- Department of Psychology, Renmin University of China, Beijing, China
Delay discounting is a common phenomenon in daily life, which refers to the subjective value of a future reward decreasing as a function of time. Previous studies have identified several cortical regions involved in delay discounting, but the neural network constructed by the cortical regions of delay discounting is less clear. In this study, we employed resting-state functional magnetic resonance imaging (RS-fMRI) to measure the spontaneous neural activity in a large sample of healthy young adults and used the Monetary Choice Questionnaire to directly measure participants’ level of delay discounting. To identify the neural network of delay discounting at rest, we used an individual difference approach to explore brain regions whose spontaneous activities were related to delay discounting across the whole brain. Then, these brain regions served as seeds to identify the neural network of delay discounting. We found that the fractional amplitude of low-frequency fluctuations (fALFF) of the left insula were positively correlated to delay discounting. More importantly, its connectivity to the anterior cingulate cortex was read out for participants’ behavioral performance in the task of delay discounting. In short, our study provides empirical evidence that insula-anterior cingulate cortex connectivity may serve as a part of the neural network for delay discounting.
Introduction
Life is full of dilemma. Your friend invites you to a big dinner, when you have just decided to lose weight; when you turn on the computer and decide to focus on work, a pop-up window reminds you that the latest episode of your favorite show has just been released. In these situations, you are faced with two options: a small but immediate reward (SIR, the big dinner, and the latest episode) or a large but delayed reward (LDR, the healthier weight, and the work). Although obviously the rational choice is LDR, we often choose SIR instead. This phenomenon is called delay discounting (Kirby and Marakovic, 1995), which refers to the subjective value of a future reward decreasing as a function of time (Kirby et al., 1999). Therefore, if the subjective value of LDR is smaller than SIR, we choose SIR. Delay discounting is found to be associated with many problematic behaviors, such as low academic performance (Kirby et al., 2005), internet addiction (Saville et al., 2010), substance abuse (Kollins, 2003), and pathological gambling (Dixon et al., 2003).
Many studies have been conducted to explore the neural mechanism of delay discounting. Neuroimaging studies have identified several brain regions involved in delay discounting, including the precuneus (McClure et al., 2007; Lim et al., 2017), the dorsolateral prefrontal cortex (Monterosso et al., 2007; Ho et al., 2016), the ventromedial prefrontal cortex (Marco-Pallares et al., 2010; Civai et al., 2016), the insula (McClure et al., 2007; Wittmann et al., 2010; Clewett et al., 2014; Grodin et al., 2017), and the anterior cingulate cortex (ACC) (Monterosso et al., 2007; Hoffman et al., 2008; Ortiz et al., 2015; Grodin et al., 2017; Dennis et al., 2020). Among all these brain areas, the insula plays a critical role in delay discounting through time perception, cognitive control, and emotion processing (Wittmann et al., 2010; Nieuwenhuys, 2012; Li et al., 2013; Noreika et al., 2013; Clewett et al., 2014), whereas the ACC has an effect on delay discounting by participating in the process of conflict detection and strategy adaptation (van Veen et al., 2004; Peters and Buechel, 2011; Li et al., 2013; Wang et al., 2017). Interestingly, both the insula and the ACC are critical nodes of the salience network (SN) identified at resting-state (Seeley et al., 2007; Menon and Uddin, 2010), and the SN is reported to influence delay discounting by playing a significant role in the process of high-level cognitive control (Li et al., 2013; Chen et al., 2017; Grodin et al., 2017; Zhu et al., 2017; Chen et al., 2018). Here, we hypothesized that the resting-state neural network constructed by the insula and the ACC may play an important role in delay discounting.
To do this, we first examined whether the insula or the ACC was associated with delay discounting at resting-state in a large population of participants (N = 264). Specifically, we measured fractional amplitude of low-frequency fluctuations (fALFF) of the insula and the ACC, along with the rest of the brain, and then correlated the fALFF values with participants’ behavioral performance in delay discounting. Having established the resting-state neural correlates at the regional level, we further investigated the role of the neural work constructed by these regions in delay discounting.
Materials and Methods
Participants
A total of 310 college students (186 women, 18–23 years of age, mean age = 20.36, SD = 0.85, 8 without age information) participated this study. Participants reported no history of neurological or psychiatric disorders. This study was approved by the Institutional Review Board of Beijing Normal University. Prior to the experiment, written informed consent was obtained from all participants.
Measures of Behaviors
Delay Discounting—Monetary Choice Questionnaire
Our study used MCQ (Kirby et al., 1999) to evaluate the degree of delay discounting for each participant. MCQ consists of 27 items, for each item, the participants chose between one SIR and one LDR. For example, “Would you prefer ¥31 today or ¥85 in 7 days?” There was no time limit for the participants to fill in the MCQ. After finishing the questionnaire, each participant received not only monetary compensation but also the reward based on their choice randomly selected from the 27 items to ensure the participants made choices based on their genuine preference. According to the MCQ (Kirby et al., 1999), we calculated k values to represent the participants’ degree of delay discounting. The larger the k value, the higher the participant’s impulsivity, which means that the participant was more likely to choose SIRs. There are 10 k values in the MCQ, each of them had a corresponding choice pattern. The k value whose choice pattern was most approximate to the participant’s was the k value indicating this participant’s degree of delay discounting. Finally, as the k values were not normally distributed, we performed log-transformation to obtain the Ln(k) values.
Intelligence—Raven’s Advanced Progressive Matrices
Previous studies indicate that there is a significant correlation between intelligence and delay discounting (Shamosh et al., 2008; Shamosh and Gray, 2008). Therefore, we included intelligence as a confounding factor which was indicated by Raven score (Raven et al., 1998). Raven’s Advanced Progressive Matrices contains 36 items, requiring the participants to select the missing figure to complete a 3×3 matrix. The number of correct answers in 30 min was taken as the Raven score of each participant.
Image Acquisition
Resting-state functional magnetic resonance imaging scanning was conducted on a 3T Siemens Trio scanner (MAGENTOM Trio, a Tim system) with a 12-channel phase-arrayed coil at Beijing Normal University Imaging Center for Brain Research, Beijing, China. During the resting-state scan, participants were instructed to keep still, remain awake, close their eyes, and not purposely think of anything. The RS-fMRI scanning consisted of 240 contiguous echo-planar imaging (EPI) volumes (TR = 2000 ms; TE = 30 ms; flip angle = 90°; number of slices = 33; matrix = 64×64; FOV = 200 × 200 mm2; acquisition voxel size = 3.125 × 3.125 × 3.6 mm3). Additionally, high-resolution T1-weighted images were obtained with a magnetization-prepared gradient echo sequence (MPRAGE: TR/TE/TI = 2530/3.39/1100 ms; flip angle = 7°; matrix = 256×256; number of slices = 128; voxel size = 1 × 1 × 1.33 mm3) for spatial registration.
Image Data Preprocessing
Resting-state functional magnetic resonance imaging image data were preprocessed with FSL (FMRIB Software Library1). The preprocessing steps included head motion correction (by aligning each volume to the middle volume of the 4-D image with MCFLIRT), spatial Gaussian smoothing (FWHM = 6 mm), realignment, intensity normalization, and the removal of linear trends. To better eliminate the influences of psychological noise on our subsequent analysis, we regressed out 18 nuisance signals from cerebrospinal fluid, white matter, global brain average, and motion correction parameters. The registration of each participant’s RS-fMRI to the anatomical images was accomplished by using FMRIB’s Liner Image Registration Tool (FLIRT) (Jenkinson and Smith, 2001; Jenkinson et al., 2002) to produce a six degree-of-freedom affine transformation matrix. The registration of each participant’s anatomical images to the Montreal Neurological Institute (MNI) space was carried out by using FLIRT to calculate a 12 degree-of-freedom linear affine matrix. Because low-frequency fluctuations are sensitive to brain activity in gray matter, we defined a gray mask with the possibility threshold of 0.5 in SPM8. In total, 128,190 voxels were included in the gray mask.
Statistical Analysis
Since 38 participants had missing scan data, 6 participants had missing age or gender information, and 2 participants’ Raven scores were outside ±3 standard deviations, 264 participants (159 women, 18–23 years of age, mean age = 21 years, SD = 2.12) were included in the following analyses. The kurtosis and skewness of age (0.05, −0.21) and Raven scores (−0.12, −0.30) were within the range from −1 to +1, which indicates the normality of age and intelligence (Marcoulides and Hershberger, 1997).
Fractional Amplitude of Low-Frequency Fluctuations-Delay Discounting Correlation Analysis
To explore the relationship between delay discounting and spontaneous neural activities, we calculated the correlation between the Ln(k) values and the fALFF values of each voxel across the whole brain, with age, gender, intelligence, and head motion parameter as the confounding factors. According to Zou et al. (2008) and Zuo et al. (2010), each participant’s fALFF value of each voxel was obtained through dividing the sum of amplitudes across the entire frequency range (0–0.25 Hz) by a fractional sum of the amplitudes within the low-frequency range (0.01–0.1 Hz). Multiple comparison correction was conducted by Gaussian random field theory (GRF) in the DPABI (Yan et al., 2016). The threshold at the voxel level was p < 0.05 and at the cluster level was p < 0.05.
Resting-State Functional Connectivity-Delay Discounting Correlation Analysis
Based on the results from the fALFF-delay discounting correlation analysis, our study examined the neural network of delay discounting at resting-state by using resting-state functional connectivity (RSFC). Firstly, we used the clusters obtained in the fALFF-delay discounting correlation analysis as the seeds. And we calculated the mean time series from all voxels in each seed for each participant. Secondly, for each participant, the RSFC of each voxel was defined as the correlation on the mean time series between the seed and other voxels. Voxel by voxel, the RSFC was calculated across the whole brain, with age, gender, intelligence, and head motion parameter as the confounding factors. Finally, for each seed, we calculated the correlation between the RSFC of each voxel and the Ln(k) values to identify the brain network involved in delay discounting. We transformed r maps to T-score maps. Multiple comparison correction was conducted by GRF in the DPABI (Yan et al., 2016) for the correlations between the RSFC of each voxel to the insula and the Ln(k) values. The threshold at the voxel level was p < 0.05 and at the cluster level was p < 0.05.
Results
The degree of delay discounting for each participant was indexed by the Ln(k) value. A higher Ln(k) suggests a greater discount on future rewards, so individuals with higher Ln(k) likely prefer immediate rewards (i.e., SIRs). The kurtosis (−0.05) and skewness (−0.04) of participants’ delay discounting were within the range from −1 to +1, which indicates the normality of the Ln(k) values (Marcoulides and Hershberger, 1997). The mean of Ln(k) values was −5.18 and the standard deviation was 1.54, which suggested that the variance of the Ln(k) values was suitable for the individual difference approach to explore the neural correlates of delay discounting.
To exhaustively investigate the brain regions which were associated with delay discounting at resting-state besides the regions of interest (i.e., the insula and the ACC), we correlated the fALFF values of each voxel with the Ln(k) values across the whole brain of each participant. After controlling for age, gender, and head motion, we found that the Ln(k) values showed a significant positive correlation with the fALFF values in the left insula (cluster size: 684; MNI coordinate: −38, −18, 18; GRF corrected, p < 0.05). This result confirmed our hypothesis on the role of the insula in delay discounting, as participants with a larger fALFF value in the left insula may discount the future value more steeply, and thus prefer to choose SIRs. In this analysis, no other significant results, including the ACC, were found.
Because the insula is a region with multiple functions—previous studies have shown that the insula is involved in intelligence as well (Takeuchi et al., 2011; Cox et al., 2019), to rule out the possibility that the observed correlation between the left insula and delay discounting may be accounted for by individual differences in intelligence, we conducted a control analysis with intelligence as a confounding factor. The results showed that the correlation between the fALFF values in the left insula and Ln(k) values was still significant after controlling for intelligence, age, gender, and head motion (cluster size: 928; MNI coordinate: −40, −16, 16; GRF corrected, p < 0.05; Figure 1). In short, the relationship between the left insula and delay discounting at resting-state was stable, unlikely ascribed to intelligence.
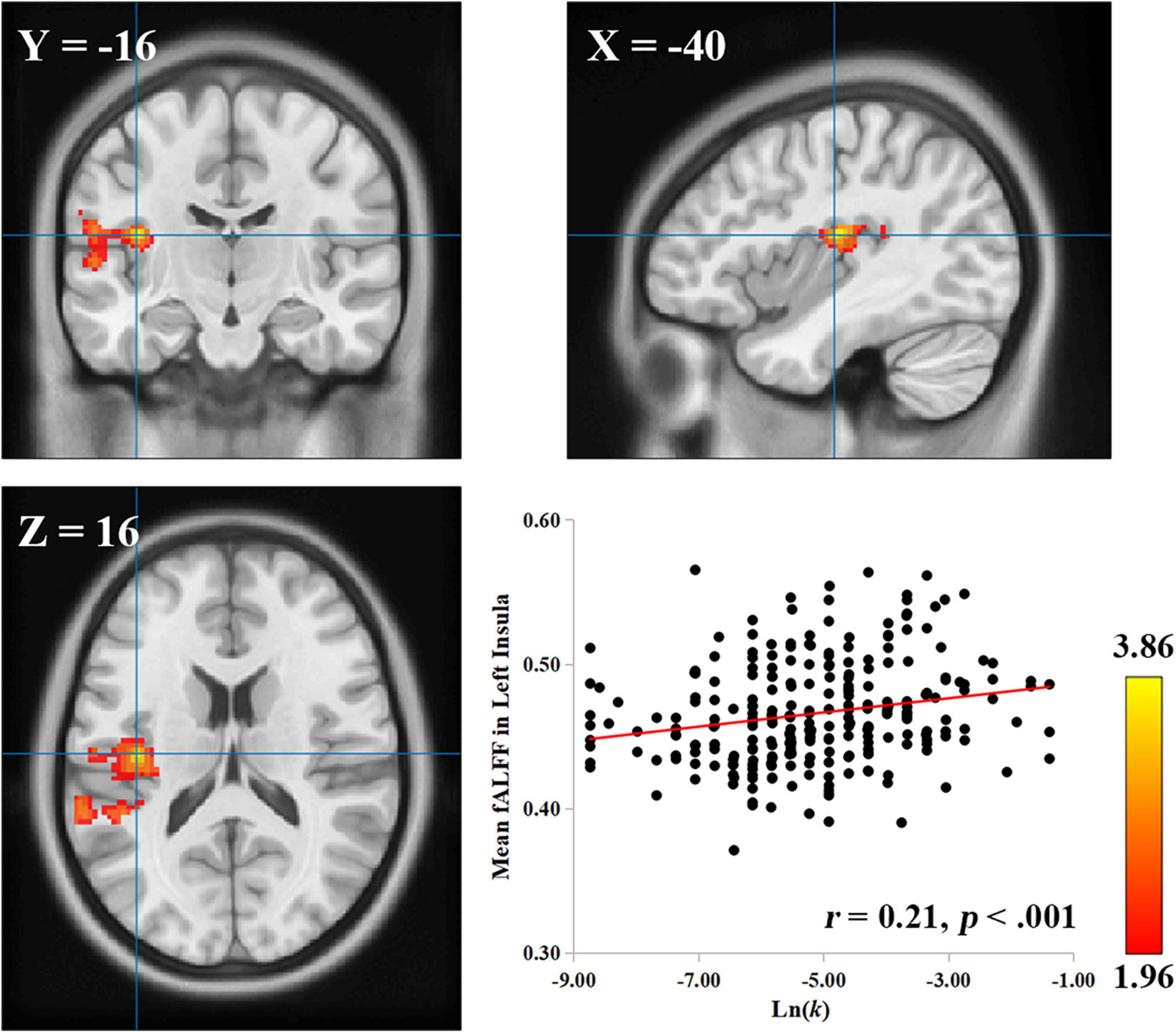
Figure 1. The left insula is related to delay discounting. A cluster with a positive correlation between the fALFF value and the Ln(k) was found in the left insula. The scatter plot between the distributions of the Ln(k) and the mean fALFF values of the cluster after controlling for intelligence, age, gender, and head motion is shown for display purposes. Each dot represents one participant.
Having identified the role of the insula during rest in delay discounting, we use the left insula as the seed ROI to construct a neural network of delay discounting with whole-brain resting-state functional connectivity (RSFC). Specifically, we correlated the RSFC of each voxel to the insula with the Ln(k) values across the whole brain of each participant. After controlling for intelligence, age, gender, and head motion, we found that the RSFC between the insula and the ACC was significantly positively correlated with participants’ Ln(k) values (r = 0.21, p < 0.001; Figure 2; the ACC cluster size: 979; MNI coordinate: 6, 4, 36; GRF corrected, p < 0.05); showing a stronger insula-ACC RSFC corresponded to a higher Ln(k) value in the task of delay discounting. That is, participants with a stronger connectivity between the insula and the ACC may discount the future value more steeply, and thus prefer to choose SIRs.
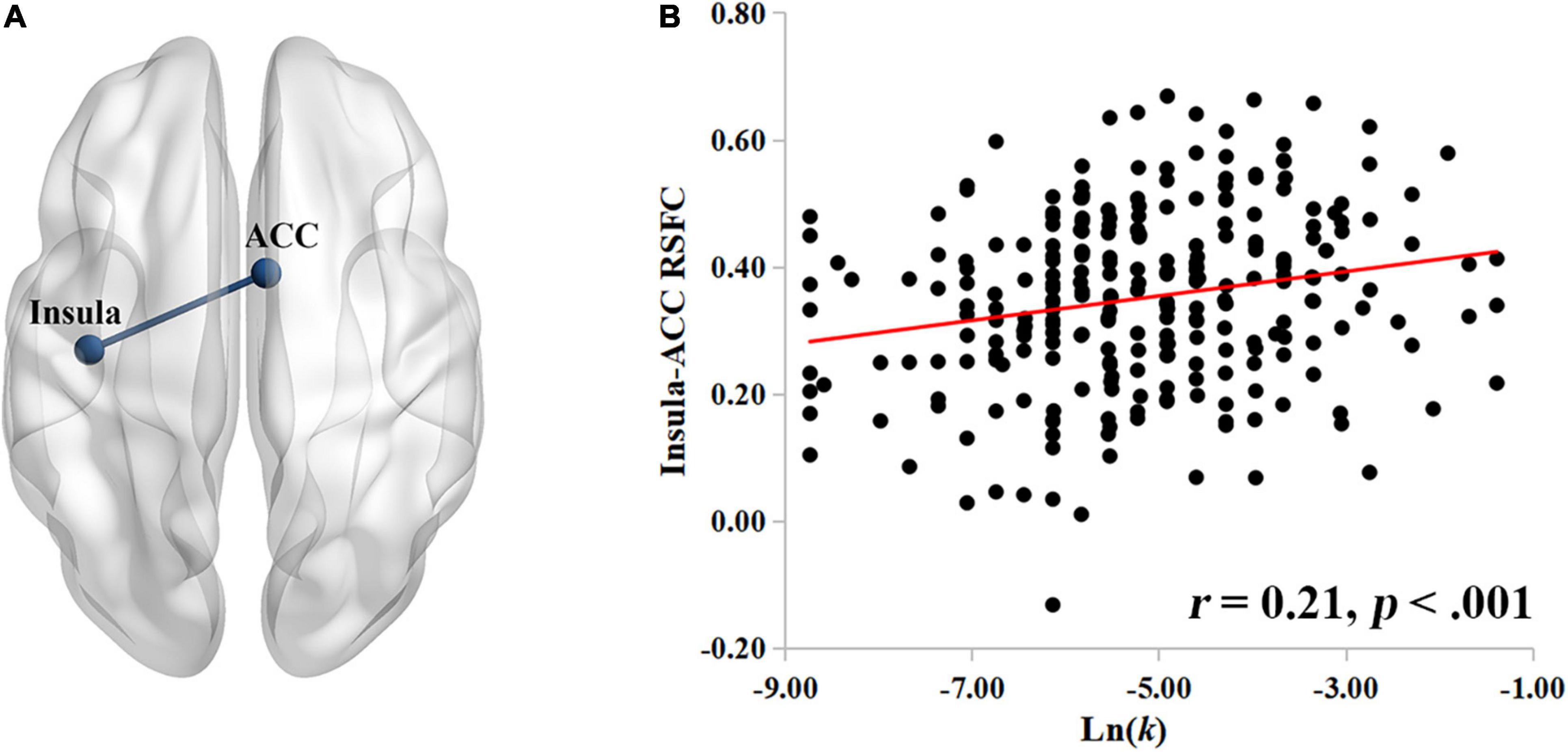
Figure 2. The insula-ACC RSFC is related to delay discounting. (A) The insula-ACC connectivity significantly correlated with the Ln(k) values. (B) The scatter plot showing the positive correlation between the insula-ACC RSFC and the Ln(k) values. Nodes and edges in the networks are overlaid on inflated surface maps generated by BrainNet Viewer (Xia et al., 2013).
Discussion
In the current study, we used RS-fMRI to explore the resting-state neural network of delay discounting in a large sample of healthy young adults. First, we found a positive correlation between the Ln(k) values and the fALFF values of the left insula, revealing that individuals with larger intensity of spontaneous neural activities in the insula discounted future rewards more sharply, and were more likely to choose SIRs over LDRs. Second, we used the delay discounting-related insula as a seed to construct the neural network for delay discounting, and found that the Ln(k) values were positively associated with the strength of the insula-ACC RSFC, suggesting the stronger the connectivity between the insula and the ACC, the more the participants preferred SIR. It is important to note that as the individual difference approach was adopted, the neural network identified in our study reflected the individual differences in delay discounting, which suggests a role for neural networks in other aspects of delay discounting. The insula and the ACC are important nodes of the salience network, and therefore our study illustrates how the salience network plays a pivotal role in delay discounting at resting-state.
In methodology, our study supplements previous studies on the resting-state neural network in three ways. First, instead of pre-selecting regions of interest to construct the neural network of delay discounting as previous studies did (Schmaal et al., 2012; Dias et al., 2013; Contreras-Rodriguez et al., 2015; Rosch et al., 2018; Holmes et al., 2020), here with a large sample of participants we had sufficient power to perform a whole-brain analysis to thoroughly explore the neural correlates of delay discounting. Second, rather than indirectly measuring delay discounting with variables affecting delay discounting (Guo and Feng, 2015; Xin et al., 2020; Zhang et al., 2021), here we used the Monetary Choice Questionnaire to directly calculate Ln(k) as an index for delay discounting. Finally, we tested heathy adult participants, which can extend the findings from previous studies based on mental health patients and adolescents (Dias et al., 2013; Contreras-Rodriguez et al., 2015; Wang et al., 2017; Zhu et al., 2017; Rosch et al., 2018; Holmes et al., 2020) to a larger population.
Our novel finding of the insula’s role in delay discounting during rest was in accordance with previous studies using task-based fMRI on healthy participants or patients (Wittmann et al., 2007; Hoffman et al., 2008; Wittmann et al., 2010; Avsar et al., 2013; Lim et al., 2017; Miranda-Olivos et al., 2021). Specifically, our finding that lower intensity of spontaneous neural activities was correlated with the preference of LDRs perfectly echoes the finding from a lesion study, where patients with damaged insulas preferred to choose LDRs (Sellitto et al., 2016). This finding also supports the hypothesis that the insula may modulate delay discounting by controlling the impulsivity of choosing immediate rewards (Menon and Uddin, 2010), not the hypothesis of uncertainty of future rewards (Paulus et al., 2003). Indeed, individuals with high intensity of resting fluctuations at rest may overshadow the neural activity in a task, which makes the inhibition of impulsivity more difficult. Without sufficient cognitive control, individuals may thus prefer immediate rewards.
The resting neural network constructed with the insula serving as a seed also showed correlations with delay discounting. Importantly, only the connectivity between the insula and the ACC reached significance. The insula and the ACC are two key nodes of the salience network (SN), consistent with previous studies showing that the SN is involved in delay discounting through the processing of high-level cognitive control (Li et al., 2013; Chen et al., 2017, 2018; Grodin et al., 2017; Zhu et al., 2017). The SN is a large network consisting of multiple cortical and subcortical regions (Seeley et al., 2007; Menon and Uddin, 2010). Our study illuminates the role of the insula and the ACC of the SN in delay discounting, which advises future studies to narrow down the scope of the interaction of these two regions in delay discounting. It is interesting to note that the insula and ACC are considered to be domain-general regions involved in a variety of processes, such as emotion (Ernst et al., 2014; Gasquoine, 2014; Nelson et al., 2015), autonomic functions (Cechetto, 2014; Rolls, 2016; Roquet and Conti, 2021), and self-referential processing (Northoff et al., 2006; Hu et al., 2016; Yoon et al., 2019). Our finding of the insula-ACC network involved in delay discounting is not contradictory to this mainstream perspective. First, our study did not support the exclusivity of the insula and ACC in delay discounting, because in the study we only ruled out the possible confounding factor of general intelligence in delay discounting. Second, delay discounting is a complex phenomenon, which consists of multiple cognitive components such as cognitive control and reward evaluation (Peters and Buechel, 2011; Miedl et al., 2012; Stanger et al., 2013; Frost and McNaughton, 2017; Patros et al., 2018). These cognitive components are likely recruited for other tasks as well. Therefore, the network identified in this study was only specific but not exclusive to delay discounting. Moreover, note that our network analysis was restricted to the seed of the insula, which was localized by its relevance to behavioral performance of delay discounting with the measure of fALFF. Therefore, we certainly missed abundant neural correlates of delay discounting, some of which may lie in the SN. Future studies shall use pre-defined SN nodes, such as the anterior insula, amygdala, ventral striatum, and hypothalamus, to investigate their relationship to delay discounting. Besides, previous studies have also reported that the interaction during rest between the SN with other large-scale neural networks (e.g., executive control network, default mode network, and frontoparietal network) is also associated with delay discounting (Li et al., 2013; Zhu et al., 2017; Chen et al., 2018). In this study, we tested a large sample of participants and performed a whole-brain analysis, but we failed to find the association between cross-network resting-state connectivity and behavioral performance in delay discounting. One possibility is that here we only explored the network centering in the insula, which might miss significant contributions from connectivity not consisting of the insula. However, we believe this is unlikely given the critical role of the insula in delay discounting, the statistical power of this study, and the direct measure of delay discounting. Future studies with more dedicate designs are needed to examine the cross-network functional connectivity in delay discounting.
In sum, our study employed RS-fMRI to demonstrate the neural correlates in the resting state of delay discounting. We first correlated the fALFF values with participants’ behavioral performance in delay discounting and established that the spontaneous activities in the insula were related to delay discounting at the regional level. We further used the delay discounting-related insula as a seed to investigate the neural work in delay discounting. Our study gives new empirical evidence that as the key nodes of the SN, the connectivity between the insula and the ACC is involved in delay discounting. An important but unaddressed issue is the cognitive components underlying delay discounting. Delay discounting consists of a variety of cognitive components, such as reward valuation, time perception, conflict detection, and cognitive control (Wittmann and Paulus, 2008; Claus et al., 2011; Frost and McNaughton, 2017; Dennis et al., 2020; Lukinova and Erlich, 2021). However, the behavioral index of the delay discounting used in our study (k value) was not able to be further decomposed into these cognitive components. On the other hand, the neural correlates of delay discounting may shed light on the cognitive components of delay discounting. For example, previous studies have shown that the ACC is an important node in the reward circuit (Breiter and Rosen, 1999; Haber and Knutson, 2010; Liu et al., 2016); therefore, the insula-ACC connectivity for delay discounting suggests the overlap between the SN and the reward circuit. That is, the function of reward evaluation may underlie delay discounting, which needs to be examined in future studies.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board of Beijing Normal University, Beijing, China. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
XL and PH designed the study. XL ran the experiments. FY analyzed the data. All authors wrote the manuscript, contributed to the article, and approved the submitted version.
Funding
This study was supported by National Social Science Foundation of China (Major Program) (19ZDA021).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Avsar, K. B., Weller, R. E., Cox, J. E., Reid, M. A., White, D. M., and Lahti, A. C. (2013). An fMRI investigation of delay discounting in patients with schizophrenia. Brain Behav. 3, 384–401. doi: 10.1002/brb3.135
Breiter, H. C., and Rosen, B. R. (1999). “Functional magnetic resonance imaging of brain reward circuitry in the human,” in Advancing from the ventral striatum to the extended amygdala: Implications for neuropsychiatry and drug use: In honor of Lennart Heimer, J. F. McGinty (New York, NY: New York Academy of Sciences), 523–547. doi: 10.1111/j.1749-6632.1999.tb09287.x
Cechetto, D. F. (2014). Cortical control of the autonomic nervous system. Exp. Physiol. 99, 326–331. doi: 10.1113/expphysiol.2013.075192
Chen, Z., Guo, Y., and Feng, T. (2017). Delay discounting is predicted by scale-free dynamics of default mode network and salience network. Neuroscience 362, 219–227. doi: 10.1016/j.neuroscience.2017.08.028
Chen, Z., Guo, Y., Suo, T., and Feng, T. (2018). Coupling and segregation of large-scale brain networks predict individual differences in delay discounting. Biol. Psychol. 133, 63–71. doi: 10.1016/j.biopsycho.2018.01.011
Civai, C., Hawes, D. R., DeYoung, C. G., and Rustichini, A. (2016). Intelligence and Extraversion in the neural evaluation of delayed rewards. J. Res. Personal. 61, 99–108. doi: 10.1016/j.jrp.2016.02.006
Claus, E. D., Kiehl, K. A., and Hutchison, K. E. (2011). Neural and Behavioral Mechanisms of Impulsive Choice in Alcohol Use Disorder. Alcohol. Clin. Exp. Res. 35, 1209–1219. doi: 10.1111/j.1530-0277.2011.01455.x
Clewett, D., Luo, S., Hsu, E., Ainslie, G., Mather, M., and Monterosso, J. (2014). Increased Functional Coupling Between the Left Fronto-Parietal Network and Anterior Insula Predicts Steeper Delay Discounting in Smokers. Hum. Brain Mapp. 35, 3774–3787. doi: 10.1002/hbm.22436
Contreras-Rodriguez, O., Albein-Urios, N., Perales, J. C., Martinez-Gonzalez, J. M., Vilar-Lopez, R., Fernandez-Serrano, M. J., et al. (2015). Cocaine-specific neuroplasticity in the ventral striatum network is linked to delay discounting and drug relapse. Addiction 110, 1953–1962. doi: 10.1111/add.13076
Cox, S. R., Ritchie, S. J., Fawns-Ritchie, C., Tucker-Drob, E. M., and Deary, I. J. (2019). Structural brain imaging correlates of general intelligence in UK Biobank. Intelligence 76, 101376. doi: 10.1016/j.intell.2019.101376
Dennis, L. E., Kohno, M., McCready, H. D., Schwartz, D. L., Schwartz, B., Lahna, D., et al. (2020). Neural correlates of reward magnitude and delay during a probabilistic delay discounting task in alcohol use disorder. Psychopharmacology 237, 263–278. doi: 10.1007/s00213-019-05364-3
Dias, T. G. C., Wilson, V. B., Bathula, D. R., Iyer, S. P., Mills, K. L., Thurlow, B. L., et al. (2013). Reward circuit connectivity relates to delay discounting in children with attention-deficit/hyperactivity disorder. Eur. Neuropsychopharmacol. 23, 33–45. doi: 10.1016/j.euroneuro.2012.10.015
Dixon, M. R., Marley, J., and Jacobs, E. A. (2003). Delay discounting by pathological gamblers. J. Appl. Behav. Anal. 36, 449–458. doi: 10.1901/jaba.2003.36-449
Ernst, J., Boker, H., Hattenschwiler, J., Schupbach, D., Northoff, G., Seifritz, E., et al. (2014). The association of interoceptive awareness and alexithymia with neurotransmitter concentrations in insula and anterior cingulate. Soc. Cogn. Affect. Neurosci. 9, 857–863. doi: 10.1093/scan/nst058
Frost, R., and McNaughton, N. (2017). The neural basis of delay discounting: a review and preliminary model. Neurosci. Biobehav. Rev. 79, 48–65. doi: 10.1016/j.neubiorev.2017.04.022
Gasquoine, P. G. (2014). Contributions of the Insula to Cognition and Emotion. Neuropsychol. Rev. 24, 77–87. doi: 10.1007/s11065-014-9246-9
Grodin, E. N., Cortes, C. R., Spagnolo, P. A., and Momenan, R. (2017). Structural deficits in salience network regions are associated with increased impulsivity and compulsivity in alcohol dependence. Drug Alcohol Depend. 179, 100–108. doi: 10.1016/j.drugalcdep.2017.06.014
Guo, Y., and Feng, T. (2015). The mediating role of LPFC-vmPFC functional connectivity in the relation between regulatory mode and delay discounting. Behav. Brain Res. 292, 252–258. doi: 10.1016/j.bbr.2015.06.035
Haber, S. N., and Knutson, B. (2010). The Reward Circuit: Linking Primate Anatomy and Human Imaging. Neuropsychopharmacology 35, 4–26. doi: 10.1038/npp.2009.129
Ho, B.-C., Koeppel, J. A., and Barry, A. B. (2016). Cerebral white matter correlates of delay discounting in adolescents. Behav. Brain Res. 305, 108–114. doi: 10.1016/j.bbr.2016.03.004
Hoffman, W. F., Schwartz, D. L., Huckans, M. S., McFarland, B. H., Meiri, G., Stevens, A. A., et al. (2008). Cortical activation during delay discounting in abstinent methamphetamine dependent individuals. Psychopharmacology 201, 183–193. doi: 10.1007/s00213-008-1261-1
Holmes, C., Owens, M., Beach, S. R. H., McCormick, M., Hallowell, E., Clark, U. S., et al. (2020). Peer influence, Frontostriatal connectivity, and delay discounting in African American emerging adults. Brain Imag. Behav. 14, 155–163. doi: 10.1007/s11682-018-9977-y
Hu, C. P., Di, X., Eickhoff, S. B., Zhang, M. J., Peng, K. P., Guo, H., et al. (2016). Distinct and common aspects of physical and psychological self-representation in the brain: a meta-analysis of self-bias in facial and self-referential judgements. Neurosci. Biobehav. Rev. 61, 197–207. doi: 10.1016/j.neubiorev.2015.12.003
Jenkinson, M., Bannister, P., Brady, M., and Smith, S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825–841. doi: 10.1006/nimg.2002.1132
Jenkinson, M., and Smith, S. (2001). A global optimisation method for robust affine registration of brain images. Med. Image Anal. 5, 143–156. doi: 10.1016/s1361-8415(01)00036-6
Kirby, K. N., and Marakovic, N. N. (1995). Modeling myopic decisions: evidence for hyperbolic delay-discounting within subjects and amounts. Organ. Behav. Hum. Decision Proc. 64, 22–30. doi: 10.1006/obhd.1995.1086
Kirby, K. N., Petry, N. M., and Bickel, W. K. (1999). Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J. Exp. Psychol.Gen. 128, 78–87. doi: 10.1037/0096-3445.128.1.78
Kirby, K. N., Winston, G. C., and Santiesteban, M. (2005). Impatience and grades: delay-discount rates correlate negatively with college GPA. Learn. Indiv. Diff. 15, 213–222. doi: 10.1016/j.lindif.2005.01.003
Kollins, S. H. (2003). Delay discounting is associated with substance use in college students. Add. Behav. 28, 1167–1173. doi: 10.1016/s0306-4603(02)00220-4
Li, N., Ma, N., Liu, Y., He, X.-S., Sun, D.-L., Fu, X.-M., et al. (2013). Resting-State Functional Connectivity Predicts Impulsivity in Economic Decision-Making. J. Neurosci. 33, 4886–4895. doi: 10.1523/jneurosci.1342-12.2013
Lim, A. C., Cservenka, A., and Ray, L. A. (2017). Effects of Alcohol Dependence Severity on Neural Correlates of Delay Discounting. Alcohol Alcohol. 52, 506–515. doi: 10.1093/alcalc/agx015
Liu, T., Li, J., Zhao, Z., Zhong, Y., Zhang, Z., Xu, Q., et al. (2016). Betel quid dependence is associated with functional connectivity changes of the anterior cingulate cortex: a resting-state fMRI study. J. Transl. Med. 14. doi: 10.1186/s12967-016-0784-1
Lukinova, E., and Erlich, J. C. (2021). Quantifying the contribution of individual variation in timing to delay-discounting. Sci. Rep. 11:18354. doi: 10.1038/s41598-021-97496-w
Marco-Pallares, J., Mohammadi, B., Samii, A., and Muente, T. F. (2010). Brain activations reflect individual discount rates in intertemporal choice. Brain Res. 1320, 123–129. doi: 10.1016/j.brainres.2010.01.025
Marcoulides, G. A., and Hershberger, S. L. (1997). Multivariate Statistical Methods: A First Course. Hillsdale: Lawrence Erlbaum Associates, Inc.
McClure, S. M., Ericson, K. M., Laibson, D. I., Loewenstein, G., and Cohen, J. D. (2007). Time discounting for primary rewards. J. Neurosci. 27, 5796–5804. doi: 10.1523/jneurosci.4246-06.2007
Menon, V., and Uddin, L. Q. (2010). Saliency, switching, attention and control: a network model of insula function. Brain Struct. Fun. 214, 655–667. doi: 10.1007/s00429-010-0262-0
Miedl, S. F., Peters, J., and Buchel, C. (2012). Altered Neural Reward Representations in Pathological Gamblers Revealed by Delay and Probability Discounting. Arch. Gen. Psychiatr. 69, 177–186. doi: 10.1001/archgenpsychiatry.2011.1552
Miranda-Olivos, R., Steward, T., Martinez-Zalacain, I., Mestre-Bach, G., Juaneda-Segui, A., Jimenez-Murcia, S., et al. (2021). The neural correlates of delay discounting in obesity and binge eating disorder. J. Behav. Add. 10, 498–507. doi: 10.1556/2006.2021.00023
Monterosso, J. R., Ainslie, G., Xu, J., Cordova, X., Domier, C. P., and London, E. D. (2007). Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Hum. Brain Mapp. 28, 383–393. doi: 10.1002/hbm.20281
Nelson, B. D., Bjorkquist, O. A., Olsen, E. K., and Herbener, E. S. (2015). Schizophrenia symptom and functional correlates of anterior cingulate cortex activation to emotion stimuli: an fMRI investigation. Psychiatr. Res. Neuroimag. 234, 285–291. doi: 10.1016/j.pscychresns.2015.11.001
Nieuwenhuys, R. (2012). The insular cortex: a review. Prog. Brain Res. 195, 123–163. doi: 10.1016/b978-0-444-53860-4.00007-6
Noreika, V., Falter, C. M., and Rubia, K. (2013). Timing deficits in attention-deficit/hyperactivity disorder (ADHD): evidence from neurocognitive and neuroimaging studies. Neuropsychologia 51, 235–266. doi: 10.1016/j.neuropsychologia.2012.09.036
Northoff, G., Heinzel, A., Greck, M., Bennpohl, F., Dobrowolny, H., and Panksepp, J. (2006). Self-referential processing in our brain - A meta-analysis of imaging studies on the self. Neuroimage 31, 440–457. doi: 10.1016/j.neuroimage.2005.12.002
Ortiz, N., Parsons, A., Whelan, R., Brennan, K., Agan, M. L. F., O’Connell, R., et al. (2015). Decreased frontal, striatal and cerebellar activation in adults with ADHD during an adaptive delay discounting task. Acta Neurobiol. Exp. 75, 326–338.
Patros, C. H. G., Sweeney, K. L., Mahone, E. M., Mostofsky, S. H., and Rosch, K. S. (2018). Greater delay discounting among girls, but not boys, with ADHD correlates with cognitive control. Child Neuropsychol. 24, 1026–1046. doi: 10.1080/09297049.2017.1359525
Paulus, M. P., Rogalsky, C., Simmons, A., Feinstein, J. S., and Stein, M. B. (2003). Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. Neuroimage 19, 1439–1448. doi: 10.1016/s1053-8119(03)00251-9
Peters, J., and Buechel, C. (2011). The neural mechanisms of inter-temporal decision-making: understanding variability. Trends Cogn. Sci. 15, 227–239. doi: 10.1016/j.tics.2011.03.002
Raven, J., Raven, J., and Court, J. (1998). Manual for Raven’s Progressive Matrices and Vocabulary Scales. Oxford: Oxford Psychologists Press.
Rolls, E. T. (2016). Functions of the anterior insula in taste, autonomic, and related functions. Brain Cogn. 110, 4–19. doi: 10.1016/j.bandc.2015.07.002
Roquet, D., and Conti, F. (2021). Disentangling the Association between the Insula and the Autonomic Nervous System. J. Neurosci. 41, 3051–3053. doi: 10.1523/jneurosci.2225-20.2021
Rosch, K. S., Mostofsky, S. H., and Nebel, M. B. (2018). ADHD-related sex differences in fronto-subcortical intrinsic functional connectivity and associations with delay discounting. J. Neurodev. Disord. 10:34. doi: 10.1186/s11689-018-9254-9
Saville, B. K., Gisbert, A., Kopp, J., and Telesco, C. (2010). Internet addiction and delay discounting in college students. Psychol. Record 60, 273–286. doi: 10.1007/bf03395707
Schmaal, L., Goudriaan, A. E., van der Meer, J., van den Brink, W., and Veltman, D. J. (2012). The association between cingulate cortex glutamate concentration and delay discounting is mediated by resting state functional connectivity. Brain Behav. 2, 553–562. doi: 10.1002/brb3.74
Seeley, W. W., Menon, V., Schatzberg, A. F., Keller, J., Glover, G. H., Kenna, H., et al. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 27, 2349–2356. doi: 10.1523/jneurosci.5587-06.2007
Sellitto, M., Ciaramelli, E., Mattioli, F., and di Pellegrino, G. (2016). Reduced Sensitivity to Sooner Reward During Intertemporal Decision-Making Following Insula Damage in Humans. Front. Behav. Neurosci. 9:367. doi: 10.3389/fnbeh.2015.00367
Shamosh, N. A., DeYoung, C. G., Green, A. E., Reis, D. L., Johnson, M. R., Conway, A. R. A., et al. (2008). Individual Differences in Delay Discounting Relation to Intelligence, Working Memory, and Anterior Prefrontal Cortex. Psychol. Sci. 19, 904–911. doi: 10.1111/j.1467-9280.2008.02175.x
Shamosh, N. A., and Gray, J. R. (2008). Delay discounting and intelligence: a meta-analysis. Intelligence 36, 289–305. doi: 10.1016/j.intell.2007.09.004
Stanger, C., Elton, A., Ryan, S. R., James, G. A., Budney, A. J., and Kilts, C. D. (2013). Neuroeconomics and Adolescent Substance Abuse: individual Differences in Neural Networks and Delay Discounting. J. Am. Acad. Child Adole. Psychiatr. 52, 747–755. doi: 10.1016/j.jaac.2013.04.013
Takeuchi, H., Taki, Y., Hashizume, H., Sassa, Y., Nagase, T., Nouchi, R., et al. (2011). Cerebral Blood Flow during Rest Associates with General Intelligence and Creativity. PLoS One 6:e25532. doi: 10.1371/journal.pone.0025532
van Veen, V., Holroyd, C. B., Cohen, J. D., Stenger, V. A., and Carter, C. S. (2004). Errors without conflict: implications for performance monitoring theories of anterior cingulate cortex. Brain Cogn. 56, 267–276. doi: 10.1016/j.bandc.2004.06.007
Wang, S., Zhou, M., Chen, T., Yang, X., Chen, G., and Gong, Q. (2017). Delay discounting is associated with the fractional amplitude of low-frequency fluctuations and resting-state functional connectivity in late adolescence. Sci. Rep. 7:10276. doi: 10.1038/s41598-017-11109-z
Wittmann, M., Leland, D. S., and Paulus, M. P. (2007). Time and decision making: differential contribution of the posterior insular cortex and the striatum during a delay discounting task. Exp. Brain Res. 179, 643–653. doi: 10.1007/s00221-006-0822-y
Wittmann, M., Lovero, K. L., Lane, S. D., and Paulus, M. P. (2010). Now or later? Striatum and insula activation to immediate versus delayed rewards. J. Neurosci. Psychol. Econ. 3, 15–26. doi: 10.1037/a0017252
Wittmann, M., and Paulus, M. P. (2008). Decision making, impulsivity and time perception. Trends Cogn. Sci. 12, 7–12. doi: 10.1016/j.tics.2007.10.004
Xia, M., Wang, J., and He, Y. (2013). BrainNet Viewer: a Network Visualization Tool for Human Brain Connectomics. PLoS One 8:e68910. doi: 10.1371/journal.pone.0068910
Xin, Y., Xu, P., Aleman, A., Luo, Y., and Feng, T. (2020). Intrinsic prefrontal organization underlies associations between achievement motivation and delay discounting. Brain Struct. Fun. 225, 511–518. doi: 10.1007/s00429-019-01982-x
Yan, C.-G., Wang, X.-D., Zuo, X.-N., and Zang, Y.-F. (2016). DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics 14, 339–351. doi: 10.1007/s12021-016-9299-4
Yoon, H. J., Seo, E. H., Kim, J. J., and Choo, I. H. (2019). Neural Correlates of Self-referential Processing and Their Clinical Implications in Social Anxiety Disorder. Clin. Psychopharmacol. Neurosci. 17, 12–24. doi: 10.9758/cpn.2019.17.1.12
Zhang, R., Chen, Z., Xu, T., and Feng, T. (2021). The neural basis underlying the relation between the action identification level and delay discounting: the medial and orbital frontal cortex functional connectivity with the precuneus. Int. J. Psychophysiol. 159, 74–82. doi: 10.1016/j.ijpsycho.2020.11.014
Zhu, X., Cortes, C. R., Mathur, K., Tomasi, D., and Momenan, R. (2017). Model-free functional connectivity and impulsivity correlates of alcohol dependence: a resting-state study. Add. Biol. 22, 206–217. doi: 10.1111/adb.12272
Zou, Q.-H., Zhu, C.-Z., Yang, Y., Zuo, X.-N., Long, X.-Y., Cao, Q.-J., et al. (2008). An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: Fractional ALFF. J. Neurosci. Methods 172, 137–141. doi: 10.1016/j.jneumeth.2008.04.012
Keywords: delay discounting, fALFF, functional connectivity, the insula, neural network
Citation: Yang F, Li X and Hu P (2022) The Resting-State Neural Network of Delay Discounting. Front. Psychol. 13:828929. doi: 10.3389/fpsyg.2022.828929
Received: 04 December 2021; Accepted: 07 February 2022;
Published: 11 March 2022.
Edited by:
Nicola Canessa, University Institute of Higher Studies in Pavia, ItalyReviewed by:
Han Lv, Capital Medical University, ChinaJulian Chase Motzkin, University of California, San Francisco, United States
Copyright © 2022 Yang, Li and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xueting Li, bGkueHVldGluZ0BydWMuZWR1LmNu; Ping Hu, aHVwaW5nQHJ1Yy5lZHUuY24=
 Fan Yang
Fan Yang Xueting Li
Xueting Li Ping Hu
Ping Hu