- 1Department of Social Work and Social Administration, The University of Hong Kong, Hong Kong, China
- 2Reproductive Medicine Center, The University of Hong Kong-Shenzhen Hospital, Shenzhen, China
- 3Kids Caring Corner, The University of Hong Kong-Shenzhen Hospital, Shenzhen, China
- 4Centre on Behavioral Health, The University of Hong Kong, Hong Kong, China
Women with polycystic ovary syndrome (PCOS) suffer significant psychological distress, which may activate the hypothalamus-pituitary-ovary axis and further affect their physiological state. They often experience elevated levels of testosterone and triglycerides. Considering reports of psychological distress among women with PCOS, this study aimed to develop a psychosocial intervention to improve their emotional and physical health, particularly in Chinese society. This pilot study employed the Integrative Body-Mind-Spirit (I-BMS) intervention model for women with PCOS in China. After a 2 h health information session, 18 participants were randomly assigned to the I-BMS group (9) or the control group (9). The intervention group received 6 weekly, 3 h I-BMS sessions. Pre- and post-blood tests and psychosocial questionnaires were collected from all participants. Retention to treatment was high with 79.6% treatment adherence gained and an overall average of five sessions completed. Compared with the control group, depression and anxiety symptoms reduced significantly for those in the intervention group (d = −1.24, p < 0.05 and d = –1.33, p < 0.01), their health-related quality of life improved significantly (d = 1.02, p < 0.01) both at post-intervention and 3 month follow-up, and their testosterone and triglycerides levels reduced significantly (d = −0.97, p < 0.001 and d = –0.41, p < 0.05) after joining the intervention. The I-BMS model is feasible and appears promising in improving psychological health, and reducing testosterone and triglyceride levels, in women with PCOS in China.
Clinical Trial Registration: www.chictr.org.cn, identifier ChiCTR1900027606.
Introduction
Polycystic ovary syndrome (PCOS) is the leading cause of anovulatory infertility, and the most common endocrine disease affecting reproductive-aged women (Balen et al., 2016). The prevalence of PCOS is 10–20% internationally (Naz et al., 2019). Its main clinical manifestations include menstrual irregularity, infertility, obesity, hirsutism, and acne (McCook et al., 2015). The Rotterdam criteria for diagnosing PCOS requires at least two of the three key features to be present: (a) polycystic ovaries on ultrasound; (b) high androgen levels/clinical hyperandrogenism; and (c) menstrual irregularity (Rotterdam Group, 2004). Between 55 and 70% of women suffering from PCOS have elevated testosterone levels compared to healthy women (Huang et al., 2010). Besides sex hormone imbalance, PCOS has been associated with other metabolic complications, such as dyslipidemia, insulin resistance, type II diabetes, and some cardiovascular diseases (Romanowski et al., 2015; Kyrou et al., 2020). PCOS sufferers have been reported as having elevated total cholesterol, triglycerides (TG) and low-density lipoprotein-cholesterol (LDL-C) levels, as well as decreased high density lipoprotein-cholesterol (HDL-C) (Castelo-Branco et al., 2010; Bilal et al., 2018). The etiology of PCOS remains unknown, and there is no current cure (Barthelmess and Naz, 2014).
As PCOS often leads to metabolic and appearance changes, the mental health and mood states of women with PCOS can be affected (Barry, 2019). Barry et al. (2018) described the pathways of testosterone leading to psychological distress, including direct and indirect effects through the unwelcome manifestations of PCOS. The association between dyslipidemia (high total cholesterol, LDL-C or TG, or low HDL-C) and anxiety and depression has also been well presented (Van Reedt Dortland et al., 2013). It has been reported that women with PCOS tend to be more emotionally labile than women who do not suffer from PCOS, and they are more likely to express feelings of anger (Barry et al., 2011a). Research also indicates that PCOS sufferers can experience a range of mental health problems. Stapinska-Syniec et al. (2018) found that 52% of 250 PCOS participants reported depressive symptoms. Significantly more women suffering with PCOS presented with severe anxiety, trait anger, and depression, compared with age-matched healthy women (Erdem et al., 2013). Mood disorders among PCOS sufferers may enhance their disease burden, as those suffering from depression and anxiety may also present with significantly higher dehydroepiandrosterone sulfate (DHEAS) levels (Annagür et al., 2013). Hyperandrogenemia and dyslipidemia in PCOS sufferers have been correlated with anxiety and depression, leading to poor health-related quality of life (HRQoL) (Dokras et al., 2016; Gul et al., 2018).
Chinese women with PCOS have recently been reported as having higher anxiety and depression than healthy women (Yin et al., 2021). This may be because they are distressed by their appearance, or the impact that PCOS may have on their fertility, which hampers their desire to fulfil their filial obligations of continuing the family name in Chinese culture (Yao et al., 2018). To date, there is no known research into psychosocial interventions focusing on holistic mental and physical health for women with PCOS in China.
There is variable evidence of the effectiveness of psychological interventions on physical and mental health of PCOS sufferers. Cooney et al. (2018) conducted a 16-week pilot randomized clinical trial to examine the effect of cognitive-behavioral therapy (CBT) on the mental health of women with PCOS. No significant biometric or metabolic changes were noted at either the week 8 or week 16 assessments. Abdollahi et al. (2018) also used CBT to improve depression among women with PCOS, reporting only slight improvement. Besides CBT, yoga, aerobic training and exercise have also been tested for the care of women with PCOS. The study by Nidhi et al. (2012, 2013a, 2013b) recruited 90 adolescent girls with PCOS, who received a 12-week daily intervention program based on holistic yoga and conventional exercise. Glucose, lipid, insulin values and HRQoL significantly improved after the yoga intervention.
The effectiveness of these interventions may be inconclusive because women with PCOS suffer from complex biological, psychosocial and spiritual pain, and no one intervention was likely to address all these elements. Moreover, although hyperandrogenemia and dyslipidemia have been associated with anxiety, depression and HRQoL, few interventions have targeted all five of these outcome areas. Thus, it is persuasive to employ an integrated method that enhances holistic health and wellbeing, and where intervention effectiveness is tested over a range of parameters.
The Integrative Body-Mind-Spirit (I-BMS) intervention model (Chan et al., 2000) is one such method. It combines Eastern philosophies and therapeutic techniques from Chinese medicine to foster a self-empowerment practice for clients experiencing various social, health and mental health issues. The I-BMS model aims to promote wellbeing of an individual: mind, body and soul. It integrates physical exercises of Tai Chi (also known as taiji), qigong, yoga…., and Eastern philosophic meditations on mindfulness, non-attachment, compassion, forgiveness, gratitude … (Lee et al., 2018). The I-BMS method has been tested on different population groups (e.g., breast cancer sufferers (Liu et al., 2008), women undergoing in vitro fertilization (Chan et al., 2012), and in different countries, such as India and Taiwan (Liu et al., 2008; Hsiao et al., 2012; Sreevani et al., 2013). It has consistently produced significant improvements in well-being and holistic health.
The I-BMS model has been adopted as empowerment training for women with PCOS in China, and this pilot study explored its feasibility, and preliminary effects on holistic health. The study aimed to: (1) reduce psychological distress, and improve holistic well-being; and (2) suppress testosterone and dyslipidemia by improving mental health.
Methods
Study Design
This was a pilot randomized controlled trial (RCT) examining the effects of the I-BMS intervention on physical and mental health of women with PCOS. A 2 (group: intervention vs. control) × 3 [time: baseline/pre-intervention (T0), post-intervention (T1), 3-month post-intervention (T2)] design was employed. This trial was registered at www.chictr.org.cn (registration number ChiCTR1900027606). The intervention was provided free of charge and participants were compensated for pre/post intervention blood tests.
Participants
PCOS sufferers were recruited at a Hospital located in Shenzhen, across the border from Hong Kong. Prior to sampling, we distributed an anonymous survey to all women attending the PCOS clinic and then invited selected participants to join the study voluntarily with written informed consent. The diagnosis of PCOS was based on the Rotterdam criteria by the medical team. Women suffering from PCOS who were pre-menopausal, aged 18–35 years old, could understand oral and written Chinese and be physically capable of simple body movements, were recruited by doctors’ referrals. Participants who were eligible and willing to participate provided written informed consent before entering the study. Women who initially agreed but later refused to undergo a full assessment were considered to be dropouts.
Women were excluded if they: (1) had taken oral contraceptives/metformin/insulin-sensitizing treatment, or received pharmacotherapy for dyslipidemia, hypertension, or diabetes/impaired glucose tolerance, or hormonal therapy in the past 4 weeks; (2) were currently suffering from other genetic or endocrine diseases, or neuropsychiatric disorders that require psychotropic substances (such as antipsychotics, antidepressants, or anticonvulsants); (3) were pregnant or who had recently given birth; (4) had participated in similar intervention programs in the past 3 months, or were currently on other exercise training programs or weight loss programs; (5) were smoking on average five or more cigarettes per day, line with the proposed association between nicotine and cortisol levels (Cohen et al., 2004).
Procedures
This pilot RCT was conducted over 10 months, from September 2019 to June 2020. After doctors’ referrals, potentially relevant individuals joined a pre-group face-to-face interview with the researchers. People’s needs and expectations were collected, and exclusion criteria were applied. Most participants were not familiar with the cardiovascular risks of PCOS. Thus, according to the interviews, a health education information session was designed and the intervention protocol was slightly modified.
All eligible participants were then invited to join a 2 h health education information session. Basic knowledge of PCOS (including manifestations, prevalence and cardiovascular risks) and usual medical treatments were introduced, and brief advice on daily diet and exercise were provided. After the session, all consenting eligible participants were randomly assigned to the intervention or the control group in a 1:1 ratio, by drawing lots. Intervention group participants then joined the I-BMS model program. Control group received no interventions apart from the health education information session.
Interventions
The intervention consisted of 6 weekly I-BMS sessions which were held on Saturdays at the group counseling center of the hospital. Each session lasted for 3 h. During each session, activities on promoting mind, body, and spiritual wellbeing were shared. The first session was focused on the introduction on mind-body relationship. As PCOS sufferers were concerned about their physical appearance as obtained from the pre-group interviews, the second session tried to help participants to recognize their self-stigma and feelings of shame about their own body. I-BMS methods for self-acceptance and self-compassion were practiced in the third and fourth sessions. The final two sessions facilitated participants to transcend the meaning of the disease, and transform challenges to opportunities. Detailed content of the intervention is provided in Table 1.

Table 1. Session content of the Integrative Body-Mind-Spirit (I-BMS) group intervention for women with Polycystic Ovary Syndrome (PCOS).
Outcome Measures
All participants provided objective blood tests at T0 and T1, and subjective outcome measures at T0-T2. Besides, feasibility and acceptability were also measured accordingly.
Overnight fasting, morning blood samples were obtained to determine fasting lipid profile (total cholesterol, LDL-C, HDL-C, and TG) and testosterone concentrations. The ratio of TG/HDL-C was also calculated as it may indicate the risk of coronary heart risk (Hadaegh et al., 2009).
Anxiety was measured by the 21-item self-report Beck Anxiety Inventory (BAI). BAI can discriminate anxiety from depression. Each item ranges from 0 to 3; higher scores indicate higher levels of anxiety. The Chinese version of BAI has been validated with excellent reliability and validity (Cheng et al., 2002).
Depression was measured by the 21-item self-report Beck Depression Inventory-II (BDI-II) (Beck et al., 1996). Each item ranges from 0 to 3; high scores correspond to high levels of depression. The Chinese version of BDI-II has been widely adopted with good reliability and validity (Dai and Feng, 2008).
Health-related quality of life (HRQoL) of PCOS sufferers was measured by the Polycystic Ovary Syndrome Questionnaire (PCOSQ). PCOSQ has 26 items, each completed using a 7-point Likert scale. The questionnaire contains five dimensions relating to manifestations of PCOS: hirsutism, obesity, infertility, menstrual irregularity, and emotions. Higher scores indicate better function and less negative influence on daily life. The Chinese version of PCOSQ has been validated demonstrating good reliability and validity (Li et al., 2015).
Feasibility was assessed by testing whether the whole intervention sessions could be completed. If any session could not be completed, the lessons would be summarized, and data analysis would not continue. Besides, screening and enrollment rate, session attendance rate, as well as reasons for absences were recoded.
Acceptability were measured by participants’ post-intervention feedback. After the last session of the intervention, every participant in the intervention group was interviewed for 10–15 min. All participants were asked: (1) did you benefit from the intervention? If yes, how much (from 1 “a few” to 10 “a lot”); (2) which part of the intervention did you remember most? Based on these two questions, participants’ feelings and feedback were collected. Acceptability was also evaluated by any complaints reported to the ethics committee and the dropout rate of the intervention group.
Data Analyses
Demographics and baseline outcome measures were compared between the intervention and control groups to test for homogeneity (to confirm appropriate randomization). Independent samples t-tests or χ2-tests were used.
Following intention-to-treat principles, all participants who provided baseline information were included in data analysis, including those who had not adhered to the allocated intervention and those lost to follow-up. Per protocol analysis was also conducted to provide more details. Paired-sample t-tests were used to determine within-group effects (T0 vs. T1 and T0 vs. T2). Repeated measures univariate analysis of variance models (ANOVA) (including over-time × group interactions) were constructed to test for between-group differences. The within-group effect size (ES) was calculated by Cohen’s d (Cohen, 1988); where values of 0.2, 0.5, and 0.8 were applied to determine small, medium, and large effect sizes, respectively. The between-group ES was calculated by partial eta square (η2), which ranges from 0 to 1, with the values of 0.01 being small, 0.08 being medium, and 0.14 being large (Moher et al., 2001).
Pearson’s correlation tests were applied to estimate the association between mental and physical health measures. All data analyses were performed by SPSS 24.0.
Ethical Considerations
This study was approved by the Medical Research Ethics Committee of the HKU-Shenzhen Hospital (Ethic [2019]167). Personal information was confidential, including names of doctors and nurses in the hospital.
Results
Sample
The sampling process is shown in Figure 1. Forty-nine PCOS sufferers were initially screened for eligibility at recruitment, with 18 participants being recruited and randomized: nine into the intervention group and nine into the control group.
Sample Descriptors and Homogeneity
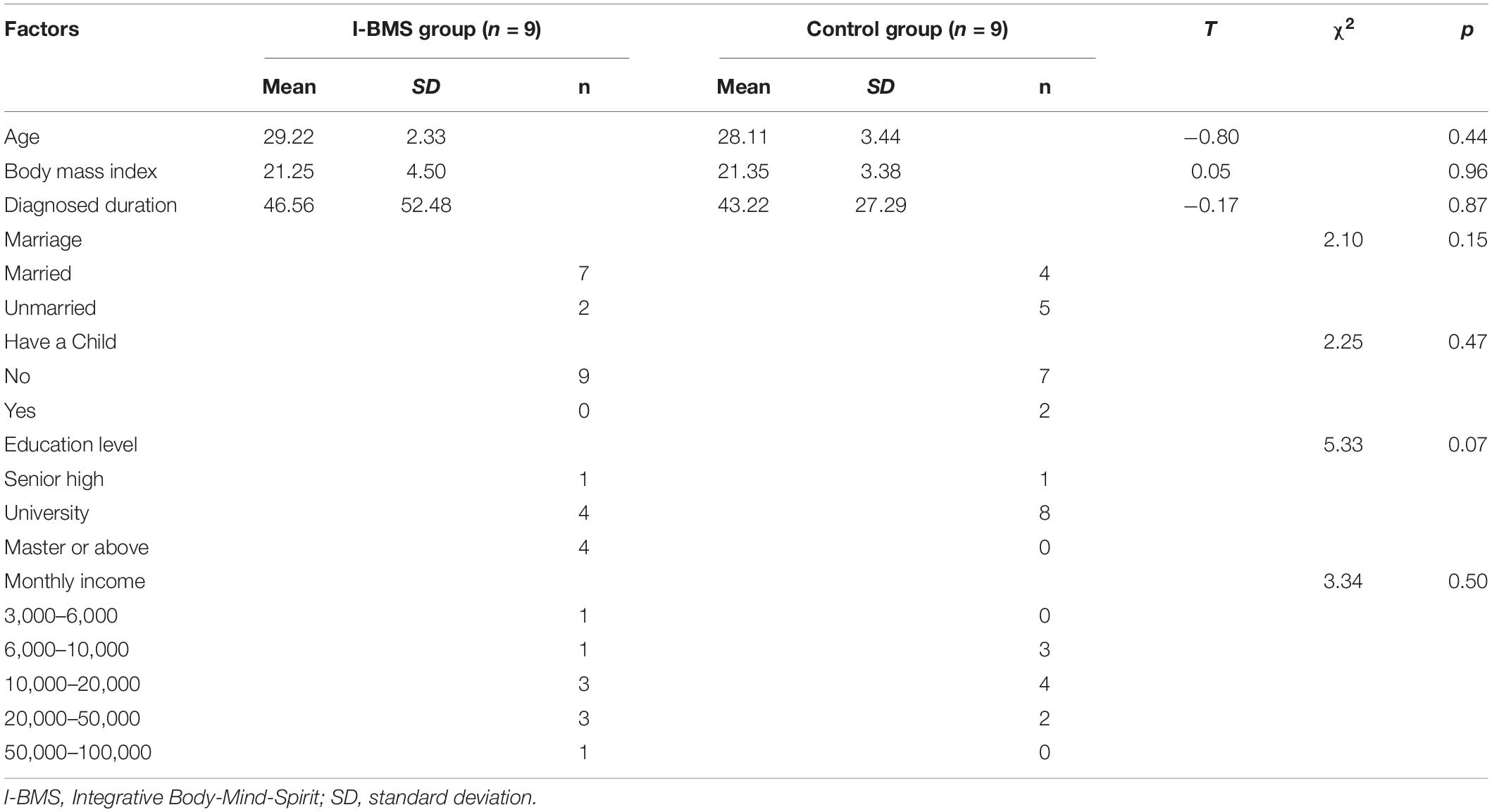
Socioeconomic characteristics of intervention and control groups are reported in Table 2. Groups did not differ in age, Body Mass Index (BMI) or diagnosis duration (p > 0.05). There was no significant difference in marriage, whether have a child or not, education level and monthly income between the two groups. No statistically significant differences were found between intervention and control groups in any mental and physical health factor at T0 (p > 0.05).
Feasibility
36.7% of the people screened were finally enrolled in the study. The whole six I-BMS intervention sessions were successfully conducted which lasted 175–218 min. Participants in the intervention group attended an average five of six sessions. The overall attendance rate was 79.6%. The reasons for being absent were time conflict with an urgent matter (72.7%) and other reasons (27.3%).
Acceptability
All participants reported they benefit a lot (seven participants rated 10, one rated 8) from the intervention and they all (100%) thought the intervention was very helpful and should be carried out to more PCOS patients. Six participants thought their internal stress got released. Five participants reflected that they felt more peace and calm after each session. Four participants noticed their mood problems more clearly and learned the I-BMS method to handle them. Two participants described their attitude change toward the program after each session and were grateful to learn about self-acceptance and an optimistic attitude toward the disease and the whole life. No complaints were received to the whole intervention and study. One participant in the intervention group stated her dropout at the end of the second session because of having a busy schedule and refused to take post-intervention measurement. Two participants in the control group lost connection in the post-test. The dropout rate was 11.1% for the intervention group and 22.2% for the control group.
Treatment Effects
Nine subjects in the intervention group and nine subjects in the control group were included in the intention-to-treat analysis (Tables 3, 4). Eight subjects in the intervention group and seven subjects in the control group were included in the per protocol analysis (Tables 4, 5). Results of intention-to-treat analysis and per protocol analysis were generally similar, but per protocol analysis provided a larger effect size. For brevity, only intention-to-treat analysis results were discussed in the following contents.
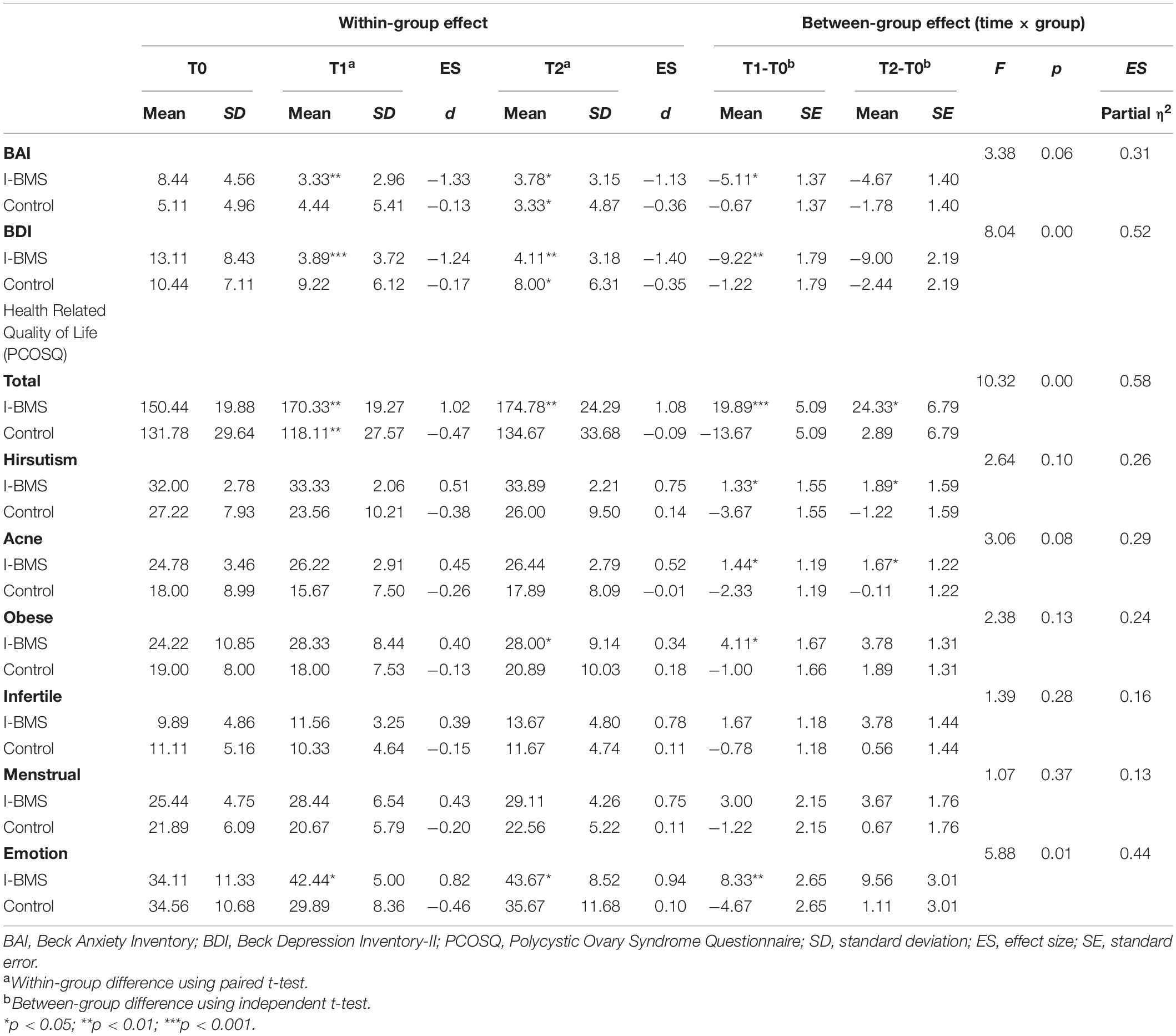
Table 3. Anxiety, depression and health-related quality of life at study time points (Intention-to-treat analysis, n = 18).
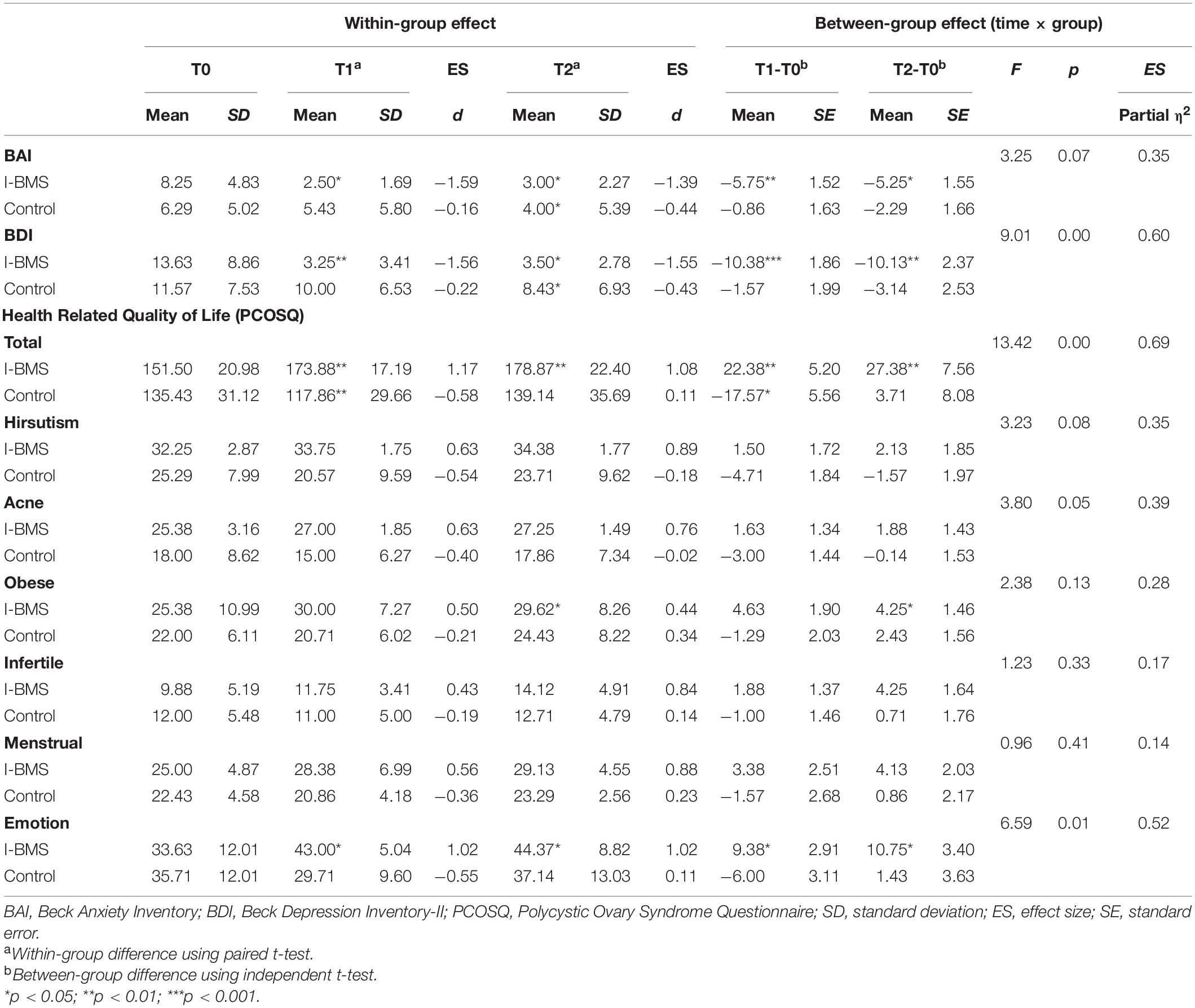
Table 5. Anxiety, depression and health-related quality of life at study time points (Per protocol analysis, n = 15).
Considering within-group effect, the mean value of participants’ depression symptoms reduced significantly (p < 0.001, d = −1.24) at T1 from moderate depression (13.11 ± 8.43) to no symptoms (3.89 ± 3.72). This effect lasted at T2 (p < 0.01, d = −1.40). There was a significant decrease in anxiety (p < 0.01, d = − 1.33), testosterone (p < 0.001, d = −0.97), TG (p < 0.05, d = −0.41), and TG/HDL-C (p < 0.05, d = −0.32) and a significant increase in emotion-related quality of life (p < 0.05, d = 0.82) and total HRQoL (p < 0.01, d = 1.02) in the intervention group at T1. The effect sizes at T1 were all medium to high according to Cohen’s d estimates. The intervention effects on anxiety (p < 0.05, d = −1.13), emotion-related quality of life (p < 0.05, d = 0.94) and total HRQoL (p < 0.01, d = 1.08) also lasted at T2. Besides, the effect on obese-related quality of life became significant at T2 (p < 0.05, d = 0.34).
The between-group effect analysis showed a significant time × group interaction in depression (F = 8.04, p < 0.01, η2 = 0.52), and testosterone (F = 23.08, p < 0.001, η2 = 0.59), TG (F = 7.91, p < 0.05, η2 = 0.33) and TG/HDL-C (F = 5.90, p < 0.05, η2 = 0.27), meaning that over time depression, testosterone, TG and TG/HDL-C significantly decreased in the intervention group than the control group. The interaction was also significant in emotion-related quality of life (F = 5.88, p < 0.05, η2 = 0.44) and total HRQoL (F = 10.32, p < 0.01, η2 = 0.58) meaning that emotion-related QoL and total HRQoL increased in the intervention group but decreased in the control group over time. The effect sizes η2 ranged from moderate to high.
Mind-Body Associations
The pre-post (T1-T0) changes in anxiety, depression, HRQoL, testosterone, TG and TG/HDL-C were calculated to examine the associations among them. Significant associations were found between changes of anxiety and depression (r = 0.77, p < 0.001), anxiety and HRQoL (r = −0.56, p < 0.05), anxiety and TG/HDL-C (r = 0.52, p < 0.05), and changes of depression and HRQoL (r = −0.69, p < 0.01), depression and testosterone (r = 0.51, p < 0.05), depression and TG/HDL-C (r = 0.49, p < 0.05). There were also significant associations between changes of HRQoL and testosterone (r = −0.54, p < 0.05), HRQoL and TG (r = −0.51, p < 0.05). Figure 2 showed the scatterplot matrix of associations between standardized residuals of changes in each outcome, which presented the mind-body associations and the significant intervention effect.
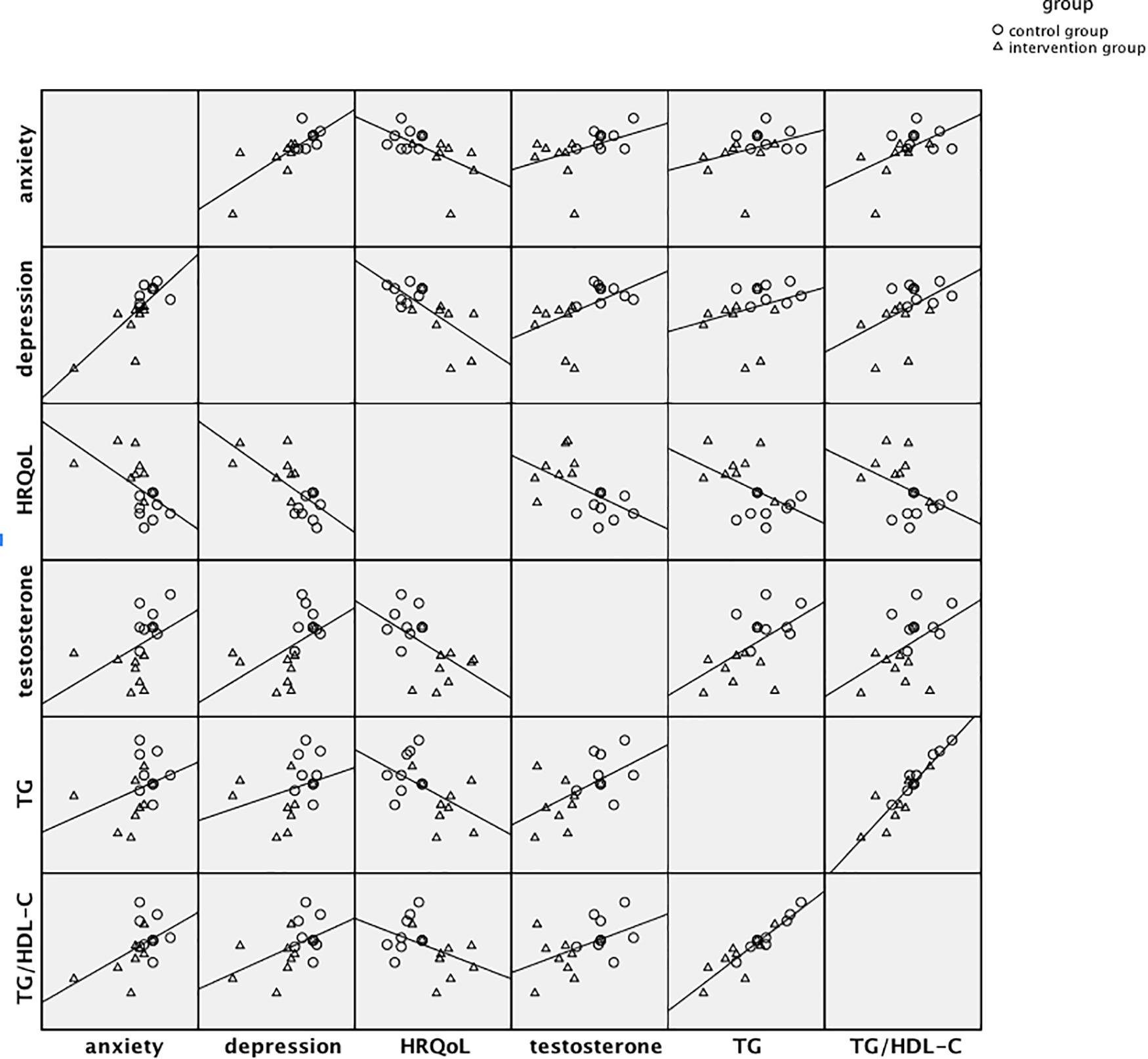
Figure 2. Scatterplot matrix of associations between standardized residuals of pre-post changes in major mental and physical factors.
Discussion
This pilot study suggested that for this small group of randomly assigned Chinese PCOS sufferers, the I-BMS intervention model improved mental and physical health, and holistic well-being compared to the control group. To the best of our knowledge, this is the first study to employ an integrative non-pharmacological intervention for Chinese PCOS patients, and the first study to examine the effect of a mind-body psychosocial intervention on testosterone and dyslipidemia of PCOS patients. The results were in line with previous findings of I-BMS interventions for other Chinese populations, supporting the interconnectivity among body and mind, and verifying the efficacy of the non-pharmaceutical I-BMS model in reducing mental and physical distresses (Chan et al., 2017). Moreover, previous psychological interventions for PCOS patients produced only limited benefits, yet the I-BMS model brought definite physical and mental improvements. Besides good feasibility, the participants in the intervention group gave positive feedback about the intervention, which helped verify its acceptability. Thus there is sufficient support from this pilot study to proceed to a larger RCT to test I-BMS applications for Chinese PCOS patients.
The mean depression and anxiety scores reduced significantly after the intervention ceased and at 3 month follow-up, showing a relatively high effect size. Mean testosterone, triglycerides and ratio of triglycerides/high-density lipoprotein cholesterol improved significantly more for the intervention group than the control group, indicating that the intervention group were functioning better after completing the 6 week intervention program. Mean scores for emotion-related QoL and total HRQoL also improved significantly in the intervention group at T1 and T2 compared to the control group, with moderate or high effect sizes. These results supported our hypothesis that the I-BMS model is likely to effectively reduce Chinese PCOS sufferers’ anxiety, depression, testosterone and triglycerides, and improve their HRQoL.
The study results were in line with previous research on the relationship between hyperandrogenemia, dyslipidemia and depression and anxiety (Nese et al., 2011; Baskind and Balen, 2016). In our study, prior to being diagnosed with PCOS, most participants reported experiencing stressful events which resulted in worsening bodily and ovarian function. This supports the negative influence of psychosocial stress and anxiety on ovarian function and hypothalamic–pituitary axis (Baskind and Balen, 2016), and on neuroendocrine and metabolic system of PCOS patients (Nese et al., 2011). The intervention fostered a happy, positive and resilient attitude toward life. As depression and anxiety symptoms improved, patients’ testosterone and triglyceride levels decreased. This supports Barry et al.’s (2018) hypothesis regarding the pathway from psychology to biology. Despite the fact that total cholesterol levels did not change significantly, the study validated the interrelationship between physiological (i.e., hyperandrogenism, triglyceride, TG/HDL-C), and psychological (i.e., depression, anxiety) factors among women with PCOS (Farrell and Antoni, 2010; Cooney and Dokras, 2017).
This pilot study has certain limitations. Firstly, the sample size was small. Although the doctors referred 49 patients during the 3 months of recruitment, only 18 participants were successfully randomized. Considering the dropout reasons and feedback from successful participants, this finding reflects potential future difficulties in the development of clinical group counseling in Chinese contexts. Secondly, all participants were not particularly over-weight nor with obvious appearance changes. Thus, modifications may be needed for future studies to adopt I-BMS to be relevant for more PCOS patients with diverse characteristics.
PCOS has been consistently associated with mental health conditions such as anxiety, depression and lowered HRQoL (Amini et al., 2012; Chaudhari et al., 2018). Metabolic changes incur bodily distress as well as psychiatric symptoms (Barry et al., 2011b); self-image dissatisfaction negatively influences mental health status, resulting in emotional frustrations (Scaruffi et al., 2019). Besides CBT, there are few psychological interventions that have been developed specifically for women with PCOS. Moreover, as the disease has no cure, self-efficacy is an important aspect of management. The I-BMS model offers a holistic empowerment intervention (Lee et al., 2018). After the program, intervention group participants were empowered to practice the self-care exercises of emotion self-management, self-acceptance and self-care, healthy lifestyle, meaning-making, acupressure, abdominal breathing, and other I-BMS techniques by themselves. Early indications from this pilot study are that I-BMS is a suitable support for women who are adjusting to living with PCOS for life. The convincing effect sizes provide the basis for calculating a robust sample size for a larger RCT to further understand how I-BMS works for women suffering from PCOS.
Conclusion
In conclusion, this pilot study suggests that the I-BMS intervention is likely to be more effective for Chinese women with PCOS, compared to other intervention methods, in improving physical and mental health symptoms. This indicates that larger-scale research is feasible to test this intervention in Chinese PCOS sufferers.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the Medical Research Ethics Committee of the HKU-Shenzhen Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
MXCY conducted the intervention and drafted the original manuscript. LBD and XNZ helped in the recruitment and measurement design, and gave advice to revise the manuscript. XNZ, YLF, and YYS gave advice on the intervention protocol and feasibility test. CHYC gave advice on the intervention protocol and revised the manuscript. CLWC gave some advice on the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was granted by Seed Fund for Basic Research of the University of Hong Kong (No. 201811159259).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abdollahi, L., Mirghafourvand, M., Kheyradin, J. B., and Mohammadi, M. (2018). The effect of cognitive behavioral therapy on depression and obesity in women with polycystic ovarian syndrome: a randomized controlled clinical trial. Iranian Red Crescent Med. J. 20, 1–8. doi: 10.5812/ircmj.62735
Amini, L., Ghorbani, B., and Seyedfatemi, N. (2012). Mental health of women with polycystic ovary syndrome (PCOS) and some of its Socio-demographic determinants. Iran J. Nursing (2008-5923) 25, 34–41.
Annagür, B. B., Tazegül, A., Uguz, F., Kerimoglu, O. S., Tekinarslan, E., and Celik, C. (2013). Biological correlates of major depression and generalized anxiety disorder in women with polycystic ovary syndrome. J. Psychosomatic Res. 74, 244–247. doi: 10.1016/j.jpsychores.2013.01.002
Balen, A. H., Morley, L. C., Misso, M., Franks, S., Legro, R. S., Wijeyaratne, C. N., et al. (2016). The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Hum. Reprod. Update 22, 687–708. doi: 10.1093/humupd/dmw025
Barry, J. A. (2019). “Psychobiological pathways of PCOS,” in Psychological Aspects of Polycystic Ovary Syndrome (Cham: Palgrave Macmillan). doi: 10.1007/978-3-030-30290-0
Barry, J. A., Hardiman, P. J., Saxby, B. K., and Kuczmierczyk, A. (2011a). Testosterone and mood dysfunction in women with polycystic ovarian syndrome compared to subfertile controls. J. Psychosomatic Obstetrics Gynecol. 32, 104–111. doi: 10.3109/0167482X.2011.568129
Barry, J. A., Kuczmierczyk, A. R., and Hardiman, P. J. (2011b). Anxiety and depression in polycystic ovary syndrome: a systematic review and meta-analysis. Hum. Reprod. 26, 2442–2451. doi: 10.1093/humrep/der197
Barry, J. A., Qu, F., and Hardiman, P. J. (2018). An exploration of the hypothesis that testosterone is implicated in the psychological functioning of women with polycystic ovary syndrome (PCOS). Med. Hypotheses 110, 42–45. doi: 10.1016/j.mehy.2017.10.019
Barthelmess, E. K., and Naz, R. K. (2014). Polycystic ovary syndrome: current status and future perspective. Front. Biosci. (Elite edition) 6:104–119. doi: 10.2741/e695
Baskind, N. E., and Balen, A. H. (2016). Hypothalamic-pituitary, ovarian and adrenal contributions. Best Practice Res. Clin. Obstetrics Gynaecol. 37, 80–97. doi: 10.1016/j.bpobgyn.2016.03.005
Beck, A. T., Steer, R. A., and Brown, G. K. (1996). Manual for the Beck Depression Inventory, 2nd Edn. San Antonio, TX: The Psychological Corporation.
Bilal, M., Haseeb, A., and Rehman, A. (2018). Relationship of polycystic ovarian syndrome with cardiovascular risk factors. Diabetes Metabolic Syndrome: Clin. Res. Rev. 12, 375–380. doi: 10.1016/j.dsx.2018.01.006
Castelo-Branco, C., Steinvarcel, F., Osorio, A., Ros, C., and Balasch, J. (2010). Atherogenic metabolic profile in PCOS patients: role of obesity and hyperandrogenism. Gynecol. Endocrinol. 26, 736–742. doi: 10.3109/09513590.2010.481025
Chan, C., Chan, C., Ng, E., Ho, P., Chan, T., Lee, G., et al. (2012). Incorporating spirituality in psychosocial group intervention for women undergoing in vitro fertilization: a prospective randomized controlled study. Psychol. Psychother. 85, 356–373. doi: 10.1111/j.2044-8341.2011.02040.x
Chan, C., Ho, P., and Chow, E. (2000). A body-mind-spirit model in health: an eastern approach. Soc. Work Health Care 34:261. doi: 10.1300/J010v34n03_02
Chan, C., Ji, X., Chan, J., Lau, B., So, K., Li, A., et al. (2017). Effects of the integrative mind-body intervention on depression, sleep disturbances and plasma IL-6. Psychother. Psychosomatics 86, 54–56. doi: 10.2307/48516317
Chaudhari, A., Mazumdar, K., and Mehta, P. (2018). Anxiety, depression, and quality of life in women with polycystic ovarian syndrome. Indian J. Psychol. Med. 40, 239–246. doi: 10.4103/IJPSYM.IJPSYM_561_17
Cheng, K. W., Wong, C. W., Wong, K. C., Chong, S. C., Wong, T. P., Chang, S. Y., et al. (2002). A study of psychometric properties, normative scores, and factor structure of the beck anxiety inventory–the Chinese version. Chinese J. Clin. Psychol. 10, 4–6.
Cohen, J. (1988). Statistical Power Analysis for the Behavioral Sciences, 2nd Edn. Hillsdale: L. Erlbaum Associates.
Cohen, L. M., Al’Absi, M., and Collins, F. (2004). Salivary cortisol concentrations are associated with acute nicotine withdrawal. Addictive Behav. 29, 1673–1678. doi: 10.1016/j.addbeh.2004.02.059
Cooney, L. G., and Dokras, A. (2017). Depression and anxiety in polycystic ovary syndrome: etiology and treatment. Curr. Psychiatry Rep. 19:83. doi: 10.1007/s11920-017-0834-2
Cooney, L. G., Milman, L. W., Hantsoo, L., Kornfield, S., Sammel, M. D., Allison, K. C., et al. (2018). Cognitive-behavioral therapy improves weight loss and quality of life in women with polycystic ovary syndrome: a pilot randomized clinical trial. Fertil. Steril. 110, 161–171. doi: 10.1016/j.fertnstert.2018.03.028
Dai, Q., and Feng, Z. Z. (2008). Research of relationship between depression and self-esteem, self-efficiency. Chinese J. Clin. Psychol. 16, 283–285.
Dokras, A., Sarwer, D. B., Allison, K. C., Milman, L., Kris-Etherton, P. M., Kunselman, A. R., et al. (2016). Weight loss and lowering androgens predict improvements in health-related quality of life in women with PCOS. J. Clin. Endocrinol. Metabolism 101, 2966–2974. doi: 10.1210/jc.2016-1896
Erdem, M., Balikci, A., Keskin, U., Bozkurt Zincir, S., Gülsün, M., Özçelik, F., et al. (2013). 2854-depression, anxiety and anger in patients with polycystic ovary syndrome. Eur. Psychiatry 28(Suppl. 1), 1. doi: 10.1016/S0924-9338(13)77435-2
Farrell, K., and Antoni, M. H. (2010). Insulin resistance, obesity, inflammation, and depression in polycystic ovary syndrome: biobehavioral mechanisms and interventions. Fertil. Steril. 94, 1565–1574. doi: 10.1016/j.fertnstert.2010.03.081
Gul, A., Gul, H., Torel-Ergur, A., and Erberk-Ozen, N. (2018). Anxiety-anger relationship in hyperandrogenemia: a comparative study with polycystic ovary syndrome (PCOS) and healthy control adolescents. Psychiatry Behav. Sci. 8, 26–31. doi: 10.5455/jmood.20180206055429
Hadaegh, F., Khalili, D., Ghasemi, A., Tohidi, M., and Azizi, F. (2009). Triglyceride/hdl-cholesterol ratio is an independent predictor for coronary heart disease in a population of Iranian men. Nutr. Metab. Cardiovascular Dis. NMCD 19, 401–408. doi: 10.1016/j.numecd.2008.09.003
Hsiao, F. H., Jow, G. M., Kuo, W. H., Chang, K. J., Liu, Y. F., Ho, T. H., et al. (2012). The effects of psychotherapy on psychological well-being and diurnal cortisol patterns in breast cancer survivors. Psychother. Psychosomatics 81, 173–182. doi: 10.1159/000329178
Huang, A., Brennan, K., and Azziz, R. (2010). Prevalence of hyperandrogenemia in the polycystic ovary syndrome diagnosed by the National Institutes of Health 1990 criteria. Fertil. Steril. 93, 1938–1941. doi: 10.1016/j.fertnstert.2008.12.138
Kyrou, I., Karteris, E., Robbins, T., Chatha, K., Drenos, F., and Randeva, H. S. (2020). Polycystic ovary syndrome (PCOS) and COVID-19: an overlooked female patient population at potentially higher risk during the COVID-19 pandemic. BMC Med. 18:220. doi: 10.1186/s12916-020-01697-5
Lee, M. Y., Chan, H. Y. C., Chan, L. W. C., Ng, S. M., and Leung, P. Y. P. (2018). Integrative Body-Mind-Spirit Social Work: An Empirically Based Approach to Assessment and Treatment. Oxford: Oxford University Press.
Li, M. Y., Ouyang, Y. Q., Wang, X. H., and Yang, L. (2015). Reliability and validity of chinese version of polycystic ovary syndrome questionnaire. J. Nursing (China) 22, 60–64.
Liu, C., Hsiung, P., Chang, K., Liu, Y., Wang, K., Hsiao, F., et al. (2008). A study on the efficacy of body-mind-spirit group therapy for patients with breast cancer. J. Clin. Nursing 17, 2539–2549. doi: 10.1111/j.1365-2702.2008.02296.x
McCook, J. G., Bailey, B. A., Williams, S. L., Anand, S., and Reame, N. E. (2015). Differential contributions of polycystic ovary syndrome (PCOS) manifestations to psychological symptoms. J. Behav. Health Services Res. 42, 1–12. doi: 10.1007/s11414-013-9382-7
Moher, D., Schulz, K. F., and Altman, D. G. (2001). The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 357, 1191–1194. doi: 10.1016/S0140-6736(00)04337-3
Naz, M., Tehrani, F. R., Majd, H. A., Ahmadi, F., Ozgoli, G., Fakari, F. R., et al. (2019). The prevalence of polycystic ovary syndrome in adolescents: a systematic review and meta-analysis. Int. J. Reprod. Biomed. 17, 533–542. doi: 10.18502/ijrm.v17i8.4818
Nese, C., Cemal, K. M., Ayla, H., Yazgan, A. D., Gurkan, B., Basaran, D., et al. (2011). Depression, anxiety and cardiometabolic risk in polycystic ovary syndrome. Hum. Reprod. 26, 3339–3345. doi: 10.1093/humrep/der338
Nidhi, R., Padmalatha, V., Nagarathna, R., and Ram, A. (2012). Effect of a yoga program on glucose metabolism and blood lipid levels in adolescent girls with polycystic ovary syndrome. Int. J. Gynecol. Obstetrics 118, 37–41. doi: 10.1016/j.ijgo.2012.01.027
Nidhi, R., Padmalatha, V., Nagarathna, R., and Ram, A. (2013a). Effects of a holistic yoga program on endocrine parameters in adolescents with polycystic ovarian syndrome: a randomized controlled trial. J. Alternat. Complementary Med. 19, 153–160. doi: 10.1089/acm.2011.0868
Nidhi, R., Padmalatha, V., Nagarathna, R., and Ram, A. (2013b). Effect of yoga program on quality of life in adolescent polycystic ovarian syndrome: a randomized control trial. Appl. Res. Qual. Life 8, 373–383. doi: 10.1007/s11482-012-9191-9
Romanowski, M. D., Parolin, M. B., Freitas, A. C., Piazza, M. J., Basso, J., and Urbanetz, A. A. (2015). Prevalence of non-alcoholic fatty liver disease in women with polycystic ovary syndrome and its correlation with metabolic syndrome. Arquivos De Gastroenterologia 52:117. doi: 10.1590/S0004-28032015000200008
Rotterdam Group. (2004). Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 8, 19–25. doi: 10.1016/j.fertnstert.2003.10.004
Scaruffi, E., Franzoi, I. G., Civilotti, C., Guglielmucci, F., La Marca, L., Tomelini, M., et al. (2019). Body image, personality profiles and alexithymia in patients with polycystic ovary syndrome (PCOS). J. Psychosomatic Obstetrics Gynecol. 40, 294–303. doi: 10.1080/0167482X.2018.1530210
Sreevani, R., Reddemma, K., Chan, C. L., Leung, P. P., Wong, V., and Chan, C. H. (2013). Effectiveness of integrated body-mind-spirit group intervention on the well-being of Indian patients with depression: a pilot study. J. Nursing Res. 21, 178–185. doi: 10.1097/jnr.0b013e3182a0b041
Stapinska-Syniec, A., Grabowska, K., Szpotanska-Sikorska, M., and Pietrzak, B. (2018). Depression, sexual satisfaction, and other psychological issues in women with polycystic ovary syndrome. Gynecol. Endocrinol. 34, 1–4. doi: 10.1080/09513590.2018.1427713
Van Reedt Dortland, A. K. B., Vreeburg, S. A., Giltay, E. J., Licht, C. M. M., Vogelzangs, N., van Veen, T., et al. (2013). The impact of stress systems and lifestyle on dyslipidemia and obesity in anxiety and depression. Psychoneuroendocrinology 38, 209–218. doi: 10.1016/j.psyneuen.2012.05.017
Yao, H., Chan, C. H. Y., and Chan, C. L. W. (2018). Childbearing importance: a qualitative study of women with infertility in china. Res. Nursing Health 41, 69–77. doi: 10.1002/nur.21846
Keywords: anxiety, depression, integrative body-mind-spirit (I-BMS), polycystic ovary syndrome (PCOS), testosterone, triglycerides
Citation: Yin MXC, Du LB, Zou XN, Fung YL, Sun YY, Chan CHY and Chan CLW (2021) Can Psychosocial Intervention Suppress Testosterone and Triglycerides Among Women With Polycystic Ovary Syndrome? A Feasibility Trial. Front. Psychol. 12:690539. doi: 10.3389/fpsyg.2021.690539
Received: 07 April 2021; Accepted: 25 June 2021;
Published: 22 July 2021.
Edited by:
Roberto Cattivelli, Istituto Auxologico Italiano (IRCCS), ItalyReviewed by:
Renana Eitan, Hebrew University Hadassah Medical School, IsraelRong Li, Peking University Third Hospital, China
Copyright © 2021 Yin, Du, Zou, Fung, Sun, Chan and Chan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Celia H. Y. Chan, Y2hhbmNlbGlhQGhrdS5oaw==
 Margaret X. C. Yin1
Margaret X. C. Yin1 Celia H. Y. Chan
Celia H. Y. Chan Cecilia L. W. Chan
Cecilia L. W. Chan