
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
HYPOTHESIS AND THEORY article
Front. Psychol., 07 March 2019
Sec. Psychoanalysis and Neuropsychoanalysis
Volume 10 - 2019 | https://doi.org/10.3389/fpsyg.2019.00470
Human beings use conscious emotions to direct their behaviors. There is some agreement in the scientific community that unconscious motivations are able to evoke conscious emotions. This manuscript focuses on Freudian motivational drives as inductors for unconscious motivation, and also on Panksepp’s framework of affective neuroscience for describing the generation of emotions. Recently, it has been suggested that imperative motor factors of Freudian drives (i.e., the hormones ghrelin, testosterone, angiotensin II and adenosine) have the ability to activate both a drive-specific brain area and brain areas of the SEEKING command system. In fact, this manuscript contends that all imperative motor factors have typical SEEKING targets (i.e., so-called receptors) in the brain areas of both nucleus accumbens and lateral hypothalamus. In addition, all imperative motor factors are able to target the central amygdala directly, a brain area classified by Panksepp as the instinctual part of the FEAR command system. Another point of interest may be the evaluation that imperative motor factors of the sexual drive, hunger and thirst can directly activate the RAGE command system by targeting the medial amygdala. Surprisingly, all imperative motor factors are able to modulate Panksepp’s granddaddy mechanism, i.e., to stimulate all seven command systems via the lateral hypothalamus. Orexinergic neurons exclusively located in the lateral hypothalamus have targets for imperative motor factors and project axons to characteristic brain areas of all seven command systems. From the fact that the imperative motor factors of the sexual drive and hunger act in an excitatory manner on orexinergic neurons whereas those of thirst and sleep inhibit such neurons, temporary termination of hunger by thirst may be understood as a very simple example of a co-regulation of Freudian drives. The author wishes to note that there are motivational drives other than the ones described by Freud. Bowlby was obviously the first in describing such drives, and Bowlbyian drive activities cannot be explained with the intermediacy of imperative motor factors. Nevertheless, the ignorance of the magnificent importance of imperative motor factors must be discarded.
At present, any consensus on a single unitary definition of the construct of motivation, derived from the Latin words movere and motivus (Gilbert, 2015; Strombach et al., 2016), is lacking in the psychological community (Gneezy et al., 2011). Panksepp noted that a motivation can be descripted as a process, “in which a bodily need is subserved by a behavior” in contrast to emotions “where no bodily need is evident” (Panksepp, 1998, p. 228). Thus, motivation can therefore be understood as an active movement of an individual initiated by a stimulus as a driving force. Such a view intrinsically predicts that the activity of a (motivational) drive will evoke a motivation. In the past, various classic psychological drive concepts – Hull’s drive reduction theory (Hull, 1943), Lorenz’s hydraulic conception of drive (Lorenz and Leyhausen, 1973), Tinbergen’s hierarchical organization of circuit nodes (Tinbergen, 1950) and Freud’s theory of motivational drives (Freud, 1905, 1915a,b,c) – were developed in order to explain drive-dependent motivations. It should be noted that (at present) a drive cannot be experimentally distinguished from a corresponding motivation when a (Freudian) drive acts as the driving force for this motivation. Adolphs and Anderson noted “The difference between ‘drive’ and ‘motivation’ is more of an operational and conceptual one than a biological one.” (Adolphs and Anderson, 2018, p. 148). In the present manuscript the construct of motivation will be nevertheless advocated but the author will distinguish metabolic-deficit-dependent motivations and metabolic-deficit-independent ones. Remarkably, Freud [a man of vast reading (Solomon, 1974)] obviously picked-up the idea of “chemical messengers”, with the first hormone identified in 1902 (Bayliss and Starling, 1902). Freud respected the intermediacy of hormones in his motivational drive theory with its known four elements somatic source, aim, object and imperative motor factor (i.e., hormones) (Freud, 1905, 1915a). Very unfortunately, persistent mistranslation of the German nouns Drang (correctly, in the Freudian sense: imperative motor factor) as “motor factor,” Trieb (correctly: drive) as “instinct” and Trieblehre (correctly in the Freudian sense: theory of motivational drives) as “theory of instincts” have given rise to a variety of misunderstandings, especially in those cases where Freud used the German word ‘Instinkt’ which was also (correctly) translated as instinct. For Freud instincts (‘Instinkte’) are “inherited mental formations” (Freud, 1915c, p. 3017) whereas drives “represent an instigation to mental activity” (Freud, 1926b, p. 4343; Holder, 1970, pp. 19). In 1923, Freud clarified that two types of Freudian motivational drives are constituent elements of Eros: “According to this view we have distinguish two classes of instincts, one of which, the sexual instinct or Eros, is by far the more conspicuous and accessible to study. It comprises not merely the uninhibited sexual instinct proper and the instinctual impulses of an aim-inhibited or sublimated nature derived from it, but also the self-preservative instinct,…” (Freud, 1923, p. 3974). Thus, according to Freud, three motivational drives (sexual drive, thirst and hunger) are constituent elements of Eros. In order to answer Freud’s question of “What instincts should we suppose there are, and how many?” (Freud, 1915a, p. 2961), we advocated three criteria for identifying a Freudian motivational drive: an imperative nature of the drive as a psychological criterion, orchestration via the lateral hypothalamus as a neurobiological cachet and a drive termination by means of the central release of 5-hydroxytryptamine as a biochemical attribute (Kirsch and Mertens, 2018). By using these criteria, we identified the sexual drive, thirst, hunger (in line with Freud’s prediction) and sleep as Freudian motivational drives with the corresponding imperative motor factors testosterone, angiotensin II, ghrelin and adenosine. These hormones address the frequently ignored problem of drive specificity – “The ability to process and ‘decide’ between the drives might be lost if each drive is not also an independent generator. In other words, we have to sustain drive-specificity…” (Wright and Panksepp, 2012, p. 18) – because they can simultaneously activate a drive-specific brain area and typical brain areas that are responsible for seeking of resources (vide infra).
In contrast to motivations, a variety of very detailed theories for describing emotions have been outlined so far, i.e., the Appraisal Theory (Scherer, 1984, 2009; Lazarus, 1991), Interoceptive Theories (Damasio, 1999; Craig, 2002; Damasio and Carvalho, 2013), Constructed Emotion Theory (Feldman Barrett, 2017), Theory of Emotion (Rolls, 1999), Higher-Order Theory of Emotion (LeDoux and Brown, 2017) and Emotion Systems (Panksepp, 1998; Panksepp and Biven, 2012). The latter framework should be attractive from the perspective of Freudian motivational drives because of the fact that Panksepp’s theory of affective neuroscience tends to emphasize motor-related representations (i.e., drives) in the development of feelings. Panksepp (Panksepp, 1998) classifies seven different types of command systems that may (but do not necessarily have to) evoke special behaviors, e.g., seeking for rewards/resources/sexual partners, lust, caring and affection, loss and panic, rage, fear and play. Special subcortical regions of the brain are involved with the processing to the corresponding conscious emotions,1,2 which are classified as so-called command systems (labeled SEEKING, RAGE, FEAR, LUST, CARE, PANIC, and PLAY) (Panksepp, 1998, 2016, 2018; Watt and Panksepp, 2009; Zellner et al., 2011; Solms and Panksepp, 2012; Wright and Panksepp, 2012; Panksepp and Yovell, 2014; Alcaro et al., 2017). Since an activated SEEKING system constantly blends well with all the other command systems by co-regulating them (Wright and Panksepp, 2012), the generation of SEEKING activities (according to Panksepp the SEEKING system is “the ‘granddaddy’ of all the emotional system.” (Panksepp and Biven, 2012, p. 86), are of central importance in the development of conscious emotions. Recently, we were able to contend the unexpected possibility that the SEEKING system can be activated by the intermediary action of Freudian imperative motor factors (Kirsch and Mertens, 2018). Since Freud explicitly noted that a motivational drive has an unconscious nature: “I am in fact of the opinion that the antithesis of conscious and unconscious is not applicable to instincts. An instinct can never become an object of consciousness – only the idea that represents the instinct can.” (Freud, 1915c, p. 3000) the question arose how an unconscious stimulus can evoke a conscious sensation? From findings of our last work it is possible to expand a fine idea introduced by Panksepp et Biven (Table 1).
Thus, according to Panksepp, the intermediacy of neuromodulators and/or classical neurotransmitters represents a mandatory premise for consciousness.3 Since it has been mentioned that there are qualitative differences of the nervous system between conscious and unconscious processes (Brakel and Shervin, 2005) and because Freudian drives have an unconscious nature (vide supra) and uses hormones as signal transporters, the unconscious action of the Freudian drives can be determined biochemically at the level of signaling codes (Table 1).4 For example, an imperative motor factor generated outside the brain [e.g., stomach derived release of ghrelin (Kojima et al., 1999; Stievenard et al., 2017)] cannot be transformed to a neurotransmitter/neuromodulator (i.e., cannot be transformed to signals necessary to achieve consciousness) as long as it circulates in the periphery. After passing the blood-brain barrier, the imperative motor factor can now induce the release of neurotransmitters/neuromodulators by occupying its hormone receptors on various pre-synapses in subcortical brain areas. This release of neuromodulators or neurotransmitters represents – from the perspective of the Global Neuronal Workspace Hypothesis (Dehaene et al., 2006) – the provision of preconscious stimuli that can (but do not necessarily have to) gain access to conscious processing.
Unfortunately, the abilities of imperative motor factors to address the subcortical brain in such a manner, was only deconvolved for the brain areas of the SEEKING system (Kirsch and Mertens, 2018). This option raises the question of whether imperative motor factors have the ability to modulate activities of other command systems, and – if so – the underlying mechanisms would be of interest. This manuscript will report on such totally underestimated capabilities, although they are quite well evaluated.
The idea of making a direct connection between Freudian drives via their imperative motor factors and Panksepp’s emotional command systems intrinsically required one to locate targets (i.e., so-called receptors) of these hormones at the brain areas of interest (Table 2).
As expected from evaluations of our earlier manuscript (Kirsch and Mertens, 2018), all imperative motor factors can directly generate SEEKING activities because, in addition to targets in their drive specific brain areas5, they all also have anchorage grounds in both the lateral hypothalamus and the nucleus accumbens (Table 2). The activation of neurons in the latter area and also in the ventral tegmental area can result in the release of the catecholamine dopamine (Naleid et al., 2005; Abizaid et al., 2006; Jerlhag et al., 2007), and that neuromodulator is a key intermediate in the activation of the SEEKING system (Panksepp, 1998; Panksepp and Biven, 2012; Watt, 2017).
Somewhat surprising was the evaluation that all imperative motor factors have receptors in the central amygdala, a brain area that was classified as a part of the FEAR command system (Panksepp, 1998; Panksepp and Biven, 2012; Watt, 2017). Thus, all imperative motor factors have direct access to both the SEEKING and the FEAR command systems. Panksepp noted that a variety of chemical messengers can activate the FEAR system (Panksepp and Biven, 2012), with the result that the view that imperative motor factors can provide a similar activation is not in conflict with the theory of affective neuroscience. In addition, Panksepp distinguished between conditional FEAR and unconditional ones, stating “Therefore, while the central nucleus of the amygdala is part of the unconditional (instinctual) FEAR system, the other nuclei are not.” (Panksepp and Biven, 2012, p. 196). Therefore, the action of the imperative motor factors on the INSTINCTUAL FEAR system may represent a phylogenetic old mechanism.6 Of course, as most Freud followers would expect, Freud was aware of a link between his motivational drives and fearful emotions: “So far we have had no occasion to regard realistic anxiety in any different light from neurotic anxiety. We know what the distinction is. A real danger is a danger which threatens a person from an external object, and a neurotic danger is one which threatens him from an instinctual demand.” (Freud, 1926a, p. 4319). The fact that imperative motor factors have direct access to the FEAR command system by targeting the central amygdala may also be an interesting finding for psychoanalysts of other schools because Bowlby noted. “No CONCEPT is more central to psychoanalytical theory than the concept of anxiety. Yet it is one about which there is little consensus of opinion, which accounts in no small measure for the divisions between different schools of thought. Put briefly, all analysts are agreed that anxiety cannot be explained simply by reference to external threat: in some way processes usually thought of as internal and instinctive seem to play a crucial role. But how these inner forces are to be conceptualized and how they give rise to anxiety has always been a puzzle.” (Bowlby, 1960). In summary, direct access of Freudian drives to the FEAR command system is not in conflict with either the theory of affective neuroscience or historical predictions by leading psychoanalysts.
Of the other command systems, only RAGE can be addressed via the medial amygdala by three imperative motor factors. The failure of adenosine, i.e., the imperative motor factor of sleep, to activate RAGE can be expected because ongoing rage is obviously counterproductive for the onset of sleep, and therefore such neurochemicals that can be elevated during RAGE (e.g., noradrenaline) are decreased during sleep and vice versa (Watson et al., 2010; Panksepp and Biven, 2012).
Although there are (beyond any doubt) direct mechanisms for the generation of command systems activities, their capability is somewhat limited, as imperative motor factors cannot evoke all types of emotion via that mechanism with the same level of efficiency. Therefore, the question arose as to whether Freud’s imperative motor factors can even do more than generate SEEKING, FEAR and RAGE activities by occupying a receptor in a typical brain region of these command systems.
Since all Freudian drives are orchestrated via the lateral hypothalamus (vide supra), the precise targets of imperative motor factors in this brain area have been analyzed (Table 3).
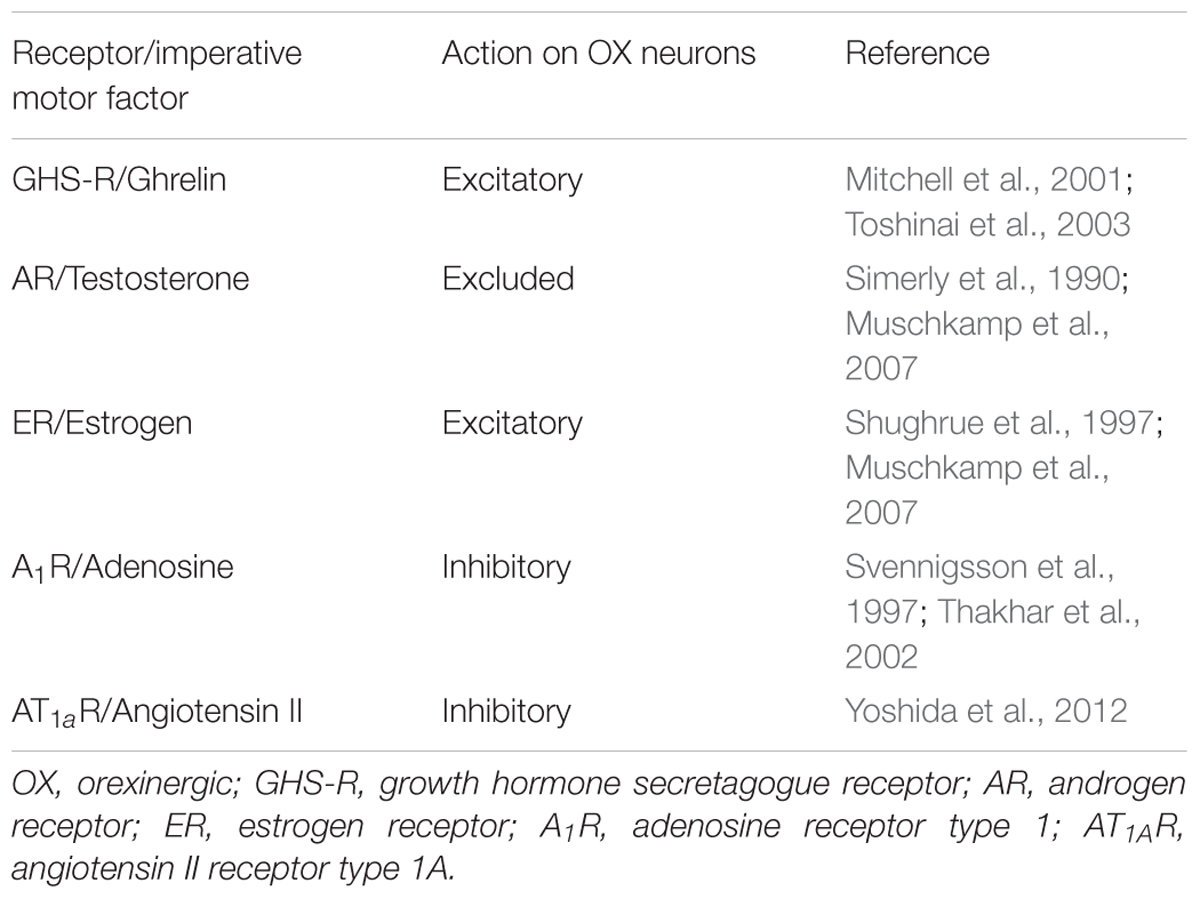
Table 3. Targets for imperative motor factors of hunger, sexual drive, sleep and thirst on orexinergic neurons located in the lateral hypothalamus.
This analysis contains two surprises. Firstly, attractive targets for testosterone on orexinergic neurons are lacking in the lateral hypothalamus (Table 3), although androgen receptors have been detected in that area (Simerly et al., 1990). Beside testosterone, its downstream product estradiol – the enzyme aromatase directly oxidizes testosterone into the estrogen derivative estradiol (Fui et al., 2014) – is also important for male sexual behavior (Cunningham et al., 2012), and estrogen receptors on orexinergic neurons are present in the lateral hypothalamus. The second surprise was the realization that receptors of all four imperative motor factors modulate (in an inhibitory or excitatory manner) the release of the neuromodulator orexin (Table 3). In 1998, two research groups independently identified peptides exclusively produced by neurons located in the lateral hypothalamus (de Lecea et al., 1998; Sakurai et al., 1998). Most scientific journals have now accepted the name “orexins” (instead of the alternative designation “hypocretins”) for these peptides, although their physiological actions are not limited to the regulation of appetite (Li et al., 2014). Very interestingly, from their location in the lateral hypothalamus, orexinergic neurons project axons to brain areas that are important for the seven command systems of affective neuroscience. In detail, orexinergic neurons project to brain areas related to SEEKING [ventral tegmental area (Marcus et al., 2001; Uramura et al., 2001; Korotkova et al., 2003; Borgland et al., 2008; Muschkamp et al., 2014) and nucleus accumbens (Trivedi et al., 1998; Marcus et al., 2001; Smith et al., 2002; Yang and Ferguson, 2003)], RAGE [medial amygdala (Trivedi et al., 1998; Marcus et al., 2001) and bed nucleus of stria terminalis (Trivedi et al., 1998; Conrad et al., 2012; Lungwitz et al., 2012)], FEAR [central amygdala (Marcus et al., 2001; Cluderay et al., 2002; Bisetti et al., 2006) and basolateral amygdala (Arendt et al., 2014)], LUST [ventromedial hypothalamus (Muroya et al., 2004)], CARE [ventral periaqueductal gray matter (Li et al., 2014)], PANIC [midline thalamic nuclei (Bayer et al., 2002; Ishibashi et al., 2005), paraventricular thalamus (a midline thalamic structure) (Peyron et al., 1998; Marcus et al., 2001; Huang et al., 2006; Li et al., 2011) and mediodorsal thalamus (Govindaiah and Cox, 2006)] and finally PLAY [parafascicular nucleus (Govindaiah and Cox, 2006)]. The postsynaptic action of orexin is generally an excitatory one on other neurons and therefore leads to the release of a variety of either neurotransmitters or neuromodulators. For example, the release of orexin in the ventral tegmental area stimulates dopaminergic (Marcus et al., 2001; Uramura et al., 2001; Korotkova et al., 2003; Muschkamp et al., 2014) as well as non-dopaminergic neurons (Korotkova et al., 2003; Borgland et al., 2008). Some of the aforementioned brain areas have projections to the lateral hypothalamus, thereby offering an afferent regulation of orexinergic neurons (as a kind of a feedback mechanism7). For instance, neurons of the medial amygdala (RAGE) project to orexinergic neurons of the lateral hypothalamus (James et al., 2017). However, knowledge of these afferent projections is currently too low to permit detailed insight into such feedback mechanisms.
The fact that orexinergic neurons of the lateral hypothalamus project to brain areas of all command systems supports Panksepp’s postulation that the SEEKING system, the lateral hypothalamus having been classified as a part of this command system (Watt, 2017), is “the ‘granddaddy’ of all the emotional system.” (Panksepp and Biven, 2012, p. 86). Since the author is unaware whether other neurons can act in a similar manner (but, of course, cannot exclude such a possibility with certainty), and because the activity of orexinergic neurons is under the control of Freud’s imperative motor factors (Table 2), it is concluded that the activity of the granddaddy mechanism, i.e., to evoke emotions via generation of SEEKING-dependent command system activities, can be under the control of Freudian motivational drives.8
It is well known that different motivations can co-regulate (conflict or support) each other (Huang and Bargh, 2014; Gilbert, 2015). Such a co-regulation of Freudian drives can be understood at a biomolecular level with the aid of Table 3 because hunger and the sexual drive can stimulate command systems activities by enhancing orexin-dependent networking, whereas thirst and sleep can operate oppositely. The sense of this mechanism should be illustrated by answering the question what drive must a hungry and thirsty person firstly satisfied? Mahatma Gandhi survived (three times) 21 days of complete starvation (Chettiar, 1943; Gandhi, 1948) but an average human being can probably survive without water for about only a few days (Kottusch et al., 2009). Thus, from a perspective of survival, the satisfaction of thirst is the more important one, and the thirst drive is able to counteract the claim of the hunger drive by downregulating orexin-dependent activation of the seven command systems. In fact, there is data to support the assertion that the claim of thirst is stronger and more stable over the day compared to the claim of hunger (Mattes, 2010). Temporary termination of the claim of the hunger drive by thirst is reminiscent of the phenomenon of a Freudian repression “the impulse then passes into the state of ‘repression’ [Verdrängung].” (Freud, 1915b, p. 2977), but albeit the expressed example is far too simple for describing such a complex psychological entity.9 Thus, the importance of the mechanism of drive co-regulation via orexin-mediated networking for psychological entities cannot be classified at present.10
Although the indirect pathway – i.e., modulation of orexin-dependent networking by targeting the lateral hypothalamus (Table 3) – expands the capabilities of imperative motor factors, they are unable to cover all possible types of motivations and (corresponding) emotions. A Freudian motivational drive is down-regulated by the cerebral release of 5-hydroxytryptamine (vide supra). Therefore, drives and corresponding motivations that would require the intermediacy of 5-hydroxytryptamine for their processing cannot be regarded as being dependent upon Freudian motivational drives. This gives rise to the puzzler: Do such drives really exist, and where are they operating in psychological situations? Most surprisingly, an entirely unexpected answer is offered by Bowlby’s attachment theory (Bowlby, 1960, 1973). The unconscious motivation of an infant to stay in close proximity to its care provider becomes measureable by expanding the distance between mother and infant for significant periods of time, resulting in distress, anxiety and fear in the infant (Bowlby, 1973). Bowlby mentioned that a drive (even suggested by Freud) is responsible for this motivation of the infant: “Our most conservative conclusion is that Freud was not wholly satisfied with his earlier accounts [i.e., theory of motivational drives]. A more radical one is that, toward the end of his life and imbued with a newly-found but vivid appreciation of the central importance of the child’s tie to his mother, Freud was not only moving away from the theory of Secondary Drive [i.e., motivational Freudian drive] but developing the notion that special drives built into the infant in the course of evolution underlie this first and unique love relationship.” (Bowlby, 1958). Of course, Bowlby’s suggestion of classifying a Freudian drive as a Secondary Drive evoked a number of heavy protests from leading Freud followers, but the one proffered by Anna Freud points to a hitherto unrecognized solution: “He [Bowlby] sets up a controversy between the tie to the mother and the action of the pleasure principle in terms of “primary and secondary drive” and criticizes us for reversing their order of importance, i.e., for regarding the tie to the mother as a secondary, the search for pleasure as a primary instinctual urge.” (Freud, 1960). Most remarkably, Anna Freud accepted Bowlby’s view that there are other drives at work as well as the motivational Freudian ones. It should therefore be helpful to classify these non-Freudian drives as Bowlbyian ones11. The dispute concerning the ranking of the drives is presumably futile since both kinds of drives are obviously essential for the survival of the human species. Thus, by accepting the view that the motivation of the child to stay in close proximity to its mother is the result of a Bowlbyian drive activity, a detailed search in literature would be of interest with regard to the possibility of whether 5-hydroxytryptamine is involved in motivations/emotion connected with attachment in general. The observation that a polymorphism of the 5-HT2A serotonin receptor gene – this receptor being one important target for 5-hydroxytryptamine in the brain – is connected with the psychological disorder referred to as ‘avoidant attachment’ (Gillath et al., 2008) currently offers the strongest proof that 5-hydroxytryptamine is in fact involved in the processing (and not in the down-regulation12) of a Bowlbyian drive. In addition, 5-hydroxytryptamine increases the secretion of oxytocin (Saydoff et al., 1991; Bagdy and Makara, 1994) and this neuromodulator is obviously highly important for the tie between an infant and its mother (Uvnäs Moberg and Prime, 2013). Since 5-hydroxytryptamine down-regulates the activity of a Freudian drive initiated by imperative motor factors and because 5-hydroxytryptamine supports via increase of oxytocin secretion the processing of a Bowlbyian drive, it can be safely concluded that imperative motor factors are not responsible for Bowlbyian drive activities. Of course, the lack of knowledge of how an impaired or down-regulated Bowlbyian drive is able to activate command system activities – according to Panksepp, an impaired attachment activates the PANIC/GRIEF system (Panksepp and Biven, 2012, pp. 312–313) – needs to be evaluated.
Experimental psychologists demonstrate that both positive emotionally valent stimuli and negative (aversive) ones can successfully enhance (the motivation) ‘attention’ in patients (Dominguez-Borras et al., 2013; Vuilleumier, 2015). From such experimental findings psychologists have concluded that emotions provide guidance for motivations and are linked with them (e.g., Gilbert, 2014). However, the conclusion that an emotion can initiate a motivation has hardly any means of little significance, given the lack of a generally accepted definition of the term ‘motivation’ in psychology. Since an emotion can inform an individual about the existence of a metabolic deficit (but cannot be responsible for such an imbalance), it is concluded that an emotion cannot initiate a Freudian drive dependent motivation. The expressed example, the putative motivation ‘attention’ is obviously independent of the intermediary action of a Freudian drive and would be (as long as any generally accepted definition of the term ‘motivation’ is lacking) tentatively classified as a metabolic deficit independent motivation that might be liberated by an emotion. The next puzzler, namely whether an emotion can provoke a Bowlbyian drive dependent motivation, cannot be answered yet because the precise architecture of this complex drive still needs to be evaluated.
This manuscript has been written under the assumption that our recently published update (Kirsch and Mertens, 2018) to Freud’s 100-year-old (but essentially accurate) theory of motivational drives needs to be conclusively expanded in order to exploit its full potential. Not just the SEEKING system, but imperative motor factors of all Freudian drives have targets in the central amygdala (Table 2), a brain area that was classified by Panksepp (Panksepp and Biven, 2012, p. 196) as the instinctual part of the FEAR command system. In addition, the imperative motor factors of the sexual drive, hunger and thirst also have targets in the medial amygdala (Table 2), an area of the brain classified as being part of the RAGE command system (Watt, 2017). Thus, besides directly generating SEEKING activities, all imperative motor factors are able to generate FEAR activities, and three of them can also directly stimulate the generation of RAGE activities. In addition, all drives can indirectly modulate all sorts of command system activities by controlling Panksepp’s ‘granddaddy’ of affective neuroscience, i.e., by modulating the activity of orexinergic neurons in the lateral hypothalamus (Table 3). Because of this, the sexual drive and hunger can stimulate affective neurophysiological activities via orexin-mediated networking, whereas sleep and thirst can inhibit such demands. The opposing actions of hunger and thirst were first used to explain the co-regulation of a Freudian drive. In order not to overrate the deconvolved impressive capabilities of Freudian drives, the astute reader needs to note that essential motivational drive activities described by Bowlby, classified here as Bowlbyian drives, cannot be explained by the intermediacy of imperative motor factors. The action of imperative motor factors is basically drafted (Figure 1).
The consideration of actual findings on Freud’s theory of motivational drives (evaluated here and in our previous manuscript) leads to the following assertions:
(1) Human beings are directed, but not determined, by Freudian drives in an unconscious manner.
(2) The satisfaction of a Freudian drive leads to the release of the neurotransmitter 5-hydroxytryptamine in order to down-regulate the drive.
(3) The sexual drive, hunger, thirst and sleep are Freudian drives with an imperative character.
(4) The imperative motor factor of a Freudian drive is a signal molecule that directly targets nucleus accumbens, lateral hypothalamus, central amygdala and a drive-specific brain area.
(5) The imperative motor factor of a Freudian drive can directly evoke generation of drive-specific SEEKING and INSTINCTUAL FEAR activities.
(6) The imperative motor factors of the sexual drive, hunger and thirst also directly target the medial amygdala, thereby evoking the generation of RAGE activities.
(7) All imperative motor factors are able to modulate, indirectly, the generation of affective neuroscience activities by targeting orexinergic neurons in the lateral hypothalamus.
In summary, it can be said that the intermediacy of Freudian imperative motor factors can explain convincingly the modulation of command system activities. Accordingly, the concept of Freudian motivational drives is somewhat underestimated even by Freudian psychoanalysts, although Boag recently mentioned the putative importance of the drives for the id and the ego, respectively (Boag, 2014).
The author wrote and designed the manuscript.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
It is a pleasure to thank Prof. Dr. Michael B. Buchholz of the International Psychoanalytic University Berlin for initiating the writing of this manuscript for a leading psychology/psychoanalysis journal. The author has benefitted from many stimulating discussions with Prof. Dr. Wolfgang Mertens (Ludwig-Maximilians-Universität München, Faculty of Psychology and Educational Sciences. Department of Psychology. Clinical Psychology and Psychotherapy). In addition, the author is also grateful to all reviewers for very useful comments on an earlier version of this manuscript. Nevertheless, any errors in this paper are entirely my own responsibility.
Abizaid, A., Liu, Z. W., Andrews, Z. B., Shanabrough, M., Borok, E., Elsworth, J. D., et al. (2006). Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J. Clin. Invest. 117, 3229–3239. doi: 10.1172/JCI29867
Adolphs, R., and Anderson, D. (2018). The Neuroscience of Emotion: A New Synthesis. Oxford: Princeton University Press.
Alcaro, A., Carta, S., and Panksepp, J. (2017). The affective core of the self: a neuro-archetypical perspective on the foundations of human (and animal) subjectivity. Front. Psychol. 8:1424. doi: 10.3389/fpsyg.2017.01424
Alvarez-Crespo, M., Skibicka, K. P., Farkas, I., Molnar, C. S., Egecioglu, E., Hrabovszky, E., et al. (2012). The amygdala as a neurobiological target for ghrelin in rats: neuroanatomical, electrophysiological and behavioral evidence. PLoS One 7:e46321. doi: 10.1371/journal.pone.0046321
Amaral, D. G., Price, J. L., Pitkanen, A., and Carmichael, S. T. (1992). Anatomical Organization of the Primate Amygdaloid Complex. New York, NY: Wiley-Liss.
Arendt, D. H., Hassell, J., Li, H., Achua, J. K., Guarnieri, D. J., Dileone, R. J., et al. (2014). Anxiolytic function of the orexin 2/hypocretin A receptor in the basolateral amygdala. Psychoneuroendocrinology 40, 17–26. doi: 10.1016/j.psyneuen.2013.10.010
Bagdy, G., and Makara, G. B. (1994). Hypothalamic paraventricular nucleus lesions differentially affect serotonin-1A (5-HT1A) and 5-HT2 receptor agonist-induced oxytocin, prolactin and corticosterone responses. Endocrinology 134, 1127–1131. doi: 10.1210/endo.134.3.8119151
Bayer, L., Eggermann, E., Saint-Mleux, B., Machard, D., Jones, B. E., Mühlethaler, M., et al. (2002). Selective action of orexin (hypocretin) on nonspecific thalamocortical projection neurons. J. Neurosci. 22, 7835–7839. doi: 10.1523/JNEUROSCI.22-18-07835.2002
Bayliss, W. M., and Starling, E. H. (1902). The mechanism of pancreatic secretion. J. Physiol. 28, 325–353. doi: 10.1113/jphysiol.1902.sp000920
Bisetti, A., Cvetkovic, V., Serafin, M., Bayer, L., Machard, D., Jones, B. E., et al. (2006). Excitatory action of hypocretin/orexin on neurons of the central medial amygdala. Neuroscience 142, 999–1004. doi: 10.1016/j.neuroscience.2006.07.018
Boag, S. (2014). Ego, drives, and the dynamics of internal objects. Front. Psychol. 5:0666. doi: 10.3389/fpsyg.2014.00666
Borgland, S. L., Storm, E., and Bonci, A. (2008). Orexin B/hypocretin 2 increases glutamatergic transmission to ventral tegmental area neurons. Eur. J. Neurosci. 28, 1545–1556. doi: 10.1111/j.1460-9568.2008.06397.x
Bowlby, J. (1960). Separation anxiety: a critical review of the literature. J. Child Psychol. Psychiatry 1, 251–269. doi: 10.1111/j.1469-7610.1960.tb01999.x
Bowlby, J. (1973). Separation, Anxiety and Anger: Attachment and Loss, Vol. 2. London: Hogarth Press.
Brakel, L. A. W., and Shervin, H. (2005). Anxiety, attributional thinking and primary process. Int. J. Psychoanal. 86, 1679–1693.
Cluderay, J. E., Harrison, D. C., and Hervieu, G. J. (2002). Protein distribution of the orexin-2 receptor in the rat central nervoius system. Regul. Pept. 104, 131–144. doi: 10.1016/S0167-0115(01)00357-3
Conrad, K. L., Davis, A. R., Silberman, Y., Sheffler, D. J., Shields, A. D., Saleh, S. A., et al. (2012). Yohimbine depresses excitatory transmission in BNST and impairs extinction of cocaine place preference through orexin-dependent, norepinephrine-independent processes. Neuropsychopharmacology 37, 2253–2266. doi: 10.1038/npp.2012.76
Craig, A. D. (2002). How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 3, 655–666. doi: 10.1038/nrn894
Cruz, M. T., Herman, M. A., Cote, D. M., Ryabinin, A. E., and Roberto, M. (2013). Ghrelin increases GABAergic transmission and interacts with ethanol actions in the rat central nucleus of the amygdala. Neuropsychopharmacology 38, 364–375. doi: 10.1038/npp.2012.190
Cunningham, R. L., Lumina, A. R., and McGinnis, Y. (2012). Andrgen receptors, sex behavior, and aggression. Neuroendocrinology 96, 131–140. doi: 10.1159/000337663
Damasio, A. R. (1999). The Feeling of What Happens: Body and Emotion in the Making of Consciousness. New York, NY: Harcourt Brace.
Damasio, A. R., and Carvalho, G. B. (2013). The nature of feelings: evolutionary and neurobiological origins. Nat. Rev. Neurosci. 14, 143–152. doi: 10.1038/nrn3403
Davis, M. (2000). “The role of the amygdala in conditioned and unconditioned fear and anxiety,” in The Amygdala, Vol. 2, ed. J. P. Aggleton (Oxford: Oxford University Press), 213–287.
de Lecea, L., Kilduff, T. S., Peyron, C., Gao, X., Foye, P. E., Danielson, P. E., et al. (1998). The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl. Acad. Sci. U.S.A. 95, 322–327. doi: 10.1073/pnas.95.1.322
Dehaene, S., Changeux, J.-P., Naccache, L., Sackur, J., and Sergent, C. (2006). Conscious, preconscious, and subliminal processing: a testable taxonomy. ıTrends Cogn. Sci. 10, 204–211. doi: 10.1016/j.tics.2006.03.007
Dominguez-Borras, J., Armony, J.-L., Maravita, A., Driver, J., and Vuilleumier, P. (2013). Partial recovery of visual extinction by pavlovian conditioning in a patient with hemispatial neglect. Cortex 49, 891–898. doi: 10.1016/j.cortex.2012.11.005
Egecioglu, E., Jerlhag, E., Salom, N., Skibicka, K. P., Haage, D., Bohlooly, Y. M., et al. (2010). Ghrelin increases intake of rewarding food in rodents. Addict. Biol. 15, 304–311. doi: 10.1111/j.1369-1600.2010.00216.x
Fanselow, M. S. (1994). Neural organization of the defensive behavior system responsible for fear. Psychon. Bull. Rev. 1, 429–438. doi: 10.3758/BF03210947
Feldman Barrett, L. (2017). The theory of constructed emotions: an active inference account of interoception and categorization. Soc. Cogn. Affect. Neurosci. 12, 17–23. doi: 10.1093/scan/nsw154
Ferre, S., Diamond, I., Goldberg, S. R., Yao, L., Hourani, S. M. O., Huang, Z. L., et al. (2007). Adenosine A2A receptors in ventral striatum, hypothalamus and nociceptive circuitry. Implications for drug addiction, sleep and pain. Prog. Neurobiol. 83, 332–347. doi: 10.1016/j.pneurobio.2007.04.002
Freud, A. (1960). Discussion of Dr. John Bowlby’s paper. Psychoanal. Study Child 15, 53–62. doi: 10.1080/00797308.1960.11822567
Freud, S. (1905). “Three essays on the theory of sexuality,” in The Standard Edition of the Complete Psychological Works of Sigmund Freud, ed. J. Strachey (London: The Hogarth Press), 1457–1552.
Freud, S. (1915a). “Instincts and their vicissitudes,” in The Standard Edition of the Complete Psychological Works of Sigmund Freud, Vol. 7, ed. J. Strachey (London: The Hogarth Press), 109–140.
Freud, S. (1915b). “Repression,” in The Standard Edition of the Complete Psychological Works of Sigmund Freud, ed. J. Strachey (London: The Hogarth Press), 2975–2988.
Freud, S. (1915c). “The unconscious,” in The Standard Edition of the Complete Psychological Works of Sigmund Freud, ed. J. Strachey (London: The Hogarth Press), 2989–3024.
Freud, S. (1923). “The Ego and the Id,” in The Standard Edition of the Complete Psychological Works of Sigmund Freud, ed. J. Strachey (London: The Hogarth Press), 3946–3992.
Freud, S. (1926a). “Inhibitions, symptoms and anxiety,” in The Standard Edition of the Complete Psychological Works of Sigmund Freud, ed. J. Strachey (London: The Hogarth Press), 77–175.
Freud, S. (1926b). “The question of lay analysis: conversations with an impartial person,” in The Standard Edition of the Complete Psychological Works of Sigmund Freud, ed. J. Strachey (London: The Hogarth Press), 4325–4398.
Fui, M. N. T., Dupuis, P., and Grossmann, M. (2014). Lowered testosterone in male obesity: mechanisms, morbidity and management. Asian J. Androl. 16, 223–231. doi: 10.4103/1008-682X.122365
Gilbert, P. (2014). The origins and nature of compassion focused therapy. Br. J. Clin. Psychol. 53, 6–41. doi: 10.1111/bjc.12043
Gilbert, P. (2015). An evolutionary approach to emotion in mental health with a focus on affiliative emotions. Emot. Rev. 7, 230–237. doi: 10.1177/1754073915576552
Gillath, O., Shaver, P. R., Baek, J.-M., and Chun, D. S. (2008). Genetic correlates of adult attachment style. Pers. Soc. Psychol. Bull. 34, 1396–1405. doi: 10.1177/0146167208321484
Gneezy, U., Meier, S., and Rey-Biel, P. (2011). When and why incentives (don’t) work to modify behavior. J. Econ. Perspect. 25, 191–210. doi: 10.1257/jep.25.4.191
Goodman, R. R., and Snyder, S. H. (1982). Auroradiographic localization of adenosine receptors in rat brain using [3H]cyclohexyladenosine. J. Neurosci. 2, 1230–1241. doi: 10.1523/JNEUROSCI.02-09-01230.1982
Govindaiah, G., and Cox, C. L. (2006). Modulation of thalamic neuron excitability by orexins. Neuropharmacology 51, 414–425. doi: 10.1016/j.neuropharm.2006.03.030
Greenwald, A. G., Draine, S. C., and Abrams, R. L. (1996). Three cognitive markers of unconscious semantic activation. Sciene 273, 1699–1702. doi: 10.1126/science.273.5282.1699
He, F., Yu, P., and Wu, R. (2013). Relationship between sexual satiety and motivation, brain androgen receptors and testosterone in male mandarin voles. Behav. Brain Res. 250, 257–263. doi: 10.1016/j.bbr.2013.05.022
Holder, A. (1970). “Instinct and drive,” in Basic Psychoanalytic Concepts on the Theory of Instincts, Vol. 3, ed. H. Nagera (London: George Allen & Unwin Ltd.), 19–22.
Huang, H., Ghosh, P., and van den Pol, A. N. (2006). Prefrontal cortex-projecting glutamatergic thalamic paraventricular nucleus-excited by hypocretin: a feedforward circuit that may enhance cognitive arousal. J. Neurophysiol. 95, 1656–1668. doi: 10.1152/jn.00927.2005
Huang, J. Y., and Bargh, J. A. (2014). The selfish goal: autonomously operating motivational structures as the proximate cause of human judgement and behavior. Behav. Brain Sci. 37, 121–135. doi: 10.1017/S0140525X13000290
Ishibashi, M., Takano, S., Yanagida, H., Takatsuna, M., Nakajima, K., Oomura, Y., et al. (2005). Effects of orexins/hypocretins on neuronal activity in the paraventricular nucleus of the thalamus in rats in vitro. Peptides 26, 471–481. doi: 10.1016/j.peptides.2004.10.014
James, M. H., Campbell, E. J., and Dayas, C. V. (2017). Role of the orexin/hypocretin system in stress-related psychiatric disorders. Curr. Topics Behav. Neurosci. 33, 197–220. doi: 10.1007/7854_2016_56
Jenkins, T. A., Chai, S. Y., and Mendelsohn, F. A. (1997). Upregulation of angiotensin II AT1 receptors in the mouse nucleus accumbens by chronic haloperidol treatment. Brain Res. 748, 137–142. doi: 10.1016/S0006-8993(96)01276-0
Jerlhag, E., Egecioglu, E., Dickson, S. L., Douhan, A., Svensson, L., and Engel, J. A. (2007). Ghrelin administration into tegmental areas stimulates locomotor activity and increases extracellular concentration of dopamine in the nucleus accumbens. Addict. Biol. 12, 6–16. doi: 10.1111/j.1369-1600.2006.00041.x
Kapp, B. S., Wilson, A., Pascoe, J. P., Supple, W. F., and Whalen, P. J. (1990). “A neuroanatomical systems analysis of conditioned bradycardia in the rabbit,” in Neurocomputations and Learning: Foundations of Adaptive Networks, eds M. Gabriel and J. Moore (New York, NY: Bradford Books), 55–90.
Kirsch, M., and Mertens, W. (2018). On the drive specificity of freudian drives for the generation of SEEKING activities: the importance of the underestimated imperative motor factor. Front. Psychol. 9:616. doi: 10.3389/fpsyg.2018.00616
Kojima, M., Hosoda, H., Date, Y., Nakazato, M., and Matsuo, H. (1999). Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402, 656–660. doi: 10.1038/45230
Korotkova, T. M., Sergeeva, O. A., Eriksson, K. S., Haas, H. L., and Brown, R. E. (2003). Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J. Neurosci. 23, 7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003
Kottusch, P., Tillman, M., and Püscheö, K. (2009). Survival time without food and drink. Arch. Kriminol. 224, 184–191.
Lang, P. J., and Bradley, M. M. (2018). “What is emotion? A natural science perspective,” in The Nature of Emotion: Fundamental Questions, eds A. S. Fox, R. C. Lapate, A. J. Shackman, and A. J. Davidson (New York, NY: Oxford University Press), 11–14.
LeDoux, J., and Brown, R. (2017). A higher-order theory of emotional consciousness. Proc. Natl. Acad. Sci. U.S.A. 114, E2016–E2025. doi: 10.1073/pnas.1619316114
LeDoux, J. E., Iwata, J., Cichettie, P., and Reis, D. J. (1988). Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J. Neurosci. 8, 2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988
Lenkei, Z., Palkovits, M., Corvol, P., and Llorens-Cortes, C. (1996). Distribution of angiotensin II type-2 receptor (AT2) mRNA expression in the adult rat brain. J. Comp. Neurol. 373, 322–339. doi: 10.1002/(SICI)1096-9861(19960923)373:3<322::AID-CNE2>3.0.CO;2-4
Li, J., Hu, Z., and de Lecea, L. (2014). The hypocretins/orexins: integrators of multiple physiological functions. Br. J. Pharmacol. 171, 332–350. doi: 10.1111/bph.12415
Li, Y., Wang, H., Qi, K., Cheng, X., Li, S., Sui, N., et al. (2011). Orexins in the midline thalamus are involved in the expression of conditioned place aversion to morphine withdrawal. Physiol. Behav. 102, 42–50. doi: 10.1016/j.physbeh.2010.10.006
Lorenz, K., and Leyhausen, P. (1973). Motivation of Human and Animal Behavior; An Ethological View. New York, NY: Van Nostrand-Reinhold.
Lungwitz, E. A., Molosh, A., Johnson, P. L., Harvey, B. P., Dirks, R. C., Dietrich, A., et al. (2012). Orexin-A induces anxiety-like behavior through interactions with glutamatergic receptors in the bed nucleus of the stria terminalis of rats. Physiol. Behav. 107, 726–732. doi: 10.1016/j.physbeh.2012.05.019
Marcus, J. N., Aschkenasi, C. J., Lee, C. E., Chemelli, R. M., Saper, C. B., Yanagisawa, M., et al. (2001). Differential expression of orexin receptors 1 ana 2 in the brain. J. Comp. Neurol. 435, 6–25. doi: 10.1002/cne.1190
Mattes, R. D. (2010). Hunger and thirst: issues in measurement and prediction of eating and drinking. Physiol. Behav. 100, 22–32. doi: 10.1016/j.physbeh.2009.1012.1026
Mendelsohn, F. A. O., Quirion, R., Saavedra, J. M., Aguilera, G., and Catt, K. J. (1984). Autoradiographic localization of angiotensin II receptors in rat brain. Proc. Natl. Acad. Sci. U.S.A. 81, 1575–1579. doi: 10.1073/pnas.81.5.1575
Mitchell, V., Bouret, S., Beauvillain, J. C., Schilling, A., Perret, M., Kordon, C., et al. (2001). Comparative distribution of mRNA encoding the growth hormone secretagogue-receptor (GHS-R) in Microcebus murinus (primate, lemurian) and rat forebrain and pituitary. J. Comp. Neurol. 429, 469–485. doi: 10.1002/1096-9861(20010115)429:3<469::AID-CNE8>3.0.CO;2
Muroya, S., Funahashi, H., Yamanaka, A., Kohno, D., Uramura, K., Nambu, T., et al. (2004). Orexins (hypocretins) directly interact with neuropeptide Y, POMC and glucose-responsive neurons to regulate Ca 2+ signaling in a reciprocal manner to leptin: orexigenic neuronal pathways in the mediobasal hypothalamus. Eur. J. Neurosci. 19, 1524–1534. doi: 10.1111/j.1460-9568.2004.03255.x
Muschkamp, J. W., Dominguez, J. M., Sato, S. M., Shen, R.-Y., and Hull, E. M. (2007). A role for hypocretin (orexin) in male sexual behavior. J. Neurosci. 27, 2837–2845. doi: 10.1523/JNEUROSCI.4121-06.2007
Muschkamp, J. W., Hollander, J. A., Thompson, J. L., Voren, G., Hassinger, L. C., Onvani, S., et al. (2014). Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proc. Natl. Acad. Sci. U.S.A. 111, E1648–E1655. doi: 10.1073/pnas.1315542111
Naleid, A. M., Grace, M. K., Cummings, D. E., and Levine, A. S. (2005). Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides 26, 2274–2279. doi: 10.1016/j.peptides.2005.04.025
Nieuwenhuys, R. (1985). Chemoarchitecture of the Brain. Berlin: Springer-Verlag. doi: 10.1007/978-3-642-70426-0
Panksepp, J. (1998). Affective Neuroscience: The Foundations of Human and Animal Emotions. Oxford: Oxford University Press.
Panksepp, J. (2016). The cross-mammalian neurophenomenology of primal emotional affects: from animal feelings to human therapeutics. J. Comp. Neurol. 524, 1624–1635. doi: 10.1002/cne.23969
Panksepp, J. (2018). “At primal levels, vast subcortical brain networks mediate instinctual emotional reactions that hepl program higher-order emotional-cognitive abilities in higher regions of the brain and mind,” in The Nature of Emotion: Fundamental Questions, eds A. S. Fox, R. C. Lapate, A. J. Shackman, and A. J. Davidson (New York, NY: Oxford University Press), 99–104.
Panksepp, J., and Yovell, Y. (2014). Preclinical modeling of primal emotional affects (SEEKING, PANIC and PLAY): gateways to the development of new treatments for depression. Psychopathology 47, 383–393. doi: 10.1159/000366208
Peyron, C., Tighe, D. K., van den Pol, A. N., de Lecea, L., Heller, H. C., Sutcliffe, J. G., et al. (1998). Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci. 18, 9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998
Rau, A. R., Ariwodola, O. J., and Weiner, J. L. (2014). Presynaptic adenosine A1 receptors modulate excitatory transmission in the rat basolateral amygdala. Neuropharmacology 77, 465–474. doi: 10.1016/j.neuropharm.2013.10.029
Rau, A. R., Ariwodola, O. J., and Weiner, J. L. (2015). Postsynaptic adenosine A2A receptors modulate intrinsic excitability of pyramidal cells in the rat basolateral amygdala. Int. J. Neuropsychopharmacol. 18:pyv017. doi: 10.1093/ijnp/pyv1017
Rosin, D. L., Hettinger, B. D., and Lee, A. (2003). Anatomy of adenosine A2A receptors in brain: morphological substrates for intergration of striatal function. Neurology 61, S12–S18. doi: 10.1212/01.WNL.0000095205.33940.99
Sakurai, T., Amemiya, A., Ishii, M., Matsuzaki, I., Chemelli, R. M., Tanaka, H., et al. (1998). Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92, 573–585. doi: 10.1016/S0092-8674(00)80949-6
Saydoff, J. A., Rittenhouse, P. A., Van de Kar, L. D., and Brownfields, M. S. (1991). Enhanced serotonergic transmission stimulates oxytocin secretion in conscious male rats. J. Pharmacol. Exp. Ther. 257, 95–99.
Schalkwijk, F. (2018). A new conceptualization of the conscience. Front. Psychol. 9:1863. doi: 10.3389/fpsyg.2018.01863
Scherer, K. R. (1984). “On the nature and function of emotion: a component process approach,” in Approaches to Emotion, eds K. R. Scherer and P. Ekman (Hilldale, NJ: L. Erlbaum Associates).
Scherer, K. R. (2009). The dynamic architecture of emotion: evidence for the component process model. Cogn. Emot. 23, 1307–1351. doi: 10.1080/02699930902928969
Shervin, H., and Fritzler, D. (1968). Visual evoked response correlates of unconscious mental processes. Science 161, 295–298. doi: 10.1126/science.161.3838.295
Shughrue, P. J., Lane, M. V., and Merchenthaler, I. (1997). Comparative distribution of Estrogen receptor-a and -b mRNA in the rat central nervous system. J. Comp. Neurol. 388, 507–525. doi: 10.1002/(SICI)1096-9861(19971201)388:4<507::AID-CNE1>3.0.CO;2-6
Simerly, R. B., Chang, C., Muramatsu, M., and Swanson, L. W. (1990). Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J. Comp. Neurol. 294, 76–95. doi: 10.1002/cne.902940107
Simoes, A. P., Machado, N. J., Goncalves, N., Kaster, M. P., Simoes, A. T., Nunes, A., et al. (2016). Adenosine A2A receptors in the amygdala control synaptic plasticity and contextual fear memory. Neuropsychopharmacology 41, 2862–2871. doi: 10.1038/npp.2016.98
Smith, B. N., Davis, S. F., van den Pol, A. N., and Xu, W. (2002). Selective enhancement of excitatory synaptic activity in the rat nucleus tractus solitarius by hypocretin 2. Neuroscience 15, 707–714. doi: 10.1016/S0306-4522(02)00488-8
Solms, M. (1997). What is consciousness? J. Am. Psychoanal. Assoc. 45, 681–778. doi: 10.1177/00030651970450031201
Solms, M. (2019). The hard problem of consciousness and the free energy principle. Front. Psychol. 9:2714. doi: 10.3389/fpsyg.2018.02714
Solms, M., and Panksepp, J. (2012). The “id” knows more than the “ego”admits: neuropsychoanalytic and primal consciousness perspectives on the interface between affective and cognitive neuroscience. Brain Sci. 2, 147–175. doi: 10.3390/brainsci2020147
Solomon, R. C. (1974). Freud and ‘unconsciouis motivation’. J. Theory Soc. Behav. 4, 191–216. doi: 10.1111/j.1468-5914.1974.tb00337.x
Stievenard, A., Mequinion, M., Andrews, Z. B., Destee, A., Chartier-Harlin, M.-C., Viltart, O., et al. (2017). Is there a role for ghrelin in central dopaminergic systems? Focus on nigrostriatal and mesocorticolimbic pathways. Neurosci. Biobehav. Rev. 73, 255–275. doi: 10.1016/j.neubiorev.2016.11.021
Strombach, T., Strang, S., Park, S. Q., and Kenning, P. (2016). “Common and distinctive approaches to motivation in different disciplines,” in Progress In Brain Research, Motivation: Theory, Neurobiology and Applications, Vol. 229, eds B. Studer and S. Knecht (Amsterdam: ELSEVIER), 3–23. doi: 10.1016/bs.pbr.2016.06.007
Svennigsson, P., Hall, H., Sedvall, G., and Fredholm, B. B. (1997). Distribution of adenosine receptors in the postmortem human brain: an extended autoradiographic study. Synapse 27, 322–335. doi: 10.1002/(SICI)1098-2396(199712)27:4<322::AID-SYN6>3.0.CO;2-E
Thakhar, M. M., Winston, S., and McCarley, R. W. (2002). Orexin neurons of the hypothalamus express adenosine A1 receptors. Brain Res. 944, 190–194. doi: 10.1016/S0006-8993(02)02873-1
Tinbergen, N. (1950). The hierarchical organization of nervous mechanisms underlying instinctive behaviour. Symp. Soc. Exp. Biol. 4, 305–312.
Toshinai, K., Date, Y., Murakami, N., Shimada, M., Monda, M., Shimbara, T., et al. (2003). Ghrelin-induced food intake is mediated via the orexin pathway. Endocrinology 144, 1506–1512. doi: 10.1210/en.2002-220788
Tracy, J. L., Robins, R. W., and Tangney, J. P. (2007). The Self-Oriented Emotions: Theory and Research. New York, NY: Guilford Press.
Trivedi, P., Yu, H., MacNeil, D. J., van der Ploeg, L. H., and Guan, X. M. (1998). Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 438, 71–75. doi: 10.1016/S0014-5793(98)01266-6
Uramura, K., Funahashi, H., Muroya, S., Shioda, S., Takigawa, M., and Yada, T. (2001). Orexin-a activates phospholipase C- and protein kinase C-mediated Ca2+ signaling in dopamine neurons of the ventral tegmental area. Neuroreport 12, 1885–1889. doi: 10.1097/00001756-200107030-00024
Uvnäs Moberg, K., and Prime, D. K. (2013). Oxytocin effects in mothers and infants during breastfeeding. Infant 9, 201–206.
Vuilleumier, P. (2015). Affective and motivational control of vision. Curr. Opin. Neurol. 28, 29–35. doi: 10.1097/WCO.0000000000000159
Watson, C. J., Baghdoyan, H. A., and Lydic, R. (2010). Neuropharmacology of sleep and wakefulness. Sleep Med. Clin. 5, 513–528. doi: 10.1016/j.jsmc.2010.08.003
Watt, D. F. (2017). Reflections on the neurosceintific legacy of Jaak Panksepp (1943–2017). Neuropsychoanalysis 19, 183–198. doi: 10.1080/15294145.2017.1376549
Watt, D. F., and Panksepp, J. (2009). Depression: an evolutionarily conserved mechanism to terminate separation distress? A review of aminergic, peptidergic, and neural network perspectives. Neuropsychoanalysis 11, 7–51. doi: 10.1016/j.neubiorev.2011.01.003
Webster, R. A., and Stanford, S. C. (2001). Neurotransmitter, Drugs and Brain Function. Chichester: John Wiley & Sons, LTD., doi: 10.1002/0470846577
Wright, J. S., and Panksepp, J. (2012). An evolutionary framework to understand foraging, wanting, and desire: the neuropsychology of the SEEKING system. Neuropsychoanalysis 14, 5–39. doi: 10.1080/15294145.2012.10773683
Yang, B., and Ferguson, A. V. (2003). Orexin-a depolarizes nucleus tractus solitarius neurons through effects on nonselective cationic and K+ conductances. J. Neurophysiol. 89, 2167–2175. doi: 10.1152/jn.01088.2002
Yoshida, T., Semprun-Prieto, L., Wainford, R. D., Sukhanov, S., Kapusta, D. R., and Delafontaine, P. (2012). Angiotensin II reduces food intake by altering orexigenic neuropeptide expression in the mouse hypothalamus. Endocrinology 153, 1411–1420. doi: 10.1210/en.2011-1764
Yoshimoto, K., Nagao, M., Watanabe, Y., Yamaguchi, T., Ueda, S., Kitamura, Y., et al. (2017). Enhanced alcohol-drinking behavior associated with active ghrelinergic and serotoninergic neurons in the lateral hypothalamus and amygdala. Pharmacol. Biochem. Behav. 153, 1–11. doi: 10.1016/j.pbb.2016.12.001
Zellner, M. R., Watt, D. F., Solms, M., and Panksepp, J. (2011). Affective neuroscientific and neuropsychoanalytic approaches to two intractable psychiatric problems: why depression feels so bad and what addicts really want. Neurosci. Biobehav. Rev. 35, 2000–2008. doi: 10.1016/j.neubiorev.2011.01.003
Keywords: SEEKING, affective neuroscience, orexin, Bowlbyian drive, unconscious
Citation: Kirsch M (2019) On the Abilities of Unconscious Freudian Motivational Drives to Evoke Conscious Emotions. Front. Psychol. 10:470. doi: 10.3389/fpsyg.2019.00470
Received: 06 December 2018; Accepted: 18 February 2019;
Published: 07 March 2019.
Edited by:
Simon Boag, Macquarie University, AustraliaReviewed by:
Patrick Connolly, Hong Kong Shue Yan University, Hong KongCopyright © 2019 Kirsch. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael Kirsch, TWljaGFlbC5raXJzY2hAdW5pLWR1ZS5kZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.