
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychol., 27 January 2021
Sec. Performance Science
Volume 11 - 2020 | https://doi.org/10.3389/fpsyg.2020.563031
This article is part of the Research TopicConnecting Music and Body Movement: Choreographic Approach of PerformanceView all 7 articles
A correction has been applied to this article in:
Corrigendum: Mirror neuron activity during audiovisual appreciation of opera performance
Opera is a performing art in which music plays the leading role, and the acting of singers has a synergistic effect with the music. The mirror neuron system represents the neurophysiological mechanism underlying the coupling of perception and action. Mirror neuron activity is modulated by the appropriateness of actions and clarity of intentions, as well as emotional expression and aesthetic values. Therefore, it would be reasonable to assume that an opera performance induces mirror neuron activity in the audience so that the performer effectively shares an embodied performance with the audience. However, it is uncertain which aspect of opera performance induces mirror neuron activity. It is hypothesized that although auditory stimuli could induce mirror neuron activity, audiovisual perception of stage performance is the primary inducer of mirror neuron activity. To test this hypothesis, this study sought to correlate opera performance with brain activity as measured by electroencephalography (EEG) in singers while watching an opera performance with sounds or while listening to an aria without visual stimulus. We detected mirror neuron activity by observing that the EEG power in the alpha frequency band (8–13 Hz) was selectively decreased in the frontal-central-parietal area when watching an opera performance. In the auditory condition, however, the alpha-band power did not change relative to the resting condition. This study illustrates that the audiovisual perception of an opera performance engages the mirror neuron system in its audience.
Opera is a performing art that employs the synergistic effect of music and acting, in which music plays the leading role. Singing and acting are under the metacognitive control of the performers (Concina, 2019). Expert musicians have a high level of metacognitive control skills, which include self-control and monitoring. Metacognitive competence is a key factor for self-evaluating one’s performance (Hart, 2014). The performance plays a critical role in expressing operatic emotions in the way that the performer had intended. Two components of opera performance, i.e., singing and acting, influence emotions in the audience primarily through visual and auditory perception. In terms of emotional and aesthetic responses, consistency between the visual perception of the stage performance and the auditory perception of singing may be a critical factor in intracerebral processing. However, to date, how visual and auditory perception modulates such processing remains unknown. It is therefore worthwhile to individually evaluate the effects of singing and acting, alongside their synergism.
According to previous studies in neuroscience, bodily expressions are translated into internal representations in observers by mirror neuron activation (Gallese et al., 2011; Rizzolatti and Fogassi, 2014). Mirror neurons are active both during self-performances and while watching others performing similar actions. The mirror neuron system “mirrors” the behaviors of others, such that the observer feels as if they are performing the actions themselves. Neuroimaging studies in humans have isolated the mirror neuron network, whose principal nodes consist of the ventral premotor cortex and inferior parietal lobule (Calvo-Merino et al., 2005; Iacoboni and Dapretto, 2006). Mirroring is not a passive visual response to actions; instead, it is considered to play important roles in social cognition, such as understanding the intentions of others’ actions, as well as emotional communication between the performer and observer (Montgomery et al., 2007; Rizzolatti and Fogassi, 2014). Facial expressions, for example, contain both motor and emotional components. Further inclusion of the insula and amygdala in the mirror neuron system (van der Gaag et al., 2007) points to the contribution of the mirror neuron system to the comprehension of facially expressed emotions in addition to the motor component of facial expressions. Therefore, the mirror neuron system is a neuronal substrate by which action representation and emotions are shared between the performer and observer.
Mirror neuron activity is detected using neuroimaging methods, such as functional magnetic resonance imaging (fMRI) and electroencephalography (EEG). While fMRI has a superior spatial resolution, high noise, and strong bodily constraints make it inadequate for experiments using music. EEG recordings, however, are quiet and bodily constraints are minimal, which are advantageous in experiments using music. In EEG studies, “mu suppression” or the suppression of alpha oscillations (8–13 Hz) over the brain’s sensorimotor region has been used as an indicator for detecting mirror neuron activity (Pineda, 2005; Fox et al., 2016; Hobson and Bishop, 2016; Wu et al., 2016; Bowman et al., 2017). Mu suppression was discovered by Gastaut and Bert (1954), and a recent meta-analysis concluded that mu suppression provides a valid means for the study of human mirror neuron activity (Fox et al., 2016). There is, however, a notion that the suppression of alpha oscillations in general confounds with several factors that are irrelevant to mirror neuron activity: attentional demands alter the amplitudes of alpha oscillations while suppression of alpha oscillations is also observed during visual processing, which occurs in the occipital regions (Bacigalupo and Luck, 2019). Source identification of mirror neuron activity is limited in studies employing EEG; however, studies with simultaneous EEG and fMRI measurements reported that mu suppression correlated with the blood-oxygen-level-dependent (BOLD) signals in the brain regions consisting of the mirror neuron network, including the dorsal premotor cortex, primary somatosensory cortex, and inferior parietal lobule (Arnstein et al., 2011; Hobson and Bishop, 2017).
One of the notable features of mirror neuron activity is its dependence on experience. Mirror neuron activity is stronger when the observed action has been previously experienced by the observer (Cannon et al., 2014; Brunsdon et al., 2020). In their EEG study, the authors compared alpha-band powers during the observation of a relatively novel action of tool handling among three groups of participants: those trained to perform this action (performers), those with only the experience of observing the action (observers), and those who were novices. The performers group exhibited the greatest alpha (mu) suppression. Previous studies suggested that dance performance induces mirror neuron activity in dancers. Compared with a visual baseline condition, the EEG power in the alpha and lower beta frequency bands (7.5–25 Hz) was significantly reduced in dancers, but not in non-dancers, while the participants were watching dance movements (Orgs et al., 2008). Poikonen et al. (2018a; 2018b) analyzed phase synchrony between EEG signals recorded while dancers, musicians, and non-experts were watching dance performance. The phase synchrony in the theta band over the frontal regions was increased in dancers, whereas alpha synchrony was decreased in all groups, suggesting that cortical communication was enhanced in dancers. Taken together, these results suggest that mirror neuron activity reflects the activation of acquired neuronal representations of actions.
Another notable feature of mirror neuron activity is its multimodality. Mirror neurons are also responsive to auditory stimuli, the sounds associated with actions (Aziz-Zadeh et al., 2004; Gazzola et al., 2006). For example, the sounds of piano melodies activate the mirror neuron network in pianists (Bangert et al., 2006; Wu et al., 2016). However, studies by Jäncke and coworkers showed an overall increase in EEG power during music listening for all frequency bands (Jäncke et al., 2015, 2018; Markovic et al., 2017), suggesting that no mirror neuron activity was elicited. The authors interpreted this result as an indication of increased internal attention so that the participants were “drawn into the music.” In any event, behind these phenomena, underlies the multisensory integration of auditory and visual stimuli. An fMRI study demonstrated that a combination of watching a choreographed dance and listening to its music together enhanced the activity in the posterior superior temporal sulcus, a multisensory area, as revealed by conjunction analysis (“audiovisual vs. auditory” and “audiovisual vs. visual”) (Jola et al., 2013). Another study reported that watching an opera performance increased emotional arousal and valence compared with only listening (Balteş et al., 2011). The emotional highlights in an opera may be products of multimodal information processing in the brain. Therefore, cautious consideration is needed when one studies opera performance, which is multimodal in nature.
Considering the previous findings, it is reasonable to assume that opera performance induces mirror neuron activity in the audience. However, the individual contributions of visual perception of acting performance and auditory perception of singing to mirror neuron activity remains unknown. Since mu suppression was found to occur when pianists passively listened to piano melodies (Wu et al., 2016), it is possible that the auditory appreciation of an opera aria elicits mirror neuron activity in the audience. Otherwise, we can conclude that opera performance elicits mirror neuron activity through audiovisual perception. To test this hypothesis, this study analyzed EEG signals when participants watched video-clips of opera scenes with sounds, when listened without visual stimulus, and when at rest.
Twenty-one right-handed healthy Japanese individuals (19 women and two men, age range: 25–50 years, mean age: 33.6 years) participated in this study. All participants were vocalists in classical music who performed in operas and concerts. None of the participants had any current or past neurological or psychiatric illnesses. This study was approved by the ethics committee of Sophia University, and all participants provided written informed consent prior to participation in the study. The participants were required to be familiar with operas, understand the lyrics, and have experience with performing onstage. This study did not include non-musicians.
The tasks employed in this study were audiovisual, auditory (listening), and resting sessions. Participants watched video-clips of opera scenes with the accompanying sounds, projected on a screen in front of them. Each participant chose an opera scene with an aria from her/his repertoires of opera performance. Participants were asked to choose, if possible, opera scenes from those we had pre-selected. Selected opera scenes were “Donde lieta usci” from La Bohème by G. Puccini (six sopranos), “Quando me’n vò” from La Bohème by G. Puccini (five sopranos), “Va! Laisse couler mes larmes” from Werther by J. Massenet (five mezzo-sopranos), “Porgi, amor, qualche ristoro” from Le nozze di Figaro by W.A. Mozart (one soprano), “Deh! tu, bell’anima” from I Capuleti e i Montecchi by V. Bellini (one mezzo-soprano), “Habanera” from Carmen by G. Bizet (one mezzo-soprano), “Quando le sere al placido” from Luisa Miller by G. Verdi (one tenor), and “Per me giunto” from Don Carlo by G. Verdi (one baritone). The participants also listened to the same videos in the absence of visual stimulus by turning off the projector. EEG recordings were taken while watching the videos with sounds, listening to the videos without visual stimuli, and, as a control, resting. EEG recordings were made for the whole sessions in the audiovisual and auditory conditions, which ranged from 2 min 10 s to 3 min 30 s, depending on the scenes. The recording time for the resting session was 60 s. In both the listening and resting sessions, participants kept their eyes open so as to be in a condition similar to that of the audiovisual task, thereby excluding the effects of closing the eyes. During all tasks, the participants were sitting on a chair and keeping still without particular movements. The participants were instructed to enjoy the scene or the aria as an audience. Evaluation of the opera performance by the participants was not required.
Scalp EEG signals were recorded using a wearable headset with 32 active dry-type gold alloy EEG electrodes (g.Nautilus, Guger Technologies or g.tec Medical Engineering GmbH., Austria), placed according to the International 10–20 System. Continuous EEG signals were sampled at 250 Hz and filtered with a 0.1–100 Hz bandpass filter. A notch filter was also used to suppress 50 Hz power-line interference. A headset with dry electrodes was mounted on the heads of the participants. We used a small, medium, or large-sized cap that fit each participant’s head. The active dry electrodes, g.Sahara, have eight pins, thereby increasing the contact surface area. Because of the round tip of pins, the participants reported a good fit without pain. Prior studies have demonstrated no significant differences in the basic characteristics of recorded EEG signals, such as waveforms and power spectra, between wet and dry electrodes (Fiedler et al., 2015; Grummett et al., 2015; Melnik et al., 2017). During the recordings, the participants were asked to be relaxed and still without particular movements.
Electroencephalography signals were preprocessed using EEGLAB version 14.1.2 (Delorme and Makeig, 2004). An independent component decomposition was performed with EEGLAB’s runica algorithm. Thirty-two components were decomposed in all participants. Components contaminated with artifacts related to eye movements and blinks were removed, and the remaining ones were back-reconstructed to EEG signals. Preprocessed data were subjected to the frequency and coherence analyses described in Section “Analysis.”
The band powers or the average powers for the four frequency bands (theta: 4–8 Hz, alpha: 8–13 Hz, beta: 13–30 Hz, and gamma: 30–45 Hz) of EEG signals in each channel were calculated using MATLAB version R2018a Update 6 (Natick, MA, United States: The MathWorks Inc.). The calculation was made by integrating the power spectral density over the corresponding frequency ranges.
The tentative criterion for detecting mirror neuron activity is to observe “mu suppression” or the suppression of alpha oscillations (8–13 Hz) in the sensorimotor region of the brain (Fox et al., 2016; Hobson and Bishop, 2016; Wu et al., 2016; Bowman et al., 2017). The alpha-band powers at individual electrodes, calculated in “Band Powers”, were subjected to paired t-tests embedded in MATLAB, to determine whether there was a statistical difference between the audiovisual and resting conditions, as well as between the listening and resting conditions. For multiple testing, a false discovery rate (FDR) corrected p-value of 0.05 was considered significant. The effect size was estimated by calculating Cohen’s d.
The analyses of the recorded EEG signals yielded the following results. Among the band powers for the four frequency bands (theta, alpha, beta, and gamma), only the alpha-band power was significantly lower in the audiovisual condition than in the resting condition mainly in the frontal, central, and parietal scalp locations. In the auditory condition, the alpha-band power did not show any significant difference at any of the electrode sites. These results are demonstrated by the scalp maps of the alpha-band power in the three experimental conditions (Figure 1). The four band powers at the central electrode site (Cz) in the three experimental conditions are shown in Figure 2. The alpha-band powers in the audiovisual and auditory conditions were significantly lower than that in the resting condition.
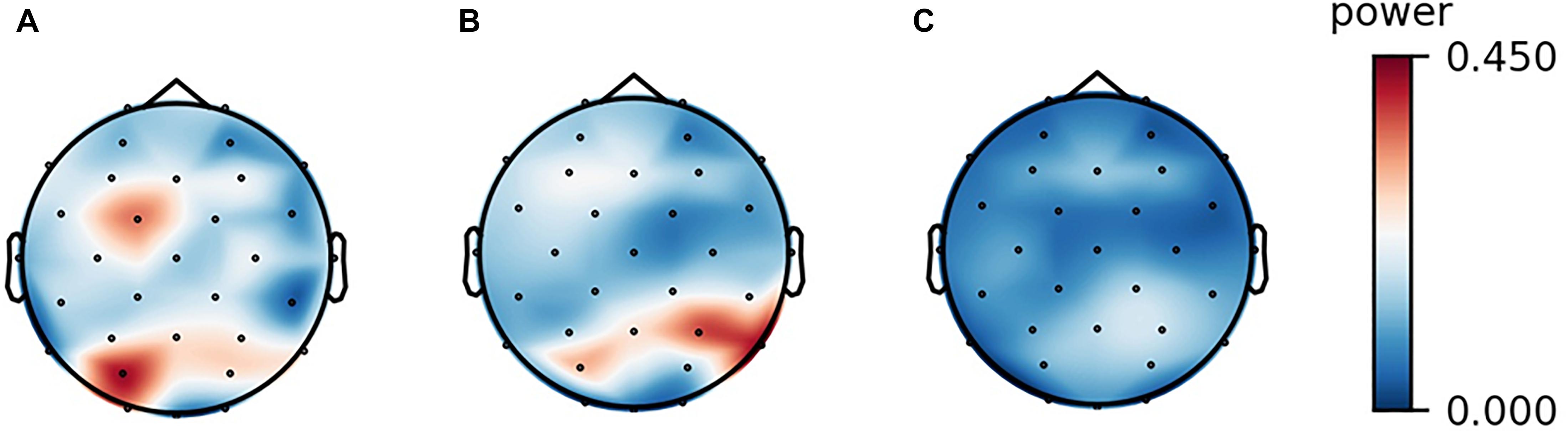
Figure 1. Scalp maps of the alpha-band power of a participant in the three experimental conditions. (A) resting, (B) listening, and (C) watching with sounds.
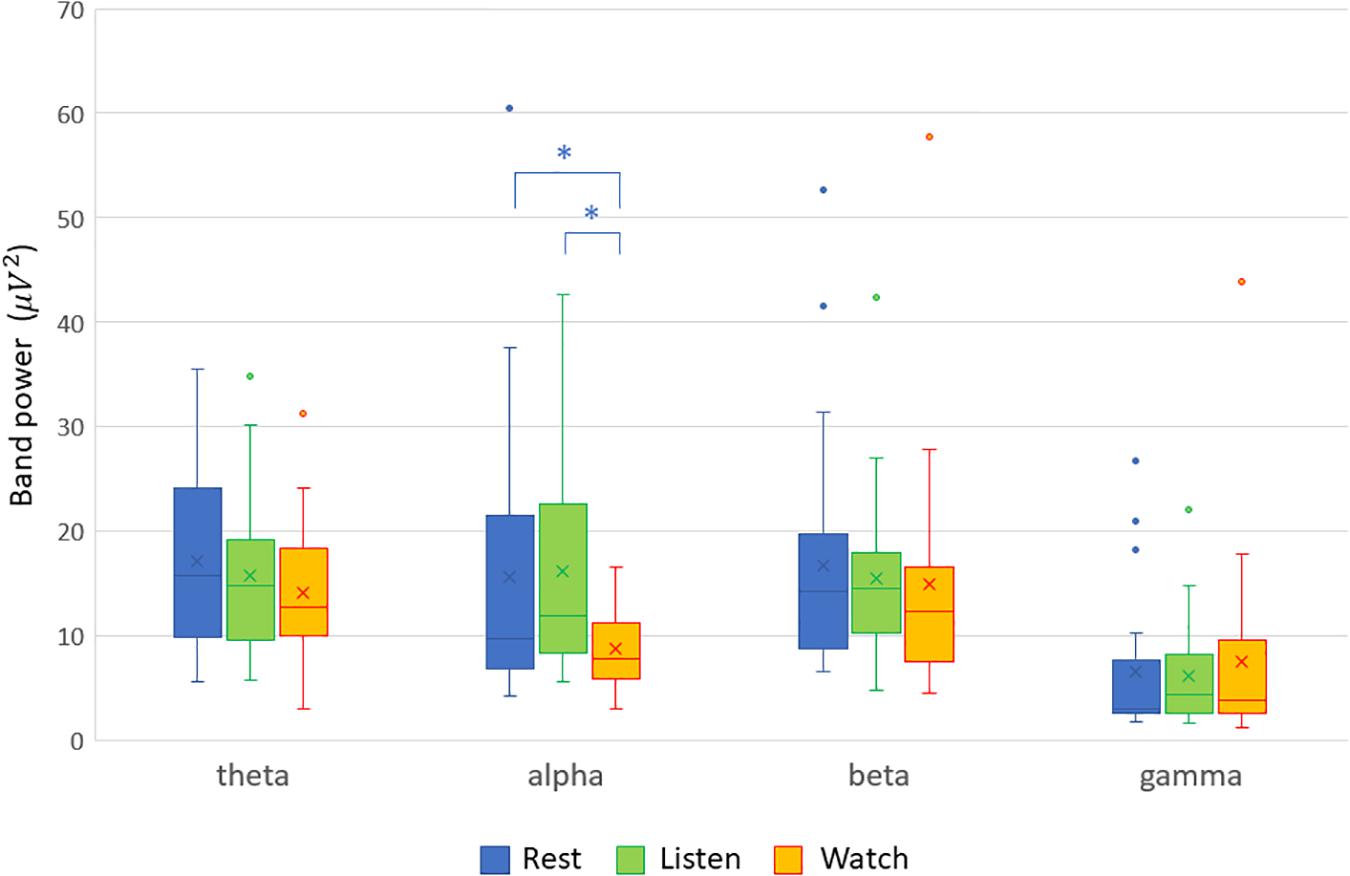
Figure 2. Band powers at the central electrode site (Cz) in the three experimental conditions (resting, listening, and watching with sounds). The alpha-band power in the audiovisual condition was significantly lower than that in the resting condition (Cohen’s d = −0.640, p = 0.0079) and that in the auditory condition (Cohen’s d = −0.774, p = 0.0019), indicated with asterisk (*).
When analyzing alpha suppression, the selection of the control condition or baseline is a matter of debate because alpha oscillations, in general, are sensitive to the attentional state (Bazanova and Vernon, 2014; Hobson and Bishop, 2017; Pagnotta et al., 2020). In this study, contrary to the resting session, which did not require any attentional demands, the auditory and audiovisual sessions made the participants attentive to stimuli. Therefore, we also analyzed the difference in the band powers between the auditory and audiovisual conditions. The differences at all the electrodes are shown in Figure 3, where it can be observed that the alpha-band powers in the audiovisual condition were lower than in the auditory condition. This figure also shows that the gamma-band powers in the audiovisual condition were slightly higher than those in the auditory condition. However, the differences were not statistically significant. Figure 4 shows the electrode locations at which the alpha-band power significantly differed between the audiovisual and auditory conditions. The alpha-band power was significantly lower in the audiovisual condition at 12 electrodes, including 4 electrodes with relatively large effect sizes at FC2 (d = −0.72; p = 0.027, FDR), Cz (d = −0.78; p = 0.027, FDR), CP1 (d = −0.86; p = 0.027, FDR), and CP6 (d = −0.72; p = 0.027, FDR). The electrodes where the alpha-band power was significantly lower in the audiovisual condition, including these four electrodes, were distributed over frontal-central-parietal regions.
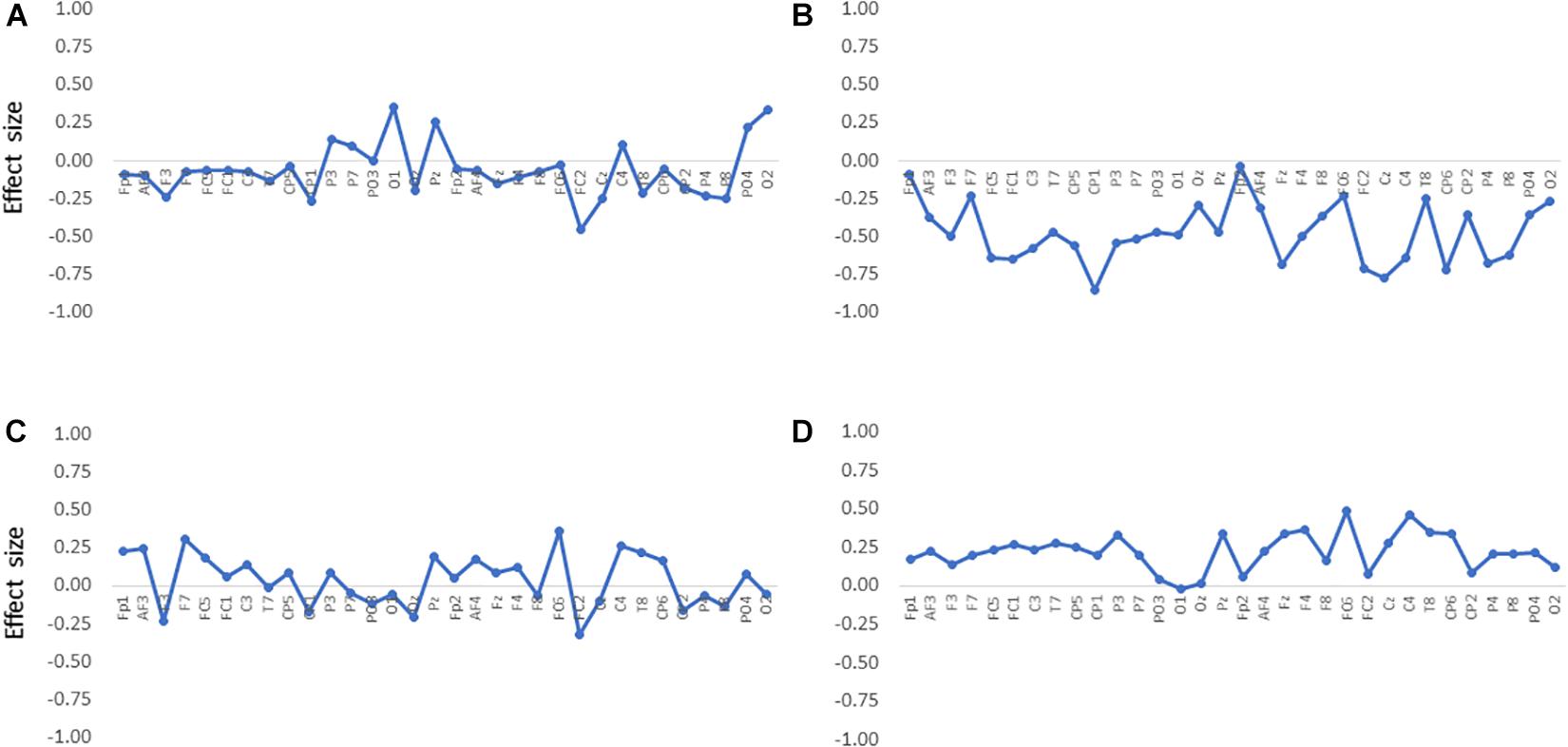
Figure 3. Differences in band powers between the audiovisual condition and the auditory condition at all electrodes in the (A) theta, (B) alpha, (C) beta, and (D) gamma bands. The effect size was estimated by calculating Cohen’s d.
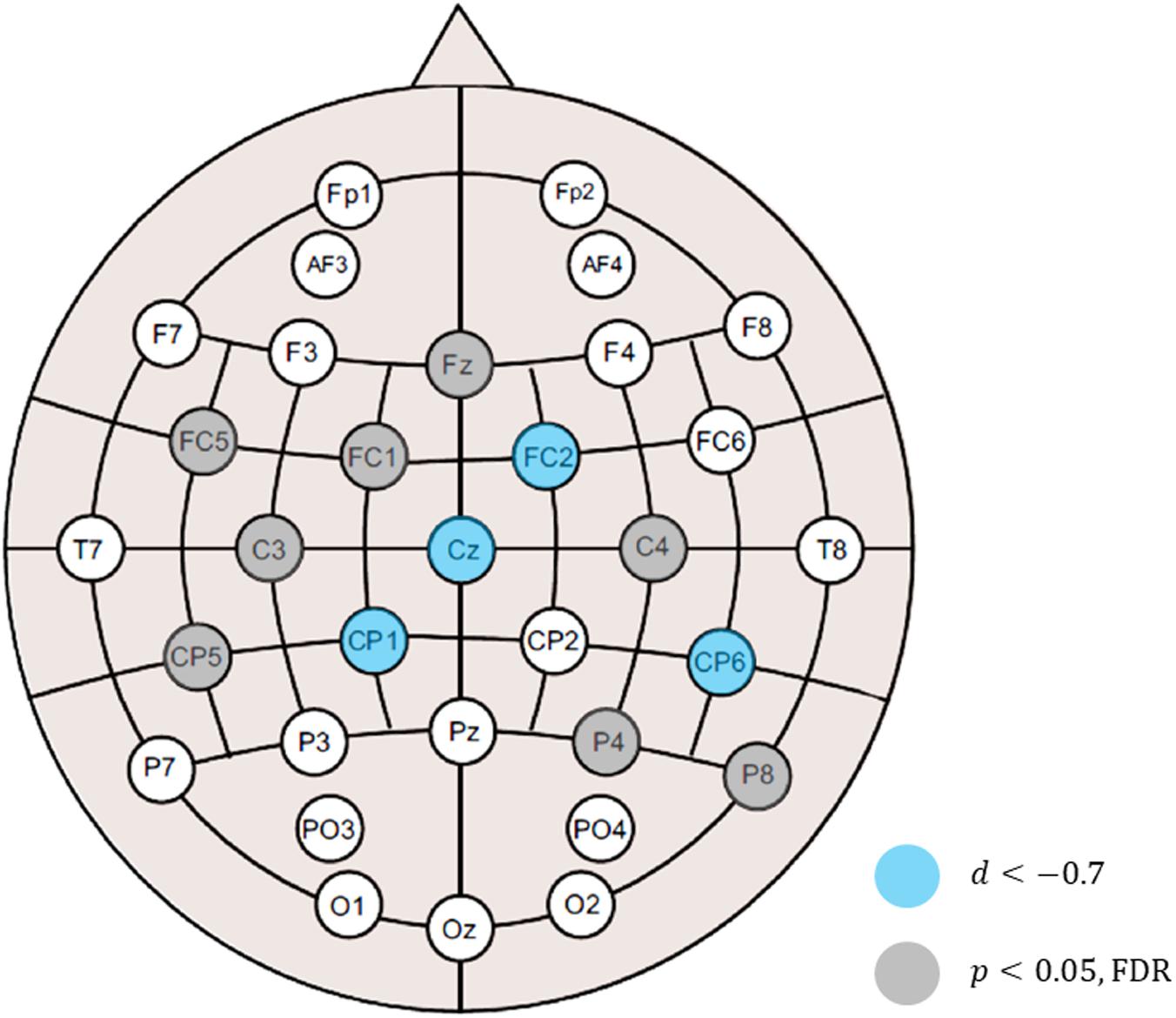
Figure 4. Electrode locations at which the alpha-band power was significantly lowered in the audiovisual condition relative to the auditory condition. Statistical significance after multiple testing correction was observed at 12 electrodes, including 4 electrodes with relatively large effect sizes at FC2 (d = –0.72; p = 0.027, FDR), Cz (d = –0.78; p = 0.027, FDR), CP1 (d = –0.86; p = 0.027, FDR), and CP6 (d = –0.72; p = 0.027, FDR).
The EEG signals analyzed in this study showed characteristic changes in the alpha frequency band during audiovisual appreciation of an opera performance. The analysis revealed marked differences in the band power between the audiovisual vs. auditory (listening) conditions. The alpha-band power was significantly decreased in the audiovisual condition relative to the resting and auditory conditions. However, only listening to an aria did not significantly change the alpha-band power compared with the resting condition.
Among the band powers for the four frequency bands (theta, alpha, beta, and gamma), only the alpha-band power was decreased in the audiovisual condition relative to the resting and auditory conditions. Since the auditory condition did not decrease alpha-band power, visual input would be crucial to this decrement. However, because the alpha-band power of EEG signals in the occipital region was not decreased during the audiovisual condition, this decrement is not attributable to visual perception per se. Moreover, the comparison of band powers between the auditory and audiovisual conditions revealed significantly lower alpha-band power in the audiovisual condition. A decrease was observed in frontal-central-parietal regions of the scalp, making it likely, therefore, that the alpha suppression observed in this study reflects mirror neuron activity associated with audiovisual integration. Notably, the present study also observed an increase of gamma-band power in the audiovisual condition at a trend level of significance. Previous studies have suggested an association between gamma oscillations and multisensory integration (Friese et al., 2016; Misselhorn et al., 2019), and that alpha oscillations coupled with gamma oscillations mediate selective attention and perceptual binding (Foxe and Snyder, 2011; Zhang et al., 2019). As such, audiovisual perception enables the mirroring of the opera performance in the observer.
This study demonstrated alpha (mu) suppression in the audiovisual condition but not in the auditory condition. In previous studies, however, auditory mirror neuron activity has been reported. For example, when pianists passively listened to piano melodies, auditory stimuli were able to activate the mirror neuron network when sounds were linked to actions (Wu et al., 2016). Even a simple auditory or motor task activated the mirror neuron network in pianists, but not in non-musicians (Bangert et al., 2006). In an fMRI study, listening to musical rhythms activated the supplementary motor area, premotor cortex, and cerebellum (Chen et al., 2008), consistent with mirror neuron activation, and generally, mirror neurons can be activated by a stimulus in the auditory domain (Aziz-Zadeh et al., 2004; Lahav et al., 2007; Chapin et al., 2010). These results, however, raise a question as to why the auditory appreciation of an aria in this study did not elicit alpha (mu) suppression. In the auditory condition, vocalists listened to arias. Unlike musical instrumentalists, such as pianists and violinists, vocalists have their own “instruments” in their bodies, and the vocalization method differs individually. The acoustic properties are unique to their instruments. Whereas instrumentalists readily associate sounds of an instrument with its manipulation, it may be unlikely that listening to an aria would be easily associated with an apparent action. Therefore, we infer that listening to an aria did not induce detectable mirror neuron activity but evoked a different intracerebral process.
During listening to an aria, the participants might have mentally constructed a “scene” by recalling a memory modified by the auditory perception of an aria. Scene construction is an internal process (Hassabis and Maguire, 2007), distinct from mirror neuron activity. In a study by Jäncke et al. (2015), the EEG powers in all frequency bands examined (theta, alpha, and beta) were increased while participants were listening to a well-known aria, leading to the suggestion that this state was characterized by increased internal attention. Whilst alpha-band power decreases when participants pay attention to an external stimulus, it increases when participants focus on internal consciousness (Magosso et al., 2019). The notion of scene construction is consistent with these results and the results of a recent study on decision-making in soccer (Fink et al., 2018). In the latter study, participants imagined themselves as acting players and were prompted to imagine a move that could lead to scoring a goal. The alpha-band power of their EEG signals was decreased at the parietal and occipital electrode sites. However, the decrease was weaker when the decisions involved a higher creative potential than when the opposite was involved. The authors suggested that the participants who generated moves that were more creative were more intensely engaged in motor imagery, which relatively increased the alpha-band power. The internal process, consisting of constructing motor imagery, had an opposite effect on alpha-band power to that of mirror neuron activity. Therefore, the present study’s results suggest that the auditory appreciation of an aria led the participants to imagine the opera scene. Note, however, that this result does not exclude the possibility that the auditory perception elicits mirror neuron activity. Since scene construction and mirror neuron activity have opposing effects, as suggested by reciprocal functional connectivity (Tanaka and Kirino, 2019), the reduction in alpha-band power reflecting mirror neuron activity might have been canceled out by the effect of the internal scene construction.
The reality or subjectivity in enacting a role may be a prerequisite for the engagement of mirror neuron activity. Mirror neuron activity allows for the matching of the internal motor representation and the perceived visual images of actions (Engel et al., 2008; Ishida et al., 2015). Furthermore, it has been demonstrated that mirror neuron activity is modulated by emotional information processing, such as facial expressions (Moore and Franz, 2017; Karakale et al., 2019) and music (McGarry et al., 2014), and possibly by the appropriateness (Caggiano et al., 2009), and clarity of intention of the actions (McGarry et al., 2014). On the other hand, there was a positive correlation between empathy self-reporting and mirror neuron activity (Baird et al., 2011). It is therefore likely that mirror neuron activity is modulated by the degree to which the observer feels the vividness and emotional expression of the action. Emotional expression in an opera needs to be realistic for the audience to interpret them as natural. Otherwise, the audience would not feel any empathy toward the characters on stage. These features of mirror neuron activity can provide useful information to evaluate opera performance. Therefore, this study proposes that analysis of mirror neuron activity during watching an opera performance is useful for its evaluation.
The detection of mirror neuron activity relied on assessing alpha (mu) suppression in this study. The finding that the alpha-band power was significantly lowered at the frontal-central-parietal scalp locations in the audiovisual condition compared with the auditory condition is supportive of its worth. However, this result is not direct evidence of mirror neuron activity. For more convincing results, comparable with those in fMRI studies, more systematic analyses, including source localization and network analysis, with a larger sample are needed.
During the audiovisual appreciation of an opera scene, audiovisual integration was anticipated to occur. Since the gamma band power was modestly increased in the audiovisual condition compared with the auditory condition, we consider that this increase is attributable to audiovisual integration. To obtain evidence of such integration, it would be theoretically conceivable to include a third condition in which the participants watch an opera scene without sounds to compare the result with those in the auditory and audiovisual conditions. However, unlike dance watching (Jola et al., 2013), watching a scene of aria singing without sounds may not produce a meaningful response, a point yet to be clarified. Therefore, employing more systematic analyses instead might be more helpful to draw a firm conclusion.
Participants were required to be familiar with operas, understand the lyrics, and have experience with performing on stage, and the opera scenes were chosen from their repertoires of opera performance. As a consequence, the chosen scenes differed across participants. Although the analyses in this study were restricted to a small group, the variability of the auditory and visual properties of individual scenes might have affected the results. Standardization of stimuli is still a challenge for future studies. For the same reason, this study did not employ non-musicians. Investigating how non-musicians respond to opera appreciation is another topic of research, which is beyond the scope of the present study. It would be meaningful to compare the responses between experts and non-musicians in a future study.
When a performance elicits mirror neuron activity in the audience, it is also of interest to see how the performance is represented in the performer’s brain. However, performance usually accompanies bodily movements. Extracting and analyzing EEG data are challenging because EEG data recorded during movement are mixed with a large number of electromyographic signals. By analyzing EEG data with movement signals, a previous study extracted the expressive components of movements from the recorded EEG data (Cruz-Garza et al., 2014). If feasible, analysis of EEG signals simultaneously recorded from a performer and an audience will provide useful information for the objective performance evaluation.
The major findings in this study are summarized as follows. First, opera appreciation induced mirror neuron activity in the participants, and audiovisual perception of the performance played a critical role in the mirroring. The neuronal processing for audiovisual perception is externally oriented. Second, different conditions of exposure, audiovisual vs. auditory, induced distinct neural processing in the participants. Auditory appreciation without visual stimulus did not seem to induce mirror neuron activity in the audience. Participants might have enjoyed mental scene construction while listening to an aria. This neuronal processing is internally oriented. Lastly, all the participants in this study were singers who had experience with opera performance. Mirror neuron activity is modulated by several factors linked to the performance, such as the level of realism, emotional expression, and aesthetic values. Therefore, performance-related features extracted from EEG data could be used as an objective indicator of opera stage performance.
The datasets generated for this study are available on request to the corresponding author.
The studies involving human participants were reviewed and approved by the Ethics Committee of Sophia University. The participants provided their written informed consent to participate in this study.
The author confirms being the sole contributor of this work and has approved it for publication.
This work was supported by the JSPS KAKENHI Grant Number 15K00380.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author acknowledges all the musicians who participated in this study.
Arnstein, D., Cui, F., Keysers, C., Maurits, N. M., and Gazzola, V. (2011). μ-suppression during action observation and execution correlates with BOLD in dorsal premotor, inferior parietal, and SI cortices. J. Neurosci. 31, 14243–14249. doi: 10.1523/JNEUROSCI.0963-11.2011
Aziz-Zadeh, L., Iacoboni, M., Zaidel, E., Wilson, S., and Mazziotta, J. (2004). Left hemisphere motor facilitation in response to manual action sounds. Eur. J. Neurosci. 19, 2609–2612. doi: 10.1111/j.0953-816X.2004.03348.x
Bacigalupo, F., and Luck, S. J. (2019). Lateralized suppression of alpha-band EEG activity as a mechanism of target processing. J. Neurosci. 39, 900–917. doi: 10.1523/JNEUROSCI.0183-18.2018
Baird, A. D., Scheffer, I. E., and Wilson, S. J. (2011). Mirror neuron system involvement in empathy: a critical look at the evidence. Soc. Neurosci. 6, 327–335. doi: 10.1080/17470919.2010.547085
Balteş, F. R., Avram, J., Miclea, M., and Miu, A. C. (2011). Emotions induced by operatic music: psychophysiological effects of music, plot, and acting: a scientist’s tribute to Maria Callas. Brain Cogn. 76, 146–157. doi: 10.1016/j.bandc.2011.01.012
Bangert, M., Peschel, T., Schlaug, G., Rotte, M., Drescher, D., Hinrichs, H., et al. (2006). Shared networks for auditory and motor processing in professional pianists: evidence from fMRI conjunction. NeuroImage 30, 917–926. doi: 10.1016/j.neuroimage.2005.10.044
Bazanova, O. M., and Vernon, D. (2014). Interpreting EEG alpha activity. Neurosci. Biobehav. Rev. 44, 94–110. doi: 10.1016/j.neubiorev.2013.05.007
Bowman, L. C., Bakermans-Kranenburg, M. J., Yoo, K. H., Cannon, E. N., Vanderwert, R. E., Ferrari, P. F., et al. (2017). The mu-rhythm can mirror: insights from experimental design, and looking past the controversy. Cortex 96, 121–125. doi: 10.1016/j.cortex.2017.03.025
Brunsdon, V. E. A., Bradford, E. E. F., Smith, L., and Ferguson, H. J. (2020). Short-term physical training enhances mirror system activation to action observation. Soc. Neurosci. 15, 98–107. doi: 10.1080/17470919.2019.1660708
Caggiano, V., Fogassi, L., Rizzolatti, G., Thier, P., and Casile, A. (2009). Mirror neurons differentially encode the peripersonal and extrapersonal space of monkeys. Science 324, 403–406. doi: 10.1126/science.1166818
Calvo-Merino, B., Glaser, D. E., Grèzes, J., Passingham, R. E., and Haggard, P. (2005). Action observation and acquired motor skills: an fMRI study with expert dancers. Cereb. Cortex 15, 1243–1249. doi: 10.1093/cercor/bhi007
Cannon, E. N., Yoo, K. H., Vanderwert, R. E., Ferrari, P. F., Woodward, A. L., and Fox, N. A. (2014). Action experience, more than observation, influences mu rhythm desynchronization. PLoS One 9:e92002. doi: 10.1371/journal.pone.0092002
Chapin, H., Jantzen, K., Kelso, J. A. S., Steinberg, F., and Large, E. (2010). Dynamic emotional and neural responses to music depend on performance expression and listener experience. PLoS One 5:e13812. doi: 10.1371/journal.pone.0013812
Chen, J. L., Penhune, V. B., and Zatorre, R. J. (2008). Listening to musical rhythms recruits motor regions of the brain. Cereb. Cortex 18, 2844–2854. doi: 10.1093/cercor/bhn042
Concina, E. (2019). The role of metacognitive skills in music learning and performing: theoretical features and educational implications. Front. Psychol. 10:1583. doi: 10.3389/fpsyg.2019.01583
Cruz-Garza, J. G., Hernandez, Z. R., Nepaul, S., Bradley, K. K., and Contreras-Vidal, J. L. (2014). Neural decoding of expressive human movement from scalp electroencephalography (EEG). Front. Hum. Neurosci. 8:188. doi: 10.3389/fnhum.2014.00188
Delorme, A., and Makeig, S. (2004). EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 134, 9–21. doi: 10.1016/j.jneumeth.2003.10.009
Engel, A., Burke, M., Fiehler, K., Bien, S., and Rösler, F. (2008). What activates the human mirror neuron system during observation of artificial movements: bottom-up visual features or top-down intentions? Neuropsychologia 46, 2033–2042. doi: 10.1016/j.neuropsychologia.2008.01.025
Fiedler, P., Pedrosa, P., Griebel, S., Fonseca, C., Vaz, F., Supriyanto, E., et al. (2015). Novel multipin electrode cap system for dry electroencephalography. Brain Topogr. 28, 647–656. doi: 10.1007/s10548-015-0435-5
Fink, A., Rominger, C., Benedek, M., Perchtold, C. M., Papousek, I., Weiss, E. M., et al. (2018). EEG alpha activity during imagining creative moves in soccer decision-making situations. Neuropsychologia 114, 118–124. doi: 10.1016/j.neuropsychologia.2018.04.025
Fox, N. A., Yoo, K. H., Bowman, L. C., Cannon, E. N., Ferrari, P. F., Bakermans-Kranenburg, M. J., et al. (2016). Assessing human mirror activity with EEG mu rhythm: a meta-analysis. Psychol. Bull. 142, 291–313. doi: 10.1037/bul0000031
Foxe, J. J., and Snyder, A. C. (2011). The role of alpha-band brain oscillations as a sensory suppression mechanism during selective attention. Front. Psychol. 2:154. doi: 10.3389/fpsyg.2011.00154
Friese, U., Daume, J., Göschl, F., König, P., Wang, P., and Engel, A. K. (2016). Oscillatory brain activity during multisensory attention reflects activation, disinhibition, and cognitive control. Sci. Rep. 6:32775. doi: 10.1038/srep32775
Gallese, V., Gernsbacher, M. A., Heyes, C., Hickok, G., Iacoboni, M., and Glenberg, A. M. (2011). Mirror neuron forum. Perspect. Psychol. Sci. 6, 369–407. doi: 10.1177/1745691611413392
Gastaut, H. J., and Bert, J. (1954). EEG changes during cinematographic presentation (moving picture activation of the EEG). Electroencephalogr. Clin. Neurophysiol. 6, 433–444. doi: 10.1016/0013-4694(54)90058-9
Gazzola, V., Aziz-Zadeh, L., and Keysers, C. (2006). Empathy and the somatotopic auditory mirror system in humans. Curr. Biol. 16, 1824–1829. doi: 10.1016/j.cub.2006.07.072
Grummett, T. S., Leibbrandt, R. E., Lewis, T. W., DeLosAngeles, D., Powers, D. M. W., Willoughby, J. O., et al. (2015). Measurement of neural signals from inexpensive, wireless and dry EEG systems. Physiol. Meas. 36, 1469–1484. doi: 10.1088/0967-3334/36/7/1469
Hart, J. T. (2014). Guided metacognition in instrumental practice. Music Educ. J. 101, 57–64. doi: 10.1177/0027432114552569
Hassabis, D., and Maguire, E. A. (2007). Deconstructing episodic memory with construction. Trends Cogn. Sci. 11, 299–306. doi: 10.1016/j.tics.2007.05.001
Hobson, H. M., and Bishop, D. V. M. (2016). Mu suppression – a good measure of the human mirror neuron system? Cortex 82, 290–310. doi: 10.1016/j.cortex.2016.03.019
Hobson, H. M., and Bishop, D. V. M. (2017). The interpretation of mu suppression as an index of mirror neuron activity: past, present and future. R. Soc. Open Sci. 4:160662. doi: 10.1098/rsos.160662
Iacoboni, M., and Dapretto, M. (2006). The mirror neuron system and the consequences of its dysfunction. Nat. Rev. Neurosci. 7, 942–951. doi: 10.1038/nrn2024
Ishida, H., Suzuki, K., and Grandi, L. C. (2015). Predictive coding accounts of shared representations in parieto-insular networks. Neuropsychologia 70, 442–454. doi: 10.1016/j.neuropsychologia.2014.10.020
Jäncke, L., Kühnis, J., Rogenmoser, L., and Elmer, S. (2015). Time course of EEG oscillations during repeated listening of a well-known aria. Front. Hum. Neurosci. 9:401. doi: 10.3389/fnhum.2015.00401
Jäncke, L., Leipold, S., and Burkhard, A. (2018). The neural underpinnings of music listening under different attention conditions. Neuroreport 29, 594–604. doi: 10.1097/WNR.0000000000001019
Jola, C., McAleer, P. M. A. P., Grosbras, M. H., Love, S. A., Morison, G., and Pollick, F. E. (2013). Uni-and multisensory brain areas are synchronised across spectators when watching unedited dance recordings. Iperception 4, 265–284. doi: 10.1068/i0536
Karakale, O., Moore, M. R., and Kirk, I. J. (2019). Mental simulation of facial expressions: mu suppression to the viewing of dynamic neutral face videos. Front. Hum. Neurosci. 13:34. doi: 10.3389/fnhum.2019.00034
Lahav, A., Saltzman, E., and Schlaug, G. (2007). Action representation of sound: audiomotor recognition network while listening to newly acquired actions. J. Neurosci. 27, 308–314. doi: 10.1523/jneurosci.4822-06.2007
Magosso, E., De Crescenzio, F., Ricci, G., Piastra, S., and Ursino, M. (2019). EEG alpha power is modulated by attentional changes during cognitive tasks and virtual reality immersion. Comput. Intell. Neurosci. 2019:7051079. doi: 10.1155/2019/7051079
Markovic, A., Kühnis, J., and Jäncke, L. (2017). Task context influences brain activation during music listening. Front. Hum. Neurosci. 11:342. doi: 10.3389/fnhum.2017.00342
McGarry, L. M., Pineda, J. A., and Russo, F. A. (2014). The role of the extended MNS in emotional and nonemotional judgments of human song. Cogn. Affect. Behav. Neurosci. 15, 32–44. doi: 10.3758/s13415-014-0311-x
Melnik, A., Legkov, P., Izdebski, K., Kärcher, S. M., Hairston, W. D., Ferris, D. P., et al. (2017). Systems, subjects, sessions: to what extent do these factors influence EEG data? Front. Hum. Neurosci. 11:150. doi: 10.3389/fnhum.2017.00150
Misselhorn, J., Schwab, B. C., Schneider, T. R., and Engel, A. K. (2019). Synchronization of sensory gamma oscillations promotes multisensory communication. eNeuro 6:ENEURO.0101-19.2019. doi: 10.1523/ENEURO.0101-19.2019
Montgomery, K. J., Isenberg, N., and Haxby, J. V. (2007). Communicative hand gestures and object-directed hand movements activated the mirror neuron system. Soc. Cogn. Affect. Neurosci. 2, 114–122. doi: 10.1093/scan/nsm004
Moore, M. R., and Franz, E. A. (2017). Mu rhythm suppression is associated with the classification of emotion in faces. Cogn. Affect. Behav. Neurosci. 17, 224–234. doi: 10.3758/s13415-016-0476-6
Orgs, G., Dombrowski, J. H., Heil, M., and Jansen-Osmann, P. (2008). Expertise in dance modulates alpha/beta event-related desynchronization during action observation. Eur. J. Neurosci. 27, 3380–3384. doi: 10.1111/j.1460-9568.2008.06271.x
Pagnotta, M. F., Pascucci, D., and Plomp, G. (2020). Nested oscillations and brain connectivity during sequential stages of feature-based attention. NeuroImage 223:117354. doi: 10.1016/j.neuroimage.2020.117354
Pineda, J. A. (2005). The functional significance of mu rhythms: translating “seeing” and “hearing” into “doing.” Brain Res. Rev. 50, 57–68. doi: 10.1016/j.brainresrev.2005.04.005
Poikonen, H., Toiviainen, P., and Tervaniemi, M. (2018a). Dance on cortex: enhanced theta synchrony in experts when watching a dance piece. Eur. J. Neurosci. 47, 433–445. doi: 10.1111/ejn.13838
Poikonen, H., Toiviainen, P., and Tervaniemi, M. (2018b). Naturalistic music and dance: cortical phase synchrony in musicians and dancers. PLoS One 13:e0196065. doi: 10.1371/journal.pone.0196065
Rizzolatti, G., and Fogassi, L. (2014). The mirror mechanism: recent findings and perspectives. Philos. Trans. R. Soc. B Biol. Sci. 369:20130420. doi: 10.1098/rstb.2013.0420
Tanaka, S., and Kirino, E. (2019). Increased functional connectivity of the angular gyrus during imagined music performance. Front. Hum. Neurosci. 13:92. doi: 10.3389/fnhum.2019.00092
van der Gaag, C., Minderaa, R. B., and Keysers, C. (2007). Facial expressions: what the mirror neuron system can and cannot tell us. Soc. Neurosci. 2, 179–222. doi: 10.1080/17470910701376878
Wu, C. C., Hamm, J. P., Lim, V. K., and Kirk, I. J. (2016). Mu rhythm suppression demonstrates action representation in pianists during passive listening of piano melodies. Exp. Brain Res. 234, 2133–2139. doi: 10.1007/s00221-016-4615-7
Keywords: action, alpha, aria, EEG, emotion, gamma, mirror neuron, music
Citation: Tanaka S (2021) Mirror Neuron Activity During Audiovisual Appreciation of Opera Performance. Front. Psychol. 11:563031. doi: 10.3389/fpsyg.2020.563031
Received: 17 May 2020; Accepted: 14 December 2020;
Published: 27 January 2021.
Edited by:
Zelia Chueke, Federal University of Paraná, BrazilReviewed by:
Lutz Jäncke, University of Zurich, SwitzerlandCopyright © 2021 Tanaka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shoji Tanaka, dGFuYWthLXNAc29waGlhLmFjLmpw; c2hvamkudGFuYWthQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.