
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Psychol., 15 April 2021
Sec. Health Psychology
Volume 12 - 2021 | https://doi.org/10.3389/fpsyg.2021.615209
This article is part of the Research TopicThe Psychological and Physiological Benefits of the ArtsView all 84 articles
Introduction: Evidence supporting the use of music interventions to maximize arousal and awareness in adults presenting with a disorder of consciousness continues to grow. However, the brain of a child is not simply a small adult brain, and therefore adult theories are not directly translatable to the pediatric population. The present study aims to synthesize brain imaging data about the neural processing of music in children aged 0-18 years, to form a theoretical basis for music interventions with children presenting with a disorder of consciousness following acquired brain injury.
Methods: We conducted a systematic review with narrative synthesis utilizing an adaptation of the methodology developed by Popay and colleagues. Following the development of the narrative that answered the central question “what does brain imaging data reveal about the receptive processing of music in children?”, discussion was centered around the clinical implications of music therapy with children following acquired brain injury.
Results: The narrative synthesis included 46 studies that utilized EEG, MEG, fMRI, and fNIRS scanning techniques in children aged 0-18 years. From birth, musical stimuli elicit distinct but immature electrical responses, with components of the auditory evoked response having longer latencies and variable amplitudes compared to their adult counterparts. Hemodynamic responses are observed throughout cortical and subcortical structures however cortical immaturity impacts musical processing and the localization of function in infants and young children. The processing of complex musical stimuli continues to mature into late adolescence.
Conclusion: While the ability to process fundamental musical elements is present from birth, infants and children process music more slowly and utilize different cortical areas compared to adults. Brain injury in childhood occurs in a period of rapid development and the ability to process music following brain injury will likely depend on pre-morbid musical processing. Further, a significant brain injury may disrupt the developmental trajectory of complex music processing. However, complex music processing may emerge earlier than comparative language processing, and occur throughout a more global circuitry.
In recent years the number of publications reporting the neural mechanisms involved in music processing has grown exponentially as scientists seek to systematically map the “implicit musical ability of the human brain” (Koelsch et al., 2000, p. 539; Koelsch, 2014, p. 170; Albusac-Jorge and Giménez-Rodríguez, 2015). The impact of music on a broad range of brain processes supports the therapeutic use of music in clinical neurologic populations; including neurorehabilitation, neuro-palliative rehabilitation, and neuro-psychiatry (Altenmüller and Schlaug, 2012; Thaut and Hoemberg, 2014; Chorna et al., 2019). However, current music neuroscience and clinical evidence pertains predominantly to the adult population.
A significant body of behavioral-based evidence indicates that fundamental auditory processing and musical communication are inherent in infants (Stewart, 2008; Trehub, 2010). It is widely accepted that the ability to meaningfully process music is acquired effortlessly from infancy without a requirement for formal training (Stewart, 2008). However the development of the neurophysiological correlates of music processing in childhood remains under-researched and poorly understood (Koelsch et al., 2003; Jeng et al., 2016a). Without a foundational knowledge of music processing in children it is not possible to understand the impact of an acquired brain injury (ABI) on the ability to process music and the potential for interruption to the ongoing development of musical processing. Additionally, a foundational knowledge is required to understand potential clinical implications of music interventions targeted to support recovery and rehabilitation following ABI.
Music is a complex auditory stimulus that consists of various components including melody, rhythm, timbre, and harmony (Altenmüller and Schlaug, 2013). When adults listen to music, these components are perceived as a phenomenological whole, however, they are processed separately through a complex bilateral network of cortical and subcortical regions (Altenmuller, 2001; Peretz and Zatorre, 2005; Levitin and Tirovolas, 2009). The neural substrates underpinning music perception and production vary depending on the musical task, as there is no single music center of the adult human brain (Tramo, 2001; Peretz and Zatorre, 2005; Altenmüller and Schlaug, 2013; Särkämö et al., 2013). This global processing supports the hypothesis that some ability to meaningfully perceive and process music will remain intact despite significant neural damage, potentially making music a unique therapeutic medium for individuals following ABI (Sihvonen et al., 2017).
A child's brain is not simply a smaller version of an adult's brain. Childhood is a period of rapid development and children's brains vary in shape and tissue composition compared to adult brains (Wilke et al., 2003). However, early plasticity theories are insufficient to describe the observed patterns of increased morbidity and cognitive impairment in young children following diffuse ABI (Crowe et al., 2012). Thus, the predominate theory of recovery following ABI in children supports that the developing brain of a child is at a significantly greater risk of adverse outcomes following severe ABI (Anderson and Yeates, 2010). Therefore, adult theories of recovery and rehabilitation cannot be directly translated to the pediatric population. This review was undertaken to develop a theoretical foundation for music-based interventions with children with a severe ABI, particularly those presenting with a disorder of consciousness (DOC).
This systematic review with narrative synthesis was conducted to address the central question “what does brain imaging data reveal about the receptive processing of music in children?” Following the development of the narrative to answer this question, the implications of the results for children with severe ABI are explored. This review is a first step in developing a theory about the neural processing of music in children to support clinical practice in music therapy.
The search strategy was developed in consultation with a medical research librarian. Systematic searches of electronic databases were conducted to retrieve peer-reviewed references for inclusion in the first round of screening. Medline (Ovid), Embase (Ovid), PsycINFO (Ovid), Cochrane, and CINAHL (Ebsco) were searched predominantly using thesaurus terms to maximize the number of retrieved studies. Pubmed was searched, using keywords only, to retrieve items not indexed in Medline. The Medline search strategy was adapted for searching all other databases (see Appendix A for Medline Search Strategy). Thesaurus terms for searching Medline were categorized around four concepts:
• Brain
• Brain/neuro imaging techniques
• Music
• Child (0-18 years of age)
The four concepts were combined using the Boolean “AND.” Results were limited to the English language and children aged 0-18 years. Additional limiters for animals and participants with neuro-developmental disorders and hearing loss were applied. Additional items were identified through hand searching reference lists of relevant retrieved articles. Results from these searches were imported to bibliographic management software (Endnote X8, Thomson Reuters).
Inclusion criteria were intentionally broad to maximize the data available for synthesis. References published in the last 20 years, reporting both electrical and hemodynamic brain imaging data, about the processing of music in neurologically healthy children, aged 0-18 years, and reported in peer-reviewed journals were included. References that reported evoked responses, neural structures, and networks and/or processes involved in the perception and processing of music and/or its individual elements were included. This included single or pure tones, as single tones are the foundation of melody. Further, the perception of music, as opposed to the production of music or active music making, was explored because music-based interventions supporting early recovery from an ABI are likely to involve passive listening rather than active music production (Rollnik and Altenmüller, 2014). See Appendix B for full inclusion and exclusion criteria, and Appendix C for the rationale for the inclusion/exclusion criteria.
Two reviewers independently screened the titles and abstracts of all retrieved references, applying the inclusion and exclusion criteria to determine inclusions for second-round review. The reviewers recorded their decision in a pre-formatted Microsoft Excel document. These results were compared, and in the instance of disagreement a third reviewer independently screened the reference. Once a consensus about reference inclusion was reached, the full-text articles were retrieved for a second round of screening. Again, two reviewers independently screened all retrieved full-text articles with a third reviewer sought for any discrepancies, to determine the full-text inclusions for data extraction and synthesis. Data were extracted from the full-text references into a pre-formatted Microsoft Excel document by the first author.
The development of the narrative synthesis was based on an adaptation of the “Guidance on the Conduct of Narrative Synthesis in Systematic Reviews” developed by Popay et al. (2006). This model supports a text-based approach to the process of data synthesis, and was suited to synthesizing data extracted from studies that report a diverse range of evidence (Popay et al., 2006). In this instance this included diverse imaging methods, diverse musical stimuli, and a broad age range. The Popay et al. (2006) guidance is best suited to synthesis of intervention studies, and given the broad intention of this review, the review was not limited to intervention studies. Therefore, only the second (developing a preliminary synthesis) and third (exploring relationships in the data) steps of the guidance were undertaken.
Database searches were conducted on May 3, 2019, and updated on June 21, 2020. A total of 3,620 references were retrieved, 543 duplicates were removed, resulting in an initial library of 3,077 references. These references were screened and 175 references were included in the second-round full-text screen. After the full-text screen, 41 references were included in the synthesis. An additional five references were located through hand searching. The synthesis included 46 references that employed the following imaging techniques (see Figure 1):
• 33 references employed EEG scanning
• 4 MEG
• 7 fMRI
• 2 fNIRS1
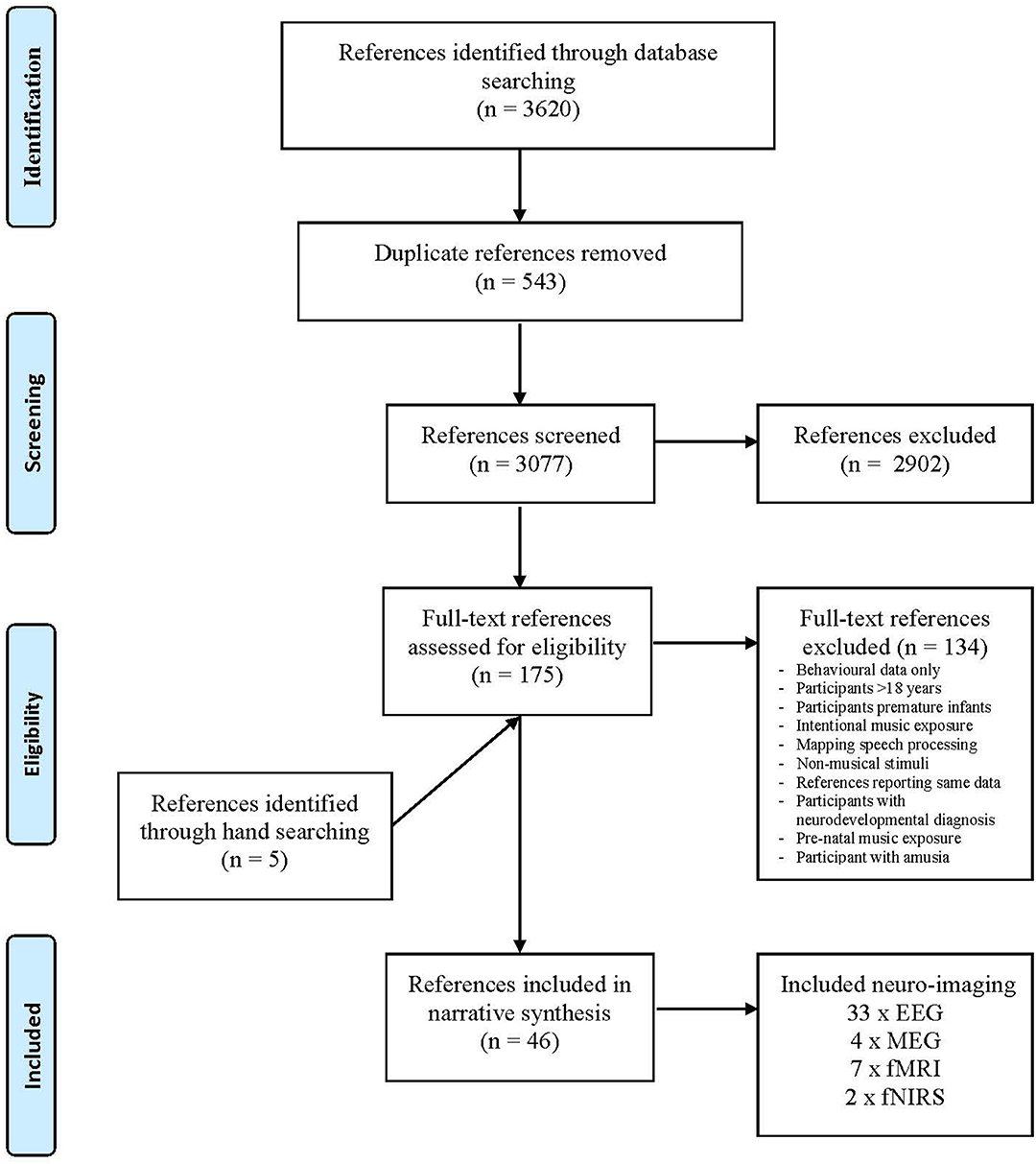
Figure 1. Reference selection process. Adapted from Moher et al. (2009).
All participants in the included references were healthy neuro-typically developing children aged 0–18 years and were not reported to have received formal musical tuition. In references where useful data about the healthy control group were presented as a comparison to a clinical population, data about the healthy controls were included. In references where the intervention group received musical training or intentional musical exposure, but there was useful data reported about the control group who did not receive musical training, data from the control group were included. In the instance where references reported the same data, the later reference was excluded, for example Overy et al. (2004, 2005).
The included references were grouped into age categorizations approximating Piaget's stages of cognitive development (see Table 1):
• Infants aged 0–24 months (24 references; 3 fMRI, 2 NIRS, 1 MEG, and 18 EEG)
• Young children aged 2–6 years (8 references; 3 fMRI, 1 MEG, and 4 EEG).
• Children aged 7–12 years (11 references; 1 fMRI, 2 MEG, and 8 EEG)
• Adolescents aged 13+ years (3 references; 3 EEG)
Where the study design was longitudinal or cross-sectional, the reference was included in the age category that includes the oldest age point in the study.
Results from the included references were initially explored in descriptive format by the first author. The narrative synthesis and discussion were then developed collaboratively with all authors, three of whom are experienced music therapists. Thus, the narrative gives consideration to data that are likely to be most pertinent to music therapists.
This narrative tracks musical processing across a timeline from full-term birth to adolescence, with the key musical milestones most relevant to clinical music therapy practice summarized below in Figure 2.
Hemodynamic data obtained from fMRI and fNIRS scanning established that the infant brain processes music throughout a bilateral network of cortical and subcortical structures (Dehaene-Lambertz et al., 2010; Perani et al., 2010; Homae et al., 2012). Despite hemodynamic responses being mixed (negative/positive) because of immature vasculature, excerpts of classical music elicited a predominantly right hemispheric activation. Peak activation in the right auditory cortex extending into the secondary auditory cortex was reported, which is similar to patterns of temporal activation observed in adult non-musicians (Anderson et al., 2001; Kotilahti et al., 2010; Perani et al., 2010). Activation in the right insula and amygdala-hippocamal complex in the subcortical limbic system was also reported. This suggests music elicits an emotional response in newborns, albeit at a sensory level (Perani et al., 2010). Increasing sensitivity of the right temporoparietal region between 3- and 6-months to chromatic tone sequences may indicate ongoing development of a lateralization effect (Homae et al., 2012).
Distinct but immature electrical responses were recorded by EEG and MEG in infants, and a significant developmental milestone of these musically evoked cortical responses occurred around 6 months of age (Cheour et al., 2002; Jing and Benasich, 2006; Stefanics et al., 2009; Hamalainen et al., 2011; Muenssinger et al., 2013). The components of the auditory-evoked potential (AEP) in infants have longer latencies and greater amplitudes compared to those of older children and adults (He et al., 2009a,b; Marie and Trainor, 2013). However, the presence of a discriminatory response, or immature mismatch negativity (MMN), from 2 days of age to duration, beat, pitch, and interval deviants suggests a fundamental capacity for auditory working memory, pitch discrimination, and melody and beat perception (Cheour et al., 2002; Stefanics et al., 2009; Winkler et al., 2009; Muenssinger et al., 2013; Háden et al., 2015). Virtala et al. (2013) further reported that newborn infants display sensitivity to major-minor and consonant-dissonant tonalities. Mismatch responses with different cortical localizations were elicited by minor and dissonant deviants (Virtala et al., 2013). While noted that these mismatch responses likely indicate automatic discrimination and not preference, they nonetheless indicate cortical sensitivity to different tonalities (Virtala et al., 2013).
The cortical change mechanism for pitch discrimination is hypothesized to emerge around 3-4 months of age (He et al., 2009a,b). Prior to this, the discriminative slow wave positive response to single tone stimuli is likely a result of different neural generators (He et al., 2007). An adult-like MMN to single tone stimuli was recorded after 3 months and had a right hemisphere dominance, with the peak amplitude increasing and the latency decreasing as a function of age (He et al., 2007). Further maturation of the discriminatory response to single tone stimuli was reported at 4 months, with an MMN response elicited that was functionally similar to the MMN of adult controls (He et al., 2009b). The MMN elicited by more complex musical stimuli was reported to have a more protracted developmental trajectory, and at 4 months an intervallic stimulus elicited an immature MMN with a longer latency and increased amplitude compared to its adult counterpart (He et al., 2009a; Stefanics et al., 2009; Tew et al., 2009; Marie and Trainor, 2014).
Further exploring more complex musical stimuli, He and Trainor (2009) utilized an oddball paradigm that was designed so that detection of the missing fundamental pitch would elicit an MMN. No MMN was elicited at 3 months of age, but the authors concluded that a major developmental shift in cortical pitch representation develops shortly thereafter (He and Trainor, 2009). An MMN was elicited in both 4- and 7-month-olds, suggesting they were able to integrate harmonics into a single pitch percept and thus perceive pitch similarly to adults (He and Trainor, 2009).
Infants were able to hold separate memory traces for polyphonic music, and displayed a high voice preference in polyphonic music from 3 months (Tew et al., 2009; Marie and Trainor, 2014). Marie and Trainor (2014) hypothesize this high voice superiority may be innately mediated at the auditory nerve level. Again the MMN-like response to this more complex polyphonic stimulus was reported to have a longer developmental trajectory than the MMN elicited by a single tone stimulus (Tew et al., 2009; Marie and Trainor, 2014).
Jing and Benasich (2006) explored the developmental trajectories of the MMN and other components of the AEP up to 2 years of age, and reported significant morphological changes of the AEP around 6 months. The P150, N250, P350, and N450 were all reliably elicited after this age and the MMN became robust and more adult-like (Jing and Benasich, 2006). From 7 months of age infants were found to have the capacity to entrain to a rhythm, however the cortical level at which this entrainment occurred was not able to be determined (Cirelli et al., 2016). Given that the deeper layers of the auditory cortex are immature in infants under 6 months, it is likely that the components of the AEP (including the MMN) have different neural generators to those of adults (He et al., 2009b). This theory was supported by Hamalainen et al. (2011) who concluded that at 6 months infants begin to utilize analogous cortical areas to adults to detect pitch change in their auditory environments, including the frontal and supratemporal cortices and anterior cingulate cortex (located in the limbic structures). After this age, the MMN component elicited by tone pairs had adult-like latency, distribution, and source locations. The positive deflection that followed this MMN was thought to resemble the adult P3a that is related to an attention-switching mechanism (Hamalainen et al., 2011). Despite the relatively rapid development of the AEP components, the perceived emotional valence of a piece of music may not significantly affect the AEP in the first 12 months of postnatal life (Schmidt et al., 2003).
Extending the knowledge of the cortically recorded AEP components, the frequency following response (FFR) component indicates that essential subcortical structures utilized for tracking pitch are functional from birth (Jeng et al., 2016a,b). Tracking the development of the FFR across time, Jeng et al. (2016b) reported improved subcortical tracking accuracy and pitch strength at 3 months, supporting the hypothesis that neural circuitry involved in processing musical stimuli is refined through development and passive exposure (Jeng et al., 2016b).
The fMRI data included in this age range extend knowledge about global cortical and subcortical hemodynamic responses to musical stimuli. Prabhakar et al. (2018) described increased bilateral hippocampi activation in sleeping toddlers, in response to a previously heard lullaby. This result indicates that music stimulates sub-cortical memory substrates in young children (Prabhakar et al., 2018). In slightly older children, activation of the bilateral temporal lobes, frontal lobes, fusiform, and cerebellum were reported in response to music (Overy et al., 2004; Guerrero Arenas et al., 2016). Wernicke's area and the cerebellum were involved in the processing of both tonal and atonal music indicating involvement of language and motor areas of the brain (Guerrero Arenas et al., 2016). Further, Overy et al. (2004) reported a small right lateralized region that displayed higher activation during melody discrimination compared to rhythm discrimination (Overy et al., 2004).
Fujioka et al. (2006) longitudinally tracked the development of cortical auditory-evoked fields (AEF) of children aged 4–6 years across a year. Maturation of the AEF was indicated by shorter peak latencies and decreased amplitudes across sessions (Fujioka et al., 2006). It was also noted that the violin tone, compared to the noise burst, consistently elicited a larger peak amplitude. This indicates that greater neural resources are required for the processing of more complex auditory stimuli (Fujioka et al., 2006). The EEG data included in this age group also track the ongoing maturation of the AEP. The MMN however, continues to be less robust than its adult counterpart and its maturational time-course may vary depending on different auditory features (Putkinen et al., 2013). No correlation was found between the MMN and informal music activities, which may indicate that development of the MMN is not exposure-dependent (Putkinen et al., 2013).
Implicit knowledge of musical syntax begins to emerge during these early childhood years (Jentschke et al., 2008, 2014; Corrigall and Trainor, 2014). The early right anterior negativity (ERAN) component of the AEP is elicited by violations of musical syntax, and is typically followed by the N5 which is thought to reflect the process of harmonic integration into a mental representation of Western tonality (Koelsch, 2009). Jentschke et al. (2014) reported that at 30 months of age, the ERAN-like response had a longer latency, smaller amplitude, and broader scalp distribution than that observed in adults (Jentschke et al., 2014). The N5 was not observed until after 4 years of age (Jentschke et al., 2008). Corrigall and Trainor (2014) also reported an immature ERAN at 4 years in response to harmonic stimuli but no significant response elicited by irregularities in a melody-only stimulus (Corrigall and Trainor, 2014). These results highlight that young children demonstrate implicit, but immature, cortical representations of Western harmonic key membership before explicit behavioral sensitivity is observed (Jentschke et al., 2008, 2014; Corrigall and Trainor, 2014).
The implicit ability to process music according to Western tonal syntax music rapidly develops during the older childhood period, and is explored in both EEG and fMRI data (Koelsch et al., 2003, 2005; Jentschke et al., 2008). Children in this age group displayed an immature ERAN to strong syntactic irregularities, or syntactic irregularity at a cadence point (Koelsch et al., 2003; Magne et al., 2006; Jentschke and Koelsch, 2009). However, an ERAN was not elicited following a weak syntactic error or a syntactic irregularity in the middle of a musical phrase (Koelsch et al., 2003; Magne et al., 2006; James et al., 2015). Further, the ERAN continued to have a long latency and different scalp distribution compared to its mature counterpart (Jentschke and Koelsch, 2009). Interestingly, at 9 years, male children displayed a left predominance for the ERAN and female children a more bilateral distribution, compared to adults' right predominance (Koelsch et al., 2003). This distribution is similar to the cortical distribution observed in the processing of some aspects of language in children at the same age (Koelsch et al., 2003). Thus there may be overlapping neural generators for music and language processing in children, and the electrical correlates of musical syntactic processing were reported to emerge earlier than those of language (Koelsch et al., 2003; Jentschke and Koelsch, 2009). A music/language overlap was further supported by James et al. (2015) who recorded a left dominant centro-posterior negativity, reminiscent of the semantic (language specific) mismatch N400 in response to an ambiguous musical syntactic error.
FMRI data demonstrated that this syntactic processing takes place in a network of frontal, fronto-temporal, and limbic structures, with a pattern of right hemisphere activation in 10-year-olds, similar to that observed in non-musically trained adults (Koelsch et al., 2005). Irregularities of musical syntax were hypothesized as having a role in generating emotions in music and stimulated a neural network that includes the pars opercularis (part of the inferior frontal gyrus) and the inferior venterolateral pre-motor cortex (located in the frontal lobe) (Koelsch et al., 2005). The included fMRI and EEG data seem to present contradictory results related to the localization of musical syntax processing. However study participants' different ages may indicate a maturational time course, or the different scanning methodologies may be impacted by temporal and spatial factors (Koelsch et al., 2003; Jentschke and Koelsch, 2009).
The majority of references included in this age group explore melodic and harmonic processing, but cortical immaturity also impacts the perception of tempo and rhythm. Parviainen et al. (2019) explored hemispheric processing of stimuli present at different inter-stimulus intervals in 7-8-year-old children. Similar to adults, tones presented monaurally elicited a stronger response in the contralateral auditory cortex. A right hemisphere preference was noted, potentially indicating immaturity of the left hemisphere at 8 years (Parviainen et al., 2019). The protracted development of rhythmic processing was further evident as stimuli presented at a fast pace (short inter-stimulus interval) did not elicit a distinct neuro-electrical response or an induced beta-band entrained oscillation in this age group (Fox et al., 2010; Cirelli et al., 2014). It is hypothesized that the absence of these evoked responses to stimuli presented with short inter-stimulus intervals may result from immaturity in both the auditory and motor cortex (Cirelli et al., 2014).
The AEP in this age cohort was predominated by the P1 and N2 peaks reflecting auditory sensory processes, with the N1 not recorded at 4 years but functional in a 9-year-old cohort to both slow and rapid stimulus presentation (Ceponiene et al., 2002). The N1 generators in the 9-year-olds were anterior to those observed in adults, and this relatively late maturation of the N1 may reflect the immaturity of the frontal lobes at this age and protracted development of the neuronal generators required for auditory sensitivity and orienting (Ceponiene et al., 2002).
The AEP continues to mature into late adolescence (Yamazaki et al., 2018). By late adolescence, right cortical hemispheric dominance for tonal stimuli resembles adult processing, as does the stability of the N1, P1, N2, and P2 components of the AEP components elicited by single tone stimuli (Shahin et al., 2010; Yamazaki et al., 2018). The presence of an audible movie soundtrack continued to have a degrading effect on the latency of the N1 and MMN peaks at 16-17 years of age, despite the stability of the amplitudes of the other AEP components (Mahajan and McArthur, 2011). The development of phase-locking to external auditory stimuli also strengthened with age, with phase-locking in the higher frequency bands (upper-beta and gamma) occurring later than lower bands. This aligns with the typical development of the power of spontaneous oscillation that shifts from lower to higher frequencies (Shahin et al., 2010).
It is evident that beyond being merely functional at birth, an infant's auditory system is sensitive to a variety of musical elements. After the immediate postnatal period, complex musical competence is acquired effortlessly throughout childhood but remains immature into adolescence (Trehub, 2003; Perani et al., 2010; Mahajan and McArthur, 2011; Jentschke et al., 2014). The following discussion explores considerations for clinical practice in music therapy with children who have sustained a severe ABI, specifically for early cognitive rehabilitation for children presenting with a disorder of consciousness (DOC).
The impact of nature vs. nurture has not been explored in this review. Indeed research on the specific effects of musical exposure and experience were excluded because it is increasingly acknowledged that formal music tuition/training in children results in neuroplastic changes observed in adulthood (See Appendix C) (Gaser and Schlaug, 2003; Putkinen et al., 2013). However, the presence of an AEP in infancy, in infants who were not reported to have had intentional pre-natal music exposure, may indicate an innate ability for musical processing (Cheour et al., 2002; Stefanics et al., 2009; Lordier et al., 2019). For example, the presence of the MMN component of the AEP to pitch deviants indicates an ability to recognize pitch change, the foundation of melody processing (Stefanics et al., 2009; Háden et al., 2015). However, the infant AEP is likely mediated by different neural pathways than its adult counterpart (Werner, 2012). Described grossly, in adults, musical stimuli project from the cochlear to the thalamus and then the auditory cortex, followed by triggering of cortical attentional resources toward musical events that may be of interest. Meaning may then be attributed to the music depending on previous experience and emotional context (Tervaniemi and Brattico, 2004). Despite the relative maturity of the auditory brainstem at birth, the cortex remains immature with the frontal lobes continuing to mature into adolescence (He and Trainor, 2009; Mahajan and McArthur, 2011; Werner, 2012). It is hypothesized that prior to maturation of the thalamo-cortical pathways after 6 months of age, AEPs likely stem from reticular formation inputs to the cortex (Werner, 2012). The reticular formation plays a fundamental role in arousal and consciousness (Mangold and Das, 2020). This may be of clinical relevance because music may support increasing arousal or potentially sustaining states of optimal arousal, through stimulating pre-attentive sensory processing.
The transmission of musically evoked response through the immature brain occurs more slowly and less efficiently than similar responses in adults (Shahin et al., 2010). The components of the infant AEP have a longer latency and variable amplitude (Cheour et al., 2002; He et al., 2007, 2009a). Increased myelination, maturation of synaptic connections, and synaptic pruning lead to increased localization and efficiency of musically elicited neuro-electrical transmissions (Magne et al., 2006; Fujioka and Ross, 2008; Giedd and Rapoport, 2010). ABI in children is also known to negatively impact the processing speed of cognitive tasks in children (Anderson et al., 2012). Children presenting with a DOC require stimulation to support optimal arousal, yet overstimulation may have a detrimental impact on recovery (Pool and Magee, 2016). The pacing and duration of musical experience should be closely monitored, particularly in children who may have a limited repertoire of behaviors to indicate distress or agitation. Repetition of musical material may support optimal processing. The impact of competing sensory stimuli needs to be considered carefully when working with children as pre-attentive processing of a single tone stimuli was reported to be less accurate when a competing auditory stimulus was present (Mahajan and McArthur, 2011).
Current evidence indicates that in healthy right-handed, non-musically trained adults, the prime musical architectures are predominantly right lateralized and are subserved by additional left hemisphere structures (Sihvonen et al., 2019). Language is somewhat the mirror of this with a left hemisphere dominance (Levman et al., 2017). Further, whilst some anatomical overlap is noted, the processing of music and speech is functionally separate in non-musically-trained adults (Abrams et al., 2011; Peretz et al., 2015). This distinction is less clear in children (see Figure 2) (Overy et al., 2004; Tervaniemi and Brattico, 2004; Putkinen et al., 2013; Jentschke et al., 2014; James et al., 2015; Jeng et al., 2016b; Sihvonen et al., 2019). While there is some evidence of a right lateralization in melodic processing, children may process music and language more similarly, and utilize a greater network of cortical structures, than adults (Koelsch et al., 2003; Perani et al., 2010; James et al., 2015). Koelsch et al. (2003) reported that at 9 years of age, males displayed a left predominance, and females a bilateral pattern, for the processing of musical syntax. It was concluded that there may be overlapping generators for the processing of musical and language syntax in children (Koelsch et al., 2003). This pattern of bilateral activation supports that a right hemispheric insult may not impair the ability to process music in children in the same way it would in adults. Further, the electrical responses elicited by musical-syntactic processing were recorded at a younger age than a comparative electrical response to language-syntactic processing (Koelsch et al., 2003; Jentschke et al., 2008). This supports a hypothesis that complex musical processing may mature earlier than language processing (Koelsch et al., 2003).
Retention of cognitive skills established before the brain insult is increasingly well-documented (Crowe et al., 2019) and it is feasible that the same retention of musical skills may occur. Gentle et al. (2015) reported the clinical case of a 5-year-old female who retained singing functioning following a profound ABI that resulted in an incomplete right hemispherectomy. The neurological resilience of song functioning was highlighted to support an argument for the potential of song in cognitive rehabilitation (Gentle et al., 2015). For the child described, it was likely that the foundational skills of musical processing were established prior to the age at insult.
The implicit understanding of melody and harmony based around Western scale structures (musical syntax) is thought to be one of the most sophisticated perceptive musical abilities, and subsequently develops later in childhood than the processing of basic musical elements (Magne et al., 2006; Jentschke and Koelsch, 2009; Trehub, 2013). Development of an implicit understanding of tonal syntax requires enculturation through exposure. Thus the discriminatory response (ERAN) elicited by syntactic incongruence does not begin to emerge until after 2 years of age (see Figure 2) (Jentschke et al., 2014). This development of a pre-attentive understanding of major-minor tonal music supports an argument for the use of melodically and harmonically regular music in the early cognitive rehabilitation for those children who have been pre-morbidly enculturated to Western tonal music. Such music may provide meaningful sensory stimulation whilst reducing the potential for overstimulation and associated cognitive fatigue. Nursery rhymes typically follow the rules of Western musical syntax, are simple, and may have emotional connections with memories of early parent-child musical interactions. Further, nursery rhymes are sung to support early cognitive, social, emotional, and motor development in infants and toddlers (Trainor, 2002). Thus, nursery rhymes may be an ideal stimulus to maximize arousal and awareness, to support optimal functional recovery in infants and children following ABI, especially in the presence of language deficits (Rosenfeld and Dun, 1999; Bower and Shoemark, 2012; Pool and Magee, 2016). Similarly, for adolescents, an argument could be made for the use of harmonically simple, pre-morbidly familiar popular music that follows the rules of Western tonal music.
Extending this evidence of enculturation and pre-attentive responses to musical syntax, emotional responses and increased arousal may result from suspension and fulfillment of musical expectancies (Koelsch, 2005; Koelsch et al., 2005; Steinbeis et al., 2006). An emerging ERAN before 3 years of age would indicate that children might also experience a similar arousal or emotional response. This has clinical implications as harmonic and melodic manipulation of pre-composed songs can be used to create expectancy and increased arousal (Bower, 2010; Bower et al., 2014). For example Bower et al. (2014) reported that a “recruitment” response in a 10-year-old child was elicited in quintessential moments in familiar songs in the early stages of recovery following profound ABI. This observed behavioral response indicated an increase in arousal and was interpreted as an intentional, cognitively mediated response from a child in an overwhelming state of low-responsiveness and agitation (Bower et al., 2014).
Studies included in this review indicate that similarly to adults, infants and children process music throughout a broad network of cortical and sub-cortical structures and that discriminatory responses to minor and dissonant music are recorded from infancy (Virtala et al., 2013). However, it is vital to note that simply being able to differentiate discrete musical elements does not equate with musical preference nor that there is any meaning attributed to this beyond that there is simply a change in the auditory stimuli (Trehub, 2013; Virtala et al., 2013). The majority of references included in this review employed “scientific reductionism” in defining music and explaining the neural structures and networks involved in its perception and processing (Tivadar and Murray, 2019, p. 85). Individual components of music (rhythm, pitch, harmony, etc.) are necessarily operationalized under experimental conditions (Hunt, 2015). While this deconstructed approach to exploring music for this review was fundamental in objectively analyzing brain responses, it potentially produces a limited definition of music as merely “organized sound” (Cross, 2003, p. 106). Further, the social or emotional meaning attached to musical experiences in children; from parental singing in infancy, to personal music listening as a leisure activity, to musical contexts for identity formation in adolescence was not captured in this review. Especially in a clinical scenario, music and musical experiences should be conceptualized as actions that occur within individual, social, and broader cultural contexts (Elliott and Silverman, 2013). Thorough considerations should be given to past musical experiences, familial music preferences, and the ecology of the clinical setting. There is also an argument for whole music (e.g., song or musical work) to be utilized in therapeutic interventions following ABI as the multiple combined elements that form music are likely to stimulate a broader range of intact cortical function than a single element alone to support increased arousal and awareness (Koelsch et al., 2005; Guerrero Arenas et al., 2016).
There are a number of limitations of the search strategy employed in this review. Firstly, the search strategy was kept intentionally broad to maximize the data included in the narrative. This has however resulted in a large number of references being included in the narrative and a subsequent reduction in the detail that could be explored. The use of the search term “EEG” rather than AEP may have reduced the number of references available for inclusion. The search terms were based around scanning technologies utilized within music therapy research, and not the elicited responses as may be the preferred search strategy in psychology and auditory sciences. Further the diverse scanning methods, ages, musical stimuli, and study designs included in the review mean it was not possible to undertake meta-analysis. Studies that research the processing of single and/or pure tone stimuli were included and it is acknowledged that some researchers and clinicians may not classify pure tone stimuli as music/musical. However, the authors argue that a single tone is the building block of melody and thus the decision was made to include these references. Additionally, in clinical practice in very early brain injury rehabilitation, single tones may be introduced to assess responsiveness to a basic musical stimuli (Magee et al., 2015).
EEG was the most utilized scanning methodology with 33/46 of the included references using this technology and this likely represents the ease of use and cost effectiveness of this method (Tivadar and Murray, 2019). It does however indicate a paucity of hemodynamic-based research (fNIRS and fMRI) that reports the neural substrates of music processing in infants and children. Further, low participant numbers were noted in a significant proportion of studies, particularly fMRI, fNIRS, and MEG. The cost, availability, and feasibility of having children remain still for extended periods of time likely explains these low participant numbers but again indicates the lack of hemodynamic-based research methodology in this area.
There is an uneven age distribution in the included references. The 0-2 years age group contained 24/46 of the included references and even within this group the majority of infants were <6 months of age. This likely reflects the pragmatic aspects of scanning methodology and ease of scanning young infants whilst asleep. Nonetheless, this age disparity represents large gaps in our understanding of musical processing in older infants, children, and adolescents. The role of gender in musical processing also warrants further exploration with only one reference exploring this (Koelsch et al., 2003). The total cerebral volume of female children peaks ~3 years earlier than males, and while correlation between volume and function is complex, it may be possible that musical syntax is not the only aspect of musical processing that is impacted by sex (Koelsch et al., 2003; Lenroot and Giedd, 2006).
The included narrative and discussion highlights the following considerations for music therapists working with pediatric neurologic populations:
• Infants are born (at term) with the neural foundations for processing basic musical elements. However, we do not know what meaning infants place on these musical experiences. It is likely that meaning is developed through interactions with an attuned partner and through ongoing exposure (Trevarthen and Aitken, 2001; Malloch and Trevarthen, 2018).
• Infants have a high voice preference when presented with polyphonic music and considerations should be given to the use of accompanying instruments, for example guitar or piano accompaniment should be pitched lower than a vocal melody.
• The responses to more complex musical stimuli have a more protracted developmental trajectory and considerations should be given to this when working with infants and young children following ABI to reduce the potential for overstimulation. Acapella singing may be optimal to stimulate intact musical processing whilst supporting fundamental interpersonal interaction.
• Following ABI, pre-morbidly attained musical skills are likely to remain intact. Given this and the emerging evidence that some elements of music processing may develop before corresponding language processing, music may be an ideal therapeutic medium to support early arousal, awareness, and cognitive stimulation for pediatric patients with an ABI.
• It is reasonable to hypothesize that an ABI will impair the ongoing maturation of music processing, and thorough consideration must be given to the age and musical development time point at which a child sustained the ABI when designing music therapy programs.
• The use of syntactically simple, melodically and harmonically regular music, that does not violate musical syntax, may reduce the potential for cognitive fatigue and negative impacts of overstimulation in children and adolescents presenting with a DOC.
• Clinicians should consider the musical manipulation of syntax and its resolution to support increased arousal in children and young people presenting in states of low arousal (including DOC) following ABI.
• It is unlikely that an ABI sustained in a childhood will impact musical processing in the same way it would an adult, and therefore adult research is not immediately translatable to the pediatric population.
A foundational understanding of the neurophysiological processing of music is essential for clinicians intending to utilize music as a therapeutic medium for infants, children, and adolescents following ABI. This knowledge encourages an understanding of what music processing skills may be present pre-morbidly, how these skills are impacted by the ABI, and finally how the ABI may alter the ongoing development of musical processing abilities. While evidence supporting the use of music interventions for adults with a DOC following ABI is expanding, the pediatric population remains severely under-represented in the current literature. The human brain reaches ~90% of adult size by 5 years but the relationships between the size of a particular brain area and its functional capacity is “staggeringly complex” as experience plays a substantial role in connectivity and functionality (Lenroot and Giedd, 2006, p. 726; Tau and Peterson, 2010). As such, key researchers promote vigilance against “adultomorphism” (Trehub, 2013, p. 1). Whilst there are some similarities in the way children and adults process music, results of this review add weight to the argument that adult knowledge is not immediately translatable to the pediatric population. Infants and children process music more slowly, use different cortical areas for this processing, and potentially lack the memory and emotional associations experienced by adults (Koelsch, 2005). Despite this, the capacity for processing the basic musical elements is present from birth and therefore may be argued to be innate. Evidence supports that rehabilitative interventions should commence in the early days and weeks following ABI to hasten emergence to consciousness and maximize functional recovery (Catroppa et al., 2016; Giacino et al., 2018). Results of this review support that music may be ideally situated to achieve this aim. It is noted that this review only explored the processing of music, and additional stimuli and/or rehabilitative therapies should be considered as part of multidisciplinary care of this vulnerable population.
There is an obvious need for further music neuroscience research with children and music therapy research with children following ABI. Research that longitudinally tracks the developmental trajectory of musical milestones would be enormously beneficial to the field. Research that maps the electrical processes and hemodynamic responses to whole music, as opposed to discrete musical elements, and explores potential emotional responses to music is also required. Exploring the processing of music in children following ABI and the potential disruption caused by an ABI to the developmental trajectory of music processing would develop clinical practice in music therapy. Finally, research that objectively describes any effect of music-based interventions targeted for consciousness rehabilitation with children following ABI is needed.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
JB ran the database searches with assistance from a medical research librarian, undertook data extraction, and drafted the manuscript. JB, WM, CC, and FB screened titles and abstracts in the first round screening, undertook full text review in the second round screening, and collaboratively developed the narrative synthesis and considerations for clinical practice. WM, CC and FB edited the manuscript. All authors contributed to the article and approved the submitted version.
JB was supported by a Research Training Grant at The University of Melbourne.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
JB would like to acknowledge the contribution of Poh Chua, Medical Research Librarian, The Royal Children's Hospital Melbourne, for her guidance with establishing the database search strategies.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyg.2021.615209/full#supplementary-material
1. ^See Supplementary Material for a description the included scanning methods.
Abrams, D. A., Bhatara, A., Ryali, S., Balaban, E., Levitin, D. J., and Menon, V. (2011). Decoding temporal structure in music and speech relies on shared brain resources but elicits different fine-scale spatial patterns. Cerebral Cortex 21, 1507–1518. doi: 10.1093/cercor/bhq198
Albusac-Jorge, M., and Giménez-Rodríguez, F. J. (2015). Citation index and scientific production on the neuroscience of music: a bibliometric study. Psychomusicology 25, 416–422. doi: 10.1037/pmu0000128
Alipour, Z. M., Mohammadkhani, S., and Khosrowabadi, R. (2019). Alteration of perceived emotion and brain functional connectivity by changing the musical rhythmic pattern. Exp. Brain Res. 237, 2607–2619. doi: 10.1007/s00221-019-05616-w
Altenmüller, E., and Schlaug, G. (2012). Music, Brain, And Health: Exploring Biological Foundations of Music's Health Effects, eds R. MacDonald, G. Kreut, & L. Mitchell. New York, NY: Oxford University Press. doi: 10.1093/acprof:oso/9780199586974.003.0002
Altenmüller, E., and Schlaug, G. (2013). Neurobiological aspects of neurologic music therapy. Music Med. 5, 210–216. doi: 10.1177/1943862113505328
Altenmuller, E. O. (2001). How many music centers are in the brain? Ann. N. Y. Acad. Sci. 930, 273–280. doi: 10.1111/j.1749-6632.2001.tb05738.x
Anderson, A. W., Marois, R., Colson, E. R., Peterson, B. S., Duncan, C. C., Ehrenkranz, R. A., et al. (2001). Neonatal auditory activation detected by functional magnetic resonance imaging. Magn. Reson. Imaging 19, 1–5. doi: 10.1016/S0730-725X(00)00231-9
Anderson, V., Godfrey, C., Rosenfeld, J. V., and Catroppa, C. (2012). 10 years outcome from childhood traumatic brain injury. Int. J. Dev. Neurosci. 30, 217–224. doi: 10.1016/j.ijdevneu.2011.09.008
Anderson, V., and Yeates, K. O. (2010). Pediatric Traumatic Brain Injury : New Frontiers in Clinical and Translational Research. Cambridge, United Kingdom: Cambridge University Press. doi: 10.1017/CBO9780511676383
Bower, J. (2010). Music therapy for a 10-year old child experiencing agitation during posttraumatic amnesia: an intrinsic mixed methods case study. (Master of Music), The University of Melbourne, Melbourne, Australia.
Bower, J., Catroppa, C., Grocke, D., and Shoemark, H. (2014). Music therapy for early cognitive rehabilitation post-childhood TBI: an intrinsic mixed methods case study. Dev. Neurorehabil. 17, 339–346. doi: 10.3109/17518423.2013.778910
Bower, J., and Shoemark, H. (2012). Music therapy for the pediatric patient experiencing agitation during posttraumatic amnesia: constructing a foundation from theory. Music Med. 4, 146–152. doi: 10.1177/1943862112442227
Catroppa, C., Anderson, V., Beauchamp, M. H., and Yeates, K. O. (2016). New Frontiers in Pediatric Traumatic Brain Injury : An Evidence Base for Clinical Practice: New York, NY: Routledge. doi: 10.4324/9780203868621
Ceponiene, R., Rinne, T., and Naatanen, R. (2002). Maturation of cortical sound processing as indexed by event-related potentials. Clin. Neurophysiol. 113, 870–882. doi: 10.1016/S1388-2457(02)00078-0
Cheour, M., Kushnerenko, E., Ceponiene, R., Fellman, V., and Naatanen, R. (2002). Electric brain responses obtained from newborn infants to changes in duration in complex harmonic tones. Dev. Neuropsychol. 22, 471–479. doi: 10.1207/S15326942DN2202_3
Chorna, O., Filippa, M., De Almeida, J. S., Lordier, L., Monaci, M. G., Hüppi, P., et al. (2019). Neuroprocessing mechanisms of music during fetal and neonatal development: a role in neuroplasticity and neurodevelopment. Neural Plast. 2019, 1–9. doi: 10.1155/2019/3972918
Cirelli, L. K., Bosnyak, D., Manning, F. C., Spinelli, C., Marie, C., Fujioka, T., et al. (2014). Beat-induced fluctuations in auditory cortical beta-band activity: using EEG to measure age-related changes. Front. Psychol. 5:742. doi: 10.3389/fpsyg.2014.00742
Cirelli, L. K., Spinelli, C., Nozaradan, S., and Trainor, L. J. (2016). Measuring neural entrainment to beat and meter in infants: Effects of music background. Front. Neurosci. 10. doi: 10.3389/fnins.2016.00229
Corrigall, K. A., and Trainor, L. J. (2014). Enculturation to musical pitch structure in young children: evidence from behavioral and electrophysiological methods. Dev. Sci. 17, 142–158. doi: 10.1111/desc.12100
Cross, I. (2003). Music as a biocultural phenomenon. Ann. N. Y. Acad. Sci. 999, 106–111. doi: 10.1196/annals.1284.010
Crowe, L. M., Arana, C. C., and Catroppa, C. (2019). “Traumatic brain injury in very early childhood,” in Handbook of Medical Neuropsychology. [electronic resource] : Applications of Cognitive Neuroscience (Second ed.), eds C. L. Armstrong and L. Morrow (New York, NY: Springer). doi: 10.1007/978-3-030-14895-9_3
Crowe, L. M., Catroppa, C., Babl, F. E., Rosenfeld, J. V., and Anderson, V. (2012). Timing of traumatic brain injury in childhood and intellectual outcome. J. Pediatr. Psychol. 37, 745–754. doi: 10.1093/jpepsy/jss070
Dehaene-Lambertz, G., Montavont, A., Jobert, A., Allirol, L., Dubois, J., Hertz-Pannier, L., et al. (2010). Language or music, mother or Mozart? Structural and environmental influences on infants' language networks. Brain Language 114, 53–65. doi: 10.1016/j.bandl.2009.09.003
Elliott, D. J., and Silverman, M. (2013). “Why music matters: philosophical and cultural foundations,” in Music, Health and Wellbeing, eds R. A. R. MacDonald, G. Kreutz, and L. Mitchell (London: Oxford University Press), 942–1447. doi: 10.1093/acprof:oso/9780199586974.003.0003
Fox, A. M., Anderson, M., Reid, C., Smith, T., and Bishop, D. V. (2010). Maturation of auditory temporal integration and inhibition assessed with event-related potentials (ERPs). BMC Neurosci. 11:49. doi: 10.1186/1471-2202-11-49
Fujioka, T., and Ross, B. (2008). Auditory processing indexed by stimulus-induced alpha desynchronization in children. Int. J. Psychophysiol. 68, 130–140. doi: 10.1016/j.ijpsycho.2007.12.004
Fujioka, T., Ross, B., Kakigi, R., Pantev, C., and Trainor, L. J. (2006). One year of musical training affects development of auditory cortical-evoked fields in young children. Brain 129, 2593–2608. doi: 10.1093/brain/awl247
Gaser, C., and Schlaug, G. (2003). Gray matter differences between musicians and nonmusicians. Ann. N. Y. Acad. Sci. 999, 514–517. doi: 10.1196/annals.1284.062
Gentle, E. C., Barker, M., and Bower, J. (2015). Preservation of singing functioning in a 5 year-old following severe right-sided traumatic brain injury: insights into the neurological resilience of song from pediatric music therapy. Music Med. 7, 14–19. doi: 10.47513/mmd.v7i3.405
Giacino, J. T., Katz, D. I., Schiff, N. D., Whyte, J., Ashman, E. J., Ashwal, S., et al. (2018). Comprehensive systematic review update summary: disorders of consciousness. Arch. Phys. Med. Rehabil. 99, 1710–1719. doi: 10.1016/j.apmr.2018.07.002
Giedd, J. N., and Rapoport, J. L. (2010). Structural MRI of pediatric brain development: what have We learned and where are we going? Neuron 67, 728–734. doi: 10.1016/j.neuron.2010.08.040
Guerrero Arenas, C., Hidalgo Tobon, S. S., Dies Suarez, P., Barragan Perez, E., Castro Sierra, E., Garcia, J., et al. (2016). Strategies for tonal and atonal musical interpretation in blind and normally sighted children: an fMRI study. Brain Behav. 6:e00450. doi: 10.1002/brb3.450
Háden, G. P., Németh, R., Török, M., and Winkler, I. (2015). Predictive processing of pitch trends in newborn infants. Brain Res. 1626, 14–20. doi: 10.1016/j.brainres.2015.02.048
Hamalainen, J. A., Ortiz-Mantilla, S., and Benasich, A. A. (2011). Source localization of event-related potentials to pitch change mapped onto age-appropriate MRIs at 6 months of age. Neuroimage 54, 1910–1918. doi: 10.1016/j.neuroimage.2010.10.016
He, C., Hotson, L., and Trainor, L. J. (2007). Mismatch response to pitch changes in early infancy. J. Cogn. Neurosci. 19, 878–892. doi: 10.1162/jocn.2007.19.5.878
He, C., Hotson, L., and Trainor, L. J. (2009a). Development of infant mismatch responses to auditory pattern changes between 2 and 4 months old. Eur. J. Neurosci. 29, 861–867. doi: 10.1111/j.1460-9568.2009.06625.x
He, C., Hotson, L., and Trainor, L. J. (2009b). Maturation of cortical mismatch responses to occasional pitch change in early infancy: effects of presentation rate and magnitude of change. Neuropsychologia 47, 218–229. doi: 10.1016/j.neuropsychologia.2008.07.019
He, C., and Trainor, L. J. (2009). Finding the pitch of the missing fundamental in infants. J. Neurosci. 29, 7718–8822. doi: 10.1523/JNEUROSCI.0157-09.2009
Homae, F., Watanabe, H., Nakano, T., and Taga, G. (2012). Functional development in the infant brain for auditory pitch processing. Hum. Brain Mapp. 33, 596–608. doi: 10.1002/hbm.21236
Hunt, A. M. (2015). Boundaries and potentials of traditional and alternative neuroscience research methods in music therapy research. Front. Hum. Neurosci. 9:342. doi: 10.3389/fnhum.2015.00342
James, C. E., Cereghetti, D. M., Roullet Tribes, E., and Oechslin, M. S. (2015). Electrophysiological evidence for a specific neural correlate of musical violation expectation in primary-school children. Neuroimage 104, 386–397. doi: 10.1016/j.neuroimage.2014.09.047
Jeng, F. C., Lin, C. D., Chou, M. S., Hollister, G. R., Sabol, J. T., Mayhugh, G. N., et al. (2016b). Development of subcortical pitch representation in three-month-old chinese infants. Perceptual Motor Skills 122, 123–135. doi: 10.1177/0031512516631054
Jeng, F. C., Lin, C. D., and Wang, T. C. (2016a). Subcortical neural representation to Mandarin pitch contours in American and Chinese newborns. J. Acoustical Soc. Am. 139:EL190. doi: 10.1121/1.4953998
Jentschke, S., Friederici, A. D., and Koelsch, S. (2014). Neural correlates of music-syntactic processing in two-year old children. Dev. Cogn. Neurosci. 9, 200–208. doi: 10.1016/j.dcn.2014.04.005
Jentschke, S., and Koelsch, S. (2009). Musical training modulates the development of syntax processing in children. Neuroimage 47, 735–744. doi: 10.1016/j.neuroimage.2009.04.090
Jentschke, S., Koelsch, S., Sallat, S., and Friederici, A. D. (2008). Children with specific language impairment also show impairment of music-syntactic processing. J. Cogn. Neurosci. 20, 1940–1951. doi: 10.1162/jocn.2008.20135
Jing, H., and Benasich, A. A. (2006). Brain responses to tonal changes in the first two years of life. Brain Dev. 28, 247–256. doi: 10.1016/j.braindev.2005.09.002
Koelsch, S. (2005). Investigating emotion with music: neuroscientific approaches. Ann. N. Y. Acad. Sci. 1060, 412–418. doi: 10.1196/annals.1360.034
Koelsch, S. (2009). Music-syntactic processing and auditory memory: similarities and differences between ERAN and MMN. Psychophysiology 46, 179–190. doi: 10.1111/j.1469-8986.2008.00752.x
Koelsch, S. (2014). Brain correlates of music-evoked emotions. Nat. Rev. Neurosci. 15, 170–180. doi: 10.1038/nrn3666
Koelsch, S., Fritz, T., Schulze, K., Alsop, D., and Schlaug, G. (2005). Adults and children processing music: an fMRI study. Neuroimage 25, 1068–1076. doi: 10.1016/j.neuroimage.2004.12.050
Koelsch, S., Grossmann, T., Gunter, T. C., Hahne, A., Schroger, E., and Friederici, A. D. (2003). Children processing music: electric brain responses reveal musical competence and gender differences. J. Cogn. Neurosci. 15, 683–693. doi: 10.1162/jocn.2003.15.5.683
Koelsch, S., Gunter, T., Friederici, A. D., and Schroger, E. (2000). Brain indices of music processing: “Non-musicians” are musical. J. Cogn. Neurosci. 12, 520–541. doi: 10.1162/089892900562183
Kotilahti, K., Nissila, I., Nasi, T., Lipiainen, L., Noponen, T., Merilainen, P., et al. (2010). Hemodynamic responses to speech and music in newborn infants. Hum. Brain Mapp. 31, 595–603. doi: 10.1002/hbm.20890
Lenroot, R. K., and Giedd, J. N. (2006). Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci. Biobehav. Rev. 30, 718–729. doi: 10.1016/j.neubiorev.2006.06.001
Levitin, D. J., and Tirovolas, A. K. (2009). Current advances in the cognitive neuroscience of music. Ann. N. Y. Acad. Sci. 1156, 211–231. doi: 10.1111/j.1749-6632.2009.04417.x
Levman, J., MacDonald, P., Lim, A. R., Forgeron, C., and Takahashi, E. (2017). A pediatric structural MRI analysis of healthy brain development from newborns to young adults. Hum. Brain Mapp. 12:5931. doi: 10.1002/hbm.23799
Lordier, L., Loukas, S., Grouiller, F., Vollenweider, A., Vasung, L., Meskaldij, D.-E., et al. (2019). Music processing in preterm and full-term newborns: a psychophysiological interaction (PPI) approach in neonatal fMRI. Neuroimage 185, 857–864. doi: 10.1016/j.neuroimage.2018.03.078
Magee, W., Ghetti, C., and Moyer, A. (2015). Feasibility of the music therapy assessment tool for awareness in disorders Of consciousness (MATADOC) for use with pediatric populations. Front. Psychol. 6:698. doi: 10.3389/fpsyg.2015.00698
Magne, C., Schon, D., and Besson, M. (2006). Musician children detect pitch violations in both music and language better than nonmusician children: behavioral and electrophysiological approaches. J. Cogn. Neurosci. 18, 199–211. doi: 10.1162/jocn.2006.18.2.199
Mahajan, Y., and McArthur, G. (2011). The effect of a movie soundtrack on auditory event-related potentials in children, adolescents, and adults. Clin. Neurophysiol. 122, 934–941. doi: 10.1016/j.clinph.2010.08.014
Malloch, S., and Trevarthen, C. (2018). The human nature of music. Front. Psychol. 9. doi: 10.3389/fpsyg.2018.01680
Mangold, S. A., and Das, M. J. (2020). Neuroanatomy, Reticular Formation. StatPearls (Internet). Retrieved from: https://www.ncbi.nlm.nih.gov/books/NBK556102/ (accessed May 20, 2020).
Marie, C., and Trainor, L. J. (2013). Development of simultaneous pitch encoding: infants show a high voice superiority effect. Cerebral Cortex 23, 660–669. doi: 10.1093/cercor/bhs050
Marie, C., and Trainor, L. J. (2014). Early development of polyphonic sound encoding and the high voice superiority effect. Neuropsychologia 57, 50–58. doi: 10.1016/j.neuropsychologia.2014.02.023
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., and Group, P. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6:e1000097. doi: 10.1371/journal.pmed.1000097
Muenssinger, J., Matuz, T., Schleger, F., Kiefer-Schmidt, I., Goelz, R., Wacker-Gussmann, A., et al. (2013). Auditory habituation in the fetus and neonate: an fMEG study. Dev. Sci. 16, 287–295. doi: 10.1111/desc.12025
Overy, K., Norton, A., Cronin, K., Winner, E., and Schlaug, G. (2005). Examining rhythm and melody processing in young children using FMRI. Ann. N. Y. Acad. Sci. 1060, 210–218. doi: 10.1196/annals.1360.014
Overy, K., Norton, A. C., Cronin, K. T., Gaab, N., Alsop, D. C., Winner, E., et al. (2004). Imaging melody and rhythm processing in young children. Neuroreport 15, 1723–1726. doi: 10.1097/01.wnr.0000136055.77095.f1
Parviainen, T., Helenius, P., and Salmelin, R. (2019). Children show hemispheric differences in the basic auditory response properties. Hum. Brain Mapp. 40, 2699–2710. doi: 10.1002/hbm.24553
Perani, D., Saccuman, M. C., Scifo, P., Spada, D., Andreolli, G., Rovelli, R., et al. (2010). Functional specializations for music processing in the human newborn brain. Proc. Natl. Acad. Sci. U.S.A. 107, 4758–4763. doi: 10.1073/pnas.0909074107
Peretz, I., Vuvan, D., Lagrois, M.-É., and Armony, J. L. (2015). Neural overlap in processing music and speech. Philosophical Transactions 370, 1–8. doi: 10.1098/rstb.2014.0090
Peretz, I., and Zatorre, R. J. (2005). Brain organization for music processing. Annu. Rev. Psychol. 56, 89–114. doi: 10.1146/annurev.psych.56.091103.070225
Pool, J., and Magee, W. L. (2016). Music in the treatment of children and youth with prolonged disorders of consciousness. Front. Psychol. 7:202. doi: 10.3389/fpsyg.2016.00202
Popay, J., Roberts, H., Sowden, A., Petticrew, M., Arai, L., Rodgers, M., et al. (2006). Guidance on the Conduct of Narrative Synthesis in Systematic Reviews. Southampton: ESRC Methods Programme.
Prabhakar, J., Johnson, E. G., Nordahl, C. W., and Ghetti, S. (2018). Memory-related hippocampal activation in the sleeping toddler. Proc. Natl. Acad. Sci. U.S.A. 115, 6500–6505. doi: 10.1073/pnas.1805572115
Putkinen, V., Tervaniemi, M., and Huotilainen, M. (2013). Informal musical activities are linked to auditory discrimination and attention in 2-3-year-old children: an event-related potential study. Eur. J. Neurosci. 37, 654–661. doi: 10.1111/ejn.12049
Rollnik, J. D., and Altenmüller, E. (2014). Music in disorders of consciousness. Front. Neurosci. 8:190. doi: 10.3389/fnins.2014.00190
Rosenfeld, J. V., and Dun, B. (1999). “Music therapy in children with severe traumatic brain injury,” in Music Medicine 3, eds R. R. Pratt and D. E. Grocke (Victoria, BC: The University of Melbourne).
Särkämö, T., Tervaniemi, M., and Huotilainen, M. (2013). Music perception and cognition: development, neural basis, and rehabilitative use of music. WIREs Cognitive Sci. 4, 441–451. doi: 10.1002/wcs.1237
Schmidt, L. A., Trainor, L. J., and Santesso, D. L. (2003). Development of frontal electroencephalogram (EEG) and heart rate (ECG) responses to affective musical stimuli during the first 12 months of post-natal life. Brain Cognition 52, 27–32. doi: 10.1016/S0278-2626(03)00006-X
Shahin, A. J., Trainor, L. J., Roberts, L. E., Backer, K. C., and Miller, L. M. (2010). Development of auditory phase-locked activity for music sounds. J. Neurophysiol. 103, 218–229. doi: 10.1152/jn.00402.2009
Sihvonen, A. J., Särkäm,ö, T., Leo, V., Tervaniemi, M., Altenmüller, E., and Soinila, S. (2017). Music-based interventions in neurological rehabilitation. Lancet Neurol. 16, 648–660. doi: 10.1016/S1474-4422(17)30168-0
Sihvonen, A. J., Särkäm,ö, T., Rodríguez-Fornells, A., Ripollés, P., Münte, T. F., and Soinila, S. (2019). Neural architectures of music – insights from acquired amusia. Neurosci. Biobehav. Rev. 107, 104–114. doi: 10.1016/j.neubiorev.2019.08.023
Stefanics, G., Haden, G. P., Sziller, I., Balazs, L., Beke, A., and Winkler, I. (2009). Newborn infants process pitch intervals. Clin. Neurophysiol. 120, 304–308. doi: 10.1016/j.clinph.2008.11.020
Steinbeis, N., Koelsch, S., and Sloboda, J. A. (2006). The role of harmonic expectancy violations in musical emotions: evidence from subjective, physiological, and neural responses. J. Cognitive Neurosci. 8:1380. doi: 10.1162/jocn.2006.18.8.1380
Stewart, L. (2008). Fractionating the musical mind: insights from congenital amusia. Curr. Opin. Neurobiol. 18, 127–130. doi: 10.1016/j.conb.2008.07.008
Tau, G. Z., and Peterson, B. S. (2010). Normal development of brain circuits. Neuropsychopharmacology 35, 147–168. doi: 10.1038/npp.2009.115
Tervaniemi, M., and Brattico, E. (2004). From sounds to music towards understanding the neurocognition of musical sound perception. J. Conscious. Stud. 11, 9–27.
Tew, S., Fujioka, T., He, C., and Trainor, L. (2009). Neural representation of transposed melody in infants at 6 months of age. Ann. N. Y. Acad. Sci. 1169, 287–290. doi: 10.1111/j.1749-6632.2009.04845.x
Thaut, M. H., and Hoemberg, V. (2014). Handbook of Neurologic Music Therpy. (Eds.). Great Britain: Oxford University Press.
Tivadar, R. I., and Murray, M. M. (2019). A primer on electroencephalography and event-related potentials for organizational neuroscience. Org. Res. Methods 22, 69–94. doi: 10.1177/1094428118804657
Tramo, M. J. (2001). Music of the hemispheres. Science 5501, 54–56. doi: 10.1126/science.10.1126/SCIENCE.1056899
Trehub, S. (2010). In the beginning: a brief history of infant music perception. Musicae Scientiae 14, 71–87. doi: 10.1177/10298649100140S206
Trehub, S. (2013). Music processing similarities between sleeping newborns and alert adults: cause for celebration or concern? Front. Psychol. 4:644. doi: 10.3389/fpsyg.2013.00644
Trehub, S. E. (2003). The developmental origins of musicality. Nat. Neurosci. 6, 669–673. doi: 10.1038/nn1084
Trevarthen, C., and Aitken, K. J. (2001). Infant intersubjectivity: research, theory, and clinical applications. J. Child Psychol. Psychiatry Allied Disciplines 42, 3–48. doi: 10.1111/1469-7610.00701
Virtala, P., Huotilainen, M., Partanen, E., Fellman, V., and Tervaniemi, M. (2013). Newborn infants' auditory system is sensitive to Western music chord categories. Front. Psychol. 4:492. doi: 10.3389/fpsyg.2013.00492
Werner, L. A. (2012). “Overview and issues in human auditory development,” in Human Auditory Development. [electronic resource], eds L. A. Werner, R. R. Fay, and A. N. Popper (New York, NY: Springer). doi: 10.1007/978-1-4614-1421-6
Wilke, M., Schmithorst, V. J., and Holland, S. K. (2003). Normative pediatric brain data for spatial normalization and segmentation differs from standard adult data. Magnetic Resonance Med. 50:749. doi: 10.1002/mrm.10606
Winkler, I., Háden, G. P., Ladinig, O., Sziller, I., Honing, H., and Purves, D. (2009). Newborn infants detect the beat in music. Proc. Natl. Acad. Sci. U.S.A. 106:2468. doi: 10.1073/pnas.0809035106
Keywords: systematic review, music, brain imaging, child, music therapy, acquired brain injury
Citation: Bower J, Magee WL, Catroppa C and Baker FA (2021) The Neurophysiological Processing of Music in Children: A Systematic Review With Narrative Synthesis and Considerations for Clinical Practice in Music Therapy. Front. Psychol. 12:615209. doi: 10.3389/fpsyg.2021.615209
Received: 08 October 2020; Accepted: 10 March 2021;
Published: 15 April 2021.
Edited by:
Changiz Mohiyeddini, Oakland University William Beaumont School of Medicine, United StatesReviewed by:
Mireille Besson, UMR7291 Laboratoire de Neurosciences Cognitives (LNC), FranceCopyright © 2021 Bower, Magee, Catroppa and Baker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Janeen Bower, amFuZWVuLmJvd2VyQHJjaC5vcmcuYXU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.