- 1 Cognitive Psychology Unit, and Leiden Institute for Brain and Cognition, Leiden University, Leiden, Netherlands
- 2 Department of Psychology, Granada University, Granada, Spain
- 3 Amsterdam Center for the Study of Adaptive Control in Brain and Behaviour, Department of Psychology, University of Amsterdam, Amsterdam, Netherlands
So far no studies have systematically looked into the cognitive consequences of khat use. This study compared the ability to inhibit and execute behavioral responses in adult khat users and khat-free controls, matched in terms of age, race, gender distribution, level of intelligence, alcohol and cannabis consumption. Response inhibition and response execution were measured by a stop-signal paradigm. Results show that users and non-users are comparable in terms of response execution but users need significantly more time to inhibit responses to stop signals than non-users.
Introduction
Leaves from the flowering evergreen khat tree (Catha edulis) have been chewed in East-Africa since ancient times to alleviate fatigue, stay alert, reduce hunger, and induce euphoria. Khat consumption has increased during the last decades in Eastern Africa and has become a global phenomenon spreading to ethnic communities all over the world, such as in The Netherlands, United Kingdom, Canada, or the United States (UNODC, World Drug Report, 2010). The airports of Amsterdam and London, where considerable amounts of khat arrive weekly, have become the European distribution points (Beckerleg, 2008; Pennings et al., 2008). In particular, in The Netherlands, it is legal to buy and sell khat bundles, whereas selling and possessing khat is illegal in other countries, such as Canada, the USA, France, Norway, or Poland.
The acute consumption of khat is associated with optimism, mild euphoria, excitation, talkativeness, increased energy, and enhanced self-esteem (Brenneisen et al., 1990; Kalix, 1996). The half-life is about 4 h, depending on the amount of chewed khat. When the acute effects vanish, users experience feelings of depletion, insomnia, numbness, depression, lack of energy, and mental fatigue. Chronic (i.e., daily) use of khat is associated with increased blood pressure, development of gastrointestinal tract problems, cytotoxic effects on liver and kidneys, and keratotic lesions at the side of chewing (Al-Habori, 2005).
Cathine (Norpseudoephedrine) and cathinone (Benzoylethanamine), the active ingredients of khat, are similar in structure and pharmacological activity to amphetamines (Wagner et al., 1982). The two alkaloids act by increasing levels of dopamine (DA), serotonin and noradrenalin (Kalix and Braenden, 1985). For this reason khat is also called a “natural amphetamine.” Cathinone increases levels of DA in the brain by acting on catecholaminergic synapses, retarding DA reuptake and/or enhancing DA release (Wagner et al., 1982; Patel, 2000), in particular in the striatum (Zelger and Carlini, 1981).
Studies addressing the neurobiological mechanism underlying the use of khat are still lacking as well as studies that have systematically looked into the long-term cognitive effects of chronic khat use. Nevertheless, given the similarity of khat and amphetamine in structure and pharmacological activity, it makes sense to assume that the long-term use of khat affects the same neurotransmitter and brain structures as the chronic use of amphetamine (see Berman et al., 2008). At a structural level, one may thus expect white matter abnormalities, lower cortical gray matter volume, and higher striatal volume. In particular, higher striatal volumes might reflect a compensation for toxicity in the dopamine-rich basal ganglia. At a functional level, in turn, chronic khat use is likely to be associated with reduced functioning of dopamine D2 (DAD2) receptors in the striatum and dysfunctions in prefrontal cortex (PFC) and orbitofrontal cortex (OFC) – areas that have been shown to play major roles in the control of goal-directed action (Miller, 2000).
Interestingly, DA plays a key role in inhibitory action control (Colzato et al., 2009, 2010). Behavioral inhibitory efficiency is commonly assessed by means of the stop-signal task (Logan and Cowan, 1984). In this task, participants are first presented with a stimulus (i.e., a go signal) prompting them to execute a particular manual response, and this stimulus may or may not be followed by a stop-signal calling for the immediate abortion of that response. Based on the mathematical considerations formulated by Logan and Cowan (1984), the stop-signal paradigm provides a direct behavioral assessment of the individual ability to stop a planned or ongoing motor response in a voluntary fashion and a quantitative estimate of the duration of the covert response-inhibition process (i.e., stop-signal reaction time or SSRT; see Figure 1). Parkinson’s patients, who suffer from loss of dopaminergic neurons in the basal ganglia, showed longer SSRTs (Gauggel et al., 2004) and impaired suppression of conflicting responses (Wylie et al., 2009, 2010) compared to matched controls. Consistent with this pattern, spontaneous eyeblink rate (EBR), a marker of dopaminergic functioning, reliably predicts the efficiency in inhibiting unwanted action tendencies in a stop-signal task (see Logan, 1994; Colzato et al., 2009; for a review). Along the same lines, Colzato et al. (2007) observed that recreational users of cocaine, who are likely to suffer from reduced DAD2 receptors in the striatum (Volkow et al., 1999), need significantly more time to inhibit responses to stop signals than non-users. Very recently, Colzato et al. (2010) found that C957T polymorphism at the DRD2 gene (polymorphisms related to striatal DA) predict individual differences in the proficiency of stopping prepotent responses.
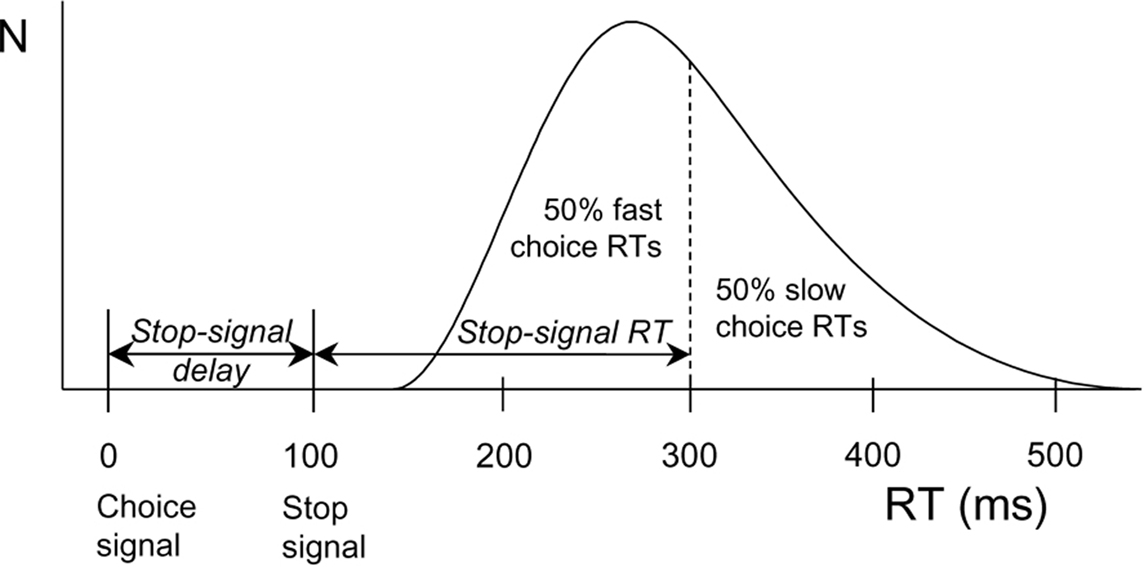
Figure 1. Calculation of stop-signal RT (SSRT) according to a race model. The curve depicts the distribution of RTs on go trials (trials without a stop signal) representing the finishing times of the response processes. Assuming independence of go and stop processes, the finishing time of the stop process bisects the go RT distribution. Given that the button-press response could be withheld in 50% of all stop trials, stop-signal RT (200 ms) is calculated by subtracting the mean stop-signal delay (100 ms) from the median go RT (300 ms).
In the present study we used the stop-signal task (Logan and Cowan, 1984) to test whether khat use produces deficiencies in inhibitory control. Given the above mentioned relation between DA and inhibitory action control on the one hand, and between DA and khat on the other, we expected impairments of inhibitory control in khat users.
Materials and Methods
Participants
Forty young healthy adults (36 men and 4 women) were compensated for their participation. They constituted the two groups of 20 khat users and 20 khat-free controls. The sample was drawn from 50 adults in the Leiden and The Hague metropolitan areas, who volunteered to participate in studies of behavioral pharmacology. Participants were recruited via ads posted on community bulletin boards and by word of mouth. We made sure that the users met the following criteria: (1) Khat consumption by chewing route for a minimum of 1 year; (2) no clinically significant medical disease and (3) no use of medication. All khat users met more than four of the seven criteria that according to the American Psychiatric Association (1994) DSM-IV and the World Health Organization (ICD-10) define addiction: tolerance, withdrawal, difficulty controlling the use, negative consequences for job, family, and health, significant time or emotional energy spent in searching/consuming the drug, put off or neglected activities because of the use, and desire to cut down the use. Khat-free controls were comparable but reported no history of past or current khat use.
Participants were asked to refrain from taking all psychoactive drugs for at least 24 h before the test, not to consume alcohol on the night before the experimental session, and to have a normal night rest. Participant’s compliance with the instruction was encouraged by taking a (no further analyzed) saliva sample test at the beginning of the session. In psychopharmacological research this deceptive method is often used and considered effective in studies addressing acute effects (Ridderinkhof et al., 2002; Colzato et al., 2004) and long-term effects of drugs (von Geusau et al., 2004; Colzato, 2009).
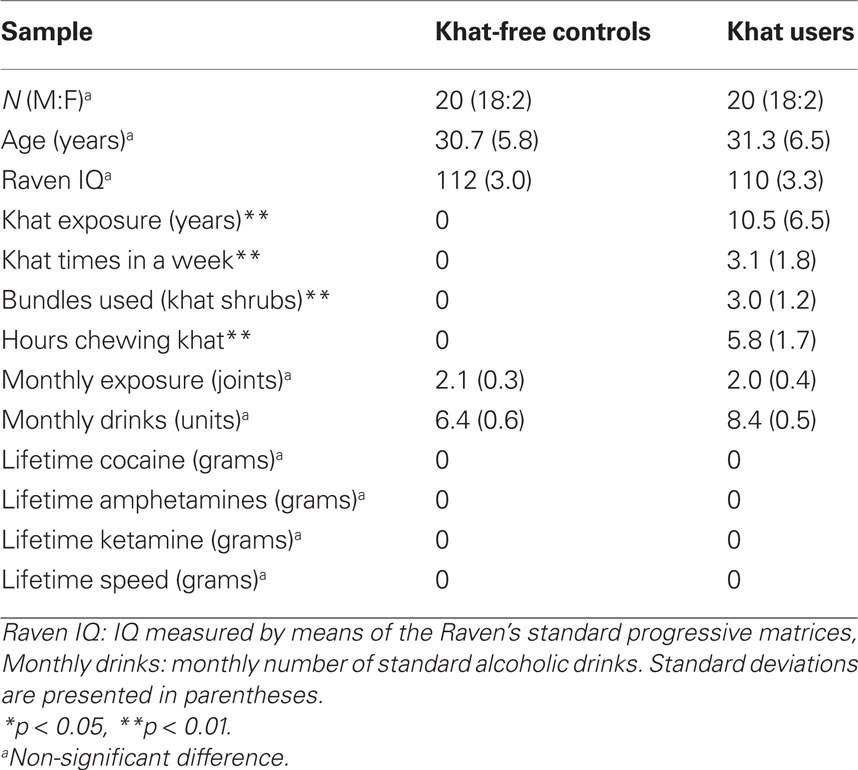
Participants in two groups were matched for ethnicity (100% African), age, sex, IQ (measured by Raven’s standard progressive matrices (SPM); Raven, 1998), and alcohol and cannabis consumption. Even if khat was the preferred drug of use for the participants, some of them drank alcohol on a weekly basis (7) and used cannabis on monthly basis (3). No participant reported to have ever used LSD, MDMA, cocaine, amphetamine, barbiturates, ketamine, GHB, or speed. Demographic and drug-use statistics are provided in Table 1. Written informed consent was obtained from all participants after the nature of the study was explained to them. The protocol and the remuneration arrangements of 25 Euro were approved by institutional review board (Leiden University, Institute for Psychological Research).
Apparatus and Stimuli
The experiment was controlled by a PC attached to a 17′ monitor (96 dpi with a refresh rate of 120 Hz). Responses were made by pressing the “Z” or “?” of the QWERTY computer keyboard with the left and right index finger, respectively. Participants were required to react quickly and accurately by pressing the left and right key in response to the direction of a left- or right-pointing green arrow (go trials) of about 3.5 × 2.0 cm with the corresponding index finger.
Procedure
All participants were tested individually. Participants completed the SPM (Raven, 1998) and performed the stop-signal task for about 30 min. Participants were allowed to take a short break (up to 5 min) between task blocks.
IQ
Individual IQs were determined by means of a 30-min reasoning-based intelligence test (SPM). The SPM assesses the individual’s ability to create perceptual relations and to reason by analogy independent of language and formal schooling; it is a standard, widely used test to measure Spearman’s g factor as well as fluid intelligence (Raven, 1998). Participants completed the SPM and subsequently performed on the behavioral task measuring inhibitory control.
Stop-Signal Task
The experimental session took about 30 min, in which participants completed a version of the stop-signal task adopted from Colzato et al. (2007), see also Colzato et al. (2009, 2010). Each trial began with the presentation of an arrow (pseudorandomly) pointing to the left or right (with a probability of 50% each). Arrows were presented pseudorandomly, with the constraint that they signaled left- and right-hand responses equally often. Arrow presentation was response-terminated. Intervals between subsequent go signals varied randomly but equiprobably, from 1250 to 1750 ms in steps of 125 ms. During these interstimulus intervals, a white fixation point (3 mm in diameter) was presented. The green arrow changed to red on 30% of the trials, upon which the choice response had to be aborted (stop trials). A staircase-tracking procedure dynamically adjusted the delay between the onset of the go signal and the onset of the stop signal to control inhibition probability (Levitt, 1971). After a successfully inhibited stop trial, stop-signal delay on the next stop trial increased by 50 ms, whereas the stop-signal delay decreased by 50 ms on the next stop trial when the participant was unable to stop. This algorithm ensured that motor actions were successfully inhibited on about half of the stop trials, which yields accurate estimates of SSRT and compensates for differences in choice RT between participants (see Band et al., 2003; Figure 1). The stop task consisted of five blocks of 104 trials each, the first of which served as a practice block to obtain stable performance.
Statistical Analysis
First, independent samples t-tests were performed to compare age, sex, and IQ across the two groups. Second, individual SSRTs for stop-signal trials were calculated to index response inhibition for all participants. SSRTs were analyzed separately by means of univariate ANOVAs with Group (khat users vs. khat-free controls) as between-subject factor. Third, to test whether the magnitude of inhibitory efficiency is proportional to the degree of exposure to khat, we computed Pearson correlation coefficients between the amount of khat consumed and SSRT. A significance level of p < 0.05 was adopted for all statistical tests.
Results
Participants
No significant group differences were obtained for age, t(38) = 0.306, p = 0.761, intelligence, t(38) = −0.973, p = 0.337, alcohol consumption, t(38) = 0.478, p = 0.521, or cannabis consumption, t(38) = 0.169, p = 1.00. Table 1 shows drug-use profiles for the two groups.
Stop-Signal Task
Analyses of mean RT to go signals showed that khat users (536 ms) tended to respond more slowly than khat-free controls (474 ms), but this difference was not significant, p > 0.055. The percentage of choice errors to go-signals was low and did not discriminate between khat users (1.8%) and khat-free users (1.0%, p > 0.30). SSRTs were computed for each participant and for each group separately. All participants were able to stop their responses successfully in about half of the stop trials (51.9% in khat users and 49.7% in khat-free controls), indicating that the dynamic tracking algorithm worked well in both groups. Most importantly, SSRTs were significantly longer for users (236 ms) than for non-users (192 ms), F(1,38) = 33.21, p < 0.001, MSE = 584.624, η2p = 0.47, see Figure 2. The group effect remained largely significant even after using RT to go signals as covariate, F(1,38) = 29.97, p < 0.001, MSE = 599.701, η2p = 0.44. To test whether the magnitude of cognitive impairments is proportional to the amount of khat consumed, we computed Pearson correlation coefficients between the individual lifetime khat exposure, hours chewing and number of bundles used in a khat session, and SSRT. No significant correlations were obtained, probably due to very little between-subject variability.
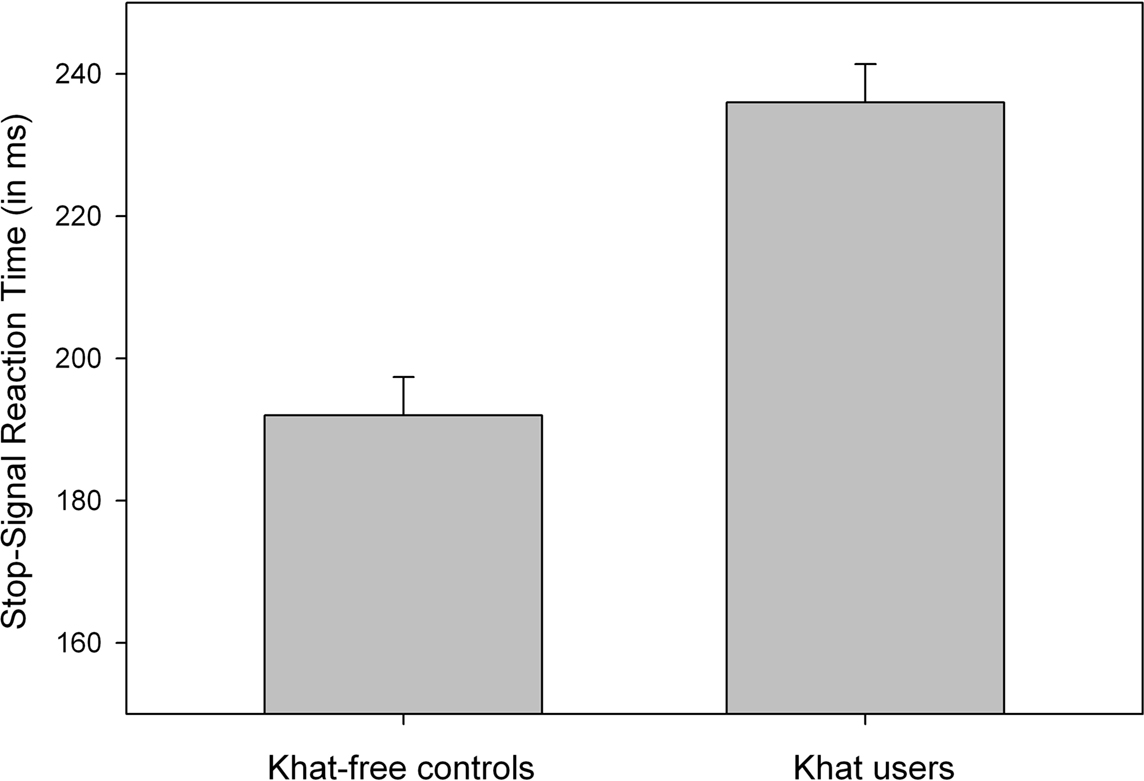
Figure 2. Mean SSRT (stopping latency) as a function of Group (khat-free controls vs. khat users). Vertical capped lines atop bars indicate standard error of the mean.
Discussion
This study tested, for the first time, whether chronic khat use is associated with a detectable selective impairment in the ability to inhibit responses. As expected, we found that khat users were roughly comparable to khat-free controls in Go RT but khat users showed significantly elevated SSRTs. In other words, chronic khat use is associated with impaired inhibitory control. We attribute this deficit to the possibility that, at long-term use, cathinone, the active ingredient of khat, is associated to dysfunctions in PFC and a reduced DA level in the striatum – the neurotransmitter that plays a crucial role in response inhibition (Gauggel et al., 2004; Colzato et al., 2007, 2009, 2010).
It may be important to note that the group difference in response execution was unreliable but approached the statistical significance level, suggesting that a larger sample might have rendered this effect reliable. On the one hand, this means that it would be premature to exclude a general performance deficit in chronic khat users until converging evidence is available. On the other hand, however, the staircase method that we used allowed separating SSRT from the general RT level, which ensures that the former cannot be explained on the basis of the latter. That is, the SSRT effect does indicate a specific impairment in inhibitory processes in khat users over and beyond a possible general performance deficit.
Even though the empirical connection between inhibitory control functions and khat use is considerable, the causal relation between the two may not be straightforward. Indeed, we cannot exclude that pre-existing neuro-developmental factors may play a mediating role and that khat users may suffer pre-existing problems in inhibitory control and impulsivity, as has been shown for cocaine users (Bechara, 2005; Verdejo-Garcia et al., 2008). Notwithstanding this caveat, the connection between inhibitory efficiency and khat use seems rather strong – the more so as a number of possible confounds were controlled for in this study: the khat users that participated in the current study were barely exposed to other drugs and the two groups were well matched in terms of age, IQ, sex, race, and alcohol and cannabis consumption. Especially the matching of age was essential: while inhibitory control seems not to be related to general intelligence, there is evidence that cognitive inhibitory processes decline in effectivity throughout the life span (Williams et al., 1999).
The present findings raise the question whether khat users, at long-term, also show impairments in other cognitive control functions, such as shifting between tasks and mental sets, and the updating and monitoring of working memory (Miyake et al., 2000). The direct effects of khat use on the brain need to be explored as well. It remains to be demonstrated, for instance, that the use of khat produces changes at the neuromodulatory (DA) and cortical level (decreased neural activity in the striatum and PFC), and that it interacts with genetic vulnerability and changes in the expression of genes. For instance, it would be informative to investigate whether, among khat users, there is also a genetic association with the Taq A1 of the DRD2 polymorphism as in the case of alcohol dependence (Blum et al., 1990) and cocaine addiction (Noble et al., 1993). This association would be useful as marker for a genetic vulnerability.
Of particular interest would be to look into the acute effects of khat. Previous research addressing the acute effect of other psychostimulant drugs – as cocaine – has shown a drug-induced facilitation of inhibitory control (Fillmore et al., 2006). Interestingly, in this study, stop-signal performance revealed a quadratic dose–response function suggesting that the improvement of inhibitory control is limited to a range of intermediate doses, while with lower and higher doses performance deficits are more likely. It also remains to be seen whether the long-term use of khat has similar effects and after-effects (after periods of abstinence) on receptor characteristics, DA sensitivity, etc., as have been observed with the long-term use of other psychostimulant drugs like cocaine (Kreek et al., 2005).
The findings of this study are rather worrying because, first, many real-life situations require the active inhibition of prepotent actions, as in the case of traffic lights turning red or of criminal actions. Consistent with this idea, the increasing number of traffic accidents in Eastern Africa countries has been related to khat chewing habits (Toennes and Kauert, 2004). Chronic khat use may indeed lead to a marked deterioration of cognitive functions (as inhibitory control) implicated in driving behavior.
Second, this impairment of inhibitory control has serious implications for personal or societal functioning. This reduced level of inhibitory control may even be involved in the emergence of addiction: the more a drug is used, the less able users are to prevent themselves from using it. In fact, socioeconomic and familial problems are common among khat consumers. Many men secure their daily portion of khat at the expense of vital needs, indicating dependence. Family life is harmed because of neglect, dissipation of family income and inappropriate behavior and in countries like Ethiopia or Kenya, khat-dependent people are the main group among persons treated for drug problems (UNODC, World Drug Report, 2010).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Lucy Chodota for her enthusiasm and invaluable assistance in recruiting the participants of this study. The research of Lorenza S. Colzato and Wery P. M. van den Wildenberg is supported by NWO (Netherlands Organization for Scientific Research). The research of Manuel J. Ruiz is supported by the Beca de Movilidad de Másters Oficiales del Ministerio de Educación y Ciencia The research of Maria Teresa Bajo is supported by grants EDU2008-01111 from the Ministry of Science/FEDER and P08-HUM-3600 (Junta de Andalucía).
References
Al-Habori, M. (2005). The potential adverse effects of habitual use of Catha edulis (khat). Expert Opin. Drug. Saf. 4, 1145–1154.
American Psychiatric Association. (1994). Diagnostic and Statistical Manual of Mental Disorders, 4th Edn. Washington, DC: American Psychiatric Association.
Band, G. P. H., van der Molen, M. W., and Logan, G. D. (2003). Horse-race model simulations of the stop-signal procedure. Acta Psychol. 112, 105–142.
Bechara, A. (2005). Decision making, impulse control and neurocognitive perspective. Nat. Neurosci. 8, 1458–1463.
Berman, S., O’Neill, J., Fears, S., Bartzokis, G., and London, E. D. (2008). Abuse of amphetamines and structural abnormalities in the brain. Ann. N. Y. Acad. Sci. 1141, 195–220.
Blum, K., Noble, E. P., Sheridan, P. J., Montgomery, A., Ritchie, T., Jagadeeswaran, P., Nogami, H., Briggs, A. H., and Cohn, J. B. (1990). Allelic association of human dopamine D2 receptor gene in alcoholism. J. Am. Med. Assoc. 263, 2055–2060.
Brenneisen, R., Fisch, H. U., Koelbing, U., Geisshüsler, S., and Kalix, P. (1990). Amphetamine-like effects in humans of the khat alkaloid cathinone. Br. J. Clin. Pharmacol. 30, 825–828.
Colzato, L. S., Erasmus, V., and Hommel, B. (2004). Moderate alcohol consumption in humans impairs feature binding in visual perception but not across perception and action. Neurosci. Lett. 360, 103–105.
Colzato, L. S., Huizinga, M., and Hommel, B. (2009). Recreational polydrug use of cocaine impairs cognitive flexibility but not working memory. Psychopharmacology 207, 225–234.
Colzato, L. S., van den Wildenberg, W., and Hommel, B. (2007). Impaired inhibitory control in recreational users of cocaine. PLoS ONE 2, e1143. doi: 10.1371/journal.pone.0001143.
Colzato, L. S., van den Wildenberg, W., van der Does, W. A. J., and Hommel, B. (2010). Genetic markers of striatal dopamine predict individual differences in dysfunctional, but not functional impulsivity. Neuroscience 170, 782–788.
Colzato, L. S., van den Wildenberg, W. P. M., van Wouwe, N. C., Pannebakker, M. M., and Hommel, B. (2009). Dopamine and inhibitory action control: evidence from spontaneous eye blink rates. Exp. Brain Res. 196, 467–474.
Fillmore, M. T., Rush, C. R., and Hays, L. (2006). Acute effects of cocaine in two models of inhibitory control: implications of non-linear dose effects. Addiction 101, 1323–1332.
Gauggel, S., Rieger, M., and Feghoff, T. A. (2004). Inhibition of ongoing responses in patients with Parkinson’s disease. J. Neurol. Neurosurg. Psychiatr. 75, 539–544.
Kalix, P., and Braenden, O. (1985). Pharmacological aspects of the chewing of khat leaves. Pharmacol. Rev. 37, 149–164.
Kreek, M. J., Bart, G., Lilly, C., LaForge, K. S., and Nielsen, D. A. (2005). Pharmacogenetics and human molecular genetics of opiate and cocaine addictions and their treatments. Pharmacol. Rev. 57, 1–26.
Levitt, H. J. (1971). Transformed up-down methods in psychoacoustics. J. Acoust. Soc. Am. 49, 467–477.
Logan, G. D. (1994). “On the ability to inhibit thought and action: a users’ guide to the stop signal paradigm,” in Inhibitory Processes in Attention, Memory and Language, eds D. Dagenbach and T. H. Carr (San Diego, CA: Academic Press), 189–239.
Logan, G. D., and Cowan, W. B. (1984). On the ability to inhibit thought and action: a theory of an act of control. Psychol. Rev. 295–327.
Miyake, A., Friedman, N. P., Emerson, M. J., Witzki, A. H., Howerter, A., and Wager, T. D. (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cogn. Psychol. 41, 49–100.
Noble, E. P., Blum, K., Khalsa, M. E., Ritchie, T., Montgomery, A., Wood, R. C., Fitch, R. J., Ozkaragoz, T., Sheridan, P. J., Anglin, M. D., Paredes, A., Treiman, L. J., and Sparkes, R. S. (1993). Allelic association of the D2 dopamine receptor gene with cocaine dependence. Drug Alcohol Depend. 33, 271–285.
Patel, N. B. (2000). Mechanism of action of cathinone: the active ingredient of khat (Catha edulis). East Afr. Med. J. 77, 329–332.
Pennings, E. J. M., Opperhuizen, A., and van Amsterdam, J. G. C. (2008). Risk assessment of khat use in the Netherlands: a review based on adverse health effects, prevalence, criminal involvement and public order. Regul. Toxicol. Pharmacol. 52, 199–207.
Raven, J. (1998). Manual for Raven’s Progressive Matrices and Vocabulary Scales. Oxford: Oxford Psychologists.
Ridderinkhof, K. R., de Vlugt, Y., Bramlage, A., Spaan, M., Elton, M., Snel, J., and Band, G. P. H. (2002). Alcohol consumption impairs detection of performance errors in mediofrontal cortex. Science 298, 2209–2211.
Toennes, S. W., and Kauert, G. F. (2004). Driving under the influence of khat – alkaloid concentrations and observations in forensic cases. Forensic Sci. Int. 140, 85–90.
Verdejo-Garcia, A. J., Lawrence, A. J., and Clarke, L. (2008). Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci. Biobehav. Rev. 32, 777–810.
Volkow, N. D., Fowler, J. S., and Wang, G. J. (1999). Imaging studies on the role of dopamine in cocaine reinforcement and addiction in humans. J. Psychopharmacol. 13, 337–345.
von Geusau, N. A., Stalenhoef, P., Huizinga, M., Snel, J., and Ridderinkhof, K. R. (2004). Impaired executive function in male MDMA (“ecstasy”) users. Psychopharmacology 175, 331–341.
Wagner, G. C., Preston, K., Ricaurte, G. A., Schuster, C. R., and Seiden, L. S. (1982). Neurochemical similarities between D,L-cathinone and D-amphetamine. Drug Alcohol Depend. 9, 279–284.
Williams, B. R., Ponesse, J. S., Schachar, R. J., Logan, G. D., and Tannock, R. (1999). Development of inhibitory control across the life-span. Dev. Psychol. 35, 205–213.
Wylie, S. A., Ridderinkhof, K. R., Bashore, T. R., and van den Wildenberg, W. P. M. (2010). The effect of Parkinson’s disease on the dynamics of online and proactive cognitive control during action selection. J. Cogn. Neurosci. 22, 2058–2073.
Wylie, S. A., van den Wildenberg, W. P. M., Ridderinkhof, K. R., Bashore, T. R., Powell, V. D., Manning, C. A., and Wooten, G. F. (2009). The effect of Parkinson’s disease on interference control during action selection. Neuropsychologia 47, 145–157.
Keywords: khat, SSRT, dopamine, stop-signal task, chronic use, long-term effect
Citation: Colzato LS, Ruiz MJ, van den Wildenberg WPM, Bajo MT and Hommel B (2011) Long-term effects of chronic khat use: impaired inhibitory control. Front. Psychology 1:219. doi: 10.3389/fpsyg.2010.00219
Received: 21 September 2010;
Accepted: 18 November 2010;
Published online: 12 January 2011.
Edited by:
Anna M. Borghi, University of Bologna and Institute of Cognitive Sciences and Technologies, Rome, ItalyReviewed by:
Frederick Verbruggen, University of Exeter, UKScott Wylie, University of Virginia Health Systems, USA
Copyright: © 2011 Colzato, Ruiz, van den Wildenberg, Bajo and Hommel. This is an open-access article subject to an exclusive license agreement between the authors and the Frontiers Research Foundation, which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and source are credited.
*Correspondence: Lorenza S. Colzato, Institute of Psychology, Department of Psychology, Cognitive Psychology Unit, Leiden University, Postbox 9555, 2300 RB Leiden, Netherlands. e-mail: colzato@fsw.leidenuniv.nl