- 1School of Wushu and Art, Nanjing Sport Institute, Nanjing, China
- 2Department of Physical Education, Southeast University, Nanjing, China
- 3Department of Physical Education, Wuhan University of Technology, Wuhan, China
Objectives: This study aims to systematically analyze the effects of mind–body exercises on post-traumatic stress disorder (PTSD) symptoms, depression, and anxiety in patients with PTSD. Furthermore, it intends to provide scientific evidence-based exercise prescriptions.
Methods: Chinese (i.e., China National Knowledge Infrastructure, VIP Database for Chinese Technical Periodicals, and Wanfang) and English (i.e., Web of Science, PubMed, the Cochrane Library, and EMBASE) databases were used as data sources to search for studies on the effects of mind–body exercises on symptoms associated with patients with PTSD from January 1980 to November 2020. After a rigorous screening, 16 eligible randomized controlled trials (RCTs) were included in the meta-analysis.
Results: Mind–body exercises exerted a significant effect on PTSD symptoms [standard mean difference (SMD) = −0.41, 95% confidence interval (CI) −0.64 to −0.19, p < 0.001], depression (SMD = −0.35, 95% CI: −0.55 to −0.15, p < 0.001), and anxiety (SMD = −0.31, 95% CI: −0.74 to −0.12, p < 0.001) among patients with PTSD. Subgroup analysis demonstrated that 60–150 min per session for 8–16 weeks of mindfulness was more effective in improving symptoms in patients with PTSD under 45 years of age compared with other subgroups. For depression, 150–180 min of yoga exercises once per week was effective. For anxiety, the frequency, timing, duration, and type of mind–body exercises that are most effective in relieving anxiety in patients with PTSD cannot be determined at this time due to the limited number of eligible RCTs.
Conclusions: Mind–body exercises were found to be significantly effective in improving PTSD symptoms, depression, and anxiety in patients with PTSD. Therefore, they can be used as an adjunct to intervention for symptoms of patients with PTSD. However, this conclusion requires further confirmation through additional scientific and objective RCTs.
Systematic Review Registration: Unique Identifier: INPLASY2020120072.
Introduction
The sudden outbreak of the coronavirus disease (COVID-19) pandemic in 2019 triggered a global mental health crisis. Internationally, the incidence of COVID-19 was related to increased depression, anxiety, excessive stress, drug abuse, and increased suicidal ideation among the general population (Czeisler et al., 2020). Moreover, the COVID-19 outbreak exerted a serious impact on life, work, and studies, which led to several negative psychological reactions (Shi et al., 2020). Specifically, the high contagiousness of COVID-19 caused widespread negative emotions, such as anxiety, helplessness, and panic (Bo et al., 2020; El-Hage et al., 2020). One of the typically negative psychological outcomes of the epidemic was post-traumatic stress disorder (PTSD) (Fekih-Romdhane et al., 2020; Liu et al., 2020). For example, the author in (Xiong et al., 2020) analyzed the literature on PTSD in the context of the new crown epidemic and found that the incidence of PTSD ranged from 7 to 53.8% (Xiong et al., 2020). Therefore, exploring the effect of mind–body exercises on the relief of PTSD symptoms despite the continued global spread of COVID-19 can not only provide effective exercise prescriptions for patients with PTSD but also alleviate anxiety and panic as a result of the epidemic. Moreover, it can even prevent the occurrence of PTSD during the COVID-19 epidemic.
Events that lead to PTSD symptoms can also occur indirectly, such as when a person hears about the details or scenes of the death or serious injury of a loved one or a friend (Forbes et al., 2007). PTSD is typically caused by extensive traumatic experiences and is, therefore, common among survivors of war or major natural disasters, people recovering from major epidemics, victims of violence, and veterans. Surveys demonstrated that the prevalence of PTSD in the general population ranges from ~5 to 10% (Forbes et al., 2007). A census in the United States illustrated that more than half of the population (60.7% of men and 51.2% of women) reported experiencing traumatic events (i.e., flood, fire, natural disaster, life-threatening accidents, rape or sexual assault, witnessing a death, or severe injury). Among these people, 7.8% suffer from PTSD throughout their lives, whereas women are twice as likely to suffer from PTSD symptoms than men (Kessler et al., 2005).
Studies have demonstrated that the effects of PTSD are multifaceted. According to the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), a diagnosis of PTSD is composed of four major symptom clusters, namely, negative cognition and emotion; reminders to avoid events; excessive arousal; and intense revisiting of traumatic events through destructive memories and nightmares (Hegberg et al., 2019). In addition to the characteristic symptoms of PTSD, changes in brain structure that lead to functional changes (Hayes et al., 2012a; Miller et al., 2017) and cognitive impairment (Hayes et al., 2012b; Woon et al., 2017) are also well-documented. People with PTSD may suffer from severe issues with social survival and interpersonal communication due to the characteristics of the diseases, such as long-term invasiveness, avoidance, and excessive arousal, which results in impaired quality of life (Schnurr et al., 2006). In addition, patients with PTSD tend to exhibit activities in the higher sympathetic nerve and lower parasympathetic nerve (Fonkoue et al., 2018). Compared with healthy people, patients with PTSP display symptoms of low heart rate variability (Streeter et al., 2012). Although psychological interventions and traditional medication were proven effective in treating symptoms associated with PTSD (Cukor et al., 2009; Lancaster et al., 2016), many problems emerged regarding the side effects and therapeutic effects of drugs. Moreover, patients with PTSD typically report several obstacles, such as treatment cost, motivation, stigma, and access to care (Sayer et al., 2009; Dickstein et al., 2010). Although exercises present hindrances and drawbacks, such as limited motivation, self-efficacy, or exercise time, they are widely available, low-cost, and low-risk forms of intervention that can effectively prevent the side effects associated with traditional pharmacological and psychological intervention methods.
In recent years, adjuvant treatments for patients with PTSD and other trauma-related diseases have increasingly received scholarly attention. In exploring methods for promoting physical and mental health, many studies have shifted their focus on mind–body exercises. They have mainly utilize physical, mental, psychological, and behavioral exercises, such as breathing adjustment, physical exercise, meditation (Wynn, 2015; Zhang et al., 2019b; Zou et al., 2019a). This form has emphasized the holy trinity of exercise, i.e., combining the body, mind, and breathing. At the same time, it has the advantage of improving physical and mental health (Chan et al., 2013; Cushing and Braun, 2018; Zou et al., 2018a,b; Zhang et al., 2019a). Recently, various modalities of integrative mind–body intervention, which are increasingly employed in the treatment of PTSD, have emerged. Moreover, an increasing body of evidence has illustrated that mind–body interventions have exerted a positive impact on the psychological status, physical health, stress reduction, and quality of life of individuals with PTSD (Gordon et al., 2004, 2008; Grodin et al., 2008; Descilo et al., 2010; Bormann et al., 2011; Staples et al., 2011; Kearney et al., 2012; Hegberg et al., 2019). In 2010, 39% of people with PTSD reported using complementary and alternative medicine interventions, which includes various mind–body exercises that combine breathing and stretching exercises, such as tai chi, yoga, qigong, and meditation (Sapolsky et al., 2000). In fact, previous studies have demonstrated that mind–body exercises may positively influence PTSD-related symptoms through psychological and neurophysiological mechanisms, such as exposure and desensitization to internal arousal cues, improved cognitive function, normalization of the hypothalamic–pituitary–adrenal (HPA) axis function, motor-induced neuroplasticity, and reduction of inflammatory markers (McEwen, 2002; Yehuda and Golier, 2009; Streeter et al., 2012). In addition, emerging evidence supports the notion that mind–body exercises can effectively improve the symptoms of stress-related diseases through neurological and biological mechanisms (Bernardi et al., 2001; Vaiva et al., 2004; Brown and Gerbarg, 2009). Therefore, mind–body exercises may improve symptoms associated with PTSD, such as depression and anxiety, and may have the potential for use as an adjunctive treatment for patients with PTSD.
This study aims to review the effects of mind–body exercises (i.e., yoga, tai chi, qigong, and meditation) on PTSD symptoms. These four intervention methods require more active participation but less environment and related facilities. Moreover, they exhibit a high degree of portability and a huge dissemination potential for easy promotion and implementation (Zhou et al., 2019). Recently, numerous studies evaluated the specific effects of mind–body exercises on the presence of mood disorders related to PTSD symptoms, depression, and anxiety in patients with PTSD. However, these studies reported varying impacts on PTSD symptoms due to variability in sample selection (i.e., content, age, and gender) and the implementation of specific interventions (i.e., timing, frequency, method, and duration). Therefore, the current meta-analysis intends to explore the intrinsic regulatory mechanisms of mind–body exercises in patients with PTSD and to assess their effects on the symptoms and mood disorders associated with patients with PTSD to provide appropriate exercise prescriptions. Meanwhile, the exercise intervention program proposed by this study is expected to alleviate the mental health burden associated with COVID-19 at the global level.
Materials and Methods
Search Strategy
The current study is registered on the INPLASY website under registration number INPLASY2020120072 (https://inplasy.com/inplasy-2020-12-0072/). The literature was obtained from English (i.e., Web of Science, EMBASE, Cochrane Library, and PubMed) and Chinese (i.e., China National Knowledge Infrastructure, Wanfang, and VIP Database for Chinese Technical Periodicals) databases. A search was conducted to identify randomized controlled trials (RCTs) published between January 1, 1980 and November 30, 2020 in any language on the effects of mind–body exercises on patients with PTSD. The search terms include “mindfulness” OR “mind–body exercise” OR “yoga” OR “stretching” OR “tai chi” OR “qigong” OR “meditation.” PTSD-related terms include “PTSD,” “post-traumatic stress disorder,” “trauma” AND “depression,” “anxiety disorder,” “mood disorder,” “depressive disorder,” AND “anxiety disorder.”
Inclusion Criteria
The criteria for the inclusion of eligible articles were as follows. (1) Studies should be categorized as RCTs. (2) The experimental sample should consist of adults (18 years or older) with a psychiatrist-confirmed DSM-Fourth Edition-Text Revision diagnosis of PTSD. The results of their physical examination should indicate that they are eligible for participation in exercise programs along with consent to participate. (3) The sample should consist of experimental groups involving mind–body exercises (e.g., mindfulness, yoga, tai chi, qigong, and meditation) vs. control groups with different interventions (e.g., treatment as usual, no physical activity, and maintenance of daily life). (4) The primary outcome measures should include relevant data on PTSD symptoms, depression, and anxiety. Lastly, (5) the language of the literature should be only Chinese or English.
Exclusion Criteria
Studies were further screened according to the following exclusion criteria: (1) repeated studies; (2) Abstract articles only (no full text available), review studies, and non-RCT studies; (3) studies with vague or no data available for analysis; (4) studies with participants with substance dependence, psychiatric disorders, or prescriptions for alpha- or beta-blocking drugs; and (5) studies on massage, acupuncture, and other similar passive mind–body interventions.
Collection of Studies
Two researchers (Lin Zhu and Long Li) first read and screened the titles and abstracts of the documents and eliminated those that did not meet the inclusion criteria. If the studies met the inclusion criteria, then their full text was further evaluated and screened. In the case of disagreement between the two researchers during the screening process, a third researcher (Lin Wang) was invited to discuss with them to further verify whether the articles under disagreement met the inclusion criteria.
Data Extraction
Details of the literature that met the inclusion criteria were extracted, which include the first author of the study, year of publication, information about the participants in the experiment group (i.e., sample size, mean age/age range, and dropout rate), the specific design of the experiment (i.e., frequency of mind–body exercises, duration of each session, intervention period, and follow-up), outcome measures and analysis of relevant indicators, and the final study results. Meanwhile, quantitative data were also extracted from the literature, such as sample size, mean and standard deviation for measurement indicators related to PTSD, depression, and anxiety in terms of mind–body exercises and control groups.
Methodological Quality Assessment
Two investigators independently assessed the quality of studies that met the inclusion criteria using the modified Physical Therapy Evidence Database scale (Zou et al., 2019b). The modified assessment criteria consisted of nine items (i.e., randomization of sample selection; hidden allocation; similarity at baseline; assessor blinding; ≤ 15% dropout rate; intention-to-treat analysis; between-group comparison of intervention and control groups; point and variability measures; and independent exercise intervention) with high final scores indicating high-quality methods used in the study.
Study Analysis Method
This study used Stata 14.0 for meta-analysis. First, an overall forest map was drafted; heterogeneity was analyzed; and further regression and subgroup analyses were conducted. According to the Cochrane Handbook for Systematic Reviews of Interventions, we selected fixed- or random-effect meta-analysis according to the potential effect of the intervention on the outcome. We calculated the SMD and 95% confidence intervals (95% CI), where SMDs were considered small, medium, and large differences at intervals of 0.2–0.49, 0.5–0.79, and 0.8, respectively. Moreover, I2 values represent low (25%), medium (50%), and high (75%) heterogeneity (Higgins et al., 2003). The random-effect model was used for meta-analysis when the heterogeneity test I2 ≥ 50%, whereas the fixed-effect model was used when I2 <50%. Moreover, regression analysis was conducted to investigate the degree of experimental heterogeneity. Categorical variables (e.g., age of the sample, type of intervention, frequency of exercises, periodicity, and duration of each) were subject to subgroup analysis with the objective of determining which subgroup was more effective in improving symptoms associated with patients with PTSD and to provide a theoretical basis for later experiments.
Results
Selection of Studies
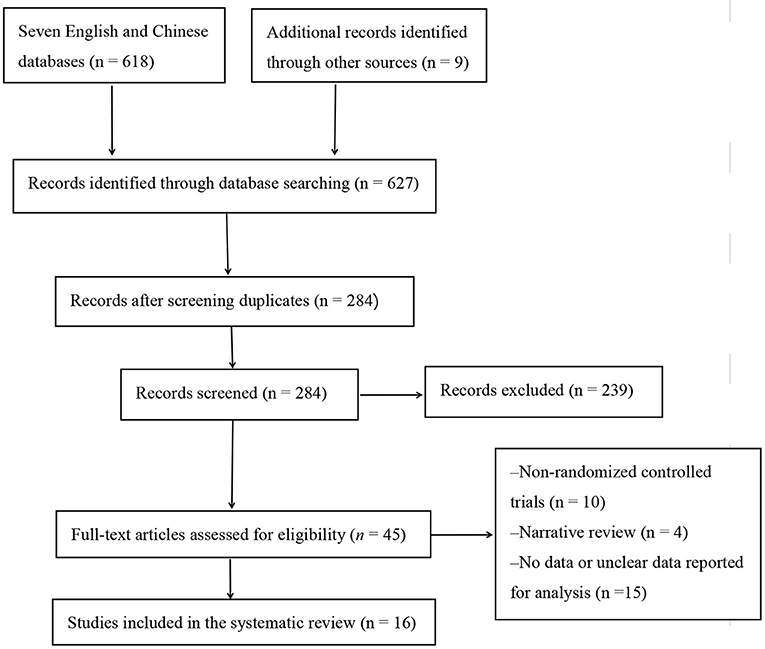
The initial literature was identified from seven English and Chinese databases (n = 618) and other resources (n = 9; Figure 1). After removing duplicate studies and those that did not meet the inclusion criteria, 284 articles remained. After a further screening, 45 papers were retained. A careful reading of the full text of the remaining 45 papers led to the exclusion of 29 studies, which included non-RCTs (n = 10), review articles (n = 4), and no or unclear measurements (n = 15). Finally, after a final rigorous screening, 16 eligible studies were included in the meta-analysis.
Characteristics of Eligible Studies
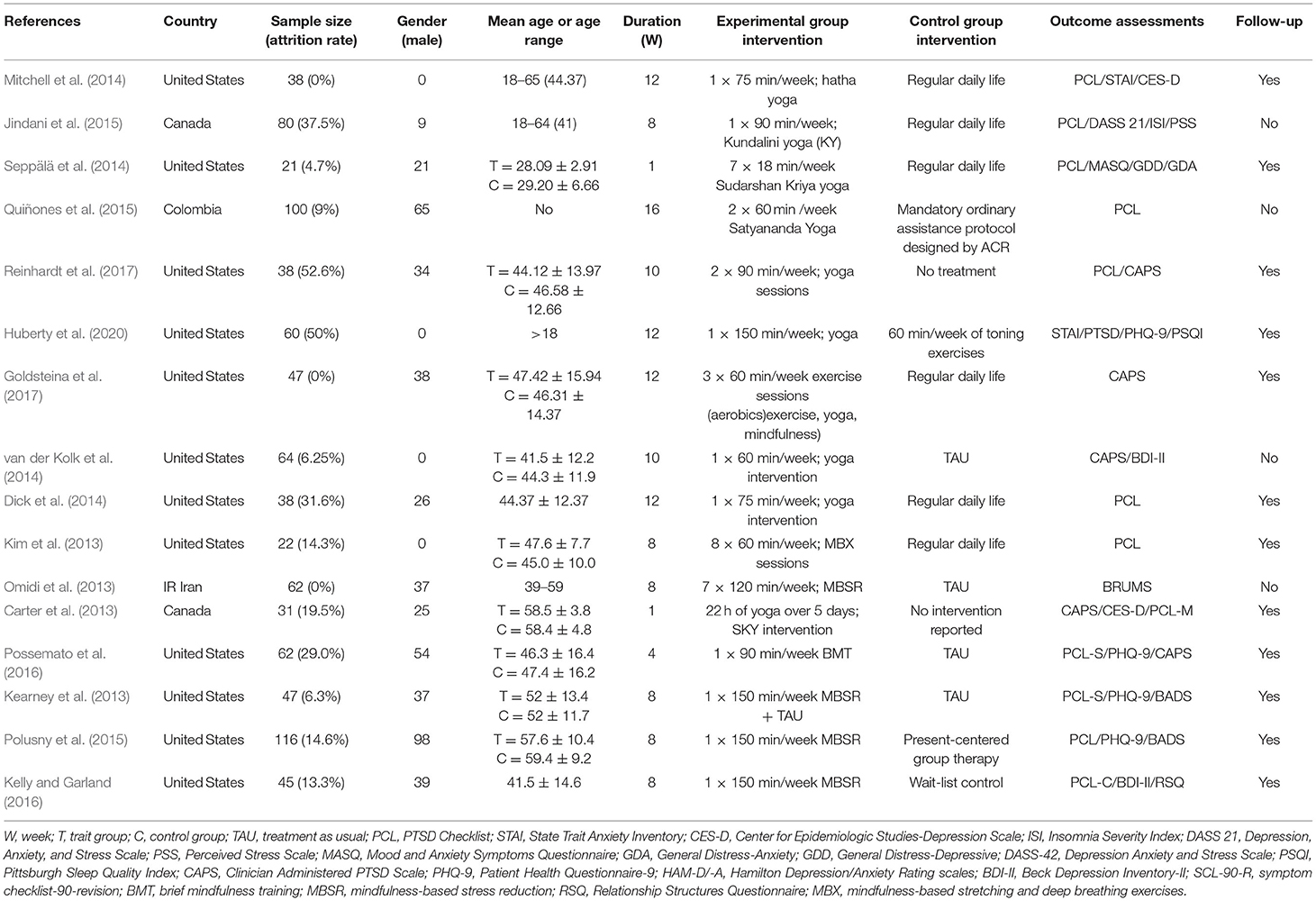
Table 1 presents the 16 eligible RCTs (Carter et al., 2013; Kearney et al., 2013; Kim et al., 2013; Omidi et al., 2013; Dick et al., 2014; Mitchell et al., 2014; Seppälä et al., 2014; van der Kolk et al., 2014; Jindani et al., 2015; Polusny et al., 2015; Quiñones et al., 2015; Kelly and Garland, 2016; Possemato et al., 2016; Goldsteina et al., 2017; Reinhardt et al., 2017; Huberty et al., 2020). In total, the selected studies included 871 participants, where the smallest and largest samples were 21 (Seppälä et al., 2014) and 116 (Polusny et al., 2015), respectively. The age of the experimental sample ranged from 18 to 65 years. The shortest and longest intervention periods were 1 and 16 weeks, respectively (Quiñones et al., 2015). The experimental group included various interventions, such as mindfulness and yoga. The control group of the experiment maintained regular daily life, usual treatment, and toning exercises (Table 1).
Methodological Quality Assessment
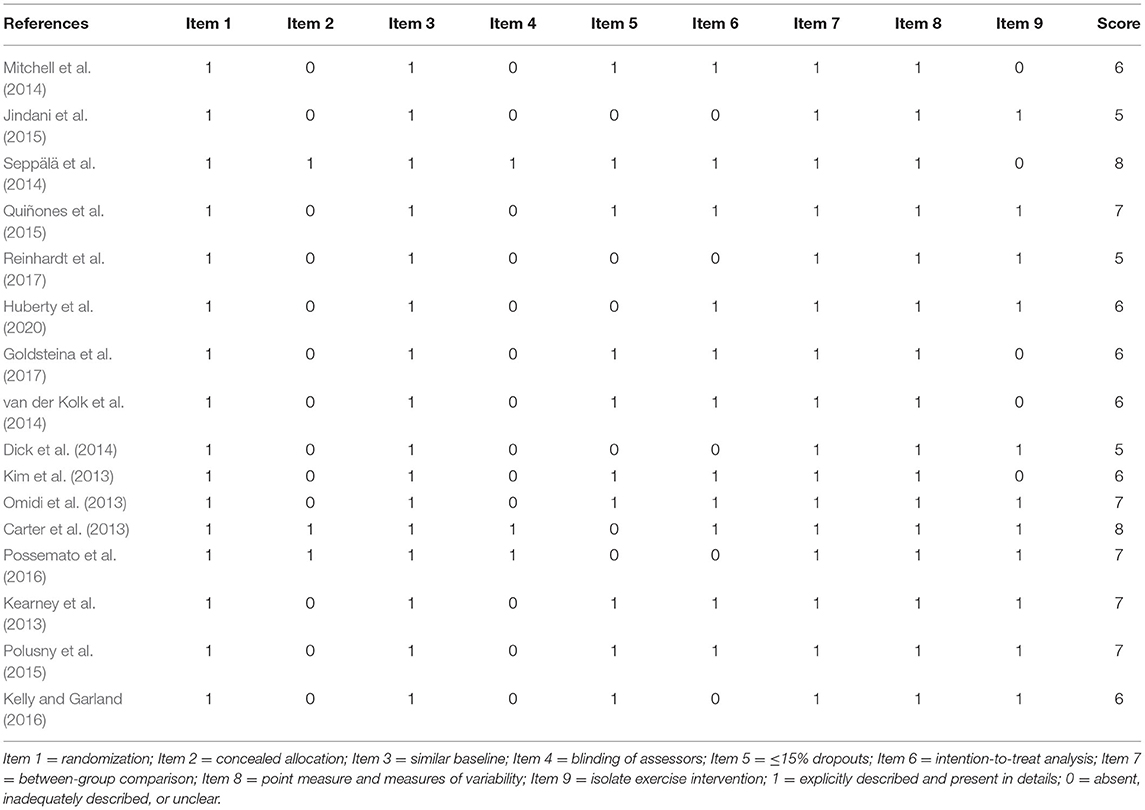
The methodological quality scores for the selected studies ranged from 5 to 8 (Table 2). All studies were eligible for the following: RCTs with similar baseline characteristics and studies with between-group comparisons; point and variable descriptive measures; and independent mind–body exercise interventions. Only three studies implemented concealed allocation and assessor blinding (Carter et al., 2013; Quiñones et al., 2015; Possemato et al., 2016). The dropout rates in all six studies were higher than 15% (Carter et al., 2013; Dick et al., 2014; Jindani et al., 2015; Possemato et al., 2016; Reinhardt et al., 2017; Huberty et al., 2020), whereas five studies did not conduct isolated exercise interventions (Kim et al., 2013; Mitchell et al., 2014; Seppälä et al., 2014; van der Kolk et al., 2014; Goldsteina et al., 2017).
Meta-Analysis of Outcome Indicators
Effects of Mind–Body Exercises on Patients With PTSD
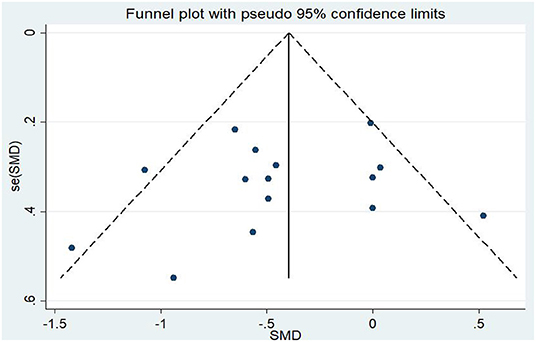
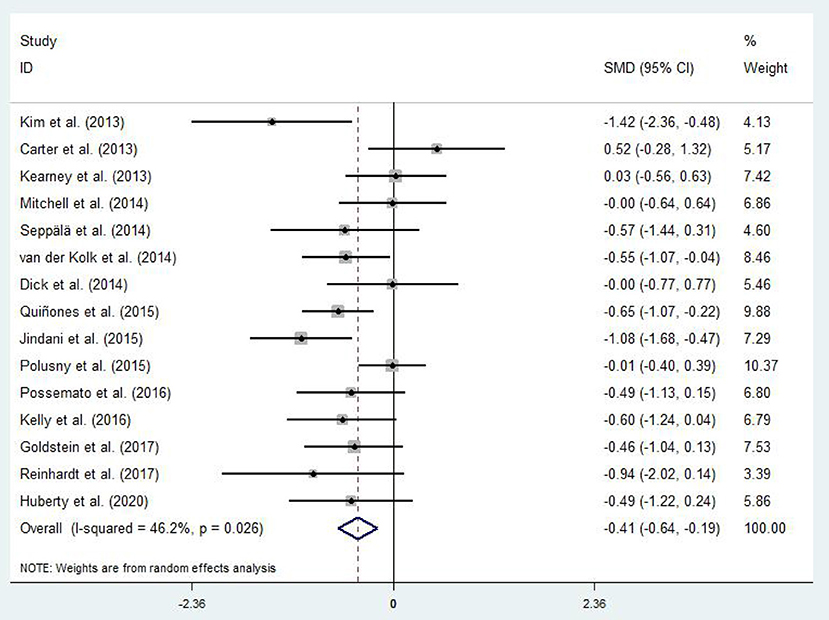
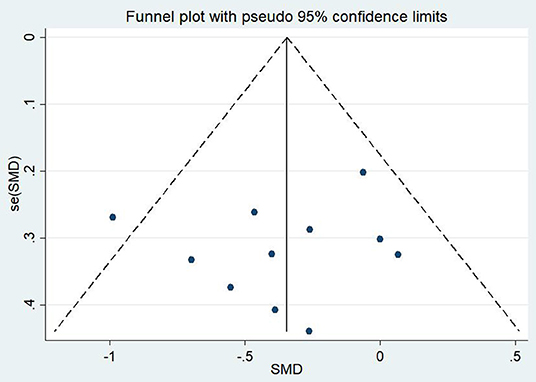
Differences in PTSD symptoms in 15 (Carter et al., 2013; Kearney et al., 2013; Kim et al., 2013; Dick et al., 2014; Mitchell et al., 2014; Seppälä et al., 2014; van der Kolk et al., 2014; Jindani et al., 2015; Polusny et al., 2015; Quiñones et al., 2015; Kelly and Garland, 2016; Possemato et al., 2016; Goldsteina et al., 2017; Reinhardt et al., 2017; Huberty et al., 2020) of the 16 articles that met the criteria were compared before and after the experimental and control groups. The asymmetric funnel plot indicates the presence of three outliers (Figure 2). The study employed the random-effect model due to the moderate heterogeneity in the literature (p = 0.026, I2 = 46.2%; Figure 3). The results of the meta-analysis of the 15 studies revealed that mind–body exercises significantly improved PTSD symptoms (SMD = −0.41, 95% CI: −0.64 to −0.19, p < 0.001).
Sensitivity analysis of heterogeneity and elimination revealed that the studies by Jindani et al. (2015), Reinhardt et al. (2017), and Kim et al. (2013) presented considerable bias. Thus, we excluded them to perform a meta-analysis of the remaining RCTs. The results demonstrated that heterogeneity was reduced (I2 = 17.5%, p = 0.282; SMD = −0.28, 95% CI: −0.46, −0.10) with significant differences between the groups (p < 0.001).
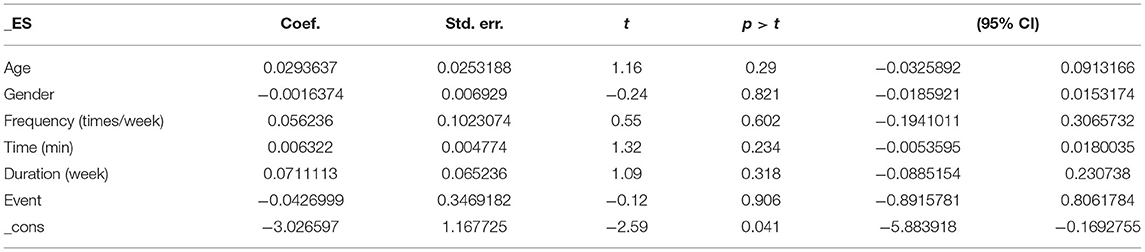
Covariates, such as age, gender, frequency, time, duration, and event are likely to be influencing factors for PTSD symptoms in patients with PTSD. Table 3 presents the results of the regression of covariates for patients with PTSD. Specifically, no significant effects were observed for age (95% CI: −0.0325892, 0.0913166, p = 0.29), gender (95% CI: −0.0185921, 0.0153174, p = 0.821), frequency (95% CI: −0.1941011, 0.3065732, p = 0.602), time (95% CI: −0.0053595, 0.8061784, p = 0.906), duration (95% CI: −0.0885154, 0.230738, p = 0.318), or event (95% CI: −0.8915781, 2.12068, p = 0.954).
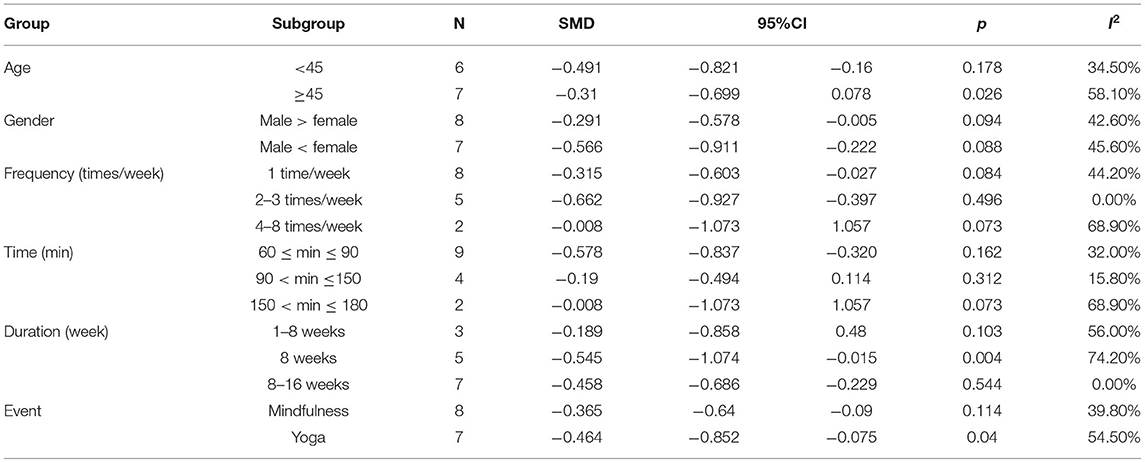
In this study, the factors influencing the experimental results were categorized into different groups according to category, such as age, gender, frequency, time, duration, and type of exercise (Table 4). The results of the subgroup analysis were as follows: (1) age: mind–body exercises were beneficial for patients with PTSD who were aged less 45 years and with PTSD symptoms. (2) Gender: when males outnumber females, mind–body exercises exerted a more significant effect on PTSD symptoms. (3) Frequency: mind–body exercises conducted 1–3 times per week was more effective in improving PTSD symptoms than 4–8 times per week. (4) Time: mind–body exercises for a duration of 60–150 min was effective in improving PTSD symptoms. (5) Duration: in the subgroup for exercise intervention duration, the improvement of symptoms in patients with PTSD was more significant with an 8–16-week intervention. (6) Event: the effect of mindfulness exerted significant effects on PTSD symptoms.
Effects of Mind–Body Exercises on Patients With PTSD and Depression
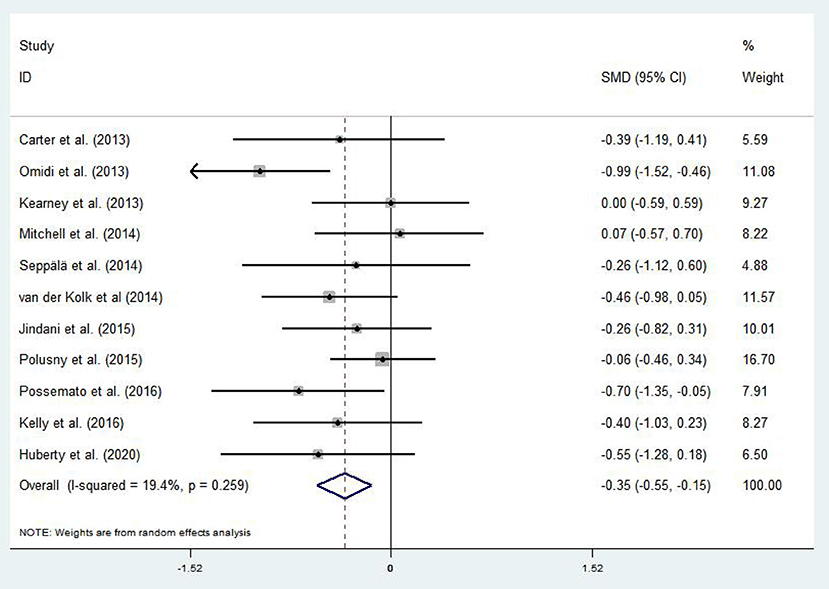
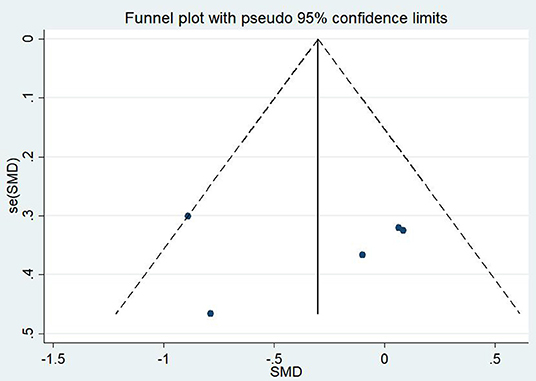
A total of 11 articles (Carter et al., 2013; Kearney et al., 2013; Omidi et al., 2013; Mitchell et al., 2014; Seppälä et al., 2014; van der Kolk et al., 2014; Jindani et al., 2015; Polusny et al., 2015; Kelly and Garland, 2016; Possemato et al., 2016; Huberty et al., 2020) evaluated the effects of mind–body exercises on depression in patients with PTSD. Figure 4 illustrates an asymmetric funnel plot and indicates the presence of one outlier. The test of heterogeneity of the included literature depicted a low level of heterogeneity (p = 0.259, I2 = 19.4%). Therefore, the meta-analysis was performed using the fixed-effect model (Figure 5). The results demonstrated that mind–body exercises significantly improved depression in patients with PTSD (SMD = −0.35, 95% CI: −0.55 to −0.15, p < 0.001).
Sensitivity analysis of heterogeneity and elimination revealed that the studies by Omidi et al. (Omidi et al., 2013) exhibited considerable bias; thus, it was excluded. Afterward, analysis was continued for the remaining RCTs. The results indicated reduced heterogeneity (I2 = 3.2%, p = 0.643, SMD = −0.26, 95% CI: −0.45, −0.07), whereas the difference between the experimental and control groups remained significant (p < 0.001).
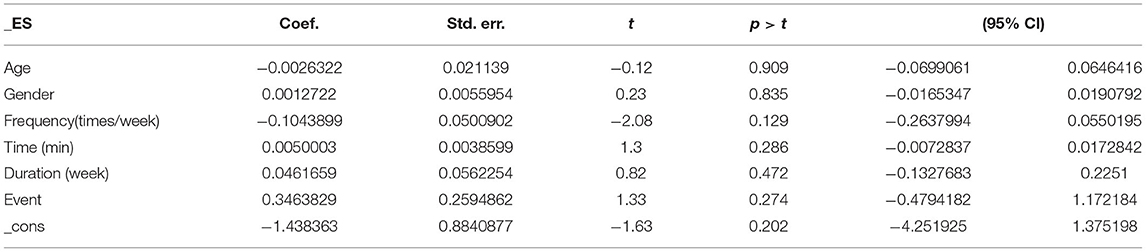
Covariates including the participants' basic profile (e.g., age and gender) and the intervention method (i.e., frequency, time, duration, and type of exercise) may be factors that influence the degree of depression in patients with PTSD. Table 5 provides the results of the covariate regression for depressive symptoms in patients with PTSD. For depression, no significant effects were observed for age (95% CI: −0.0699061, 0.0646416, p = 0.909), gender (95% CI: −0.0165347, 0.0190792, p = 0.835), frequency (95% CI: −0.2637994, 0.0550195, p = 0.129), time (95% CI: −0.0072837, 0.0172842, p = 0.286), duration (95% CI: −0.1327683, 0.2251, p = 0.472), or event (95% CI: −0.4794182, 1.172184, p = 0.274).
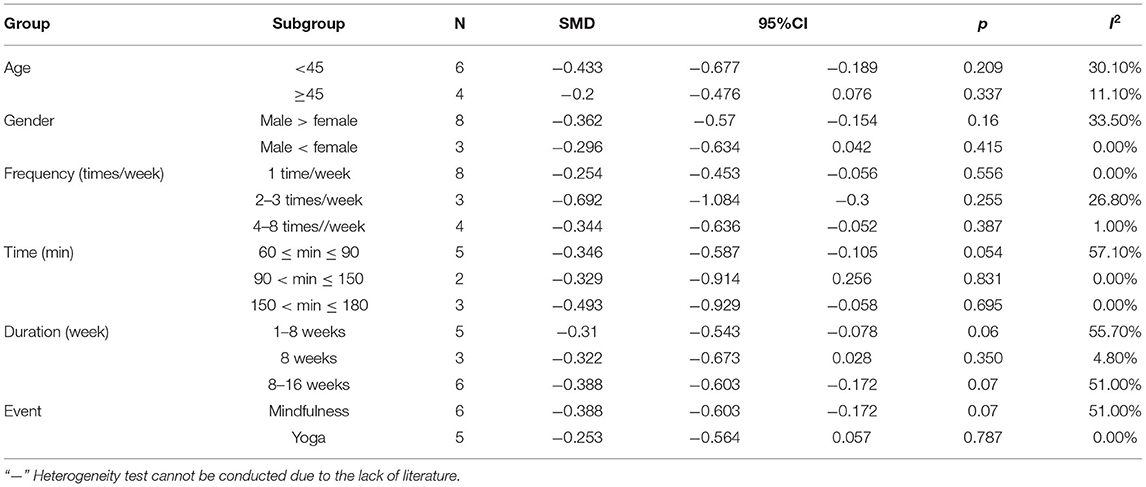
In all eligible studies, we performed a subgroup analysis based on the underlying profile of the participants and the intervention method (Table 6). The results are as follows. (1) Age: mind–body exercises were beneficial for the improvement of depressive symptoms in patients with PTSD aged more than 45 years. (2) Gender: when females outnumbered the males, the improvement in depressive symptoms in patients with PTSD is more significant. (3) Frequency: the effect of performing mind–body exercises once per week or 4–8 times per week was more effective for depression symptoms in patients with PTSD. (4) Time: Exercises for 60–90 min or 150–180 min per session were beneficial for depressive conditions in patients with PTSD. (5) Duration: An intervention period of 1–8 or 8–16 weeks exerted a significant effect on depressive symptoms in patients with PTSD. (6) Event: For patients with PTSD and depression, the effect of yoga exerted significant effects.
Effect of Mind–Body Exercises on Patients With PTSD and Anxiety
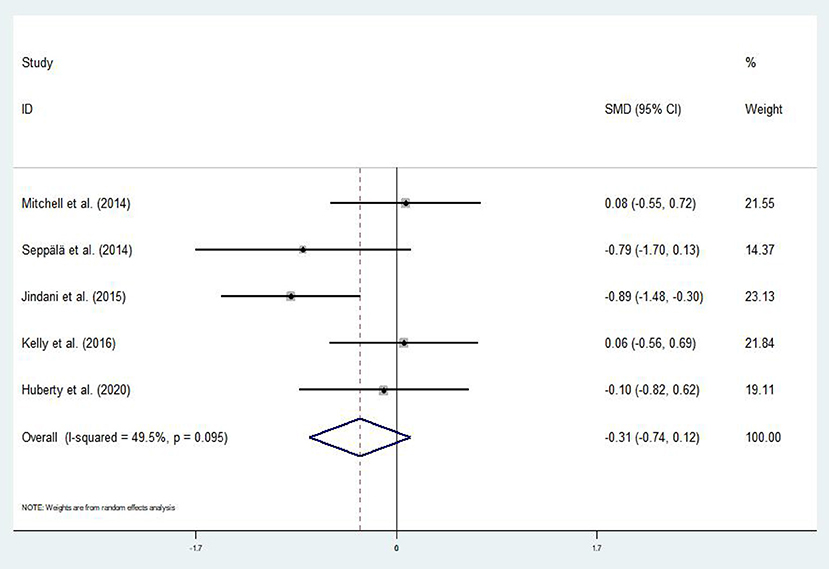
Out of the 16 eligible articles, only five (Mitchell et al., 2014; Seppälä et al., 2014; Jindani et al., 2015; Kelly and Garland, 2016; Huberty et al., 2020) assessed the effect of mind–body exercises on anxiety symptoms in patients with PTSD. The asymmetric funnel plot in Figure 6 indicated no outliers. The results of the heterogeneity test displayed moderate heterogeneity (p = 0.095, I2 = 49.5%); thus, we employed the random-effects model (Figure 7). Meta-analysis revealed that mind–body exercises significantly improved anxiety symptoms in patients with PTSD (SMD = −0.31, 95% CI: −0.74 to −0.12, p < 0.001).
Discussion
This systematic review and meta-analysis aimed to analyze the effects of mind–body exercises on PTSD symptoms, depression, and anxiety in patients with PTSD and to lay the foundation for the development of rational exercise prescriptions. The results suggested that mind–body exercises can improve PTSD symptoms, depression, and anxiety in patients with PTSD. When a patient's physical condition permits, medical staff can use mind–body exercises as an adjunct to conventional treatments to further optimize the treatment plans for patients with PTSD.
The findings of this systematic review are consistent with those of published articles that concluded that mind–body exercises can improve PTSD symptoms, depression, and anxiety in patients with PTSD. Specifically, the effect size of PTSD, depression, and anxiety ranged from −0.64 to −0.19 (p < 0.001), from −0.5 to −0.15 (p < 0.001), and from −0.74 to −0.12 (p < 0.001), respectively. In addition, a high level of heterogeneity may exist in the study of Kim et al. (2013), who recruited only 22 participants and exhibited an attrition rate as high as 14.3%. Thus, the lower sample content of the final experiment may be the major reason for the heterogeneity.
Subgroup analysis indicated that mindfulness exercises for 60–150 min per session over a span of 8–16 weeks resulted in further significant improvements in PTSD symptoms in patients aged <45 years. In the subgroup analysis for frequency, exercises conducted 1–3 times per week were found significant. In addition, mind–body exercises may be more effective in improving PTSD symptoms among males. For depression, once per week, 4–8 times per week, 60–90 min, or 150–180 min of yoga displayed larger effects on patients with PTSD who were aged more than 45 years. Unfortunately, RCTs on the effect of mind–body exercises on anxiety symptoms in patients with PTSD are limited. Therefore, we were unable to determine the most effective frequency, duration, experimental period, and type of intervention. However, the exercise prescription should follow the recommendations provided by the International Organization of Physical Therapists in Mental Health for the generally healthy population. These recommendations include 150 min of moderate-intensity exercises or 75 min of vigorous exercises per week (Vancampfort et al., 2012). Notably, in the subgroup analysis of PTSD and depression, yoga exhibited differences in heterogeneity for the PTSD (I2 = 54.5%) and depression (I2 = 0.0%) subgroups. The mechanisms by which PTSD symptoms and depression occur in patients with PTSD may be the main source of the outcome bias.
PTSD symptoms can inflict a serious impact on a patient's normal social and daily life. However, low-to-moderate-intensity mind–body exercise intervention exerts a positive impact on PTSD symptoms (Telles et al., 2007).
Unhealthy lifestyles and personal traumatic experiences are expected to exert adverse effects on the body, which leads to an imbalance in the HPA axis (McEwen, 2002). Dysfunction in the HPA axis, which is characterized by abnormally high levels of corticotropin-releasing hormone (CRH) and low cortisol levels, is one of the unique neuroendocrine characteristics that distinguish PTSD from other mental diseases (Yehuda, 2002; Yehuda and Golier, 2009). Many studies have demonstrated that cortisol plays a crucial role in the pathophysiology of PTSD. However, among healthy individuals exposed to an acute stressor, the HPA axis can respond to the stressor by secreting relevant hormones, such as the CRH from the hypothalamus, adrenocorticotropic hormone (ACTH) from the anterior pituitary, and glucocorticoids from the adrenal cortex (Sapolsky et al., 2000). Increased basal levels of cortisol can improve declarative memory and performance and are associated with the normalization of glucose metabolism in the limbic system (Twamley et al., 2004). Patients with PTSD symptoms exhibit low basic levels of cortisol (Boscarino, 1996). Compared with individuals without PTSD, the sensitivity of the negative feedback inhibition of cortisol in the HPA axis is higher (Yehuda, 2002). In patients with PTSD, elevated basal cortisol levels are associated with improvement in symptoms (Aerni et al., 2004). Several related studies indicated that exercise is related to the transient increase in plasma cortisol (Brandenberger and Follenius, 1975) and improvement in cognitive function (Twamley et al., 2004). Conversely, CRH secretion is abnormally high in patients with PTSD (Bremner et al., 1997), but the level of ACTH is low, which is the hormone that causes the adrenal cortex to secrete glucocorticoids (Smith et al., 1989). The slow response of ACTH to CRH may lead to a decrease in ACTH secretion in the pituitary, which leads to a decrease in the production of cortisol in the adrenal cortex (Smith et al., 1989). Dehydroepiandrosterone sulfate (DHEAS) is an anabolic steroid molecule that enhances an individual's resistance to stress by preventing cell death and behavioral deficits through neuroprotective effects. Relevant studies have illustrated that reasonable exercise can effectively increase DHEAS levels (Johnson, 1997; Rasmusson et al., 2004). Therefore, mind–body exercises can effectively improve PTSD symptoms in patients with PTSD.
The main psychiatric abnormalities in patients with PTSD are typically symptoms of depression and anxiety (Javidi and Yadollahie, 2012). An increasing number of studies demonstrate that proper exercise is beneficial for depression and anxiety symptoms, including those in patients with PTSD (Rebar et al., 2015; Schuch et al., 2016). Evidence exists that impaired adult hippocampal neurogenesis (AHN) is related to various neurological diseases, including PTSD, depression, and Alzheimer's disease (Gao et al., 2017; Barnett et al., 2019). Thus, enhancing AHN can effectively reduce the mood symptoms of the depression model, improve memory resolution, and avoid the recovery of panic experience (Sahay et al., 2011; Hill et al., 2015). In the pathogenesis of PTSD, a dysfunction in the AHN leads to the onset of cognitive impairment and related psychiatric symptoms (Malberg, 2004; Catlow et al., 2013; Besnard and Sahay, 2016). Therefore, improving AHN can effectively alleviate cognitive dysfunction and emotional problems related to patients with PTSD.
The tight regulation of AHN is derived from the interaction between environmental factors and endogenous ecotropic regulators (Toda and Gage, 2018). For example, the PI3K/Akt pathway plays an important role in linking pro-neurogenic gene transcription factors with various neurotrophic and growth factors, such as brain-derived neurotrophic factor and epithelial growth factor signaling (Yin H. et al., 2019; Yin L. et al., 2019). Alternatively, several studies provide evidence that highlight the importance of Akt signaling in regulating AHN. In addition, Akt1 is highly expressed in the adult hippocampus and may influence the synaptic plasticity of the CA1 sub-region (Levenga et al., 2017). Scaffold protein disrupted-in-schizophrenia 1 can regulate AHN via the Akt pathway (Wu et al., 2013). Moreover, exercise-mediated Akt signaling may also play a role in cognitive and emotional regulation and promote synaptic plasticity and synapse formation at the same time (Qian et al., 2015; Levenga et al., 2017). Hippocampal neurogenesis is an important neuroplasticity that provides adaptability under stress and regulates cognitive function and antidepressant behavior (Gao et al., 2017, 2018). Thus, a certain density of immature neurons also contributes to the clearance of fear memory, which, in turn, improves memory resolution (Pereira et al., 2014; Johnston et al., 2016).
Previous studies have demonstrated that as a mature non-drug treatment method, psychosomatic movement is reliant on the activation of Akt by AHN to play a role in improving cognition/anti-depression, in promoting AHN, and in improving neurobehavior. In the medical field, scholars are increasingly interested in studying the physical and psychological effects of mind–body interventions, such as mindfulness and yoga. Mind–body exercises can enhance the ability of patients with PTSD to endure unpleasant emotions, which reduces mental stress (Brown and Gerbarg, 2005; Rocha et al., 2012; West et al., 2014). Yoga focuses on a combination of physical exercise and breathing, where the main exercises include body movement, muscle relaxation, and meditation (Granath et al., 2006). Slow and rhythmic breathing in conjunction with yoga body movements can help to activate the parasympathetic nervous system and stimulate vagus nerve activity (Papp et al., 2013), which can effectively alleviate the symptoms of overexcitement. In this manner, this process is beneficial to patients with PTSD. Other studies depicted that yoga can help restore balance to the HPA axis by lowering the levels of the stress hormone cortisol (Gothe et al., 2016). Streeter et al. (2012) proposed that yoga can reduce a stress-induced unsteady state load using three stress response systems, namely, the HPA axis, the autonomic nervous system, and the GABA-ergic system. Therefore, yoga also may be effective in improving the symptoms of patients with PTSD, including depression and anxiety (Kjellgren et al., 2017). Through mindfulness practice, yoga provides practitioners with a strategy for concentration and for enhancing mind and body awareness (Iverson et al., 2011). Specifically, enhancing mindfulness can increase one's acceptance of one's emotions, improve regulation, and reduce avoidance (Mitchell et al., 2014).
In general, patients with PTSD undergo a lengthy disease duration and a high relapse rate; thus, long-term treatment can place a serious financial burden on individuals and families. Estimates suggest that trauma-related disorders cost over $45 billion USD per year in medical and related costs (Tanielian et al., 2016; von der Warth et al., 2020). Thus, mind–body exercises as a complementary alternative therapy can effectively reduce medical expenditures and reduce the burden on society and families. PTSD symptoms may include, but are not limited to, rapid heartbeat, difficulty in breathing, muscle tightness, hyper-arousal, inability to relax, chronic pain, mood issues, racing thoughts, and substance abuse (Briere and Spinazzola, 2005). Low levels of self-esteem, lack of coping skills, and lack of interpersonal skills constitute a range of symptoms that can impact a patient's quality of life and relationships (Van der Kolk, 2006). In this regard, mind–body exercises can effectively relieve muscle tension and improve breathing problems and other related symptoms through physical activities with breathing exercises, which can improve the quality of life of patients to a certain extent.
In the present review, all articles indicated the significant effect of mind–body exercises on PTSD symptoms among patients with PTSD. Specifically, Reinhardt et al. (2017) presented a sample attrition rate of as high as 52.6% and a sample type of veterans. Therefore, many studies face the challenge of retaining veterans for treatment. Moreover, Carter et al. (2013) and Mitchell et al. (2014) suggested that yoga is feasible as a complementary treatment for veterans with PTSD despite the fact that the results of the intervention for PTSD symptoms among veterans failed to consistently indicate significant changes. In summary, additional RCTs are required to demonstrate the effects of mind–body exercises on PTSD symptoms, depression, and anxiety in patients with PTSD. Therefore, mind–body exercises can be used as an alternative treatment for patients with mild forms of PTSD, as well as an adjunct to recovery and home care for patients with moderate or even severe PTSD.
Limitations
Although the current literature review tentatively established the benefits of mind–body exercises for patients with PTSD, the limitations of the existing studies should be noted. The reliability of the results may have been influenced by age; gender; duration of the disease; frequency, duration, and intensity of the experimental intervention; and type of exercise. Several qualitative differences were observed for the studies included in the review. (1) Several studies employed small sample sizes with very limited age range and/or race and region (many participants were predominantly white). (2) Only three studies indicated that the sample allocation used hidden allocation, which could be a cause of systematic bias in the treatment effect. (3) Only three studies used assessor blinding in measuring the outcomes. (4) The absence of the descriptions of disease duration and degree of illness in the majority of the literature relatively influences the specific analysis of the effectiveness of mind–body exercises on symptom improvement in patients with PTSD. (5) For anxiety, we were unable to determine the optimal frequency, timing, duration, and type of exercise for mind–body exercises for improving anxiety symptoms in patients with PTSD due to the limited number of eligible RCTs. (6) Lastly, methodological differences in the timing, frequency, duration, and outcome measures of the interventions contributed to the differences in outcomes and led to the difficulties in interpretation. Although these issues limit the generalizability of the studies to different populations, the positive correlation between mind–body exercises and PTSD-related symptoms in these studies provides evidence for the generalizability of the findings.
Conclusions
Research has proven that mind–body exercises can effectively improve PTSD symptoms, depression, and anxiety in patients with PTSD and can be used as an alternative or adjunctive treatment according to the unique situation of each patient. In cases where patients with PTSD can exercise without adverse effects, then medical staff may use mind–body exercises as a supplemental regimen to conventional treatment. In addition, the conclusion of this research requires further confirmation using additional high-quality RCTs with large samples. Meanwhile, the current review can also provide an exercise prescription for relieving stress and traumatic stress disorders for people experiencing PTSD symptoms due to COVID-19.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
LL and LW presented the conceptualization and design of the study protocol. LZ, LW, and LL applied the search strategy and screened the literature based on the selection criteria. LZ and X-zL assessed the risk of bias for the screened literature. All researchers were involved in the collation and analysis of the data and interpreted the results of the data analysis. LZ wrote this manuscript, which was further revised and edited by LW, LL, and X-zL. All authors have read the final revised manuscript and agree to its publication.
Funding
This work was supported by the Ministry of education of Humanities and Social Science project (17YJA890025); the Fundamental Research Funds for the Central Universities (WUT: 2020VI001).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aerni, A., Traber, R., and Hock, C. (2004). Low-dose cortisol for symptoms of posttraumatic stress disorder. Am. J. Psychiatry 161, 1488–1490. doi: 10.1176/appi.ajp.161.8.1488
Barnett, B. R., Anderson, J. M., Torres-Velazquez, M., Yi, S. Y., Rowley, P. A., and Yu, J. J. (2019). Exercise ameliorates deficits in neural microstructure in a Disc1 model of psychiatric illness. Magn. Reson. Imaging 61, 90–96. doi: 10.1016/j.mri.2019.05.021
Bernardi, L., Gabutti, A., Porta, C., and Spicuzza, L. (2001). Slow breathing reduces chemoreflex response to hypoxia and hypercapnia, and increases baroreflex sensitivity. J. Hypertens. 19, 2221–2229. doi: 10.1097/00004872-200112000-00016
Besnard, A., and Sahay, A. (2016). Adult hippocampal neurogenesis, fear generalization, and stress. Neuropsychopharmacology41, 24–44. doi: 10.1038/npp.2015.167
Bo, H. X., Li, W., Yang, Y., Wang, Y., Zhang, Q., and Cheung, T. (2020). Posttraumatic stress symptoms and attitude toward crisis mental health services among clinically stable patients with COVID-19 in China. Psychol. Med. 51, 1052–1053. doi: 10.1017/S0033291720000999
Bormann, J. E., Liu, L., Thorp, S. R., and Lang, A. J. S. (2011). Spiritual wellbeing mediates PTSD change in veterans with military-related PTSD. Int. J. Behav. Med. 8:28. doi: 10.1007/s12529-011-9186-1
Boscarino, J. A (1996). Posttraumatic stress disorder, exposure to combat, and lower plasma cortisol among Vietnam veterans: findings and clinical implications. J. Consult. Clin. Psychol. 64, 191–201. doi: 10.1037/0022-006X.64.1.191
Brandenberger, G., and Follenius, M. (1975). Influence of timing and intensity of muscular exercise on temporal patterns of plasma cortisol levels. J. Clin. Endocrinol. Metab. 40, 845–849. doi: 10.1210/jcem-40-5-845
Bremner, J. D., Licinio, J., Darnell, A., Krystal, J. H., Owens, M. J., and Southwick, S. M. (1997). Elevated CSF corticotropinreleasing factor concentrations in posttraumatic stress disorder. Am. J. Psychiatry. 154, 624–629. doi: 10.1176/ajp.154.5.624
Briere, J., and Spinazzola, J. (2005). Phenomenology and psychological assessment of complex posttraumatic states. J. Trauma. Stress 18, 401–412. doi: 10.1002/jts.20048
Brown, R. P., and Gerbarg, P. L. (2005). Sudarshan Kriya Yogic breathing in the treatment of stress, anxiety, and depression: part II- clinical applications and guidelines. J. Altern. Complement. Med. 11, 711–717. doi: 10.1089/acm.2005.11.711
Brown, R. P., and Gerbarg, P. L. (2009). Yoga breathing, meditation, and longevity. Ann. N. Y. Acad. Sci. 1172, 54–62. doi: 10.1111/j.1749-6632.2009.04394.x
Carter, J., Gerbarg, P. L., Brown, R. P., Ware, R. S., D'Ambrosio, C., Anand, L., et al. (2013). Multi-component yoga breath program for Vietnam veteran post traumatic stress disorder: randomized controlled trial. J. Traum. Stress Disord. Treat. 2:1000108. doi: 10.4172/2324-8947.1000108
Catlow, B. J., Song, S., Paredes, D. A., Kirstein, C. L., and Sanchez-Ramos, J. (2013). Effects of psilocybin on hippocampal neurogenesis and extinction of trace fear conditioning. Exp. Brain Res. 228, 481–491. doi: 10.1007/s00221-013-3579-0
Chan, A. S., Sze, S. L., Siu, N. Y., Lau, E. M., and Cheung, M. C. A. C. (2013). A Chinese mind-body exercise improves selfcontrol of children with autism: a randomized controlled trial. PloSONE 8:e68184. doi: 10.1371/journal.pone.0068184
Cukor, J., Spitalnick, J., Difede, J., Rizzo, A., and Rothbaum, B. O. (2009). Emerging treatments for PTSD. Clin. Psychol. Rev. 29, 715–726. doi: 10.1016/j.cpr.2009.09.001
Cushing, R. E., and Braun, K. L. (2018). Mind-body therapy for military veterans with post-traumatic stress disorder: a systematic review. J Altern Complem Med. 24, 106–114. doi: 10.1089/acm.2017.0176
Czeisler, M. É., Lane, R. I., Petrosky, E., Wiley, J. F., Christensen, A., Njai, R., et al. (2020). Mental health, substance use, and suicidal ideation during the COVID-19 pandemic-United States. MMWR 69, 1049–1057. doi: 10.15585/mmwr.mm6932a1
Descilo, T., Vedamurtachar, A., and Gerbarg, P. L. (2010). Effects of a yoga breath intervention alone and in combination with an exposure therapy for post-traumatic stress disorder and depression in survivors of the 2004 South-East Asia tsunami. Acta Psychiatr. Scand. 121, 289–300. doi: 10.1111/j.1600-0447.2009.01466.x
Dick, A. M., Niles, B. L., Street, A. E., DiMartino, D.M., and Mitchell, K.S. (2014). Examining mechanisms of change in a yoga intervention for women: the influence of mindfulness, psychological flexibility, and emotion regulation on PTSD symptoms. J. Clin. Psychol. 70, 1170–1182. doi: 10.1002/jclp.22104
Dickstein, B. D., Vogt, D. S., Handa, S., and Litz, B. T. (2010). Targeting self-stigma in returning military personnel and veterans: a review of intervention strategies. Mil. Psychol. 22:224. doi: 10.1080/08995600903417399
El-Hage, W., Hingray, C., Lemogne, C., Yrondi, A., Brunault, P., and Bienvenu, T. (2020). Health professionals facing the coronavirus disease 2019 (COVID-19) pandemic: What are the mental health risks? L'Encephale 46, s73–s80. doi: 10.1016/j.encep.2020.04.008
Fekih-Romdhane, F., Ghrissi, F., Abbassi, B., and Cherif, W. (2020). Prevalence and predictors of PTSD during the COVID-19 pandemic: findings from a Tunisian community sample. Psychiatry Res. 290:113131. doi: 10.1016/j.psychres.2020.113131
Fonkoue, I. T., Norrholm, S. D., Marvar, P. J., Li, Y., Kankam, M. L., and Rothbaum, B. O. (2018). Elevated resting blood pressure augments autonomic imbalance in posttraumatic stress disorder. Am J Physiol. Reg I. 315, R1272–R1280. doi: 10.1152/ajpregu.00173.2018
Forbes, D., Creamer, M., Phelps, A., Bryant, R., McFarlane, A., Devilly, G. J., et al. (2007). Australian guidelines for the treatment of adults with acute stress disorder and post-traumatic stress disorder. Australas. Psychiatry 41, 637–648. doi: 10.1080/00048670701449161
Gao, C., Chen, X., Xu, A., Cheng, K., and Shen, J. (2018). Adaptor protein APPL2 affects adult antidepressant behaviors and hippocampal neurogenesis via regulating the sensitivity of glucocorticoid receptor. Mol. Neurobiol. 55, 5537–5547. doi: 10.1007/s12035-017-0785-y
Gao, C., Wang, Q., Chung, S. K., and Shen, J. (2017). Crosstalk of metabolic factors and neurogenic signaling in adult neurogenesis: implication of metabolic regulation for mental and neurological diseases. Neurochem. Int. 106, 24–36. doi: 10.1016/j.neuint.2017.02.001
Goldsteina, L. A., Mehling, W. E., Metzler, T. J., Cohen, B. E., and Barnes, D. E. (2017). Veterans group exercise: a randomized pilot trial of an Integrative Exercise program for veterans with posttraumatic stress. J. Affect. Disord. 227, 345–352. doi: 10.1016/j.jad.2017.11.002
Gordon, J. S., Staples, J. K., Blyta, A., and Bytyqi, M. (2004). Treatment of posttraumatic stress disorder in Postwar Kosovo high school students using mind-body skills groups: a pilot study. J. Trauma. Stress. 17, 143–147. doi: 10.1023/B:JOTS.0000022620.13209.a0
Gordon, J. S., Staples, J. K., Blyta, A., Bytyqi, M., and Wilson, A. T. (2008). Treatment of posttraumatic stress disorder in postwar Kosovar adolescents using mind-body skills groups: a randomized controlled trial. J. Clin. Psychiatry. 69, 1469–1476. doi: 10.4088/JCP.v69n0915
Gothe, N. P., Keswani, R. K., and McAuley, E. (2016). Yoga practice improves executive function by attenuating stress levels. Biol. Psychol. 121, 109–16. doi: 10.1016/j.biopsycho.2016.10.010
Granath, J., Ingvarsson, S., von Thiele, U., and Lundberg, U. (2006). Stress management: a randomized study of cognitive behavioural therapy and yoga. Cogn. Behav. Ther. 35, 3–10. doi: 10.1080/16506070500401292
Grodin, M. A., Piwowarczyk, L., Fulker, D., Bazazi, A. R., and Saper, R. B. (2008). Treating survivors of torture and refugee trauma: a preliminary case series using qigong and tai chi. J. Altern. Complement. Med. 14, 801–806. doi: 10.1089/acm.2007.0736
Hayes, J. P., Hayes, S. M., and Mikedis, A. M. (2012a). Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biol. Mood Anxiety Disord. 2:9. doi: 10.1186/2045-5380-2-9
Hayes, J. P., Vanelzakker, M. B., and Shin, L. M. (2012b). Emotion and cognition interactions in PTSD: a review of neurocognitive and neuroimaging studies. Front. Integr. Neurosci. 6:89. doi: 10.3389/fnint.2012.00089
Hegberg, N. J., Hayes, J. P., and Hayes, S. M. E. (2019). A narrative review and rationale for implementation. Front. Psychiatry 10:133. doi: 10.3389/fpsyt.2019.00133
Higgins, J. P. T., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in metaanalyses. BMJ Br Med J. 327, 557–560. doi: 10.1136/bmj.327.7414.557
Hill, A. S., Sahay, A., and Hen, R. (2015). Increasing adult hippocampal neurogenesis is sufficient to reduce anxiety and depression-like behaviors. Neuropsychopharmacology 40, 2368–2378. doi: 10.1038/npp.2015.85
Huberty, J., Sullivan, M., Green, J., Kurka, J., Leiferman, J., Gold, K., et al. (2020). Online yoga to reduce post traumatic stress in women who have experienced stillbirth: a randomized control feasibility trial. BMC Complement. Med. Ther. 20, 2–19. doi: 10.1186/s12906-020-02926-3
Iverson, K. M., Hendricks, A. M., Kimerling, R., Krengel, M., Meterko, M., Stolzmann, K. L., et al. (2011). Psychiatric diagnoses and neurobehavioral symptom severity among OEF/OIF VA patients with deploymentrelated traumatic brain injury: a gender comparison. Womens Health Issues 21, S210–S217. doi: 10.1016/j.whi.2011.04.019
Javidi, H., and Yadollahie, M. (2012). Post-traumatic stress disorder. Int. J. Occup. Environ. Med. 3, 2–9.
Jindani, F., Turner, N., and Khalsa, S. B. S. (2015). A yoga intervention for posttraumatic stress: a preliminary randomized control trial. eCAM 2015:351746. doi: 10.1155/2015/351746
Johnson, L. G. K (1997). Effects of estrogen replacement therapy on dehydroepiandrosterone, dehydroepiandrosterone sulfate, and cortisol responses to exercise in postmenopausal women. Fertil. Steril. 68, 836–843. doi: 10.1016/S0015-0282(97)00369-5
Johnston, S. T., Shtrahman, M., Parylak, S., Goncalves, J.T., and Gage, F.H. (2016). Paradox of pattern separation and adult neurogenesis: a dual role for new neurons balancing memory resolution and robustness. Neurobiol. Learn. Mem. 129, 60–68. doi: 10.1016/j.nlm.2015.10.013
Kearney, D. J., McDermott, K., Malte, C., Martinez, M., and Simpson, T.L. (2013). Effects of participation in a mindfulness program for veterans with posttraumatic stress disorder: a randomized controlled pilot study. J. Clin. Psychol. 69, 14–27. doi: 10.1002/jclp.21911
Kearney, D. J., McDermott, K., Malte, C., Martinez, M., and Simpson, T. L. (2012). Association of participation in a mindfulness program with measures of PTSD, depression and quality of life in a veteran sample. J. Clin. Psychol. 68, 101–116. doi: 10.1002/jclp.20853
Kelly, A., and Garland, E. L. (2016). Trauma-informed mindfulness-based stress reduction for female survivors of interpersonal violence: results from a stage I RCT. J. Clin. Psychol. 72, 311–328. doi: 10.1002/jclp.22273
Kessler, R., Berglund, P., Dember, O., Jin, R., Merikangas, K., and Walters, E. (2005). Lifetime prevalence of age of onset distributions of DSM-V disorders in the national comorbidity survey replication. Arch. Gen. Psychiatry 62, 593–603. doi: 10.1001/archpsyc.62.6.593
Kim, S. H., Schneider, S. M., Bevans, M., Kravitz, L., Mermier, C., Qualls, C., et al. (2013). PTSD symptom reduction with mindfulness-based stretching and deep breathing exercise: Randomized controlled clinical trial of efficacy. J. Clin. Endocrinol. Metabol. 98, 2984–2992. doi: 10.1210/jc.2012-3742
Kjellgren, A., Bood, S. A., Axelsson, K., Norlander, T., and Saatcioglu, F. (2017). Wellness through a comprehensive yogic breathing program-a controlled pilot trial. BMC Complement. Altern. Med. 7:43. doi: 10.1186/1472-6882-7-43
Lancaster, C. L., Teeters, J. B., Gros, D. F., and Back, S. E. (2016). Posttraumatic stress disorder: overview of evidence-based assessment and treatment. J. Clin. Med. 5:105. doi: 10.3390/jcm5110105
Levenga, J., Wong, H., Milstead, R. A., Keller, B. N., LaPlante, L.E., and Hoeffer, C.A. (2017). AKT isoforms have distinct hippocampal expression and roles in synaptic plasticity. Elife 6:e30640. doi: 10.7554/eLife.30640.021
Liu, C. H., Zhang, E., and Wong, G. T. F. (2020). Factors associated with depression, anxiety, and PTSD symptomatology during the COVID-19 pandemic: clinical implications for U.S. young adult mental health. Psychiatry Res. 290:113172. doi: 10.1016/j.psychres.2020.113172
Malberg, J. E (2004). Implications of adult hippocampal neurogenesis in antidepressant action. J. Psychiatry Neurosci. 29, 196–205.
McEwen, B.S (2002). The neurobiology and neuroendocrinology of stress. Implications for post-traumatic stress disorder from a basic science perspective. Psychiatr. Clin. North Am. 25, 469–494. doi: 10.1016/S0193-953X(01)00009-0
Miller, D. R., Hayes, S. M., Hayes, J. P., Spielberg, J. M., Laflfleche, G., and Verfaellie, M. (2017). Default mode network subsystems are difffferentially disrupted in posttraumatic stress disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2, 363–71. doi: 10.1016/j.bpsc.2016.12.006
Mitchell, K. S., Dick, A. M., DiMartino, D.M., Smith, B.N., Niles, B., Koenen, K.C., et al. (2014). A pilot study of a randomized controlled trial of yoga as an intervention for PTSD symptoms in women. J. Trauma Stress 27, 121–128. doi: 10.1002/jts.21903
Omidi, A., Mohammadi, A., Zargar, F., and Akbari, H. (2013). Efficacy of mindfulness based stress reduction on mood states of veterans with post-traumatic stress disorder. Arch Trauma Res. 1, 151–154. doi: 10.5812/atr.8226
Papp, M. E., Lindfors, P., Storck, N., and Wändell, P. E. (2013). Increased heart rate variability but no effect on blood pressure from 8 weeks of Hatha yoga:a pilot study. BMC Res. Notes 6:59. doi: 10.1186/1756-0500-6-59
Pereira, D. G., Hollywood, R., Bevilaqua, M. C., da Luz, A. C., Hindges, R., Nardi, A. E., et al. (2014). Consequences of cancer treatments on adult hippocampal neurogenesis: implications for cognitive function and depressive symptoms. Neurooncology 16, 476–492. doi: 10.1093/neuonc/not321
Polusny, M. A., Erbes, C. R., Thuras, P., Moran, A., Lamberty, G. J., Collins, R. C., et al. (2015). Mindfulness-based stress reduction for posttraumatic stress disorder among veterans: a randomized clinical trial. JAMA 314, 456–465. doi: 10.1001/jama.2015.8361
Possemato, K., Bergen-Cico, D., Treatman, S., Allen, C., Wade, M., and Pigeon, W. A. R. (2016). A randomized clinical trial of of primary care brief mindfulness training for veterans with PTSD. J. Clin. Psychol. 72, 179–193. doi: 10.1002/jclp.22241
Qian, Y., Huang, M., Guan, T., Chen, L., Cao, L., Han, X. J., et al. (2015). Maslinic acid promotes synaptogenesis and axon growth via Akt/GSK-3beta activation in cerebral ischemia model. Eur. J. Pharmacol. 764, 298–305. doi: 10.1016/j.ejphar.2015.07.028
Quiñones, N., Maquet, Y. G., Vélez, D. M. A., and López, M. A. (2015). Efficacy of a Satyananda Yoga intervention for reintegrating adults diagnosed with posttraumatic stress disorder. Int. J. Yoga Therap. 25, 89–99. doi: 10.17761/1531-2054-25.1.89
Rasmusson, A. M., Vasek, J., and Lipschitz, D. S. (2004). An increased capacity for adrenal DHEA release is associated with decreased avoidance and negative mood symptoms in women with PTSD. Neuropsychopharmacology 29, 1546–1557. doi: 10.1038/sj.npp.1300432
Rebar, A. L., Stanton, R., Geard, D., Short, C., Duncan, M. J., and Vandelanotte, C. A. M. (2015). Meta-meta-analysis of the effect of physical activity on depression and anxiety in non-clinical adult populations. Health Psychol. Rev. 9, 366–378. doi: 10.1080/17437199.2015.1022901
Reinhardt, K. M., Taylor, J. J. N., Johnston, J., Zameer, A., and Cheema, S. (2017). Kripalu yoga for military veterans with PTSD: a randomized trial. J. Clin. Psychol. 74, 93–108. doi: 10.1002/jclp.22483
Rocha, K. K. F., Ribeiro, A. M., and Rocha, K. C. F. (2012). Improvement in physiological and psychological parameters after 6 months of yoga practice. Conscious. Cogn. 21, 843–850. doi: 10.1016/j.concog.2012.01.014
Sahay, A., Scobie, K. N., Hill, A. S., O'Carroll, C. M., Kheirbek, M. A., Burghardt, N. S., et al. (2011). Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 472, 466–470. doi: 10.1038/nature09817
Sapolsky, R. M., Romero, L. M., and Munck, A. U. (2000). How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 21, 55–89. doi: 10.1210/edrv.21.1.0389
Sayer, N. A., Friedemann-Sanchez, G., Spoont, M., Murdoch, M., Parker, L. E., and Chiros, C. (2009). A qualitative study of determinants of PTSD treatment initiation in veterans. Psychiatry. 72, 238–255. doi: 10.1521/psyc.2009.72.3.238
Schnurr, P. P., Hayes, A. F., Lunney, C. A., McFall, M., and Uddo, M. (2006). Longitudinal analysis of the relationship between symptoms and quality of life in veterans treated for posttraumatic stress disorder. J. Consult. Clin. Psych. 74, 707–713. doi: 10.1037/0022-006X.74.4.707
Schuch, F. B., Vancampfort, D., Richards, J., Rosenbaum, S., Ward, P. B., and Stubbs, B. (2016). Exercise as a treatment for depression: a meta-analysis adjusting for publication bias. J. Psychiatr. Res. 77, 42–51. doi: 10.1016/j.jpsychires.2016.02.023
Seppälä, E. M., Nitschke, J. B., Tudorascu, D. L., Hayes, A., Goldstein, M. R., Nguyen, D. T., et al. (2014). Breathing-based meditation decreases posttraumatic stress disorder symptoms in US military veterans: a randomized controlled longitudinal study. J. Trauma. Stress 27, 397–405. doi: 10.1002/jts.21936
Shi, L., Lu, Z. A., Que, J. Y., Huang, X. L., Liu, L., and Ran, M. S. (2020). Prevalence of and risk factors associated with mental health symptoms among the general population in China during the coronavirus disease 2019 pandemic. JAMA Network Open 3:e2014053. doi: 10.1001/jamanetworkopen.2020.14053
Smith, M. A., Davidson, J., and Ritchie, J. C. (1989). The corticotropin-releasing hormone test in patients with posttraumatic stress disorder. Biol. Psychiatry 26, 349–355. doi: 10.1016/0006-3223(89)90050-4
Staples, J. K., Abdel Atti, J. A., and Gordon, J. S. (2011). Mind-body skills groups for posttraumatic stress disorder and depression symptoms in Palestinian children and adolescents in Gaza. Int. J. Stress Manag. 18, 246–262. doi: 10.1037/a0024015
Streeter, C. C., Gerbarg, P. L., Saper, R. B., Ciraulo, D. A., and Brown, R. P. (2012). Effects of yoga on the autonomic nervous system, gamma-aminobutyric-acid, and allostasis in epilepsy, depression, and posttraumatic stress disorder. Med. Hypotheses. 78, 571–579. doi: 10.1016/j.mehy.2012.01.021
Tanielian, T., Woldetsadik, M. A., Jaycox, L. H., Batka, C., Moen, S., and Farmer, C. (2016). Barriers to engaging service members in mental health care within the US military health system. Psychiat. Serv. 67, 718–727. doi: 10.1176/appi.ps.201500237
Telles, S., Naveen, K. V., and Dash, M. (2007). Yoga reduces symptoms of distress in tsunami survivors in the Andaman islands. Evid. Based Complement. Alternat. Med. 4, 503–509. doi: 10.1093/ecam/nem069
Toda, T., and Gage, F. H. (2018). Review: adult neurogenesis contributes to hippocampal plasticity. Cell Tissue Res. 373, 693–709. doi: 10.1007/s00441-017-2735-4
Twamley, E. W., Hami, S., and Stein, M. B. (2004). Neuropsychological function in college students with and without posttraumatic stress disorder. Psychiatry Res. 126, 265–274. doi: 10.1016/j.psychres.2004.01.008
Vaiva, G., Thomas, P., and Ducrocq, F. (2004). Low posttrauma GABA plasma levels as a predictive factor in the development of acute posttraumatic stress disorder. Biol. Psychiatry 55, 250–254. doi: 10.1016/j.biopsych.2003.08.009
van der Kolk, B., Stone, L., West, J., Rhodes, A., Emerson, D., Suvak, M., et al. (2014). Yoga as an adjunctive treatment for posttraumatic stress disorder: a randomized controlled trial. J. Clin. Psychiatry 75, 559–565. doi: 10.4088/JCP.13m08561
Van der Kolk, B. A. C (2006). Clinical implications of neuroscience research in PTSD. Ann. N. Y. Acad. Sci. 1071, 277–293. doi: 10.1196/annals.1364.022
Vancampfort, D., De Hert, M., Skjerven, L., Gyllensten, A., Parker, A., Mulders, N., et al. (2012). International organization of physical therapy in mental health consensus on physical activity within multidisciplinary rehabilitation programmes for minimising cardio-metabolic risk in patients with schizophrenia. Disabil. Rehabil. 34, 1–12. doi: 10.3109/09638288.2011.587090
von der Warth, R., Dams, J., and Grochtdreis, T. (2020). Economic evaluations and cost analyses in posttraumatic stress disorder: a systematic review. EJPT 11, 1–20. doi: 10.1080/20008198.2020.1753940
West, J., Otte, C., Geher, K., Johnson, J., and Mohr, D. C. (2014). Effects of Hatha yoga and African dance on perceived stress, affect, and salivary cortisol. Ann. Behav. Med. 28, 114–118. doi: 10.1207/s15324796abm2802_6
Woon, F. L., Farrer, T. J., Braman, C. R., Mabey, J. K., and Hedges, D. W. (2017). A metaanalysis of the relationship between symptom severity of posttraumatic stress disorder and executive function. Cogn. Neuropsychiatry 22, 1–16. doi: 10.1080/13546805.2016.1255603
Wu, Q., Li, Y., and Xiao, B. (2013). DISC1-related signaling pathways in adult neurogenesis of the hippocampus. Gene 518, 223–230. doi: 10.1016/j.gene.2013.01.015
Wynn, G. H (2015). Complementary and alternative medicine approaches in the treatment of PTSD. Curr. Psychiatry Rep. 17:62. doi: 10.1007/s11920-015-0600-2
Xiong, J., Lipsitz, O., Nasri, F., Lui, L. M., Gill, H., and Phan, L. (2020). Impact of COVID-19 pandemic on mental health in the general population: a systematic review. J. Affect. Disord. 277, 55–64. doi: 10.1016/j.jad.2020.08.001
Yehuda, R (2002). Post-traumatic stress disorder. N. Engl. J. Med. 346, 108–114. doi: 10.1056/NEJMra012941
Yehuda, R., and Golier, J. (2009). Is there a rationale for cortisol-based treatments for PTSD? Expert Rev. Neurother. 9, 1113–1115. doi: 10.1586/ern.09.79
Yin, H., Jiang, Y., Zhang, Y., Ge, H., and Yang, Z. (2019). The inhibition of BDNF/TrkB/PI3K/Akt signal mediated by AG1601 promotes apoptosis in malignant glioma. J. Cell. Biochem. 120, 18771–18781. doi: 10.1002/jcb.29190
Yin, L., Gao, S., Shi, H., Wang, K., Yang, H., and Peng, B. (2019). TIP-B1 promotes kidney clear cell carcinoma growth and metastasis via EGFR/AKT signaling. Aging 11, 7914–7937. doi: 10.18632/aging.102298
Zhang, S., Zou, L. Y., Chen, L. Z., Yao, Y., Loprinzi, P. D., Siu, P. M., et al. (2019a). The effect of Tai Chi Chuan on negative emotions in non-clinical populations: a meta-analysis and systematic review. Int. J. Environ. Res. Public Health 16:E3033. doi: 10.3390/ijerph16173033
Zhang, Y. J., Loprinzi, P. D., Yang, L., Liu, J., Liu, S. J., and Zou, L. Y. (2019b). The beneficial effects of traditional chinese exercises for adults with low back pain: a meta-analysis of randomized controlled trials. Med. Lithuania 55:UNSP118. doi: 10.3390/medicina55050118
Zhou, S. W., Zhang, Y. J., Kong, Z. W., Loprinzi, P. D., Hu, Y., Ye, J. J., et al. (2019). The effects of tai chi on markers of atherosclerosis, lower-limb physical function, and cognitive ability in adults aged over 60: a randomized controlled trial. Int. J. Environ. Res. Public Health 16:753. doi: 10.3390/ijerph16050753
Zou, L. Y., Han, J., Li, X. C., Yeung, A., Hui, S. C., Tsang, W. N., et al. (2019b). The effects of Tai Chi on lower limb proprioception in adults aged over 55: a systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 100, 1102–1113. doi: 10.1016/j.apmr.2018.07.425
Zou, L. Y., Yeung, A. S., Li, C. X., Wei, G. X., Chen, K. W., Kinser, P. A., et al. (2018a). Effects of meditative movements on major depressive disorder: a systematic review and meta-analysis of randomized controlled trials. J. Clin. Med. 7:195. doi: 10.3390/jcm7080195
Zou, L. Y., Yeung, A. S., Zeng, N., Wang, C. Y., Sun, L., Tomas, G. A., et al. (2018b). Effects of mind-body exercises for mood and functional capabilities in patients with stroke: an analytical review of randomized controlled trials. Int. J. Environ. Res. Public Health 15:721. doi: 10.3390/ijerph15040721
Keywords: mind-body exercise, depression, anxiety, exercise prescription, PTSD, HPA axis
Citation: Zhu L, Li L, Li X-z and Wang L (2022) Mind–Body Exercises for PTSD Symptoms, Depression, and Anxiety in Patients With PTSD: A Systematic Review and Meta-Analysis. Front. Psychol. 12:738211. doi: 10.3389/fpsyg.2021.738211
Received: 08 July 2021; Accepted: 14 December 2021;
Published: 18 January 2022.
Edited by:
Wanhong Zheng, West Virginia University, United StatesReviewed by:
Kai Wang, The University of Iowa, United StatesSheri Oduola, University of East Anglia, United Kingdom
Copyright © 2022 Zhu, Li, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Long Li, bGlsb25nQHN1ZGEuZWR1LmNu; Lin Wang, d2FuZ2xpbjEyM0AxMjYuY29t
 Lin Zhu
Lin Zhu Long Li1*
Long Li1* Lin Wang
Lin Wang