- 1Department of Child Psychiatry of Shenzhen Kangning Hospital, Shenzhen Mental Health Center, School of Mental Health, Shenzhen University, Shenzhen, China
- 2National Clinical Research Center for Mental Disorders, Department of Psychiatry, The Second Xiangya Hospital of Central South University, Changsha, China
- 3Hunan Key Laboratory of Psychiatry and Mental Health, Changsha, China
- 4Mental Health Zhejiang University School of Medicine, Hangzhou Seventh People’s Hospital, Hangzhou, China
- 5Unit of Psychiatry, Department of Public Health and Medicinal Administration, Institute of Translational Medicine, Faculty of Health Sciences, University of Macau, Macao, SAR China
Background: The relationship of events occurring during pregnancy and perinatal period with attention deficit/hyperactivity disorder (ADHD) is not clear. Thus, the focus of the current study was to examine the effects of events occurring during pregnancy and perinatal period on ADHD.
Methods: A two-phase cross-sectional study was performed across 13 schools in Changsha and Yiyang cities from March to December, 2014. We preliminarily screened all students using CBCL and established the diagnosis using Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID) and the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). A total of 3,418 questionnaires were effectively completed in this study.
Results: History of threatened abortion (TA) [odds ratio (OR): 1.707 (1.201–2.426)] (vs. No-TA) and neonatal asphyxia (NA) [OR: 2.497(1.225–5.09)] (vs. health) showed a positive association with ADHD. On subgroup analysis, TA [OR: 2.216 (1.458–3.369)] (vs. No-TA) was a risk factor for ADHD without comorbidity; instrumental delivery [OR: 2.748 (1.057–7.142)] (vs. natural birth) and NA [OR: 2.789 (1.222–6.361)] (vs. health) were risk factors for ADHD in the subgroup of ADHD with comorbidity; TA (vs. no-TA) and NA (vs. health) were risk factors for ADHD among male students [ORs: 2.232 (1.439–3.462) and 2.808 (1.115–7.068), respectively], while low birth weight (LBW) (vs. normal birth weight) was a risk factor [OR: 2.054 (1.063–3.967)] for ADHD among female students.
Conclusion: TA was a risk factor for ADHD in the absence of comorbid conditions; instrumental delivery and NA were risk factors for ADHD in the subgroup of ADHD with comorbidity; TA and NA were risk factors for ADHD among male students. LBW was a risk factor for ADHD among female students.
Background
Attention deficit/hyperactivity disorder (ADHD) is a common neurodevelopmental disorder characterized by hyperactivity, impulsive behavior, and poor attention (Choi et al., 2017). According to a meta-analysis, an estimated 5.9–7.1% of children and adolescents are affected by ADHD globally; the estimates were based on ratings by parents/teachers or on clinical diagnosis (Willcutt, 2012). In our previous study, the estimated prevalence of ADHD among children and adolescent students in Hunan province, China was found to be 4.64–5.29%, according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) (Shen et al., 2018a). Based on the assumption that ADHD may persist to adulthood in a certain proportion of individuals, we also investigated the prevalence of ADHD among Chinese medical college students and found similar prevalence rate (3.02–3.98%) (Shen et al., 2018b); this indicates that ADHD is not limited to children and adolescents. Students with ADHD may have poor executive function (Capri et al., 2020; Fabio et al., 2020), experience academic difficulties (Martin, 2014) and are at a higher risk of injuries (Amiri et al., 2017). In addition, individuals with ADHD often have comorbid psychiatric disorders, such as anxiety disorder, disruptive behavioral disorders, oppositional defiant disorder, sleep disorder, and substance use disorder (Bilgiç et al., 2013; Miguel et al., 2016; Reale et al., 2017). Generally, individuals with ADHD who have comorbid psychiatric disorders have poorer mental health. For example, Miguel et al. (2016) found that subjects with ADHD who had concomitant substance use disorder had poorer cognitive function as compared with ADHD without substance use disorder. In a study, adult subjects with ADHD who had concomitant substance use disorder showed lower gray matter volume in striatum as compared to their counterparts with only ADHD, which may be an underlying pathogenic mechanism (van Wingen et al., 2013). Therefore, ADHD is a major global public health problem that imposes a considerable socioeconomic burden. Doshi et al. (2012) found that the overall national annual incremental costs attributable to ADHD in the United States were in the range of $143–$266 billion.
However, the pathogenic mechanism of ADHD is not clear. Previous studies have found linkage of ADHD with many factors including genetic and environmental factors. In previous studies, some genes related to the dopamine pathway were shown to be associated with ADHD (Nyman et al., 2007). With respect to environmental factors, change in household income, home environment, and parental rearing style were shown to be associated with ADHD (Tully et al., 2004; Rodriguez and Bohlin, 2005; Mulligan et al., 2013; Arijit et al., 2016; Dadds et al., 2016). In addition, the role of certain gestation-related risk factors in the pathogenesis of ADHD has attracted attention. For example, maternal stress, anxiety, depression, sleep disorder, infection, lower maternal levels of vitamin D, and smoking during pregnancy showed a significant association with ADHD (Rodriguez and Bohlin, 2005; Ronald et al., 2010; Zhu et al., 2014; Morales et al., 2015; He et al., 2017; Laugesen et al., 2017; Ginsberg et al., 2018; Vizzini et al., 2018). Such associations pertaining to the perinatal period are also of particular concern. In addition, perinatal period is associated with a greater risk of brain injury due to causes such as intrapartum hypoxic ischaemia and brain injury during assisted modes of delivery. For example, Rennie et al. (2007) found an association between intrapartum hypoxic ischaemia and a range of childhood disabilities, including cognitive deficit. Moreover, neonatal asphyxia has also been shown to be a risk factor for psychiatric disorders such as ADHD and autism (Li et al., 2011; Duan et al., 2014). In addition, low birth weight and threatened abortion [before the fetus would be viable ex utero, vaginal bleed with a closed cervix (Greene, 2019)] may also be risk factors for psychiatric disorders (Hanć et al., 2015; Wang et al., 2017). Although the above studies found an association of ADHD with some events occurring during pregnancy and the perinatal period, some other studies have yielded inconsistent results (Oudin et al., 2019; O’Callaghan and Harvey, 1997). This may be attributable to small sample size in some studies which may limit the statistical power of the analysis. Secondly, subjects with ADHD frequently have other comorbid psychiatric disorders, which may have affected the results of some of the studies.
Based on the limitation of prior studies, we hypothesized that certain pregnant and perinatal events were independent risk factors for ADHD. To test this hypothesis, we assessed the relationship between ADHD and events occurring during pregnancy and perinatal period in a large sample after controlling for other possible confounding factors such as parental rearing style and demographic characteristics. In addition, we hypothesized that risk factors for ADHD pertaining to pregnancy and the perinatal period may vary depending on the sex as well as the clinical profile of ADHD (e.g., ADHD alone versus ADHD with other comorbid mental disorders). To test this hypothesis, we performed subgroup analysis in subgroups of ADHD disaggregated by comorbidity and sex to further explore the risk factors for ADHD.
Materials and Methods
Design and Population
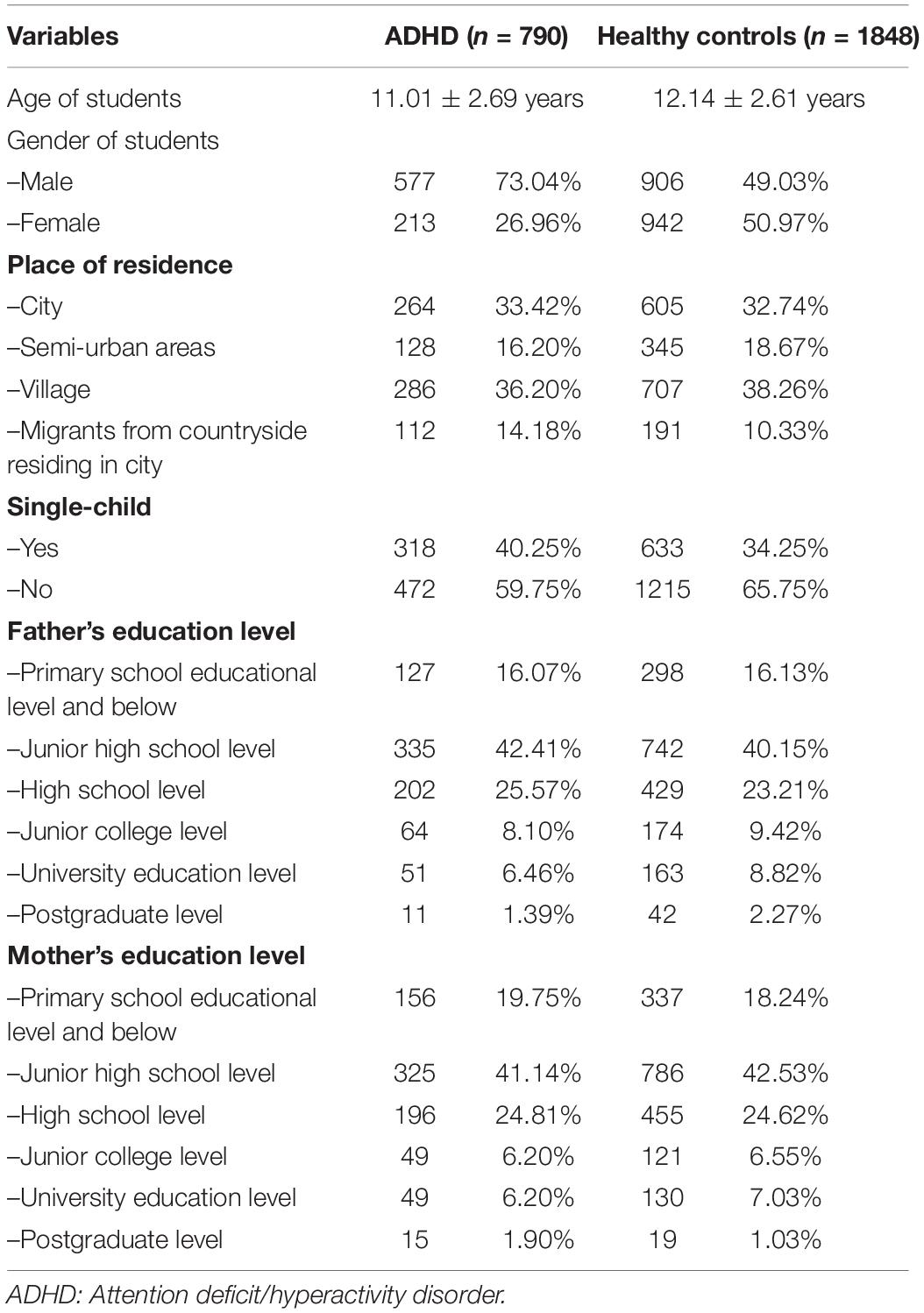
This study used data from the epidemiological investigation of Hunan children and adolescent psychiatry disorders (age range of subjects: 6–16 years) from March to December, 2014 (Shen et al., 2018a). This study is a two-stage study, including screening of children with Child Behavior Checklist (CBCL) and establishment of diagnosis using the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID) and DSM-IV. We screened all students (n = 18,778) in 13 primary and junior high schools of Changsha and Yiyang cities using the CBCL. A total of 17,071 students qualified the selection criteria and were included in the study, while 1707 students were excluded. The reasons for exclusion were: refusal to complete the questionnaire or incomplete questionnaire (n = 782), questionnaire with >10% missing data (n = 522), and age outside the range of 6–16 years (n = 403). If the score of any subscale in CBCL is higher than the norm, it is regarded as CBCL positive. According to the results of CBCL, students who received a positive CBCL score (n = 3465) and matched students who received a negative CBCL score (n = 3465) were selected from the 17,071 students; these students were asked to complete the investigation of parental rearing attitude and behavior and their parents were asked to complete the investigation of events related to pregnancy and the perinatal period of their children. Finally, a total of 3,418 questionnaires were effectively completed for the investigation of events related to pregnancy and the perinatal period and the egma minen bardoms uppfostran (response rate: 49.29%) among students who received a positive CBCL score (n = 3465) and matched students who received a negative CBCL score (n = 3465). Further, students who received a positive CBCL score (n = 3465) and 10% of students who were CBCL negative (randomly sampled) among 17071 students were diagnosed using MINI-KID and DSM-IV; of these, 1,663 students (age range, 6–16 years) were confirmed to have a diagnosis of a psychiatric disorder. Among students (n = 3418) for whom the investigation of events related to pregnancy and the perinatal period and the egma minen bardoms uppfostran was effectively completed, 790 students were diagnosed with ADHD (including 383 who were diagnosed with ADHD comorbid with other mental disorders), 780 students were diagnosed with mental disorders other than ADHD, and 1,848 students were identified as healthy based on MINI-KID and DSM-IV. The Ethics Committee at the Second Xiangya Hospital approved this study. All parents or guardians and students in this study gave written informed consent.
The Screening and Diagnosis Tool
Measurement of Demographic Variables and Variables Related to Pregnancy and the Perinatal Period
Demographic variables include age of students, gender of students (male/female), place of residence (city/semi-urban areas/village/migrants from countryside residing in city), single-child (yes/no), father’s education level and mother’s education level (Primary school educational level and below/Junior high school level/High school level/Junior college level/University education level/Postgraduate level). Events related to pregnancy and the perinatal period (from parent reports) included maternal health status during pregnancy (healthy/hypertension of pregnancy/other), threatened abortion (no/yes), mode of delivery (natural birth/Cesarean delivery/instrumental delivery), newborn health (healthy/neonatal asphyxia/other), birth weight (normal birth weight/low birth weight/macrosomia), and feeding patterns (breastfeeding/artificial feeding/mixed feeding).
The Child Behavior Checklist
In this study, the section of the child behavior checklist (CBCL) (a 113-item standardized scale) pertaining to behavioral problems was used to quantify behavioral problems (including internalization and externalization) of children in the age-group of 6–16 years. Each item in this section is scored as 0, 1 or 2. The Chinese version of the CBCL has been validated with satisfactory psychometric properties (retest reliability: 0.77–0.79; criterion validity: 0.61–0.76) (Su and Li, 1996).
The Mini International Neuropsychiatric Interview for Children and Adolescents 5.0 (MINI-KID)
The MINI-KID, a structured interview instrument to diagnose psychiatric disorders among subjects in the age-group of 6–16 years, was designed according to DSM-IV and the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10). Both the Chinese parent and child versions of the MINI-KID have been shown to have satisfactory validity (Liu et al., 2010, 2011). In this study, ADHD identified based on either parent version or child version was further verified clinically to confirm the diagnosis as per the DSM-IV.
Egma Minnen Barndoms Uppfostran
The Egma Minnen Barndoms Uppfostran (EMBU) is an 81-item self-reported scale that is widely used to assess the subject’s memories of parental rearing attitude and behavior. The items are rated on a four-point scale ranging from 1 (no, never) to 4 (yes, always) for the father and the mother separately. The EMBU consists of 11 factors including father rearing attitude and behavior (6 factors) and mother rearing attitude and behavior (5 factors). The Chinese version of EMBU used in this study has been validated in Chinese adolescents with correlation coefficient between factors and total scale scores ranging from 0.34 to 0.65; the Cronbach’s alpha ranges from 0.65 to 0.86 (Lai et al., 2013).
Statistical Analysis
Data analysis was conducted using SPSS version 21 (IBM, Armonk, NY, United States). Continuous variables are presented as mean (M) ± standard deviation (SD), and categorical variables are presented as frequency (%). The association between each research variable and ADHD was tested individually using univariate analyses (logistic regression analysis) and odds ratios (OR) with 95% confidence intervals (CIs) calculated. We also performed multivariate analysis (logistic regression analysis) by including variables that qualified the following criteria: variables that showed a significant association with ADHD (p < 0.1) in univariate analyses; variables meeting clinical plausibility despite not showing a significant association in univariate analyses. For multivariate analysis, we employed two models. In the first model, we included events occurring during pregnancy and perinatal period in the logistic regression model. In the second model, we included demographic variables and parental rearing style on the basis of the first model. In addition, subgroup analysis was also performed by disaggregating subjects with ADHD by comorbidity and gender to explore the association of different subgroups with events occurring during pregnancy and perinatal period in the above two models. P-values in all analyses were two sided, with a significance level of 0.05.
Results
Demographic Characteristics and Univariate Analysis
The demographic characteristics are summarized in Table 1. On univariate analysis, female gender, younger age of students, maternal age 40-49 years (vs. 20-29 years), father’s emotional warmth, and mother’s emotional warmth were found to protect against ADHD compared with the reference group (p < 0.05 for all). Further, variables such as migrants from countryside living in cities (vs. city), father punishment, father interference, father rejection, father overprotection, mother emotional warmth, mother interference, mother rejection, mother punishment, single child (vs. non-single child), hypertension of pregnancy (vs. health), threatened abortion (TA) (vs. no-TA), cesarean delivery (vs. natural birth), instrumental delivery (vs. natural birth), neonatal asphyxia (NA) (vs. health), and low birth weight (LBW) (vs. normal birth weight) were found to be risk factors for ADHD (all p < 0.05) (Table 2).

Table 2. Results of univariate analysis (logistic regression analysis) for predicting ADHD in the total study population (n = 2638).
Multivariate Analysis for the Relationship Between ADHD and Variables of Maternal Pregnancy and Perinatal Period in the Total Study Population
We included all maternal events occurring during pregnancy and perinatal period in the logistic regression model 1; the results showed that TA [OR: 1.922 (1.399–2.64)] (vs. no-TA), instrumental delivery [OR: 2.238 (1.087–4.606)] (vs. natural birth) and NA [OR: 2.34 (1.234–4.436)] (vs. health) were risk factors for ADHD (Supplementary Table 1). In model 2, we further controlled for demographic variables and parental rearing style variables based on model 1; the logistic regression model shown that TA [OR: 1.707 (1.201–2.426)] (vs. no-TA) and NA [OR: 2.497 (1.225–5.09)] (vs. health) were risk factors for ADHD (Table 3).
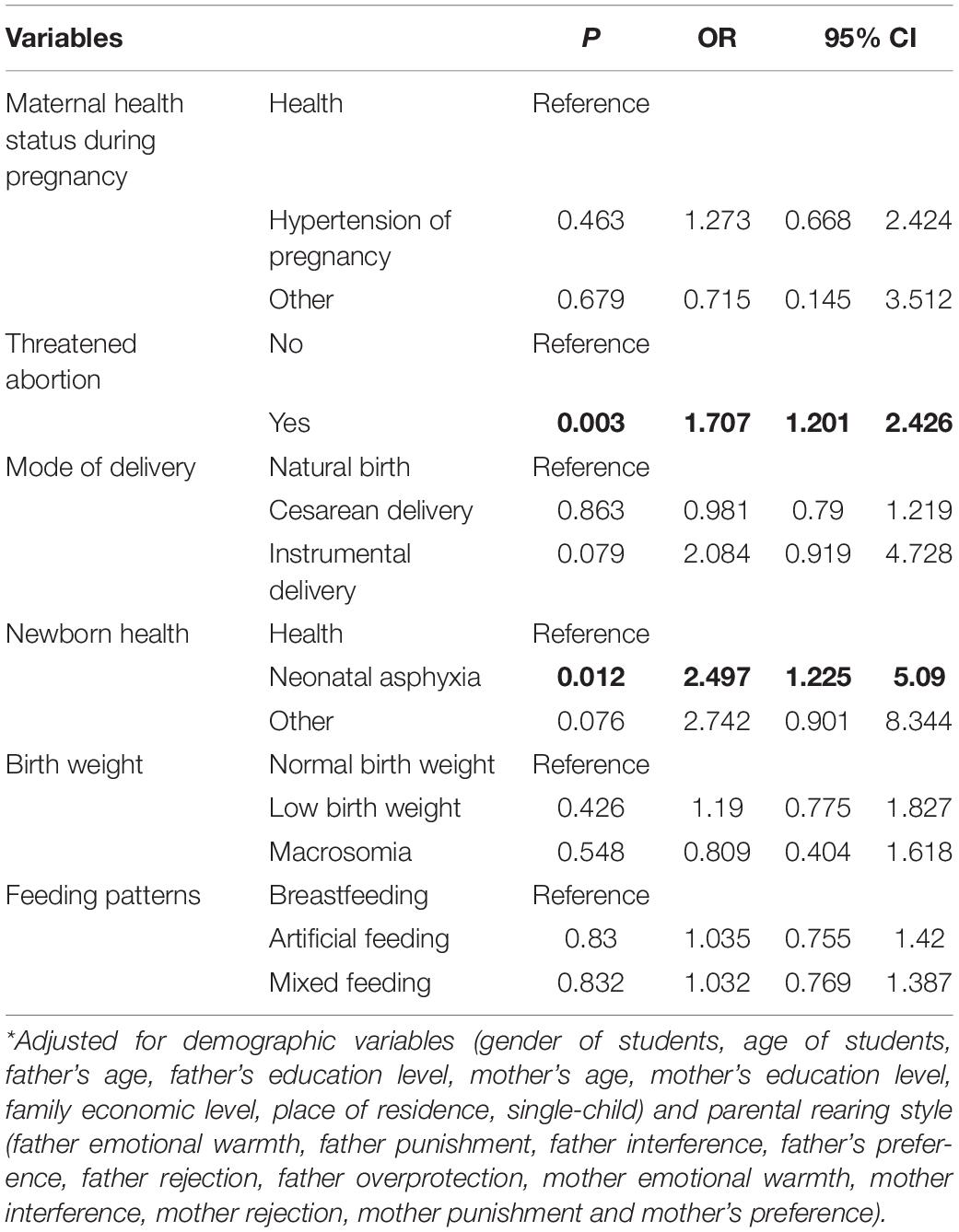
Table 3. Results of multivariate analysis (logistic regression analysis) showing relationship between ADHD and maternal events occurring during pregnancy and perinatal period in the second model (n = 2638).
Subgroup Analysis in Subgroups of Subjects With ADHD Disaggregated by Comorbidity (ADHD Comorbid With/Without Other Mental Disorders) and Gender
In subgroup analysis, we employed univariate analysis and multivariate analysis in the first model (Supplementary Tables 2–9). In the first model, TA [OR: 2.433 (1.682–3.518)] (vs. no-TA) and artificial feeding [OR: 1.511 (1.082–2.11)] (vs. breast feeding) were risk factors for ADHD without comorbidity (Supplementary Table 3); instrumental delivery [OR: 2.738 (1.182–6.343)] (vs. natural birth) and NA [OR: 2.781 (1.306–5.92)] (vs. health) were risk factors for ADHD with comorbidity (Supplementary Table 5); TA [OR: 2.096 (1.409–3.118)] (vs. no-TA) and NA [OR: 2.69(1.175–6.156)] (vs. health) were risk factors for male ADHD (Supplementary Table 7), while low birth weight [OR: 1.915 (1.079–3.4)] (vs. normal birth weight) was a risk factor for female ADHD (Supplementary Table 9).
Multivariate analysis in the subgroup of ADHD without/with comorbidity and gender were adjusted for demographic variables, and parental rearing style. The results of final models showed that TA [OR: 2.216 (1.458–3.369)] (vs. no-TA) was a risk factor for ADHD without comorbidity; instrumental delivery [OR: 2.748 (1.057–7.142)] (vs. natural birth) and NA [OR: 2.789 (1.222–6.361)] (vs. health) were risk factors for ADHD with comorbidity; TA (vs. no-TA) and NA (vs. health) were risk factors for ADHD among male groups [ORs: 2.232 (1.439–3.462) and 2.808 (1.115–7.068), respectively], while low birth weight (LBW) [OR: 2.054 (1.063–3.967)] (vs. normal birth weight) was a risk factor for ADHD among females groups (Table 4).
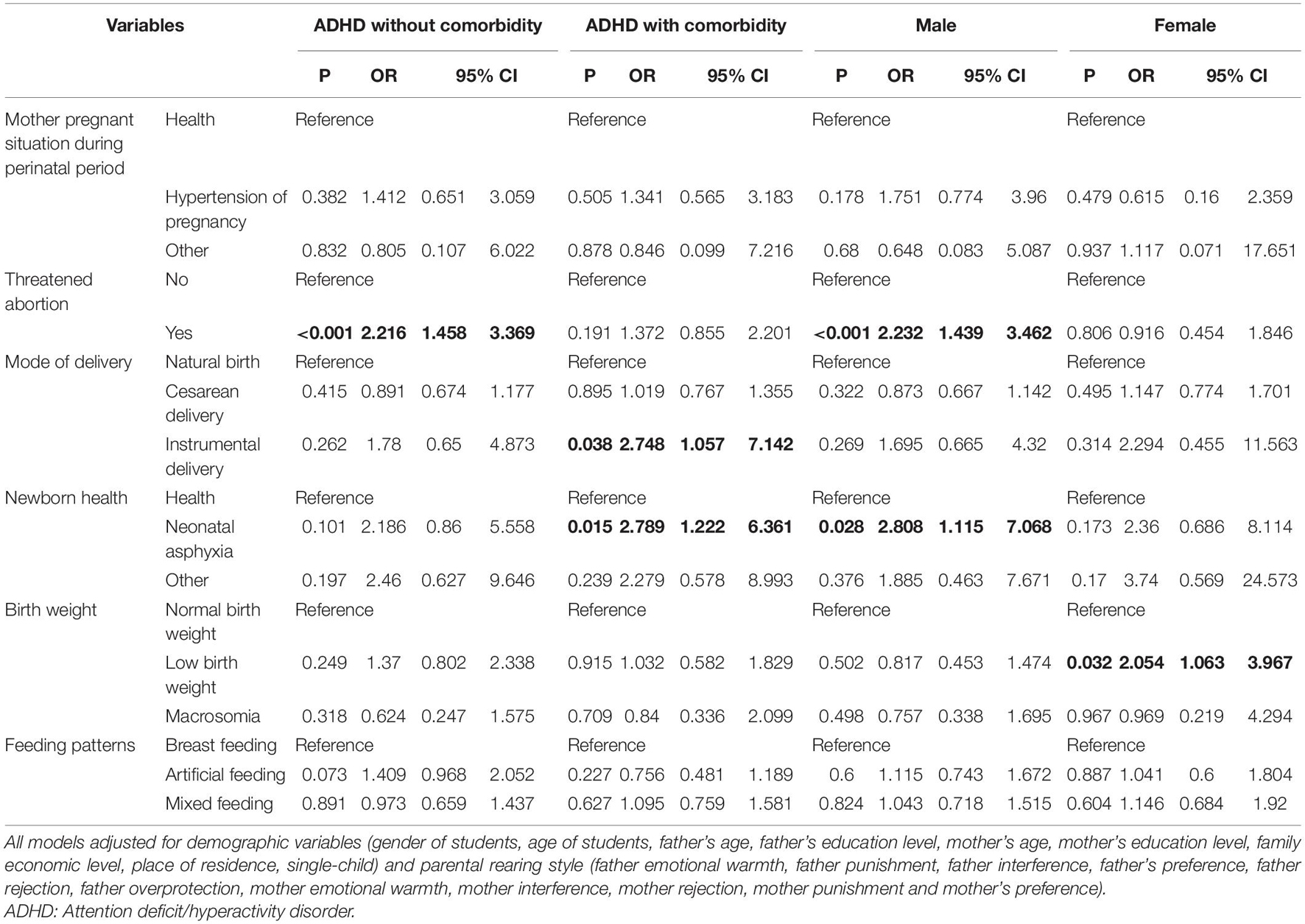
Table 4. Results of multivariate analysis (logistic regression analysis) for the subgroup of ADHD with comorbidity, disaggregated by gender in the second model.
Discussion
The etiopathogenesis of ADHD is not clear; however, both genetic (Yuan et al., 2017) and environmental factors (pre-, peri-, and postnatal environmental factors) are believed to be involved (Mick et al., 2002; Howe, 2010; Silva et al., 2014). In some studies, the interaction between genetic and environmental factors was also found to increase the risk of ADHD (Howe, 2010). Among the environmental factors, risk factors pertaining to pregnancy and perinatal period, such as, LBW, NA, and delivery by caesarean section (Mick et al., 2002; Li et al., 2011; Curran et al., 2015; Franz et al., 2018), have attracted much attention. However, some studies have yielded conflicting results (Mimounibloch et al., 2013; Silva et al., 2014). In addition, few studies have explored the risk factors pertaining to pregnancy and perinatal period in different ADHD subgroups. In this study, we evaluated the stability of results related to the association between risk factors pertaining to pregnancy and perinatal period and ADHD in a large sample and in different ADHD subgroups. Our results indicated that TA, NA, LBW, and instrumental delivery are risk factors for ADHD.
First, we found a strong correlation between TA and ADHD in the total study population, which is not consistent with the results of previous studies (Dongol et al., 2011). This inconsistency is likely attributable to the different sample characteristics. Sliva’s study included children, adolescents, and adults; however, our study only included students in the age-group of 6–16 years (Silva et al., 2014). Additionally, we controlled for parental rearing style, which is an important postnatal environmental factor. However, Sliva’s study only included risk factors pertaining to pregnancy (Silva et al., 2014). TA is a common occurrence during pregnancy (reported incidence rate: 20–25%) (Dongol et al., 2011); it is associated with many poor pregnancy outcomes including placental abruption, preterm delivery, intrauterine growth restriction, and LBW (Basama and Crosfill, 2004; Weiss et al., 2004); all of these developments may affect brain development.
Second, in the present study, NA was found to increase the risk of ADHD in the total population, which is consistent with previous findings. For example, Linnet et al. (2005) found that Apgar score <7 at 5 min increases the risk of ADHD. Getahun et al. (2013) also found similar results on multivariate analysis. Neonatal asphyxia is a serious perinatal event that causes brain injury. Prior studies (Pagida et al., 2013; Tapia-Bustos et al., 2016) have found that hypoxia can cause long-term disturbances of dopaminergic system, and even decrease the expression of dopamine D2 receptor (Kaewsuk et al., 2009), which in turn increases the risk of ADHD. In addition, recent studies have found that asphyxia can alter Catechol-O-methyltransferase (COMT) gene expression; COMT gene was shown to be associated with ADHD or ADHD traits (Park et al., 2015; Akutagavamartins et al., 2016). All these findings support the notion that NA is a risk factor for ADHD.
Finally, in order to test the stability of results, we performed subgroup analysis after disaggregating the ADHD population by presence or absence of comorbidity and gender. In the subgroup analysis of ADHD population with/without comorbidity, we found that TA and NA were associated with ADHD without other comorbid mental disorders and ADHD with other comorbid mental disorders, respectively, this finding is consistent with the findings observed in the total study population. In addition, we also found that ADHD with other comorbid mental disorders was associated with more events during pregnancy and perinatal period. Instrumental delivery also increased the risk of ADHD with other comorbid mental disorders, which is a novel finding of our study. Prior studies have shown that instrumental delivery increases the risk of fetal injury, included head injuries (Doumouchtsis and Arulkumaran, 2006); however, further studies are required to explore the underlying mechanisms. On subgroup analysis disaggregated by gender, we found that TA and NA increased the risk of ADHD in male subjects, which is consistent with the results in the total ADHD population. However, only LBW was a risk factor for ADHD in female subjects. In the total ADHD population, LBW did not appear as a significant risk factor; however, it was found to be a risk factor for ADHD among females. This may be attributable to the higher proportion of males with ADHD in this study population. No study has explained the gender-based differences in the relationship between LBW and ADHD. For example, Sauver et al. (2004) and Mick et al. (2002) found no sex-based differences with respect to the association of LBW with ADHD. The association between LBW and ADHD can be explained by several reasons. First, newborns with LBW are often borne of preterm deliveries, which reduces the time for maturation of brain; this assertion is based on functional magnetic resonance imaging studies (Niwa et al., 2017). Moreover, a recent study has found that preterm birth injury affects the cholinergic system (Grothe et al., 2016), which in turn is linked to ADHD. Second, newborns with LBW are at a higher risk of morbidity, which may affect the parental rearing style and the overall growth. Third, overlapping risk gene has been found between ADHD and LBW. MTHFR gene is associated with infant birth weight (Wu et al., 2017) as well as with ADHD (Gokcen et al., 2011). Lastly, LBW affects gene expression, such as that of hippocampal gene (Buschdorf et al., 2016), which is a potential molecular basis for mental disorder. Some studies have found other events of ADHD related to pregnancy and the perinatal period, such as, caesarean delivery, feeding styles, and hypertension of pregnancy (Curran et al., 2015; Say et al., 2015; Pohlabeln et al., 2017). However, these findings were not confirmed in this study probably because those studies used only univariate analysis or were based on a small sample size.
Limitations
Several limitations of this study should be acknowledged. First, despite the large sample size in this study, the cross-sectional design of our study does not permit causal inferences. Therefore, a large cohort study is required to further examine the relationship of events occurring during pregnancy and ADHD. Second, the possibility of recall bias for parental rearing attitude and behavior, and events related to pregnancy and the perinatal period cannot be ruled out. Third, parental smoking during the perinatal period has been shown to be associated with a higher risk of ADHD in the progeny (Linnet et al., 2005); however, we did not control for parental smoking during pregnancy and perinatal period in the analysis. Finally, only 3,418 questionnaires were effectively completed with respect to events related to pregnancy and the perinatal period; the lower response rate may have introduced an element of selection bias.
Conclusion
In this study, TA, NA, instrumental delivery, and LBW were found to be associated with an increased risk of ADHD in the total study population or in subgroups of subjects with ADHD disaggregated by comorbidity and gender. Among these, TA was found to increase the risk of ADHD without other comorbid mental disorders. Instrumental delivery and NA were found to increase the risk of ADHD with other comorbid mental disorders. TA and NA were found to be risk factors for ADHD among males, but not among females. LBW was a risk factor for ADHD among females.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee at the Second Xiangya Hospital. All parents or guardians and students in this study gave written informed consent.
Author Contributions
JBL was responsible for data processing and writing of the manuscript. YQH participated in the investigation and writing of the manuscript. YMS participated in the design, investigation, and evaluation of the study. YYZ participated in data collection and analysis. TTM participated in the investigation and evaluation of the study. BX and XLC participated in the investigation and evaluation of the study. YMF participated in data processing. XRL participated in the design, investigation, and evaluation of the study and contributed to critical revision. YTX contributed to critical revision. JPL contributed to critical revision and evaluation of the study. All authors have read and approved the manuscript.
Funding
This study was supported by the National Key Research and Development Program of China (No. 2016YFC1306204), Shenzhen Double Chain [No. (2018)256], and Sanming Project of Medicine in Shenzhen (SZSM201612079).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to acknowledge all respondents who participated in the study and the research assistants who had contributed to the recruitment process and data collection.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyg.2021.707500/full#supplementary-material
References
Akutagavamartins, G. C., Salatinooliveira, A., Kieling, C., Genro, J. P., Polanczyk, G. V., Anselmi, L., et al. (2016). COMT and DAT1 genes are associated with hyperactivity and inattention traits in the 1993 pelotas birth cohort: evidence of sex-specific combined effect. J. Psychiatry Neurosci. 41:150270. doi: 10.1503/jpn.150270
Amiri, S., Sadeghi-Bazargani, H., Nazari, S., Ranjbar, F., and Abdi, S. (2017). Attention deficit/hyperactivity disorder and risk of injuries: a systematic review and meta-analysis. J. Inj. Violence Res. 9, 95–105. doi: 10.5249/jivr.v9i2.858
Arijit, K., Subhamita, M., Barnali, C., Deepak, V., Swagata, S., Mohanakumar, K. P., et al. (2016). Monoamine oxidase B gene variants associated with attention deficit hyperactivity disorder in the Indo-Caucasoid population from West Bengal. BMC Genet. 17:92. doi: 10.1186/s12863-016-0401-6
Basama, F. M. S., and Crosfill, F. (2004). The outcome of pregnancies in 182 women with threatened miscarriage. Arch. Gynecol. Obstet. 270:86. doi: 10.1007/s00404-003-0475-z
Bilgiç, A., Türkoğlu, S., Özcan, Ö, Tufan, A. E., Yılmaz, S., and Yüksel, T. (2013). Relationship between anxiety, anxiety sensitivity and conduct disorder symptoms in children and adolescents with attention-deficit/hyperactivity disorder (ADHD). Eur. Child Adolesc. Psychiatry 22, 523–532. doi: 10.1007/s00787-013-0392-z
Buschdorf, J. P., Ong, M. L., Ong, S. X., Macisaac, J. L., Chng, K., Kobor, M. S., et al. (2016). Low birth weight associates with hippocampal gene expression. Neuroscience 318, 190–205. doi: 10.1016/j.neuroscience.2016.01.013
Capri, T., Santoddi, E., and Fabio, R. A. (2020). Multi-source interference task paradigm to enhance automatic and controlled processes in ADHD. Res. Dev. Disabil. 97:103542. doi: 10.1016/j.ridd.2019.103542
Choi, Y., Shin, J., Cho, K. H., and Park, E. C. (2017). Change in household income and risk for attention deficit hyperactivity disorder during childhood: a nationwide population-based cohort study. J. Epidemiol. 27, 56–62. doi: 10.1016/j.je.2016.09.004
Curran, E. A., O’Neill, S. M., Cryan, J. F., Kenny, L. C., Dinan, T. G., Khashan, A. S., et al. (2015). Research review: birth by caesarean section and development of autism spectrum disorder and attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. J. Child Psychol. Psychiatry 56:500. doi: 10.1111/jcpp.12351
Dadds, M. R., Schollar-Root, O., Lenroot, R., Moul, C., and Hawes, D. J. (2016). Epigenetic regulation of the DRD4 gene and dimensions of attention-deficit/hyperactivity disorder in children. Eur. Child Adolesc. Psychiatry 25, 1081–1089. doi: 10.1007/s00787-016-0828-3
Dongol, A., Mool, S., and Tiwari, P. (2011). Outcome of pregnancy complicated by threatened abortion. Kathmandu Univ. Med. J. 9:41. doi: 10.3126/kumj.v9i1.6261
Doshi, J. A., Hodgkins, P., Kahle, J., Sikirica, V., Cangelosi, M. J., Setyawan, J., et al. (2012). Economic impact of childhood and adult attention-deficit/hyperactivity disorder in the United States. J. Am. Acad. Child Adolesc. Psychiatry 51, 990–1002. doi: 10.1016/j.jaac.2012.07.008
Doumouchtsis, S. K., and Arulkumaran, S. (2006). Head injuries after instrumental vaginal deliveries. Curr. Opin. Obstet. Gynecol. 18, 129–134. doi: 10.1097/01.gco.0000192983.76976.68
Duan, G., Yao, M., Ma, Y., and Zhang, W. (2014). Perinatal and background risk factors for childhood autism in central China. Psychiatry Res. 220, 410–417. doi: 10.1016/j.psychres.2014.05.057
Fabio, R. A., Bianco, M., Caprì, T., Marino, F., Ruta, L., Vagni, D., et al. (2020). Working memory and decision making in children with ADHD: an analysis of delay discounting with the use of the dual-task paradigm. BMC Psychiatry 20:272. doi: 10.1186/s12888-020-02677-y
Franz, A. P., Bolat, G. U., Bolat, H., Matijasevich, A., Santos, I. S., Silveira, R. C., et al. (2018). Attention-deficit/hyperactivity disorder and very preterm/very low birth weight: a meta-analysis. Pediatrics 141:e20171645. doi: 10.1542/peds.2017-1645
Getahun, D., Rhoads, G. G., Demissie, K., Lu, S. E., Quinn, V. P., Fassett, M. J., et al. (2013). In utero exposure to ischemic-hypoxic conditions and attention-deficit/hyperactivity disorder. Pediatrics 131:e53. doi: 10.1542/peds.2012-1298
Ginsberg, Y., D’Onofrio, B., Rickert, M., Class, Q., Rosenqvist, M., Almqvist, C., et al. (2018). Maternal infection requiring hospitalization during pregnancy and attention-deficit hyperactivity disorder in offspring: a quasi-experimental family-based study. J. Child Psychol. Psychiatry 60, 160–168. doi: 10.1111/jcpp.12959
Gokcen, C., Kocak, N., and Pekgor, A. (2011). Methylenetetrahydrofolate reductase gene polymorphisms in children with attention deficit hyperactivity disorder. Int. J. Med. Sci. 8, 523–528. doi: 10.7150/ijms.8.523
Greene, M. F. (2019). Progesterone for threatened abortion. N. Engl. J. Med. 380, 1867–1868. doi: 10.1056/NEJMe1903069
Grothe, M. J., Scheef, L., Bäuml, J., Meng, C., Daamen, M., Baumann, N., et al. (2016). Reduced cholinergic basal forebrain integrity links neonatal complications and adult cognitive deficits after premature birth. Biol. Psychiatry 82, 119–126. doi: 10.1016/j.biopsych.2016.12.008
Hanć, T., Słopień, A., Wolańczyk, T., Dmitrzak-Wêglarz, M., Szwed, A., Czapla, Z., et al. (2015). ADHD and overweight in boys: cross-sectional study with birth weight as a controlled factor. Eur. Child Adolesc. Psychiatry 24, 41–53. doi: 10.1007/s00787-014-0531-1
He, Y., Chen, J., Zhu, L., Hua, L., and Ke, F. (2017). Maternal smoking during pregnancy and ADHD: results from a systematic review and meta-analysis of prospective cohort studies. J. Atten. Disord. 24, 1637–1647. doi: 10.1177/1087054717696766
Howe, D. (2010). ADHD and its comorbidity: an example of gene–environment interaction and its implications for child and family social work. Child Fam. Soc. Work 15, 265–275. doi: 10.1111/j.1365-2206.2009.00666.x
Kaewsuk, S., Tannenberg, R. K., Kuo, S. W., Björkman, S. T., Govitrapong, P., Stadlin, A., et al. (2009). Regional expression of dopamine D1 and D2 receptor proteins in the cerebral cortex of asphyxic newborn infants. J. Child Neurol. 24, 183–193. doi: 10.1177/0883073808322669
Lai, S. R., Ling, S. U., and Wen, J. U. (2013). The factorial structure study on parental rearing scale(EMBU)in adolescents. Strait J. Prev. Med. 19, 13–15.
Laugesen, K., Byrjalsen, A., Frøslev, T., Olsen, M. S., and Sørensen, H. T. (2017). Use of glucocorticoids during pregnancy and risk of attention-deficit/hyperactivity disorder in offspring: a nationwide Danish cohort study. BMJ Open 7:e016825. doi: 10.1136/bmjopen-2017-016825
Li, J., Olsen, J., Vestergaard, M., and Obel, C. (2011). Low apgar scores and risk of childhood attention deficit hyperactivity disorder. J. Pediatr. 158:775. doi: 10.1016/j.jpeds.2010.10.041
Linnet, K. M., Wisborg, K. C., Secher, N. J., Thomsen, P. H., Agerbo, E., and Henriksen, T. B. (2005). Smoking during pregnancy and the risk for hyperkinetic disorder in offspring. Pediatrics 116, 462–467. doi: 10.1542/peds.2004-2054
Liu, Y. X., Liu, J., and Wang, Y. (2010). Reliability and validity of Chinese version of the Mini International Neuropsychiatric Interview for Children and Adolescents(Parent Version). Chin. Ment. Health J. 24, 921–925. doi: 10.3969/j.issn.1000-6729.2010.12.009
Liu, Y. X., Liu, J., and Wang, Y. (2011). Reliability and validity of Chinese version of the Mini International Neuropsychiatric Interview for Children and Adolescents(Child Version). Chin. Ment. Health J. 25, 8–13. doi: 10.3969/j.issn.1000-6729.2011.01.003
Martin, A. (2014). The role of ADHD in academic adversity: disentangling ADHD effects from other personal and contextual factors. Sch. Psychol. Q. 29, 395–408.
Mick, E., Biederman, J., Prince, J., Fischer, M. J., and Faraone, S. V. (2002). Impact of low birth weight on attention-deficit hyperactivity disorder. J. Dev. Behav. Pediatr. 23, 16–22. doi: 10.1097/00004703-200202000-00004
Miguel, C., Martins, P., Moleda, N., Klein, M., Chaim-Avancini, T., Gobbo, M., et al. (2016). Cognition and impulsivity in adults with attention deficit hyperactivity disorder with and without cocaine and/or crack dependence. Drug Alcohol Depend. 160, 97–104. doi: 10.1016/j.drugalcdep.2015.12.040
Mimounibloch, A., Kachevanskaya, A., Mimouni, F. B., Shuper, A., Raveh, E., and Linder, N. (2013). Breastfeeding may protect from developing attention-deficit/hyperactivity disorder. Breastfeed. Med. 8, 363–367. doi: 10.1089/bfm.2012.0145
Morales, E., Julvez, J., Torrent, M., Ballester, F., Rodrã-Guez-Bernal, C. L., Andiarena, A., et al. (2015). Vitamin D in pregnancy and attention deficit hyperactivity disorder-like symptoms in childhood. Epidemiology 26, 458–465. doi: 10.1097/EDE.0000000000000292
Mulligan, A., Anney, R., Butler, L., O’Regan, M., Richardson, T., Tulewicz, E. M., et al. (2013). Home environment: association with hyperactivity/impulsivity in children with ADHD and their non-ADHD siblings. Child Care Health Dev. 39, 202–212. doi: 10.1111/j.1365-2214.2011.01345.x
Niwa, T., Suzuki, K., Sugiyama, N., and Imai, Y. (2017). Regional volumetric assessment of the brain in moderately preterm infants (30-35 gestational weeks) scanned at term-equivalent age on magnetic resonance imaging. Early Hum. Dev. 111:36. doi: 10.1016/j.earlhumdev.2017.05.009
Nyman, E., Ogdie, M. N., Loukola, A., Varilo, T., Taanila, A., Hurtig, T., et al. (2007). ADHD candidate gene study in a population-based birth cohort: association with DBH and DRD2. J. Am. Acad. Child Adolesc. Psychiatry 46, 1614–1621. doi: 10.1097/chi.0b013e3181579682
O’Callaghan, M. J., and Harvey, J. M. (1997). Biological predictors and co-morbidity of attention deficit and hyperactivity disorder in extremely low birth weight infants at school. J. Paediatr. Child Health 33, 491–496. doi: 10.1111/j.1440-1754.1997.tb01657.x
Oudin, A., Frondelius, K., Haglund, N., Källén, K., Forsberg, B., Gustafsson, P., et al. (2019). Prenatal exposure to air pollution as a potential risk factor for autism and ADHD. Environ. Int. 133(Pt A):105149. doi: 10.1016/j.envint.2019.105149
Pagida, M. A., Konstantinidou, A. E., Tsekoura, E., Mangoura, D., Patsouris, E., and Panayotacopoulou, M. T. (2013). Vulnerability of the mesencephalic dopaminergic neurons of the human neonate to prolonged perinatal hypoxia: an immunohistochemical study of tyrosine hydroxylase expression in autopsy material. J. Neuropathol. Exp. Neurol. 72, 337–350. doi: 10.1097/NEN.0b013e31828b48b3
Park, S., Park, J. E., Yoo, H. J., Kim, J. W., Cheong, J. H., Han, D. H., et al. (2015). Association of the catechol O-Methyltransferase Val158-met polymorphism and reduced interference control in korean children with attention-deficit hyperactivity disorder. Psychiatry Investig. 12, 563–565. doi: 10.4306/pi.2015.12.4.563
Pohlabeln, H., Rach, S., Henauw, S. D., Eiben, G., Gwozdz, W., Hadjigeorgiou, C., et al. (2017). Further evidence for the role of pregnancy-induced hypertension and other early life influences in the development of ADHD: results from the IDEFICS study. Eur. Child Adolesc. Psychiatry 26, 957–967. doi: 10.1007/s00787-017-0966-2
Reale, L., Bartoli, B., Cartabia, M., Zanetti, M., Costantino, M., Canevini, M., et al. (2017). Comorbidity prevalence and treatment outcome in children and adolescents with ADHD. Eur. Child Adolesc. Psychiatry 26, 1443–1457. doi: 10.1007/s00787-017-1005-z
Rennie, J., Hagmann, C., and Robertson, N. (2007). Outcome after intrapartum hypoxic ischaemia at term. Semin. Fetal Neonatal Med. 12, 398–407. doi: 10.1016/j.siny.2007.07.006
Rodriguez, A., and Bohlin, G. (2005). Are maternal smoking and stress during pregnancy related to ADHD symptoms in children? J. Child Psychol. Psychiatry 46, 246–254. doi: 10.1111/j.1469-7610.2004.00359.x
Ronald, A., Pennell, C. E., and Whitehouse, A. J. (2010). Prenatal maternal stress associated with ADHD and autistic traits in early childhood. Front. Psychol. 1:223. doi: 10.3389/fpsyg.2010.00223
Sauver, J. L. S., Barbaresi, W. J., Katusic, S. K., Colligan, R. C., Weaver, A. L., and Jacobsen, S. J. (2004). Early life risk factors for attention-deficit/hyperactivity disorder: a population-based cohort study. Mayo Clin. Proc. 79, 1124–1131.
Say, G. N., Babadağı, Z., and Karabekiroðlu, K. (2015). Breastfeeding history in children with autism and attention deficit hyperactivity disorder. Breastfeed. Med. 10:283. doi: 10.1089/bfm.2015.0033
Shen, Y., Chan, B., Liu, J., Zhou, Y., Cui, X., He, Y., et al. (2018a). The prevalence of psychiatric disorders among students aged 6~16 years old in central Hunan, China. BMC Psychiatry 18:243. doi: 10.1186/s12888-018-1823-7
Shen, Y., Chan, B. S. M., Liu, J., Meng, F., Yang, T., He, Y., et al. (2018b). Estimated prevalence and associated risk factors of attention deficit hyperactivity disorder (ADHD) among medical college students in a Chinese population. J. Affect. Disord. 241, 291–296. doi: 10.1016/j.jad.2018.08.038
Silva, D., Colvin, L., Hagemann, E., and Bower, C. (2014). Environmental risk factors by gender associated with attention-deficit/hyperactivity disorder. Pediatrics 133, 14–22. doi: 10.1542/peds.2013-1434
Su, L., and Li, X. (1996). The norms of achenbach child behavior checklist in hunan province. Chin. J. Clin. Psychol. 4, 24–28.
Tapia-Bustos, A., Perez-Lobos, R., Vío, V., Lespay-Rebolledo, C., Palacios, E., Chiti-Morales, A., et al. (2016). Modulation of postnatal neurogenesis by perinatal asphyxia: effect of D1 and D2 dopamine receptor agonists. Neurotox. Res. 31, 109–121. doi: 10.1007/s12640-016-9669-6
Tully, L. A., Arseneault, L., Caspi, A., Moffitt, T. E., and Morgan, J. (2004). Does maternal warmth moderate the effects of birth weight on twins’ attention-deficit/hyperactivity disorder (ADHD) symptoms and low IQ? J Consult. Clin. Psychol. 72, 218–226. doi: 10.1037/0022-006X.72.2.218
van Wingen, G., van den Brink, W., Veltman, D., Schmaal, L., Dom, G., Booij, J., et al. (2013). Reduced striatal brain volumes in non-medicated adult ADHD patients with comorbid cocaine dependence. Drug Alcohol Depend. 131, 198–203. doi: 10.1016/j.drugalcdep.2013.05.007
Vizzini, L., Popovic, M., Zugna, D., Vitiello, B., Trevisan, M., Pizzi, C., et al. (2018). Maternal anxiety, depression and sleep disorders before and during pregnancy, and preschool ADHD symptoms in the NINFEA birth cohort study. Epidemiol. Psychiatr. Sci. 28, 521–531. doi: 10.1017/S2045796018000185
Wang, C., Geng, H., Liu, W., and Zhang, G. J. M. (2017). Prenatal, perinatal, and postnatal factors associated with autism: a meta-analysis. Medicine (Baltimore) 96:e6696. doi: 10.1097/MD.0000000000006696
Weiss, J. L., Malone, F. D., Vidaver, J., Ball, R. H., Nyberg, D. A., Comstock, C. H., et al. (2004). Threatened abortion: a risk factor for poor pregnancy outcome, a population-based screening study. Am. J. Obstet. Gynecol. 190, 745–750. doi: 10.1016/j.ajog.2003.09.023
Willcutt, E. G. (2012). The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics 9, 490–499. doi: 10.1007/s13311-012-0135-8
Wu, H., Zhu, P., Geng, X., Liu, Z., Cui, L., Gao, Z., et al. (2017). Genetic polymorphism of MTHFR C677T with preterm birth and low birth weight susceptibility: a meta-analysis. Arch. Gynecol. Obstet. 295, 1105–1118. doi: 10.1007/s00404-017-4322-z
Yuan, F. F., Xue, G., Xin, H., Yan, Z., and Jing, W. (2017). SLC6A1 gene involvement in susceptibility to attention-deficit/hyperactivity disorder: a case-control study and gene-environment interaction. Prog. Neuropsychopharmacol. Biol. Psychiatry 77, 202–208. doi: 10.1016/j.pnpbp.2017.04.015
Keywords: ADHD, threatened abortion, low birth weight, neonatal asphyxia, instrumental delivery
Citation: Liu J, He Y, Shen Y, Zhou Y, Meng T, Xiao B, Cui X, Fang Y, Lu J, Xiang Y-T and Luo X (2021) Association of Attention Deficit/Hyperactivity Disorder With Events Occurring During Pregnancy and Perinatal Period. Front. Psychol. 12:707500. doi: 10.3389/fpsyg.2021.707500
Received: 10 May 2021; Accepted: 25 August 2021;
Published: 21 September 2021.
Edited by:
Gian Marco Marzocchi, University of Milano-Bicocca, ItalyReviewed by:
Tindara Caprì, Institute for Biomedical Research and Innovation, National Research Council, Consiglio Nazionale delle Ricerche, ItalySeyed Mohammad Mahdi Moshirian Farahi, Carleton University, Canada
Copyright © 2021 Liu, He, Shen, Zhou, Meng, Xiao, Cui, Fang, Lu, Xiang and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuerong Luo, bHVveHVlcm9uZ0Bjc3UuZWR1LmNu; Jianping Lu, c3psdWppYW5waW5nQDEyNi5jb20=
†These authors share first authorship
 Jianbo Liu1†
Jianbo Liu1† Yuqiong He
Yuqiong He Yanmei Shen
Yanmei Shen Xilong Cui
Xilong Cui Yu-Tao Xiang
Yu-Tao Xiang Xuerong Luo
Xuerong Luo