
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychol. , 24 November 2011
Sec. Cognition
volume 2 - 2011 | https://doi.org/10.3389/fpsyg.2011.00336
This article is part of the Research Topic Cognitive and Affective Control View all 22 articles
The present study focused on the interplay between arousal, valence, and cognitive control. To this end, we investigated how arousal and valence associated with affective stimuli influenced cognitive flexibility when switching between tasks voluntarily. Three hypotheses were tested. First, a valence hypothesis that states that the positive valence of affective stimuli will facilitate both global and task-switching performance because of increased cognitive flexibility. Second, an arousal hypothesis that states that arousal, and not valence, will specifically impair task-switching performance by strengthening the previously executed task-set. Third, an attention hypothesis that states that both cognitive and emotional control ask for limited attentional resources, and predicts that arousal will impair both global and task-switching performance. The results showed that arousal affected task-switching but not global performance, possibly by phasic modulations of the noradrenergic system that reinforces the previously executed task. In addition, positive valence only affected global performance but not task-switching performance, possibly by phasic modulations of dopamine that stimulates the general ability to perform in a multitasking environment.
For many years, research on cognitive control has been conducted without taking into account that goal-directed behavior takes place in an environment consisting of a multitude of stimuli, some of which are emotional. Yet, research not only has shown that cognitive control modulates emotions (e.g., Gross, 2002) but also that emotions influence cognitive control (e.g., Gray, 1999; for a review see Pessoa, 2009). Gray et al. (2002) found that activation in the dorso-lateral pre-frontal cortex (DLPFC), which is part of the cognitive control system, also depends on the presentation of emotional information. Furthermore, the anterior cingulate cortex (ACC) is both connected to the DLPFC and to the limbic system (Bush et al., 2000), which is important for emotional control. Although the connections between emotional and cognitive control are obvious at the neuro-functional level, at the process level it is not always clear how both systems interact. In the present study, we investigated different mechanisms underlying this interplay by testing the influence of irrelevant affective pictures on cognitive flexibility.
Different mechanisms have been proposed that can account for the relation between cognitive and emotional control. A first mechanism is related to the function of the neurotransmitter dopamine (DA). Ashby et al. (1999, 2002) argued that positive information can lead to an increase of DA, resulting in an enhancement of cognitive control (see also Braver et al., 1999; Braver and Cohen, 2000; Cohen et al., 2002; Savine and Braver, 2010). In line with this account, it has been shown that positive information stimulates cognitive flexibility (e.g., Isen and Daubman, 1984; Greene and Noice, 1988; Isen et al., 1992; Kuhl and Kazén, 1999; Bolte et al., 2003; Dreisbach and Goschke, 2004; Dreisbach, 2006). More recently, this account has been adjusted in two ways (Cools et al., 2001, 2007, 2009; Cools and Robbins, 2004). First, phasic and tonic modulations of DA have been dissociated (see also Cools and Robbins, 2004; Cools et al., 2009). While high tonic DA favors reward-based learning, low tonic DA favors punishment-based learning. Second, Cools et al. (2001, 2007) suggested that the influence of phasic increases of DA on cognitive control depends on the demands of the task and on the neural structure in which the DA levels are changed (see also Frank et al., 2004; Maia and Frank, 2011). While phasic increases of DA in the striatum lead to more flexible behavior, and can thus enhance cognitive flexibility, phasic increases of DA in the PFC lead to less distractible behavior, which improves protection from irrelevant information, but deteriorates cognitive flexibility. In order to test this so-called valence hypothesis, we manipulated the valence of affective stimuli on trial basis, presumably resulting in phasic modulations of DA.
A second mechanism that can explain the interplay between emotional and cognitive control is related to the function of the neurotransmitter noradrenalin (NA) in the locus coeruleus (LC). As for DA modulations, Aston-Jones and Cohen (2005) argued that there are two modes of LC–NA function: a phasic mode that stimulates behavioral stability and a tonic mode that stimulates more flexible, but also more distractible behavior. Based on this original model, Verguts and Notebaert (2009) introduced a binding account for cognitive control. This account was based on the observation that when a response conflict arises during the execution of a particular task on trial n-1, a response conflict on trial impairs performance less severely than when no response conflict was present on trial n-1 (Gratton et al., 1992). Verguts and Notebaert (2009) argued that the experience of a conflict causes arousal that triggers an immediate boost of levels of NA in the LC. This phasic increase of LC–NA function stimulates Hebbian learning (Hebb, 1949), which binds stimulus and response features into a task-set or event file (Hommel, 2004) and, as a result, task-sets prone to arousal become strengthened (see also Braem et al., 2011). As a consequence, performance based on such task-sets is impaired less by new response conflicts and can thus be interpreted as more stable and less flexible behavior (Aston-Jones and Cohen, 2005). In sum, the arousal hypothesis predicts that arousing affective stimuli, irrespective of their valence, affect cognitive control by strengthening the components of a particular task-set, leading to a decreased cognitive flexibility.
A third more cognitive mechanism for the interplay between emotional and cognitive control is related to the competition between both systems for the limited attentional resources (Schimmack, 2005). In line with this so-called attention hypothesis, it has been shown that arousal related to an affective picture makes it harder to withhold a pre-potent response in a stop-signal task (Verbruggen and De Houwer, 2007) and on no-go trials in a go/no-go task (De Houwer and Tibboel, 2010). In sum, this hypothesis entails that affective information interferes strongly with behavior that asks for cognitive control, such as processes responsible for withholding a pre-potent response, because arousal induced by affective information taxes cognitive resources.
The three aforementioned hypotheses for the interplay between cognitive and emotional control were investigated within the task-switching paradigm, which offers a lab-analog for cognitive flexibility. Task-switching is a well-established tool for studying cognitive control in a setting in which participants are frequently imposed to switch from one task to another (for reviews see Monsell, 2003; Kiesel et al., 2010; Vandierendonck et al., 2010). A typical finding is that switching tasks elicits a switch cost, which is indicated by longer RTs and more errors on task switches than on task repetitions. The switch cost is considered as an index of processes that cope with the reconfiguration of the cognitive system from one task to another but also with the interference this brings along (e.g., Allport et al., 1994; Rogers and Monsell, 1995; Meiran, 1996, 2008; Mayr and Kliegl, 2000; Waszak et al., 2003). In the present study we favored to use the voluntary task-switching (VTS) procedure over more traditional task-switching procedures for three reasons. First, traditional task-switching procedures only have a limited ecological validity because they impose tasks to the participants, resulting in a rather artificial situation (see Vandierendonck et al., 2010). We argue that VTS offers are more complete view of cognitive control, since participants can make free task choices. Second, recent studies have shown that switch costs in VTS are more likely to reflect cognitive control than switch costs observed in traditional task-switching procedures (e.g., Liefooghe et al., 2009, 2010; but see Yeung, 2010). Third, some studies have found evidence that the selection component and the execution component in VTS are underlain by distinct sets of processes and are taxing different sets of control processes (see also Arrington and Yates, 2009; Butler et al., 2011). Thus, besides a switch cost, this procedure also offers an additional index of choice behavior and thus of cognitive flexibility. Typically, participants prefer repeating tasks. This phenomenon is called the task-repetition bias and is thought to result from a difficulty to disengage from a previously executed task (Demanet et al., 2010; Vandamme et al., 2010).
In order to investigate the immediate influence of valence and arousal on VTS, in the present study, affective pictures were presented. Three types of pictures were used: (a) pictures with a positive valence and high arousal; (b) pictures with a neutral valance and low arousal; and (c) pictures with a negative valence and high arousal. These pictures were task-irrelevant and were presented within the interval separating two consecutive VTS trials. This procedure allowed us to investigate the influence of these pictures on consecutive behavior.
Based on the valence hypothesis one could predict that information with a positive valence influences the ability to switch tasks. On the basis of this hypothesis, we predict that positive information will enhance cognitive flexibility (e.g., Isen and Daubman, 1984; Greene and Noice, 1988; Isen et al., 1992; Kuhl and Kazén, 1999; Bolte et al., 2003; Dreisbach and Goschke, 2004; Dreisbach, 2006), possibly by increased levels of DA in the striatum (Aarts et al., 2011), resulting not only in improved global performance but also in an improvement of the ability to switch tasks. In addition, because the size of the task-repetition bias is inversely related to the efficiency of cognitive control to disengage from a previously executed task (e.g., Mayr and Bell, 2006; Demanet et al., 2010), this account predicts that the repetition bias will be smaller following positive affective pictures.
According to the arousal hypothesis one would predict that arousing stimuli strengthen the activated task-sets, irrespective to the valence of these stimuli (e.g., Aston-Jones and Cohen, 2005; Verguts and Notebaert, 2009). Consequently, a strengthened task-set should lead to more facilitation on task repetitions and more interference on task switches, resulting in an increased switch cost (Wylie and Allport, 2000; Yeung and Monsell, 2003). With respect to the task-repetition bias, this hypothesis may imply that because task-sets have been strengthened by arousal, their higher activation may encourage participants to re-select the previously executed task. In other words, a higher task-repetition bias is expected.
According to the attention hypothesis (Schimmack, 2005; Verbruggen and De Houwer, 2007; De Houwer and Tibboel, 2010), it is predicted that arousing stimuli will occupy the cognitive resources needed to switch tasks. As a result, this will lead to higher switch costs. In contrast to the arousal hypothesis, the attention hypothesis predicts that the higher switch cost following arousing pictures will only be caused by slower task switches and not by faster task repetitions. According to the attention hypothesis, all behavior, thus even behavior that requires less attentional resources, such as during task repetitions, will be impaired following arousing pictures, although to a smaller extent than during task switches (e.g., De Houwer and Tibboel, 2010). Therefore, this hypothesis entails that, next to a higher switch cost and task-repetition bias, arousal will lead to an impairment of global performance, which should for instance be reflected in a global increase of reaction times and error rates following arousing pictures.
The three hypotheses seem to suggest that manipulations that affect the size of the switch cost also automatically affect the size of the task-repetition bias. Although evidence was reported that both are related (e.g., Mayr and Bell, 2006), recent studies have shown that the underlying processes are not identical (Arrington and Yates, 2009; Butler et al., 2011) and thus may measure different aspects of cognitive control. Therefore it is difficult to predict whether both will be influenced in a similar way by the affective information.
Sixty students of Ghent University participated for course requirements. Sixteen were excluded from analysis because they exceeded the threshold of 80% task repetitions (for a similar cut-off procedure see Arrington and Logan, 2004, 2005). All 44 participants (23 females) had normal or corrected-to-normal vision and were naïve to the purpose of the experiment.
The target stimuli were the symbols “#” and “%” presented in the colors purple and green. Participants either categorized the identity of the symbol (symbol task) or the color (color task) of the symbol. Participants performed both tasks manually and responded on a QWERTY keyboard. Both tasks were assigned to a different hand. The symbol task was performed with the left hand with the response keys “D” for symbol “#” and “F” for symbol “%.” The color task was performed with the right hand with the response keys “J” for purple symbols and “K” for green symbols. Fifteen pictures (448/336 pixels) were selected from the IAPS. Because previous studies found sex differences in the ratings for the IAPS pictures (e.g., Bradley et al., 2001), this was done for male and female participants separately. These pictures were categorized in three conditions of five pictures (Table A1 in Appendix): positive, neutral and negative. As shown in Table A1 in Appendix the positive and negative pictures were matched in function of arousal. The mean arousal score was higher for positive and negative pictures than for neutral pictures. Important to mention is that in order to match the arousal level of the positive pictures with the level of the negative pictures we had to include erotic pictures for the male subjects. This was not the case for female subjects.
Participants were tested individually by means of a Pentium III personal computer with a 17-inch color monitor running Tscope (Stevens et al., 2006). Instructions were presented on screen and paraphrased if necessary. The instructions stated that participants were free to select which task to perform on each trial, as long as they performed each task an approximate equal number of times and the pattern of task choices was not predictable. In order to explain unpredictability, we translated the coin-flipping metaphor of Arrington and Logan’s (2004, 2005) studies into Dutch. This metaphor entails that subjects sometimes will have to repeat the same task and sometimes have to switch between tasks and that they have to choose the tasks as if flipping the coin has decided which task to perform.
The experiment started with a practice block of 60 trials, followed by 10 test blocks of 60 trials. There was a short break of approximately 30 s following each block. On each trial, a task-irrelevant affective picture was presented for 900 ms and disappeared. The picture type (positive, negative, neutral) varied from trial to trial and followed an unpredictable pattern. In order to investigate the immediate influence of valence and arousal on VTS, we presented the task-irrelevant affective pictures at the beginning of each trial. Subsequently, a neutral target stimulus was presented in the center of a black screen in ARIAL font, size 108. When a response was given or a maximum presentation time of 6000 ms had elapsed, the target stimulus disappeared. For incorrect responses the word “FOUT” (error) was presented for 1000 ms before the ITI of 100 ms started (for a schematic overview see Figure 1).
First, trials were categorized according to the task that was chosen on the basis of the response hands. Next, trials were classified as a task repetition or a task switch. First trials of a block, trials following an error and trials with RTs shorter than 50 ms were excluded from analysis (data loss: 13.3%). Because we were interested in immediate effects of valence and arousal on the ability to switch tasks, we wanted to avoid that our measures were contaminated by the influence of the affective picture presented on the previous trial. Therefore, we focused on trials following trials in which a neutral picture was presented. By comparing task choice and task performance on positive pictures, neutral, and negative pictures, we tested for the influence of valence, and by comparing positive and negative pictures with neutral pictures we tested for the influence of arousal.
RTs and error rates were subjected to a 2 (task transition: task-repetition or task switch) by 3 (trial type: positive, neutral, or negative) repeated measures ANOVA with an alpha-level of 0.05. RTs and accuracies of each cell of the design are shown in Figures 2 and 3 respectively. For RTs, the main effect of task transition was significant, F(1,43) = 60.11, MSE = 19031, ηp2 = 0.58, indicating that RTs were higher on task switches (M = 767 ms, SE = 28) than on task repetitions (M = 635 ms, SE = 26). The main effect of trial type was significant, F(2,86) = 2.57, MSE = 4904, p = 0.08, ηp2 = 0.06. Planned comparisons showed that RTs on positive trials (M = 689 ms, SE = 23) were faster than on negative trials (M = 713 ms, SE = 28), F(1,43) = 5.17, MSE = 4871, ηp2 = 0.11, but did not differ from neutral trials (M = 700 ms, SE = 28), F(1,43) = 1.14, MSE = 4849, ηp2 = 0.03. Also the RTs on neutral trials did not differ reliably from the RTs on negative trials, F(1,43) = 1.42, MSE = 4992, ηp2 = 0.03. The difference between positive and negative trials indicates that positive valence facilitates general task performance. The interaction between task transition and trial type was significant1, F(2,86) = 3.12, MSE = 4402, ηp2 = 0.07 (see Figure 2). Planned comparisons showed that the switch cost was larger on positive (139 ms) than on neutral trials (104 ms), F(1,43) = 2.85, MSE = 4927, p < 0.10, ηp2 = 0.06, but this difference was only marginally significant. Also on negative trials (152 ms) the switch cost was higher than on neutral trials, F(1,43) = 5.48, MSE = 4646, ηp2 = 0.11. There was no difference between switch costs on positive and negative trials, F < 1, indicating that the valence of the affective picture did not affect the switch cost. In order to investigate the effect of arousal on the switch cost we collapsed both high arousal conditions (positive and negative trials) and compared the mean RTs with the mean RT of the low arousal condition (neutral trials). As the individual contrasts already suggested, this analysis showed that the switch cost was significantly larger with high-arousing pictures than with low-arousing pictures, F(1,43) = 4.98, MSE = 5170, ηp2 = 0.10. Additional analyses showed that this higher switch cost was primarily caused by faster task repetitions, F(1,43) = 4.33, MSE = 2768, ηp2 = 0.09, and not by slower task switches following arousing pictures, F(1,43) = 1.88, MSE = 7340, p = 0.18, ηp2 = 0.04.
On the error rates we found that participants made more errors on task switches (M = 0.07, SE = 0.008) than on task repetitions (M = 0.05, SE = 0.004), F(1,43) = 14.59, MSE = 0.0025, ηp2 = 0.25. The main effect of trial type was not significant, F(2,86) = 1.54, MSE = 0.0020, p = 0.22, ηp2 = 0.03. The interaction between task transition and trial type was marginally significant, F(2,86) = 2.55, MSE = 0.0020, p = 0.08, ηp2 = 0.06 (see Figure 3). Planned comparisons showed that the switch cost was marginally significantly larger on positive (0.027) than on neutral trials (0.016), F(1,43) = 2.95, MSE = 0.0017, p < 0.10, ηp2 = 0.06. The switch cost on negative trials (0.047) was also higher than on neutral trials, F(1,43) = 5.17, MSE = 0.0020, ηp2 = 0.11. No difference in switch cost was observed between the positive and negative trials, F < 1, again suggesting that the valence of the affective picture did not affect the switch cost. After collapsing positive and negative high-arousing pictures, an additional analysis showed that the switch cost was higher with high-arousing than with neutral pictures, F(1,43) = 6.40, MSE = 0.002, ηp2 = 0.13. Additional analyses showed that this higher switch cost was caused by more errors during task switches, F(1,43) = 6.88, MSE = 0.002, ηp2 = 0.09, and not by fewer errors during task-repetitions, F < 1. In order to differentiate between the arousal and the attention hypothesis it is important to mention that global performance, both on RTs and error rates, was never impaired following high-arousing pictures compared to neutral low-arousing pictures.
In addition, the task choices were analyzed. For each trial type, the proportion of task repetitions and switches was calculated. Because the proportion of repetitions and switches are complementary, namely p(switches) = 1-p(repetitions), we only focused on the proportion of task repetitions. On these proportions we conducted a repeated measures ANOVA with trial type (positive, neutral, or negative) as single factor. The main effect of trial type was not significant, F(2,86) = 1.40, MSE = 0.0035, ηp2 = 0.03, indicating that the proportion of task repetitions did not differ between positive (M = 0.603, SE = 0.020), neutral (M = 0.595, SE = 0.020), and negative trials (M = 0.616, SE = 0.018). After collapsing positive and negative high-arousing pictures, an additional analysis showed that the task-repetition bias did not differ between high-arousing and neutral pictures, F(1,43) = 1.70, MSE = 0.004, ηp2 = 0.04.
In the present study we focused on the relation between emotional and cognitive control by investigating the influence of the valence and arousal of task-irrelevant pictures on cognitive flexibility during VTS. It was found that arousal specifically affected the switch cost and that valence affected global performance but did not affect the switch cost. The preference to repeat or switch was not affected by the arousal neither by the valence of the affective pictures.
The results support the arousal hypothesis as it showed that the switch cost increased following arousing stimuli. Both findings of a larger switch cost caused by facilitated task repetitions, as found for the response latencies, and a larger switch cost caused by impaired task switches, as found for the error rates, are in line with the predictions of the arousal hypothesis (Verguts and Notebaert, 2009). According to Verguts and Notebaert (2009) high-arousing pictures lead to larger switch costs because the stimulus-response associations related to the previously executed task-set become strengthened through increased Hebbian learning. As such, switching toward the alternative task becomes more difficult, while repeating the same task is facilitated (see also Figure 4 for a schematic presentation). The pattern of results of the present study is remarkably similar to the findings of Braem et al. (2011) that showed that switch costs increased following conflict trials. The present finding that arousal induced by affective pictures can have a similar influence on the switch cost can be considered as indirect support for the hypothesis of Braem et al. (2011) stating that reductions in switch costs following conflict trials are related to the level of arousal triggered by the experience of a response conflict, facilitating task repetitions, and impairing task switches.
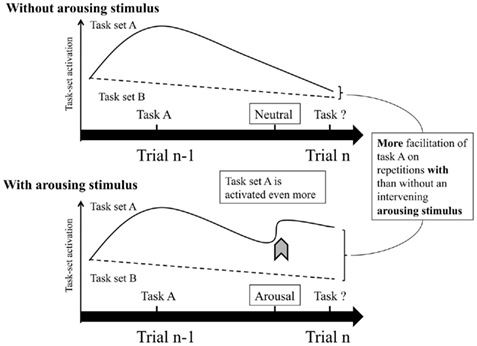
Figure 4. Scheme of the application of the noradrenalin account of Verguts and Notebaert (2009) on the switch cost in VTS.
At first sight, the finding that the switch cost was higher following arousing stimuli also seems to be in line with the attention hypothesis as proposed by Schimmack (2005) and De Houwer and colleagues (Verbruggen and De Houwer, 2007; De Houwer and Tibboel, 2010). These authors argued that arousal interferes with cognitively controlled behaviour, because arousal occupies the necessary cognitive resources. In the context of task switching this hypothesis entails that processes such as task-reconfiguration processes or processes necessary for interference control, which are especially important on task switches, suffer from a lack of available attentional resources caused by the arousing stimulus. As a consequence this hypothesis predicts that especially the ability to switch tasks should suffer, leading to higher switch costs. In addition, this hypothesis entails that performance on task repetitions also should suffer because during task repetitions in a task-switching context, cognitive control is also important, although not as important as during task switches (e.g., Braver et al., 2003). However, the results showed that task repetitions following high-arousing pictures were never impaired, not on the response latencies and error rates. The results even showed that the higher switch costs observed on the response latencies were driven mainly by facilitated task repetitions. Both these findings cannot be accounted for by the attention hypothesis, since this hypothesis only predicts impaired performance in cognitively demanding situations (e.g., De Houwer and Tibboel, 2010). It is important to mention that, although the present data support the arousal hypothesis, we do not state that competition for attentional resources did not take place, since this effect is widely accepted and replicated in a large amount of studies (e.g., Fox et al., 2001; Schimmack, 2005; Wyble et al., 2008). We simply argue that the attention hypothesis cannot account for the observed facilitation on task repetitions following arousing pictures and that an additional mechanism, possibly related to the LC–NA system, also played an important role in the observed interaction between emotional and cognitive control.
In addition, we found that pictures with a positive valence improved global performance, but we did not observe that valence had an influence on the ability to switch tasks, both on the switch cost and the task-repetition bias. As already mentioned in the introduction, this finding does not correspond with a large amount of studies in which was reported that positive information affects cognitive flexibility (e.g., Isen and Daubman, 1984; Greene and Noice, 1988; Isen et al., 1992; Kuhl and Kazén, 1999; Bolte et al., 2003; Dreisbach and Goschke, 2004; Dreisbach, 2006). However, we believe it is difficult to compare the findings of these studies directly with the results of the present study, since in most of these studies the effects of arousal were not controlled for. In addition, as Cools et al. (2001) already pointed out, the effects of DA modulations also strongly depend on the task demands. In fact, most effects of valence have been reported in studies in which subjects were not asked to switch between tasks and cognitive flexibility was measured with different paradigms. In the single study that was designed to dissociate the effects of valence and arousal of affective information on the ability to switch tasks, performed by Dreisbach and Goschke (2004), was found that subjects were more able to adapt to a task switch when positive information was presented. However, the procedure used in that study differed strongly from the currently used procedure in three important aspects. First, they used the so-called intermittent instruction procedure, in which subjects had no free task choice and tasks were only switched occasionally (once in each block). Second, they did not investigate the impact of affective pictures on the switch cost directly, but they investigated the difference in performing the five tasks before a task switch and the five tasks following a task switch, which was indicated by a task cue. Therefore, we think it is possible that the study of Dreisbach and Goschke (2004) taps on a different component of cognitive control, not on the ability to switch tasks from trial to trial, but on the ability to adapt to changing task demands over a longer period of time. We found indirect evidence for this explanation by showing that the general performance improved following positive than negative affective pictures. In addition, this finding suggests that the valence of an affective picture affects multitasking ability and cognitive control on a more general level, possibly by phasic modulations of DA (Ashby et al., 1999; Cools et al., 2009). Third, in comparison with the present study, Dreisbach and Goschke (2004) did not mix positive and negative trials with neutral trials in a single block. This difference could have caused the failure in the present study to find an influence of valence, since it is possible that mixing positive and negative affect cancels out the short-term effects of valence. In sum, the inconsistencies between findings in the study of Dreisbach and Goschke (2004) and the present study concerning the effects of emotional valence suggest that more research is necessary in order to capture the critical conditions in which emotional valence has an influence on the ability to adapt flexibly to a changing environment.
The influence of arousal on the switch cost, and the hypothesis that this effect is caused by task-set strengthening, converges with the more recent assumption that the switch cost is mainly related to interference control that is needed to cope with persisting task-set activation (Wylie and Allport, 2000; Yeung and Monsell, 2003), and not by switch-specific task-reconfiguration processes (e.g., Rogers and Monsell, 1995). Interestingly, we found that the switch cost but not the proportion of task repetitions varied with arousal. In convergence with recent research on VTS, this suggests that the selection component and the execution component in VTS are underlain by distinct sets of processes and are tapping on different aspects of cognitive control (see also Arrington and Yates, 2009; Butler et al., 2011). More importantly, this finding suggests an important feature of the interplay between emotional and cognitive control, namely that not every aspect of cognitive control is necessarily influenced by arousal. More precisely, it seems that arousal only affects those aspects of cognitive control related to behavioral stability and reduced distractibility, such as processes responsible for interference control. Arousal helps avoiding interference of irrelevant task-sets by strengthening the currently relevant task-set. This hypothesis can be related to a VTS study of Butler et al. (2011) where it was observed that individual differences in working-memory capacity affected task performance but not task choice in VTS. Based on the finding that a key feature of working-memory capacity is the ability to cope with interference of irrelevant information (Kane et al., 2001), it thus seems that task performance is affected by arousal because it comprises interference control, while task choice does not.
In conclusion, the present study was the first to investigate the impact of arousal and valence of a task-irrelevant stimulus on the ability to switch tasks voluntarily. We found that presenting affective pictures affected the ability to switch between tasks. The data showed that arousal related to an affective stimulus made it more difficult to switch between tasks. This result is in line with the binding account of Verguts and Notebaert (2009) that states that phasic modulation of NA plays an important role in the functional overlap between emotional and cognitive control because an arousing signal triggers the noradrenergic system that reinforces the previously executed task. In addition, we observed that presenting positive pictures only improved global performance but did not affect the ability to switch between tasks compared to negative pictures. This finding suggests that positive valence affects general multitasking performance but does not have an immediate influence on the efficiency of the processes that are necessary to switch between tasks from trial to trial.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank Senne Braem for valuable discussions about this research project.
Aarts, E., van Holstein, M., and Cools, R. (2011). Striatal dopamine and the interface between motivation and cognition. Front. Psychol. 2, 163. doi: 10.3389/fpsyg.2011.00163
Allport, A., Styles, E. A., and Hsieh, S. (1994). “Shifting intentional set: Exploring the dynamic control of tasks,” in Attention and performance XV, eds C. Umilta, and M. Moscovitch (Cambridge, MA: MIT Press), 421–452.
Arrington, C. M., and Logan, G. D. (2004). The cost of a voluntary task switch. Psychol. Sci. 15, 610–615.
Arrington, C. M., and Logan, G. D. (2005). Voluntary task switching: Chasing the elusive homunculus. J. Exp. Psychol. Learn. Mem. Cogn. 31, 683–702.
Arrington, C. M., and Yates, M. M. (2009). The role of attentional networks in voluntary task switching. Psychon. Bull. Rev. 16, 660–665.
Ashby, F. G., Isen, A. M., and Turken, U. (1999). A neuropsychological theory of positive affect and its influence on cognition. Psychol. Rev. 106, 529–550.
Ashby, F. G., Valentin, V. V., and Turken, A. U. (2002). “The effects of positive affect and arousal on working memory and executive attention: neurobiology and computational models,” in Emotional Cognition: From Brain to Behavior, eds S. Moore, and M. Oaksford (Amsterdam: John Benjamins), 245–287.
Aston-Jones, G., and Cohen, J. D. (2005). An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci. 28, 403–450.
Bolte, A., Goschke, T., and Kuhl, J. (2003). Emotion and intuition: effects of positive and negative mood on implicit judgments of semantic coherence. Psychol. Sci. 14, 416–421.
Bradley, M. M., Codispoti, M., Sabatinelli, D., and Lang, P. J. (2001). Emotion and motivation: II. Sex differences in picture processing. Emotion 1, 300–319.
Braem, S., Verguts, T., and Notebaert, W. (2011). Conflict adaptation by means of associative learning. J. Exp. Psychol. Hum. Percept. Perform. 37, 1662–1666.
Braver, T. S., Barch, D. M., and Cohen, J. D. (1999). Cognition and control in schizophrenia: a computational model of dopamine and prefrontal function. Biol. Psychiatry 46, 312–328.
Braver, T. S., and Cohen, J. D. (2000). “On the control of control: the role of dopamine in regulating prefrontal function and working memory,” in Attention and Performance XVIII: Control of cognitive processes, eds S. Monsell, and J. Driver (Cambridge, MA: MIT Press), 713–737.
Braver, T. S., Reynolds, J. R., and Donaldson, D. I. (2003). Transient and sustained cognitive control during task switching. Neuron 39, 713–726.
Bush, G., Luu, P., and Posner, M. I. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. (Regul. Ed.) 4, 215–222.
Butler, K. M., Arrington, C. M., and Weywadt, C. (2011). Working memory capacity modulates task performance but has little influence on task choice. Mem. Cognit. 39, 708–724.
Cohen, J. D., Braver, T. S., and Brown, J. W. (2002). Computational perspectives on dopamine function in prefrontal cortex. Curr. Opin. Neurobiol. 12, 223.
Cools, R., Barker, R. A., Sahakian, B. J., and Robbins, T. W. (2001). Enhanced or impaired cognitive function in Parkinson’s disease as a function of dopaminergic medication and task demands. Cereb. Cortex 11, 1136–1143.
Cools, R., Frank, M. F., Gibbs, S. E., Miyakawa, A., Jagust, W., and D’Esposito, M. (2009). Striatal dopamine predicts outcome-specific reversal learning and its sensitivity to dopaminergic drug administration. J. Neurosci. 29, 1538–1543.
Cools, R., and Robbins, T. W. (2004). Chemistry of the adaptive mind. Philos. Transact. A Math. Phys. Eng. Sci. 362, 2871–2888.
Cools, R., Sheridan, M., Jacobs, E., and D’Esposito, M. (2007). Impulsive personality predicts dopamine-dependent changes in frontostriatal activity during component processes of working memory. J. Neurosci. 27, 5506–5514.
De Houwer, J., and Tibboel, H. (2010). Stop what you are not doing! Emotional pictures interfere with the task not to respond. Psychon. Bull. Rev. 17, 699–703.
Demanet, J., Verbruggen, F., Liefooghe, B., and Vandierendonck, A. (2010). Voluntary task switching under load: contribution of top-down and bottom-up factors in goal-directed behavior. Psychon. Bull. Rev. 17, 387–393.
Dreisbach, G. (2006). How positive affect modulates cognitive control: the costs and benefits of reduced maintenance capability. Brain Cogn. 60, 11–19.
Dreisbach, G., and Goschke, T. (2004). How positive affect modulates cognitive control: reduced perseveration at the cost of increased distractibility. J. Exp. Psychol. Learn. Mem. Cogn. 30, 343–353.
Fox, E., Russo, R., Bowles, R. J., and Dutton, K. (2001). Do threatening stimuli draw or hold visual attention in sub-clinical anxiety? J. Exp. Psychol. Gen. 130, 681–700.
Frank, M. J., Seeberger, L., and O’Reilly, R. C. (2004). By carrot or by stick: cognitive reinforcement learning in Parkinsonism. Science 306, 1940–1943.
Gratton, G., Coles, M. G. H., and Donchin, E. (1992). Optimizing the use of information: strategic control of activation and responses. J. Exp. Psychol. Gen. 121, 480–506.
Gray, J. R. (1999). A bias toward short-term thinking in threat-related negative emotional states. Pers. Soc. Psychol. Bull. 25, 65–75.
Gray, J. R., Braver, T. S., and Raichle, M. E. (2002). Integration of emotion and cognition in the lateral prefrontal cortex. Proc. Natl. Acad. Sci. U.S.A. 99, 4115–4120.
Greene, T. R., and Noice, H. (1988). Influence of positive affect upon creative thinking and problem solving in children. Psychol. Rep. 63, 895–898.
Gross, J. J. (2002). Emotion regulation: affective, cognitive, and social consequences. Psychophysiology 39, 281–291.
Hommel, B. (2004). Event files: feature binding in and across perception and action. Trends Cogn. Sci. (Regul. Ed.) 8, 494–500.
Isen, A. M., and Daubman, K. A. (1984). The influence of affect on categorization. J. Pers. Soc. Psychol. 47, 1206–1217.
Isen, A. M., Niedenthal, P., and Cantor, N. (1992). The influence of positive affect on social categorization. Motiv. Emot. 16, 65–78.
Kane, M. J., Bleckley, M. K., Conway, A. R. A., and Engle, R. W. (2001). A controlled-attention view of working-memory capacity. J. Exp. Psychol. Gen. 130, 169–183.
Kiesel, A., Steinhauser, M., Wendt, M., Falkenstein, M., Jost, K., Phillip, A., and Koch, I. (2010). Control and interference in task switching – a review. Psychol. Bull. 136, 849–874.
Kuhl, J., and Kazén, M. (1999). Volitional facilitation of difficult intentions: joint activation of intention memory and positive affect removes Stroop interference. J. Exp. Psychol. Gen. 128, 382–399.
Liefooghe, B., Demanet, J., and Vandierendonck, A. (2009). Is advance reconfiguration in voluntary task switching affected by the design employed? Q. J. Exp. Psychol. 65, 850–857.
Liefooghe, B., Demanet, J., and Vandierendonck, A. (2010). Persisting activation in voluntary task switching: it all depends on the instructions. Psychon. Bull. Rev. 17, 381–386.
Maia, T. V., and Frank, M. J. (2011). From reinforcement learning models to psychiatric and neurological disorders. Nat. Neurosci. 14, 154–162.
Mayr, U., and Bell, T. (2006). On how to be unpredictable: evidence from the voluntary task-switching paradigm. Psychol. Sci. 9, 774–780.
Mayr, U., and Kliegl, R. (2000). Task-set Switching and Long-Term Memory Retrieval. J. Exp. Psychol. Learn. Mem. Cogn. 26, 1124–1140.
Meiran, N. (1996). Reconfiguration of processing mode prior to task performance. J. Exp. Psychol. Learn. Mem. Cogn. 22, 1423–1442.
Meiran, N. (2008). The dual implication of dual affordance: stimulus-task binding and attentional focus changing during task preparation. Exp. Psychol. 55, 251–259.
Pessoa, L. (2009). How do emotion and motivation direct executive control. Trends Cogn. Sci. 13, 160–166.
Rogers, R. D., and Monsell, S. (1995). Costs of a predictable switch between simple cognitive tasks. J. Exp. Psychol. Gen. 124, 207–231.
Savine, A. C., and Braver, T. S. (2010). Motivated cognitive control: reward incentives modulate preperatory neural activity during task-switching. J. Neurosci. 30, 294–305.
Schimmack, U. (2005). Response latencies of pleasure and displeasure ratings: further evidence for mixed feelings. Cogn. Emot. 19, 671–691.
Stevens, M., Lammertyn, J., Verbruggen, F., and Vandierendonck, A. (2006). Tscope: a C library for programming cognitive experiments on the MS Windows platform. Behav. Res. Methods 38, 280–286.
Vandamme, K., Szmalec, A., Liefooghe, B., and Vandierendonck, A. (2010). Are voluntary switches corrected repetitions? Psychophysiology 47, 1176–1181.
Vandierendonck, A., Liefooghe, B., and Verbruggen, F. (2010). Task switching: interplay of reconfiguration and interference. Psychol. Bull. 136, 601–626.
Verbruggen, F., and De Houwer, J. (2007). Do emotional stimuli interfere with response inhibition? Evidence from the stop signal paradigm. Cogn. Emot. 21, 391–403.
Verguts, T., and Notebaert, W. (2009). Adaptation by binding: a learning account of cognitive control. Trends Cogn. Sci. (Regul. Ed.) 13, 252–257.
Waszak, F., Hommel, B., and Allport, A. (2003). Task switching and long-term priming: role of episodic S-R bindings in task-shift costs. Cogn. Psychol. 46, 361–413.
Wyble, B., Sharma, D., and Bowman, H. (2008). Strategic regulation of cognitive control by emotional salience, a neural network model. Cogn. Emot. 22, 1019–1051.
Wylie, G., and Allport, A. (2000). Task switching and the measurement of “switch costs.” Psychol. Res. 63, 212–233.
Yeung, N. (2010). Bottom-up influences on voluntary task switching: the elusive homunculus escapes. J. Exp. Psychol. Learn. Mem. Cogn. 36, 348–362.
Keywords: task-switching, voluntary task-switching, emotional control, cognitive control, affective stimuli, IAPS
Citation: Demanet J, Liefooghe B and Verbruggen F (2011) Valence, arousal, and cognitive control: a voluntary task-switching study. Front. Psychology 2:336. doi: 10.3389/fpsyg.2011.00336
Received: 01 July 2011; Paper pending published: 31 July 2011;
Accepted: 31 October 2011; Published online: 24 November 2011.
Edited by:
Wim Notebaert, Ghent University, BelgiumReviewed by:
Guido P. H. Band, Leiden University, NetherlandsCopyright: © 2011 Demanet, Liefooghe and Verbruggen. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Jelle Demanet, Department of Experimental Psychology, Ghent University, Henri-Dunantlaan 2, Ghent 9000, Belgium. e-mail:amVsbGUuZGVtYW5ldEB1Z2VudC5iZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.