
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychol. , 22 August 2017
Sec. Psychology of Language
Volume 8 - 2017 | https://doi.org/10.3389/fpsyg.2017.01277
This article is part of the Research Topic The Janus-Face of language: Where are the emotions in words and the words in emotions? View all 26 articles
According to embodiment theories, language and emotion affect each other. In line with this, several previous studies investigated changes in bodily responses including facial expressions, heart rate or skin conductance during affective evaluation of emotional words and sentences. This study investigates the embodiment of emotional word processing from a social perspective by experimentally manipulating the emotional valence of a word and its personal reference. Stimuli consisted of pronoun-noun pairs, i.e., positive, negative, and neutral nouns paired with possessive pronouns of the first or the third person (“my,” “his”) or the non-referential negation term (“no”) as controls. Participants had to quickly evaluate the word pairs by key presses as either positive, negative, or neutral, depending on the subjective feelings they elicit. Hereafter, they elaborated the intensity of the feeling on a non-verbal scale from 1 (very unpleasant) to 9 (very pleasant). Facial expressions (M. Zygomaticus, M. Corrugator), heart rate, and, for exploratory purposes, skin conductance were recorded continuously during the spontaneous and elaborate evaluation tasks. Positive pronoun-noun phrases were responded to the quickest and judged more often as positive when they were self-related, i.e., related to the reader’s self (e.g., “my happiness,” “my joy”) than when related to the self of a virtual other (e.g., “his happiness,” “his joy”), suggesting a self-positivity bias in the emotional evaluation of word stimuli. Physiologically, evaluation of emotional, unlike neutral pronoun-noun pairs initially elicited an increase in mean heart rate irrespective of stimulus reference. Changes in facial muscle activity, M. Zygomaticus in particular, were most pronounced during spontaneous evaluation of positive other-related pronoun-noun phrases in line with theoretical assumptions that facial expressions are socially embedded even in situation where no real communication partner is present. Taken together, the present results confirm and extend the embodiment hypothesis of language by showing that bodily signals can be differently pronounced during emotional evaluation of self- and other-related emotional words.
Theoretical considerations have long been emphasizing the independence of language and emotion. Semantic network models of language, for instance, consider language mainly as a cognitive phenomenon, its representation bearing no direct relation to sensory, sensorimotor or affective processes in the brain or the body (e.g., Semin and Smith, 2008; or Winkielman et al., 2015 for an overview). However, neurophysiologic research has proven otherwise: language and emotion processing affect each other. This has been shown for the processing of simple words (e.g., Martín-Loeches et al., 2001; Tabert et al., 2001; Kuchinke et al., 2005; Kissler et al., 2007; Herbert et al., 2008, 2009; Scott et al., 2009) and sentences (e.g., Bayer et al., 2010; Jiménez-Ortega et al., 2012). Regarding the processing of single words, rapid serial presentation of words with emotional content, in several studies, facilitated both initial stimulus processing in the visual cortex and subsequent recall performance of emotional words (e.g., Kissler et al., 2007; Herbert et al., 2008). Moreover, in several studies, reading emotional words activated emotional brain structures such as the amygdala (Hamann, 2001; Tabert et al., 2001; Kuchinke et al., 2005; Hazlett et al., 2007; Herbert et al., 2009) and induced changes in affective behavior including priming of approach and avoidance including defensive responses like the startle-reflex (e.g., Herbert et al., 2006; Herbert and Kissler, 2010; Citron et al., 2016). Presentation of emotional words also influences the perception and appraisal of non-verbal emotional signals: on a behavioral (e.g., Lindquist et al., 2006) as well as on a neural or physiological level (Lieberman et al., 2007; Moseley et al., 2012; Herbert et al., 2013a,c), having implications for the treatment of clinical and neurological disorders (Roberson et al., 2007; Kircanski et al., 2012).
Thus, preferential processing of emotional words, activation of emotional brain structures as well as changes in affective behavior during word processing could be taken as evidence for theories of embodiment arguing that written language is able to elicit, modulate and regulate emotional processes in the brain and the body (see Niedenthal, 2007; Glenberg et al., 2009).
This, however, raises the question about the social relevance of embodied language processing. Expressing one’s own emotions to others as well as inducing emotions in others is a key function of spoken and written language. This holds true even in situations in which no direct face-to-face communication is possible: for instance, we text, blog, and tweet our sentiments to others and “like/dislike” others for their affection. But to what extent is language processing embodied when we assess and appraise emotional content related to one’s own self (e.g., “my fear”) or the self of another person (e.g., “his fear”), especially in contexts and situations where input from non-verbal modalities is not readily available to the perceiver of the message? In other words, will bodily, peripheral-physiological reactions differ as a function of the valence or as a function of the self-other reference of a word? Crucially, what does this mean theoretically for the embodiment of language and more generally for the embodiment of emotional communication?
Regarding emotional communication, the human face has been considered an important “socio-emotional signal detector,” even in the absence of direct face-to-face communication (e.g., Fridlund, 1991; Buck, 1994; Hess et al., 1995). Whether facial expressions are, however, more important for understanding one’s own rather than other people’s emotions is still under scientific debate. For instance, Hess et al. (1992) could demonstrate that spontaneous elicitation of facial expressions influences primarily the perception of one’s own subjective emotional experiences. Other studies found that people spontaneously mimic other people’s emotional expressions (Chartrand and Bargh, 1999), even if the other is only imagined as a virtual other (Fridlund, 1991). In these latter views, spontaneous simulations of emotions via facial expressions are not just reflexive readouts of one’s own emotions (e.g., Buck, 1994 for an overview) but may preferentially occur in response to other-related emotional stimuli, in particular to positive stimuli (Fridlund, 1991). One purpose of this “sociality effect” (Fridlund, 1991) could be to help evaluate the hedonic quality of other-related stimuli by using one’s own facial expressions as proxy.
Regarding language processing, involvement of facial expressions has been reported in several recent studies recording facial muscle activity during emotional evaluation of words and sentences (electromyography, EMG; e.g., Foroni and Semin, 2009; Niedenthal et al., 2009; Havas et al., 2010). These studies revealed that reading positive words or sentences is accompanied by activation of the main facial muscle used for smiling, M. Zygomaticus, whereas reading words, sentences, or statements with negative content is accompanied by activation of the main facial muscle used for frowning, M. Corrugator (see also Foroni and Semin, 2009; Niedenthal et al., 2009; Foroni and Semin, 2011). Moreover, negating the emotional meaning of a positive statement has been found to be associated with attenuated M. Zygomaticus activity (Foroni and Semin, 2013), suggesting that changes in facial expressions during emotional word processing are related to semantic processing and word comprehension. This is also suggested by recent observations about physiological or experimental manipulation of facial muscle activity, including studies on facial Botox treatment or suppression of the facial musculature by holding a pen with the lips or teeth indicating that inhibiting facial expressions impairs specifically the comprehension (Havas et al., 2010) and emotional evaluation of emotional statements (Niedenthal et al., 2009; also see Strack et al., 1988 using cartoons). In addition, changes in facial muscle activity have been found to be more pronounced in language tasks affording emotional instead of cognitive evaluation (Niedenthal et al., 2009) and for concrete compared to abstract emotional words (Foroni and Semin, 2009, 2013), although, overall, changes in facial muscle activity seem to be less pronounced for written words than for pictures or scenes (Larsen et al., 2003), probably due to the lower arousal of word as compared to picture stimuli.
Taken together, the aforementioned findings support the idea of facial expressions being paramount for the decoding and appraisal of the emotional meaning of language stimuli. However, previous language studies have not considered the influence social factors may have on emotional language processing, leaving open the theoretical question of whether participants will be mimicking more during emotional evaluation of other-related than self-related emotional words, or vice versa.
Regarding the perception of one’s own emotions, historically (see e.g., Sorabji, 1992; Höffe et al., 2005) and metaphorically, the heart has been proposed as the central core of one’s own feelings. In fact, individuals who are able to accurately detect their own heart beats experience emotions with heightened intensity (Wiens et al., 2000). They also seem to intuitively make use of their cardio-visceral reactions for decision making (Dunn et al., 2006; Werner et al., 2009) although this does not always promote favorable decisions (Dunn et al., 2010). Additionally, changes in cardiac cycle as well as in parasympathetic tone, as measured by heart rate variability (HRV), can influence social cognition, emotional stimulus processing, and later semantic memory retrieval (Wallentin et al., 2011; Quintana et al., 2012; Garfinkel et al., 2013). Even though these studies do show that interactions between emotional, mental, and cognitive processing are accompanied by cardiac changes, regarding emotion and language processing, only a few studies have investigated stimulus-driven changes in mean HR during processing of emotional words. The studies available used a mix of spoken or written (synthesized) words, sentences, and stories, in combination with autobiographical imagery, recall, or cognitive instructions (Vrana et al., 1986; Ilves and Surakka, 2012), or presented highly selective self-relevant stimulus materials such as threat words or body words to particular samples of individuals at risk for anxiety (Thayer et al., 2000) or eating disorders (Herbert et al., 2013b), impeding the generalizability of the results.
Crucially, with a few exceptions, previous studies did not explicitly control for the words’ personal reference, i.e., whether the emotional content of a word was related to the reader’s own self or the self of another person. In an earlier study by Cacioppo et al. (1985), positive and negative trait adjectives were presented to healthy students who were asked to judge each word according to orthographic and grammatical rules, or to evaluate the words for hedonic pleasure (“is this word good?”) and self-descriptiveness (“does this trait describe you?”). Mean HR differed during semantic (emotional and self-related) and non-semantic (orthographic and grammatical) evaluation. However, mean HR did not differ significantly between the emotional and self-referential evaluation tasks, suggesting no specific influence of the self-relatedness of the task on changes in HR during word processing. This observation contrasts with findings from text-driven imagery where often considerably strong HR acceleration patterns were reported during imagery of autobiographic, self-related emotional scenes (Vrana and Rollock, 2002). Thus, so far, no clear picture has emerged with regard to whether HR varies as a function of the personal reference of a word (i.e., self- vs. other-reference), or whether during word processing changes in HR indicate differences in emotional content (positive, negative, or neutral) and depth of stimulus elaboration (e.g., Cacioppo et al., 1985), regardless of the word’s personal reference.
Regarding neurophysiological processes in the brain, self-reference seems to be uniquely linked to emotional processing (e.g., Northoff et al., 2006; D’Argembeau et al., 2012). Regarding verbal stimuli (e.g., Esslen et al., 2008; Herbert et al., 2011c), there is evidence that processing of emotional words related to the reader’s self increases activity in anterior cortical midline structures (medial prefrontal cortex, including the anterior cingulate cortex and the ventromedial prefrontal cortex), i.e., brain structures involved in self-referential processing of emotional stimuli (Northoff et al., 2006 for an overview). In addition, electrophysiological studies reported sustained cortical processing and better free recall performance of especially self-related positive words (Watson et al., 2007; Herbert et al., 2011b). Mood congruent processing has been proposed as the possible underpinning of this prioritized processing of positive stimuli related to the self; mildly positive mood being the norm in healthy Western subjects (see Herbert et al., 2011d for modulation with depression; Mezulis et al., 2004; Shi et al., 2016 for cross-cultural findings; Taylor and Brown, 1988).
In view of the observations outlined above, the present study’s aims are to contribute to the so far fragmentary understanding of self-other reference and bodily involvement in language and emotion processing. To this end, a novel paradigm (see Herbert et al., 2011b,c) is deployed to investigate peripheral physiological responses to self- and other-related words with emotional and neutral content. Unlike many previous studies summarized above, in the present paradigm, the emotional valence and the personal reference of a word are altered simultaneously by using pronoun-noun pairs that are related to the reader’s self (e.g., “my fear,” “my joy”) or other-related, i.e., related to the self of a virtual other (e.g., “his fear,” “his joy”). Physiological responses are measured while participants read and quickly judge the pronoun-noun phrases for hedonic pleasure/displeasure and then evaluate them with respect to the intensity of their subjective feelings. An additional set of stimuli consisting of negated emotional and neutral words (e.g., “no fear,” “no happiness,” or “no book”) is included as a control condition to determine whether participants’ spontaneous judgments and their initial physiological reactions will be based on the evaluation of the words’ semantic meaning as proposed by previous research (e.g., Foroni and Semin, 2013). The physiological measures include recording facial muscle activity (fEMG), HR, and skin conductance (electrodermal activity, EDA), the latter being included for exploratory purposes to control for physiological arousal.
Extending previous research, the following questions are addressed: How does self- versus other-reference influence emotional word processing on a behavioral, subjective and peripheral-physiological level? Is the processing of self-related positive words prioritized on a behavioral level indicating better access to one’s own positive emotions in healthy subjects? Is this preference also reflected at a physiological level and associated with changes of fEMG or HR? In particular, do participants respond with differential fEMG to emotional words depending on whether the emotional content is self- or other- related? Lastly, is HR variation during emotional word evaluation sensitive to the emotional valence of a word, the self-reference of a word, or both?
In total, twenty-nine young healthy adults (five males, M = 22.8 years, SD = 2.6; range: 18–28 years), all students of the University of Tübingen, native speakers of German, with normal or corrected to normal vision, and normal depression scores (see Table 1 for an overview) were included in the study. Twenty-eight subjects were non-smokers and one subject reported occasional smoking with less than half a cigarette a day. Caffeine intake was controlled at the day of testing. In addition, habitual drinking habits were assessed by self-report scales. Three subjects were left-handed. Participants were to report that they are currently taking no medication that might affect emotional functioning or interact with the acetylcholinergic system. They provided written informed consent prior to participation and were compensated with an hourly wage of eight Euros in return for participation. The study was approved by the local Ethics Committee (https://www.medizin.uni-tuebingen.de/Forschung/Ethik\_Kommission.html).
Participants were familiarized with the laboratory setting and the experiment was explained to them in general terms before giving informed consent. Participants were asked about social demographics and handedness (German version of Oldfield, 1971), and received written instructions. In particular, participants were instructed that words paired with the possessive pronoun of the third person “his” are related to a virtual other whereas words paired with the possessive pronoun of the first person “my” are related to themselves, e.g., describing the reader’s own emotions. Participants received practice trials and had to repeat the instructions to the experimenter in their own words prior to the start of the experiment to ensure that they had understood the instructions. The main experiment, following the practice trials lasted approximately 60 min. After experimental testing participants completed questionnaires as described subsequently. The state scale of the Positive and Negative Affect Schedule (PANAS state; Watson et al., 1988) and the Beck Depression Inventory II (BDI-II; Hautzinger et al., 2006) were administered to control for mood effects and possible risk for depression. The State-Trait Anxiety Inventory (STAI; Laux et al., 1981) was used to control for state and trait anxiety. The Toronto Alexithymia Scale (TAS-20; Bagby et al., 1994) enables identification of alexithymic individuals who should only have attenuated access to their evoked feelings. The Mehrfachwahl-Wortschatz-Intelligenztest (MWT-B, a verbal IQ test; Lehrl, 2005) allows quantification of familiarity with German language which is relevant for correct processing of the presented stimulus material. Although self-report data was assessed primarily to exclude participants scoring high on alexithymia, depression or anxiety, alexithymia, depression and anxiety scores as well as scores of positive and negative affect were later on also used in exploratory analyses assessing potential interindividual differences in behavioral and physiological measures. At the very end, subjects were asked about potential strategies they might have used during the main experiment and were debriefed if desired.
Stimuli were presented on a computer screen. Participants’ task was to read the words silently and to spontaneously judge the words for hedonic pleasure/displeasure (i.e., “is this word eliciting a positive, negative, or neutral feeling?”) before evaluating each word in detail with respect to the intensity of the subjectively experienced feeling (i.e., “how intense is the feeling elicited by the word?”). Participants were instructed to base their judgments solely on their gut feelings and decide as quickly and as spontaneously as possible. Spontaneous judgments included a quick button press for a coarse valence judgment (negative, neutral, or positive) for which participants had to press one of three keyboard buttons. The response assignment to keys was counterbalanced across participants with the middle button remaining the neutral response for all participants and the left and right buttons altering in response assignment between ‘negative/unpleasant’ and ‘positive/pleasant.’ The subsequent elaborate evaluation, following the spontaneous judgment, included a voice response. For the voice response, the valence scale of the nine-point self-assessment manikin (SAM; Lang, 1980) was presented to participants before the start of the experiment, to familiarize them with the scale, and also after each stimulus block during the experiment as reminder. Participants were told to evaluate the intensity of their stimulus-evoked feelings by naming a number corresponding to the manikin that fits best to the evoked feeling. Number assignments always started with ‘1’ at the outermost left manikin counting up to ‘9’ at the right outermost manikin.
Each trial started with the presentation of a pronoun-noun pair. The pair was presented in upper case in the middle of the computer screen for 4000 ms. The button response had to be given while the stimulus remained at the display. Subsequently, a microphone icon was presented for 4000 ms indicating the voice response interval, in which participants were asked to elaborate the intensity of the feeling elicited by the stimulus during the spontaneous appraisal. Interstimulus intervals were uniformly distributed between 3000 and 4000 ms. An overview of the experimental task is provided in Figure 1.
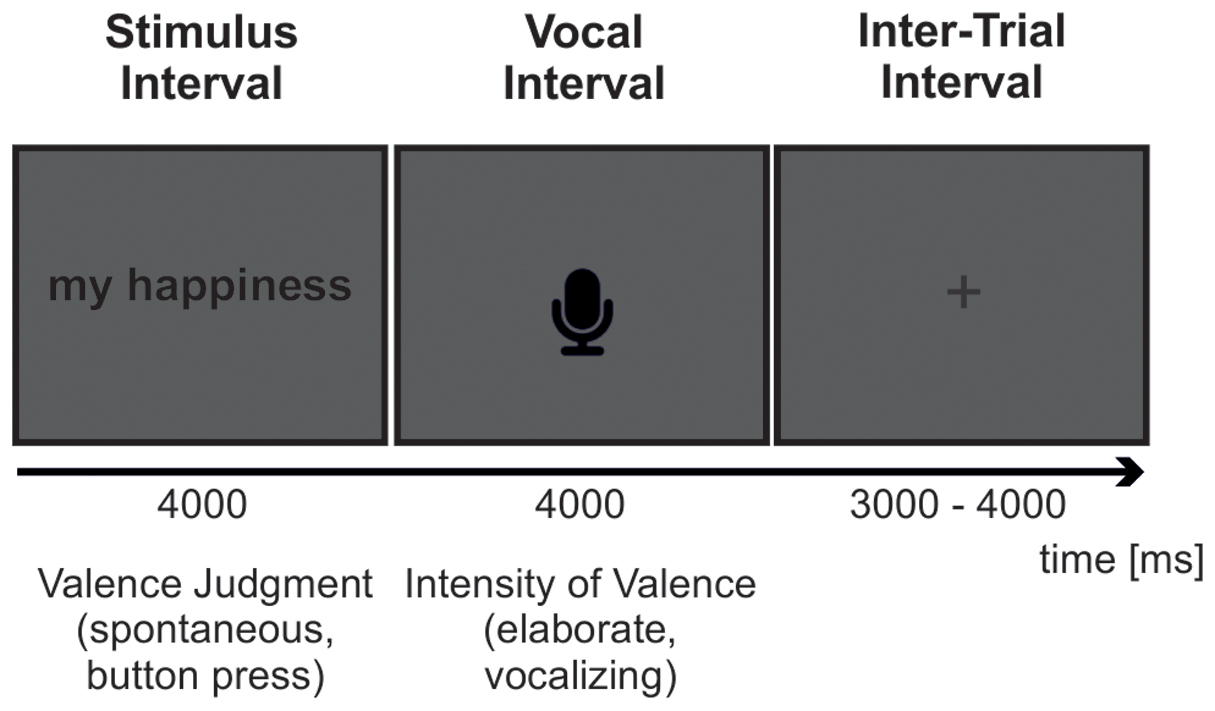
FIGURE 1. Trial Sequence. Each trial consisted of a 4000 ms stimulus interval followed by a 4000 ms vocal response interval and an inter-trial interval uniformly distributed between 3000 and 4000 ms. Subjects had to coarsely judge the pronoun-noun phrases in reference to their feelings using one of three keyboard buttons (spontaneous valence judgments). The button press had to occur during the 4000 ms time window of stimulus presentation. As soon as the microphone icon appeared, subjects had to elaborate the intensity of the evoked feelings by speaking out the numbers ‘one’ to ‘nine’ corresponding to the nine-point self assessment manikin scale of emotional valence (elaborate valence judgment).
Each stimulus consisted of one out of three different pronouns (“mein/e,” German for “my,” “sein/e,” German for “his,” or “kein/e,” German for “no”) and one out of 84 nouns categorized into three different valence categories (negative, neutral, or positive), resulting in a 3∗3 (reference∗valence) design. Nouns were used for valence manipulation; pronouns were used for reference manipulation (“my” for self-reference, “his” for other-reference, and “no” for no reference). Only the male version of the third person German possessive pronoun was used for other-reference because in German language the female version of the third person possessive pronoun could be ambiguous (referring either to “her” or “their”). Each of the 84 nouns was paired with all possible pronouns (e.g., “my fear,” “his fear,” and “no fear”), resulting in 252 trials in total. Trials were presented in blocks, each block consisting of four trials with the same pronoun and the same valence category. A randomized block-design was chosen in order to avoid changes in physiology due to an increase in cognitive load or mental effort which might have been likely to occur when switching one’s evaluation from trial to trial. Therefore, pronoun order was randomized while the following rule was considered: within three consecutive blocks, each pronoun (self-related, other-related, or no-reference) was used once and there were no adjacent blocks with the same pronoun. Valence order was randomized while the following rule was considered: within every nine blocks, every noun valence category was paired with every pronoun exactly once and adjacent blocks never had the same valence.
Nouns were taken from the German affective word list BAWL-R (Võ et al., 2009) and were matched on several dimensions including stimulus valence (-3: very negative to 3: very positive), arousal (1: low arousal to 5: high arousal), imageability (1: low imageability to 7: high imageability), and total frequency of appearance per million words (FTOT). Out of the over 800 nouns included in the BAWL-R with either negative (valence < -1.5), neutral (0.2 ≥ valence ≥-0.2) or positive (valence > 1.5) valence, 28 were selected for each emotional category. The selection procedure was based on matching between valence groups for arousal, FTOT, word length, gender, and compatibility with the used pronouns. To control for compatibility effects between pronouns and nouns, we assessed whether the respective pronouns occurred as significant left occurrences of each of the 84 nouns in our list. For this procedure, a German linguistic corpus (Wortschatz Universität Leipzig1) was used. The procedure used for extracting significant left occurrences within the Wortschatz Universität Leipzig was described by Biemann et al. (2004).
Positive, negative, and neutral nouns differed significantly in arousal, F(2,81) = 38.66, p < 0.001. There was no difference in arousal between positive and negative nouns, T(54) = 0.25, p = 0.81, but between positive and neutral, T(54) = 6.86, p < 0.001, and between negative and neutral, T(54) = 7.90, p < 0.001, nouns. There was no difference in word length, F(2,81) = 0.04, p = 0.96, FTOT, F(2,81) < 0.01, p > 0.99, or imageability, F(2,81) = 2.16, p = 0.12, between valence groups. The clustering into different valence categories was successful, F(2,81) = 18.62, p < 0.001. Negative nouns differed in valence from neutral, T(54) = 3.49, p = 0.002, and positive, T(54) = 4.98, p < 0.001, nouns. Positive nouns differed in valence from neutral nouns, T(54) = 3.59, p = 0.001. Means and standard deviations of all parameters, depending on valence group, are summarized in Table 2.
Visual stimuli were presented at a distance of 100 cm on a 22-inch monitor (Eizo SX2262W, 1650 × 1024 pixel resolution, 60 Hz frame rate) using MATLAB version R2011a (The Mathworks, Inc., Natick, MA, United States) and the Psychophysics Toolbox (Brainard, 1997; Pelli, 1997). Button press responses were recorded using a PS/2 connected standard keyboard. Voice responses were recorded using a standard interfacial microphone (Samson Technologies, Hauppauge, NY, United States). Physiological data, i.e., data from electrocardiogram (ECG), EDA, and fEMG at M. Corrugator and M. Zygomaticus regions, was registered using the mobile Varioport amplifier (Becker Meditec, Karlsruhe, Germany), which allows data sampling at a rate of 512 Hz. The EMG electrodes were placed in accordance with methodological guidelines (Fridlund and Cacioppo, 1986). For electrocardiography, one-way electrodes were used which were placed at the upper sternum, lower sternum, and left lateral margin of the chest. This placement is thought to lead to minimal movement artifacts (Jennings et al., 1981). Skin conductance including EDA was registered using direct current stimulation with Ag/AgCl electrodes of a diameter of 4 mm. Due to hardwired settings of the amplifier, the physiological signals were filtered online. The ECG-signal was band-pass filtered at 0.9–100 Hz (-3 db). The fEMG-signal was band-pass filtered at 70–400 Hz (-3 db). While the attenuation of the fEMG-signal in the low frequency range leads to a reduction of power line noise, it also decreases a sizable part of the surface EMG signal which has most of the energy between 10 and 200 Hz (Tassinary et al., 2007). Usage of the 70 to 400 Hz passband attenuates especially weak signals originating from single motor unit firing as opposed to aggregated motor unit activity (Tassinary et al., 2007). The usage of the described online filter may thus lead to a distortion of the fEMG-signal, especially when interested in periods of low fEMG, which, however, was not analyzed in the present study.
Only reaction times in the time window of 100 to 3900 ms after stimulus onset were considered. Trials with multiple key presses were excluded from analysis resulting on average in a loss of 1.3–2% of trials per stimulus condition. Key presses related to participants’ spontaneous evaluations of the stimuli were coded offline from -1 (negative) to 0 (neutral) to +1 (positive). Participants’ elaborate evaluations were coded offline in line with the SAM from 1 to 9 (1: very unpleasant, 9: very pleasant).
Electromyography raw data was high-pass filtered to reduce movement and blink artifacts and subsequently full-wave rectified. Continuous data was visually inspected and epochs with remaining artifacts were rejected. On average, 5.1% of trials per condition had to be rejected. Afterwards, data was segmented into epochs from -1000 ms to 11000 ms with relation to stimulus onset and 1000 ms pre-stimulus baseline-correction was performed.
R-spikes in the raw ECG signal were extracted using the QRStool (Allen et al., 2007) and raw ECG data was inspected for artifacts. Between 9.8 and 13.5% of the trials were rejected in each of the nine conditions, suggesting a uniform distribution of rejections. After artifact rejection, each condition for each individual subjects included more than 70% of trials. Data was segmented into epochs starting 1000 ms before stimulus onset until 11000 ms after stimulus onset. Epochs were baseline-corrected using the 1000 ms interval before stimulus onset. The epoch length was chosen to fit the maximal length of a trial with the shortest jittering possible (i.e., 4000 ms stimulus interval, 4000 ms voice response interval, and 3000 ms inter-trial interval).
Electrodermal activity was visually inspected and epochs with noisy baseline or activity that could not be classified as stimulus-elicited SCR with regard to the criteria described by Boucsein (2012) were rejected. Afterward, data was segmented into epochs from -1000 ms to 11000 ms with relation to stimulus onset and 1000 ms pre-stimulus baseline-correction was performed. Data of eight subjects showed no reliable responses time locked to the onset of the stimulus (non-responders). Conservative inspection of artifacts resulted in the rejection of another eight subjects, such that finally only a sample size of 13 subjects was included into the analysis. Therefore, the interpretation of the exploratory EDA data may have only limited validity and generalizability and results from preliminary EDA analysis are reported only in the Supplementary Material.
After the experiment participants were asked for potential processing strategies, which revealed no differences, confirming that all participants followed the instruction given. In particular, all participants stated that they used similar encoding or appraisal strategies for words with self-related, other-related and negated pronouns.
Arithmetic means of reaction times, spontaneous judgments (given via key press), and elaborate judgments (given via voice) were analyzed in separate repeated measurement analyses of variance (ANOVAs) using the factors reference (self-reference, other-reference, no reference) and valence (positive, negative, neutral). Participants’ key presses were coded as -1 (if associated with a ‘negative/unpleasant’ response), 0 (if associated with a ‘neutral’ response), and +1 (if associated with a ‘positive/pleasant’ response) and, to obtain index scores of response accuracy (ranging from -1 to +1), individual responses (positive, negative, or neutral key presses) were averaged for each word category separately2. Dependent t-tests were conducted as post hoc tests.
Changes in mean M. Corrugator and mean M. Zygomaticus activity (fEMG), HR, and EDA were statistically analyzed with repeated measures ANOVAs. For fEMG and HR, activity between 0 and 4000 ms after stimulus onset was analyzed. EDA activity was analyzed between 0 and 11000 ms after stimulus onset. The longer analysis window is due to the slow reactivity of this measure, i.e., changes in mean skin conductance are characterized by slow wave drifts lasting about 10 up to 16 s.
All physiological signals were also assessed across time to determine stimulus-locked fluctuations across the whole stimulus presentation period from 0 to 4000 ms. To this end, the continuously recorded ECG and fEMG-signals were clustered into time bins of 500 ms; EDA data was clustered into 1000 ms bins and analyzed from 0 to 11000 ms after stimulus onset. Ultimately, each repeated measures ANOVA contained the factors reference (self-related, other-related, negated), valence (pleasant, unpleasant, neutral valence), and time. The results of the time series analyses are reported in the Supplementary Material (beneath the respective time × amplitude plots in Supplementary Figures 1B, 2B, 3B, 5B).
Analyses of variance results are reported Greenhouse-Geisser corrected where appropriate. Significant main and interaction effects were further analyzed by dependent t-tests. P-values of post hoc tests were controlled for multiple comparisons according to the procedure suggested by Benjamini and Hochberg (1995) which controls for false discovery rate (FDR).
Reaction time showed a main effect of valence, F(2,56) = 18.12, p < 0.001, η2 = 0.39. Reaction times were significantly shorter for positive and negative words than for neutral words [negative vs. neutral: T(28) = -4.18, p < 0.001; positive vs. neutral: T(28) = -5.20, p < 0.001]. The main effect of reference was not significant, F(2,56) = 2.47, p = 0.094, η2 = 0.08. However, a significant interaction effect of valence × reference, F(4,112) = 5.62, p < 0.001, η2 = 0.17, was observed, supporting the hypothesis of a self-positivity bias. As shown in Figure 2, participants responded to self-related positive words significantly faster than to self-related negative or self-related neutral words and significantly faster than to other-related positive words [all three comparisons |T(28)| > 2.8, p < 0.01]. For self- and other-related negative or neutral words no difference in reaction times was found. Moreover, negated words without any personal reference were not responded to slower than other-related words [other-related vs. negated: T(28) = 0.405, p = 0.689], suggesting no considerable increase in task difficulty for the evaluation of negated compared to other-related stimuli.
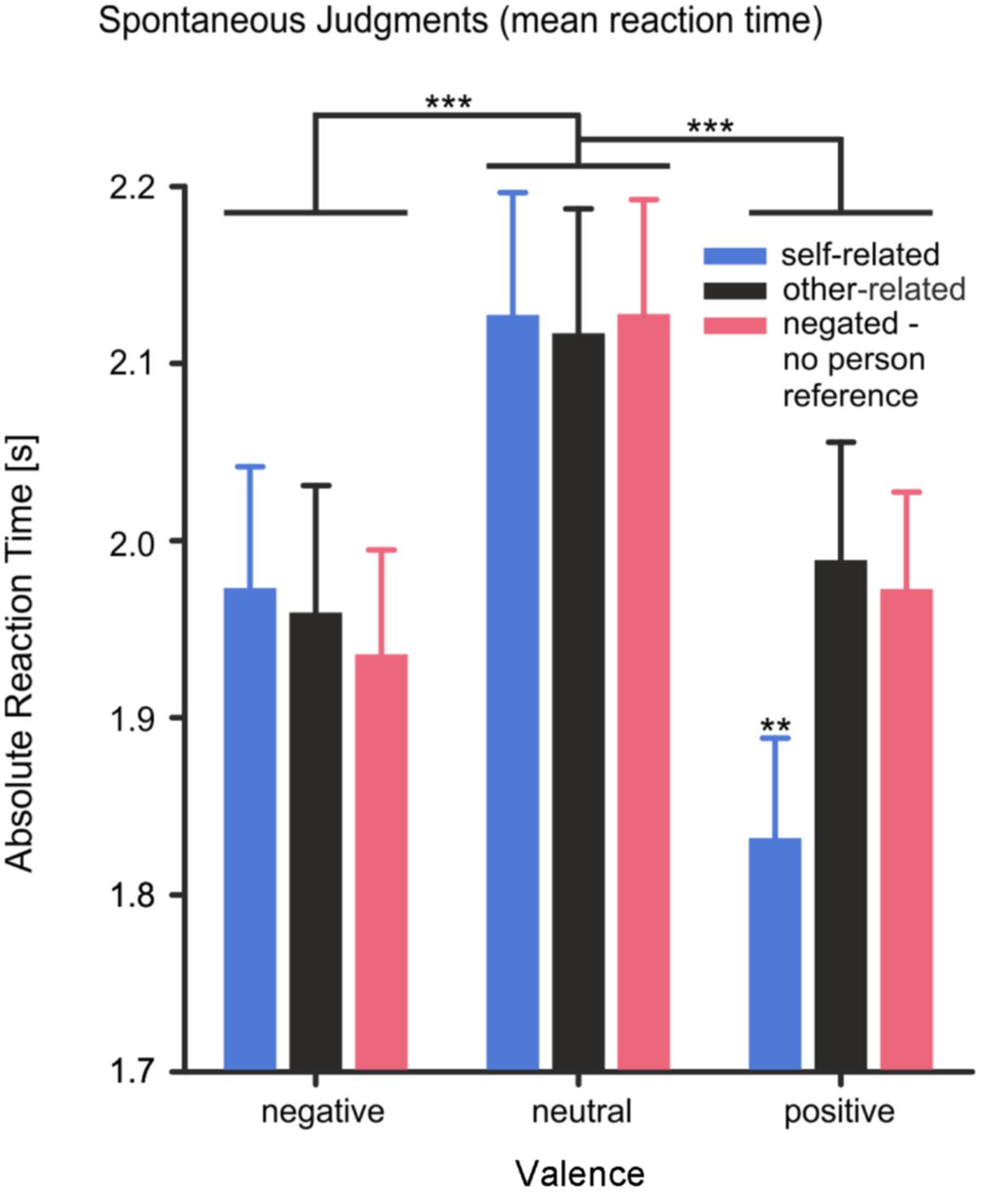
FIGURE 2. Mean reaction times of spontaneous judgments (absolute values). The reaction time represents the time subjects needed to spontaneously judge the valence of the pronoun-noun pairs. Error bars depict SEM. ∗∗p < 0.01, ∗∗∗p < 0.001, FDR corrected.
Spontaneous judgments were modulated by valence, F(2,56) = 88.97, p < 0.001, η2 = 0.76, and reference, F(2,56) = 19.86, p < 0.001, η2 = 0.42, and by a significant interaction effect of valence x reference, F(4,112) = 170.54, p < 0.001, η2 = 0.86. Post hoc tests revealed that positive words were judged more often as positive when they were self-related than when they were other-related, T(28) = 4.38, p < 0.001. Participants responded also more often with a positive key press to self-related neutral words compared to other-related neutral words, T(28) > 4.5, p < 0.001. For self- vs. other-related negative words no such response bias could be observed: as shown in Figure 3A, participants did not judge self-related negative words more often as negative than other-related negative words, T(28) = 1.01, p = 0.334. Negated positive words were more often judged as negative compared to negated neutral words, T(28) = 6.75, p < 0.001, and negated negative words were more often judged as positive than negated neutral words, T(28) = 8.46, p < 0.001, indicating that participants’ spontaneous emotional judgments were based on the semantics of the words.
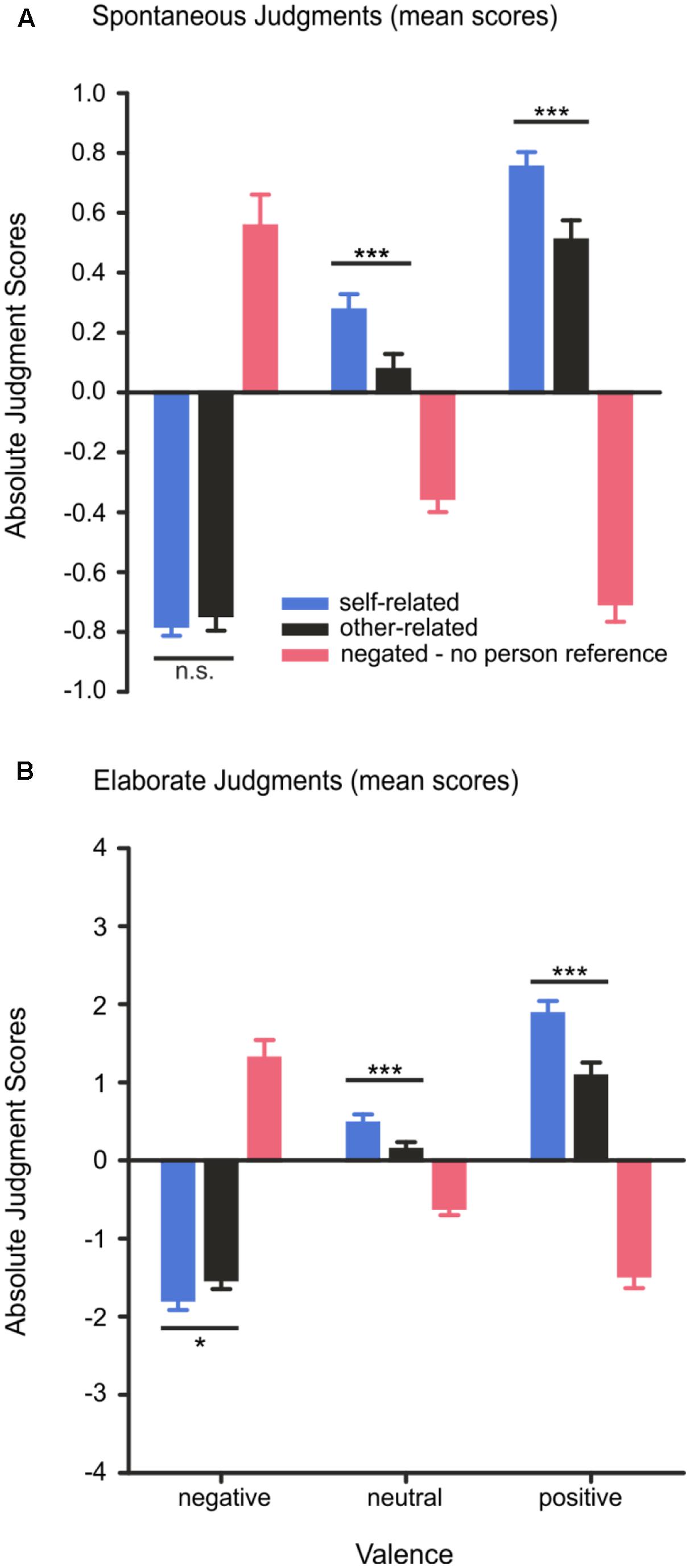
FIGURE 3. (A,B) Spontaneous and elaborate valence judgments. For the spontaneous judgment, subjects had to press one of three buttons corresponding to either negative (–1), neutral (0), or positive (1) valence. In the elaborate judgment, subjects had to verbalize a number on a 9-point scale for their rating to indicate the intensity of their feelings. The number ‘one’ represents very negative, the number ‘five’ neutral and the number ‘nine’ very positive. Error bars depict SEM. n.s. p > 0.1, ∗p < 0.05, ∗∗∗p < 0.001, FDR corrected.
Elaborate judgments showed a significant main effect of valence, F(2,56) = 86.01, p < 0.001, η2 = 0.75, of reference, F(2,56) = 16.97, p < 0.001, η2 = 0.38, as well as a significant interaction effect of valence × reference, F(4,112) = 154.27, p < 0.001, η2 = 0.85. Post hoc tests showed that subjective feelings elicited by positive and negative word pairs were judged as more intense, i.e., more positive, T(28) = 6.32, p < 0.001, or more negative, T(28) = 9.85, p < 0.001, respectively, compared to neutral word pairs. Moreover, positive feelings elicited by self-related positive words (e.g., “my joy”) were rated higher in intensity than were feelings elicited by other-related positive words (e.g., “his joy”), T(28) = 5.24, p < 0.001. Feelings elicited by negative words were also rated as significantly higher in intensity when they were related to the self (e.g., “my death”) than when they were other-related (e.g., “his death”), T(28) = 2.61, p = 0.020, suggesting that self-reference enhances the intensity of subjective feelings for positive and negative words during elaborate judgments. Feelings elicited by negated positive words (e.g., “no joy”) were rated as more negative in intensity than were feelings elicited by negated neutral words, T(28) = 6.72, p < 0.001. Likewise, feelings elicited by negated unpleasant words (e.g., “no death”) were rated as more positive than were negated neutral words, T(28) = 8.90, p < 0.001, confirming that the negating pronoun reversed the valence of negative and positive words. Results are depicted in Figure 3B. An overview of the behavioral results is provided in Table 3.
Mean M. Corrugator activity (0–4000 ms, see Figure 4) showed a main effect of the factor valence, F(2,56) = 12.84, p < 0.001, η2 = 0.31, as well as an interaction of the factors valence × reference, F(4,112) = 5.75, p < 0.001, η2 = 0.17. Mean M. Corrugator activity was significantly attenuated during the presentation of positive compared to neutral words, T(28) = 4.22, p < 0.001, or negative words, T(28) = 3.55, p = 0.002. This attenuation was observed particularly for positive other-related words in comparison to positive self-related words, T(28) = 3.67, p = 0.002, and trending for other-related positive words in comparison to negated positive words, T(28) = 2.08, p = 0.050.
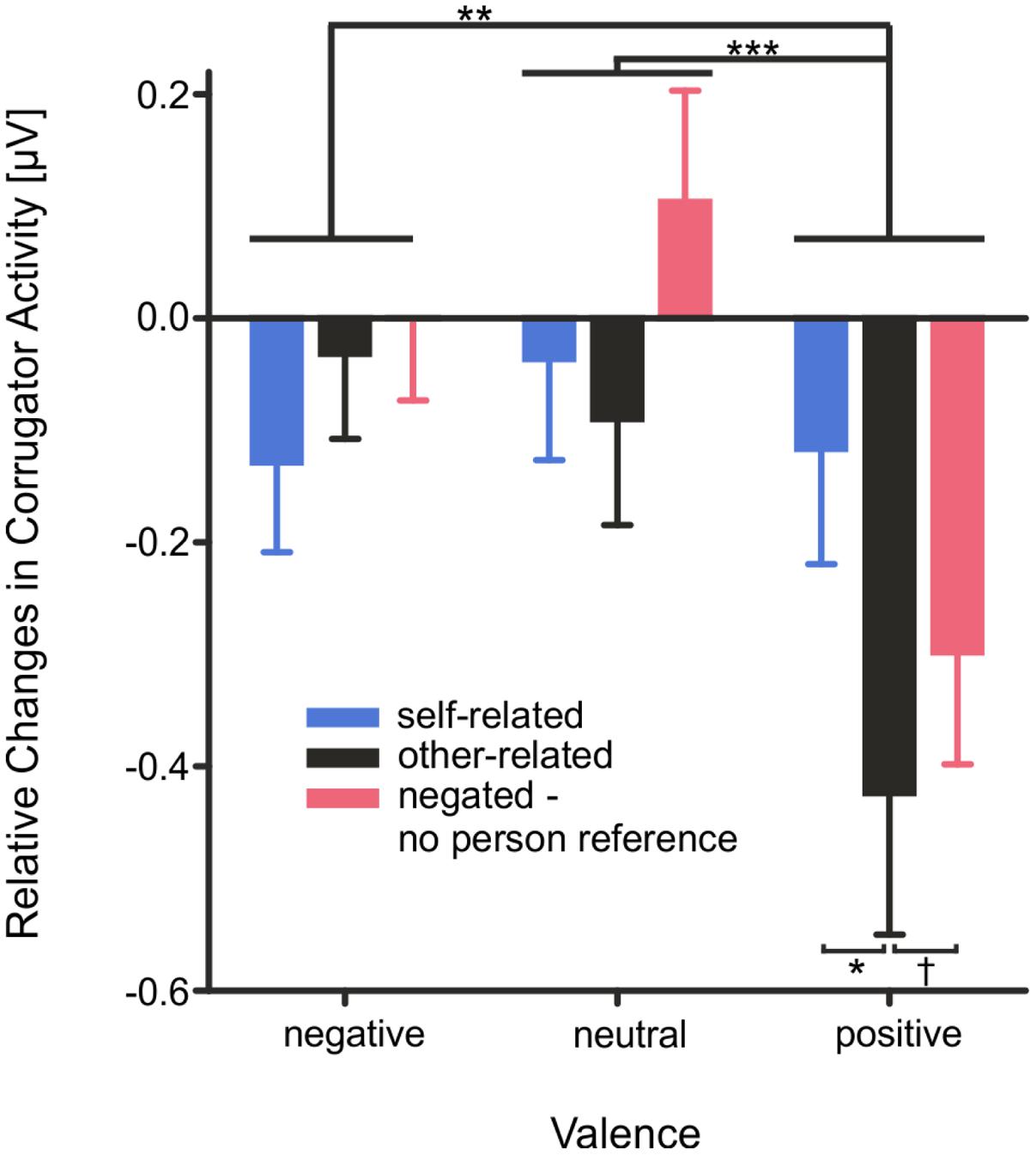
FIGURE 4. Changes in mean M. Corrugator activity as measured by fEMG depicted as changes in mean activity during the stimulus interval from 0 to 4000 ms. Error bars depict SEM. Changes are illustrated as relative changes from baseline. n.s. p > 0.1, †p = 0.05, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, FDR corrected.
Mean M. Zygomaticus activity (0–4000 ms) showed a significant main effect of valence, F(2,56) = 4.17, p = 0.039, η2 = 0.13, and of reference, F(2,56) = 4.76, p = 0.012, η2 = 0.15, as well as a significant interaction of valence × reference, F(4,112) = 3.46, p = 0.043, η2 = 0.11. Mean M. Zygomaticus activity was significantly more pronounced for positive than for negative words, T(28) = 2.51, p = 0.022. No difference was found between positive and neutral words T(28) = 1.11, p = 0.278. Also, changes in Zygomaticus activity were more pronounced for other-related than for self-related, T(28) = 2.48, p = 0.023, or negated words, T(28) = 2.41, p = 0.026. Crucially, changes in mean Zygomaticus activity were more pronounced for other-related than for self-related positive words, T(28) = 2.52, p = 0.022, or negated positive words, T(28) = 2.83, p = 0.013; see Figure 5.
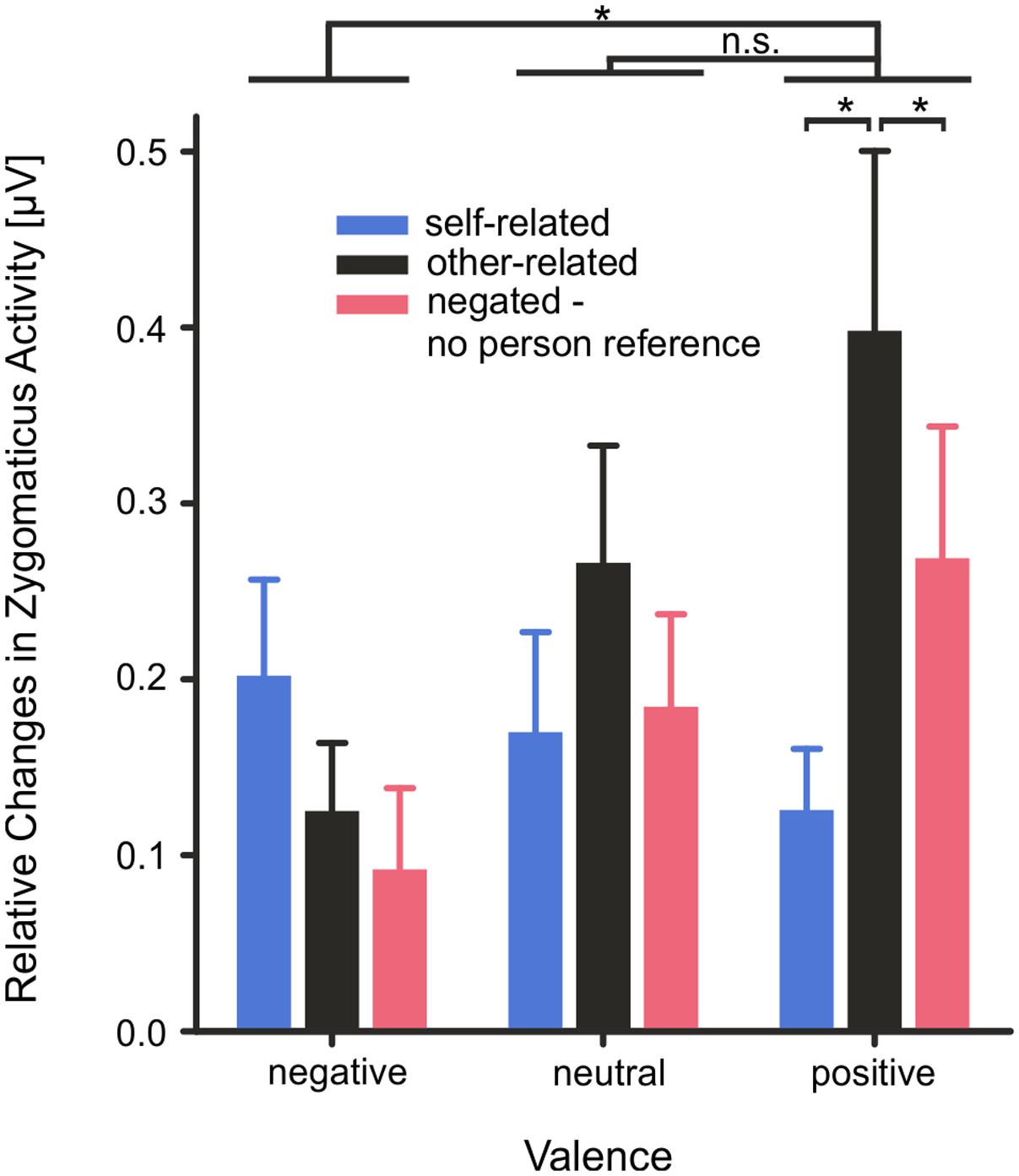
FIGURE 5. Changes in mean M. Zygomaticus activity as measured by fEMG depicted as mean activity during the stimulus interval from 0 to 4000 ms. Error bars depict SEM. Changes are illustrated as relative changes from baseline. n.s. p > 0.1, ∗p < 0.05, FDR corrected.
Changes in mean M. Corrugator and mean M. Zygomaticus activity were not significantly correlated, irrespective of whether self- or other-related words or control stimuli were presented (all N = 29, all p > 0.1).
Changes in mean HR (0–4000 ms) were significantly modulated by the factor valence, F(2,56) = 7.99, p < 0.001, η2 = 0.22, but not by reference, F(2,56) = 2.53, p = 0.089, η2 = 0.08. Mean HR increased significantly during presentation of positive, T(28) = 3.57, p = 0.002, and negative words, T(29) = 2.66, p = 0.018, compared to neutral words. The interaction of the factors valence × reference was not significant, F(4,112) = 0.72, p = 0.579, η2 = 0.02, (see Figure 6).
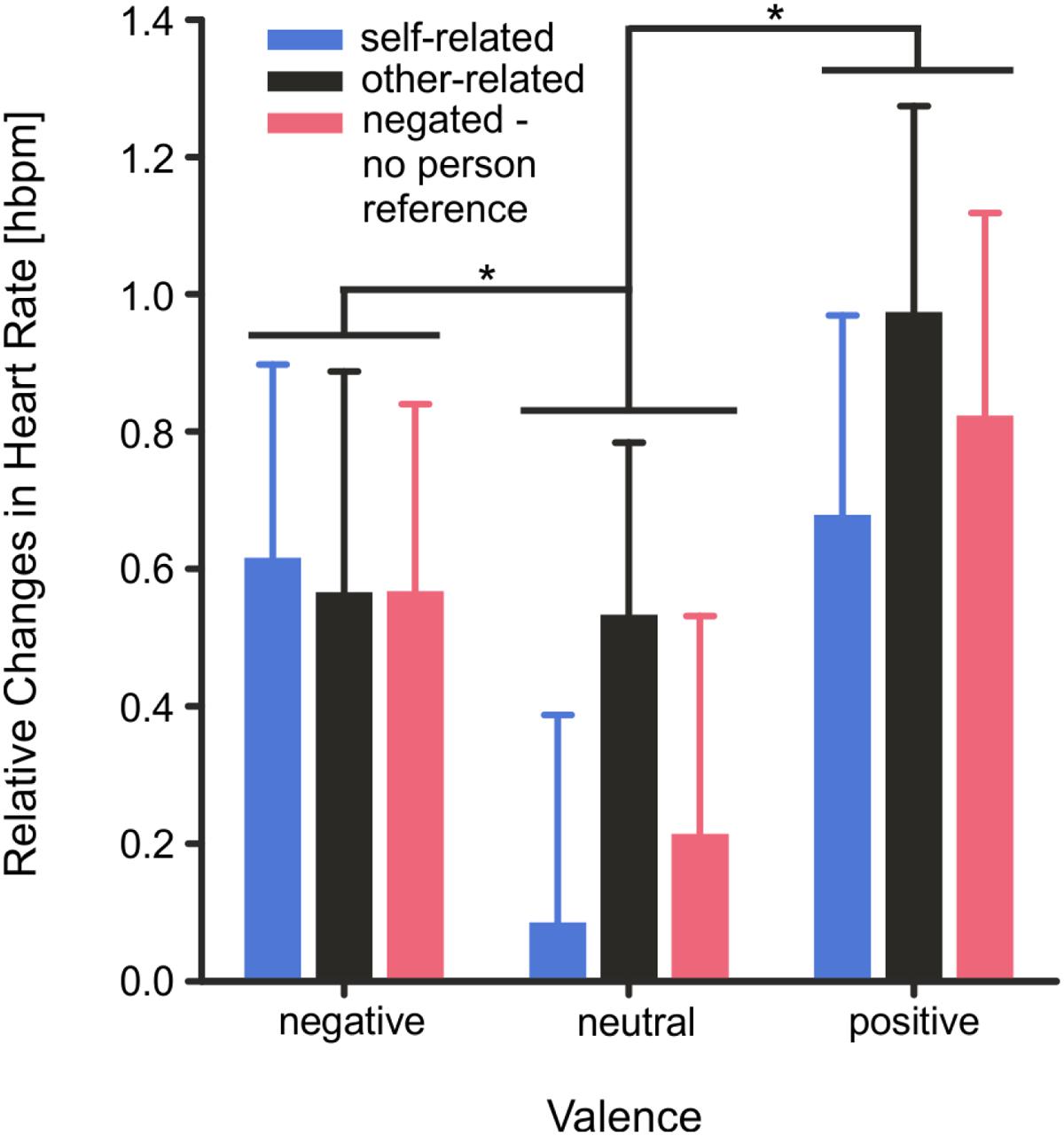
FIGURE 6. Heart rate activity as measured by 3-Lead ECG and depicted as mean changes during the stimulus interval from 0 to 4000 ms. Error bars depict SEM. Changes are illustrated as relative changes from baseline for all stimulus categories. However, as described in detail in the text, mean heart rate was significantly modulated by the factor valence and not by the factor reference and the interaction of the factors valence × reference was not significant. ∗p < 0.05, FDR corrected.
Analysis of electrodermal activity (exploratory analysis, N = 13 subjects) is reported in the Supplement.
Correlation analyses between behavioral, physiological, and self-reported data (depression, alexithymia, anxiety, and positive and negative affect) revealed no consistent pattern of interactions. Regarding behavioral data, spontaneous judgments of self-related positive words showed a negative correlation with depression (r = -0.045, p = 0.008, one-tailed) and a positive correlation with self-reported positive affect (r = 0.36, p = 0.028, one-tailed).
This study investigated reaction times, emotional judgments, and changes in affective physiology, fEMG and HR in particular, during emotional evaluation of words varying in emotional valence and personal reference (self-other reference). Extending previous research supporting an embodied view of language, the present study was aimed at investigating the differential sensitivity of each of these measures to changes in valence (positive, neutral, negative) and personal reference (self, other).
Participants’ behavioral data indicated preferential processing of positive pronoun-noun phrases, particularly when these were self-related. The preferential processing of self-related positive words was observed during spontaneous judgments and associated with faster reaction times and significantly higher response accuracy. This self-positivity bias was evident when comparing self-related positive words to self-related negative or self-related neutral words and in comparison to other-related positive words as well as control items (i.e., negated words). Elaborate judgments revealed that subjective feelings were significantly more intense when positive and negative words were self-related than when they were other-related.
The self-positivity bias in reaction times is in line with recent EEG studies reporting a processing bias for positive words in designs in which the valence of a word and its personal reference (self-other reference) were experimentally manipulated or controlled for (e.g., Watson et al., 2007; Herbert et al., 2008, 2011b; Fields and Kuperberg, 2012, 2015). The present results confirm these findings on a behavioral level and suggest that participants have faster access to self-related positive information than to self-related negative information in support of mood congruent processing, mildly positive mood being the norm in healthy subjects (Diener et al., 1997; Mezulis et al., 2004). Crucially, the present results attest that it is the self-reference of a stimulus that improves the bias toward positive information, facilitating spontaneous judgments to positive words when their content is related to the reader’s self.
In general, mean reaction times appeared to be slower than reaction times reported in word processing studies using, for instance, lexical decision tasks. However, reaction times greater than one second have been reported in previous studies using emotional evaluation task (see for instance, Niedenthal et al., 2009). Moreover, previous EEG-ERP studies using similar stimulus material as the present one (e.g., Herbert et al., 2011b,d) found a processing advantage for self- versus other-related emotional words specifically at later cortical processing stages in the time windows of the N400 (e.g., Herbert et al., 2011d) or the late positive potential, LPP (e.g., Herbert et al., 2011b; see also Fields and Kuperberg, 2012, 2015). Hence, for abstract stimuli such as words, discrimination between self and other might appear earliest at the level of semantic stimulus integration, and thus temporally after the initial emotional content conveyed by nouns and its personal relatedness (conveyed by pronouns) have been integrated into one semantic concept. Thus, a certain degree of semantic processing is required to discriminate emotional stimuli related to the self from those related to the other. That judgments were based on semantics and thus on the meaning of the word phrases was confirmed by the judgments of the control stimuli: pronoun-noun pairs containing a negation (e.g., “no joy,” “no death”) reversed the direction of the valence judgment for negative and positive words, which is possible only if the negation term is semantically taken into consideration (see e.g., Kaup and Zwaan, 2003; Herbert et al., 2011a).
Interestingly, despite a processing advantage of self-related positive words in the behavioral and subjective measures, this bias was not accompanied by activity changes in fEMG or HR data. Physiological data did by no means point toward stronger embodiment of self-related positive words in comparison to other-related positive words.
Changes in HR were modulated by the emotional valence of the stimuli with significantly stronger HR acceleration patterns for positive and negative than neutral words during the first 4 s of spontaneous word evaluation. Basic changes in HR in response to the a word’s emotional tone (positive or negative vs. neutral) may occur in anticipation of approach or defense (Bradley and Lang, 2007) and may be larger for emotional stimuli rated higher in emotional arousal than neutral words (Bradley et al., 2008). Of note, the positive and negative nouns chosen for the experiment differed not only in emotional valence but also in emotional arousal from neutral nouns. The observed HR changes therefore fit well with previous reports showing that increases in HR evoked by positive and negative stimuli are modulated by emotional arousal (Bradley et al., 2008) and by preparation for action (Bradley and Lang, 2007).
Mean M. Zygomaticus as well as mean M. Corrugator activity revealed significantly stronger activity changes during emotional evaluation of positive words. In particular, changes were more pronounced for other-related than self-related positive words. Whereas M. Zygomaticus activity showed a significant activity increase, M. Corrugator activity showed a significant decrease particularly during the evaluation of other-related positive words as compared to self-related positive words. While activity increases in mean M. Zygomaticus activity are reliable indicators of positive emotions, decreases in M. Corrugator activity below baseline have also been observed in previous studies in response to positive stimuli eliciting relaxation or surprise (Neta et al., 2009). In the present study, changes in mean activity of the M. Zygomaticus and the M. Corrugator muscles were not significantly correlated during word evaluation, suggesting that changes in both muscles reflect different facets of emotion processing.
Regarding the processing of concrete emotional stimuli such as faces, response peaks in fEMG have been reported to occur quickly after stimulus presentation (e.g., Dimberg, 1982; Dimberg et al., 2000), which may indicate spontaneous readout of the reader’s own emotions. Regarding previous studies using single emotional words the earliest changes in fEMG activity were occasionally observed as early as 500 ms after word presentation (Foroni and Semin, 2009). However, Niedenthal et al. (2009), for instance, in an emotional evaluation task reported considerably longer latencies. Moreover, previous EEG studies outlined above (e.g., Herbert et al., 2011b; Fields and Kuperberg, 2015) suggest that discrimination between self-related and other-related emotional words may occur during later stimulus processing stages for why changes in facial responses may also occur later for pronoun-noun pairs than for single words. Future research investigating the time course of changes in fEMG during the evaluation of self- vs. other-related emotional words is needed to answer this question (please see the Supplement for a first exploratory and descriptive overview regarding changes in biosignals across the time window of word presentation).
Nevertheless, positive words elicited stronger emotion-congruent changes in mean M. Zygomaticus activity when other-related than when self-related. Differential facial responses to positive words support the assumption that people spontaneously and preferentially mimic in relation to others, even if the other is only a virtual other (Fridlund, 1991). In line with this hypothesis and the present observations, it has been suggested that facial expressions as well as feedback from motor and action units of the face are considered particularly important for understanding other people’s actions and emotional states (Rizzolatti and Craighero, 2004; Niedenthal, 2007; Gallese, 2009). The present data might therefore support the view that – as far as verbal input is concerned – facial expressions preferentially occur in response to other-related emotional stimuli; in particular to positive stimuli (Fridlund, 1991), at least when the intention is to evaluate other-related emotional words for their hedonic pleasure. Crucially, participants were not instructed to feel into the emotions of others or empathize with them during evaluation of other-related words, suggesting that it is unlikely that empathy or individual differences in empathy have influenced the results. Personality traits and interindividual differences in mood and affect may modulate facial responsivity (e.g., Ferri et al., 2010). In the present study, participants scoring high in self-report measures of depression or alexithymia were excluded from participation, which reduced the chance of finding strong correlations between these self-report, physiological and behavioral measures. Nevertheless, regarding spontaneous judgments, appraisal of self-related positive words was negatively correlated with depression scores and positively correlated with self-reported positive affect. Although, these correlations do support the hypothesis of mood congruent processing being the cause of the self-positivity bias in emotional judgments in healthy subjects, these correlations should be treated with caution and need to be validated in larger sample sizes.
Taken together, the observed fEMG results could be a challenge for traditional associative network models of language processing (Lang, 1979; Bower, 1981). According to these models, fEMG activity during word reading would be the result of activation spread after memory activation. For instance, activation of the words happiness or joy would lead to spread of activation to associated concepts (e.g., smile), thereby leading to changes in the associated parts of the peripheral nervous system, e.g., the neurons controlling facial musculature (see e.g., Lang, 1979; Bower, 1981). Viewed from this perspective, the fEMG findings would imply that nodes are more strongly interrelated in memory for other-related than for self-related positive information. This conclusion contrasts with the behavioral results as well as with several previous findings predicting overall better memory and prioritized processing for self-related information (self-reference effect; for a meta-study, see Symons and Johnson, 1997).
Differences in cognitive versus affective appraisal strategies could be one reason for differential facial involvement in the evaluation of other- versus self-related emotional words in the present study (e.g., Niedenthal et al., 2009). This speculation is, however, unlikely because the instruction was the same for all words. In addition, in the manipulation check participants did not self-report any processing differences between word categories (self, other, no reference). Thus, possible differences in appraisal strategies (cognitive versus affective) cannot explain why participants “frowned” less and particularly “smiled” more when evaluating other- versus self-related positive words. Moreover, abstractness has been shown to affect the magnitude of facial expressions: for instance, fEMG is larger for emotion-related action words (e.g., smiling, crying, etc.) than for emotional words (e.g., adjectives such as happy, funny etc.; e.g., Foroni and Semin, 2009; Fino et al., 2016). However, abstractness cannot account for the differential effects in fEMG during evaluation of self- and other-related pronoun-noun pairs: words were carefully matched on this dimension and the same set of nouns was presented in each condition such that stimulus-reference was the critical dimension signaling whether nouns were self-related or other-related. However, gender might have played a role as N = 24 out of N = 29 of the participants were females and physiological signals including fEMG activity has been reported to be more pronounced in women than in men (Greenwald et al., 1989; Bradley et al., 2001). However, previous studies using similar material (Herbert et al., 2011b,d) as well as pronouns to induce self- or other-reference in Western and Asian participant samples (Li and Zhou, 2010; Zhou et al., 2010; Blume and Herbert, 2014) did not report any gender effects. Nevertheless, gender differences should be examined further in future studies using larger sample sizes.
In the present study, the personal reference (self-other reference) and the emotional valence of words were experimentally manipulated to assess the impact of these dimensions on behavioral, subjective, and physiological responses during an emotional word evaluation task. Whereas behavioral responses indicated preferential processing of self-related positive words, facial responses were most pronounced during evaluation of other-related positive words. Moreover, changes in HR occurred during evaluation of emotional compared to neutral words regardless of their personal reference. Thus, behavioral responses support a self-positivity bias in emotional judgments whereas changes in fEMG seem to support sociality effects (Fridlund, 1991). Physiologically, bodily signals may contribute differently to the emotional evaluation of verbal content with facial expressions, M. Zygomaticus activity in particular, being most pronounced during the evaluation of other-related emotional content, positive in particular (Fridlund, 1991), and HR being modulated by differences in emotional content irrespective of whom the information may refer to. Crucially, further studies are needed to scrutinize and validate these assumptions in different settings including actual conversations taking place in both laboratory and real-life settings. The paradigm used in the present study might be especially fruitful to this end.
This manuscript is a shared first authorship of PW and CH. CH wrote main parts of the manuscript, designed the study, supervised the study, and analyzed the data together with PW (master student supervised by CH). The study is part of a project funded by the German Research Foundation (HE5880/3-1), awarded to CH. PW helped writing the manuscript including the method section, conducted the study (programmed the design, recorded the data) and analyzed the data together with CH.
This study was funded by the German Research Foundation (DFG), grant HE 5880/3-1, awarded to CH.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpsyg.2017.01277/full#supplementary-material
Allen, J. J., Chambers, A. S., and Towers, D. N. (2007). The many metrics of cardiac chronotropy: a pragmatic primer and a brief comparison of metrics. Biol. Psychol. 74, 243–262. doi: 10.1016/j.biopsycho.2006.08.005
Bagby, R. M., Parker, J. D., and Taylor, G. J. (1994). The twenty-item toronto alexithymia scale-I. Item selection and cross-validation of the factor structure. J. Psychosom. Res. 38, 23–32. doi: 10.1016/0022-3999(94)90005-1
Bayer, M., Sommer, W., and Schacht, A. (2010). Reading emotional words within sentences: the impact of arousal and valence on event-related potentials. Int. J. Psychophysiol. 78, 299–307. doi: 10.1016/j.ijpsycho.2010.09.004
Benjamini, Y., and Hochberg, Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Roy. Statist. Soc. Ser. B 57, 289–300.
Biemann, C., Bordag, S., Heyer, G., Quasthoff, U., and Wolff, C. (2004). “Language-independent methods for compiling monolingual lexical data,” in Computational Linguistics and Intelligent Text Processing, ed. A. Gelbukh (Berlin: Springer), 217–228.
Blume, C., and Herbert, C. (2014). The HisMine-paradigm: a new paradigm to investigate self-awareness employing pronouns. Soc. Neurosci. 9, 289–299. doi: 10.1080/17470919.2014.886616
Bradley, M. M., Codispoti, M., Sabatinelli, D., and Lang, P. J. (2001). Emotion and motivation II: sex differences in picture processing. Emotion 1, 300–319. doi: 10.1037/1528-3542.1.3.300
Bradley, M. M., and Lang, P. J. (2007). “Emotion and motivation,” in Handbook of Psychophysiology, 3rd Edn, eds J. T. Cacioppo, L. G. Tassinary, and G. G. Berntson (Cambridge: Cambridge University Press), 581–607.
Bradley, M. M., Miccoli, L., Escrig, M. A., and Lang, P. J. (2008). The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology 45, 602–627. doi: 10.1111/j.1469-8986.2008.00654.x
Brainard, D. H. (1997). The psychophysics toolbox. Spat. Vis. 10, 433–436. doi: 10.1163/156856897x00357
Buck, R. (1994). Social and emotional functions in facial expression and communication: the readout hypothesis. Biol. Psychol. 38, 95–115. doi: 10.1016/0301-0511(94)90032-9
Cacioppo, J. T., Petty, R. E., and Morris, K. J. (1985). Semantic, evaluative, and self-referent processing: memory, cognitive effort, and somatovisceral activity. Psychophysiology 22, 371–384. doi: 10.1111/j.1469-8986.1985.tb01618.x
Chartrand, T. L., and Bargh, J. A. (1999). The chameleon effect: the perception-behavior link and social interaction. J. Pers. Soc. Psychol. 76, 893–910. doi: 10.1037/0022-3514.76.6.893
Citron, F. M. M., Abugaber, D., and Herbert, C. (2016). Approach and withdrawal tendencies during written word processing: effects of task, emotional valence, and emotional arousal. Front. Psychol. 6:1935. doi: 10.3389/fpsyg.2015.01935
D’Argembeau, A., Jedidi, H., Balteau, E., Bahri, M., Phillips, C., and Salmon, E. (2012). Valuing one’s self: medial prefrontal involvement in epistemic and emotive investments in self-views. Cereb. Cortex 22, 659–667. doi: 10.1093/cercor/bhr144
Diener, E., Suh, E., and Oishi, S. (1997). Recent findings on subjective well-being. Ind. J. Clin. Psychol. 24, 25–41.
Dimberg, U. (1982). Facial reactions to facial expressions. Psychophysiology 19, 643–647. doi: 10.1111/j.1469-8986.1982.tb02516.x
Dimberg, U., Thunberg, M., and Elmehed, K. (2000). Unconscious facial reactions to emotional facial expressions. Psychol. Sci. 11, 86–89. doi: 10.1111/1467-9280.00221
Dunn, B. D., Dalgleish, T., and Lawrence, A. D. (2006). The somatic marker hypothesis: a critical evaluation. Neurosci. Biobehav. Rev. 30, 239–271.
Dunn, B. D., Galton, H. C., Morgan, R., Evans, D., Oliver, C., Meyer, M., et al. (2010). Listening to your heart how interoception shapes emotion experience and intuitive decision making. Psychol. Sci. doi: 10.1177/0956797610389191
Esslen, M., Metzler, S., Pascual-Marqui, R., and Jancke, L. (2008). Pre-reflective and reflective self-reference: a spatiotemporal EEG analysis. Neuroimage 42, 437–449. doi: 10.1016/j.neuroimage.2008.01.060
Ferri, F., Stoianov, I. P., Gianelli, C., D’Amico, L., Borghi, A. M., and Gallese, V. (2010). When action meets emotions: how facial displays of emotion influence goal-related behavior. PLoS ONE 5:e13126. doi: 10.1371/journal.pone.0013126
Fields, E. C., and Kuperberg, G. R. (2012). It’s all about you: an erp study of emotion and self-relevance in discourse. Neuroimage 62, 562–574. doi: 10.1016/j.neuroimage.2012.05.003
Fields, E. C., and Kuperberg, G. R. (2015). Loving yourself more than your neighbor: ERPs reveal online effects of a self-positivity bias. Soc. Cogn. Affect. Neurosci. 10, 1202–1209.
Fino, E., Menegatti, M., Avenanti, A., and Monica, R. (2016). Enjoying vs. smiling: facial muscular activation in response to emotional language. Biol. Psychol. 118, 126–135.
Foroni, F., and Semin, G. R. (2009). Language that puts you in touch with your bodily feelings the multimodal responsiveness of affective expressions. Psychol. Sci. 20, 974–980. doi: 10.1111/j.1467-9280.2009.02400.x
Foroni, F., and Semin, G. R. (2011). When does mimicry affect evaluative judgment? Emotion 11, 687–690. doi: 10.1037/a0023163
Foroni, F., and Semin, G. R. (2013). Comprehension of action negation involves inhibitory simulation. Front. Hum. Neurosci. 7:209. doi: 10.3389/fnhum.2013.00209
Fridlund, A. J. (1991). Sociality of solitary smiling: potentiation by an implicit audience. J. Pers. Soc. Psychol. 60, 229–240. doi: 10.1037/0022-3514.60.2.229
Fridlund, A. J., and Cacioppo, J. T. (1986). Guidelines for human electromyographic research. Psychophysiology 23, 567–589. doi: 10.1111/j.1469-8986.1986.tb00676.x
Gallese, V. (2009). Mirror neurons, embodied simulation, and the neural basis of social identification. Psychoanal. Dial. 19, 519–536. doi: 10.1080/10481880903231910
Garfinkel, S. N., Barrett, A. B., Minati, L., Dolan, R. J., Seth, A. K., and Critchley, H. D. (2013). What the heart forgets: cardiac timing influences memory for words and is modulated by metacognition and interoceptive sensitivity. Psychophysiology 50, 505–512.
Glenberg, A. M., Webster, B. J., Mouilso, E., Havas, D., and Lindeman, L. M. (2009). Gender, emotion, and the embodiment of language comprehension. Emot. Rev. 1, 151–161. doi: 10.1177/1754073908100440
Greenwald, M. K., Cook, E. W., and Lang, P. J. (1989). Affective judgment and psychophysiological response: dimensional covariation in the evaluation of pictorial stimuli. J. Psychophysiol. 3, 51–64.
Hamann, S. (2001). Cognitive and neural mechanisms of emotional memory. Trends Cogn. Sci. 5, 394–400. doi: 10.1016/S1364-6613(00)01707-1
Hautzinger, M., Keller, F., and Kühner, C. (2006). The Beck Depression Inventory II. German Version and Manual for the BDI II. Frankfurt: Harcourt Test Services.
Havas, D. A., Glenberg, A. M., Gutowski, K. A., Lucarelli, M. J., and Davidson, R. J. (2010). Cosmetic use of botulinum toxin-a affects processing of emotional language. Psychol. Sci. 21, 895–900. doi: 10.1177/0956797610374742
Hazlett, E. A., Speiser, L. J., Goodman, M., Roy, M., Carrizal, M., Wynn, J. K., et al. (2007). Exaggerated affect-modulated startle during unpleasant stimuli in borderline personality disorder. Biol. Psychiatry 62, 250–255. doi: 10.1016/j.biopsych.2006.10.028
Herbert, C., Deutsch, R., Platte, P., and Pauli, P. (2013a). No fear, no panic: probing negation as a means for emotion regulation. Soc. Cogn. Affect. Neurosci. 8, 654–661. doi: 10.1093/scan/nss043
Herbert, C., Deutsch, R., Sütterlin, S., Kübler, A., and Pauli, P. (2011a). Negation as a means for emotion regulation? Startle reflex modulation during processing of negated emotional words. Cogn. Affect. Behav. Neurosci. 11, 199–206. doi: 10.3758/s13415-011-0026-1
Herbert, C., Ethofer, T., Anders, S., Junghofer, M., Wildgruber, D., Grodd, W., et al. (2009). Amygdala activation during reading of emotional adjectives-an advantage for pleasant content. Soc. Cogn. Affect. Neurosci. 4, 35–49. doi: 10.1093/scan/nsn027
Herbert, C., Herbert, B. M., Ethofer, T., and Pauli, P. (2011b). His or mine? The time course of self-other discrimination in emotion processing. Soc. Neurosci. 6, 277–288. doi: 10.1080/17470919.2010.523543
Herbert, C., Herbert, B. M., and Pauli, P. (2011c). Emotional self-reference: brain structures involved in the processing of words describing one’s own emotions. Neuropsychologia 49, 2947–2956. doi: 10.1016/j.neuropsychologia.2011.06.026
Herbert, C., Junghofer, M., and Kissler, J. (2008). Event related potentials to emotional adjectives during reading. Psychophysiology 45, 487–498. doi: 10.1111/j.1469-8986.2007.00638.x
Herbert, C., and Kissler, J. (2010). Motivational priming and processing interrupt: startle reflex modulation during shallow and deep processing of emotional words. Int. J. Psychophysiol. 76, 64–71. doi: 10.1016/j.ijpsycho.2010.02.004
Herbert, C., Kissler, J., Junghöfer, M., Peyk, P., and Rockstroh, B. (2006). Processing of emotional adjectives: evidence from startle EMG and ERPs. Psychophysiology 43, 197–206. doi: 10.1111/j.1469-8986.2006.00385.x
Herbert, C., Kübler, A., and Vögele, C. (2013b). Risk for eating disorders modulates startle-responses to body words. PLoS ONE 8:e53667. doi: 10.1371/journal.pone.0053667
Herbert, C., Pauli, P., and Herbert, B. M. (2011d). Self-reference modulates the processing of emotional stimuli in the absence of explicit self-referential appraisal instructions. Soc. Cogn. Affect. Neurosci. 6, 653–661. doi: 10.1093/scan/nsq082
Herbert, C., Sfärlea, A., and Blumenthal, T. (2013c). Your emotion or mine: labeling feelings alters emotional face perception—an ERP study on automatic and intentional affect labeling. Front. Hum. Neurosci. 7:378. doi: 10.3389/fnhum.2013.00378
Hess, U., Banse, R., and Kappas, A. (1995). The intensity of facial expression is determined by underlying affective state and social situation. J. Pers. Soc. Psychol. 69:280.
Hess, U., Kappas, A., McHugo, G. J., Lanzetta, J. T., and Kleck, R. E. (1992). The facilitative effect of facial expression on the self-generation of emotion. Int. J. Psychophysiol. 12, 251–265. doi: 10.1016/0167-8760(92)90064-I
Höffe, O., Geiger, R., Brüllmann, P., and Rapp, C., (eds). (2005). Aristoteles-Lexikon, Deutschland: Kröner, 427–436.
Ilves, M., and Surakka, V. (2012). Heart rate responses to synthesized affective spoken words. Advan. Hum. Comput. Interact. 2012:14. doi: 10.1155/2012/158487
Jennings, J., Richard, W., Bberg, K., J. Hutcheson, O. P., Porges, S., and Turpin, G. (1981). Publication guidelines for heart rate studies in man. Psychophysiology 18, 226–231. doi: 10.1111/j.1469-8986.1981.tb03023.x
Jiménez-Ortega, L., Martín-Loeches, M., Casado, P., Sel, A., Fondevila, S., de Tejada, P. H., et al. (2012). How the emotional content of discourse affects language comprehension. PLoS ONE 7:e33718. doi: 10.1371/journal.pone.0033718
Kaup, B., and Zwaan, R. A. (2003). Effects of negation and situational presence on the accessibility of text information. J. Exp. Psychol. Learn. Mem. Cogn. 29, 439–446. doi: 10.1037/0278-7393.29.3.439
Kircanski, K., Lieberman, M. D., and Craske, M. G. (2012). Feelings into words: contributions of language to exposure therapy. Psychol. Sci. 23, 1086–1091. doi: 10.1177/0956797612443830
Kissler, J., Herbert, C., Peyk, P., and Junghofer, M. (2007). Buzzwords early cortical responses to emotional words during reading. Psychol. Sci. 18, 475–480. doi: 10.1111/j.1467-9280.2007.01924.x
Kuchinke, L., Jacobs, A. M., Grubich, C., Melissa, L.-H., Conrad, M., and Herrmann, M. (2005). Incidental effects of emotional valence in single word processing: an fMRI study. Neuroimage 28, 1022–1032. doi: 10.1016/j.neuroimage.2005.06.050
Lang, P. (1980). “Behavioral treatment and bio-behavioral assessment: computer applications,” in Technology in Mental Health Care Delivery Systems, eds J. B. Sidowski, J. H. Johnson, and T. A. Williams (Key: Citeulike).
Lang, P. J. (1979). A bio-informational theory of emotional imagery. Psychophysiology 16, 495–512. doi: 10.1111/j.1469-8986.1979.tb01511.x
Larsen, J. T., Norris, C. J., and Cacioppo, J. T. (2003). Effects of positive and negative affect on electromyographic activity over zygomaticus major and corrugator supercilii. Psychophysiology 40, 776–785. doi: 10.1111/1469-8986.00078
Laux, L., Glanzmann, P., Schaffner, P., and Spielberger, S. (1981). Das State-Trait-Angstinventar. Weinheim: Beltz.
Li, X., and Zhou, X. (2010). Who is Ziji? ERP responses to the chinese reflexive pronoun during sentence comprehension. Brain Res. 1331, 96–104. doi: 10.1016/j.brainres.2010.03.050
Lieberman, M. D., Eisenberger, N. I., Crockett, M. J., Tom, S. M., Pfeifer, J. H., and Way, B. M. (2007). Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychol. Sci. 18, 421–428. doi: 10.1111/j.1467-9280.2007.01916.x
Lindquist, K. A., Barrett, L. F., Bliss-Moreau, E., and Russell, J. A. (2006). Language and the perception of emotion. Emotion 6, 125–138. doi: 10.1037/1528-3542.6.1.125
Martín-Loeches, M., Hinojosa, J. A., Gómez-Jarabo, G., and Rubia, F. J. (2001). An early electrophysiological sign of semantic processing in basal extrastriate areas. Psychophysiology 38, 114–124. doi: 10.1111/1469-8986.3810114
Mezulis, A. H., Abramson, L. Y., Hyde, J. S., and Hankin, B. L. (2004). Is there a universal positivity bias in attributions? A meta-analytic review of individual, developmental, and cultural differences in the self-serving attributional bias. Psychol. Bull. 130, 711–747. doi: 10.1037/0033-2909.130.5.711
Moseley, R., Carota, F., Hauk, O., Mohr, B., and Pulvermüller, F. (2012). A role for the motor system in binding abstract emotional meaning. Cereb. Cortex. 22, 1634–1647.
Neta, M., Norris, C. J., and Whalen, P. J. (2009). Corrugator muscle responses are associated with individual differences in positivity-negativity bias. Emotion 9, 640–648. doi: 10.1037/a0016819
Niedenthal, P. M., Winkielman, P., Mondillon, L., and Vermeulen, N. (2009). Embodiment of emotion concepts. J. Pers. Soc. Psychol. 96, 1120–1136. doi: 10.1037/a0015574
Norman, G. (2010). Likert scales, levels of measurement and the ‘laws’ of statistics. Adv. Health Sci. Educ. 15, 625–632. doi: 10.1007/s10459-010-9222-y
Northoff, G., Heinzel, A., De Greck, M., Bermpohl, F., Dobrowolny, H., and Panksepp, J. (2006). Self-referential processing in our brain-a meta-analysis of imaging studies on the self. Neuroimage 31, 440–457. doi: 10.1016/j.neuroimage.2005.12.002
Oldfield, R. C. (1971). The assessment and analysis of handedness: the edinburgh inventory. Neuropsychologia 9, 97–113. doi: 10.1016/0028-3932(71)90067-4
Pelli, D. G. (1997). The videotoolbox software for visual psychophysics: transforming numbers into movies. Spat. Vis. 10, 437–442. doi: 10.1163/156856897X00366
Quintana, D. S., Guastella, A. J., Outhred, T., Hickie, I. B., and Kemp, A. H. (2012). Heart rate variability is associated with emotion recognition: direct evidence for a relationship between the autonomic nervous system and social cognition. Int. J. Psychophysiol. 86, 168–172. doi: 10.1016/j.ijpsycho.2012.08.012
Rizzolatti, G., and Craighero, L. (2004). The mirror-neuron system. Annu. Rev. Neurosci. 27, 169–192. doi: 10.1146/annurev.neuro.27.070203.144230
Roberson, D., Damjanovic, L., and Pilling, M. (2007). Categorical perception of facial expressions: evidence for a ‘category adjustment’ model. Mem. Cogn. 35, 1814–1829. doi: 10.3758/BF03193512
Scott, G. G., O’Donnell, P. J., Leuthold, H., and Sereno, S. C. (2009). Early emotion word processing: evidence from event-related potentials. Biol. Psychol. 80, 95–104. doi: 10.1016/j.biopsycho.2008.03.010
Semin, G. R., and Smith, E. R. (2008). Embodied Grounding: Social, Cognitive, Affective, and Neuroscientific Approaches. Cambridge: Cambridge University Press.
Shi, Y., Sedikides, C., Cai, H., Liu, Y., and Yang, Z. (2016). Disowning the self: the cultural value of modesty can attenuate self-positivity. Q. J. Exp. Psychol. 70, 1023–1032. doi: 10.1080/17470218.2015.1099711
Sorabji, R. (1992). “Intentionality and physiological processes: aristotle’s theory of sense-perception,” in Essays on Aristotle’s de Anima, eds M. C. Nussbaum and A. O. Rorty (Wotton-under-Edge: Clarendon Press), 195–225.
Strack, F., Martin, L. L., and Stepper, S. (1988). Inhibiting and facilitating conditions of the human smile: a nonobtrusive test of the facial feedback hypothesis. J. Pers. Soc. Psychol. 54, 768–777. doi: 10.1037/0022-3514.54.5.768
Symons, C. S., and Johnson, B. T. (1997). The self-reference effect in memory: a meta-analysis. Psychol. Bull. 121, 371–394.
Tabert, M. H., Borod, J. C., Tang, C. Y., Lange, G., Wei, T. C., Johnson, R., et al. (2001). Differential amygdala activation during emotional decision and recognition memory tasks using unpleasant words: an fMRI study. Neuropsychologia 39, 556–573. doi: 10.1016/S0028-3932(00)00157-3
Tassinary, L. G., Cacioppo, J. T., and Vanman, E. J. (2007). “The skeletomotor system: surface electromyogrphy,” in Handbook of Psychophysiology, 3rd Edn, eds J. T. Cacioppo, L. G. Tassinary, and G. G. Berntson (Cambridge: Cambridge University Press), 267–299.
Taylor, S. E., and Brown, J. D. (1988). Illusion and well-being: a social psychological perspective on mental health. Psychol. Bull. 103, 193–210. doi: 10.1037/0033-2909.103.2.193
Thayer, J. F., Friedman, B. H., Borkovec, T. D., Johnsen, B. H., and Molina, S. (2000). Phasic heart period reactions to cued threat and nonthreat stimuli in generalized anxiety disorder. Psychophysiology 37, 361–368. doi: 10.1017/S0048577200982192
Võ, M. L. H., Conrad, M., Kuchinke, L., Urton, K., Hofmann, M. J., and Jacobs, A. M. (2009). The berlin affective word list reloaded (BAWL-R). Behav. Res. Methods 41, 534–538. doi: 10.3758/BRM.41.2.534
Vrana, S. R., Cuthbert, B. N., and Lang, P. J. (1986). Fear imagery and text processing. Psychophysiology 23, 247–253. doi: 10.1111/j.1469-8986.1986.tb00626.x
Vrana, S. R., and Rollock, D. (2002). The role of ethnicity, gender, emotional content, and contextual differences in physiological, expressive, and self-reported emotional responses to imagery. Cogn. Emot. 16, 165–192. doi: 10.1080/02699930143000185
Wallentin, M., Nielsen, A. H., Vuust, P., Dohn, A., Roepstorff, A., and Lund, T. E. (2011). Amygdala and heart rate variability responses from listening to emotionally intense parts of a story. Neuroimage 58, 963–973. doi: 10.1016/j.biopsycho.2006.08.005
Watson, D., Clark, L. A., and Tellegen, A. (1988). Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Soc. Psychol. 54, 1063–1070. doi: 10.1037/0022-3514.54.6.1063
Watson, L. A., Dritschel, B., Obonsawin, M. C., and Jentzsch, I. (2007). Seeing yourself in a positive light: brain correlates of the self-positivity bias. Brain Res. 1152, 106–110. doi: 10.1016/j.brainres.2007.03.049
Werner, N. S., Jung, K., Duschek, S., and Schandry, R. (2009). Enhanced cardiac perception is associated with benefits in decision-making. Psychophysiology 46, 1123–1129. doi: 10.1111/j.1469-8986.2009.00855.x
Wiens, S., Mezzacappa, E. S., and Katkin, E. S. (2000). Heartbeat detection and the experience of emotions. Cogn. Emot. 14, 417–427. doi: 10.1080/026999300378905
Winkielman, P., Niedenthal, P., Wielgosz, J., Eelen, J., and Kavanagh, L. C. (2015). “Embodiment of cognition and emotion,” in APA Handbook of Personality and Social Psychology: Attitudes and Social Cognition, Vol. 1, eds M. Mikulincer and P. R. Shaver (Worcester, MA: American Psychological Association), 151–175.
Keywords: emotion, self, language, embodiment, facial expression, heart rate, skin conductance, emotional communication
Citation: Weis PP and Herbert C (2017) Bodily Reactions to Emotional Words Referring to Own versus Other People’s Emotions. Front. Psychol. 8:1277. doi: 10.3389/fpsyg.2017.01277
Received: 17 August 2016; Accepted: 12 July 2017;
Published: 22 August 2017.
Edited by:
Jerker Rönnberg, Linköping University, SwedenReviewed by:
Ivilin Peev Stoianov, Centre National de la Recherche Scientifique (CNRS), FranceCopyright © 2017 Weis and Herbert. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cornelia Herbert, Y29ybmVsaWEuaGVyYmVydEB1bmktdWxtLmRl
†These authors have contributed equally to this work and shared first authorship.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.