- 1School of Public Health, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2JC School of Public Health and Primary Care, Chinese University of Hong Kong, Hong Kong, Hong Kong SAR, China
- 3MOE-Shanghai Key Laboratory of Children’s Environmental Health, Xinhua Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 4School of Nursing, The Hong Kong Polytechnic University, Hong Kong, Hong Kong SAR, China
- 5School of Global Health, Chinese Center for Tropical Diseases Research, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Background: Concerning the changes in the prevalence of neurodevelopmental disorders (NDDs), we estimate the prevalence of attention-deficit/hyperactivity disorder (ADHD), autism spectrum disorder (ASD), intellectual disorder (ID), and learning disability (LD) among US children and adolescents aged 3–17 years in 2019 and 2020.
Methods: The study includes 14,983 US children and adolescents aged 3–17 years in 2019 and 2020 from the National Health Interview Survey (NHIS). Parents were interviewed about whether their children ever and/or currently had NDDs diagnosed. Prevalence estimates of NDDs were calculated with a survey-based weighting scheme. Logistic regression models were used to estimate the associations between NDDs prevalence and subgroups.
Results: The weighted prevalence of ADHD, ASD, ID, and LD was 8.5% (95% CI: 7.9–9.2%), 2.9% (95% CI: 2.6–3.4%), 1.4% (95% CI: 1.2–1.7%), and 6.4% (95% CI: 5.8–7.0%), respectively. A higher prevalence of ADHD, ASD, ID, and LD was observed in boys, those who ever had anxiety or depression symptoms, those with lower family income, those living in a rented house, ever been bullied, and ever lived with anyone mentally ill.
Conclusion: The study found the prevalence of ADHD, ASD, ID, and LD was different by demographics, comorbidity/mental problems, household/parental characteristics, and stressful life events.
Introduction
Children and adolescent neurodevelopmental disorders (NDDs), including attention-deficit/hyperactivity disorder (ADHD), autism spectrum disorder (ASD), intellectual disorder (ID), learning disability (LD), and others, may result in serious delay or irregularity in growth, especially functional, structural, and cognitive maturation (Thapar et al., 2017; Antshel and Russo, 2019; Nickel et al., 2019). Belated diagnosis and treatments of NDDs would predispose life-long disabilities to individuals and bring heavy burdens to families and society (Lai et al., 2014; Posner et al., 2020). In addition, children with NDDs are likely to be diagnosed with various mental or psychiatric problems in adulthood, which may lead to extreme events or secondary negative impacts on individuals, families, and society (Du Rietz et al., 2021).
Continued monitoring of the prevalence of NDDs in children and adolescents is necessary to evaluate the current disease burdens and predict future impacts. According to the early evidence before 2018, the prevalence of ADHD and ASD was steadily rising among US children and adolescents (Xu et al., 2018; Zablotsky et al., 2019a; Zablotsky and Black, 2020). The observed increase may partly attribute to the improved perception and alternative diagnosis methods for ADHD and ASD, while unknown factors cannot be ignored and warrant further investigation (Lai et al., 2014; Posner et al., 2020). The trends in the prevalence of ID and LD seemed to plateau in the last decades (Zablotsky et al., 2019a). However, the NDDs prevalence may be overestimated because previous studies in US children and adolescents counted cases by ever-diagnosed NDDs rather than currently had NDDs, which might mix up with those who were ever diagnosed with NDDs but no longer meet the criteria. Recent studies showed that NDD symptoms could be reduced to a non-clinical level by early non-pharmaceutical or pharmaceutical interventions (Cramer et al., 2011; Berry-Kravis et al., 2018). Prevalence based on those who currently have NDDs rather than ever had NDDs would mirror the accurate level (Kogan et al., 2018).
Interactions between genetic heritability and environmental factors contribute to the onset of children and adolescent NDDs (Bourgeron, 2015; Kasparek et al., 2015). Previous studies focused more on the prevalent distribution of demography and socioeconomic status (Kogan et al., 2018; Xu et al., 2018; Zablotsky et al., 2019a). Few studies investigated the potential entanglement of NDDs with stressful life events, comorbidity/psychiatric symptoms, and household/parental characteristics. Although cross-sectional studies are limited in drawing causal inferences, this study attempted to detail the prevalence of NDDs on more population characteristics among US children and adolescents to underly diversified materials for risk factors.
Materials and methods
Data source
We retrieved data from the National Health Interview Survey (NHIS) in 2019 and 2020. NHIS, conducted by the National Center for Health Statistics (NCHS), is an annual, nationally representative, multistage-sampling interview survey to collect health-related information on the civilian non-institutionalized US population (National Center for Health Statistics, 2020, 2021). NHIS was approved by the research ethics review board of the NCHS and has been widely used to estimate the nationwide prevalence of various diseases in the US (Xu et al., 2018; Gu et al., 2021). Compared with previous versions, NHIS in 2019 and 2020 was redesigned to minimize respondent burden and enhance data quality. During the sampling procedure of NHIS, one sample adult (≥18 years old) and one sample child (≤17 years old, if any children live in the household) were randomly selected from each household. Information about the sample child was collected from a parent or adult who was knowledgeable about and responsible for the healthcare of the sample child. This study only retrieved children’s data for analysis. A total of 9,193 and 5,790 interviewed sample child questionnaires have been collected in 2019 and 2020, respectively, and the corresponding final sample child response rates were approximately 59.1 and 47.8%. The weighting process for 2019 and 2020 was also updated since multilevel regression models, including predictive variables of both survey response and key health outcomes, were used for non-response adjustment.
Data collection
The NDD outcomes were ascertained by asking the parents of sampled children whether their children ever had ADHD, ASD, ID, or LD told by a representative of a school or a health professional. The parents were also questioned if their child currently had these NDDs. Information on demographics, comorbidities, or psychological disorders, as well as household or parental factors, were collected and grouped. The stratification included age (3–11 and 12–17 years), sex (boy and girl), race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, and non-Hispanic other and multiple races), health insurance (uninsured, private only, and any public), urbanicity (urban and rural), geographic region (Northeast, Midwest, South, and West), ever had asthma (yes and no), ever had anxiety symptoms (yes and no), ever had depressive symptoms (yes and no), household structure (single parent, married parents, and others), number of children (1 child and ≥ 2 children), living with an elder/elders (yes and no), the highest level of education (below high school, high school, and beyond high school), family income to poverty ratio (IPR, grouped into < 100, 100–199, 200–399, and ≥ 400%), and home ownership (owned/being bought and rented/other arrangements). Questions of ever had anxiety symptoms and ever had depression symptoms only include children and adolescents aged 5–17 years old. Stressful life events of the child or his/her family members were included in the dataset of 2019 but unavailable for 2020. Relevant information included experience as a victim of/witnessed violence (yes and no), bullied by others (yes and no), ever lived with a parent who was incarcerated (yes and no), ever lived with anyone mentally ill (yes and no), and ever lived with anyone with an alcohol problem (yes and no).
Statistical analysis
Prevalence estimates of NDDs were stratified by demographics, stressful life events, comorbidities or psychological disorders, and household or parental factors. Prevalence estimates with corresponding 95% confidence intervals (95% CIs) of ADHD, ASD, ID, and LD were weighted using survey procedures by “survey” and “srvyr” packages in the R environment (version 3.6.1). The non-response weights were calculated by controlling for age, sex, race/ethnicity, education level, census division, and Metropolitan Statistical Area status. The 95% CI was asymmetric about the point estimate due to the logit transformation. The P-values showing the statistical difference across strata were calculated by the Rao-Scott χ2-test with adjusted F-statistic, considering the complex sample design. Multivariable logistic regression models were used to estimate the associations between NDDs prevalence and the subgroups after controlling for age, sex, race, health insurance, urbanicity, and geographic regions, considering the various potential confounders. Statistical significance was claimed when P-value < 0.05.
Results
Based on the NHIS data in 2019 and 2020, the estimated population of 61.3 million children and adolescents aged over 3–17 years can be represented in the analysis, where we identified 5.2 million individuals with ADHD, 1.8 million with ASD, 0.9 million with ID, and 3.9 million with LD. Data filtering and the unweighted number of NDDs are shown in Figure 1. The weighted prevalence of currently had NDDs was 8.5% (95% CI: 7.9–9.2%) for ADHD, 2.9% (95% CI: 2.6–3.4%) for ASD, 1.4% (95% CI: 1.2–1.7%) for ID, and 6.4% (95% CI: 5.8–7.0%) for LD (Table 1). Boys had a weighted prevalence of 11.1% (95% CI: 10.2–12.4%) for ADHD and 4.4% (95% CI: 3.8–5.0%) for ASD, compared with 5.8% (95% CI: 5.0–6.6%) for ADHD and 1.4% (95% CI: 1.1–1.9%) for ASD in girls. Higher prevalence of ID and LD were also observed in boys than girls. The prevalence of ADHD and ASD was higher among those who ever had anxiety than those without anxiety (ADHD among those with anxiety 14.8%, 95% CI: 13.5–16.1% vs. ADHD among those without anxiety 5.1%, 95% CI: 4.4–5.9%; ASD among those with anxiety 5.2%, 95% CI: 4.4–6.1% vs. ASD among those without anxiety 1.3%, 95% CI: 0.9–1.8%). Similarly, ADHD and ASD were more prevalent among those who ever had depression than those not (ADHD with depression 15.9%, 95% CI: 14.5–17.5% vs. ADHD without depression 7.1%, 95% CI: 6.3–7.9%; ASD with depression 4.9, 95% CI: 4.0–6.0% vs. ASD without depression 2.4%, 95% CI: 2.0–2.8%). The weighted prevalence of ID and LD showed similar patterns in anxiety and depression. Higher prevalence of ADHD, ASD, ID, and LD were also shown among children or adolescents who lived in rented houses. In addition, the prevalence of ADHD and LD was higher among those without health insurance (ADHD 8.7%, 95% CI: 8.0–9.4%; LD 6.6%, 95% CI: 6.0–7.2%), ever had asthma (ADHD 13.4%, 95% CI: 11.5–15.5%; LD 11.5%, 95% CI: 9.6–13.7%), living with a single parent (ADHD 11.7%, 95% CI: 10.3–13.4%; LD 8.3%, 95% CI: 7.0–9.7%), and family income to poverty ratio less than 100% (ADHD 11.8%, 95% CI: 9.9–14.0%; LD 11.0%, 95% CI: 9.2–13.0%) (Table 1).
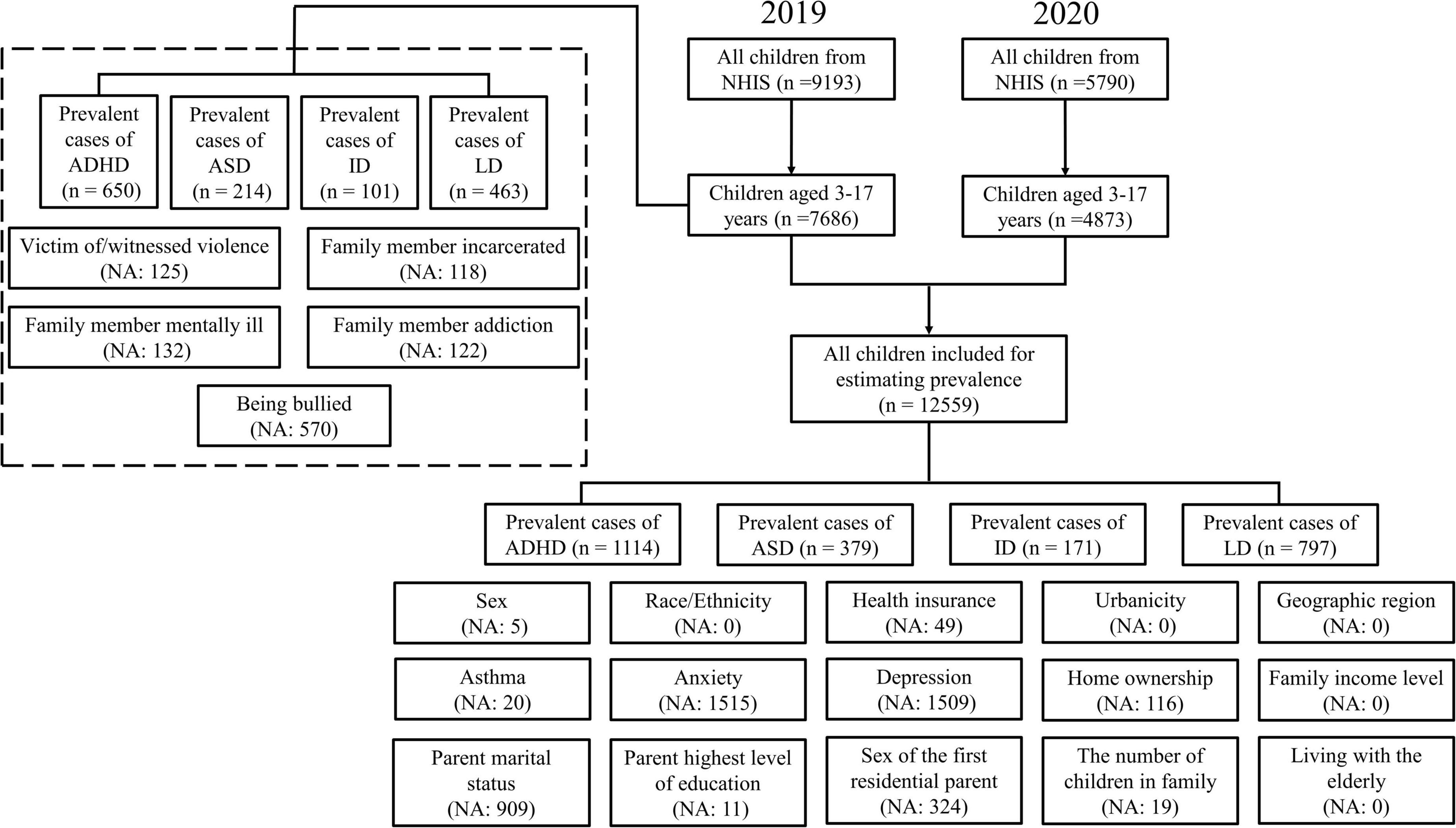
Figure 1. A flowchart of children and adolescents included in the study and the number of missing values for each variable.
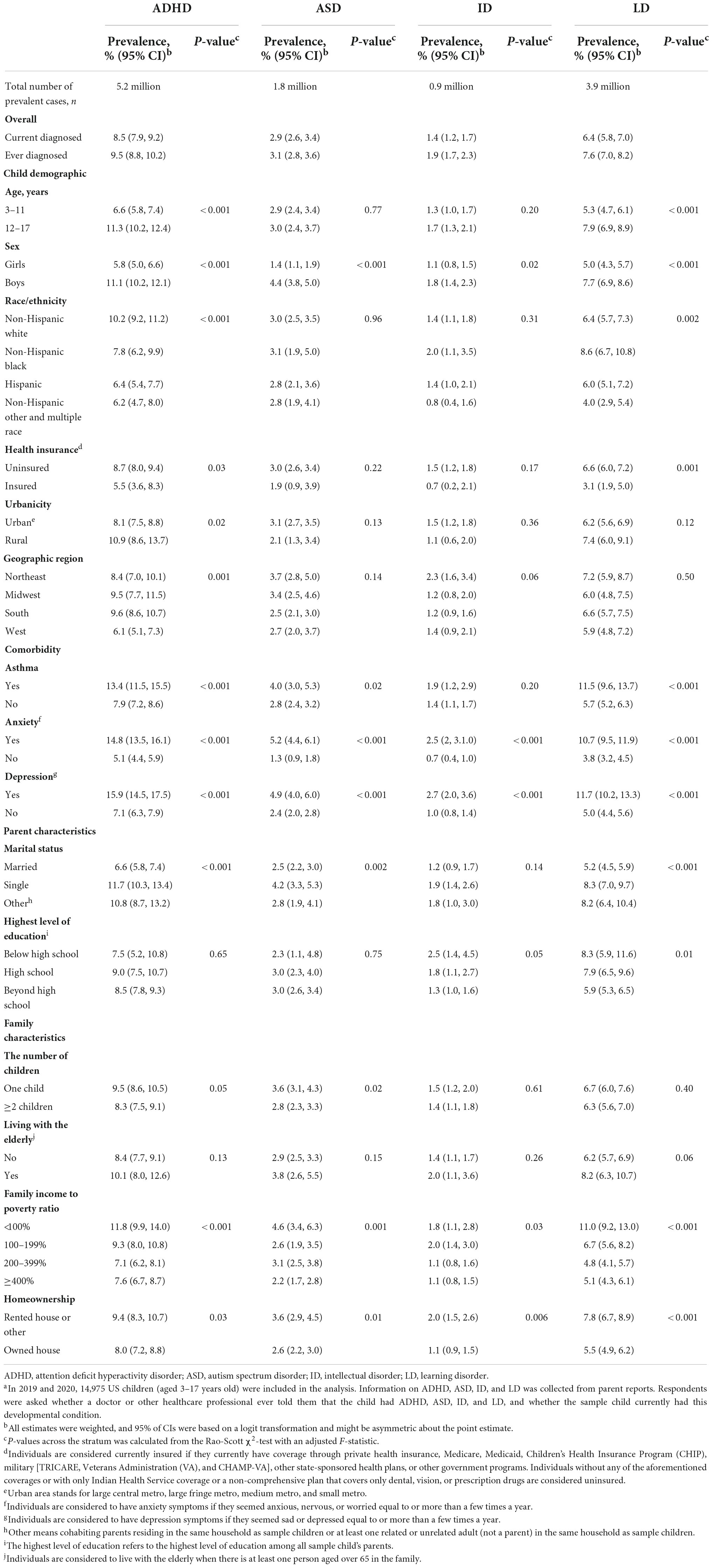
Table 1. Prevalence of cause-specific neurodevelopmental disorders among US children and adolescents aged 3–17 years in 2019 and 2020a.
The prevalence of NDDs among US children and adolescents was different from their stressful life experiences. We found a higher prevalence of ADHD, ASD, ID, and LD among those who were the victim of/witnessed violence (ADHD 20.3%, 95% CI: 16.4–24.9%; ASD 3.7%, 95% CI: 2.1–6.4%; ID 3.5%, 95% CI: 2.0–6.1%; LD 14.8%, 95% CI: 11.3–19.1%), being bullied by others (ADHD 18.2%, 95% CI: 15.7–21.0%; ASD 6.6%, 95% CI: 5.0–8.7%; ID 2.5%, 95% CI: 1.6–4.1%; LD 14.8%, 95% CI: 12.4–17.5%), and ever lived with anyone mentally ill (ADHD 20.0%, 95% CI: 16.6–23.9%; ASD 4.4%, 95% CI: 2.9–6.6%; ID 3.6%, 95% CI: 2.3–5.4%; LD 12.6%, 95% CI: 10.0–15.7%) (Table 2). Higher prevalence of ADHD (18.0%, 95% CI: 14.3–22.3%), ID (2.77%, 95% CI: 1.47, 5.21), and LD (11.4%, 95% CI: 8.8–14.7%) were also found among individuals with incarcerated family members. Using logistic regression models, we found that the prevalence of ADHD, ASD, ID, and LD was all associated with sex, psychological problems, family income levels, homeownership, and stressful life events, including ever being a victim of/witnessed violence (except for ASD), bullied by others, ever lived with a parent who was incarcerated (except for ASD), ever lived with anyone mentally ill, and ever lived with anyone addicting to alcohol/drug. In addition, children and adolescents without health insurance, ever had asthma, living with a single parent, or others have higher risks for ADHD and LD (Table 3).
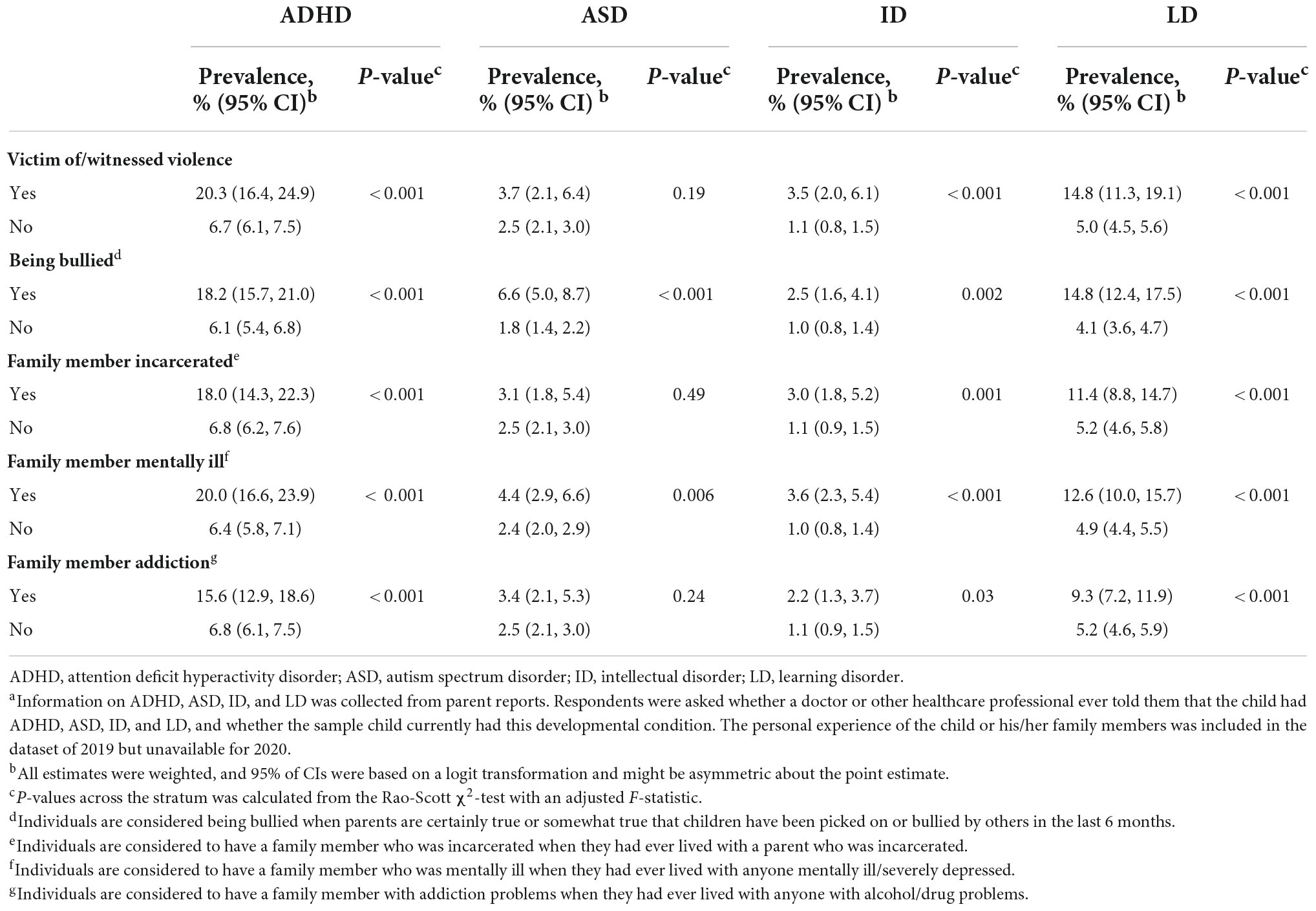
Table 2. Subgroup prevalence by stressful life events among US children and adolescents aged 3–17 years in 2019a.
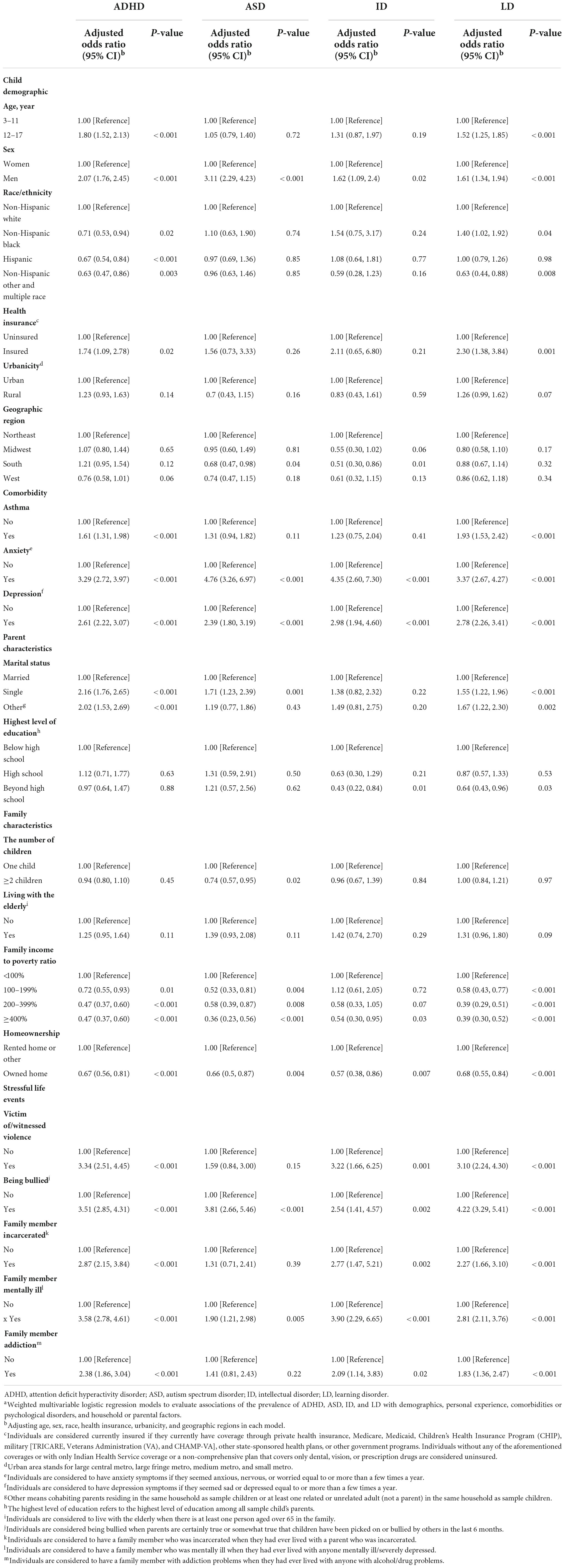
Table 3. Weighted logistic regression models for ADHD, ASD, ID, and LD among US children and adolescents aged 3–17 yearsa.
Discussion
Based on the counts of currently having NDDs from NHIS in 2019 and 2020, we estimated that the prevalence of ADHD, ASD, ID, and LD was 8.5, 2.9, 1.4, and 6.4%, respectively. Our estimates on NDDs prevalence were lower than previous estimates, where cases were counted by ever had NDDs in the numerator calculation (Zablotsky et al., 2019b; Zablotsky and Black, 2020; Khan et al., 2021; Yang et al., 2021). Since NDDs symptoms could alleviate through behavioral and educational therapies or after maturation (Magiati et al., 2014), the prevalence might be overestimated if counting all with historical NDDs (Kogan et al., 2018; Xu et al., 2018; Zablotsky et al., 2019a). On the other hand, the prevalence estimates of ADHD and ASD have been continuously growing in recent decades (Visser et al., 2014; Zablotsky et al., 2019a; Chiarotti and Venerosi, 2020). On the other hand, our results are higher than those in earlier studies. In the US, the prevalence estimates of ADHD and ASD have been continuously growing (Visser et al., 2014; Zablotsky et al., 2019a; Chiarotti and Venerosi, 2020). The reasons could be in part explained by the modified diagnosis criteria and the increase in health awareness (American Psychiatric Association, 2013; Polanczyk et al., 2014). The difference in data collection may also affect the prevalence estimation. Studies that used hospital-reported prescription data may underestimate the prevalence estimates due to missing part of children who lack medical support or were not suitable for the prescription because medications are not always required in the intervention and treatment for NDDs (Cairncross and Miller, 2020). With the new diagnosis standards, our estimates might be closer to the true prevalence among US children and adolescents. In addition, we further estimated the prevalent differentiation by demographics, parental characteristics, comorbidities/mental problems, as well as household and stressful life events, which led to more clues about susceptible populations or risk factors.
In line with previous studies, we found a significant difference in the NDDs prevalence by sex (Thapar et al., 2017). In our study, boys are more likely to have ADHD, ASD, ID, and LD than girls. There could be a phenotypic difference in the relevant symptoms between boys and girls, while they have the same diagnostic standard. The plausible explanation for the sex difference could be relevant symptoms in boys can be onset earlier and are less likely to be concealed compared with girls because of less externalizing behavior in girls and potential chromosome-linked genetic difference (Thapar et al., 2017; Mowlem et al., 2019; Dillon et al., 2021; Walsh et al., 2021). More studies are expected to enrich the mechanism explanation. Furthermore, ADHD and LD could be initiated based on cumulative effects of childhood-onset negative events; hence, the related symptoms could appear at an older age in children and adolescents (Arora et al., 2018). The coverage of public health insurance can raise parents’ concern for the early growth of children and thus improve the detection rate of NDDs, which is consistent with previous findings (Danielson et al., 2018).
Evidence showed that NDDs are frequently comorbid with other psychiatric disorders and medical conditions. Although there are some overlapping symptoms among these NDDs, the current diagnostic procedure is mainly based on the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5) (American Psychiatric Association). There are some gold-standard diagnostic measures for NDDs, such as the Autism Diagnostic Interview-Revised and the Autism Diagnostic Observation Schedule, the second edition for ASD and the standardized ADHD rating scales, structured interviews such as the KSADS-PL, and behavioral observations for ADHD. The diagnostic procedure of DSM-5 also emphasizes the core symptoms and distinguishes different disorders by specific scales or based on parents’ and teachers’ reports on children’s behavior (American Psychiatric Association; Nickel et al., 2019). Prevalence estimates of ADHD, ASD, ID, and LD were found higher in individuals with depressive and anxiety symptoms or individuals living with anyone mentally ill, suggesting hereditation of psychiatric disorders across generations, which is parallel with previous findings (Chandra et al., 2016). However, since psychiatric symptoms are highly heritable, further studies are warranted to investigate if parental psychiatric care can bring a positive influence on the alleviation of psychiatric severity of the next generation. The observed relationship between asthma and ADHD in our study is consistent with the previous investigation (Zheng et al., 2016; Cortese et al., 2018; Kim et al., 2020), and the relationship between asthma and LD is also significant. Effects of allergic inflammatory cytokines and elevated psychological stress were broadly recognized as contributors to the neuro-immunological and psychological mechanisms between asthma and NDDs (Lord et al., 2018; Papi et al., 2018; Kim et al., 2020; Posner et al., 2020). In addition, the stress increased by NDDs may contribute to poor adherence to treatment, and worse asthma control, thus raising more stress (Papi et al., 2018). Such vicious circles may also apply to NDDs treatments as well as raise the anxiety and depression levels of children.
Living with married parents and other children may be associated with a lower chance of NDD symptoms among US children and adolescents. The reason could be family companionship and motivation which bring positive effects on the sense of achievement and mental development for individuals (Karst and Van Hecke, 2012; Deater-Deckard, 2017). It might be also related to the higher income in the family with married parents and more children (Lord et al., 2018; Posner et al., 2020). It is of note that observations in the cross-sectional study might not build the causal relationship between family/sibling accompany and the onset of NDDs. Reverse causality is also likely since parents may pay more attention to children already with NDD symptoms. In addition, having a child with NDDs may contribute to a higher rate of divorces due to parenting stress. More epidemiological evidence is highly demanded in further studies. Children and adolescents in low-income families were more likely to have NDDs, which is in line with the observation that cases of NDDs were less prevalent among those living in houses owned by their families than their counterparts. Lower family income may cause less companionship from working parents and a lack of medical care or early intervention before diagnosis. Thus, a lack of financial support would cause a higher prevalence of NDDs and reduce the chance of adulthood independence and symptoms decreasing (Lord et al., 2018; Posner et al., 2020; Zuvekas et al., 2021). Moreover, although house ownership is entangled with the family’s economic level, a stable living environment should be valuable in the neurodevelopment of children.
We found that victims of/witnessed violence or those living with anyone ever incarcerated were associated with a higher prevalence of ADHD, ID, and LD. It might be partly explained by genetic factors as violent family members may also suffer from NDDs. On the other hand, experiencing violent crime may cause disrupted cortisol patterns among children, which may increase their stress and lead to mental health problems (Heissel et al., 2018). Moreover, aggression or defensiveness in daily interactions due to heightening stress may obstruct getting support from communities and society, and thus, accelerate the deterioration of neurodevelopmental or psychiatric disorders (Cuartas and Leventhal, 2020). Parents with alcohol or drug problems may be associated with the initiation of ADHD, ID, and LD in children and adolescents. Most parents with serious alcohol/drug problems may also misuse alcohol or drugs during pregnancy or breastfeeding. The cognition and mental health of offspring might be influenced by parental drinking/drug use in sensitive periods though mechanisms remain unclear (Gibson and Porter, 2018; Syed and Gilbert, 2019). In addition, because of the inequality of income or other reasons, bullying is a prevalent and preventable risk factor for children and adolescence (Elgar et al., 2019; Zhang et al., 2020). In our study, experiencing bully was associated with the prevalence of ADHD, ASD, ID, and LD. Based on the risk of long-term emotional and behavioral problems due to posttraumatic stress disorder, experiencing bullying may have effects on the progress of ASD rather than initiation because most ASD is detected before school age (Lai et al., 2014).
The findings in our study can be interpreted to represent national estimates among current US children and adolescents because of the large sample size, high response rate, and systematic weighting procedure of NHIS in 2019 and 2020. There are several limitations of the study. First, information on diagnosed NDDs was collected by parent reporting rather than clinical evaluation or educational records. Although non-differential misclassification is inevitable due to recall bias or conceptual confusion, previous evidence showed a high concordance rate (93–98%) between parent reports (such as ASD) and clinician’s diagnosis in verbal children (Daniels et al., 2012), which may reflect the validation of parent reports. Second, the prevalence estimates in those without healthcare insurance might be unreliable due to the high relative standard error (>30%). The sample size for specific subgroups could be deficient because only NHIS in 2019 was leveraged in our study. Prevalence estimates across multiple years are in need to generate results with higher reliability. Third, non-response bias and selection bias were also likely that families without a settled household or who tend to ignore phone calls can result in a lower response. Moreover, the COVID-19 pandemic in 2020 led to a smaller sample size than in previous years and a change of investigation method from mainly in-person interviews to phone interviews. However, the survey attempted to mitigate any differential non-response and selection bias by non-response weighting adjustments and post-stratification adjustments (National Center for Health Statistics, 2021).
Conclusion
Knowledge about the national-wide prevalence of NDDs serves for the assessment of disease burden and informing the healthcare management strategies. Our study updated nationwide estimates for the prevalence of ADHD, ASD, ID, and LD among US children and adolescents aged 3–17 years in 2019 and 2020, and also detected the stratification among subgroups of demographics, comorbidity/mental problems, household/parental characteristics, and stressful life events. Future studies are in demand to explore the risk factors and corresponding bio-mechanisms for the onset and progression of NDDs, especially ADHD and ASD.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm.
Author contributions
JR conceived the idea and designed the study. YY and MZ acquired the data. SZ and JR cleaned and analyzed the data. YY and LH interpreted the results. YY, LH, and JR drafted the manuscript. JR, LH, SZ, MX, JZ, SC, and HW revised the manuscript. All authors contributed to the content and critical revision of the manuscript and approved the final version.
Funding
This work was supported by the Shanghai Science and Technology Development Foundation (Grant No. 22YF1421100 to JR) and the Startup Fund for Youngman Research at SJTU (Grant No. 21 010501093 to JR).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
American Psychiatric Association (2013). DSM-V Diagnostic and Statistical Manual of Mental Disorders, 5th Edn. Virginia, VA: APA.
Antshel, K. M., and Russo, N. (2019). Autism spectrum disorders and ADHD: overlapping phenomenology, diagnostic issues, and treatment considerations. Curr. Psychiatry Rep. 21:34. doi: 10.1007/s11920-019-1020-5
Arora, N. K., Nair, M. K. C., Gulati, S., Deshmukh, V., Mohapatra, A., Mishra, D., et al. (2018). Neurodevelopmental disorders in children aged 2-9 years: population-based burden estimates across five regions in India. PLoS Med. 15:e1002615. doi: 10.1371/journal.pmed.1002615
Berry-Kravis, E. M., Lindemann, L., Jønch, A. E., Apostol, G., Bear, M. F., Carpenter, R. L., et al. (2018). Drug development for neurodevelopmental disorders: lessons learned from fragile X syndrome. Nat. Rev. Drug Discov. 17, 280–299. doi: 10.1038/nrd.2017.221
Bourgeron, T. (2015). From the genetic architecture to synaptic plasticity in autism spectrum disorder. Nat. Rev. Neurosci. 16, 551–563. doi: 10.1038/nrn3992
Cairncross, M., and Miller, C. J. (2020). The effectiveness of mindfulness-based therapies for ADHD: a meta-analytic review. J. Attent. Disord. 24, 627–643. doi: 10.1177/1087054715625301
Chandra, S., Biederman, J., and Faraone, S. V. (2016). assessing the validity of the age at onset criterion for diagnosing ADHD in DSM-5. J. Attent. Disord. 25, 143–153. doi: 10.1177/1087054716629717
Chiarotti, F., and Venerosi, A. (2020). Epidemiology of autism spectrum disorders: a review of worldwide prevalence estimates since 2014. Brain Sci. 10:274. doi: 10.3390/brainsci10050274
Cortese, S., Sun, S., Zhang, J., Sharma, E., Chang, Z., Kuja-Halkola, R., et al. (2018). Association between attention deficit hyperactivity disorder and asthma: a systematic review and meta-analysis and a Swedish population-based study. Lancet Psychiatry 5, 717–726. doi: 10.1016/S2215-0366(18)30224-4
Cramer, S. C., Sur, M., Dobkin, B. H., O’Brien, C., Sanger, T. D., Trojanowski, J. Q., et al. (2011). Harnessing neuroplasticity for clinical applications. Brain 134, 1591–1609. doi: 10.1093/brain/awr039
Cuartas, J., and Leventhal, T. (2020). Exposure to community violence and children’s mental health: a quasi-experimental examination. Soc. Sci. Med. 246:112740. doi: 10.1016/j.socscimed.2019.112740
Daniels, A. M., Rosenberg, R. E., Anderson, C., Law, J. K., Marvin, A. R., Law, P. A., et al. (2012). Verification of parent-report of child autism spectrum disorder diagnosis to a web-based autism registry. J. Autism Dev. Disord. 42, 257–265. doi: 10.1007/s10803-011-1236-7
Danielson, M. L., Bitsko, R. H., Ghandour, R. M., Holbrook, J. R., Kogan, M. D., Blumberg, S. J., et al. (2018). Prevalence of parent-reported ADHD diagnosis and associated treatment among U.S. Children and Adolescents, 2016. J. Clin. Child Adolescent Psychol. 47, 199–212. doi: 10.1080/15374416.2017.1417860
Deater-Deckard, K. (2017). Parents’ and children’s ADHD in a family system. J. Abnorm. Child Psychol. 45, 519–525. doi: 10.1007/s10802-017-0276-7
Dillon, E. F., Kanne, S., Landa, R. J., Annett, R., Bernier, R., Bradley, C., et al. (2021). Sex differences in autism: examining intrinsic and extrinsic factors in children and adolescents enrolled in a national ASD cohort. J. Autism Dev. Disord. Online ahead of print. doi: 10.1007/s10803-021-05385-y
Du Rietz, E., Brikell, I., Butwicka, A., Leone, M., Chang, Z., Cortese, S., et al. (2021). Mapping phenotypic and aetiological associations between ADHD and physical conditions in adulthood in Sweden: a genetically informed register study. Lancet Psychiatry 8, 774–783. doi: 10.1016/S2215-0366(21)00171-1
Elgar, F. J., Gariepy, G., Dirks, M., Walsh, S. D., Molcho, M., Cosma, A., et al. (2019). Association of early-life exposure to income inequality with bullying in adolescence in 40 countries. JAMA Pediatr. 173:e191181.
Gibson, L., and Porter, M. (2018). Drinking or smoking while breastfeeding and later cognition in children. Pediatrics 142:e20174266. doi: 10.1542/peds.2017-4266
Gu, J. K., Charles, L. E., Fekedulegn, D., Allison, P., Ma, C. C., Violanti, J. M., et al. (2021). Temporal trends in prevalence of cardiovascular disease (CVD) and CVD risk factors among U.S. older workers: NHIS 2004–2018. Ann. Epidemiol. 55, 78–82. doi: 10.1016/j.annepidem.2020.10.002
Heissel, J. A., Sharkey, P. T., Torrats-Espinosa, G., Grant, K., Adam, E. K., et al. (2018). Violence and vigilance: the acute effects of community violent crime on sleep and cortisol. Child Dev. 89, e323–e331. doi: 10.1111/cdev.12889
Karst, J. S., and Van Hecke, A. V. (2012). Parent and family impact of autism spectrum disorders: a review and proposed model for intervention evaluation. Clin. Child Fam. Psychol. Rev. 15, 247–277. doi: 10.1007/s10567-012-0119-6
Kasparek, T., Theiner, P., and Filova, A. (2015). Neurobiology of ADHD from childhood to adulthood: findings of imaging methods. J. Atten. Disord. 19, 931–943. doi: 10.1177/1087054713505322
Khan, M. U. G., Naz, Z., Khan, J., Saba, T., Abunadi, I., Rehman, A., et al. (2021). Cognitive skill enhancement system using neuro-feedback for ADHD patients. CMC-Comp. Mater. Continua 68, 2363–2376. doi: 10.32604/cmc.2021.014550
Kim, J. H., Kim, J. Y., Lee, J., Jeong, G. H., Lee, E., Lee, S., et al. (2020). Environmental risk factors, protective factors, and peripheral biomarkers for ADHD: an umbrella review. Lancet Psychiatry 7, 955–970. doi: 10.1016/S2215-0366(20)30312-6
Kogan, M. D., Vladutiu, C. J., Schieve, L. A., Ghandour, R. M., Blumberg, S. J., Zablotsky, B., et al. (2018). The prevalence of parent-reported autism spectrum disorder among US children. Pediatrics 142:e20174161. doi: 10.1542/peds.2017-4161
Lai, M.-C., Lombardo, M. V., and Baron-Cohen, S. (2014). Autism. Lancet 383, 896–910. doi: 10.1016/S0140-6736(13)61539-1
Lord, C., Elsabbagh, M., Baird, G., and Veenstra-Vanderweele, J. (2018). Autism spectrum disorder. Lancet 392, 508–520. doi: 10.1016/S0140-6736(18)31129-2
Magiati, I., Tay, X. W., and Howlin, P. (2014). Cognitive, language, social and behavioural outcomes in adults with autism spectrum disorders: a systematic review of longitudinal follow-up studies in adulthood. Clin. Psychol. Rev. 34, 73–86. doi: 10.1016/j.cpr.2013.11.002
Mowlem, F. D., Rosenqvist, M. A., Martin, J., Lichtenstein, P., Asherson, P., and Larsson, H. (2019). Sex differences in predicting ADHD clinical diagnosis and pharmacological treatment. Eur. Child Adolescent Psychiatry 28, 481–489. doi: 10.1007/s00787-018-1211-3
National Center for Health Statistics (2020). National Health Interview Survey, 2019: Survey Description. Hyattsville, MD: National Center for Health Statistics.
National Center for Health Statistics (2021). National Health Interview Survey, 2020: Survey Description. Hyattsville, MD: National Center for Health Statistics.
Nickel, K., Maier, S., Endres, D., Joos, A., Maier, V., Tebartz, et al. (2019). Systematic review: overlap between eating, autism spectrum, and attention-deficit/hyperactivity disorder. Front. Psychiatry 10:708. doi: 10.3389/fpsyt.2019.00708
Papi, A., Brightling, C., Pedersen, S. E., and Reddel, H. K. (2018). Asthma. Lancet 391, 783–800. doi: 10.1016/S0140-6736(17)33311-1
Polanczyk, G. V., Willcutt, E. G., Salum, G. A., Kieling, C., and Rohde, L. A. (2014). ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. Int. J. Epidemiol. 43, 434–442. doi: 10.1093/ije/dyt261
Posner, J., Polanczyk, G. V., and Sonuga-Barke, E. (2020). Attention-deficit hyperactivity disorder. Lancet 395, 450–462. doi: 10.1016/S0140-6736(19)33004-1
Syed, S., and Gilbert, R. (2019). Parental alcohol misuse has major effects on children’s health and development. BMJ 364:l912. doi: 10.1136/bmj.l912
Thapar, A., Cooper, M., and Rutter, M. (2017). Neurodevelopmental disorders. Lancet Psychiatry 4, 339–346. doi: 10.1016/S2215-0366(16)30376-5
Visser, S. N., Danielson, M. L., Bitsko, R. H., Holbrook, J. R., Kogan, M. D., Ghandour, R. M., et al. (2014). Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003-2011. J. Am. Acad. Child Adolescent Psychiatry 53, 34–46.e2. doi: 10.1016/j.jaac.2013.09.001
Walsh, M. J. M., Wallace, G. L., Gallegos, S. M., and Braden, B. B. (2021). Brain-based sex differences in autism spectrum disorder across the lifespan: a systematic review of structural MRI, fMRI, and DTI findings. Neuroimage-Clinical 31:102719. doi: 10.1016/j.nicl.2021.102719
Xu, G., Liu, B., Sun, Y., Du, Y., Snetselaar, L. G., Hu, F. B., et al. (2018). Prevalence of diagnosed type 1 and type 2 diabetes among US adults in 2016 and 2017: population based study. BMJ 362:k1497. doi: 10.1136/bmj.k1497
Yang, W., Liu, B., Gao, R., Snetselaar, L. G., Strathearn, L., Bao, W., et al. (2021). Association of anemia with neurodevelopmental disorders in a nationally representative sample of US children. J. Pediatrics 228, 183–189.e2. doi: 10.1016/j.jpeds.2020.09.039
Zablotsky, B., Black, L. I., Maenner, M. J., Schieve, L. A., Danielson, M. L., Bitsko, R. H., et al. (2019a). Prevalence and trends of developmental disabilities among children in the United States: 2009-2017. Pediatrics 144:e20190811. doi: 10.1542/peds.2019-0811
Zablotsky, B., Maenner, M. J., and Blumberg, S. J. (2019b). Geographic disparities in treatment for children with autism spectrum disorder. Acad. Pediatr. 19, 740–747. doi: 10.1016/j.acap.2019.02.013
Zablotsky, B., and Black, L. I. (2020). Prevalence of Children Aged 3–17 years with developmental disabilities, by urbanicity: United States, 2015–2018. Natl. Health Stat. Rep. 139, 1–7.
Zhang, L. M., Ellis, R. J., Ma, M., Cheung, E. O., Hoyt, D. B., Bilimoria, K. Y., et al. (2020). Prevalence, types, and sources of bullying reported by US general surgery residents in 2019. JAMA 323, 2093–2095. doi: 10.1001/jama.2020.2901
Zheng, Z., Zhang, L., Zhu, T., Huang, J., Qu, Y., and Mu, D. (2016). Association between asthma and autism spectrum disorder: a meta-analysis. PLoS One 11:e0156662. doi: 10.1371/journal.pone.0156662
Keywords: neurodevelopmental disorders, attention-deficit/hyperactivity disorder, autism spectrum disorder, nationwide prevalence, United States
Citation: Yang Y, Zhao S, Zhang M, Xiang M, Zhao J, Chen S, Wang H, Han L and Ran J (2022) Prevalence of neurodevelopmental disorders among US children and adolescents in 2019 and 2020. Front. Psychol. 13:997648. doi: 10.3389/fpsyg.2022.997648
Received: 21 July 2022; Accepted: 31 October 2022;
Published: 24 November 2022.
Edited by:
Pamela Bryden, Wilfrid Laurier University, CanadaReviewed by:
Guiomar Gonçalves Oliveira, University of Coimbra, PortugalIleana Ratiu, Arizona State University, United States
Copyright © 2022 Yang, Zhao, Zhang, Xiang, Zhao, Chen, Wang, Han and Ran. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lefei Han, bGZoYW5Ac2p0dS5lZHUuY24=; Jinjun Ran, amluanVuckBzanR1LmVkdS5jbg==
†These authors have contributed equally to this work
 Yiwei Yang
Yiwei Yang Shi Zhao
Shi Zhao Meihui Zhang1
Meihui Zhang1 Jian Zhao
Jian Zhao Shucheng Chen
Shucheng Chen Hui Wang
Hui Wang Lefei Han
Lefei Han Jinjun Ran
Jinjun Ran