- 1Department of Psychiatry, The Third People's Hospital of Foshan, Foshan, Guangdong, China
- 2Department of Psychiatry, National Clinical Research Center for Mental Disorders, The Second Xiangya Hospital of Central South University, Changsha, Hunan, China
Background: This study aimed to investigate the relationships between regional neural activity and multiple related indicators in patients with major depressive disorder (MDD).
Methods: Forty-two patients and 42 healthy controls (HCs) were enrolled. Pearson/Spearman correlation analyses were applied to examine the associations between abnormal regional homogeneity (ReHo) and different indicators in the patients.
Results: Compared with HCs, patients with MDD had increased ReHo in the left inferior temporal gyrus (ITG) and decreased ReHo values in the left putamen, anterior cingulate cortex (ACC), and precentral gyrus. The ReHo of the left putamen was positively correlated with the PR interval, Repeatable Battery for the Assessment of Neuropsychological Status 4A, and Discriminant analysis (D), and negatively correlated with Ae (block) and Ae (total) in the patients. The ReHo value of the left ACC was positively correlated with the severity of depression, Stroop Color Word Test of C − 2B + 100 in reaction time, and negatively correlated with Ce (Missay) and Perseverative Responses in the patients. The ReHo of the left ITG was positively correlated with the Neuroticism scores and negatively correlated with the Lie scores in the patients.
Conclusion: These results suggested that the decreased ReHo of the salience network might be the underpinning of cognitive impairments in patients with MDD.
Introduction
Major depressive disorder (MDD) is a highly prevailing and disabling mental disorder associated with high morbidity and mortality (Fagiolini et al., 2013). About 20 to 30% of individuals with MDD evolve into chronic disease (Angst et al., 2009; Murphy and Byrne, 2012). It affects about 350 million people around the world. By 2030, MDD will be the leading cause of the burden of disease worldwide (Collins et al., 2011). Plenty of studies have shown that patients with MDD have aberrant brain imaging (Zhang et al., 2018; Liang et al., 2020; Yang et al., 2021), clinical (Caldwell and Steffen, 2018; Kircanski et al., 2019), event-related potentials (ERPs; Landes et al., 2018; Kim et al., 2020; Fan et al., 2021; Xin et al., 2021), and cognitive indicators (Pan et al., 2019). MDD is a heterogeneous disease (Harald and Gordon, 2012), and its etiology remains vague. Combining clinical factors, ERPs, cognitive indicators and other factors to explore the changes of brain functional activity in patients with MDD may provide valuable evidence for understanding the etiology of depression.
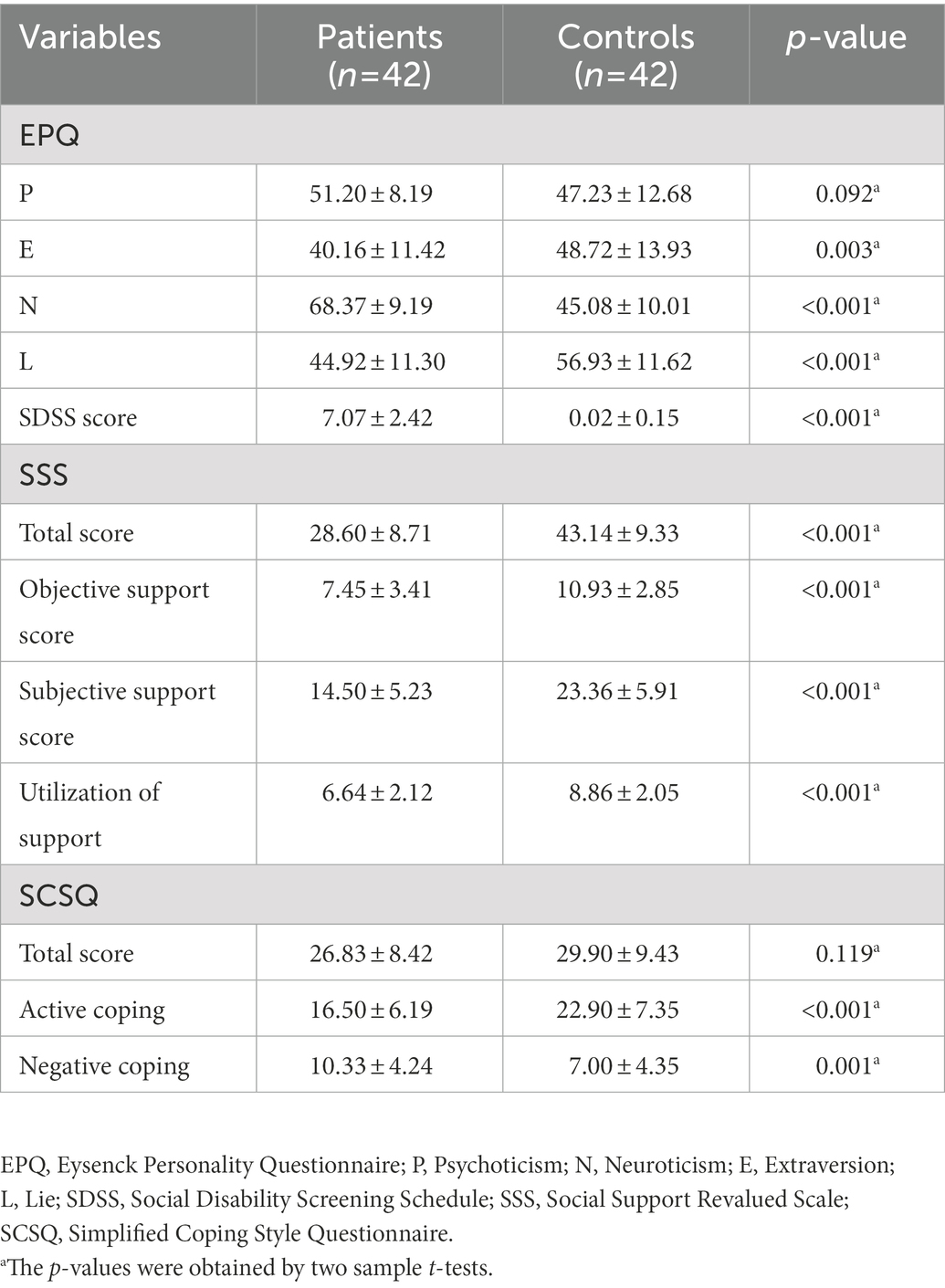
MDD is a psychological abnormality concerning mood dysregulation, including neuroendocrine changes, autonomic nervous system (ANS) disorder, immune system activation rhythm disturbances, and oxidative stress (Halaris, 2017). The Hamilton Depression Rating Scale (HAMD; Gibbons et al., 1993; Stefanis and Stefanis, 2001) and Hamilton Anxiety Rating Scale (HAMA; Clark and Donovan, 1994) have been frequently used to assess the severity of depression and anxiety separately to help clarify clinical diagnosis and degree division of mood disorders. The social disability screening schedule (SDSS; Yang et al., 2013), social support rating scale (SSRS; Qin et al., 2021), simplified coping style questionnaire (SCSQ; Lin et al., 2020), and Eysenck personality questionnaire (EPQ) are widely used to assess the psychological status of patients with MDD. Studies have shown that patients with MDD have abnormal EPQ scores (Małyszczak et al., 2019; Wu et al., 2019; Otsubo et al., 2021). Many investigations have reported that patients with MDD exhibit a variety of abnormal biological indexes during outpatient and hospitalization. Heart rate in patients with MDD is significantly increased at rest (Williams et al., 2011). The co-morbidity between MDD and cardiovascular disease (CVD) is a matter of public knowledge. A meta-analytic study found that MDD was recognized as a major risk element for mortality in coronary heart disease (CHD; Barth et al., 2004). And heart rate variability(HRV)pertains to the variation in heart rate and measures the interplay between the sympathetic and parasympathetic nervous systems (Kidwell and Ellenbroek, 2018). Previous researches have established the key element in the link between MDD and CVD with a declining HRV (Musselman et al., 1998; Stapelberg et al., 2012). Several meta-analysis studies have reported that MDD is associated with increased total triglyceride (TG) and cholesterol (CHOL), and low high density lipoprotein (HDL), low density lipoprotein (LDL), and omega-3 polyunsaturated fatty acids (PUFA; Lin et al., 2010; Pan et al., 2012; Persons and Fiedorowicz, 2016). Besides, the thyroid hormones in patients with MDD were lower than those in HCs (Zhou Y. et al., 2021), and thyroid hormones were applied as a supplementary treatment for MDD (Mcintyre, 2016). Moreover, decreased antioxidant uric acid was observed in patients with MDD (Bartoli et al., 2018). A large number of studies have described the relationship between Hypercortisolemia and MDD (Parker et al., 2003; Stetler and Miller, 2011; Nobis et al., 2020). Hypercortisolemia has been linked to depression with ruminations (Stewart et al., 2013), psychogenic depression (Schatzberg et al., 1984), and melancholic and psychotic depressive subtypes (Krishnan and Nestler, 2008; Kunugi et al., 2015). Hypercortisolemia was also depicted as a possible biomarker for treatment-resistant depression (TRD) and persisted after remission (Markopoulou et al., 2009).
A systematic review and meta-analysis by Rock and Roiser et al. suggested that cognitive impairment was a central feature of MDD (Rock et al., 2014). About two-thirds of patients with MDD have cognitive impairment (Afridi et al., 2011). Studies have shown that cognitive impairment persists beyond the acute episode of MDD, with a third to half of the remissive patients with MDD still having a cognitive impairment (Reppermund et al., 2009).
Notably, There is a good deal of tools widely used in measuring cognitive function in MDD, including the Wisconsin Card sorting test (WCST; Nakano et al., 2008; Liao et al., 2021), Stroop Color Word Test (SCWT; Shi et al., 2020; Duan et al., 2021; Zhou Q. et al., 2021), Repeatable Battery for the Assessment of Neuropsychological Status (RBANS; Guan et al., 2021; Teng et al., 2021), eye-tracking test (Takahashi et al., 2021), and ERP (Bruder et al., 2011; Jaworska and Protzner, 2013; Klawohn et al., 2021; Tseng et al., 2021; White et al., 2021), which have proved that the cognitive domains including executive functioning, learning and memory, processing speed, decision-making, concentration, and attention are notably impaired in patients with MDD (Pan et al., 2019; Wang et al., 2020).
Most of the research theories on the causes of MDD comprise of psychological, biological, and social factors (Beck and Bredemeier, 2016; Brouwer et al., 2019), but few studies have combined these factors in a single study (Kennis et al., 2019). Many of them mainly discuss the relationship between a certain index of patients with MDD and the whole brain activity, whereas there are few studies on exploring local brain activity in patients with MDD by combining multiple indicators. Brain activity in the bilateral orbital frontal cortex (OFC) of patients with MDD was reduced, resulting in decreased ability of patients with MDD to inhibit negative stimuli (Zhang et al., 2016). A structural neuroimaging study has shown decreased volume of the caudate nucleus and lateral orbitofrontal cortex (LOC) and a thinning of the bilateral insula cortex, which are associated with decreased working memory performance and processing speed in MDD (Saleh et al., 2017). The dynamic functional network connectivity in MDD showed that stronger links existed among sensory-related regions than those in HCs (HCs), which were connected with extroversion and neuroticism of the EPQ in the patients (Wu et al., 2019). A study combining ERP and functional magnetic resonance imaging(fMRI) showed that ventral striatum activation and feedback negativity amplitude decreased in patients with MDD, and there was a significant correlation between feedback negativity amplitude and ventral striatum activation (Foti et al., 2014). Hence, it has a pivotal role in discussing the correlation between brain function changes and functional impairment in patients with MDD.
Regional Homogeneity (ReHo) is a voxel-based analysis, which is according to the synchronicity between the time sequences of a given voxel and its neighboring voxels. ReHo is computed by the Kendall consistency coefficient (KCC) of blood oxygen level-dependent (BOLD) time series. ReHo has a good test–retest reliability (Zuo and Xing, 2014), and it can show the local features of cerebral activity. Higher ReHo values indicate higher coherence and centrality of local brain activity (Lv et al., 2018). ReHo is usually calculated in the low-frequency range, with low frequencies (0.01–0.08 Hz) being sensitive to cortical activity (Song et al., 2014). Many studies have found abnormal ReHo in patients with MDD, such as MDD with gastrointestinal symptoms (Yan et al., 2021a), melancholic MDD patients (Yan et al., 2021b), treatment-resistant depression (Guo et al., 2011b), and first-episode and treatment-naive depression (Guo et al., 2011a). Up to now, it is still unclear whether ReHo is related to various clinical indicators in MDD.
Previous studies separately report that patients with MDD have a diversity of brain structural and functional connectivity abnormalities, which are associated with manifold abnormal indicators. Nonetheless, there is an inconsistency with the results on abnormal functional synchronization of brain regions at rest in patients with MDD, and the interconnection with multifarious indicators remains unclear. Therefore, we collected fasting blood samples, HAMA, HAMD, SDSS, SSRS, SCSQ, EPQ, WCST, SCWT, RBANS, eye-tracking test, ERP and resting state MRI data of MDD patients and healthy subjects for comparison, and conducted correlation analysis with abnormal ReHo value of depression. This study aims to examine the alterations of brain functional synchronization in patients with MDD and their correlations with various indicators including biological, clinical, psychological, and cognitive indicators. We hypothesized that patients with MDD would exhibit abnormal ReHo in multiple brain regions, which were associated with clinical and cognitive parameters.
Materials and methods
Participants
Because of head movement and data deficiency, we excluded 4 patients with MDD and 3 HCs. Finally, 42 patients with MDD and 42 HCs were enrolled in the analysis. We recruited patients with MDD from the outpatient and inpatient departments of Foshan Third People’s Hospital, which are aged between 18 and 60 years old. According to the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5; American Psychiatric Association, 2013), the diagnosis was determined independently by two psychiatrists. All patients met the following inclusion criteria: (1) patients with first-episode or recurrent MDD; (2) right-handed; and (3) patients with first-episode MDD were drug-naïve and patients with recurrent MDD were drug-free for at least 2 weeks. Exclusion criteria were: (1) serious physical diseases especially organic cardiopathy or substance abuse; (2) other serious mental diseases, including schizophrenia, bipolar disorders, intellectual disability, and dementia; and (3) patients currently undergoing or preparing to undergo other clinical studies.
HCs were recruited from the local community through posters. They were excluded if they suffered from any medical and neurological disorders, psychotic symptoms, and substance abuse. All subjects obtained relevant information through a written informed consent and this study was approved by the Ethics Committee of Foshan Third People’s Hospital.
Collection of related indicators
Twelve milliliters of peripheral venous blood was collected from all subjects at a fasting state. Blood lipid, thyroid hormone, cortisol, and uric acid were detected by the enzymatic method with the automatic biochemical analyzer. Electrocardiography (ECG) data were collected by a 12-lead ECG machine. HAMA and HAMD were used to evaluate emotional states. EPQ, SDSS, SSRS, and SCSQ were applied to evaluate the psychological status of the subjects. The RBANS, SCWT, and WCST were utilized to assess cognitive function. Eye movement analysis was performed using an eye movement analyzer, and event-related brain potentials were analyzed using an evoked potentiometer.
Image capture and processing
Resting-state functional magnetic resonance imaging was performed using a 3.0 T GE scanner (GE 3.0 T Signa Pioneer). During image collection, subjects were required to keep quiet and still and stay awake with closing their eyes. Foam pads were used to reduce the head movement of the subjects, and soft earplugs were used to reduce the noise of the scanner. The parameter setting of repetition time (TR)/echo time (TE) in this study is 2000/30 ms, the number of layers is 36, the number of rows and columns in the MR image layer is 64*64 matrix, the flip Angle is 90°, the field of view (FOV) is 24 cm, the thickness of the exciting layer is 4 mm, no gap, and a total of 250 volumes (500 s).
Data Processing Assistant for Resting-State fMRI (DPARSF) software package (Chao-Gan and Yu-Feng, 2010) was used to preprocess the collected image data. Since the signal was unstable at the beginning of the collection and subjects needed to adapt to the environment, the first 10 time points of each subject were removed. Subjects with a maximum translation of no more than 2 mm in the X, Y, and Z axes and a maximum rotation of no more than 2° in each axis were included in the subsequent analysis. The 240 left volumes underwent slice timing and head motion correction. Then, the data of different subjects were registered to the standard MNI space using the echo plane imaging (EPI) template to solve the problem of brain morphology differences between different subjects and spatial location inconsistency during scanning. The data were resampled to 3*3*3 mm3 resolution. The acquired images were bandpass filtered (0.01~0.08 Hz) and linearly detrended.
ReHo analysis
We performed ReHo analysis using the DPARSF toolbox to investigate the functional synchronization of spontaneous neural activity. ReHo describes the synchronization between a voxel and its neighboring voxel time series. The calculation formula of KCC has been stated in a previous study (Zang et al., 2004). In the voxel-based analysis method, the ReHo maps of subjects are obtained according to the KCC value of the time series of a given voxel and its nearest voxel (26 voxels). To reduce the influence of individual differences on KCC values, it is necessary to divide the KCC of each voxel by the average KCC of the whole brain to obtain a standardized ReHo profile. The generated imaging data were spatially smoothed and a Gaussian kernel with a full width of 4 mm was used to achieve a half-maximum value.
Statistical analysis
A Chi-square test was used to analyze the difference in gender of patients with MDD and HCs. Two-sample t-tests were used to analyze the blood biochemical, ECG, psychological status, and cognitive indicators of the two groups. The significance level was set at p < 0.05.
For the voxel-based ReHo map, the differences between patients with MDD and HCs were compared by two-sample t-tests with education level, gender, age and mean framewise displacement (FD) as covariates. The significance level was corrected for multiple comparisons based on Gaussian Random Field (GRF) theory (voxel significance: p < 0.001, cluster significance: p < 0.05).
Correlation analysis
Pearson/Spearman correlation analyses were performed to clarify the correlation between ReHo values and various indicators in the patients. p < 0.05 was considered as the significant threshold. The Bonferroni correction was performed for several dependent or independent statistical tests that were performed simultaneously.
Results
Participants and clinical baselines
There was no significant difference in years of education and gender between patients with MDD and HCs (Table 1). But there was a statistical difference in age (p = 0.001). There were significant differences in HAMD and HAMA (p < 0.001).
Biological indexes differences between patients with MDD and HCs
In terms of thyroxine, we collected data from 42 individuals for each group of patients with MDD and HCs. There was a statistical difference in FT3 (p = 0.006), and no statistical difference was detected in the rest parameters (Table 1). Forty patients with MDD and 42 HCs had the ECG data. There were significant differences in HR (p = 0.006), PR interval (p = 0.001), and QTc (p < 0.001), except for QRS width (Table 1).
There were no statistically significant differences in TG, CHOL, HDL, and LDL between the two groups. In terms of cortisol, 41 data were collected from patients with MDD, and 42 data were collected from HCs. There was no statistically significant difference between the two groups. In terms of uric acid, 41 data were collected from patients with MDD or healthy subjects. There was no statistical difference between them (Supplementary Table S1).
Psychological status differences between patients with MDD and HCs
As shown in Table 2, both 42 Patients with MDD and 42 HCs completed the psychological status assessment. There were significant differences in the scores of Extraversion (E; p < 0.001), Neuroticism (N; p < 0.001), and Lie (L; p = 0.038), except for Psychoticism (P) in the EPQ. There were significant statistical differences in the total scores of SDSS between patients with MDD and HCs (p < 0.001). In terms of SSS, there were significant differences in total scores (p < 0.001), objective support scores (p < 0.001), subjective support scores (p < 0.001), and utilization of support (p < 0.001) between the two groups. In terms of SCSQ, except for the total scores, there was a statistical difference between active coping (p < 0.001) and negative coping (p = 0.001).
Cognitive status differences between patients with MDD and HCs
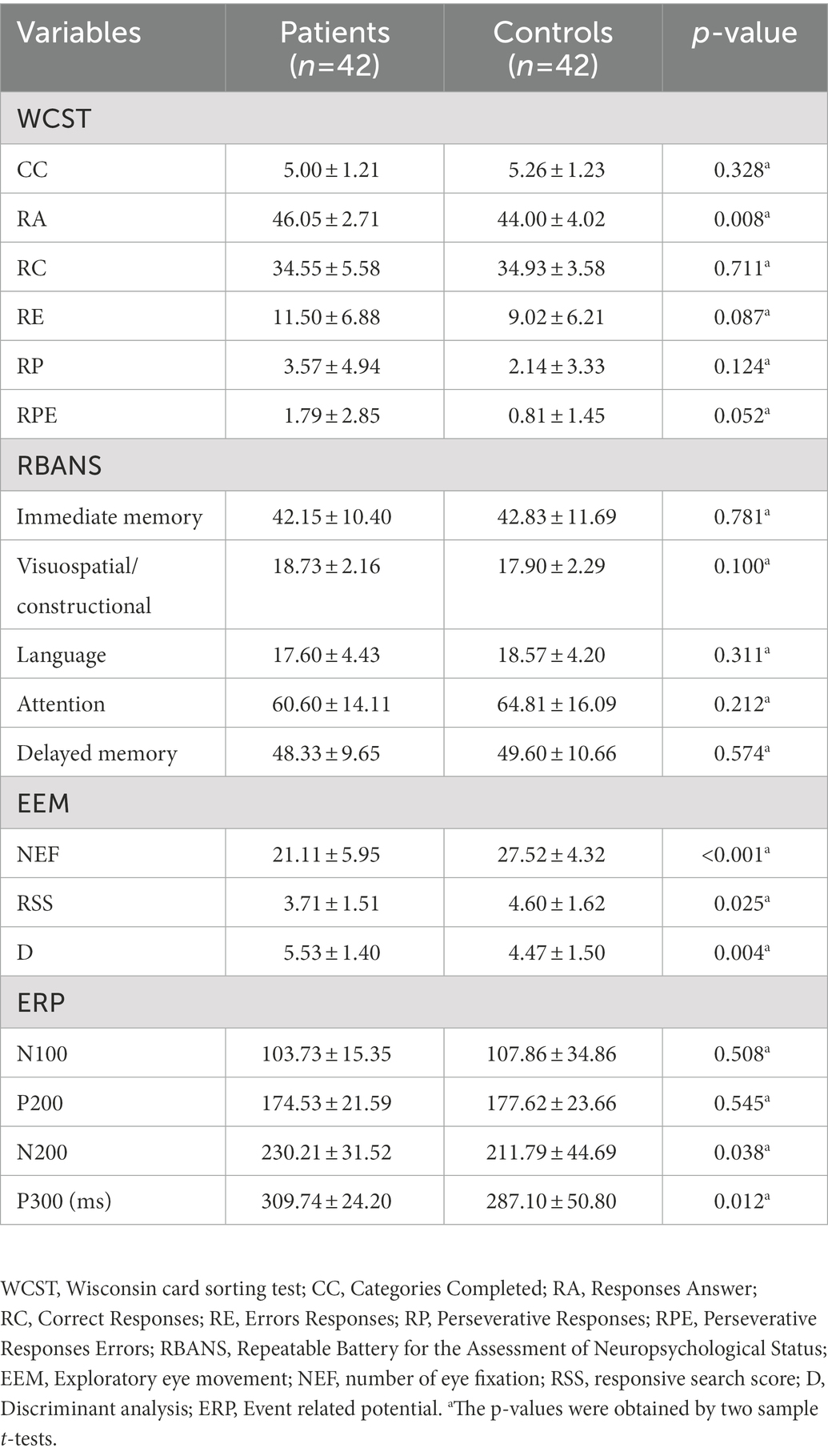
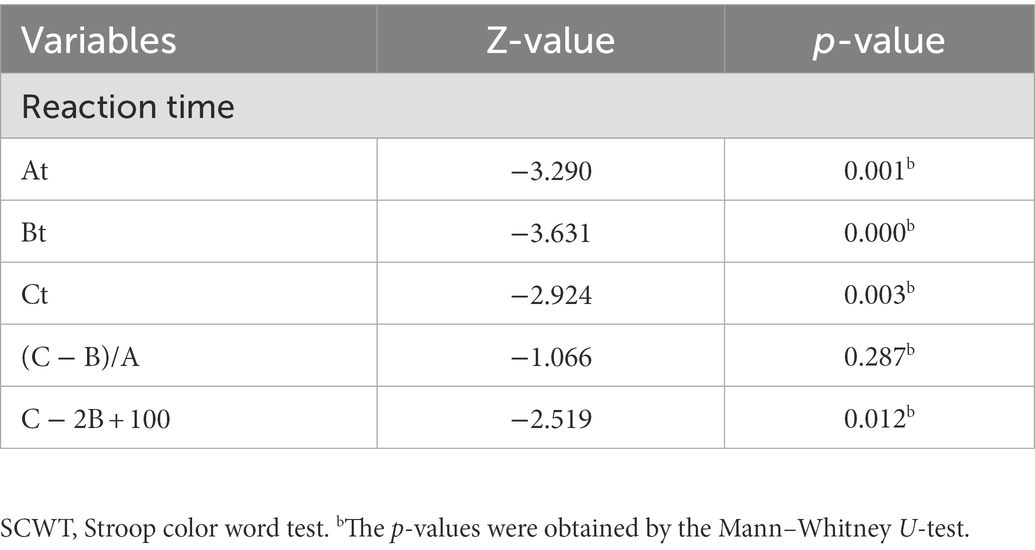
As shown in Table 3 and Supplementary Table S2, only Responses Answer (RA) showed significant differences between patients with MDD and HCs (p = 0.008) in the WCST examination, whereas Categories Completed (CC), Correct Responses (RC), Errors Responses (RE), Perseverative Responses (RP), and Perseverative Responses Errors (RPE) showed no significant differences. In the RBANS test, there was no significant difference between the two groups. In eye movement examination, there were statistical differences in the Number of Eye Fixation (NEF; p < 0.001), Responsive Search Score (RSS; p = 0.025), and Discriminant analysis (D; p = 0.004) between the two groups. In the ERP examination, there was no statistical difference between the two groups in N100 and P200, but there was a statistical difference in N200 (p = 0.038) and P300 (p = 0.012). To the reaction time of SCWT, there were significant differences in At, Bt, Ct, and C − 2B + 100 between patients with MDD and HCs (Table 4), but no significant differences in (C − B)/A, and in the error reaction.
ReHo: Group comparisons
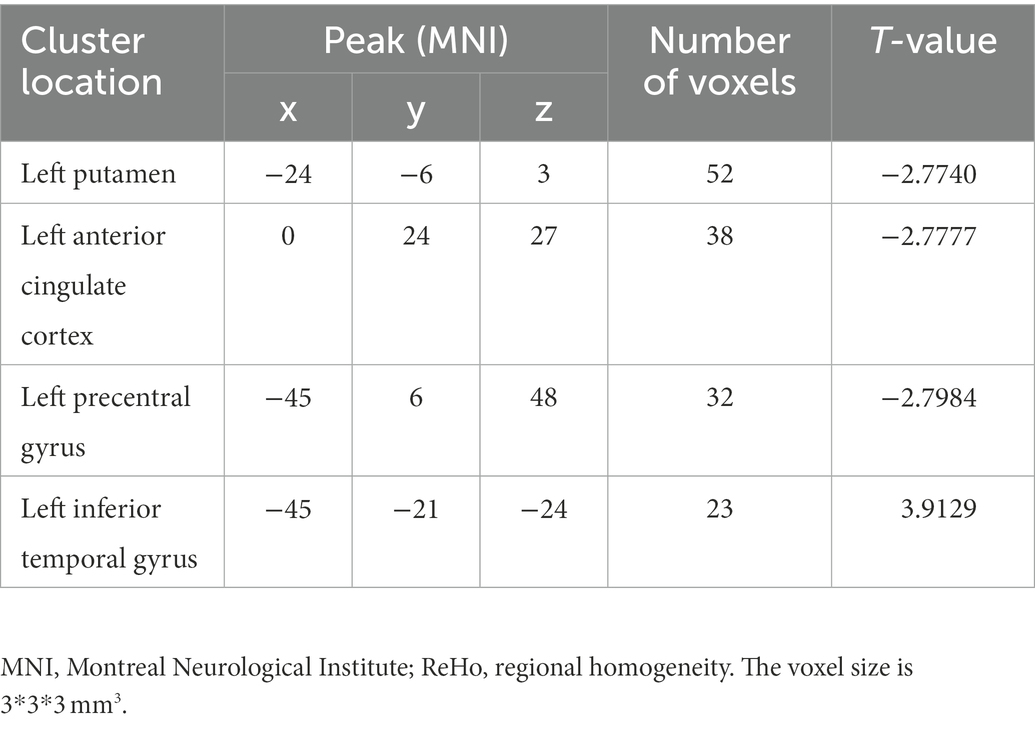
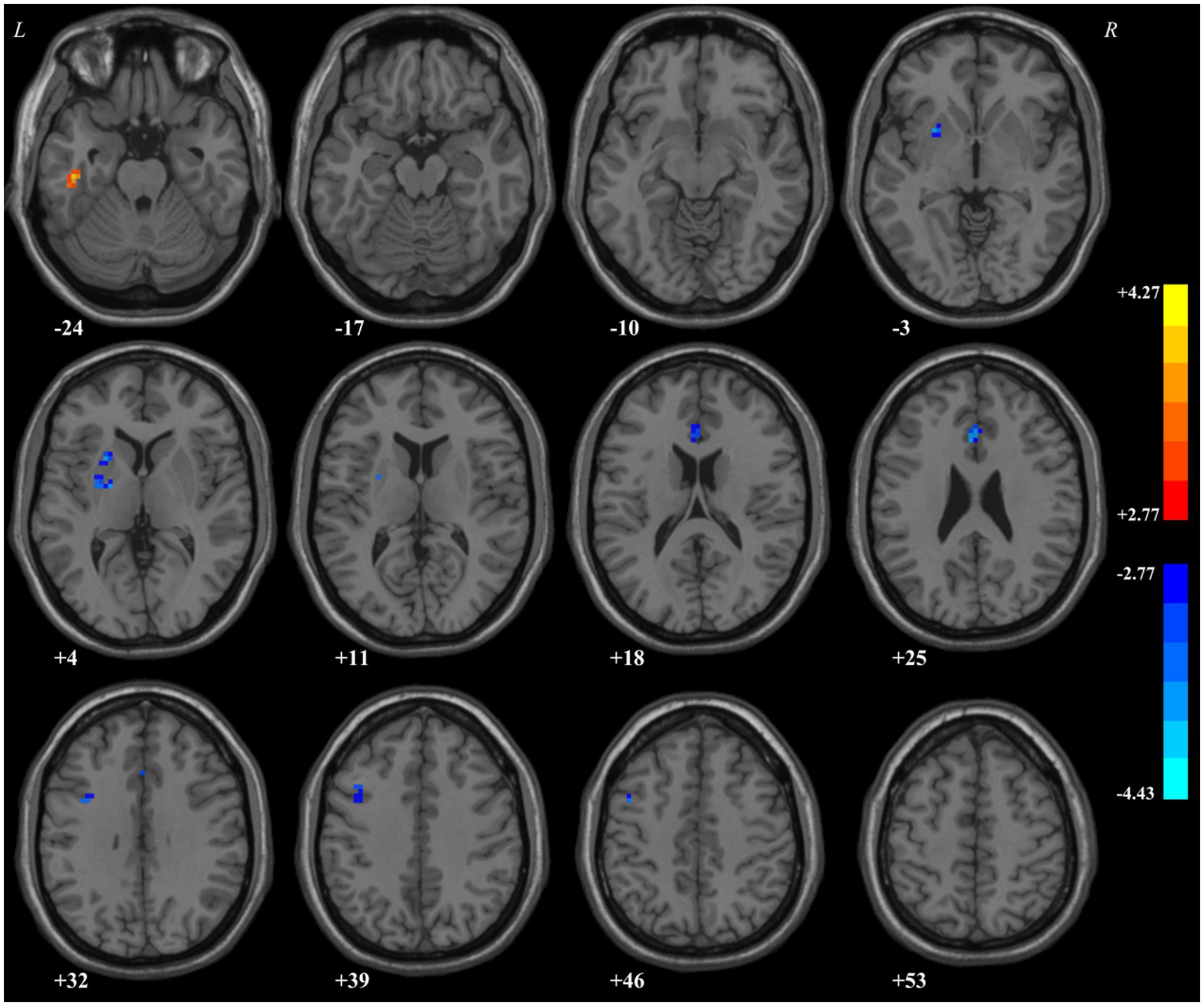
ReHo in the left ITG of patients with MDD significantly increased compared with that of HCs. By contrast, decreased ReHo values were found in the left putamen, anterior cingulate cortex (ACC), and precentral gyrus (Table 5; Figure 1).
The correlations between ReHo values and symptoms and related factors
As shown in Figure 2, the ReHo value of the left putamen was positively correlated with the PR interval of ECG (r = 0.365, p = 0.021), RBANS 4A (r = 0.334, p = 0.035), and D of eye movement examination (r = 0.428, p = 0.023), and negatively correlated with Ae (block; r = −0.397, p = 0.010) and Ae (total) of Stroop word color test (r = −0.327, p = 0.037) in the patients. The ReHo value of left ACC was positively correlated with HAMD score (r = 0.316, p = 0.047), C − 2B + 100 of Stroop word color test (r = 0.326, p = 0.037); and negatively correlated with Ce (Missay) of Stroop word color test (r = −0.315, p = 0.045) and RP of WCST (r = −0.346, p = 0.025) in the patients. The ReHo value of the left precentral gyrus was positively correlated with the N of EPQ (r = 0.318, p = 0.040) and negatively correlated with the L of EPQ (r = −0.446, p = 0.003) in the patients. However, no correlation could survive the Bonferroni correction. In addition, ReHo values of the left putamen, ACC, ITG, and precentral gyrus were not correlated with scores of HAMA, blood biochemistry, SSS, SDSS, and SCSQ in the patients.

Figure 2. Pearson/Spearman correlation analyses showed that correlations between (1) the ReHo values in the left putamen and PR interval (r = 0.365, p = 0.021, df = 39); (2) the ReHo values in the left putamen and Ae (block; r = −0.397, p = 0.010, df = 40); (3) the ReHo values in the left putamen and Ae (total; r = −0.327, p = 0.037, df = 40); (4) the ReHo values in the left putamen and RBANS 4A (r = 0.334, p = 0.035, df = 39); (5) ReHo values in the left putamen and D (r = 0.428, p = 0.023, df = 27); (6) the ReHo values in the left ACC and HAMD score (r = 0.316, p = 0.047, df = 39); (7) the ReHo values in the left ACC and C − 2B + 100 (r = 0.326, p = 0.037, df = 40); (8) the ReHo values in the left ACC and Ce (missay; r = −0.315, p = 0.045, df = 40); (9) the ReHo values in the left ACC and RP (r = −0.346, p = 0.025, df = 41); (10) the ReHo values in the left precentral gyrus and EPQ-N score (r = 0.318, p = 0.040, df = 41); and (11) the ReHo values in the left precentral gyrus and EPQ-L score (r = −0.446, p = 0.003, df = 41). ReHo, regional homogeneity; ACC, anterior cingulate cortex; HAMD, Hamilton Depression Rating Scale; EPQ, Eysenck Personality Questionnaire.
Discussion
This study was designed to detect the alterations of ReHo of patients with MDD and its relationship with a lot of clinical indicators. We observed notable differences between patients with MDD and HCs, with ReHo values increased in the left ITG; and decreased in the left putamen, ACC, and precentral gyrus. In the meanwhile, the ReHo values of left PG, putamen, and ACC are interconnected with multitudinous indicators in patients with MDD.
The results showed that abnormal ReHo values in patients with MDD were concentrated to the left side of the brain. Previous studies on the dominant cerebral hemisphere had shown that the left hemisphere had a dominant role in coordinating the hands to carry out complex movements and processing tools, as well as using language to communicate (Goodglass and Kaplan, 1963). Therefore, we speculated that the left cerebral hemisphere had a key role in MDD.
Brain network dysfunction is thought to underlie the cognitive and emotional abnormalities in MDD (Kaiser et al., 2015). Current researches have shown that MDD is caused by the interaction of three key networks (the central executive network, default mode network, and salience network) and the deficits in the functional connections between them and other brain regions (Culpepper, 2015). In this study, brain regions with abnormal ReHo values in patients with MDD were mainly involved in the salience network. The regional brain components of the salience network contain the dorsal ACC, putamen, anterior insula, and mid-cingulate, which mediate emotional regulation, monitoring for salient events, interoceptive awareness, and motivational behaviors (Yun and Kim, 2021).
Consistent with previous studies (Yang et al., 2015; Fu et al., 2018; Liu et al., 2021), the ReHo values of the left putamen were reduced in patients with MDD. Previous studies have revealed that the left putamen volume was smaller in patients with MDD than that in HCs, and the left putamen was smaller in melancholic MDD compared with non-melancholic MDD (Sachs-Ericsson et al., 2018), suggesting that abnormal spontaneous neural activity in the left putamen of patients with MDD has an anatomical basis. The results of correlation analysis showed that the ReHo values of the left putamen were positively correlated with D of the eye tracking test and RBANS 4A, and negatively correlated with Ae (block) and Ae (total) of SCWT. All of them are cognitive indicators, which are mainly related to attention and semantic retrieval. A study showed that the left anterior putamen operated in conjunction with classical language regionals in the dominant (left) hemisphere, directly related to semantic retrieval and comprehension by using the meta-analytic connectivity modeling (MACM) technique (Vinas-Guasch and Wu, 2017). In addition, other studies have shown that the left putamen is associated with the dorsal prefrontal cortex (DLPFC) by using probabilistic tractography, indicating that DLPFC may aid individuals to maintain mental health at both cognitive and emotional levels (Brosch et al., 2021). The putamen is an important node of the salience network, and DLPFC is an important component of the executive control network (Gunning et al., 2021). Therefore, reduced ReHo values in the left putamen may lead to dysfunctions of the salience network and cognitive impairments in MDD. The DLPFC played a role in goal-driven attention, working memory, task switching, problem-solving, planning, and novelty seeking (Jones and Graff-Radford, 2021). Therefore, abnormal ReHo value in the left putamen leading to cognitive dysfunction in patients with MDD might be directly affecting semantic retrieval and understanding, and further leading to cognitive dysfunction by affecting DLPFC.
As for the correlation between ReHo values and clinical indicators in patients with MDD, abnormal ReHo values of the left putamen were positively correlated with the PR interval. Valenza G et al. demonstrated that brain regions, such as the frontal gyrus, insula, paracingulate and cingulate cortex, lateral occipital cortex, and precuneus cortex, as well as subcortical structures (putamen, thalamus, globus pallidus, amygdala, hippocampus, brainstem, and right caudate nucleus) were involved in the regulation of ANS and mediated cardiovascular control (Valenza et al., 2017). Patients with ANS dysfunction can give rise to sympathoadrenal (SA) hyperactivity, which can cause elevated heart rates (Otte et al., 2005). Meanwhile, coupling with the decrease in parasympathetic tone may contribute to ventricular arrhythmias, and likely interpret the reason for higher cardiovascular mortality in patients with MDD and CVD (Halaris, 2017). Therefore, abnormal ReHo values in the left putamen may be involved in the regulation of ANS, and then affected the PR interval.
In line with a previous study (Xue et al., 2016), the ReHo values of the left ACC were decreased in patients with MDD compared with HCs. A VBM analysis found a decrease in gray matter volume in the left ACC of MDD compared with HCs (Chen et al., 2018). The ACC, a part of the neocortex, is involved in various cognitive functions such as misrecognition, emotional control, and adaptation to change (Allman et al., 2001; Gasquoine, 2013). ACC is involved in executive control in semantic processing through extensive connectivity with the sensory and motor cortices, and impaired connectivity with the ACC may lead to deficits in semantic performance (Zhao et al., 2017). In the present study, correlation analysis showed that the reduced ReHo values of left ACC were positively correlated with HAMD scores, C − 2B + 100 at reaction time in SCWT, and negatively correlated with Ce of SCWT and RP of WCST in the patients. Except for HAMD, they are cognitive indicators. RP is one of the best indicators of WCST to indicate cognitive flexibility (Heaton, 1981). SCWT tests the ability of individuals to overcome the occurrence of interference between two different dimensions of a stimulus through inhibitory control and selective attention mechanisms (Macleod, 1991). Previous studies have demonstrated that the DLPFC, ACC, and striatum are involved in SCWT tasks (Nee et al., 2007), and ACC is thought to monitor conflicts or errors during the SCWT tasks (Milham et al., 2001; Liu et al., 2006). Therefore, reduced ReHo values of the left ACC may contribute to cognitive impairment in MDD by directly affecting the salience network.
Decreased ReHo values were observed in the left precentral gyrus and were positively correlated with EPQ-N scores and negatively correlated with EPQ-L scores in the patients. Reduced ReHo values of the left precentral gyrus in patients with MDD have been found in the published literature (Zhang et al., 2021; Song et al., 2022). Previous studies have shown that patients with MDD who attempted suicide had fewer hemodynamic responses in the left precentral gyrus than patients with MDD without suicidal ideation and HCs (Tsujii et al., 2017). For EPQ, neuroticism has been identified as a risk factor for depression (Grav et al., 2012). Taking into account the higher score of the Lie subscale, the more stable and mature personality, Lie subscale is conducive to the development of behavior (Cao and Su, 2007).
The ITG plays an important role in visual object recognition, decision-making, and attentional impulse (Herath et al., 2001; Li and Kong, 2017). Some studies have shown that the left ITG is essential in lexical and phonological decision-making (Mechelli et al., 2003). In this study, the ReHo values of left ITG in patients with MDD increased, but no correlation with other indicators was observed. Kocsis K et al. found that the more severe depressive symptoms, the higher level of the asymmetry of ITG in patients with MDD (Kocsis et al., 2021). The results of a fractional Amplitude of Low Frequency Fluctuation (fALFF) study showed that the fALFF values of the left ITG were significantly increased in patients with MDD, and the fALFF values of the left ITG were correlated with the score of the Continuous Performance Test (CPT) second subtest (Huang et al., 2017). A voxel-based morphometry (VBM) study has shown a reduction in gray matter volume (GMV) in the right inferior temporal gyrus (Guo et al., 2014). Increased ReHo in the left ITG of patients with MDD may represent the presence of left–right hemisphere asymmetry, indicating that our results are consistent with those of previous studies.
Although there are valuable findings, there are still some limitations in this study. First, the sample size is relatively small. Second, the age of the two groups was mismatched, and mismatched age might have effects on our results although it was applied as a covariate in the analyses. Third, all correlations could not survive the Bonferroni correction although there were several correlations between abnormal ReHo and clinical and cognitive parameters in the patients. Thus, these results should be interpreted with caution. Finally, changes in ReHo values in patients with MDD after treatment were not tracked. Future studies with a view of the dynamic changes in ReHo values in patients with clinically cured MDD are needed to clarify whether the changes in ReHo values in patients with MDD are a trait phenomenon or a state marker.
Overall, this study is the first to evaluate the correlations between ReHo values and numerous indicators of MDD, including biological, clinical, psychological, and cognitive indicators. We found that the ReHo values were mainly significantly correlated with the cognitive indicators of patients with MDD, suggesting that the reduced ReHo values in brain regions of the salience network might be the underpinning of cognitive impairments in patients with MDD.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Foshan Third People’s Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
YW, XL, and HY: methodology, data curation, formal analysis, and writing and editing. YO, WeW, WS, WC, YY, JL, WaW, HL, ZL, and XM: conceptualization and data curation. GX and WG: methodology, data curation, writing—review and editing, and funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by grants from the “The 14th Five-Year” Medical High-level Key Medical Specialty Development Project of Foshan (grant no. FSGSP145069), the project of Foshan Science and Technology Bureau (grant no. 2020001005608), and National Natural Science Foundation of China (grant no. 82171508).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyg.2022.1090181/full#supplementary-material
References
Afridi, M. I., Hina, M., Qureshi, I. S., and Hussain, M. (2011). Cognitive disturbance comparison among drug-naïve depressed cases and healthy controls. J. Coll. Physicians Surg. Pak. 21, 351–355.
Allman, J. M., Hakeem, A., Erwin, J. M., Nimchinsky, E., and Hof, P. (2001). The anterior cingulate cortex. The evolution of an interface between emotion and cognition. Ann. N. Y. Acad. Sci. 935, 107–117. doi: 10.1111/j.1749-6632.2001.tb03476.x
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders: DSM-5™, 5th Edn. Arlington, VA, US: American Psychiatric Publishing, Inc.
Angst, J., Gamma, A., Rössler, W., Ajdacic, V., and Klein, D. N. (2009). Long-term depression versus episodic major depression: results from the prospective Zurich study of a community sample. J. Affect. Disord. 115, 112–121. doi: 10.1016/j.jad.2008.09.023
Barth, J., Schumacher, M., and Herrmann-Lingen, C. (2004). Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom. Med. 66, 802–813. doi: 10.1097/01.psy.0000146332.53619.b2
Bartoli, F., Trotta, G., Crocamo, C., Malerba, M. R., Clerici, M., and Carra, G. (2018). Antioxidant uric acid in treated and untreated subjects with major depressive disorder: a meta-analysis and meta-regression. Eur. Arch. Psychiatry Clin. Neurosci. 268, 119–127. doi: 10.1007/s00406-017-0817-7
Beck, A. T., and Bredemeier, K. (2016). A unified model of depression: integrating clinical, cognitive, biological, and evolutionary. Perspectives 4, 596–619. doi: 10.1177/2167702616628523
Brosch, K., Stein, F., Meller, T., Schmitt, S., Yuksel, D., Ringwald, K. G., et al. (2021). DLPFC volume is a neural correlate of resilience in healthy high-risk individuals with both childhood maltreatment and familial risk for depression. Psychol. Med. 52, 1–7. doi: 10.1017/S0033291721001094
Brouwer, M. E., Williams, A. D., Kennis, M., Fu, Z., Klein, N. S., Cuijpers, P., et al. (2019). Psychological theories of depressive relapse and recurrence: a systematic review and meta-analysis of prospective studies. Clin. Psychol. Rev. 74:101773. doi: 10.1016/j.cpr.2019.101773
Bruder, G. E., Kayser, J., and Tenke, C. E. (2011). “Event-related brain potentials in depression: clinical, cognitive, and neurophysiological implications” in The Oxford handbook of event-related potential components. eds. E. S. Kappenman and S. J. Luck (Oxford University Press).
Caldwell, Y. T., and Steffen, P. R. (2018). Adding HRV biofeedback to psychotherapy increases heart rate variability and improves the treatment of major depressive disorder. Int. J. Psychophysiol. 131, 96–101. doi: 10.1016/j.ijpsycho.2018.01.001
Cao, F., and Su, L. (2007). Internet addiction among Chinese adolescents: prevalence and psychological features. Child Care Health Dev. 33, 275–281. doi: 10.1111/j.1365-2214.2006.00715.x
Chao-Gan, Y., and Yu-Feng, Z. (2010). DPARSF: a MATLAB toolbox for "pipeline" data analysis of resting-state fMRI. Front. Syst. Neurosci. 4:13. doi: 10.3389/fnsys.2010.00013
Chen, L., Wang, Y., Niu, C., Zhong, S., Hu, H., Chen, P., et al. (2018). Common and distinct abnormal frontal-limbic system structural and functional patterns in patients with major depression and bipolar disorder. Neuroimage Clin. 20, 42–50. doi: 10.1016/j.nicl.2018.07.002
Clark, D. B., and Donovan, J. E. (1994). Reliability and validity of the Hamilton anxiety rating scale in an adolescent sample. J. Am. Acad. Child Adolesc. Psychiatry 33, 354–360. doi: 10.1097/00004583-199403000-00009
Collins, P. Y., Patel, V., Joestl, S. S., March, D., Insel, T. R., Daar, A. S., et al. (2011). Grand challenges in global mental health. Nature 475, 27–30. doi: 10.1038/475027a
Culpepper, L. (2015). Neuroanatomy and physiology of cognition. J. Clin. Psychiatry 76:e900. doi: 10.4088/JCP.13086tx3c
Duan, Y., Wei, J., Geng, W., Jiang, J., Zhao, X., Li, T., et al. (2021). Research on cognitive function in anxious depression patients in China. J. Affect. Disord. 280, 121–126. doi: 10.1016/j.jad.2020.11.078
Fagiolini, A., Forgione, R., Maccari, M., Cuomo, A., Morana, B., Dell'osso, M. C., et al. (2013). Prevalence, chronicity, burden and borders of bipolar disorder. J. Affect. Disord. 148, 161–169. doi: 10.1016/j.jad.2013.02.001
Fan, L., Kong, X., Zhang, P., Lin, P., Zhao, J., Ji, X., et al. (2021). Hypersensitivity to negative feedback during dynamic risky-decision making in major depressive disorder: An event-related potential study. J. Affect. Disord. 295, 1421–1431. doi: 10.1016/j.jad.2021.09.019
Foti, D., Carlson, J. M., Sauder, C. L., and Proudfit, G. H. (2014). Reward dysfunction in major depression: multimodal neuroimaging evidence for refining the melancholic phenotype. Neuroimage 101, 50–58. doi: 10.1016/j.neuroimage.2014.06.058
Fu, C., Zhang, H., Xuan, A., Gao, Y., Xu, J., and Shi, D. (2018). A combined study of (18) F-FDG PET-CT and fMRI for assessing resting cerebral function in patients with major depressive disorder. Exp. Ther. Med. 16, 1873–1881. doi: 10.3892/etm.2018.6434
Gasquoine, P. G. (2013). Localization of function in anterior cingulate cortex: from psychosurgery to functional neuroimaging. Neurosci. Biobehav. Rev. 37, 340–348. doi: 10.1016/j.neubiorev.2013.01.002
Gibbons, R. D., Clark, D. C., and Kupfer, D. J. (1993). Exactly what does the Hamilton depression rating scale measure? J. Psychiatr. Res. 27, 259–273. doi: 10.1016/0022-3956(93)90037-3
Goodglass, H., and Kaplan, E. (1963). Disturbance of gesture and pantomime in APHASIA1. Brain 86, 703–720. doi: 10.1093/brain/86.4.703
Grav, S., Stordal, E., Romild, U. K., and Hellzen, O. (2012). The relationship among neuroticism, extraversion, and depression in the HUNT study: in relation to age and gender. Issues Ment. Health Nurs. 33, 777–785. doi: 10.3109/01612840.2012.713082
Guan, L. Y., Hou, W. L., Zhu, Z. H., Cao, J. Q., Tang, Z., Yin, X. Y., et al. (2021). Associations among gonadal hormone, triglycerides and cognitive decline in female patients with major depressive disorders. J. Psychiatr. Res. 143, 580–586. doi: 10.1016/j.jpsychires.2020.11.022
Gunning, F. M., Oberlin, L. E., Schier, M., and Victoria, L. W. (2021). Brain-based mechanisms of late-life depression: implications for novel interventions. Semin. Cell Dev. Biol. 116, 169–179. doi: 10.1016/j.semcdb.2021.05.002
Guo, W. B., Liu, F., Xue, Z. M., Yu, Y., Ma, C. Q., Tan, C. L., et al. (2011a). Abnormal neural activities in first-episode, treatment-naive, short-illness-duration, and treatment-response patients with major depressive disorder: a resting-state fMRI study. J. Affect. Disord. 135, 326–331. doi: 10.1016/j.jad.2011.06.048
Guo, W., Liu, F., Yu, M., Zhang, J., Zhang, Z., Liu, J., et al. (2014). Functional and anatomical brain deficits in drug-naive major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 54, 1–6. doi: 10.1016/j.pnpbp.2014.05.008
Guo, W. B., Sun, X. L., Liu, L., Xu, Q., Wu, R. R., Liu, Z. N., et al. (2011b). Disrupted regional homogeneity in treatment-resistant depression: a resting-state fMRI study. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 1297–1302. doi: 10.1016/j.pnpbp.2011.02.006
Halaris, A. (2017). Inflammation-associated co-morbidity between depression and cardiovascular disease. Curr. Top. Behav. Neurosci. 31, 45–70. doi: 10.1007/7854_2016_28
Harald, B., and Gordon, P. (2012). Meta-review of depressive subtyping models. J. Affect. Disord. 139, 126–140. doi: 10.1016/j.jad.2011.07.015
Heaton, R. K. (1981). A manual for the Wisconsin card sorting test. Psychological Assessment Resources.
Herath, P., Kinomura, S., and Roland, P. E. (2001). Visual recognition: evidence for two distinctive mechanisms from a PET study. Hum. Brain Mapp. 12, 110–119. doi: 10.1002/1097-0193(200102)12:2<110::AID-HBM1008>3.0.CO;2-0
Huang, M., Lu, S., Yu, L., Li, L., Zhang, P., Hu, J., et al. (2017). Altered fractional amplitude of low frequency fluctuation associated with cognitive dysfunction in first-episode drug-naive major depressive disorder patients. BMC Psychiatry 17:11. doi: 10.1186/s12888-016-1190-1
Jaworska, N., and Protzner, A. (2013). Electrocortical features of depression and their clinical utility in assessing antidepressant treatment outcome. Can. J. Psychiatry 58, 509–514. doi: 10.1177/070674371305800905
Jones, D. T., and Graff-Radford, J. (2021). Executive dysfunction and the prefrontal cortex. Continuum 27, 1586–1601. doi: 10.1212/CON.0000000000001009
Kaiser, R. H., Andrews-Hanna, J. R., Wager, T. D., and Pizzagalli, D. A. (2015). Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiat. 72, 603–611. doi: 10.1001/jamapsychiatry.2015.0071
Kennis, M., Gerritsen, L., Van Dalen, M., Williams, A., Cuijpers, P., and Bockting, C. (2019). Prospective biomarkers of major depressive disorder: a systematic review and meta-analysis. Mol. Psychiatry 25, 321–338. doi: 10.1038/s41380-019-0585-z
Kidwell, M., and Ellenbroek, B. A. (2018). Heart and soul: heart rate variability and major depression. Behav. Pharmacol. 29, 152–164. doi: 10.1097/FBP.0000000000000387
Kim, E. J., Kwon, Y. J., Lee, H. Y., Yoon, H. J., Kim, J. S., and Shim, S. H. (2020). The relationship between response-inhibitory event-related potentials and symptoms of attention-deficit/hyperactivity disorder in adult patients with major depressive disorder. Psychiatry Investig. 17, 996–1005. doi: 10.30773/pi.2020.0074
Kircanski, K., Williams, L. M., and Gotlib, I. H. (2019). Heart rate variability as a biomarker of anxious depression response to antidepressant medication. Depress. Anxiety 36, 63–71. doi: 10.1002/da.22843
Klawohn, J., Burani, K., Bruchnak, A., Santopetro, N., and Hajcak, G. (2021). Reduced neural response to reward and pleasant pictures independently relate to depression. Psychol. Med. 51, 741–749. doi: 10.1017/S0033291719003659
Kocsis, K., Holczer, A., Kazinczi, C., Boross, K., Horváth, R., Németh, L. V., et al. (2021). Voxel-based asymmetry of the regional gray matter over the inferior temporal gyrus correlates with depressive symptoms in medicated patients with major depressive disorder. Psychiatry Res. Neuroimaging 317:111378. doi: 10.1016/j.pscychresns.2021.111378
Krishnan, V., and Nestler, E. J. (2008). The molecular neurobiology of depression. Nature 455, 894–902. doi: 10.1038/nature07455
Kunugi, H., Hori, H., and Ogawa, S. (2015). Biochemical markers subtyping major depressive disorder. Psychiatry Clin. Neurosci. 69, 597–608. doi: 10.1111/pcn.12299
Landes, I., Bakos, S., Kohls, G., Bartling, J., Schulte-Körne, G., and Greimel, E. (2018). Altered neural processing of reward and punishment in adolescents with major depressive disorder. J. Affect. Disord. 232, 23–33. doi: 10.1016/j.jad.2018.01.017
Li, J., and Kong, X. Z. (2017). Morphological connectivity correlates with trait impulsivity in healthy adults. PeerJ 5:e3533. doi: 10.7717/peerj.3533
Liang, S., Xue, K., Wang, W., Yu, W., Ma, X., Luo, S., et al. (2020). Altered brain function and clinical features in patients with first-episode, drug naive major depressive disorder: a resting-state fMRI study. Psychiatry Res. Neuroimaging 303:111134. doi: 10.1016/j.pscychresns.2020.111134
Liao, X., Lai, S., Zhong, S., Wang, Y., Zhang, Y., Shen, S., et al. (2021). Interaction of serum copper and Neurometabolites on executive dysfunction in Unmedicated patients with major depressive disorder. Front. Psych. 12:564375. doi: 10.3389/fpsyt.2021.564375
Lin, P. Y., Huang, S. Y., and Su, K. P. (2010). A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol. Psychiatry 68, 140–147. doi: 10.1016/j.biopsych.2010.03.018
Lin, J., Su, Y., Lv, X., Liu, Q., Wang, G., Wei, J., et al. (2020). Perceived stressfulness mediates the effects of subjective social support and negative coping style on suicide risk in Chinese patients with major depressive disorder. J. Affect. Disord. 265, 32–38. doi: 10.1016/j.jad.2020.01.026
Liu, X., Banich, M. T., Jacobson, B. L., and Tanabe, J. L. (2006). Functional dissociation of attentional selection within PFC: response and non-response related aspects of attentional selection as ascertained by fMRI. Cereb. Cortex 16, 827–834. doi: 10.1093/cercor/bhj026
Liu, S., Ma, R., Luo, Y., Liu, P., Zhao, K., Guo, H., et al. (2021). Facial expression recognition and ReHo analysis in major depressive disorder. Front. Psychol. 12:688376. doi: 10.3389/fpsyg.2021.688376
Lv, H., Wang, Z., Tong, E., Williams, L. M., Zaharchuk, G., Zeineh, M., et al. (2018). Resting-state functional MRI: everything that nonexperts have always wanted to know. AJNR Am. J. Neuroradiol. 39, 1390–1399. doi: 10.3174/ajnr.A5527
Macleod, C. M. (1991). Half a century of research on the Stroop effect: an integrative review. Psychol. Bull. 109, 163–203. doi: 10.1037/0033-2909.109.2.163
Małyszczak, K., Inglot, M., Frydecka, D., Hadryś, T., and Pawłowski, T. (2019). Biological and psychological components of depression in patients receiving IFN-alpha therapy for hepatitis C. Adv. Clin. Exp. Med. 28, 1217–1222. doi: 10.17219/acem/104617
Markopoulou, K., Papadopoulos, A., Juruena, M. F., Poon, L., Pariante, C. M., and Cleare, A. J. (2009). The ratio of cortisol/DHEA in treatment resistant depression. Psychoneuroendocrinology 34, 19–26. doi: 10.1016/j.psyneuen.2008.08.004
Mcintyre, R. S. (2016). Implementing treatment strategies for different types of depression. J. Clin. Psychiatry 77, 9–15. doi: 10.4088/JCP.14077su1c.02
Mechelli, A., Gorno-Tempini, M. L., and Price, C. J. (2003). Neuroimaging studies of word and pseudoword reading: consistencies, inconsistencies, and limitations. J. Cogn. Neurosci. 15, 260–271. doi: 10.1162/089892903321208196
Milham, M. P., Banich, M. T., Webb, A., Barad, V., Cohen, N. J., Wszalek, T., et al. (2001). The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Brain Res. Cogn. Brain Res. 12, 467–473. doi: 10.1016/S0926-6410(01)00076-3
Murphy, J. A., and Byrne, G. J. (2012). Prevalence and correlates of the proposed DSM-5 diagnosis of chronic depressive disorder. J. Affect. Disord. 139, 172–180. doi: 10.1016/j.jad.2012.01.033
Musselman, D. L., Evans, D. L., and Nemeroff, C. B. (1998). The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch. Gen. Psychiatry 55, 580–592. doi: 10.1001/archpsyc.55.7.580
Nakano, Y., Baba, H., Maeshima, H., Kitajima, A., Sakai, Y., Baba, K., et al. (2008). Executive dysfunction in medicated, remitted state of major depression. J. Affect. Disord. 111, 46–51. doi: 10.1016/j.jad.2008.01.027
Nee, D. E., Wager, T. D., and Jonides, J. (2007). Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cogn. Affect. Behav. Neurosci. 7, 1–17. doi: 10.3758/CABN.7.1.1
Nobis, A., Zalewski, D., and Waszkiewicz, N. (2020). Peripheral markers of depression. J. Clin. Med. 9. doi: 10.3390/jcm9123793
Otsubo, T., Hokama, C., Sano, N., Watanabe, Y., Kikuchi, T., and Tanaka, K. (2021). How significant is the assessment of the DSM-5 'anxious distress' specifier in patients with major depressive disorder without comorbid anxiety disorders in the continuation/maintenance phase? Int. J. Psychiatry Clin. Pract. 25, 385–392. doi: 10.1080/13651501.2021.1907415
Otte, C., Neylan, T. C., Pipkin, S. S., Browner, W. S., and Whooley, M. A. (2005). Depressive symptoms and 24-hour urinary norepinephrine excretion levels in patients with coronary disease: findings from the heart and soul study. Am. J. Psychiatry 162, 2139–2145. doi: 10.1176/appi.ajp.162.11.2139
Pan, A., Keum, N., Okereke, O. I., Sun, Q., Kivimaki, M., Rubin, R. R., et al. (2012). Bidirectional association between depression and metabolic syndrome: a systematic review and meta-analysis of epidemiological studies. Diabetes Care 35, 1171–1180. doi: 10.2337/dc11-2055
Pan, Z., Park, C., Brietzke, E., Zuckerman, H., Rong, C., Mansur, R. B., et al. (2019). Cognitive impairment in major depressive disorder. CNS Spectr. 24, 22–29. doi: 10.1017/S1092852918001207
Parker, K. J., Schatzberg, A. F., and Lyons, D. M. (2003). Neuroendocrine aspects of hypercortisolism in major depression. Horm. Behav. 43, 60–66. doi: 10.1016/S0018-506X(02)00016-8
Persons, J. E., and Fiedorowicz, J. G. (2016). Depression and serum low-density lipoprotein: a systematic review and meta-analysis. J. Affect. Disord. 206, 55–67. doi: 10.1016/j.jad.2016.07.033
Qin, X., Wang, M., Lu, X., Sun, J., Dong, Q., Zhang, L., et al. (2021). Childhood emotional neglect is associated with low social support in Chinese patients with major depressive disorder. Front. Psych. 12:781738. doi: 10.3389/fpsyt.2021.781738
Reppermund, S., Ising, M., Lucae, S., and Zihl, J. (2009). Cognitive impairment in unipolar depression is persistent and non-specific: further evidence for the final common pathway disorder hypothesis. Psychol. Med. 39, 603–614. doi: 10.1017/S003329170800411X
Rock, P. L., Roiser, J. P., Riedel, W. J., and Blackwell, A. D. (2014). Cognitive impairment in depression: a systematic review and meta-analysis. Psychol. Med. 44, 2029–2040. doi: 10.1017/S0033291713002535
Sachs-Ericsson, N. J., Hajcak, G., Sheffler, J. L., Stanley, I. H., Selby, E. A., Potter, G. G., et al. (2018). Putamen volume differences among older adults: depression status, melancholia, and age. J. Geriatr. Psychiatry Neurol. 31, 39–49. doi: 10.1177/0891988717747049
Saleh, A., Potter, G. G., Mcquoid, D. R., Boyd, B., Turner, R., Macfall, J. R., et al. (2017). Effects of early life stress on depression, cognitive performance and brain morphology. Psychol. Med. 47, 171–181. doi: 10.1017/S0033291716002403
Schatzberg, A. F., Rothschild, A. J., Bond, T. C., and Cole, J. O. (1984). The DST in psychotic depression: diagnostic and pathophysiologic implications. Psychopharmacol. Bull. 20, 362–364.
Shi, Y., Li, J., Feng, Z., Xie, H., Duan, J., Chen, F., et al. (2020). Abnormal functional connectivity strength in first-episode, drug-naive adult patients with major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 97:109759. doi: 10.1016/j.pnpbp.2019.109759
Song, Y., Huang, C., Zhong, Y., Wang, X., and Tao, G. (2022). Abnormal Reginal homogeneity in left anterior Cingulum cortex and Precentral Gyrus as a potential neuroimaging biomarker for first-episode major depressive disorder. Front. Psych. 13:924431. doi: 10.3389/fpsyt.2022.924431
Song, X., Zhang, Y., and Liu, Y. (2014). Frequency specificity of regional homogeneity in the resting-state human brain. PLoS One 9:e86818. doi: 10.1371/journal.pone.0086818
Stapelberg, N. J., Hamilton-Craig, I., Neumann, D. L., Shum, D. H., and Mcconnell, H. (2012). Mind and heart: heart rate variability in major depressive disorder and coronary heart disease - a review and recommendations. Aust. N. Z. J. Psychiatry 46, 946–957. doi: 10.1177/0004867412444624
Stetler, C., and Miller, G. E. (2011). Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom. Med. 73, 114–126. doi: 10.1097/PSY.0b013e31820ad12b
Stewart, J. G., Mazurka, R., Bond, L., Wynne-Edwards, K. E., and Harkness, K. L. (2013). Rumination and impaired cortisol recovery following a social stressor in adolescent depression. J. Abnorm. Child Psychol. 41, 1015–1026. doi: 10.1007/s10802-013-9740-1
Takahashi, J., Hirano, Y., Miura, K., Morita, K., Fujimoto, M., Yamamori, H., et al. (2021). Eye movement abnormalities in major depressive disorder. Front. Psych. 12. doi: 10.3389/fpsyt.2021.673443
Teng, Z., Wang, L., Li, S., Tan, Y., Qiu, Y., Wu, C., et al. (2021). Low BDNF levels in serum are associated with cognitive impairments in medication-naïve patients with current depressive episode in BD II and MDD. J. Affect. Disord. 293, 90–96. doi: 10.1016/j.jad.2021.06.018
Tseng, Y. J., Nouchi, R., and Cheng, C. H. (2021). Mismatch negativity in patients with major depressive disorder: a meta-analysis. Clin. Neurophysiol. 132, 2654–2665. doi: 10.1016/j.clinph.2021.06.019
Tsujii, N., Mikawa, W., Tsujimoto, E., Adachi, T., Niwa, A., Ono, H., et al. (2017). Reduced left precentral regional responses in patients with major depressive disorder and history of suicide attempts. PLoS One 12:e0175249. doi: 10.1371/journal.pone.0175249
Valenza, G., Duggento, A., Passamonti, L., Diciotti, S., Tessa, C., Barbieri, R., et al. (2017). Resting-state brain correlates of instantaneous autonomic outflow. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2017, 3325–3328. doi: 10.1109/EMBC.2017.8037568
Vinas-Guasch, N., and Wu, Y. J. (2017). The role of the putamen in language: a meta-analytic connectivity modeling study. Brain Struct. Funct. 222, 3991–4004. doi: 10.1007/s00429-017-1450-y
Wang, X., Zhou, H., and Zhu, X. (2020). Attention deficits in adults with major depressive disorder: a systematic review and meta-analysis. Asian J. Psychiatr. 53:102359. doi: 10.1016/j.ajp.2020.102359
White, E. J., Nacke, M., Akeman, E., Cannon, M. J., Mayeli, A., Touthang, J., et al. (2021). P300 amplitude during a monetary incentive delay task predicts future therapy completion in individuals with major depressive disorder. J. Affect. Disord. 295, 873–882. doi: 10.1016/j.jad.2021.08.106
Williams, L. M., Rush, A. J., Koslow, S. H., Wisniewski, S. R., Cooper, N. J., Nemeroff, C. B., et al. (2011). International study to predict optimized treatment for depression (iSPOT-D), a randomized clinical trial: rationale and protocol. Trials 12:4. doi: 10.1186/1745-6215-12-4
Wu, X., He, H., Shi, L., Xia, Y., Zuang, K., Feng, Q., et al. (2019). Personality traits are related with dynamic functional connectivity in major depression disorder: a resting-state analysis. J. Affect. Disord. 245, 1032–1042. doi: 10.1016/j.jad.2018.11.002
Xin, W., Yu, R., and Zhao, L. (2021). Event-related-potential based evidence of cognitive dysfunction of processing emotional faces in major depressive disorder patients. Neurosci. Lett. 742:135545. doi: 10.1016/j.neulet.2020.135545
Xue, S., Wang, X., Wang, W., Liu, J., and Qiu, J. (2016). Frequency-dependent alterations in regional homogeneity in major depression. Behav. Brain Res. 306, 13–19. doi: 10.1016/j.bbr.2016.03.012
Yan, M., Chen, J., Liu, F., Li, H., Huang, R., Tang, Y., et al. (2021a). Disrupted regional homogeneity in major depressive disorder with gastrointestinal symptoms at rest. Front. Psych. 12:636820. doi: 10.3389/fpsyt.2021.636820
Yan, M., He, Y., Cui, X., Liu, F., Li, H., Huang, R., et al. (2021b). Disrupted regional homogeneity in melancholic and non-melancholic major depressive disorder at rest. Front. Psych. 12:618805. doi: 10.3389/fpsyt.2021.618805
Yang, H., Chen, X., Chen, Z. B., Li, L., Li, X. Y., Castellanos, F. X., et al. (2021). Disrupted intrinsic functional brain topology in patients with major depressive disorder. Mol. Psychiatry 26, 7363–7371. doi: 10.1038/s41380-021-01247-2
Yang, L., Lin, F., Zhou, Y., Xu, J., Yu, C., Pan, W. J., et al. (2013). Iterative cross-correlation analysis of resting state functional magnetic resonance imaging data. PLoS One 8:e58653. doi: 10.1371/journal.pone.0058653
Yang, X., Ma, X., Li, M., Liu, Y., Zhang, J., Huang, B., et al. (2015). Anatomical and functional brain abnormalities in unmedicated major depressive disorder. Neuropsychiatr. Dis. Treat. 11, 2415–2423. doi: 10.2147/NDT.S93055
Yun, J. Y., and Kim, Y. K. (2021). Graph theory approach for the structural-functional brain connectome of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 111:110401. doi: 10.1016/j.pnpbp.2021.110401
Zang, Y., Jiang, T., Lu, Y., He, Y., and Tian, L. (2004). Regional homogeneity approach to fMRI data analysis. Neuroimage 22, 394–400. doi: 10.1016/j.neuroimage.2003.12.030
Zhang, Z., Chen, Y., Wei, W., Yang, X., Meng, Y., Yu, H., et al. (2021). Changes in regional homogeneity of medication-free major depressive disorder patients with different onset ages. Front. Psych. 12:713614. doi: 10.3389/fpsyt.2021.713614
Zhang, X., Di, X., Lei, H., Yang, J., Xiao, J., Wang, X., et al. (2016). Imbalanced spontaneous brain activity in orbitofrontal-insular circuits in individuals with cognitive vulnerability to depression. J. Affect. Disord. 198, 56–63. doi: 10.1016/j.jad.2016.03.001
Zhang, F. F., Peng, W., Sweeney, J. A., Jia, Z. Y., and Gong, Q. Y. (2018). Brain structure alterations in depression: Psychoradiological evidence. CNS Neurosci. Ther. 24, 994–1003. doi: 10.1111/cns.12835
Zhao, Y., Song, L., Ding, J., Lin, N., Wang, Q., Du, X., et al. (2017). Left anterior temporal lobe and bilateral anterior cingulate cortex are semantic hub regions: evidence from behavior-nodal degree mapping in brain-damaged patients. J. Neurosci. 37, 141–151. doi: 10.1523/JNEUROSCI.1946-16.2016
Zhou, Q., Lv, X., Zhou, S., Liu, Q., Tian, H., Zhang, K., et al. (2021). Inflammatory cytokines, cognition, and response to antidepressant treatment in patients with major depressive disorder. Psychiatry Res. 305:114202. doi: 10.1016/j.psychres.2021.114202
Zhou, Y., Ma, Y., Wu, Q., Wang, Q., Yang, W. F. Z., Wang, Y., et al. (2021). Comparison of thyroid hormone levels between patients with major depressive disorder and healthy individuals in China. Front. Psych. 12:750749. doi: 10.3389/fpsyt.2021.750749
Keywords: major depressive disorder, regional homogeneity, support vector machine, salience network, cognition
Citation: Wang Y, Li X, Yan H, Zhang Q, Ou Y, Wu W, Shangguan W, Chen W, Yu Y, Liang J, Wu W, Liao H, Liu Z, Mai X, Xie G and Guo W (2023) Multiple examinations indicated associations between abnormal regional homogeneity and cognitive dysfunction in major depressive disorder. Front. Psychol. 13:1090181. doi: 10.3389/fpsyg.2022.1090181
Edited by:
Bao-Liang Zhong, Wuhan Mental Health Center, ChinaReviewed by:
Daihui Peng, Shanghai Jiao Tong University, ChinaJiajia Zhu, First Affiliated Hospital of Anhui Medical University, China
Wenhao Jiang, Southeast University, China
Copyright © 2023 Wang, Li, Yan, Zhang, Ou, Wu, Shangguan, Chen, Yu, Liang, Wu, Liao, Liu, Mai, Xie and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guojun Xie, ✉ xiegjfs@126.com; Wenbin Guo, ✉ guowenbin76@csu.edu.cn
†These authors have contributed equally to this work
 Yun Wang1†
Yun Wang1†