- Department of Biopsychology, Institute of Cognitive Neuroscience, Ruhr-University Bochum, Bochum, Germany
Hemispheric asymmetries play an important role in almost all cognitive functions. For more than a century, they were considered to be uniquely human but now an increasing number of findings in all vertebrate classes make it likely that we inherited our asymmetries from common ancestors. Thus, studying animal models could provide unique insights into the mechanisms of lateralization. We outline three such avenues of research by providing an overview of experiments on left–right differences in the connectivity of sensory systems, the embryonic determinants of brain asymmetries, and the genetics of lateralization. All these lines of studies could provide a wealth of insights into our own asymmetries that should and will be exploited by future analyses.
Introduction
The two hemispheres of the human brain are not equivalent. Relative functional differences between the left and the right side of the brain, so-called functional hemispheric asymmetries, have been observed for several cognitive functions (Corballis, 2009). For example, most individuals show a right-hemispheric dominance for visuo-spatial processing (e.g., Vogel et al., 2003) and a left-hemispheric dominance for production and processing of language (e.g., Bethmann et al., 2007; Ocklenburg et al., 2011a). In addition to these functional hemispheric asymmetries, anatomical differences between the two sides of the brain (e.g., in volume or size of a certain area), so-called structural hemispheric asymmetries, have can be found in a wide range of brain regions (e.g., Amunts, 2010). Several explanations for the emergence of hemispheric asymmetries have been given, including an enhancement of an individual’s ability to perform two different tasks at the same time (Rogers et al., 2004), an increase in neural capacity due to an avoidance of unnecessary duplication of neural networks (Vallortigara, 2006) and the greater speed of uni-hemispheric processing since no interhemispheric transfer via the corpus callosum is needed (Ringo et al., 1994). Historically, the scientific exploration of hemispheric asymmetries started with a seminal paper by a French surgeon called Broca (1861), who described a patient called Monsieur Tan because the only syllable he was able to generate was “tan.” Post-mortem analysis of this massively speech-impaired patient’s brain revealed a large lesion in the left posterior inferior frontal gyrus, an area now known as Broca’s area. This result indicated for the first time that the left hemisphere is highly relevant for language production. After this initial discovery in the language system, hemispheric asymmetries were thought to be uniquely human. In contrast to this view, left–right asymmetries of brain and behavior have now been observed in all vertebrate classes including mammals (Corballis, 2009), birds (Rogers, 2008; George, 2010; Güntürkün and Manns, 2010), reptiles (Bisazza et al., 1998; Bonati et al., 2008, 2010; Csermely et al., 2010, 2011), amphibians (Bisazza et al., 1998; Vallortigara, 2006), bony fishes (Vallortigara and Rogers, 2005; Lippolis et al., 2009; Dadda et al., 2010a), as well as cartilaginous, and jawless fishes (Concha and Wilson, 2001). Recent evidence for asymmetrical organization in only distantly related invertebrate species, ranging from Octopus vulgaris (Byrne et al., 2002) to the honey bee Apis mellifera (Rogers and Vallortigara, 2008; Frasnelli et al., 2010) and the nematode Caenorhabditis elegans (Taylor et al., 2010) – just to name a few examples – revealed that lateralization is indeed not restricted to humans, but constitutes a fundamental principle of nervous system organization. Lateralization is highly relevant for animal behavior and possibly survival. For example, chicks recognize familiar birds better with the left than with the right eye (Vallortigara, 1992) and react faster to a predator approaching from the left than from the right side (Vallortigara, 2006), while most species fish show a consistent tendency to turn preferentially to one side when facing an obstacle while fleeing from a predator (Bisazza et al., 2000). These discoveries yield tremendous possibilities regarding the employment of model species in order to investigate the ontogenesis and phylogenesis of human brain asymmetry. Unfortunately, there has never been a strong integration of research in humans and non-human animals in the field of hemispheric asymmetries, a circumstance that may be rooted in the assumption of human exceptionalism that dominated the field from early on (Taylor et al., 2010). In the present review, we argue that an interdisciplinary comparative approach, combining findings from psychology, biology, neuroscience, and genetics, provides a uniquely powerful tool in order to advance understanding of the ontogenetic and phylogenetic processes responsible for lateralization.
For example, one field of research in which the integration of findings from diverse animal species has influenced current views about evolution and development of human lateralization is the study of language lateralization. About 95% of right-handers and 75% of left-handers show left-hemispheric language dominance (Bethmann et al., 2007), a feature that was widely thought to be uniquely human (Corballis, 2009). Contradictory to this view, evidence suggesting a left-hemispheric dominance for conspecific communication has now been observed not only in a wide variety of mammals like chimpanzees (Taglialatela et al., 2008), rhesus monkeys (Hauser and Andersson, 1994), gray mouse lemurs (Leliveld et al., 2010), dogs (Siniscalchi et al., 2008), mice (Ehret, 1987), and sea lions (Böye et al., 2005) but also in some non-mammalian vertebrate species like frogs (Bauer, 1993). Moreover, a left hypoglossal dominance has been reported in canaries (Nottebohm and Nottebohm, 1976). Interestingly, it has been shown that animal communication asymmetries are modulated by the emotional content of the communicative sounds, with a greater involvement of the right hemisphere during production or perception of communicative sounds expressing or eliciting fear (Hook-Costigan and Rogers, 1998; Siniscalchi et al., 2008). These findings parallel the right-hemispheric dominance for negative emotions in humans (e.g., Onal-Hartmann et al., 2011). Taken together, while the evidence remains sparse for most vertebrate orders and certainly more research addressing this topic is needed, a recent cladographic comparative study (Ocklenburg et al., 2011b) concluded that there is convincing evidence for lateralization of production and perception of conspecific vocalization in several mammalian species, especially within the order of Primates. These findings suggest a phylogenetically early emergence of vocal communication asymmetries. Thus, human language lateralization might not be due to a dominance of the left hemisphere for language as such, but rather due to a left-hemispheric dominance for more basic features of species-typical communicative sounds or their production (Böye et al., 2005). Hence, lateralization of cognitive functions in the human brain did not necessarily emerge during human evolution. Instead, it may have been incorporated into the functional architecture of cognitive functions of which some, like language, are unique to Homo sapiens (MacNeilage et al., 2009). Therefore, we will now focus on several key questions of human lateralization and will outline how a comparative approach could possibly help to elucidate them.
Connections Matter: The Role of White Matter Tracts in Lateralized Functioning
What exactly causes functional hemispheric asymmetries? A common conception is that functional asymmetries are a consequence of structural asymmetries in the brain (Wada, 2009). Traditionally, research regarding this question has focused on macroscopic gray matter asymmetries such as volume or shape of certain brain areas (Amunts, 2010). However, it has been surprisingly difficult to find any clear-cut links between structural gray matter asymmetries in the human brain and left–right differences of behavior (Dos Santos Sequeira et al., 2006). Interestingly, evidence from recent studies in animal models suggests that structural asymmetries in connectivity patterns of homologous regions in the two hemispheres may be of greater functional relevance than asymmetries in region size or volume. One of the major animal models to investigate the neuronal foundations of hemispheric asymmetries is the visual system of birds. The two hemispheres of birds display a complementary pattern of visual analysis. The left hemisphere is specialized for detailed object analysis, attends to local features and excels in the categorization of visual stimuli (Vallortigara et al., 1996; Yamazaki et al., 2007). In contrast, the right hemisphere extracts relational configurations of visual stimuli that can be relevant during spatial orientation (Vallortigara et al., 2004; Yamazaki et al., 2007; Rugani et al., 2011). Additionally, the right hemisphere is in charge of visually guided social interactions (Rosa Salva et al., 2010), fear and escape responses (Rogers, 2000; Koboroff et al., 2008), sexual contacts (Gülbetekin et al., 2007), and encoding of relational spatial information (Tommasi and Vallortigara, 2001). Thus, each hemisphere seems to process visual stimuli in a different way. Anatomical and physiological studies support this dissociation and demonstrated that asymmetrical projections of the ascending visual pathways underlie parts of these lateralized visual behaviors.
Like mammals, birds process visual information within two ascending pathways, the thalamofugal, and the tectofugal system (see Figure 1).
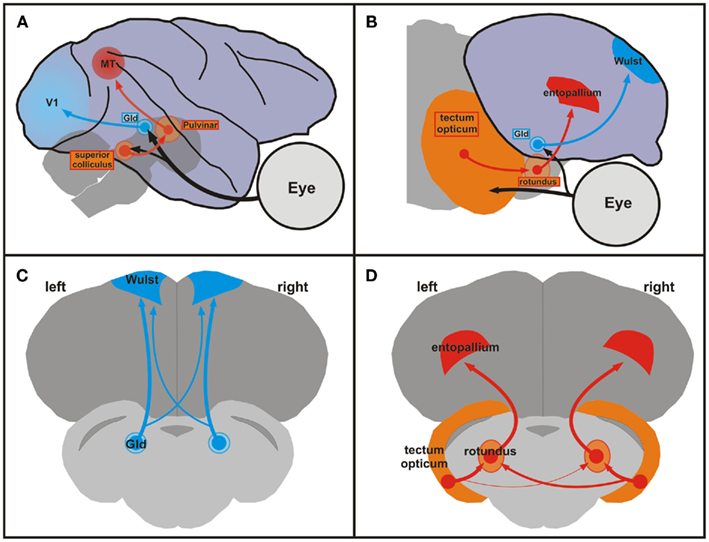
Figure 1. Comparison of mammalian and avian ascending visual pathways and asymmetries of the tectofugal pathway in pigeons. (A) Schematic sagittal view of the geniculostriate (blue) and extrageniculostriate (orange, red) projections in the monkey brain. Brainstem and thalamic structures are depicted as transparent to visualize their position under the cortex. (B) Schematic sagittal view of the thalamofugal (blue) and tectofugal (orange, red) pathways in the pigeon brain. These pathways correspond to the geniculostriate and extrageniculostriate system, respectively. (A,B) Dorsal is upward and rostral is to the right. (C,D) Schematic frontal views of the forebrain and brainstem of the pigeon brain showing the thalamofugal (C) and the tectofugal (D) pathways. Note the larger right-to-left projection of the tectorotundal efferents in the tectofugal system (D). The organization of the sections in (C,D) shows all relevant components within the same plane and is not anatomically correct. Abbreviations: GLd, nucleus geniculatus lateralis pars dorsalis; MT, middle temporal visual area (also V5); V1, primary visual cortex.
The thalamofugal pathway corresponds to the mammalian geniculostriate system and transfers retinal information via the contralateral geniculate complex (GLd) bilaterally onto the telencephalic visual Wulst. The tectofugal system corresponds to the mammalian extrageniculostriate pathway and projects via the contralateral midbrain optic tectum and the thalamic nucleus rotundus to the telencephalic entopallium (Manns and Güntürkün, 2009). A wealth of studies has revealed connectional asymmetries in the thalamofugal and tectofugal pathways of both chicks and pigeons (Rogers, 2008). For example, Rajendra and Rogers (1993) retrogradely traced projections from the dorsolateral anterior thalamus to the hyperpallium apicale (old nomenclature: hyperstriatum accessorium) in chicks and found that the ratio of labeled cells in the side of the thalamus contralateral to the injection site compared to the number of labeled cells in the side of the thalamus ipsilateral to the injection site was significantly greater for tracer injections in the right hemisphere compared to injections in the left hemisphere. In pigeons, the tectofugal pathway is the most important pathway for visually guided behavior. Since the optic nerve of birds is essentially crossed and since pigeons have laterally placed eyes, retinal fibers of the tectofugal system create a uni-hemispheric representation of the contralateral visual field in the midbrain tectum. From there, tectal neurons project bilaterally onto the thalamic rotundus (Güntürkün et al., 1993). Accordingly, rotundal and entopallial neurons often respond to visual stimulation from both eyes (Folta et al., 2004). However, the tectorotundal pathway has an asymmetry in its crossing component: more fibers cross from right tectum to left rotundus than from left tectum to right rotundus (Güntürkün et al., 1998). In line with the stronger bilateral input toward the left half of the brain, electrophysiological studies demonstrated that a higher number of left rotundal neurons respond to contralateral as well as ipsilateral visual input (Folta et al., 2004). Thus, the left tectofugal pathway predominantly integrates input from both eyes and possibly enables a more complete representation of the visual scenery. This assumption was tested psychophysically by Güntürkün and Hahmann (1999) with unilateral rotundus lesions. They demonstrated that damages to the left rotundus led to a bilateral decrease in visual acuity whereas right-sided lesions only had a minor contralateral impact. In a further study, Valencia-Alfonso et al. (2009) trained pigeons in a task where each eye was exposed to different color pairs. Accordingly, each hemisphere had only direct experience with one pair of colors. As a consequence, there was a pair of “known” (learned with the contralateral eye) and “unknown” (learned ipsilaterally) colors for each hemisphere. Then, each eye/hemisphere was separately tested with a mixture of known and unknown color pairs. While discriminating known color pairs evinced no asymmetry, the left hemisphere demonstrated better performance in discriminating the unknown stimulus pair. Thus, the left hemisphere had more access to information from the ipsilateral eye than the right hemisphere. This is a strong argument for the left hemisphere having a more bilateral representation of the visual input compared to the right hemisphere. In sum, the ascending tectofugal pathway displays a neuronal organization that creates an asymmetrical representation of the visual scene at the forebrain level. This enables the left hemisphere to process and to compare visual objects irrespective of their location within the whole visual scenery. This functional asymmetry results from left to right differences of white matter projections at the junction between midbrain and thalamus.
In humans, much less is known about the relation between structural asymmetries in white matter projections and functional lateralization. A first clue comes from a recent diffusion tensor tractography study (Barrick et al., 2007) in which two asymmetric white matter pathways were identified in the human brain: firstly, a rightward-asymmetric pathway connecting the posterior temporal lobe to the superior parietal lobule and secondly a leftward-asymmetric pathway connecting the parietal and frontal lobes to the temporal lobe. The authors suggest that the rightward-asymmetric pathway may be related to a rightward functional lateralization of auditory spatial attention and working memory whereas the leftward-asymmetric pathway may be related to leftward functional lateralization for language, but they did not test this assumption on a behavioral level. More direct evidence comes from a combined diffusion tensor imaging tractography and functional magnetic resonance imaging study in alcoholics and healthy controls (Schulte et al., 2010) were it was observed that white matter fiber degradation in the Corpus Callosum due to alcoholism leads to an attenuated pattern of functional visuo-motor asymmetries. While these studies are only a first step, they nevertheless show that, parallel to the work that has been conducted in birds, it may indeed be a promising approach to further investigate the role of structural white matter asymmetries in the human brain in order to reveal the underlying neurophysiological processes of human functional hemispheric asymmetries.
It all Starts in the Egg: The Role of Early Ontogenetic Signals for Asymmetry Formation
Do non-genetic factors play a role in human asymmetry formation or not? For handedness, some evidence suggests so, including the frequent observation of discordant handedness in monozygotic twins (Gurd, 2006), the lower incidence of left-handedness in countries where the left hand is associated with uncleanliness (Zverev, 2006), the higher incidence of left-handers among individuals born in spring and ensuing months than among individuals born during the rest of the year (Jones and Martin, 2008) as well as parental influences on handedness (Laland, 2008). For other types of functional hemispheric asymmetries, not much is known in humans. In birds, however, early ontogenetic signals have repeatedly been shown to play a crucial role for asymmetry formation and similar findings have also been reported for zebrafish (Andrew et al., 2009). Avian embryos consistently keep their head turned such that the right eye is close to the egg shell and the left eye is occluded by the body (Kuo, 1932). Since breeding birds regularly turn their eggs and intermittently leave the nest, eggs are frequently exposed to light which traverses the egg shell and primarily stimulates the right eye (Buschmann et al., 2006). As a consequence, most chickens and pigeons develop right eye superiority in visual discrimination (Güntürkün et al., 2000; Deng and Rogers, 2002). Incubation in the dark prevents development of functional asymmetries (Rogers, 1982; Zappia and Rogers, 1983; Deng and Rogers, 2002), and abolishes anatomical asymmetries within the visual pathways (Manns and Güntürkün, 1999). Experimentally induced embryonic bilateral light exposure creates symmetrical posthatch performance (Deng and Rogers, 2002). Embryonic (Rogers, 1990) or posthatch (Manns and Güntürkün, 1999) visual stimulation of the left eye can even reverse behavioral asymmetry, in chicks and pigeons respectively. Thus, normal rearing conditions correspond to right eye stimulation, resulting in left hemisphere superiority for visual object discrimination. This population bias is not genetically determined by factors within the visual system but by the lateralized epigenetic light factor that results from the genetically determined body position.
The resulting asymmetry in visual object discrimination is mediated through activity differences between left and right retinal ganglion cells. Since synaptic maturation of visual pathways is regulated by retinal activity (Ruthazer and Cline, 2004), transiently blocking right eye retinal activity in pigeons reverses visual asymmetry for the entire life (Prior et al., 2004). The lateralized retinal activation asymmetrically regulates tectal neurons, which in turn possibly release tectal brain derived neurotrophic factor (BDNF) asymmetrically (Manns et al., 2008). BDNF affects synaptic transmission and controls neurite sprouting and maintenance (Cohen-Cory and Lom, 2004). BDNF and the signaling cascade of its high-affinity receptor TrkB are asymmetrically activated in response to embryonic light stimulation (Manns et al., 2005). The small G protein p21Ras is a critical molecular switch for relaying neurotrophic actions into morphological changes. Its amount within the pigeon’s optic tectum depends on photic stimulation and consequently shows profound left–right differences (Manns et al., 2005). It is likely that BDNF, TrkB, and p21Ras represent one of the biochemical pathways that translate a transient embryonic visual stimulation asymmetry into structural left–right differences of the tectofugal system that then determine lateralized visually guided behavior for the entire lifespan of the animal. The biased embryonic photic input ignites several asymmetries (object discrimination: Rogers, 1990; left–right discrimination: Chiandetti and Vallortigara, 2009), while leaving others unaffected (visual reaction to novelty: Chiandetti et al., 2005; Rogers, 2008). Thus, the neuronal effects of lateralized embryonic visual stimulation only affect some of the visually guided functions.
The development of visual object discrimination asymmetries in birds demonstrates that the establishment of a functional asymmetry can proceed along the same principles of synaptic plasticity that are already well known from other sensory systems. Avian visual asymmetry results from an interaction between an epigenetic event (left–right differences of light stimulation) and a genetic factor (embryonic right-turn of the head; see Figure 2; Manns, 2006; Manns and Güntürkün, 2009).
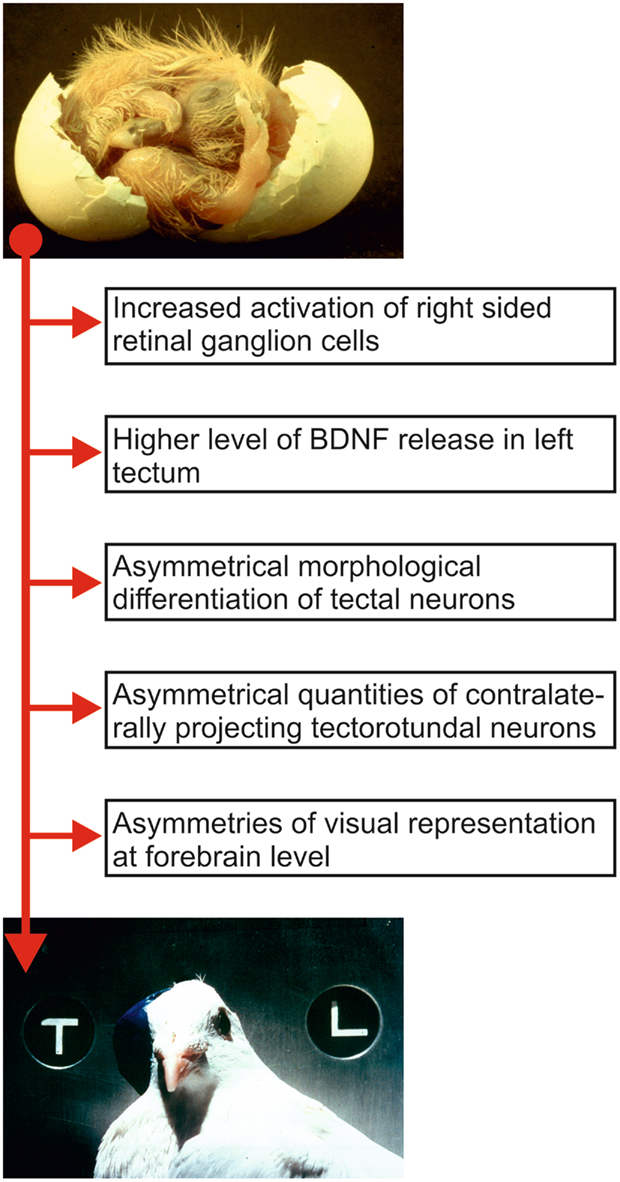
Figure 2. Sequence of relevant ontogenetic events that possibly constitute components of the development of visual asymmetry in pigeons. The top picture shows a pigeon embryo during hatch. Note the position of the head that is turned to the right such that the right eye is positioned close to the eggshell. The resulting biased light input before hatch is translated into morphological asymmetries of ascending visual pathways that then results in left–right differences of behavior. The bottom picture shows an adult pigeon wearing an eye cap and participating in a pattern discrimination task.
Comparable events may have relevance also in the mammalian and thus the human brain. An asymmetrical environmental stimulation is able to induce the formation of structural and physiological left–right differences within the ascending sensory pathways. It is conceivable that such a critical role of a lateralized experience is not confined to sensory systems but also applies to the development of motor asymmetries as in the case of human handedness. For example, the ability of spinally controlled motor asymmetries to influence the cerebral cortex may represent a human corollary to the avian system (Ververs et al., 1994). In this case, early spinal asymmetries could act as lateralized “precursors” of asymmetrical cortical motor functions (Hiscock and Kinsbourne, 1995). But early motor asymmetries could also shape sensorimotor circuits of hand control in a lateralized way. Like birds, humans have an early bias to turn the head to the right (Ververs et al., 1994). This early prenatal bias not only persists into adulthood (Güntürkün, 2003) but also correlates with right handedness (Ocklenburg and Güntürkün, 2009). This relation between head position and hand use could result from a higher probability of visuo-motor coupling between gaze position and the right hand during early childhood. To test the causal nature of this link, Ocklenburg et al. (2010) studied children with torticollis, a condition that causes a subtle pathological tilt of the head to the left or to the right, in combination with a contralateral rotation of face and chin. The resulting head posture leads to an increased visual experience of the hand contralateral to the head-tilt and had a strong effect on handedness. Relative to controls, children with torticollis had a higher probability of right- or left-handedness when having a head-tilt to the opposite side. Thus, early biased sensory input or motor preference could modify lateralized systems of humans. The physiological mechanism underlying this modulation may be not identical to the impact of early visual stimulation of one eye in birds. Nevertheless, these findings show that, comparable to birds, a non-genetic, experience-based factor can influence human lateralization.
The Genetics of Asymmetry: From Zebrafish to Human
Which genetic factors play a role in human asymmetry formation? While there is very little doubt that handedness and language lateralization, the two most obvious examples of functional lateralization in humans, are, at least to some extent, genetically determined (Corballis, 2009), the answer to this question proved to be surprisingly difficult to find. Based on indirect statistical evidence, several authors suggested a common monogenetic background for these two traits (e.g., Annett, 2002). This view has repeatedly been questioned during recent years and it has been suggested that at least partly independent polygenic mechanisms for the inheritance of language lateralization and handedness exist (e.g., Medland et al., 2009; Tzourio-Mazoyer et al., 2010). The biggest problem of monogenic theories of handedness and language lateralization is that, despite continuous efforts to do so, no single gene has ever been identified that explains even remotely enough variance in behavioral lateralization data to qualify for a single gene explanation. Moreover, a recent study found an effect of LRRTM1 on chromosome 2p12 (a gene that is possibly involved in neural differentiation in the brain) on handedness in a sample of dyslexic siblings, but not in a sample of healthy siblings (Francks et al., 2007). The authors therefore suggest that the effect of LRRTM1 on behavioral lateralization depends on other genetic and environmental factors in order to manifest and they concluded that handedness and brain lateralization are likely to be etiologically complex traits that are influenced by multiple genetic and environmental factors (Francks et al., 2007). This conclusion was also recently supported by a genome-wide association study that reported an association of another gene (PCSK6) with handedness in as dyslexic sample (Scerri et al., 2011).
Comparative studies on the genetics of brain lateralization also indicate that it is indeed highly unlikely that handedness and brain lateralization are determined by a single gene. The most widely used vertebrate model species in research on the genetic background of hemispheric asymmetries is the zebrafish (Danio rerio). The epithalamus of the zebrafish, a brain region consisting of the left and right habenula as well as the medial pineal organ and the parapineal organ, shows pronounced structural hemispheric asymmetries regarding its neuronal organization (Dadda et al., 2010b). Most notably, the parapineal organ lies to the left of the pineal organ in most individuals (see Figure 3).
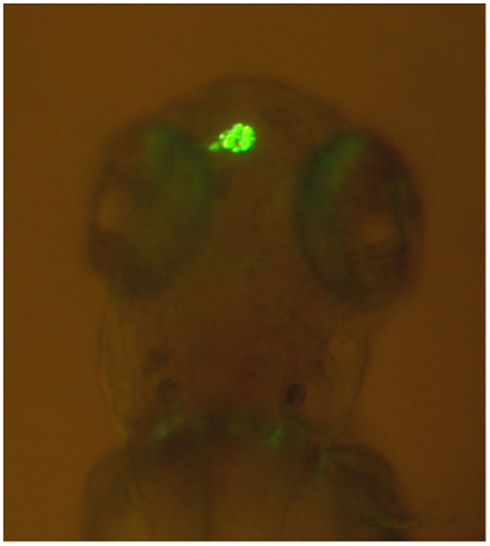
Figure 3. Leftward asymmetry of the parapineal organ in a zebrafish made visible by green fluorescent protein expression in a transgenic tg(foxD3:GFP)zf15 zebrafish (Modified from Dadda et al., 2010b). Rostral is upward and caudal is downward.
These epithalamic asymmetries are regulated by several genes in the Nodal signaling pathway with the exact mechanisms having been reviewed elsewhere (Snelson and Gamse, 2009; Taylor et al., 2010; Roussigné et al., 2011). Interestingly, when Nodal genes are not expressed at all, epithalamic asymmetries are not absent, but their direction is determined at random (Concha et al., 2000). This shows that Nodal genes only determine the direction of asymmetries but not their initial establishment (Concha et al., 2000), possibly indicating that another signaling pathway is relevant for initial symmetry breaking. Recently, several studies have reported a link between the genetically controlled structural asymmetry in the zebrafish epithalamus and functional lateralization. For example, the commonly observed reversal of heart, gut, and structural diencephalic asymmetries in the frequent-situs-inversus (fsi) line of zebrafish is related to a reversal of functional asymmetries in several behavioral laterality tests, including mirror viewing and approaching a target to bite (Barth et al., 2005). These findings suggest that at least two different genetic mechanisms influence different forms of functional lateralization in the zebrafish (Barth et al., 2005). More recently, it has been reported that zebrafishes with a left or right parapineal organ show significant differences in several behavioral laterality tests, including eye preference for viewing their own reflection, eye use in predator inspection, rotational preference, and turning direction in the dark (Dadda et al., 2010b), indicating a clear link between structural and functional asymmetry. Apart from zebrafish, similar findings regarding habenular and behavioral asymmetry have also been observed in two different cichlid species (Reddon et al., 2009; Gutiérrez-Ibáñez et al., 2011). Not much is known about the relation of epithalamus structure and functional lateralization in humans, and human functional asymmetries are most likely driven by different genetic mechanisms. However, functional hemispheric lateralization is a conserved feature of the central nervous system in vertebrates (Vallortigara et al., 1999; Vallortigara and Rogers, 2005; Bianco et al., 2008) and, as such, the findings in the zebrafish could possibly help to understand why no single gene determining handedness and language lateralization in humans has been found yet. From a comparative point of view, it is highly questionable that a trait like brain asymmetry which is determined by two complex polygenic signaling pathways in one vertebrate species is determined by only a single gene in another vertebrate species. Thus, when viewing lateralization in H. sapiens from this perspective, it becomes clear that it is necessary to develop polygenic instead of monogenic theories for its ontogenesis. Particularly, the idea that two different signaling pathways may control for the initial establishment and direction of asymmetries could fundamentally change theoretical approaches to asymmetry formation in humans.
Concluding Remarks
Comparative approaches have greatly enhanced our understanding of several different human cognitive domains (de Waal and Ferrari, 2010; Haun et al., 2010). In line with these scientific success stories, comparative neuroscience also allows for unique insights into ontogenetic and phylogenetic processes responsible for human brain lateralization. These insights, however, are only parts of the whole story and it becomes increasingly clear that we are still far away from having a complete understanding of the complex interactions of non-genetic and genetic factors that underlie the neurophysiological processes that drive human functional hemispheric asymmetries. Genetic and neuroscientific methods are rapidly advancing but we need to integrate the resulting insights into a broader comparative schema. To this end, it is fundamentally important to understand that human lateralization is not unique, but a trait that is shared with a multitude of other vertebrates. The idea of human exceptionalism had and still has a strong impact on lateralization research. Only by abandoning this approach and viewing H. sapiens as one vertebrate species among many, we will be able to solve the riddle of functional hemispheric asymmetries in humans and other animals.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Figure 3 is reprinted from Behavioral Brain Research, 206(2), Dadda M, Domenichini A, Piffer L, Argenton F, Bisazza A. Early differences in epithalamic left-right asymmetry influence lateralization and personality of adult zebrafish. 208–215, Copyright (2010), with permission from Elsevier. We like to thank Dr. Marco Dadda for providing this figure to us.
References
Amunts, K. (2010). “Structural indices of asymmetry,” in The Two Halves of the Brain, eds K. Hughdahl and R. Westerhausen (Cambridge, MA: The MIT Press), 145–176.
Andrew, R. J., Osorio, D., and Budaev, S. (2009). Light during embryonic development modulates patterns of lateralization strongly and similarly in both zebrafish and chick. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 983–989.
Barrick, T. R., Lawes, I. N., Mackay, C. E., and Clark, C. A. (2007). White matter pathway asymmetry underlies functional lateralization. Cereb. Cortex 17, 591–598.
Barth, K. A., Miklosi, A., Watkins, J., Bianco, I. H., Wilson, S. W., and Andrew, R. J. (2005). Fsi zebrafish show concordant reversal of laterality of viscera, neuroanatomy, and a subset of behavioral responses. Curr. Biol. 15, 844–850.
Bauer, R. H. (1993). Lateralization of neural control for vocalization by the frog (Rana pipiens). Psychobiology 21, 243–248.
Bethmann, A., Tempelmann, C., De Bleser, R., Scheich, H., and Brechmann, A. (2007). Determining language laterality by fMRI and dichotic listening. Brain Res. 1133, 145–157.
Bianco, I. H., Carl, M., Russell, C., Clarke, J. D., and Wilson, S. W. (2008). Brain asymmetry is encoded at the level of axon terminal morphology. Neural Dev. doi:10.1186/1749-8104-3–9
Bisazza, A., Cantalupo, C., Capocchiano, M., and Vallortigara, G. (2000). Population lateralisation and social behaviour: a study with 16 species of fish. Laterality 5, 269–284.
Bisazza, A., Rogers, L. J., and Vallortigara, G. (1998). The origins of cerebral asymmetry: a review of evidence of behavioural and brain lateralization in fishes, reptiles and amphibians. Neurosci. Biobehav. Rev. 22, 411–426.
Bonati, B., Csermely, D., López, P., and Martín, J. (2010). Lateralization in the escape behaviour of the common wall lizard (Podarcis muralis). Behav. Brain Res. 207, 1–6.
Bonati, B., Csermely, D., and Romani, R. (2008). Lateralization in the predatory behaviour of the common wall lizard (Podarcis muralis). Behav. Processes 79, 171–174.
Böye, M., Güntürkün, O., and Vauclair, J. (2005). Right ear advantage for conspecific calls in adults and subadults, but not infants, California sea lions (Zalophus californianus): hemispheric specialization for communication? Eur. J. Neurosci. 21, 1727–1732.
Broca, P. (1861). Remarques sur le sie’ge de la faculte’ du language articule’, suivies d’une observation d’aphe’mie (perte de la parole). Bull. Soc. Anat. 6, 330–357.
Buschmann, J. U., Manns, M., and Güntürkün, O. (2006). “Let there be light!” Pigeon eggs are naturally exposed to light during breeding. Behav. Processes 73, 62–67.
Byrne, R. A., Kuba, M., and Griebel, U. (2002). Lateral asymmetry of eye use in Octopus vulgaris. Anim. Behav. 64, 461–468.
Chiandetti, C., Regolin, L., Rogers, L. J., and Vallortigara, G. (2005). Effects of light stimulation of embryos on the use of position-specific and object-specific cues in binocular and monocular domestic chicks (Gallus gallus). Behav. Brain Res. 163, 10–17.
Chiandetti, C., and Vallortigara, G. (2009). Effects of embryonic light stimulation on the ability to discriminate left from right in the domestic chick. Behav. Brain Res. 198, 240–246.
Cohen-Cory, S., and Lom, B. (2004). Neurotrophic regulation of retinal ganglion cell synaptic connectivity: from axons and dendrites to synapses. Int. J. Dev. Biol. 48, 947–956.
Concha, M. L., Burdine, R. D., Russell, C., Schier, A. F., and Wilson, S. W. (2000). A nodal signaling pathway regulates the laterality of neuroanatomical asymmetries in the zebrafish forebrain. Neuron 28, 399–409.
Concha, M. L., and Wilson, S. W. (2001). Asymmetry in the epithalamus of vertebrates. J. Anat. 199, 63–84.
Corballis, M. C. (2009). The evolution and genetics of cerebral asymmetry. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 867–879.
Csermely, D., Bonati, B., Lopez, P., and Martin, J. (2011). Is the Podarcis muralis lizard left-eye lateralised when exploring a new environment? Laterality 16, 240–255.
Csermely, D., Bonati, B., and Romani, R. (2010). Lateralisation in a detour test in the common wall lizard (Podarcis muralis). Laterality 15, 535–547.
Dadda, M., Koolhaas, W. H., and Domenici, P. (2010a). Behavioural asymmetry affects escape performance in a teleost fish. Biol. Lett. 6, 414–417.
Dadda, M., Domenichini, A., Piffer, L., Argenton, F., and Bisazza, A. (2010b). Early differences in epithalamic left-right asymmetry influence lateralization and personality of adult zebrafish. Behav. Brain Res. 206, 208–215.
de Waal, F. B., and Ferrari, P. F. (2010). Towards a bottom-up perspective on animal and human cognition. Trends Cogn. Sci. (Regul. Ed.) 14, 201–207.
Deng, C., and Rogers, L. J. (2002). Prehatch visual experience and lateralization in the visual Wulst of the chick. Behav. Brain Res. 134, 375–385.
Dos Santos Sequeira, S., Woerner, W., Walter, C., Kreuder, F., Lueken, U., Westerhausen, R., Wittling, R. A., Schweiger, E., and Wittling, W. (2006). Handedness, dichotic-listening ear advantage, and gender effects on planum temporale asymmetry – a volumetric investigation using structural magnetic resonance imaging. Neuropsychologia 44, 622–636.
Ehret, G. (1987). Left hemisphere advantage in the mouse brain for recognizing ultrasonic communication calls. Nature 325, 249–251.
Folta, K., Diekamp, B., and Güntürkün, O. (2004). Asymmetrical modes of visual bottom-up and top-down integration in the thalamic nucleus rotundus of pigeons. J. Neurosci. 24, 9475–9485.
Francks, C., Maegawa, S., Laurén, J., Abrahams, B. S., Velayos-Baeza, A., Medland, S. E., Colella, S., Groszer, M., McAuley, E. Z., Caffrey, T. M., Timmusk, T., Pruunsild, P., Koppel, I., Lind, P. A., Matsumoto-Itaba, N., Nicod, J., Xiong, L., Joober, R., Enard, W., Krinsky, B., Nanba, E., Richardson, A. J., Riley, B. P., Martin, N. G., Strittmatter, S. M., Möller, H. J., Rujescu, D., St Clair, D., Muglia, P., Roos, J. L., Fisher, S. E., Wade-Martins, R., Rouleau, G. A., Stein, J. F., Karayiorgou, M., Geschwind, D. H., Ragoussis, J., Kendler, K. S., Airaksinen, M. S., Oshimura, M., DeLisi, L. E., and Monaco, A. P. (2007). LRRTM1 on chromosome 2p12 is a maternally suppressed gene that is associated paternally with handedness and schizophrenia. Mol. Psychiatry 12, 1129–1139.
Frasnelli, E., Anfora, G., Trona, F., Tessarolo, F., and Vallortigara, G. (2010). Morpho-functional asymmetry of the olfactory receptors of the honeybee (Apis mellifera). Behav. Brain Res. 209, 221–225.
George, I. (2010). “Hemispheric asymmetry of songbirds,” in The Two Halves of the Brain, eds K. Hughdahl and R. Westerhausen (Cambridge, MA: The MIT Press), 91–120.
Gülbetekin, E., Güntürkün, O., Dural, S., and Cetinkaya, H. (2007). Asymmetry of visually guided sexual behaviour in adult Japanese quail (Coturnix japonica). Laterality 12, 321–331.
Güntürkün, O., Diekamp, B., Manns, M., Nottelmann, F., Prior, H., Schwarz, A., and Skiba, M. (2000). Asymmetry pays: visual lateralization improves discrimination success in pigeons. Curr. Biol. 10, 1079–1081.
Güntürkün, O., and Hahmann, U. (1999). Functional subdivisions of the ascending visual pathways in the pigeon. Behav. Brain Res. 98, 193–201.
Güntürkün, O., Hellmann, B., Melsbach, G., and Prior, H. (1998). Asymmetries of representation in the visual system of pigeons. Neuroreport 9, 4127–4130.
Güntürkün, O., and Manns, M. (2010). “The embryonic development of visual asymmetry in the pigeon,” in The Two Halves of the Brain, eds K. Hughdahl and R. Westerhausen (Cambridge, MA: The MIT Press), 121–143.
Güntürkün, O., Melsbach, G., Hörster, W., and Daniel, S. (1993). Different sets of afferents are demonstrated by the fluorescent tracers fast blue and rhodamine. J. Neurosci. Methods 49, 103–111.
Gurd, J. M. (2006). Hand preference and performance in 20 pairs of monozygotic twins with discordant handedness. Cortex 42, 934–945.
Gutiérrez-Ibáñez, C., Reddon, A. R., Kreuzer, M. B., Wylie, D. R., and Hurd, P. L. (2011). Variation in asymmetry of the habenular nucleus correlates with behavioural asymmetry in a cichlid fish. Behav. Brain Res. 221, 189–196.
Haun, D. B., Jordan, F. M., Vallortigara, G., and Clayton, N. S. (2010). Origins of spatial, temporal and numerical cognition: insights from comparative psychology. Trends Cogn. Sci. (Regul. Ed.) 14, 552–560.
Hauser, M. D., and Andersson, K. (1994). Left hemisphere dominance for processing vocalizations in adult, but not infant, rhesus monkeys: field experiments. Proc. Natl. Acad. Sci. U.S.A. 91, 3946–3948.
Hiscock, M., and Kinsbourne, M. (1995). “Phylogeny and ontogeny of cerebral lateralizations” in Brain Asymmetry, eds R. J. Davidson and K. Hugdahl (Cambridge, MA: The MIT Press), 535–578.
Hook-Costigan, M. A., and Rogers, L. J. (1998). Lateralized use of the mouth in production of vocalizations by marmosets. Neuropsychologia 36, 1265–1273.
Koboroff, A., Kaplan, G., and Rogers, L. J. (2008). Hemispheric specialization in Australian magpies (Gymnorhina tibicen) shown as eye preferences during response to a predator. Brain Res. Bull. 76, 304–306.
Kuo, Z. Y. (1932). Ontogeny of embryonic behavior in aves. III. The structural and environmental factors in embryonic behavior. J. Comp. Psychol. 13, 245–271.
Laland, K. N. (2008). Exploring gene-culture interactions: insights from handedness, sexual selection and niche-construction case studies. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363, 3577–3589.
Leliveld, L. M., Scheumann, M., and Zimmermann, E. (2010). Effects of caller characteristics on auditory laterality in an early primate (Microcebus murinus). PLoS ONE 5, e9031. doi:10.1371/journal.pone.0009031
Lippolis, G., Joss, J. M., and Rogers, L. J. (2009). Australian lungfish (Neoceratodus forsteri): a missing link in the evolution of complementary side biases for predator avoidance and prey capture. Brain Behav. Evol. 73, 295–303.
MacNeilage, P. F., Rogers, L. J., and Vallortigara, G. (2009). Origins of the left & right brain. Sci. Am. 301, 60–67.
Manns, M. (2006). “The epigenetic control of asymmetry formation: lessons from the avian visual system,” in Behavioural and Morphological Asymmetries in Vertebrates, eds Y. Malashichev and A. W. Deckel (Austin, TX: Landes Bioscience), 613–618.
Manns, M., Freund, N., and Güntürkün, O. (2008). Development of the diencephalic relay structures of the thalamofugal system in pigeons. Brain Res. Bull. 75, 424–427.
Manns, M., and Güntürkün, O. (1999). Monocular deprivation alters the direction of functional and morphological asymmetries in the pigeon’s visual system. Behav. Neurosci. 113, 1–10.
Manns, M., and Güntürkün, O. (2009). Dual coding of visual asymmetries in the pigeon brain – the interaction of bottom-up and top-down systems. Exp. Brain Res. 199, 323–332.
Manns, M., Güntürkün, O., Heumann, R., and Blöchl, A. (2005). Photic inhibition of TrkB/Ras activity in the pigeon’s tectum during development: impact on brain asymmetry formation. Eur. J. Neurosci. 22, 2180–2186.
Medland, S. E., Duffy, D. L., Wright, M. J., Geffen, G. M., Hay, D. A., Levy, F., van-Beijsterveldt, C. E., Willemsen, G., Townsend, G. C., White, V., Hewitt, A. W., Mackey, D. A., Bailey, J. M., Slutske, W. S., Nyholt, D. R., Treloar, S. A., Martin, N. G., and Boomsma, D. I. (2009). Genetic influences on handedness: data from 25,732 Australian and Dutch twin families. Neuropsychologia 47, 330–337.
Nottebohm, F., and Nottebohm, M. E. (1976). Left hypoglossal dominance in the control of canary and white-crowned sparrow song. J. Comp. Physiol. A 108, 171–192.
Ocklenburg, S., Bürger, C., Westermann, C., Schneider, D., Biedermann, H., and Güntürkün, O. (2010). Visual experience affects handedness. Behav. Brain Res. 207, 447–451.
Ocklenburg, S., and Güntürkün, O. (2009). Head-turning asymmetries during kissing and their association with lateral preference. Laterality 14, 79–85.
Ocklenburg, S., Güntürkün, O., and Beste, C. (2011a). Lateralized neural mechanisms underlying the modulation of response inhibition processes. Neuroimage 55, 1771–1778.
Ocklenburg, S., Ströckens, F., and Güntürkün, O. (2011b). Lateralization of conspecific vocalization in non-human vertebrates. Laterality. (in press).
Onal-Hartmann, C., Pauli, P., Ocklenburg, S., and Güntürkün, O. (2011). The motor side of emotions: investigating the relationship between hemispheres, motor reactions and emotional stimuli. Psychol. Res. doi: 10.1007/s00426-011-0337-4. [Epub ahead of print].
Prior, H., Diekamp, B., Güntürkün, O., and Manns, M. (2004). Post-hatch activity-dependent modulation of visual asymmetry formation in pigeons. Neuroreport 15, 1311–1314.
Rajendra, S., and Rogers, L. J. (1993). Asymmetry is present in the thalamofugal visual projections of female chicks. Exp. Brain Res. 92, 542–544.
Reddon, A. R., Gutiérrez-Ibáñez, C., Wylie, D. R., and Hurd, P. L. (2009). The relationship between growth, brain asymmetry and behavioural lateralization in a cichlid fish. Behav. Brain Res. 201, 223–228.
Ringo, J. L., Doty, R. W., Demeter, S., and Simard, P. Y. (1994). Time is of the essence: a conjecture that hemispheric specialization arises from interhemispheric conduction delay. Cereb. Cortex 4, 331–343.
Rogers, L. J. (1982). Light experience and asymmetry of brain function in chickens. Nature 297, 223–225.
Rogers, L. J. (1990). Light input and the reversal of functional lateralization in the chicken brain. Behav. Brain Res. 38, 211–221.
Rogers, L. J. (2000). Evolution of hemispheric specialization: advantages and disadvantages. Brain Lang. 73, 236–253.
Rogers, L. J. (2008). Development and function of lateralization in the avian brain. Brain Res. Bull. 76, 235–244.
Rogers, L. J., and Vallortigara, G. (2008). From antenna to antenna: lateral shift of olfactory memory recall by honeybees. PLoS ONE 3, e2340. doi:10.1371/journal.pone.0002340
Rogers, L. J., Zucca, P., and Vallortigara, G. (2004). Advantages of having a lateralized brain. Proc. Biol. Sci. 271(Suppl. 6), 420–422.
Rosa Salva, O., Daisley, J. N., Regolin, L., and Vallortigara, G. (2010). Time-dependent lateralization of social learning in the domestic chick (Gallus gallus domesticus): effects of retention delays in the observed lateralization pattern. Behav. Brain Res. 212, 152–158.
Roussigné, M., Blader, P., and Wilson, S. W. (2011). The zebrafish epithalamus clears a path through the complexity of brain lateralization. Dev. Neurobiol. doi:10.1002/dneu.20885. [Epub ahead of print].
Rugani, R., Vallortigara, G., Vallini, B., and Regolin, L. (2011). Asymmetrical number-space mapping in the avian brain. Neurobiol. Learn. Mem. 95, 231–238.
Ruthazer, E. S., and Cline, H. T. (2004). Insights into activity-dependent map formation from the retinotectal system: a middle-of-the-brain perspective. J. Neurobiol. 59, 134–146.
Scerri, T. S., Brandler, W. M., Paracchini, S., Morris, A. P., Ring, S. M., Richardson, A. J., Talcott, J. B., Stein, J., and Monaco, A. P. (2011). PCSK6 is associated with handedness in individuals with dyslexia. Hum. Mol. Genet. 20, 608–614.
Schulte, T., Müller-Oehring, E. M., Rohlfing, T., Pfefferbaum, A., and Sullivan, E. V. (2010). White matter fiber degradation attenuates hemispheric asymmetry when integrating visuomotor information. J. Neurosci. 30, 12168–12178.
Siniscalchi, M., Quaranta, A., and Rogers, L. J. (2008). Hemispheric specialization in dogs for processing different acoustic stimuli. PLoS ONE 3, e3349. doi:10.1371/journal.pone.0003349
Snelson, C. D., and Gamse, J. T. (2009). Building an asymmetric brain: development of the zebrafish epithalamus. Semin. Cell Dev. Biol. 20, 491–497.
Taglialatela, J. P., Russell, J. L., Schaeffer, J. A., and Hopkins, W. D. (2008). Communicative signaling activates ‘Broca’s’ homolog in chimpanzees. Curr. Biol. 18, 343–348.
Taylor, R. W., Hsieh, Y. W., Gamse, J. T., and Chuang, C. F. (2010). Making a difference together: reciprocal interactions in C. elegans and zebrafish asymmetric neural development. Development 137, 681–691.
Tommasi, L., and Vallortigara, G. (2001). Encoding of geometric and landmark information in the left and right hemispheres of the avian brain. Behav. Neurosci. 115, 602–613.
Tzourio-Mazoyer, N., Simon, G., Crivello, F., Jobard, G., Zago, L., Perchey, G., Hervé, P. Y., Joliot, M., Petit, L., Mellet, E., and Mazoyer, B. (2010). Effect of familial sinistrality on planum temporale surface and brain tissue asymmetries. Cereb. Cortex 20, 1476–1485.
Valencia-Alfonso, C. E., Verhaal, J., and Güntürkün, O. (2009). Ascending and descending mechanisms of visual lateralization in pigeons. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 955–963.
Vallortigara, G. (1992). Right hemisphere advantage for social recognition in the chick. Neuropsychologia 30, 761–768.
Vallortigara, G. (2006). The evolutionary psychology of left and right: costs and benefits of lateralization. Dev. Psychobiol. 48, 418–427.
Vallortigara, G., Pagni, P., and Sovrano, V. A. (2004). Separate geometric and non-geometric modules for spatial reorientation: evidence from a lopsided animal brain. J. Cogn. Neurosci. 16, 390–400.
Vallortigara, G., Regolin, L., Bortolomiol, G., and Tommasi, L. (1996). Lateral asymmetries due to preferences in eye use during visual discrimination learning in chicks. Behav. Brain Res. 74, 135–143.
Vallortigara, G., and Rogers, L. J. (2005). Survival with an asymmetrical brain: advantages and disadvantages of cerebral lateralization. Behav. Brain Sci. 28, 575–589.
Vallortigara, G., Rogers, L. J., and Bisazza, A. (1999). Possible evolutionary origins of cognitive brain lateralization. Brain Res. Brain Res. Rev. 30, 164–175.
Ververs, I. A., de Vries, J. I., van Geijn, H. P., and Hopkins, B. (1994). Prenatal head position from 12-38 weeks. I. Developmental aspects. Early Hum. Dev. 39, 83–91.
Vogel, J. J., Bowers, C. A., and Vogel, D. S. (2003). Cerebral lateralization of spatial abilities: a metaanalysis. Brain Cogn. 52, 197–204.
Wada, J. A. (2009). Is functional hemispheric lateralization guided by structural cerebral asymmetry? Can. J. Neurol. Sci. 36(Suppl. 2), 25–31.
Yamazaki, Y., Aust, U., Huber, L., Hausmann, M., and Güntürkün, O. (2007). Lateralized cognition: asymmetrical and complementary strategies of pigeons during discrimination of the ‘human concept.’ Cognition 104, 315–344.
Zappia, J. V., and Rogers, L. J. (1983). Light experience during development affects asymmetry of forebrain function in chickens. Brain Res. 11, 93–106.
Keywords: lateralization, pigeon, tectofugal pathway, thalamofugal pathway, white matter, genetics, environmental factors, ontogenesis
Citation: Ocklenburg S and Güntürkün O (2012) Hemispheric asymmetries: the comparative view. Front. Psychology 3:5. doi: 10.3389/fpsyg.2012.00005
Received: 24 October 2011;
Accepted: 05 January 2012;
Published online: 26 January 2012.
Edited by:
Simon Matthew Reader, Utrecht University, NetherlandsReviewed by:
Lesley J. Rogers, University of New England, AustraliaChristian Agrillo, University of Padova, Italy
Isabelle George, Rennes 1 University, France
Angelo Bisazza, Università di Padova, Italy
Copyright: © 2012 Ocklenburg and Güntürkün. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Onur Güntürkün, Department of Biopsychology, Faculty of Psychology, Institute of Cognitive Neuroscience, Ruhr-University Bochum, Universitätsstraße 150, D-44780 Bochum, Germany. e-mail:b251ci5ndWVudHVlcmt1ZW5AcnViLmRl

