, S. T., and Grady, K. (1991). Work disability and the experience of pain and depression in rheumatoid arthritis. Soc. Sci. Med. 33, 579–585. doi: 10.1016/0277-9536(91)90215-x
Fordyce, W. E. (1994). Pain and suffering: What is the unit? Qual. Life Res. 3, S51–S56. doi: 10.1007/BF00433377
Fullwood, D., Gomez, R. N., Huo, Z., Cardoso, J. S., Bartley, E. J., Booker, S. Q., et al. (2021). A mediation appraisal of catastrophizing, pain-related outcomes, and race in adults with knee osteoarthritis. J. Pain 22, 1452–1466. doi: 10.1016/j.jpain.2021.04.018
Gibson, G. O., and Craig, J. C. (2002). Relative roles of spatial and intensive cues in the discrimination of spatial tactile stimuli. Percept. Psychophys. 64, 1095–1107. doi: 10.3758/BF03194759
Good, M., and Ahn, S. (2008). Korean and American music reduces pain in Korean women after gynecologic surgery. Pain Manag. Nurs. 9, 96–103. doi: 10.1016/j.pmn.2008.02.002
Goodin, B. R., Bulls, H. W., Herbert, M. S., Schmidt, J., King, C. D., Glover, T. L., et al. (2014). Temporal summation of pain as a prospective predictor of clinical pain severity in adults aged 45 years and older with knee osteoarthritis: Ethnic differences. Psychosom. Med. 76, 302–310. doi: 10.1097/PSY.0000000000000058
Greenspan, J. D., Slade, G. D., Bair, E., Dubner, R., Fillingim, R. B., Ohrbach, R., et al. (2011). Pain sensitivity risk factors for chronic TMD: Descriptive data and empirically identified domains from the OPPERA case control study. J. Pain 12, T61–T74. doi: 10.1016/j.jpain.2011.08.006
Grimby-Ekman, A., Ahlstrand, C., Gerdle, B., Larsson, B., and Sandén, H. (2020). Pain intensity and pressure pain thresholds after a light dynamic physical load in patients with chronic neck-shoulder pain. BMC Musculoskelet. Disord. 21:266. doi: 10.1186/s12891-020-03298-y
Im, E. O., Liu, Y., Kim, Y. H., and Chee, W. (2008). Asian American cancer patients’ pain experience. Cancer Nurs. 31, E17–E23. doi: 10.1097/01.NCC.0000305730.95839.83
Jensen, B. (2016). Chronic pain assessment from bench to bedside: Lessons along the translation continuum. Transl. Behav. Med. 6, 596–604. doi: 10.1007/s13142-015-0370-8
Jung-Choi, K., Park, J., Kim, N., and Park, H. (2014). Status of chronic pain prevalence in the Korean adults. Public Health Wkly. Rep. 8, 728–734. doi: 10.1016/j.ajog.2019.12.010
Kamonseki, D. H., Christenson, P., Rezvanifar, S. C., and Calixtre, L. B. (2021). Effects of manual therapy on fear avoidance, kinesiophobia and pain catastrophizing in individuals with chronic musculoskeletal pain: Systematic review and meta-analysis. Musculoskelet. Sci. Pract. 51:102311. doi: 10.1016/j.msksp.2020.102311
Kawi, J., Reyes, A. T., and Arenas, R. A. (2019). Exploring pain management among Asian immigrants with chronic pain: Self-management and resilience. J. Immigr. Minor. Health 21, 1123–1136. doi: 10.1007/s10903-018-0820-8
Kim, S. (2015). Ppcor: An R package for a fast calculation to semi-partial correlation coefficients. Commun. Stat. Appl. Methods 22, 665–674. doi: 10.5351/CSAM.2015.22.6.665
Kim, H. J., Chang, S. J., Park, H., Choi, S. W., Juon, H. S., Lee, K. E., et al. (2020). Intra-ethnic differences in chronic pain and the associated factors: An exploratory, comparative design. J Nurs. Scholarsh 52, 389–396. doi: 10.1111/jnu.12564
Kim, H. J., Greenspan, J. D., Ohrbach, R., Fillingim, R. B., Maixner, W., Renn, C. L., et al. (2019). Racial/ethnic differences in experimental pain sensitivity and associated factors - cardiovascular responsiveness and psychological status. PLoS One 14:e0215534. doi: 10.1371/journal.pone.0215534
Kim, H., Mawla, I., Lee, J., Gerber, J., Walker, K., Kim, J., et al. (2020). Reduced tactile acuity in chronic low back pain is linked with structural neuroplasticity in primary somatosensory cortex and is modulated by acupuncture therapy. Neuroimage 217:116899. doi: 10.1016/j.neuroimage.2020.116899
Kim, H. J., Park, H., and Juon, H. S. (2021). The mediating role of pain catastrophizing on the association between depression and pain severity and interference among elderly Asian immigrants with chronic pain. J. Pain Res. 14, 737–745. doi: 10.2147/JPR.S304440
Kim, H. J., Yang, G. S., Greenspan, J. D., Downton, K. D., Griffith, K. A., Renn, C. L., et al. (2017). Racial and ethnic differences in experimental pain sensitivity: Systematic review and meta-analysis. Pain 158, 194–211. doi: 10.1097/j.pain.0000000000000731
Kong, J., Spaeth, R. B., Wey, H. Y., Cheetham, A., Cook, A. H., Jensen, K., et al. (2013). S1 is associated with chronic low back pain: A functional and structural MRI study. Mol. Pain 9:1744-8069-9-43. doi: 10.1186/1744-8069-9-43
Machado, G. C., Maher, C. G., Ferreira, P. H., Pinheiro, M. B., Lin, C. W., Day, R. O., et al. (2015). Efficacy and safety of paracetamol for spinal pain and osteoarthritis: Systematic review and meta-analysis of randomised placebo controlled trials. BMJ 350:h1225. doi: 10.1136/bmj.h1225
Magerl, W., and Treede, R. D. (2004). Secondary tactile hypoesthesia: A novel type of pain-induced somatosensory plasticity in human subjects. Neurosci. Lett. 361, 136–139. doi: 10.1016/j.neulet.2003.12.001
Meeker, T. J., Emerson, N. M., Chien, J. H., Saffer, M. I., Bienvenu, O. J., Korzeniewska, A., et al. (2021). During vigilance to painful stimuli: Slower response rate is related to high trait anxiety, whereas faster response rate is related to high state anxiety. J. Neurophysiol. 125, 305–319. doi: 10.1152/jn.00492.2020
Mills, S. E. E., Nicolson, K. P., and Smith, B. H. (2019). Chronic pain: A review of its epidemiology and associated factors in population-based studies. Br. J. Anaesth. 123, e273–e283. doi: 10.1016/j.bja.2019.03.023
Mogil, J. S., and Bailey, A. L. (2010). Sex and gender differences in pain and analgesia. Prog. Brain Res. 186, 141–157. doi: 10.1016/B978-0-444-53630-3.00009-9
Moseley, G. L., Zalucki, N. M., and Wiech, K. (2008). Tactile discrimination, but not tactile stimulation alone, reduces chronic limb pain. Pain 137, 600–608. doi: 10.1016/j.pain.2007.10.021
Nguyen, A. L., and Seal, D. W. (2014). Cross-cultural comparison of successful aging definitions between Chinese and Hmong elders in the United States. J. Cross Cult. Gerontol. 29, 153–171. doi: 10.1007/s10823-014-9231-z
Orhan, C., Van Looveren, E., Cagnie, B., Mukhtar, N. B., Lenoir, D., and Meeus, M. (2018). Are pain beliefs, cognitions, and behaviors influenced by race, ethnicity, and culture in patients with chronic musculoskeletal pain: A systematic review. Pain Physician 21, 541–558. doi: 10.36076/ppj.2018.6.541
Perrot, S., Cohen, M., Barke, A., Korwisi, B., Rief, W., Treede, R. D., et al. (2019). The IASP classification of chronic pain for ICD-11: chronic secondary musculoskeletal pain. Pain 160, 77–82. doi: 10.1097/j.pain.0000000000001389
Rolke, R., Magerl, W., Campbell, K. A., Schalber, C., Caspari, S., Birklein, F., et al. (2006). Quantitative sensory testing: A comprehensive protocol for clinical trials. Eur. J. Pain 10, 77–88. doi: 10.1016/j.ejpain.2005.02.003
Ryan, C., Harland, N., Drew, B. T., and Martin, D. (2014). Tactile acuity training for patients with chronic low back pain: A pilot randomised controlled trial. BMC Musculoskelet. Disord. 15:59. doi: 10.1186/1471-2474-15-59
Seltzer, S. F., and Seltzer, J. L. (1986). Tactual sensitivity of chronic pain patients to non-painful stimuli. Pain 27, 291–295. doi: 10.1016/0304-3959(86)90156-9
Shin, J., Park, S., Cho, S., Chiu, Y., Bang, H., and Bernstein, K. S. (2010). Validation of patient health questionnaire-9 Korean version (PHQ-9K) scale for screening depression among Korean Americans in community settings. J. Theory Constr. Test. 14, 45–50.
Shraim, M. A., Sluka, K. A., Sterling, M., Arendt-Nielsen, L., Argoff, C., Bagraith, K. S., et al. (2022). Features and methods to discriminate between mechanism-based categories of pain experienced in the musculoskeletal system: A Delphi expert consensus study. Pain 163, 1812–1828. doi: 10.1097/j.pain.0000000000002577
Slade, G. D., Sanders, A. E., Ohrbach, R., Fillingim, R. B., Dubner, R., Gracely, R. H., et al. (2014). Pressure pain thresholds fluctuate with, but do not usefully predict, the clinical course of painful temporomandibular disorder. Pain 155, 2134–2143. doi: 10.1016/j.pain.2014.08.007
Smit, T., Rogers, A. H., Garey, L., Allan, N. P., Viana, A. G., and Zvolensky, M. J. (2020). Anxiety sensitivity and pain intensity independently predict opioid misuse and dependence in chronic pain patients. Psychiatry Res. 294:113523. doi: 10.1016/j.psychres.2020.113523
Sullivan, M. J. L., Bishop, S. R., and Pivik, J. (1995). The pain catastrophizing scale: Development and validation. Psychol. Assess. 7, 524–532. doi: 10.1037/1040-3590.7.4.524
Sullivan, M. D., Sturgeon, J. A., Lumley, M. A., and Ballantyne, J. C. (2022). Reconsidering Fordyce’s classic article, “pain and suffering: What is the unit?” to help make our model of chronic pain truly biopsychosocial. Pain 164, 271–279. doi: 10.1097/j.pain.0000000000002748
Tung, W.-C., and Li, Z. (2015). Pain beliefs and behaviors among Chinese. Home Health Care Manag. Pract. 27, 95–97. doi: 10.1177/1084822314547962
Uddin, Z., and MacDermid, J. C. (2016). Quantitative sensory testing in chronic musculoskeletal pain. Pain Med. 17, 1694–1703. doi: 10.1093/pm/pnv105
van Baal, K., Allofs, J., Ehrenbrusthoff, K., Grüneberg, C., Hering, T., Kopkow, C., et al. (2020). Effects of a movement control and tactile acuity training in patients with nonspecific chronic low back pain and control impairment - a randomised controlled pilot study. BMC Musculoskelet. Disord. 21:794. doi: 10.1186/s12891-020-03727-y
Varallo, G., Suso-Ribera, C., Ghiggia, A., Veneruso, M., Cattivelli, R., Guerrini Usubini, A., et al. (2022). Catastrophizing, kinesiophobia, and acceptance as mediators of the relationship between perceived pain severity, self-reported and performance-based physical function in women with fibromyalgia and obesity. J. Pain Res. 15, 3017–3029. doi: 10.2147/JPR.S370718
Yun, Y. H., Mendoza, T. R., Heo, D. S., Yoo, T., Heo, B. Y., Park, H. A., et al. (2004). Development of a cancer pain assessment tool in Korea: A validation study of a Korean version of the brief pain inventory. Oncology 66, 439–444. doi: 10.1159/000079497
Zingaretti, P., Petta, A. M., Cruciani, G., and Spitoni, G. F. (2019). Tactile sensitivity, tactile acuity, and affective touch: From childhood to early adolescence. Somatosens. Mot. Res. 36, 90–96. doi: 10.1080/08990220.2019.1604334
Zvolensky, M. J., Rogers, A. H., Garey, L., Ditre, J. W., Shepherd, J. M., Viana, A. G., et al. (2020). The role of anxiety sensitivity in the relation between pain intensity with substance use and anxiety and depressive symptoms among smokers with chronic pain. Int. J. Behav. Med. 27, 668–676. doi: 10.1007/s12529-020-09914-4
Keywords: chronic pain, pain catastrophizing, depression, rheumatic disease, biopsychosocial, musculoskeletal, sex differences
Citation: Kim HJ, Meeker TJ, Jung J-Y, Kim J-W and Kim H-A (2023) Biological sex influences psychological aspects of the biopsychosocial model related to chronic pain intensity and interference among South Korean patients with chronic secondary musculoskeletal pain in rheumatic diseases. Front. Psychol. 14:1063164. doi: 10.3389/fpsyg.2023.1063164
Edited by:
Tushar Singh, Banaras Hindu University, IndiaReviewed by:
Jillian Vinall Miller, University of Calgary, CanadaGiorgia Varallo, University of Parma, Italy
Aishwarya Jaiswal, Banaras Hindu University, India
Copyright © 2023 Kim, Meeker, Jung, Kim and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hyoun-Ah Kim, bmFraGFkYUBuYXZlci5jb20=
 Hee Jun Kim
Hee Jun Kim Timothy J. Meeker
Timothy J. Meeker Ju-Yang Jung3
Ju-Yang Jung3 Ji-Won Kim
Ji-Won Kim Hyoun-Ah Kim
Hyoun-Ah Kim