- 1Department of Community Dentistry and Behavioral Science, University of Florida, Gainesville, FL, United States
- 2Pain Research and Intervention Center of Excellence, University of Florida, Gainesville, FL, United States
- 3Department of Clinical and Health Psychology, University of Florida, Gainesville, FL, United States
- 4Center for Pain Research and Behavioral Health, University of Florida, Gainesville, FL, United States
Evidence supports the benefits of resilience among older adults with chronic pain. While numerous factors confer resilience, research has largely examined these measures in isolation, despite evidence of their synergistic effects. Conceptualizing resilience from a multisystem perspective may provide a deeper understanding of adaptive functioning in pain. Sixty adults (ages 60+ years) with chronic low back pain completed measures of physical function, pain intensity, disability, and a performance-based task assessing back-related physical functioning and movement-evoked pain (MEP). Depressive symptoms, quality of life, and general resilience were also evaluated. To examine multisystem resiliency, principal components analysis (PCA) was conducted to create composite domains for psychological (positive affect, hope, positive well-being, optimism), health (waist–hip ratio, body mass index, medical comorbidities), and social (emotional, instrumental, informational support) functioning measures, followed by cluster analysis to identify participant subgroups based upon composites. Results yielded four clusters: Cluster 1 (high levels of functioning across psychological, health, and social support domains); Cluster 2 (optimal health and low psychosocial functioning); Cluster 3 (high psychological function, moderate-to-high social support, and poorer health); and Cluster 4 (low levels of functioning across the three domains). Controlling for sociodemographic characteristics, individuals with a more resilient phenotype (Cluster 1) exhibited lower levels of disability, higher quality of life and psychological functioning, and greater functional performance when compared to those with a lower degree of personal resources (Cluster 4). No significant cluster differences emerged in self-reported pain intensity or MEP. These findings signify the presence of resiliency profiles based upon psychological, social, and health-related functioning. Further examination of the additive effects of multiple adaptive behaviors and resources may improve our understanding of resilience in the context of pain, informing novel interventions for older adults.
Introduction
Older adults represent the fastest growing population in the United States. As such, increased attention on enhancing the health and well-being of this cohort is imperative. Among health complaints and chronic medical conditions, pain remains a significant area of concern in aging adults, with approximately 18.7 million (53%) adults ages 65 years and older (Patel et al., 2013) reporting they experience bothersome pain (Helme and Gibson, 2001). Further, chronic low back pain (cLBP) impacts 36% of this population and is the leading cause of disability in older adults (Weiner et al., 2003; Molton and Terrill, 2014). In spite of the significant burden of chronic pain in older adults, this group is often subjected to inadequate assessment and suboptimal treatment of pain (Gibson and Lussier, 2012; Molton and Terrill, 2014).
Traditionally, aging has been viewed as a period of frailty, vulnerability, and decline. However, there is considerable variability in the aging process. Indeed, the importance of considering the role of adaptive constructs in promoting successful aging (characterized by decreased disability, greater health-related functioning, and better life engagement) has been highlighted (Rowe and Kahn, 1997, 2015). Understanding factors that could delay or prevent aging-related illnesses and support successful aging would allow for the development of approaches that attenuate disability related to these health conditions. Thus, in the context of functional limitations and decreased quality of life associated with chronic pain, greater emphasis should be placed on identifying factors that ultimately inform targeted interventions for pain in older adults. These investigations should account for the multidimensional nature of pain and the myriad biopsychosocial elements that influence it.
Diminished functioning (e.g., physical disability and work-related interference) and psychosocial interference (e.g., depressed mood, anxiety, pain-related fear, and limited social support) that often accompany chronic pain play a role in disrupted quality of life in individuals with pain. To date, existing research has primarily focused on risk and vulnerability factors related to the maintenance and exacerbation of pain. For example, negative psychological factors (e.g., negative affective states) have consistently been shown to facilitate pain and disability; depression and anxiety are highly comorbid with chronic pain and can significantly impact the pain experience, leading to greater pain severity, impaired functioning, and reduced quality of life (Bair et al., 2003; Lerman et al., 2015). In fact, evidence suggests that in older adults, depression can uniquely contribute to increased risk of developing disabling back pain (Reid et al., 2003). Similarly, reciprocal relationships between symptoms of anxiety and depression and greater pain interference have been demonstrated in the aging population (Arola et al., 2010).
Additionally, negative pain beliefs (e.g., pain catastrophizing and fear-avoidance) are known to adversely influence pain-related outcomes. Consistent evidence suggests that pain catastrophizing (pain-associated rumination, magnification, and helplessness) leads to enhanced pain and greater affective disturbance (Turner and Clancy, 1986; Sullivan et al., 2001). Likewise, individuals may develop a fear of pain and movement that facilitates avoidance of certain activities following a painful injury, when they view these activities as having the potential to cause re-injury and subsequent pain (Vlaeyen and Crombez, 1999; Vlaeyen and Linton, 2000; Crombez et al., 2012). These fear-avoidance beliefs can increase pain and functional impairment, such as physical deconditioning arising from limited mobility (Rainville et al., 2011; Wertli et al., 2014). Although informative, an emphasis on pathology/vulnerability does not capture the impact of additional contributors on the pain experience, including the potentially protective role of positive, adaptive factors on chronic pain.
While aging has been regarded as a period of loss, this view has been contrasted by mounting evidence that older adults have the capacity for resilience; evidenced by high levels of reported well-being, quality of life, and self-rated successful aging, despite worsening health and substantive physical challenges (i.e., pain) (MacLeod et al., 2016). Although there are competing approaches to the conceptualization and measurement of resilience, it has largely been characterized as a trajectory of positive adaptation in response to significant risk or adversity (Ong et al., 2009). Resilience has also been delineated as a trait-like construct, consisting of personality characteristics and stable psychosocial factors that contribute to adaptive functioning; however, it is argued that this definition lacks precision as it overlooks time-varying and contextually dependent aspects of resilient responding. Further, characterization of resilience as purely dispositional fails to account for the malleability of human functioning or the consideration of how resilience can be promoted through therapeutic intervention. More recent theoretical models have conceptualized resilience as a dynamic process, characterized as an interplay between trait-based resources (e.g., personality factors) and active mechanisms (e.g., cognitive and affective states) that influence adaptive coping responses to pain. This process, in turn, promotes sustainability in meaningful and valued activities, personal growth as a result of one’s experience with chronic pain, and the capacity to recover or rebound from disruptions in physiological, emotional, or cognitive functioning (e.g., pain flare-up) (Sturgeon and Zautra, 2010, 2013).
Abundant literature has identified multiple psychological contributors to resilience. For example, optimism (Ferreira and Sherman, 2007; Goodin and Bulls, 2013; Goodin et al., 2013; Cousins et al., 2014), hope (Berg et al., 2008; Howell et al., 2015; Bartley et al., 2019b), positive affect (PA) (Zautra et al., 2005; Finan and Garland, 2015; Hassett and Finan, 2016), self-efficacy (Wright et al., 2008; Wylde et al., 2012; Brembo et al., 2017; Martinez-Calderon et al., 2018; Karasawa et al., 2019), and pain acceptance (McCracken, 1998; Kratz et al., 2007; Jensen et al., 2016) have been associated with adaptive changes across a number of pain and mental health outcomes. Perceived social support also shows benefits in individuals with chronic pain, which may be particularly relevant for older adults as social engagement provides a means of coping with pain (Molton and Terrill, 2014). In fact, perceptions of support are associated with fewer depressive symptoms (Ferreira and Sherman, 2007; Lopez-Martinez et al., 2008; Lee et al., 2016; McKillop et al., 2017), greater quality of life (Ethgen et al., 2004), lower pain intensity (Lopez-Martinez et al., 2008), and improvements in postsurgical (i.e., lower-limb amputation) functioning (Hanley et al., 2004).
Together with psychological and social functioning, numerous lifestyle and health factors also contribute to resilience. Tobacco usage is associated with a greater incidence and prevalence of pain (Goldberg et al., 2000; Shiri et al., 2010b), while multimorbidity has profound consequences on the occurrence (Schneider et al., 2007) and worsening of pain and physical functioning (Calders and Van Ginckel, 2018). Similarly, sleep has been posited as a key regulator of pain modulation, with effects on somatosensory sensitivity (Campbell et al., 2015; Schrimpf et al., 2015), pain severity (Gerhart et al., 2017), and interference (Kothari et al., 2015). Although sleep and pain are temporally related, sleep quality appears to have a more robust influence on pain symptomatology than vice versa (Finan et al., 2013; Gerhart et al., 2017), and may even serve as a risk factor for pain development and chronification (Gupta et al., 2007; Finan et al., 2013). Likewise, intervening on sleep may have salutary effects on pain, with recent evidence highlighting the influence that treatment-related sleep improvements have on pain intensity (de la Vega et al., 2019). Exercise as a therapeutic modality also confers many health benefits but can be especially potent for pain symptomatology. Increasing evidence suggests that sedentary behavior is inversely associated with functional performance (Lee et al., 2015), with greater physical activity predicting more optimal long-term outcomes in pain and disability (Pinto et al., 2014). Also, acute bouts of exercise yield analgesic effects on pain-evoked laboratory measures (Burrows et al., 2014), yet appear to be differentially influenced by physical activity behavior (e.g., sedentarism and level of physical activity) (Naugle et al., 2017; Ohlman et al., 2018). In turn, sedentary behavior may promote greater adiposity (i.e., body mass index and waist–hip ratio) which can be a risk factor for pain and functional disability (Fanuele et al., 2002; Shiri et al., 2010a; Walsh et al., 2018), presumably through a myriad of pathways such as increased joint loading, biochemical mediators, and mood disturbance (Okifuji and Hare, 2015). The association between pain and obesity is likely reciprocal, however, with chronic pain also potentiating risk for weight gain. Given that obesity is a potentially modifiable factor, some studies have highlighted the efficacy of weight loss interventions in reducing the incidence and severity of pain (Hughes et al., 2018; Dunlevy et al., 2019).
Taken together, there is a wealth of literature supporting the protective effects of psychological, social, and lifestyle factors in the experience of pain. However, much of research has examined these factors in isolation, with limited consideration of their additive contributions. Even more, while existing conceptualizations of resilience have varied widely, it has commonly been defined as a trait-based construct comprised primarily of psychological facets (Windle et al., 2011). Thus, prevailing approaches to the study of resilience may not fully capture the multidimensionality of the construct or how resilient functioning can be promoted through various systems. Extending our current conceptual models may carry important implications in terms of explicating the resources and mechanisms that promote adaptive pain outcomes. Only a modest literature has addressed the notion of multisystem resiliency. For instance, Agrigoroaei and Lachman (2011) found that a protective composite of psychosocial and behavioral factors (i.e., control beliefs, social support quality, and physical activity) predicted cognitive functioning, above and beyond the effects of sociodemographics, physical health, and cognitive activity engagement. Further, the combination of low-risk lifestyle factors (i.e., smoking, physical activity, adiposity, alcohol use, and diet) was more robustly associated with longer leukocyte telomere length (a marker of cellular aging) in women, as compared to the independent effects of each factor (Sun et al., 2012). Similarly, Johnson et al. (2019) found that a psychosocial and behavioral index of resilience [i.e., optimism, PA, negative affect (NA), active coping, perceived stress, social support, tobacco use, and waist–hip ratio] had a stronger association with telomere length in older adults with knee pain, relative to a composite comprised solely of psychological functioning measures. Overall, these findings provide compelling support for an integrative approach to studying resilience and underscore the importance of exploring these contributions in chronic pain.
The current study sought to address this gap in the literature by examining the association of multisystem resiliency with pain and psychological outcomes in a sample of older adults with cLBP. Given the dimensionality of resilience, several psychosocial resources (i.e., PA, hope, positive well-being, optimism, and social support) and health/lifestyle variables (i.e., waist–hip ratio, body mass index, physical health comorbidities, and smoking status) were considered for inclusion. These measures were selected as they represent modifiable factors with strong, empirical support for their impact on pain and health-related processes. Therefore, the primary aims were to: (1) empirically identify domains of resilience based upon psychological, social, and health-related factors and (2) using cluster analysis, explore whether resiliency phenotypes differ across measures of physical function, pain intensity, disability, and psychological functioning. It was hypothesized that: (1) homogenous subgroups would emerge from patterns of psychological, health, and social resiliency and (2) individuals with more resilient phenotypes (i.e., higher in protective resources) would exhibit higher physical function, lower self-reported pain and disability, and greater psychological functioning.
Materials and Methods
Participants and Procedures
This was a cross-sectional study based on a secondary data analysis from the Adaptability and Resilience in Aging Adults (ARIAA) study, a project evaluating the effects of resilience mechanisms on pain modulatory capacity among individuals with cLBP. Sample size estimations were based upon previous pilot data (Bartley et al., 2019b) establishing that 60 participants would provide power of 0.80 at 0.05 (two-tailed) for detecting moderate to large effect sizes between measures of resilience and pain.
Older adults (ages 60+ years) with cLBP (N = 69) were recruited from the community via posted fliers, media announcements, and word-of-mouth referral. All participants provided verbal and written informed consent. Participants were included if they reported at least mild LBP (≥2/10) occurring on at minimum half of the days during the preceding 3 months. Enrollment in the study was not limited to LBP (due to the presence of medical comorbidities in this population) as long as LBP was an individual’s primary pain condition. Exclusion criteria were as follows: recent vertebral fracture; back surgery within the past 6 months; diagnosis of cauda equina syndrome; uncontrolled hypertension (≥150/90); severe cardiovascular disease (e.g., recent heart attack); neurological disease associated with somatosensory abnormalities (e.g., neuropathy, seizures, and Parkinson’s disease); current major medical illness (e.g., metastatic or visceral disease); chronic opioid use; and systemic inflammatory disease (e.g., spondylarthropathies such as ankylosing spondylitis and systemic lupus erythematosus). Participants were provided up to $100 compensation upon completion of the study.
The University of Florida Institutional Review Board approved all study procedures. Initially, participants were evaluated for study inclusion and exclusion through a brief telephone screen. The following sociodemographic and health data were obtained as part of the screening: self-reported sex, age, and a brief health history including the presence of major medical illnesses, recent back-related injuries or surgeries, and LBP symptoms. If eligible, participants attended two, 2–3.5-h appointments scheduled approximately 1 week apart. During Session 1, eligibility criteria were verified through a self-reported demographic and medical history assessment, and participants completed anthropometric tests, psychosocial questionnaires, and functional performance measures. During the time in between Sessions 1 and 2, participants completed several questionnaires at home. Sensory pain testing was conducted during Session 2 (data not reported), and additional psychosocial questionnaires were also completed at that visit.
Measures
Predictors of Multisystem Resilience
Positive and negative affect schedule
The 20-item Positive and Negative Affect Schedule (PANAS) was used to examine PA and NA (Watson et al., 1988). Respondents were presented with 10 positively valenced and 10 negatively valenced terms that are rated on a five-point scale ranging from 1 (very slightly or not at all) to 5 (extremely) resulting in scale scores for PA and NA, with higher scores indicating increased positive and NA, respectively (only PA scores were included in the current analysis). Reliability tests indicated high internal consistency of items on the PA scale (α = 0.90).
Adult dispositional hope scale
The Adult Dispositional Hope Scale (ADHS) is a 12-item questionnaire that includes eight statements measuring two aspects of hope: pathways (e.g., “There are lots of ways around a problem.”) and agency (e.g., “I energetically pursue my goals.”), as well as four “filler” statements that are not included in scoring (Snyder et al., 1991). Items are rated on a scale ranging from 1 (definitely false) to 8 (definitely true) and respondents select the number that best describes them for each statement. Higher scores indicate greater trait levels of hope. Reliability analyses from the current investigation revealed Cronbach’s α for the ADHS = 0.92, indicating high internal consistency for this measure.
PROMIS positive affect and well-being scale
The Patient-Reported Outcomes Measurement Information System (PROMIS) PA and Well-Being Scale was used to measure PA and overall sense of satisfaction with life (Salsman et al., 2013). This scale consists of 23 items rated on a 1 (never) to 5 (always) scale to indicate how often respondents experienced positive emotion and/or purpose/meaning in life (e.g., “[Lately], I had a sense of balance in my life.”). Higher scores reflect greater PA and well-being (Cronbach’s α = 0.97).
Life-orientation test-revised
Dispositional optimism was evaluated using the Life-Orientation Test-Revised (LOT-R), which consists of 10 items (including four unscored items and three reverse-scored items). Participants were asked to use a 5-point scale ranging from 0 (strongly disagree) to 4 (strongly agree) and rate the degree to which they agreed with the presented statements (e.g., “In uncertain times, I usually expect the best.”) (Herzberg et al., 2006). Higher LOT-R scores indicate greater optimism. This measure demonstrated adequate reliability in the sample (α = 0.73).
PROMIS support (emotional, instrumental, informational)
To measure social functioning, the short forms of the PROMIS emotional (eight items; e.g., “I have someone who makes me feel appreciated.”), instrumental (four items; e.g., “Do you have someone to take you to the doctor if you need it?”), and informational (four items; e.g., “I have someone to turn to for suggestions about how to deal with a problem.”) support scales were administered (Hahn et al., 2014). Items are rated on a 1 (Never) to 5 (Always) scale for all three domains, with higher scores indicating greater social support. All three scales were found to have high internal consistency and were also highly reliable with each other: emotional (α = 0.97), instrumental (α = 0.96), informational (α = 0.96), all support measures combined (α = 0.97).
Anthropometric tests: body composition
During Session 1, participants’ waist (5 cm above the navel) and hip circumferences (widest part of the hips) were calculated (in cm) using a measuring tape, with waist–hip ratio determined by dividing the waist circumference by the hip circumference. Body weight was measured to the nearest 0.1 kg using a digital scale (Healthometer) and height was assessed to the nearest centimeter using a wall stadiometer. Calculation of BMI was determined by weight in kilograms divided by height in meters squared.
Health comorbidities
To determine the presence of physical health comorbidities, participants completed a health status questionnaire whereby they were asked to place an “X” next to any current medical conditions (i.e., high blood pressure, heart disease, diabetes, asthma/breathing problems, kidney/renal disease, thyroid problem, neurological disorder, or other self-reported health conditions). Medical diagnoses were placed into ICD-10 diagnostic categories for reporting purposes.
Smoking status
Current cigarette smoking status was assessed using the following question: “How would you describe your cigarette smoking?” Possible responses included: “never smoked,” “used to smoke but have now quit,” and “current smoker,” and individuals were categorized as either current smokers (yes) or non-smokers (no).
Study Outcomes
Back performance scale
Functional performance and movement-evoked pain (MEP) were measured using the Back Performance Scale (BPS). The BPS consists of a series of tasks (i.e., Sock Test, Pick-up Test, Roll-up Test, Fingertip-to-Floor Test, and Lift Test) that are designed to measure functional capacity during completion of mobility-oriented activities that have been deemed to be particularly difficult for individuals with back pain (Magnussen et al., 2004; Strand, 2017). An evaluator assesses the degree to which these tasks are completed. Physical functioning scores range from 0 to 3 for each test (total scale score = 0–15), with increasing scores indicating greater difficulty with task performance. MEP was measured by asking participants to rate their current LBP from 0 (no pain) to 100 (most intense pain imaginable) immediately after completion of each of the five tasks on the BPS. MEP was determined from an average of the five pain ratings. Internal consistency was good for this measure (α = 0.83).
PROMIS physical function
To evaluate the general physical functioning, the short form of the PROMIS Physical Function measure was administered (Rose et al., 2008, 2014). This scale includes four questions (e.g., “Are you able to do chores such as vacuuming or yard work?”) to examine the difficulty with which an individual is able to complete certain functional tasks. Ratings are made from 5 (without any difficulty) to 1 (unable to do) and lower scores indicate greater difficulty with task performance. This measure demonstrated high reliability among the sample (α = 0.85).
PROMIS pain intensity
The three-item PROMIS Pain Intensity short form measure was used to evaluate pain intensity over the past week (Cella et al., 2010). This scale asks respondents to report their average and worst pain during the past 7 days, as well as pain at the time of questionnaire completion by providing a 1 (no pain) to 5 (very severe) pain rating. The PROMIS Pain Intensity scale demonstrated good reliability (α = 0.81).
Roland-morris disability questionnaire
The Roland-Morris Disability Questionnaire (RMDQ) is a self-report measure that assesses health status and disability related to LBP (Roland and Morris, 1983). The RMDQ is comprised of 24 statements such as “I stay at home most of the time because of my back” and “I only walk short distances because of my back.” Respondents are instructed to indicate which of the statements describe their current experience. The number of endorsed items is summed to obtain a total score (more items endorsed = greater disability). Internal consistency was high for this measure (α = 0.87).
PROMIS depression scale
The eight-item short form of the PROMIS Depression Scale was used to assess depressive symptoms (e.g., “I felt worthless.”) (Pilkonis et al., 2011). Respondents rate the frequency of their experience of each symptom in the past 7 days from 1 (never) to 5 (always), with higher scores indicating a greater presence of depressive symptoms. The PROMIS Depression Scale demonstrated high reliability (α = 0.93).
Brief resilience scale
Trait resilience was examined using the Brief Resilience Scale (BRS), which is a six-item measure examining the ability to bounce back and recover from stressful events and challenges (e.g., “I tend to bounce back quickly after hard times.”) (Smith et al., 2008). Responses are provided using a five-point scale (1 = strongly disagree, 5 = strongly agree), with total scores ranging from 6 to 30. Higher scores on the BRS indicate greater resilience (Cronbach’s α = 0.84).
World health organization quality of life-brief
The World Health Organization Quality of Life-Brief (WHOQOL-BREF) is a 26-item questionnaire designed to measure quality of life across four domains over the past 2 weeks: physical health, psychological health, social relationships, and environment (Skevington et al., 2004). The first item of the WHOQOL-BREF (i.e., “How would you rate your quality of life?”) was used to examine overall quality of life. This item is rated from 1 (very poor) to 5 (very good).
Statistical Analysis
All analyses were conducted using SPSS 24 and significance level was set at p ≤ 0.05 (two-tailed). Means, standard deviations, and counts for demographic characteristics were calculated using descriptive statistics. Zero-order correlations were conducted between sociodemographic characteristics and outcome variables (i.e., physical function, MEP, pain intensity, back-related disability, depressive symptoms, general resilience, and quality of life). Demographic variables that were significantly related to outcome variables were controlled for in cluster analyses. The following 11 variables were entered into a PCA to characterize the dimensionality of each resilience measure: PA, dispositional hope, positive well-being, optimism, waist–hip ratio, body mass index, physical health comorbidities, smoking status, emotional support, instrumental support, and informational support. PCA with oblique rotation was used to allow for correlation between factors, with the recommendation that at least three items load on a factor and a difference of ≥0.20 was present between cross-loadings (Howard, 2016). Components with eigenvalues >1 were selected for further analysis and the scree plot was inspected to confirm the number of factors to be retained. Hierarchical cluster analysis employing Ward’s clustering method with squared Euclidean distances as the similarity measures was conducted to identify subgroups of individuals that differed across empirically derived resilience domains. Agglomeration coefficients were examined to identify the cluster solution that best represented the data, with the optimal number being chosen based upon the point at which the percentage change was the largest between the clusters (Milligan and Cooper, 1985). Chi-square analysis for categorical variables or analysis of variance (ANOVA) for continuous variables was employed to examine cluster group differences across demographic composition. Differences across physical function, pain, and psychological outcomes were assessed using multivariate ANOVA’s, controlling for the effects of relevant sociodemographic characteristics. Significant findings on multivariate analyses were followed by Sidak-corrected post hoc comparisons. To obtain effect size estimates associated with F-tests, partial eta-squared () was calculated (small = 0.01, medium = 0.06, and large = 0.14).
Results
Participant Characteristics
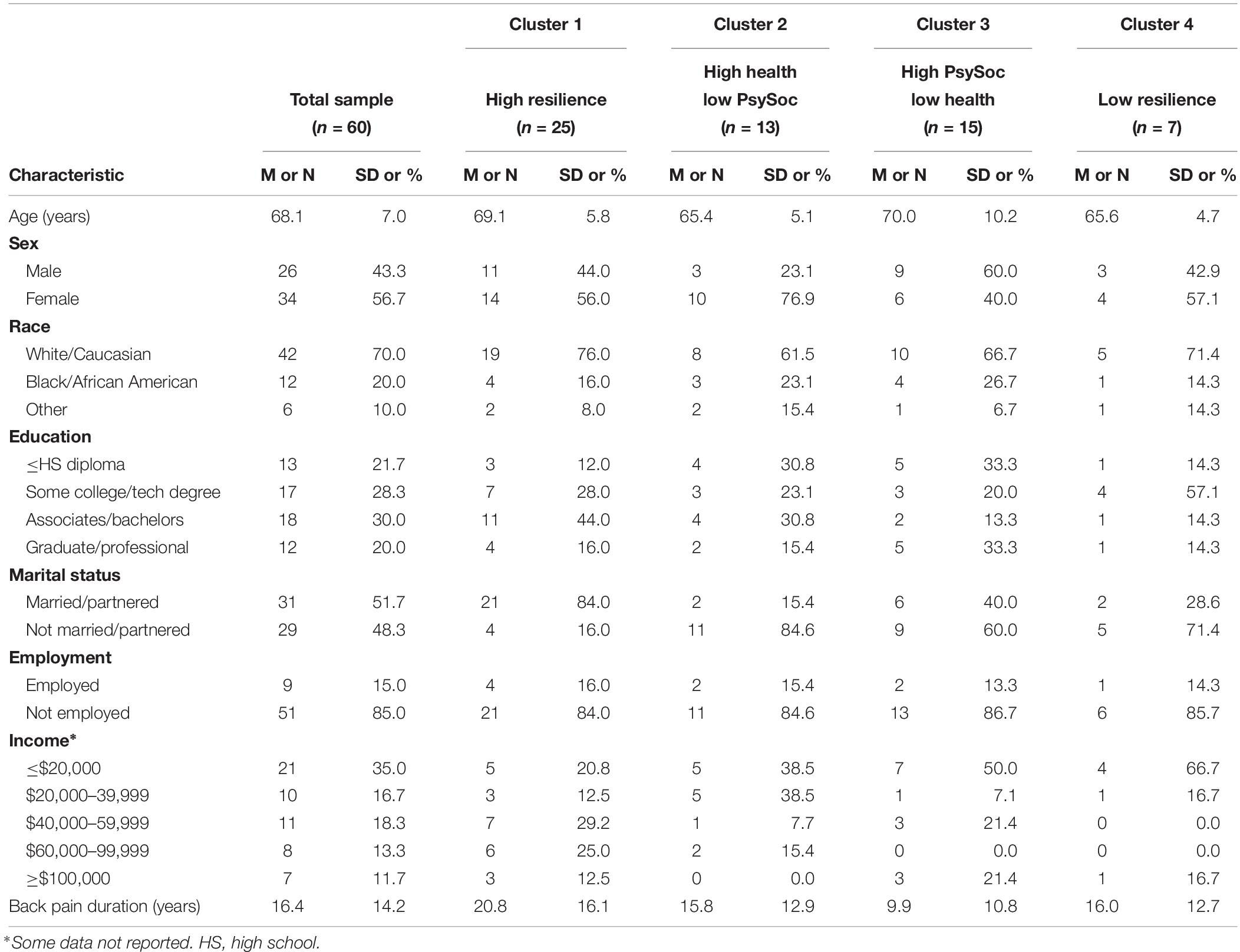
Demographic characteristics (means and SDs) are reported in Table 1. Participants were mostly female (57%), White/Caucasian (70%), had a college degree (50%), were married or partnered (52%), and were not employed (85%). Average age was 68 years (range: 60–93 years), duration of back pain was 16.4 years (range: 1–56 years), and participants reported back pain of moderate intensity during the initial session (M = 5.5, range = 2–10). Two of the 69 participants discontinued after the first session due to time constraints, and 7 participants who were initially eligible were excluded during their first appointment (n = 1 use of exclusion medications, n = 3 exclusionary medical condition, n = 3 not meeting pain duration criteria), thus leaving 60 participants. Based on ICD-10 classifications (World Health Organization, 2016), the following medical comorbidities/diseases were reported: circulatory and respiratory (n = 27, 45.0%), metabolic and endocrine (n = 14, 23.3%), genitourinary and renal (n = 4, 6.7%), digestive (n = 3, 5.0%), skin/subcutaneous tissue (n = 3, 5.0%), eye (n = 3, 5.0%), musculoskeletal (n = 1, 1.6%), nervous system (n = 1, 1.6%), infectious disease (n = 3, 5.0%), and sleep disorders (n = 2, 3.3%). Current smoking was reported among 16.7% (n = 10) of the sample.
Zero-Order Correlations
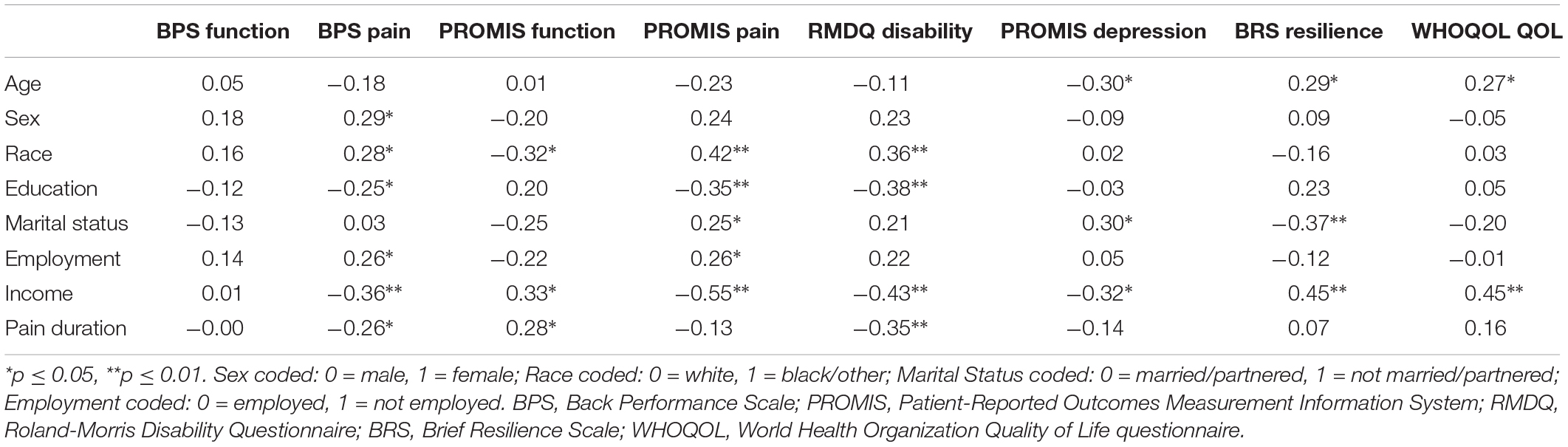
To identify potential study covariates, zero-order correlations were analyzed across sociodemographic variables and study outcomes (Table 2). In general, age, sex, race, education, marital status, employment, income, and back pain duration were associated with physical function, pain, and psychological outcomes (all ps < 0.04). Hence, analyses assessing cluster group differences across study outcomes included these sociodemographic variables as statistical covariates.
Principal Components Analysis
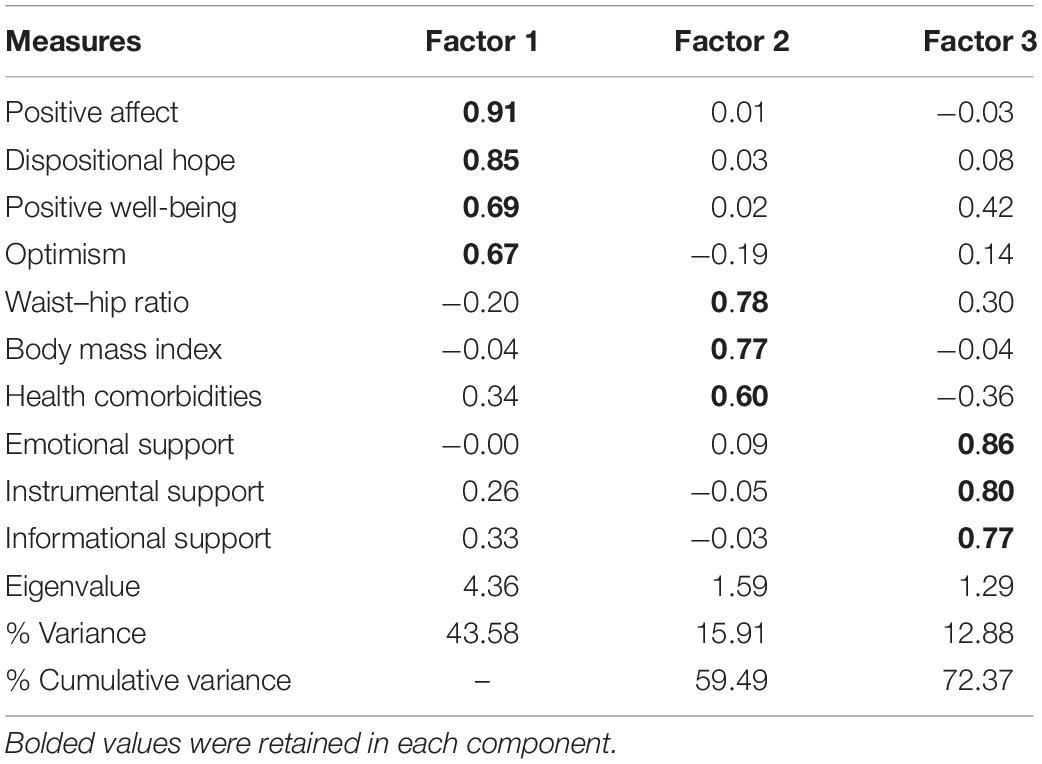
A PCA was conducted with all 11 items using oblique rotation (direct oblimin), resulting in a four-factor solution. However, on the basis of our item selection criteria (i.e., more than or equal to three items load on a factor), this solution was eliminated as it returned one component containing smoking status. This variable was therefore removed from the model. The resulting analysis revealed the presence of a three-factor solution with eigenvalues over Kaiser’s criterion of 1, accounting for 72.4% of the variance in scores. The Kaiser–Meyer–Olkin measure verified the sampling adequacy for the analysis (Kaiser–Meyer–Olkin = 0.78; Bartlett’s test of sphericity = <0.001) and all KMO values were above the acceptable limit of 0.50 (Field, 2013). Inspection of the scree plot confirmed inflexions that would justify retaining three factors. Table 3 reports the factor loadings after rotation, with Component 1 representing positive, psychological factors (factor loadings 0.67–0.91), Component 2 denoting health-related functioning (factor loadings 0.60–0.78), and Component 3 reflecting social support (factor loadings 0.77–0.86). The factor loadings from each domain were used in subsequent cluster analysis.
Cluster Analysis Across Resilience Domains
The three composite domains were subjected to Cluster Analysis to identify empirically derived classifications based upon profiles of psychological, health, and social resiliency (Figure 1 and Supplementary Table 1). For ease of interpretation, the health domain was reverse scored, such that lower scores reflected higher waist–hip ratio, body mass index, and health comorbidities. Four clusters were revealed and characterized by the following: (1) Cluster 1: High Resilience group (n = 25, 41.7%): high levels of psychological, health, and social support functioning; (2) Cluster 2: High Health/Low Psychosocial group (n = 13, 21.7%): optimal health-related functioning and low levels of psychosocial function; (3) Cluster 3: High Psychosocial/Low Health group (n = 15, 25.0%): poor health functioning, high psychological functioning, and moderate-to-high social support; and (4) Cluster 4: Low Resilience group (n = 7, 11.7%): low levels of functioning across psychological, social, and health-related factors. There were no sociodemographic differences across cluster groups, with the exception of the High Resilience group (Cluster 1) having the highest proportion of participants who were married or partnered (Table 1); thereby, consistent with previous research (Mun et al., 2019).
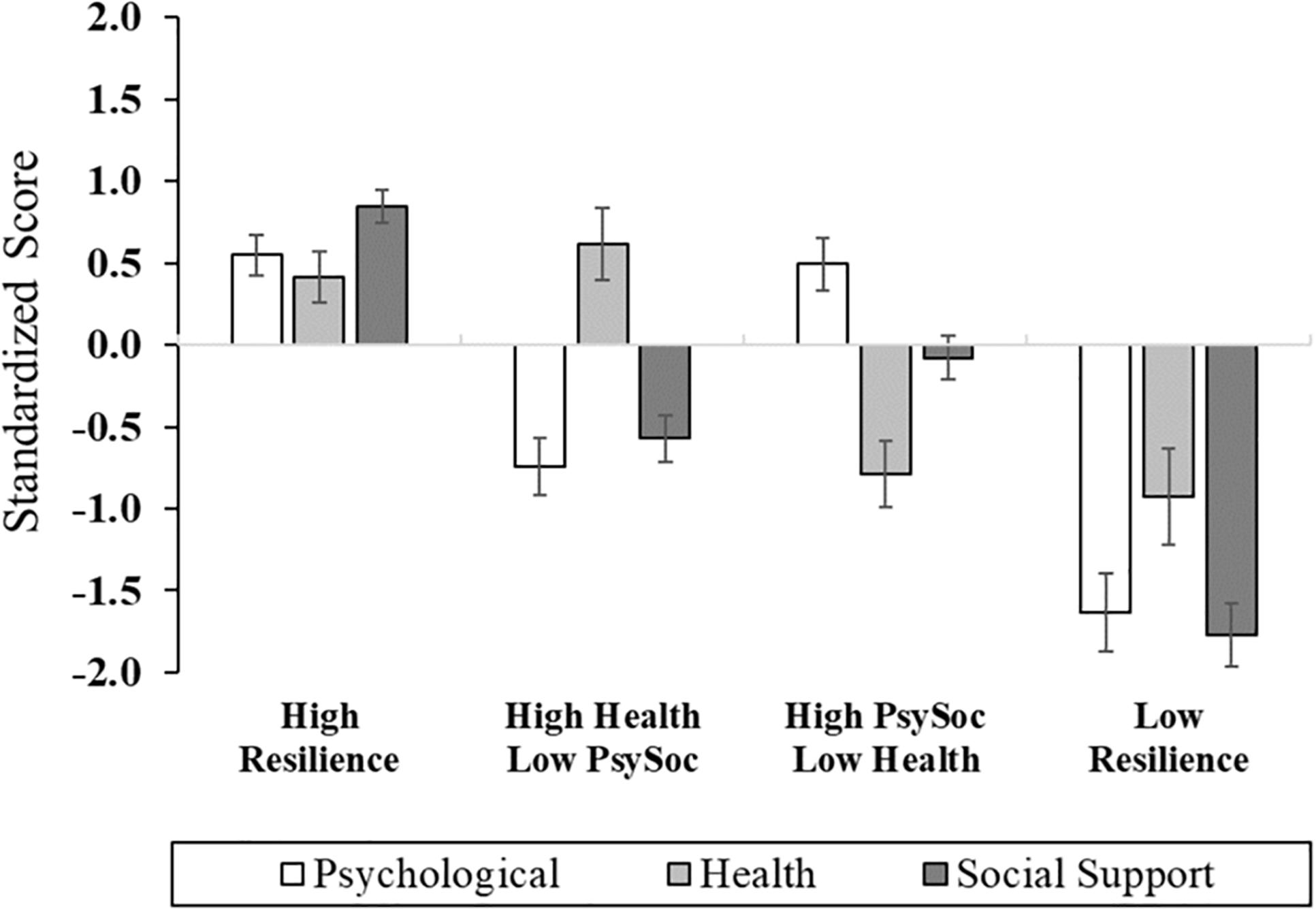
Figure 1. Cluster group differences across multisystem resilience domains comprised of psychological, health, and social functioning.
Psychosocial Profiles Across Cluster Group
After adjusting for age, sex, race, education, marital status, employment, income, and back pain duration, significant differences across cluster membership emerged in functional performance, physical function, back-related functional disability, depression, general resilience, and quality of life (Figures 2, 3 and Supplementary Table 2). In particular, functional performance and functional disability due to LBP were poorest among the Low Resilience group, relative to individuals in the High Resilience and High Health/Low Psychosocial cluster groups (ps < 0.05). Post hoc comparisons were non-significant across cluster groups for self-reported physical function, although the difference between the High Health/Low Psychosocial and Low Resilience groups approached significance with a large effect size ( = 0.22). For psychological outcomes, individuals in the Low Resilience group reported the highest levels of depression (ps ≤ 0.001) and lowest quality of life (ps ≤ 0.04), relative to all other groups. Depression was also lower in the High Resilience group (p = 0.05), when compared to individuals with low psychosocial resources (Cluster 2). The High Resilience (p = 0.04) and High Psychosocial/Low Health (p = 0.02) clusters had greater general resilience than the High Health/Low Psychosocial group. In addition, while the Low Resilience group reported statistically lower levels of general resilience relative to the High Psychosocial/Low Health group (p = 0.04), these effects only approached significance (p = 0.07) when compared with the High Resilience group. No differences in MEP (p = 0.08) or self-reported pain intensity (p = 0.33) were detected across cluster groups.
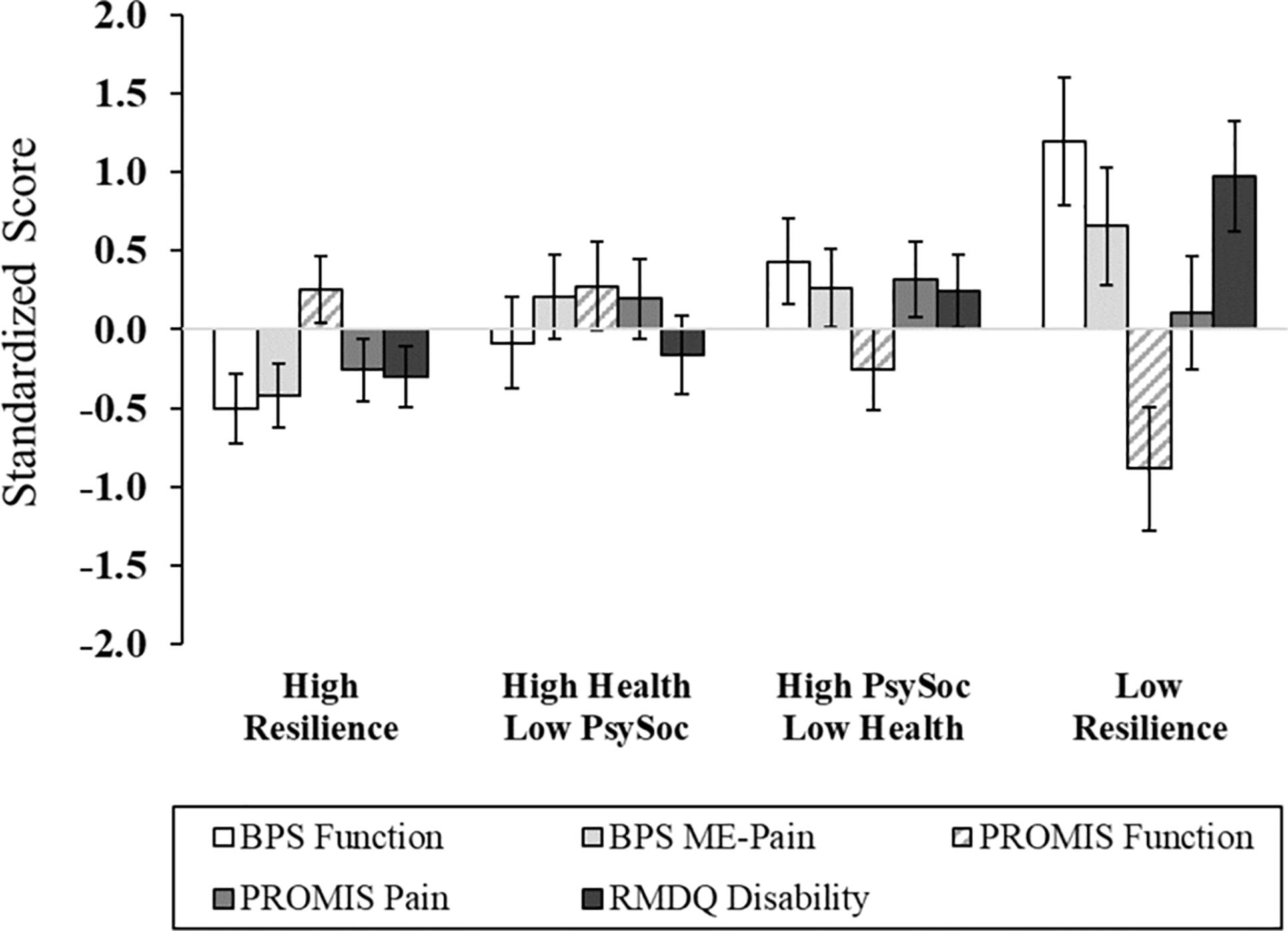
Figure 2. Pain and physical functioning outcomes across multisystem resilience profiles. Relative to Cluster 4 (Low Resilience group), individuals with a greater degree of protective resources had higher functional performance and self-reported physical function, as well as lower disability. There were no group differences in movement-evoked pain or pain intensity. Higher scores on PROMIS function, better physical functioning; BPS, Back Performance Scale; ME, Movement-Evoked; PROMIS, Patient-Reported Outcomes Measurement Information System; RMDQ, Roland-Morris Disability Questionnaire.
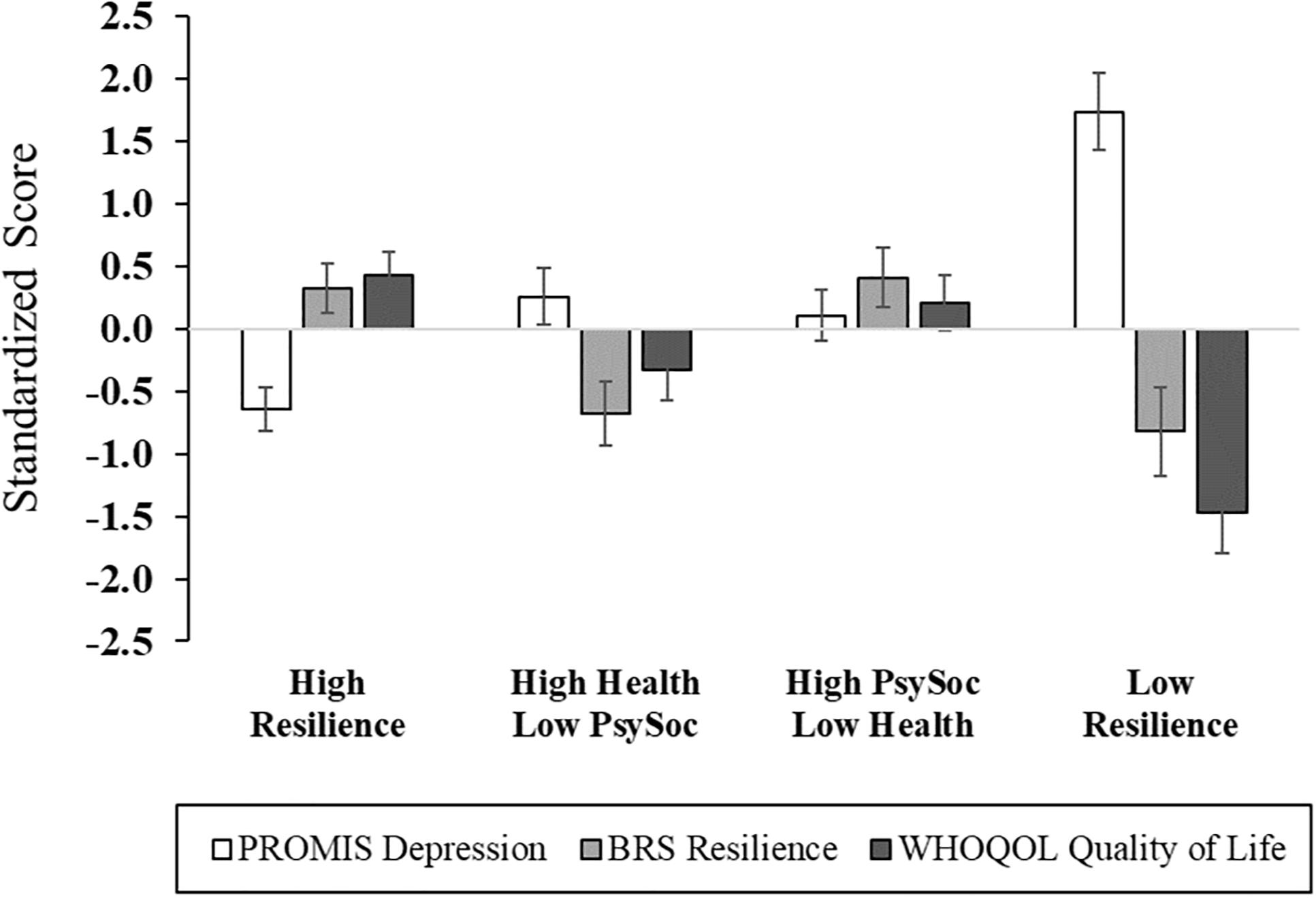
Figure 3. Psychological functioning across multisystem resilience profiles. Compared to Cluster 4 (Low Resilience group), individuals with more resilient phenotypes exhibited lower depressive symptoms, and higher general resilience and quality of life. PROMIS, Patient-Reported Outcomes Measurement Information System; BRS, Brief Resilience Scale; WHOQOL, World Health Organization Quality of Life questionnaire.
Discussion
Although risk factors have been extensively studied in respect to pain, there is a burgeoning literature supporting the role of resilience mechanisms in promoting adaptive pain outcomes. Mounting evidence signifies that a multitude of psychological, social, and physical/biological factors confer resilience; however, much of the extant literature has focused on psychological resources. Moreover, resilience factors have predominantly been examined in isolation, thus overlooking their potentially synergistic and additive effects. Due to the exponential growth of older adults and global burden of chronic pain in this population, explicating the mechanisms that protect against pain and disability is of critical importance. While a modest literature has considered the cumulative effects of a broad range of personal resources (Agrigoroaei and Lachman, 2011; Sun et al., 2012; Johnson et al., 2019), we have extended previous research by capturing the multifaceted nature of resilience and exploring its influence on pain outcomes among older adults with cLBP.
Aligning with study hypotheses, we found evidence of phenotypic patterns of resilience based upon psychological, social, and health-related functioning. In particular, individuals with a higher array of protective factors exhibited more optimal outcomes in physical function, disability, and psychological processes (despite similar levels of pain), suggesting potentially important benefits of multiple adaptive resources. Overall, these findings signify that individuals with a more resilient phenotype may have a greater sense of coherence that allows them to mobilize resources to successfully navigate the ongoing challenges associated with pain. This would align with Antonovsky’s salutogenic model of health (Antonovsky, 1996) which highlights the importance of coping strengths in fostering one’s capacity for optimal health and well-being.
While a number of studies have classified patients according to negative psychological and lifestyle variables (Rabey et al., 2016; Almeida et al., 2018), limited research has stratified subgroups according to sources of resilience. In fibromyalgia, Mun et al. (2019) found that individuals with a higher degree of personal resources (i.e., pain acceptance, resilience, social support, sleep quality) exhibited lower levels of morning pain and depressive symptoms, as well as afternoon pain interference (although effects varied according to level of depression). Similar findings were also observed among patients with chronic neurological/neuromuscular disease, as those with a more resilient profile reported lower interference from pain (Mun et al., 2019). Furthermore, our findings echo a previous study in knee osteoarthritis (Cruz-Almeida et al., 2013), whereby a subgroup characterized by high optimism and low NA exhibited the lowest degree of pain, disability, and somatosensory sensitivity.
Results also suggest that health and psychosocial factors are differentially expressed across older adults with LBP. In particular, when compared to individuals with low resilience (Cluster 4), those with a higher degree of protective resources exhibited lower depression and higher quality of life. However, the findings for general resilience were more robust among individuals with higher social support and positive, psychological function (Cluster 3), thus underscoring the protective nature of psychosocial resources in coping with stress and adversity. Likewise, more favorable outcomes in functional performance and disability were not only observed among individuals with higher overall resilience, but also among those with more adaptive health-related function. This is not entirely surprising as higher disease burden and adiposity may facilitate decrements in functional capacity through mechanisms linked to frailty, psychological comorbidities, physiological dysregulation, increased joint loading, cardiopulmonary reserve, and activity restriction, among others (Fried et al., 2004; Kalyani et al., 2010; Calders and Van Ginckel, 2018). What is more, the influence of these health factors is likely not independent; rather, their effects are interactive and systemic, impacting multiple homeostatic processes to potentiate downstream effects on disability and function (Chapman et al., 2008).
Importantly, our findings have important clinical value as the protective resources we examined are modifiable. For instance, a greater emphasis on enhancing social support and positive, psychological processes may be particularly advantageous for improving adaptive coping, and to some extent, attenuating depressive symptoms in Cluster 2 individuals. Interventions with empirical support for their efficacy (e.g., cognitive-behavioral therapy and spouse-assisted training) (Edwards et al., 2016) are likely to derive some benefit; however, therapies focusing on harnessing resilience through positive, psychological resources [e.g., positive activity interventions (PAIs)] have also shown promise in chronic pain populations (Hausmann et al., 2014, 2017; Muller et al., 2016; Peters et al., 2017). For individuals with poorer health-related functioning (Cluster 3), minimizing the severity of multimorbidity and reducing weight burden through diet and exercise promotion may mitigate functional decline and disability. Ultimately, the development of strategies for the prevention of obesity and medical comorbidities is a critical directive. Results also suggest that individuals with a low resilient profile would likely benefit from a multimodal approach that optimizes both psychosocial and health-related resources. In particular, combining psychotherapy with lifestyle modification may yield protective benefits in physical and emotional functioning. For individuals in Cluster 1 who appear to be adapting well despite the presence of cLBP, these treatments may be less justified. Surprisingly, while indices of pain intensity (i.e., PROMIS pain intensity, MEP) were lower among individuals with a greater degree of resources (Cluster 1), these effects failed to reach significance across cluster groups. Although it is conceivable that other unmeasured resources may have a more robust influence on pain severity, it is also possible our study was underpowered to detect pain-specific effects. On the basis of the effect sizes observed ( = 0.07 to 0.14), a power analysis revealed that a sample size of 72 to 150 participants would be adequate to detect significant effects in MEP and self-reported pain intensity, respectively. Thus, consideration of these findings in a larger sample is warranted.
Multiple resources shape the expression and development of resilience in chronic pain. While the measurement of resilience is inherently complex (Southwick et al., 2014) due to varying definitions and multiple methods by which to assess this construct, our current models lack precision and fail to account for the multifaceted nature of resilience. Indeed, recent theoretical literature (Liu et al., 2017) posits that resilience should be conceptualized from various levels of analysis that includes intraindividual (e.g., physiological, health behaviors), interpersonal (e.g., personality correlates, coping appraisals), and socio-ecological factors (e.g., socioeconomic status, group membership). While exploring the independent determinants that buffer against negative pain sequelae has clinical utility, recognition of resilience from a multidimensional perspective will likely provide a greater understanding of adaptive capacity. Expanding our current models of resilience and considering new approaches, both theoretically and statistically, in how resilience is conceptualized and assessed in the context of pain will be an important future direction.
Strengths and Limitations
Several strengths of our study merit acknowledgment. To our knowledge, the current investigation is the first to examine resilience from a multidimensional perspective in older adults with cLBP. Participants were phenotyped according to several adaptive resources, offering a novel opportunity to explore how pain, disability, and psychological functioning differ across resiliency profiles. We used an empirical approach to characterize our resilience indices, which provides a statistical, data-driven, and robust method for classification of subgroups. A number of valid and reliable measures were also utilized across the assessment of psychological, social, and health-related functioning. Further, despite the small sample (N = 60), large effect sizes (ranging from = 0.17 to 0.45) were observed for pain and psychological outcomes.
In spite of these strengths, a few limitations are worth noting in the interpretation of results. First, given the nature of cluster analysis our findings should be considered exploratory. In addition, our relatively modest sample may have influenced our classification of individuals using cluster analysis, with few individuals categorized into particular profiles (e.g., only seven participants comprised the “Low Resilience” cluster). This may have impacted the external validity of the study, thereby compromising generalizability. Future studies with larger sample sizes are warranted to confirm these findings. In light of these limitations, results should be interpreted cautiously. Although we employed a robust, empirical approach to devise our resilience domains, smoking status was eliminated from the analyses due to its retention as an independent factor. Despite smoking being a relevant lifestyle behavior with tremendous health consequences, we have confidence that the omission of this variable did not alter our findings, as it only contributed a small amount of additional variance (4%) to our model. Furthermore, analyses revealed that smoking status did not differ across cluster groups (p = 0.18). Nevertheless, there are strengths and challenges to various statistical methods (e.g., factor analysis, z-scores, and median split approach) and future studies should consider the comparison of these approaches, as well as their clinical relevance. Of note, some cross-loadings (>0.30) were observed across psychological, health, and social support factors. Although this may be a limitation, this phenomenon is also anticipated given the natural correlation among these constructs (e.g., health-related constructs correlate with many other variables). Also, medical comorbidities were determined via participant self-report, which may not provide a complete representation of individuals’ health histories. Medical records should be obtained for verification of health history in future endeavors. Related, because we excluded individuals experiencing major medical conditions, these results may not generalize to those who experience more severe health comorbidities.
Although multiple psychosocial and health constructs were used to derive subgroups, this did not reflect an exhaustive list of protective resources. Indeed, the small sample limited our ability to examine several factors, including relevant physiological/biological markers (e.g., inflammatory cytokines) (Khan et al., 2017), or demographic characteristics such as sex and race/ethnicity. Moreover, there is a need to replicate these findings in a more diverse sample, especially given growing evidence of the impact of race/ethnicity on resilience and pain-related outcomes (Bartley et al., 2019a). Inclusion of these and other diversity variables will be important considerations for future studies. Employing additional measures of health/lifestyle factors (e.g., physical activity, sleep, diet, alcohol, and drug consumption), as well as other positive psychological (e.g., self-efficacy and sense of coherence) and social support-related indices (e.g., quantity versus quality of support) will be key to improving our understanding of resilience. Likewise, given the importance of external determinants of resilience, several other contextual and social/environmental factors, including socioeconomic status and access to healthcare, are critical areas to examine (Liu et al., 2017). And finally, it may be beneficial to use a dual-focus approach to closely examine both risk and resilience factors and how this interplay influences multisystem resiliency in respect to pain (perhaps even considering the “degree” of negative factors, such as the impact of lower NA, less catastrophic thinking, etc.). For example, personal resources (such as optimism or PA) may broaden an individual’s coping repertoire by facilitating engagement in adaptive behaviors that mitigate the narrowing effects of pain catastrophizing (Fredrickson, 2001). This would align with predominant risk-resilience models that highlight the consideration of both vulnerability and protective mechanisms in understanding individual adaptation to pain (Sturgeon and Zautra, 2013; Goubert and Trompetter, 2017).
Conclusion
In sum, our findings support the contribution of protective factors in the context of pain and suggest that examining resilience from a multisystem perspective may have significant clinical utility. Importantly, homogenous subgroups emerged from psychological, social, and health-related processes, with lower disability, better functional performance, and higher psychological functioning observed among individuals with a more resilient phenotype. Consideration of the multiple resources that harness resilience, including their additive effects, may improve our understanding of adaptive function among older adults with chronic pain and ultimately facilitate the development of more targeted clinical care.
Data Availability
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the University of Florida Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
EB and SP contributed to the conception and design of the study, and wrote the first draft of the manuscript. EB performed the statistical analysis. All authors reviewed the text critically and approved the submitted version.
Funding
This work was supported by the National Institutes of Health (NIH)/the National Institute on Aging (NIA) grant (K99AG052642) awarded to EB and the NIH/NIA grant (T32AG049673) provided to the University of Florida (SP). Dr. EB is currently funded by the NIH/NIA grant (R00AG052642).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Ralisa Pop, Stephanie Hersman, Morgan Ingram, Jordan McGee, Kylie Broskus, Paige McKenzie, and Michelle Jacomino for their assistance with data collection.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyg.2019.01932/full#supplementary-material
References
Agrigoroaei, S., and Lachman, M. E. (2011). Cognitive functioning in midlife and old age: combined effects of psychosocial and behavioral factors. J. Gerontol. B Psychol. Sci. Soc. Sci. 66(Suppl. 1), i130–i140. doi: 10.1093/geronb/gbr017
Almeida, S. C., George, S. Z., Leite, R. D. V., Oliveira, A. S., and Chaves, T. C. (2018). Cluster subgroups based on overall pressure pain sensitivity and psychosocial factors in chronic musculoskeletal pain: differences in clinical outcomes. Physiother. Theory Pract. doi: 10.1080/09593985.2018.1474512 [Epub ahead of print].
Antonovsky, A. (1996). The salutogenic model as a theory to guide health promotion. Health Promot. Int. 11, 11–18. doi: 10.1093/heapro/11.1.11
Arola, H. M., Nicholls, E., Mallen, C., and Thomas, E. (2010). Self-reported pain interference and symptoms of anxiety and depression in community-dwelling older adults: can a temporal relationship be determined? Eur. J. Pain 14, 966–971. doi: 10.1016/j.ejpain.2010.02.012
Bair, M. J., Robinson, R. L., Katon, W., and Kroenke, K. (2003). Depression and pain comorbidity: a literature review. Arch. Intern. Med. 163, 2433–2445. doi: 10.1001/archinte.163.20.2433
Bartley, E. J., Hossain, N. I., Gravlee, C. C., Sibille, K. T., Terry, E. L., Vaughn, I. A., et al. (2019a). Race/ethnicity moderates the association between psychosocial resilience and movement-evoked pain in knee osteoarthritis. ACR Open Rheumatol. 1, 16–25. doi: 10.1002/acr2.1002
Bartley, E. J., LaGattuta, N. R., Robinson, M. E., and Fillingim, R. B. (2019b). Optimizing resilience in orofacial pain: a randomized controlled pilot study on hope. Pain Rep. 4:e726. doi: 10.1097/PR9.0000000000000726
Berg, C. J., Snyder, C. R., and Hamilton, N. (2008). The effectiveness of a hope intervention in coping with cold pressor pain. J. Health Psychol. 13, 804–809. doi: 10.1177/1359105308093864
Brembo, E. A., Kapstad, H., Van Dulmen, S., and Eide, H. (2017). Role of self-efficacy and social support in short-term recovery after total hip replacement: a prospective cohort study. Health Qual Life Outcomes 15:68. doi: 10.1186/s12955-017-0649-1
Burrows, N. J., Booth, J., Sturnieks, D. L., and Barry, B. K. (2014). Acute resistance exercise and pressure pain sensitivity in knee osteoarthritis: a randomised crossover trial. Osteoarthr. Cartil. 22, 407–414. doi: 10.1016/j.joca.2013.12.023
Calders, P., and Van Ginckel, A. (2018). Presence of comorbidities and prognosis of clinical symptoms in knee and/or hip osteoarthritis: a systematic review and meta-analysis. Semin. Arthritis Rheum. 47, 805–813. doi: 10.1016/j.semarthrit.2017.10.016
Campbell, C. M., Buenaver, L. F., Finan, P., Bounds, S. C., Redding, M., McCauley, L., et al. (2015). Sleep, pain catastrophizing, and central sensitization in knee osteoarthritis patients with and without insomnia. Arthritis Care Res. 67, 1387–1396. doi: 10.1002/acr.22609
Cella, D., Riley, W., Stone, A., Rothrock, N., Reeve, B., Yount, S., et al. (2010). The patient-reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J. Clin. Epidemiol. 63, 1179–1194. doi: 10.1016/j.jclinepi.2010.04.011
Chapman, C. R., Tuckett, R. P., and Song, C. W. (2008). Pain and stress in a systems perspective: reciprocal neural, endocrine, and immune interactions. J. Pain 9, 122–145. doi: 10.1016/j.jpain.2007.09.006
Cousins, L. A., Cohen, L. L., and Venable, C. (2014). Risk and resilience in pediatric chronic pain: exploring the protective role of optimism. J. Pediatr. Psychol. 40, 934–942. doi: 10.1093/jpepsy/jsu094
Crombez, G., Eccleston, C., Van Damme, S., Vlaeyen, J. W., and Karoly, P. (2012). Fear-avoidance model of chronic pain: the next generation. Clin. J. Pain 28, 475–483. doi: 10.1097/AJP.0b013e3182385392
Cruz-Almeida, Y., King, C. D., Goodin, B. R., Sibille, K. T., Glover, T. L., Riley, J. L., et al. (2013). Psychological profiles and pain characteristics of older adults with knee osteoarthritis. Arthritis Care Res. 65, 1786–1794. doi: 10.1002/acr.22070
de la Vega, R., Racine, M., Castarlenas, E., Sole, E., Roy, R., Jensen, M. P., et al. (2019). The role of sleep quality and fatigue on the benefits of an interdisciplinary treatment for adults with chronic pain. Pain Pract. 19, 354–362. doi: 10.1111/papr.12746
Dunlevy, C., MacLellan, G. A., O’Malley, E., Blake, C., Breen, C., Gaynor, K., et al. (2019). Does changing weight change pain? Retrospective data analysis from a national multidisciplinary weight management service. Eur. J. Pain doi: 10.1002/ejp.1397 [Epub ahead of print].
Edwards, R. R., Dworkin, R. H., Sullivan, M. D., Turk, D. C., and Wasan, A. D. (2016). The role of psychosocial processes in the development and maintenance of chronic pain. J. Pain 17 9(Suppl.), T70–T92. doi: 10.1016/j.jpain.2016.01.001
Ethgen, O., Vanparijs, P., Delhalle, S., Rosant, S., Bruyere, O., and Reginster, J. Y. (2004). Social support and health-related quality of life in hip and knee osteoarthritis. Qual. Life Res. 13, 321–330. doi: 10.1023/B:QURE.0000018492.40262.d1
Fanuele, J. C., Abdu, W. A., Hanscom, B., and Weinstein, J. N. (2002). Association between obesity and functional status in patients with spine disease. Spine 27, 306–312. doi: 10.1097/00007632-200202010-00021
Ferreira, V. M., and Sherman, A. M. (2007). The relationship of optimism, pain and social support to well-being in older adults with osteoarthritis. Aging Ment. Health 11, 89–98. doi: 10.1080/13607860600736166
Field, A. (2013). Discovering Statistics Using IBM SPSS Statistics, 4th Edn. Thousand Oaks, CA: SAGE Publications Ltd.
Finan, P. H., and Garland, E. L. (2015). The role of positive affect in pain and its treatment. Clin. J. Pain 31, 177–187. doi: 10.1097/ajp.0000000000000092
Finan, P. H., Goodin, B. R., and Smith, M. T. (2013). The association of sleep and pain: an update and a path forward. J. Pain 14, 1539–1552. doi: 10.1016/j.jpain.2013.08.007
Fredrickson, B. L. (2001). The role of positive emotions in positive psychology. The broaden-and-build theory of positive emotions. Am. Psychol. 56, 218–226. doi: 10.1037/0003-066X.56.3.218
Fried, L. P., Ferrucci, L., Darer, J., Williamson, J. D., and Anderson, G. (2004). Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J. Gerontol. A Biol. Sci. Med. Sci. 59, 255–263. doi: 10.1093/gerona/59.3.m255
Gerhart, J. I, Burns, J. W., Post, K. M., Smith, D. A., Porter, L. S., Burgess, H. J., et al. (2017). Relationships between sleep quality and pain-related factors for people with chronic low back pain: tests of reciprocal and time of day effects. Ann. Behav. Med. 51, 365–375. doi: 10.1007/s12160-016-9860-2
Gibson, S. J., and Lussier, D. (2012). Prevalence and relevance of pain in older persons. Pain. Med. 13(Suppl. 2), S23–S26. doi: 10.1111/j.1526-4637.2012.01349.x
Goldberg, M. S., Scott, S. C., and Mayo, N. E. (2000). A review of the association between cigarette smoking and the development of nonspecific back pain and related outcomes. Spine 25, 995–1014. doi: 10.1097/00007632-200004150-00016
Goodin, B. R., and Bulls, H. W. (2013). Optimism and the experience of pain: benefits of seeing the glass as half full. Curr. Pain Headache Rep. 17:329. doi: 10.1007/s11916-013-0329-8
Goodin, B. R., Kronfli, T., King, C. D., Glover, T. L., Sibille, K., and Fillingim, R. B. (2013). Testing the relation between dispositional optimism and conditioned pain modulation: does ethnicity matter? J. Behav. Med. 36, 165–174. doi: 10.1007/s10865-012-9411-7
Goubert, L., and Trompetter, H. (2017). Towards a science and practice of resilience in the face of pain. Eur. J. Pain 21, 1301–1315. doi: 10.1002/ejp.1062
Gupta, A., Silman, A. J., Ray, D., Morriss, R., Dickens, C., MacFarlane, G. J., et al. (2007). The role of psychosocial factors in predicting the onset of chronic widespread pain: results from a prospective population-based study. Rheumatology 46, 666–671. doi: 10.1093/rheumatology/kel363
Hahn, E. A., DeWalt, D. A., Bode, R. K., Garcia, S. F., DeVellis, R. F., Correia, H., et al. (2014). New english and Spanish social health measures will facilitate evaluating health determinants. Health Psychol. 33, 490–499. doi: 10.1037/hea0000055
Hanley, M. A., Jensen, M. P., Ehde, D. M., Hoffman, A. J., Patterson, D. R., and Robinson, L. R. (2004). Psychosocial predictors of long-term adjustment to lower-limb amputation and phantom limb pain. Disabil. Rehabil. 26, 882–893. doi: 10.1080/09638280410001708896
Hassett, A. L., and Finan, P. H. (2016). The role of resilience in the clinical management of chronic pain. Curr. Pain Headache Rep. 20:39. doi: 10.1007/s11916-016-0567-7
Hausmann, L. R., Parks, A., Youk, A. O., and Kwoh, C. K. (2014). Reduction of bodily pain in response to an online positive activities intervention. J. Pain 15, 560–567. doi: 10.1016/j.jpain.2014.02.004
Hausmann, L. R. M., Youk, A., Kwoh, C. K., Ibrahim, S. A., Hannon, M. J., Weiner, D. K., et al. (2017). Testing a positive psychological intervention for osteoarthritis. Pain. Med. 18, 1908–1920. doi: 10.1093/pm/pnx141
Helme, R. D., and Gibson, S. J. (2001). The epidemiology of pain in elderly people. Clin. Geriatr. Med. 17, 417–431. doi: 10.1016/S0749-0690(05)70078-1
Herzberg, P. Y., Glaesmer, H., and Hoyer, J. (2006). Separating optimism and pessimism: a robust psychometric analysis of the revised life orientation test (LOT-R). Psychol. Assess. 18, 433–438. doi: 10.1037/1040-3590.18.4.433
Howard, M. C. (2016). A review of exploratory factor analysis decisions and overview of current practices: what we are doing and how can we improve? Int. J. Hum. Comput. Interact. 32, 51–62. doi: 10.1080/10447318.2015.1087664
Howell, A. J., Jacobson, R. M., and Larsen, D. J. (2015). Enhanced psychological health among chronic pain clients engaged in hope-focused group counseling. Couns. Psychol. 43, 586–613. doi: 10.1177/0011000014551421
Hughes, S. L., Tussing-Humphreys, L., Schiffer, L., Smith-Ray, R., Marquez, D. X., DeMott, A. D., et al. (2018). Fit & strong! plus trial outcomes for obese older adults with osteoarthritis. Gerontologist doi: 10.1093/geront/gny146 [Epub ahead of print].
Jensen, M. P., Smith, A. E., Alschuler, K. N., Gillanders, D. T., Amtmann, D., and Molton, I. R. (2016). The role of pain acceptance on function in individuals with disabilities: a longitudinal study. Pain 157, 247–254. doi: 10.1097/j.pain.0000000000000361
Johnson, A. J., Terry, E., Bartley, E. J., Garvan, C., Cruz-Almeida, Y., Goodin, B., et al. (2019). Resilience factors may buffer cellular aging in individuals with and without chronic knee pain. Mol. Pain 15, 1–11. doi: 10.1177/1744806919842962
Kalyani, R. R., Saudek, C. D., Brancati, F. L., and Selvin, E. (2010). Association of diabetes, comorbidities, and A1C with functional disability in older adults: results from the national health and nutrition examination survey (NHANES), 1999-2006. Diabetes Care 33, 1055–1060. doi: 10.2337/dc09-1597
Karasawa, Y., Yamada, K., Iseki, M., Yamaguchi, M., Murakami, Y., Tamagawa, T., et al. (2019). Association between change in self-efficacy and reduction in disability among patients with chronic pain. PLoS One 14:e0215404. doi: 10.1371/journal.pone.0215404
Khan, A. N., Jacobsen, H. E., Khan, J., Filippi, C. G., Levine, M., and Lehman, R. A. Jr., et al. (2017). Inflammatory biomarkers of low back pain and disc degeneration: a review. Ann. N. Y. Acad. Sci. 1410, 68–84. doi: 10.1111/nyas.13551
Kothari, D. J., Davis, M. C., Yeung, E. W., and Tennen, H. A. (2015). Positive affect and pain: mediators of the within-day relation linking sleep quality to activity interference in fibromyalgia. Pain 156, 540–546. doi: 10.1097/01.j.pain.0000460324.18138.0a
Kratz, A. L., Davis, M. C., and Zautra, A. J. (2007). Pain acceptance moderates the relation between pain and negative affect in female osteoarthritis and fibromyalgia patients. Ann. Behav. Med. 33, 291–301. doi: 10.1080/08836610701359860
Lee, J., Chang, R. W., Ehrlich-Jones, L., Kwoh, C. K., Nevitt, M., Semanik, P. A., et al. (2015). Sedentary behavior and physical function: objective evidence from the osteoarthritis initiative. Arthritis. Care Res. 67, 366–373. doi: 10.1002/acr.22432
Lee, J. E., Kahana, B., and Kahana, E. (2016). Social support and cognitive functioning as resources for elderly persons with chronic arthritis pain. Aging Ment. Health 20, 370–379. doi: 10.1080/13607863.2015.1013920
Lerman, S. F., Rudich, Z., Brill, S., Shalev, H., and Shahar, G. (2015). Longitudinal associations between depression, anxiety, pain, and pain-related disability in chronic pain patients. Psychosom. Med. 77, 333–341. doi: 10.1097/psy.0000000000000158
Liu, J. J. W., Reed, M., and Girard, T. A. (2017). Advancing resilience: an integrative, multi-system model of resilience. Pers. Individ. Dif. 111, 111–118. doi: 10.1016/j.paid.2017.02.007
Lopez-Martinez, A. E., Esteve-Zarazaga, R., and Ramirez-Maestre, C. (2008). Perceived social support and coping responses are independent variables explaining pain adjustment among chronic pain patients. J. Pain 9, 373–379. doi: 10.1016/j.jpain.2007.12.002
MacLeod, S., Musich, S., Hawkins, K., Alsgaard, K., and Wicker, E. R. (2016). The impact of resilience among older adults. Geriatr Nurs 37, 266–272. doi: 10.1016/j.gerinurse.2016.02.014
Magnussen, L., Strand, L. I., and Lygren, H. (2004). Reliability and validity of the back performance scale: observing activity limitation in patients with back pain. Spine 29, 903–907. doi: 10.1097/00007632-200404150-00017
Martinez-Calderon, J., Zamora-Campos, C., Navarro-Ledesma, S., and Luque-Suarez, A. (2018). The role of self-efficacy on the prognosis of chronic musculoskeletal pain: a systematic review. J. Pain 19, 10–34. doi: 10.1016/j.jpain.2017.08.008
McCracken, L. M. (1998). Learning to live with the pain: acceptance of pain predicts adjustment in persons with chronic pain. Pain 74, 21–27. doi: 10.1016/S0304-3959(97)00146-2
McKillop, A. B., Carroll, L. J., Jones, C. A., and Battie, M. C. (2017). The relation of social support and depression in patients with chronic low back pain. Disabil. Rehabil. 39, 1482–1488. doi: 10.1080/09638288.2016.1202335
Milligan, G. W., and Cooper, M. C. (1985). An examination of procedures for determining the number of clusters in a data set. Psychometrika 50, 159–179. doi: 10.1007/bf02294245
Molton, I. R., and Terrill, A. L. (2014). Overview of persistent pain in older adults. Am. Psychol. 69, 197–207. doi: 10.1037/a0035794
Muller, R., Gertz, K. J., Molton, I. R., Terrill, A. L., Bombardier, C. H., Ehde, D. M., et al. (2016). Effects of a tailored positive psychology intervention on well-being and pain in individuals with chronic pain and a physical disability: a feasibility trial. Clin. J. Pain 32, 32–44. doi: 10.1097/ajp.0000000000000225
Mun, C. J., Davis, M. C., Molton, I. R., Karoly, P., Suk, H. W., Ehde, D. M., et al. (2019). Personal resource profiles of individuals with chronic pain: sociodemographic and pain interference differences. Rehabil. Psychol. 64, 245–262. doi: 10.1037/rep0000261
Naugle, K. M., Ohlman, T., Naugle, K. E., Riley, Z. A., and Keith, N. R. (2017). Physical activity behavior predicts endogenous pain modulation in older adults. Pain 158, 383–390. doi: 10.1097/j.pain.0000000000000769
Ohlman, T., Miller, L., Naugle, K. E., and Naugle, K. M. (2018). Physical activity levels predict exercise-induced hypoalgesia in older adults. Med. Sci. Sports Exerc. 50, 2101–2109. doi: 10.1249/mss.0000000000001661
Okifuji, A., and Hare, B. D. (2015). The association between chronic pain and obesity. J. Pain Res. 8, 399–408. doi: 10.2147/jpr.s55598
Ong, A. D., Bergeman, C. S., and Boker, S. M. (2009). Resilience comes of age: defining features in later adulthood. J. Pers. 77, 1777–1804. doi: 10.1111/j.1467-6494.2009.00600.x
Patel, K. V., Guralnik, J. M., Dansie, E. J., and Turk, D. C. (2013). Prevalence and impact of pain among older adults in the United States: findings from the 2011 national health and aging trends study. Pain 154, 2649–2657. doi: 10.1016/j.pain.2013.07.029
Peters, M. L., Smeets, E., Feijge, M., van Breukelen, G., Andersson, G., Buhrman, M., et al. (2017). Happy despite pain: a randomized controlled trial of an 8-week internet-delivered positive psychology intervention for enhancing well-being in patients with chronic pain. Clin. J. Pain 33, 962–975. doi: 10.1097/ajp.0000000000000494
Pilkonis, P. A., Choi, S. W., Reise, S. P., Stover, A. M., Riley, W. T., and Cella, D. (2011). Item banks for measuring emotional distress from the patient-reported outcomes measurement information system (PROMIS(R)): depression, anxiety, and anger. Assessment 18, 263–283. doi: 10.1177/1073191111411667
Pinto, R. Z., Ferreira, P. H., Kongsted, A., Ferreira, M. L., Maher, C. G., and Kent, P. (2014). Self-reported moderate-to-vigorous leisure time physical activity predicts less pain and disability over 12 months in chronic and persistent low back pain. Eur. J. Pain 18, 1190–1198. doi: 10.1002/j.1532-2149.2014.00468.x
Rabey, M., Smith, A., Beales, D., Slater, H., and O’Sullivan, P. (2016). Differing psychologically derived clusters in people with chronic low back pain are associated with different multidimensional profiles. Clin. J. Pain 32, 1015–1027. doi: 10.1097/ajp.0000000000000363
Rainville, J., Smeets, R. J., Bendix, T., Tveito, T. H., Poiraudeau, S., and Indahl, A. J. (2011). Fear-avoidance beliefs and pain avoidance in low back pain–translating research into clinical practice. Spine J. 11, 895–903. doi: 10.1016/j.spinee.2011.08.006
Reid, M. C., Williams, C. S., Concato, J., Tinetti, M. E., and Gill, T. M. (2003). Depressive symptoms as a risk factor for disabling back pain in community-dwelling older persons. J. Am. Geriatr. Soc. 51, 1710–1717. doi: 10.1046/j.1532-5415.2003.51554.x
Roland, M., and Morris, R. (1983). A study of the natural history of back pain. Part I: development of a reliable and sensitive measure of disability in low-back pain. Spine 8, 141–144.
Rose, M., Bjorner, J. B., Becker, J., Fries, J. F., and Ware, J. E. (2008). Evaluation of a preliminary physical function item bank supported the expected advantages of the patient-reported outcomes measurement information system (PROMIS). J. Clin. Epidemiol. 61, 17–33. doi: 10.1016/j.jclinepi.2006.06.025
Rose, M., Bjorner, J. B., Gandek, B., Bruce, B., Fries, J. F., and Ware, J. E. Jr., et al. (2014). The PROMIS physical function item bank was calibrated to a standardized metric and shown to improve measurement efficiency. J. Clin. Epidemiol. 67, 516–526. doi: 10.1016/j.jclinepi.2013.10.024
Rowe, J. W., and Kahn, R. L. (1997). Successful aging. Gerontologist 37, 433–440. doi: 10.1093/geront/37.4.433
Rowe, J. W., and Kahn, R. L. (2015). Successful aging 2.0: conceptual expansions for the 21st century. J. Gerontol. B Psychol. Sci. Soc. Sci. 70, 593–596. doi: 10.1093/geronb/gbv025
Salsman, J. M., Victorson, D., Choi, S. W., Peterman, A. H., Heinemann, A. W., Nowinski, C., et al. (2013). Development and validation of the positive affect and well-being scale for the neurology quality of life (Neuro-QOL) measurement system. Qual. Life Res. 22, 2569–2580. doi: 10.1007/s11136-013-0382-0
Schneider, S., Mohnen, S. M., Schiltenwolf, M., and Rau, C. (2007). Comorbidity of low back pain: representative outcomes of a national health study in the federal republic of germany. Eur. J. Pain 11, 387–397. doi: 10.1016/j.ejpain.2006.05.005
Schrimpf, M., Liegl, G., Boeckle, M., Leitner, A., Geisler, P., and Pieh, C. (2015). The effect of sleep deprivation on pain perception in healthy subjects: a meta-analysis. Sleep Med. 16, 1313–1320. doi: 10.1016/j.sleep.2015.07.022
Shiri, R., Karppinen, J., Leino-Arjas, P., Solovieva, S., and Viikari-Juntura, E. (2010a). The association between obesity and low back pain: a meta-analysis. Am. J. Epidemiol. 171, 135–154. doi: 10.1093/aje/kwp356
Shiri, R., Karppinen, J., Leino-Arjas, P., Solovieva, S., and Viikari-Juntura, E. (2010b). The association between smoking and low back pain: a meta-analysis. Am. J. Med. 123, 87.e7–87.e35. doi: 10.1016/j.amjmed.2009.05.028
Skevington, S. M., Lotfy, M., and O’Connell, K. A. (2004). The world health organization’s WHOQOL-BREF quality of life assessment: psychometric properties and results of the international field trial. A report from the WHOQOL group. Qual. Life Res. 13, 299–310. doi: 10.1023/b:qure.0000018486.91360.00
Smith, B. W., Dalen, J., Wiggins, K., Tooley, E., Christopher, P., and Bernard, J. (2008). The brief resilience scale: assessing the ability to bounce back. Int. J. Behav. Med. 15, 194–200. doi: 10.1080/10705500802222972
Snyder, C. R., Harris, C., Anderson, J. R., Holleran, S. A., Irving, L. M., Sigmon, S. T., et al. (1991). The will and the ways: development and validation of an individual-differences measure of hope. J. Pers. Soc. Psychol. 60, 570–585. doi: 10.1037/0022-3514.60.4.570
Southwick, S. M., Bonanno, G. A., Masten, A. S., Panter-Brick, C., and Yehuda, R. (2014). Resilience definitions, theory, and challenges: interdisciplinary perspectives. Eur. J. Psychotraumatol. 5, 1–14. doi: 10.3402/ejpt.v5.25338
Strand, L. I. (2017). Back performance scale. J. Physiother. 63:262. doi: 10.1016/j.jphys.2017.07.004
Sturgeon, J. A., and Zautra, A. J. (2010). Resilience: a new paradigm for adaptation to chronic pain. Curr. Pain Headache Rep. 14, 105–112. doi: 10.1007/s11916-010-0095-9
Sturgeon, J. A., and Zautra, A. J. (2013). Psychological resilience, pain catastrophizing, and positive emotions: perspectives on comprehensive modeling of individual pain adaptation. Curr. Pain Headache Rep. 17:317. doi: 10.1007/s11916-012-0317-4
Sullivan, M. J., Thorn, B., Haythornthwaite, J. A., Keefe, F., Martin, M., Bradley, L. A., et al. (2001). Theoretical perspectives on the relation between catastrophizing and pain. Clin. J. Pain 17, 52–64. doi: 10.1097/00002508-200103000-00008
Sun, Q., Shi, L., Prescott, J., Chiuve, S. E., Hu, F. B., De Vivo, I., et al. (2012). Healthy lifestyle and leukocyte telomere length in U.S. women. PLoS One 7:e38374. doi: 10.1371/journal.pone.0038374
Turner, J. A., and Clancy, S. (1986). Strategies for coping with chronic low back pain: relationship to pain and disability. Pain 24, 355–364. doi: 10.1016/0304-3959(86)90121-1
Vlaeyen, J. W., and Crombez, G. (1999). Fear of movement/(re)injury, avoidance and pain disability in chronic low back pain patients. Man. Ther. 4, 187–195. doi: 10.1054/math.1999.0199
Vlaeyen, J. W., and Linton, S. J. (2000). Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain 85, 317–332. doi: 10.1016/S0304-3959(99)00242-0
Walsh, T. P., Arnold, J. B., Evans, A. M., Yaxley, A., Damarell, R. A., and Shanahan, E. M. (2018). The association between body fat and musculoskeletal pain: a systematic review and meta-analysis. BMC Musculoskelet. Disord. 19:233. doi: 10.1186/s12891-018-2137-0
Watson, D., Clark, L. A., and Tellegen, A. (1988). Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Soc. Psychol. 54, 1063–1070. doi: 10.1037/0022-3514.54.6.1063
Weiner, D. K., Haggerty, C. L., Kritchevsky, S. B., Harris, T., Simonsick, E. M., Nevitt, M., et al. (2003). How does low back pain impact physical function in independent, well-functioning older adults? Evidence from the Health ABC Cohort and implications for the future. Pain. Med. 4, 311–320. doi: 10.1111/j.1526-4637.2003.03042.x
Wertli, M. M., Rasmussen-Barr, E., Held, U., Weiser, S., Bachmann, L. M., and Brunner, F. (2014). Fear-avoidance beliefs-a moderator of treatment efficacy in patients with low back pain: a systematic review. Spine J. 14, 2658–2678. doi: 10.1016/j.spinee.2014.02.033
Windle, G., Bennett, K. M., and Noyes, J. (2011). A methodological review of resilience measurement scales. Health Qual. Life Outcomes 9:8. doi: 10.1186/1477-7525-9-8
World Health Organization (2016). International Statistical Classification of Diseases and Related Health Problems 10th Revision. Available at: https://icd.who.int/browse10/2016/en (accessed July 29, 2019).
Wright, L. J., Zautra, A. J., and Going, S. (2008). Adaptation to early knee osteoarthritis: the role of risk, resilience, and disease severity on pain and physical functioning. Ann. Behav. Med. 36, 70–80. doi: 10.1007/s12160-008-9048-5
Wylde, V., Dixon, S., and Blom, A. W. (2012). The role of preoperative self-efficacy in predicting outcome after total knee replacement. Musculoskeletal Care 10, 110–118. doi: 10.1002/msc.1008
Keywords: resilience, multisystem, low back pain, aging, psychological, health, social support
Citation: Bartley EJ, Palit S, Fillingim RB and Robinson ME (2019) Multisystem Resiliency as a Predictor of Physical and Psychological Functioning in Older Adults With Chronic Low Back Pain. Front. Psychol. 10:1932. doi: 10.3389/fpsyg.2019.01932
Received: 17 May 2019; Accepted: 06 August 2019;
Published: 22 August 2019.
Edited by:
Rocio de la Vega, Seattle Children’s Research Institute, United StatesReviewed by:
Bárbara Oliván Blázquez, University of Zaragoza, SpainVolker Max Perlitz, Simplana GmbH, Germany
Copyright © 2019 Bartley, Palit, Fillingim and Robinson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Emily J. Bartley, RUJhcnRsZXlAZGVudGFsLnVmbC5lZHU=
 Emily J. Bartley
Emily J. Bartley Shreela Palit
Shreela Palit Roger B. Fillingim1,2
Roger B. Fillingim1,2 Michael E. Robinson
Michael E. Robinson