
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychol., 31 August 2021
Sec. Eating Behavior
Volume 12 - 2021 | https://doi.org/10.3389/fpsyg.2021.716010
This article is part of the Research TopicNeurocognitive and Translational Science of Binge Eating: Understanding Mechanisms of Change.View all 8 articles
 Kristi R. Griffiths1*†
Kristi R. Griffiths1*† Leonor Aparício1,2†
Leonor Aparício1,2† Taylor A. Braund1,3,4
Taylor A. Braund1,3,4 Jenny Yang1
Jenny Yang1 Grace Harvie1
Grace Harvie1 Anthony Harris1,5
Anthony Harris1,5 Phillipa J. Hay6
Phillipa J. Hay6 Stephen Touyz7,8
Stephen Touyz7,8 Michael R. Kohn9,10
Michael R. Kohn9,10High trait impulsivity is thought to contribute to the sense of loss of control over eating and impulses to binge eat experienced by those with binge eating disorder (BED). Lisdexamfetamine dimesylate (LDX), a drug approved for treatment of moderate to severe BED, has been shown to decrease impulsive features of BED. However, the relationship between LDX-related reductions of binge eating (BE) episodes and impulsivity has not yet been explored. Forty-one adults aged 18–40years with moderate to severe BED completed questionnaires and tasks assessing impulsivity at baseline and after 8weeks of 50–70mg of LDX. Twenty age-matched healthy controls were also assessed at two timepoints for normative comparison. Data were analysed using linear mixed models. BED participants exhibited increased self-reported motor, non-planning, cognitive and food-related impulsivity relative to controls but no differences in objective task-based measures of impulsivity. Food-related and non-planning impulsivity was significantly reduced by LDX, but not to normative levels. Individuals with higher baseline levels of motor and non-planning impulsivity, and loss of control over eating scores experienced the greatest reduction in BE frequency after 8weeks of LDX. Further, there were significant associations between the degree to which subjective loss of control over eating, non-planning impulsivity and BE frequency reduced after 8weeks of LDX. These data suggest that specific subjective measures of impulsivity may be able to predict who will have the greatest benefit from LDX treatment and that reductions in BE frequency may be moderated by concurrent reductions in non-planning impulsivity.
Binge eating disorder (BED) is one of the most prevalent eating disorders worldwide with a lifetime prevalence estimate of 1.9% (Kessler et al., 2013). It is characterised by recurrent episodes of excessive food consumption together with a perceived lack of behavioural control. According to the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5; American Psychiatric Association, 2013), these binge eating (BE) episodes occur at least once a week for 3months. Furthermore, compensatory purging behaviours to reduce caloric intake as seen in bulimia nervosa are not engaged in regularly in BED. It is generally accepted that the sense of loss of control while eating is the most important and consistent feature of a binge eating episode and leads to marked distress among individuals with BED (Brownstone et al., 2013; Li et al., 2019).
Impulsivity may underlie the loss of control experienced during BE episodes in BED (Dawe and Loxton, 2004; Kessler et al., 2016). Patients suffering from BED show higher general trait impulsivity compared to healthy normal-weight individuals, but potentially also compared to body mass index (BMI)-matched individuals (Giel et al., 2017). Indeed, this trait has been described as a possible hallmark of binge eating behaviour, which is present even in the absence of weight or full-blown eating disorders (Oliva et al., 2019). As a multidimensional and complex construct, impulsivity has distinct neuronal and behavioural components that are differentially disturbed in BED (Dawe and Loxton, 2004; Giel et al., 2017). According to Dawe and Loxton (2004), the main components of impulsivity consist of reward sensitivity and rash-spontaneous impulsiveness. Reward sensitivity refers to the enhanced reward value and selective attention (i.e., attention bias) attributed to food-related cues that prompt the individual to seek appetitive stimuli (Dawe and Loxton, 2004; Hou et al., 2011; Schag et al., 2013). Combined with a reduced delay and probabilistic tolerance (i.e., increased preference for smaller immediate rewards delivered with higher probability over larger delayed rewards delivered with smaller or variable probabilities (Manwaring et al., 2011; Voon et al., 2015), this frequently leads to disadvantageous and impatient decision-making in BED patients. Conversely, rash-spontaneous impulsivity reflects the poor cognitive and motor inhibitory control observed leading up to and during binge episodes (Dawe and Loxton, 2004), which constitutes the diagnostic criteria for BED. More recently, a third domain of impulsivity characterised as the ‘impulsive personality trait’ relates to the persisting underlying tendency to behave impulsively (MacKillop et al., 2016). This differs from ‘state impulsivity’, which can be modulated by external influences (Yeo et al., 2020).
While heightened impulsivity in BED is typically thought to be food-specific, there is evidence of increased impulsive tendencies independent of food cues in people with BED (Schag et al., 2013; Oliva et al., 2020). In an experimental study involving a dice game, Svaldi et al. (2010) concluded that women with BED made more choices that involved larger monetary gains with lower winning probabilities, which reflects higher non-food-specific probabilistic discounting. They further mentioned that women with BED changed their game strategy significantly less often than healthy controls in response to negative feedback after a risky choice (Svaldi et al., 2010), which is consistent with the persistent tendency to make impulsive choices observed in BED. Similarly, individuals with BED chose more often to receive immediate shorter massage time over the same delayed longer reward, also reflecting higher non-food-specific delayed reward discounting (Manwaring et al., 2011). In a motor inhibition task, Mobbs et al. (2011) compared response inhibition towards food- and body-related targets. They found that individuals with BED and a high BMI have a general inhibition problem and difficulty focusing their attention when compared with individuals of normal-weight and without BED, a cognitive deficit that was independent of stimuli type.
There is also a relatively high rate of comorbidity with other impulse control disorders, such as substance use disorders and attention deficit hyperactivity disorder (ADHD; Dawe and Loxton, 2004; Nazar et al., 2016), suggesting common neurobiological underpinnings. Indeed, similar executive function deficits are described in all three aforementioned disturbances: the increased activation of mesolimbic dopaminergic pathway and prefrontal cortex circuits underlies enhanced reward sensitivity and rash spontaneous behaviour (Dawe and Loxton, 2004; Reinblatt, 2015).
Lisdexamfetamine dimesylate (LDX; Vyvanse®) is a prodrug of D-amphetamine that is proposed to improve impulse control via modulation of corticostriatal circuits, which are broadly involved in reward sensitivity and inhibitory control. LDX was the first approved drug for the treatment of moderate to severe BED in adults and has been shown to not only reduce the intake of highly palatable food in BED models (Presby et al., 2020) but also decrease global binge eating severity and trait impulsive features of BED (McElroy et al., 2016). This is promising, as it suggests that LDX may aid in reducing additional impulse control issues beyond binge eating.
To date, McElroy et al.’s (2016) study is the only study to examine the effects of LDX on impulsivity in BED. While their study provided significant advances in the field, important outstanding questions remain. Firstly, there has been no direct examination of the relationship between LDX-related changes in BE frequency and impulsivity. Second, there has been no comparison with healthy controls to determine whether LDX not only reduces impulsive features, but also normalises them. Finally, McElroy et al. (2016) used self-report measures of trait impulsivity and food-specific impulsivity/compulsivity but no objective task-based measures of impulsivity. Given the recognition of impulsivity as a complex and multifaceted construct, it is important to use various tools to examine the different aspects.
In the present study, we analyse the effects of LDX on different sub-domains of impulsivity in individuals with moderate to severe BED enrolling an open-label phase 4 clinical trial, comparing with healthy controls (HC; Griffiths et al., 2019). Impulsivity was assessed with both subjective and objective measures (Barratt Impulsiveness Scale-11; The Brief Loss of Control over Eating Scale (B-LCOES); and Cued Go No-Go (cGNG) task, Monetary Incentive Delay Task, respectively) that focused on food-specific or non-food-specific aspects. We hypothesised that individuals with BED would have higher levels of impulsivity relative to HC and that LDX would ‘normalise’ impulsivity levels. Given impulsivity is reflective of underlying neurobiology, and LDX treatment targets neurobiology associated with impulsivity; then, greater impulsivity may reflect neurobiological functioning that is more responsive to the benefits of LDX treatment. Therefore, in addition to expecting LDX to reduce binge eating frequency, we hypothesised that impulsivity would moderate the degree of change in binge eating frequency, whereby greater impulsivity would be associated with greater binge eating frequency reductions.
Forty-one individuals aged 18–40years, with moderate to severe BED, were recruited via referral from participating clinicians or self-referral through online advertisements. All BED participants met the DSM-5 criteria for moderate to severe disease, confirmed by Module I of the Structural Clinical interview for DSM-5 Research Version (First et al., 2014). This requires a BE frequency of at least 3days per week in the month prior to the baseline assessment and a minimum score of 4 on the Clinical Global Impression-Severity (CGI-S) scale (Busner and Targum, 2007). Inclusion criteria included a BMI between 20 and 45kg/m2 and medical approval for LDX commencement.
Twenty age and gender-matched healthy controls (HC) were recruited from the community. They were screened for psychiatric disorders using the MINI International Neuropsychiatric Interview Version 7.0.2 for DSM-5 (Sheehan et al., 1998; MINI) and excluded if they had any current or past eating disorders. Participants from both groups were excluded if they had certain comorbid psychiatric disorders, such as anorexia nervosa, bulimia nervosa, psychosis, mania and substance dependence; a neurological condition or history of physical brain injury that might interfere with the assessments to be made; and psychostimulant use in the past 6months. Recruitment and testing of all participants occurred from May 2018 to January 2021.
The study was approved by the Human Research Ethics Committee of the Western Sydney Local Health District, and all participants provided written informed consent. The trial was registered at the Australian and New Zealand Clinical Trials Registry (anzctr.org.au) #ACTRN12618000623291.
A description of full trial protocol has been previously reported (Griffiths et al., 2019). Each participant attended a baseline session to complete (1) a clinical interview and health check (2) self-report questionnaires relating to general and food-specific impulsivity, and (3) a series of cognitive tasks. BED participants were provided with a self-monitoring diary and instructed to start LDX 30mg/day. After 2weeks of treatment, the study clinician evaluated them to determine whether it was safe to titrate the dose to 50mg/day. At week 4 of treatment, they were assessed by a study clinician to determine whether the dose should remain at 50mg/day or increase to 70mg/day. At week 8 of LDX treatment, research assessments were repeated, while BED participants were on LDX. HC completed the cognitive tasks at week 8, in order to control for practise effects.
Cognitive tasks were programmed using Inquisit 5 Lab (2018; millisecond.com), and self-report questionnaire data were recorded on RedCap.
BE frequency was obtained from daily self-monitoring binge eating diaries and confirmed at the baseline and week 8 clinical interviews.
Reward sensitivity was objectively assessed with the Monetary Incentive Delay Task (Knutson et al., 2000). This task consists of multiple trials that require participants to press a button as quickly as possible during the presentation of a visual target, under different monetary reward conditions (potential earning, potential punishment, or no monetary outcome). Incentive task difficulty was calibrated to participants’ mean reaction time (collected before the beginning of the task), so that each participant succeeded on approximately 60% of the incentive trials. Performance feedback appeared immediately after the response and reaction time and accuracy of response (expressed as the percentage of correct responses) were recorded on all trials. Two measures extracted for analysis were as follows: (1) reaction time difference between reward incentive trials and control non-incentive trials, and (2) proportion of accurate reward trials.
Rash-spontaneous behaviour was objectively examined with the Cued Go No-Go task (Fillmore, 2003), during which participants were asked to quickly respond by pressing a button to go targets and inhibit responding to no-go targets. The task induces response prepotency by presenting a preliminary go or no-go cue before the actual go or no-go target is displayed. The cue-target relationship is manipulated so that in 20% of trials the cue incorrectly signals the target (invalid cue). Percent commission errors (i.e., failure to inhibit response) following a go cue were used to assess the subject’s inhibitory control over a prepotent response.
The Barratt Impulsiveness Scale (Patton et al., 1995) is the most widely administered instrument for the assessment of impulsiveness in both research and clinical settings (Stanford et al., 2009). It is a self-report questionnaire that measures both personality and behavioural aspects of impulsivity based on three sub-traits: motor (acting without thinking and inability to concentrate), cognitive (making quick cognitive decisions) and non-planning impulsiveness (lack of forethought; Patton et al., 1995). Each item is answered on a 4-point scale, and then, the sum of the 30 items yields a total impulsivity score that ranges from 30 to 120 (Stanford et al., 2009).
The Brief Loss of Control over Eating Scale (Latner et al., 2014) is a 7-item self-reported scale that assesses behavioural, cognitive/dissociative and positive/euphoric aspects related to the loss of control over eating. Each item is rated on a 1–5-point scale with higher scores indicating greater severity of this condition. This measure’s reliability and construct validity are supported by its strong content validity, internal consistency (α=0.93), high test–retest reliability (r=0.82), and convergent and discriminant validity, when compared to the full 24-item scale (Latner et al., 2014).
Statistical analyses were designed to address four primary study questions (1) Do individuals with BED have higher levels of impulsivity relative to HC? (2) Does LDX normalise any aberrant measures of impulsivity for individuals with BED? (3) Are LDX-related changes in BE frequency associated with concurrent changes in impulsivity measures? and (4) Do baseline impulsivity levels impact the degree to which LDX reduces BE frequency.
Independent-samples t-tests and ANCOVAs were used first to test whether BED and HC groups differed on baseline demographic and impulsivity measures. Welch two-sample t-tests were conducted in instances with unequal variance between groups.
To evaluate the effects of LDX on outcome measures in the BED group, a linear mixed model was performed separately for each measure, with the impulsivity measure or BE frequency as a dependent variable, individual as a random effect, and timepoint (week 0 and week 8) as a fixed effect. As per previous literature (McElroy et al., 2015), BE frequency was log-transformed to reduce skewness (number of binge eating days per week +1). To determine whether LDX normalised self-report impulsivity measures, independent-samples t-tests were conducted between BED at week 8 and HC at week 0. This was due to self-report questionnaires not being collected for healthy controls at week 8. For cognitive measures, mixed-effect group (BED and HC) by timepoint (week 0 and week 8) interactions were tested to identify if normalisation occurred, while accounting for practise effects.
To assess associations between concurrent changes in impulsivity measures and BE frequency, we included an interaction term to the timepoint model (change in impulsivity × timepoint), with BE frequency as the dependent variable. This model was tested both with and without baseline levels of the impulsivity measures included. Simple effects analyses were used to follow up significant interactions.
To assess whether baseline levels of impulsivity were associated with change in BE frequency, we tested baseline impulsivity × timepoint interactions for each measure, with BE frequency as the dependent variable.
To further explore the relationships between baseline measures of BE frequency and impulsivity, post-hoc correlation analyses were conducted in the BED group. Benjamini-Hochberg corrections were applied to control the false discovery rate.
All analyses were conducted with and without the covariates age, years of education and BMI (covariate-adjusted models reported in supplementary materials). As groups were not matched on BMI, supplementary analyses were also conducted in a BMI-matched sub-sample to determine whether results held. Analyses were performed using R 3.5.1 (Team, 2020). The full sample with baseline data was used to answer question 1 which related to group differences, while questions relating to treatment effects (2,3,4) used only treatment completers. Mixed linear models were tested using the ‘lme4’ package in R (Bates et al., 2015). p values for mixed linear models were calculated using the ‘lmerTest’ package in R (Kuznetsova et al., 2017). Cohen’s d effect sizes were calculated using the ‘effectsize’ package in R (Ben-Shachar et al., 2020). Post-hoc effect sizes were calculated using the eff_size function within the ‘emmeans’ package in R (Lenth et al., 2019).
Forty-one individuals with moderate to severe BED and 20 healthy controls (HC) were assessed at baseline. Thirty-three of the BED participants and 14 HC were assessed at the 8-week follow-up. Baseline demographic and clinical characteristics of recruited individuals in both arms are provided in Table 1. At week 8, 20 BED participants were taking 50mg of LDX, while 13 were taking 70mg. In the BED group, log BE frequency reduced from week 0 (M=0.70, SD 0.10; mean BE frequency 4.27/week) to week 8 (M=0.31, SD 0.20; mean BE frequency 1.33/week), [t(32)=−9.83, p<0.001, d=1.71]. This effect remained significant after controlling for baseline log BE frequency, [t(69)=−11.98, p<0.001].
At baseline, BED participants had higher scores than HC on the B-LOCES [BED, M 27.95, SD 2.98; HC, M 9.89, SD 2.62; t(58)=22.62, p<0.001, d=6.43], BIS-11 motor [BED, M 24.61, SD 5.21; HC, M 20.63, SD 3.37; t(58)=3.04, p=0.004, d=0.91], BIS-11 cognitive [BED, M 18.98, SD 5.05; HC, M 14.74, SD 2.85; t(55.59)=4.14, p<0.001, d=1.03] and BIS-11 non-planning scales [BED, M 28.07, SD 5.64; HC, M 22.11, SD 4.32; t(58)=4.09, p<0.001, d=1.19; see Figure 1].
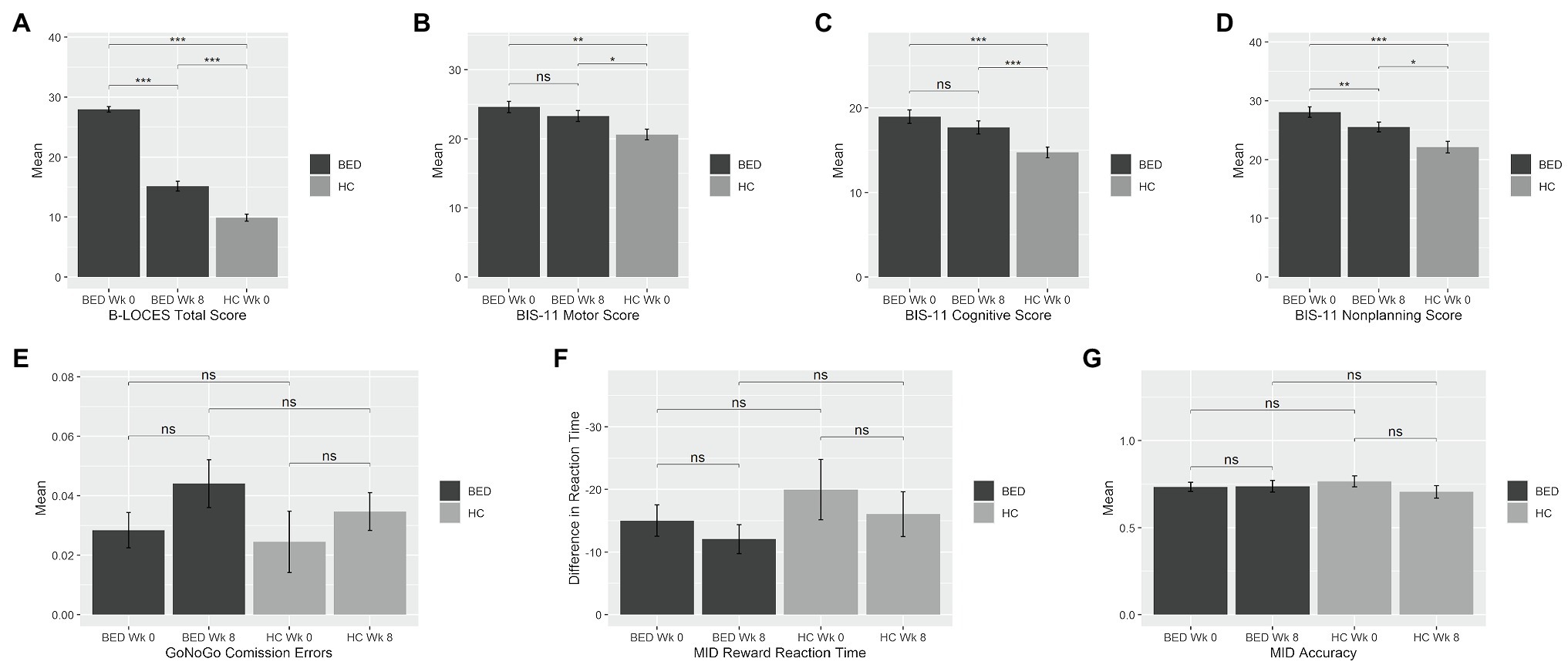
Figure 1. Mean impulsivity measures at week 0 and week 8 for the binge eating disorder (BED) and healthy control (HC) groups. Bars represent means, and error bars represent standard error from the mean. BED, Binge Eating Disorder; HC, healthy control; Wk, week; BIS, Barratt Impulsiveness Scale; MID, Monetary Incentive Delay. BED, Binge Eating Disorder; HC, healthy control; Wk, week; BIS, Barratt Impulsiveness Scale; MID, Monetary Incentive Delay. *p=0.05; **p=0.001; ***p<0.001.
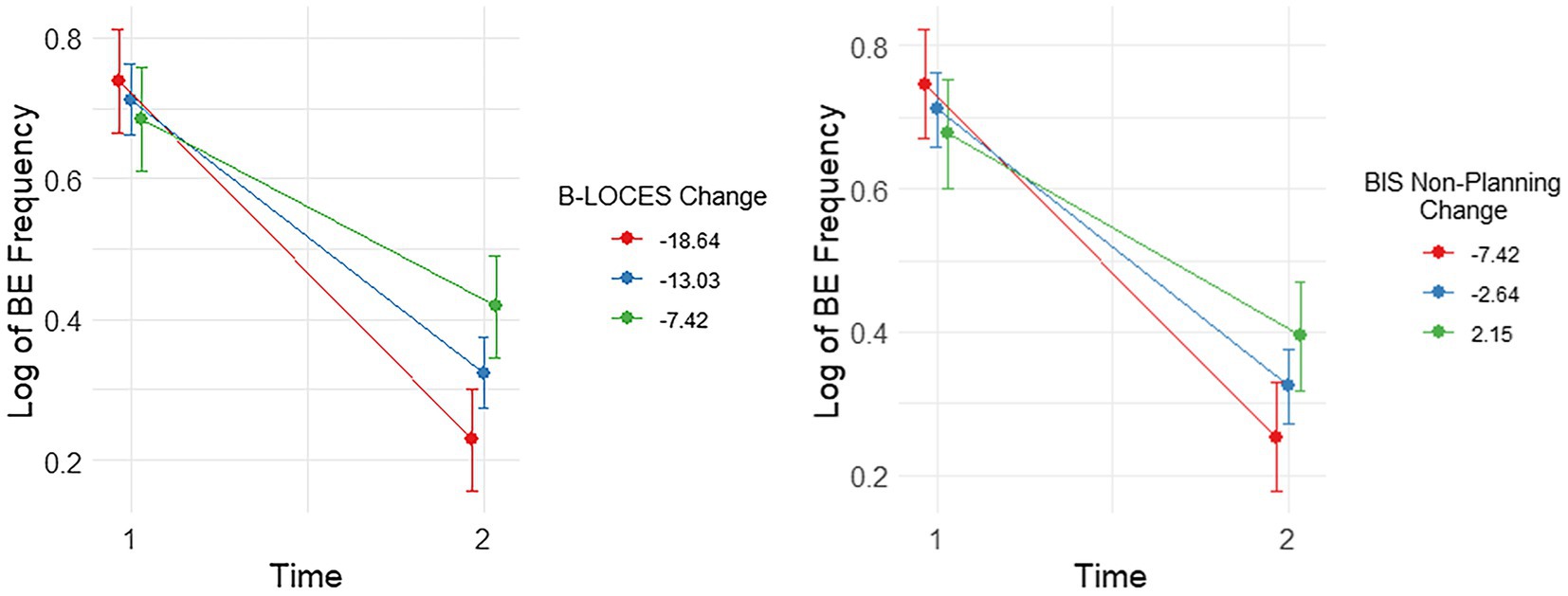
Figure 2. Plots showing significant interactions between change in Log Binge Eating (BE) Frequency and Brief Loss of Control over Eating Scores (B-LOCES) and BIS non-planning from time 1 to 2. Following the convention suggested by Aiken and West (1991), we used the mean value of the moderators (i.e. change in BIS non-planning and B-LOCES scores) as well as one standard deviation below and above the mean value to plot the moderating effect of these measures on BE frequency between baseline and week 8.
Groups did not significantly differ in cGNG percent commission errors during go-cue/nogo-target trials [BED, M 2.84, SD 3.82; HC, M 2.45, SD 4.61; t(56)=0.35, p=0.731], in MIDT reward reaction time[(BED, M -15.02, SD 15.97; HC, M -19.98, SD 21.60; t(55)=0.99, p=0.328] or reward trial accuracy [BED, M 0.73, SD 0.17; HC, M 0.77, SD 0.14; t(55)=−0.72, p=0.475].
After 8weeks of LDX, the BED group reported reductions in B-LOCES [week 0, M 27.95, SD 2.98; week 8, M 15.15, SD 5.30; t(32)=−13.24, p<0.001, d=2.3] and BIS-11 non-planning [week 0, M 28.07, SD 5.64; week 8, M 25.55, SD 5.33; t(32.64)=−3.14, p=0.004, d=0.55] relative to baseline; however, both measures remained elevated relative to HC [B-LOCES, t(49.18)=4.77, p<0.001, d=1.26; BIS-11 non-planning, t(50)=2.39, p=0.021, d=0.71].
There were no significant LDX-related changes in BIS-11 motor [week 0, M 24.61, SD 5.21; Week 8, M 23.30, SD 4.97; t(32)=−1.87, p=0.071] or BIS-11 cognitive [week 0, M 18.98, SD 5.05; week 8, M 17.70, SD 5.03; t(32)=−1.51, p=0.139], and these measures remained elevated relative to HC [BIS-11 motor, t(50)=2.08, p=0.043, d=0.63; BIS-11 cognitive, t(50)=2.71, p=0.009, d=0.73].
Cued go no-go task percent commission errors did not change significantly [week 0, M 2.84, SD 3.82; week 8, M 4.40, SD 5.16; t(31)=−1.69, p=0.101]. Similarly, the BED group did not experience significant change in MIDT reward reaction time from week 0 to week 8 [week 0, M -15.02, SD 15.97; week 8, M -12.06, SD 14.88; t(35.72)=0.78, p=0.440] or reward trial accuracy [week 0, M 0.73, SD 0.17; week 8, M 0.74, SD 0.21; t(32.65)=−0.19, p=0.852]. There were no significant timepoint x group interactions in MIDT reward reaction time [t(53.55)=0.32, p=0.752] or reward trial accuracy [t(48.88)=−1.27, p=0.212], or cGNG percent commission errors [t(55.60)=−0.32, p=0.753].
The inclusion of age, education and BMI as covariates did not alter any of these findings.
There were significant interactions between change in log BE frequency and change in BIS non-planning, [t(31.00)=2.96, p=0.006] and change in B-LOCES, [t(31.00)=3.59, p=0.001]. These effects remained significant after controlling for baseline BIS non-planning, [t(31.00)=2.96, p=0.006] and baseline B-LOCES, [t(31.00)=3.59, p=0.001], respectively.
Simple effects analysis showed that reductions in log BE frequency from week 0 to week 8 were most pronounced for those with the largest reductions in BIS-11 non-planning (i.e., around −7.42 reduction, b=−0.50, t(31)=−9.78, p<0.001, es=3.43), though still quite pronounced with smaller reductions (i.e., around −2.64 reduction, b=−0.39, t(31)=−10.97, p<0.001, es=2.70) and small increases (i.e., around 2.15 increase, b=−0.28, t(31)=−5.57, p<0.001, es=1.96; Figure 2).
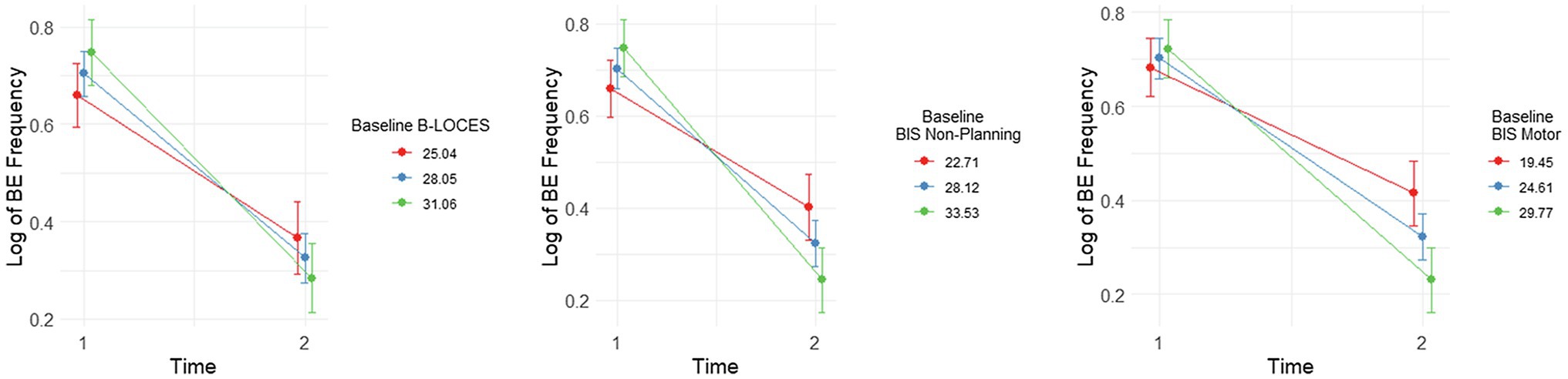
Figure 3. Plots showing significant interactions between change in Log Binge Eating (BE) Frequency from time 1 to 2 and baseline (i.e., time 1) levels of Brief Loss of Control over Eating Scores (B-LOCES), BIS non-planning and BIS motor scores. Following the convention suggested by Aiken and West (1991), we used the mean value of the moderators (i.e., baseline B-LOCES, BIS non-planning and BIS motor scores) as well as one standard deviation below and above the mean value to plot the moderating effect of these measures on BE frequency between baseline and week 8.
Simple effects analysis also showed that the reduction in log BE frequency from week 0 to week 8 was most pronounced for those with the largest reductions in B-LOCES [i.e. around −18.64, b=−0.51, t(31)=−10.67, p<0.001, es=3.72], but also for those with average reductions in B-LOCES [i.e. around −13.03, b=−0.39, t(31)=−11.53, p<0.001, es=2.84], and small reductions in B-LOCES [i.e. -7.42, b=0.27, t(31)=5.57, p<0.001, es=1.95; Figure 2.]
There were no significant interactions between change in log BE frequency and change in BIS motor [t(31.00)=1.52, p=0.139], change in BIS cognitive [t(31.00)=1.97, p=0.058], change in cGNG commission errors [t(30.00)=0.78, p=0.442], change in MIDT reward reaction time [t(56.00)=0.11, p=0.915] or MIDT reward trial accuracy [t(56)=−0.38, p=0.703]. These results did not change when controlling for baseline BIS motor, [t(61)=1.53, p=0.139], baseline BIS cognitive score, [t(31.00)=1.97, p=0.058], baseline cGNG commission errors, [t(30.00)=0.78, p=0.442], baseline MIDT reward reaction time [t(28.00)=0.11, p=0.915] or baseline MIDT reward accuracy [t(28.00)=−0.38, p=0.710], respectively.
There was a significant interaction between change in log BE frequency and baseline BIS motor score, t(38.56)=−3.48, p=0.001, BIS non-planning score, t(41.05)=−3.84, p<0.001, and B-LOCES, t(38.07)=−2.48, p=0.018.
Simple effects analysis also showed that reduction in log BE frequency from week 0 to week 8 was most pronounced for those with the highest BIS non-planning scores at baseline [i.e. around 33.53, b=−0.50, t(37.3)=−11.20, p<0.001, es= 3.80], followed by those with average BIS non-planning scores [i.e. around 28.12, b=−0.38, t(36.1)=−12.10, p<0.001, es= 2.87] and those with the lowest BIS non-planning scores [i.e. 22.70, b=−0.26, t(38.0)=−5.68, p<0.001, es= 1.94; Figure 3].
Similarly, reduction in log BE frequency from week 0 to week 8 was most pronounced for those with the highest baseline B-LOCES scores [i.e. around 31.06, b=−0.46, t(34.6.0)=−9.59, p<0.001, es= 3.18], followed by those with average B-LOCES scores [i.e. around 28.05, b=−0.39, t(36.6)=−11.00, p<0.001, es= 2.59] and those with the lowest B-LOCES scores [i.e. 25.04, b=−0.29, t(37.5)=−5.98, p<0.001, es= 2.00; Figure 3.]
Finally, reduction in log BE frequency from week 0 to week 8 was also most pronounced for those with the highest BIS motor scores [i.e. around 29.77, b=−0.49, t(36.6)=−10.75, p<0.001, es= 3.60], followed by those with average BIS motor scores [i.e. around 24.61, b=−0.38, t(36.5)=−11.78, p<0.001, es= 2.78] and those with the lowest BIS motor scores [i.e. 19.45, b=−0.27, t(36.2)=−5.83, p<0.001, es= 1.95; Figure 3.]
Change in BE frequency did not interact significantly with baseline BIS cognitive scores, t(40.53)=−1.42, p=0.164, cGNG commission errors, t(38.05)=−1.26, p=0.214, MIDT reward reaction time, t(65)=−0.49, p=0.638, or MIDT reward trial accuracy, t(65)=−0.24, p=0.816.
In the BED group, log BE frequency was positively correlated with B-LOCES (r=0.44, p=0.004) and BIS-11 non-planning (r=0.46, p=0.002). B-LOCES was positively correlated with BIS-non-planning (r=0.421, p=0.006). No other correlations survived correction for multiple comparisons (q=0.0062). No correlations were observed in the baseline impulsivity measures for the HC group.
In the current study, we investigated impulsivity in moderate to severe BED and its relationship with LDX efficacy. We found that individuals with BED reported increased food-specific and general impulsivity on self-report measures relative to controls, but no differences in task-based measures. Eight weeks of LDX treatment reduced food-specific and a ‘non-planning’ scale of impulsivity, but did not normalise these measures. However, the degree of reduction in these two measures was associated with the level of concurrent reductions in BE frequency after LDX treatment. Finally, individuals with higher baseline levels of food-related, non-planning and motor impulsivity experienced the greatest reductions in BE frequency after 8weeks of LDX.
Consistent with previous research, the BED group had elevated levels of self-reported impulsivity relative to HC in both food-specific and general measures of impulsivity (Meule, 2013; Bodell et al., 2018). This increase in more general impulsivity is particularly interesting as it suggests that individuals with BED may experience challenges with behaviours beyond eating. Indeed, there is a body of research into the increased co-occurrence of binge eating with ADHD (Cortese et al., 2007; Derefinko et al., 2008), problem gambling (Jiménez-Murcia et al., 2013; Farstad et al., 2015) and substance abuse (Bahji et al., 2019), which may all stem from this impulsive behavioural phenotype. This is further supported by descriptions of shared pathophysiological mechanisms underlying these psychiatric entities (Dawe and Loxton, 2004; Schreiber et al., 2013; Reinblatt, 2015), which opens up opportunities to study and manage patients with co-morbid and interrelated impulsivity disturbances with a single treatment.
Despite some previous research reporting that people with BED have an increased tendency to act rashly and spontaneously in inhibitory control tasks (Schag et al., 2013; Wu et al., 2013; Giel et al., 2017), we did not find differences in measures assessed from the Go-NoGo and monetary incentive delay tasks. One possible reason for this discrepancy is that most previous studies focused on people with BED and a BMI over 30 (Monica et al., 2010; Mobbs et al., 2011; Loeber et al., 2012; Wu et al., 2013; Hege et al., 2015), whereas 70% of our BED cohort were in the normal or overweight BMI range (BMI <30kg/m2). Nonetheless, controlling for BMI statistically did not alter our findings. In addition, a number of studies used food-related versions of the Go-NoGo, which may increase task salience in the BED group, thereby strengthening group differences. However, other studies also found no differences in food-related response inhibition between groups (Svaldi et al., 2015), revealing the inconsistent nature of inhibitory control task results in people with BED. As suggested by Kollei et al. (2018), it is possible that state-based factors, such as hunger or stress, could moderate performance, and should be measured in future studies.
Impulsivity is not a unidimensional concept itself, so different modalities of measurement (e.g., self-report versus objective behavioural tasks) may assess different aspects of inhibitory control (Dawe and Loxton, 2004; Giel et al., 2017). The proposed ‘trait versus state dichotomy of impulsivity’ (Yeo et al., 2020) suggests that self-report measures, like the BIS-11, capture a more trait-like representation of impulsivity, while behavioural tasks may be more dependent on state-dependent factors, such as stress. Further, neuroimaging research from children with ADHD shows that psychostimulants may be most effective at ameliorating aberrances in reward and inhibitory control circuits (Solanto, 1998), which are more likely to present as changes in trait-like impulsivity than environmentally-induced state factors. In line with previous studies demonstrating low correlations between trait and state measures of impulsivity (Wingrove and Bond, 1997; Aichert et al., 2012), it seems that cGNG and BIS-11, especially the motor subscale, do not measure the same aspects of impulsivity. This difference may thus reflect an active compensatory effort to slow down responses in behavioural tasks after realising that they tend to behave impulsively (Wingrove and Bond, 1997).
After 8weeks of LDX intervention, participants with BED reported a significant reduction not only in BE frequency, but also in B-LOCES total and BIS-11 non-planning scores. This largely supports the findings by McElroy et al. (2016); however, we did not replicate their finding of reductions in the BIS motor subscale. This may be due to McElroy et al. assessing BED patients after 11weeks of LDX treatment relative to 8weeks of treatment in the current study. Despite the significant reduction that was found, both measures remained elevated compared to controls, which suggests a decreasing trend in general and food-specific self-reported impulsivity measures that does not reach normalised levels after 8weeks of LDX treatment. McElroy et al. (2016) did show continued reductions in most measures between weeks 8 and 11 in their LDX efficacy trial; therefore, it is plausible that continued use would have led to significantly reduced BIS motor scores. Overall, these results demonstrate that LDX plays an important role in self-perceived impulsiveness beyond that relating to food. Further research would be beneficial in evaluating the broader impact of LDX in the subset of patients with comorbid issues relating to impulse control.
Two measures stood out in their strong correlation with BE frequency, both at baseline and with regard to treatment-related change: B-LOCES and BIS non-planning. This is somewhat unsurprising for the B-LOCES and is consistent with the aforementioned role of the sense of loss of control while eating in BED characterisation and severity. However, it is less obvious why BIS non-planning is so tightly coupled to BE frequency in a BED sample. The fact that participants with BED scored higher in items like ‘I do things without thinking’ and ‘I am more interested in the present than in the future’ suggests that they have a present orientation that interferes with eating patterns. This is supported by the positive correlation between BIS-11 non-planning and B-LOCES, suggesting that individuals who are less able to plan ahead may end up experiencing greater loss of control during binges. Together with the positively correlated BIS-11 non-planning and BE frequency, it reinforces the idea that BED severity is influenced by different time-spaced components of impulsivity.
Despite the overall high efficacy of LDX in reducing BE frequency, there was some individual variability in the degree to which this change occurred. Our data showed that individuals with higher baseline levels of motor and non-planning impulsivity and B-LOCES scores experienced the greatest reduction in BE frequency during 8weeks of LDX. This has important clinical implications, as it means that two brief and easy to administer questionnaires may be useful for predicting who will benefit the most from LDX treatment. It is known that psychological therapies do not have the same level of efficacy for all people with BED (Hilbert et al., 2019). Individuals with particularly high levels of motor and non-planning impulsivity may represent a subgroup of people with BED who would benefit from LDX as an adjunct to psychological therapy.
Some limitations of this study should be acknowledged. First, healthy control participants were not matched to BED group in terms of BMI. This is potentially important given that previous research has reported increased impulsivity in people with obesity, in the absence of BED (Derefinko et al., 2008). However, the inclusion of BMI as a covariate of no interest in all analyses (supplementary materials) did not alter any of the results, suggesting that group differences were due to psychological rather than weight-based factors. Second, this trial was not designed to be an efficacy study and therefore does not include a placebo arm. As such, we cannot conclusively say that reductions in BE frequency and impulsivity measures are due to LDX treatment alone. It does however replicate the real-world setting where placebo and physician contact effects contribute to the overall clinical response. Further, this study focussed on the relationships between impulsivity and BE frequency measures, which we can assume were equally affected by placebo effects. Third, the BIS-11 and B-LOCES were not collected at week 8 for control participants. Although no major changes in self-perceived impulsivity were expected to have happened during this period for healthy controls, we cannot rule out that effects seen in the BED group were in part due to time-related factors rather than LDX. Given the significant correlations between baseline measures, this seems unlikely. Finally, only one male BED participant was recruited into the study, despite the fact that BED has the highest male-to-female ratio of any eating disorder (Hudson et al., 2007). This can likely be attributed to the increased stigma around BED in men (Thapliyal et al., 2018), and lower health-seeking behaviour rates of men relative to women (Kessler et al., 2013). There are documented differences between males and females in impulsive action and choice (Weafer and de Wit, 2014), which unfortunately limits the generalisability of these results to male BED patients.
To conclude, self-perceived impulsivity features are significantly associated with BED psychopathology and are susceptible to LDX-induced changes. This has broad clinical implications, such as the ability to predict response to LDX in moderate to severe BED, and potential use in concurrently treating a range of comorbid impulse control disorders or addictive behaviours. Finally, future research should be conducted to investigate whether LDX-induced reductions in trait impulsivity may enhance treatment outcomes for additional treatments, such as cognitive behavioural therapy.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Western Sydney Local Health District. The patients/participants provided their written informed consent to participate in this study.
KG designed the study protocol, collected and analysed the data, and wrote the paper. LA collected and analysed the data and wrote the paper. TB analysed the data and wrote the paper. JY and GH collected the data and wrote the paper. AH assessed the clinical patients and wrote the paper. PH and ST designed the study protocol and wrote the paper. MK designed the study protocol, assessed the clinical patients and wrote the paper. All authors contributed to the article and approved the submitted version.
This study was funded by an investigator-initiated research grant (IIR-AUS-001429) from Takeda Pharmaceutical International AG Singapore Branch. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication. KG was supported by a NHMRC Early Career Fellowship (GNT1122842).
KG has been the recipient of honoraria from Shire (Takeda group of companies) for public speaking engagements. PH receives/has received sessional fees and lecture fees from the Australian Medical Council, Therapeutic Guidelines publication, and HETI (New South Wales and the former NSW Institute of Psychiatry) and royalties/honoraria from Hogrefe and Huber, McGraw Hill Education, and Blackwell Scientific Publications, Biomed Central and PlosMedicine, and she has received research grants from the NHMRC and ARC. She is Chair of the National Eating Disorders Collaboration Steering Committee in Australia (2019-) and was Member of the ICD-11 Working Group for Eating Disorders and was Chair Clinical Practice Guidelines Project Working Group (Eating Disorders) of RANZCP (2012–2015). She has prepared a report under contract for Takeda (formerly Shire) Pharmaceuticals in regard to binge eating disorder (July 2017) and is a consultant to Takeda Pharmaceuticals. All views in this paper are her own. ST has been the recipient of honoraria from Shire (Takeda group of companies) for public speaking engagements and commissioned reports. He has been the Chair of their Clinical Advisory Group for binge eating disorder. He receives honoraria for books/book chapters from McGraw Hill. Hogrefe and Huber and Taylor and Francis. He is the co-director of the Inside Out Institute at the University of Sydney. ST is a mental health consultant to BUPA. He is a member of the Department of Health Technical Advisory Group for Eating Disorders (Commonwealth Government). MK has been the recipient of honoraria from Shire (Takeda group of companies) for public speaking engagements. LA, TB, JY, GH and AH have no potential conflicts of interest to declare.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Dr. Jagadeesh Andepalli for his generous help with clinical interviews and Nancy Tom for her wonderful assistance with booking in appointments. Finally, we wish to thank the participants of this trial for volunteering their time and making this research possible.
Abbreviations, B-LOCES, Brief Loss of Control Over Eating Scale; BE, binge eating; BED, binge eating disorder; BIS-11, Barratt Impulsiveness Scale-11; cGNG, Cued Go No-Go; LDX, Lisdexamfetamine dimesylate; MIDT, Monetary Incentive Delay Task.
Aichert, D. S., Wöstmann, N. M., Costa, A., Macare, C., Wenig, J. R., Möller, H.-J., et al. (2012). Associations between trait impulsivity and prepotent response inhibition. J. Clin. Exp. Neuropsychol. 34, 1016–1032. doi: 10.1080/13803395.2012.706261
American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders. 5th Edn. Arlington, VA: American Psychiatric Publising.
Bahji, A., Mazhar, M. N., Hudson, C. C., Nadkarni, P., MacNeil, B. A., and Hawken, E. (2019). Prevalence of substance use disorder comorbidity among individuals with eating disorders: a systematic review and meta-analysis. Psychiatry Res. 273, 58–66. doi: 10.1016/j.psychres.2019.01.007
Bates, D., Mächler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48.
Ben-Shachar, M. S., Lüdecke, D., and Makowski, D. (2020). Effectsize: estimation of effect size indices and standardized parameters. J. Open Source Soft. 5, 2815. doi: 10.21105/joss.02815
Bodell, L. P., Forney, K. J., Chavarria, J., Keel, P. K., and Wildes, J. E. (2018). Self-report measures of loss of control over eating: psychometric properties in clinical and non-clinical samples. Int. J. Eat. Disord. 51, 1252–1260. doi: 10.1002/eat.22957
Brownstone, L. M., Bardone-Cone, A. M., Fitzsimmons-Craft, E. E., Printz, K. S., Le Grange, D., Mitchell, J. E., et al. (2013). Subjective and objective binge eating in relation to eating disorder symptomatology, negative affect, and personality dimensions. Int. J. Eat. Disord. 46, 66–76. doi: 10.1002/eat.22066
Busner, J., and Targum, S. D. (2007). The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry 4, 28–37.
Cortese, S., Bernardina, B. D., and Mouren, M.-C. (2007). Attention-deficit/hyperactivity disorder (ADHD) and binge eating. Nutr. Rev. 65, 404–411. doi: 10.1111/j.1753-4887.2007.tb00318.x
Dawe, S., and Loxton, N. J. (2004). The role of impulsivity in the development of substance use and eating disorders. Neurosci. Biobehav. Rev. 28, 343–351. doi: 10.1016/j.neubiorev.2004.03.007
Derefinko, K. J., Adams, Z. W., Milich, R., Fillmore, M. T., Lorch, E. P., and Lynam, D. R. (2008). Response style differences in the inattentive and combined subtypes of attention-deficit/hyperactivity disorder. J. Abnorm. Child Psychol. 36, 745–758. doi: 10.1007/s10802-007-9207-3
Farstad, S. M., von Ranson, K. M., Hodgins, D. C., El-Guebaly, N., Casey, D. M., and Schopflocher, D. P. (2015). The influence of impulsiveness on binge eating and problem gambling: A prospective study of gender differences in Canadian adults. Psychol. Addict. Behav. 29:805. doi: 10.1037/adb0000069
Fillmore, M. T. (2003). Drug abuse as a problem of impaired control: current approaches and findings. Behav. Cogn. Neurosci. Rev. 2, 179–197. doi: 10.1177/1534582303257007
First, M., Williams, J., Karg, R., and Spitzer, R. (2014). Structured Clinical Interview for DSM-5 Disorders–Research Version (SCID-5-RV). Arlington: American Psychiatric Assocation.
Giel, K. E., Teufel, M., Junne, F., Zipfel, S., and Schag, K. (2017). Food-related impulsivity in obesity and binge eating disorder—A systematic update of the evidence. Nutrients 9:1170. doi: 10.3390/nu9111170
Griffiths, K. R., Yang, J., Touyz, S. W., Hay, P. J., Clarke, S. D., Korgaonkar, M. S., et al. (2019). Understanding the neural mechanisms of lisdexamfetamine dimesylate (LDX) pharmacotherapy in binge eating disorder (BED): a study protocol. J. Eat. Disord. 7:23. doi: 10.1186/s40337-019-0253-3
Hege, M. A., Stingl, K. T., Kullmann, S., Schag, K., Giel, K. E., Zipfel, S., et al. (2015). Attentional impulsivity in binge eating disorder modulates response inhibition performance and frontal brain networks. Int. J. Obes. 39, 353–360. doi: 10.1038/ijo.2014.99
Hilbert, A., Petroff, D., Herpertz, S., Pietrowsky, R., Tuschen-Caffier, B., Vocks, S., et al. (2019). Meta-analysis of the efficacy of psychological and medical treatments for binge-eating disorder. J. Consult. Clin. Psychol. 87, 91–105. doi: 10.1037/ccp0000358
Hou, R., Mogg, K., Bradley, B. P., Moss-Morris, R., Peveler, R., and Roefs, A. (2011). External eating, impulsivity and attentional bias to food cues. Appetite 56, 424–427. doi: 10.1016/j.appet.2011.01.019
Hudson, J. I., Hiripi, E., Pope, H. G. Jr., and Kessler, R. C. (2007). The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol. Psychiatry 61, 348–358. doi: 10.1016/j.biopsych.2006.03.040
Jiménez-Murcia, S., Granero, R., Stinchfield, R., Fernández-Aranda, F., Penelo, E., Savvidou, L. G., et al. (2013). Typologies of young pathological gamblers based on sociodemographic and clinical characteristics. Compr. psychiatry, 54, 1153–1160. doi: 10.1016/j.comppsych.2013.05.017
Kessler, R. C., Berglund, P. A., Chiu, W. T., Deitz, A. C., Hudson, J. I., Shahly, V., et al. (2013). The prevalence and correlates of binge eating disorder in the World Health Organization world mental health surveys. Biol. Psychiatry 73, 904–914. doi: 10.1016/j.biopsych.2012.11.020
Kessler, R. M., Hutson, P. H., Herman, B. K., and Potenza, M. N. (2016). The neurobiological basis of binge-eating disorder. Neurosci. Biobehav. Rev. 63, 223–238. doi: 10.1016/j.neubiorev.2016.01.013
Knutson, B., Westdorp, A., Kaiser, E., and Hommer, D. (2000). FMRI visualization of brain activity during a monetary incentive delay task. NeuroImage 12, 20–27. doi: 10.1006/nimg.2000.0593
Kollei, I., Rustemeier, M., Schroeder, S., Jongen, S., Herpertz, S., and Loeber, S. (2018). Cognitive control functions in individuals with obesity with and without binge‐eating disorder. Int. J. Eat. Disord. 51, 233–240. doi: 10.1002/eat.22824
Kuznetsova, A., Brockhoff, P. B., and Christensen, R. H. (2017). lmerTest package: tests in linear mixed effects models. J. Stat. Softw. 82, 1–26. doi: 10.18637/jss.v082.i13
Latner, J. D., Mond, J. M., Kelly, M. C., Haynes, S. N., and Hay, P. J. (2014). The loss of control over eating scale: development and psychometric evaluation. Int. J. Eat. Disord. 47, 647–659. doi: 10.1002/eat.22296
Lenth, R., Singmann, H., Love, J., and Buerkner, P. (2019). Emmeans: estimated marginal means, aka least-squares means. R package v. 1.3. 4. In.
Li, N., Mitchison, D., Touyz, S., and Hay, P. (2019). Cross-sectional comparison of health-related quality of life and other features in people with and without objective and subjective binge eating using a general population sample. BMJ Open 9:e024227. doi: 10.1136/bmjopen-2018-024227
Loeber, S., Grosshans, M., Korucuoglu, O., Vollmert, C., Vollstädt-Klein, S., Schneider, S., et al. (2012). Impairment of inhibitory control in response to food-associated cues and attentional bias of obese participants and normal-weight controls. Int. J. Obes. 36, 1334–1339. doi: 10.1038/ijo.2011.184
MacKillop, J., Weafer, J., Gray, J. C., Oshri, A., Palmer, A., and de Wit, H. (2016). The latent structure of impulsivity: impulsive choice, impulsive action, and impulsive personality traits. Psychopharmacology 233, 3361–3370. doi: 10.1007/s00213-016-4372-0
Manwaring, J. L., Green, L., Myerson, J., Strube, M. J., and Wilfley, D. E. (2011). Discounting of various types of rewards by women with and without binge eating disorder: evidence for general rather than specific differences. Psychol. Rec. 61, 561–582. doi: 10.1007/BF03395777
McElroy, S. L., Hudson, J. I., Mitchell, J. E., Wilfley, D., Ferreira-Cornwell, M. C., Gao, J., et al. (2015). Efficacy and safety of lisdexamfetamine for treatment of adults with moderate to severe binge-eating disorder: a randomized clinical trial. JAMA Psychiat. 72, 235–246. doi: 10.1001/jamapsychiatry.2014.2162
McElroy, S. L., Mitchell, J. E., Wilfley, D., Gasior, M., Ferreira-Cornwell, M. C., McKay, M., et al. (2016). Lisdexamfetamine dimesylate effects on binge eating behaviour and obsessive-compulsive and impulsive features in adults with binge eating disorder. Eur. Eat. Disord. Rev. 24, 223–231. doi: 10.1002/erv.2418
Meule, A. (2013). Impulsivity and overeating: a closer look at the subscales of the Barratt impulsiveness scale. Front. Psychol. 4:177. doi: 10.3389/fpsyg.2013.00177
Mobbs, O., Iglesias, K., Golay, A., and Van der Linden, M. (2011). Cognitive deficits in obese persons with and without binge eating disorder. Investigation using a mental flexibility task. Appetite 57, 263–271. doi: 10.1016/j.appet.2011.04.023
Monica, D., Paulo, M., Appolinário, J. C., Freitas, S. R. D., Coutinho, G., Santos, C., et al. (2010). Assessment of executive functions in obese individuals with binge eating disorder. Braz. J. Psychiatry 32, 381–388.
Nazar, B. P., Bernardes, C., Peachey, G., Sergeant, J., Mattos, P., and Treasure, J. (2016). The risk of eating disorders comorbid with attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. Int. J. Eat. Disord. 49, 1045–1057. doi: 10.1002/eat.22643
Oliva, R., Morys, F., Horstmann, A., Castiello, U., and Begliomini, C. (2019). The impulsive brain: neural underpinnings of binge eating behavior in normal-weight adults. Appetite 136, 33–49. doi: 10.1016/j.appet.2018.12.043
Oliva, R., Morys, F., Horstmann, A., Castiello, U., and Begliomini, C. (2020). Characterizing impulsivity and resting-state functional connectivity in normal-weight binge eaters. Int. J. Eat. Disord. 53, 478–488. doi: 10.1002/eat.23212
Patton, J. H., Stanford, M. S., and Barratt, E. S. (1995). Factor structure of the Barratt impulsiveness scale. J. Clin. Psychol. 51, 768–774. doi: 10.1002/1097-4679(199511)51:6<768::AID-JCLP2270510607>3.0.CO;2-1
Presby, R. E., Rotolo, R. A., Yang, J. H., Correa, M., and Salamone, J. D. (2020). Lisdexamfetamine suppresses instrumental and consummatory behaviors supported by foods with varying degrees of palatability: exploration of a binge-like eating model. Pharmacol. Biochem. Behav. 189:172851. doi: 10.1016/j.pbb.2020.172851
Reinblatt, S. P. (2015). Are eating disorders related to attention deficit/hyperactivity disorder? Curr. Treat Options Psychiatry 2, 402–412. doi: 10.1007/s40501-015-0060-7
Schag, K., Schönleber, J., Teufel, M., Zipfel, S., and Giel, K. E. (2013). Food-related impulsivity in obesity and binge eating disorder—a systematic review. Obes. Rev. 14, 477–495. doi: 10.1111/obr.12017
Schreiber, L. R., Odlaug, B. L., and Grant, J. E. (2013). The overlap between binge eating disorder and substance use disorders: diagnosis and neurobiology. J. Behav. Addict. 2, 191–198. doi: 10.1556/JBA.2.2013.015
Sheehan, D. V., Lecrubier, Y., Sheehan, K. H., Amorim, P., Janavs, J., Weiller, E., et al. (1998). The mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59(Suppl. 20), 22–33.
Solanto, M. V. (1998). Neuropsychopharmacological mechanisms of stimulant drug action in attention-deficit hyperactivity disorder: a review and integration. Behav. Brain Res. 94, 127–152. doi: 10.1016/S0166-4328(97)00175-7
Stanford, M. S., Mathias, C. W., Dougherty, D. M., Lake, S. L., Anderson, N. E., and Patton, J. H. (2009). Fifty years of the Barratt impulsiveness scale: an update and review. Personal. Individ. Differ. 47, 385–395. doi: 10.1016/j.paid.2009.04.008
Svaldi, J., Brand, M., and Tuschen-Caffier, B. (2010). Decision-making impairments in women with binge eating disorder. Appetite 54, 84–92. doi: 10.1016/j.appet.2009.09.010
Svaldi, J., Naumann, E., Biehl, S., and Schmitz, F. (2015). Impaired early-response inhibition in overweight females with and without binge eating disorder. PLoS One 10:e0133534. doi: 10.1371/journal.pone.0133534
Team, R. C. (2020). R: A language and environment for statistical computing (3.5. 1)[computer software]. R Foundation for Statistical Computing.
Thapliyal, P., Hay, P., and Conti, J. (2018). Role of gender in the treatment experiences of people with an eating disorder: a metasynthesis. J. Eat. Disord. 6, 1–16. doi: 10.1186/s40337-018-0207-1
Voon, V., Morris, L. S., Irvine, M. A., Ruck, C., Worbe, Y., Derbyshire, K., et al. (2015). Risk-taking in disorders of natural and drug rewards: neural correlates and effects of probability, valence, and magnitude. Neuropsychopharmacology 40, 804–812. doi: 10.1038/npp.2014.242
Weafer, J., and de Wit, H. (2014). Sex differences in impulsive action and impulsive choice. Addict. Behav. 39, 1573–1579. doi: 10.1016/j.addbeh.2013.10.033
Wingrove, J., and Bond, A. J. (1997). Impulsivity: a state as well as trait variable. Does mood awareness explain low correlations between trait and behavioural measures of impulsivity? Personal. Individ. Differ. 22, 333–339. doi: 10.1016/S0191-8869(96)00222-X
Wu, M., Giel, K. E., Skunde, M., Schag, K., Rudofsky, G., de Zwaan, M., et al. (2013). Inhibitory control and decision making under risk in bulimia nervosa and binge-eating disorder. Int. J. Eat. Disord. 46, 721–728. doi: 10.1002/eat.22143
Wu, M., Hartmann, M., Skunde, M., Herzog, W., and Friederich, H.-C. (2013). Inhibitory control in bulimic-type eating disorders: a systematic review and meta-analysis. PLoS One 8:e83412. doi: 10.1371/journal.pone.0083412
Keywords: clinical trial, drug therapy, lisdexamfetamine dimesylate, impulsivity, binge eating disorder
Citation: Griffiths KR, Aparício L, Braund TA, Yang J, Harvie G, Harris A, Hay PJ, Touyz S and Kohn MR (2021) Impulsivity and Its Relationship With Lisdexamfetamine Dimesylate Treatment in Binge Eating Disorder. Front. Psychol. 12:716010. doi: 10.3389/fpsyg.2021.716010
Received: 28 May 2021; Accepted: 27 July 2021;
Published: 31 August 2021.
Edited by:
Kelly Costello Allison, University of Pennsylvania, United StatesReviewed by:
Christine Peat, University of North Carolina at Chapel Hill, United StatesCopyright © 2021 Griffiths, Aparício, Braund, Yang, Harvie, Harris, Hay, Touyz and Kohn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kristi R. Griffiths, a3Jpc3RpLmdyaWZmaXRoc0BzeWRuZXkuZWR1LmF1
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.