
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Psychol. , 14 November 2022
Sec. Cognition
Volume 13 - 2022 | https://doi.org/10.3389/fpsyg.2022.1045217
Background: Over-general autobiographical memory (AM) retrieval is proposed to have a causal role in the maintenance of psychological disorders like depression and PTSD. As such, the identification of drugs that modulate AM specificity may open up new avenues of research on pharmacological modeling and treatment of psychological disorders.
Aim: The current review summarizes randomized, placebo-controlled studies of acute pharmacological modulation of AM specificity.
Method: A systematic search was conducted of studies that examined the acute effects of pharmacological interventions on AM specificity in human volunteers (healthy and clinical participants) measured using the Autobiographical Memory Test.
Results: Seventeen studies were identified (986 total participants), of which 16 were judged to have low risk of bias. The presence and direction of effects varied across drugs and diagnostic status of participants (clinical vs. healthy volunteers). The most commonly studied drug—hydrocortisone—produced an overall impairment in AM specificity in healthy volunteers [g = −0.28, CI (−0.53, −0.03), p = 0.03], although improvements were reported in two studies of clinical participants. In general, studies of monoamine modulators reported no effect on specificity.
Conclusion: Pharmacological enhancement of AM specificity is inconsistent, although monaminergic modulators show little promise in this regard. Drugs that reduce AM specificity in healthy volunteers may be useful experimental-pharmacological tools that mimic an important transdiagnostic impairment in psychological disorders.
Systematic review registration: PROSPERO, identifier CRD42020199076, https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020199076.
Autobiographical memory (AM) refers to the store of self-related knowledge and memories for personal experiences (Conway and Pleydell-Pearce, 2000; Tulving, 2002). It is central to an individual's sense of self, identity, and their capacity to understand their place in the world. Dysfunctions in AM processing may therefore have serious implications for mental wellbeing. Much of the interest in AM has focused on the (deficits in) retrieval of specific AMs (i.e., spatiotemporally unique memories of personally experienced events). Although particularly well-characterized in depressive (Williams et al., 2007) and traumatic stress disorders (Moore and Zoellner, 2007; Ono et al., 2016), reduced retrieval of specific AMs, otherwise known as overgeneral AM, is found in a range of psychological disorders (e.g., Jones et al., 1999; Berna et al., 2016; Barry et al., 2021). In sufferers of depression, for example, maladaptive processing of memories for personal experiences and self-related knowledge may include overgeneral AM, reduced recollection of positive AM, and enhanced (repetitive and involuntary) access to negative self-representations and AMs (Dalgleish and Werner-Seidler, 2014). While these particular biases represent distinct phenomena, they all rely on dysfunctional or biased memory retrieval processes (Brewin, 2006), which are likely further compounded by negative affective biases (e.g., Hitchcock et al., 2020) and impaired or overgeneralized encoding of experiences (Dillon and Pizzagalli, 2018; Kumar et al., 2018; Murphy et al., 2019; Ai et al., 2020).
Pharmacological agents have been used to model neuropsychological dysfunctions in encoding or consolidation stages of memory processing (e.g., Kamboj and Curran, 2006). Such approaches have been invaluable in the study of organic brain disorders like Alzheimer's disease through the ability to replicate the commonly observed memory encoding/consolidation impairments that characterize these disorders (e.g., Haider et al., 2016). Psychological disorders, however, are characterized by more complex patterns of hyper- and/or hypomnesia that reflect dysfunctional memory storage and retrieval, yet evidence suggests that acute impairments to episodic memory retrieval can also be induced pharmacologically (e.g., Strange et al., 2003; Kuhlmann et al., 2005; Hurlemann et al., 2007; Diekelmann et al., 2011; Rimmele et al., 2015; Kroes et al., 2016). Retrieval dysfunction has largely been induced by agents targeting stress-related hormones and neurotransmitters [i.e., endogenous regulators of glucocorticoids and (nor)adrenaline]. These findings highlight the potential for developing pharmacological models of and, by extension, potential therapeutic targets for dysfunctional retrieval. However, unlike episodic encoding impairments in Alzheimer's, for example, there are currently no accepted experimental-pharmacological models of the transdiagnostic dysfunctional retrieval processes observed in common psychological disorders like depression, anxiety, and PTSD.
In Conway and Pleydell-Pearce's (2000) influential model of AM, which aims to account for variation in AM specificity, specific AM retrieval involves the reactivation of self-related knowledge from a hierarchical store. Executive processes evaluate the current contents of memory in relation to their relevance to current goals and are involved in terminating the search when the search goals (to retrieve a specific episode) are achieved. The ability to recall specific AMs is most commonly assessed using the Autobiographical Memory Test (AMT; Williams and Broadbent, 1986). Cued memories are considered specific if they contain unique temporal (specific event lasting < 1 day) or spatial (a specific location and arrangement of objects and people) episodic details. Reliability and validity of the AMT has been assessed across test re-test intervals ranging from 1 to 5 months (Raes et al., 2009b), and a study of 40,000 memories in young teenagers found that the AMT operates well over a wide range of scores and delivers a reliable continuous measure of overgeneral (i.e., non-specific) AM (Heron et al., 2012). The AMT reliably measures the single factor of AM specificity in both healthy (Heron et al., 2012) and clinical samples (Griffith et al., 2012).
AMT performance in psychological disorders is characterized by a tendency to retrieve a larger number of general relative to specific AMs in response to word cues. Based on this pattern of AMT performance, Williams (2006) and Williams et al. (2007) adapted Conway and Pleydell-Pearce's (2000) model to account for the overgeneral AM retrieval observed in emotional disorders (Williams et al., 2007; see also Ono et al., 2016). The Williams model emphasizes three critical mechanisms of cognitive maladaptation which, alone or in combination, contribute to overgeneral AM retrieval: capture and rumination (a tendency to perseverate on a negative, analytic form of memory processing), functional avoidance (a tendency for memory search to be truncated to prevent full affective activation accompanying specific retrieval), and “executive control” (impairment in tracking of specificity of AMs), the so called CaRFAX model.
Overgeneral AM has consistently been associated with depression, PTSD, acute stress disorder, and other psychological disorders (Moore and Zoellner, 2007; Williams et al., 2007; Barry et al., 2021). Overgeneral AM has been found to lessen following natural remission or successful psychological treatment (Sutherland and Bryant, 2007; Ahern and Semkovska, 2017), suggesting that reduced specific AM retrieval may represent a state marker of psychological disorder. However, longitudinally, overgeneral AM also predicts PTSD symptoms (Bryant et al., 2007), while prepartum AM non-specificity (i.e., overgeneral AM) has been associated with more posttraumatic symptoms in those who had experienced a complicated pregnancy (Hauer et al., 2009). In an fMRI study of adolescents with or without documented experience of childhood maltreatment, AM-related brain activity was not a significant predictor of future psychosocial functioning, whereas, in those with maltreatment histories, greater overgeneral AM (assessed using the AMT) at baseline predicted reduced prosocial behavior at follow-up (Puetz et al., 2021).
Dysfunctional AM specificity has been suggested to be a consequence of trauma or depression (the scarring hypothesis, e.g., Stokes et al., 2004; Crane et al., 2014), or, alternatively, an antecedent trait, increasing the likelihood of developing depression or PTSD following a negative experience (the vulnerability hypothesis, e.g., Hauer et al., 2009). Overgeneral AM, assessed using the AMT is both associated with, and a predictor of, higher depressive symptoms at follow-up in clinical samples (Sumner et al., 2010; Warne et al., 2020; Hallford et al., 2021).
Understanding the factors that contribute to and potentially ameliorate dysfunction in AM specificity using the AMT and pharmacological tools could have implications for the treatment (or prevention) of several psychological disorders. In particular, if overgeneral AM has a causal role in symptom maintenance or deterioration (Hallford et al., 2021), (psycho)pharmacological treatments that reverse this dysfunction could be employed in the treatment of personality disorders (Startup et al., 2001), schizophrenia (Corcoran and Frith, 2003; Barry et al., 2019; Zhang et al., 2019), obsessive compulsion (Spinhoven et al., 2009), depression (Dalgleish and Werner-Seidler, 2014), and traumatic stress disorders (Schönfeld and Ehlers, 2006; Schönfeld et al., 2007).
A number of behavioral interventions potentially target memory specificity in neuropsychiatric disorders (e.g., life review/reminiscence therapy; Arean et al., 1993; Memory Specificity Training; Raes et al., 2009a) based on Williams's conceptualization of the causal role of overgeneral memory in emotional disorders (particularly depression) and in the latter case is specifically designed to reverse this cognitive phenotype. A meta-analytic review of the effect of Memory Specificity Training on symptoms in emotional disorders (Barry et al., 2019) found a substantial reduction in depressive symptoms, although these benefits appeared to be relatively short-lived. This might suggest that a maintenance form of this training is required in order to achieve lasting effects. In any case, memory specificity treatment development has significantly benefited from advances in our understanding of basic cognitive processes underlying symptoms of psychopathology (e.g., studies of the CaRFAX model), whereas progress in the development of pharmacological treatments targeting overgenerality has been slower. Understanding the neuropharmacological systems underlying memory specificity might therefore provide key insights into fruitful avenues for future pharmacological intervention development.
Research on the neurobiology of stress and memory has provided significant insights into the neuropharmacology of AM by delineating the role of the HPA-axis in modulation of hippocampal-dependent (i.e., episodic) retrieval. Persistent hyperactivity of the HPA-axis due to prolonged stress, depression, or experiences of trauma can lead to significant structural and functional changes to key regions in emotional memory processing regions (i.e., the hippocampus, amygdala, and prefrontal cortex (PFC) (van Eijndhoven et al., 2009; Schmaal et al., 2016; Wellman and Moench, 2019), which may then influence episodic memory, including AM.
The hippocampus and amygdala are modulated by glucocorticoid and adrenergic regulators of the stress response system (Wang et al., 2013, 2014) and thought to critically support AM function. In AM retrieval, the amygdala has been shown to support emotional experience or the reinstatement of emotion at autobiographical retrieval (Bocchio et al., 2017; Ford and Kensinger, 2019), while hippocampal structures are thought to be responsible for accessing and integrating information into a spatiotemporally contextualized memory trace (for reviews see Cabeza and St Jacques, 2007; Sheldon and Levine, 2016; Sheldon et al., 2019). This integration (during encoding and retrieval) occurs across a broad network of brain structures involved in self-referential and emotional processing, autonoetic awareness, scene reconstruction and accuracy monitoring, which may at least partially explain why dysfunctional AM specificity has emerged as a transdiagnostic cognitive impairment in emotional disorders.
Abnormal HPA-axis functioning a common feature across psychological disorders including major depressive disorder (MDD; Stetler and Miller, 2011; Iob et al., 2020), PTSD (Steudte et al., 2011), schizophrenia (Aas et al., 2019) and borderline personality disorder (BPD; Drews et al., 2019). Hence, pharmacological research into the neurobiological correlates of depression and traumatic stress disorders (e.g., dysregulated HPA-axis functioning) may provide some clues as to the nature of the neurophysiological and neurotransmitter/neuromodulator disruption underlying overgeneral AM.
Stress-related episodic elevations in cortisol, analogous to those seen in depression (Bhagwagar et al., 2005; Herane-Vives et al., 2018) can be mimicked by experimentally elevating the stress-hormone cortisol, either endogenously (through stress induction procedures) or pharmacologically (through exogenous hydrocortisone administration). When cortisol elevation follows memory encoding this commonly results in improved delayed recall performance for emotionally salient aspects of memory (e.g., Smeets et al., 2008; Cunningham et al., 2021). However, when levels are endogenously or exogenously elevated prior to retrieval this has a deleterious effect on immediate recall (Smeets et al., 2008; Terfehr et al., 2011; Schwabe and Wolf, 2014; Merz et al., 2018). Both of these effects may further follow a non-linear (inverted U) dose-response relationship, producing seemingly discrepant findings and highlighting the complex interaction between episodic stress, hormonal concentrations, and memory processing.
D-cycloserine (DCS), a partial agonist at the glycine binding site of the NMDA receptor, has long been thought to facilitate memory consolidation and retrieval (Quartermain et al., 1994), and has shown promising results in some studies when combined with behavioral (exposure-based) therapy (e.g., Inslicht et al., 2021; for reviews see Schade and Paulus, 2016; Mataix-Cols et al., 2017; Rosenfield et al., 2019). Typically, probing of monoaminergic, glutamatergic and oxytocin signaling can only be achieved pharmacologically.
Studies of serotoninergic modulation are of similar relevance to AM retrieval given the central role of serotonin in emotional processing and cognitive functioning (e.g., Hornboll et al., 2018; Knorr et al., 2019), as well as the proposed disturbance in serotoninergic functioning in some biological theories of depression (e.g., Ruhé et al., 2007) and serotonergic basis of its most common treatments. Acute tryptophan depletion has been shown to impair episodic retrieval (McAllister-Williams et al., 2002) yet evidence is mixed (e.g., van der Veen et al., 2006).
The dorsal hippocampus has been linked to an oxytocin-sensitive forebrain stress circuit, and as a central regulator (via suppression) of stress-induced neuroendocrine and molecular responses. As such, oxytocin may warrant investigation in AM retrieval dysfunction (Windle et al., 2004). In healthy volunteers, a single dose of oxytocin prior to learning (Herzmann et al., 2013) and prior to both learning and retrieval (Weigand et al., 2013) has been found to improve retrieval of negative emotional material. Both the HPA-axis and locus coeruleus (LC) -noradrenergic (NA) system are known to regulate the physiological stress response, yet the impact of LC-NA system dysregulation on AM specificity remains comparatively understudied. However, in similar studies of the acute effects of antidepressant treatments, monoaminergic modulators have been shown to improve positive information retrieval, in the absence of generalized improvements in cognitive performance (Harmer et al., 2003, 2008; Arnone et al., 2009). In extending these effects to clinical samples, Harmer et al. (2009) provided initial evidence that MDD patients may be more sensitive than healthy controls to episodic memory improvements following noradrenergic stimulation, as the acute administration of a noradrenaline reuptake inhibitor reboxetine resulted in an improvement in positive episodic retrieval in depressed patients only.
Our understanding of the cognitive neuroscience of AM specificity has improved considerably in recent years (Barry et al., 2018). However, the neuropsychopharmacology of AM is less well understood. The goal of the current review is to systematically review findings from published placebo-controlled studies of the pharmacological modulation of AM specificity assessed using the AMT. We aim to address a gap in the AM with the aim of identify the most promising pharmacological systems for future AM research.
The review methodology was preregistered on the PROSPERO prospective register of systematic reviews (reg. no: CRD42020199076). PsycInfo, PsycArticles, Ovid MEDLINE®, Embase, Scopus and Web of Science Core Collection databases, the Cochrane trial registry, and the OpenGrey and Open Access Theses and Dissertations databases were searched using the following terms: (Autobiographic* OR ((personal* or self* or event*) adj2 memor*) OR ((real*life or personal*) adj1 (experience* or episodic* or event*))) AND ((tryptophan or Levotryptophan or l*tryptophan) OR (placebo* or sham)). Note, “tryptophan” was included as a specific term since initial scoping searches failed to identify relevant tryptophan depletion studies known to the authors. The use of “placebo” was expected to capture placebo-controlled studies of pharmaceutical preparations. However, the term “sham” is typically used in tryptophan depletion studies, and inclusion of this term did indeed capture the TD studies. Proximity operators were adapted for each database. The ClinicalTrials.gov registry and World Health Organization International Clinical Trials Registry Platform were searched using the terms “autobiographical OR episodic” and “memory”. The Open Access Theses and Dissertations search was restricted to English language and doctorate theses only. The above listed searches were originally run on 2nd July 2020 and up-dated on 5th May 2022. One additional study was identified between these searches (Wong et al., 2021). No other restrictions (e.g., date restrictions) were placed on the search criteria. A pre-registered aim of the review was to perform a meta-analysis on effects of various pharmacological treatments on AM retrieval. However, upon review, we could not justify such a quantitative synthesis because of the small number of studies (usually only a single study) that examined each specific drug class, and the lack of a biological rationale for pooling studies across drug classes that targeted different neurotransmitter/neuromodulatory systems with different predicted directional effects on AM. As such, this aim could not be implemented in full, although a small number of methodologically homogeneous studies of hydrocortisone were combined to obtain a provisional effect size for this drug alone.
Search, screening, and selection processes were conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (Page et al., 2021; Figure 1). Titles and abstracts were independently reviewed by the author (E.C) and another researcher (G.P). There were no disagreements on final study inclusions. Eligibility was restricted to randomized, placebo-controlled studies of adult participants, that including a pharmacological manipulation administered prior to AM retrieval. Eligibility was also restricted to studies where AM retrieval was elicited and scored for specificity using either the original or a modified version of the AMT to provide a continuous measure of the number of specific memories retrieved (Williams and Broadbent, 1986).
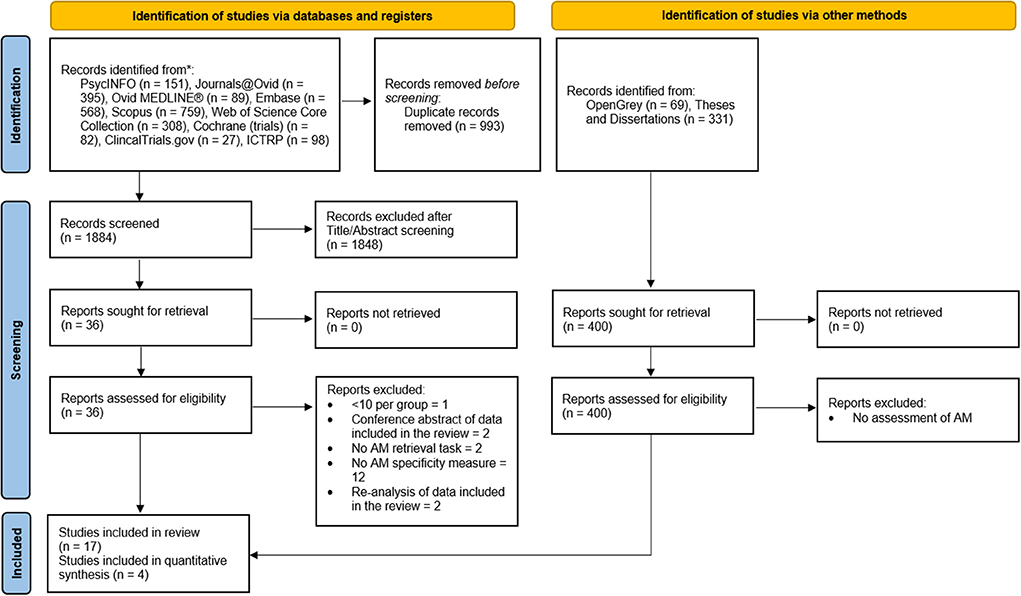
Figure 1. Page et al. (2021) flowchart of the study selection process.
The included studies were diverse in terms of participant characteristics and the neurotransmitter/neuromodulator system(s) being targeted. Only within-subjects crossover studies that examined the effects of low dose hydrocortisone in healthy volunteers could be used to calculate a pooled effect size estimate (Buss et al., 2004; Schlosser et al., 2010; Wingenfeld et al., 2012a, 2013).
The following study characteristics were independently extracted by two researchers (EC and GP): study design, nature of participant group(s), pharmacological treatment characteristics (drug, dose, route of administration), biological assessment of systemic drug levels, author-specified predictions on the effects of the drug on retrieval (i.e., improvement or reduction in specificity), AMT protocols, AMT performance (number of specific memories), other outcome and explanatory variables (Table 1). For outcomes included the pooled analysis of the effects of hydrocortisone in healthy volunteers, one author provided means and standard deviations since numerical values because these were not reported in the publication (Wingenfeld et al., 2012a). One study was not included in the pooled analysis because we were unable to obtain the means and standard deviations required (Fleischer et al., 2017). The methods sections of included studies were independently reviewed by EC and GP and the Cochrane risk of bias tool (Higgins et al., 2016) was used to evaluate methodological features of the reviewed studies.
Effect sizes on the number of specific AMs generated after drug and placebo administration were calculated by dividing mean differences by pooled SDs and applying a correction for small sample bias (i.e., Hedges' g). A pooled ES was determined via a random effects model using maximum likelihood estimation for the four studies that used low dose hydrocortisone in healthy volunteers using SPSS (IBM Corp. Released 2020. IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp).
The literature search yielded a total of 17 studies from 11 publications. A total of n = 986 participants (mean age = 27.65, SD = 4.33) were included across all reviewed studies. This included data from six studies of clinical participants involving n = 108 participants with a diagnosis of BPD, n = 80 with MDD, and n = 44 with PTSD (Schlosser et al., 2010; Wingenfeld et al., 2012a,b, 2013; Kuffel et al., 2014; Fleischer et al., 2015; see Table 1). Clinical participants were commonly recruited via psychiatric centers and the comparator control participants via local advertising. Diagnoses in studies of clinical participants were established using the Structured Clinical Interviewing for the DSM-IV which were also used to screen and exclude control participants (i.e., based on current or historic psychiatric diagnoses) in the studies of clinical groups. Two studies examined participants who were in remission but at risk of MDD (n = 43) (Haddad et al., 2009; Alhaj et al., 2012), while the remaining nine studies only included healthy volunteers (n = 711) (Park et al., 1994; Buss et al., 2004; Carvalho et al., 2006; Young et al., 2011, 2016; Cardoso et al., 2014; Fleischer et al., 2017; Chen et al., 2020; Wong et al., 2021).
Key design and methodological features of the included studies are summarized in Table 1. Common methodological strengths included double blinding of drug administration; (except for Wingenfeld et al., 2013; only participant-blinded), and counterbalancing of drug conditions in within subject crossover designs; (except for Haddad et al., 2009). Inclusion and exclusion criteria were generally well reported in all of the reviewed studies. With the exception of Haddad et al. (2009) no other studies explicitly reported on the absence or presence of missing data in the AMT. Using information published in each study, it was determined that thirteen publications had no missing AMT data. However, missingness could not be determined using the information provided in the three remaining included studies (Park et al., 1994; Carvalho et al., 2006; Fleischer et al., 2015).
The AMT protocol and scoring procedures were generally well-described in all of the reviewed studies. Small inconsistencies in memory scoring procedures may be a minor methodological weakness to the overall quality of the reviewed research, as the type and frequency of details required for a memory to be rated as “specific” varied slightly across studies. Only one study indicated that rating of AM responses was blind (Young et al., 2016). Ten studies reported using independent raters of participant responses (in most cases using a subset of memory descriptions; Buss et al., 2004; Schlosser et al., 2010; Young et al., 2011, 2016; Wingenfeld et al., 2012a; Cardoso et al., 2014; Fleischer et al., 2015, 2017; Chen et al., 2020; Wong et al., 2021). Of the ten studies using independent rating, seven reported indices of inter-rater agreement of AM scoring which were generally high (0.75–0.97; Buss et al., 2004; Schlosser et al., 2010; Young et al., 2011, 2016; Cardoso et al., 2014; Chen et al., 2020; Wong et al., 2021).
Nine studies included biochemical assays of drug levels (Park et al., 1994; Buss et al., 2004; Haddad et al., 2009; Young et al., 2011, 2016; Alhaj et al., 2012; Wingenfeld et al., 2012b; Kuffel et al., 2014; Fleischer et al., 2017; see Table 1). In all of these studies, the expected changes in drug (or amino acid) level were observed.
Sixteen of the included studies were evaluated to have a low risk of bias based in items from the commonly used Cochrane risk of bias tool. One study was found to have a moderate risk of bias due to not including details on participant or experimenter blinding to placebo vs. drug treatment (Wingenfeld et al., 2013). A summarized figure of the risk of bias assessment is provided in the Supplementary material. It should be noted that none of the studies were “clinical trials” and most pre-date the increasingly common practice of pre-registration of experimental studies.
Two studies were funded by industry sponsors, yet in both cases the authors reported no influence of sponsorship on either study (Wingenfeld et al., 2012b; Young et al., 2016). The contributions of a single author to one study (C. Otte; Fleischer et al., 2015), and of two authors in another study (K. Wingenfeld and B. Lowe; Kuffel et al., 2014) were supported by industry sponsors. However, it should be reiterated that none of the studies were clinical trials investigating the efficacy of a medicinal product for a specific indication.
Studies targeted the glucocorticoid (k = 6), mineralocorticoid (k = 2), monoaminergic (k = 6), oxytocinergic (k = 2), and glutamatergic systems (k = 1; Table 1). It is noteworthy that no studies on cholinergic or GABAergic modulators, which include the classic amnestic agents (anticholinergics and benzodiazepines, respectively), were identified in the search.
Of the seven studies targeting glucocorticoid modulation, five had methodological similarities, particularly in relation to the use of a single low oral dose (10 mg) of hydrocortisone (Buss et al., 2004; Schlosser et al., 2010; Wingenfeld et al., 2012a, 2013; Fleischer et al., 2017). Among these, three non-clinical studies reported statistically significantly impairment of AM specificity relative to placebo (Buss et al., 2004; Schlosser et al., 2010; Wingenfeld et al., 2013). However, this effect was not observed in two other non-clinical studies (Wingenfeld et al., 2012a; Fleischer et al., 2017). In the only study of intravenous hydrocortisone in non-clinical participants, a high dose (0.45 mg/kg) led to a reduction in the percentage of specific AMs and simultaneous increase in categorical (i.e., non-specific) AM retrieval relative to placebo (Young et al., 2011; see Table 1; this latter effect was also seen with a three-fold lower dose).
Three of these five low-dose oral hydrocortisone studies were conducted in participants with a psychiatric disorder (Schlosser et al., 2010; Wingenfeld et al., 2012a, 2013). In a small study with MDD patients, no effect of hydrocortisone was found (Schlosser et al., 2010), although a larger study with PTSD patients showed significantly higher AM retrieval relative to placebo (Wingenfeld et al., 2012a; see Table 1). A similar specificity-enhancement effect was observed in a larger sample of BPD patients although this did not reach statistical significance (Wingenfeld et al., 2013; see Table 1). In both cases, the authors suggest this result may be due to improved reactivity to exogenous cortisol in these particular clinical populations, but this cannot be confirmed in either study due to a lack of additional measures of HPA axis functioning (e.g., basal cortisol levels).
In a within-subjects crossover study of glucocorticoid antagonism by mifepristone on AM retrieval (in a non-clinical sample), reduced glucocorticoid receptor activation significantly increased the percentage of specific AMs retrieved relative to placebo, albeit in a small, all-male sample (Young et al., 2016; see Table 1).
The general impairing effect of hydrocortisone on retrieval specificity among healthy participants in the studies described above is consistent with the notion of divergent acute effects of glucocorticoids on encoding vs. retrieval (Roozendaal, 2003). Data from four within-subject crossover studies using the same oral dose of hydrocortisone (10 mg) and following the same AMT protocol were pooled to determine an overall effect size (Buss et al., 2004; Schlosser et al., 2010; Wingenfeld et al., 2012a, 2013; see Table 1). Although the pooled ES was large, it was estimated with low precision [g = −0.89, CI (−1.93, 0.16)] and hence, was not significant (p = 0.10) (Figure 2). Heterogeneity statistics indicated a very large degree of between study heterogeneity (I2 = 94%), consistent with the small number of studies and the single outlier study (Buss et al., 2004). Removing Buss et al. (2004) reduced heterogeneity (I2 = 0%) and the aggregate ES [g = −0.28, CI (−0.53, −0.03)], (p = 0.03) (Figure 3). However, in this second analysis, the general impairing effect of hydrocortisone on healthy participants' AM retrieval specificity was statistically significant.
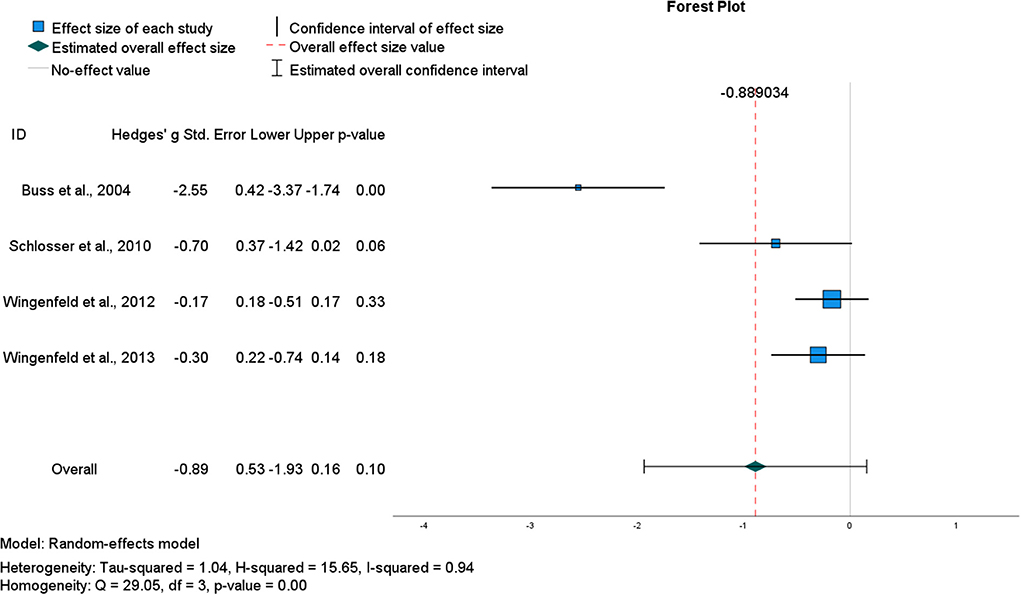
Figure 2. Forest plot for pooled studies of the (acute) effect of oral hydrocortisone (10 mg) administration in healthy participants on the number of specific AMs recalled.
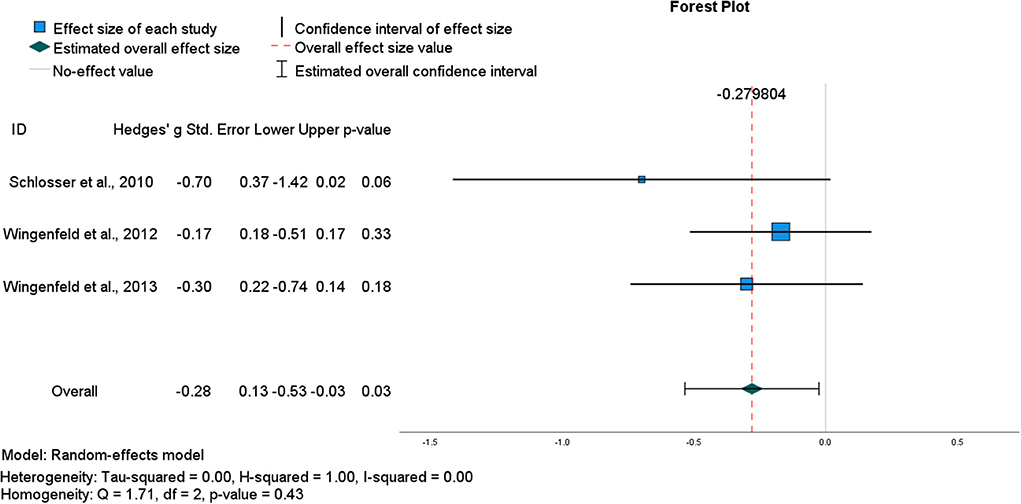
Figure 3. Forest plot for final pooled studies of the (acute) effect of oral hydrocortisone (10 mg) administration in healthy participants on the number of specific AMs recalled.
Two studies have investigated drugs with a preference for mineralocorticoid receptors. Fludrocortisone (0.4 mg, oral), a selective mineralocorticoid receptor agonist had no effect on specificity in healthy women or in women with MDD and BPD (Fleischer et al., 2015). However, in a small sample of healthy men, AM specificity was impaired during mineralocorticoid receptor antagonism using spironolactone (Young et al., 2016; see Table 1).
Wingenfeld et al. (2012b) hypothesized that blocking the presynaptic alpha-2-adrenoceptor to increase NA activity using a low-dose (5 mg) yohimbine would improve AM memory performance at retrieval. Harmer et al. (2009) predicted larger effects in MDD patients vs. non-clinical controls following reboxetine. Although a general improvement was observed on delayed (24 h) free recall of a wordlist in healthy and depressed participants, the effect was stronger in MDD. Yohimbine had no effect on AM specificity at retrieval in healthy or depressed participants.
Similarly, a single dose of the NE–DA reuptake inhibitor bupropion or the selective serotonin reuptake inhibitor sertraline produced no detectable effects on AM specificity in non-clinical volunteers (Carvalho et al., 2006; see Table 1). Kuffel et al. (2014) proposed a potential upregulation of alpha-2 receptors in depressed participants and predicted that clonidine, an alpha 2 agonist, would impair specificity in both non-clinical and MDD samples. However, they found no treatment effect on AM specificity.
Three studies have assessed the effect of low-dose tryptophan depletion on AM specificity and included analysis of plasma concentrations which confirmed that the treatment (i.e., acute tryptophan depletion) was successful in all three. One reported a significant reduction in specificity due to the treatment but in negative cue words only (Haddad et al., 2009), while the other two found no effects of treatment on AM specificity (Park et al., 1994; Alhaj et al., 2012; see Table 1).
A moderate dose of oxytocin (intranasal) improved AM specificity relative to the high dose and placebo in a single, small-scale, study of healthy men (Cardoso et al., 2014; see Table 1). Wong et al. (2021) examined the effect of a moderate oxytocin dose on AM specificity in two studies of healthy volunteers and found no effect on the number of specific or overgeneral AMs retrieved in either study (see Table 1).
In the only identified placebo-controlled study of glutamatergic regulation of AM, Chen et al. (2020) reported that pre-retrieval d-cycloserine improved AM specificity, and this effect persisted for 24 h following drug administration (see Table 1). No concurrent effects were reported on subjective mood, emotional facial expression recognition, or word recall.
This review provides the first synthesis of studies examining pharmacological modulation of AM specificity. The systematic search identified a relatively small number of studies in this area, commensurate with our limited understanding of the neuropsychopharmacology of AM retrieval. We hope that this review will motivate further research on this topic, which has potential implications for our understanding of, and the development of treatments for, a range of psychological disorders. Given the included studies' diversity in terms of the neurotransmitter or neuromodulatory systems targeted, it was generally not possible to quantitatively synthesize the effects. A critical mass of studies on each primary drug group discussed here will allow researchers to undertake such quantitative analyses in the future.
Where a preliminary quantitative synthesis was possible (on the effects of low dose oral hydrocortisone in non-clinical participants), study effects were heterogeneous, largely due to an outlying single study (Buss et al., 2004). Following sensitivity analysis, the pooled effect size was modest yet statistically significant, and this impairing effect of hydrocortisone in non-clinical participants was in the direction predicted by each study's authors (impaired specificity). Lower AM specificity following hydrocortisone administration is consistent with literature that reports broad retrieval impairments with both endogenous and exogenously elevated cortisol, for example, stimulus-response tasks in rodents and in emotional word list, and probabilistic classification learning in humans (de Quervain et al., 2007; Atsak et al., 2016; Zerbes et al., 2019). The current results tentatively suggest that low dose hydrocortisone could be used to model dysfunctional AM specificity in non-clinical participants.
In the single study using intravenous hydrocortisone in healthy volunteers, only a high dose (~31.8 mg/kg) produced the expected increase in plasma cortisol, corticosteroid binding globulin concentrations, and impairment of AM specificity (Young et al., 2011). The importance of including biological indicators of treatment efficacy is underscored by Young et al.'s (2011) study, and so where possible, physiological baseline differences relevant to the neuromodulator system under assessment should be recorded in future studies (e.g., baseline cortisol levels; Young et al., 2011) and any intended elevation in cortisol by drug verified biologically. Based on these findings, future research should aim to replicate these enhancing and impairing effects at the suggested doses and extend them to include female and clinical samples. The combination of sex and sex-hormone levels can influence the effect of exogenous hydrocortisone on retrieval of aversive memories in healthy volunteers (e.g., Hennessy et al., 2022), and sex differences are well-established in PTSD (for reviews see Li and Graham, 2017; Kornfield et al., 2018) and episodic memory (Ertman et al., 2011; Soni et al., 2013).
In a large study of PTSD patients, most of whom reported repeated experiences of childhood trauma, low dose (10 mg) hydrocortisone resulted in significantly improved specific AM retrieval –opposite to the effect seen in healthy participants (Wingenfeld et al., 2012a). An enhancing effect of exogenous hydrocortisone on AM specificity was also observed in BPD patients (Wingenfeld et al., 2013; see Table 1), implying a potential common glucocorticoid-mediated disorder in AM retrieval (and possible treatment target) across these two disorders. In a similar placebo-controlled crossover study with healthy controls, PTSD and BPD patients, and using low dose hydrocortisone, the same research team found that in both PTSD and BPD patient groups, childhood trauma and symptom severity were negatively correlated with functional connectivity between regions associated with successful autobiographical retrieval (the hippocampus and dorsomedial PFC) (Metz et al., 2019).
Considering this evidence, it is possible that the distinct effects of hydrocortisone in PTSD and BPD vs. healthy volunteers reflects a developmental-stress-mediated change in glucocorticoid receptor functioning in chronic and complex PTSD and BPD, the neuropsychological symptoms of which can be partially remediated by the administration of exogenous cortisol. PTSD with and without MDD is associated with lower daily cortisol output relative to non-trauma exposed controls, with general trauma exposure associated with enhanced HPA feedback (Morris et al., 2012; Rauch et al., 2020). Moreover, stress-induced (endogenous) elevation in cortisol has no such effect on AM specificity in both BPD patients and healthy controls (Duesenberg et al., 2019).
It is likely that glucocorticoid receptor expression/sensitivity is adaptively downregulated in the presence of chronically elevated cortisol; potentially explaining the opposing effect of elevated cortisol in clinical samples vs. healthy volunteers. In the studies reviewed, BPD and MDD patient volunteers were overrepresented relative to those with PTSD. No manipulation effects were observed on the AMT for MDD patients, however, the potential effect of low dose hydrocortisone on AMT performance in MDD was not investigated in the reviewed research and may therefore represent a novel avenue for future study. Childhood (and adult) psychological trauma is associated with dysregulated cortisol secretion and blunted cortisol responding, as well as dendritic atrophy (McGowan et al., 2009; Wellman and Moench, 2019), and experience of childhood trauma appears to induce glucocorticoid resistance in those with depression (Nikkheslat et al., 2020). Therefore, this subgroup of MDD patients (with childhood trauma exposure-induced glucocorticoid receptor downregulation) may selectively benefit from a pre-retrieval low dose hydrocortisone administration to enhance AM specificity similar to those with PTSD and BPD.
A U-shaped dose-response relationship was reported for the effects of oxytocin on AM specificity (Cardoso et al., 2014), as only the moderate dose had an impact (positive) on specificity. The authors suggest that oxytocin may enhance self-referential processing with downstream beneficial effects on AM specificity, and that diminished effects at higher doses may be due to partial occupation of arginine vasopressin receptors by oxytocin (Manning et al., 2008, 2012). In a larger sample of men who underwent Pavlovian fear conditioning, a subsequent 24 IU dose of oxytocin was shown to enhance fear memory extinction, and this was associated with a general upregulation of PFC responding and downregulation in the amygdala (Eckstein et al., 2015). Thus, moderate doses of oxytocin appear to generally enhance emotional memory retrieval processes. It is therefore unclear whether studies of oxytocin will provide any special insights into the neuropharmacology of AM.
Chen et al. (2020) found that in healthy volunteers, a single pre-retrieval administration of d-cycloserine (250 mg) improved AM specificity, and this improvement persisted for at least 24 h. Research on emotional and declarative memory has recently focused on the relationship between stress and stress-related hormonal and neurochemical mediators (particularly glucocorticoids) and increased glutamatergic transmission in the PFC. This stress-induced increase in glutamatergic function has been implicated in beneficial effects on cognition and emotional processes (e.g., enhanced memory consolidation), yet also dysfunction in such processes through over-activation of the stress response system in neuropsychiatric disorders (Popoli et al., 2012; Wellman and Moench, 2019).
There are common methodological strengths across the reviewed studies despite their limited number and the range of neuromodulator systems targeted. Overall, the Cochrane Risk of Bias tool suggested that the studies had a low risk of bias. On the other hand, our risk of bias assessment was limited because AMT-specific methodological details were not captured by the Cochrane tool. While these specific methodological aspects were generally strong, no study was pre-registered. Preregistration and standardization of AMT scoring procedures will be key to providing like-for-like comparisons of drug effects in this field going forward.
The overall numbers of clinical participants contributing data to the current review was small. Furthermore, considering the limited available data for the reviewed drug class categories, it is not yet possible to determine the effect (size) of each drug class on AM specificity or possible moderation by clinical diagnoses. In general, based on the limited data, hydrocortisone, d-cycloserine, and oxytocin in particular appear to warrant further investigation in healthy volunteers. Pre-retrieval hydrocortisone should also be investigated to potentially improve specificity in clinical populations, particularly PTSD and BPD patients. While “positive” findings with these drugs should motivate replication and extension, further research on the other drug groups outlined here should not be foreclosed simply because of a small number of null results. As noted above, sex/sex hormones play a critical role in emotional memory, and critically, in the sensitivity to drug effects on emotional memory. An absence of evidence for pharmacological modulation may simply reflect an inability of some study designs to detect such modulation because of a failure to account for sex (hormones). As such, future studies should incorporate sex as an explanatory factor into their study design (potentially as a moderator in the pre-registered data analysis plan).
Where possible, future pharmacological research probing the neurobiology of AM specificity (e.g., using low dose hydrocortisone) should assess baseline endogenous neuromodulator functioning. Significant baseline (endogenous) differences in targeted modulators may alter cognitions (e.g., biases toward negative retrieval and/or reduced overall AM retrieval) and potential efficacy of a pharmacological intervention on specific AM retrieval. For example, baseline hair cortisol is positively correlated with PTSD symptoms and sleep disturbances in those with subsequent trauma exposure (Sopp et al., 2021).
Depending on the drug class category investigated, exploring the role of neuromodulator systems in specific AM retrieval may also benefit from the concurrent assessment of cue-response latency and physiological arousal. Such measures could be used to help model the potential influence of general cognitive performance and to assess corroborating physiological effects of a pharmacological manipulation alongside AMT performance. Phenomenological experience during retrieval (e.g., subjective happiness vs. distress) should also be considered. For example, the subjective severity of traumatic events (peritraumatic stress) and distress during retrieval of an associated negative AM can have a significant impact on the development and course of PTSD (Vance et al., 2018) and subjectively positive AMs may be protective against symptom development (e.g., Hamlat et al., 2015).
There are four clinical implications of the current findings that warrant further investigation in larger and/or more diverse samples. First, low dose hydrocortisone may be used to model overgeneral AM retrieval deficits seen in psychopathology in healthy samples. Such pharmacological approaches to “symptom provocation” are essential for furthering our understanding of the contribution of specific neuropsychological phenotypes to psychopathology. They also have advantages over behavioral methods, which mimic symptoms for relatively brief periods. Secondly, low dose hydrocortisone may also be used to improve AM retrieval deficits in psychological disorders characterized by overgeneral AM memory. Third, a more comprehensive analysis of divergence in tonic cortisol concentrations between healthy and clinical samples, and interaction between baseline levels and drug effects could improve treatment tailoring. Finally, that drugs that target oxytocin and glutamatergic systems may enhance AM specificity in healthy samples.
Dysfunctional (i.e., overgeneral) AM retrieval is a transdiagnostic symptom in psychopathology and therefore may be an ideal target for the development of novel pharmacotherapies. Psychotherapies and trauma-focused CBT are the current most effective treatments for MDD and PTSD, respectively (McPherson and Hengartner, 2019; Mavranezouli et al., 2020). The beneficial effects of these treatments has been suggested to be rooted in AM processing, in either the formation and strengthening of competing adaptive AM representations (i.e., self-related knowledge and memories for personal experiences) (Brewin, 2006), or through a “rewriting” process that adaptively alters and/or updates maladaptive AM representations via memory reconsolidation (Lane et al., 2015). The development of more-effective psychological interventions specifically targeting AM retrieval (e.g., Memory Specificity Training; Raes et al., 2009a) is likely to benefit from pharmacological approaches, as these represent a novel pathway to strengthening our mechanistic understanding of AM specificity and potential catalysts for psychological approaches.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
EC and SK designed and conducted the systematic review and wrote the article for publication. EC and GP extracted data and assessed risk of bias. SK supervised the study. All authors contributed to the article and approved the submitted version.
This research was conducted at UCL and funded by the Sir Bobby Charlton Foundation (Registered Charity No. 114091).
This systematic review was carried out as part of EC's Ph.D., at University College London.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyg.2022.1045217/full#supplementary-material
Aas, M., Pizzagalli, D. A., Laskemoen, J. F., Reponen, E. J., Ueland, T., Melle, I., et al. (2019). Elevated hair cortisol is associated with childhood maltreatment and cognitive impairment in schizophrenia and in bipolar disorders. Schizophr. Res. 213, 65–71. doi: 10.1016/j.schres.2019.01.011
Ahern, E., and Semkovska, M. (2017). Cognitive functioning in the first-episode of major depressive disorder: a systematic review and meta-analysis. Neuropsychology 31, 52. doi: 10.1037/neu0000319
Ai, H., Opmeer, E. M., Marsman, J. B. C., Veltman, D. J., van der Wee, N. J., Aleman, A., and van Tol, M. J. (2020). Longitudinal brain changes in MDD during emotional encoding: effects of presence and persistence of symptomatology. Psychol. Med. 50, 1316–1326. doi: 10.1017/S0033291719001259
*Alhaj, H. A., Selman, M., Jervis, V., Rodgers, J., Barton, S., and McAllister-Williams, R. H. (2012). Effect of low-dose acute tryptophan depletion on the specificity of autobiographical memory in healthy subjects with a family history of depression. Psychopharmacology 222, 285–292. doi: 10.1007/s00213-012-2644-x
American Psychiatric Association (2000). Diagnostic and Statistical Manual of Mental Disorders Fourth Edition Text Revision (DSM-IV-TR). Washington, DC: American Psychiatric Association. doi: 10.1176/appi.books.9780890423349
Anderson, N. H. (1968). Likableness ratings of 555 personality-trait words. J. Pers. Soc. Psychol. 9, 272–279.
Arean, P. A., Perri, M. G., Nezu, A. M., Schein, R. L., Christopher, F., and Joseph, T. X. (1993). Comparative effectiveness of social problem-solving therapy and reminiscence therapy as treatments for depression in older adults. J. Consult. Clin. Psychol. 61, 1003–1010. doi: 10.1037/0022-006X.61.6.1003
Arnone, D., Horder, J., Cowen, P. J., and Harmer, C. J. (2009). Early effects of mirtazapine on emotional processing. Psychopharmacology 203, 685. doi: 10.1007/s00213-008-1410-6
Atsak, P., Guenzel, F. M., Kantar-Gok, D., Zalachoras, I., Yargicoglu, P., Meijer, O. C., et al. (2016). Glucocorticoids mediate stress-induced impairment of retrieval of stimulus-response memory. Psychoneuroendocrinology 67, 207–215. doi: 10.1016/j.psyneuen.2016.02.006
Baddeley, A., and Emslie, H. Nimmo-Smith, I. (1993). The Spot-the-Word test: A robust estimate of verbal intelligence based on lexical decision. Br. J. Clin. Psychol. 32, 55–65.
Barry, T. J., Chiu, C. P., Raes, F., Ricarte, J., and Lau, H. (2018). The neurobiology of reduced autobiographical memory specificity. Trends Cogn. Sci. 22, 1038–1049. doi: 10.1016/j.tics.2018.09.001
Barry, T. J., Hallford, D. J., and Takano, K. (2021). Autobiographical memory impairments as a transdiagnostic feature of mental illness: a meta-analytic review of investigations into autobiographical memory specificity and overgenerality among people with psychiatric diagnoses. Psychol. Bull. 147, 1054. doi: 10.1037/bul0000345
Barry, T. J., Sze, W. Y., and Raes, F. (2019). A meta-analysis and systematic review of Memory Specificity Training (MeST) in the treatment of emotional disorders. Behav. Res. Ther. 116, 36–51. doi: 10.1016/j.brat.2019.02.001
Beck, A. T., Steer, R. A., and Hautzinger, M. (1994). Beck-Depressions-Inventar:(bdi); Testhandbuch. Huber.
Beck, A. T., Ward, C. H., Mendelson, M., Mock, J., and Erbaugh, J. (1961). An inventory for measuring depression. Arch. Gen. Psychiatr. 4, 561–571.
Berna, F., Potheegadoo, J., Aouadi, I., Ricarte, J. J., Allé, M. C., Coutelle, R., et al. (2016). A meta-analysis of autobiographical memory studies in schizophrenia spectrum disorder. Schizophr. Bull. 42, 56–66. doi: 10.1093/schbul/sbv099
Bernstein, D. P., Stein, J. A., Newcomb, M. D., Walker, E., Pogge, D., Ahluvalia, T., et al. (2003). Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Neglect. 27, 169–190. doi: 10.1016/s0145-2134(02)00541-0
Bhagwagar, Z., Hafizi, S., and Cowen, P. J. (2005). Increased salivary cortisol after waking in depression. Psychopharmacology 182, 54–57. doi: 10.1007/s00213-005-0062-z
Bocchio, M., Nabavi, S., and Capogna, M. (2017). Synaptic plasticity, engrams, and network oscillations in amygdala circuits for storage and retrieval of emotional memories. Neuron 94, 731–743. doi: 10.1016/j.neuron.2017.03.022
Brewin, C. R. (2006). Understanding cognitive behaviour therapy: a retrieval competition account. Behav. Res. Ther. 44, 765–784. doi: 10.1016/j.brat.2006.02.005
Bryant, R. A., Sutherland, K., and Guthrie, R. M. (2007). Impaired specific autobiographical memory as a risk factor for posttraumatic stress after trauma. J. Abnorm. Psychol. 116, 837doi: 10.1037/0021-843X.116.4.837
*Buss, C., Wolf, O. T., Witt, J., and Hellhammer, D. H. (2004). Autobiographic memory impairment following acute cortisol administration. Psychoneuroendocrinology 29, 1093–1096. doi: 10.1016/j.psyneuen.2003.09.006
Cabeza, R., and St Jacques, P. (2007). Functional neuroimaging of autobiographical memory. Trends Cogn. Sci. 11, 219–227. doi: 10.1016/j.tics.2007.02.005
*Cardoso, C., Orlando, M. A., Brown, C. A., and Ellenbogen, M. A. (2014). Oxytocin and enhancement of the positive valence of social affiliation memories: an autobiographical memory study. Soc. Neurosci. 9, 186–195. doi: 10.1080/17470919.2013.873079
*Carvalho, A. F., Köhler, C. A., Cruz, E. P., Stürmer, P. L., Reichman, B. P., Barea, B. M., et al. (2006). Acute treatment with the antidepressants bupropion and sertraline do not influence memory retrieval in man. Eur. Arch. Psychiatry Clin. Neurosci. 256, 320–325. doi: 10.1007/s00406-006-0640-z
*Chen, R., Capitão, L. P., Cowen, P. J., and Harmer, C. J. (2020). Effect of the NMDA receptor partial agonist, d-cycloserine, on emotional processing and autobiographical memory. Psychol. Med. 51, 2657–2665. doi: 10.1017/S0033291720001221
Conway, M. A., and Pleydell-Pearce, C. W. (2000). The construction of autobiographical memories in the self-memory system. Psychol. Rev. 107, 261. doi: 10.1037/0033-295X.107.2.261
Corcoran, R., and Frith, C. D. (2003). Autobiographical memory and theory of mind: evidence of a relationship in schizophrenia. Psychol. Med. 33, 897. doi: 10.1017/S0033291703007529
Crane, C., Heron, J., Gunnell, D., Lewis, G., Evans, J., and Williams, J. M. G. (2014). Childhood traumatic events and adolescent overgeneral autobiographical memory: findings in a UK cohort. J. Behav. Ther. Exp. Psychiatry 45, 330–338. doi: 10.1016/j.jbtep.2014.02.004
Cunningham, T. J., Mattingly, S. M., Tlatenchi, A., Wirth, M. M., Alger, S. E., Kensinger, E. A., et al. (2021). Higher post-encoding cortisol benefits the selective consolidation of emotional aspects of memory. Neurobiol. Learn. Mem. 180, 107411. doi: 10.1016/j.nlm.2021.107411
Dalgleish, T., and Werner-Seidler, A. (2014). Disruptions in autobiographical memory processing in depression and the emergence of memory therapeutics. Trends Cogn. Sci. 18, 596–604. doi: 10.1016/j.tics.2014.06.010
de Quervain, D. J. F., Aerni, A., and Roozendaal, B. (2007). Preventive effect of β-adrenoceptor blockade on glucocorticoid-induced memory retrieval deficits. Am. J. Psychiatry 164, 967–969. doi: 10.1176/ajp.2007.164.6.967
Delis, D. C., Kramer, J. H., Kaplan, E., and Ober, B. A. (1987). California Verbal Learning Test–Assessment.
Diekelmann, S., Wilhelm, I., Wagner, U., and Born, J. (2011). Elevated cortisol at retrieval suppresses false memories in parallel with correct memories. J. Cogn. Neurosci. 23, 772–781. doi: 10.1162/jocn.2010.21493
Dillon, D. G., and Pizzagalli, D. A. (2018). Mechanisms of memory disruption in depression. Trends Neurosci. 41, pp.137–149. doi: 10.1016/j.tins.2017.12.006
Drews, E., Fertuck, E. A., Koenig, J., Kaess, M., and Arntz, A. (2019). Hypothalamic-pituitary-adrenal axis functioning in borderline personality disorder: a meta-analysis. Neurosci. Biobehav. Rev. 96, 316–334. doi: 10.1016/j.neubiorev.2018.11.008
Duesenberg, M., Wolf, O. T., Metz, S., Roepke, S., Fleischer, J., Elias, V., et al. (2019). Psychophysiological stress response and memory in borderline personality disorder. Eur. J. Psychotraumatol. 10, 1568134. doi: 10.1080/20008198.2019.1568134
Eckstein, M., Becker, B., Scheele, D., Scholz, C., Preckel, K., Schlaepfer, T. E., et al. (2015). Oxytocin facilitates the extinction of conditioned fear in humans. Biol. Psychiatry 78, 194–202. doi: 10.1016/j.biopsych.2014.10.015
Endicott, J. (1978). Family History: Research Diagnostic Criteria:(FH-RDC). New York, NY: New York State Psychiatric Institute.
Ertman, N., Andreano, J. M., and Cahill, L. (2011). Progesterone at encoding predicts subsequent emotional memory. Learn. Memory 18, 759–763. doi: 10.1101/lm.023267.111
Eysenck, H. J., and Eysenck, S. B. (1976). Eysenck Personality Questionnaire. Educational and Industrial Testing Service.
*Fleischer, J., Weber, J., Hellmann-Regen, J., Düsenberg, M., Wolf, O. T., Otte, C., et al. (2017). The effect of cortisol on autobiographical memory retrieval depends on remoteness and valence of memories. Biol. Psychol. 123, 136–140. doi: 10.1016/j.biopsycho.2016.12.010
*Fleischer, J., Wingenfeld, K., Kuehl, L. K., Hinkelmann, K., Roepke, S., and Otte, C. (2015). Does fludrocortisone influence autobiographical memory retrieval? A study in patients with major depression, patients with borderline personality disorder and healthy controls. Stress 18, 718–722. doi: 10.3109/10253890.2015.1087504
Ford, J. H., and Kensinger, E. A. (2019). The role of the amygdala in emotional experience during retrieval of personal memories. Memory 27, 1362–1370. doi: 10.1080/09658211.2019.1659371
Griffith, J. W., Kleim, B., Sumner, J. A., and Ehlers, A. (2012). The factor structure of the Autobiographical Memory Test in recent trauma survivors. Psychol. Assess. 24, 640. doi: 10.1037/a0026510
*Haddad, A. D., Williams, J. M. G., McTavish, S. F., and Harmer, C. J. (2009). Low-dose tryptophan depletion in recovered depressed women induces impairments in autobiographical memory specificity. Psychopharmacology 207, 499–508. doi: 10.1007/s00213-009-1693-2
Haider, S., Tabassum, S., and Perveen, T. (2016). Scopolamine-induced greater alterations in neurochemical profile and increased oxidative stress demonstrated a better model of dementia: a comparative study. Brain Res. Bull. 127, 234–247. doi: 10.1016/j.brainresbull.2016.10.002
Hallford, D. J., Rusanov, D., Yeow, J. J. E., and Barry, T. J. (2021). Overgeneral and specific autobiographical memory predict the course of depression: an updated meta-analysis. Psychol. Med. 51, 909–926. doi: 10.1017/S0033291721001343
Hamlat, E. J., Connolly, S. L., Hamilton, J. L., Stange, J. P., Abramson, L. Y., and Alloy, L. B. (2015). Rumination and overgeneral autobiographical memory in adolescents: an integration of cognitive vulnerabilities to depression. J. Youth Adolesc. 44, 806–818. doi: 10.1007/s10964-014-0090-2
Harmer, C. J., Heinzen, J., O'Sullivan, U., Ayres, R. A., and Cowen, P. J. (2008). Dissociable effects of acute antidepressant drug administration on subjective and emotional processing measures in healthy volunteers. Psychopharmacology 199, 495–502. doi: 10.1007/s00213-007-1058-7
Harmer, C. J., Hill, S. A., Taylor, M. J., Cowen, P. J., and Goodwin, G. M. (2003). Toward a neuropsychological theory of antidepressant drug action: increase in positive emotional bias after potentiation of norepinephrine activity. Am. J. Psychiatry 160, 990–992. doi: 10.1176/appi.ajp.160.5.990
Harmer, C. J., O'Sullivan, U., Favaron, E., Massey-Chase, R., Ayres, R., Reinecke, A., et al. (2009). Effect of acute antidepressant administration on negative affective bias in depressed patients. Am. J. Psychiatry 166, 1178–1184. doi: 10.1176/appi.ajp.2009.09020149
Hauer, B. J., Wessel, I., Engelhard, I. M., Peeters, L. L., and Dalgleish, T. (2009). Prepartum autobiographical memory specificity predicts post-traumatic stress symptoms following complicated pregnancy. Memory 17, 544–556. doi: 10.1080/09658210902953836
Hennessy, V. E., Troebinger, L., Iskandar, G., Das, R. K., and Kamboj, S. K. (2022). Accelerated forgetting of a trauma-like event in healthy men and women after a single dose of hydrocortisone. Transl. Psychiatry 12, 1–9. doi: 10.1038/s41398-022-02126-2
Herane-Vives, A., Fischer, S., de Angel, V., Wise, T., Cheung, E., Chua, K. C., et al. (2018). Elevated fingernail cortisol levels in major depressive episodes. Psychoneuroendocrinology 88, 17–23. doi: 10.1016/j.psyneuen.2017.10.026
Heron, J., Crane, C., Gunnell, D., Lewis, G., Evans, J., and Williams, J. M. G. (2012). 40,000 memories in young teenagers: psychometric properties of the Autobiographical Memory Test in a UK cohort study. Memory 20, 300–320. doi: 10.1080/09658211.2012.656846
Herzmann, G., Bird, C. W., Freeman, M., and Curran, T. (2013). Effects of oxytocin on behavioral and ERP measures of recognition memory for own-race and other-race faces in women and men. Psychoneuroendocrinology 38, 2140–2151. doi: 10.1016/j.psyneuen.2013.04.002
Higgins, J. P., Sterne, J., Savović, J., Page, M., Hróbjartsson, A., Boutron, I., et al. (2016). “A revised tool for assessing risk of bias in randomized trials,” in Cochrane Database Systematic Reviews, eds J. Chandler, J. McKenzie, I. Boutron, and V. Welch, Vol. 10, Cochrane Methods, 29–31.
Hitchcock, C., Newby, J., Timm, E., Howard, R. M., Golden, A. M., Kuyken, W., et al. (2020). Memory category fluency, memory specificity, and the fading affect bias for positive and negative autobiographical events: performance on a good day–bad day task in healthy and depressed individuals. J. Exp. Psychol. General 149, 198. doi: 10.1037/xge0000617
Hornboll, B., Macoveanu, J., Nejad, A., Rowe, J., Elliott, R., Knudsen, G. M., et al. (2018). Neuroticism predicts the impact of serotonin challenges on fear processing in subgenual anterior cingulate cortex. Sci. Rep. 8, 1–10. doi: 10.1038/s41598-018-36350-y
Hurlemann, R., Matusch, A., Hawellek, B., Klingmuller, D., Kolsch, H., Maier, W., et al. (2007). Emotion-induced retrograde amnesia varies as a function of noradrenergic-glucocorticoid activity. Psychopharmacology 194, 261–269. doi: 10.1007/s00213-007-0836-6
Inslicht, S. S., Niles, A. N., Metzler, T. J., Lipshitz, S. A. L., Otte, C., Milad, M. R., et al. (2021). Randomized controlled experimental study of hydrocortisone and D-cycloserine effects on fear extinction in PTSD. Neuropsychopharmacology 47, 1945–1952. doi: 10.1038/s41386-021-01222-z
Iob, E., Kirschbaum, C., and Steptoe, A. (2020). Persistent depressive symptoms, HPA-axis hyperactivity, and inflammation: the role of cognitive-affective and somatic symptoms. Mol. Psychiatry 25, 1130–1140. doi: 10.1038/s41380-019-0501-6
Jones, B., Heard, H., Startup, M., Swales, M., Williams, J. M. G., and Jones, R. S. P. (1999). Autobiographical memory and dissociation in borderline personality disorder. Psychol. Med. 29, 1397–1404. doi: 10.1017/S0033291799001208
Kamboj, S. K., and Curran, H. V. (2006). Neutral and emotional episodic memory: global impairment after lorazepam or scopolamine. Psychopharmacology 188, 482–488. doi: 10.1007/s00213-006-0552-7
Knorr, U., Madsen, J. M., and Kessing, L. V. (2019). The effect of selective serotonin reuptake inhibitors in healthy subjects revisited: a systematic review of the literature. Exp. Clin. Psychopharmacol. 27, 413. doi: 10.1037/pha0000264
Kornfield, S. L., Hantsoo, L., and Epperson, C. N. (2018). What does sex have to do with it? The role of sex as a biological variable in the development of posttraumatic stress disorder. Curr. Psychiatry Rep. 20, 1–8. doi: 10.1007/s11920-018-0907-x
Kroes, M. C., Tona, K. D., den Ouden, H. E., Vogel, S., van Wingen, G. A., and Fernández, G. (2016). How administration of the beta-blocker propranolol before extinction can prevent the return of fear. Neuropsychopharmacology 41, 1569–1578. doi: 10.1038/npp.2015.315
*Kuffel, A., Eikelmann, S., Terfehr, K., Mau, G., Kuehl, L. K., Otte, C., et al. (2014). Noradrenergic blockade and memory in patients with major depression and healthy participants. Psychoneuroendocrinology 40, 86–90. doi: 10.1016/j.psyneuen.2013.11.001
Kuhlmann, S., Kirschbaum, C., and Wolf, O. T. (2005). Effects of oral cortisol treatment in healthy young women on memory retrieval of negative and neutral words. Neurobiol. Learn. Mem. 83, 158–162. doi: 10.1016/j.nlm.2004.09.001
Kumar, P., Goer, F., Murray, L., Dillon, D. G., Beltzer, M. L., Cohen, A. L., Brooks, N. H., and Pizzagalli, D. A. (2018). Impaired reward prediction error encoding and striatal-midbrain connectivity in depression. Neuropsychopharmacology 43, 1581–1588. doi: 10.1038/s41386-018-0032-x
Lane, R. D., Ryan, L., Nadel, L., and Greenberg, L. (2015). Memory reconsolidation, emotional arousal, and the process of change in psychotherapy: new insights from brain science. Behav. Brain Sci. 38, e1. doi: 10.1017/S0140525X14000041
Li, S. H., and Graham, B. M. (2017). Why are women so vulnerable to anxiety, trauma-related and stress-related disorders? The potential role of sex hormones. Lancet Psychiatry 4, 73–82. doi: 10.1016/S2215-0366(16)30358-3
Manning, M., Misicka, A., Olma, A., Bankowski, K., Stoev, S., Chini, B., et al. (2012). Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. J. Neuroendocrinol. 24, 609–628. doi: 10.1111/j.1365-2826.2012.02303.x
Manning, M., Stoev, S., Chini, B., Durroux, T., Mouillac, B., and Guillon, G. (2008). Peptide and non-peptide agonists and antagonists for the vasopressin and oxytocin V1a, V1b, V2 and OT receptors: research tools and potential therapeutic agents. Prog. Brain Res. 170, 473–512. doi: 10.1016/S0079-6123(08)00437-8
Mataix-Cols, D., De La Cruz, L. F., Monzani, B., Rosenfield, D., Andersson, E., Pérez-Vigil, A., et al. (2017). D-cycloserine augmentation of exposure-based cognitive behavior therapy for anxiety, obsessive-compulsive, and posttraumatic stress disorders: a systematic review and meta-analysis of individual participant data. JAMA Psychiatry 74, 501–510. doi: 10.1001/jamapsychiatry.2016.3955
Mavranezouli, I., Megnin-Viggars, O., Daly, C., Dias, S., Welton, N. J., Stockton, S., et al. (2020). Psychological treatments for post-traumatic stress disorder in adults: a network meta-analysis. Psychol. Med. 50, 542–555. doi: 10.1017/S0033291720000070
McAllister-Williams, R., Massey, A., and Rugg, M. (2002). Effects of tryptophan depletion on brain potential correlates of episodic memory retrieval. Psychopharmacology 160, 434–442. doi: 10.1007/s00213-001-0996-8
McGowan, P. O., Sasaki, A., D'alessio, A. C., Dymov, S., Labont,é, B., Szyf, M., et al. (2009). Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat. Neurosci. 12, 342–348. doi: 10.1038/nn.2270
McNair, D. M., Lorr, M., and Droppleman, L. F. (1971). Manual for the Profile of Mood States. San Diego, CA: Educational & Industrial Testing Service.
McNair, D. M., Lorr, M., and Droppleman, L. F. (1992). Revised manual for the Profile of Mood States. San Diego, CA: Educational & Industrial Testing Services.
McPherson, S., and Hengartner, M. P. (2019). Long-term outcomes of trials in the National Institute for Health and Care Excellence depression guideline. BJPsych Open 5, e81. doi: 10.1192/bjo.2019.65
Merz, C. J., Hamacher-Dang, T. C., Stark, R., Wolf, O. T., and Hermann, A. (2018). Neural underpinnings of cortisol effects on fear extinction. Neuropsychopharmacology 43, 384–392. doi: 10.1038/npp.2017.227
Metz, S., Fleischer, J., Grimm, S., Gärnter, M., Golde, S., Duesenberg, M., et al. (2019). Resting-state functional connectivity after hydrocortisone administration in patients with post-traumatic stress disorder and borderline personality disorder. Eur. Neuropsychopharmacol. 29, 936–946. doi: 10.1016/j.euroneuro.2019.05.008
Moore, S. A., and Zoellner, L. A. (2007). Overgeneral autobiographical memory and traumatic events: an evaluative review. Psychol. Bull. 133, 419. doi: 10.1037/0033-2909.133.3.419
Morris, M. C., Compas, B. E., and Garber, J. (2012). Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: a systematic review and meta-analysis. Clin. Psychol. Rev. 32, 301–315. doi: 10.1016/j.cpr.2012.02.002
Murphy, O. W., Hoy, K. E., Wong, D., Bailey, N. W., Fitzgerald, P. B., and Segrave, R. A. (2019). Individuals with depression display abnormal modulation of neural oscillatory activity during working memory encoding and maintenance. Biol. Psychol. 148, 107766. doi: 10.1016/j.biopsycho.2019.107766
Nikkheslat, N., McLaughlin, A. P., Hastings, C., Zajkowska, Z., Nettis, M. A., Mariani, N., et al. (2020). Childhood trauma, HPA axis activity and antidepressant response in patients with depression. Brain Behav. Immun. 87, 229–237. doi: 10.1016/j.bbi.2019.11.024
Ono, M., Devilly, G. J., and Shum, D. H. (2016). A meta-analytic review of overgeneral memory: the role of trauma history, mood, and the presence of posttraumatic stress disorder. Psychol. Trauma Theory Res. Pract. Policy 8, 157. doi: 10.1037/tra0000027
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int. J. Surgery 88, 105906. doi: 10.1016/j.ijsu.2021.105906
*Park, S. B., Coull, J. T., McShane, R. H., Young, A. H., Sahakian, B. J., Robbins, T. W., et al. (1994). Tryptophan depletion in normal volunteers produces selective impairments in learning and memory. Neuropharmacology 33, 575–588. doi: 10.1016/0028-3908(94)90089-2
Popoli, M., Yan, Z., McEwen, B. S., and Sanacora, G. (2012). The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat. Rev. Neurosci. 13, 22–37. doi: 10.1038/nrn3138
Puetz, V. B., Viding, E., Hoffmann, F., Gerin, M. I., Sharp, M., Rankin, G., et al. (2021). Autobiographical memory as a latent vulnerability mechanism following childhood maltreatment: association with future depression symptoms and prosocial behavior. Dev. Psychopathol. 33, 1300–1307. doi: 10.1017/S0954579420000504
Quartermain, D., Mower, J., Rafferty, M. F., Herting, R. L., and Lanthorn, T. H. (1994). Acute but not chronic activation of the NMDA-coupled glycine receptor with D-cycloserine facilitates learning and retention. Eur. J. Pharmacol. 257, 7–12. doi: 10.1016/0014-2999(94)90687-4
Raes, F., Williams, J. M. G., and Hermans, D. (2009a). Reducing cognitive vulnerability to de- pression: A preliminary investigation of MEmory Specificity Training (MEST) in inpatients with depressive symptomatology. J. Behav. Ther. Exp. Psychiatry 40, 24–38. doi: 10.1016/j.jbtep.2008.03.001
Raes, F., Williams, M., and Hermans, D. (2009b). “Short communications: On the test-retest reliability of the autobiographical memory test,” in Personality Assessment: New Research. p. 391–397.
Rauch, S. A., King, A., Kim, H. M., Powell, C., Rajaram, N., Venners, M., et al. (2020). Cortisol awakening response in PTSD treatment: Predictor or mechanism of change. Psychoneuroendocrinology 118, 104714. doi: 10.1016/j.psyneuen.2020.104714
Rimmele, U., Besedovsky, L., Lange, T., and Born, J. (2015). Emotional memory can be persistently weakened by suppressing cortisol during retrieval. Neurobiol. Learn. Mem. 119, 102–107. doi: 10.1016/j.nlm.2015.01.010
Roozendaal, B. (2003). Systems mediating acute glucocorticoid effects on memory consolidation and retrieval. Prog. Neuro-Psychopharmacol. Biol. Psychiatr. 27, 1213–1223. doi: 10.1016/j.pnpbp.2003.09.015
Rosenfield, D., Smits, J. A., Hofmann, S. G., Mataix-Cols, D., de la Cruz, L. F., Andersson, E., et al. (2019). Changes in dosing and dose timing of D-cycloserine explain its apparent declining efficacy for augmenting exposure therapy for anxiety-related disorders: an individual participant-data meta-analysis. J. Anxiety Disord. 68, 102149. doi: 10.1016/j.janxdis.2019.102149
Ruhé, H. G., Mason, N. S., and Schene, A. H. (2007). Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol. Psychiatry 12, 331–359. doi: 10.1038/sj.mp.4001949
Schade, S., and Paulus, W. (2016). D-cycloserine in neuropsychiatric diseases: a systematic review. Int. J. Neuropsychopharmacol. 19, pyv102. doi: 10.1093/ijnp/pyv102
*Schlosser, N., Wolf, O. T., Fernando, S. C., Riedesel, K., Otte, C., Muhtz, C., et al. (2010). Effects of acute cortisol administration on autobiographical memory in patients with major depression and healthy controls. Psychoneuroendocrinology 35, 316–320. doi: 10.1016/j.psyneuen.2009.06.015
Schmaal, L., Veltman, D. J., van Erp, T. G., Sämann, P. G., Frodl, T., Jahanshad, N., et al. (2016). Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol. Psychiatry 21, 806–812. doi: 10.1038/mp.2015.69
Schönfeld, S., and Ehlers, A. (2006). Overgeneral memory extends to pictorial retrieval cues and correlates with cognitive features in posttraumatic stress disorder. Emotion 6, 611. doi: 10.1037/1528-3542.6.4.611
Schönfeld, S., Ehlers, A., Böllinghaus, I., and Rief, W. (2007). Overgeneral memory and suppression of trauma memories in post-traumatic stress disorder. Memory 15, 339–352. doi: 10.1080/09658210701256571
Schwabe, L., and Wolf, O. T. (2014). Timing matters: temporal dynamics of stress effects on memory retrieval. Cognit. Affect. Behav. Neurosci. 14, 1041–1048. doi: 10.3758/s13415-014-0256-0
Sheehan, D. V., Lecrubier, Y., Sheehan, K. H., Amorim, P., Janavs, J., Weiller, E., et al. (1998). The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatr. 59, 22–33.
Sheldon, S., Fenerci, C., and Gurguryan, L. (2019). A neurocognitive perspective on the forms and functions of autobiographical memory retrieval. Front. Syst. Neurosci. 13, 4. doi: 10.3389/fnsys.2019.00004
Sheldon, S., and Levine, B. (2016). The role of the hippocampus in memory and mental construction. Ann. N. Y. Acad. Sci. 1369, 76–92. doi: 10.1111/nyas.13006
Smeets, T., Otgaar, H., Candel, I., and Wolf, O. T. (2008). True or false? Memory is differentially affected by stress-induced cortisol elevations and sympathetic activity at consolidation and retrieval. Psychoneuroendocrinology 33, 1378–1386. doi: 10.1016/j.psyneuen.2008.07.009
Soni, M., Curran, V. H., and Kamboj, S. K. (2013). Identification of a narrow post-ovulatory window of vulnerability to distressing involuntary memories in healthy women. Neurobiol. Learn. Mem. 104, 32–38. doi: 10.1016/j.nlm.2013.04.003
Sopp, M. R., Michael, T., Lass-Hennemann, J., Haim-Nachum, S., and Lommen, M. J. (2021). Longitudinal associations between hair cortisol, PTSD symptoms, and sleep disturbances in a sample of firefighters with duty-related trauma exposure. Psychoneuroendocrinology 134, 105449. doi: 10.1016/j.psyneuen.2021.105449
Spielberger, C. D., Gonzalez-Reigosa, F., Martinez-Urrutia, A., Natalicio, L. F., and Natalicio, D. S. (1971). The state-trait anxiety inventory. Revista Interamericana de Psicologia/Interamerican J. Psychol. 5(3 & 4). doi: 10.30849/rip/ijp.v5i3&4.620
Spinhoven, P., Bamelis, L., Molendijk, M., Haringsma, R., and Arntz, A. (2009). Reduced specificity of autobiographical memory in Cluster C personality disorders and the role of depression, worry, and experiential avoidance. J. Abnorm. Psychol. 118, 520. doi: 10.1037/a0016393
Startup, M., Heard, H., Swales, M., Jones, B., Williams, J. M. G., and Jones, R. S. (2001). Autobiographical memory and parasuicide in borderline personality disorder. Br. J. Clin. Psychol. 40, 113–120. doi: 10.1348/014466501163535
Stetler, C., and Miller, G. E. (2011). Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom. Med. 73, 114–126. doi: 10.1097/PSY.0b013e31820ad12b
Steudte, S., Kolassa, I. T., Stalder, T., Pfeiffer, A., Kirschbaum, C., and Elbert, T. (2011). Increased cortisol concentrations in hair of severely traumatized Ugandan individuals with PTSD. Psychoneuroendocrinology 36, 1193–1200. doi: 10.1016/j.psyneuen.2011.02.012
Stokes, D. J., Dritschel, B. H., and Bekerian, D. A. (2004). The effect of burn injury on adolescents autobiographical memory. Behav. Res. Ther. 42, 1357–1365. doi: 10.1016/j.brat.2003.10.003
Strange, B. A., Hurlemann, R., and Dolan, R. J. (2003). An emotion-induced retrograde amnesia in humans is amygdala-and β-adrenergic-dependent. Proc. Nat. Acad. Sci. 100, 13626–13631. doi: 10.1073/pnas.1635116100
Sumner, J. A., Griffith, J. W., and Mineka, S. (2010). Overgeneral autobiographical memory as a predictor of the course of depression: a meta-analysis. Behav. Res. Ther. 48, 614–625. doi: 10.1016/j.brat.2010.03.013
Sutherland, K., and Bryant, R. A. (2007). Autobiographical memory in posttraumatic stress disorder before and after treatment. Behav. Res. Ther. 45, 2915–2923. doi: 10.1016/j.brat.2007.08.009
Terfehr, K., Wolf, O. T., Schlosser, N., Fernando, S. C., Otte, C., Muhtz, C., et al. (2011). Hydrocortisone impairs working memory in healthy humans, but not in patients with major depressive disorder. Psychopharmacology 215, 71–79. doi: 10.1007/s00213-010-2117-z
Tulving, E. (2002). Episodic memory: from mind to brain. Annu. Rev. Psychol. 53, 1–25. doi: 10.1146/annurev.psych.53.100901.135114
van der Veen, F. M., Evers, E. A., van Deursen, J. A., Deutz, N. E., Backes, W. H., and Schmitt, J. A. (2006). Acute tryptophan depletion reduces activation in the right hippocampus during encoding in an episodic memory task. Neuroimage 31, 1188–1196. doi: 10.1016/j.neuroimage.2006.01.014
van Eijndhoven, P., van Wingen, G., van Oijen, K., Rijpkema, M., Goraj, B., Verkes, R. J., et al. (2009). Amygdala volume marks the acute state in the early course of depression. Biol. Psychiatry 65, 812–818. doi: 10.1016/j.biopsych.2008.10.027
Vance, M. C., Kovachy, B., Dong, M., and Bui, E. (2018). Peritraumatic distress: a review and synthesis of 15 years of research. J. Clin. Psychol. 74, 1457–1484. doi: 10.1002/jclp.22612
Wang, Q., Van Heerikhuize, J., Aronica, E., Kawata, M., Seress, L., Joels, M., et al. (2013). Glucocorticoid receptor protein expression in human hippocampus; stability with age. Neurobiol. Aging 34, 1662–1673. doi: 10.1016/j.neurobiolaging.2012.11.019
Wang, Q., Verweij, E. W. E., Krugers, H. J., Joels, M., Swaab, D. F., and Lucassen, P. J. (2014). Distribution of the glucocorticoid receptor in the human amygdala; changes in mood disorder patients. Brain Struct. Function 219, 1615–1626. doi: 10.1007/s00429-013-0589-4
Warne, N., Caseras, X., and Rice, F. (2020). The cross-sectional and longitudinal relationship between overgeneral autobiographical memory and adolescent depression in a UK population-based cohort. J. Affect. Disord. 266, 621–625. doi: 10.1016/j.jad.2020.02.011
Watson, D., Clark, L. A., and Tellegen, A. (1988). Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Soc. Psychol. 54, 1063–70.
Weigand, A., Feeser, M., Gärtner, M., Brandt, E., Fan, Y., Fuge, P., et al. (2013). Effects of intranasal oxytocin prior to encoding and retrieval on recognition memory. Psychopharmacology 227, 321–329. doi: 10.1007/s00213-012-2962-z
Wellman, C. L., and Moench, K. M. (2019). Preclinical studies of stress, extinction, and prefrontal cortex: intriguing leads and pressing questions. Psychopharmacology 236, 59–72. doi: 10.1007/s00213-018-5023-4
Williams, J. M., and Broadbent, K. (1986). Autobiographical memory in suicide attempters. J. Abnorm. Psychol. 95, 144. doi: 10.1037/0021-843X.95.2.144
Williams, J. M. G. (2006). Capture and rumination, functional a voidance, and executive control (CaRFAX): three processes that underlie overgeneral memory. Cogn. Emot. 20, 548–568. doi: 10.1080/02699930500450465
Williams, J. M. G., Barnhofer, T., Crane, C., Herman, D., Raes, F., Watkins, E., et al. (2007). Autobiographical memory specificity and emotional disorder. Psychol. Bull. 133, 122. doi: 10.1037/0033-2909.133.1.122
Windle, R. J., Kershaw, Y. M., Shanks, N., Wood, S. A., Lightman, S. L., and Ingram, C. D. (2004). Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo–pituitary–adrenal activity. J. Neurosci. 24, 2974–2982. doi: 10.1523/JNEUROSCI.3432-03.2004
*Wingenfeld, K., Driessen, M., Terfehr, K., Schlosser, N., Fernando, S. C., Otte, C., et al. (2012a). Cortisol has enhancing, rather than impairing effects on memory retrieval in PTSD. Psychoneuroendocrinology 37, 1048–1056. doi: 10.1016/j.psyneuen.2011.12.002
*Wingenfeld, K., Driessen, M., Terfehr, K., Schlosser, N., Fernando, S. C., Otte, C., et al. (2013). Effects of cortisol on memory in women with borderline personality disorder: role of co-morbid post-traumatic stress disorder and major depression. Psychol. Med. 43, 495. doi: 10.1017/S0033291712001961
*Wingenfeld, K., Kuffel, A., Uhlmann, C., Terfehr, K., Schreiner, J., Kuehl, L. K., et al. (2012b). Effects of noradrenergic stimulation on memory in patients with major depressive disorder. Stress 16, 191–201. doi: 10.3109/10253890.2012.708951
*Wong, S. F., Cardoso, C., Orlando, M. A., Brown, C. A., and Ellenbogen, M. A. (2021). Depressive symptoms and social context modulate oxytocin's effect on negative memory recall. Soc. Cogn. Affect. Neurosci. 16, 1234–1243. doi: 10.1093/scan/nsab072
*Young, K., Drevets, W. C., Schulkin, J., and Erickson, K. (2011). Dose-dependent effects of hydrocortisone infusion on autobiographical memory recall. Behav. Neurosci. 125, 735. doi: 10.1037/a0024764
*Young, K. D., Preskorn, S. H., Victor, T., Misaki, M., Bodurka, J., and Drevets, W. C. (2016). The effect of mineralocorticoid and glucocorticoid receptor antagonism on autobiographical memory recall and amygdala response to implicit emotional stimuli. Int. J. Neuropsychopharmacol. 19, 1–11. doi: 10.1093/ijnp/pyw036
Zerbes, G., Kausche, F. M., Müller, J. C., Wiedemann, K., and Schwabe, L. (2019). Glucocorticoids, noradrenergic arousal, and the control of memory retrieval. J. Cogn. Neurosci. 31, 288–298. doi: 10.1162/jocn_a_01355
Zhang, Y., Kuhn, S. K., Jobson, L., and Haque, S. (2019). A review of autobiographical memory studies on patients with schizophrenia spectrum disorders. BMC Psychiatry 19, 1–36. doi: 10.1186/s12888-019-2346-6
Zimmerman, M., and Coryell, W. (1987). The Inventory to Diagnose Depression (IDD): a self-report scale to diagnose major depressive disorder. J. Consult. Clin. Psychol. 55, 55–59.
*^ References marked with an asterisk indicate studies included in the review.
Keywords: autobiographical, memory, specificity, pharmacological, modulation
Citation: Cawley E, Piazza G, Das RK and Kamboj SK (2022) A systematic review of the pharmacological modulation of autobiographical memory specificity. Front. Psychol. 13:1045217. doi: 10.3389/fpsyg.2022.1045217
Received: 15 September 2022; Accepted: 27 October 2022;
Published: 14 November 2022.
Edited by:
Valerio Santangelo, University of Perugia, ItalyReviewed by:
Daniel Greenberg, College of Charleston, United StatesCopyright © 2022 Cawley, Piazza, Das and Kamboj. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Emma Cawley, ZW1tYS5jYXdsZXlAdWNsLmFjLnVr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.