
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Psychol. , 28 April 2015
Sec. Cognitive Science
Volume 6 - 2015 | https://doi.org/10.3389/fpsyg.2015.00475
This article is part of the Research Topic Neuro-education and neuro-rehabilitation View all 17 articles
In the last decade, important advances in the field of cognitive science, psychology, and neuroscience have largely contributed to improve our knowledge on brain functioning. More recently, a line of research has been developed that aims at using musical training and practice as alternative tools for boosting specific perceptual, motor, cognitive, and emotional skills both in healthy population and in neurologic patients. These findings are of great hope for a better treatment of language-based learning disorders or motor impairment in chronic non-communicative diseases. In the first part of this review, we highlight several studies showing that learning to play a musical instrument can induce substantial neuroplastic changes in cortical and subcortical regions of motor, auditory and speech processing networks in healthy population. In a second part, we provide an overview of the evidence showing that musical training can be an alternative, low-cost and effective method for the treatment of language-based learning impaired populations. We then report results of the few studies showing that training with musical instruments can have positive effects on motor, emotional, and cognitive deficits observed in patients with non-communicable diseases such as stroke or Parkinson Disease. Despite inherent differences between musical training in educational and rehabilitation contexts, these results favor the idea that the structural, multimodal, and emotional properties of musical training can play an important role in developing new, creative and cost-effective intervention programs for education and rehabilitation in the next future.
Recently, the Organisation for Economic Co-operation and Development published the “2012 PISA report” providing strong evidence of the dramatic drop in the scholar level of 15-year-old French pupils over the past 10 years (OECD, 2014). Compared to data collected in 2003, 15 years old French children exhibited a drop of 15 points (from 511 to 495) in mathematics and the number of pupils in difficulty increased dramatically. In addition, learning disorders are very frequently diagnosed during childhood with the prevalence of developmental dyslexia being of 7–10% of the general population (Démonet et al., 2004; Collective expertise INSERM, 2007). These data point toward the urge of building and testing efficient learning tools to optimize the learning trajectories of typically developing young pupils and even more importantly to remediate specific disabilities found in children with language-based learning impairments. In addition to the burden in the young population, the demographic changes in life expectancy will lead to a significant increase of the population aged over 65 years old in Europe. This population is at high risk of suffering from neurologic age-related diseases (Salomon et al., 2012). For instance, the incidence of stroke is expected to grow by 36% from 2000 to 2025 (Truelsen et al., 2006). The last study of the World Health Organization Global Burden of Disease project is eloquent in showing that stroke remains the second cause of worldwide mortality (Lozano et al., 2012). Although advances in the acute medical management of stroke patients have reduced mortality in high-income countries (Feigin et al., 2014), stroke is still a major cause of disability-adjusted life-years (Murray et al., 2012). Beyond cardiovascular diseases, the rapid aging in Europe will also increase other non-communicable disorders such as neurodegenerative diseases. For instance, Parkinson’s disease affects 2.4 per 100 inhabitants of more than 65 years and its prevalence is expected to double by year 2030 (Dorsey et al., 2007). In this context, the need to develop innovative and effective rehabilitation evidence-based techniques is a challenge for the next years in the field of neuro-rehabilitation.
During the last decade, the neuroscientific community has developed a line of research on music perception and on the musician’s brain. Results obtained have largely contributed to increase our knowledge on the brain functioning in general and have allowed delineating the positive impact of playing a musical instrument on brain plasticity. In the first part of the review, we report evidence from cross-sectional and longitudinal studies showing that learning to play a musical instrument can induce substantial neuro-plastic changes in cortical and subcortical regions of motor, auditory and speech processing networks. The second part focuses on music to language transfer effect and on the necessary conditions for enhancing language processing in healthy participants. We follow by reporting an overview of the evidence showing that musical training can be an alternative, low-cost, and effective method for the remediation of language-based learning impaired populations. We then focus on neuro-rehabilitation by presenting the results of studies showing that music interventions can enhance motor recovery and neuroplasticity after stroke and can ameliorate motor deficits observed in Parkinson disease. Finally we discuss some of the important similarities and differences between musical training for neuro-education and for rehabilitation purposes.
In the last decade, the neuroscientific community has concentrated a great amount of effort to explore the positive impact of playing a musical instrument on the brain. Those efforts have provided converging evidence that the musician’s brain is an excellent model of neuroplasticity, specifically in the sensory-motor system (Münte et al., 2002; Zatorre, 2013). Indeed, playing in a symphony orchestra requires a large amount of practice: 20-year-old orchestra musicians typically spend more than 10,000 h of musical practice (Krampe and Ericsson, 1996). During all those hours, the musician will develop and ultimately master many different competences involving sensory-motor, mnesic, cognitive control, and attentional processes. As a consequence of the repetition of this complex activity, the underlying neural substrates will be eventually modified due to functional and structural neuroplastic mechanisms (see for a recent review, Kolb and Muhammad, 2014).
The neuroplastic changes induced by specific training can be studied using both cross-sectional and longitudinal approaches. While longitudinal studies generally use a test-training-retest procedure with naïve participants before training, cross-sectional studies compare a group of experts to a group of laymen participants. In the case of cross-sectional studies in which eventual pre-existing inter-individual differences might account for the differences observed between the groups (Zatorre, 2013) so that causality between music training and the observed effects cannot be demonstrated. By contrast, longitudinal studies with pseudo-random assignment of the participants to a training group and a non-training group allow determining that musical training is the cause of the differences (Schellenberg, 2004). Using a cross-sectional approach, Bangert and Schlaug (2006) conducted a study comparing the anatomical structure of the primary motor cortices in professional musicians and non-musicians. Using magnetic resonance imaging (MRI) these authors compared a group of non-musicians to pianists and violinists. While pianists showed similar structural modifications over both hemispheres, violinists showed a modification only over the right hemisphere. Indeed, playing the piano or the violin involves different type of bimanual control: pianists require fast and accurate finger movements of both hands whereas violinists use both hands asymmetrically favoring fine motor control of left hand fingers and gross motor control of the right hand thus leading to such a structural asymmetry. It is interesting to note that these studies provided evidence on how practicing particular cognitive and motor induce structural plastic brain alterations and improved level of performance for instance in motor areas (Schlaug et al., 1995a; Schlaug, 2001; Schmithorst and Wilke, 2002; Gaser and Schlaug, 2003; Hutchinson et al., 2003; see also Elbert et al., 1995; Draganski et al., 2004; Bengtsson et al., 2005).
Interestingly, the level of musical practice seems to be positively correlated with the increase in gray matter (GM) over motor regions (Gaser and Schlaug, 2003). Nonetheless, a recent study using particularly well controlled and highly selected pianists challenged the results mentioned above by showing a more complex pattern with decreasing GM density in peri-rolandic and striatal areas together with increasing GM density over areas involved in higher order processing such as the right fusiform gyrus, the right mid orbital gyrus, and the left inferior frontal gyrus (James et al., 2014). Increases in GM volume in the putamen have also been associated with timing variability and irregularity of scale playing in professional pianists (Granert et al., 2011). In this study, it was also observed that patients with musical dystonia presented more volume of GM in the right middle putamen. These surprising results may relate to the age of onset of musical practice and to excessive training, a potentially important factor for influencing plastic mechanisms and neural efficiency (Amunts et al., 1997). For instance, the age of onset of musical training is correlated with the level of motor performance in a rhythm synchronization task (Bailey et al., 2014) and may be decisive for the non-linear dynamic of structural brain changes induced by musical practice (Steele et al., 2013; Groussard et al., 2014).
Musical practice can also modify the strength of the connections between distant areas via white matter modifications. Compared to non-musicians, professional pianists exhibit a larger anterior portion of the Corpus Callosum, the main white matter fiber bundle connecting the two hemispheres (Schlaug et al., 1995b). Using diffusion tractography imaging method (DTI), it has been recently reported that musicians exhibit stronger white matter connectivity in the left and right supplementary motor areas (Li et al., 2014), in the corticospinal tract (Imfeld et al., 2009) and importantly, between auditory and motor areas (Oechslin et al., 2010; Halwani et al., 2011). The strong coupling of motor and auditory networks has been confirmed by further functional imaging studies showing activation of motor areas during the listening to musical rhythms in musicians (Haueisen and Knösche, 2001; Grahn and Brett, 2007; Bengtsson et al., 2009; Grahn and Rowe, 2009). Nevertheless, non-musicians also show such audio–motor co-activation (Baumann et al., 2007).
Moreover, short-term training programs also induce functional plastic changes. When beginners learn to play simple melodies (Bangert and Altnemüller, 2003). For instance, Lahav et al. (2007) trained non-musicians to play melodies by ear during five consecutive days and found activations in premotor regions when participants passively listened to the trained melodies (see also Meister et al., 2005). By contrast, Chen et al. (2012) observed a reduction of activation in both dorsal and ventral premotor cortices during musical training of naïve participants. This pattern of reduced activation over motor areas in musicians may reflect functional efficiency changes induced by musical training (Jäncke et al., 2000). In this context, Pascual-Leone et al. (1995) were the first in observing functional reorganization during piano learning using transcranial magnetic stimulation (TSM). After 4 weeks of training, participants showed a reduction of the motor map while showed an increase during the first week. This reorganization process over cortical motor maps highlight three important aspects. Firstly, rapid functional changes can occur after a rather short period of training. Secondly, the plastic changes induced by short-term training disappear when the training ends (see for example also Draganski et al., 2004). Thirdly, long-term training can lead to more efficient reduced patterns of activation. Aside from motor regions, plastic changes have also been reported at the level of the somatosensory cortices: musicians are more sensitive to tactile stimulations of the fingers than non-musicians, suggesting that musical practice can modify the size of the somatosensory receptive fields (Elbert et al., 1995).
Playing a musical instrument requires a clear motor component for a good performance. Nonetheless, the auditory dimension is also crucial in order to generate error feedback that might correct or adjust movements in case of errors (Maidhof, 2013; Maidhof et al., 2013; Pfordresher and Beasley, 2014) and to accurately perceive the pertinent acoustical parameters of the auditory input. Therefore, music induced plastic modifications over auditory areas might also be expected to occur.
The positive effects of musical practice on auditory processing have been evidenced by several studies showing lower discrimination thresholds for frequency, duration, silences, or time intervals in experts than in laymen (Jones et al., 1995; Kishon-Rabin et al., 2001; Micheyl et al., 2006; Rammsayer and Altenmüller, 2006; Mishra et al., 2014). In terms of structural plasticity, as in the case of the motor system, musical practice induces structural changes in the cortical auditory network: GM changes have been observed in both longitudinal and cross-sectional studies with musicians exhibiting enlarged AC compared to non-musicians (Schlaug et al., 1995a; Keenan et al., 2001; Schneider et al., 2002, 2005; Bermudez and Zatorre, 2005; Hyde et al., 2009). Moreover, compared to non-musicians, musicians also show more developed superior longitudinal fasciculus and arcuate fasciculus, the fiber bundles connecting the AC to Broca’s area (Oechslin et al., 2010; Wan and Schlaug, 2010; Halwani et al., 2011).
In line with these findings, functional changes have been reported in musicians at almost every single step of the auditory pathways: from the cochlea to the inferior colliculus (IC) and finally to the auditory cortices (AC). Musicians show functional changes already at the very peripheral level with enhanced activity of the Medial olivocochlear complex, responsible for controlling the cochlear micromechanics (Perrot et al., 1999; see for a review Perrot and Collet, 2014). Since almost 10 years, the systematic work of Nina Kraus and her group have provided exciting evidence that musical practice also induces substantial neuroplastic changes over subcortical structures. The IC, a subcortical structure in the brainstem receiving both bottom-up inputs from the cochlea and top-down inputs via the corticofugal pathway (Kraus and Chandrasekaran, 2010), encodes specific characteristics of the auditory input (Russo et al., 2004). For instance, very brief acoustic events such as stop consonants (“b,” “p,” “g,” “d,” “t”) are reflected by the transient response of the auditory brainstem responses (ABRs) whereas sustained acoustic events such as vowels are reflected by the frequency following response (FFR). Compared to non-musicians, adult musicians show more robust transient response and FFR to both musical and speech sounds (Musacchia et al., 2007; Parbery-Clark et al., 2012) suggesting that at the level of the brainstem, the representations of the sounds are more elaborated and more accurate in musicians than in non-musicians. The enhancement of ABRs is already visible at age three suggesting that very few years of musical training might be sufficient to elicit such consistent plastic changes in the IC (Strait et al., 2013). Moreover, the benefit of musical training received during childhood appears to remain during adulthood as revealed by a correlation between ABR amplitude and how recently participants quitted the training (Skoe and Kraus, 2012; see also Strait and Kraus, 2011).
Finally, musical practice fosters functional brain plasticity in cortical areas: compared to non-musicians, adult and child musicians show enhanced response of the AC as reflected by larger N1/P2 amplitude to complex sounds (Shahin et al., 2003, 2004; Trainor et al., 2003). Moreover, this enhanced N1/P2 is particularly visible when the stimulus presented is the instrument played by the participants (Pantev et al., 1998, 2001). Again, these results suggest that musicians have a more elaborated representation of the auditory input and better encode fine-grained features of sounds than non-musically trained individuals.
Interestingly, musical practice also increases the neural sensitivity to the statistical regularities found in the auditory input (François and Schön, 2014). For instance, a deviant sound rarely occurring within a sequence of repeated standard sounds elicit a specific event-related potential (ERP) component, the mismatch negativity (MMN), reflecting the pre-attentive detection of an auditory change (Näätänen et al., 2005; Grimm and Escera, 2011). Adult and child musicians often show larger and/or earlier MMNs to changes in several features of the sounds such as frequency, duration, intensity, or spatial localization than non-musicians (Tervaniemi et al., 2001, 2005; Van Zuijen et al., 2005; Vuust et al., 2005, 2011; Brattico et al., 2009; Marie et al., 2011; Putkinen et al., 2014). Additional evidence shows that musicians exhibit enhanced MMN to change in longer sequences of structured sounds (Tervaniemi et al., 2001; Trainor et al., 2002; Fujioka et al., 2004; Habermeyer et al., 2009).
After having delineated the positive effects of playing music on brain plasticity in the auditory and motor networks, we now turn to the topic of neuro-education We aim to give an overview of evidence showing that language-based learning impaired children often show deficits in the specific processes that are boosted by musical practice. We then present the hypothesis of music to language transfer effect, which allow grounding the subsequent evidence from both cross-sectional and longitudinal studies showing the beneficial effects of musical practice on speech processing in typically developing children. We finally summarize the few studies testing music-training programs in language-based learning impaired populations.
Children with language based learning impairments such as developmental dyslexia present a specific deficit in reading despite conventional instruction, socio-cultural level, normal intelligence and the absence of sensory deficits (Lyon et al., 2003; Vellutino et al., 2004; Collective expertise INSERM, 2007). Phonological awareness is crucial for normal language development (Ramus, 2003; Serniclaes et al., 2004) and relies on the ability to categorize speech sounds on the basis of extremely short timing differences. The voice onset time (VOT) is an acoustic parameter largely used to study phonological awareness as it allows differentiating the sound “ba” from the sound “pa,” a task in which dyslexic children have difficulties (Serniclaes et al., 2004). In line with these findings, Chobert et al. (2012) were able to demonstrate that children with dyslexia are impaired in the pre-attentive processing of VOT and duration in syllables based on MMN data. Children with language learning difficulties have also difficulties in extracting speech sounds when presented in a background noise (Ziegler et al., 2005, 2009) and show degraded neural responses to speech in noise stimuli (Warrier et al., 2004; Wible et al., 2004; Anderson et al., 2010).
These children might present a general deficit in the processing of timing information (Goswami et al., 2011). This timing hypothesis would explain why they exhibit impaired phonological processing (Ziegler and Goswami, 2005) and impaired general rhythmic processing (Thomson and Goswami, 2008; Corriveau and Goswami, 2009). Huss et al. (2011) showed that children with dyslexia present a deficit in the perception of rise time, an acoustic parameter important to extract the periodic and eventually metrical structures of speech (Cummins and Port, 1998; Quené and Port, 2005; Goswami, 2010; Huss et al., 2011). Growing evidence converge on the idea that rhythmic skills are crucial for the development of literacy skills in typically developing children (Tierney and Kraus, 2013; Woodruff Carr et al., 2014) and that children with dyslexia are impaired at tapping to a rhythm, and in perceiving tempo (Thomson and Goswami, 2008; Corriveau and Goswami, 2009). These results also confirm findings showing a link between literacy skills, phonological abilities, and musical aptitudes in typically developing child and in adult participants (Anvari et al., 2002; Slevc and Miyake, 2006; Tierney and Kraus, 2013). Following this line, a recent study with 48 children with dyslexia shows that temporal auditory processing strongly predicts phonological processing and reading abilities (Flaugnacco et al., 2014).
Transfer of skills generally occurs when a specific skill acquired in one specific domain influences processes in another supposedly unrelated domain. Several observations have lead to the hypothesis that musical practice could transfer to language and more specifically to speech processing (Kraus and Chandrasekaran, 2010; Besson et al., 2011; Patel, 2011, 2014). Firstly, as presented above, there is a whole body of literature showing enhanced auditory processing in musicians. Secondly, music and speech share similarities: both involve the processing of similar acoustic cues (such as pitch, intensity, timbre, and duration) and both involve maintaining sequences of sounds that are unfolding in time in a structured manner. Thirdly, music and speech processing show a clear overlap in their cortical and subcortical neural substrates (Koelsch et al., 2005; Vigneau et al., 2006; Schön et al., 2010), suggesting shared neural resources (Patel, 2011, 2014).
In the specific context of education, it is now clearly demonstrated that noisy environments in classroom settings with higher than normal ranges level of noise negatively impacts pupils’ performance (Knecht et al., 2002; Shield and Dockrell, 2008). Indeed, the level of performance in a syllable discrimination task dramatically drops as the level of noise increases (Neuman et al., 2010). The amplitude and latency of ABRs are also clearly reduced in the presence of acoustic noise (Burkard and Sims, 2002; Russo et al., 2004). Children with language based learning impairment show impaired speech in noise perception and altered neural responses to speech sounds presented in a background noise. Musical practice seems to be a good tool to prevent this deleterious effect of noise as adult musicians exhibit more preserved ABRs in noise than non-musicians (Parbery-Clark et al., 2009a,b; Bidelman and Krishnan, 2010; Strait et al., 2012). Furthermore, a recent longitudinal study has revealed that musical training in high school music classes can induce these changes (Tierney et al., 2013), which appear to maintain during lifespan (Zendel and Alain, 2012, 2013).
If musicians are better in perceiving speech in noise they also have refined representations of syllables (Degé and Schwarzer, 2011; Zuk et al., 2013; see also Moreno et al., 2009) at both subcortical and cortical levels (Chobert et al., 2011, 2014; Strait et al., 2012; see Figure 1 for an illustration of the experimental design). All together, these results show that musicians have better neural encoding of speech sounds, which might help to develop a greater sensitivity to the metrical structure of speech (Port, 2003). Compared to non-musicians, adult and child musicians showed increased sensitivity to subtle pitch modifications inserted in the prosodic contour of sentences being uttered in their native or in a foreign language (Schön et al., 2004; Magne et al., 2006; Marques et al., 2007). Adult musicians are also more sensitive to the metrical structures of speech and to anomalous durational modifications in sentences (Marie et al., 2011).
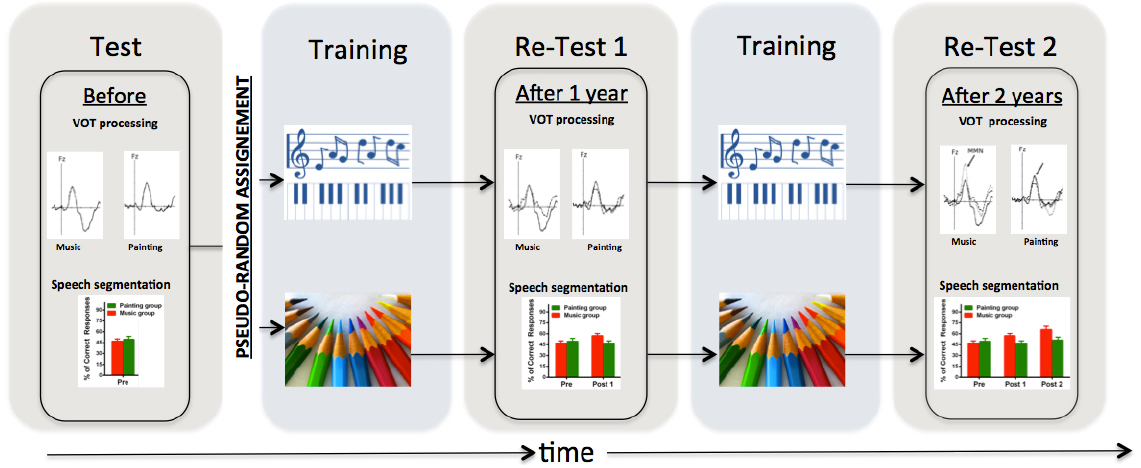
Figure 1. Illustration of the experimental design used in Chobert et al. (2014) and François et al. (2013). Using a similar design over 2 school years with test-training-retest-training-retest procedure over 2 years, 8-year-old children who followed a musical training program exhibited behavioral and electrophysiological evidence of increasing VOT processing and speech segmentation skills than children who followed a painting training program. Note that, (i) the pseudo-random assignment of the participants is crucial to control for possible confounds including, socio-economic, educational, cognitive, and linguistic measures; (ii) that the two training programs must be equally motivating, engaging, diverse; and that (iii) the training programs were provided in collective groups and not in individual.
Further evidence of better speech processing skills in musicians than in non-musicians was also provided by cross-sectional and longitudinal studies exploring speech segmentation ability in adults and children (François and Schön, 2011; François et al., 2013, 2014). Speech segmentation is one of the mandatory steps for acquiring a new language, which requires the ability to extract words from continuous speech. When presented with 2 min of an artificial stream of statistically structured syllables, infants and adults are able to segment and discriminate syllables sequences that are part of the stream (i.e., familiar sequences) from new sequences (i.e., unfamiliar sequences; Saffran et al., 1996; Aslin et al., 1998). While adult musicians barely outperformed non-musicians (François and Schön, 2011), musically trained children largely outperformed their non-musician counterparts after 1 year and even more after 2 years of musical training (François et al., 2013). Moreover, neural responses in both adult and child musicians differentiated familiar from unfamiliar sequences during the behavioral test. In a further study, François et al. (2014) provided evidence that the ability to differentiate familiar from unfamiliar items was correlated with how fast a fronto-central negative ERP component emerged during the exposition to the artificial speech stream.
The mounting evidence of the beneficial effects of musical training on speech auditory processing in typically developing children as well as the timing deficits found in children with language based learning impairments led researchers to test the idea that musical training could be used as a remediation tool.
Overy (2000, 2003) were the first evaluating the efficiency of playing music in children with dyslexia. Despite a small group and no clear matched-control, the results showed improved phonological awareness and spelling performance after a rhythmic training program. Along these lines, short rhythmic priming sequences have be used to enhance phonological processing in typically developing children and in prelingually deaf children (Cason and Schön, 2012; Cason et al., 2015) and to improve syntactic processing in children with language impairments (Przybylski et al., 2013). Despite a clear lack of controlled trials in children and adolescents with dyslexia (Cogo-Moreira et al., 2012), a recently published study confirms that musical training can improve reading skills and educational achievement in those children (Cogo-Moreira et al., 2013). Moreover, a recent study from Bishop-Liebler et al. (2014) provides clear evidence of the positive influence of musical practice on the timing deficits found in dyslexics.
Finally, recent data suggest that an adapted musical training program can enhance auditory, phonological, and cognitive processes in 8-year-old deaf children (Rochette et al., 2014) and that musical rhythmic priming can enhance phonological production in prelingually deaf children (Cason et al., 2015). These results are important for future studies in cochlear-implanted users who show profound deficits in speech in noise perception, in the perception of complex rhythms, timbres, and melodies with somewhat preserved tempo and simple rhythms perception (McDermott, 2004; Drennan and Rubinstein, 2008).
Music playing requires the processing of multimodal information and entails high neural demands. Multimodality is an excellent opportunity to adapt different musical activities such as playing an instrument, moving in synchrony along a rhythm or listening to music with a therapeutic purpose (Pantev and Herholz, 2011). In this section, we present rehabilitative techniques using music as a key feature to remediate motor deficits in neurological conditions.
Motor impairment after stroke refers to a weakness of the muscles mainly affecting the control and performance of voluntary movements (Mohr et al., 2011). This deficit is the most common outcome after stroke with paresis of the upper and lower extremities being found in almost 70% of cases (Rathore et al., 2002). While paresis of the legs impedes functional mobility such as walking, the limitations of arm and hand paresis extend to several daily-living activities (Langhorne et al., 2011).
Music-supported therapy (MST) aims to restore paresis of the upper limb through musical instrument playing (Schneider et al., 2007; see Table 1). In order to enhance fine and gross movements, patients are trained on playing melodies on a midi piano and/or electronic drum pads. MST relies on four basic principles: massive repetition, audio–motor coupling, shaping, and emotion-motivation effects (Rodríguez-Fornells et al., 2012). Firstly, MST requires massive repetition of simple sequences of movements through the intervention. Importantly, high-intensity practice is a basic and well-accepted principle in neuro-rehabilitation (Langhorne et al., 2009, 2011). Secondly, multimodal integration may enhance audio–motor coupling where the musical sound serves as a feedback to reinforce the movement, to correct the errors, to adjust the timing and to refine motor representations. Thirdly, the therapy is adapted to the level of impairment and to the progression of the patient. Fourthly, the emotional-motivational aspects of music may regulate emotional responses through the playfulness of learning a new skill.
Music-supported therapy is successful in reducing the motor deficits in subacute stroke patients. Altenmüller et al. (2009) and Schneider et al. (2007) compared the effectiveness of MST to conventional treatment. Only patients in the MST group improved in frequency, velocity and smoothness of fingers and hand tapping movements. Moreover, those patients obtained greater scores over time on standardized clinical tests assessing motor functions. However, to what extent is the presence of music responsible for the observed gains and associated plasticity? In a single-case study (Rojo et al., 2011), a patient performed a passive listening task with unfamiliar and trained melodies. Interestingly, while before the application of MST the patient exhibited only activation of the AC, the motor regions were also activated after the training. This phenomenon of audio–motor coupling provided evidence that the auditory feedback is an essential part of the therapy by contributing to enhance activations over motor regions (Rodríguez-Fornells et al., 2012). In a further study, Amengual et al. (2013) used TMS to demonstrate changes in the excitability of the sensorimotor cortex due to MST. Participants recovered from their motor deficits and exhibited an increased excitability of the sensorimotor cortex in the affected hemisphere after 4 weeks of MST. Moreover, a lateral shift in the motor map of chronic patients was evidenced after the training and was associated with motor gains. Interestingly, similar results have been recently reported in subacute patients (Grau-Sánchez et al., 2013). Taken together these studies suggest that MST can induce functional changes associated to brain reorganization processes.
Recent studies aimed at modifying different aspects of the MST protocol. Van Vugt et al. (2014) implemented MST in two groups of subacute stroke patients in which participants received the therapy in pairs instead of individual sessions. One group had to play together while the other group played in turns. Although both groups improved their performance in a motor task, results indicated a positive trend favoring the in-turn group. Besides, the in-turn group improved more their mood as well as their feelings about their partner. The idea that music playing is a shared experience (Overy, 2012) is interesting to consider because patients can feel emphatic and understood by others individuals presenting similar difficulties. This could in turn enhance the mood and reduce depressive symptoms (Gillen, 2010). MST has also been evaluated in a home-training protocol (Villeneuve et al., 2014) showing improvements in the paresis of chronic patients that were maintained over time. During nine 1-h sessions, patients played musical sequences using Synthesia (Synthesia LLC) a software that provides visual cues to guide them. The sessions were complemented with at home exercises on a roll-up piano. Home sessions in chronic stages may be an appropriate cost-effective approach once patients have gained a certain degree of improvements and stabilization. In this vein, the use of new technologies and adapted software with rehabilitative purposes opens new directions in the field of neuro-rehabilitation. The recently developed MusicGlove (Friedman et al., 2014) may be an alternative tool to treat paresis in chronic stages. The MusicGlove is an instrumented glove that produces notes during gripping movements while being guided with visual stimuli displayed on a screen. An exploratory study with 12 chronic patients has revealed improvements in motor functions compared to conventional therapy or isometric training (Friedman et al., 2014).
Importantly, all subacute patients involved in theses studies receive a rehabilitation program in a hospital or outpatient setting that includes physiotherapy and occupational therapy to train the affected extremity (Gillen, 2010). This may limit the interpretation of the positive effects of MST over conventional treatments because participants cannot be excluded from the standard rehabilitation program (Oremus et al., 2012). Moreover, natural brain processes of recovery take place in acute and subacute stages which may be a confounding factor (Cramer et al., 2011; Johansson, 2011; Zeiler and Krakauer, 2013). In order to control for spontaneous recovery, it is important to have comparable groups in terms of age, severity of the deficits and time since stroke. Chronic stages are characterized by a stabilization of the deficits via compensatory mechanisms at both the behavioral and neural levels (Cramer et al., 2011; Langhorne et al., 2011) and thus, it might be more appropriate to perform proper randomized controlled trials (RCT) using within-participant designs combined together with disease progression models.
The main symptom in Parkinson’s disease is motor impairment in gait, which is characterized by a decreased speed, shorter stride length, and asymmetries in stride times for both lower limbs, all in turn increasing cadence of steps. This pronounced reduction in speed and amplitude is accompanied by a difficulty in initiating voluntary movements in later stages of the disease, with patients experiencing a freezing of the movements (Okuma and Yanagisawa, 2008). This limitation in walking will in turn impair balance and postural control and lead patients to a reduced activity and high risk of falls (Kim et al., 2013). A dysfunction of the basal ganglia is responsible for the mentioned symptoms (Stoessl et al., 2014) and the efficacy of pharmacological treatment reduces with time. Thus, the development of behavioral therapies may help in coping with the impairment and may be an alternative to be combined with pharmacological therapy.
One approach to enhance intrinsically rhythmical movements consists in using external sensory cues to entrain the movements (Thaut and Abiru, 2010; Thaut et al., 2014). Rhythm auditory stimulation (RAS, Thaut et al., 1996) aims to facilitate gait using metronome beats. The patient is first trained to move to the beat and then the tempo is increased from 5 to 10% over the baseline to accomplish faster movements. A first study observed that 3 weeks of RAS could improve gait velocity, stride length, and step cadence more than no treatment or self-paced training program (Thaut et al., 1996). Further studies have confirmed the positive effects of RAS on gait parameters such as overcoming freezing of gate (McIntosh et al., 1997, 1998; Thaut et al., 1997; Freedland et al., 2002; Fernandez del Olmo and Cudeiro, 2003, 2005; Arias and Cudeiro, 2010; for a review, see Nombela et al., 2013). It also improves stride length, gait velocity, cadence, and asymmetry in patients with stroke (Thaut et al., 1997, 2007; for a review in other neurological conditions, see Wittner et al., 2013). The neuroplastic mechanisms beyond the effectiveness of RAS may be due to an increased activity in the cerebellum, trying to compensate the dysfunctional pathway of the basal ganglia to regions of the premotor cortex (Fernandez del Olmo and Cudeiro, 2003). However, two studies have showed more benefits in the advanced than in the early stages of the disease suggesting that the effectiveness of RAS depends on the stage of the disease (Willems et al., 2006; Arias and Cudeiro, 2008). Moreover, some studies have explored variations in RAS, manipulating the tempo (Fernandez del Olmo and Cudeiro, 2003, 2005) as well as the rhythm with respect to the individual’s baseline (Willems et al., 2006; Ledger et al., 2008). These studies have evidenced that beats presented at 20% slower than the baseline cadence does not benefit gait (Willems et al., 2006). Although other types of therapy have examined the use of other sensorial modalities (visual and proprioceptive cues), the auditory modality seems to be the best to improve gait in Parkinson’s disease (Nombela et al., 2013).
Beyond motor impairment, neurological patients are at high risk for suffering psychological consequences. Around one third of stroke patients suffer from depression in the following months and years (Hackett et al., 2005; Ayerbe et al., 2013) and apathy and anxiety can also be found as a frequent neuropsychiatric consequence (Campbell Burton et al., 2011; Caeiro et al., 2013). These neuropsychiatric symptoms are thought to impact health-related quality of life, increase morbidity and worsen the cognitive impairments (Whyte and Mulsant, 2002; Aarsland et al., 2012; Ayerbe et al., 2013). Psychological factors can also have a negative effect on recovery and can affect the engagement in the rehabilitation program. Pharmacological interventions have small effects on treating depression and reducing its symptoms in stroke and Parkinson’s disease and can also lead to negative side effects (Hackett et al., 2008, 2010; Aarsland et al., 2012). Some studies have reported that MST can reduce depression and fatigue and can improve the quality of life in stroke patients (Grau-Sánchez et al., 2013; Van Vugt et al., 2014). Music playing could be an alternative approach to target depression and neuropsychiatric symptoms through emotion regulation. However, there is little research studying the effectiveness of music therapy in the emotional domain. Compared to listening to audio books or auditory intervention, the daily listening to self-selected music during 2 months improves mood in the following months. Listening to music can also improve verbal memory and focused attention (Särkämö et al., 2008). Importantly, listening to music also induced structural changes with an increase of GM in frontal and limbic structures (Särkämö et al., 2014). Participants reported that music was helpful for relaxing and sleeping, influenced their mood and evoked memories and reflexive thoughts (Forsblom et al., 2009). Musical activities may be a tool to modulate the emotional reactions and cope with them through playfulness activity.
We focused our review on MST, RAS, and listening to music as standardized therapies to treat motor deficits and improve mood. Further research should aim to standardize interventions to build a strong dataset in this field. Moreover, the majority of studies refer to conventional treatment as the current rehabilitation program provided by the hospital. However, the content of the rehabilitation program may vary depending upon the facilities or the countries. Thus, an accurate description of the exercises performed by the control group is needed. Randomization of participants and blind evaluations of the treatment constitute RCTs and are necessary to implement interventional programs in clinical practice.
Fundamental differences may exist between musical training for education or rehabilitation. Firstly, conceptual differences on the aim of musical training remain in the two cases. In neuro-education, musical training generally aims to boost a “typical” developmental trajectory (Kraus and Chandrasekaran, 2010), whereas in neuro-rehabilitation, musical training is rather used to normalize or compensate sensory, motor or cognitive deficits induced by a pathological condition (Cramer et al., 2011). Nonetheless, in the case of children with dyslexia, musical training will also aim to normalize the learning trajectories in order to facilitate speech and language processing which in turn may have a positive impact on educational achievement (Tierney and Kraus, 2013).
Secondly, the neuro-plastic mechanisms induced by musical training during childhood or in neurological population may be different. This might be due to the intrinsic physiological differences at play in these two cases. Animal and human studies showed that auditory stimulation received early in life enhances both sub-cortical and cortical electrophysiological responses to sounds (Sanes and Constantine-Paton, 1985; Kraus and Chandrasekaran, 2010) and persist in adult development (Zhang et al., 2001; Skoe and Kraus, 2012; White-Schwoch et al., 2013). Early in life, the neural system is immature and capitalizes on plastic changes such as myelination, neurogenesis, or dendritic growth (Kolb and Telskey, 2012; Kolb et al., 2012). On the contrary, stroke generally occurs in a mature neural system in which the insult has induced the death of neurons from a specific region and the disruption of neural networks. The plastic mechanisms underpinning recovery after stroke rely on different processes than in typical development such as restitution and substitution (Albert and Kesselring, 2012). Studies exploring the benefits of MST have shown that musical training induced functional reorganization of cortical motor maps (Amengual et al., 2013). In Parkinson disease, the aim of the therapy is to compensate the deficits in internally generated movements using music as an external cue that engages a different motor pathway to achieve the same goal, which is the initiation and normalization of gait (Nombela et al., 2013).
Thirdly, qualitative and quantitative practical differences in the type of training administered also remain. In neuro-rehabilitation settings, the training is generally provided during a relatively short period of time together with a high intensity (Albert and Kesselring, 2012). Interestingly, the use of the affected upper extremity during sessions of conventional stroke rehabilitation is minimal (Birkenmeier et al., 2010; Krakauer et al., 2012), suggesting that rehabilitation protocols should increase the doses of practice. In this context, musical training protocols for motor rehabilitation may be a good choice as they involve the massive repetition of movements with a high-intensity. Moreover, MST and RAS are most of the time individually administered and the complexity of the tasks is adapted to the progression of each patient. In the case of MST and particularly in middle to low-income countries, the training may become rather expensive due to the individualized administration of the therapy. However and importantly, when patients are discharged from the rehabilitation unit, they are most of the time physically inactive, exhibiting sedentary behaviors and have poor social interactions (Särkämö et al., 2008; Dontje et al., 2013; Tieges et al., 2015). The use home-based MST and RAS therapies may be important to encourage these patients to continue the rehabilitation and to maintain active. Moreover, listening to music could be good alternative not only to be applied at home but also in the rehabilitation unit, where most of the time patients remain in their rooms with no social interaction (Särkämö et al., 2008). Music making in neuro-education settings is generally provided in group settings with a lower intensity than in neuro-rehabilitation but importantly, the training is administered during longer periods of time thus allowing reaching a higher degree of musical complexity than in the context of rehabilitation. The group settings used with children may be more cognitively demanding than the ones used with neurologic patients. Most of the time, children have to play in synchrony with each other and ultimately create new musical pieces whereas patients will generally listen and reproduce simple familiar musical pieces.
Despite obvious differences, fundamental similarities might relate to the emotional, sensory, motor, cognitive, and social demands of music making per se (Herholz and Zatorre, 2012). In both fields, musical training is selected for its unique characteristics involving complex interactions between different domains and systems. Due to its multimodal aspect, musical training represents a good activity to develop audio–motor interactions, for cognitive stimulation and mood regulation. Moreover, music making as well as music listening are generally pleasant activities that are most likely to induce motivated behaviors. This is particularly the case for patients who may be more committed toward an enjoyable activity with a specific purpose rather than to a repetitive training with different rehabilitative tools. Musical training is also able to reinforce social cohesion or bonding through repetitive inter-individual interactions (Huron, 2001). Another similarity may also reside on the fact that musical practice will induce positive side effects: by enhancing language processing for the educational side and by boosting spared functions for the rehabilitation side. Finally, the permanence in time of the benefits of music making is clearly observed and is probably the most meaningful aspect for both purposes.
The neural mechanisms of music-induced plasticity are still not perfectly understood (Fukui and Toyoshima, 2008), but the evidence for a positive effect of musical practice are growing and could justify the use of music both in the context of neuro-education (Caine and Caine, 1990) and of neuro-rehabilitation (Särkämö et al., 2014). The findings showing that the age of onset of musical training influences the dynamic of training induced plastic changes (Steele et al., 2013; Groussard et al., 2014) leads to the idea that multiple sensitive periods for specific functions and specific brain networks may co-exist in typical development (Penhune, 2011). This opens interesting perspectives to study the benefit of musical training in the developing brain as well as to study its consequences on speech perception and scholar achievement. The recent findings showing that listening to music is a rewarding experience for most of the people (Mas-Herrero et al., 2014) and that simple music listening activates the rewarding dopaminergic system (Salimpoor et al., 2013) give even more support to the idea that musical practice may be the perfect tool for neuro-education and Rehabilitation by fostering plastic changes in the healthy or pathological brains. Despite these growing evidence, the educational and health systems generally seem to be refractory to the idea of developing musical training programs. We hope that both teachers and therapists will keep on believing and applying alternative methods based on musical practice.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Financial support for the present project has been received by the Spanish government (Ministry of Economy and Competitiveness [MINECO] Grant PSI2011-29219 to AF) and from the Fyssen Foundation to CF (http://www.fondationfyssen.fr/). We are grateful to three reviewers and to Dr. Mireille Besson for helping us improving the quality of the manuscript. The research leading to these results has received funding from RecerCaixa.
Aarsland, D., Påhlhagen, S., Ballard, C. G., Ehrt, U., and Svenningsson, P. (2012). Depression in Parkinson disease-epidemiology, mechanisms and management. Nat. Rev. Neurol. 8, 35–47. doi: 10.1038/nrneurol.2011.189
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Albert, S. J., and Kesselring, J. (2012). Neurorehabilitation of stroke. J. Neurol. 259, 817–832. doi: 10.1007/s00415-011-6247-y
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Altenmüller, E., Marco-Pallares, J., Münte, T. F., and Schneider, S. (2009). Neural reorganization underlies improvement in stroke-induced motor dysfunction by music-supported therapy. Annu. N. Y. Acad. Sci. 1169, 395–405. doi: 10.1111/j.1749-6632.2009.04580.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Amengual, J. L., Rojo, N., Veciana de las Heras, M., Marco-Pallarés, J., Grau-Sánchez, J., Schneider, S., et al. (2013). Sensorimotor plasticity after music-supported therapy in chronic stroke patients revealed by transcranial magnetic stimulation. PLoS ONE 8:e61883. doi: 10.1371/journal.pone.0061883
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Amunts, K., Schlaug, G., Jäncke, L., Steinmetz, H., Schleicher, A., Dabringhaus, A., et al. (1997). Motor cortex and hand motor skills: structural compliance in the human brain. Hum. Brain Mapp. 5, 206–215. doi: 10.1002/(SICI)1097-0193(1997)5:3<206::AID-HBM5>3.0.CO;2-7
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Anderson, S., Skoe, E., Chandrasekaran, B., and Kraus, N. (2010). Neural timing is linked to speech perception in noise. J. Neurosci. 30, 4922–4926. doi: 10.1523/JNEUROSCI.0107-10.2010
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Anvari, S., Trainor, L., Woodside, J., and Levy, B. (2002). Relations among musical skills, phonological processing, and early reading ability in preschool children. J. Exp. Child Psychol. 83, 111–130. doi: 10.1016/S0022-0965(02)00124-8
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Arias, P., and Cudeiro, J. (2008). Effects of rhythmic sensory stimulation (auditory visual) on gait in Parkinson’s disease patients. Exp. Brain Res. 186, 589–601. doi: 10.1007/s00221-007-1263-y
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Arias, P., and Cudeiro, J. (2010). Effect of rhythmic auditory stimulation on gait in Parkinsonian patients with and without freezing of gait. PLoS ONE 5:e9675. doi: 10.1371/journal.pone.0009675
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Aslin, R. N., Saffran, J. R., and Newport, E. L. (1998). Computation of conditional probability statistics by 8-month-old infants. Psychol. Sci. 9, 321–324. doi: 10.1111/1467-9280.00063
Ayerbe, L., Ayis, S., Wolfe, C. D., and Rudd, A. G. (2013). Natural history, predictors and outcomes of depression after stroke: systematic review and meta-analysis. Br. J. Psychiatry 202, 14–21. doi: 10.1192/bjp.bp.111.107664
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bailey, J. A., Zatorre, R. J., and Penhune, V. B. (2014). Early musical training is linked to gray matter structure in the ventral premotor cortex and auditory-motor rhythm synchronization performance. J. Cogn. Neurosci. 26, 755–767. doi: 10.1162/jocn_a_00527
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bangert, M., and Altnemüller, E. (2003). Mapping perception to action in piano practice: a longitudinal DC-EEG study. BMC Neurosci. 4:26. doi: 10.1186/1471-2202-4-26
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bangert, M., and Schlaug, G. (2006). Specialization of the specialized in features of external human brain morphology. Eur. J. Neurosci. 24, 1832–1834. doi: 10.1111/j.1460-9568.2006.05031.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Baumann, S., Koeneke, S., Schmidt, C. F., Meyer, M., Lutz, K., and Jancke, L. (2007). A network for audio-motor coordination in skilled pianists and non-musicians. Brain Res. 1161, 65–78. doi: 10.1016/j.brainres.2007.05.045
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bengtsson, S. L., Nagy, Z., Skare, S., Forsman, L., Forssberg, H., and Ullén, F. (2005). Extensive piano practicing has regionally specific effects on white matter development. Nat. Neurosci. 8, 1148–1150. doi: 10.1038/nn1516
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bengtsson, S. L., Ullén, F., Ehrsson, H. H., Hashimoto, T., Kito, T., Naito, E., et al. (2009). Listening to rhythms activates motor and premotor cortices. Cortex 45, 62–71. doi: 10.1016/j.cortex.2008.07.002
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bermudez, P., and Zatorre, R. J. (2005). Differences in gray matter between musicians and nonmusicians. Ann. N. Y. Acad. Sci. 1060, 395–399. doi: 10.1196/annals.1360.057
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Besson, M., Chobert, J., and Marie, C. (2011). Transfer of training between music and speech: common processing, attention, and memory. Front. Psychol. 2:94. doi: 10.3389/fpsyg.2011.00094
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bidelman, G. M., and Krishnan, A. (2010). Effects of reverberation on brainstem representation of speech in musicians and non-musicians. Brain Res. 1355, 112–125. doi: 10.1016/j.brainres.2010.07.100
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Birkenmeier, R. L., Prager, E. M., and Lang, C. E. (2010). Translating animal doses of task-specific training to people with chronic stroke in 1-hour therapy sessions: a proof-of-concept study. Neurorehabil. Neural Repair 24, 620–635. doi: 10.1177/1545968310361957
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bishop-Liebler, P., Welch, G., Huss, M., Thomson, J. M., and Goswami, U. (2014). Auditory temporal processing skills in musicians with dyslexia. Dyslexia 20, 261–279. doi: 10.1002/dys.1479
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Burkard, R. F., and Sims, D. (2002). A comparison of the effects of broadband masking noise on the auditory brainstem response in young and older adults. Am. J. Audiol. 11, 13–22. doi: 10.1044/1059-0889(2002/004)
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Brattico, E., Pallesen, K. J., Varyagina, O., Bailey, C., Anourova, I., Järvenpää, et al. (2009). Neural discrimination of nonprototypical chords in music experts and laymen: an MEG study. J. Cogn. Neurosci. 21, 2230–2244. doi: 10.1162/jocn.2008.21144
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Caeiro, L., Ferro, J. M., and Costa, J. (2013). Apathy secondary to stroke: a systematic review and meta-analysis. Cerebrovasc. Dis. 35, 23–39. doi: 10.1159/000346076
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Caine, R., and Caine, G. (1990). Understanding a brain-based approach to learning and teaching. Educ. Leadersh. 48, 66–70.
Campbell Burton, C. A., Holmes, J., Murray, J., Gillespie, D., Lightbody, C. E., Watkins, C. L., et al. (2011). Interventions for treating anxiety after stroke. Cochrane Database Syst. Rev 12:CD008860. doi: 10.1002/14651858.CD008860.pub2
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Carroll, D. (1965). A quantitative test of upper extremity function. J. Chronic Dis. 18, 479–491. doi: 10.1016/0021-9681(65)90030-5
Cason, N., Hidalgo, C., Isoard, F., Roman, S., and Schön, D. (2015). Rhythmic priming enhances speech production abilities: evidence from prelingually deaf children. Neuropsychology 29, 102–107. doi: 10.1037/neu0000115
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cason, N., and Schön, D. (2012). Rhythmic priming enhances the phonological processing of speech. Neuropsychologia 50, 2652–2658. doi: 10.1016/j.neuropsychologia.2012.07.018
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Chen, J. L., Rae, C., and Watkins, K. E. (2012). Learning to play a melody: an fmri study examining the formation of auditory-motor associations. Neuroimage 59, 1200–1208. doi: 10.1016/j.neuroimage.2011.08.012
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Chobert, J., François, C., Habib, M., and Besson, M. (2012). Deficit in the preattentive processing of syllabic duration and VOT in children with dyslexia. Neuropsychologia 50, 2044–2055. doi: 10.1016/j.neuropsychologia.2012.05.004
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Chobert, J., François, C., Velay, J. L., and Besson, M. (2014). Twelve months of active musical training in 8- to 10-year-old children enhances the preattentive processing of syllabic duration and voice onset time. Cereb. Cortex 24, 956–967. doi: 10.1093/cercor/bhs377
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Chobert, J., Marie, C., François, C., Schön, D., and Besson, M. (2011). Enhanced passive and active processing of syllables in musician children. J. Cogn. Neurosci. 23, 3874–3887. doi: 10.1162/jocn_a_00088
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Collective expertise INSERM. C. N. D. R. S. D. I. (2007). Dyslexie, dysorthographie, dyscalculie: Bilan des données scientifiques. Paris: INSERM.
Cogo-Moreira, H., Andriolo, R. B., Yazigi, L., Ploubidis, G. B., Brandão de Ávila, C. R., and Mari, J. J. (2012). Music education for improving reading skills in children and adolescents with dyslexia. Cochrane Database. Syst. Rev 8:CD009133. doi: 10.1002/14651858.CD009133.pub2
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cogo-Moreira, H., Brandão de Ávila, C. R., Ploubidis, G. B., and Mari, J. J. (2013). Effectiveness of music education for the improvement of reading skills and academic achievement in young poor readers: a pragmatic cluster-randomized, controlled clinical trial. PLoS ONE 8:e59984. doi: 10.1371/journal.pone.0059984
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Corriveau, K. H., and Goswami, U. (2009). Rhythmic motor entrainment in children with speech and language impairments: tapping to the beat. Cortex 45, 119–130. doi: 10.1016/j.cortex.2007.09.008
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cramer, S. C., Sur, M., Dobkin, B. H., O’Brien, C., Sanger, T. D., Trojanowski, J. Q., et al. (2011). Harnessing neuroplasticity for clinical applications. Brain 134, 1591–1609. doi: 10.1093/brain/awr039
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cummins, F., and Port, R. F. (1998). Rhythmic constraints on stress timing in English. J. Phon. 26, 145–171. doi: 10.1006/jpho.1998.0070
Degé, F., and Schwarzer, G. (2011). The effect of a music program on phonological awareness in preschoolers. Front. Psychol. 2:124. doi: 10.3389/fpsyg.2011.00124
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Démonet, J. F., Taylor, M. J., and Chaix, Y. (2004). Developmental dyslexia. Lancet 363, 1451–1460. doi: 10.1016/S0140-6736(04)16106-0
Dontje, M. L., de Greef, M. H., Speelman, A. D., van Nimwegen, M., Krijnen, W. P., Stolk, R. P., et al. (2013). Quantifying daily physical activity and determinants in sedentary patients with Parkinson’s disease. Parkinsonism Relat. Disord. 19, 878–882. doi: 10.1016/j.parkreldis.2013.05.014
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dorsey, E. R., Constantinescu, R., Thompson, J. P., Biglan, K. M., Holloway, R. G., Kieburtz, K., et al. (2007). Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 68, 384–386. doi: 10.1212/01.wnl.0000247740.47667.03
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Draganski, B., Gaser, C., Busch, V., Schuierer, G., Bogdahn, U., and May, A. (2004). Neuroplasticity: changes in grey matter induced by training. Nature 427, 311–312. doi: 10.1038/427311a
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Drennan, W. R., and Rubinstein, J. T. (2008). Music perception in cochlear implant users and its relationship with psychophysical capabilities. J. Rehabil. Res. Dev. 45, 779–789. doi: 10.1682/JRRD.2007.08.0118
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Elbert, T., Pantev, C., Wienbruch, C., Rockstroh, B., and Taub, E. (1995). Increased cortical representation of the fingers of the left hand in string players. Science 270, 305–307. doi: 10.1126/science.270.5234.305
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Feigin, V. L., Forouzanfar, M. H., Krishnamurthi, R., Mensah, G. A., Connor, M., Bennet, D. A., et al. (2014). Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet 383, 245–254. doi: 10.1016/S0140-6736(13)61953-4
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fernandez del Olmo, M., and Cudeiro, J. (2003). A simple procedure using auditory stimuli to improve movement in Parkinson’s disease: a pilot study. Neurol. Clin. Neurophysiol. 2003, 1–7.
Fernandez del Olmo, M., and Cudeiro, J. (2005). Temporal variability of gait in Parkinson disease: effects of a rehabilitation programme based on rhythmic sound cues. Parkinsonism Relat. Disord. 11, 25–33. doi: 10.1016/j.parkreldis.2004.09.002
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Flaugnacco, E., Lopez, L., Terribili, C., Zoia, S., Buda, S., Tilli, S., et al. (2014). Rhythm perception and production predict reading abilities in developmental dyslexia. Front. Hum. Neurosci. 8:392. doi: 10.3389/fnhum.2014.00392
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Forsblom, A., Laitinen, S., Särkämö, T., and Tervaniemi, M. (2009). Therapeutic role of music listening in stroke rehabilitation. Ann. N. Y. Acad. Sci. 1169, 426–430. doi: 10.1111/j.1749-6632.2009.04776.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
François, C., Chobert, J., Besson, M., and Schön, D. (2013). Music training for the development of speech segmentation. Cereb. Cortex 23, 2038–2043. doi: 10.1093/cercor/bhs180
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
François, C., Jaillet, S., Takerkart, S., and Schön, D. (2014). Faster word segmentation in musicians than in nonmusicians. PLoS ONE 9:e101340. doi: 10.1371/journal.pone.0101340
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
François, C., and Schön, D. (2011). Musical expertise boosts implicit learning of both musical and linguistic structures. Cereb. Cortex 21, 2357–2365. doi: 10.1093/cercor/bhr022
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
François, C., and Schön, D. (2014). Neural sensitivity to statistical regularities as a fundamental biological process that underlies auditory learning: the role of musical practice. Hear. Res. 308, 122–128. doi: 10.1016/j.heares.2013.08.018
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Freedland, R. L., Festa, C., Sealy, M., McBean, A., Elghazaly, P., Capan, A., et al. (2002). The effects of pulsed auditory stimulation on various gait measurements in persons with Parkinson’s disease. NeuroRehabilitation 17, 81–87.
Friedman, N., Chan, V., Reinkensmeyer, A. N., Beroukhim, A., Zambrano, G. J., Bachman, M., et al. (2014). Retraining and assessing hand movement after stroke using the MusicGlove: comparison with conventional therapy and isometric grip training. J. Neuroeng. Rehabil. 11, 76. doi: 10.1186/1743-0003-11-76
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fujioka, T., Trainor, L. J., Ross, B., Kakigi R., and Pantev, C. (2004). Musical training enhances automatic encoding of melodic contour and interval structure. J. Cogn. Neurosci. 16, 1010–1021. doi: 10.1162/0898929041502706
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fukui, H., and Toyoshima, K. (2008). Music facilitates the neurogenesis, regeneration and repair of neurons. Med. Hypotheses 71, 765–769. doi: 10.1016/j.mehy.2008.06.019
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gaser, C., and Schlaug, G. (2003). Brain structures differ between musicians and non-musicians. J. Neurosci. 23, 9240–9245.
Goswami, U. (2010). “Language, music and children’s brains: a rhythmic timing perspective on language and music as cognitive systems,” in Language and Music as Cognitive Systems, eds P Rebuschat, M. Rohrmeier, J. Hawkins, and I. Cross (Oxford: Oxford University Press), 292–301.
Goswami, U., Wang, H. L. S., Cruz, A., Fosker, T., Mead, N., and Huss, M. (2011). Language universal sensory deficits in developmental dyslexia: English, Spanish, and Chinese. J. Cogn. Neurosci. 23, 325–337. doi: 10.1162/jocn.2010.21453
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Grahn, J. A., and Brett, M. (2007). Rhythm and beat perception in motor areas of the brain. J. Cogn. Neurosci. 19, 893–906. doi: 10.1162/jocn.2007.19.5.893
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Grahn, J. A., and Rowe, J. B. (2009). Feeling the beat: premotor and striatal interactions in musicians and nonmusicians during beat perception. J. Neurosci. 29, 7540–7548. doi: 10.1523/JNEUROSCI.2018-08.2009
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Granert, O., Peller, M., Gaser, C., Groppa, S., Hallett, M., Knutzen, et al. (2011). Manual activity shapes structure and function in contralateral human motor hand area. Neuroimage 54, 32–41. doi: 10.1016/j.neuroimage.2010.08.013
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Grau-Sánchez, J., Amengual, J. L., Rojo, N., Veciana de las Heras, M., Montero, J., Rubio, F., et al. (2013). Plasticity in the sensorimotor cortex induced by Music-supported therapy in stroke patients: a TMS study. Front. Hum. Neurosci. 7:494. doi: 10.3389/fnhum.2013.00494
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Grimm, S., and Escera, C. (2011). Auditory deviance detection revisited: evidence for a hierarchical novelty system. Int. J. Psychophysiol. 85, 88–92. doi: 10.1016/j.ijpsycho.2011.05.012
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Groussard, M., Viader, F., Landeau, B., Desgranges, B., Eustache, F., and Platel, H. (2014). The effects of musical practice on structural plasticity: the dynamics of grey matter changes. Brain Cogn. 90, 174–180. doi: 10.1016/j.bandc.2014.06.013
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Habermeyer, B., Herdener, M., Esposito, F., Hilti, C. C., Klarhöfer, M., di Salle, F., Wetzel, S., et al. (2009). Neural correlates of pre-attentive processing of pattern deviance in professional musicians. Hum. Brain Mapp. 30, 3736–3747. doi: 10.1002/hbm.20802
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hackett, M. L., Anderson, C. S., House, A., and Xia, J. (2008). Interventions for treating depression after stroke. Cochrane Database Syst. Rev. 4:CD003437. doi: 10.1002/14651858.CD003437.pub3
Hackett, M. L., Yapa, C., Parag, V., and Anderson, C. S. (2005). Frequency of depression after stroke: a systematic review of observational studies. Stroke 36, 1330–1340. doi: 10.1161/01.STR.0000165928.19135.35
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hackett, M. L., Yang, M., Anderson, C. S., Horrocks, J. A., and House, A. (2010). Pharmaceutical interventions for emotionalism after stroke. Cochrane Database Syst. Rev. 2:CD003690. doi: 10.1002/14651858.CD003690.pub3
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Halwani, G. F., Loui, P., Rüber, T., and Schlaug, G. (2011). Effects of practice and experience on the arcuate fasciculus: comparing singers, instrumentalists, and non-musicians. Front. Psychol. 2:156. doi: 10.3389/fpsyg.2011.00156
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Haueisen, J., and Knösche, T. R. (2001). Involuntary motor activity in pianists evoked by music perception. J. Cogn. Neurosci. 13, 786–792. doi: 10.1162/08989290152541449
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Herholz, S. C., and Zatorre, R. J. (2012). Musical training as a framework for brain plasticity: behavior, function, and structure. Neuron 76, 486–502. doi: 10.1016/j.neuron.2012.10.011
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Huron, D. (2001). Is music an evolutionary adaptation? Ann. N. Y. Acad. Sci. 930, 43–61. doi: 10.1111/j.1749-6632.2001.tb05724.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Huss, M., Verney, J. P., Fosker, T., Mead, N., and Goswami, U. (2011). Music, rhythm, rise time perception and developmental dyslexia: perception of musical meter predicts reading and phonology. Cortex 47, 674–689. doi: 10.1016/j.cortex.2010.07.010
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hutchinson, S., Lee, L. H. L., Gaab, N., and Schlaug, G. (2003). Cerebellar volume: gender and musicianship effects. Cereb. Cortex 13, 943–949. doi: 10.1093/cercor/13.9.943
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hyde, K. L., Lerch, J., Norton, A., Forgeard, M., Winner, E., Evans, A. C., et al. (2009). Musical training shapes structural brain development. J. Neurosci. 29, 3019–3025. doi: 10.1523/JNEUROSCI.5118-08.2009
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Imfeld, A., Oechslin, M. S., Meyer, M., Loenneker, T., and Jäncke, L. (2009). White matter plasticity in the corticospinal tract of musicians: a diffusion tensor imaging study. Neuroimage 46, 600–607. doi: 10.1016/j.neuroimage.2009.02.025
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jäncke, L., Shah, N. J., and Peters, M. (2000). Cortical activations in primary and secondary motor areas for complex bimanual movements in professional pianists. Brain Res. Cogn. Brain Res. 10, 177–183. doi: 10.1016/S0926-6410(00)00028-8
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
James, C. E., Oechslin, M. S., Van De Ville, D., Hauert, C. A., Descloux, C., and Lazeyras, F. (2014). Musical training intensity yields opposite effects on grey matter density in cognitive versus sensorimotor networks. Brain Struct. Funct. 219, 353–366. doi: 10.1007/s00429-013-0504-z
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jebsen, R. H., Taylor, N., Trieschmann, R. B., Trotter, M. J., and Howard, l. A. (1969). An objective and standardized test of hand function. Arch. Phys. Med. Rehabil. 50, 311–319.
Johansson, B. B. (2011). Current trends in stroke rehabilitation. A review with focus on brain plasticity. Acta Neurol. Scand. 123, 147–159. doi: 10.1111/j.1600-0404.2010.01417.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jones, M. R., Jagacinski, R. J., Yee, W., Floyd, R. L., and Klapp, S. T. (1995). Tests of attentional flexibility in listening to polyrhythmic patterns. J. Exp. Psychol. Hum. Percept. Perform. 21, 293–307. doi: 10.1037/0096-1523.21.2.293
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keenan, J. P., Thangaraj, V., Halpern, A. R., and Schlaug, G. (2001). Absolute pitch and planum temporale. Neuroimage 14, 1402–1408. doi: 10.1006/nimg.2001.0925
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kim, S. D., Allena, N. E., Canning, C. G., and Fung, V. S. (2013). Postural instability in patients with Parkinson’s disease. Epidemiology, pathophysiology and management. CNS Drugs 27, 97–112. doi: 10.1007/s40263-012-0012-3
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kishon-Rabin, L., Amir, O., Vexler, Y., and Zaltz, Y. (2001). Pitch discrimination: are professional musicians better than non-musicians? J. Basic Clin. Physiol. Pharmacol. 12(Suppl. 2), 125–143. doi: 10.1515/JBCPP.2001.12.2.125
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Knecht, H. A., Nelson, P. B., Whitelaw, G. M., and Feth, L. L. (2002). Background noise levels and reverberation times in unoccupied classrooms: predictions and measurements. Am. J. Audiol. 11, 65–71. doi: 10.1044/1059-0889(2002/009)
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Koelsch, S., Fritz, T., Schulze, K., Alsop, D., and Schlaug, G. (2005). Adults and children processing music: an fMRI study. Neuroimage 25, 1068–1076. doi: 10.1016/j.neuroimage.2004.12.050
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kolb, B., and Muhammad, A. (2014). Harnessing the power of neuroplasticity for intervention. Front. Hum. Neurosci. 8:377. doi: 10.3389/fnhum.2014.00377
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kolb, B., Mychasiuk, R., Muhammad, A., Li, Y., Frost, D. O., and Gibb, R. (2012). Experience and the developing prefrontal cortex. Proc. Natl. Acad. Sci. U.S.A. 109, 17186–17193. doi: 10.1073/pnas.1121251109
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kolb, B., and Telskey, G. C. (2012). Age, experience, injury, and the changing brain. Dev. Psychobiol. 54, 311–325. doi: 10.1002/dev.20515
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Krakauer, J. W., Carmichael, S. T., Corbett, D., and Wittenberg, G. F. (2012). Getting neurorehabilitation right: what can be learned from animal models? Neurorehabil. Neural Repair 26, 923–931. doi: 10.1177/1545968312440745
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Krampe, R. T., and Ericsson, K. A. (1996). Maintaining excellence: deliberate practice and elite performance in young and older pianists. J. Exp. Psychol. Gen. 125, 331–359. doi: 10.1037/0096-3445.125.4.331
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kraus, N., and Chandrasekaran, B. (2010). Music training for the development of auditory skills. Nat. Rev. Neurosci. 11, 599–605. doi: 10.1038/nrn2882
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lahav, A., Saltzman, E., and Schlaug, G. (2007). Action representation of sound: audiomotor recognition network while listening to newly acquired actions. J. Neurosci. 27, 308–314. doi: 10.1523/JNEUROSCI.4822-06.2007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Langhorne, P., Bernhardt, J., and Kwakkel, G. (2011). Stroke rehabilitation. Lancet 377, 1693–1702. doi: 10.1016/S0140-6736(11)60325-5
Langhorne, P., Coupar, F., and Pollock, A. (2009). Motor recovery after stroke: a systematic review. Lancet Neurol. 8, 741–754. doi: 10.1016/S1474-4422(09)70150-4
Ledger, S., Galvin, R., Lynch, D., and Stokes, E. K. (2008). A randomised controlled trial evaluating the effect of an individual auditory cueing device on freezing and gait speed in people with Parkinson’s disease. BMC Neurol. 8:46. doi: 10.1186/1471-2377-8-46
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Li, J., Luo, C., Peng, Y., Xie, Q., Gong, J., Dong, L., et al. (2014). Probabilistic diffusion tractography reveals improvement of structural network in musicians. PLoS ONE 9:e105508. doi: 10.1371/journal.pone.0105508
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lozano, R., Naghavi, M., Foreman, K., Lim, S., Shibuya, K., Aboyans, V., et al. (2012). Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2095–2128. doi: 10.1016/S0140-6736(12)61728-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lyle, R. C. (1981). A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int. J. Rehab. Res. 4, 483–492. doi: 10.1097/00004356-198112000-00001
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lyon, R., Shaywitz, S. E., and Shaywitz, B. A. (2003). Defining dyslexia, comorbidity, teachers’ knowledge of language and reading. Ann. Dyslexia 53, 1–14. doi: 10.1007/s11881-003-0001-9
Magne, C., Schön D., and Besson, M. (2006). Musician children detect pitch violations in both music and language better than nonmusician children: behavioral and electrophysiological approaches. J. Cogn. Neurosci. 18, 199–211. doi: 10.1162/jocn.2006.18.2.199
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Maidhof, C. (2013). Error monitoring in musicians. Front. Hum. Neurosci. 7:401. doi: 10.3389/fnhum.2013.00401
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Maidhof, C., Pitkäniemi, A., and Tervaniemi, M. (2013). Predictive error detection in pianists: a combined ERP and motion capture study. Front. Hum. Neurosci. 7:587. doi: 10.3389/fnhum.2013.00587
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Marie, C., Magne, C., and Besson, M. (2011). Musicians and the metric structure of words. J. Cogn. Neurosci. 23, 294–305. doi: 10.1162/jocn.2010.21413
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Marques, C., Moreno, S., Castro, S. L., and Besson, M. (2007). Musicians detect pitch violation in a foreign language better than nonmusicians: behavioral and electrophysiological evidence. J. Cogn. Neurosci. 19, 1453–1463. doi: 10.1162/jocn.2007.19.9.1453
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mas-Herrero, E., Zatorre, R. J., Rodriguez-Fornells, A., and Marco-Pallarés, J. (2014). Dissociation between musical and monetary reward responses in specific musical anhedonia. Curr. Biol. 24, 699–704. doi: 10.1016/j.cub.2014.01.068
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mathiowetz, V., Volland, G., Kashman, N., and Weber, K. (1985). Adult norms for the Box and Block Test of manual dexterity. Am. J. Occup. Ther. 39, 386–391. doi: 10.5014/ajot.39.6.386
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
McDermott, H. J. (2004). Music perception with cochlear implants: a review. Trends Amplif. 8, 49–82. doi: 10.1177/108471380400800203
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
McIntosh, G. C., Brown, S. H., Rice, R. R., and Thaut, M. H. (1997). Rhythmic auditory-motor facilitation of gait patterns in patients with Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 62, 22–26. doi: 10.1136/jnnp.62.1.22
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
McIntosh, G. C., Rice, R. R., Hurt, C. P., and Thaut, M. H. (1998). Long-term training effects of rhythmic auditory stimulation on gait in patients with Parkinson’s disease. Mov. Disord. 13, 212.
Meister, I., Krings, T., Foltys, H., Boroojerdi, B., Müller, M., Töpper, R., et al. (2005). Effects of long-term practice and task complexity in musicians and nonmusicians performing simple and complex motor tasks:implications for cortical motor organization. Hum. Brain Mapp. 25, 345–352. doi: 10.1002/hbm.20112
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Micheyl, C., Delhommeau, K., Perrot, X., and Oxenham, A. J. (2006). Influence of musical and psychoacoustical training on pitch discrimination. Hear. Res. 219, 36–47. doi: 10.1016/j.heares.2006.05.004
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mishra, S. K., Panda, M. R., and Herbert, C. (2014). Enhanced auditory temporal gap detection in listeners with musical training. J. Acoust. Soc. Am. 136, EL173–EL178. doi: 10.1121/1.4890207
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mohr, J. P., Grotta, J. C., Wolf, P. A., Moskowitz, M. A., Mayberg, M. R., and Von Kummer, R. (2011). Stroke: Pathophysiology, Diagnosis, and Management. Phyladelphia: Saunders, Elsevier.
Moreno, S., Marques, C., Santos, A., Santos, M., Castro, S. L., and Besson, M. (2009). Musical training influences linguistic abilities in 8-year-old children: more evidence for brain plasticity. Cereb. Cortex 19, 712–713. doi: 10.1093/cercor/bhn120
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Münte, T. F., Altenmüller, E., and Jäncke, L. (2002). The musician’s brain as a model of neuroplasticity. Nat. Rev. Neurosci. 3, 473–478. doi: 10.1038/nrn843
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Murray, C. J. L., Vos, T., Lozano, R., Naghavi, M., Flaxman, A. D., Michaud, C., et al. (2012). Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2197–2223. doi: 10.1016/S0140-6736(12)61689-4
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Musacchia, G., Sams, M., Skoe, E., and Kraus, N. (2007). Musicians have enhanced subcortical auditory and audiovisual processing of speech and music. Proc. Natl. Acad. Sci. U.S.A. 104, 15894–15898. doi: 10.1073/pnas.0701498104
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Näätänen, R., Jacobsen, T., and Winkler, I. (2005). Memory-based or afferent processes in mismatch negativity (MMN): a review of the evidence. Psychophysiology 42, 25–32. doi: 10.1111/j.1469-8986.2005.00256.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Neuman, A. C., Wroblewski, M., Hajicek, J., and Rubinstein, A. (2010). Combined effects of noise and reverberation on speech recognition performance of normal-hearing children and adults. Ear Hear. 31, 336–344. doi: 10.1097/AUD.0b013e3181d3d514
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nombela, C., Hughes, L. E., Owen, A. M., and Grahn, J. A. (2013). Into the groove: can rhythm influence Parkinson’s disease? Neurosci. Biobehav. Rev. 37, 2564–2570. doi: 10.1016/j.neubiorev.2013.08.003
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
OECD. (2014), PISA 2012 Results: What Students Know and Can Do–Student Performance in Mathematics, Reading and Science, Vol. 1, Revised Edn, February 2014. PISA: OECD Publishing.
Oechslin, M. S., Imfeld, A., Loenneker, T., Meyer, M., and Jäncke, L. (2010). The plasticity of the superior longitudinal fasciculus as a function of musical expertise: a diffusion tensor imaging study. Front. Hum. Neurosci. 3:76. doi: 10.3389/neuro.09.076.2009
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Okuma, Y., and Yanagisawa, N. (2008). The clinical spectrum of freezing of gait in Parkinson’s disease. Mov. Disord. 25, 149–156. doi: 10.1002/mds.21934
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Oremus, M., Santaguida, P., Walker, K., Wishart, L. R., Siegel, K. L., and Raina, P. (2012). Studies of stroke rehabilitation therapies should report blinding and rationalize use of outcome measurement instruments. J. Clin. Epidemiol. 65, 368–374. doi: 10.1016/j.jclinepi.2011.10.013
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Overy, K. (2000). Dyslexia, temporal processing and music: the potential of music as an early learning aid for dyslexic children. Psychol. Music 28, 218–229. doi: 10.1177/0305735600282010
Overy, K. (2003). Dyslexia and music: from timing deficits to musical intervention. Ann. N. Y. Acad. Sci. 999, 497–505. doi: 10.1196/annals.1284.060
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Overy, K. (2012). Making music in a group: synchronization and shared experience. Ann. N. Y. Acad. Sci. 1252, 65–68. doi: 10.1111/j.1749-6632.2012.06530.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pantev, C., and Herholz, S. C. (2011). Plasticity of the human auditory cortex related to musical training. Neurosci. Biobehav. Rev. 35, 2140–2154. doi: 10.1016/j.neubiorev.2011.06.010
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pantev, C., Oostenveld, R., Engelien, A., Ross, B., Roberts, L. E., and Hoke, M. (1998). Increased auditory cortical representation in musicians. Nature 392, 811–814. doi: 10.1038/33918
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pantev, C., Roberts, L. E., Schulz, M., Engelien, A., and Ross B. (2001). Timbre specific enhancement of auditory cortical representations in musicians. Neuroimage 12, 169–174. doi: 10.1097/00001756-200101220-00041
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Parbery-Clark, A., Skoe, E., and Kraus, N. (2009a). Musical experience limits the degradative effects of background noise on the neural processing of sound. J. Neurosci. 29, 14100–14107. doi: 10.1523/JNEUROSCI.3256-09.2009
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Parbery-Clark, A., Skoe, E., Lam, C., and Kraus, N. (2009b). Musician enhancement for speech in noise. Ear Hear. 30, 653–661. doi: 10.1097/AUD.0b013e3181b412e9
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Parbery-Clark, A., Tierney, A., Strait, D. L., and Kraus, N. (2012). Musicians have fine-tuned neural distinction of speech syllables. Neuroscience 219, 111–119. doi: 10.1016/j.neuroscience.2012.05.042
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Parker, V. M., Wade, D. T., and Langton-Hewer, R. (1986). Loss of arm function after stroke: measurement, frequency and recovery. Int. Rehabil. Med. 8, 69–73. doi: 10.3109/03790798609166178
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pascual-Leone, A., Nguyet, D., Cohen, L. G., Brasil-Nieto, J. P., Cammarota, A., and Hallett, M. (1995). Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J. Neurophysiol. 74, 1037–1045.
Patel, A. D. (2011). Why would musical training benefit the neural encoding of speech? The OPERA Hypothesis. Front. Psychol. 2:142. doi: 10.3389/fpsyg.2011.00142
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Patel, A. D. (2014). Can nonlinguistic musical training change the way the brain processes speech? The expanded OPERA hypothesis. Hear. Res. 308, 98–108. doi: 10.1016/j.heares.2013.08.011
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Penhune, V. (2011). Sensitive periods in human development: evidence from musical training. Cortex 47, 1126–1137. doi: 10.1016/j.cortex.2011.05.010
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Perrot, X., and Collet, L. (2014). Function and plasticity of the medial olivocochlear system in musicians: a review. Hear. Res. 308, 27–40. doi: 10.1016/j.heares.2013.08.010
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Perrot, X., Micheyl, C., Khalfa, S., and Collet, L. (1999). Stronger bilateral efferent influences on cochlear biomechanical activity in musicians than in non-musicians. Neurosci. Lett. 262, 167–170. doi: 10.1016/S0304-3940(99)00044-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pfordresher, P. Q., and Beasley, R. T. (2014). Making and monitoring errors based on altered auditory feedback. Front. Psychol. 5:l914. doi: 10.3389/fpsyg.2014.00914
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Przybylski, L., Bedoin, N., Krifi-Papoz, S., Herbillon, V., Roch, D., Léculier, L., et al. (2013). Rhythmic auditory stimulation influences syntactic processing in children with developmental language disorders. Neuropsychology 27, 121–131. doi: 10.1037/a0031277
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Putkinen, V., Tervaniemi, M., Saarikivi, K., Ojala, P., and Huotilainen, M. (2014). Enhanced development of auditory change detection in musically trained school-aged children: a longitudinal event-related potential study. Dev. Sci. 17, 282–297. doi: 10.1111/desc.12109
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Quené, H., and Port, R. F. (2005). Effects of timing regularity and metrical expectancy on spoken-word perception. Phonetica 62, 1–13. doi: 10.1159/000087222
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Rammsayer, T., and Altenmüller, E. (2006). Temporal information processing in musicians and nonmusicians. Music Percept. 24, 37–48. doi: 10.1525/mp.2006.24.1.37
Ramus, F. (2003). Developmental dyslexia: specific phonological deficit or general sensorimotor dysfunction? Curr. Opin. Neurobiol. 13, 212–218. doi: 10.1016/S0959-4388(03)00035-7
Rathore, S. S., Hinn, A. R., Cooper, L. S., Tyroler, H. A., and Rosamond, W. D. (2002). Characterization of incident stroke signs and symptoms: findings from the atherosclerosis risk in communities study. Stroke 33, 2718–2721. doi: 10.1161/01.STR.0000035286.87503.31
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Rochette, F., Moussard, A., and Bigand, E. (2014). Music lessons improve auditory perceptual and cognitive performance in deaf children. Front. Hum. Neurosci. 8:488. doi: 10.3389/fnhum.2014.00488
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Rodríguez-Fornells, A., Rojo, N., Amengual, J. L., Ripollés, P., Altenmüller, E., and Münte, T. (2012). The involvement of audio-motor coupling in the music-supported therapy applied to stroke patients. Ann. N. Y. Acad. Sci. 1252, 282–293. doi: 10.1111/j.1749-6632.2011.06425.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Rojo, N., Amengual, J. L., Juncadella, M., Rubio, F., Camara, E., Marco-Pallares, J., et al. (2011). Music-supported therapy induces plasticity in the sensorimotor cortex in chronic stroke: a single-case study using multimodal imaging (fMRI-TMS). Brain Inj. 25, 787–793. doi: 10.3109/02699052.2011.576305
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Russo, N., Nicol, T., Musacchia, G., and Kraus, N. (2004). Brainstem responses to speech syllables. Clin. Neurophysiol. 115, 2021–2030. doi: 10.1016/j.clinph.2004.04.003
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Saffran, J. R., Newport, E. L., and Aslin, R. N. (1996). Word segmentation: the role of distributional cues. J. Mem. Lang. 35, 606–621. doi: 10.1006/jmla.1996.0032
Salimpoor, V. N., van den Bosch, I., Kovacevic, N., McIntosh, A. R., Dagher, A., and Zatorre, R. J. (2013). Interactions between the nucleus accumbens and auditory cortices predict music reward value. Science 340, 216–219. doi: 10.1126/science.1231059
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Salomon, J. A., Wang, H., Freeman, M. K., Vos, T., Flaxman, A. D., Lopez, A. D., et al. (2012). Healthy life expectancy for 187 countries, 1990-2010: a systematic analysis for the Global Burden Disease Study 2010. Lancet 380, 2144–2162. doi: 10.1016/S0140-6736(12)61690-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sanes, D. H., and Constantine-Paton, M. (1985). The sharpening of frequency tuning curves requires patterned activity during development in the mouse, Mus musculus. J. Neurosci. 5, 1152–1166.
Särkämö, T., Ripollés, P., Vepsäläinen, H., Autti, T., Silvennoinen, H. M., Salli, E., et al. (2014). Structural changes induced by daily music listening in the recovering brain after middle cerebral artery stroke: a voxel-based morphometry study. Front. Hum. Neurosci. 8:245. doi: 10.3389/fnhum.2014.00245
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Särkämö, T., Tervaniemi, M., Laitinen, S., Forsblom, A., Soinila, S., Mikkonen, M., et al. (2008). Music listening enhances cognitive recovery and mood after middle cerebral artery stroke. Brain 131, 866–876. doi: 10.1093/brain/awn013
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Schellenberg, E. G. (2004). Music lessons enhance IQ. Psychol. Sci. 15, 511–514. doi: 10.1111/j.0956-7976.2004.00711.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Schlaug, G., Jäncke, L., Huang, Y., and Steinmetz, H. (1995a). In vivo evidence of structural brain asymmetry in musicians. Science 267, 699–701. doi: 10.1126/science.7839149
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Schlaug, G., Jäncke, L., Huang, Y., Staiger, J. F., and Steinmetz, H. (1995b). Increased corpus callosum size in musicians. Neuropsychologia 33, 1047–1055. doi: 10.1016/0028-3932(95)00045-5
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Schlaug, G. (2001). The brain of musicians. A model for functional and structural adaptation. Ann. N. Y. Acad. Sci. 930, 281–299. doi: 10.1111/j.1749-6632.2001.tb05739.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Schmithorst, V. J., and Wilke, M. (2002). Differences in white matter architecture between musicians and non-musicians: a diffusion tensor imaging study. Neurosci. Lett. 321, 57–60. doi: 10.1016/S0304-3940(02)00054-X
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Schneider, P., Scherg, M., Dosch, H. G., Specht, H. J., Gutschalk, A., and Rupp, A. (2002). Morphology of Heschl’s gyrus reflects enhanced activation in the auditory cortex of musicians. Nat. Neurosci. 5, 688–694. doi: 10.1038/nn871
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Schneider, P., Sluming, V., Roberts, N., Scherg, M., Goebel, R., Specht, H. J., et al. (2005). Structural and functional asymmetry of lateral Heschl’s gyrus reflects pitch perception preference. Nat. Neurosci. 8, 1241–1247. doi: 10.1038/nn1530
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Schneider, S., Schönle, P. W., Altenmüller, E., and Münte, T. F. (2007). Using musical instruments to improve motor skill recovery following a stroke. J. Neurol. 254, 1339–1346. doi: 10.1007/s00415-006-0523-2
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Schön, D., Gordon, R., Campagne, A., Magne, C., Astésano, C., Anton, J. L., et al. (2010). Similar cerebral networks in language, music and song perception. Neuroimage 51, 450–461. doi: 10.1016/j.neuroimage.2010.02.023
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Schön, D., Magne, C., and Besson, M. (2004). The music of speech: music training facilitates pitch processing in both music and language. Psychophysiology 41, 341–349. doi: 10.1111/1469-8986.00172.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Serniclaes, W., Van Heghe, S., Mousty, P., Carré, R., and Sprenger-Charolles, L. (2004). Allophonic mode of speech perception in dyslexia. J. Exp. Child Psychol. 87, 336–361. doi: 10.1016/j.jecp.2004.02.001
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Shahin, A., Bosnyak, D. J., Trainor, L. J., and Roberts, L. E. (2003). Enhancement of neuroplastic P2 and N1c auditory evoked potentials in musicians. J. Neurosci. 23, 5545–5552.
Shahin, A., Roberts, L. E., and Trainor, L. J. (2004). Enhancement of auditory cortical development by musical experience in children. Neuroimage 15, 1917–1921. doi: 10.1097/00001756-200408260-00017
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Shield, B. M., and Dockrell, J. E. (2008). The effects of environmental and classroom noise on the academic attainments of primary school children. J. Acoust. Soc. Am. 123, 133–144. doi: 10.1121/1.2812596
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Skoe, E., and Kraus, N. (2012). A little goes a long way: how the adult brain is shaped by musical training in childhood. J. Neurosci. 32, 11507–11510. doi: 10.1523/JNEUROSCI.1949-12.2012
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Slevc, L. R., and Miyake, A. (2006). Individual differences in second-language proficiency: does musical ability matter? Psychol. Sci. 17, 675–681. doi: 10.1111/j.1467-9280.2006.01765.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Steele, C. J., Bailey, J. A., Zatorre, R. J., and Panhune, V. B. (2013). Early musical training and white-matter plasticity in the corpus callosum: evidence for a sensitive period. J. Neurosci. 33, 1282–1290. doi: 10.1523/JNEUROSCI.3578-12.2013
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Stoessl, A. J., Lehericy, S., and Strafella, A. P. (2014). Imaging insights into basal ganglia function, Parkinson’s disease, and dystonia. Lancet 384, 532–544. doi: 10.1016/S0140-6736(14)60041-6
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Strait, D. L., and Kraus, N. (2011). Playing music for a smarter ear: cognitive, perceptual and neurobiological evidence. Music Percept. 29, 133–146. doi: 10.1525/mp.2011.29.2.133
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Strait, D. L., O’Connell, S., Parbery-Clark, A., and Kraus, N. (2013). Musicians’ enhanced neural differentiation of speech sounds arises early in life: developmental evidence from ages 3–30. Cereb. Cortex 24, 2512–2521. doi: 10.1093/cercor/bht103
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Strait, D. L., Parbery-Clark, A., Hittner, E., and Kraus, N. (2012). Musical training during early childhood enhances the neural encoding of speech in noise. Brain Lang. 123, 191–201. doi: 10.1016/j.bandl.2012.09.001
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tervaniemi, M., Just, V., Koelsch, S., Widmann, A., and Schröger, E. (2005). Pitch discrimination accuracy in musicians vs nonmusicians: an event-related potential and behavioral study. Exp. Brain Res. 171, 1–10. doi: 10.1007/s00221-004-2044-5
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tervaniemi, M., Rytkönen, M., Schröger, E., Ilmoniemi, R. J., and Näätänen, R. (2001). Superior formation of cortical memory traces for melodic patterns in musicians. Learn. Mem. 8, 295–300. doi: 10.1101/lm.39501
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Thaut, M. H., and Abiru, M. (2010). Rhythmic auditory stimulation in rehabilitation of movement disorders: a review of current research. Music Percept. 27, 263–269. doi: 10.1525/mp.2010.27.4.263
Thaut, M. H., Leins, A. K., Rice, R. R., Argstatter, H., Kenyon, G. P., McIntosh, G. C., et al. (2007). Rhythmic auditory stimulation improves gait more than NDT/Bobath training in near-ambulatory patients early poststroke: a single- blind, randomized trial. Neurorehabil. Neural Repair 21, 455–459. doi: 10.1177/1545968307300523
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Thaut, M. H., McIntosh, G. C., and Hoemberg, V. (2014). Neurobiological foundations of neurologic music therapy: rhythmic entrainment and the motor system. Front. Psychol. 5:1185. doi: 10.3389/fpsyg.2014.01185
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Thaut, M. H., McIntosh, G. C., and Rice, R. R. (1997). Rhythmic facilitation of gait training in hemiparetic stroke rehabilitation. J. Neurol. Sci. 151, 207–212. doi: 10.1016/S0022-510X(97)00146-9
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Thaut, M. H., McIntosh, G. C., Rice, R. R., Miller, R. A., Rathbun, J., and Brault, J. M. (1996). Rhythmic auditory stimulation in gait training for Parkinson’s disease patients. Mov. Disord. 11, 193–200. doi: 10.1002/mds.870110213
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Thomson, J. M., and Goswami, U. (2008). Rhythmic processing in children with developmental dyslexia: auditory and motor rhythms link to reading and spelling. J. Physiol. Paris 102, 120–129. doi: 10.1016/j.jphysparis.2008.03.007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tieges, Z., Mead, G., Allerhand, M., Duncan, F., van Wijck, F., Fitzsimons, C., et al. (2015). Sedentary behaviour in the first year after stroke: a longitudinal cohort study with objective measures. Arch. Phys. Med. Rehabil. 96, 15–23. doi: 10.1016/j.apmr.2014.08.015
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tierney, A., and Kraus, N. (2013). Music training for the development of reading skills. Prog. Brain Res. 207, 209–241. doi: 10.1016/B978-0-444-63327-9.00008-4
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tierney, A., Krizman, J., Skoe, E., Johnston, K., and Kraus, N. (2013). High school music classes enhance the neural processing of speech. Front. Educ. Psychol. 4:855. doi: 10.3389/fpsyg.2013.00855
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Trainor, L. J., McDonald, K. L., and Alain, C. (2002). Automatic and controlled processing of melodic contour and interval information measured by electrical brain activity. J. Cogn. Neurosci. 14, 1–13. doi: 10.1162/089892902317361949
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Trainor, L. J., Shahin, A., and Roberts, L. E. (2003). Effects of musical training on the auditory cortex in children. Ann. N. Y. Acad. Sci. 999, 506–513. doi: 10.1196/annals.1284.061
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Truelsen, T., Piechowski-Józwiak, B., Bonita, R., Mathers, C., Bogousslavsky, J., and Boysen, G. (2006). Stroke incidence and prevalence in Europe: a review of available data. Eur. J. Neurol. 13, 581–598. doi: 10.1111/j.1468-1331.2006.01138.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Van Vugt, F. T., Ritter, J., Rollnik, J. D., and Altenmüller, E. (2014). Music-supported motor training after stroke reveals no superiority of synchronization in group therapy. Front. Hum. Neurosci. 8:315. doi: 10.3389/fnhum.2014.00315
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Van Zuijen, T., Sussman, E., Winkler, I., Näätänen, R., and Tervaniemi, M. (2005). Auditory organization of sound sequences by a temporal or numerical regularity—a mismatch negativity study sounds comparing musicians and non-musicians. Cogn. Brain Res. 23, 270–276. doi: 10.1016/j.cogbrainres.2004.10.007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Vellutino, F. R., Fletcher, J. M., Snowling, M. J., and Scanlon, D. M. (2004). Specific reading disability (dyslexia): what have we learned in the past four decades? J. Child Psychol. Psychiatry 45, 2–40. doi: 10.1046/j.0021-9630.2003.00305.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Vigneau, M., Beaucousin, V., Herve, P. Y., Duffau, H., Crivello, F., Houde, O., et al. (2006). Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage 30, 1414–1432. doi: 10.1016/j.neuroimage.2005.11.002
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Villeneuve, M., Penhune, V., and Lamontagne, A. (2014). A piano training program to improve manual dexterity and upper extremity function in chronic stroke survivors. Front. Hum. Neurosci. 8:662. doi: 10.3389/fnhum.2014.00662
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Vuust, P., Brattico, E., Glerean, E., Seppänen, M., Pakarinen, S., Tervaniemi, M., et al. (2011). New fast mismatch negativity paradigm for determining the neural prerequisites for musical ability. Cortex 47, 1091–1098. doi: 10.1016/j.cortex.2011.04.026
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Vuust, P., Pallesen, K. J., Bailey, C., van Zuijen, T. L., Gjedde, A., and Roepstor, V. A. (2005). To musicians, the message is in the meter—preattentive neuronal responses to incongruent rhythm are left lateralized in musicians. Neuroimage 24, 560–564. doi: 10.1016/j.neuroimage.2004.08.039
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wade, D. T., Langton-Hewer, R., Wood, V. A., Skilbeck, C. E., and Ismail, H. M. (1983). The hemiplegic arm after stroke: measurement and recovery. J. Neurol. Neurosurg. Psychiatry 46, 521–524. doi: 10.1136/jnnp.46.6.521
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wan, C. Y., and Schlaug, G. (2010). Music making as a tool for promoting brain plasticity across the life span. Neuroscientist 16, 566–577. doi: 10.1177/1073858410377805
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Warrier, C. M., Johnson, K. L., Hayes, E. A., Nicol, T., and Kraus, N. (2004). Learning impaired children exhibit timing deficits and training-related improvements in auditory cortical responses to speech in noise. Exp. Brain Res. 157, 431–441. doi: 10.1007/s00221-004-1857-6
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
White-Schwoch, T., Carr, K. W., Anderson, S., Strait, D. L., and Kraus, N. (2013). Older adults benefit from music training early in life: biological evidence for long-term training-driven plasticity. J. Neurosci. 33, 17667–17674. doi: 10.1523/JNEUROSCI.2560-13.2013
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Whyte, E. M., and Mulsant, B. H. (2002). Post-stroke depression: epidemiology, pathophysiology, and biological treatment. Biol. Psychiatry 52, 253–264. doi: 10.1016/S0006-3223(02)01424-5
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wible, B., Nicol, T., and Kraus, N. (2004). Atypical brainstem representation of onset and formant structure of speech sounds in children with language-based learning problems. Biol. Psychol. 67, 299–317. doi: 10.1016/j.biopsycho.2004.02.002
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Willems, A. M., Nieuwboer, A., Chavret, F., Desloovere, K., Dom, R., Rochester, L., et al. (2006). The use of rhythmic auditory cues to influence gait in patients with Parkinson’s disease, the differential effect for freezers and non-freezers, an explorative study. Disabil. Rehabil. 28, 721–728. doi: 10.1080/09638280500386569
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wittner, J. E., Webster, K. E., and Hill, K. (2013). Rhythmic auditory cueing to improve walking in patients with neurological conditions other than Parkinson’s disease-what is the evidence? Dissabil. Rehabil. 35, 164–176. doi: 10.3109/09638288.2012.690495
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Woodruff Carr, K., White-Schwoch, T., Tierney, A. T., Strait, D. L., and Kraus, N. (2014). Beat synchronization predicts neural speech encoding and reading readiness in preschoolers. Proc. Natl. Acad. Sci. U.S.A. 111, 14559–14564. doi: 10.1073/pnas.1406219111
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zatorre, R. J. (2013). Predispositions and plasticity in music and speech learning: neural correlates and implications. Science 342, 585–589. doi: 10.1126/science.1238414
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zeiler, S. R., and Krakauer, J. W. (2013). The interaction between training and plasticity in the poststroke brain. Curr. Opin. Neurol. 26, 609–616. doi: 10.1097/WCO.0000000000000025
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zendel, B. R., and Alain, C. (2012). Musicians experience less age-related decline in central auditory processing. Psychol. Aging. 27, 410–417. doi: 10.1037/a0024816
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zendel, B. R., and Alain, C. (2013). The influence of lifelong musicianship on neurophysiological measures of concurrent sound segregation. J. Cogn. Neurosci. 25, 503–516. doi: 10.1162/jocn_a_00329
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhang, L. I., Bao, S., and Merzenich, M. M. (2001). Persistent and specific influences of early acoustic environments on primary auditory cortex. Nat. Neurosci. 4, 1123–1130. doi: 10.1038/nn745
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ziegler, J. C., and Goswami, U. (2005). Reading acquisition, developmental dyslexia, and skilled reading across languages: a psycholinguistic grain size theory. Psychol. Bull. 131, 3–29. doi: 10.1037/0033-2909.131.1.3
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ziegler, J. C., Pech-Georgel, C., George, F., Alario, F. X., and Lorenzi, C. (2005). Deficits in speech perception predict language learning impairment. Proc. Natl. Acad. Sci. U.S.A. 102, 14110–14115. doi: 10.1073/pnas.0504446102
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ziegler, J. C., Pech-Georgel, C., George, F., and Lorenzi, C. (2009). Speech-perception-in-noise deficits in dyslexia. Dev. Sci. 12, 732–745. doi: 10.1111/j.1467-7687.2009.00817.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zuk, J., Andrade, P. E., Andrade, O. V., Gardiner, M., and Gaab, N. (2013). Musical, language, and reading abilities in early Portuguese readers. Front. Psychol. 4:288. doi: 10.3389/fpsyg.2013.00288
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keywords: neuro-rehabilitation, neuro-education, music training, music therapy, stroke rehabilitation, language development disorders
Citation: François C, Grau-Sánchez J, Duarte E and Rodriguez-Fornells A (2015) Musical training as an alternative and effective method for neuro-education and neuro-rehabilitation. Front. Psychol. 6:475. doi: 10.3389/fpsyg.2015.00475
Received: 03 December 2014; Accepted: 02 April 2015;
Published: 28 April 2015.
Edited by:
Mireille Besson, Centre National de la Recherche Scientifique, Institut de Neurosciences Cognitives de la Méditerranée, FranceReviewed by:
Yun Nan, Beijing Normal University, ChinaCopyright © 2015 François, Grau-Sánchez, Duarte and Rodriguez-Fornells. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Clément François and Jennifer Grau-Sánchez, Department of Basic Psychology, University of Barcelona, Campus de Bellvitge, Pavelló de Govern, L’Hospitalet de Llobregat, 08908 Barcelona, SpainZmNsZW1lbnQyNEBob3RtYWlsLmNvbQ==;amVubnlfZ3JhdUBob3RtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.