- Division of Yoga and Life Sciences, Cognitive Neuroscience Laboratory, Swami Vivekananda Yoga Anusandhana Samsthana (S-VYASA), Bangalore, KA, India
This study investigated the immediate effect of slow yoga breathing (SYB) at 6 breaths per minute (bpm) simultaneously on working memory performance and heart rate variability (HRV) in yoga practitioners. A total of 40 healthy male volunteers performed a working memory task, ‘n-back’, consisting of three levels of difficulty, 0-back, 1-back, and 2-back, separately, before and after three SYB sessions on different days. The SYB sessions included alternate nostril breathing (ANB), right nostril breathing (RNB), and breath awareness (BAW). Repeated measures analysis of variance showed a significant reduction in reaction time (ms) in 2-back condition immediately after ANB (−8%), RNB (−8%) and BAW (−5%) practices. Similarly, the accuracy was improved in the 0-back condition after RNB (4%), and in the 2-back condition after ANB (6%) and RNB (6%) practices. These results suggest that SYB practice enhances cognitive abilities (8–9%) related to memory load and improves the functioning of cardiac autonomic activity, which is required for the successful completion of mental tasks.Trial registered in the Clinical Trials Registry of India (CTRI/2018/01/011132).
Introduction
Cognitive abilities affect everyday life and being able to perform mental tasks efficiently, is important for psychological well-being. These abilities include mental workload, attention, multitasking ability, reasoning, etc. (Tucker-Drob, 2009). Excessive stress affects autonomic and cardiovascular function, which is connected to impaired cognitive and mental functions (da Silva et al., 2009; Delliaux et al., 2019). However, for intended results in task performance, mental faculties and cognitive performance necessitate a certain amount of stress (Atkinson, 1957). Previous research suggested that elevated heart rate variability (HRV) mitigates the effect of mental stress on frontal lobe functions (McIntosh et al., 2021). The HRV is a promising index of cardiac autonomic activity that reflects oscillatory intervals between consecutive heartbeats. The beat-to-beat interactions (i.e., R-R interval) reflect the parasympathetic nervous system (PNS) and the sympathetic nervous system (SNS) input from sinoatrial nodes (Shaffer and Ginsberg, 2017). The dynamic interaction of R-R intervals has frequently been linked with various mind–body interventions that include yoga breathing practices also (Raghuraj and Telles, 2008; Pal et al., 2014; Bhagat et al., 2017). A recent study has shown that slow yoga breathing increases parasympathetic activity and baroreflex sensitivity in yoga practitioners compared to naïve (Anasuya et al., 2020).
Yoga breathing is a three-step method that manipulates nostril breathing: deep inhalation (puraka), complete exhalation (rechaka), and the cessation of breath (khumbhaka). Hatha yogic books have described several slow yoga breathing techniques (Muktibodhananda, 1998; Saraswati, 2012) and in the current study, we focused on the most convenient and popular type of yoga breathing techniques, i.e., (i) alternate nostril breathing (Nadisodhana; ANB), (ii) right nostril breathing (Suryanuloma-viloma; RNB), and (iii) breath awareness (BAW). The manipulations in yoga breathing allow alteration in autonomic activity and higher-order cognitive functions that are yet to be fully understood. The relationship between the R-R interval of autonomic measures following RNB and ANB has been explored in previous investigations (Raghuraj and Telles, 2008; Pal et al., 2014). The authors reported that RNB practice has sympathomimetic effects that were expressed by increased heart rate (HR), metabolism, and O2 consumption (Telles et al., 1994; Sarang and Telles, 2006), while the practice of ANB at a fixed rate of 5 breaths per min (BPM) showed higher low frequency (LF) power, LF/HF ratio, and a lower high frequency (HF) power of HRV measures (Sharma et al., 2014; Vinay et al., 2016). In contrast, Srivastava et al. reported that practice of 15 min of ANB for 8 weeks reduces HR, indicating a parasympathetic activity (Srivastava et al., 2005). Overall studies suggest that RNB practice increases SNS and ANB increases PNS activity (Lee and Ghiya, 2012; Bhavanani et al., 2014). However, there is a scarcity of research that has explored how slow yoga breathing techniques influence neurocognitive activities. A few research investigations suggest that yoga breathing affects autonomic activity, which may also alter brain functions that include attention and memory (Raghuraj et al., 1998; Santhanam Kumar et al., 2020). A previous investigation has revealed that the practice of ANB improves the performance in the target detection task (evaluated through the P300 oddball task) compared to the BAW session (Telles et al., 2013). According to the studies cited above, yoga breathing not only alters sympathetic or parasympathetic autonomic activity but also enhances mental performance. As far as we know there is no study that has explored the influence of slow yoga breathing (SYB) simultaneously on mental load during working memory and changes in autonomic indices of HRV. Hence, the present study aimed to evaluate the effect of slow yoga breathing practices (SYB) at a fixed rate of approx. 6 bpm simultaneously on: (1) working memory, (2) HRV in different working memory conditions, and (3) HRV during SYB sessions.
Materials and Methods
Participants
We recruited 40 young right-hand dominant males to participate in the present study, 27 undergraduate and 13 postgraduate students, from a yoga University, in South India (aged between 18 and 30 years, mean ± SD; 22.3 ± 4.2 years). The sample size was calculated with G-Power software (Erdfelder et al., 1996) by fixing the alpha at 0.05, power at 0.95, and an effect size of 0.71 based on the LF power in normalized unite (n.u.) mean and SD of the ANB session of an earlier study (Pal et al., 2014). The calculated sample size was 37, but due to possible dropout, we considered 40 participants in the study. The study was conducted between August 2016 and July 2018. Each participant was selected based on the following criteria, these were (i) Participants should have a minimum of 6 months of yoga practice, (ii) participants should not take any medication which influences cognitive functions, (iii) participants should not be involved in any other ongoing research activities, (iv) only male participants were enrolled in the study because cognitive, autonomic, and respiratory variables are known to change with the menstrual cycle (Ramírez et al., 2015; Anjana et al., 2017), and (v) participants who are willing to participate and adhere to the study protocol. The exclusion criteria were (i) addicted to a drug or on medicine, (ii) surgery in the last 6 months, and (iii) any type of known impairment.
The study was approved by the Institutional Ethics Committee (IEC) of the S-VYASA University, Bangalore, and a signed consent form was obtained from each participant. The trial was registered at Clinical Trial Registry—India (CTRI/2018/01/011132).
Design of the study
Even though participants had prior experience of yoga breathing, the participants were given a 30-min orientation to practice SYB at 6 bpm for 1 month (5 days/week) under the guidance of an experienced yoga trainer before data acquisition in the laboratory. The purpose of the training was to maintain uniformity in SYB sessions during data acquisition in the laboratory. The SYB sessions included (i) right-nostril breathing (suryanuloma viloma; RNB), (ii) alternate nostril breathing (nadishodhana; ANB), and (iii) breath awareness (spontaneous breathing from both nostrils with awareness; BAW) on different days but at the same time for each participant. All eligible participants who completed the orientation program were given a practice session of a neurocognitive task.
Before and after each SYB session, participants were asked to perform a neurocognitive task, i.e., the visual n-back task consisted of three conditions (0-back, 1-back, and 2-back) as described in assessments. The electrocardiogram (ECG) and respiration were recorded during the baseline (BS), neurocognitive (Pre and Post n-back), and during SYB sessions. The schematic presentation of the study design and the neurocognitive task is given in Figure 1A and Figure 1B, respectively. The order of SYB sessions was random as presented in Figure 1C.
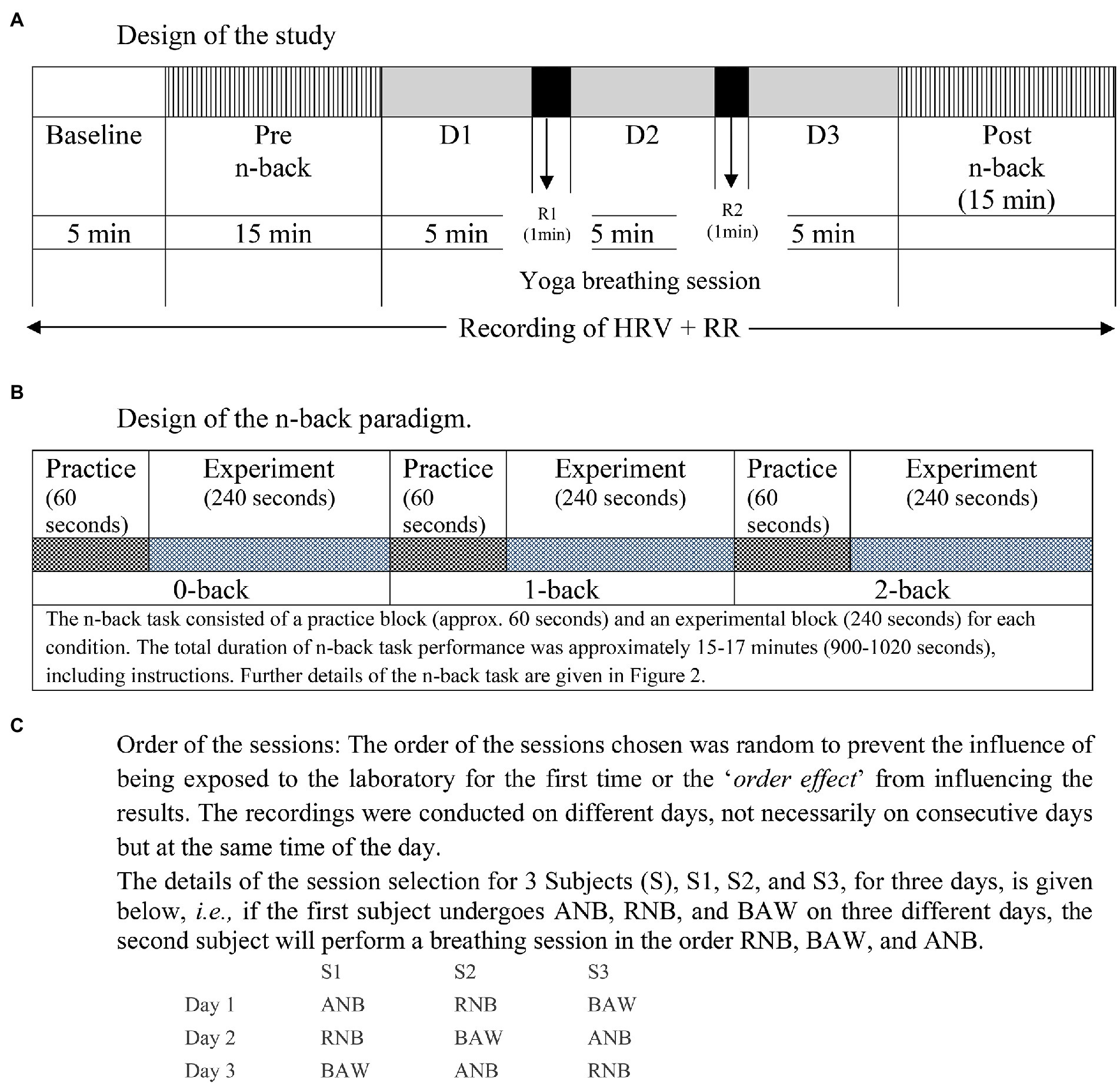
Figure 1. Schematic representation of the design of the study. (A) Design of the study, (B) Design of the n-back paradigm, (C) Order of the sessions.
Procedure
All assessments were done in the Cognitive Neuroscience laboratory of the S-VYASA University. The demographic information (age, education, physical activity, and socioeconomic status) and medical history were obtained before administering the working memory task. Before recordings, each participant was asked about the previous and current day activities such as physical exertion, diet, and quality of sleep because these activities are known to influence autonomic and cognitive functions. The recording was done at 5: 00–8: 30 AM and 4: 00–6: 30 PM. The assessments included the administration of a visual n-back task (0-back, 1-back, and 2-back) during which continuous ECG and respiration were recorded.
Participants were seated comfortably in a light dampening and sound attenuated chamber on a straight back armchair with elbows flexed at an angle of 90°. Each participant performed a visual-n back task before and after yoga breathing sessions.
Visual N-back task
Working memory (WM; Kirchner, 1958) was assessed using a computerized visual n-back task, which consisted of three conditions, i.e., 0-back, 1-back, and 2-back. Alphabet stimuli were presented in pseudorandom sequences in a fixed central location on a Dell computer using SuperLab™ 5.0 (Cedrus Corp, San Pedro, CA, United States). All participants used their right-hand index and middle fingers to press one of two buttons, denoting “target” and “nontarget” on a response pad. In the 0-back condition, the target was any letter that matched a pre-specified letter (i.e., “X”). Thus, this condition required sustained attention but no WM demand. In the 1-back condition, the target was any letter identical to the letter immediately preceding it (i.e., the letter presented one trial back). In the 2-back condition, the target was any letter that was identical to the letter presented two trials back. Each stimulus appeared on the screen for 500 ms, followed by a blank screen for another 500 ms in 0-back, 700 in 1-back, and 1,500 in 2-back for responses. Further, for preparation, 1,000 ms was given for the interstimulus interval (ISI) between each alphabet. Participants completed one block of trials with each of the three conditions and each block consisted of 150 trials. A short break (5–20 s) between blocks was provided to allow participants to rest. Before the actual task, participants were given practice blocks (of 50 trials each) before each condition with feedback on their performance until they demonstrated that they understood the task and their performance stabilized. Reaction times (RTs) and error scores were obtained for each trial.
Autonomic changes using HRV
The ECG and respiration were recorded throughout a session, which lasted for 55–60 min (before, during n-back, and breathing epochs were used for analysis) using a 16-channel polygraph system (MP 100 BIOPAC, Acknowledge software, BIOPAC System Inc., United States). The ECG was recorded using Ag/AgCl pre-gelled electrodes (Tyco Healthcare, Germany), and recordings were made with Standard Limb Lead I configuration. Data were acquired at the sampling rate of 1,024 Hz and were analyzed offline to obtain the HRV, which gives an indication of the balance in the autonomic innervation to the heart. The HRV can be modified by complex factors, including respiration. Noise-free data were included for analysis. A sample of original traces of ECG, and respiration during spontaneous breathing at baseline, during yoga breathing, and during the n-back performance is presented in Figure 2.
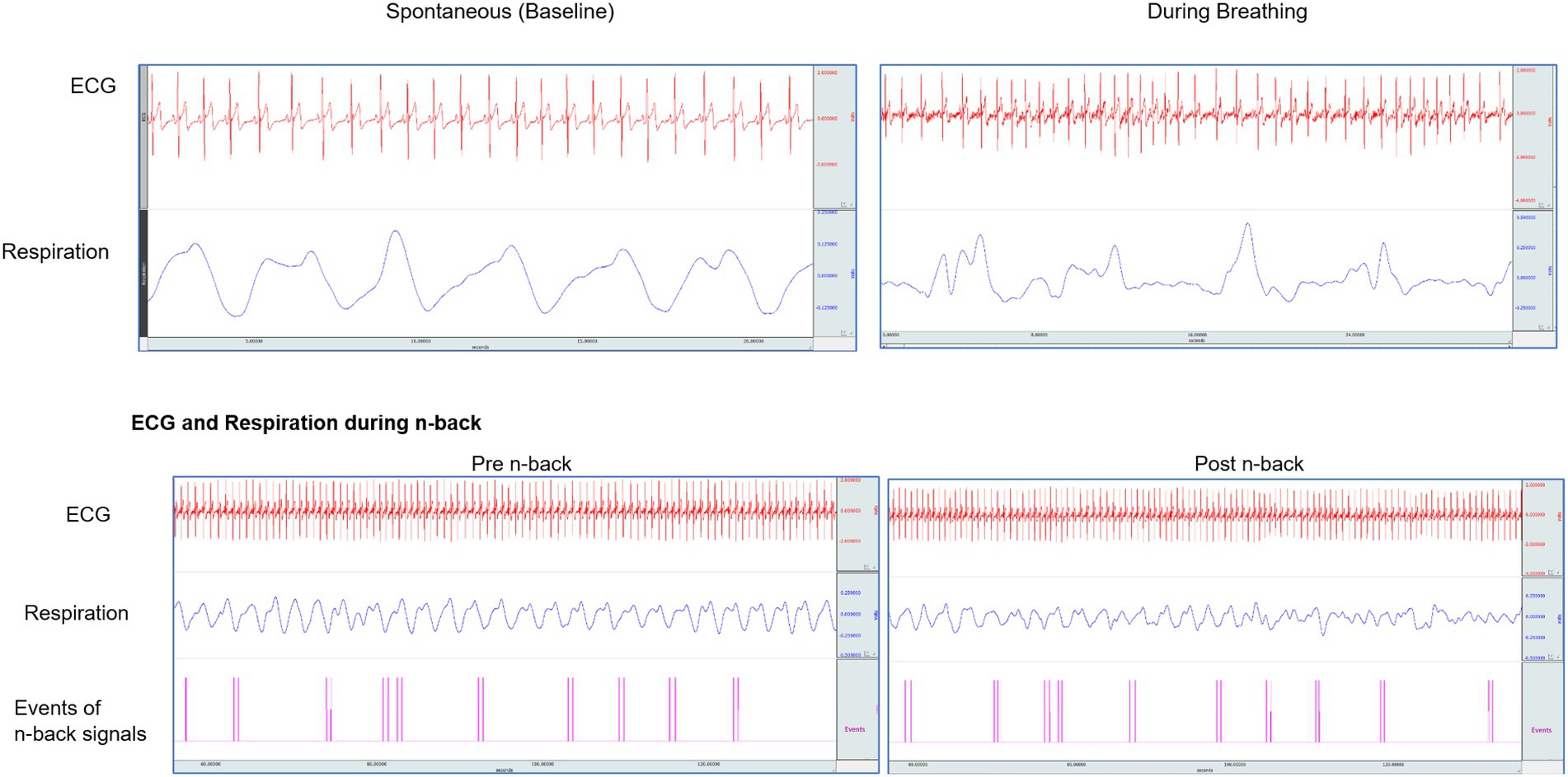
Figure 2. Original traces of ECG, respiration in one participant while breathing at their baseline, during slow yoga breathing, and in n-back task performance.
Respiration rate
Respiration was concurrently monitored in an attempt to remove the contribution of respiration to the HRV, especially lower frequencies (around 0.1 Hz) as the interaction between the HRV and respiration is traditionally known. The respiratory rate was recorded using a volumetric pressure transducer fixed around the trunk about 8 cm below the lower costal margin as the participants sat erect.
Slow yoga breathing (SYB) sessions
There were three SYB sessions given to each participant as described below.
Right nostril yoga breathing (RNB) or Suryanuloma viloma pranayama practice involves breathing exclusively inhalation and exhalation through the right nostril while the left nostril is gently occluded. Whereas, alternate nostril yoga breathing (ANB) practice involves breathing through left and right nostrils alternately. In this practice, the thumb and ring finger of the right hand were used to manipulate or occlude the nostril through the gesture known as nasika mudra. This helps to manipulate breath through nostrils with ease during pranayama.
During the active control BAW session, participants focused their awareness on the breath. Each participant was asked to observe the flow of air passing through nasal passages with closed eyes.
Data extraction
The simultaneous HRV and respiration were recorded during 4 states, i.e., (i) baseline (5 min), (ii) pre-n-back (12–15 min), (iii) SYB session (15 min), and (iv) post-n-back (12–15 min). Before analysis, the acquired data were visually reviewed offline, and only noise-free data were considered for further analysis. The original traces of ECG and respiration during the entire sitting of one participant are represented in Figure 2. The Fast Fourier Transform (FFT) was used to obtain the HRV power spectrum. The following HRV variables were studied in frequency domain measures: (i) low frequency (LF) band (0.05–0.15 Hz), (ii) high frequency (HF) band (0.15–1.50 Hz), and (iii) the LF/HF ratio. The low frequency and high-frequency band values were expressed as normalized units. The following components of time-domain HRV were analyzed: (i) mean RR interval (the mean of the intervals between the instantaneous heart rate), (ii) RMSSD (square root of the squared difference of successive NN intervals; where NN = normal to normal intervals), (iii) NN50 (the number of successive NN intervals with differences greater than 50 ms), and (iv) pNN50 (proportion derived by dividing NN50 by the total number of NN intervals; where NN50 is the number of interval differences of successive NN intervals greater than 50 ms).
Data analysis
The raw data of continuous variables were inspected visually with histograms and tested with the Kolmogorov–Smirnov test. Statistical analysis was done using Statistical Package for Social Science (SPSS) version 21. The effect of SYB sessions on reaction time and accuracy of the n-back task was analyzed with repeated measures of ANOVA with two Within-subject factors: Factor 1-SYB sessions (ANB vs. RNB vs. BAW), and Factor 2-time (pre n-back-vs post n-back). Further, SYB sessions’ effect on different conditions of the n-back task was analyzed with a repeated-measures ANOVA. A similar analysis was done for HRV during SYB sessions with a repeated-measures ANOVA with two Within-subject factors: Factor 1-SYB sessions (ANB, RNB, and BAW) and Factor 2-State (Baseline, During 1, During 2, and During 3 (D1, D2, D3)) for each parameter.
Results
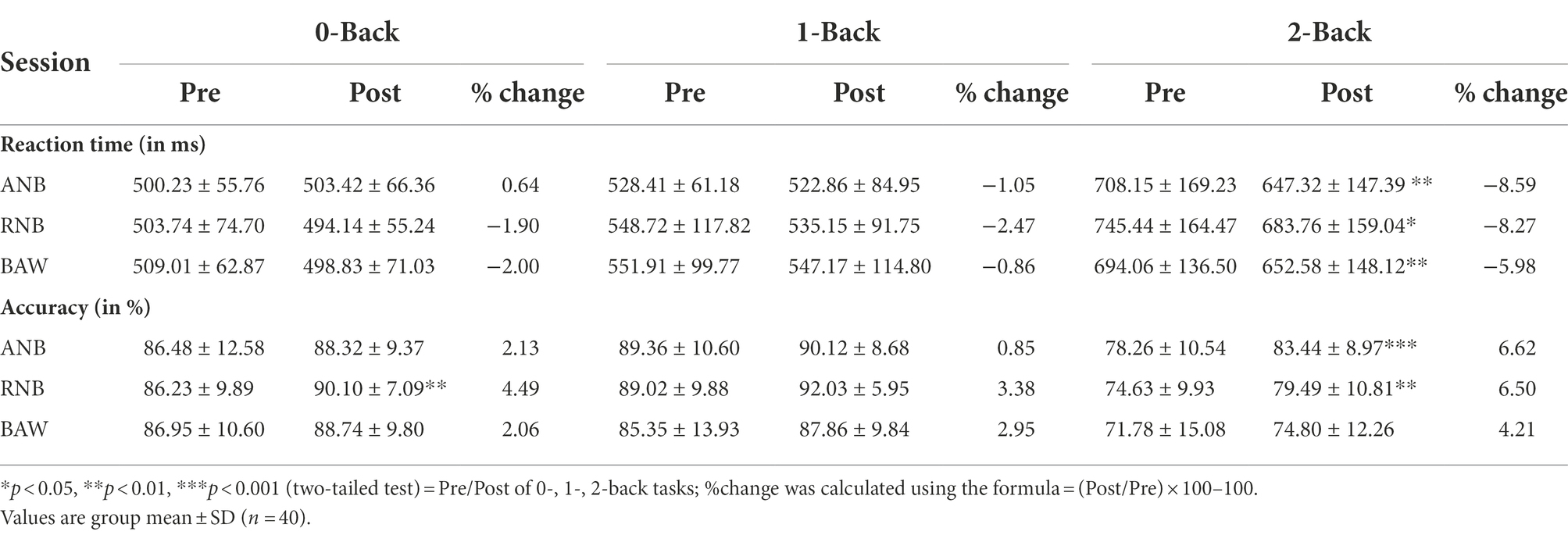
There were no dropouts in the study. The group mean values ± S.D. reported for (1) the reaction time and accuracy of the n-back task, (2) HRV during n-back, and (3) HRV during SYB sessions are given in Tables 1,2,3, respectively.
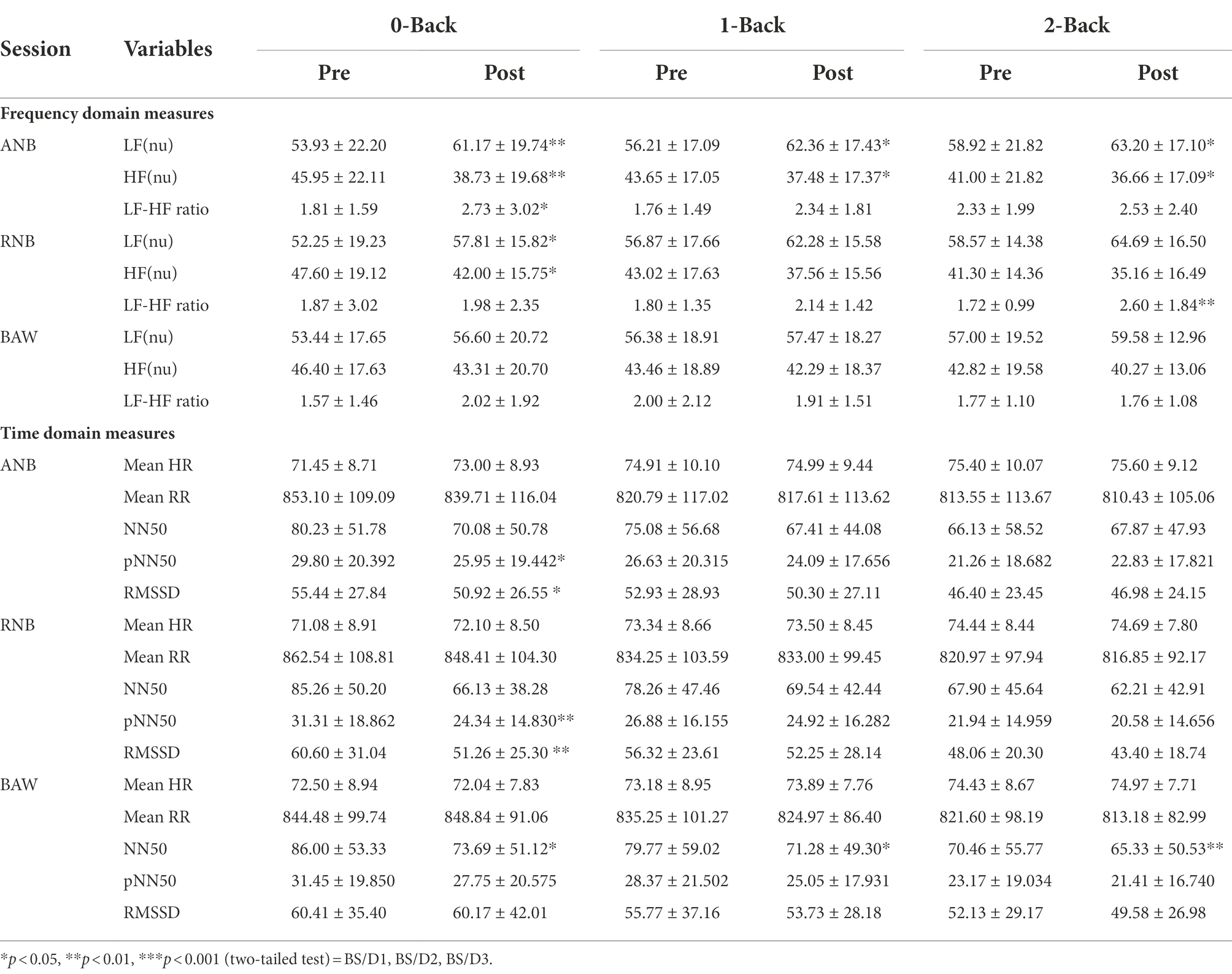
Table 2. HRV measures during an n-back performance before and after yoga breathing. Values are group mean ± SD (n = 40).
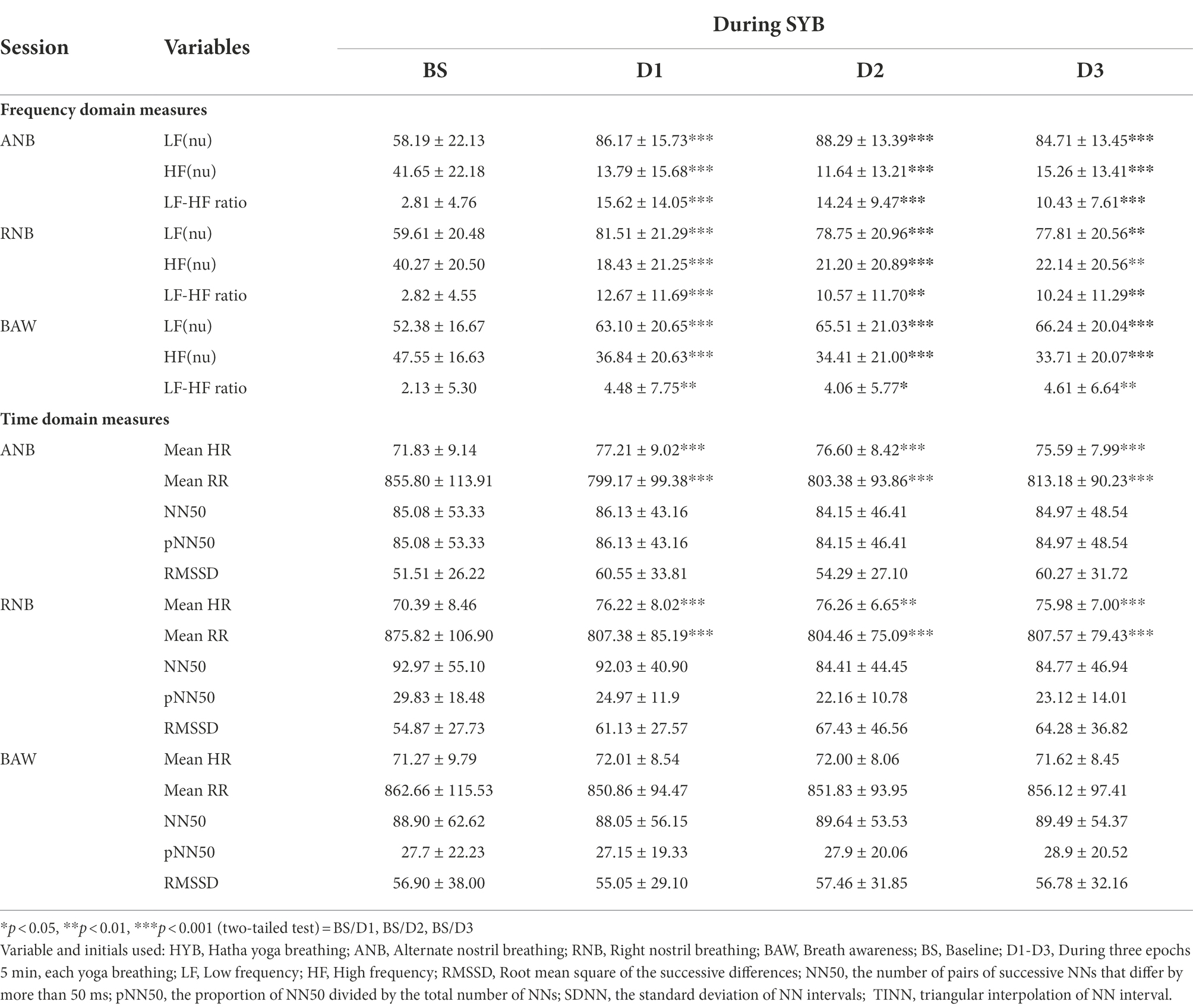
Table 3. HRV measures at baseline and during yoga breathing practices. Values are group mean ± SD (n = 40).
N-back task
The ANOVA results for reaction time showed a significant main effect on Time [F(1,39) = 12, p < 0.001, ηp2 = 0.36]. Similarly, in accuracy, there was a significant main effect on session [F(2,78) = 6.99, p < 0.01, ηp2 = 0.15] and time [F(1,39) = 22.14, p < 0.001, ηp2 = 0.24].
Post-hoc test with Bonferroni adjustment was performed for each condition of n-back. The results showed a significant decrease in the reaction time of 2-back after ANB (p < 0.01), RNB (p < 0.05), and BAW (p < 0.01) practices. However, participants showed a better reaction time after ANB compared to other practices. Participants showed a significant improvement in accuracy of the 2-back condition (p < 0.01) after ANB practice, and significant improvement in 0-back (p < 0.01) and 2-back (p < 0.01) conditions after RNB practice, while there was no change in accuracy of the n-back performance after BAW practice when compared to pre-scores.
HRV during n-back
The ANOVA results for measures of frequency domain analysis showed a significant main effect on time in LF [F(1,37) = 17.39, p < 0.001, ηp2 = 0.32], HF [F(1,37) = 17.51, p < 0.001, ηp2 = 0.32], and LF-HF ratio [F(1,38) = 9.46, p < 0.01, ηp2 = 0.20]. Similarly, in measures of time-domain analysis, there was a significant main effect on time in RMSSD [F(1,37) = 7.9, p < 0.01, ηp2 = 0.18], NN50 [F(1,37) = 9.58, p < 0.01, ηp2 = 0.21], and pNN50 [F(1,37) = 7.5, p < 0.01, ηp2 = 0.17].
Post-hoc analysis with Bonferroni correction showed that after ANB practice there was a significant increase in the LF power during 0-back (p < 0.01), 1-back (p < 0.05), and 2-back (p < 0.05), and in the LF/HF ratio during 0-back (p < 0.05). And there was a decrease in the HF power during 0-back (p < 0.01), 1-back (p < 0.05) and 2-back (p < 0.05). Results on post RNB practice showed that there was a significant increase in LF power during 0-back (p < 0.05) and LF/HF ratio during 2-back (p < 0.01) and a decrease in the HF power in 0-back (p < 0.05).
ANB practice revealed a significant decrease in pNN50 and RMSSD (p < 0.05) in time-domain HRV measurements during the post-0-back condition compared to the pre-condition. There was a significant decrease in pNN50 (p < 0.01), and RMSSD (p < 0.01) when compared to pre 0-back condition following the RNB session. After BAW practice, there was a significant decrease in NN50 during post 0-back (p < 0.05), post 1-back (p < 0.05) and post 2-back (p < 0.01) when compared to respective pre-conditions.
HRV during SYB sessions
The ANOVA results of HRV measures of frequency domain analysis during SYB showed significant main effect on sessions in LF [F(2, 76) = 25.63, p < 0.001, ηp2 = 0.40], HF [F(2,76) = 27.7, p < 0.001, ηp2 = 0.41], and LF-HF ratio [F(2,76) = 19.46, p < 0.001, ηp2 = 0.34]. Likewise, main effect on during in LF [F(1.68, 63.80) = 52.30, p < 0.001, ηp2 = 0.58], HF [F(1.68, 63.80) = 51.92, p < 0.001, ηp2 = 0.58], and LF-HF ratio [F(2.44, 92.59) = 26.17, p < 0.001, ηp2 = 0.42].
Also, there was a significant interaction effect on sessions × during in LF [F(3.73, 141.61) = 4.37, p < 0.01, ηp2 = 0.10], HF[F(3.73, 141.65) = 4.32, p < 0.01, ηp2 = 0.10], LF-HF ratio [F(5.41, 201.68) = 6.34, p < 0.001, ηp2 = 0.14]. Similarly, HRV measures of time domain analysis during SYB showed a significant main effect on sessions in Mean HR [F(2, 76) = 7.05, p < 0.05, ηp2 = 0.16] and Mean RR [F(1, 74) = 6.08, p < 0.01, ηp2 = 0.14]; and interaction effect between session × during in Mean HR [F(5.30, 196.16) = 10.34, p < 0.001, ηp2 = 0.22], Mean RR [F(4.28, 158.4) = 6.55, p < 0.001, ηp2 = 0.21], and RMSSD [F(4.5, 166.5) = 4.58, p < 0.001, ηp2 = 0.11].
Post-hoc analysis with Bonferroni correction in frequency domain analysis showed that there was a significant increase in LF power, LF/HF ratio, and decrease in HF power during ANB, RNB, and BAW sessions (p < 0.05; p < 0.01; p < 0.001). Similarly, time-domain measures also showed a significant increase in Mean HR, and Mean RR, during ANB and RNB sessions (p < 0.05; p < 0.01; p < 0.001). However, there were no changes in time-domain measures of the BAW session.
The graphical presentation of LF power, HF power, and LF/HF ratio during baseline, n-back, and yoga breathing are given in Figure 3A. Similarly, the changes in reaction time and accuracy of n-back conditions before and after SYB sessions are given in Figure 3B.
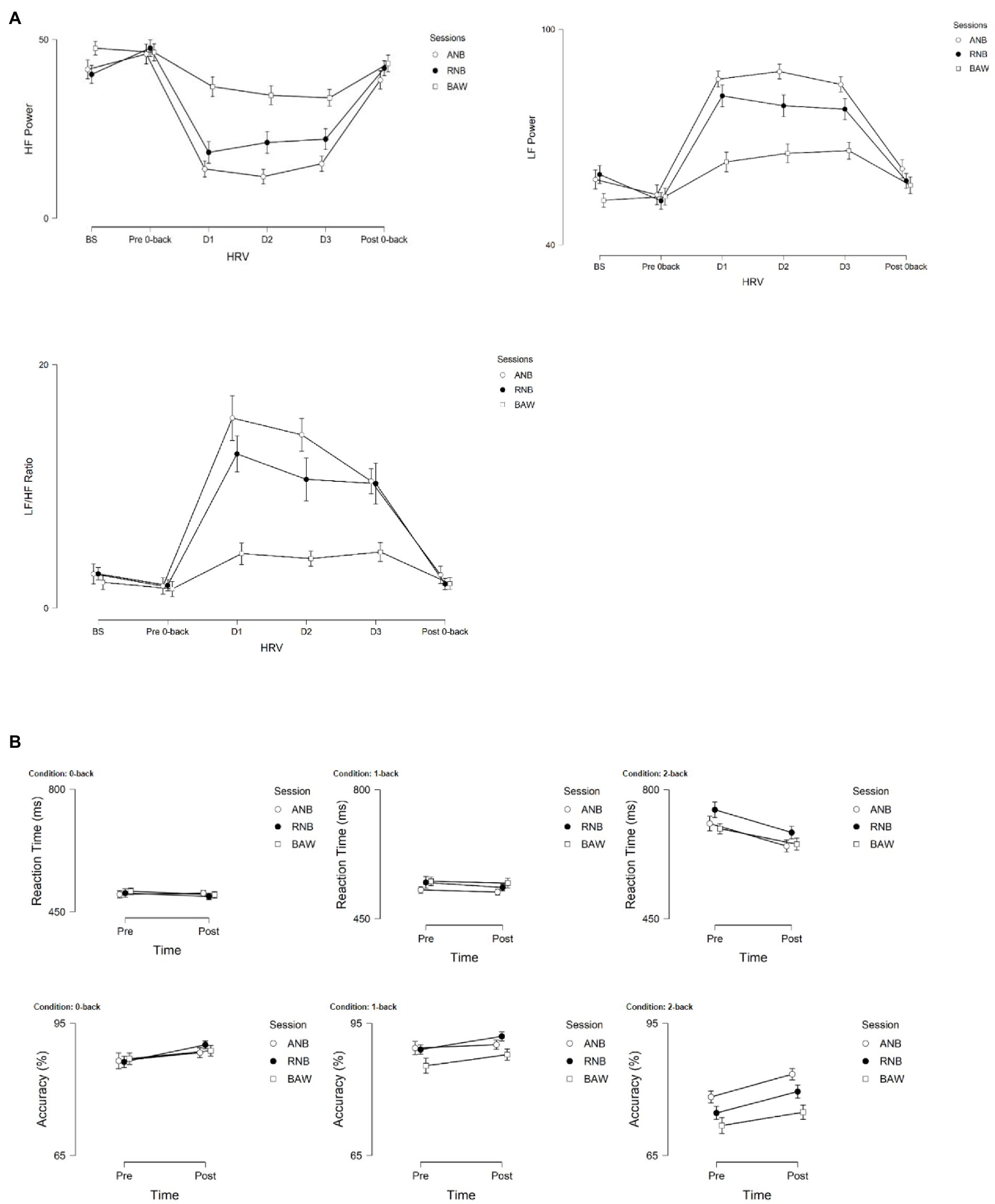
Figure 3. (A) Graphical representations of LF power, HF power, and LF/HF ratio during baseline, n-back (pre-post), and during SYB (n = 40). (B) Reaction time (ms) and accuracy (%) of n-back conditions (0-, 1-, and 2-back) following SYB.
The breathing rate did not show any significant change during the session, and is therefore not reported in the results.
Discussion
To our knowledge, this is the first study to investigate the immediate effect of SYB practice simultaneously on mental workload with working memory tasks and changes in the measures of HRV. The study results revealed that SYB at 6 bpm significantly increases SNS activity and improves mental processes during working memory performance. Our findings indicate that practicing yoga breathing can increase the physiological load, i.e., linked to the significant increase in SNS activity. SNS activity was moderately elevated during the mental load in the present study which is required to complete working memory tasks efficiently (Knight et al., 2020). This has been reflected in the results by faster reaction time (ms) in working memory performance with better accuracy, suggesting that slow yoga breathing through one nostril may improve mental functioning in young adults (Giuliano et al., 2018). Therefore, it can be suggested that the SYB practice may enhance processing speed and the ability to discriminate between target and non-target events in the complex n-back task, particularly in the 2-back condition.
According to previous research, cognitive tasks require significant levels of sympathetic activity or parasympathetic inhibition to complete the task (Berntson et al., 1996; Melis and Van Boxtel, 2001; Melis and van Boxtel, 2007). Parasympathetic withdrawal is associated with “feed-forward” input from higher brain centers to the medullary cardiovascular center that triggers a rapid increase in heart rate which is mediated by temporarily reduced cardiac parasympathetic neural activity. However, sympathetic activation is triggered with moderate to maximal intensity of practice and mediated by the systemic sympathoadrenal activation (Billman, 2013; White and Raven, 2014). During working memory performance, HRV results indicate parasympathetic withdrawal after ANB and RNB breathing practice. That may be linked to improving performance on a task that needs more mental effort. In addition to this, after BAW there was a decrease in reaction time for the 2-back task without any statistically significant changes in accuracy.
An earlier study demonstrated that participants practicing yoga breathing at a rate of 6 bpm showed sympathetic arousal which might be due to an increase in physiological load during yogic breathing (Raghuraj and Telles, 2008). Overall, the findings showed that slow breathing considerably raises the LF power and LF/HF ratio, reflecting the predominance of sympathetic activity and sympathovagal balance, respectively. Slow yoga breathing directly influences vagal tone or baroreflex sensitivity that modulate the sinoatrial node, thereby increasing HR oscillation and HRV (Bernardi et al., 2001). The ANB and RNB showed a temporary withdrawal of parasympathetic activity and a concomitant increase in HR that can be linked to higher baroreflex sensitivity and oxygen saturation (Mason et al., 2013). Further, slow breathing studies have demonstrated beneficial effects on cardiorespiratory functions in patients with cardiac surgery, prehypertension, asthma, and major depressive disorder (Karavidas et al., 2007; Lin et al., 2012). The findings of the present study are consistent with the previous study (Lin et al., 2014) which implies that slow yoga breathing practices may be beneficial for the physical and mental health of clinically healthy individuals, mainly due to improvements in the subjective feeling of relaxation. However, the mechanism of action of yoga breathing on autonomic and cognition is not fully understood.
In the neurovisceral integration model, greater parasympathetic activity is linked to selective attention, whereas heightened sympathetic activity is associated with an efficient attention-related processing (Giuliano et al., 2018). In line with the aforementioned findings, we observed that cognitive task performance was improved after slow and controlled SYB (ANB and RNB) sessions, and even after a simple BAW session. The SYB practice may activate specific brain regions such as the dorsal pons, periaqueductal grey matter, cerebellum, hypothalamus, thalamus, lateral and anterior insular cortices in the human brain as revealed in the volitional controlled slow breathing study (Nagai et al., 2015). A recent study reported that yoga breathing and meditation increase synchronization between respiratory and attentional systems via LC activity thereby improving attentional, emotional, and physiological functioning (Melnychuk et al., 2018). Moreover, unilateral yoga breathing at a slow rate (5–8 bpm) demonstrated increased oxygenation and blood volume in the left prefrontal cortex (PFC) after the RNB session as compared to the BAW session (Singh et al., 2015). Though speculation, the above-mentioned brain areas may be associated with the enhancement of autonomic functions during yoga breathing and while performing a neurocognitive task. There might be several other pathways involved in yoga breathing practice that directly or indirectly influence autonomic activity and cognitive functions that needs to be explored in future studies.
Limitations
A number of limiting factors are likely to account for the current study: the sample size was relatively small, also the lack of a control group (those never exposed to yoga practice), and study outcomes are limited to male yoga practitioners only. Further, during the neurocognitive task, the remnant effect of the practice block could not be ruled out due to technical limitations. The results cannot be extrapolated to generalize the neurocognitive performance as a whole because they are restricted to the immediate impact of yoga breathing practice on neurocognitive function. Moreover, the autonomic changes during pre n-back task might have a carryover effect on HRV in breathing sessions, which should be controlled in a further study. Furthermore, the study findings can only be generalized to healthy individuals and future studies could concentrate on participants with depression or people with mental health issues. Future research should extend the present methodology to a wider array of experimental tasks and examine simultaneous assessments of HRV, and brain imaging techniques (fMRI, fNIRS, or EEG) related to the long-term practice of meditation and yoga breathing. However, within our study, SYB sessions increased parasympathetic withdrawal which was reflected in the improvement of working memory performance when compared with spontaneous breathing in the BAW session.
Conclusion
The present study empirically showed cardiac autonomic arousal during SYB among yoga practitioners. Additionally, moderate SNS activation may support physiological arousal, which is necessary for efficient mental functioning and working memory performance. The outcome of the study suggests that yoga breathing may have the potential to enhance coping abilities by optimizing heightened physiological arousal and facilitating attentional processes. Therefore, SYB may be useful for bringing positive changes in the autonomic activity related to clinical conditions (dysautonomia, attention deficit hyperactive disorder [ADHD], and major depressive disorder [MDD]), which should be investigated in future studies. Furthermore, SYB immediate practice prior to a mental task may facilitate better responses to autonomic activity that is associated with neurocognitive performance.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Ethics Committee of SVYASA - RES/IEC-SVYASA/009/2015. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SD: conceptualization, funding acquisition, methodology, project administration, formal analysis, data curation, validation, interpretation of the data, and writing—original drafting of the work. RB: acquisition, resources, software for analysis, supervision, and validation. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Science and Engineering Research Board (SERB) [YSS/2015/000734], Department of Science and Technology (DST), New Delhi, Government of India.
Acknowledgments
The authors are thankful to S-VYASA administrative staff, course coordinators for their support, and students for their participation in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Anasuya, B., Deepak, K. K., Jaryal, A. K., and Narang, R. (2020). Effect of slow breathing on autonomic tone & baroreflex sensitivity in yoga practitioners. Indian J. Med. Res. 152, 638–647. doi: 10.4103/ijmr.IJMR_559_19
Anjana, R. M., Deepa, M., Pradeepa, R., Mahanta, J., Narain, K., Das, H. K., et al. (2017). Prevalence of diabetes and prediabetes in 15 states of India: results from the ICMR–INDIAB population-based cross-sectional study. Lancet Diabetes Endocrinol. 5, 585–596. doi: 10.1016/S2213-8587(17)30174-2
Atkinson, J. W. (1957). Motivational determinants of risk-taking behavior. Psychol. Rev. 64, 359–372. doi: 10.1037/h0043445
Bernardi, L., Gabutti, A., Porta, C., and Spicuzza, L. (2001). Slow breathing reduces chemoreflex response to hypoxia and hypercapnia, and increases baroreflex sensitivity. J. Hypertens. 19, 2221–2229. doi: 10.1097/00004872-200112000-00016
Berntson, G. G., Cacioppo, J. T., and Fieldstone, A. (1996). Illusions, arithmetic, and the bidirectional modulation of vagal control of the heart. Biol. Psychol. 44, 1–17. doi: 10.1016/S0301-0511(96)05197-6
Bhagat, O. L., Kharya, C., Jaryal, A., and Deepak, K. K. (2017). Acute effects on cardiovascular oscillations during controlled slow yogic breathing. Indian J. Med. Res. 145, 503–512. doi: 10.4103/ijmr.IJMR_830_15
Bhavanani, A., Pushpa, D., Ramanathan, M., and Balaji, R. (2014). Differential effects of uninostril and alternate nostril pranayamas on cardiovascular parameters and reaction time. Int. J. Yoga 7, 60–65. doi: 10.4103/0973-6131.123489
Billman, G. E. (2013). The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front. Physiol. 4, 26. doi: 10.3389/fphys.2013.00026
da Silva, T. L., Ravindran, L. N., and Ravindran, A. V. (2009). Yoga in the treatment of mood and anxiety disorders: a review. Asian J. Psychiatry 2, 6–16. doi: 10.1016/j.ajp.2008.12.002
Delliaux, S., Delaforge, A., Deharo, J. C., and Chaumet, G. (2019). Mental workload alters heart rate variability, Lowering non-linear dynamics. Front. Physiol. 10, 565. doi: 10.3389/fphys.2019.00565
Erdfelder, E., Faul, F., and Buchner, A. (1996). GPOWER: a general power analysis program. Behav. Res. Methods Instrum. Comput. 28, 1–11. doi: 10.3758/BF03203630
Giuliano, R. J., Karns, C. M., Bell, T. A., Petersen, S., Skowron, E. A., Neville, H. J., et al. (2018). Parasympathetic and sympathetic activity are associated with individual differences in neural indices of selective attention in adults. Psychophysiology 55, e13079. doi: 10.1111/psyp.13079
Karavidas, M. K., Lehrer, P. M., Vaschillo, E., Vaschillo, B., Marin, H., Buyske, S., et al. (2007). Preliminary results of an open label study of heart rate variability biofeedback for the treatment of major depression. Appl. Psychophysiol. Biofeedback 32, 19–30. doi: 10.1007/s10484-006-9029-z
Kirchner, W. K. (1958). Age differences in short-term retention of rapidly changing information. J. Exp. Psychol. 55, 352–358. doi: 10.1037/h0043688
Knight, E. L., Giuliano, R. J., Shank, S. W., Clarke, M. M., and Almeida, D. M. (2020). Parasympathetic and sympathetic nervous systems interactively predict change in cognitive functioning in midlife adults. Psychophysiology 57:e13622. doi: 10.1111/psyp.13622
Lee, C., and Ghiya, S. (2012). Influence of alternate nostril breathing on heart rate variability in non-practitioners of yogic breathing. Int. J. Yoga 5, 66–69. doi: 10.4103/0973-6131.91717
Lin, I. M., Tai, L. Y., and Fan, S. Y. (2014). Breathing at a rate of 5.5breaths per minute with equal inhalation-to-exhalation ratio increases heart rate variability. Int. J. Psychophysiol. 91, 206–211. doi: 10.1016/j.ijpsycho.2013.12.006
Lin, G., Wang, T., Wang, S., Fu, X., Xiang, Q., Chen, S., et al. (2012). Heart rate variability biofeedback decreases blood pressure in Prehypertensive subjects by improving autonomic function and Baroreflex. J. Altern. Complement. Med. 18, 143–152. doi: 10.1089/acm.2010.0607
Mason, H., Vandoni, M., Debarbieri, G., Codrons, E., Ugargol, V., and Bernardi, L. (2013). Cardiovascular and respiratory effect of yogic slow breathing in the yoga beginner: what is the best approach? Evid. Based Complement. Alternat. Med. 2013, 1–7. doi: 10.1155/2013/743504
McIntosh, R. C., Khambaty, T., Llabre, M. M., Perreira, K. M., Gonzalez, H. M., Kansal, M. M., et al. (2021). Paradoxical effect of cumulative stress exposure on information processing speed in Hispanics/Latinos with elevated heart rate variability. Int. J. Psychophysiol. 164, 1–8. doi: 10.1016/j.ijpsycho.2021.01.019
Melis, C., and Van Boxtel, A. (2001). Differences in autonomic physiological responses between good and poor inductive reasoners. Biol. Psychol. 58, 121–146. doi: 10.1016/S0301-0511(01)00112-0
Melis, C., and van Boxtel, A. (2007). Autonomic physiological response patterns related to intelligence. Intelligence 35, 471–487. doi: 10.1016/j.intell.2006.09.007
Melnychuk, M. C., Dockree, P. M., O’Connell, R. G., Murphy, P. R., Balsters, J. H., and Robertson, I. H. (2018). Coupling of respiration and attention via the locus coeruleus: effects of meditation and pranayama. Psychophysiology 55:e13091. doi: 10.1111/psyp.13091
Muktibodhananda, S. Swami. (1998). Hatha Yoga Pradipika: Light on Hatha Yoga. 4th Edn. Bihar, India: Bihar School of Yoga.
Nagai, Y., Minati, L., Gray, M. A., Chiesa, P. A., Bernardi, L., Critchley, H. D., et al. (2015). Slow breathing and hypoxic challenge: cardiorespiratory consequences and their central neural substrates. PLoS One 10:e0127082. doi: 10.1371/journal.pone.0127082
Pal, G. K., Agarwal, A., Karthik, S., Pal, P., and Nanda, N. (2014). Slow yogic breathing through right and left nostril infl uences sympathovagal balance, heart rate variability, and cardiovascular risks in young adults. North Am. J. Med. Sci. 6, 145–151. doi: 10.4103/1947-2714.128477
Raghuraj, P., Ramakrishnan, A. G., Nagendra, H. R., and Telles, S. (1998). Effect of two selected yogic breathing techniques on heart rate variability. Indian J. Physiol. Pharmacol. 42, 467–472.
Raghuraj, P., and Telles, S. (2008). Immediate effect of specific nostril manipulating yoga breathing practices on autonomic and respiratory variables. Appl. Psychophysiol. Biofeedback 33, 65–75. doi: 10.1007/s10484-008-9055-0
Ramírez, E., Ortega, A. R., and Reyes Del Paso, G. A. (2015). Anxiety, attention, and decision making: The moderating role of heart rate variability. Int. J. Psychophysiol. 98, 490–496. doi: 10.1016/j.ijpsycho.2015.10.007
Santhanam Kumar, S. S., Kamath, A., and Poojary, S. (2020). Effect of unilateral left nostril breathing (Chandra Anga pranayama) on cognitive function in healthy yoga-Naïve individuals: A randomized, controlled, pilot study. Complement. Med. Res. 27, 319–327. doi: 10.1159/000506972
Sarang, P. S., and Telles, S. (2006). Oxygen consumption and respiration during and after two yoga relaxation techniques. Appl. Psychophysiol. Biofeedback 31, 143–153. doi: 10.1007/s10484-006-9012-8
Saraswati, N. (2012). Gheranda Samhita: commentary on the yoga teachings of sage Gheranda. Yoga Publications Trust. Available at: https://books.google.co.in/books?id=qLHEoQEACAAJ (Accessed August 24, 2022).
Shaffer, F., and Ginsberg, J. P. (2017). An overview of heart rate variability metrics and norms. Front. Public Health 5, 258. doi: 10.3389/fpubh.2017.00258
Sharma, V. K., Rajajeyakumar, M., Velkumary, S., Subramanian, S. K., Bhavanani, A. B., Madanmohan,, et al. (2014). Effect of fast and slow pranayama practice on cognitive functions in healthy volunteers. J. Clin. Diagn. Res. 8, 10–13. doi: 10.7860/JCDR/2014/7256.3668
Singh, K., Bhargav, H., and Srinivasan, T. (2015). Effect of uninostril yoga breathing on brain hemodynamics: a functional near-infrared spectroscopy study. Int. J. Yoga 9, 12–19. doi: 10.4103/0973-6131.171711
Srivastava, R. D., Jain, N., and Singhal, A. (2005). Influence of alternate nostril breathing on cardiorespiratory and autonomic functions in healthy young adults. Indian J. Physiol. Pharmacol. 49,475–483.. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16579403 (Accessed August 24, 2022).
Telles, S., Nagarathna, R., and Nagendra, H. R. (1994). Breathing through a particular nostril can alter metabolism and autonomic activities. Indian J. Physiol. Pharmacol. 38, 133–137.
Telles, S., Singh, N., and Puthige, R. (2013). Changes in P300 following alternate nostril yoga breathing and breath awareness. Biopsychosoc. Med. 7, 11. doi: 10.1186/1751-0759-7-11
Tucker-Drob, E. M. (2009). Differentiation of cognitive abilities Across the life span. Dev. Psychol. 45, 1097–1118. doi: 10.1037/a0015864
Vinay, A., Venkatesh, D., and Ambarish, V. (2016). Impact of short-term practice of yoga on heart rate variability. Int. J. Yoga 9, 62–66. doi: 10.4103/0973-6131.171714
Keywords: pranayama, working memory, attention, heart rate variability (HRV), autonomic functions, yoga breathing
Citation: Deepeshwar S and Budhi RB (2022) Slow yoga breathing improves mental load in working memory performance and cardiac activity among yoga practitioners. Front. Psychol. 13:968858. doi: 10.3389/fpsyg.2022.968858
Edited by:
Costantino Balestra, Haute École Bruxelles-Brabant (HE2B), BelgiumReviewed by:
Iffat Jahan, Eastern Medical College and Hospital, BangladeshMaria A. Zayas, Brenau University, United States
Lisza Gaiswinkler, Folkwang Institute, Research & education center for holistic lifestyles, Austria
Copyright © 2022 Deepeshwar and Budhi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Singh Deepeshwar, ZGVlcGVzaHdhci5zaW5naEBzdnlhc2EuZWR1Lmlu
†ORCID: Singh Deepeshwar, https://orcid.org/0000-0002-9867-1405
 Singh Deepeshwar
Singh Deepeshwar Rana Bal Budhi
Rana Bal Budhi