
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Psychol. , 24 July 2020
Sec. Neuropsychology
Volume 11 - 2020 | https://doi.org/10.3389/fpsyg.2020.01732
This article is part of the Research Topic Unawareness of Illness in Neurological Disorders: A Focussed Neurocognitive Approach shedding light on Neuropsychological Deficits and Neural Underpinnings Potential Association View all 8 articles
 Umberto Bivona1*
Umberto Bivona1* Paola Ciurli1
Paola Ciurli1 Giulia Ferri1
Giulia Ferri1 Tiziana Fontanelli1
Tiziana Fontanelli1 Susanna Lucatello1
Susanna Lucatello1 Teresa Donvito1
Teresa Donvito1 Dolores Villalobos2,3
Dolores Villalobos2,3 Laura Cellupica1
Laura Cellupica1 Fabiana Mungiello1
Fabiana Mungiello1 Paola Lo Sterzo1
Paola Lo Sterzo1 Amalia Ferraro1
Amalia Ferraro1 Eleonora Giandotti1
Eleonora Giandotti1 Giorgio Lombardi1
Giorgio Lombardi1 Eva Azicnuda1
Eva Azicnuda1 Carlo Caltagirone1,4
Carlo Caltagirone1,4 Rita Formisano1
Rita Formisano1 Alberto Costa1,5
Alberto Costa1,5Self-awareness (SA) is frequently impaired after severe acquired brain injury (sABI) and may lead to reduced subject’s compliance to treatment, worse functional outcome, and high caregiver distress. Considering the multifaceted nature of SA, a specific and effective assessment is crucial to address treatment of impairment of SA (ISA). Many tools can currently assess ISA; however, they have some important limits. In the present study, we proposed the Self-Awareness Multilevel Assessment Scale (SAMAS), a new scale for assessment of SA at different levels (i.e., declarative, emergent, and anticipatory) across all domains of functioning. The SAMAS has been designed to be administered by the cognitive/behavioral therapist with the involvement of a patient’s relative. Findings showed that the SAMAS allowed specifically assessing SA at a declarative level and on all possible functional domains. More interestingly, it seems also able to assess both emergent and anticipatory SA, thus overcoming some important limits of other current assessment methods. Our findings are consistent with a holistic perspective of the patient with sABI because thanks to the combined use of assessing tools, the SAMAS can provide an accurate diagnosis of ISA, thus better addressing the neurorehabilitation treatment and, accordingly, reducing the possible occurrence of its primary and secondary implications.
Self-awareness (SA), defined by Prigatano and Schacter (1991) as “the capacity to perceive the ‘self’ in relatively ‘objective’ terms whilst maintaining a sense of subjectivity,” is frequently impaired after a severe acquired brain injury (sABI) (Levy et al., 1998; Andersson and Finset, 2000; Gainotti and Marra, 2002), with a prevalence of impairment varying from 76 to 97% depending on the method of measurement adopted (Sherer et al., 1998).
Impaired self-awareness (ISA) has been associated to apathy or anosodiaphoria (Babinski, 1914; Tranel, 2002; Heilman and Harciarek, 2010; Gasquoine, 2016; Bivona et al., 2019), reduced subject’s compliance to treatment, worse functional outcome (Lam et al., 1988; Pollens et al., 1988; Ezrachi et al., 1991; Prigatano and Leathem, 1993; Fleming et al., 1996; Sherer et al., 1998, 2003; Prigatano and Wong, 1999; Bivona et al., 2014), and caregiver distress (Prigatano, 2005). Thus, a careful and early assessment of ISA after sABI is an important clinical issue.
However, some issues are still debated.
First: which is the functional architecture of SA? Crosson et al. (1989) posited a pyramidal model consisting of three interdependent and hierarchical levels: (a) intellectual awareness, that is, the subject’s ability to understand (mostly thanks to relatives’ or clinicians’ feedback) and refer that a function is impaired; (b) emergent awareness (a subsequent level of the former), the subject’s ability to recognize problems when they happen; and finally (at the top of the pyramid) (c) anticipatory awareness, that is, the ability to anticipate that a problem will occur because of a deficit (Crosson et al., 1989). Toglia and Kirk (2000), instead, proposed the Dynamic Comprehensive Model of Awareness (DCMA), which views the relationship between different aspects of metacognition and awareness as a dynamic process, rather than as a series of hierarchical levels (Toglia and Kirk, 2000). The DCMA differentiates between (a) metacognitive awareness, that is, knowledge of task characteristics and knowledge of one’s own capabilities (similarly to – even if broader than – the concept of intellectual SA of Crosson et al.’s model); and b) online awareness (activated during a task), which consists of the self-monitoring and recognition of errors (similarly to emergent SA of Crosson et al.’s model), as well as of the person’s appraisal of current task demands (comparable with anticipatory SA of Crosson et al.’s model). For the purpose of the present study, we will adopt the term “declarative” SA, referring to intellectual/metacognitive levels of SA.
Second: does SA affect different domains homogeneously? In fact, an individual may recognize some specific deficits (e.g., motor impairment), but being unaware of deficits in other domains (i.e., everyday problem solving or social situations) (Toglia and Kirk, 2000). In general, patients tend to show more severe ISA for behavioral and affective functions, moderate for cognitive functions, and less severe for motor and sensory functions (Sherer et al., 1998; Hart et al., 2003, 2004).
Third: how to best assess ISA after sABI? Several methods have been proposed: (a) clinical observation (Langer and Samuels, 2008), even by structured rating scales such as the “Clinician’s Rating Scale” (Prigatano and Klonoff, 1998); (b) structured and semi-structured interviews, such as the “Self-Awareness Deficits Interview” (SADI; Fleming et al., 1996) or the “Self-Regulation Skills Interview” (Ownsworth et al., 2000); (c) by comparing patient’s self-assessment and their performance on neuropsychological tests, such as the “Awareness Interview” (Anderson and Tranel, 1989) and the “Assessment of Awareness of Disability” (Tham et al., 1999); and (d) the comparison between patient’s self-report and clinician/relative’s report, such as the “Patient Competency Rating Scale” (PCRS; Prigatano et al., 1986), the “Awareness Questionnaire” (Sherer et al., 1998), and the Head Injury Behavior Scale (Godfrey et al., 1993).
However, these methods have some limits. For example, they cannot be administered to patients who suffer from aphasia, as well as from severe memory deficits or reduced reasoning and judgment abilities. Moreover, to our knowledge, currently used inventories and interviews can assess solely declarative awareness. In fact, to assess emergent and anticipatory awareness, patient’s performance has to be evaluated in a variety of situations by a trained professional (Barco et al., 1991). In this regard, the limits of studies investigating emergent (Ownsworth et al., 2000, 2002; Abreu et al., 2001; O’Keeffe et al., 2007; Krasny-Pacini et al., 2014; Dockree et al., 2015) or anticipatory (Fleming et al., 1996; O’Keeffe et al., 2007) SA after sABI are the use of tasks sensitive only to some specific cognitive or behavioral functions. Importantly, as anticipatory awareness allows the implementation of correct future behavior, it needs to be objectively assessed. Indeed, the offline collection of the patient’s report itself, reflecting just a declarative awareness on possible future difficulties in relation to the post-injury difficulties, is not a reliable method: only an external report (e.g., by an informal caregiver of the care recipient) about the patient’s real behavior during the activities of daily life can evidence more reliably that the patient has actually gained anticipatory SA (Stuss, 1991; Flashman et al., 1998).
Given this background, the aim of the present study was to examine the validity of the Self-Awareness Multilevel Assessment Scale (SAMAS) to assess ISA after sABI. The SAMAS assesses different levels of SA (i.e., declarative, emergent, and anticipatory) across all domains of functioning (i.e., physical, cognitive, emotional/behavioral). It has been designed to be completed by the cognitive/behavioral therapist, with the involvement of the patients’ relative, as well as of other members of the inter-professional neurorehabilitation team, when necessary. Furthermore, to examine the potential added value of SAMAS to assess ISA after sABI in respect to extant tools, the PCRS and the SADI, two of the most currently used scales to assess ISA in this population, were also administered.
Twenty-five patients with sABI, consecutively admitted to the Post-Coma Unit of Santa Lucia Foundation in Rome (Italy) from March 2018 to September 2019, were recruited. The study was approved by the local Ethics Committee, and all participants were included in the study after providing their (or by one legal surrogate) informed consent.
Patients with sABI were recruited according to the following inclusion criteria: (a) age ≥ 16 years; (b) diagnosis of severe ABI (Glasgow Coma Scale score ≤ 8 in the acute phase); (c) score at the Level of Cognitive Functioning Scale ≥ 6, with inclusion of the patient according to the judgment of the neuropsychologist involved in the study; d) capacity to undergo a formal psychological evaluation; (e) availability of informed consent.
Exclusion criteria for patients recruited in this study were (a) history of drug and alcohol addiction, (b) psychiatric diseases, and (c) repeated sABI and/or other neurological disorders.
Socio-demographic and clinical characteristic of patients were 19 males and 6 females, with a mean age of 43.4 years (SD = 16.1); mean educational level of 12.4 years (SD = 4.2); time since injury from 45 to 472 days, with a mean of 131.2 days (SD = 97.1); and etiology of sABI: Traumatic Brain Injury (TBI) (n = 15), stroke (n = 9), and other causes (n = 1).
A functional assessment was performed by means of the following scales: (1) Glasgow Outcome Scale (Jennett and Bond, 1975); (2) Level of Cognitive Functioning (LCF) scale (Hagen et al., 1972); (3) Disability Rating Scale (Rappaport et al., 1982).
To date, no measures useful to assess concurrent validity regarding emergent and anticipatory SA are available; thus, evaluation of both levels of SA requires observations of the patient’s task performance in a variety of situations, accompanied by timely questions by a trained professional (Barco et al., 1991). Accordingly, in line with other studies who underline the role of the clinician as a rater of the level of SA (Fleming et al., 1996), in the present study we adopted as a gold standard measure of SA the clinical judgment of a neuropsychologist (P.C.) expert for around 30 years in the field of severe ABI. It is worth noting that she judged SA in the context of a complete neuropsychological assessment performed by herself, which enhanced the reliability of her global assessment of SA. Indeed, by a careful observation of the patients’ behavior and self-monitoring during the test administration, completed by a clinical interview to the patients and their caregiver, and by information gathered together with other professional of the rehabilitation team (i.e., nurses, occupational therapists, physiotherapists), the neuropsychologist assessed at best all the levels of SA taken into account in the present work, in all the possible domains.
In particular, for each level, the neuropsychologist assessed patients in blind with respect to the other professional included in the study, scoring between 0 (i.e., “good SA”) and 4 (“severe ISA”) on each one of the several domains of interest (i.e., motor/sensitive, cognitive, behavioral/affective, and other) and levels of SA (i.e., declarative, emergent, and anticipatory SA). Accordingly, the maximum possible score on each domain was 12, being the maximum possible total score equal to 36.
In the present study, we developed this new scale with the main purpose of assessing, by a single and comprehensive tool, the aforementioned different levels of SA (i.e., declarative, emergent, and anticipatory) along with the following domains: motor, cognitive, psycho-behavioral, and others (i.e., phoniatric, dysphagic). Within each level, the scores for each domain can range from 0 (i.e., “good SA”) to 2 (“relevant ISA”). In particular, score 0 for each level and domain is index of patients’ ability to spontaneously recognize their possible difficulties; score 1, index of their ability to recognize possible difficulties only after receiving a cue by the therapist; and score 2, index of a severe impairment in recognizing their possible difficulties even after such cue. When a patient does not present with any problem in one or more of the domains, SAMAS in that or those domains is scored as “not applicable” (see Supplementary Annex for more details on the scale).
The declarative level consisted of two items: patient’s recognition of the presence of current difficulties, and the functional implications of such difficulties. The emergent level is composed of one item referring to the patient’s online recognition of difficulties, if and when they occur in each domain. Finally, as for the anticipatory level, the SAMAS contains five items: the patient’s ability to recognize the problematic nature of a task with respect to his/her own deficits; the patient’s ability to set realistic goals in relation to his own difficulties; the patient’s expression of strategies to avoid having difficulties; the patient’s effective use of such strategies; the patient’s ability to generalize such strategies (when they are used) to all the contexts in which he/she acts.
The SAMAS can be completed by a cognitive/behavioral therapist as soon as he/she has a clear and complete picture of the patients’ SA at the different levels and on the different domains. In particular, in the present study the SAMAS has been completed by a cognitive/behavioral therapist within 30 min maximum, and within 10 observation sessions in all cases. The SAMAS has been conceived to be completed with the support of the physiotherapist and informal caregivers, to obtain a clear and complete picture of the patients’ SA. In particular, both physiotherapists and informal caregivers allowed verifying two main aspects: (a) the emergent SA even in other contexts (for instance, if the patients are able to recognize motor difficulties when they occur during the sessions of physiotherapy, or in their hospital room or at home); (b) the patients’ anticipatory SA beyond its declarative level, that is, verifying if it actually corresponds to a real anticipatory level of SA, thanks to an external report on patients’ behaviors outside the cognitive/behavioral setting. Accordingly, in assessing both emergent and anticipatory SA, each cognitive/behavioral therapist involved in the present study scored the SAMAS only after having collected and accurately weighed the information reported by both physiotherapists and patients’ informal caregivers.
The PCRS (Prigatano et al., 1986; Ciurli et al., 2010) is a 30-item self-report questionnaire that requires patients and their relatives to make an independent judgment of perceived degree of competency demonstrated in several behavioral, cognitive, and emotional situations. To assess declarative SA, it is important to consider both the patient self-report and the magnitude of the difference between patients’ report and the relative report of patients’ functional competency, that is, the PCRS discrepancy score (PCRS-DS) (Prigatano et al., 1990; Prigatano, 2014; Bivona et al., 2019). Higher PCRS-DS mean higher ISA.
The SADI (Fleming et al., 1996) is a clinician-rated measure in a semi-structured interview format. It includes items addressing three domains: SA of deficits, SA of the functional implication of the deficits, and ability to set realistic goals. Higher scores mean more impaired SA.
After admission to the Post-Coma Unit of the Santa Lucia Foundation IRCCS, a neurorehabilitation hospital in Rome, the whole SA assessment was conducted to all patients as soon as they were diagnosed by our expert neuropsychologist (P.C.) as emerged from the level 5 of LCF scale, that is, when their responses, even if they might have been incorrect because of memory problems, were appropriate to the situation; or when they showed beginning immediate awareness of personal situation; or when they no longer wandered and were, even inconsistently, oriented to time and place.
Within the same week, for each patient, the same neuropsychologist, a cognitive/behavioral therapist, and a clinical psychologist completed his/her assessment in blind with respect to each other, as follows: (a) the neuropsychologist began, in a quiet room, the administration of the neuropsychological test battery and, by observing the patients in that context, with the support of the patient’s informal caregiver, physiotherapist, and nurses of the Post-Coma Unit, she completed the assessment of SA (i.e., the gold standard assessment in the present study); (b) a cognitive/behavioral therapists (G.F., S.L., L.C., F.M., or P.L.) completed both the SADI and the SAMAS in the context of the cognitive/behavioral neurorehabilitation setting (i.e., in another room of the Post-Coma Unit); (c) a clinical psychologist (T.D. or G.L.) administered, in a third room, the PCRS to each patient and (separately) to his/her caregiver.
Data analysis was carried out using SPSS software (version 22). Preliminarily, we described the study variables in terms of means and SDs to illustrate the characteristics of the patients.
To investigate whether participants’ score on SAMAS predict clinical judgment of SA, the forward linear regression model was applied. In particular, the specific aim of the study is to explore whether SAMAS can improve the clinical diagnosis of ISA in respect to some of the extant tools. Indeed, SAMAS directly assesses two SA factors (i.e., anticipatory and emergent SA) that are not fully taken into account by currently used tools. Accordingly, in the regression model, the clinical judgment (score range 1–4) was entered in the model as dependent variable and global scores on SAMAS, PCRS-DS, and SADI were entered as independent variables. Therefore, four forward linear regression analyses were run in which the independent variables were those indicated above. As for the dependent variables, they were as follows: in the first analysis, the global clinical judgment of SA; in the second, the clinical judgment of declarative SA; in the third, the clinical judgment of emergent SA; in the fourth, the clinical judgment of anticipatory SA, respectively. Pearson’s r correlations were executed to investigate the association between the clinical judgment on each of the three dimensions of SA and the score on the corresponding subscale of the SAMAS. Pearson’s r correlations were also performed to investigate the association between participants’ global scores on SAMAS, PCRS-DS, and SADI.
Descriptive statistics for the sample including demographic, functional, and SA are reported in Table 1.
In the first step of this analysis, the SAMAS total score entered the regression equation [F(1, 24) = 25.9; p < 0.001; R2 = 0.53] with a positive correlation with the dependent variable (Beta = 0.73; t = 5.09; p < 0.001). This shows that higher scores on SAMAS are associated with worse SA according to the global clinical judgment. In the second step, the score on the PCRS-DS significantly contributed to the model [R2 change = 0.10; F(2, 24) = 18.9; p < 0.001], also in this case with a positive correlation with the dependent variable (Beta = 0.35; t = 2.48; p = 0.021). This documents that higher PCRS-DS are associated with global clinical judgment of more severe ISA. SADI score, instead, did not enter the regression equation (Beta = −0.29; t = −0.15; p > 0.80) (Table 2).
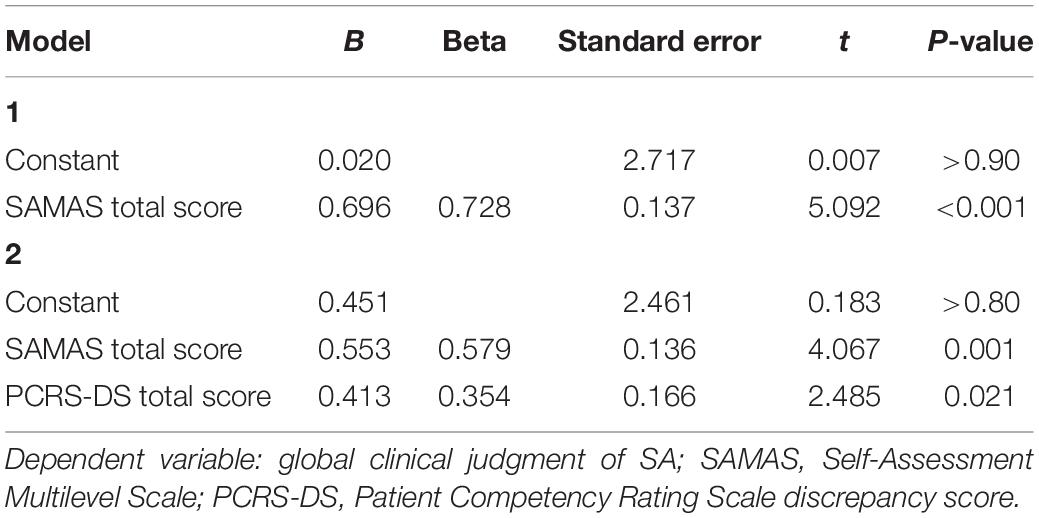
Table 2. Results of the forward linear regression analysis performed to investigate whether participants’ score on SAMAS predicted global clinical judgment of self-awareness.
Results of this analysis are similar to the results of analysis presented previously. In the first step, the SAMAS total score entered the regression equation [F(1, 24) = 23.2; p < 0.001; R2 = 0.50] with a positive correlation with the criterion (Beta = 0.71; t = 4.82; p < 0.001). This shows that higher scores on SAMAS are associated with more severe impairment in declarative SA according to the clinical judgment. In the second step the PCRS-DS score also entered the regression equation [R2 change = 0.17; F(2, 24) = 24.3; p < 0.001], showing a positive correlation with the dependent variable (Beta = 0.47; t = 3.62; p = 0.002). This documents that worse declarative SA according to PCRS-DS are associated with global clinical judgment of more severe declarative SA. Also in this case, SADI score did not significantly contribute to the model (Beta = 0.15; t = 0.85; p > 0.30) (Table 3).
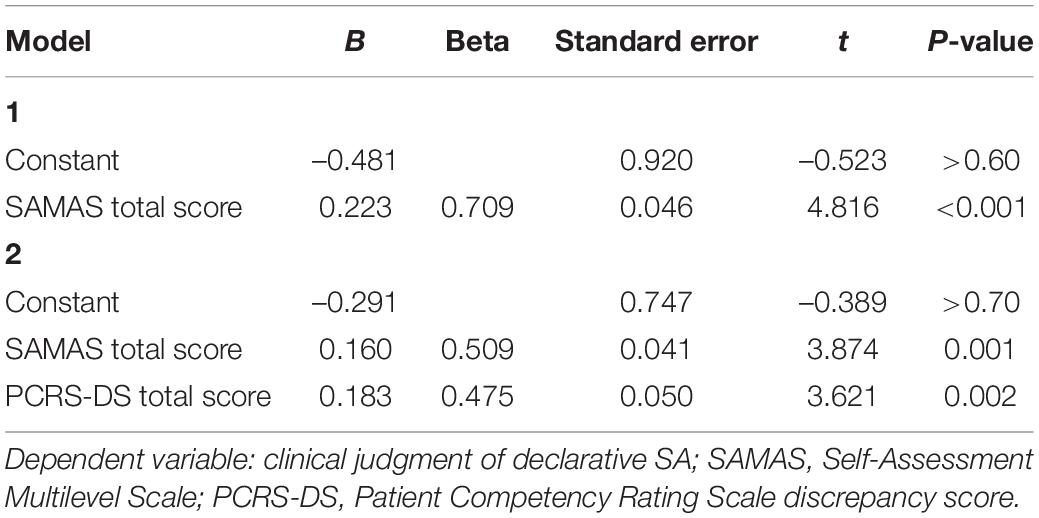
Table 3. Results of the forward linear regression analysis performed to investigate whether participants’ score on SAMAS predicted the clinical judgment of declarative self-awareness.
Results of this analysis show that the only independent variable entering the regression equation was the SAMAS score [F(1, 24) = 27.3; p < 0.001; R2 = 0.54] with a positive correlation with the criterion (Beta = 0.74; t = 5.22; p < 0.001). This result documents that higher scores on SAMAS are associated with more reduced emergent SA according to the clinical judgment. In this case, neither PCRS-DS (Beta = 0.27; t = 1.84; p = 0.08) nor SADI scores (Beta = 0.01; t = 0.05; p > 0.90) significantly contributed to the model (Table 4).
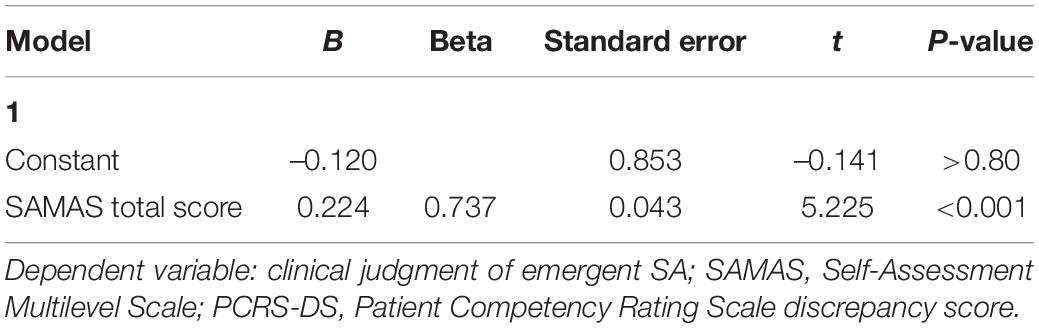
Table 4. Results of the forward linear regression analysis performed to investigate whether participants’ score on SAMAS predicted the clinical judgment of emergent self-awareness.
Results of this analysis also show that SAMAS score was the only independent variable entering the regression equation [F(1, 24) = 19.3; p < 0.001; R2 = 0.46], also in this case showing a positive correlation with the dependent variable (Beta = 0.68; t = 4.39; p < 0.001). This result indicates that higher scores on SAMAS are associated to worse clinical judgment of anticipatory SA. Both PCRS-DS (Beta = 0.29; t = 1.77; p = 0.09) and SADI scores (Beta = 0.03; t = 0.16; p > 0.80) were excluded from the regression model (Table 5).

Table 5. Results of the forward linear regression analysis performed to investigate whether participants’ score on SAMAS predicted the clinical judgment of anticipatory self-awareness.
Results of Pearson’s r correlation analyses show a highly significant positive correlation between the declarative score obtained on the SAMAS and the clinical judgment of declarative SA (r = 0.67; p < 0.001) as well as between score for the anticipatory items of the SAMAS and the clinical judgment of anticipatory SA (r = 0.62; p < 0.001). As for the correlation between emergent score obtained on the SAMAS and the clinical judgment of emergent SA in this case, instead, we found a tendency toward a significant effect (r = 0.33; p = 0.053).
Results from these analyses document that participants’ global scores on SAMAS, PCRS-DS, and SADI are significantly correlated with each other. In particular, scores on SAMAS positively correlated with scores on both PCRS-DS (r = 0.42; p = 0.036) and SADI (r = 0.66; p < 0.001). In turn, scores on PCRS-DS and SADI also showed a significant positive correlation (r = 0.52; p = 0.008). These results clearly indicate that scores on the three scales are congruently associated.
This study aimed at investigating the validity of a new tool, the SAMAS, to assess ISA in people with sABI. First, ISA was assessed by an expert neuropsychologist who rated a clinical judgment on a 4-point scale on the declarative, anticipatory, and emergent dimensions of SA. Then, regression analyses were performed to examine the predictive value of SAMAS score on the clinical judgment earlier, taking into account the weight of the PCRS-DS and SADI score. Main results show that SAMAS scores significantly predicted all dimensions of SA. Interestingly, the SAMAS score was the unique variable entering the regression equation in the analyses, including the clinical judgment of anticipatory and emergent ISA as dependent variables. Moreover, results document a highly significant positive correlation between the declarative score obtained on the SAMAS and the clinical judgment of declarative SA as well as between score for the anticipatory items of the SAMAS and the clinical judgment of anticipatory SA, whereas for the correlation between emergent score obtained on the SAMAS and the clinical judgment of emergent SA, we found a tendency toward a significant effect.
These results indicate that SAMAS is able to specifically and broadly assess both emergent and (actual) anticipatory SA. Indeed, although SAMAS showed significant positive correlations with both PCRS-DS and SADI, thus indicating that high scores on the three scales coherently outline low global levels of SA, as from results of regression analyses its score was independently associated to the clinical judgment on anticipatory and emergent SA (i.e., PCRS-DS and SADI scores did not enter the regression equation). This represents an important and innovative contribution of the present study allowing overcoming some important limits of other current methods of assessment of SA. Indeed, the extant tools that assessed emergent SA (Ownsworth et al., 2000, 2002; Abreu et al., 2001; O’Keeffe et al., 2007; Krasny-Pacini et al., 2014; Dockree et al., 2015) in the field of ABI utilize only a few number of specific tasks, on limited cognitive or behavioral domains. Conversely, the SAMAS (emergent section) has been completed by the cognitive/behavioral therapists after an accurate online observation of patients’ behavior and report during several performances within the neurorehabilitation context, as well as thanks to collecting information from the patients’ physiotherapist and caregiver in other contexts (for instance, during the sessions of physiotherapy, or in the hospital room or at home). These series of measures correlated with the clinical judgment of our expert neuropsychologist who, in parallel and blindly, assessed emergent SA within the neuropsychological assessment context. In fact, this can be considered an important index of concurrent validity. In this regard, as reported previously, it should be noted that although we found an association between the score on emergent subscale of SAMAS and the clinical judgment of emergent SA (r = 0.33; p = 0.053), such an association only approached statistical significance, likely as a result of the relatively limited sample size.
Similarly, the studies in the literature which aimed at assessing anticipatory SA (Fleming et al., 1996; O’Keeffe et al., 2007) considered, as a matter of fact, only its declarative aspects, without providing a confirmation of a real anticipatory SA, such as, for instance, the fact that patients avoided dangerous or dysfunctional behaviors in their daily life. Even in this case, SAMAS allowed overcoming this limit because therapists completed the anticipatory section of the scale only when patients’ report were consistent with the parallel interview to their physiotherapists and informal caregivers regarding their real behaviors outside the cognitive/behavioral neurorehabilitation context and, more generally, during daily life. Only this comparison allowed therapist claiming whether the patients effectively gained an actual anticipatory SA, beyond its declarative aspect.
A final comment should be deserved to the finding of a highly significant correlations we found between the three subscales of SAMAS.
We would underline that our study must be considered just as preliminary, owing to the limited sample size, as well as to not having investigated the inter-rater reliability of the SAMAS between the different cognitive/behavioral therapists who took part in the study. Nevertheless, taken together, our findings are consistent with the main aim of the present study that is proposing a new clinical tool to deeply and quantitatively assess SA at its different levels (at least according to the main theoretical models) (Crosson et al., 1989; Toglia and Kirk, 2000), and on the possible functional domains.
In conclusion, it is worth noting that this preliminary study is part of a study still in progress because, by enlarging the sample size, we are aiming at investigating also the inter-rater reliability of the SAMAS.
However, our current results suggest that the SAMAS can be conceived as a useful scale to broadly assess SA and, in particular, to quantify some relevant information on patients’ levels of SA, which usually remain only as a part of a qualitative clinical observation. Indeed, the great advantage of SAMAS is that it allows the cognitive/behavioral therapist to systematically quantify what is usually observed within the rehabilitation setting regarding the different levels of self-awareness on each functional domain. Accordingly, a careful use of SAMAS would allow a better monitoring of ISA within the neurorehabilitation process, as well as a more reliable comparison between different professionals in rehabilitation. In particular, to date, it seems to be the only tool in the literature that allows the assessment of emergent and (really, not just declaratively) anticipatory SA.
However, we would also underline that the SAMAS can be a thorough and effective assessing tool of ISA as long as (a) it is completed within the context of an accurate clinical observation, (b) if it is accompanied by an accurate interview to the informal caregivers, and (c) if it is supported by the necessary information gained by the other members of the inter-professional neurorehabilitation team; accordingly, an adequate experience with team work is mandatory to achieve a correct coding of the SAMAS. Moreover, to better assess declarative level of SA, we also recommend the combined use of the SAMAS with some of the traditional questionnaires or interviews, to enhance the reliability of all measures used. Indeed, only a holistic approach to the patient with sABI, thanks to the combined use of clinical observation, interviews and scales, can allow obtaining an early and accurate diagnosis of ISA. Accordingly, it is possible also to better address the ISA treatment and reduce the possible occurrence of its primary (e.g., poor motivation and compliance; hostility toward therapy, and risk of failure of rehabilitation) and secondary (e.g., poor ability of risk evaluation, ineffective behaviors, poor social and work re-entry) implications, that so often make ISA an everlasting problem not only for the patients but sometimes even more for their whole family and social systems.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Comitato Etico of the IRCCS Fondazione Santa Lucia di Roma (It) – Santa Lucia Foundation local Ethics Committee. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
UB, GF, and TF conceived the study. AC designed and supervised the project and analyzed the data. PC collected the data as a gold standard. GF, SL, LC, FM, and PL collected the data on the SAMAS and SADI scales. TD, TF, AF, and EG collected the data on PCRS scale. DV, GL, and EA managed the database and contributed to the general organization and realization of the project. CC and RF supervised and gave an intellectual contribution to the project. UB wrote the first draft of the manuscript. All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are very grateful to Francesca Amadori for some important cues at an early stage of the study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyg.2020.01732/full#supplementary-material
Abreu, B. C., Seale, G., Scheibel, R. S., Zhang, L., and Ottenbacher, K. J. (2001). Levels of self-awareness after acute brain injury: how patients’ and rehabilitation specialists’ perceptions compare. Arch. Phys. Med. Rehabil. 82, 49–56. doi: 10.1053/apmr.2001.9167
Anderson, S. W., and Tranel, D. (1989). Awareness of disease states following cerebral infarction, dementia, and head trauma: standardized assessment. Clin. Neuropsychol. 3, 327–339. doi: 10.1080/13854048908401482
Andersson, A., and Finset, S. (2000). Coping strategies in patients with acquired brain injury: relationships between coping, apathy, depression and lesion location. Brain Inj. 14, 887–905. doi: 10.1080/026990500445718
Babinski, J. (1914). Contribution à l’étude des troubles mentaux dans l’hémiplégie organique cérébrale (Anosognosie). Revue Neurologique 61, 845–848.
Barco, P., Crosson, B., Bolesta, M., Wets, D., and Stout, R. (1991). “Training awareness and compensation in postacute head injury rehabilitation,” in Cognitive Rehabilitation for Persons with Traumatic Brain Injury. A Functional Approach, eds J. S. Kreutzer and P. H. Wehman (Batimore: Paul H. Brookes Publishing), 129–146.
Bivona, U., Costa, A., Contrada, M., Silvestro, D., Azicnuda, E., Aloisi, M., et al. (2019). Depression, apathy and impaired self-awareness following severe traumatic brain injury: a preliminary investigation. Brain Inj. 33, 1245–1256. doi: 10.1080/02699052.2019.1641225
Bivona, U., Riccio, A., Ciurli, P., Carlesimo, G., Donne, V., Pizzonia, E., et al. (2014). Low self-awareness of individuals with severe traumatic brain injury can lead to reduced ability to take another person’s perspective. J. Head Trauma Rehabil. 29, 157–171. doi: 10.1097/HTR.0b013e3182864f0b
Ciurli, P., Bivona, U., Barba, C., Onder, G., Silvestro, D., Azicnuda, E., et al. (2010). Metacognitive unawareness correlates with executive function impairment after severe traumatic brain injury. J. Int. Neuropsychol. Soc. 16, 360–368. doi: 10.1017/S135561770999141X
Crosson, B., Barco, P. P., Velozo, C. A., Bolesta, M. M., Cooper, P. V., Werts, D., et al. (1989). Awareness and compensation in postacute head injury rehabilitation. J. Head Trauma Rehabil. 4, 46–54. doi: 10.1097/00001199-198909000-00008
Dockree, P. M., Tarleton, Y. M., Carton, S., and FitzGerald, M. C. C. (2015). Connecting Self-Awareness and Error-Awareness in Patients with Traumatic Brain Injury. J. Int. Neuropsychol. Soc. 21, 473–482. doi: 10.1017/S1355617715000594
Ezrachi, O., Ben-Yishay, Y., Kay, T., Diller, L., and Rattok, J. (1991). Predicting employment in traumatic brain injury following neuropsychological rehabilitation. J. Head Trauma Rehabil. 6, 71–84. doi: 10.1097/00001199-199109000-00010
Flashman, L. A., Amador, X., and McAllister, T. W. (1998). Lack of awareness of deficits in traumatic brain injury. Semin. Clin. Neuropsychiatry 3, 201–210.
Fleming, J., Strong, J., and Ashton, R. (1996). Self-awareness of deficits in adults with traumatic brain injury: how best to measure? Brain Inj. 10, 1–15. doi: 10.1080/026990596124674
Gainotti, G., and Marra, C. (2002). Determinants and consequences of post-stroke depression. Curr. Opin. Neurol. 15, 85–89. doi: 10.1097/00019052-200202000-00013
Gasquoine, P. G. (2016). Blissfully unaware: anosognosia and anosodiaphoria after acquired brain injury. Neuropsychol. Rehabil. 26, 261–285. doi: 10.1080/09602011.2015.1011665
Godfrey, H. P. D., Partridge, F. M., Knight, R. G., and Bishara, S. (1993). Course of insight disorder and emotional dysfunction following closed head injury: a controlled cross-sectional follow-up study. J. Clin. Exp. Neuropsychol. 15, 503–515. doi: 10.1080/01688639308402574
Hagen, C., Malkmus, D., and Durham, P. (1972). Levels of Cognitive Functioning. Downey, CA: Rancho de los Amigos Hospital.
Hart, T., Sherer, M., Whyte, J., Polansky, M., and Novack, T. A. (2004). Awareness of behavioral, cognitive, and physical deficits in acute traumatic brain injury. Arch. Phys. Med. Rehabil. 85, 1450–1456. doi: 10.1016/j.apmr.2004.01.030
Hart, T., Whyte, J., Polansky, M., Millis, S., Hammond, F. M., Sherer, M., et al. (2003). Concordance of patient and family report of neurobehavioral symptoms at 1 year after traumatic brain injury. Arch. Phys. Med. Rehabil. 84, 204–213. doi: 10.1053/apmr.2003.50019
Heilman, K. M., and Harciarek, M. (2010). “Anosognosia and anosodiaphoria of weakness,” in The Study of Anosognosia, ed. G. P. Prigatano (Oxford: Oxford University Press), 89–112.
Jennett, B., and Bond, M. (1975). Assessment of outcome after severe brain damageS. A practical scale. Lancet 305, 480–484. doi: 10.1016/S0140-6736(75)92830-5
Krasny-Pacini, A., Chevignard, M., and Evans, J. (2014). Goal Management Training for rehabilitation of executive functions: a systematic review of effectivness in patients with acquired brain injury. Disabil. Rehabil. 36, 105–116. doi: 10.3109/09638288.2013.777807
Lam, C. S., Mcmahon, B. T., Priddy, D. A., and Gehred-Schultz, A. (1988). Deficit awareness and treatment performance among traumatic head injury adults. Brain Inj. 2, 235–242. doi: 10.3109/02699058809150947
Langer, K. G., and Samuels, M. C. (2008). Unawareness of disability in CVA: a comparison study with musculoskeletal patients. Cogn. Behav. Neurol. 21, 206–213. doi: 10.1097/WNN.0b013e3181864a4b
Levy, M. L., Cummings, J. L., Fairbanks, L. A., Masterman, D., Miller, B. L., Craig, A. H., et al. (1998). Apathy is not depression. J. Neuropsychiatry Clin. Neurosci. 10, 314–319. doi: 10.1176/jnp.10.3.314
O’Keeffe, F., Dockree, P., Moloney, P., Carton, S., and Robertson, I. H. (2007). Awareness of deficits in traumatic brain injury: a multidimensional approach to assessing metacognitive knowledge and online-awareness. J. Int. Neuropsychol. Soc. 13, 38–49. doi: 10.1017/S1355617707070075
Ownsworth, T. L., McFarland, K., and Young, R. M. (2002). The investigation of factors underlying deficits in self-awareness and self-regulation. Brain Inj. 16, 291–309. doi: 10.1080/02699050110103986
Ownsworth, T. L., McFarland, K. M., and Young, R. M. (2000). Development and standardization of the Self-regulation Skills Interview (SRSI): a new clinical assessment tool for acquired brain injury. Clin. Neuropsychol. 14, 76–92. doi: 10.1076/1385-4046(200002)14:1;1-8;FT076
Pollens, R. D., McBratnie, B. P., and Burton, P. L. (1988). Beyond cognition: executive functions in closed head injury. Cogn. Rehabil. 6, 23–32.
Prigatano, G. P. (2005). Distrubances of self-awareness and rehabilitation of patients with traumatic brain injury: a 20-year perspective. J. Head Trauma Rehabil. 20, 19–29. doi: 10.1097/00001199-200501000-00004
Prigatano, G. P. (2014). Anosognosia and patterns of impaired self-awareness observed in clinical practice. Cortex 61, 81–92. doi: 10.1016/j.cortex.2014.07.014
Prigatano, G. P., Altman, I. M., and O’Brien, K. P. (1990). Behavioral limitations that traumatic-brain-injured patients tend to underestimate. Clin. Neuropsychol. 4, 163–176. doi: 10.1080/13854049008401509
Prigatano, G. P., Fordyce, D. J., Zeiner, H. K., Roueche, J. R., Pepping, M., and Wood, B. C. (1986). Neuropsychological Rehabilitation After Brain Injury. Baltimore: John Hopkins Univ. Press.
Prigatano, G. P., and Klonoff, P. S. (1998). A clinician’s rating scale for evaluating impaired self-awareness and denial of disability after brain injury. Clin. Neuropsychol. 12, 56–67. doi: 10.1076/clin.12.1.56.1721
Prigatano, G. P., and Leathem, J. M. (1993). Awareness of behavioral limitations after traumatic brain injury: a cross- cultural study of New Zealand Maoris and non-Maoris. Clin. Neuropsychol. 7, 123–135. doi: 10.1080/13854049308401514
Prigatano, G. P., and Schacter, D. L. (1991). Awareness of Deficit After Brain Injury: Clinical and Theoretical Issues. New York: Oxford University Press.
Prigatano, G. P., and Wong, J. L. (1999). Cognitive and affective improvement in brain dysfunctional patients who achieve inpatient rehabilitation goals. Arch. Phys. Med. Rehabil. 80, 77–84. doi: 10.1016/S0003-9993(99)90311-8
Rappaport, M., Hall, K. M., Hopkins, K., Belleza, T., and Cope, D. N. (1982). Disability rating scale for severe head trauma: coma to community. Arch. Phys. Med. Rehabil. 63, 118–123.
Sherer, M., Bergloff, P., Boake, C., High, W., and Levin, E. (1998). The Awareness Questionnaire: factor structure and internal consistency. Brain Inj. 12, 63–68. doi: 10.1080/026990598122863
Sherer, M., Hart, T., and Nick, T. G. (2003). Measurement of impaired self-awareness after traumatic brain injury: a comparison of the patient competency rating scale and the awareness questionnaire. Brain Inj. 17, 25–37. doi: 10.1080/0269905021000010113
Stuss, D. T. (1991). “Disturbance of self-awareness after frontal system damage,” in Awareness of Deficits After Brain Injury: Clinical and Theoretical Implications, eds G. P. Prigatano and D. L. Schacter (New York: Oxford University Press), 63–83.
Tham, K., Bernspång, B., and Fisher, A. G. (1999). Development of the assessment of awareness of disability. Scand. J. Occup. Ther. 6, 184–190. doi: 10.1080/110381299443663
Toglia, J., and Kirk, U. (2000). Understanding awareness deficits following brain injury. NeuroRehabilitation 15, 57–70. doi: 10.3233/nre-2000-15104
Tranel, D. (2002). “Functional neuroanatomy. Neuropsychological correlates of cortical and subcortical damage,” in The American Psychiatric Association Publishing Textbook of Neuropsychiatry and Clinical Neurosciences, eds D. B. Arciniegas, S. C. Yudofsky, and R. E. Hales (Washington, DC: American Psychiatric Association), 93.
Keywords: severe acquired brain injury, anosognosia, self-awareness multilevel assessment, neurorehabilitation, functional deficit
Citation: Bivona U, Ciurli P, Ferri G, Fontanelli T, Lucatello S, Donvito T, Villalobos D, Cellupica L, Mungiello F, Lo Sterzo P, Ferraro A, Giandotti E, Lombardi G, Azicnuda E, Caltagirone C, Formisano R and Costa A (2020) The Self-Awareness Multilevel Assessment Scale, a New Tool for the Assessment of Self-Awareness After Severe Acquired Brain Injury: Preliminary Findings. Front. Psychol. 11:1732. doi: 10.3389/fpsyg.2020.01732
Received: 20 April 2020; Accepted: 23 June 2020;
Published: 24 July 2020.
Edited by:
Sara Palermo, University of Turin, ItalyReviewed by:
Stefano Zago, Foundation IRCCS Ca’ Granda Ospedale Maggiore Policlinico, ItalyCopyright © 2020 Bivona, Ciurli, Ferri, Fontanelli, Lucatello, Donvito, Villalobos, Cellupica, Mungiello, Lo Sterzo, Ferraro, Giandotti, Lombardi, Azicnuda, Caltagirone, Formisano and Costa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Umberto Bivona, dS5iaXZvbmFAaHNhbnRhbHVjaWEuaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.