- 1Department of Psychiatry, Amsterdam Neuroscience and Amsterdam Public Health, Amsterdam University Medical Center, University of Amsterdam, Amsterdam, Netherlands
- 2Department of Child and Adolescent Psychiatry, Amsterdam University Medical Center, Amsterdam, Netherlands
- 3Department of Epidemiology and Data Science, Amsterdam Public Health Research Institute, Amsterdam University Medical Center, University of Amsterdam, Amsterdam, Netherlands
- 4Department of Internal and Vascular Medicine, Amsterdam Cardiovascular Sciences, Amsterdam University Medical Center, University of Amsterdam, Amsterdam, Netherlands
- 5Department of Public Health, Amsterdam University Medical Center, University of Amsterdam, Amsterdam, Netherlands
- 6Department of Rheumatology and Clinical Immunology, Amsterdam Rheumatology and Immunology Center, Amsterdam University Medical Center, University of Amsterdam, Amsterdam, Netherlands
- 7Sinai Center, Amstelveen, Netherlands
- 8Department of Psychiatry, Amsterdam University Medical Center, Vrije University Amsterdam, Amsterdam, Netherlands
- 9Department of Epidemiology, Health Promotion and Care Innovation, Public Health Service Amsterdam, Amsterdam, Netherlands
- 10Section of Epidemiology, Department of Public Health, University of Copenhagen, Copenhagen, Denmark
- 11Department of Public and Occupational Health, Amsterdam University Medical Center, University of Amsterdam, Amsterdam, Netherlands
Background: Child maltreatment is a common negative experience and has potential long-lasting adverse consequences for mental and physical health, including increased risk for major depressive disorder (MDD) and metabolic syndrome. In addition, child maltreatment may increase the risk for comorbid physical health conditions to psychiatric conditions, with inflammation as an important mediator linking child maltreatment to poor adult health. However, it remains unresolved whether experiencing child maltreatment increases the risk for the development of comorbid metabolic syndrome to MDD. Therefore, we investigated whether child maltreatment increased the risk for comorbid metabolic syndrome to depressed mood. Subsequently, we examined whether C-reactive protein (CRP), as an inflammatory marker, mediated this association. In addition, we investigated whether effects differed between men and women.
Methods: Associations were examined within cross-sectional data from the multiethnic HELIUS study (N = 21,617). Adult residents of Amsterdam, Netherlands, self-reported on child maltreatment (distinct and total number of types experienced before the age of 16 years) as well as current depressed mood (PHQ-9 score ≥ 10), and underwent physical examination to assess metabolic syndrome. The CRP levels were assessed in N = 5,998 participants. Logistic and linear regressions were applied for binary and continuous outcomes, respectively. All analyses were adjusted for relevant demographic, socioeconomic, and lifestyle characteristics, including ethnicity.
Results: A higher number of maltreatment types as well as distinct types of emotional neglect, emotional abuse, and sexual abuse were significantly associated with a higher risk for current depressed mood. Child maltreatment was not significantly associated with the risk for metabolic syndrome in the whole cohort, nor within individuals with depressed mood. As child maltreatment was not significantly associated with the CRP levels, subsequent mediation analyses were not performed. No significant moderating effects by sex were observed.
Conclusion: In this multiethnic urban cohort, child maltreatment was associated with a higher risk for depressed mood. Contrary to our expectations, child maltreatment was not significantly associated with an increased risk for metabolic syndrome, neither in the whole cohort nor as a comorbid condition in individuals with depressed mood. As the data were cross-sectional and came from a non-clinical adult population, longitudinal perspectives in relation to various stages of the investigated conditions were needed with more comprehensive assessments of inflammatory markers.
Introduction
During childhood, biopsychological development occurs relatively fast, leading to vulnerability to lifelong adverse health effects of negative influences from within the environment (Hensch, 2005; Fumagalli et al., 2007). Child maltreatment, as reported by 34–62% of healthy adults during their childhood in Western countries, is such a common negative experience that may have potential long-lasting adverse consequences for mental and physical health (Felitti et al., 1998; Gilbert et al., 2015; Hughes et al., 2017; Merrick et al., 2018). Child maltreatment involves both direct (i.e., sexual, physical, emotional abuse, or neglect) and indirect (i.e., parental conflict and parental substance abuse) sources of stress (Felitti et al., 1998; Gilbert et al., 2015; Merrick et al., 2018; World Health Organization [WHO], 2017). Health may be either directly or indirectly influenced by maltreatment, due to abnormal functioning of biological systems leading to disturbances in emotional, cognitive, physical, and social functioning (Pechtel and Pizzagalli, 2011; Danese and McEwen, 2012) or by reinforcement of abnormal behavior (e.g., smoking, physical inactivity, and unhealthy diet), respectively (Anda et al., 1999; Ford et al., 2011; Midei and Matthews, 2011; Al Odhayani et al., 2013; Bellis et al., 2014; Danese and Tan, 2014; Hughes et al., 2017; Elkins et al., 2019).
Child maltreatment has been shown to increase the risk for major depressive disorder (MDD) and metabolic syndrome (Scott et al., 2011; Danese and Tan, 2014; Mandelli et al., 2015; Otte et al., 2016; Hughes et al., 2017). MDD, defined as experiencing a depressive episode lasting at least 2 weeks, is the most prevalent psychiatric disorder affecting 163,000 million people in 2017 worldwide (American Psychiatric Association, 2013; Otte et al., 2016; Gbd 2017 Disease and Injury Incidence and Prevalence Collaborators, 2018). Metabolic syndrome is defined as the presence of a specific set of components that constitute risk factors for cardiovascular disease and type 2 diabetes, including increased abdominal obesity, triglyceride levels, blood pressure, fasting glucose levels, and low high-density lipoprotein (HDL) cholesterol. Metabolic syndrome forms a leading public health concern as it affects approximately one-quarter of the population worldwide in 2018 (Alberti et al., 2005; Gheshlagh et al., 2016; Saklayen, 2018). According to the World Health Organization (WHO), the number of people affected by MDD and metabolic syndrome will increase significantly in the next decades, resulting in an expected higher contribution to overall morbidity and mortality (World Health Organization; Vaccarino et al., 2008; Dzherieva et al., 2011). According to a recent meta-analysis, the prevalence of the co-occurrence of MDD and metabolic syndrome is 30.5% (Vancampfort et al., 2014). Individuals with MDD are at 1.5 times higher odds ratio of developing metabolic syndrome than individuals without MDD (Moradi et al., 2021). Metabolic syndrome thus is a common comorbid condition of MDD (Muhtz et al., 2009; Foley et al., 2010; Pan et al., 2012; Penninx et al., 2013; Whooley and Wong, 2013; Gheshlagh et al., 2016; Hiles et al., 2016; Moradi et al., 2021; Vancampfort et al., 2014). This comorbidity is associated with higher societal costs and a further increase of the burden of disease compared to the isolated occurrence of both disorders (Alberti et al., 2005; Penninx et al., 2013).
Child maltreatment has been found to increase the risk for comorbid physical health conditions to psychiatric conditions, including MDD (Widom et al., 2007; Scott et al., 2011). Three previous studies investigated the association between child maltreatment and comorbid (components of) metabolic syndrome to MDD (McIntyre et al., 2012; Wingenfeld et al., 2017; Hosang et al., 2018). These previous studies showed inconsistent findings regarding the association between child maltreatment and risk for comorbid metabolic syndrome to MDD (McIntyre et al., 2012; Wingenfeld et al., 2017; Hosang et al., 2018). It thus remains unresolved whether experiencing child maltreatment increases the risk for the development of comorbid metabolic syndrome to MDD.
Inflammation may be an important mediator linking child maltreatment to poor adult mental and physical health (Danese et al., 2007, 2009; Widom et al., 2007; Capuron et al., 2008; Berens et al., 2017). Several studies have found that child maltreatment significantly impacts inflammatory markers, including higher C-reactive protein (CRP) (Baumeister et al., 2016). CRP is an acute-phase protein synthesized by the liver. Increased circulating CRP is an indicator of ongoing inflammation and is considered a risk factor for developing metabolic syndrome (Sluzewska et al., 1996; Berk et al., 1997; Hornig et al., 1998; Festa et al., 2000; Han et al., 2002; Coelho et al., 2014). Rethorst et al. (2014) previously demonstrated a positive association between the MDD and CRP levels. In addition, previous studies have shown that CRP, as an inflammatory marker, impacts the association between MDD and metabolic syndrome (Rethorst et al., 2014; Chirinos et al., 2017; Lamers et al., 2020). For instance, a former study has found a positive association between CRP and the presence of comorbid metabolic syndrome within individuals with depressed mood (Rethorst et al., 2014). As a result of the previous research, CRP as an inflammatory marker could be a potential mediator in the assumed association between child maltreatment and the development of comorbid metabolic syndrome to MDD.
Against this background, our primary focus was to investigate whether child maltreatment increased the risk of comorbid occurrence of metabolic syndrome and its individual components to the current depressed mood as a self-reported proxy for MDD. Subsequently, the role of CRP as a mediator for these associations was examined. In all analyses, we investigated potential moderating effects by sex, as an increasing amount of literature shows that associations between child maltreatment and adverse health outcomes can differ between men and women (Garad et al., 2017; Ehrlich et al., 2021). This study used data from the Healthy Life in an Urban Setting (HELIUS) study which is a multiethnic urban cohort including several ethnic groups living in Amsterdam, Netherlands. The design of the HELIUS study forms a representative cross-section of this Western European capital’s population in terms of demographic factors (e.g., socioeconomic status, educational level, and ethnicity) (Stronks et al., 2013; Snijder et al., 2017). With this study, we extend on previous findings within the same population that individual child maltreatment types were associated with a higher risk for current depressed mood, without significant moderating effects of sex (Sunley et al., 2020).
The association between the number of child maltreatment types experienced and the risk for current depressed mood was not yet investigated in this population, nor were associations between child maltreatment, metabolic syndrome, and CRP.
Materials and Methods
Participants and Procedure
This study describes baseline data from the HELIUS study which is a multiethnic cohort study performed in Amsterdam, Netherlands. It aims to investigate the impact of ethnicity on common major physical and mental health problems. Between 2011 and 2015, baseline data were collected among N = 23,942 Amsterdam residents of South-Asian Surinamese, African Surinamese, Turkish, Moroccan, Ghanaian, and Dutch origin. Full details on the study and recruitment method are described elsewhere (Stronks et al., 2013; Snijder et al., 2017). In brief, individuals between the ages of 18 and 70 years were randomly sampled, stratified by ethnicity, from the municipality register of Amsterdam, and invited to participate. We were able to contact 55% of those invited, either by response card or after a home visit by an ethnically matched interviewer. Of those, 50% agreed to participate (participation rate). Therefore, the overall response rate was 28% with some variations across ethnic groups (33% among Dutch, 31% among Surinamese, 22% among Turks, 21% among Moroccans, and 35% among Ghanaians).
Previous non-response analyses indicated that the HELIUS cohort is representative of the investigated ethnic groups in Amsterdam, as only minor differences in socioeconomic characteristics were observed between participants, non-participants, and non-invited eligible individuals. Written informed consent was obtained prior to any study procedures. Participants completed an extensive questionnaire and a physical examination that included the collection of biological samples. The study was approved by the Institutional Review Board of the Amsterdam University Medical Centers located at Academic Medical Center.
For this study, baseline questionnaire and physical examination data were available for n = 22,165 participants. We excluded participants of Javanese Surinamese origin (n = 233), other/unknown Surinamese origin (n = 267), and participants with unknown ethnic background (n = 48) due to their comparatively small sample sizes. This resulted in a total sample size of n = 21,617. The CRP levels were determined in random subsamples of n = 1,000 participants from each ethnic group (total n = 6,000), for whom complete data on cardiovascular measurements and stored blood samples were available. The CRP levels could not be assessed in two participants due to very less material, resulting in n = 5,998 participants.
Measures
Child Maltreatment
Participants self-reported on experienced child maltreatment using a short self-report questionnaire derived from the NEMESIS Trauma questionnaire (De Graaf et al., 2010). It contains 4 items, each reflecting a specific type of maltreatment experienced before the age of 16 years, namely, emotional neglect (ignored or unsupported), physical abuse (kicked, hit, bitten, or hurt), emotional abuse (yelled at, insulted, or threatened), and sexual abuse (any unwanted sexual experience). Items were scored on a Likert scale (never-once-sometimes-often-would rather not say). The items inquiring about emotional neglect, physical abuse, and emotional abuse were considered endorsed when experienced sometimes or often. The item inquiring about sexual abuse was considered endorsed when experienced at least once (De Graaf et al., 2010). Subsequently, the total number of endorsed maltreatment types was calculated (range: 0–4). Missing data were imputed (refer to below details on the applied imputation strategy).
Depressed Mood
Depressed mood was determined using the self-report questionnaire Patient Health Questionnaire-9 (PHQ-9), which inquiries about the presence of depressive symptoms over the past 2 weeks (Kroenke et al., 2010). Its cross-cultural validity across the ethnic groups in the HELIUS study was previously demonstrated (Galenkamp et al., 2017). The questionnaire consists of nine items, with a Likert response scale ranging from never (0) to nearly every day (3). A maximum of one missing item was allowed, in which case the mean score on the other eight items was used to replace it. The cutoff score ≥ 10 indicated a depressed mood (Kroenke et al., 2001). Both the sensitivity and specificity for current MDD at this cutoff score were previously established to be 0.88 (Kroenke et al., 2001).
Metabolic Syndrome
Metabolic syndrome was determined using the harmonized definition of the International Diabetes Federation (Alberti et al., 2009), which considers metabolic syndrome to be present if a minimum of three of five criteria are met: (a) elevated fasting glucose (≥ 5.6 mM, or current use of glucose-lowering medication); (b) elevated blood pressure (systolic ≥ 130 and/or diastolic ≥ 85 mm Hg, or current use of blood pressure-lowering medication); (c) reduced HDL cholesterol (< 1.0 mM for men, < 1.3 mM for women, or current use of lipid-lowering medication); (d) elevated triglycerides (≥ 1.7 mM, or current use of lipid-lowering medication); and (e) elevated waist circumference (ethnic-specific cutoff values; for all women ≥ 80 cm, South-Asian men ≥ 90 cm, and other men ≥ 94 cm).
To assess these criteria, blood samples were collected during the physical examination after overnight fasting. Glucose was determined using spectrophotometry with hexokinase as the primary enzyme, and triglycerides and HDL-C were determined using colorimetric spectrophotometry (Roche Diagnostics, Tokyo, Japan) from heparin plasma. Blood pressure was measured in a seated position using a semiautomatic sphygmomanometer (Microlife WatchBP Home; Microlife AG, Widnau, Switzerland) using appropriate cuff sizes after being seated for at least 5 min. The mean of duplicate measurements was used. Waist circumference was measured during the physical examination, also determined as the mean of duplicate measurements. If the difference between the two measurements was greater than 1.0 cm, a third measurement was obtained and the mean of the two measurements that were closest together was calculated.
C-Reactive Protein
Blood samples for CRP determination were collected during the physical examination after overnight fasting. Highly sensitive CRP was determined from heparin plasma using particle-enhanced immunoturbidimetric assay (Cobas 702c analyzer; Roche Diagnostics, Mannheim, Germany).
Covariates
Socioeconomic and demographic covariates were age, sex, ethnicity, and educational level. Ethnicity was defined according to the countries of birth of the participant and his/her parents. Participants were considered to be of Dutch origin if the person and both parents were born in Netherlands. Participants were considered to be of non-Dutch origin if the person was either born abroad with at least one parent born abroad (first generation) or was born in Netherlands with both his/her parents born abroad (second generation). Surinamese subgroups (i.e., African and South-Asian) were classified according to self-reported ethnic origin. Dummy variables were created contrasting each ethnic minority group (i.e., South-Asian Surinamese, African Surinamese, Ghanaian, Turkish, and Moroccan) to the Dutch group.
Educational level was used as an indicator of socioeconomic status and was defined as the highest qualification obtained, either in Netherlands or in the country of origin. For educational level, we created dummy variables for lower vocational or general secondary education; intermediate vocational or higher secondary education; and higher vocational education or university, contrasting each to no completed or elementary schooling only as the reference group.
The following information regarding health-behavioral and chronic stress characteristics was also included as they represent potential mediating pathways in the associations under investigation, and we aimed to investigate additional effects of child maltreatment over and above these previously described mediating pathways: smoking (in estimated pack-years), alcohol use in the past 12 months (yes/no), and negative life events experienced in the past 12 months (yes/no), all determined by the self-report questionnaire. In addition, the current use of any of the following psychopharmacological medication was included as covariate: antipsychotics (ATC code N05A), anxiolytics (ATC codes N05BA and N03AE), hypnotics (ATC coded N05C and R06AD02), and antidepressants (ATC coded N06AB, N06AA, N03AF, N03AG, N05AN, and N03AX or without ATC code).
Statistical Analyses
All analyses were performed using SPSS version 26.0. The total number of endorsed types of maltreatment initially could not be calculated for 8.8% of participants because of one or more missing child maltreatment items (either left at least one of the items blank or answered as “would rather not say,” refer to Supplementary Table 1). Missingness was significantly correlated with all dependent and covariate variables, except smoking. To avoid biased estimations of the investigated associations concerning child maltreatment, missing data for the 4 child maltreatment items were imputed using Markov Chain Monte Carlo imputation with fully conditional specification using predictive mean matching. We included auxiliary variables to account for the fact that missingness was not at random (main effects and two-way interactions among categorical predictors). All variables included in our final analyses were included as auxiliary variables (Alberti et al., 2009). In total, 10 imputations were needed to obtain adequate imputation efficiency. All findings reported concern the pooled results from these 10 imputed datasets (refer to Supplementary Table 1 for a comparison of the child maltreatment prevalence in the pooled imputed vs. non-imputed data). PHQ-9 scores to determine the current depressed mood were missing for n = 211 (0.98%), and information on metabolic syndrome diagnosis was missing for n = 120 (0.56%); these data were pair-wise deleted from the analyses.
Stepwise logistic regression models were applied to assess the association between the number of experienced maltreatment types and the presence of current depressed mood and metabolic syndrome (diagnosis and its 5 individual components) in the full sample and to assess the association between the number of maltreatment types and the presence of co-morbid metabolic syndrome (diagnosis and its 5 individual components) in participants with current depressed mood only. To aid in the interpretation of observed effects, the number of child maltreatment types was included as an ordinal categorical variable, using indicator contrasts with 0 types experienced as the reference group. These analyses were subsequently repeated including the effects of the four distinct child maltreatment types within one analysis, instead of the total number of types experienced.
For each outcome, the first step included the main effect of the number of experienced maltreatment types, sex, and interaction terms between the number of maltreatment types and sex. The next step included all covariates regarding demographic, socioeconomic, health-behavioral, and chronic stress characteristics and current medication use as described earlier. We refer to these models as the simple and full models, respectively. In the latter model, we assess the associations between child maltreatment and the outcomes of interest over and above these previously described potential confounders and mediators. If the interaction effects between child maltreatment and sex were non-significant, these effects were removed from the respective step of the model and the analysis was repeated including only the main effects of child maltreatment and sex.
The Bonferroni corrections were applied to correct for K = 26 regressions performed, k = 14 in the full sample (1: depressed mood, 2: metabolic syndrome diagnosis, and 3–7: individual metabolic syndrome components), k = 12 in participants with current depressed mood (1: metabolic syndrome diagnosis and 2–6: individual metabolic syndrome components), resulting in α < 0.002.
Subsequently, we investigated potential mediation by CRP. As CRP was not normally distributed, a log10(+1) transformation was applied. We excluded N = 27 outliers (0.005%) with standardized log-transformed values > ± 3.29. We first performed a linear regression analysis on the association between the number of maltreatment types endorsed and CRP, followed by a regression including the effects of the four distinct child maltreatment types. For these analyses, we applied the same stepwise approach as described earlier, with an applied Bonferroni correction for K = 2 analyses, resulting in α < 0.025. As the association between child maltreatment and CRP proved non-significant, no further analyses regarding this potential mediation effect were performed.
Results
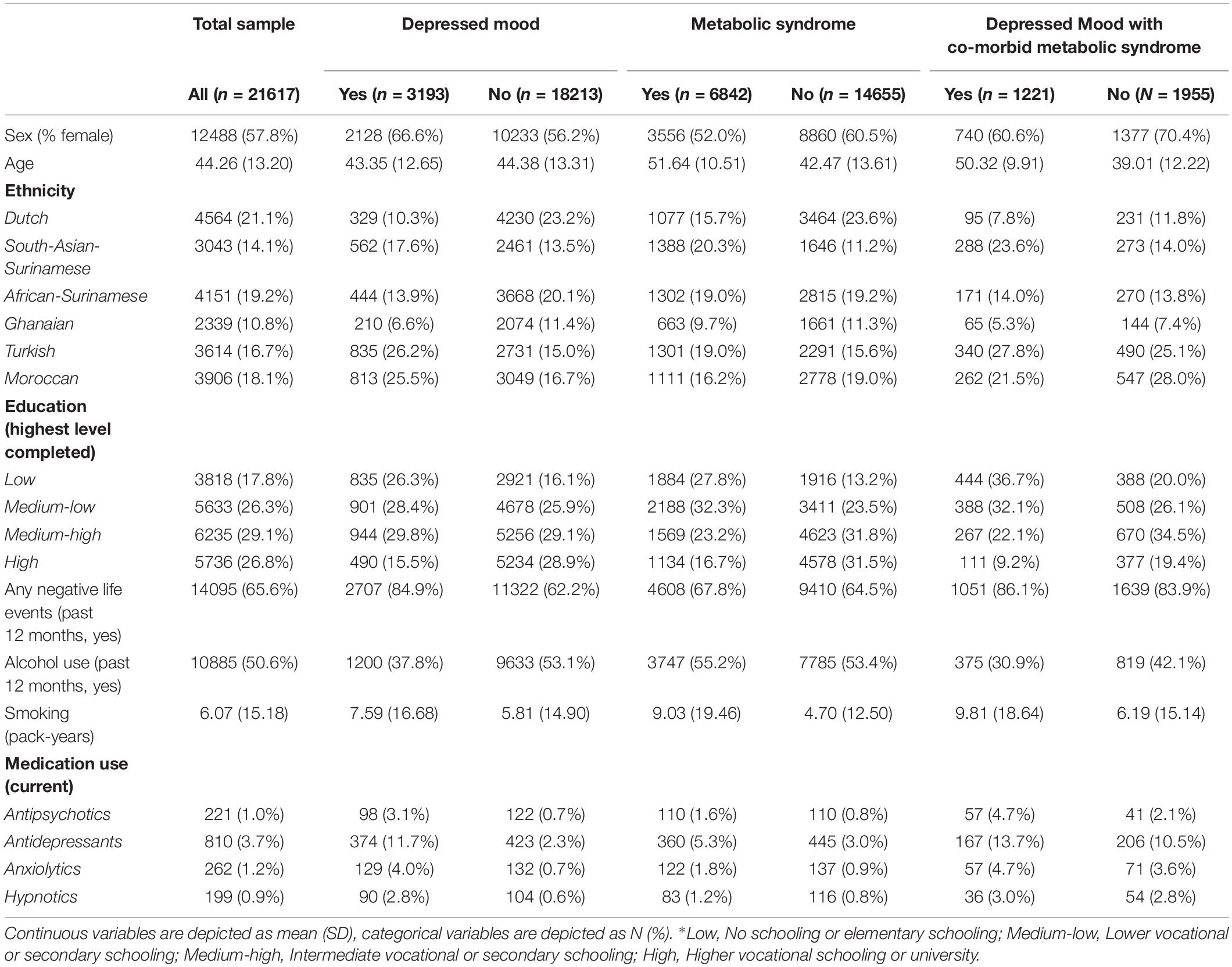
Sample characteristics of the full sample are shown in Tables 1, 2. On average, participants were 44.3 (SD = 13.2) years old. Women constituted 57.8% (n = 12,488) of the sample. Within the total sample, the prevalence of current depressed mood was 14.8% (n = 3,193). In addition, 31.8% (n = 6,482) of the participants within the total sample met the diagnostic criteria for metabolic syndrome. Of the n = 3,193 participants with current depressed mood, 38.4% (n = 1,221) of the participants also met the criteria for comorbid metabolic syndrome. The average number of experienced maltreatment types was 0.60 (SD = 1.06): 31.3% (n = 6,174) of participants reported to have experienced at least one child maltreatment type.
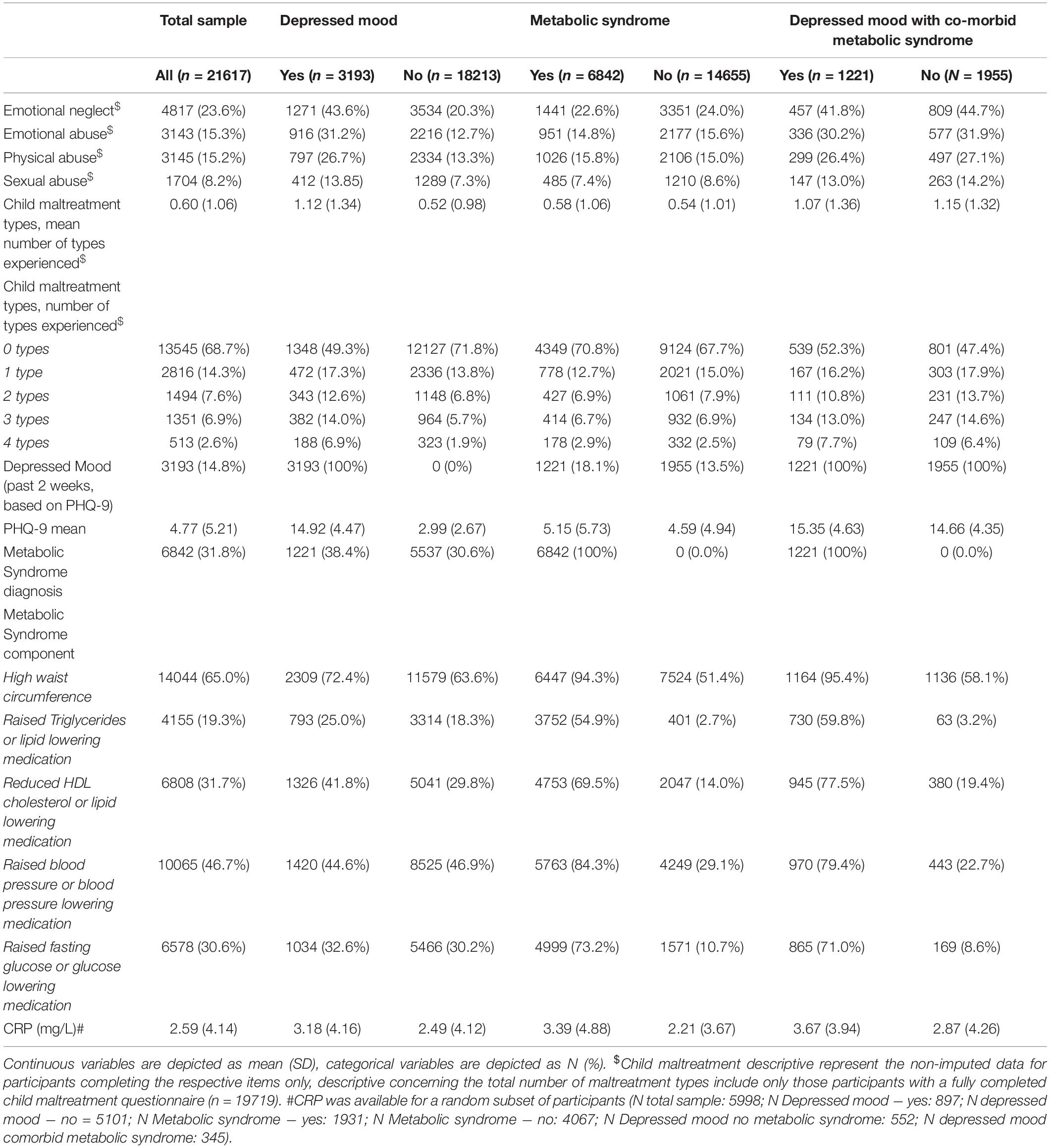
Table 2. Descriptive information regarding child maltreatment, depressed mood, metabolic syndrome, and C-reactive protein (CRP).
Association Between Child Maltreatment and Depressed Mood
Women were at increased risk for depressed mood, but sex did not significantly moderate the association between the number of child maltreatment types and depressed mood (Supplementary Table 2). Therefore, the interaction effects were removed from the models. The number of experienced child maltreatment types was significantly associated with the risk for current depressed mood, both in the simple and full models (Table 3). Similarly, for the four distinct child maltreatment types, sex did not significantly moderate the association with depressed mood (all p-Values > 0.002). The experience of emotional neglect [simple: OR: 1.966 (95% CI: 1.763–2.192) p < 0.001; full: OR: 1.929 (95% CI: 1.707–2.180) p < 0.001] and emotional abuse [simple: OR: 1.621 (95% CI: 1.429–1.838) p < 0.001; full: OR: 1.599, 95% CI: 1.400–1.826, p < 0.001] was significantly associated with depressed mood in both the simple and full models. The effect of sexual abuse was only significant within the full model [simple: OR: 1.211 (95% CI: 1.069–1.371) p = 0.003; full: OR: 1.434, (95% CI 1.246–1.650), p < 0.001], while the effect of physical abuse was non-significant in both the simple and full models [simple: OR: 1.192 (95% CI: 1.051–1.354) p = 0.007; full: OR: 1.149 (95% CI: 1.007–1.312) p = 0.039].
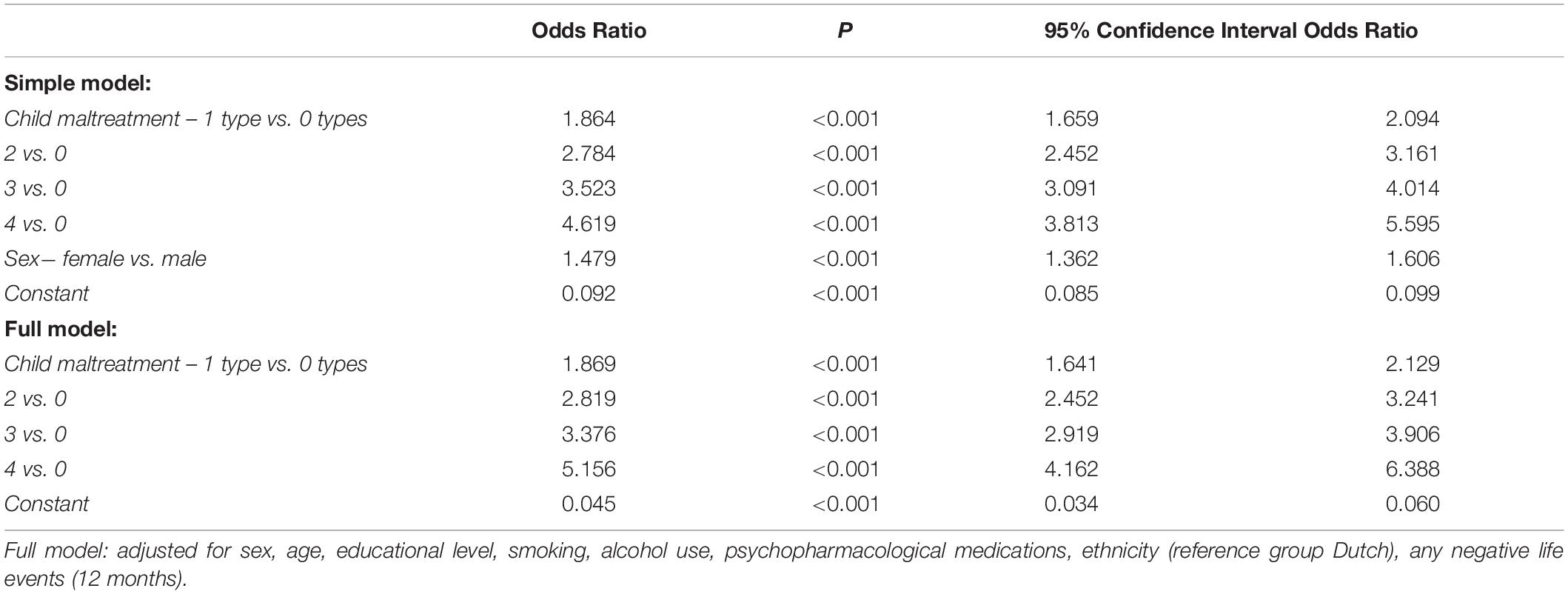
Table 3. Results of the logistic regression analyses assessing associations between the number of experienced child maltreatment types and the presence of current depressed mood in N = 20,740 participants.
Association Between Child Maltreatment and Metabolic Syndrome
Men were at increased risk for metabolic syndrome, but sex did not significantly moderate the association between child maltreatment and metabolic syndrome (Supplementary Table 3). The number of experienced maltreatment types was not significantly associated with the risk for metabolic syndrome diagnosis, both in the simple and full models (Table 4).
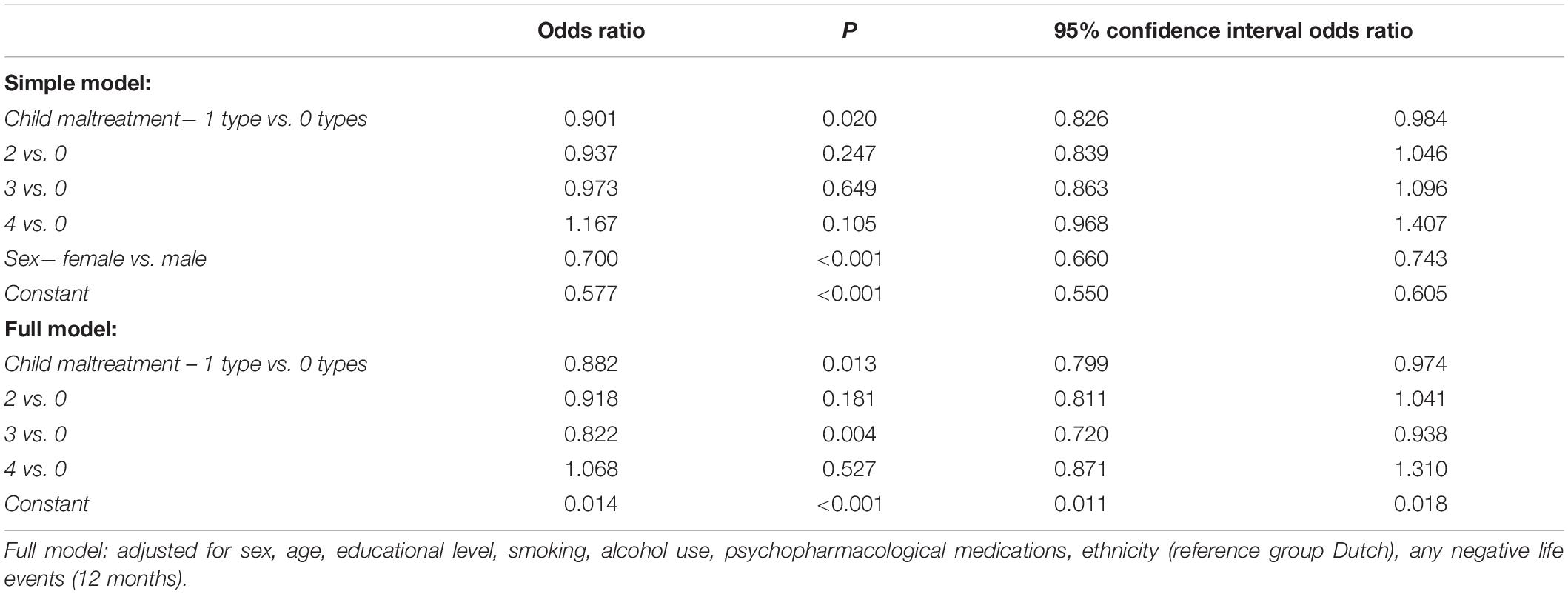
Table 4. Results of the logistic regression analysis assessing the association between the number of experienced child maltreatment types and metabolic syndrome diagnosis in N = 20,714 participants.
Similarly, for the four distinct child maltreatment types, sex did not significantly moderate the association with metabolic syndrome, nor were distinct maltreatment types significantly associated with the risk for metabolic syndrome diagnosis, both in the simple and full models (all p-Values > 0.002).
The number of experienced maltreatment types (either as main effect or interaction effect with sex) was also not significantly associated with the risk for the presence of the individual components of the metabolic syndrome, except for a significantly decreased risk for the presence of elevated blood pressure upon reporting child maltreatment (refer to Supplementary Tables 4A−E).
Regarding the distinct maltreatment types, the only effect that remained significant within the full model was a significantly decreased risk for the presence of elevated blood pressure upon reporting emotional neglect specifically [simple model: OR: 0.814 (95% CI: 0.747–0.887) p < 0.001; full model: OR: 0.807 (95% CI: 0.732–0.889), p < 0.001].
The effect of physical abuse was also significant in the simple model, but no longer within the full model [simple: OR: 1.275 (95% CI: 1.160–1.402) p < 0.001; full: OR: 1.051 (95% CI: 0.994–1.171) p = 0.363]. All other effects were non-significant, including the moderation effects by sex (all p-Values > 0.002).
Association Between Child Maltreatment and Comorbid Metabolic Syndrome to Depressed Mood
Men were at increased risk for comorbid metabolic syndrome, but sex did not significantly moderate the impact of the number of child maltreatment types on metabolic syndrome (Supplementary Table 5). The number of experienced maltreatment types was not significantly associated with the risk for comorbid metabolic syndrome in participants with current depressed mood, both in the simple and full models (Table 5). The number of experienced maltreatment types was also not significantly associated with the individual components of the metabolic syndrome in participants with current depressed mood, either as main effects or interaction effects with sex (refer to Supplementary Tables 6A−E).
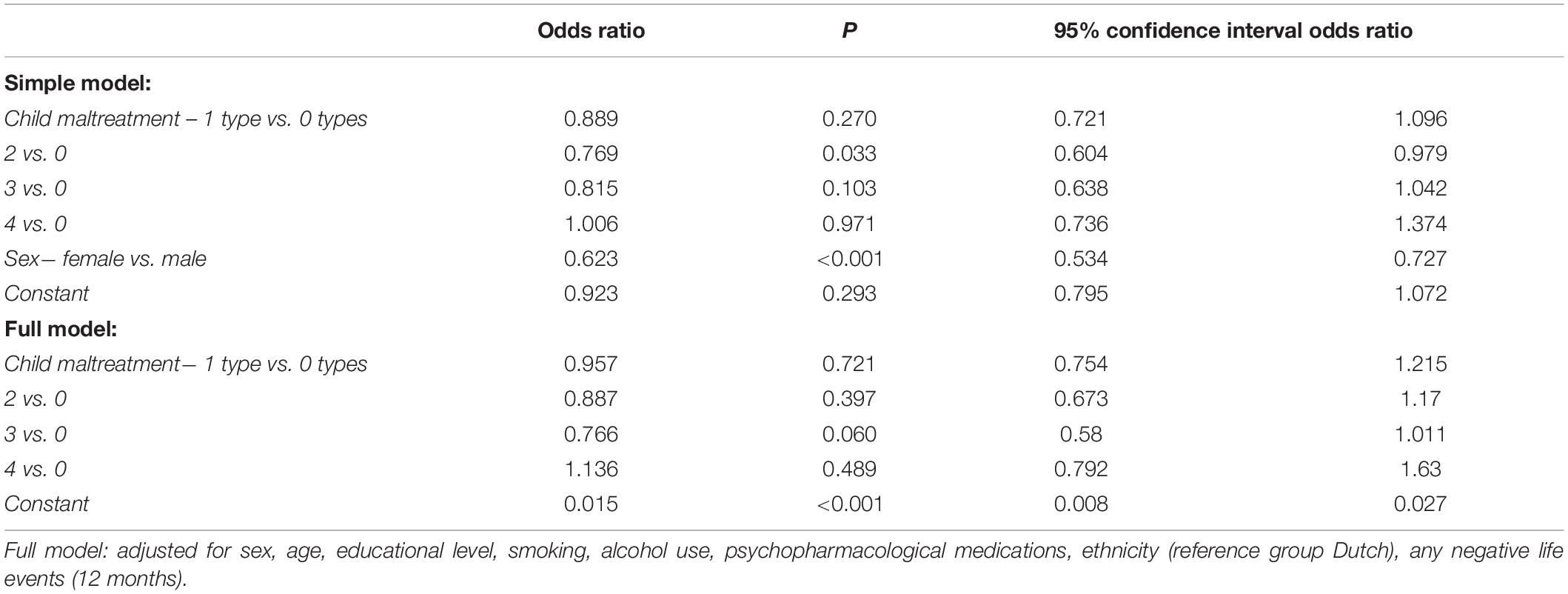
Table 5. Results of the logistic regression analysis assessing the association between the number of experienced child maltreatment types and co-morbid metabolic syndrome diagnosis to current depressed mood in N = 3,061 participants.
Similarly, non-significant results regarding the moderation by sex and main effects on comorbid metabolic syndrome were observed for the four distinct child maltreatment types (all p-Values > 0.002).
Association Between Child Maltreatment and C-Reactive Protein
Women had significantly higher CRP levels, but sex did not significantly moderate the associations between child maltreatment and CRP (Supplementary Table 7). Therefore, the interaction effects were removed from the models. The number of experienced maltreatment types was not significantly associated with circulating CRP levels in the simple and full models (Table 6). There were also no significant associations between the four distinct maltreatment types and CRP, nor significant moderation effects by sex (all p-Values > 0.002) Therefore, mediation analyses for the associations between maltreatment, depressed mood, and (co-morbid) metabolic syndrome were not performed.
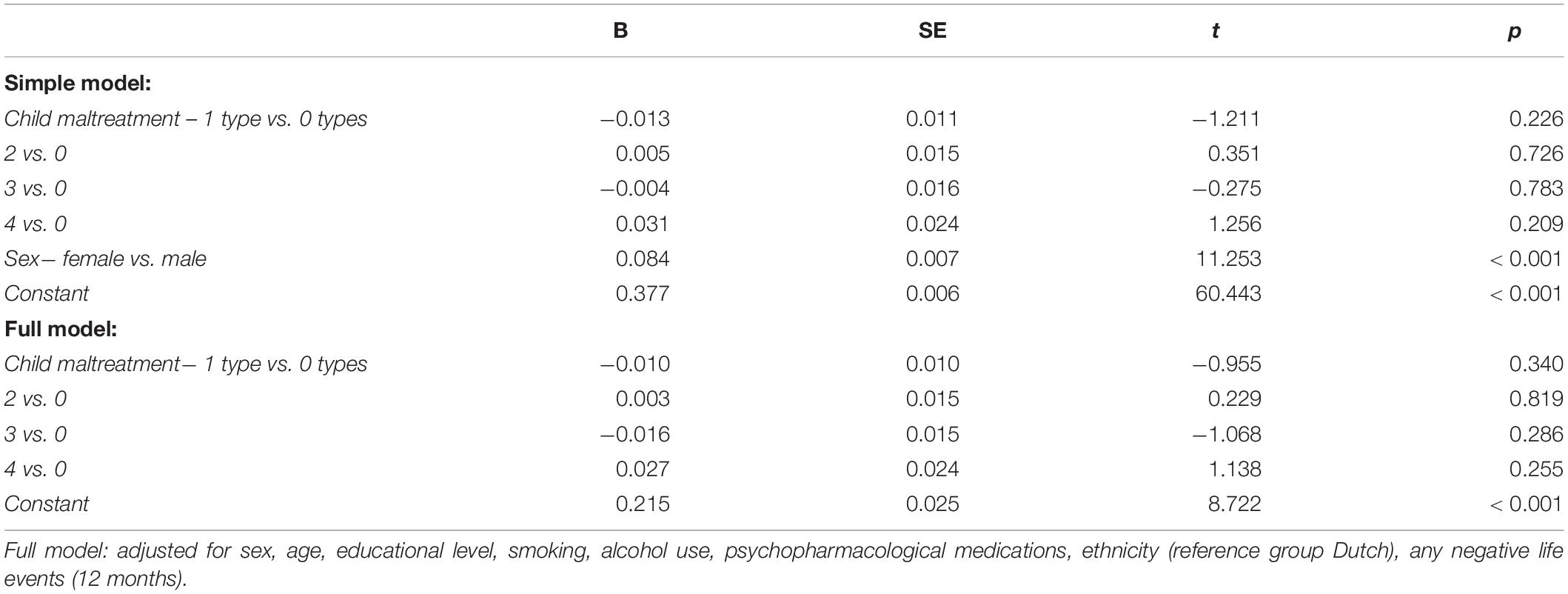
Table 6. Results of the linear regression analysis assessing the association between the number of experienced child maltreatment types and circulating CRP in N = 5,879 participants.
Discussion
In this study, we investigated whether child maltreatment increases the risk for the presence of co-morbid metabolic syndrome to the current depressed mood among a representative multiethnic urban cohort from Amsterdam, Netherlands. Extending previous research within the cohort in which we showed that individual types of child maltreatment were associated with a higher risk for current depressed mood (Sunley et al., 2020), we observed that a higher number of experienced child maltreatment types were positively associated with a higher risk for current depressed mood. Contrary to our expectations, child maltreatment was not significantly associated with increased risk for metabolic syndrome, neither in the whole sample nor as a comorbid condition to depressed mood. Subsequently, we aimed to investigate whether CRP as an inflammatory marker mediated these associations. However, this mediation analysis was not performed since the association between child maltreatment and CRP was not significant.
We did not observe significant associations between child maltreatment and increased risk for metabolic syndrome or its separate components within the whole sample, in either men or women. Our findings regarding the absent association between the number of child maltreatment types and metabolic syndrome diagnosis are in line with findings from Li et al. (2019) who neither observed an association between the number of experienced child maltreatment types, as reported both in childhood and adulthood, nor a risk for metabolic syndrome at mid-adulthood in women and men from a birth cohort that included both British-born residents and immigrants. Yet, Li et al. (2019) observed specific associations between distinct types of maltreatment and increased risk for specific components of the metabolic syndrome, with the largest effects observed for physical abuse and childhood neglect (Li et al., 2019), while in our study the distinct child maltreatment types were not associated with increased risk for the metabolic syndrome components. We did, however, unexpectedly observe negative associations between distinct maltreatment types as well as the total number of maltreatment types and the presence of elevated blood pressure. The effects of emotional abuse and having experienced once vs. no types of child maltreatment remained significant in the full model including demographic, socioeconomic, health-behavioral, and chronic stress characteristics and current medication use, and warrant further investigation.
In addition, Midei et al. (2013) also did not observe an association between exposure to physical, sexual, and emotional abuse and risk for metabolic syndrome in premenopausal or early perimenopausal women in midlife. Yet, they did observe that physical abuse specifically increased the risk for incident metabolic syndrome in the following 7 years. This effect was specifically related to two components of metabolic syndrome, namely high waist circumference and high fasting glucose (Midei et al., 2013). Contrastingly, Lee et al. (2014) observed that child maltreatment was associated with increased risk for the presence of a higher number of metabolic syndrome components as well as metabolic syndrome diagnosis in both women and men in mid-adulthood within a US study. Moreover, in this study, emotional and physical abuse increased the risk of developing metabolic syndrome across women and men, whereas sexual abuse only increased the risk for metabolic syndrome in women (Lee et al., 2014). Together these studies suggest that there is no general association between childhood maltreatment and metabolic syndrome, rather associations might differ based on interactions between sex, maltreatment type, participant age, and type of cohort. Furthermore, all these studies including this study were cross-sectional, except for Midei and Matthews (2011) who investigated both cross-sectional and longitudinal data. This hampers the interpretation of the nature of the observed associations. It would, therefore, be of interest to investigate longitudinal associations between the various distinct child maltreatment types and the prospective incidence of metabolic syndrome in the planned follow-up assessments within our cohort, especially since the mean age of our cohort is 44.3 years and the associations between child maltreatment and adverse outcome may strengthen with age (Midei et al., 2013).
Within the participants with current depressed mood, childhood maltreatment was not significantly associated with the risk for the presence of co-morbid metabolic syndrome, nor with its separate components. Again, these effects did not differ between men and women. In concordance with this study, the study by Wingenfeld et al. (2017), Hosang et al. (2018) also did not find an association between self-reported maltreatment and medical comorbidities related to metabolic syndrome (e.g., diabetes type 1 and 2, heart problems, and hypertension) in people with recurrent and current unipolar depression, respectively. However, a cross-sectional study by McIntyre et al. (2012) reported that a history of any self-reported childhood trauma was associated with increased risk for one component of the metabolic syndrome in people with MDD, i.e., lower HDL levels. In our study, we used self-reported current depressed mood in the past 2 weeks as a proxy for probable current MDD, whereas McIntyre et al. (2012), Wingenfeld et al. (2017), Hosang et al. (2018) included a clinical MDD diagnosis. Although it is not completely substitutable for a diagnostic interview, the PHQ-9 has been repeatedly found to be a valid diagnostic screener to measure current depressed mood and has good sensitivity and specificity for the detection of current MDD (Kroenke et al., 2001). Nonetheless, potentially our use of a more lenient definition of depressed mood might explain the contrasting findings between studies, and this should be further investigated in the future.
In support of our approach to investigating comorbid metabolic syndrome to depressed mood, a meta-analysis by Blaine (2008) showed that people with depression were more likely to develop obesity over time than people without depression. Conversely, in a 5-year-longitudinal study, Roberts et al. (2003) showed that the presence of obesity at baseline predicted the subsequent development of a major depressive episode among middle-aged men and women. A recent meta-analysis extends this evidence, as they observed that three surrogate measures of insulin resistance at baseline and development of prediabetes within the first 2 years were associated with a greater risk of incident MDD among healthy adults with no history of MDD or anxiety in a 9-year follow-up (Watson et al., 2021). These findings emphasize the need to also investigate the association between child maltreatment and comorbid depressed mood to metabolic syndrome, preferably in a design that allows for drawing temporal causal inferences. Again, longitudinal studies could help to determine the direction of the effect in the relationship between depression and metabolic syndrome and potential effects of child maltreatment.
We also aimed to investigate whether circulating CRP levels mediated the association between child maltreatment and comorbid metabolic syndrome in participants with current depressed mood. The actual mediation analysis was not performed since the main association between child maltreatment and CRP levels was not significant. Our finding of the absence of an association between childhood maltreatment and CRP is in line with a recent systematic review (Kerr et al., 2021) that showed that previous retrospective studies with retrospective reporting on child maltreatment in adulthood including non-clinical samples have also found no association between maltreatment and CRP. Yet, in contrast, three prospective studies using objective assessment of maltreatment already in childhood, including non-clinical samples, all observed that maltreatment was associated with elevated CRP levels (Danese et al., 2007; Nikulina and Widom, 2014; Osborn and Widom, 2020).
Baldwin et al. (2019) previously investigated the validity of self-report vs. objective measurements of maltreatment. They emphasize that retrospective self-report measures should be used with caution since it may not accurately reflect the experiences of maltreatment due to memory biases which can result in overreporting or underreporting of actual experiences (Baldwin et al., 2019). This might be especially relevant for individuals with depressed mood or MDD, as this might influence the recall and interpretation of previous negative events such as child maltreatment (negative cognitive bias) (Łosiak et al., 2019). Therefore, future longitudinal studies should ideally use a combination of retrospective self-report and prospective objective measures to capture the experiences of maltreatment.
xsx Furthermore, we measured inflammation with just one single marker, i.e., CRP. Although CRP is an established indicator of ongoing inflammation and is commonly used as a diagnostic biomarker for inflammation, individual inflammatory markers in general do not adequately reflect all aspects of inflammatory processes (Del Giudice and Gangestad, 2018). Therefore, future studies should ideally include a more comprehensive assessment of inflammatory markers for a more detailed investigation of the potential mediating role of inflammatory processes.
Moreover, in our multiethnic cohort, the minority groups were previously found to have an increased risk for multiple diseases, including cardiovascular diseases and asthma (Aarab et al., 2019; Perini et al., 2019). Therefore, the observed elevated CRP levels in four out of the five minority groups compared to the ethnic Dutch reference group are not unexpected. Yet, it is well conceivable that these larger effects confounded more subtle associations between child maltreatment and CRP, even though we accounted for ethnicity in our analyses.
Strengths and Limitations
This study investigated child maltreatment and comorbid metabolic syndrome to current depressed mood in a large representative urban multiethnic cohort in Western Europe (Snijder et al., 2017). The assumed generalizability of the findings is further supported by the fact that the self-reported prevalence rate of child maltreatment of approximately 30% is similar to that previously reported in the general Dutch population (De Graaf et al., 2010).
Nevertheless, our study has several limitations that should be addressed. First, as already mentioned earlier as this study had a cross-sectional design, it is not possible to draw causal or inferences on directionality of effects. Our study suggests that child maltreatment is not associated with increased risk for comorbid metabolic syndrome to depressed mood. However, there is a possibility that the effects of child maltreatment do exist at a more nuanced level and will only come to light when depressed mood and metabolic syndrome development are investigated longitudinally. Next, this study used self-report questionnaires which could have led to decreased reporting reliability. However, in contrast, self-report questionnaires may also represent the respondent’s perspective more reliably and stimulate disclosing as compared to interview situations, specifically on sensitive topics, i.e., child maltreatment and depressed mood (McNeeley, 2012). In addition, with respect to child maltreatment, recall bias may have occurred, as child maltreatment may happen at a young age in which children do not have the cognitive ability to recall these events. Moreover, our measure of child maltreatment only included four types of maltreatment (i.e., emotional neglect, psychological abuse, physical abuse, and sexual abuse), which obviously does not cover all the various types of child maltreatment. The measurement of child maltreatment used was developed for use in a Dutch community population. Unfortunately, the questionnaire’s cross-cultural validity has not yet been investigated, and we cannot exclude differences in the reliability of retrospective child maltreatment reporting between the different ethnic groups included in our study population, nor in the interpretation of what constitutes child maltreatment and was, therefore, endorsed as such in the questionnaire. Furthermore, in line with previous investigations on the associations between child maltreatment and adverse physical health outcomes within the HELIUS study (Bakema et al., 2020), we solely investigated the associations between the occurrence of the distinct child maltreatment types and the total number of experienced maltreatment types. The developmental timing and chronicity of maltreatment was also not assessed, which could have had an impact on the results, as previous studies have shown that the exact timing of maltreatment within childhood impacts the occurrence of physical and mental health and neurobiological correlates in adulthood (Pechtel et al., 2014).
Finally, while we included ethnicity as a covariate in our analyses, we did not explicitly investigate whether there were differences in the associations between child maltreatment and the various outcomes of interest between our included ethnic groups, nor in the associations of these outcomes with the included covariates on demographic, socioeconomic, health-behavioral, and chronic stress characteristics and current medication use in the statistical models.
Conclusion and Future Implications
This multiethnic urban cohort study provides insights into the association between child maltreatment and comorbid metabolic syndrome in people with current depressed mood, and the potential mediating effect of the inflammatory marker CRP. No significant association was found between child maltreatment and comorbid metabolic syndrome in people with current depressed mood. As child maltreatment was not significantly associated with circulating CRP, mediation was excluded. The observed associations did not differ between men and women. Although our cohort was cross-sectional and consisted of a non-clinical adult population, our study findings provide additional support for the notion that although child maltreatment has debilitating effects on wellbeing and many health outcomes, it does not unequivocally increase the risk for all adverse health outcomes at any moment throughout life. In future research, it is essential to gain prospective longitudinal perspectives regarding different stages of the examined conditions with more comprehensive measures of maltreatment, depressed mood, metabolic syndrome, and inflammation.
Data Availability Statement
The data that support the findings of this study are available upon request from the HELIUS research cohort, but restrictions apply to the availability of these data which were used under license for the current study, and so are not publicly available. Galenkamp is the Scientific Coordinator of HELIUS and may be contacted with further questions (aC5nYWxlbmthbXBAYW1zdGVyZGFtdW1jLm5s).
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of the Amsterdam University Medical Centers, location Academic Medical Center. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
MZ and AL conceptualized and designed the current study. FW, SR, B-JB, AH, KT, MS, and LE contributed to the conceptualization and interpretation of results. MZ organized the database and performed the statistical analyses. FW and MZ wrote the first draft of the manuscript. AL and JZ wrote sections of the manuscript. All authors contributed to manuscript revision, read and approved the submitted version.
Funding
The Amsterdam UMC, located at the Academic Medical Center (AMC) of Amsterdam and the Public Health Service Amsterdam (GGD Amsterdam), provided core financial support for HELIUS. The HELIUS study was also funded by research grants from the Dutch Heart Foundation (Hartstichting; Grant No. 2010T084), The Netherlands Organization for Health Research and Development (ZonMw; Grant No. 200500003), the European Integration Fund (EIF; Grant No. 2013EIF013), and the European Union (Seventh Framework Program, FP-7; Grant No. 278901).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyg.2022.787029/full#supplementary-material
References
Aarab, R., Vijverberg, S. J. H., Prins, M., Snijder, M. B., Van Ree, M. B., Fokkens, W. J., et al. (2019). Prevalence of and factors associated with adult-onset asthma in different ethnic groups: the HELIUS study. Respir. Med. 150, 113–119. doi: 10.1016/J.RMED.2019.02.018
Al Odhayani, A., Watson, W. J., and Watson, L. (2013). Conséquences comportementales de la violence faite aux enfants. Can. Fam. Phys. 59, e350–e356.
Alberti, K. G. M., Zimmet, P., and Shaw, J. (2005). The metabolic syndrome—a new worldwide definition. Lancet 366, 1059–1062. doi: 10.1016/S0140-6736(05)67402-8
Alberti, K. G., Eckel, R. H., Grundy, S. M., Zimmet, P. Z., Cleeman, J. I., Donato, K. A., et al. (2009). Harmonizing the metabolic syndrome: a joint interim statement of the International diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; International. Circulation 120, 1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644
American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders: DSM 5, 5th Edn. Washington, D.C: American Psychiatric Association.
Anda, R. F., Croft, J. B., Felitti, V. J., Nordenberg, D., Giles, W. H., Williamson, D. F., et al. (1999). Adverse childhood experiences and smoking during adolescence and adulthood. J. Am. Med. Assoc. 282, 1652–1658. doi: 10.1001/jama.282.17.1652
Bakema, M. J., van Zuiden, M., Collard, D., Zantvoord, J. B., de Rooij, S. R., Elsenburg, L. K., et al. (2020). Associations between child maltreatment, autonomic regulation, and adverse cardiovascular outcome in an urban population: the HELIUS study. Front. Psychiatry 11:69. doi: 10.3389/fpsyt.2020.00069
Baldwin, J. R., Reuben, A., Newbury, J. B., and Danese, A. (2019). Agreement between prospective and retrospective measures of childhood maltreatment: a systematic review and meta-analysis. JAMA Psychiatry 76:584. doi: 10.1001/JAMAPSYCHIATRY.2019.0097
Baumeister, D., Akhtar, R., Ciufolini, S., Pariante, C. M., and Mondelli, V. (2016). Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Mol. Psychiatry 21, 642–649. doi: 10.1038/mp.2015.67
Bellis, M. A., Lowey, H., Leckenby, N., Hughes, K., and Harrison, D. (2014). Adverse childhood experiences: retrospective study to determine their impact on adult health behaviours and health outcomes in a UK population. J. Public Heal (United Kingdom) 36, 81–91. doi: 10.1093/pubmed/fdt038
Berens, A. E., Jensen, S. K. G., and Nelson, C. A. (2017). Biological embedding of childhood adversity: from physiological mechanisms to clinical implications. BMC Med. 15:135. doi: 10.1186/S12916-017-0895-4
Berk, M., Wadee, A. A., Kuschke, R. H., and O’Neill-Kerr, A. (1997). Acute-phase proteins in major depression. J. Psychosom. Res. 43, 529–534.
Blaine, B. (2008). Does depression cause obesity?: A meta-analysis of longitudinal studies of depression and weight control. J. Health Psychol. 13, 1190–1197. doi: 10.1177/1359105308095977
Capuron, L., Su, S., Miller, A. H., Bremner, J. D., Goldberg, J., Vogt, G. J., et al. (2008). Depressive symptoms and metabolic syndrome: is inflammation the underlying link? Biol. Psychiatry 64, 896–900. doi: 10.1016/J.BIOPSYCH.2008.05.019
Chirinos, D. A., Murdock, K. W., LeRoy, A. S., and Fagundes, C. (2017). Depressive symptom profiles, cardio-metabolic risk and inflammation: results from the MIDUS study. Psychoneuroendocrinology 82, 17–25. doi: 10.1016/j.psyneuen.2017.04.011
Coelho, R., Viola, T. W., Walss-Bass, C., and Brietzke, E. G.-O. R. (2014). Childhood maltreatment and inflammatory markers: a systematic review. Acta Psychiatr. Scand. 129, 180–192.
Danese, A., and McEwen, B. S. (2012). Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol. Behav. 106, 29–39. doi: 10.1016/j.physbeh.2011.08.019
Danese, A., and Tan, M. (2014). Childhood maltreatment and obesity: systematic review and meta-analysis. Mol. Psychiatry 19, 544–554. doi: 10.1038/mp.2013.54
Danese, A., Moffitt, T. E., Harrington, H., Milne, B. J., Polanczyk, G., Pariante, C. M., et al. (2009). Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Arch. Pediatr. Adolesc. Med. 163:1135. doi: 10.1001/ARCHPEDIATRICS.2009.214
Danese, A., Pariante, C. M., Caspi, A., Taylor, A., and Poulton, R. (2007). Childhood maltreatment predicts adult inflammation in a life-course study. Proc. Natl. Acad. Sci. U.S.A. 104, 1319–1324. doi: 10.1073/PNAS.0610362104
De Graaf, R., Ten Have, M., and Van Dorsselaer, S. (2010). The Netherlands mental health survey and incidence study-2 (NEMESIS-2): design and methods. Int. J. Methods Psychiatr. Res. 19, 125–141. doi: 10.1002/mpr.317
Del Giudice, M., and Gangestad, S. W. (2018). Rethinking IL-6 and CRP: why they are more than inflammatory biomarkers, and why it matters. Brain Behav. Immun. 70, 61–75. doi: 10.1016/J.BBI.2018.02.013
Dzherieva, I. S., Volkova, N. I., and Panfilova, N. S. (2011). Depressive disorders in males with metabolic syndrome. J. Biomed. Clin. Res. 4, 46–49.
Ehrlich, K. B., Miller, G. E., Rogosch, F. A., and Cicchetti, D. (2021). “Maltreatment exposure across childhood and low-grade inflammation: considerations of exposure type, timing, and sex differences,” in Developmental Psychobiology, Vol. 63, ed. E. M. Blass (Hoboken, NJ: John Wiley and Sons Inc), 529–537. doi: 10.1002/dev.22031
Elkins, J., Miller, K. M., Briggs, H. E., Kim, I., Mowbray, O., and Orellana, E. R. (2019). Associations between adverse childhood experiences, major depressive episode and chronic physical health in adolescents: moderation of race/ethnicity. Soc. Work Public Health 34, 444–456. doi: 10.1080/19371918.2019.1617216
Felitti, V. J., Anda, R. F., Nordenberg, D., Williamson, D. F., Spitz, A. M., Edwards, V., et al. (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: the adverse childhood experiences (ACE) study. Am. J. Prev. Med. 14, 245–258. doi: 10.1016/S0749-3797(98)00017-8
Festa, A., D’Agostino, R. Jr., Howard, G., Mykkaänen, L., Tracy, R. P., and Haffner, S. M. (2000). Chronic subclinical inflammation as part of the insulin resistance syndrome. Circulation 102, 42–47. doi: 10.1161/01.CIR.102.1.42
Foley, D. L., Morley, K. I., Madden, P. A., Heath, A. C., Whitfield, J. B., and Martin, N. G. (2010). Major depression and the metabolic syndrome. Twin Res. Hum. Genet. 13:347. doi: 10.1375/TWIN.13.4.347
Ford, E. S., Anda, R. F., Edwards, V. J., Perry, G. S., Zhao, G., Li, C., et al. (2011). Adverse childhood experiences and smoking status in five states. Prev. Med. (Baltim) 53, 188–193. doi: 10.1016/j.ypmed.2011.06.015
Fumagalli, F., Molteni, R., Racagni, G., and Riva, M. A. (2007). Stress during development: Impact on neuroplasticity and relevance to psychopathology. Prog. Neurobiol. 81, 197–217. doi: 10.1016/j.pneurobio.2007.01.002
Galenkamp, H., Stronks, K., Snijder, M. B., and Derks, E. M. (2017). Measurement invariance testing of the PHQ-9 in a multi-ethnic population in Europe: the HELIUS study. BMC Psychiatry 17:349. doi: 10.1186/s12888-017-1506-9
Garad, Y., Maximova, K., MacKinnon, N., McGrath, J. J., Kozyrskyj, A. L., and Colman, I. (2017). Sex-specific differences in the association between childhood adversity and cardiovascular disease in adulthood: evidence from a national cohort study. Can. J. Cardiol. 33, 1013–1019. doi: 10.1016/j.cjca.2017.05.008
Gbd 2017 Disease and Injury Incidence and Prevalence Collaborators (2018). Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1789–1858. doi: 10.1016/S0140-6736(18)32279-7
Gheshlagh, R. G., Parizad, N., and Sayehmiri, K. (2016). The relationship between depression and metabolic syndrome: systematic review and meta-analysis study. Iran Red Crescent Med. J. 18:26523. doi: 10.5812/IRCMJ.26523
Gilbert, L. K., Breiding, M. J., Merrick, M. T., Thompson, W. W., Ford, D. C., Dhingra, S. S., et al. (2015). Childhood adversity and adult chronic disease: an update from ten states and the district of Columbia, 2010. Am. J. Prev. Med. 48, 345–349. doi: 10.1016/j.amepre.2014.09.006
Han, T. S., Sattar, N., Williams, K., Gonzalez-Villalpando, C., Lean, M. E. J., and Haffner, S. M. (2002). Prospective study of c-reactive protein in relation to the development of diabetes and metabolic syndrome in the mexico city diabetes study. Diabetes Care 25, 2016–2021. doi: 10.2337/DIACARE.25.11.2016
Hensch, T. K. (2005). Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci. 6, 877–888. doi: 10.1038/nrn1787
Hiles, S. A., Révész, D., Lamers, F., Giltay, E., and Penninx, B. W. J. H. (2016). Bidirectional prospective associations of metabolic syndrome components with depression, anxiety, and antidepressant use. Depress Anxiety 33:754. doi: 10.1002/DA.22512
Hornig, M., Goodman, D. B. P., Kamoun, M., and Amsterdam, J. D. (1998). Positive and negative acute phase proteins in affective subtypes. J. Affect. Disord. 49, 9–18. doi: 10.1016/s0165-0327(97)00180-8
Hosang, G. M., Fisher, H. L., Hodgson, K., Maughan, B., and Farmer, A. E. (2018). Childhood maltreatment and adult medical morbidity in mood disorders: comparison of unipolar depression with bipolar disorder. Br. J. Psychiatry 213:645. doi: 10.1192/BJP.2018.178
Hughes, K., Bellis, M. A., Hardcastle, K. A., Sethi, D., Butchart, A., Mikton, C., et al. (2017). The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. Lancet Public Heal. 2, e356–e366. doi: 10.1016/S2468-2667(17)30118-4
Kerr, D. M., McDonald, J., and Minnis, H. (2021). The association of child maltreatment and systemic inflammation in adulthood: a systematic review. PLoS One 16:e0243685. doi: 10.1371/journal.pone.0243685
Kroenke, K., Spitzer, R. L., and Williams, J. B. W. (2001). The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 16, 606–613. doi: 10.1046/j.1525-1497.2001.016009606.x
Kroenke, K., Spitzer, R. L., Williams, J. B., and Löwe, B. (2010). The patient health questionnaire somatic, anxiety, and depressive symptom scales: a systematic review. Gen. Hosp. Psychiatry 32, 345–359. doi: 10.1016/J.GENHOSPPSYCH.2010.03.006
Lamers, F., Milaneschi, Y., Vinkers, C. H., Schoevers, R. A., Giltay, E. J., and Penninx, B. W. J. H. (2020). Depression profilers and immuno-metabolic dysregulation: longitudinal results from the NESDA study. Brain Behav. Immun. 88, 174–183. doi: 10.1016/J.BBI.2020.04.002
Lee, C., Tsenkova, V., and Carr, D. (2014). Childhood trauma and metabolic syndrome in men and women. Soc. Sci. Med. 105:122. doi: 10.1016/J.SOCSCIMED.2014.01.017
Li, L., Pinto Pereira, S. M., and Power, C. (2019). Childhood maltreatment and biomarkers for cardiometabolic disease in mid-adulthood in a prospective British birth cohort: associations and potential explanations. BMJ Open 9:e024079. doi: 10.1136/bmjopen-2018-024079
Łosiak, W., Blaut, A., Kłosowska, J., and Łosiak-Pilch, J. (2019). Stressful life events, cognitive biases, and symptoms of depression in young adults. Front. Psychol. 10:2165. doi: 10.3389/FPSYG.2019.02165
Mandelli, L., Petrelli, C., and Serretti, A. (2015). The role of specific early trauma in adult depression: a meta-analysis of published literature. Childhood trauma and adult depression. Eur. Psychiatry 30, 665–680. doi: 10.1016/J.EURPSY.2015.04.007
McIntyre, R. S., Soczynska, J. K., Liauw, S. S., Woldeyohannes, H. O., Brietzke, E., Nathanson, J., et al. (2012). The association between childhood adversity and components of metabolic syndrome in adults with mood disorders: results from the international mood disorders collaborative project. Int. J. Psychiatry Med. 43, 165–177. doi: 10.2190/PM.43.2.E
McNeeley, S. (2012). “Sensitive issues in surveys: reducing refusals while increasing reliability and quality of responses to sensitive survey items,” in Handbook of Survey Methodology for the Social Sciences, ed. L. Gideon (New York, NY: Springer), 377–396. doi: 10.1007/978-1-4614-3876-2_22
Merrick, M. T., Ford, D. C., Ports, K. A., and Guinn, A. S. (2018). Prevalence of adverse childhood experiences from the 2011-2014 behavioral risk factor surveillance system in 23 States. JAMA Pediatr. 172, 1038–1044. doi: 10.1001/jamapediatrics.2018.2537
Midei, A. J., and Matthews, K. A. (2011). Interpersonal violence in childhood as a risk factor for obesity: a systematic review of the literature and proposed pathways. Obes. Rev. 12, e159–e172. doi: 10.1111/j.1467-789X.2010.00823.x
Midei, A. J., Matthews, K. A., Chang, Y.-F., and Bromberger, J. T. (2013). Childhood physical abuse is associated with incident metabolic syndrome in mid-life women. Health Psychol. 32, 121–127. doi: 10.1037/a0027891
Moradi, Y., Albatineh, A. N., Mahmoodi, H., and Gheshlagh, R. G. (2021). The relationship between depression and risk of metabolic syndrome: a meta-analysis of observational studies. Clin. Diabetes Endocrinol. 7:4. doi: 10.1186/S40842-021-00117-8
Muhtz, C., Zyriax, B. C., Klähn, T., Windler, E., and Otte, C. (2009). Depressive symptoms and metabolic risk: effects of cortisol and gender. Psychoneuroendocrinology 34, 1004–1011. doi: 10.1016/J.PSYNEUEN.2009.01.016
Nikulina, V., and Widom, C. S. (2014). Do race, neglect, and childhood poverty predict physical health in adulthood? A multilevel prospective analysis. Child Abus. Negl. 38, 414–424. doi: 10.1016/j.chiabu.2013.09.007
Osborn, M., and Widom, C. S. (2020). Do documented records and retrospective reports of childhood maltreatment similarly predict chronic inflammation? Psychol. Med. 50, 2406–2415. doi: 10.1017/S0033291719002575
Otte, C., Gold, S. M., Penninx, B. W., Pariante, C. M., Etkin, A., Fava, M., et al. (2016). Major depressive disorder. Nat. Rev. Dis. Prim. 2:16065. doi: 10.1038/nrdp.2016.65
Pan, A., Keum, N., Okereke, O. I., Sun, Q., Kivimaki, M., Rubin, R. R., et al. (2012). Bidirectional association between depression and metabolic syndrome: a systematic review and meta-analysis of epidemiological studies. Diabetes Care 35, 1171–1180. doi: 10.2337/DC11-2055
Pechtel, P., and Pizzagalli, D. A. (2011). Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology (Berl) 214, 55–70. doi: 10.1007/s00213-010-2009-2
Pechtel, P., Lyons-Ruth, K., Anderson, C. M., and Teicher, M. H. (2014). Sensitive periods of amygdala development: the role of maltreatment in preadolescence. Neuroimage 97:236. doi: 10.1016/J.NEUROIMAGE.2014.04.025
Penninx, B. W. J. H., Milaneschi, Y., Lamers, F., and Vogelzangs, N. (2013). Understanding the somatic consequences of depression: biological mechanisms and the role of depression symptom profile. BMC Med. 11:129. doi: 10.1186/1741-7015-11-129
Perini, W., Kunst, A. E., Snijder, M. B., Peters, R. J. G., and van Valkengoed, I. G. M. (2019). Ethnic differences in metabolic cardiovascular risk among normal weight individuals: Implications for cardiovascular risk screening. The HELIUS study. Nutr. Metab. Cardiovasc. Dis. 29, 15–22. doi: 10.1016/J.NUMECD.2018.09.004
Rethorst, C. D., Bernstein, I., and Trivedi, M. H. (2014). Inflammation, obesity and metabolic syndrome in depression: analysis of the 2009–2010 National health and nutrition survey (NHANES). J. Clin. Psychiatry 75:e1428. doi: 10.4088/JCP.14M09009
Roberts, R. E., Deleger, S., Strawbridge, W. J., and Kaplan, G. A. (2003). Prosepctive association between obesity and depression: evidence rom the Alameda county study. Int. J. Obes. 27, 514–521. doi: 10.1038/sj.ijo.0802204
Saklayen, M. G. (2018). The global epidemic of the metabolic syndrome. Curr. Hypertens Rep. 20:12. doi: 10.1007/s11906-018-0812-z
Scott, K. M., Von Korff, M., Angermeyer, M. C., Benjet, C., Bruffaerts, R., De Girolamo, G., et al. (2011). Association of childhood adversities and early-onset mental disorders with adult-onset chronic physical conditions. Arch. Gen. Psychiatry 68, 838–844. doi: 10.1001/archgenpsychiatry.2011.77
Sluzewska, A., Rybakowski, L., Bosmans, E., Sobieska, M., Berghmans, R., Maes, M., et al. (1996). Indicators of immune activation in major depression. Psychiatry Res. 64, 161–167.
Snijder, M. B., Galenkamp, H., Prins, M., Derks, E. M., Peters, R. J. G., Zwinderman, A. H., et al. (2017). Cohort profile: the healthy life in an Urban setting (HELIUS) study in Amsterdam, the Netherlands. BMJ Open 7:e017873. doi: 10.1136/bmjopen-2017-017873
Stronks, K., Snijder, M. B., Peters, R. J., Prins, M., Schene, A. H., and Zwinderman, A. H. (2013). Unravelling the impact of ethnicity on health in Europe: the HELIUS study. BMC Public Health 13:402. doi: 10.1186/1471-2458-13-402
Sunley, A. K., Lok, A., White, M. J., Snijder, M. B., Van Zuiden, M., Zantvoord, J. B., et al. (2020). Ethnic and sex differences in the association of child maltreatment and depressed mood. The HELIUS study. Child Abuse Negl. 99:104239. doi: 10.1016/J.CHIABU.2019.104239
Vaccarino, V., McClure, C., Johnson, B. D., Sheps, D. S., Bittner, V., Rutledge, T., et al. (2008). Depression, the metabolic syndrome and cardiovascular risk. Psychosom. Med. 70, 40–48. doi: 10.1097/PSY.0B013E31815C1B85
Vancampfort, D., Correll, C., Wampers, M., Sienaert, P., Mitchell, A., De Herdt, A., et al. (2014). Metabolic syndrome and metabolic abnormalities in patients with major depressive disorder: a meta-analysis of prevalences and moderating variables. Psychol. Med. 44, 2017–2028. doi: 10.1017/S0033291713002778
Watson, K. T., Simard, J. F., Henderson, V. W., Nutkiewicz, L., Lamers, F., Nasca, C., et al. (2021). Incident major depressive disorder predicted by three measures of insulin resistance: a dutch cohort study. Am. J. Psychiatry 178, 914–920. doi: 10.1176/APPI.AJP.2021.20101479
World Health Organization [WHO] (2017). Number of People with Depression Increases. Geneva: World Health Organization.
Whooley, M. A., and Wong, J. M. (2013). Depression and cardiovascular disorders. Annu. Rev. Clin. Psychol. 9, 327–354. doi: 10.1146/annurev-clinpsy-050212-185526
Widom, C. S., DuMont, K., and Czaja, S. J. (2007). A prospective investigation of major depressive disorder and comorbidity in abused and neglected children grown up. Arch. Gen. Psychiatry 64, 49–56. doi: 10.1001/ARCHPSYC.64.1.49
Wingenfeld, K., Kuehl, L. K., Boeker, A., Schultebraucks, K., Schulz, A., Stenzel, J., et al. (2017). Are adverse childhood experiences and depression associated with impaired glucose tolerance in females? An experimental study. J. Psychiatr. Res. 95, 60–67. doi: 10.1016/J.JPSYCHIRES.2017.07.028
Keywords: child maltreatment, depressed mood, metabolic syndrome, CRP, HELIUS study
Citation: Willemen FEM, van Zuiden M, Zantvoord JB, de Rooij SR, van den Born B-JH, Hak AE, Thomaes K, Segeren M, Elsenburg LK and Lok A (2022) Associations Between Child Maltreatment, Inflammation, and Comorbid Metabolic Syndrome to Depressed Mood in a Multiethnic Urban Population: The HELIUS Study. Front. Psychol. 13:787029. doi: 10.3389/fpsyg.2022.787029
Received: 30 September 2021; Accepted: 17 June 2022;
Published: 14 July 2022.
Edited by:
Xiaofei Xie, Peking University, ChinaReviewed by:
Ulrich Schweiger, Helios Hanseklinikum, GermanyIlaria Riboldi, University of Milano-Bicocca, Italy
Copyright © 2022 Willemen, van Zuiden, Zantvoord, de Rooij, van den Born, Hak, Thomaes, Segeren, Elsenburg and Lok. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anja Lok, YS5sb2tAYW1zdGVyZGFtdW1jLm5s
†These authors share first authorship
 Fabienne E. M. Willemen
Fabienne E. M. Willemen Mirjam van Zuiden
Mirjam van Zuiden Jasper B. Zantvoord
Jasper B. Zantvoord Susanne R. de Rooij
Susanne R. de Rooij Bert-Jan H. van den Born4,5
Bert-Jan H. van den Born4,5 Menno Segeren
Menno Segeren Leonie K. Elsenburg
Leonie K. Elsenburg Anja Lok
Anja Lok