- 1College of Grassland Science and Technology, Sichuan Agricultural University, Chengdu, China
- 2Institute of Soil Fertilizer and Water Saving Agriculture, Gansu Academy of Agricultural Sciences, Lanzhou, China
- 3State Key Laboratory of Crop Gene Exploration and Utilization in Southwest China, Sichuan Agricultural University, Chengdu, China
Diethyl aminoethyl hexanoate (DA-6) serving as a non-toxic and low-cost plant growth regulator is used for improving plant growth and stress tolerance, but the DA-6-mediated organic metabolites remodeling in relation to drought tolerance is not well documented in crops. The aims of the present study were to evaluate impacts of DA-6 on physiological functions including osmotic adjustment, photochemical efficiency, oxidative damage, and cell membrane stability as well as organic metabolites remodeling in white clover (Trifolium repens) leaves based on the analysis of metabolomics. Plants were foliarly treated with or without DA-6 and subsequently exposed to drought stress for 8 days. Results demonstrated that foliar application of DA-6 (1.5 mM) could significantly ameliorate drought tolerance, which was linked with better leaf water status, photosynthetic performance, and cell membrane stability as well as lower oxidative injury in leaves. Metabolic profiling of organic metabolites identified a total of 59 metabolites including 17 organic acids, 20 sugars, 12 alcohols, and 10 other metabolites. In response to drought stress, the DA-6 induced accumulations of many sugars and sugar alcohols (erythrulose, arabinose, xylose, inosose, galactose, talopyranose, fucose, erythritol, and ribitol), organic acids (propanoic acid, 2,3-dihydroxybutanoic acid, palmitic acid, linolenic acid, and galacturonic acid), and other metabolites (2-oxazoline, silane, and glycine) in white clover. These altered metabolites induced by the DA-6 could perform critical functions in maintenances of osmo-protection, osmotic adjustment, redox homeostasis, cell wall structure and membrane stability when white clover suffered from water deficit. In addition, the campesterol and stigmasterol significantly accumulated in all plants in spite of the DA-6 pretreatment under drought stress, which could be an important adaptive response to water deficit due to beneficial roles of those two metabolites in regulating cell membrane stability and antioxidant defense. Present findings provide new evidence of DA-6-regulated metabolic homeostasis contributing to drought tolerance in leguminous plants.
1 Introduction
Due to global warming, the world is experiencing serious environmental problems including increased CO2 level and drought (Gosling and Arnell, 2016; Jackson et al., 2017). Among these, drought is deliberated as the chief environmental issue with severe agricultural penalties (Fahad et al., 2017), because water scarcity significantly disrupts plant water status and also induces stomatal closure resulting in decreased CO2 assimilation, carbon fixation, and photosynthesis for ATP production (França et al., 2000; Flexas and Medrano, 2002; Wu et al., 2008; Liang et al., 2019). In addition, drought stress disturbs the redox homeostasis leading to excessive reactive oxygen species (ROS), which induces severe oxidative injury to functional biomolecules such as nucleic acids, chlorophylls, proteins, and membrane lipids, thereby promoting programmed cell death (Gill and Tuteja, 2010; Sharma et al., 2019). However, undesirable consequences induced by drought stress depend on various factors such as the duration or intensity of the stress, plant growth stage, and species or genotype (Okçu et al., 2005; Hassan et al., 2022). Plants tend to acclimatize hazardous conditions by regulating morphological, biochemical, and physiological responses at genetic and metabolic levels (Gong et al., 2020; Li et al., 2021; Zhang et al., 2021).
By metabolomics analysis, biologically important and significantly different metabolites are identified and separated from specific cells or tissues, and different metabolic processes as well as response mechanisms occurring in organisms are studied (Sumner et al., 2003). Plant metabolomics has become one of the main research hot spots in plant-based studies and serves as an indispensable bridge linking plant phenotypes and genotypes (Hall, 2006). Many studies have shown that the accumulation and reprogramming of comprehensive metabolites are not only related to quality and quantity of crops, but also contributed to alteration of stress tolerance (Khan et al., 2019; Patel et al., 2022; Zhou et al., 2023). Some of them are advantageous for increased stress tolerance such as soluble sugars and multiple amino acids including proline, glycine, and γ-aminobutyric acid etc., while other metabolites such as aldehydes and quinones in massive quantity are harmful for plants under unfavorable environmental conditions (Srivastava et al., 2017; Martínez-Noël and Tognetti, 2018; Batista-Silva et al., 2019). Global metabolites reprogramming has been reported to be associated with drought tolerance in different plant species based on comparative metabolomics (Sanchez et al., 2012). Moreover, spermine-regulated accumulation of organic metabolites such as galactose, sucrose-6-phosphate, mannose, and maltose enhanced heat or drought tolerance of creeping bentgrass (Agrostis stolonifera) by using non-targeted metabolomics (Li et al., 2022). Thus, study on relationship between alterations in metabolites profile and stress tolerance is crucial for broad-spectrum insight about stress responsive mechanisms in different plant species.
Over the last decade, the use of plant growth regulators (PGRs) for ameliorating crop yield and stress tolerance in the agricultural sector has become a common practice worldwide (Khan et al., 2020). Diethyl aminoethyl hexanoate (DA-6) is an important synthetic tertiary amine with multiple beneficial effects in plants under normal and stressed conditions (Hassan et al., 2021a). For example, DA-6 has been reported to enhance germination and seedling establishment of aged soybean (Glycine max) seeds via regulation of fatty acid metabolism and glycometabolism (Zhou et al., 2019). Foliar application of DA-6 after anthesis significantly mediated wheat (Triticum aestivum) grain filling, hence contributing to improved crop production (Wen et al., 2019). DA-6 also improved grain yield of summer maize (Zea mays) by increasing leaf photosynthetic functions and defense-related enzymes activities under field conditions (Nie et al., 2010). Moreover, the DA-6-regulated mechanisms of the tolerance to abiotic stress have been shown in many plant species. For example, the study of (Hassan et al., 2022) found that DA-6 ameliorated white clover (Trifolium repens) seedlings growth by regulating oxidative injury, photosynthetic performance, and lipids reprogramming under water deficient condition. DA-6 significantly strengthened antioxidant defense system in plants under different abiotic stresses such as low temperature and heavy metal toxicity (Fu et al., 2011; He et al., 2013; Li et al., 2018). Exogenous application of DA-6 effectively alleviated salt-induced oxidative damage in Cassia obtusifolia (Zhang et al., 2016). Foliar supplementation of DA-6 performed a positive function in increasing cadmium-extraction efficiency, thereby alleviating cadmium stress (He et al., 2014). The study of (Hassan et al., 2021a) also reported that DA-6 enhanced germination of white clover seeds by enhancing osmotic adjustment (OA), hormonal homeostasis, and accumulation of dehydrins under drought stress. Regardless of these previous studies, the mediatory function of DA-6 in drought tolerance in relation to organic metabolites remodeling still requires to be further investigated in plants.
White clover occupies a core position among global cool-season forage crops because of highly palatable, easily digestible, and nutritionally rich traits as well as tremendous ability to fix nitrogen in soils, hence contributing significantly to soil fertility and livestock industry (Cranston et al., 2015; Caradus et al., 2023; Sawicka et al., 2023). It’s also used as an important ornamental grass and ground cover plant for urban landscape (Sincik and Acikgoz, 2007). However, ineffective transpirational regulation and shallow root system make white clover highly vulnerable to water stress (Annicchiarico and Piano, 2004). Thus, the improvement of drought tolerance of legume crops like white clover is crucial to increase the production and quality of forage and ruminant’s performance. Aims of the study were to elucidate the impact of foliar DA-6 spray on oxidative damage, water status, and photochemical efficiency and to further reveal metabolic balance associated with organic metabolites reprogramming based on metabolomics in white clover plants exposed to drought stress. Current findings will supply vital information about regulatory roles and mechanisms of the DA-6 in legume species in response to drought stress.
2 Materials and methods
2.1 Planting materials & treatments
White clover seeds cv. ‘Haifa’ were sterilized with mercuric chloride solution (0.1%) for 4 min and washed twice with deionized water. Sterile 0.05 g of seeds were uniformly sown in each plastic box (18 cm breadth, 24 cm length, or 9 cm deep) containing sterilized quartz sands in a controlled environment (700 μmol photon m−2 s–1 PAR, 23/19°C day/night temperature, 12 h photoperiod cycle, and a relative humidity of 65%). Seeds were irrigated initially with distilled water for 7 days of germination, and then the distilled water was replaced by half-strength Hoagland solution (Hoagland and Arnon, 1950) until the second leaf expanded completely. Before being exposed to drought stress, one-month-old seedlings were pretreated by foliar application of DA-6 (1.5 mM) or deionized water once a day for 3 consecutive days to ensure that plants could absorb adequate quantity of DA-6 or deionized water through leaves. DA-6-treated and untreated plants were then exposed to Hoagland’s solution (control) or drought stress (-0.3 Mpa) induced by 17% polyethylene glycol (PEG) 6000 which was dissolved in Hoagland’s solution for the next 8 days. All solutions were refreshed daily to prevent any change in concentration. Four different treatments were used for this experiment: 1) C, plants grown in Hoagland’s solution as well-watered control; 2) C+DA-6, DA-6-pretreated plants grown in Hoagland’s solution; 3) PEG, plants were cultivated in -0.3 Mpa PEG solution as drought stress; 4) PEG+DA-6, DA-6-pretreated plants were cultivated in -0.3 Mpa PEG solution. All treatments comprised of four biological replicates and were arranged in a completely randomized design (CRD) in growth chamber. Leaf samples were taken on the 8th day of drought stress for analyses of physiological parameters and metabolomics. The optimum dose of DA-6 (1.5mM) was chosen based on the result of our previous study (Hassan et al., 2022).
2.2 Measurements of leaf water status & osmotic adjustment
To determine leaf relative water content (RWC), fresh leaves (0.1g) were cut and promptly weighed to note the fresh weight (FW). Afterwards, leaves were submerged in deionized water for 1 day to attain the turgid weight (TW). Samples were then dried by placing them in an oven at 80°C for three consecutive days, and dry weight (DW) was measured. The formula (RWC (%) = 100×[(FW-DW)/(TW-DW)]) devised by (Barrs and Weatherley, 1962) was utilized to calculate leaf RWC. To evaluate osmotic potential (OP), leaf tissues were detached and dipped in deionized water for 8 h to ensure sufficient hydration. The hydrated leaves were taken out, blotted dry, and kept in liquid nitrogen for further analysis. After being thawed in an ice bath, leaf sap was extricated. A 10 μl leaf sap was taken and injected into an osmometer (Wescor, Inc., Logan, UT) to estimate osmolality (mmol kg−1). OP was calculated by using the following formula: MPa = −osmolality × 0.001 × 2.58 (Blum, 1989).
2.3 Measurements of chlorophyll content and photochemical efficiency
For chlorophyll (Chl) content, fresh leaf samples (0.1 g) were dipped in 15 ml of dimethyl sulphoxide and placed in dark environment for two days. Afterwards, leaf extract was collected, and the absorbance value was noticed spectrophometrically at 663 or 645 nm. The formula defined by (Arnon, 1949) was used for computing contents of Chl a, chl b, and total Chl. For determination of photochemical efficiency (Fv/Fm) and performance index on an absorption basis (PIBAS), fresh leaves were subjected to dark conditions with leaf clips for half an hour. Later, the Fv/Fm ratio and PIABS was noticed by using a fluorescence meter (Pocket PEA, Hansatech, United Kingdom).
2.4 Measurements of electrolyte leakage & oxidative damage
Electrolyte leakage (EL) was determined by using the protocols of (Blum and Ebercon, 1981) with slight changes. Fresh leaves (0.1 g) were detached and dipped in centrifuge tubes filled with deionized water (35 ml). The tubes were then placed on a rotary shaker for 1 day, and initial conductance (Ci) of solutions was recorded. Afterwards, the tubes were kept in an autoclave (140°C) for half an hour, and the maximum conductance (Cm) of the solutions was noted. EL was computed as the percentage (%) of Ci to Cm. For assays of superoxide anion (O2.-) content, malondialdehyde (MDA) content, and hydrogen peroxide (H2O2) content, 0.1 g of leaf tissue was ground in 4 ml cold phosphate buffer (50 µM, pH 7.8) containing polyvinylpyrrolidone (1%, w/v). After following centrifugation at 12000 g for 30 min, the supernatant was collected. The O2.- was examined with sulfanilamide method (Elstner and Heupel, 1976), and the absorbance of reaction solution was spectrophotometrically noted at 530 nm. The H2O2 content was estimated according to the potassium iodide (KI) protocol. The absorbance of oxidation product was noticed at 390 nm (Velikova et al., 2000). MDA content was estimated by using the procedure illustrated by (Dhindsa et al., 1981). The supernatant (0.5 ml) and reaction solution (1 ml) comprising of trichloroacetic acid (20%, w/v) and thiobarbituric acid (0.5%, w/v) were mixed and shaken thoroughly. The homogenate was placed in a high temperature water bath (95°C) for 15 min and cooled instantly by using an ice water bath. After centrifugation at 8000 g for 10 min, the supernatant was taken, and the absorbance was read at 600 and 532 nm spectrophotometrically.
2.5 Analysis of metabolomics
The content of different metabolites was detected by using gas chromatography-time of flight mass spectrography (GC-TOFMS). The procedure described by (Li et al., 2019a) was followed for the extraction, separation, and quantification of metabolites. A total of 20 mg of the fine dry leaf powders were mixed with 100 μL of double distilled water, and subsequently the 500 μL of aqueous methanol was added. The mixture in the tube was subjected to sonication for 20 min. Later, the centrifugation was performed at 12000 g for 10 min. Then, 300 μL of the supernatant was mixed with 10 μL of 0.3 mg/mL chlorophenylalanine (an internal standard) before desiccation in a CentriVap benchtop centrifugal concentrator (Labconco, Kansas City, MO). After being fully desiccated, the samples were reconstituted in 80 μL of methoxyamine hydrochloride and incubated at 30°C for 90 min. The 80 μL of N-methyl-N-(trimethylsilyl) trifluoroacetamide containing 1% trimethylchlorosilane was added into the mixture which was then incubated at 70°C for 60 min. The treated samples were analyzed by utilizing GC-TOFMS. The initial GC temperature was held at 80°C for 0.2 min and then increased to 180°C at a rate of 10°C min-1. The metabolite identification was accomplished with ChromaTOF software (v. 4.50.8.0, LECO, St. Joseph, MI, USA) and commercially available compound libraries: NIST 2005 (PerkinElmer Inc., Waltham, MS, USA), Wiley 7.0 (John Wiley and Sons Ltd., Hoboken, NJ, USA).
2.6 Statistical analysis
Data was arranged by using Microsoft Excel 2016 software (Microsoft Corp., Redmond, WA, United States) and figures were made by using GraphPad Prism 8.3.0 (538). Significant differences were examined with two-way ANOVA in combination with Tukey’s test at p ≤ 0.05.
3 Results
3.1 Effects of foliar spray of DA-6 on water status & photosynthetic functions
Under normal condition, foliar application of DA-6 exhibited no significant changes in RWC and OP in leaves, but white clover plants pretreated with DA-6 showed 28.48% higher RWC and 17.43% lower OP when compared with untreated plants under drought stress (Figures 1A, B). Exogenous DA-6 pretreatment significantly increased contents of total Chl and Chl a under non-stress condition, however, Chl b, Chl a/b, Fv/Fm, and PIABS remained unaffected by the DA-6 under normal condition (Figures 2C–F). Drought stress substantially reduced the total Chl content, Chl a content, Chl b content, Fv/Fm, and PIABS in both DA-6-treated and untreated plants, but did not affect the ratio of Chl a to Chl b (Figures 2A–F). DA-6-pretreated plants demonstrated a 16%, 16%, 7%, or 32% significantly higher total Chl content, Chl a content, Fv/Fm, or PIABS than non-treated plants exposed to drought stress (Figures 2A, B, E, F). The DA-6 had no significant effect on Chl b content and Chl a/b ratio under water deficient condition as shown in Figures 2C, D.
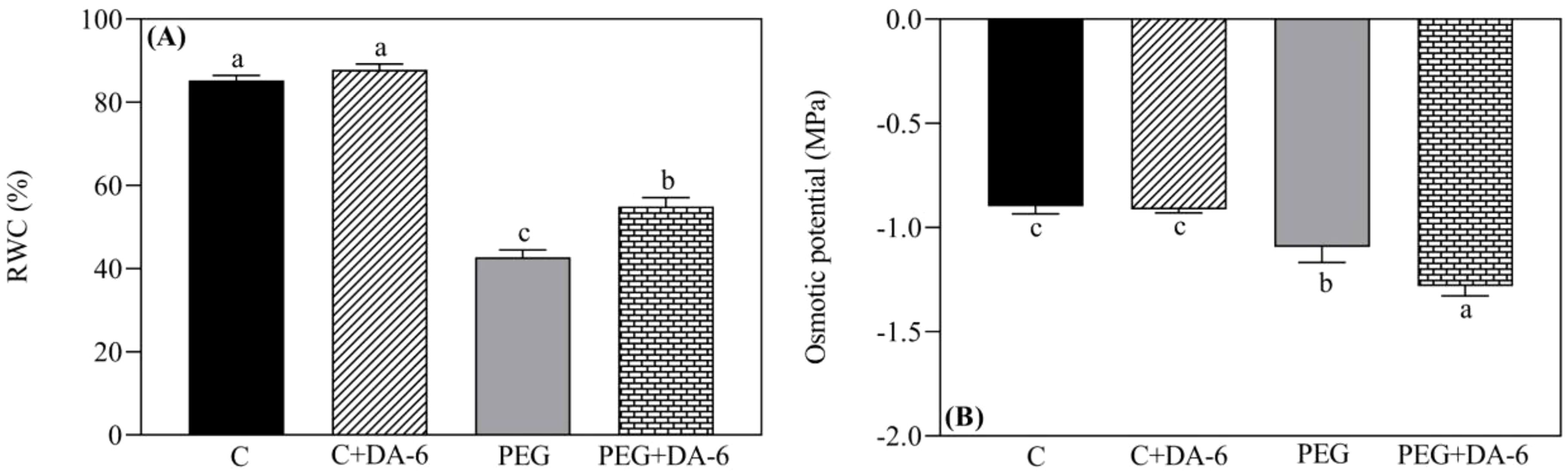
Figure 1. Impacts of foliar application of DA-6 on (A) relative water content (RWC), and (B) osmotic potential (OP) in leaves of white clover under normal and drought conditions. Different letters above or below the vertical columns demonstrate significant differences among different treatments based on the two-way ANOVA in combination with Tukey’s test at p ≤ 0.05. Vertical bars represent the ± standard error (SE) of mean (n = 4). C, well-watered control; C+DA-6, well-watered plants supplemented with DA-6; PEG, 17% PEG-induced drought stress (-0.3 Mpa); PEG+DA-6, 17% PEG-induced drought stress plus exogenous application of DA-6.
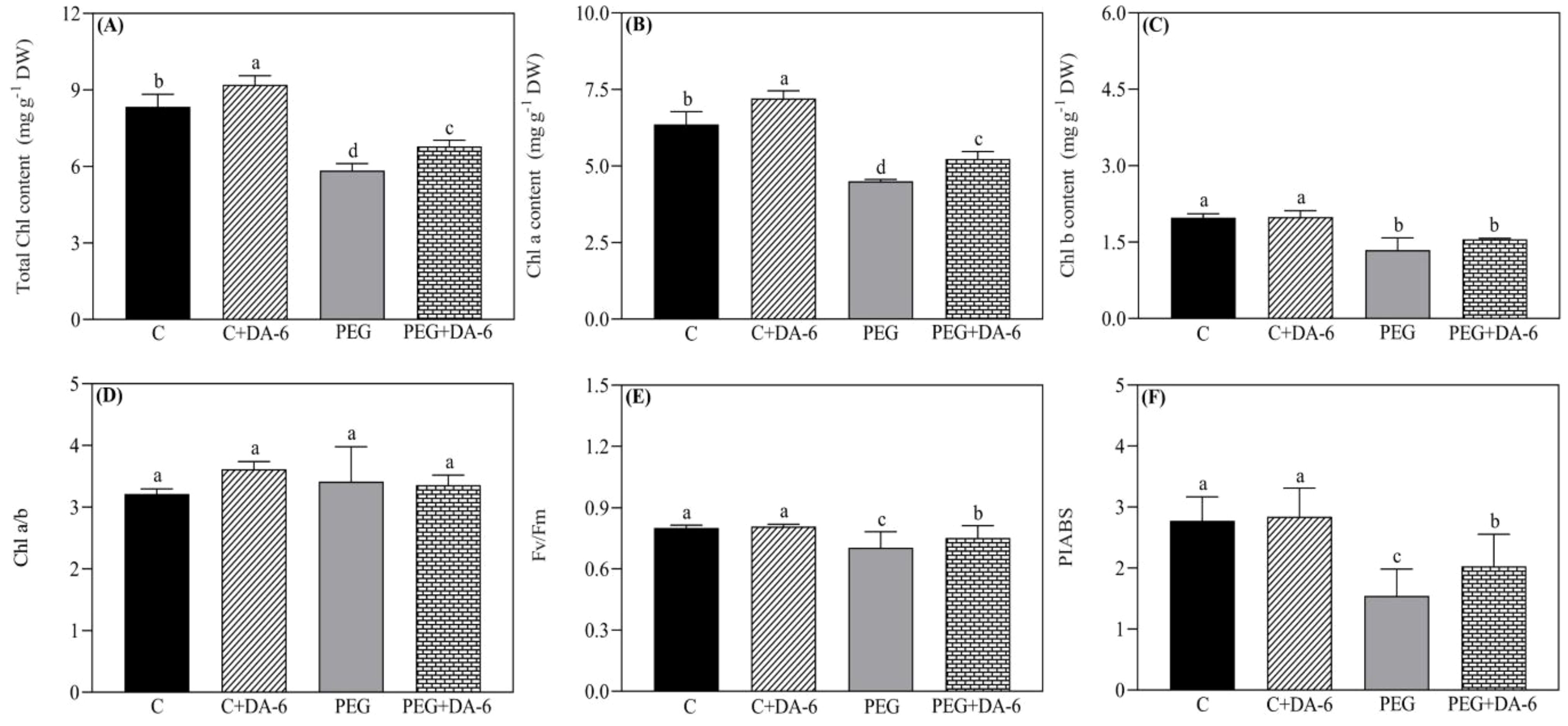
Figure 2. Impacts of foliar application of DA-6 on (A) total chlorophyll (Chl) content, (B) Chl a content, (C) Chl b content, (D) Chl a/b ratio, (E) photochemical efficiency (Fv/Fm), and (F) performance index on an absorption basis (PIABS) in leaves of white clover under normal and drought conditions. Different letters above the vertical columns demonstrate significant differences among different treatments based on the two-way ANOVA in combination with Tukey’s test at p ≤ 0.05. Vertical bars represent the ± standard error (SE) of mean (n = 4). C+DA-6, well-watered plants supplemented with DA-6; PEG, 17% PEG-induced drought stress (-0.3 Mpa); PEG+DA-6, 17% PEG-induced drought stress plus exogenous application of DA-6.
3.2 Effects of foliar spray of DA-6 on cell membrane stability & oxidative damage
Leaf EL, ROS (O2.- and H2O2) contents, and MDA content were not significantly influenced by the DA-6 pretreatment under non-stressed condition as shown in Figures 3A–D. Drought stress significantly increased the above-mentioned four parameters in DA-6-treated and untreated white clover plants. However, foliar DA-6 application markedly reduced the drought-stimulated an increase in EL, O2.- content, H2O2 content, or MDA content by 15%, 15.55%, 21%, or 7.60% when compared with untreated plants, respectively (Figures 3A–D).
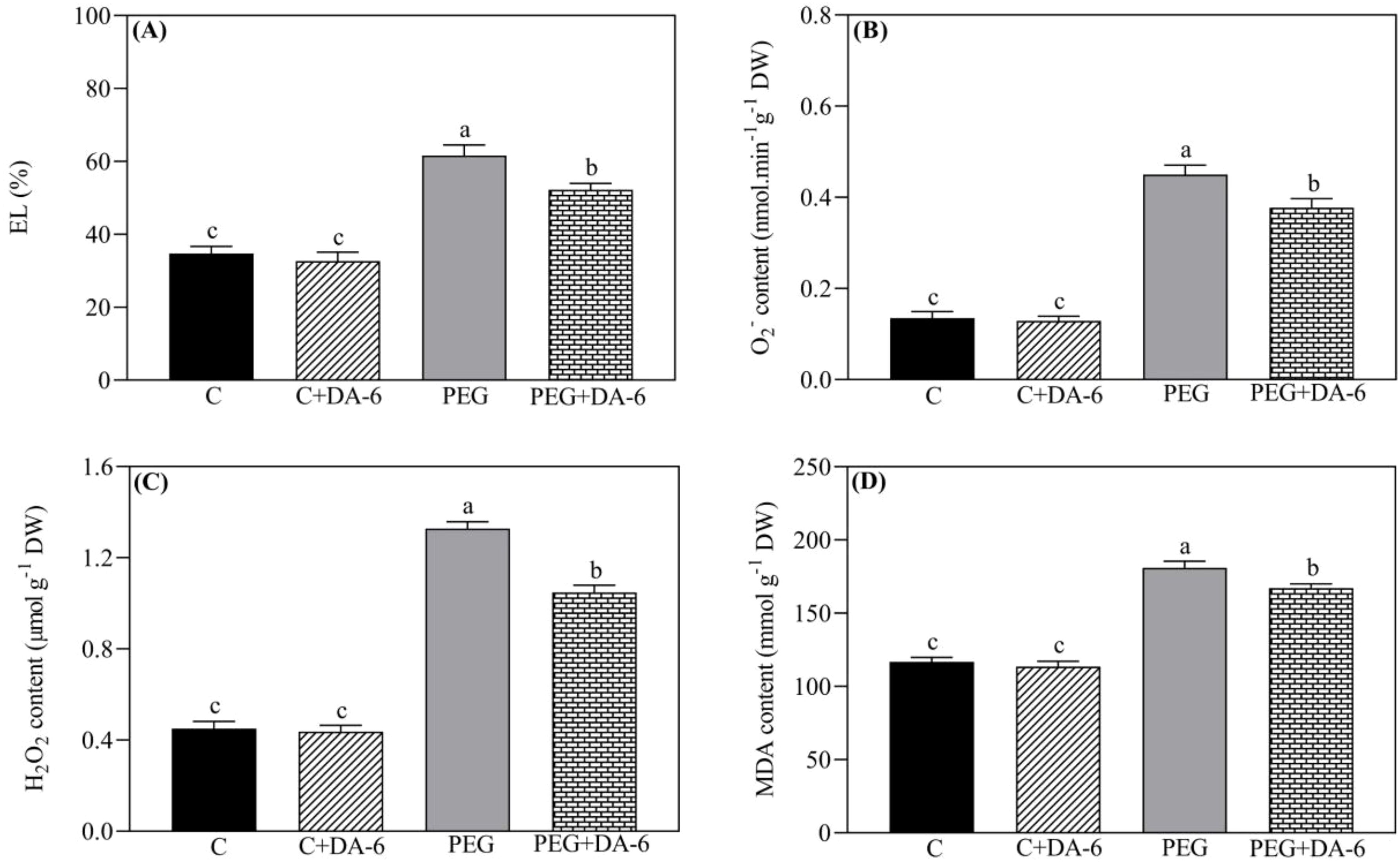
Figure 3. Impacts of foliar application of DA-6 on (A) electrolyte leakage (EL), (B) superoxide anion (O2.-) content, (C) hydrogen peroxide (H2O2) content, and (D) malondialdehyde (MDA) content in leaves of white clover under normal and drought conditions. Different letters above the vertical columns demonstrate significant differences among different treatments based on the two-way ANOVA in combination with Tukey’s test at p ≤ 0.05. Vertical bars represent the ± standard error (SE) of mean (n = 4). C+DA-6, well-watered plants supplemented with DA-6; PEG, 17% PEG-induced drought stress (-0.3 Mpa); PEG+DA-6, 17% PEG-induced drought stress plus exogenous application of DA-6.
3.3 Metabolites profiling in white clover plants influenced by foliar application of DA-6
A total of 59 metabolites comprising of 17 organic acids, 20 sugars, 12 alcohols, and 10 other metabolites were detected and quantified in leaves of white clover (Figure 4A). Heat map of metabolites demonstrated that these metabolites were differentially regulated by foliar application of DA-6 and water stress. Majority of these metabolites remained unaffected by the DA-6 pretreatment under well-watered condition as shown by C+DA-6 Vs. C (Figure 4B). Under drought stress, 29% or 15% metabolites were significantly up-regulated or down-regulated by the DA-6, as depicted by PEG+DA-6 vs. PEG (Figure 4B). Only 24% or 19% metabolites were significantly decreased in PEG vs. C or PEG+DA-6 vs. C, respectively. In addition, a 56% upsurge in metabolites was noticed in PEG vs. C or PEG+DA-6 vs. C, respectively (Figure 4B). In contrast to control, drought stress significantly enhanced the accumulation of sugars and alcohols, but did not significantly affect the accumulation of organic acids in both DA-6-treated and untreated plants (Figure 4C). Drought stress did not induce significant effects on contents of organic acids, alcohols, and other metabolites between DA-6-pretreated and untreated plants (Figure 4C). However, drought stress significantly increased the accumulation of sugar in DA-6-pretreated plants when compared with non-treated plants (Figure 4C).
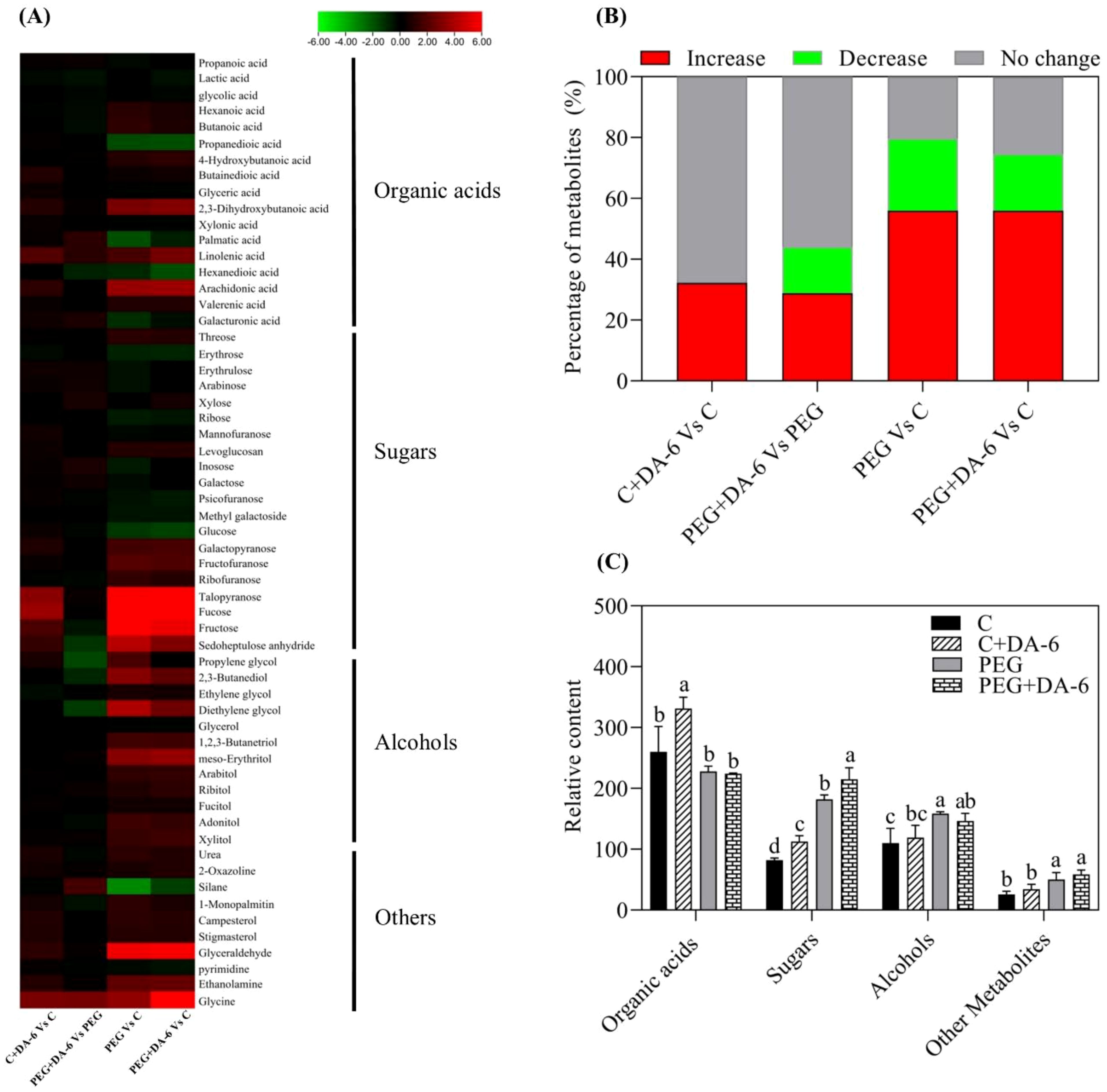
Figure 4. Impacts of foliar application of DA-6 on changes in (A) heat map of 59 identified metabolites, (B) percentage of unchanged, down-regulated, and up-regulated metabolites, and (C) relative contents of organic acids, sugars, alcohols, and other metabolites in leaves of white clover under normal and drought conditions. Different letters above the vertical columns demonstrate significant differences among different treatments based on the two-way ANOVA in combination with Tukey’s test at p ≤ 0.05. Vertical bars represent the ± standard error (SE) of mean (n = 4). C, well-watered control; C+DA-6, well-watered plants supplemented with DA-6; PEG, 17% PEG-induced drought stress (-0.3 Mpa); PEG+DA-6, 17% PEG-induced drought stress plus exogenous application of DA-6.
3.4 Differentially accumulated metabolites influenced by foliar application of DA-6
Exogenous application of DA-6 significantly enhanced accumulations of multiple sugars including erythrulose, arabinose, mannofuranose, levoglucosan, psicofuranose, glucose, and galactopyranose under normal condition (Figures 5A, B). Under drought stress, the application of DA-6 significantly ameliorated drought-induced decreases in erythrulose, arabinose, xylose, inosose, and galactose, and also further increased contents of talopyranose and fucose (Figures 5A, B). In term of changes in different alcohols, drought stress significantly enhanced accumulations of 2,3-butanediol, diethylene glycol, 1,2,3-butanetriol, meso-erythritol, arabitol, ribitol, fucitol, adonitol, and xylitol in both DA-6-pretreated and non-pretreated plants (Figure 6). The DA-6 induced significant increases in meso-erythritol and ribitol contents under drought stress (Figure 6). For changes in organic acids, the PEG + DA-6 treatment exhibited significantly higher propanoic acid, 2,3-dihydroxybutanoic acid, palmitic acid, linolenic acid, and galacturonic acid contents than the PEG treatment (Figures 7A, B). In addition, drought stress significantly induced accumulations of campesterol and stigmasterol, and the foliar pretreatment of DA-6 significantly increased contents of 2-oxazoline, silane, and glycine under drought stress (Figure 8). Figure 9 demonstrated that metabolites reprogramming related to energy metabolism, osmotic adjustment, ROS homeostasis, and cell wall and membrane stability was mediated by foliar application of DA-6 in white clover under drought stress.
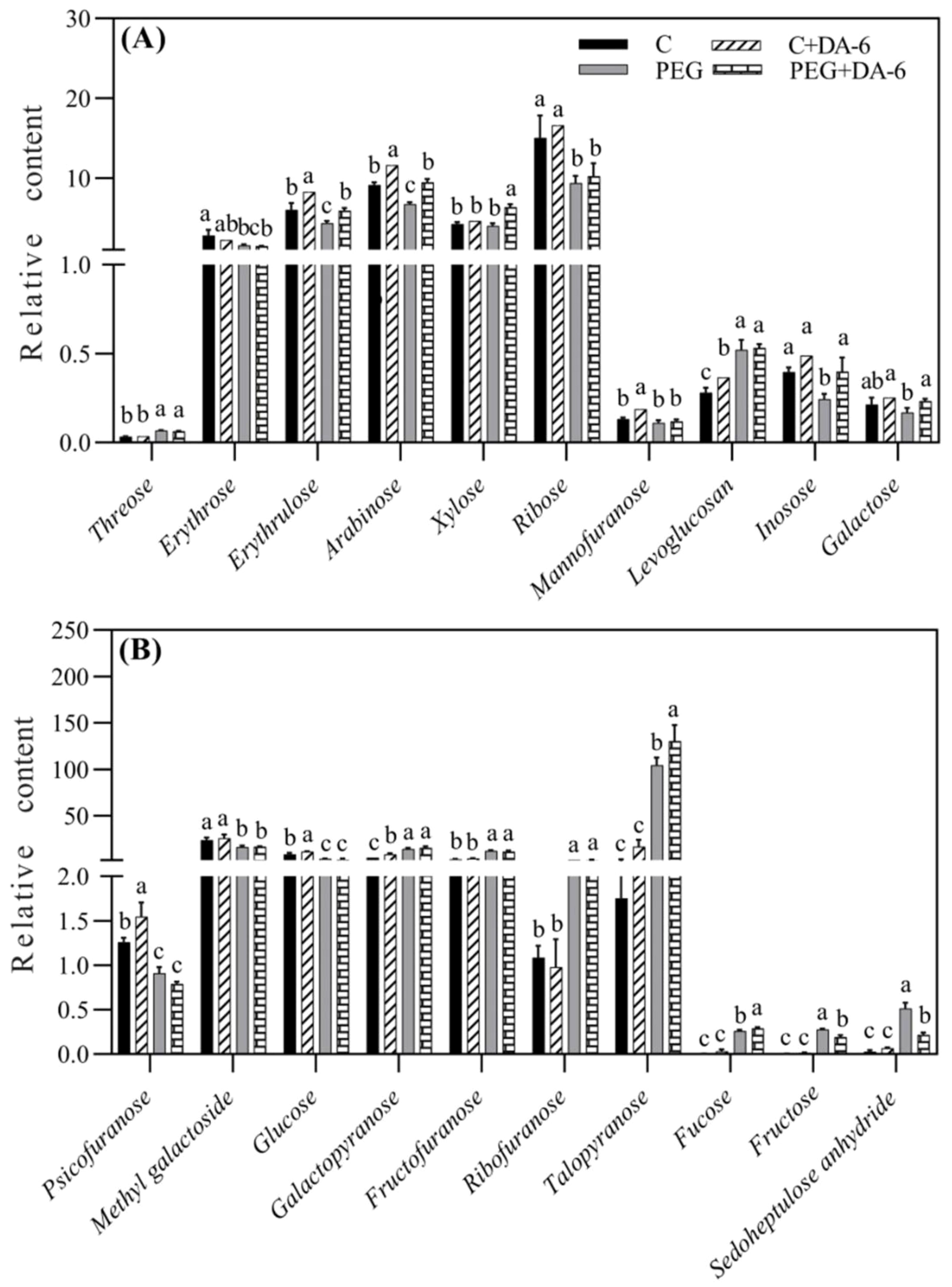
Figure 5. Impacts of foliar application of DA-6 on (A, B) sugars in leaves of white clover under normal and drought conditions. Different letters above the vertical columns demonstrate significant differences among different treatments based on the two-way ANOVA in combination with Tukey’s test at p ≤ 0.05. Vertical bars represent the ± standard error (SE) of mean (n = 4). C+DA-6, well-watered plants supplemented with DA-6; PEG, 17% PEG-induced drought stress (-0.3 Mpa); PEG+DA-6, 17% PEG-induced drought stress plus exogenous application of DA-6.
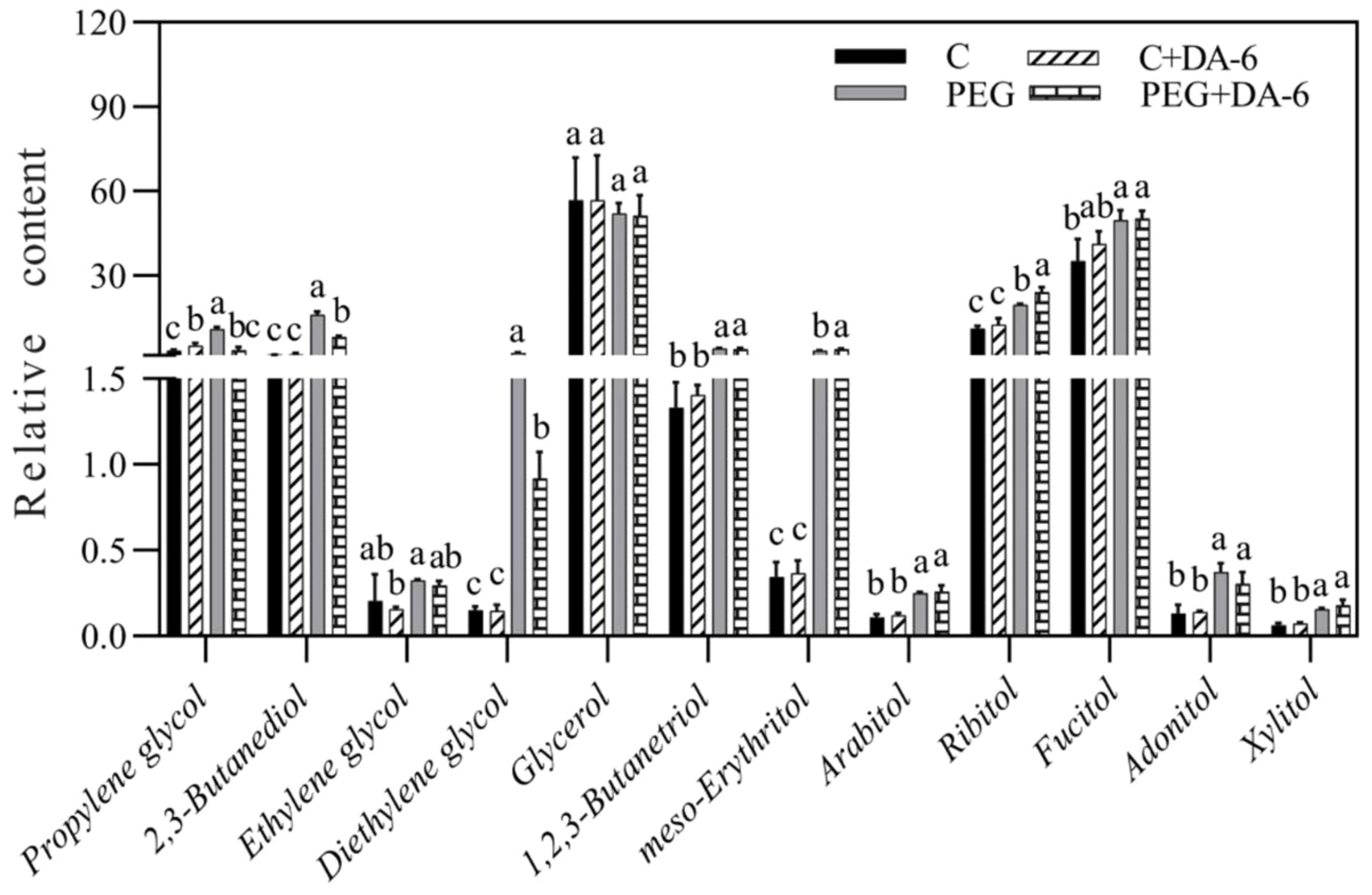
Figure 6. Impacts of foliar application of DA-6 on alcohols in leaves of white clover under normal and drought conditions. Different letters above the vertical columns demonstrate significant differences among different treatments based on the two-way ANOVA in combination with Tukey’s test at p ≤ 0.05. Vertical bars represent the ± standard error (SE) of mean (n = 4). C, well-watered control; C+DA-6, well-watered plants supplemented with DA-6; PEG, 17% PEG-induced drought stress (-0.3 Mpa); PEG+DA-6, 17% PEG-induced drought stress plus exogenous application of DA-6.

Figure 7. Impacts of foliar application of DA-6 on (A, B) organic acids in leaves of white clover under normal and drought conditions. Different letters above the vertical columns demonstrate significant differences among different treatments based on the two-way ANOVA in combination with Tukey’s test at p ≤ 0.05. Vertical bars represent the ± standard error (SE) of mean (n = 4). C, well-watered control; C+DA-6, well-watered plants supplemented with DA-6; PEG, 17% PEG-induced drought stress (-0.3 Mpa); PEG+DA-6, 17% PEG-induced drought stress plus exogenous application of DA-6.
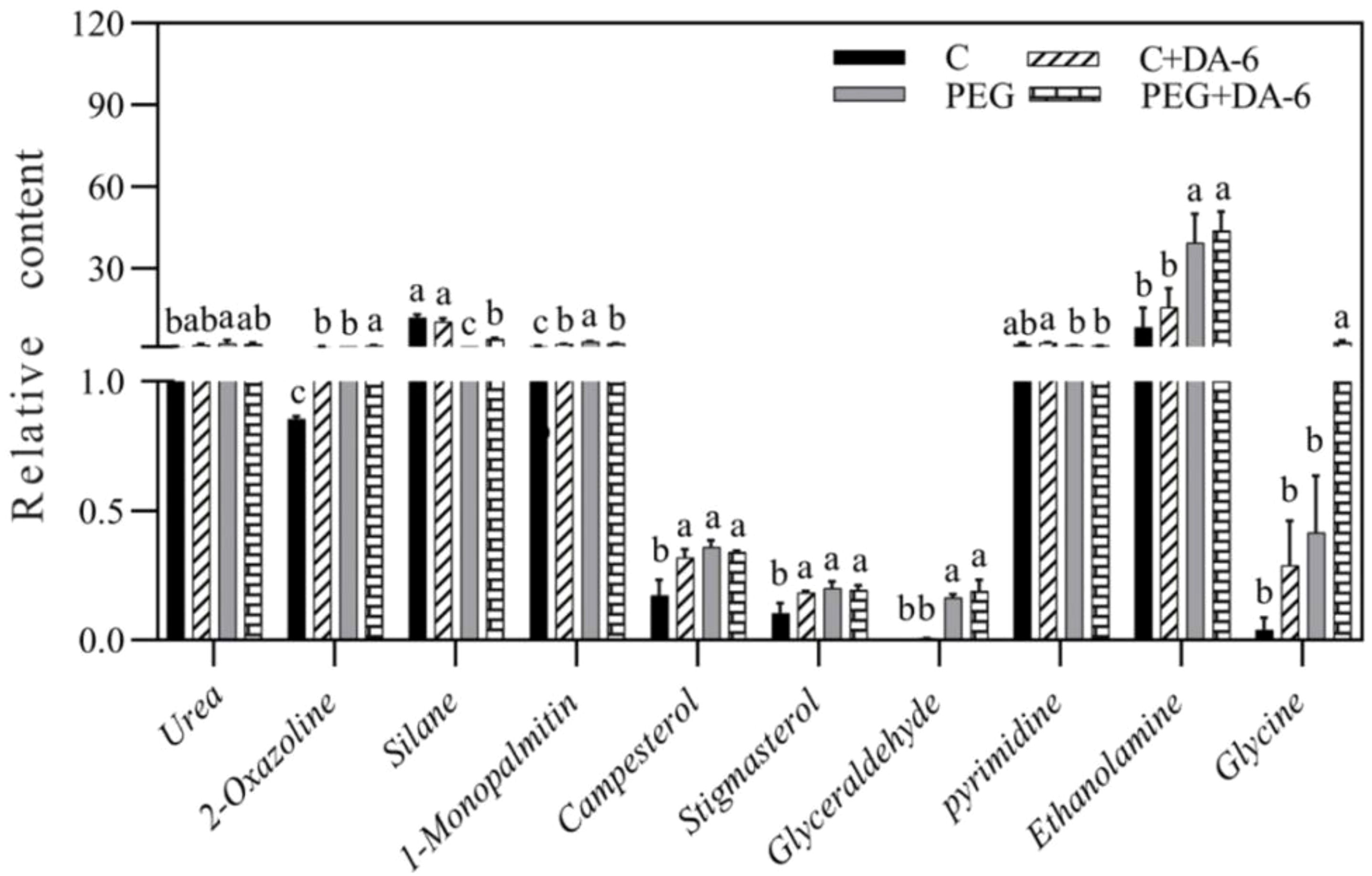
Figure 8. Impacts of foliar application of DA-6 on other metabolites in leaves of white clover under normal and drought conditions. Different letters above the vertical columns demonstrate significant differences among different treatments based on the two-way ANOVA in combination with Tukey’s test at p ≤ 0.05. Vertical bars represent the ± standard error (SE) of mean (n = 4). C, well-watered control; C+DA-6, well-watered plants supplemented with DA-6; PEG, 17% PEG-induced drought stress (-0.3 Mpa); PEG+DA-6, 17% PEG-induced drought stress plus exogenous application of DA-6.
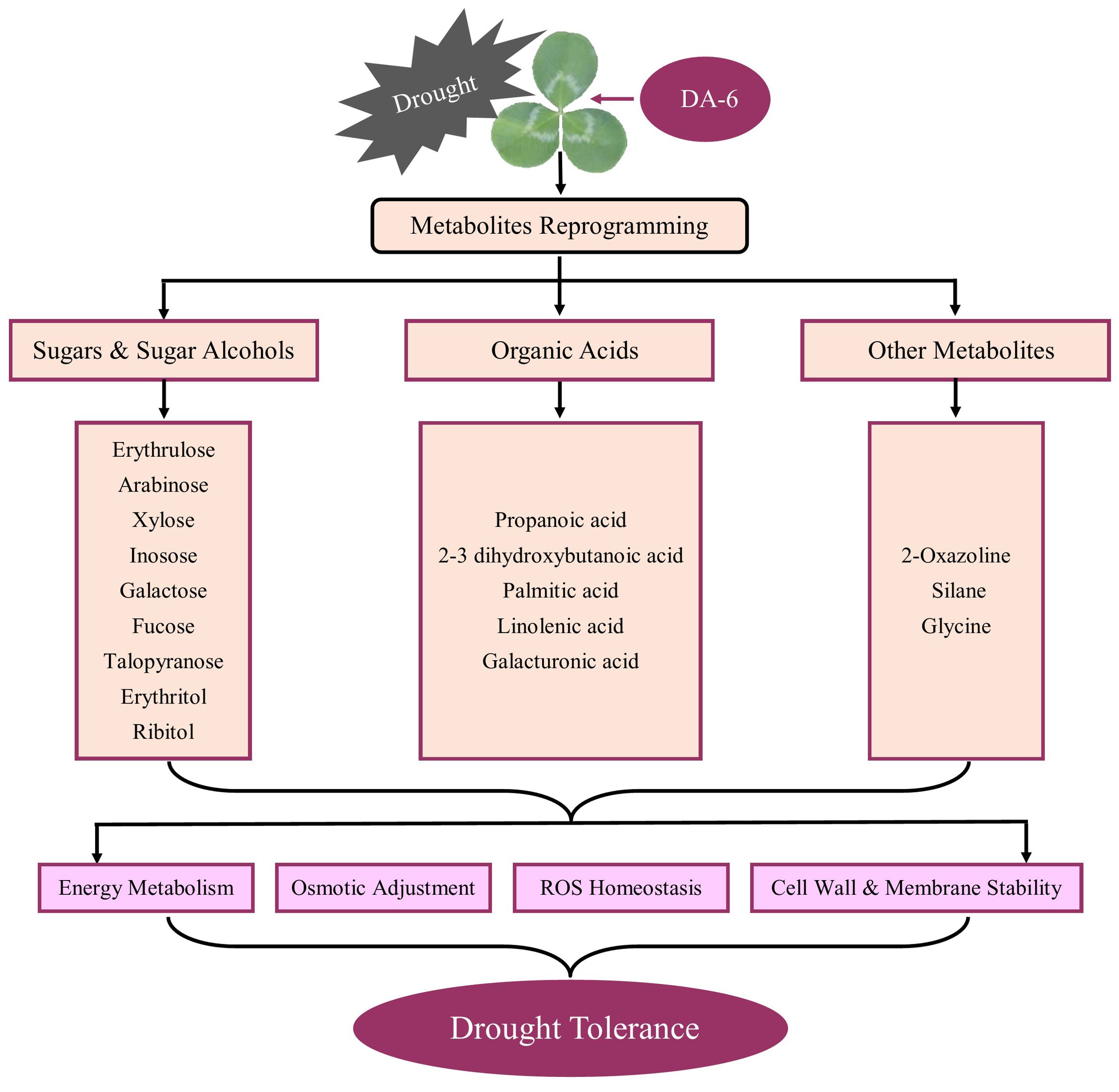
Figure 9. A schematic diagram illustrating DA-6-regulated metabolites reprogramming associated with adaptive response to drought stress in white clover.
4 Discussion
Growth retardation, leaf wilting, and senescence are the most obvious phenotypic symptoms when plants undergo water deficiency (Farooq et al., 2009). However, plants have naturally developed different adaptive strategies including hormonal regulation, OA, and antioxidant defense system to acclimatize drought stress (Seki et al., 2007; Jiao et al., 2012). Beneficial effects of the DA-6 have been reported widely in plants associated with delayed seed aging, improved photosynthesis, and enhanced antioxidant defense system and OA under normal or stressful conditions (Zhou et al., 2019; Hassan et al., 2021a). Our current study found that the foliar pretreatment of appropriate dose of DA-6 significantly ameliorated water status and photosynthetic functions of white clover leaves, as demonstrated by improved Chl content, Fv/Fm, PIABS, RWC, and OA under PEG-induced drought stress (17% PEG and -0.3 Mpa) (Figures 1, 2). Moreover, DA-6-treated white clover plants also demonstrated reduced oxidative damage which was characterized by declines in contents of O2.-, H2O2, and MDA and improved membrane stability than non-treated plants exposed to drought stress (Figure 3). Early study of (Fu et al., 2011) found that the DA-6 application could increase tolerance of strawberry (Fragaria ananassa) seedlings to chilling-induced oxidative injury by enhancing antioxidant capacity and photosynthesis. Recent study of (Huang et al., 2023) also demonstrated that the DA-6 treatment effectively alleviated drought-induced oxidative damage through enhancing enzymatic antioxidant defense system in Ananas comosus plants. Current findings suggested a promising regulatory role of the DA-6 in drought tolerance of white clover contributing to ameliorated water balance, photosynthetic function, and cellular membrane stability. The DA-6 could be used as a potential PGR for regulating crop senescence and stress tolerance due to low cost and nontoxic character.
Stress-triggered metabolites reprograming played key roles in mediating tolerance to various abiotic stresses in plants (Patel et al., 2022). As important primary metabolites, sugars and sugar alcohols with fundamental functions of energy supply, OA, and signaling transduction are responsible for plant growth and stress defense (Bhattacharya and Kundu, 2020). Previous studies related to metabolic profiling proved advantageous effects of massive accumulations of sugars and sugar alcohols on mitigating abiotic stresses in different plant species (Shi et al., 2015; Martínez-Noël and Tognetti, 2018). Our results showed that foliar application of the DA-6 significantly induced accumulations of sugars (erythrulose, xylose, galactose, arabinose, fucose, inosose, and talopyranose) and sugar alcohols (erythritol and ribitol) in white clover after being subjected to PEG-induced drought stress (Figures 5, 6). Erythrulose, xylose, and galactose are reductive monosaccharides and perform critical roles in mediating cellular metabolic homeostasis under drought stress (Li et al., 2017; Lu et al., 2023). Combined heat and drought stress resulted in higher galactose content contributing to better OA in poplar (Populus) leaves (Jia et al., 2016). In plants, arabinose is an essential constituent of various cell wall polysaccharides, glycoproteins, flavonoids, and signaling peptides (Rautengarten et al., 2017). Fucose has been found to be an integral component in the biosynthesis of cell wall polymers as well as sugar-regulated proteins (Reiter et al., 1997; Zentella et al., 2017). Significant increases in arabinose biosynthesis and fucose accumulation have been reported to be associated with salt tolerance in Arabidopsis thaliana and white clover (Zhao et al., 2019; Cheng et al., 2022). In addition, myo-inositol acting as a derivative of inosose positively regulated drought tolerance due to its roles in OA and antioxidant (Li et al., 2019b). Exogenous pretreatment of myo-inositol effectively decreased drought damage to creeping bentgrass via improvement in OA and elimination of oxidative damage (Li et al., 2020). Moreover, the positive role of erythritol and ribitol as essential osmolytes has been well documented in plants under adverse environmental conditions (Fàbregas and Fernie, 2019; Zhou et al., 2023). Our findings suggested that the DA-6-regulated drought tolerance of white clover could be associated with higher accumulation of these sugars and sugar alcohols leading to better cell wall and plasma membrane stability, OA and osmoprotection, signaling transduction, and antioxidant defense for redox homeostasis.
Various organic acids were generally or differentially regulated by DA-6 in white clover. Main functions of organic acids including pH regulation, elimination of ionic toxicity, OA, and energy metabolism have been elucidated in different plant species under unfavorable environmental conditions (Lecoeur et al., 1992; Jones, 1998; Ma et al., 2001). As crucial fatty acids, palmitic acid and linolenic acid are involved in various cellular functions such as constituents of cellular membranes, carbon and energy source in triacylglycerol, precursors of bioactive molecules, and stress signaling in plants (Zhukov, 2015; He and Ding, 2020; Hassan et al., 2022). Galacturonic acid that is an essential component of pectins in cell wall performs imperative roles in plant growth and defense system (Pourcelot et al., 2011; Kuivanen et al., 2015). Significant increases in contents of palmitic acid, linolenic acid, or galacturonic acid by the DA-6 and β-sitosterol pretreatment were also linked with ameliorated drought tolerance of white clover (Li et al., 2019a; Hassan et al., 2022), which is in accordance with our present findings. The DA-6-mediated increases in palmitic acid, linolenic acid, and galacturonic acid could be associated with improved lipids reprograming, therefore resulting in superior cell membrane and wall stability under water-limited condition. In addition, the DA-6 application also induced accumulations of propanoic acid and 2,3-dihydroxybutanoic acid in white clover leaves in response to PEG-induced drought stress. However, regulatory roles and mechanisms of short chain fatty acids propanoic acid and 2,3-dihydroxybutanoic acid in stress tolerance have rarely been reported in plants so far and still demand an in-depth investigation in our future studies.
Apart from sugars, alcohols and organic acids, other metabolites such as 2-oxazoline, silane, and glycine also responded to the DA-6 differentially in white clover under PEG-stimulated drought stress (Figure 8). The 2-oxazoline is a heterocyclic organic compound for the biosynthesis of complex oxazolines which have potential roles in protecting carboxylic acids (Wenker, 1938). Various 2-oxazolines such as 2-ethyl-2-oxazoline perform living cationic ring-opening polymerization to produce poly(2-oxazoline)s (Kobayashi and Uyama, 2002; Hoogenboom, 2009). A recent study reported the synergistic role of biochar and poly (2-ethyl-2-oxazoline) hydrogels in improving carrot (Daucus carota) production under saline condition (Abdeen et al., 2023). Silane serves as a precursor in the synthesis of elemental silicon. The beneficial role of silicon in morphological, physiological and biochemical functions of plants has been well documented under different abiotic stresses including drought (Luyckx et al., 2017; Zargar et al., 2019). In addition, silanes possessing inorganic or organic attachments are coupling agents and adhesion promoters (London et al., 2013). Glycine performs vital roles in amino acids metabolism, signaling as well as plant stress responses (Depeint et al., 2006). The study of (Li et al., 2019b) reported that exogenous application of γ-aminobutyric acid significantly enhanced the glycine accumulation in favor of amino acids homeostasis in white clover seedlings under drought stress. Significant upsurge in the accumulation of glycine was also associated with improved thermo-tolerance of creeping bentgrass (Li et al., 2016). These findings indicated that the DA-6-regulated adaptability to PEG-stimulated drought stress could be connected with increased accumulations of 2-oxazoline, silane, and glycine in leaves of white clover plants. However, functions of 2-oxazoline associated with drought tolerance could not be fully explained in our current study and deserves to be further studied, since little information is available so far.
Interestingly, PEG-induced drought stress significantly induced accumulation of campesterol and stigmasterol in both of DA-6-pretreated and untreated white clover plants, and the DA-6 application also increased their accumulations in leaves under normal condition. Campesterol and stigmasterol are key regulators of plasma membrane fluidity and integrity as components of the phospholipid bilayer membrane in plants (Saffan, 2008). OsFes1A-transgenic A. thaliana with a significant increase in endogenous campesterol content exhibited significantly higher drought tolerance than wild type (Xu et al., 2024). Both of drought-sensitive and drought-tolerant rice (Oryza sativa) cultivars could significantly accumulate compesterol and stigmasterol for better maintenances of membrane lipids homeostasis and the integrity of cell membranes in response to drought stress (Kumar et al., 2018). Higher accumulation of phytosterols such as campesterol and stigmasterol as well as better membrane stability during drought stress were observed in drought-tolerant rice genotype as compared to drought-sensitive one (Kumar et al., 2015). On the contrary, barley (Hordeum vulgare) genotype with the lowest level of campesterol was susceptible to drought stress (Kuczyńska et al., 2019). In addition, a previous study reported that heat-tolerant hard fescue (Festuca trachyphylla) genotype Reliant IV demonstrated higher stigmasterol content when compared with the heat-sensitive Predator (Wang et al., 2017). Exogenous stigmasterol mitigated negative impact of drought stress on flax (Linum usitatissimum) plants through activating antioxidant defense to relieve oxidative damage (Hassan et al., 2021b; Nemat Alla et al., 2022). Recent study of Hanafy and Sadak also found that foliar application of stigmasterol could significantly improve growth and productivity of sunflower (Helianthus annuus) related to enhanced antioxidant metabolism (Hanafy and Sadak, 2023). Based on these previous reports and our current study, the accumulations of campesterol and stigmasterol could be important adaptive responses to drought stress in white clover due to beneficial roles of campesterol and stigmasterol in regulating cell membrane stability and antioxidant defense.
5 Conclusion
Exogenous application of DA-6 significantly alleviated drought-induced oxidative damage and also improved water balance, photosynthetic function and cell membrane stability when white clover suffered from drought stress. Metabolic profiling demonstrated that a total of 59 metabolites were generally or differentially regulated by the DA-6 under water-limited condition. The DA-6 induced the accumulation of sugars and sugar alcohols including erythrulose, arabinose, xylose, inosose, galactose, talopyranose, fucose, erythritol and ribitol. In addition, foliar pretreatment of DA-6 also significantly enhanced the accumulation of various organic acids and amino acids such as propanoic acid, 2,3-dihydroxybutanoic acid, palmitic acid, linolenic acid, D-glucuronic acid, lactone, and glycine. The DA-6-induced increases in these organic metabolites could be contributed to improved drought tolerance of white clover due to their potential roles in OA, osmo-protection, ROS homeostasis, cell wall structure and membrane stability. The present findings provide new evidence of DA-6-regulated metabolic homeostasis related to drought tolerance in leguminous plants.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
MH: Methodology, Data curation, Formal analysis, Investigation, Writing – original draft. AN: Investigation, Writing – review & editing, Formal analysis. MZ: Data curation, Formal analysis, Writing – review & editing. MR: Formal analysis, Investigation, Writing – review & editing. UA: Formal analysis, Investigation, Writing – review & editing. BC: Investigation, Methodology, Resources, Supervision, Writing – review & editing. YL: Writing – review & editing, Methodology, Supervision, Conceptualization, Project administration, Funding acquisition, Resources. ZL: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by Ya’an Science and Technology Program (24ZDYF0040). The funding agency had no role in the design of the study, collection, analysis, and interpretation of data, or in writing the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdeen, S. A., Hefni, H. H., Awadallah-F, A., El-Rahman, N. R. A. (2023). The synergistic effect of biochar and poly (2-ethyl-2-oxazoline)/poly (2-hydroxyethylmethacarylate)/chitosan) hydrogels on saline soil properties and carrot productivity. Chem. Biol. Technol. Agric. 10, 68. doi: 10.1186/s40538-023-00435-2
Annicchiarico, P., Piano, E. (2004). Indirect selection for root development of white clover and implications for drought tolerance. J. Agron. Crop Sci. 190, 28–34. doi: 10.1046/j.0931-2250.2003.00070.x
Arnon, D. I. (1949). Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 24, 1–14. doi: 10.1104/pp.24.1.1
Barrs, H., Weatherley, P. (1962). A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 15, 413–428. doi: 10.1071/bi9620413
Batista-Silva, W., Heinemann, B., Rugen, N., Nunes-Nesi, A., Araújo, W. L., Braun, H. P., et al. (2019). The role of amino acid metabolism during abiotic stress release. Plant Cell Environ. 42, 1630–1644. doi: 10.1111/pce.13518
Bhattacharya, S., Kundu, A. (2020). “Sugars and sugar polyols in overcoming environmental stresses,” in Protective chemical agents in the amelioration of plant abiotic stress: Biochemical and molecular perspectives, 71–101. doi: 10.1002/9781119552154.ch4
Blum, A. (1989). Osmotic adjustment and growth of barley genotypes under drought stress. Crop Sci. 29, 230–233. doi: 10.2135/cropsci1989.0011183x002900010052x
Blum, A., Ebercon, A. (1981). Cell membrane stability as a measure of droughtand heat tolerance in wheat 1. Crop Sci. 21, 43–47. doi: 10.2135/cropsci1981.0011183X002100010013x
Caradus, J., Roldan, M., Voisey, C., Woodfield, D. (2023). “White clover (Trifolium repens L.) benefits in grazed pastures and potential improvements,” in Production and utilization of legumes-progress and prospects, 1–28. doi: 10.5772/intechopen.109625
Cheng, B., Hassan, M. J., Feng, G., Zhao, J., Liu, W., Peng, Y., et al. (2022). Metabolites reprogramming and Na+/K+ transportation associated with putrescine-regulated white clover seed germination and seedling tolerance to salt toxicity. Front. Plant Sci. 13, 856007. doi: 10.3389/fpls.2022.856007
Cranston, L., Kenyon, P., Morris, S., Kemp, P. (2015). A review of the use of chicory, plantain, red clover and white clover in a sward mix for increased sheep and beef production. J. New Z. Grasslands 77, 89–94. doi: 10.33584/jnzg.2015.77.475
Depeint, F., Bruce, W. R., Shangari, N., Mehta, R., O’Brien, P. J. (2006). Mitochondrial function and toxicity: role of B vitamins on the one-carbon transfer pathways. Chemico-biological Interact. 163, 113–132. doi: 10.1016/j.cbi.2006.05.010
Dhindsa, R. S., Plumb-Dhindsa, P., Thorpe, T. A. (1981). Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 32, 93–101. doi: 10.1093/jxb/32.1.93
Elstner, E. F., Heupel, A. (1976). Inhibition of nitrite formation from hydroxylammoniumchloride: a simple assay for superoxide dismutase. Analytical Biochem. 70, 616–620. doi: 10.1016/0003-2697(76)90488-7
Fàbregas, N., Fernie, A. R. (2019). The metabolic response to drought. J. Exp. Bot. 70, 1077–1085. doi: 10.1093/jxb/ery437
Fahad, S., Bajwa, A. A., Nazir, U., Anjum, S. A., Farooq, A., Zohaib, A., et al. (2017). Crop production under drought and heat stress: plant responses and management options. Front. Plant Sci. 8, 1147. doi: 10.3389/fpls.2017.01147
Farooq, M., Wahid, A., Kobayashi, N., Fujita, D., Basra, S. (2009). “Plant drought stress: effects, mechanisms and management,” in Sustainable agriculture, 153–188. doi: 10.1007/978-90-481-2666-8_12
Flexas, J., Medrano, H. (2002). Drought-inhibition of photosynthesis in C3 plants: stomatal and non-stomatal limitations revisited. Ann. Bot. 89, 183–189. doi: 10.1093/aob/mcf027
França, M. G. C., Thi, A. T. P., Pimentel, C., Rossiello, R. O. P., Zuily-Fodil, Y., Laffray, D. (2000). Differences in growth and water relations among Phaseolus vulgaris cultivars in response to induced drought stress. Environ. Exp. Bot. 43, 227–237. doi: 10.1016/s0098-8472(99)00060-x
Fu, X., Maimaiti, A., Mou, H., Yang, Q., Liu, G. (2011). Hexanoic acid 2-(diethylamino) ethyl ester enhances chilling tolerance in strawberry seedlings by impact on photosynthesis and antioxidants. Biol. Plantarum 55, 793–796. doi: 10.1007/s10535-011-0190-8
Gill, S. S., Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48, 909–930. doi: 10.1016/j.plaphy.2010.08.016
Gong, Z., Xiong, L., Shi, H., Yang, S., Herrera-Estrella, L. R., Xu, G., et al. (2020). Plant abiotic stress response and nutrient use efficiency. Sci. China Life Sci. 63, 635–674. doi: 10.1007/s11427-020-1683-x
Gosling, S. N., Arnell, N. W. (2016). A global assessment of the impact of climate change on water scarcity. Climatic Change 134, 371–385. doi: 10.1007/s10584-013-0853-x
Hall, R. D. (2006). Plant metabolomics: from holistic hope, to hype, to hot topic. New Phytol. 169, 453–468. doi: 10.1111/j.1469-8137.2005.01632.x
Hanafy, R. S., Sadak, M. S. (2023). Foliar spray of stigmasterol regulates physiological processes and antioxidant mechanisms to improve yield and quality of sunflower under drought stress. J. Soil Sci. Plant Nutr. 23, 2433–2450. doi: 10.1007/s42729-023-01197-4
Hassan, N. M., Budran, I. G., El-Bastawisy, Z. M., El-Harary, E. H., Nemat Alla, M. M. (2021b). Stigmasterol relieves the negative impact of drought on flax through modulation of redox homeostasis. Egyptian J. Bot. 61, 623–635. doi: 10.21608/ejbo.2021.57628.1607
Hassan, M. J., Geng, W., Zeng, W., Raza, M. A., Khan, I., Iqbal, M. Z., et al. (2021a). Diethyl aminoethyl hexanoate priming ameliorates seed germination via involvement in hormonal changes, osmotic adjustment, and dehydrins accumulation in white clover under drought stress. Front. Plant Sci. 12, 709187. doi: 10.3389/fpls.2021.709187
Hassan, M. J., Qi, H., Cheng, B., Hussain, S., Peng, Y., Liu, W., et al. (2022). Enhanced adaptability to limited water supply regulated by diethyl aminoethyl hexanoate (DA-6) associated with lipidomic reprogramming in two white clover genotypes. Front. Plant Sci. 13, 879331. doi: 10.3389/fpls.2022.879331
He, M., Ding, N. (2020). Plant unsaturated fatty acids: multiple roles in stress response. Front. Plant Sci. 11, 562785. doi: 10.3389/fpls.2020.562785
He, S., Wu, Q., He, Z. (2013). Effect of DA-6 and EDTA alone or in combination on uptake, subcellular distribution and chemical form of Pb in Lolium perenne. Chemosphere 93, 2782–2788. doi: 10.1016/j.chemosphere.2013.09.037
He, S., Wu, Q., He, Z. (2014). Synergetic effects of DA-6/GA3 with EDTA on plant growth, extraction and detoxification of Cd by Lolium perenne. Chemosphere 117, 132–138. doi: 10.1016/j.chemosphere.2014.06.015
Hoagland, D. R., Arnon, D. I. (1950). The Water Culture Method for Growing Plants Without Soil. Berkeley, CA: California Agricultural Experiment Station Circular.
Hoogenboom, R. (2009). Poly (2-oxazoline) s: a polymer class with numerous potential applications. Angewandte Chemie Int. Edition 48, 7978–7994. doi: 10.1002/anie.200901607
Huang, X., Rao, G., Peng, X., Xue, Y., Hu, H., Feng, N., et al. (2023). Effect of plant growth regulators DA-6 and COS on drought tolerance of pineapple through bromelain and oxidative stress. BMC Plant Biol. 23, 180. doi: 10.1186/s12870-023-04200-3
Jackson, R., Le Quéré, C., Andrew, R., Canadell, J., Peters, G., Roy, J., et al. (2017). Warning signs for stabilizing global CO2 emissions. Environ. Res. Lett. 12, 110202. doi: 10.1088/1748-9326/aa9662
Jia, J., Li, S., Cao, X., Li, H., Shi, W., Polle, A., et al. (2016). Physiological and transcriptional regulation in poplar roots and leaves during acclimation to high temperature and drought. Physiologia plantarum 157, 38–53. doi: 10.1111/ppl.12400
Jiao, Z., Li, Y., Li, J., Xu, X., Li, H., Lu, D., et al. (2012). Effects of exogenous chitosan on physiological characteristics of potato seedlings under drought stress and rehydration. Potato Res. 55, 293–301. doi: 10.1007/s11540-012-9223-8
Khan, N., Bano, A. M., Babar, A. (2020). Impacts of plant growth promoters and plant growth regulators on rainfed agriculture. PloS One 15, e0231426. doi: 10.1371/journal.pone.0231426
Khan, N., Bano, A., Rahman, M. A., Rathinasabapathi, B., Babar, M. A. (2019). UPLC-HRMS-based untargeted metabolic profiling reveals changes in chickpea (Cicer arietinum) metabolome following long-term drought stress. Plant Cell Environ. 42, 115–132. doi: 10.1111/pce.13195
Kobayashi, S., Uyama, H. (2002). Polymerization of cyclic imino ethers: From its discovery to the present state of the art. J. Polymer Sci. Part A: Polymer Chem. 40, 192–209. doi: 10.1002/pola.10090
Kuczyńska, A., Cardenia, V., Ogrodowicz, P., Kempa, M., Rodriguez-Estrada, M. T., Mikołajczak, K. (2019). Effects of multiple abiotic stresses on lipids and sterols profile in barley leaves (Hordeum vulgare L.). Plant Physiol. Biochem. 141, 215–224. doi: 10.1016/j.plaphy.2019.05.033
Kuivanen, J., Penttilä, M., Richard, P. (2015). Metabolic engineering of the fungal D-galacturonate pathway for L-ascorbic acid production. Microbial Cell Factories 14, 1–9. doi: 10.1186/s12934-014-0184-2
Kumar, M. S., Ali, K., Dahuja, A., Tyagi, A. (2015). Role of phytosterols in drought stress tolerance in rice. Plant Physiol. Biochem. 96, 83–89. doi: 10.1016/j.plaphy.2015.07.014
Kumar, M. S., Mawlong, I., Ali, K., Tyagi, A. (2018). Regulation of phytosterol biosynthetic pathway during drought stress in rice. Plant Physiol. Biochem. 129, 11–20. doi: 10.1016/j.plaphy.2018.05.019
Lecoeur, J., Wery, J., Turc, O. (1992). Osmotic adjustment as a mechanism of dehydration postponement in chickpea (Cicer arietinum L.) leaves. Plant Soil 144, 177–189. doi: 10.1007/bf00012874
Li, Z., Cheng, B., Wu, X., Zhang, Y., Feng, G., Peng, Y. (2022). Spermine-mediated metabolic homeostasis improves growth and stress tolerance in creeping bentgrass (Agrostis stolonifera) under water or high-temperature stress. Front. Plant Sci. 13, 944358. doi: 10.3389/fpls.2022.944358
Li, Z., Cheng, B., Yong, B., Liu, T., Peng, Y., Zhang, X., et al. (2019a). Metabolomics and physiological analyses reveal β-sitosterol as an important plant growth regulator inducing tolerance to water stress in white clover. Planta 250, 2033–2046. doi: 10.1007/s00425-019-03277-1
Li, Z., Fu, J., Shi, D., Peng, Y. (2020). Myo-inositol enhances drought tolerance in creeping bentgrass through alteration of osmotic adjustment, photosynthesis, and antioxidant defense. Crop Sci. 60, 2149–2158. doi: 10.1002/csc2.20186
Li, Z., Yong, B., Cheng, B., Wu, X., Zhang, Y., Zhang, X., et al. (2019b). Nitric oxide, γ-aminobutyric acid, and mannose pretreatment influence metabolic profiles in white clover under water stress. J. Integr. Plant Biol. 61, 1255–1273. doi: 10.1111/jipb.12770
Li, Z., Yu, J., Peng, Y., Huang, B. (2016). Metabolic pathways regulated by γ-aminobutyric acid (GABA) contributing to heat tolerance in creeping bentgrass (Agrostis stolonifera). Sci. Rep. 6, 30338. doi: 10.1038/srep30338
Li, Z., Zhang, R., Zhang, H. (2018). Effects of plant growth regulators (DA-6 and 6-BA) and EDDS chelator on phytoextraction and detoxification of cadmium by Amaranthus hybridus Linn. Int. J. phytoremediation 20, 1121–1128. doi: 10.1080/15226514.2017.1365348
Li, Z., Zhang, Y., Zhang, X., Merewitz, E., Peng, Y., Ma, X., et al. (2017). Metabolic pathways regulated by chitosan contributing to drought resistance in white clover. J. Proteome Res. 16, 3039–3052. doi: 10.1021/acs.jproteome.7b00334
Li, Z., Zou, Y., Li, Y., Sun, C., Liu, Y., Cui, L., et al. (2021). Exploring the salt-and drought-tolerant genes of alfalfa through expression library screening strategy. Grass Res. 1, 1–14. doi: 10.48130/gr-2021-0011
Liang, D., Ni, Z., Xia, H., Xie, Y., Lv, X., Wang, J., et al. (2019). Exogenous melatonin promotes biomass accumulation and photosynthesis of kiwifruit seedlings under drought stress. Scientia Hortic. 246, 34–43. doi: 10.1016/j.scienta.2018.10.058
London, G., Carroll, G. T., Feringa, B. L. (2013). Silanization of quartz, silicon and mica surfaces with light-driven molecular motors: construction of surface-bound photo-active nanolayers. Organic Biomolecular Chem. 11, 3477–3483. doi: 10.1039/c3ob40276b
Lu, S., Chen, Y., Wang, S., Han, B., Zhao, C., Xue, P., et al. (2023). Combined metabolomic and transcriptomic analysis reveals key components of OsCIPK17 overexpression improves drought tolerance in rice. Front. Plant Sci. 13, 1043757. doi: 10.3389/fpls.2022.1043757
Luyckx, M., Hausman, J.-F., Lutts, S., Guerriero, G. (2017). Silicon and plants: current knowledge and technological perspectives. Front. Plant Sci. 8, 411. doi: 10.3389/fpls.2017.00411
Ma, J. F., Ryan, P. R., Delhaize, E. (2001). Aluminium tolerance in plants and the complexing role of organic acids. Trends Plant Sci. 6, 273–278. doi: 10.1016/s1360-1385(01)01961-6
Martínez-Noël, G. M. A., Tognetti, J. A. (2018). “Sugar signaling under abiotic stress in plants,” in Plant metabolites and regulation under environmental stress, 397–406. doi: 10.1016/B978-0-12-812689-9.00022-4
Nemat Alla, M. M., Hassan, N. M., Budran, I. G., El-Bastawisy, Z. M., El-Harary, E. H. (2022). Stigmasterol alleviates the impacts of drought in flax and improves oil yield via modulating efficient antioxidant and ROS homeostasis. Iranian J. Plant Physiol. 12, 3973–3984. doi: 10.30495/ijpp.2022.689073
Nie, L., Jiang, X., Wu, S., Zhang, J., Liu, P. (2010). Effects of DA-6 on leaf photosynthetic carboxylase and protective enzyme activities and grain yield of high-yielding summer maize. J. Appl. Ecol. 21, 2558–2564.
Okçu, G., Kaya, M. D., Atak, M. (2005). Effects of salt and drought stresses on germination and seedling growth of pea (Pisum sativum L.). Turkish J. Agric. forestry 29, 237–242. doi: 10.3906/tar-0407-2
Patel, J., Khandwal, D., Choudhary, B., Ardeshana, D., Jha, R. K., Tanna, B., et al. (2022). Differential physio-biochemical and metabolic responses of peanut (Arachis hypogaea L.) under multiple abiotic stress conditions. Int. J. Mol. Sci. 23, 660. doi: 10.3390/ijms23020660
Pourcelot, M., Barbier, M., Landoni, M., S Couto, A., Grand, E., Kovensky, J. (2011). Synthesis of galacturonate containing disaccharides and protein conjugates. Curr. Organic Chem. 15, 3523–3534. doi: 10.2174/138527211797374706
Rautengarten, C., Birdseye, D., Pattathil, S., McFarlane, H. E., Saez-Aguayo, S., Orellana, A., et al. (2017). The elaborate route for UDP-arabinose delivery into the Golgi of plants. Proc. Natl. Acad. Sci. 114, 4261–4266. doi: 10.1073/pnas.1701894114
Reiter, W. D., Chapple, C., Somerville, C. R. (1997). Mutants of Arabidopsis thaliana with altered cell wall polysaccharide composition. Plant J. 12, 335–345. doi: 10.1046/j.1365-313x.1997.12020335.x
Saffan, S. E.-S. (2008). Effect of heat stress on phytochemical composition of peanut seedlings. Res. J. Agric. Biol. Sci. 4, 167–174.
Sanchez, D. H., Schwabe, F., Erban, A., Udvardi, M. K., Kopka, J. (2012). Comparative metabolomics of drought acclimation in model and forage legumes. Plant Cell Environ. 35, 136–149. doi: 10.1111/j.1365-3040.2011.02423.x
Sawicka, B., Krochmal-Marczak, B., Sawicki, J., Skiba, D., Pszczółkowski, P., Barbaś, P., et al. (2023). White clover (Trifolium repens L.) cultivation as a means of soil regeneration and pursuit of a sustainable food system model. Land 12, 838. doi: 10.3390/land12040838
Seki, M., Umezawa, T., Urano, K., Shinozaki, K. (2007). Regulatory metabolic networks in drought stress responses. Curr. Opin. Plant Biol. 10, 296–302. doi: 10.1016/j.pbi.2007.04.014
Sharma, A., Shahzad, B., Rehman, A., Bhardwaj, R., Landi, M., Zheng, B. (2019). Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 24, 2452. doi: 10.3390/molecules24132452
Shi, H., Jiang, C., Ye, T., Tan, D., Reiter, R. J., Zhang, H., et al. (2015). Comparative physiological, metabolomic, and transcriptomic analyses reveal mechanisms of improved abiotic stress resistance in Bermudagrass [Cynodon dactylon (L). Pers.] by exogenous melatonin. J. Exp. Bot. 66, 681–694. doi: 10.1093/jxb/eru373
Sincik, M., Acikgoz, E. (2007). Effects of white clover inclusion on turf characteristics, nitrogen fixation, and nitrogen transfer from white clover to grass species in turf mixtures. Commun. Soil Sci. Plant Anal. 38, 1861–1877. doi: 10.1080/00103620701435621
Srivastava, S., Brychkova, G., Yarmolinsky, D., Soltabayeva, A., Samani, T., Sagi, M. (2017). Aldehyde oxidase 4 plays a critical role in delaying silique senescence by catalyzing aldehyde detoxification. Plant Physiol. 173, 1977–1997. doi: 10.1104/pp.16.01939
Sumner, L. W., Mendes, P., Dixon, R. A. (2003). Plant metabolomics: large-scale phytochemistry in the functional genomics era. Phytochemistry 62, 817–836. doi: 10.1016/s0031-9422(02)00708-2
Velikova, V., Yordanov, I., Edreva, A. (2000). Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci. 151, 59–66. doi: 10.1016/S0168-9452(99)00197-1
Wang, J., Juliani, H. R., Jespersen, D., Huang, B. (2017). Differential profiles of membrane proteins, fatty acids, and sterols associated with genetic variations in heat tolerance for a perennial grass species, hard fescue (Festuca trachyphylla). Environ. Exp. Bot. 140, 65–75. doi: 10.1016/j.envexpbot.2017.05.014
Wen, D., Li, Y., He, L., Zhang, C. (2019). Transcriptome analysis reveals the mechanism by which spraying diethyl aminoethyl hexanoate after anthesis regulates wheat grain filling. BMC Plant Biol. 19, 1–10. doi: 10.1186/s12870-019-1925-5
Wenker, H. (1938). Syntheses from ethanolamine. V. Synthesis of Δ2-oxazoline and of 2, 2’-Δ2-dioxazoline. J. Am. Chem. Soc. 60, 2152–2153. doi: 10.1021/ja01276a036
Wu, F., Bao, W., Li, F., Wu, N. (2008). Effects of drought stress and N supply on the growth, biomass partitioning and water-use efficiency of Sophora davidii seedlings. Environ. Exp. Bot. 63, 248–255. doi: 10.1016/j.envexpbot.2007.11.002
Xu, M., Zhang, M., Tu, Y., Zhang, X. (2024). Overexpression of the OsFes1A increased the phytosterols content and enhanced drought and salt stress tolerance in Arabidopsis. Planta 259, 1–14. doi: 10.1007/s00425-024-04346-w
Zargar, S. M., Mahajan, R., Bhat, J. A., Nazir, M., Deshmukh, R. (2019). Role of silicon in plant stress tolerance: opportunities to achieve a sustainable cropping system. 3 Biotech. 9, 73. doi: 10.1007/s13205-019-1613-z
Zentella, R., Sui, N., Barnhill, B., Hsieh, W. P., Hu, J., Shabanowitz, J., et al. (2017). The Arabidopsis O-fucosyltransferase SPINDLY activates nuclear growth repressor DELLA. Nat. Chem. Biol. 13, 479–485. doi: 10.1038/nchembio.2320
Zhang, C., He, P., Li, Y., Li, Y., Yao, H., Duan, J., et al. (2016). Exogenous diethyl aminoethyl hexanoate, a plant growth regulator, highly improved the salinity tolerance of important medicinal plant Cassia obtusifolia L. J. Plant Growth Regul. 35, 330–344. doi: 10.1007/s00344-015-9536-3
Zhang, J., Zhang, Q., Xing, J., Li, H., Miao, J., Xu, B. (2021). Acetic acid mitigated salt stress by alleviating ionic and oxidative damages and regulating hormone metabolism in perennial ryegrass (Lolium perenne L.). Grass Res. 1, 1–10. doi: 10.48130/gr-2021-0003
Zhao, C., Zayed, O., Zeng, F., Liu, C., Zhang, L., Zhu, P., et al. (2019). Arabinose biosynthesis is critical for salt stress tolerance in Arabidopsis. New Phytol. 224, 274–290. doi: 10.1111/nph.15867
Zhou, W., Chen, F., Zhao, S., Yang, C., Meng, Y., Shuai, H., et al. (2019). DA-6 promotes germination and seedling establishment from aged soybean seeds by mediating fatty acid metabolism and glycometabolism. J. Exp. Bot. 70, 101–114. doi: 10.1093/jxb/ery247
Zhou, M., Yuan, Y., Lin, J., Lin, L., Zhou, J., Li, Z. (2023). γ-Aminobutyric acid priming alleviates acid-aluminum toxicity to creeping bentgrass by regulating metabolic homeostasis. Int. J. Mol. Sci. 24, 14309. doi: 10.3390/ijms241814309
Keywords: water deficit, metabolomics, organic acids, sugars, physiological function, photosynthetic performance
Citation: Hassan MJ, Najeeb A, Zhou M, Raza MA, Ali U, Cheng B, Ling Y and Li Z (2024) Diethyl aminoethyl hexanoate reprogramed accumulations of organic metabolites associated with water balance and metabolic homeostasis in white clover under drought stress. Front. Plant Sci. 15:1430752. doi: 10.3389/fpls.2024.1430752
Received: 10 May 2024; Accepted: 23 September 2024;
Published: 11 October 2024.
Edited by:
Weiqiang Li, Chinese Academy of Sciences (CAS), ChinaReviewed by:
Yuguang Wang, Heilongjiang University, ChinaAdrian A. Moreno, Universidad Andres Bello, Chile
Copyright © 2024 Hassan, Najeeb, Zhou, Raza, Ali, Cheng, Ling and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhou Li, bGl6aG91MTk4NjgxNEAxNjMuY29t; Yao Ling, OTcyOTc1MkBxcS5jb20=
 Muhammad Jawad Hassan
Muhammad Jawad Hassan Atiqa Najeeb1
Atiqa Najeeb1 Min Zhou
Min Zhou Muhammad Ali Raza
Muhammad Ali Raza Zhou Li
Zhou Li