- 1Department of Psychology, University of Turin, Turin, Italy
- 2European Innovation Partnership on Active and Healthy Ageing, Brussels, Belgium
Frailty is an age-related dynamic status, characterized by a reduced resistance to stressors due to the cumulative decline of multiple physiological systems. Several researches have highlighted a relationship between physical frailty and cognitive decline; however, the role of specific cognitive domains has not been deeply clarified yet. Current studies have hypothesized that physical frailty and neuropsychological deficits may share systemic inflammation and increased oxidative stress in different neurodegenerative disorders, such as Alzheimer’s and Parkinson’s disease. However, the role of the executive dysfunction should be investigated in a more detailed way using a multidimensional approach. With this aim, we conducted a review of the literature on the few experimental articles published to discuss the existence of a relationship between frailty and cognitive impairment in neurocognitive disorders, particularly focusing on the domain of executive dysfunction. The data suggest that physical frailty and cognitive decline, especially executive dysfunction, are two aspects strongly linked in mild and major neurocognitive disorders due to Alzheimer’s and Parkinson’s disease. In light of this, a new framework linking aging, cognitive decline, and neurodegenerative diseases is needed. In order to analyze the effects that aging processes have on neural decline and neurocognitive disease, and to identify relevant groups of users and patients, future longitudinal studies should adopt a multidimensional approach, in the field of primary prevention and in the continuum from mild to major neurocognitive disorder.
Introduction
Frailty is a complex and heterogeneous clinical status described as the loss of harmonic interactions among various dimensions, such as biological, genetic, functional, psychological, cognitive, and social domains (Pilotto et al., 2020), that lead to homeostatic instability. Although the relationship between this issue and poor outcomes has been highlighted, currently there is no gold standard on how to define measure and diagnose frailty (Richards et al., 2018).
Nowadays, there are at least three main models to study frailty in aging subjects (Figure 1): the phenotypic model (Fried et al., 2001), the deficit accumulation model (Rockwood et al., 2005; Rockwood and Mitnitski, 2007b), and the bio-psycho-social model (Gobbens et al., 2010); the first two characterize the biomedical approach.
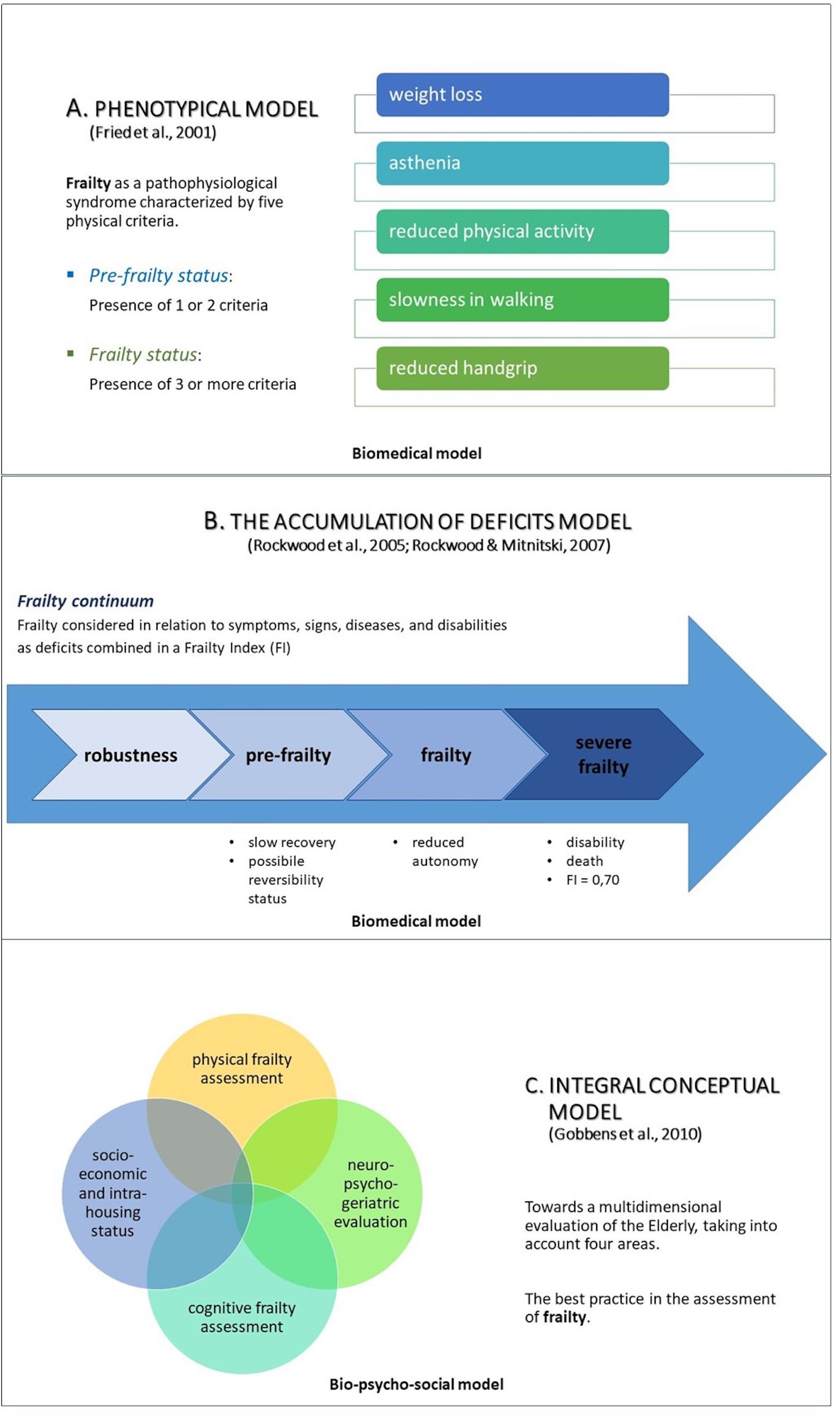
Figure 1. The three main approaches to study frailty: the phenotypical model (Fried et al., 2001; A), the accumulation of deficits model (Rockwood et al., 2005; Rockwood and Mitnitski, 2007b; B), and the integral conceptual model, based on a bio-psycho-social approach (Gobbens et al., 2010; C).
The biomedical approach highlights how a reduction in the ability to preserve homeostasis from a physiological point of view, and to respond to environmental changes appropriately, implies a loss of functional autonomy (Xue, 2011).
The phenotypic model (Fried et al., 2001) considers frailty in terms of a physiopathological syndrome composed of five physical determinants: slowness in walking, a decrease in hand-grip-strength, unintentional weight loss, low physical activity, and asthenia. The presence of one or two criteria identifies a pre-frailty status; instead, the presence of three or more, a frailty condition (see Figure 1A).
The deficit accumulation model (Rockwood et al., 2005; Rockwood and Mitnitski, 2007b), may be interpreted in line with a Frailty Index (FI) characterized by age-related deficits, which configure an augmented vulnerability resulting from age-related decline across several body organs and physiological systems. Considering this model of clinical frailty syndrome, the higher the FI, the frailer the individual (see Figure 1B).
Although Rockwood’s model allows for a more extensive evaluation compared to Fried’s one, also demonstrating greater sensitivity in predicting poor outcomes (Rockwood et al., 2007a), it did not fully take into account the psycho-social aspects that may affect the development of frailty.
Over time, the biomedical approach has been criticized (Canevelli et al., 2015) for different reasons: (1) frailty assessment was carried out above all by adopting Fried’s criteria (Fried et al., 2001), as they focused mainly on physical frailty; (2) the majority of these studies evaluated the global cognitive functioning only through the Mini Mental State Examination (MMSE: Folstein et al., 1975), lacking of a full neuropsychological screening; (3) most of the participants were community-dwelling elderly people, compromising the applicability of the results to different types of patients, such as those with neurodegenerative disorders. Therefore, a new concept of frailty has emerged in relation to its applicability in clinical practice. According to this view, frailty can be interpreted as an integrated and multidimensional condition in which multiples domains (such as biological, functional, psychological, and social dimension) interact together, determining and characterizing a frailty status. The above led to the development of the third model, represented by the bio-psycho-social paradigm (Gobbens et al., 2010). Since the interaction of the different “dimensions” is likely to be the basis of the bio-psycho-social and clinical complexity of the frail elderly, multidimensional evaluation is the most suitable choice for frailty detection; it allows to explore not only the physical/medical symptoms but also other important variables that must complete this complex picture (see Figure 1C).
Lately, the importance of a multidimensional approach has been emphasized to better comprehend frailty, not only as a physiopathological syndrome (Amanzio et al., 2017). According to this approach, the multidimensional prognostic index (MPI) could be considered a more comprehensive evaluation tool (Pilotto et al., 2013, 2020; Angleman et al., 2015), useful for the assessment of subjects with neurodegenerative disorders, from minor to major neurocognitive decline, with different frailty status (Amanzio et al., 2017).
Frailty and Cognitive Functions: What Kind of Association?
Originally, the concept of frailty referred only to a physical condition; recently, it includes also a cognitive status, which could be related to a reduction of neurophysiological reserves. At present, cognition is considered a relevant domain for frailty comprehension and a novel target for the prevention of elderly dependency (Ruan et al., 2015). Indeed, cognitive frailty seems to be both an effect and a cause of physical frailty.
Physical frailty is considered a risk factor for Mild Cognitive Impairment (Boyle et al., 2010). In a 10-year longitudinal study, Raji et al. (2010) explored whether cognitive impairment could predict frailty risk in robust elderly. The authors suggested that robust older people with cognitive dysfunctions had a 9% higher chance to become frail per year, compared to the individuals with preserved cognition. 30.9% of the elderly with cognitive impairment fulfilled the criteria for weight loss from the first to the second follow-up, while the 25% fulfilled the criteria for slowness from the second to the last follow-up (Raji et al., 2010).
More recently, data from the Italian Longitudinal Study on Aging (ILSA) suggested that cognitive frailty increased risk of all common cause of mortality in older people, over a 3.5-year and 7-year follow-up (Solfrizzi et al., 2017). Cognitive impairment was found to be associated in a higher risk of adverse health outcomes also in The Singapore Longitudinal Aging Studies (SLAS), for which cognitive impairment resulted implicated in the increased prevalence and incidence of functional disability, poor quality of life, and mortality (Feng et al., 2017).
Cognitive impairment can be easily detected by administering neuropsychological cognitive tests, such as the MMSE. Exceeding the limit of the exclusive use of the MMSE, a small number of studies examined the association between specific cognitive functions and physical frailty (Canevelli et al., 2015), pointing out a relationship between a reduction in gait speed or grip strength and an impairment of attention and executive functions (Woollacott and Shumway-Cook, 2002; O’Halloran et al., 2014; Canevelli et al., 2015). These findings were supported by the results of a 9-year longitudinal study of 331 healthy women, which showed the association of executive functioning with frailty progression, suggesting that both impairments and declines in executive functioning were associated with risk of frailty onset (Gross et al., 2016). More recently, data from The Toledo Study for Healthy Aging (TSHA) demonstrated that deficit in executive functioning is a powerful predictor of frailty (increased risk of 13%), disability (increased risk of 11%), and mortality (increased risk of 7%) (Rosado-Artalejo et al., 2017).
Executive Functions (EFs) are a set of abilities that control thoughts and behaviors (Miyake and Friedman, 2012). They can be categorized into “cool” EFs, which involve conscious control of thoughts and actions in non-emotional conditions, and “hot” EFs, concerning goal-directed and future-oriented cognitive processes in contexts that elicit emotions, motivation, and tension (Poon, 2018). Although there is still no consensus regarding which are the cognitive functions that may or may not be included in the EFs (Poon, 2018), there is a general agreement that shifting, updating/monitoring, and inhibition are the core EFs (Diamond, 2013), which play a different and complementary role in performing complex executive tasks (Miyake et al., 2000). In order to comprehend the unity but also the heterogeneity of EFs, Miyake et al. (2000) proposed a structural model characterized by mental shifting, information monitoring and updating, and inhibition of preponderant responses. From these, higher-order EFs arise such as problem solving and planning (Lunt et al., 2012).
On the other hand, the term “executive dysfunction” refers to the inability to formulate, organize, and plan goal-directed behaviors and novel cognitive tasks (Parker et al., 2013).
Executive deficits are related to frontal network disruption and can occur in various diseases, including neurodegenerative disorders (Elliott, 2003). Several executive dysfunctions, evaluated by different methodologies and tools, have been reported in literature. The most common concern deficits in inhibitory control (inability to initiate an action or inhibit a predominant response and maintain attention), cognitive flexibility (shifting from a cognitive task to another), and monitoring (maintaining, organizing information and planning behavior) (Diamond, 2013).
Prefrontally mediated attentional-executive functions have been previously related to motor and other important features of physical frailty (Rosano et al., 2008; Amboni et al., 2013). Specific executive functions (EFs) associated with the medial prefrontal cortex - such as “action monitoring”—have been also considered in pre-frailty status in neurocognitive disorders due to Alzheimer’s disease (Amanzio et al., 2017).
Frailty and Cognitive Impairment: The Need to Study the Case of Neurodegenerative Disorders
The first studies on frailty analyzed the association with cognitive impairment through the biomedical model (see Figures 1A,B). They emphasized how physical frailty, combined with cognitive impairment, is predictive of an increased risk of a poor prognosis. One of the first studies analyzed the association between physical frailty and a progressive cognitive decline (Samper-Ternent et al., 2008). In particular, 1370 subjects were studied and baseline values for physical frailty (according to Fried’s paradigm) and MMSE were observed after 3, 5, and 10 years. The results showed a substantial reduction of the mean of MMSE among frail individuals compared to pre-frail and robust ones.
Subsequent studies, while analyzing the presence of frailty with Fried’s paradigm, began to investigate different cognitive sub-domains, widening the focus of observation. This new approach, characterized by the assessment of the cognitive dimension of frailty, enabled to outline the neuropsychological profile of the elderly people analyzed. Some studies tried to investigate more deeply the relationship between cognitive domains and physical frailty (Canevelli et al., 2015). The authors pointed out that the best neuropsychological model to study the presence of frailty associated with cognitive impairment was the paradigms of attentional and executive functions (Canevelli et al., 2015). Interestingly, attention domain and executive functions seemed to be associated with frailty; on physical side, gait speed and grip strength were mainly related to cognitive impairment, with a particular role played by executive dysfunction (Lundin-Olsson et al., 1998; Patrick et al., 2002; Woollacott and Shumway-Cook, 2002; Harley et al., 2009; Kang et al., 2009; O’Halloran et al., 2011, 2014; Langlois et al., 2012; McGough et al., 2013; Shimada et al., 2013; Delrieu et al., 2016; Hooghiemstra et al., 2017; Sargent and Brown, 2017). In this direction, subjects in a pre-frail and frail status were less able to perform the “Sustained Attention to Response Task” (SART), compared to robust ones. Frailty patients made more commission errors and omissions, suggesting that their response monitoring ability could be impaired (Langner and Eickhoff, 2013; O’Halloran et al., 2014; Robertson et al., 2014).
Robertson et al. (2014), carried out a study on a community population of 50 years and older to analyze the association between frailty and different cognitive domains. The authors investigated the effect of each frailty indicator (according to the phenotypic model) on each cognitive domain analyzed (i.e., global cognition, memory, attention, executive functions, processing speed, and self-rated memory) through a multivariate linear regression. Results showed that asthenia was associated with global cognitive functioning, as was the decrease in handgrip strength. The latter was also associated with executive functioning, assessed by neuropsychological tests concerning reasoning, verbal fluency (phonemic) and response inhibition. Finally, walking speed was associated with different cognitive domains, such as attention, processing speed and executive functions.
Some other studies investigated the role played by mood changes on frailty (Mezuk et al., 2012; Espinoza et al., 2013; Paulson and Lichtenberg, 2013). Their results showed a possible association between depressive symptoms and frailty. In particular, depression could be both a cause and an effect of frailty (Robertson et al., 2013).
Even if these studies represent a first important attempt to describe the association between cognitive functions and physical frailty, there is still a need to assess frailty with a multidimensional approach (Pilotto and Ferrucci, 2011; Avelino-Silva et al., 2014; Sternberg and Bentur, 2014) (see Figure 1, C). Indeed, as underlined by the bio-psycho-social model, frailty is composed not only of physical aspects but also by cognitive and social components, which interact and influence each other (Mantovani et al., 2020).
Future studies should clarify the type of association between cognitive impairment and frailty, in order to implement effective treatments. It also remains to be determined whether this association is causal or shares aging-related mechanisms, such as neurodegeneration. To understand which one is predominant on the other, longitudinal studies should be set up in the field of primary prevention and in the continuum from MCI to major neurocognitive disorder. As well highlighted by Lyreskog (2018), a new framework that connects aging, cognitive decline, and neurodegenerative disease is needed. This new paradigm would be useful for “(a) adequately account for the effects that the processes of aging have on neural decline and disease, and (b) be helpful in identifying relevant groups of users and patients” (Lyreskog, 2018; page 57).
The progression of cognitive frailty towards neurodegenerative disorders is not currently clear. However, several longitudinal studies have investigated the possible association (Gómez-Gómez and Zapico, 2019). It has been suggested that classic aging mechanisms, such as oxidative stress, mitochondrial malfunction, and systemic inflammation could play a role in the pathogenesis of cognitive frailty and other associated neurodegenerative diseases (such as Alzheimer’s and Parkinson’ diseases) (Buchman et al., 2007; Ahmed et al., 2008; Robertson et al., 2013; Gómez-Gómez and Zapico, 2019). Frailty prevalence in neurodegenerative disorders was explored by The Comprehensive Assessment of Neurodegeneration and Dementia (COMPASS-ND) Study (Burt et al., 2019), which verified a prevalence rate equal to 11% and 14% according to the Frailty Index and the frailty phenotype, respectively. The prevalence of frailty in Alzheimer’s disease varied with a wide range from 11.1% to 50.0% (with a pooled prevalence of 31.9% in mild-moderate stages) (Kojima et al., 2017). A similar prevalence was found by Borda et al. (2019), who also observed a rate of 37.14% in a sample of patients with Lewy body dementia.
In Parkinson’s disease (PD) many motor and non-motor symptoms are difficult to explain in terms of a purely ascending degeneration process (Diederich and Parent, 2012), suggesting the need to consolidate the multidimensional elements of PD. In this perspective, the frailty model can be applied to motor disorders albeit with some caution. Frailty and PD may clinically overlap and screening PD patients for frailty may be warranted. Roland et al. (2012) found that correlation coefficients described relationships between PD-related characteristics and physical frailty according to the phenotype criteria. Indeed, frailty is common in PD (prevalence rate = 22.2%) and is associated with a more adverse clinical outcome (Peball et al., 2019). A review by Smith et al. (2019) also provided data in this direction: authors found a prevalence of frailty, which ranged from 29% to 67%.
All together, these findings suggest that the influence of underlying frailty should be considered when managing neurological conditions.
Therefore, a better understanding of cognitive factors, associated with multidisciplinary caring, will form the basis of assistance to frail elderly, with the following possible clinical relapses: slowing of functional decline, reduction of mortality/morbidity, improvement of the quality of life, and reduction of re-hospitalizations. Despite this, very few studies investigated the impact of cognitive functions (more specifically on executive functions) as a precipitating and perpetuating factor of frailty in subjects suffering from neurodegenerative disorders. The proposed mini-review focuses on common points characterizing executive dysfunction, neurocognitive and neurobiological factors potentially involved in frailty in such patients. In particular, the present study aims to investigate and address the following issues:
1. Since physical frailty and cognitive decline (in particular executive dysfunction) are two aspects strongly connected within neurodegenerative disorders (i.e., Alzheimer’s disease and Parkinson’s disease), are the latter duly taken into consideration in the literature?
2. Which of the frailty models are referred to in these studies (biomedical, bio-psycho-social)?
3. What kind of executive dysfunction are considered and with what neuropsychological tools are they detected?
Selection of Studies
A systematic search strategy was implemented to identify studies on frailty, published until 31st March 2020, across the online database most frequently used in the international literature (Medline database with PubMed literature search1). We used a single set of query terms: Frailty AND Executive Functions [ALL].
We adopted the “PRISMA Statement” in order to make the selection and data collection process clear (Liberati et al., 2009).
With this aim, we reviewed the relevant literature in order to ensure to select only papers regarding patients with mild or major neurocognitive disorders (DSM 5; American Psychiatric Association [APA], 2013) due to neurodegenerative disorders. We only selected original studies. Moreover, descriptive reviews, systematic reviews or meta-analyses were excluded.
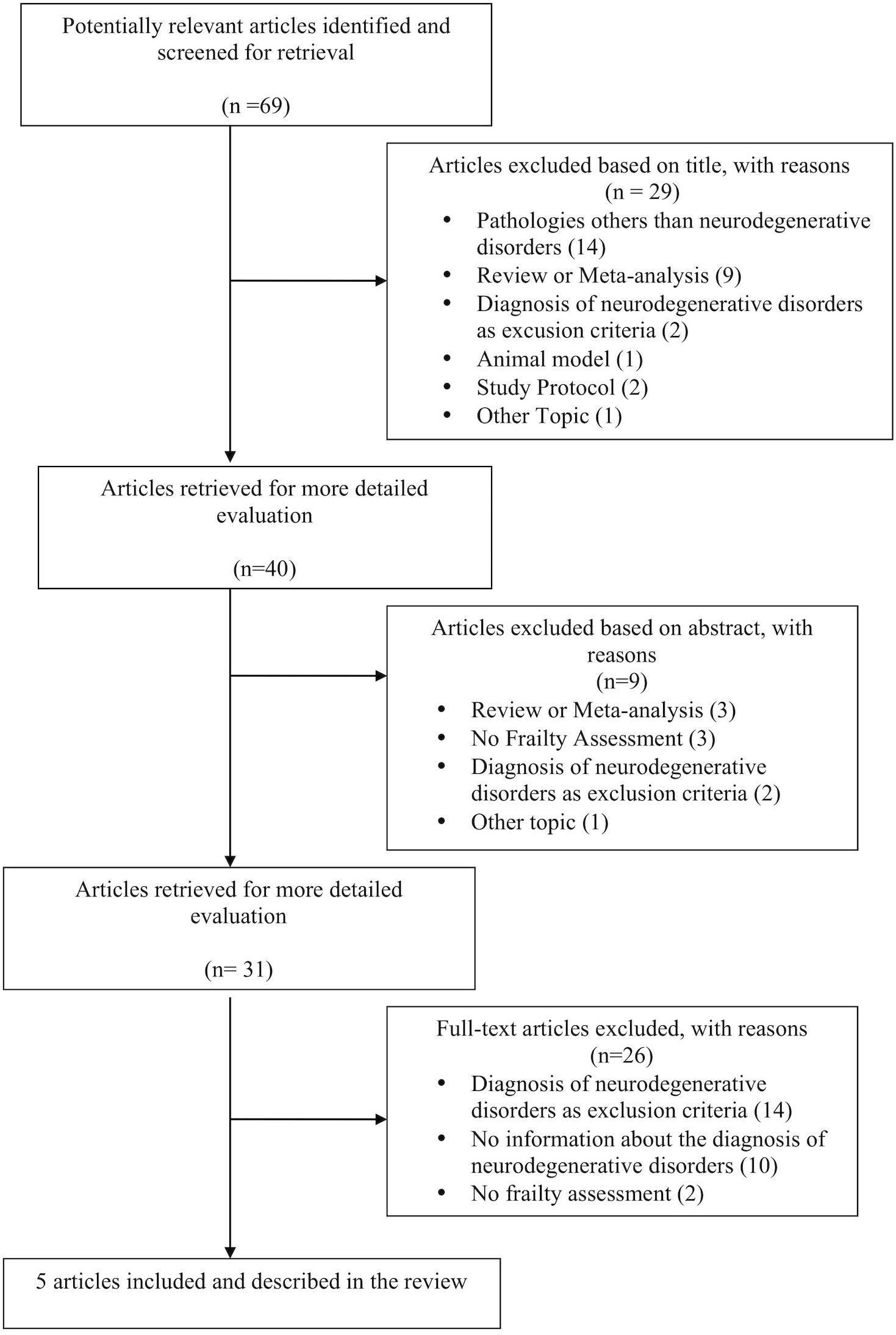
During the selection phase, we found 69 studies analyzing frailty in the above-mentioned pathologies. 64 studies were excluded because not consistent with the purpose of the review, while 5 were selected (see the flow chart in Figure 2 and the Supplementary Material for the selection of the articles and the reason for the exclusion).
Description of the Selected Studies
The selected studies mainly concerned subjects with AD and PD, focusing in particular on the two most common neurodegenerative disorders (Xie et al., 2014). Four out of the five selected studies assessed frailty through the biomedical paradigm. In particular, three of those (Shimada et al., 2013; Chen et al., 2019; Lin et al., 2019) adopted Fried’s criteria, while one (Dutzi et al., 2017) used the model proposed by Rockwood et al. (2005) and Rockwood and Mitnitski (2007b).
Shimada et al. (2013) analyzed the relationship between physical frailty and Mild Cognitive Impairment (MCI) in 5104 community-dwelling persons aged 65 years and older (mean age 71 years).
The criteria used to define mild cognitive impairment are those reported by Petersen et al. (1999, 2001) for the “MCI-amnestic” type, which presents a high risk of conversion into a major neurocognitive disorder due to Alzheimer’s disease (Petersen et al., 1999; Grundman et al., 2004; Petersen and Negash, 2008);.
By adopting the phenotype model, Shimada et al. (2013) subdivided participants in respect of frailty status and level of cognitive impairment using the MMSE and 8 cognitive tests on memory, attention and executive functions, processing speed, and visuospatial skills. Particularly, the executive functioning – in terms of cognitive flexibility – was assessed through the trail making test (part A and B; Reitan and Wolfson, 1994).
The authors reported the presence of a frailty status in about 11% of the subjects and a MCI in about 19% of the participants. Considering the two aspects together, about 3% of subjects had both, frailty status and MCI, i.e., a cognitive frailty status (Kelaiditi et al., 2013).
Moreover, authors found that the subjects at higher risk for frailty were 80 years and older, with 9 years or less of education. As for cognitive impairment, the subjects with a higher probability of developing MCI were men, with 9 years or less of education. Finally, the co-occurrence of frailty and MCI (cognitive frailty) increased in relation with age and lower level of education.
The other two selected studies adopting the phenotypic model analyzed the relationship between physical frailty and cognitive impairment in patients with PD (Chen et al., 2019; Lin et al., 2019).
Chen et al. (2019) investigated structural brain changes in relation to physical frailty and cognitive decline in sixty-one PD patients (mean age 62.61 ± 8.59 years), by using MRI. Voxel-wise multiple linear regression analyses were carried out in order to identify the overlapping areas of gray matter volume decrease concerning such aspects.
Frailty was assessed by adopting Fried’s criteria. Several cognitive domains, such as attention, memory, language, visuospatial skills, and executive functions, were neuropsychologically evaluated. In particular, EFs were investigated, as indicated by the authors, by using some Wechsler Adult Intelligence Scale-III subtests (picture arrangement, arithmetic, digit symbol coding, and matrix reasoning) (Wechsler et al., 2002), and by the abstract thinking scores from the Cognitive Ability Screening Instrument (Lin et al., 2012).
The authors identified the lateral occipital cortex as an overlapping region of physical frailty and cognitive impairment. Specifically, an overlapping region was observed in the left lateral occipital cortex for every cognitive domain in relation to frailty. This cerebral region is part of the ventral object-based visual pathway (Mishkin et al., 1983), whose decrease in thickness had previously been identified in PD patients in relation to impaired cognitive functioning, in particular visuospatial skills, memory, and executive functions (Pereira et al., 2014).
Moreover, an additional overlapping region relating to the superior frontal gyrus had been identified in connection with executive functioning and frailty. These findings highlighted how frailty and cognitive decline are connected in the brain (Chen et al., 2019).
As a precaution, considering the elements of difficult disambiguation between frailty and PD, it is appropriate to consider the correlations between frailty and cognitive impairments observed in the study by Chen and collaborators related to the pathophysiology (e.g., alpha synuclein in the brain) rather than a sign of frailty.
Finally, by adopting Fried’s criteria, Lin et al. (2019) divided their sample of 76 PD patients (mean age 62.64 ± 9.23 years) into two groups: “with physical frailty” (38.2%) and “without physical frailty” (61.8%). PD patients with frailty were significantly older, showed worse disease severity, and poorer cognitive functions compared to robust ones. The neuropsychological assessment was the same carried out in Chen et al.’s study (2019).
A stepwise logistic regression analysis indicated how impaired executive functions increased considerably the risk of physical frailty.
In light of these results, the authors suggested that assessing cognitive functions in PD patients might be a useful approach to identify the subjects at greatest risk of developing frailty and to prevent negative outcomes through targeted strategies of intervention (Lin et al., 2019).
Dutzi et al. (2017) assessed frailty by using the model proposed by Rockwood et al. (2005). The authors investigated cognitive changes following hospital rehabilitation in 154 patients (mean age 83.7 ± 5.9) with mild and major neurocognitive disorder, with different etiopathogenesis [Alzheimer’s disease (AD) prevalently]. They considered several aspects that could affect rehabilitation, including cognitive functioning, independence in basic activities of daily living (bADL), and frailty status. Particularly, frailty was evaluated using the Clinical Frailty Scale (CFS) (Rockwood et al., 2005), which allows the clinician to assess the patient’s degree of frailty through clinical data. This tool correlates strongly with FI but is faster and easier to administer (Rockwood et al., 2005). The executive functioning was evaluated by the verbal fluency and the modified version of the trail making test, from Nuremberg Gerontopsychological Inventory (Oswald and Fleischmann, 1985). The verbal fluency test is considered a task for the assessment of cognitive flexibility (Diamond, 2013), as well as the trail making test (Lezak et al., 2004).
The authors found that patients presenting a worse frailty status and lower functional independence during the admission were those who did not benefit from cognitive rehabilitation.
They suggested that frailty and deficit in the bADL may have played an important role in the worsening of cognitive decline and in the ineffectiveness of the rehabilitation intervention (Dutzi et al., 2017).
As previously mentioned, 4 out of the 5 selected studies analyzed frailty by adopting the biomedical models. Only one study (Amanzio et al., 2017) provided for the assessment of frailty through the bio-psycho-social model, highlighting its multidimensionality (Pilotto et al., 2020). Amanzio et al. (2017) investigated the association among a multidimensional assessment of frailty, executive dysfunction, and specific cognitive and behavioral changes, using an overall neuropsychological battery in sixty patients with mild and major neurocognitive disorders due to AD (mean age 66.62 ± 6.80).
The authors used the MPI for a comprehensive assessment of frailty (EIP-AHA–European Innovation Partnership on Active and Healthy Ageing, 2013; Pilotto et al., 2013, 2020; Angleman et al., 2015). This tool not only takes into consideration the clinical, functional, neuropsychological, and nutritional status, but also gives information on the associated pathologies and pharmacological therapies, and on the social support network (Pilotto and Ferrucci, 2011, Pilotto et al., 2013, 2020). Executive functions, in terms of self-monitoring, were assessed through the metacognitive version of the Wisconsin Card Sorting test (m-WCST: Koren et al., 2006). This version differs from the original one as the subject is asked to answer two questions: to assess his or her online self-monitoring (“What is your degree of confidence in this answer?”) and to control abilities (“Do you want to take this response into account in your total score?”) (see Amanzio et al., 2017).
These findings suggested that also a pre-frailty status was associated with metacognitive-executive dysfunction, in terms of action monitoring in MCI-likely due to AD and AD patients. Specifically, it was observed a significant association between the MPI and monitoring resolution at the m-WCST, where patients failed to distinguish between correct and incorrect sorts. These results were specific and not influenced by other cognitive functions such as global cognition, memory, language comprehension, and non-verbal reasoning, with the exception of the selective attention task that reached a near significance level. Moreover, taking into account the MPI scores, this study demonstrated an involvement of mood depression changes, apathy, disinhibition, and a reduced awareness of IADL, associated with a higher frailty status (Amanzio et al., 2017).
Since apathy, disinhibition, and executive dysfunction seemed to be attributable to the malfunction of the same brain network (Masterman and Cummings, 1997; Bonelli and Cummings, 2007), the authors hypothesized that pre-frailty might also be due to a dysfunction of the medial prefrontal-ventral striatal network (Amanzio et al., 2017).
Conclusion
The studies analyzed in this mini-review highlighted how physical frailty and cognitive decline, particularly executive dysfunction, are two aspects heavily connected within neurodegenerative disorders (i.e., AD and PD).
Several cognitive domains have been taken into account in the selected studies due to the lack of a univocal definition of EFs, assessed by different neuropsychological instruments.
The analyzed studies showed that frailty is related to executive dysfunction, in terms of cognitive flexibility (Shimada et al., 2013; Dutzi et al., 2017) and self-monitoring (Amanzio et al., 2017) in neurocognitive disorders.
In our opinion, the Wechsler Adult Intelligence Scale-III (WAIS-III) subtests, used by Chen et al. (2019) and Lin et al. (2019), are not the gold-standard instruments to assess EFs, as WAIS-III was created for the evaluation of reasoning and intellectual abilities (Wechsler, 1997).
However, as reported by Robertson et al. (2014), several cognitive functions such as global cognition, attention, executive functions—including reasoning—and memory are associated with frailty status. These results confirm the hypothesis that there is a relation between frailty and cognitive decline in different domains, even within neurodegenerative disorders (such as PD).
Previous researches had shown a strong association between physical frailty and the incident of neurocognitive disorders, such as AD, MCI (Panza et al., 2015; Xu et al., 2015; Kojima et al., 2016), and cerebral vascular diseases (Avila-Funes et al., 2012). Frailty and cognitive impairment share several risk factors such as age-related chronic diseases, inflammation or cardiovascular problems (Robertson et al., 2013).
In a recent work of systematic review and meta-analysis, Borges et al. (2019) investigated the relationship between physical frailty and cognitive impairment, highlighting how frailty seemed to be one of the greatest risk factors for the development of major neurocognitive disorders.
However, it is important to underline how, to date, the studies have not clarified the direction of the association between frailty and the presence of a cognitive impairment yet. In particular, it is the presence of frailty that determines cognitive impairment or vice versa?
In our opinion, given the multidimensional nature of frailty, the bio-psycho-social model is the most appropriate paradigm for the evaluation and management of frail older people with cognitive decline.
Longitudinal studies may be the most correct approach to assess the presence of cognitive disorders many years before the development of frailty itself. Further studies will be important to better characterized this association over time and replicate these findings in a larger group of patients. Analyzing the association between frailty and cognitive dysfunction in this at-risk population, would allow to develop specific physical and/or cognitive empowerment and rehabilitation measures.
Author Contributions
MB wrote the manuscript, developed the search strategy, revised the images and figures, and took part in the review and critique processes. SP wrote the manuscript and took part in the review and critique processes. GEC wrote and edited the manuscript, revised the images and figures. MA conceived the content of the review, developed the search strategy, wrote the first draft of the manuscript, proceeded to extend the theoretical models of frailty associated with executive functions, supervised subsequent changes and took part in the critique processes. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyg.2020.554307/full#supplementary-material
Footnotes
References
Ahmed, N. N., Sherman, S. J., and Vanwyck, D. (2008). Frailty in Parkinson’s disease and its clinical implications. Parkinsonism. Relat. Disord. 14, 334–337. doi: 10.1016/j.parkreldis.2007.10.004
Amanzio, M., Palermo, S., Zucca, M., Rosato, R., Rubino, E., Leotta, D., et al. (2017). Neuropsychological correlates of pre-frailty in neurocognitive disorders: a possible role for metacognitive dysfunction and mood changes. Front. Med. 15:199. doi: 10.3389/fmed.2017.00199
Amboni, M., Barone, P., and Hausdorff, J. M. (2013). Cognitive contributions to gait and falls: evidence and implications. Mov. Disord. 28, 1520–1533. doi: 10.1002/mds.25674
American Psychiatric Association [APA] (2013). Diagnostic and Statistical Manual of Mental Disorders, 5th Edn. Washington, DC: American Psychiatric Association Publishing.
Angleman, S. B., Santoni, G., Pilotto, A., Fratiglioni, L., Welmer, A. K., and Mpi_Age Project Investigators (2015). Multidimensional prognostic index in association with future mortality and number of hospital days in a population-based sample of older adults: results of the EU funded MPI_AGE project. PLoS One 10:e0133789. doi: 10.1371/journal.pone.0133789
Avelino-Silva, T. J., Farfel, J. M., Curiati, J. A., Amaral, J. R., Campora, F., and Jacob-Filho, W. (2014). Comprehensive geriatric assessment predicts mortality and adverse outcomes in hospitalized older adults. BMC Geriatr. 14:129. doi: 10.1186/1471-2318-14-129
Avila-Funes, J. A., Carcaillon, L., Helmer, C., Carrière, I., Ritchie, K., Rouaud, O., et al. (2012). Is frailty a prodromal stage of vascular dementia? Results from the Three-City Study. J. Am. Geriatr. Soc. 60, 1708–1712. doi: 10.1111/j.1532-5415.2012.04142.x
Bonelli, R. M., and Cummings, J. L. (2007). Frontal-subcortical circuitry and behavior. Dialogues Clin. Neurosci. 9:141.
Borda, M. G., Soennesyn, H., Steves, C. J., Osland Vik-Mo, A., Pérez-Zepeda, M. U., and Aarsland, D. (2019). Frailty in older adults with mild dementia: dementia with lewy bodies and Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. Extra. 9, 176–183. doi: 10.1159/000496537
Borges, M. K., Canevelli, M., Cesari, M., and Aprahamian, I. (2019). Frailty as a predictor of cognitive disorders: a systematic review and meta-analysis. Front. Med. 6:26. doi: 10.3389/fmed.2019.00026
Boyle, P. A., Buchman, A. S., Wilson, R. S., Leurgans, S. E., and Bennett, D. A. (2010). Physical frailty is associated with incident mild cognitive impairment in community-based older persons. J. Am. Geriatr. Soc. 58, 248–255. doi: 10.1111/j.1532-5415.2009.02671.x
Buchman, A. S., Boyle, P. A., Wilson, R. S., Tang, Y., and Bennett, D. A. (2007). Frailty is associated with incident Alzheimer’s disease and cognitive decline in the elderly. Psychosom. Med. 69, 483–489. doi: 10.1097/psy.0b013e318068de1d
Burt, J. R., Godin, J., Filion, J., Montero-Odasso, M., Rockwood, K., Andrew, M. K., et al. (2019). Frailty prevalence in the COMPASS-ND study of neurodegenerative disorders. CGJ 22, 205–212. doi: 10.5770/cgj.22.392
Canevelli, M., Cesari, M., and van Kan, G. A. (2015). Frailty and cognitive decline: how do they relate? Curr. Opin. Clin. Nutr. Metab. Care 18, 43–50. doi: 10.1097/MCO.0000000000000133
Chen, Y. S., Chen, H. L., Lu, C. H., Chen, M. H., Chou, K. H., Tsai, N. W., et al. (2019). Reduced lateral occipital gray matter volume is associated with physical frailty and cognitive impairment in Parkinson’s disease. Eur. Radiol. 29, 2659–2668. doi: 10.1007/s00330-018-5855-7
Delrieu, J., Andrieu, S., Pahor, M., Cantet, C., Cesari, M., Ousset, P. J., et al. (2016). Neuropsychological profile of “cognitive frailty” subjects in MAPT study. J. Prev. Alzheimers Dis. 3:151. doi: 10.14283/jpad.2016.94
Diamond, A. (2013). Executive functions. Annu. Rev. Psychol. 64, 135–168. doi: 10.1146/annurev-psych-113011-143750
Diederich, N. J., and Parent, A. (2012). Parkinson’s disease: acquired frailty of archaic neural networks? J. Neurol. Sci. 314, 143–151. doi: 10.1016/j.jns.2011.10.003
Dutzi, I., Schwenk, M., Kirchner, M., Bauer, J. M., and Hauer, K. (2017). Cognitive change in rehabilitation patients with dementia: prevalence and association with rehabilitation success. J. Alzheimers. Dis. 60, 1171–1182. doi: 10.3233/JAD-170401
EIP-AHA–European Innovation Partnership on Active and Healthy Ageing (2013). A Compilation of Good Practices – Prevention and Early Diagnosis of Frailty and Functional Decline, both Physical and Cognitive in Older People. Brussels: European Commission.
Elliott, R. (2003). Executive functions and their disorders: imaging in clinical neuroscience. Br. Med. Bull. 65, 49–59. doi: 10.1093/bmb/65.1.49
Espinoza, S. E., Jung, I., and Hazuda, H. (2013). The Hispanic paradox and predictors of mortality in an aging biethnic cohort of Mexican Americans and European Americans: the San Antonio longitudinal study of aging. J. Am. Geriatr. Soc. 61, 1522–1529. doi: 10.1111/jgs.12421
Feng, L., Nyunt, M. S. Z., Gao, Q., Feng, L., Yap, K. B., and Ng, T. P. (2017). Cognitive frailty and adverse health outcomes: findings from the singapore longitudinal ageing studies (SLAS). J. Am. Med. Dir. Assoc. 18, 252–258. doi: 10.1016/j.jamda.2016.09.015
Folstein, M. F., Folstein, S. E., and McHugh, P. R. (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198. doi: 10.1016/0022-3956(75)90026-6
Fried, L. P., Tangen, C. M., Walston, J., Newman, A. B., Hirsch, C., Gottdiener, J., et al. (2001). Frailty in older adults: evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 56, M146–M156. doi: 10.1093/gerona/56.3.m146
Gobbens, R. J., van Assen, M. A., Luijkx, K. G., Wijnen-Sponselee, M. T., and Schols, J. M. (2010). Determinants of frailty. J. Am. Med. Dir. Assoc. 11, 356–364. doi: 10.1016/j.jamda.2009.11.008
Gómez-Gómez, M. E., and Zapico, S. C. (2019). Frailty, cognitive decline, neurodegenerative diseases and nutrition interventions. Int. J. Mol. Sci. 20:2842. doi: 10.3390/ijms20112842
Gross, A. L., Xue, Q. L., Bandeen-Roche, K., Fried, L. P., Varadhan, R., McAdams-DeMarco, M. A., et al. (2016). Declines and impairment in executive function predict onset of physical frailty. J. Gerontol. A Biol. Sci. Med. Sci. 71, 1624–1630. doi: 10.1093/gerona/glw067
Grundman, M., Petersen, R. C., Ferris, S. H., Thomas, R. G., Aisen, P. S., Bennett, D. A., et al. (2004). Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch. Neurol. 61, 59–66. doi: 10.1001/archneur.61.1.59
Harley, C., Wilkie, R. M., and Wann, J. P. (2009). Stepping over obstacles: attention demands and aging. Gait Posture 29, 428–432. doi: 10.1016/j.gaitpost.2008.10.063
Hooghiemstra, A. M., Ramakers, I. H. G. B., Sistermans, N., Pijnenburg, Y. A. L., Aalten, P., Hamel, R. E. G., et al. (2017). Gait speed and grip strength reflect cognitive impairment and are modestly related to incident cognitive decline in memory clinic patients with subjective cognitive decline and mild cognitive impairment: findings from the 4C study. J. Gerontol. A Biol. Sci. Med. Sci. 72, 846–854. doi: 10.1093/gerona/glx003
Kang, H. G., Costa, M. D., Priplata, A. A., Starobinets, O. V., Goldberger, A. L., Peng, C. K., et al. (2009). Frailty and the degradation of complex balance dynamics during a dual-task protocol. J. Gerontol. A Biol. Sci. Med. Sci. 64, 1304–1311. doi: 10.1093/gerona/glp113
Kelaiditi, E., Cesari, M., Canevelli, M., Van Kan, G. A., Ousset, P. J., Gillette-Guyonnet, S., et al. (2013). Cognitive frailty: rational and definition from an (IANA/IAGG) international consensus group. J. Nutr. Health Aging 17, 726–734. doi: 10.1007/s12603-013-0367-2
Kojima, G., Liljas, A., Iliffe, S., and Walters, K. (2017). Prevalence of frailty in mild to moderate Alzheimer’s disease: a systematic review and meta-analysis. Curr. Alzheimer Res. 14, 1256–1263. doi: 10.2174/1567205014666170417104236
Kojima, G., Taniguchi, Y., Iliffe, S., and Walters, K. (2016). Frailty as a predictor of Alzheimer disease, vascular dementia, and all dementia among community-dwelling older people: a systematic review and meta-analysis. J. Am. Med. Dir. Assoc. 17, 881–888. doi: 10.1016/j.jamda.2016.05.013
Koren, D., Seidman, L. J., Goldsmith, M., and Harvey, P. D. (2006). Real-world cognitive—and metacognitive—dysfunction in schizophrenia: a new approach for measuring (and remediating) more “right stuff”. Schizophr. Bull. 32, 310–326. doi: 10.1093/schbul/sbj035
Langlois, F., Vu, T. T., Kergoat, M. J., Chassé, K., Dupuis, G., and Bherer, L. (2012). The multiple dimensions of frailty: physical capacity, cognition, and quality of life. Int. Psychogeriatr. 24, 1429–1436. doi: 10.1017/S1041610212000634
Langner, R., and Eickhoff, S. B. (2013). Sustaining attention to simple tasks: a meta-analytic review of the neural mechanisms of vigilant attention. Psychol. Bull. 139, 870–900. doi: 10.1037/a0030694
Lezak, M. D., Howieson, D. B., and Loring, D. W. (2004). Neuropsychological Assessment, 4th Edn. New York, NY: Oxford University Press.
Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gøtzsche, P. C., Ioannidis, J. P., et al. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. BMJ 339:b2700. doi: 10.1136/bmj.b2700
Lin, K. N., Wang, P. N., Liu, H. C., and Teng, E. L. (2012). Cognitive abilities screening instrument, Chinese version 2.0 (CASI C-2.0): administration and clinical application. Acta Neurol. Taiwan 21, 180–189.
Lin, W. C., Huang, Y. C., Leong, C. P., Chen, M. H., Chen, H. L., Tsai, N. W., et al. (2019). Associations between cognitive functions and physical frailty in patients with Parkinson’s disease. Front. Aging Neurosci. 30:283. doi: 10.3389/fnagi.2019.00283
Lundin-Olsson, L., Nyberg, L., and Gustafson, Y. (1998). Attention, frailty, and falls: the effect of a manual task on basic mobility. J. Am. Geriatr. Soc. 46, 758–761. doi: 10.1111/j.1532-5415.1998.tb03813.x
Lunt, L., Bramham, J., Morris, R. G., Bullock, P. R., Selway, R. P., Xenitidis, K., et al. (2012). Prefrontal cortex dysfunction and ‘Jumping to Conclusions’: bias or deficit? J. Neuropsychol. 6, 65–78. doi: 10.1111/j.1748-6653.2011.02005.x
Lyreskog, D. M. (2018). Frailty and neurodegenerative disease: anticipating the future, expanding the framework. J. Frailty Aging 7, 57–59. doi: 10.14283/jfa.2017.46
Mantovani, E., Zucchella, C., Schena, F., Romanelli, M. G., Venturelli, M., and Tamburin, S. (2020). Towards a redefinition of cognitive frailty. J. Alzheimers Dis. 76, 831–843. doi: 10.3233/JAD-200137
Masterman, D. L., and Cummings, J. L. (1997). Frontal-subcortical circuits: the anatomic basis of executive, social and motivated behaviors. J. Psychopharmacol. 11, 107–114. doi: 10.1177/026988119701100203
McGough, E. L., Cochrane, B. B., Pike, K. C., Logsdon, R. G., McCurry, S. M., and Teri, L. (2013). Dimensions of physical frailty and cognitive function in older adults with amnestic mild cognitive impairment. Ann. Phys. Rehabil. Med. 56, 329–341. doi: 10.1016/j.rehab.2013.02.005
Mezuk, B., Edwards, L., Lohman, M., Choi, M., and Lapane, K. (2012). Depression and frailty in later life: a synthetic review. Int. J. Geriatr. Psychiatry 27, 879–892. doi: 10.1002/gps.2807
Mishkin, M., Ungerleider, L. G., and Macko, K. A. (1983). Object vision and spatial vision: two cortical pathways. Trends Neurosci. 6, 414–417. doi: 10.1016/0166-2236(83)90190-X
Miyake, A., and Friedman, N. P. (2012). The nature and organization of individual differences in executive functions: four general conclusions. Curr. Dir. Psychol. Sci. 21, 8–14. doi: 10.1177/0963721411429458
Miyake, A., Friedman, N. P., Emerson, M. J., Witzki, A. H., Howerter, A., and Wager, T. D. (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cogn. Psychol. 41, 49–100. doi: 10.1006/cogp.1999.0734
O’Halloran, A. M., Fan, C. W., Kenny, R. A., Pénard, N., Galli, A., and Robertson, I. H. (2011). Variability in sustained attention and risk of frailty. J. Am. Geriatr. Soc. 59, 2390–2392. doi: 10.1111/j.1532-5415.2011.03706.x
O’Halloran, A. M., Finucane, C., Savva, G. M., Robertson, I. H., and Kenny, R. A. (2014). Sustained attention and frailty in the older adult population. J. Gerontol. B Psychol. Sci. Soc. Sci. 69, 147–156. doi: 10.1093/geronb/gbt009
Oswald, W. D., and Fleischmann, U. M. (1985). Psychometrics in aging and dementia: advances in geropsychological assessments. Arch. Gerontol. Geriatr. 4, 299–309. doi: 10.1016/0167-4943(85)90037-8
Panza, F., Solfrizzi, V., Barulli, M. R., Santamato, A., Seripa, D., Pilotto, A., et al. (2015). Cognitive frailty: a systematic review of epidemiological and neurobiological evidence of an age-related clinical condition. Rejuvenat. Res. 18, 389–412. doi: 10.1089/rej.2014.1637
Parker, K. L., Lamichhane, D., Caetano, M. S., and Narayanan, N. S. (2013). Executive dysfunction in Parkinson’s disease and timing deficits. Front. Integr. Neurosci. 7:75. doi: 10.3389/fnint.2013.00075
Patrick, L., Gaskovski, P., and Rexroth, D. (2002). Cumulative illness and neuropsychological decline in hospitalized geriatric patients. Clin. Neuropsychol. 16, 145–156. doi: 10.1076/clin.16.2.145.13239
Paulson, D., and Lichtenberg, P. A. (2013). Vascular depression: an early warning sign of frailty. Aging Ment. Health 17, 85–93. doi: 10.1080/13607863.2012.692767
Peball, M., Mahlknecht, P., Werkmann, M., Marini, K., Murr, F., Herzmann, H., et al. (2019). Prevalence and associated factors of sarcopenia and frailty in Parkinson’s disease: a cross-sectional study. Gerontology 65, 216–228. doi: 10.1159/000492572
Pereira, J. B., Svenningsson, P., Weintraub, D., Brønnick, K., Lebedev, A., Westman, E., et al. (2014). Initial cognitive decline is associated with cortical thinning in early Parkinson disease. Neurology 82, 2017–2025. doi: 10.1212/wnl.0000000000000483
Petersen, R. C., Doody, R., Kurz, A., Mohs, R. C., Morris, J. C., Rabins, P. V., et al. (2001). Current concepts in mild cognitive impairment. Arch. Neurol. 58, 1985–1992. doi: 10.1001/archneur.58.12.1985
Petersen, R. C., and Negash, S. (2008). Mild cognitive impairment: an overview. CNS Spectr. 13, 45–53. doi: 10.1017/s1092852900016151
Petersen, R. C., Smith, G. E., Waring, S. C., Ivnik, R. J., Tangalos, E. G., and Kokmen, E. (1999). Mild cognitive impairment: clinical characterization and outcome. Arch. Neurol. 56, 303–308. doi: 10.1001/archneur.56.3.303
Pilotto, A., Custodero, C., Maggi, S., Polidori, M. C., Veronese, N., and Ferrucci, L. (2020). A multidimensional approach to frailty in older people. Ageing Res. Rev. 60:101047. doi: 10.1016/j.arr.2020.101047
Pilotto, A., and Ferrucci, L. A. (2011). A clinical definition of frailty: usefulness of the multidimensional assessment. G Gerontol. 59, 125–129.
Pilotto, A., Gallina, P., Fontana, A., Sancarlo, D., Bazzano, S., Copetti, M., et al. (2013). Development and validation of a multidimensional prognostic index for mortality based on a standardized multidimensional assessment schedule (MPI-SVaMA) in community-dwelling older subjects. J. Am. Med. Dir. Assoc. 14, 287–292. doi: 10.1016/j.jamda.2013.01.005
Poon, K. (2018). Hot and cool executive functions in adolescence: development and contributions to important developmental outcomes. Front. Psychol. 8:2311. doi: 10.3389/fpsyg.2017.02311
Raji, M. A., Al Snih, S., Ostir, G. V., Markides, K. S., and Ottenbacher, K. J. (2010). Cognitive status and future risk of frailty in older Mexican Americans. J. Gerontol. A Biol. Sci. Med. Sci. 65, 1228–1234. doi: 10.1093/gerona/glq121
Reitan, R. M., and Wolfson, D. (1994). A selective and critical review of neuropsychological deficits and the frontal lobes. Neuropsychol. Rev. 4, 161–198. doi: 10.1007/bf01874891
Richards, S. J., Frizelle, F. A., Geddes, J. A., Eglinton, T. W., and Hampton, M. B. (2018). Frailty in surgical patients. Int. J. Colorectal. Dis. 33, 1657–1666. doi: 10.1007/s00384-018-3163-y
Robertson, D. A., Savva, G., Coen, R. F., and Kenny, R. A. (2014). Cognitive function in the prefrailty and frailty syndrome. J. Am. Geriatr. Soc. 62, 2118–2124. doi: 10.1111/jgs.13111
Robertson, D. A., Savva, G. M., and Kenny, R. A. (2013). Frailty and cognitive impairment. A review of the evidence and causal mechanisms. Ageing Res. Rev. 12, 840–851. doi: 10.1016/j.arr.2013.06.004
Rockwood, K., Andrew, M., and Mitnitski, A. (2007a). A comparison of two approaches to measuring frailty in elderly people. J. Gerontol. A Biol. Sci. Med. Sci. 62, 738–743. doi: 10.1093/gerona/62.7.738
Rockwood, K., and Mitnitski, A. (2007b). Frailty in relation to the accumulation of deficits. J. Gerontol. A Biol. Sci. Med. Sci. 62, 722–727. doi: 10.1093/gerona/62.7.722
Rockwood, K., Song, X., MacKnight, C., Bergman, H., Hogan, D. B., McDowell, I., et al. (2005). A global clinical measure of fitness and frailty in elderly people. CMAJ 173, 489–495. doi: 10.1503/cmaj.050051
Roland, K. P., Cornett, K. M., Theou, O., Jakobi, J. M., and Jones, G. R. (2012). Concurrence of frailty and Parkinson’s disease. J. Frailty Aging 1, 123–127. doi: 10.14283/jfa.2012.20
Rosado-Artalejo, C., Carnicero, J. A., Losa-Reyna, J., Guadalupe-Grau, A., Castillo-Gallego, C., Gutierrez-Avila, G., et al. (2017). Cognitive performance across 3 frailty phenotypes: toledo study for healthy aging. J. Am. Med. Dir. Assoc. 18, 785–790. doi: 10.1016/j.jamda.2017.04.008
Rosano, C., Aizenstein, H., Brach, J., Longenberger, A., Studenski, S., and Newman, A. B. (2008). Special article: gait measures indicate underlying focal gray matter atrophy in the brain of older adults. J. Gerontol. A Biol. Sci. Med. Sci. 63, 1380–1388. doi: 10.1093/gerona/63.12.1380
Ruan, Q., Yu, Z., Chen, M., Bao, Z., Li, J., and He, W. (2015). Cognitive frailty, a novel target for the prevention of elderly dependency. Ageing Res. Rev. 20, 1–10. doi: 10.1016/j.arr.2014.12.004
Samper-Ternent, R., Al Snih, S., Raji, M. A., Markides, K. S., and Ottenbacher, K. J. (2008). Relationship between frailty and cognitive decline in older Mexican Americans. J. Am. Geriatr. Soc. 56, 1845–1852. doi: 10.1111/j.1532-5415.2008.01947.x
Sargent, L., and Brown, R. (2017). Assessing the current state of cognitive frailty: measurement properties. J. Nutr. Health Aging 21, 152–160. doi: 10.1007/s12603-016-0735-9
Shimada, H., Makizako, H., Doi, T., Yoshida, D., Tsutsumimoto, K., Anan, Y., et al. (2013). Combined prevalence of frailty and mild cognitive impairment in a population of elderly Japanese people. J. Am. Med. Dir. Assoc. 14, 518–524. doi: 10.1016/j.jamda.2013.03.010
Smith, N., Brennan, L., Gaunt, D. M., Ben-Shlomo, Y., and Henderson, E. (2019). Frailty in Parkinson’s disease: a systematic review. J. Parkinsons Dis. 9, 517–524. doi: 10.3233/JPD-191604
Solfrizzi, V., Scafato, E., Lozupone, M., Seripa, D., Giannini, M., Sardone, R., et al. (2017). Additive role of a potentially reversible cognitive frailty model and inflammatory state on the risk of disability: the Italian longitudinal study on aging. Am. J. Geriatr. Psychiatry 25, 1236–1248. doi: 10.1016/j.jagp.2017.05.018
Sternberg, S. A., and Bentur, N. (2014). The contribution of comprehensive geriatric assessment to primary care physicians. ISR J. Health Policy Res. 3:44. doi: 10.1186/2045-4015-3-44
Wechsler, D. (1997). Administration and scoring manual for the Wechsler Adult Intelligence Scale, Third Edn. San Antonio, TX: The Psychological Corporation.
Wechsler, D., Chen, Y., and Chen, X. (2002). WAIS-III Chinese Version Technical Manual. San Antonio, TX: The Psychological Corporation.
Woollacott, M., and Shumway-Cook, A. (2002). Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture 16, 1–14. doi: 10.1016/S0966-6362(01)00156-4
Xie, A., Gao, J., Xu, L., and Meng, D. (2014). Shared mechanisms of neurodegeneration in Alzheimer’s disease and Parkinson’s disease. Biomed. Res. Int. 2014:648740. doi: 10.1155/2014/648740
Xu, W., Tan, L., Wang, H. F., Jiang, T., Tan, M. S., Tan, L., et al. (2015). Meta-analysis of modifiable risk factors for Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatr. 86, 1299–1306. doi: 10.1136/jnnp-2015-310548
Keywords: frailty, mild cognitive impairment, neurocognitive disorders, Alzheimer’s disease, Parkinson’s disease, executive functions, mini-review
Citation: Bartoli M, Palermo S, Cipriani GE and Amanzio M (2020) A Possible Association Between Executive Dysfunction and Frailty in Patients With Neurocognitive Disorders. Front. Psychol. 11:554307. doi: 10.3389/fpsyg.2020.554307
Received: 21 April 2020; Accepted: 23 October 2020;
Published: 11 November 2020.
Edited by:
Claudia Gianelli, University Institute of Higher Studies in Pavia, ItalyReviewed by:
Matias M. Pulopulos, Ghent University, BelgiumAntonio Daniele, Catholic University of the Sacred Heart, Italy
Copyright © 2020 Bartoli, Palermo, Cipriani and Amanzio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Massimo Bartoli, bWFzc2ltby5iYXJ0b2xpQHVuaXRvLml0
 Massimo Bartoli
Massimo Bartoli Sara Palermo
Sara Palermo Giuseppina Elena Cipriani
Giuseppina Elena Cipriani Martina Amanzio
Martina Amanzio