
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychol. , 04 November 2020
Sec. Movement Science
Volume 11 - 2020 | https://doi.org/10.3389/fpsyg.2020.558849
This article is part of the Research Topic Physical Activity: An Optimizer of the Neurophysiological System? View all 11 articles
Background: Women with FM have a reduced ability to perform two simultaneous tasks. However, the impact of dual task (DT) on the neurophysiological response of women with FM has not been studied.
Objective: To explore both the neurophysiological response and physical performance of women with FM and healthy controls while performing a DT (motor–cognitive).
Design: Cross-sectional study.
Methods: A total of 17 women with FM and 19 age- and sex-matched healthy controls (1:1 ratio) were recruited. The electroencephalographic (EEG) activity was recorded while participants performed two simultaneous tasks: a motor (30 seconds arm-curl test) and a cognitive (remembering three unrelated words). Theta (4–7 Hz), alpha (8–12 Hz), and beta (13–30) frequency bands were analyzed by using EEGLAB.
Results: Significant differences were obtained in the healthy control group between single task (ST) and DT in the theta, alpha, and beta frequency bands (p-value < 0.05). Neurophysiological differences between ST and DT were not found in women with FM. In addition, between-group differences were found in the alpha and beta frequency bands between healthy and FM groups, with lower values of beta and alpha in the FM group. Therefore, significant group∗condition interactions were detected in the alpha and beta frequency bands. Regarding physical condition performance, between groups, analyses showed that women with FM obtained significantly worse results in the arm curl test than healthy controls, in both ST and DT.
Conclusion: Women with FM showed the same electrical brain activity pattern during ST and DT conditions, whereas healthy controls seem to adapt their brain activity to task commitment. This is the first study that investigates the neurophysiological response of women with FM while simultaneously performing a motor and a cognitive task.
Fibromyalgia (FM) is characterized by chronic, widespread, and persistent pain and its prevalence is around 2% to 3% worldwide (Helmick et al., 2008). Nevertheless, FM is accompanied by other symptoms, such as stiffness, mobility problems, sleep disorders, anxiety, depression, or cognitive impairments (Wolfe et al., 2010). The study of cognition and FM is a growing field of research since previous studies have reported cognitive impairments in several domains of people with FM, including long-term memory (Canovas et al., 2009), short-term memory (Park et al., 2001), working memory (Coppieters et al., 2015), processing speed (Del Paso et al., 2015), or even an abnormal EEG signal at rest (Villafaina et al., 2019a,b).
Due to the impact of FM symptoms, people with FM showed a diminished quality of life and reduced ability to perform activities of daily living (Burckhardt et al., 1993; Huijnen et al., 2015). In this regard, activities of daily living are commonly presented under the dual-task paradigm (where two or even more tasks are simultaneously required) (Yuan et al., 2015). People with FM have shown a diminished physical performance when two tasks are simultaneously presented during postural control (de Gier et al., 2003), balance (Villafaina et al., 2019c) or when compared with healthy controls in physical fitness tests (Villafaina et al., 2018, 2019d).
Progress in mobile and wireless technologies have allowed studying the impact of DT on the electroencephalography (EEG) (Wagner et al., 2012; Winslow et al., 2016) during ecological scenarios. In this regard, previous studies have explored brain dynamics during dual-task conditions in young and older people (De Sanctis et al., 2014; Malcolm et al., 2015; Bogost et al., 2016). However, the impact of DT on neurophysiological measures has not been investigated. Therefore, the present study aimed to explore the neurophysiological response of women with FM and healthy controls while performing a DT (motor–cognitive). We hypothesized that DT would engage brain areas related to executive function (prefrontal cortex) (Ford et al., 2002; Giraud et al., 2007) and areas related to sensorimotor integration (sensorimotor cortex and posterior parietal cortex) (Sipp et al., 2013; Beurskens et al., 2016; Bradford et al., 2016). Moreover, neurophysiological differences between healthy controls and people with FM could emerge since people with FM reported an attention deficit disorder (van Rensburg et al., 2018; Yilmaz and Tamam, 2018). This is because people with FM have impaired the ability to perform two simultaneous tasks (Huijnen et al., 2015; Villafaina et al., 2018, 2019d).
Twenty-five women with fibromyalgia participated in this study with a cross-sectional design. They were divided into two groups: (1) women with fibromyalgia (N = 17; age = 51.88 [7.30]) and (2) age- and gender-matched healthy controls (N = 19; age = 50.95 [6.83]) (Table 1). The Extremadura Association of Fibromyalgia (Spain) recruited the women with FM by telephone calls in April 2018.
The inclusion criteria for participants were as follows: (a) be a female and aged between 30 and 75 years, (b) be able to communicate with technicians, and (c) have read and signed the written informed consent. In addition, women with FM have to be diagnosed by a rheumatologist, according to the American College of Rheumatology’s criteria (Wolfe et al., 2010). However, the following exclusion criteria were defined as follows: (a) had contraindications for physical activity, (b) have been suffering from a psychiatric or neurological diagnoses according to their current medical history, and (c) were pregnant. Moreover, healthy controls were excluded if they suffered from any pain that lasted for more than three months in the last six months.
The University of Extremadura bioethical research committee approved the procedures (approval number: 62/2017), following the updated Declaration of Helsinki. All the participants read and signed the informed consent prior to the first assessment.
The body composition measurement was measured using the using the Tanita Body Composition Analyzer BC-418 MA. Moreover, the impact of the disease was evaluated with the (Bennett, 2005; Esteve-Vives et al., 2007) Spanish version of the Fibromyalgia Impact Questionnaire (FIQ), which evaluates the impact of symptoms (in several domains such as pain, fatigue, rested, stiffness, anxiety, depression, physical impairment, feeling good, or work missed). Furthermore, the pain intensity was measured through the VAS for pain (0–100) (Boonstra et al., 2008; Hawker et al., 2011), referring to the day they were evaluated. Body composition was evaluated in both FM and healthy groups whereas the impact of the disease and pain intensity were assessed (through an interview) exclusively in the FM group.
EEG was recorded while participants performed the arm-curl test (Ariadna Aparicio et al., 2015). Therefore, participants have to be seated in a chair holding a 2.3-kg weight and encouraged to perform, as many times as possible for 30 s, arm curls (to lift the weight and return to the starting position) through a full range of motion. This physical fitness test was selected since it could potentially discriminate women with fibromyalgia from healthy women (Ariadna Aparicio et al., 2015) and the performance is associated with the severity of the symptoms (Soriano-Maldonado et al., 2015). The best of the two trials, for each arm, was chosen for analyses purposed.
Participants performed the physical fitness test in both single-task (ST) and DT conditions. The simultaneous cognitive task consisted of remembering three random words. Therefore, participants were encouraged to think in these words while the arm-curl test was being performed. The cognitive performance and correct responses were registered. Conditions (ST and DT) were randomized.
The EEG signal was record using the Enobio device (Neuroelectrics, Cambridge, MA, United States) (Ruffini et al., 2007). The EEG signal was recorded in a total of 19 scalp locations according to the International 10–20 system (Homan, 1988) in different brain areas such as frontal (Fz, Fp1, Fp2, F3, F4, F7, and F8), central (Cz, C3, and C4), temporal (T3, T4, T5, and T6), parietal (Pz, P3, and P4), and occipital (O1 and O2).
Two electrodes placed in each mastoid served as reference. Moreover, the impedance was kept below 5 KΩ and a sampling rate of 500 Hz was used. Preprocessing steps and data analyses were conducted with the EEGlab toolbox (MatLab) (Delorme and Makeig, 2004). A 1-Hz high-pass filter was used, and the line noise was removed using the CleanLine algorithm in EEGlab. In order to reject bad channels and correct continuous data, the Artifact Subspace Reconstruction (ASR) was used. In this regard, if a channel is correlated with the surrounding channels less than 0.8, the channel was rejected. Moreover, principal components (PCs) were classified into high variance (in this case, with a standard deviation of 8 from the calibration data) or normal variance. A window rejection criterion of 0.25 was set, meaning that if more than 0.25 of channels are judged to be bad even after ASR, the window will be rejected. Then, bad channels were interpolated and the data was re-referenced to average. In addition, the independent component analysis (ICA) was conducted (Jung et al., 2000) and single equivalent current dipoles estimated, looking for the symmetrically constrained bilateral dipoles. Dipoles located outside the brain were removed using the independent components (ICs) when the dipoles’ residual variance was larger than 15%. Moreover, a visual inspection of dipoles located inside or outside the brain was performed. Lastly, the fast Fourier transform (FFT) method was used to compute spectral decomposition after splitting continuous data in 1-s epochs. Therefore, theta (4–7 Hz), alpha (8–12), and beta (13–30) power spectrums were computed.
Additionally, in order to report EEG sources analysis, evoke-related potentials (ERPs) were generated with a 7000-ms window time-locked, and dipoles were then estimated utilizing DIPFIT. Thus, a Kmeans cluster procedure (k = 10) was performed for clustering dipoles, using dipole location. Different clusters were performed according to the group and condition. Thus, for within- and between-group analysis purposes, clusters were obtained taking into account their group (fibromyalgia or healthy control) and condition (dual or single task). Therefore, ERSP were extracted to observe the amplitude and latency as well as the time–frequency analysis for source analyses. For analysis purposes, only clusters which contain the signal of the majority of the sample were selected. Results from these analyses are presented in Supplementary File 1.
A 2 × 2 design using the EEGLAB study design was used to explore the EEG data during ST versus DT in both healthy and women with FM. Permutation statistics with 2000 repetitions and the false discovery rate (FDR) correction were applied for EEG analyses.
In addition, the SPSS statistical package (version 22.0; SPSS, Inc., Chicago, IL, United States) was used to analyze the arm-curl test performance in both ST and DT conditions. Moreover, non-parametric analyses were conducted taking into account the results of Shapiro–Wilk and Kolmogorov–Smirnov tests. Therefore, the Wilcoxon signed-rank test was used to assess differences within groups, whereas Mann–Whitney U or chi-squared tests (when appropriate) were used to explore between-group differences in both ST and DT conditions. Additionally, the dual-task cost (DTC), which measures the losses of performance due to motor–cognitive interference, was calculated as follows:
DTC = (Result of DT condition – Result of ST condition)/Result of ST condition.
Effect size [r] was calculated for each the ST and DT comparisons (Fritz et al., 2012). Values of 0.37, 0.24, and 0.10 represent large, medium, and small effect sizes, respectively (McGrath and Meyer, 2006). The alpha level of significance (0.05) was adjusted according to the Benjamini–Hochberg procedure to avoid type I error derived from multiple comparisons (Benjamini and Hochberg, 1995).
Table 2 shows within- and between-group differences. Regarding within group analyses, differences were not found in women with FM nor healthy controls (p-value > 0.05). Nevertheless, between groups, analyses showed that women with FM obtained significantly worse results in the arm curl test than healthy controls in both DT (p-value < 0.001) and ST (p-value < 0.001) conditions.
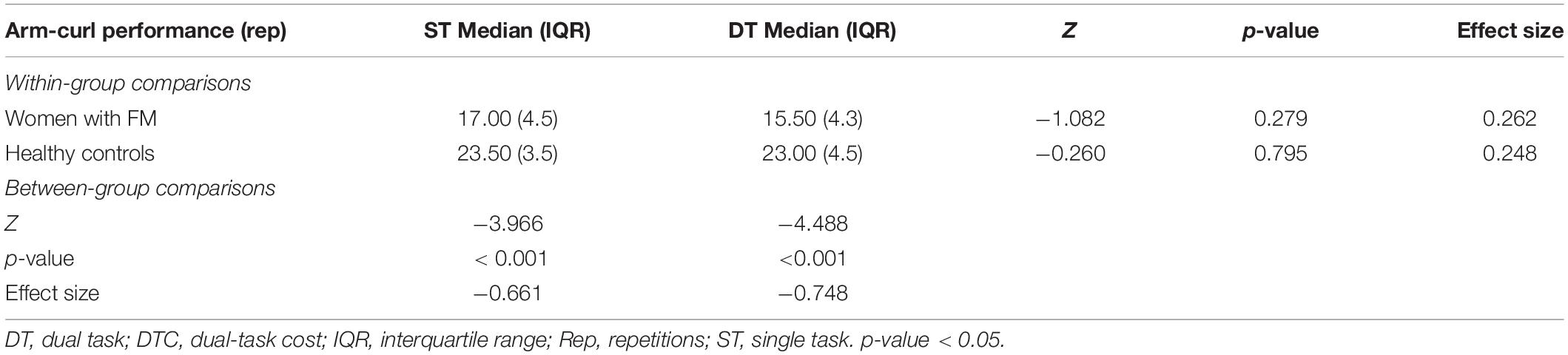
Table 2. Within- and between-group comparisons in the arm-curl test during ST and DT in women with FM and healthy controls.
Table 3 shows the between-group differences in the arm-curl test DTC and cognitive performance (successful responses). Differences between healthy controls and women with FM were not observed in the DTC.

Table 3. Between-group comparisons in the dual-task cost and cognitive performance in the arm-curl test in women with FM and healthy controls.
Figure 1 shows the theta power spectrum (4–7 Hz) topographic maps in the women with FM and healthy controls during ST and DT conditions. Differences (p-value < 0.05) were only observed in healthy controls when compared ST and DT conditions. Significant differences between groups (p-value < 0.005) were located in the frontal (Fz, F3, F4, F7, and F8), central (Cz, C3, and C4), temporal (T3, T4, T5, and T6), parietal (Pz, P3, and P4), and occipital (O1 and O2). Higher theta power spectrum values were found in the DT compared to ST values. However, significant between-group differences or group∗condition interactions were not found.
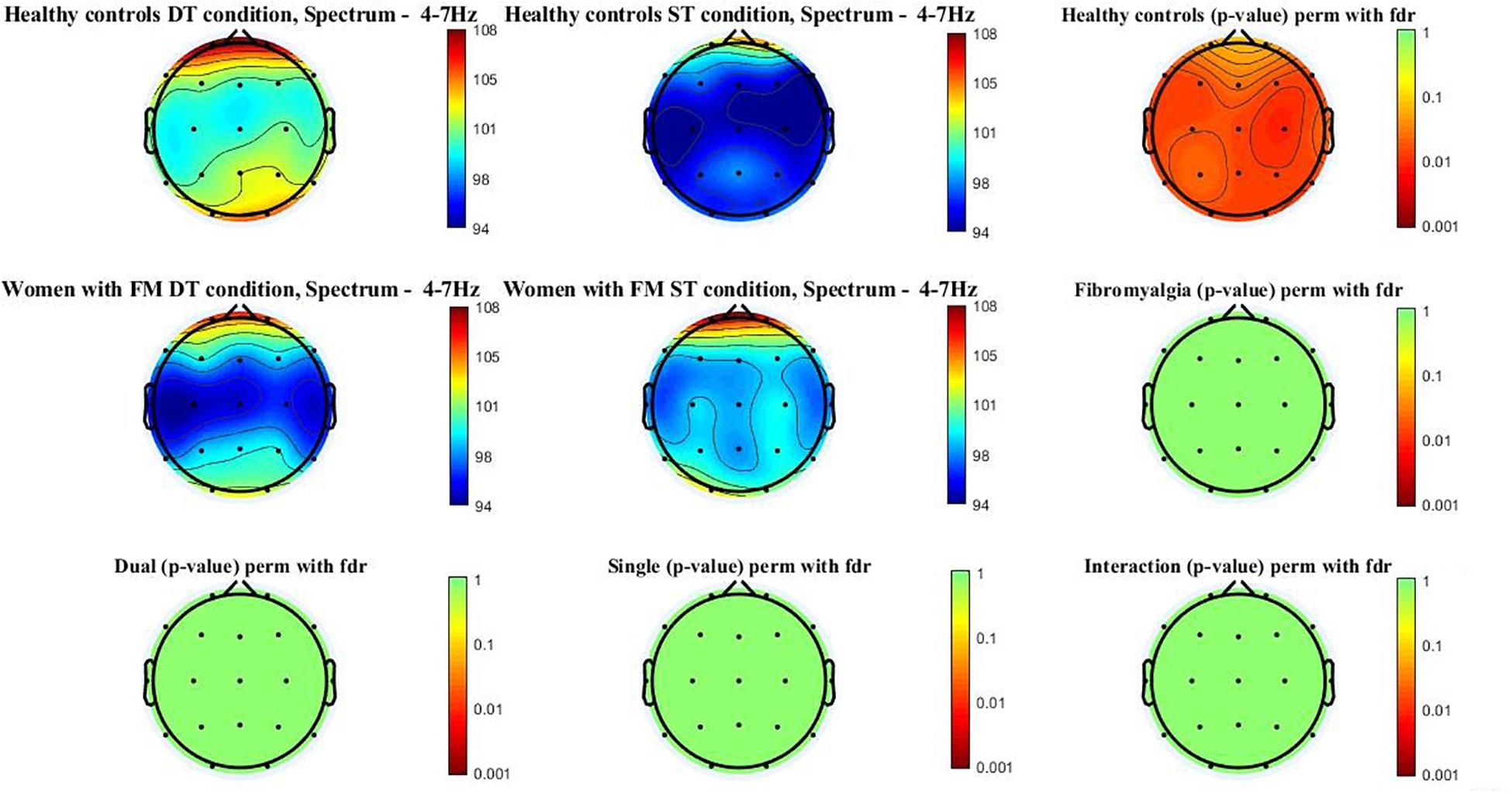
Figure 1. Theta power spectrum (4–7 Hz) topographic maps in women with FM and healthy controls. Differences were only located (p < 0.05) between ST and DT conditions in healthy controls in the following scalp locations: frontal (Fz, F3, F4, F7, and F8), central (Cz, C3, and C4), temporal (T3, T4, T5, and T6), parietal (Pz, P3, and P4), and occipital (O1 and O2).
Figure 2 shows the alpha power spectrum (8–12 Hz) topographic maps in the women with FM and healthy controls during ST and DT conditions. Differences (p-value < 0.05) were observed in healthy control when compared ST and DT conditions [scalp locations: frontal (Fz, Fp1, Fp2, F3, F4, F7, and F8), central (Cz, C3, and C4), temporal (T3, T4, T5, and T6), parietal (Pz, P3, and P4), and occipital (O1 and O2)]. Moreover, significant between-group differences (p-value < 0.005) were found when comparing healthy controls and women with FM in the DT condition [scalp locations: frontal (F7), central (Cz, C3, and C4), temporal (T3, T5, and T6), parietal (Pz and P4), and occipital (O2)]. The group∗condition interactions were also significant in the frontal (Fp1, F4, F7, and F8), central (Cz, C3, and C4), temporal (T3, T5, and T6), and occipital (O1).
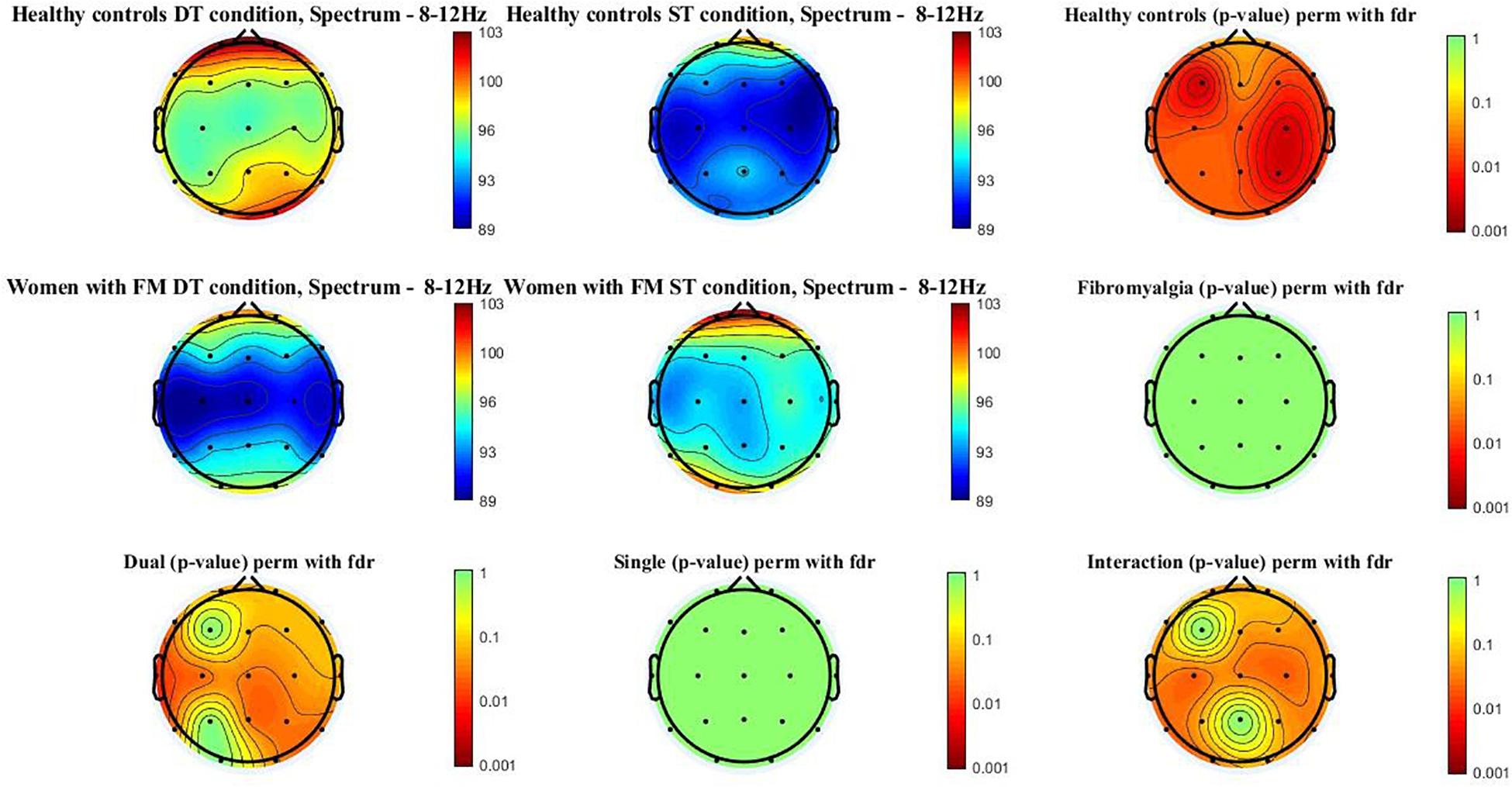
Figure 2. Alpha power spectrum (8–12 Hz) topographic maps in women with FM and healthy controls. Differences were only located (p < 0.05) between ST and DT conditions in healthy controls and between healthy controls and women with FM in the DT condition. In addition, the interactions group*condition is significant in the following scalp locations: central (Cz, C3, and C4), temporal (T5 and T6), and occipital (O1).
Figure 3 shows the beta power spectrum (13–30 Hz) topographic maps in the women with FM and healthy controls during ST and DT conditions. Differences (p-value < 0.05) were observed in healthy control when comparing ST and DT conditions [scalp locations: frontal (Fz, Fp1, Fp2, F3, F4, F7, and F8), central (Cz, C3, and C4), temporal (T3, T4, T5, and T6), parietal (Pz, P3, and P4), and occipital (O1 and O2)]. Moreover, significant between-group differences (p-value < 0.005) were found when comparing healthy controls and women with FM in the DT condition [scalp locations: frontal (F7, F8, Fp1, and Fp2), central (Cz, C3, and C4), temporal (T3, T4, T5, and T6), parietal (Pz and P4), and occipital (O2)]. The group∗condition interactions were also significant in the frontal (Fp1, Fp2, Fz, F4, F7, and F8), central (Cz, C3, and C4), temporal (T3, T4, T5, and T6), parietal (P3 and P4), and occipital (O1).
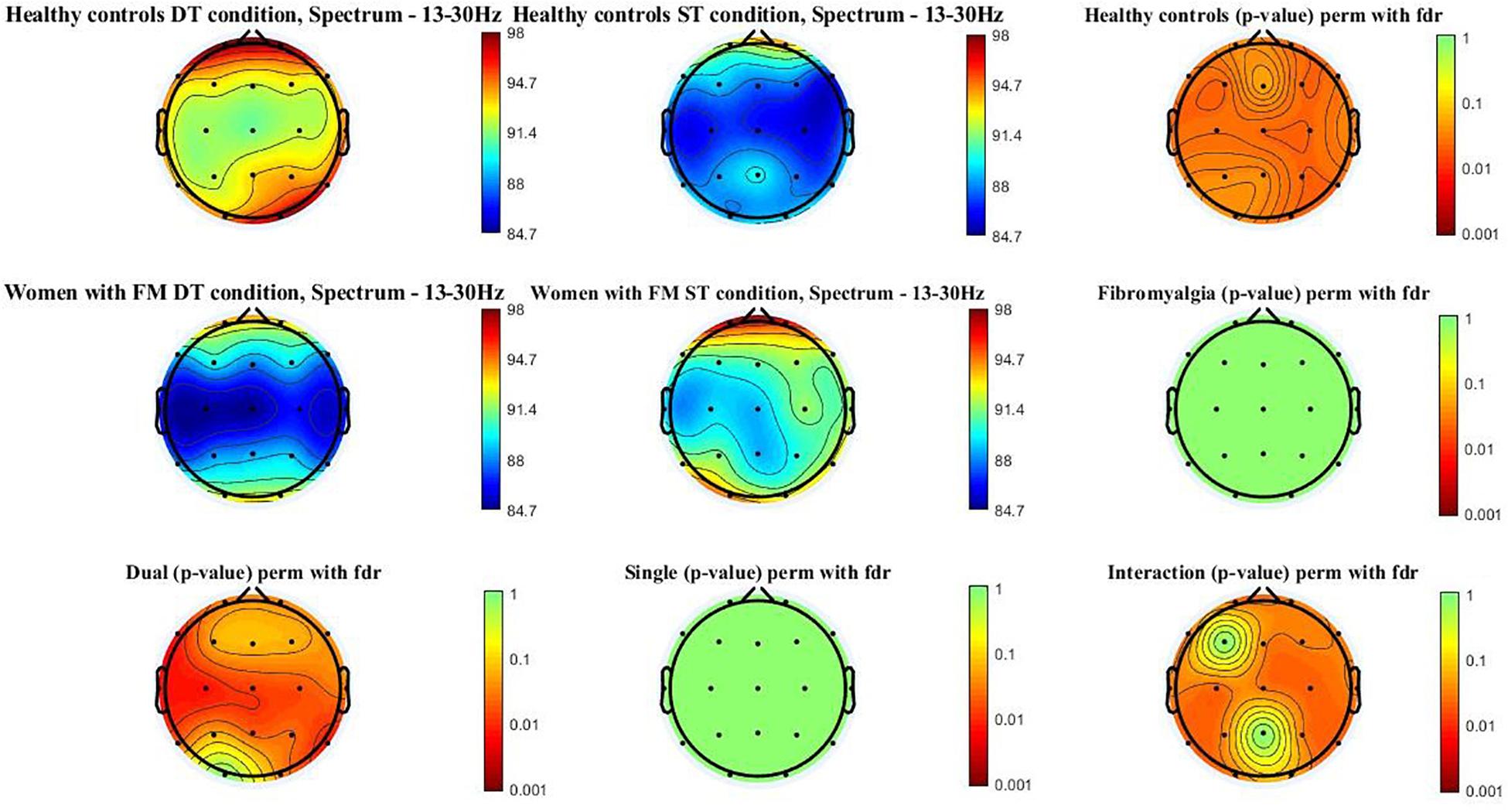
Figure 3. Beta power spectrum (13–30 Hz) topographic maps in women with FM and healthy controls. Differences were only located (p < 0.05) between ST and DT conditions in healthy controls and between healthy controls and women with FM in the DT condition. In addition, the interactions group*condition is significant in the following scalp locations: frontal (Fp1, Fp2, Fz, F4, F7, and F8), central (Cz, C3, and C4), temporal (T3, T4, T5, and T6), parietal (P3 and P4), and occipital (O1).
Supplementary File 1 shows the results of the complementary ERSP sources analyses. Differences within-group (ST vs. DT) were found in both healthy and FM group in one cluster for each group. In the healthy control group, the centroid dipole was located in the left medial frontal gyrus (Talairach coordinates: X = −4, Y = 56, Z = −5), whereas in the fibromyalgia group, the centroid dipole was located in the right middle temporal gyrus (Talairach coordinates: X = 67, Y = −23, Z = −5).
Between-group differences (fibromyalgia vs. healthy control group) showed differences during dual- and single-task conditions. During dual-task conditions, the centroid dipoles were located in the right cuneus (Talairach coordinates: X = 11, Y = −73, Z = 15) and right medial frontal gyrus (Talairach coordinates: X = 1, Y = 58, Z = −12). During single-task conditions, the centroid dipole was located in the right medial frontal gyrus (Talairach coordinates: X = 8, Y = 48, Z = 7).
The main purpose of this study was to explore the neurophysiological response of women with FM and healthy controls while performing a DT (motor–cognitive). Significant differences were obtained in the healthy control group between ST and DT in the theta, alpha, and beta frequency bands. Nevertheless, significant within-group differences were not obtained in women with FM between ST and DT in any of the frequency bands. In addition, between-group differences were found in the alpha and beta frequency bands between healthy and FM groups, with lower values of beta and alpha in the FM group. Therefore, significant group∗condition interactions were detected in the alpha and beta frequency bands. Regarding physical condition performance, between groups, analyses showed that women with FM obtained significantly worse results in the arm curl test than healthy controls, in both ST and DT.
Previous studies have reported differences between healthy controls and women with FM in the EEG signal at rest (Fallon et al., 2017; Villafaina et al., 2019a). However, this is the first study reporting differences between healthy controls and women with FM during DT conditions. Previous investigations have explored the brain activity while dual-tasking. Most of these studies have used the functional near-infrared spectroscopy (fNIRS) (Holtzer et al., 2011, 2015; Al-Yahya et al., 2016; Lin and Lin, 2016; Maidan et al., 2017) over the prefrontal cortex to investigate this topic. In this regard, increases in oxygen levels in the prefrontal cortex have been reported when performed any type of DT (Holtzer et al., 2015; Al-Yahya et al., 2016; Lin and Lin, 2016). However, results from fNIRS studies are limited to the prefrontal area due to the restricted number of channels that could be recorded. Thus, previous studies have employed the EEG to study the impact of DT on brain activity (De Sanctis et al., 2014; Malcolm et al., 2015; Bogost et al., 2016). Our results are consistent with a previous study where an increase in theta and beta power spectrum were observed between ST and DT (Pizzamiglio et al., 2017) in the frontal and parietal regions in healthy adults.
However, in the study of Pizzamiglio et al. (2017), a decrease in the alpha power spectrum during the DT was detected when compared with the ST condition. This is inconsistent with our results, where a significant increase in the DT was found in the healthy control group when compared with the ST condition. However, hypothetically, it could be due to the type of DT, which is presented in this study (a memory-based DT, which consisted of remembering three random unrelated words while the women were performing the tests). In this regard, findings of alpha power have not been consistent across experimental studies (Wianda and Ross, 2019). In our study, participants were encouraged to keep in mind these unrelated words while performing the motor task. This could be connected with the findings of a previous study where an alpha increase during the retention interval in a short-term memory task was reported (Jensen et al., 2002). Interestingly, another study has suggested that the increase in alpha power spectrum plays an active role in preventing the distracting information into areas which retain the memory items (Mazaheri et al., 2014). Moreover, previous studies have linked beta band to short-term memory (Tallon-Baudry et al., 1999; Palva et al., 2011), elevated mental workload (Coelli et al., 2015), or concentration (Kakkos et al., 2019) as well as increasing in working memory (Spitzer and Haegens, 2017). In the same line, the theta band is associated to increases in cognitive workload (Fuentes-García et al., 2019; Diaz-Piedra et al., 2020). Therefore, further investigation is needed to clarify the role of alpha, beta, and theta bands in different types of DT conditions.
The arm curl provides useful information in people with fibromyalgia since it could potentially be used to discriminate (in ST conditions) women with fibromyalgia from healthy women (Ariadna Aparicio et al., 2015) or even the severity of the symptoms (Soriano-Maldonado et al., 2015). Our results showed differences between women with FM and healthy controls in both ST and DT in this physical fitness test. Nevertheless, within-group differences were not observed in women with FM nor healthy controls in the ST or DT conditions. These results are consistent with previous investigations where similar results using both the arm-curl test and the same simultaneous cognitive task were observed (Villafaina et al., 2019d). These results, as previously suggested by Villafaina et al. (2018), could be due to a low complexity of the motor task. However, since the arm curl test has not been deeply studied under DT conditions in women with FM, further studies are necessary to confirm this hypothesis.
However, neurophysiological results showed that healthy controls modified their brain activity between ST and DT in order to adapt their brain activity to the task commitment. These changes were not observed in the women with FM between the ST and the DT conditions. This could be derived from the attention deficit disorder (van Rensburg et al., 2018; Yilmaz and Tamam, 2018), which is common in this population. Besides, people with FM usually showed depression or cognitive impairment (Wolfe et al., 2010) in several domains such as long-term memory (Canovas et al., 2009), short-term memory (Park et al., 2001), or working memory (Coppieters et al., 2015). These comorbidities could have an impact on EEG, showing a left hemisphere hypoactivation (Villafaina et al., 2019e) or a reduced theta power at rest (Musaeus et al., 2018; Villafaina et al., 2019b). However, taking into account the simultaneous cognitive task, the attention deficit disorder could have a significant impact on the EEG patter during the DT condition. The attention deficit disorder could lead to difficulties in focusing their attention on three unrelated words provided before the test started, “forgetting” to think in these words during the DT condition. This, hypothetically, may explain that changes in the neurophysiological measures were not found. For instance, it could be expected, as occurred in the healthy group, that beta and theta bands would increase during the DT condition due to higher cognitive demands or workload (Fuentes-García et al., 2019; Diaz-Piedra et al., 2020). This is because increases in beta and theta bands, due to increases in cognitive workload, could be expected. Thus, EEG measurement could be an interesting tool to enhance the knowledge about the DT paradigm as well as help to interpret the reasons behind differences between healthy controls and women with FM in the physical performance in both DT and ST conditions.
Source analysis shows differences within group (ST vs. DT) in both healthy and FM groups. In the healthy control group, the centroid dipole was located in the right anterior cingulate, whereas in the fibromyalgia group, the centroid dipole was located in the left cingulate gyrus. In this regard, source analysis has been used to investigate the electrocortical source during ST and DT paradigms (Lin et al., 2011; Bogost et al., 2016). A previous study showed a reduction in the mean absolute N1 ERP peak amplitude in the DT compared with the ST in the N1 between ST and DT conditions (Bogost et al., 2016). However, the dual-task employed in this investigation (visual working memory) does not allow to compare the results. Thus, further investigation in this field is required.
This study has some limitations. First, all the participants were women, so results cannot be extrapolated to men with FM. In the same line, the relatively small sample size (17 women with FM and 19 healthy controls) could make that only greater differences would reach the significance level. Thus, results cannot be generalized and further research in this field is necessary. Third, the lack of test–retest reliability, validity, and variability of the EEG results should be considered. Therefore, an EEG register longer than 30 s should be recommended as well as the inclusion of a baseline assessment. Lastly, according to their current medical history any psychiatric or neurological diagnoses were present in their medical history. However, due to the high rate of comorbidities with several psychiatric disorders in FM (such as mood, anxiety, or depression) (Sancassiani et al., 2017) could not be discarded that some of the participants might be suffering from these disorders when women with FM were assessed. Taking into account these limitations, results should be taken with caution.
Neurophysiological differences between women with FM and healthy controls were found during DT condition. This is the first study which investigates the neurophysiological response of women with FM while simultaneously performing a motor and a cognitive task. Women with FM showed the same brain activity pattern during ST and DT conditions, whereas healthy controls seem to adapt their brain activity to task commitment.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the University of Extremadura Bioethical Committee. The patients/participants provided their written informed consent to participate in this study.
JF-G, SV, and NG conceived the study. SV and RC-P collected the data. SV and RC-P analyzed the data. JF-G, NG, and SV designed the figures and tables. SV, JF-G, and NG wrote the manuscript. NG, SV, JF-G, and RC-P provided critical revisions on the successive drafts. All authors approved the manuscript in its final form.
This study was cofunded by the Spanish Ministry of Economy and Competitiveness (reference no. DEP2015-70356) in the framework of the Spanish National R + D + I Plan. Moreover, this study has been supported by the Biomedical Research Networking Center on Frailty and Healthy Aging (CIBERFES) and FEDER funds from the European Union (CB16/10/00477), Ministry of Economy and Infrastructure of the Junta de Extremadura through the European Regional Development Fund, and a way to make Europe (GR18129 and GR18155). SV was supported by a grant from the Regional Department of Economy and Infrastructure of the Government of Extremadura and the European Social Fund (PD16008). The funders played no role in the study design, the data collection and analysis, the decision to publish, or the preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We acknowledge the Extremadura Association of Fibromyalgia (AFIBROEX) in Cáceres for helping to recruit the participants for this study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyg.2020.558849/full#supplementary-material
Al-Yahya, E., Johansen-Berg, H., Kischka, U., Zarei, M., Cockburn, J., and Dawes, H. (2016). Prefrontal cortex activation while walking under dual-task conditions in stroke: a multimodal imaging study. Neurorehabil. Neural Repair 30, 591–599. doi: 10.1177/1545968315613864
Ariadna Aparicio, V., Segura-Jimenez, V., Alvarez-Gallardo, I. C., Soriano-Maldonado, A., Castro-Pinero, J., Delgado-Fernandez, M., et al. (2015). Fitness testing in the fibromyalgia diagnosis: the al-Andalus project. Med. Sci. Sports Exerc. 47, 451–459. doi: 10.1249/mss.0000000000000445
Benjamini, Y., and Hochberg, Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 57, 289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x
Bennett, R. (2005). The fibromyalgia impact questionnaire (FIQ): a review of its development, current version, operating characteristics and uses. Clin. Exp. Rheumatol. 23, S154–S162.
Beurskens, R., Steinberg, F., Antoniewicz, F., Wolff, W., and Granacher, U. (2016). Neural correlates of dual-task walking: effects of cognitive versus motor interference in young adults. Neural Plast. 2016:8032180.
Bogost, M. D., Burgos, P. I., Little, C. E., Woollacott, M. H., and Dalton, B. H. (2016). Electrocortical sources related to whole-body surface translations during a single- and dual-task paradigm. Front. Hum. Neurosci. 10:524. doi: 10.3389/fnhum.2016.00524
Boonstra, A. M., Schiphorst Preuper, H. R., Reneman, M. F., Posthumus, J. B., and Stewart, R. E. (2008). Reliability and validity of the visual analogue scale for disability in patients with chronic musculoskeletal pain. Int. J. Rehabil. Res. 31, 165–169. doi: 10.1097/mrr.0b013e3282fc0f93
Bradford, J. C., Lukos, J. R., and Ferris, D. P. (2016). Electrocortical activity distinguishes between uphill and level walking in humans. J. Neurophysiol. 115, 958–966. doi: 10.1152/jn.00089.2015
Burckhardt, C. S., Clark, S. R., and Bennett, R. M. (1993). Fibromyalgia and quality of life: a comparative analysis. J. Rheumatol. 20, 475–479.
Canovas, R., Leon, I., Roldan, M. D., Astur, R., and Cimadevilla, J. M. (2009). Virtual reality tasks disclose spatial memory alterations in fibromyalgia. Rheumatology 48, 1273–1278. doi: 10.1093/rheumatology/kep218
Coelli, S., Sclocco, R., Barbieri, R., Reni, G., Zucca, C., and Bianchi, A. M. (2015). “EEG-based index for engagement level monitoring during sustained attention,” in Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, (New Orleans, LA: IEEE), 1512–1515.
Coppieters, I., Ickmans, K., Cagnie, B., Nijs, J., De Pauw, R., Noten, S., et al. (2015). Cognitive performance is related to central sensitization and health-related quality of life in patients with chronic whiplash-associated disorders and fibromyalgia. Pain Physician 18, E389–E401.
de Gier, M., Peters, M. L., and Vlaeyen, J. W. S. (2003). Fear of pain, physical performance, and attentional processes in patients with fibromyalgia. Pain 104, 121–130. doi: 10.1016/s0304-3959(02)00487-6
De Sanctis, P., Butler, J. S., Malcolm, B. R., and Foxe, J. J. (2014). Recalibration of inhibitory control systems during walking-related dual-task interference: a mobile brain-body imaging (MOBI) study. Neuroimage 94, 55–64. doi: 10.1016/j.neuroimage.2014.03.016
Del Paso, G. A. R., Montoro, C. I., and Duschek, S. (2015). Reaction time, cerebral blood flow, and heart rate responses in fibromyalgia: evidence of alterations in attentional control. J. Clin. Exp. Neuropsychol. 37, 414–428. doi: 10.1080/13803395.2015.1023265
Delorme, A., and Makeig, S. (2004). EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 134, 9–21. doi: 10.1016/j.jneumeth.2003.10.009
Diaz-Piedra, C., Sebastián, M. V., and Di Stasi, L. L. (2020). EEG theta power activity reflects workload among army combat drivers: an experimental study. Brain Sci. 10:199. doi: 10.3390/brainsci10040199
Esteve-Vives, J., Redondo, J. R., Salvat, M. I. S., De Gracia Blanco, M., and De Miquele, C. A. (2007). Proposal for a consensus version of the fibromyalgia impact questionnaire (FIQ) for the Spanish population. Reumatol. Clín. 3, 21–24. doi: 10.1016/s2173-5743(07)70204-6
Fallon, N., Chiu, Y., Nurmikko, T., and Stancak, A. (2017). Altered theta oscillations in resting EEG of fibromyalgia syndrome patients. Eur. J. Pain 22, 49–57. doi: 10.1002/ejp.1076
Ford, J. M., Mathalon, D. H., Whitfield, S., Faustman, W. O., and Roth, W. T. (2002). Reduced communication between frontal and temporal lobes during talking in schizophrenia. Biol. Psychiatry 51, 485–492. doi: 10.1016/s0006-3223(01)01335-x
Fritz, C. O., Morris, P. E., and Richler, J. J. (2012). Effect size estimates: current use, calculations, and interpretation. J. Exp. Psychol. Gen. 141, 2–18. doi: 10.1037/a0024338
Fuentes-García, P. J., Pereira, T., Castro, A. M., Carvalho Santos, A., and Villafaina, S. (2019). Heart and brain responses to real versus simulated chess games in trained chess players: a quantitative EEG and HRV study. Int. J. Environ. Res. Public Health 16:5021. doi: 10.3390/ijerph16245021
Giraud, A.-L., Kleinschmidt, A., Poeppel, D., Lund, T. E., Frackowiak, R. S. J., and Laufs, H. (2007). Endogenous cortical rhythms determine cerebral specialization for speech perception and production. Neuron 56, 1127–1134. doi: 10.1016/j.neuron.2007.09.038
Hawker, G. A., Mian, S., Kendzerska, T., and French, M. (2011). Measures of adult pain: visual analog scale for pain (VAS Pain), numeric rating scale for pain (NRS Pain), McGill pain questionnaire (MPQ), short-form McGill pain questionnaire (SF-MPQ), chronic pain grade scale (CPGS), short form-36 bodily pain scale (SF-36 BPS), and measure of intermittent and constant osteoarthritis pain (ICOAP). Arthritis Care Res. 63, S240–S252.
Helmick, C. G., Felson, D. T., Lawrence, R. C., Gabriel, S., Hirsch, R., Kwoh, C. K., et al. (2008). Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Arthritis Rheum. 58, 15–25. doi: 10.1002/art.23177
Holtzer, R., Mahoney, J. R., Izzetoglu, M., Izzetoglu, K., Onaral, B., and Verghese, J. (2011). fNIRS study of walking and walking while talking in young and old individuals. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 66, 879–887. doi: 10.1093/gerona/glr068
Holtzer, R., Mahoney, J. R., Izzetoglu, M., Wang, C., England, S., and Verghese, J. (2015). Online fronto-cortical control of simple and attention-demanding locomotion in humans. Neuroimage 112, 152–159. doi: 10.1016/j.neuroimage.2015.03.002
Homan, R. W. (1988). The 10-20 electrode system and cerebral location. Am. J. EEG Technol. 28, 269–279. doi: 10.1080/00029238.1988.11080272
Huijnen, I. P. J., Verbunt, J. A., Meeus, M., and Smeets, R. (2015). Energy expenditure during functional daily life performances in patients with fibromyalgia. Pain Pract. 15, 748–756. doi: 10.1111/papr.12245
Jensen, O., Gelfand, J., Kounios, J., and Lisman, J. E. (2002). Oscillations in the alpha band (9–12 Hz) increase with memory load during retention in a short-term memory task. Cereb. Cortex 12, 877–882. doi: 10.1093/cercor/12.8.877
Jung, T. P., Makeig, S., Westerfield, M., Townsend, J., Courchesne, E., and Sejnowski, T. J. (2000). Removal of eye activity artifacts from visual event-related potentials in normal and clinical subjects. Clin. Neurophysiol. 111, 1745–1758. doi: 10.1016/s1388-2457(00)00386-2
Kakkos, I., Dimitrakopoulos, G. N., Gao, L., Zhang, Y., Qi, P., Matsopoulos, G. K., et al. (2019). Mental workload drives different reorganizations of functional cortical connectivity between 2D and 3D simulated flight experiments. IEEE Trans. Neural Syst. Rehabil. Eng. 27, 1704–1713. doi: 10.1109/tnsre.2019.2930082
Lin, C.-T., Chen, S.-A., Chiu, T.-T., Lin, H.-Z., and Ko, L.-W. (2011). Spatial and temporal EEG dynamics of dual-task driving performance. J. Neuroeng. Rehabil. 8:11. doi: 10.1186/1743-0003-8-11
Lin, M.-I. B., and Lin, K.-H. (2016). Walking while performing working memory tasks changes the prefrontal cortex hemodynamic activations and gait kinematics. Front. Behav. Neurosci. 10:92. doi: 10.3389/fnbeh.2016.00092
Maidan, I., Bernad-Elazari, H., Giladi, N., Hausdorff, J. M., and Mirelman, A. (2017). When is higher level cognitive control needed for locomotor tasks among patients with Parkinson’s disease? Brain Topogr. 30, 531–538. doi: 10.1007/s10548-017-0564-0
Malcolm, B. R., Foxe, J. J., Butler, J. S., and De Sanctis, P. (2015). The aging brain shows less flexible reallocation of cognitive resources during dual-task walking: a mobile brain/body imaging (MoBI) study. Neuroimage 117, 230–242. doi: 10.1016/j.neuroimage.2015.05.028
Mazaheri, A., Van Schouwenburg, M. R., Dimitrijevic, A., Denys, D., Cools, R., and Jensen, O. (2014). Region-specific modulations in oscillatory alpha activity serve to facilitate processing in the visual and auditory modalities. Neuroimage 87, 356–362. doi: 10.1016/j.neuroimage.2013.10.052
McGrath, R. E., and Meyer, G. J. (2006). When effect sizes disagree: the case of r and d. Psychol. Methods 11, 386–401. doi: 10.1037/1082-989x.11.4.386
Musaeus, C. S., Engedal, K., Høgh, P., Jelic, V., Mørup, M., Naik, M., et al. (2018). EEG theta power is an early marker of cognitive decline in dementia due to Alzheimer’s disease. J. Alzheimers Dis. 64, 1359–1371. doi: 10.3233/jad-180300
Palva, S., Kulashekhar, S., Hämäläinen, M., and Palva, J. M. (2011). Localization of cortical phase and amplitude dynamics during visual working memory encoding and retention. J. Neurosci. 31, 5013–5025. doi: 10.1523/jneurosci.5592-10.2011
Park, D. C., Glass, J. M., Minear, M., and Crofford, L. J. (2001). Cognitive function in fibromyalgia patients. Arthritis Rheum. 44, 2125–2133. doi: 10.1002/1529-0131(200109)44:9<2125::aid-art365>3.0.co;2-1
Pizzamiglio, S., Naeem, U., Abdalla, H., and Turner, D. L. (2017). Neural correlates of single-and dual-task walking in the real world. Front. Hum. Neurosci. 11:460. doi: 10.3389/fnhum.2017.00460
Ruffini, G., Dunne, S., Farres, E., Cester, I., Watts, P. C. P., Silva, S. R. P., et al. (2007). “ENOBIO dry electrophysiology electrode; first human trial plus wireless electrode system,” in Proceedings of the 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, (IEEE: Lyon), 6690–6694.
Sancassiani, F., Machado, S., Ruggiero, V., Cacace, E., Carmassi, C., Gesi, C., et al. (2017). The management of fibromyalgia from a psychosomatic perspective: an overview. Int. Rev. Psychiatry 29, 473–488. doi: 10.1080/09540261.2017.1320982
Sipp, A. R., Gwin, J. T., Makeig, S., and Ferris, D. P. (2013). Loss of balance during balance beam walking elicits a multifocal theta band electrocortical response. J. Neurophysiol. 110, 2050–2060. doi: 10.1152/jn.00744.2012
Soriano-Maldonado, A., Henriksen, M., Segura-Jiménez, V., Aparicio, V. A., Carbonell-Baeza, A., Delgado-Fernández, M., et al. (2015). Association of physical fitness with fibromyalgia severity in women: the al-Ándalus project. Arch. Phys. Med. Rehabil. 96, 1599–1605. doi: 10.1016/j.apmr.2015.03.015
Spitzer, B., and Haegens, S. (2017). Beyond the status quo: a role for beta oscillations in endogenous content (re) activation. eNeuro 4:ENEURO.0170-17.2017.
Tallon-Baudry, C., Kreiter, A., and Bertrand, O. (1999). Sustained and transient oscillatory responses in the gamma and beta bands in a visual short-term memory task in humans. Vis. Neurosci. 16, 449–459. doi: 10.1017/s0952523899163065
van Rensburg, R., Meyer, H. P., Hitchcock, S. A., and Schuler, C. E. (2018). Screening for adult ADHD in patients with fibromyalgia syndrome. Pain Med. 19, 1825–1831. doi: 10.1093/pm/pnx275
Villafaina, S., Collado-Mateo, D., Domínguez-Muñoz, F. J., Fuentes-García, J. P., and Gusi, N. (2018). Impact of adding a cognitive task while performing physical fitness tests in women with fibromyalgia: a cross-sectional descriptive study. Medicine 97:e13791. doi: 10.1097/md.0000000000013791
Villafaina, S., Collado-Mateo, D., Fuentes-García, J. P., Cano-Plasencia, R., and Gusi, N. (2019a). Impact of fibromyalgia on alpha-2 EEG power spectrum in the resting condition: a descriptive correlational study. Biomed Res. Int. 2019:7851047.
Villafaina, S., Collado-Mateo, D., Fuentes-García, J. P., Domínguez-Muñoz, F. J., and Gusi, N. (2019b). Duration of the symptoms and brain aging in women with fibromyalgia: a cross-sectional study. Appl. Sci. 9:2106. doi: 10.3390/app9102106
Villafaina, S., Gusi, N., Rodriguez-Generelo, S., Martin-Gallego, J. D. D., Fuentes-García, J. P., and Collado-Mateo, D. (2019c). Influence of a cell-phone conversation on balance performance in women with fibromyalgia: a cross-sectional descriptive study. Biomed Res. Int. 2019:5132802.
Villafaina, S., Polero, P., Collado-Mateo, D., Fuentes-García, J. P., and Gusi, N. (2019d). Impact of adding a simultaneous cognitive task in the elbow’s range of movement during arm curl test in women with fibromyalgia. Clin. Biomech. 65, 110–115. doi: 10.1016/j.clinbiomech.2019.04.006
Villafaina, S., Sitges, C., Collado-Mateo, D., Fuentes-García, J. P., and Gusi, N. (2019e). Influence of depressive feelings in the brain processing of women with fibromyalgia: an EEG study. Medicine 98:e15564. doi: 10.1097/md.0000000000015564
Wagner, J., Solis-Escalante, T., Grieshofer, P., Neuper, C., Müller-Putz, G., and Scherer, R. (2012). Level of participation in robotic-assisted treadmill walking modulates midline sensorimotor EEG rhythms in able-bodied subjects. Neuroimage 63, 1203–1211. doi: 10.1016/j.neuroimage.2012.08.019
Wianda, E., and Ross, B. (2019). The roles of alpha oscillation in working memory retention. Brain Behav. 9:e01263. doi: 10.1002/brb3.1263
Winslow, A. T., Brantley, J., Zhu, F., Vidal, J. L. C., and Huang, H. (2016). Corticomuscular coherence variation throughout the gait cycle during overground walking and ramp ascent: a preliminary investigation. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2016, 4634–4637.
Wolfe, F., Clauw, D. J., Fitzcharles, M. A., Goldenberg, D. L., Katz, R. S., Mease, P., et al. (2010). The American college of rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 62, 600–610. doi: 10.1002/acr.20140
Yilmaz, E., and Tamam, L. (2018). Attention-deficit hyperactivity disorder and impulsivity in female patients with fibromyalgia. Neuropsychiatr. Dis. Treat. 14, 1883–1889. doi: 10.2147/ndt.s159312
Keywords: dual task, pain, physical fitness, EEG, strength
Citation: Villafaina S, Fuentes-García JP, Cano-Plasencia R and Gusi N (2020) Neurophysiological Differences Between Women With Fibromyalgia and Healthy Controls During Dual Task: A Pilot Study. Front. Psychol. 11:558849. doi: 10.3389/fpsyg.2020.558849
Received: 04 May 2020; Accepted: 24 September 2020;
Published: 04 November 2020.
Edited by:
Ana-Maria Cebolla, Université libre de Bruxelles, BelgiumReviewed by:
Pablo Ignacio Burgos, University of Chile, ChileCopyright © 2020 Villafaina, Fuentes-García, Cano-Plasencia and Gusi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan Pedro Fuentes-García, anBmdWVudEB1bmV4LmVz
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.