- 1Faculty of Health and Social Care, Edge Hill University, Ormskirk, United Kingdom
- 2Liverpool Head and Neck Centre, Liverpool University Hospital Aintree, Liverpool, United Kingdom
- 3School of Medicine, Medical and Biological Sciences, North Haugh, St. Andrews, United Kingdom
- 4Astraglobe Ltd., Congleton, United Kingdom
- 5Leeds Teaching Hospitals and St. James Institute of Oncology, Leeds Dental Institute and Leeds General Infirmary, Leeds, United Kingdom
Background: Fear of cancer recurrence (FCR) is recognized as a common concern for patients with head and neck cancer (HNC). The aim of this study is to describe in greater detail the demographic and clinical characteristics of HCN patients who indicate a high level of FCR in their review consultation.
Methods: A pragmatic cluster-controlled trial was conducted between January 2017 and December 2018 at two UK HNC centers (Leeds and Liverpool) to test the efficacy of a prompt tool called the Patient Concerns Inventory (PCI). Patients completed the PCI and the UW-QOLv4 which included a single 5 category rating of FCR. Secondary statistical analyses focused on variables associated with high FCR.
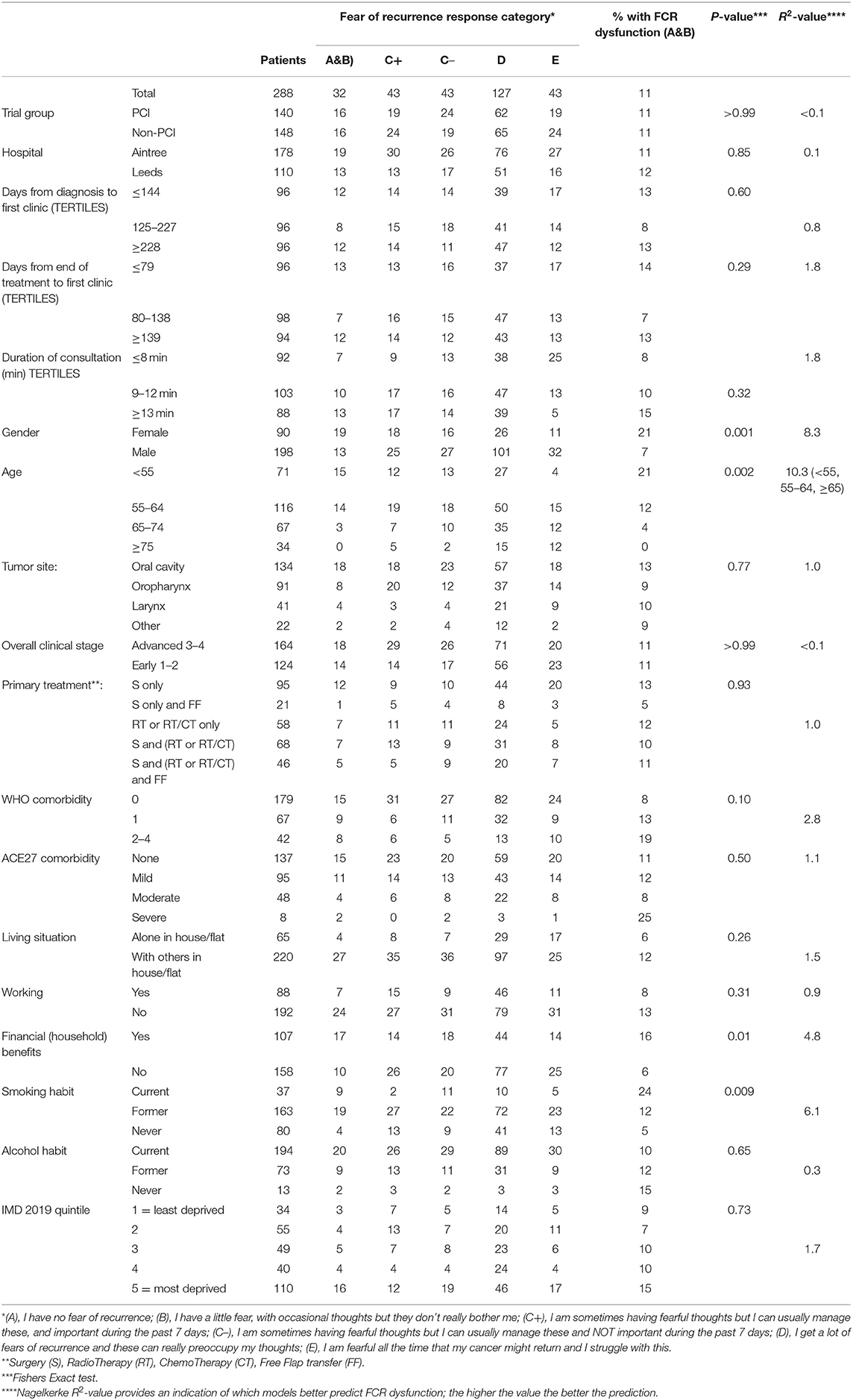
Results: Two hundred and eighty-eight trial patients were recruited in this trial. At a median of 194 days after diagnosis and 103 days after the end of treatment 8% stated (n = 24) “I get a lot of fears of recurrence and these can really preoccupy my thoughts” and 3% (n = 8) “I am fearful all the time that my cancer might return, and I struggle with this.” Thus, 11% (n = 32) responded in the worst two categories, 95% Confidence interval 7.7–15.3% for high FCR. Stepwise logistic regression resulted in female gender (p < 0.001), age (p = 0.007), and receiving financial benefits (p = 0.01) as independent predictors.
Conclusions: Around one in ten HNC patients attending routine outpatient follow-up consultations report high FCR, however for female patients under the age of 55 the rate was one in three. This group requires specialist attention and could be the focus of a multicenter intervention trial.
Introduction
Head and neck cancer (HNC) is the eighth most commonly diagnosed cancer in the UK (Cancer Research UK, 2017) with approximately 12,200 new cases each year. Risk factors include smoking and alcohol and recently the Human Papilloma virus (HPV) (Cooper et al., 2009; Dhull et al., 2018). The 5-year disease specific survival is around 60% for both men and women (Cadoni et al., 2017). Most HNC recurrences occur within the first 2 years following diagnosis (Kissun et al., 2006) and have a poor prognosis with survival in terms of months (Fullarton et al., 2016). With this backdrop it is hardly surprising that Fear of Cancer Recurrence (FCR) is (Humphris and Ozakinci, 2008) one of the most frequent issues patients wish to talk about in their out-patient review consultations (Kanatas et al., 2012). Given the likelihood of early recurrence, clinicians often stress the importance of vigilance and adherence to follow-up visits. One of the greatest challenges is carefully balancing the degree of emphasis on recurrence without alarming the patient. Fear of Cancer Recurrence is of particular interest as this fear holds considerable psychological stress which in turn negatively affects the patient's quality of life (QoL) (Smith et al., 2006; Dunne et al., 2017), daily functioning (Lee-Jones et al., 1997), relationship with carers (Hodges and Humphris, 2009), and mental well-being (Humphris et al., 2003). HNC survivors with inadequate health literacy have increased FCR compared to those with adequate health literacy (Clarke et al., 2021).
The issue of FCR can be hard to elicit in the follow-up clinic, whether this be face to face or virtual, as imposed by restrictions due to the COVID-19 pandemic. The Patients Concerns Inventory (PCI) is a condition specific prompt list devised in collaboration with patients as a means by which they can raise issues of concern with the clinician (Rogers et al., 2009) (Supplementary Material). A systematic review and content comparison of unmet needs self-report measures favored the PCI over 13 other tools (Shunmugasundaram et al., 2019). In a randomized trial the PCI has been shown to be a low-cost intervention which is feasible in routine clinical practice and is associated with a positive effect on QoL and socio-emotional dysfunction (Rogers et al., 2020).
Although the PCI can help to identify the frequency of FCR (Rogers et al., 2010a; Kanatas et al., 2012; Ghazali et al., 2013), thus far specific details of which kind of patient is most at risk are lacking. The PCI randomized trial (Rogers et al., 2020) has given an opportunity to evaluate in much greater depth the issue of FCR and this is of particular merit as the trial is based in standard practice and involved 15 consultant HNC surgeons. Hence, the aim of this study is to describe the demographic background and clinical characteristics for those HCN patients who indicate a high level of FCR in their review consultation. This may allow prompt identification of those patients in need of further support during their clinical consultations.
Materials and Methods
Setting and Participants
The methodology of this trial has been described in detail (Rogers et al., 2018a). Briefly, this is a pragmatic cluster-controlled trial, with consultants (clusters) randomized to “using” or “not using” an intervention incorporating the PCI prompt list at all their trial clinics, i.e., at both baseline and at all follow-up clinics. Two centers participated, Aintree and Leeds, with 15 consultants of whom 8 used the PCI and 7 did not. We report results from the first “baseline” trial clinic and focus on findings regarding fear of recurrence (FCR). We also report FCR results after 12 months of follow-up. Eligible patients were treated curatively for primary HNC, and all sites, stages of disease and treatments were included, and second primary tumors were later accepted. Patients treated palliatively or with a recurrence or with a history of cognitive impairment, psychoses or dementia were excluded.
Measures
The UW-QOL v4 questionnaire comprises 12 single item domains, with between 3 and 5 response options scaled evenly from 0 (worst) to 100 (best) according to response hierarchy (Rogers et al., 2002). UW-QOL domains are presented within two subscales, physical function and social-emotional function (Rogers et al., 2010b) with each subscale score being the mean of six domain scores (http://www.hancsupport.com/professionals/quality-life/qol-questionnaires/university-washington). The physical function score is the mean of the appearance, swallowing, chewing, speech, taste and saliva domain scores, while the social-emotional score is the mean of the pain, activity, recreation, shoulder, mood, and anxiety domain scores. Criteria derived from earlier work was used to highlight domains in which patients have a significant problem or dysfunction (Rogers and Lowe, 2009). There was also a single item overall QOL question on the UWQOL v4 for which patients are asked to consider not only physical and mental health, but also other factors, such as family, friends, spirituality, or personal leisure activities important to their enjoyment of life. The study HRQOL data also included the Distress Thermometer score (Hegel et al., 2008) and EQ-5D-5L (Rogers et al., 2016).
The FCR question has five response options: (A) I have no fear of recurrence, (B) I have a little fear, with occasional thoughts but they don't really bother me, (C) I am sometimes having fearful thoughts, but I can usually manage these, (D) I get a lot of fears of recurrence and these can really preoccupy my thoughts, (E) I am fearful all the time that my cancer might return and I struggle with this. There is also a separate question asking patients whether FCR had been important to them (Yes/No) during the past 7 days. From earlier work (Rogers et al., 2016) it was postulated that those patients responding in the worst two categories should be considered for added assessment and support; we define these patients as having a significant problem or dysfunction with FCR.
The PCI is a condition-specific item prompt list (Rogers et al., 2009) comprising 56 items, which patients select from before seeing their consultant, to help guide the outpatient consultation, and it covers a range of symptoms and potential problems patients may face after treatment. It helps focus the consultation, aids doctor–patient communication, and helps route patients to other professionals for advice and support. It can be integrated into routine clinical practice (Rogers et al., 2018b).
Pre-consultation questionnaires including the PCI prompt list were completed electronically (desktop, tablet, iPAD) apart from one Liverpool hospital (non-PCI consultant) that used paper. PCI patients took into their consultations a summary sheet of paper that listed (a) all PCI items they selected for discussion, (b) any University of Washington (UWQOL) questionnaire domains in which there was a significant problem or dysfunction, (c) their overall QOL response, (d) their Distress Thermometer score, and (e) health professionals they wanted to see. This one page paper summary printout was the visible difference between trial arms as far as contact between consultant and patient was concerned. Control patients completed exactly the same pre-clinic information apart from the PCI prompt list but neither they nor their consultant saw any summary sheet. Both groups completed the EQ-5D-5L for purposes of health economic assessment.
Baseline clinical and demographic data were collected using a baseline clinic questionnaire based on that of the Head and Neck 5000 project (Ness et al., 2014), or by extraction from baseline clinical records. Information was collected as to whether patients lived alone or with others, whether they were working and whether they lived in a household that received UK state financial benefits. Lifestyle factors about tobacco and alcohol use were also collected, as was patient gender and age. Clinical details about primary tumor site, grade, treatment, WHO, and ACE27 comorbidity were obtained from clinical records. Index of Multiple Deprivation (English Indices of Deprivation–IMD, 2019) scores were derived from patient postcodes using publicly available data and these provide a relative measure of deprivation at a small area level across England.
Statistical Analyses
The statistical analysis focused on variables associated with FCR dysfunction. We considered patient and clinical casemix variables and also a wide range of HRQOL measures. Fishers Exact test was used to compare patient groups regarding FCR dysfunction (Yes/No). Logistic regression was used to form predictive models of FCR dysfunction. Univariate models with each predictor variable were run and the Nagelkerke R square value (R2) was noted for each model, these values providing an indication of which models better predict FCR dysfunction; the higher the value the better the prediction. Multivariable logistic regression modeling was done using significant univariate casemix variables (Table 1) and a stepwise regression approach with p < 0.01 for inclusion was adopted for the consideration of the many HRQOL predictor variables (Table 2). Given the number of tests performed for this paper, statistical significance was regarded as p < 0.01.
Results
Patients for the trial were first discussed at MDT meetings between January 2017 and December 2018, with first trial clinics between April 2017 and October 2019. Characteristics of the 288 trial patients can be determined from Table 1. Median (IQR) age at baseline was 62 (55–69) and 69% (198) were male. Baseline clinics were a median (IQR) of 194 (125–249) days after diagnosis and 103 (71–162) days after the end of treatment. Regarding FCR, 15% (43) stated “I have no fear of recurrence,” 44% (127) “I have a little fear, with occasional thoughts but they don't really bother me,” 30% (86) “I am sometimes having fearful thoughts but I can usually manage these,” 8% (24) “I get a lot of fears of recurrence and these can really preoccupy my thoughts,” and 3% (8) “I am fearful all the time that my cancer might return and I struggle with this.” Thus 11% (32) responded in the worst two categories, 95% Confidence interval 7.7–15.3% for FCR dysfunction, and this rate was notably higher in females and in patients younger than 65 years (Table 1). No significant variation was seen regarding tumor site, clinical stage, or treatment. It was also higher in those still smoking and in those with households receiving financial benefits. Stepwise logistic regression with gender, age (<55/55–64/≥65), financial benefits (Yes/No), and smoking habit (Current/Ex/Never) resulted in gender (p < 0.001), age (p = 0.007), and financial benefits (p = 0.01) as independent predictors, n = 265. The first two predictors, gender and age, were retained to preserve the full sample size. In female patients under the age of 55 the rate was 36% (10/28), while for those aged 55–64 it was 29% (8/28) and for those ≥65 it was 3% (1/34); for males the rates were 12% (5/43), 7% (6/88), and 3% (2/67), respectively.
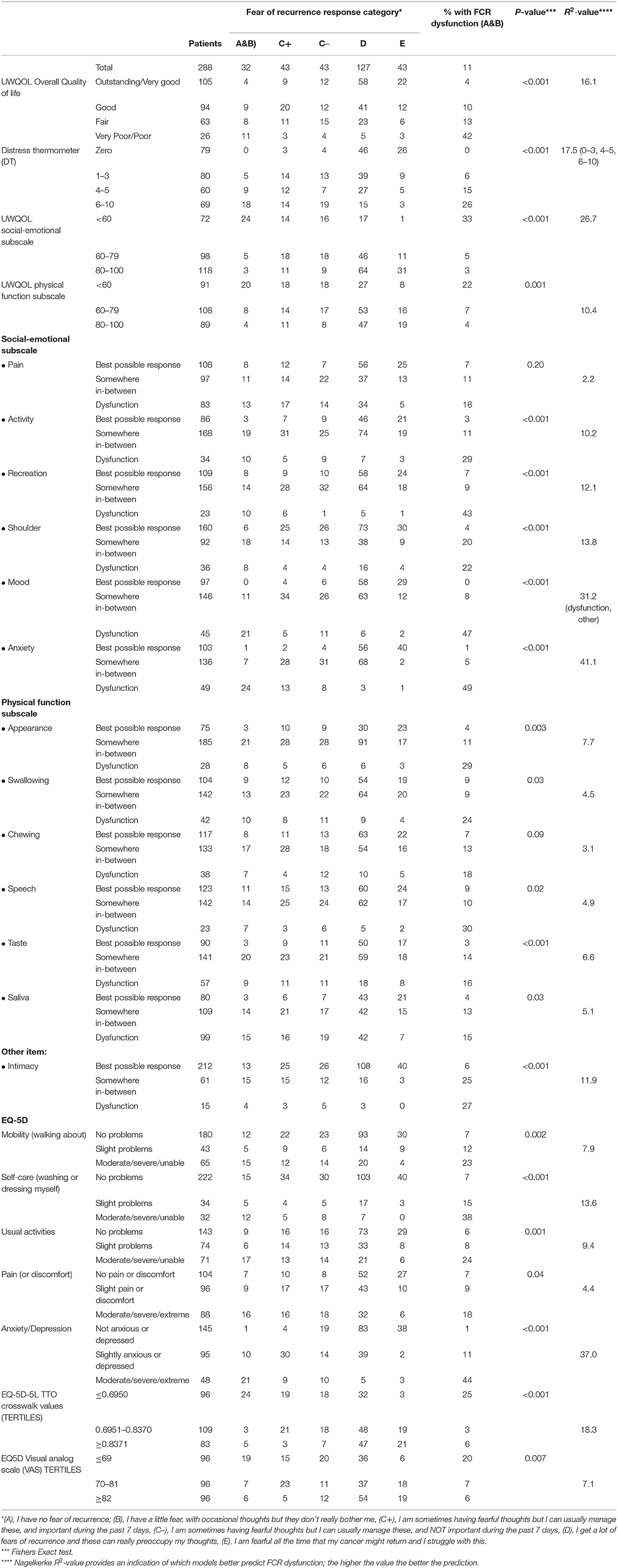
Most of the HRQOL measures in this study were associated with FCR (Table 2) and with each other (results not shown). Fear of Cancer Recurrence dysfunction was present in 49% (24/49) of those with dysfunction in anxiety (UWQOL), in 47% (21/45) of those with dysfunction in mood (UWQOL), in 44% (21/48) with moderate, severe, or extreme problems with anxiety/depression (EQ5D), in 43% (10/23) with dysfunction in recreation (UWQOL) and 42% (11/26) with poor or very poor overall quality of file (UWQOL). Comparison of the R2 values indicated that the most predictive variables from Table 2 were UWQOL anxiety (R2 = 41.1), EQ5D anxiety/depression (37.0), UWQOL Mood (31.2) and UWQOL social-emotional function subscale score (26.9). In comparison the R2-value for the model with gender and age group was 18.2. When gender and age and all the variables from Table 2 were considered within a stepwise regression at p < 0.001 for entry then UWQOL anxiety (p < 0.001, R2 = 41.1) was selected first, followed by UWQOL Mood (p < 0.001, R2 = 46.4 combined) and then gender (p = 0.002, R2 = 52.1 combined). If patients had both UWQOL anxiety and mood dysfunction then 66% (19/29) also had FCR dysfunction (males: 61%, 11/18; females: 73%, 8/11). If patients had UWQOL anxiety and mood dysfunction but not both then 19% (7/36) had FCR dysfunction (males; 9%, 2/22; females: 36%, 5/14). For other patients only 3% (6/223) had FCR dysfunction (males: 0%, 0/158; females: 9%, 6/65).
One-third (97/288) of patients said that FCR had been important to them in the past week, and this varied from 81% (26/32) of those with FCR dysfunction, 50% (43/86) of those sometimes having fearful thoughts, 19% (24/127) of those having a little fear and 9% (4/43) of those without fear at the time of data entry. In the group who sometimes had fearful thoughts, there were no significant differences in patient characteristics between the 43 patients regarding FCR as important and the 43 patients for whom it was not important (Tables 1, 2) apart from an association with EQ5D anxiety/depression (p < 0.001) in Table 2 which only affected the balance between not being anxious or depressed and being slightly anxious or depressed. Patients in the PCI intervention group could select the FCR item from the PCI prompt list if they wanted to discuss this during their consultation, and 34% (48/140) did select this item. Of the 16 patients with FCR dysfunction then 8 wanted to discuss their fears and 8 did not (Table 3). About half (53%, 23/43) of patients who sometimes had fearful thoughts but could usually manage them wanted to discuss their fears, 74% (14/19) if FCR had been important to them during the past week and 38% (9/24) if FCR had not been not important. About one quarter (27%, 17/62) with just a little fear wanted to discuss FCR, 56% (5/9) if FCR had been important, and 23% (12/53) if not important. None of the 19 patients without FCR wanted to discuss the issue. Of those wanting to discuss their fears, most (40/48) did not have FCR dysfunction.
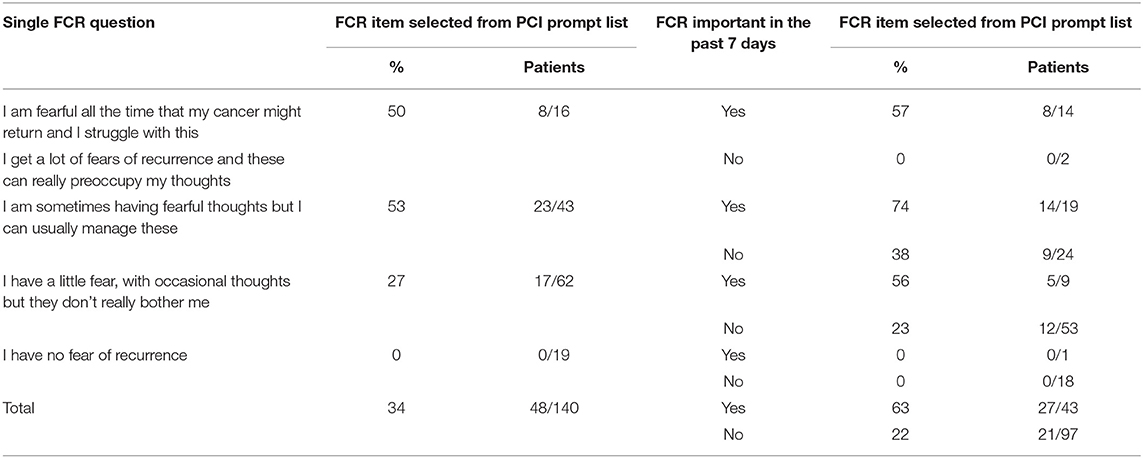
Table 3. Selection of FCR item from the PCI prompt list for 140 PCI patients, by self-reported level of fear of recurrence (FCR) and by whether FCR had been important over the past 7 days.
During the trial patients attended a median (IQR) of 4 (3–5) clinics, range 1–10. Follow-up results at around 12 months were available for 205 patients, at a median (IQR) of 357 (329–391) days from baseline. The percentages with FCR dysfunction were 8% (17/205) at the first study clinic and 6% (13/205) after about 12 months. Five patients had FCR dysfunction on both occasions, 12 only at first, 8 only later, and 180 on neither occasion. In female patients under the age of 65 the rates for FCR dysfunction were 25% (10/40) and 13% (5/40), while for older females they were 0% (0/22) and 5% (1/22); for males 6% (6/96), 7% (7/96), and 2% (1/47), 0% (0/47), respectively. Of 83 lost to the study between baseline and follow-up, 18% (15) had significant fears at the first clinic. The FCR item from the PCI prompt list was selected by 36% (36/100) at baseline and 17% (17/100) at follow-up.
Discussion
Fear of Cancer Recurrence is an issue that is common in HNC and one that is amenable to intervention (Humphris and Rogers, 2012). Its prevalence rate overall was in the region of one in ten patients and this is similar to previous studies (Llewellyn et al., 2008). A more recent survey in the Netherlands using the Cancer Worry Scale has reported “approximately one in two of all patients newly diagnosed with HNC had FCR levels above the validated cut-off for high FCR shortly after diagnosis (Mirosevic et al., 2019).” There may be differences with the proportion of patients categorized as stating they have high FCR according to the measure employed. For example, the Mirosevic et al. article (Mirosevic et al., 2019) set their cut-off against the validated Fears of Cancer Recurrence Inventory (FCRI). A review of the FCRI has raised concerns about the low cut-off on this measure (Smith et al., 2020). Hence, careful attention needs to be paid to the actual wording and psychometric properties of the measures used. Our single item follows the principles of keeping the assessment of FCR focused and with face valid wording, expressed by Costa (2017). Furthermore, our group subscribe to the view that moderate levels of FCR are probably of value, and not regarded as dysfunctional, as the patient remains reasonably vigilant to changes to symptom experience. The caveat would be for patients to have the support from the H&N team should these fears become heightened, and maintained, and for the staff to monitor and discuss their FCR to reverse back to a moderate level. Among the variables that clinicians can use to identify patients with H&N cancer and high FCR indicated by this data set, include the following:
Age
A significant relationship between the younger age of these patients and dysfunctional FCR was found. This was 21% in those under 55, 14% in those aged 55–64 and only 3% in those over 65. The median age in this study was 62 implying that nearly half of the patient population is at higher risk. This association is in line with previous research indicating age was a predictor of FCR (Humphris et al., 2003; Lim and Humphris, 2020). There are several potential explanations as to why age plays a significant role in fears of recurrence. Researchers (Hutton and Williams, 2001) have argued that older patients are not always able to differentiate the interference caused by treatment and the natural degeneration of aging, with a cancer diagnosis becoming increasingly more predictable with older age. In comparison, younger patients find the diagnoses of cancer much more unanticipated and abrupt as it threatens their ability to fulfill major life events, such as having grandchildren or marriage of children (Simard et al., 2013).
Gender
The finding that gender is associated with FCR has been highlighted in a recent systematic review (Pang and Humphris, 2021). One explanation as to why females experience higher FCR is because they tend to experience more symptoms of dysfunctional anxiety (Faravelli et al., 2013). A Norwegian and Swedish HNC study found that those who were under 65 and female were the most likely to develop anxiety (Hammerlid et al., 1999). In this analysis' parent study, 17% of patients experienced dysfunctional anxiety symptoms according to the UWQOL social-emotional subscale. As anxiety levels were not included in their secondary analysis, we must leave room to consider whether present FCR are a surface level manifestation of other psychiatric comorbidities that have been previously found to be significant within this sub-population.
Financial Benefits
In this study, it was found that a significant predictor of developing FCR was living in a household in receipt of financial benefits. Head and neck cancer has a serious impact on patient finances and is associated with poor HRQOL (Rogers et al., 2012). Previous evidence has shown that rates of financial instability are the highest within HNC in comparison to all other cancer types (Avis et al., 2005). This is exemplified within this study with 37% of participants in households receiving financial benefits. A systematic review of FCR found that of the 10 studies investigating financial difficulties and FCR, the association was only found to be significant in three (Simard et al., 2013). However, previous studies regarding FCR and financial position should be applied carefully as this fear could be more prominent in countries whose national health programme relies solely on private hospitals. In a previous study within our group, it was found that FCR had a weak relationship with deprivation which would partially support this finding of patients reporting to receive state benefits (Rylands et al., 2016). This mirrors the findings of a study by Clarke et al. (2021) in which HNC survivors with inadequate health literacy reported lower levels of self-management behaviors, lower functional HRQOL, and increased FCR.
Health Related Quality of Life
Functional/symptom items on H&N cancer HRQOL questionnaires have potential utility in respect to FCR as symptoms that patients experience might trigger anxiety regarding the possibility of recurrence. This is especially so in the field of H&N cancer care where previous reports of patient experience of physical symptoms have focused our group to build on the FCR model proposed by Lee-Jones et al. (1997) and promoted the AFTER intervention (Humphris and Ozakinci, 2008) to reduce high FCR. Most of the HRQOL measures in this study were associated with FCR, for example, 42% in those with poor or very poor overall QOL had dysfunctional FCR. There was anxiety or mood dysfunction in 65 patients (23% of the whole sample) and this group accounted for 81% (26/32) of all those with FCR dysfunction. These relatively broad-brush HRQOL questionnaires, such as the UW-QOL mood and anxiety items and, interestingly a similar amount of variance explained with another well-developed measure, namely: the EQ-5D, together with the EORTCc30, these assessments have shown the ability to identify patients with high FCR. Due to the high degree of overlap between these HRQOL measures, only one or, at most, two would be required to assist with identifying patients with possible high FCR. Within England the NHS England QOL Metric study outcome measures include the EQ-5D and EORTCc30 (NHS England Cancer Data, 2021). Hence, it would enable all cancer patients in England to be given the opportunity to complete the questionnaires at the 18 month post diagnosis window. Those scoring badly, for example, on the EQ-5D will have possibly high FCR which would need to be further assessed. These assessment approaches are part of a possible development to construct stepped-care programmes of support and intervention for moderate to high scorers of FCR which is being explored in patients with melanoma (Lynch et al., 2020).
The regression R2-values (Table 2) indicated that the age and gender associations with FCR were dwarfed relatively by the associations between the HRQOL measures and FCR. Logistic regression on FCR using all variables in Table 2 as predictors selected anxiety, mood, and gender. For patients with anxiety or mood dysfunction or both—there was dysfunctional FCR for 56% of females (13/25) and 32% (13/40) of males; for patients without anxiety of mood dysfunction—there was dysfunctional FCR for 9% (6/65) of females and 0% (0/158) of males.
Our results have included a split of the FCR patient responses to the middle category of the rating scale. The split was whether, or not, FCR had been “important” to the patient within the previous 7 days. We found on close inspection little difference about this split—in either patient/clinical characteristics or across the HRQOL measures. Hence the important message that this reinforces is that dysfunction in FCR is best measured by the worst two FCR response options only and that the middle option if important should not be added to this. This recommendation was postulated in a previous paper (Rogers et al., 2016). In addition, it is worth emphasizing that a degree of FCR is “normal” and to be expected. In our sample, three quarters reported “I have a little fear, with occasional thoughts but they don't really bother me” (44%) and “I am sometimes having fearful thoughts but I can usually manage these” (30%).
Exploring the longitudinal data, FCR severe rates remain relatively unchanged over the year with evidence of a slight improvement 8–6%. This serves to highlight how imbeded this fear can be for patients. Patients still wish to talk about FCR involving one in three at the start of the trial and one in five at the last consultation. The PCI prompt is potentially a useful adjunct to the conversation with the health professional as even with the passage of time it helps the patients to broach what can be a sensitive and distressing issue and this provides an opportunity to reassure on repeated occasions as prompted by the patient themselves.
There are several potential limitations of this work. Firstly, in the trial of those approached to be recruited, around one in five declined to participate. It might be that patients with an anxiety disorder are less likely to take part and that these are more likely to experience high FCR. Thus, this current analysis might underestimate the rates of FCR. The findings should have a reasonable level of generalisability, as although involving only two centers, 15 different consultants were involved, and the sample comprised 80% of eligible patients. The second limitation relates to the single FCR item question used as part of the HRQOL survey. It is recognized that this question will not be as reliable as other more comprehensive FCR measures (Thewes et al., 2012). However, this together with the PCI item on FCR gives a unique opportunity to analyse characteristics that might be predictive of higher FCR dysfunction. Because of the variety of different criteria and definitions of FCR, direct comparisons to this research with other studies should be treated with caution (Mirosevic et al., 2019). This should also be considered in any future work.
The potential value of using a prompt list (PCI) as part of the consultation is that patients might be more amenable to raise the FCR issue through this prompt, and also to discuss their fear in preference to discussing other psychological issues such as depression or anxiety. Fear of Cancer Recurrence is perceived as an understandable concern that both the patient and the clinician can relate to. When talking through natural and health concerns the clinician can gauge the extent of the fear and can offer additional support. A specific FCR intervention could be considered for those most distressed. Considerable work is now being conducted to develop interventions for those with high levels of FCR (Tauber et al., 2019). Further research is needed to carefully construct and evaluate the cost effectiveness of a tiered programme of support. However, a straightforward means to highlight the issue of FCR in routine oncology clinics, either by clinical characteristics, questionnaire caseness, or a prompt from the patient themselves, or a combination of these, could be a suitable starting point for both informal and formal Interventions, with the desired outcome being less distress and improved HRQOL.
Data Availability Statement
The raw data can be made available by the authors, without undue reservation, to any qualified researcher through due process.
Ethics Statement
The studies involving human participants were reviewed and approved by Liverpool. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SR, DL, GH, and AK: study design and critical revision of the manuscript. SR, DL, and AK: data collection. All authors analysis, interpretation, drafting of the manuscript, and approved the final version for publication.
Funding
This paper presents independent research funded by the National Institute for Health Research (NIHR) under its Research for Patient Benefit (RfPB) Programme (Grant Reference Number PB-PG-0215-36047).
Disclaimer
The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. In addition, this research was supported by the National Institute for Health Research (NIHR) infrastructure at Leeds (DenTCRU∣).
Conflict of Interest
DL was employed by company Astraglobe Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank all the patients and clinicians that contributed to the trail and to the trials team.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyg.2021.671366/full#supplementary-material
References
Avis, N. E., Smith, K. W., McGraw, S., Smith, R. G., Petronis, V. M., and Carver, C. S. (2005). Assessing quality of life in adult cancer survivors (QLACS). Qual. Life Res. 14, 1007–1023. doi: 10.1007/s11136-004-2147-2
Cadoni, G., Giraldi, L., Petrelli, L., Pandolfini, M., Giuliani, M., Paludetti, G., et al. (2017). Prognostic factors in head and neck cancer: a 10-year retrospective analysis in a single-institution in Italy. Acta Otorhinolaryngol. Ital. 37, 458–466. doi: 10.14639/0392-100x-1246
Cancer Research UK (2017). Available online at: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/head-and-neck-cancers (accessed January 28, 2021).
Clarke, N., Dunne, S., Coffey, L., Sharp, L., Desmond, D., O'Conner, J., et al. (2021). Health literacy impacts self-management, quality of life and fear of recurrence in head and neck cancer survivors. J. Cancer Surviv. doi: 10.1007/s11764-020-00978-5. [Epub ahead of print].
Cooper, J. S., Porter, K., Mallin, K., Hoffman, H. T., Weber, R. S., Ang, K. K., et al. (2009). National Cancer Database report on cancer of the head and neck: 10-year update. Head Neck. 31, 748–758. doi: 10.1002/hed.21022
Costa, D. S. J. (2017). Screening for clinical levels of fear of cancer recurrence. Psychooncology 26, 2002–2003. doi: 10.1002/pon.4390
Dhull, A. K., Atri, R., Dhankhar, R., Chauhan, A. K., and Kaushal, V. (2018). Major risk factors in head and neck Cancer: a retrospective analysis of 12-year experiences. World J. Oncol. 9, 80–84. doi: 10.14740/wjon1104w
Dunne, S., Mooney, O., Coffey, L., Sharp, L., Desmond, D., Timon, C., et al. (2017). Psychological variables associated with quality of life following primary treatment for head and neck cancer: a systematic review of the literature from 2004 to 2015. Psychooncology 26, 149–160. doi: 10.1002/pon.4109
English Indices of Deprivation–IMD (2019). Available online at: http://imd-by-postcode.opendatacommunities.org (accessed January 11, 2021).
Faravelli, C., Alessandra Scarpato, M., Castellini, G., and Lo Sauro, C. (2013). Gender differences in depression and anxiety: the role of age. Psychiatry Res. 210, 1301–1303. doi: 10.1016/j.psychres.2013.09.027
Fullarton, M., Pybus, S., Mayland, C., and Rogers, S. N. (2016). Analysis of deaths between 2007 and 2012 of patients with cancer of the head and neck on a surgical ward at a regional centre and in an independent hospice. Br. J. Oral Maxillofac. Surg. 54, 62–67. doi: 10.1016/j.bjoms.2015.10.014
Ghazali, N., Cadwallader, E., Lowe, D., Humphris, G., Ozakinci, G., and Rogers, S. N. (2013). Fear of recurrence among head and neck cancer survivors: longitudinal trends. Psychooncology. 22, 807–813. doi: 10.1002/pon.3069
Hammerlid, E., Ahlner-Elmqvist, M., Bjordal, K., Biörklund, A., Evensen, J., Boysen, M., et al. (1999). A prospective multicentre study in Sweden and Norway of mental distress and psychiatric morbidity in head and neck cancer patients. Br. J. Cancer 80, 766–774. doi: 10.1038/sj.bjc.6690420
Hegel, M. T., Collins, E. D., Kearing, S., Gillock, K. L., Moore, C. P., and Ahles, T. A. (2008). Sensitivity and specificity of the Distress Thermometer for depression in newly diagnosed breast cancer patients. Psychooncology 17, 556–560. doi: 10.1002/pon.1289
Hodges, L. J., and Humphris, G. M. (2009). Fear of recurrence and psychological distress in head and neck cancer patients and their carers. Psychoocology 18, 841–848. doi: 10.1002/pon.1346
Humphris, G., and Ozakinci, G. (2008). The AFTER intervention: a structured psychological approach to reduce fears of recurrence in patients with head and neck cancer. Br. J. Health Psychol. 13, 223–230. doi: 10.1348/135910708x283751
Humphris, G. M., and Rogers, S. N. (2012). AFTER and beyond: cancer recurrence fears and test of an intervention in oropharyngeal patients. Soc. Sci. Dent. 2, 29–38. Available online at: ssdopen.com
Humphris, G. M., Rogers, S. N., McNally, D., Lee-Jones, C., Brown, J., and Vaughan, D. (2003). Fear of recurrence and possible cases of anxiety and depression in orofacial cancer patients. Int. J. Oral Maxillofac. Surg. 32, 486–491. doi: 10.1054/ijom.2002.0399
Hutton, J. M., and Williams, M. (2001). An investigation of psychological distress in patients who have beentreated for head and neck cancer. Br. J. Oral Maxillofac. Surg. 39, 333–339. doi: 10.1054/bjom.2001.0645
Kanatas, A., Ghazali, N., Lowe, D., Udberg, M., Heseltine, J., O'Mahony, E., et al. (2012). Issues patients would like to discuss at their review consultation: variation by early and late stage oral, oropharyngeal and laryngeal subsites. Eur. Arch. Otorhinolaryngol. 270, 1067–1074. doi: 10.1007/s00405-012-2092-6
Kissun, D., Magennis, P., Lowe, D., Brown, J. S., Vaughan, E. D., and Rogers, S. N. (2006). Timing and presentation of recurrent oral and oropharyngeal squamous cell carcinoma and awareness in the outpatient clinic. Br. J. Oral Maxillofac. Surg. 44, 371–376. doi: 10.1016/j.bjoms.2005.08.010
Lee-Jones, C., Humphris, G., Dixon, R., and Bebbington Hatcher, M. (1997). Fear of cancer recurrence—a literature review and proposed cognitive formulation to explain exacerbation of recurrence fears. PsychoOncology 6, 95–105. doi: 10.1002/(SICI)1099-1611(199706)6:2<95
Lim, E., and Humphris, G. (2020). The relationship between fears of cancer recurrence and patient age: a systematic review and meta-analysis. Cancer Rep (Hoboken) 3:e1235. doi: 10.1002/cnr2.1235
Llewellyn, C. D., Weinman, J., McGurk, M., and Humphris, G. (2008). Can we predict which head and neck cancer survivors develop fears of recurrence?. J. Psychosom. Res. 65, 525–532. doi: 10.1016/j.jpsychores.2008.03.014
Lynch, F. A., Katona, L., Jefford, M., Smith, A. B., Shaw, J., Dhillon, H. M., et al. (2020). Feasibility and acceptability of fear-less: a stepped-care program to manage fear of cancer recurrence in people with metastatic melanoma. J. Clin. Med. 9:2969. doi: 10.3390/jcm9092969
Mirosevic, S., Thewes, B., van Herpen, C., Kaanders, J., Merkx, T., Humphris, G., et al. (2019). Prevalence and clinical and psychological correlates of high fear of cancer recurrence in patients newly diagnosed with head and neck cancer. Head Neck 41, 3187–3200. doi: 10.1002/hed.25812
Ness, A. R., Waylen, A., Hurley, K., Jeffreys, M., Penfold, C., Pring, M., et al. (2014). Establishing a large prospective clinical cohort in people with head and neck cancer as a biomedical resource: head and neck 5000. BMC Cancer 14:973. doi: 10.1186/1471-2407-14-973
NHS England Cancer Data (2021). Available online at: http://www.cancerqol.england.nhs.uk (accessed June 9, 2019).
Pang, C., and Humphris, G. M. (2021). The relationship between fears of cancer recurrence and patient gender: a systematic review and meta-analysis. Front Psychol. 12:640866. doi: 10.3389/fpsyg.2021.640866
Rogers, S. N., Allmark, C., Bekiroglu, F., Edwards, R. T., Fabbroni, G., Flavel, R., et al. (2020). Improving quality of life through the routine use of the patient concerns inventory for head and neck cancer patients: main results of a cluster preference randomised controlled trial. Eur. Arch. Otorhinolaryngol. 21, 1–15. doi: 10.1007/s00405-020-06533-3
Rogers, S. N., Cross, B., Talwar, C., Lowe, D., and Humphris, G. (2016). A single-item screening question for fear of recurrence in head and neck cancer. Eur. Arch. Otorhinolaryngol. 273:1235–1242. doi: 10.1007/s00405-015-3585-x
Rogers, S. N., El-Sheikha, J., and Lowe, D. (2009). The development of a patients concerns inventory (PCI) to help reveal patients concerns in the head and neck clinic. Oral Oncol. 45:555–556. doi: 10.1016/j.oraloncology.2008.09.004
Rogers, S. N., Gwanne, S., Lowe, D., Humphris, G., Yueh, B., and Weymuller, E. A. Jr. (2002). The addition of mood and anxiety domains to the University of Washington quality of life scale. Head. Neck. 24, 521–529. doi: 10.1002/hed.10106
Rogers, S. N., Harvey-Woodworth, C. N., Hare, J., Leong, P., and Lowe, D. (2012). Patients' perception of the financial impact of head and neck cancer and the relationship to health related quality of life. Br. J. Oral Maxillofac. Surg. 50, 410–416. doi: 10.1016/j.bjoms.2011.07.026
Rogers, S. N., and Lowe, D. (2009). Screening for dysfunction to promote multidisciplinary intervention by using the University of Washington Quality of Life Questionnaire. Arch. Otolaryngol. Head Neck Surg. 135, 369–375. doi: 10.1001/archoto.2009.7
Rogers, S. N., Lowe, D., Lowies, C., Yeo, S. T., Allmark, C., Mcavery, D., et al. (2018a). Improving quality of life through the routine use of the patient concerns inventory for head and neck cancer patients: a cluster preference randomized controlled trial. BMC Cancer 18:444. doi: 10.1186/s12885-018-4355-0
Rogers, S. N., Lowe, D., Yueh, B., and Weymuller, E. A. Jr. (2010b). The physical function and social-emotional function subscales of the University of Washington Quality of Life Questionnaire. Arch. Otolaryngol. Head Neck Surg. 136:352–357. doi: 10.1001/archoto.2010.32
Rogers, S. N., Scott, B., Lowe, D., Ozakinci, G., and Humphris, G. M. (2010a). Fear of recurrence following head and neck cancer in the outpatient clinic. Eur. Arch. Otorhinolaryngol. 267, 1943–1949. doi: 10.1007/s00405-010-1307-y
Rogers, S. N., Thomson, F., and Lowe, D. (2018b). The Patient Concerns Inventory integrated as part of routine head and neck cancer follow-up consultations: frequency, case-mix, and items initiated by the patient. Ann. R Coll. Surg. Engl. 100, 209–215. doi: 10.1308/rcsann.2017.0215
Rylands, J., Lowe, D., and Rogers, S. N. (2016). Influence of deprivation on health-related quality of life of patients with cancer of the head and neck in Merseyside and Cheshire. Br. J. Oral Maxillofac. Surg. 54, 669–76. doi: 10.1016/j.bjoms.2016.03.030
Shunmugasundaram, C., Rutherford, C., Butow, P. N., Sundaresan, P., and Dhillon, H. M. (2019). Content comparison of unmet needs self report measures used in patients with head and neck cancer: a systematic review. Psychooncology 28, 2295–2306. doi: 10.1002/pon.5257
Simard, S., Thewes, B., Humphris, G., Dixon, M., Hayden, C., Mireskandari, S., et al. (2013). Fear of cancer recurrence in adult cancer survivors: a systematic review of quantitative studies. J. Cancer Survivor. 7, 300–322. doi: 10.1007/s11764-013-0272-z
Smith, A. B., Costa, D., Galica, J., Lebel, S., Tauber, N., van Helmondt, S. J., et al. (2020). Spotlight on the Fear of Cancer Recurrence Inventory (FCRI). Psychol. Res. Behav. Manag. 13, 1257–1268. doi: 10.2147/PRBM.S231577
Smith, G. I., Yeo, D., Clark, J., Choy, E. T., Gao, K., Oates, J., et al. (2006). Measures of health-related quality of life and functional status in survivors of oral cavity cancer who have had defects reconstructed with radial forearm free flaps. Brit. J. Oral Maxillofac. Sur. 44, 187–192. doi: 10.1016/j.bjoms.2005.06.022
Tauber, N. M., O'Toole, M. S., Dinkel, A., Galica, J., Humphris, G., Lebel, S., et al. (2019). Effect of psychological intervention on fear of cancer recurrence: a systematic review and meta-analysis. J. Clin. Oncol. 37, 2899–2915. doi: 10.1200/JCO.19.00572
Keywords: fear of cancer recurrence, quality of life, patient concerns inventory, head and neck cancer, randomized trial
Citation: Rogers SN, Monssen C, Humphris GM, Lowe D and Kanatas A (2021) Which Head and Neck Cancer Patients Are Most at Risk of High Levels of Fear of Cancer Recurrence. Front. Psychol. 12:671366. doi: 10.3389/fpsyg.2021.671366
Received: 23 February 2021; Accepted: 07 June 2021;
Published: 16 July 2021.
Edited by:
Eun-Jung Shim, Pusan National University, South KoreaReviewed by:
Paul Van De Heyning, University of Antwerp, BelgiumSeockhoon Chung, University of Ulsan, South Korea
Copyright © 2021 Rogers, Monssen, Humphris, Lowe and Kanatas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Simon N. Rogers, c2ltb25uLnJvZ2Vyc0BhaW50cmVlLm5ocy51aw==
†ORCID: Simon N. Rogers orcid.org/0000-0002-5989-6142
Anastasios Kanatas orcid.org/0000-0003-2025-748X
 Simon N. Rogers1,2*†
Simon N. Rogers1,2*† Gerald M. Humphris
Gerald M. Humphris Derek Lowe
Derek Lowe Anastasios Kanatas
Anastasios Kanatas