- 1Department of Genetics, Development and Cell Biology, Iowa State University, Ames, IA, United States
- 2Roy J. Carver Department of Biochemistry, Biophysics and Molecular Biology, Iowa State University, Ames, IA, United States
Introduction: VPS45 belongs to the Sec1/Munc18 family of proteins, which interact with and regulate Qa-SNARE function during membrane fusion. We have shown previously that Arabidopsis thaliana VPS45 interacts with the SYP61/SYP41/VTI12 SNARE complex, which locates on the trans-Golgi network (TGN). It is required for SYP41 stability, and it functions in cargo trafficking to the vacuole and in cell expansion. It is also required for correct auxin distribution during gravitropism and lateral root growth.
Results: As vps45 knockout mutation is lethal in Arabidopsis, we identified a mutant, vps45-3, with a point mutation in the VPS45 gene causing a serine 284-to-phenylalanine substitution. The VPS45-3 protein is stable and maintains interaction with SYP61 and SYP41. However, vps45-3 plants display severe growth defects with significantly reduced organ and cell size, similar to vps45 RNAi transgenic lines that have reduced VPS45 protein levels. Root hair and pollen tube elongation, both processes of tip growth, are highly compromised in vps45-3. Mutant root hairs are shorter and thicker than those of wild-type plants, and are wavy. These root hairs have vacuolar defects, containing many small vacuoles, compared with WT root hairs with a single large vacuole occupying much of the cell volume. Pollen tubes were also significantly shorter in vps45-3 compared to WT.
Discussion: We thus show that VPS45 is essential for proper tip growth and propose that the observed vacuolar defects lead to loss of the turgor pressure needed for tip growth.
1 Introduction
The endomembrane system consists of membrane bound organelles that exchange protein and lipid cargo by vesicle trafficking (Wang and Hussey, 2015; Rout and Field, 2017). Vesicle trafficking proteins mediate vesicle budding from the donor compartment, movement, and docking and fusion with a target organelle (Bonifacino and Glick, 2004). These proteins include SNAREs (soluble N-ethylmaleimide sensitive factor adaptor protein receptors), Rab GTPases, tethers, and regulatory Sec1/Munc18 (SM) proteins (Kim and Brandizzi, 2012; Hong and Lev, 2014; Zhang and Hughson, 2021), which cooperate to drive membrane fusion. The cargo contained within a vesicle is therefore delivered to a cellular compartment such as the Golgi or, in the case of exocytosis, released from the cell (Baker and Hughson, 2016).
SNARE proteins catalyze vesicle fusion in all eukaryotes, with formation of a trans-SNARE complex between SNAREs on opposing membranes. The complex involves three target SNAREs (t-SNAREs) anchored on the target membrane and a vesicle SNARE (v-SNARE), anchored on the destination membrane (Jahn and Scheller, 2006; Baker and Hughson, 2016). SNAREs are also classified as Qa, Qb, Qc (usually t-SNAREs) and R (usually v-SNARES), depending on the presence of a conserved central glutamine or arginine residue in the SNARE motif (Ungar and Hughson, 2003; Zhang and Hughson, 2021). SNARE-mediated membrane fusion is regulated by SM-family proteins, which are peripheral membrane proteins that interact with Qa-SNAREs (Carr and Rizo, 2010; Rizo and Südhof, 2012). The SM protein VPS (Vacuolar Protein Sorting) 45 localizes to the trans-Golgi network and early endosomes and functions in vesicle fusion with these organelles (Koumandou et al., 2007).
In Arabidopsis thaliana, homozygous null mutations in VPS45 are lethal, showing that VPS45 is critical for plant growth. VPS45 interacts with the SYP (Syntaxin of Plants) 41/SYP61/VTI (Vps ten interacting) 12 SNARE complex at the TGN (Zouhar et al., 2009), which regulates trafficking at the TGN for vacuolar cargo sorting, secretion of cell wall components, auxin homeostasis and abiotic/biotic stress responses (Bassham et al., 2000; Zhu et al., 2002; Surpin et al., 2003; Uemura et al., 2012; Tanaka et al., 2013). RNA interference lines with greatly reduced VPS45 protein levels also had decreased SYP41 protein (Zouhar et al., 2009), reminiscent of the situation in yeast, in which Tlg2p, a putative ortholog of SYP41, is unstable in a vps45 mutant (Bryant and James, 2001). This further underscores the role of VPS45 in regulating the activity of the SYP41/SYP61/VTI12 SNARE complex. VPS45 silencing resulted in mis-sorting of vacuolar sorting receptors (VSRs), membrane-bound receptors that recognize cargo for transport to vacuoles, and interfered with the sorting of cargo containing C-terminal vacuolar sorting determinants (ctVSDs) (Zouhar et al., 2009). Atben2, containing a point mutation in VPS45 resulting in an aspartate-to-asparagine substitution at the 129th amino acid position, revealed a role for VPS45 in auxin transporter recycling and endocytic uptake of membrane cargo from the plasma membrane (Tanaka et al., 2013). Together, these results suggest a critical role for VPS45, along with the SYP41/SYP61/VTI12 complex, at the TGN in endocytic and vacuolar cargo sorting.
Here, we identified vps45-3, a VPS45 mutant harboring a serine to phenylalanine substitution at the 284th position of the polypeptide. vps45-3 plants have a dwarf phenotype, with reduced organ size and cell expansion defects, consistent with the previously reported RNAi lines (Zouhar et al., 2009). The mutants also have reduced growth of root hairs and pollen tubes, potentially caused by fragmented vacuoles. VPS45 is therefore important for cell expansion in both diffusely growing and tip growing cells.
2 Materials and methods
2.1 Arabidopsis thaliana genotypes and plant growth conditions
Arabidopsis thaliana genotypes used are WT (Columbia-0), vps45-3 (TILLING mutant), vps45-3 COM (complemented line), WT-EYFP-RabF2a, vps45-3-EYFP-RabF2a. All genotypes were grown at 22°C either on soil in growth chambers or on sterile nutrient media under light racks. Soil-grown plants were kept in long day (16 hr light/8 hr dark) conditions. For growth on nutrient media, seeds were surface sterilized in 33% (v/v) bleach and 0.1% (v/v) Triton X-100 (Thermo Scientific, AAA16046AP) solution for 10 minutes and washed with sterile water at least five times. After two days of cold treatment in the dark, the seeds were plated on solid 1/2-strength Murashige-Skoog (MS) medium with vitamins (Caisson Labs, MSP09), 1% (w/v) sucrose (IBI scientific, IB37160), 2.4 mM 2-morphinolino-ethanesulfonic acid pH 5.7 (Sigma-Aldrich, M3671) and 0.8% (w/v) Phytoagar (Caisson Labs, PTP01). vps45-3 was generated by TILLING (Colbert et al., 2001) and mutants identified using forward primer 5’ -TGGCGTTGAAACGAAGACCTGTCA-3’ and reverse primer 5’-GAGCAGGACTTGGCTTGCAATGGT-3’ as described (de Vere et al., 2015). The point mutation introduces a novel MseI restriction site in a 998bp or 587bp gDNA or cDNA region respectively. Homozygous point mutants were identified by PCR amplification of this 998bp region containing the novel restriction site followed by digestion with MseI restriction enzymes at 37 °C for 1 hr. Upon gel electrophoresis, WT gDNA results in 3 bands of 422, 333 and 243 bp while vps45-3 gDNA results in 4 bands of 422, 243,213 and 120 bp. Restriction digestion of amplified cDNA results in two bands of 328bp and 259 bp for vps45-3 cDNA while the WT cDNA lacks this site and thus results in a single 587 bp band.
Complementation of vps45-3 mutants was performed by introducing a binary vector containing the VPS45 coding sequence driven by the VPS45 endogenous promoter described in (Zouhar et al., 2009). Plants were transformed using Agrobacterium tumefaciens by the floral dip method (Clough and Bent, 1998). Complemented lines were identified by resistance to hygromycin (30 mg L−1) and MseI restriction digestion as described above. Homozygous transformant lines were identified by appearance of all three bands in the restriction profile and resistance to hygromycin in subsequent progeny of the primary transformants.
EYFP- RabF2a (Preuss et al., 2004) constructs were generously provided by Dr. Erik Nielsen. All constructs were introduced into Arabidopsis by the floral dip method (Clough and Bent, 1998). Transgenic plants were selected on kanamycin and were imaged by confocal microscopy using a YFP filter at excitation and emission wavelengths of 488 nm and 528 nm respectively.
2.2 In vitro pollen germination and pollen tube length measurement
Flowers were collected from Arabidopsis plants 1 to 2 weeks after bolting and dehydrated at room temperature for at least 2 hrs. Pollen was germinated on an agar medium containing 18% sucrose (IBI Scientific, IB37160), 0.01% boric acid, 1 mM MgSO4, 1 mM CaCl2, 1 mM Ca(NO3)2, and 0.5% agar, pH 7 (Li et al., 1999) at room temperature for 12 hours. It was then examined under a Zeiss MacroZoom light microscope (Carl Zeiss Inc., Jena, Germany) and photographed with a 35 mm camera. Pollen tube lengths were measured as the distance from the pollen grain to the pollen tube tip, using segmented line and length measurements with the ImageJ software (Schneider et al., 2012). Average length and standard deviations for 100 pollen tubes were calculated for 3 independent biological replicates, n = 100.
2.3 Root hair phenotyping
Five-day-old seedlings were mounted on a slide and imaged using a Zeiss AxioImager microscope (Carl Zeiss Inc., Jena, Germany) with a 20X objective with bright field and differential interference contrast (DIC). Root hair length quantification was carried out by using segmented line and length measurements with the ImageJ software. Average length and standard deviations among at least 100 root hairs were calculated. Root hairs were imaged in the root elongation zone and neighboring cells in the early maturation zone, while excluding the older maturation zone cells.
2.4 FM4-64 staining and brefeldin A treatment
FM4-64 staining was modified from (Dettmer et al., 2006). To test bulk endocytosis, 4-day-old seedlings were transferred to MS liquid medium containing 4 μM FM4-64 (Invitrogen, T3166) for 2 min and subsequently washed twice for 30 s each time in 0.5× MS liquid medium before visualization. For analyzing arrival of FM4-64 at Brefeldin A (BFA) bodies, 4-day-old seedlings were transferred to 0.5× MS liquid medium containing 35 μM BFA (Sigma-Aldrich, B7651) for an hour followed by a 10 min treatment with 4 μM FM4-64 plus 35 μM BFA and two subsequent washes of 30 s each. The root tips were visualized using a Leica SP5 confocal laser scanning microscope (Leica Microsystems, Wetzlar, Germany) at the Iowa State University Roy J Carver High Resolution Microscopy Facility, using a 63× oil immersion objective lens and excitation and emission wavelengths of 558 and 734 nm. Images were acquired under identical conditions for both genotypes with equal exposure, scan frequency and line average settings. A total of 15 seedlings from at least three independent replicates were observed for each treatment and genotype.
2.5 Subcellular fractionation
0.8 g of WT and vps45-3 7-day-old seedlings were collected and ground in 1 mL of cold extraction buffer (0.3 M Sucrose, 0.1 M Tris-HCl, 1 mM EDTA, pH 7.5), with protease inhibitor cocktail (Roche). This was followed by centrifugation at 2,800g for 5 minutes at 4oC. A 100 μl portion of the supernatant was kept as the total protein fraction and the rest of the supernatant was transferred to a new tube followed by centrifugation at 13,000g for 30 minutes at 4oC. The pellet was resuspended in 100 μl of extraction buffer and represents the P13 fraction, and the supernatant was transferred to ultra-centrifuge tubes and centrifuged at 100,000g for 30 minutes at 4oC. The supernatant was transferred to a new tube and represents the SUP fraction, and the pellet was resuspended in 100 μl of extraction buffer and represents the P100 fraction.
Protein fractions were dissolved in SDS loading buffer and analyzed by immunoblotting using the indicated antibodies (Zouhar et al., 2009).
2.6 Immunoprecipitation
Immunoprecipitation was done as previously described (Bassham et al., 2000) using antibodies also previously described (Zouhar et al., 2009). 5 grams of 4 - to 6 - week-old Arabidopsis leaves were ground in 15 ml cold extraction buffer (0.3 M Sucrose, 0.1 M Tris-HCl, 1 mM EDTA, pH 7.5) with protease inhibitor cocktail (Roche, 11836153001). The crude extract was passed through Miracloth to remove debris, followed by centrifugation at 1000g for 5 min at 4°C. To dissolve membrane proteins, 0.5% Triton X-100 (v/v) was added to the supernatant, followed by rocking at 4°C for 2-3 hours. The protein extract was then transferred to ultra-centrifuge tubes followed by centrifugation at 100,000g to pellet the insoluble material. The supernatant was transferred to new 15 mL tubes and anti-SYP41 (1:200) antibodies were added to the samples followed by 2 hours rocking at 4°C. Protein A Sepharose CL-4B (Sigma-Aldrich, GE17-0780-01) was prepared according to the manufacturer’s protocol. The samples were further rocked with 50 μl suspended prepared protein A Sepharose overnight at 4°C. Beads were collected by centrifugation at 200g for 5 minutes at 4°C and washed 3 times with PBS buffer with 0.1% (v/v) Triton X-100. Proteins were eluted in SDS loading buffer (62.5 mM Tris-HCl (pH 6.8), 2% (w/v) sodium dodecyl sulfate, 25% (v/v) glycerol, and 0.01% bromophenol blue). Eluted proteins were analyzed by immunoblotting using the indicated antibodies.
2.7 FDA and MDY-64 and propidium iodide staining
Fluorescein diacetate (FDA) staining was performed as previously described (Saedler et al., 2009). Seedlings were submerged in a solution of 40 μg FDA in water for 5 min, and then mounted on a slide. Confocal microscopic images of root hairs were obtained using a Leica confocal microscope (Leica Microsystems, Wetzlar, Germany) using 63× oil immersion objective lens after excitation of the dye at 488 nm and emission was detected between 520 and 560 nm.
MDY-64 staining was performed as described (Scheuring et al., 2015). Seedlings were submerged in a solution of 0.25 μM MDY-64 (Invitrogen, Y7536) in 0.5X liquid MS medium for 5 min. The seedlings were then rinsed in 0.5X liquid MS medium and mounted on a slide. Confocal images of root hairs were obtained using a 63× oil immersion objective lens after excitation of the dye at 451 nm using an Ar/Kr laser, and emission was detected at 497 nm.
2.8 Visualization of YFP localization
Five-day-old seedlings were transferred to a slide and imaged using a Zeiss AxioImager microscope (Carl Zeiss Inc., Jena, Germany) with a 40X objective with differential interference contrast and confocal microscope using an EYFP-specific filter.
3 Results
3.1 vps45-3 mutant has a severe dwarf phenotype
Arabidopsis VPS45 is essential for plant growth and development, as homozygous null mutants are inviable (Zouhar et al., 2009). As an alternative approach to determine the physiological roles of VPS45, a point mutation in VPS45 was recovered by a TILLING approach (Colbert et al., 2001) and designated as vps45-3. The mutation is a C-to-T substitution at the 851st nucleotide of the VPS45 coding sequence, leading to a serine-to-phenylalanine substitution at the 284th amino acid position (Figure 1A). This mutation introduces a new MseI restriction site in the vps45-3 coding sequence, allowing differentiation between mutant and wild-type alleles (Figure 1B).
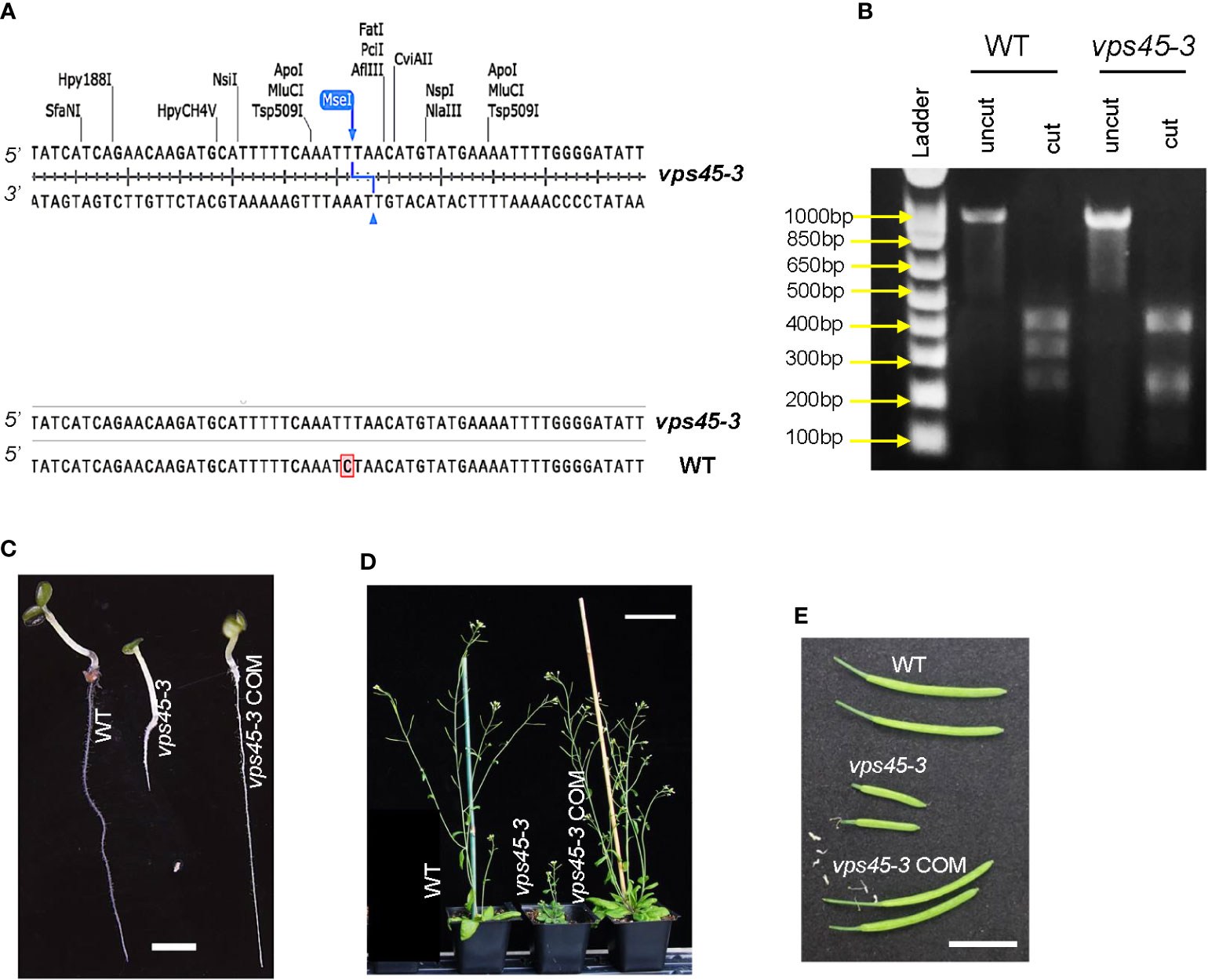
Figure 1 vps45-3 mutants display severe phenotypic defects. (A) Coding sequence alignment of VPS45 and vps45-3 displaying the site of nucleotide substitution for vps45-3. vps45-3 harbors a C-to-T substitution at the 851st nucleotide of the VPS45 coding sequence and this results in a novel MseI restriction site. (B) MseI restriction digest profiles of a 998 bp amplified gDNA fragment containing the mutation. vps45-3 gDNA gives four bands of 422, 243, 213 and 120 bp upon MseI digest while the WT cDNA lacks this site and thus results in three bands of 422, 333, 243 bp upon digestion. (C–E) Phenotypes of WT, vps45-3 and complemented (COM) vps45-3. (C) 7-day-old seedlings, grown on vertical plates on 0.5X MS medium, pH 6. Scale bar = 5 mm. (D) 30-day-old plants grown under long days showing the severe dwarf phenotype of vps45-3 compared to the WT and vps45-3 COM. Scale bar = 70 mm. (E) Siliques from 40-day-old long day-grown plants, Scale bar = 10 mm.
vps45-3 plants were dwarfed, with highly reduced sizes of many organs (Figures 1C–E, S1). similar to the previously reported VPS45 RNAi lines (Zouhar et al., 2009), although less severe. To confirm that the observed phenotype results from mutation of VPS45, we introduced the VPS45 cDNA driven by the native VPS45 promoter into the vps45-3 mutant to generate complementation lines (vps45-3 COM) and assessed the plant phenotype. Growth and organ size defects of vps45-3 plants were ameliorated by complementation with the VPS45 transgene (Figures 1C-E, S1), confirming that the defects were caused by mutation of the VPS45 gene and suggesting that the VPS45-3 protein has reduced function. The vps45-3 plants were fertile and produced flowers and viable seeds without any noticeable abnormalities. This confirms that VPS45 is important for plant growth and that the vps45-3 mutant is valuable to further analyze the function of VPS45.
3.2 VPS45 is important for cell expansion
We reasoned that the dwarf phenotype of vps45-3 plants could be due to reduced cell size, similar to RNAi plants. To analyze this possibility, 4-day-old seedling roots of WT, vps45-3 and vps45-3 COM were stained with propidium iodide (PI), which stains cell walls (Scheuring et al., 2015), and imaged by confocal microscopy. Cell size was significantly reduced in vps45-3 compared to WT and vps45-3 COM roots (Figures 2A–D). This was further visualized by using an agar imprinting method (Mathur and Koncz, 1997) to analyze the hypocotyl and root cells (Figure S2). Thus, a single ser-to-phe change in VPS45 causes cell expansion defects.
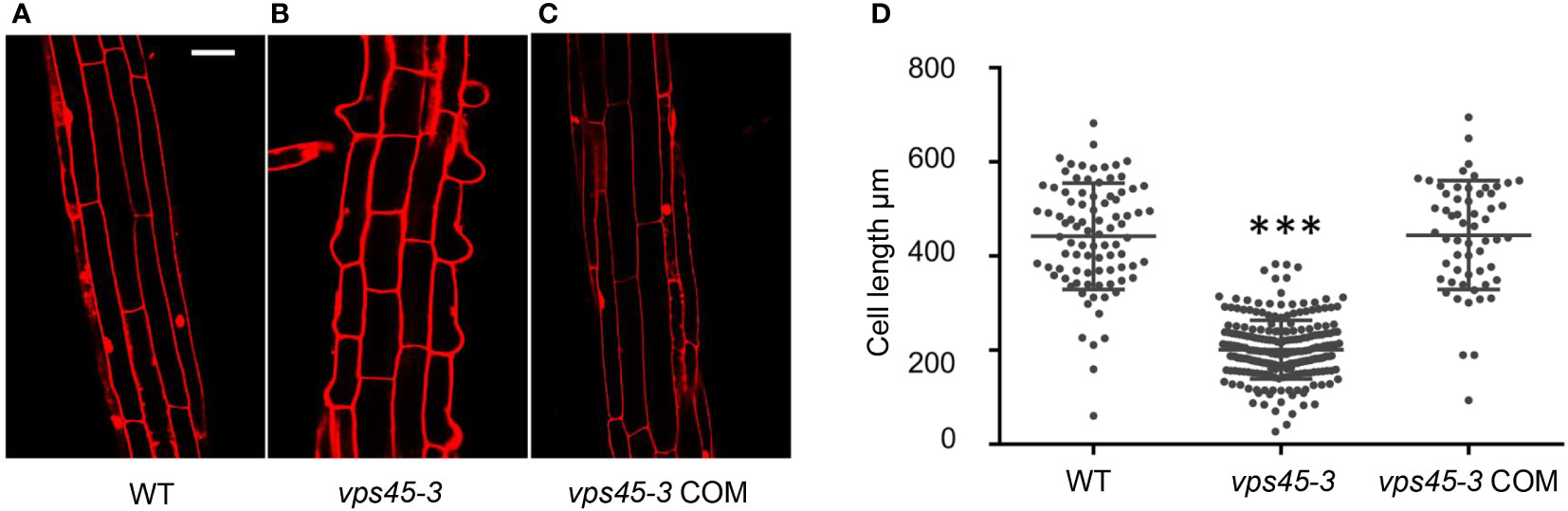
Figure 2 vps45-3 exhibits reduced cell size. (A–C) WT, vps45-3 and vps45-3 COM roots were stained with propidium iodide (PI) and imaged by confocal microscopy. Scale bar =50 μm. (D) Plot of average cell size for WT, n = 88, vps45-3, n = 231, and vps45-3 COM, n = 59. Cell length was computed using Image J software. Data are measurements of each individual cell, error bars show standard deviations, * shows statistically significant differences (P < 0.05) as determined by one-way ANOVA.
3.3 vps45-3 mutants have reduced growth of root hairs and pollen tubes
We observed that vps45-3 had a shorter main root compared to WT and had root hair defects (Figures 3A-C). Mutant root hairs were significantly shorter, wider and wavy compared with the root hairs of WT (Figures 3D, E). Complementation of the mutant rescued the root hair elongation defects (Figures 3F, G) confirming that the point mutation causes the observed root hair defects and that VPS45 is required for root hair cell expansion.
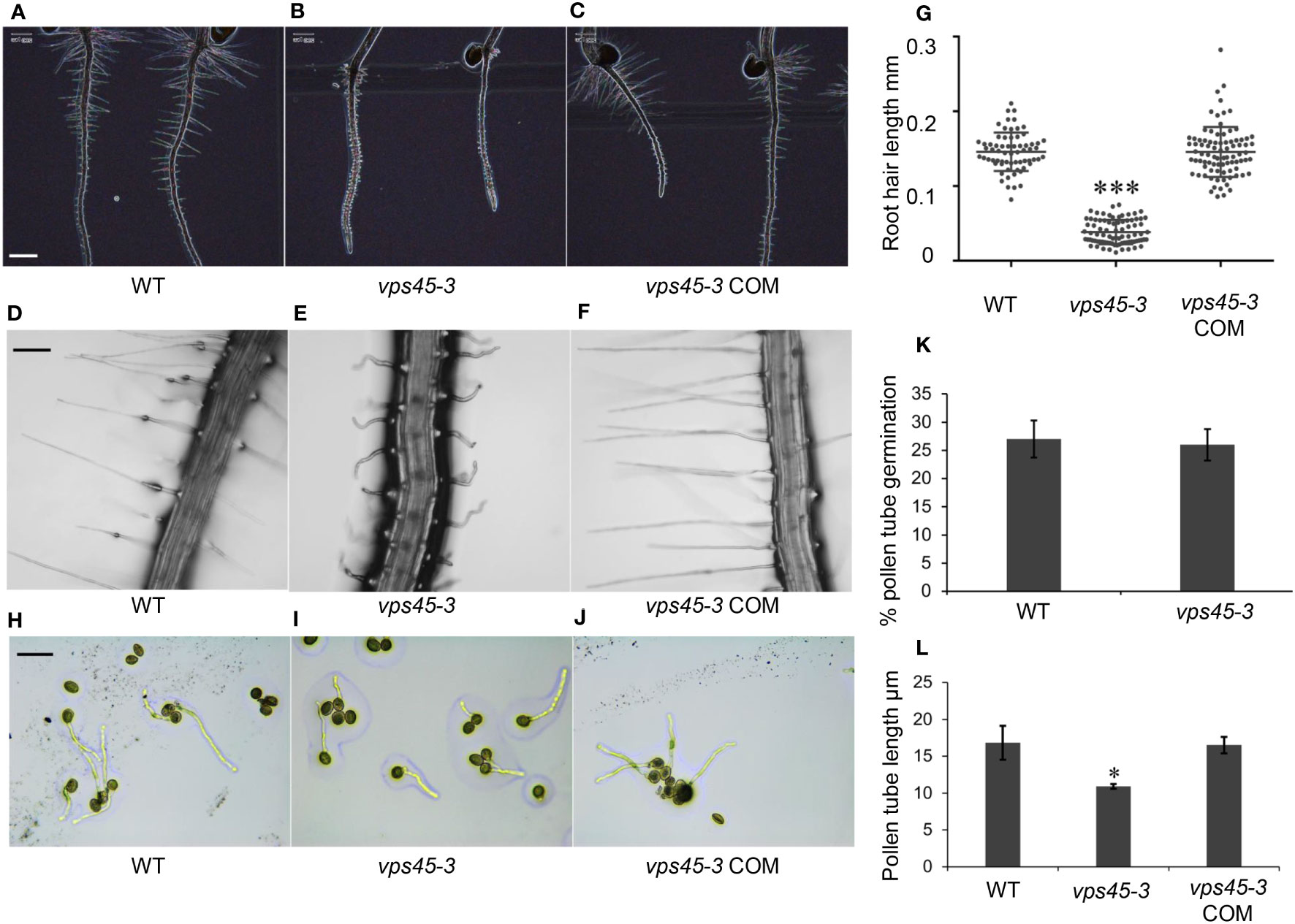
Figure 3 vps45-3 has root hair and pollen tube defects. (A–C) Color inverted images of 5-day-old roots of WT, vps45-3 and vps45-3 COM to show root hair silhouettes. Scale bar = 500 μm. (D–F) Representative light microscopic images of 7-day old WT, vps45-3 and vps45-3 COM root hairs. Scale bar = 50 μm. (G) Root hair length of 7-day old WT, n= 66, vps45-3, n= 83 and vps45-3 COM, n = 86 seedlings. Data are measurements of each individual root hair. Error bars show standard deviations, * shows statistically significant differences (P < 0.05), determined by one-way ANOVA. (H–J) Pollen grains from WT, vps45-3 and vps45-3 COM plants were germinated on medium overnight and examined under a light microscope. Scale bar = 50 μm (K) Percentage pollen germination comparison for WT and vps45-3. (L) Average pollen tube length comparison for WT, vps45-3 and vps45-3 COM. Data are measurements of average pollen tube length for three independent replicates. Error bars show standard deviations, * indicates statistically significant differences (P < 0.05), determined by one-way ANOVA.
Root hairs and pollen tubes undergo tip growth, in contrast to other cells which undergo diffuse growth (Mathur and Hülskamp, 2001). Tip growth involves development of apical-basal polarity of the endomembrane system and rapid secretion at the tip of the developing root hairs and pollen tubes (Cole and Fowler, 2006; Rounds and Bezanilla, 2013; Šamaj et al., 2006). To determine if vps45-3 has general defects in tip growth, we analyzed the growth of pollen tubes in vitro. Pollen from WT and vps45-3 plants was plated onto pollen germination medium (Li et al., 1999) and incubated overnight to allow germination (Figures 3H-J). While no differences could be seen in the extent of germination between pollen from WT and mutant plants (Figure 3K), pollen tubes were significantly shorter in vps45-3 compared to WT, and this defect was rescued in vps45-3 COM lines (Figure 3L). These data suggest that VPS45 may play a role in tip growth in Arabidopsis.
3.4 The vps45-3 mutation has no effect on interaction with and stability of the SYP41 SNARE complex
Our results indicate that vps45-3 plants are dwarf with significant reduction in organ sizes, have defects in root hairs and pollen tubes, and show cell expansion defects. This suggests that the substitution of the serine to a phenylalanine, i.e. a polar to non-polar substitution, affects VPS45 function. Unlike a previously described VPS45 point mutant (ben2) (Tanaka et al., 2013), amino acid sequence alignment showed that the substituted amino acid in vps45-3 is not conserved across different organisms (Figure S3), and that this amino acid may be important for VPS45 function in plants only.
VPS45 has been implicated in regulating the stability and localization of its cognate SNARE complex (VTI12/SYP41/SYP61), as the levels of SYP41 were reduced in parallel to the levels of VPS45 in RNAi-silenced lines (Zouhar et al., 2009). To test the stability of both the SM protein and the SNARE complex we carried out subcellular fractionation of organelles from WT and vps45-3 seedlings, followed by immunoblotting of different fractions with VPS45, SYP41 and SYP61 antibodies. The amount of SYP41, SYP61 and VPS45 was similar in WT and vps45-3 in all fractions (Figure 4A), suggesting that both VPS45 and the SNARE proteins are stable in the mutant and that VPS45-3 can still associate with membranes.

Figure 4 VPS45-3 maintains stability and interaction with SYP41 SNARE complex. (A) VPS45-3 maintains proper subcellular distribution and stability of SYP41 and SYP61. Total protein samples from 7-day-old wild-type and vps45-3 plants were subjected to subcellular fractionation by centrifugation to obtain a total fraction (TOT) after centrifugation at 5,000g, low speed pellet (P13) after centrifugation at 13,000g, soluble fraction (SUP) and high-speed pellet (P100) after centrifugation at 100,000g. Aliquots of fractions were analyzed by SDS-PAGE followed by immunoblotting with the indicated antibodies. (B) Homology model of the predicted Arabidopsis VPS45-3 protein sequence modeled onto the crystal structure of c6MX1 from Chaetomium thermophilum (RCSB PDB). The mutated residue is shown in red and labeled and is in the 3a domain as shown by the black outline. (C) VPS45-3, SYP41, and SYP61 coimmunoprecipitate with SYP41 antibodies. Detergent solubilized membrane preparations from Arabidopsis leaves were subjected to immuno-isolation using SYP41 antibodies. Aliquots of total extracts and the eluate from the antibody column were analyzed by SDS-PAGE and immunoblotting with the indicated antibodies.
To further understand the potential effect of the point mutation, we used homology modeling to fit the predicted Arabidopsis VPS45-3 protein sequence onto the crystal structure of c6MX1 from Chaetomium thermophilum, with a confidence score of 100.0 among all available protein structures. The mutation is in domain 3a of the protein (Figure 4B) and in close proximity to a region that is important for VPS45 interaction with other proteins (Eisemann et al., 2020).
Since binding of a SM protein to its cognate SNARE is required for SNARE complex function (Furgason et al., 2009; Shanks et al., 2012), we assessed whether defects in the mutant might be caused by altered interaction of VPS45-3 with SYP41 and SYP61. To test the interaction of VPS45-3 with SYP41 and SYP61, SYP41 was immunoprecipitated from WT or vps45-3 plants using anti-SYP41 antibodies, and co-immunoprecipitation of VPS45 and SYP61 was assessed by immunoblotting (Figure 4C). The amount of VPS45 and SYP61 that co-precipitated with SYP41 was equivalent in WT and vps45-3 mutant. This implies that the point mutation does not affect the interaction of VPS45-3 with SYP41 and SYP61 (Figure 4C).
3.5 Endocytosis and membrane arrival at the TGN are unaffected in the mutants
Given that other vps45 mutants have endocytic defects (Tanaka et al., 2013), we assessed whether endocytosis and recycling are affected in the vps45-3 plants. This could explain the cell expansion phenotype owing to slower trafficking at the TGN during cell expansion (Gendre et al., 2015), particularly during root hair growth. We stained root cells with a FM4-64, a lipophilic styryl dye that is used as an endocytic tracer (Dettmer et al., 2006). The uptake of the FM4-64 was similar in both vps45-3 and WT cells (Figure 5A), suggesting no major effect of the vps45-3 mutation on internalization from the plasma membrane. We also tested whether membrane arrival at the TGN was affected in the mutants. We stimulated formation of TGN-endosomal aggregates by treating roots of 4-day old seedlings with Brefeldin A (BFA), a vesicle trafficking inhibitor, followed by incubation with FM4-64 and imaging with confocal microscopy. Labeling of the BFA compartments with the dye occurred at similar times in mutant and WT, suggesting that BFA body formation and arrival of membrane cargo at the TGN is not affected in the mutant (Figure 5B).
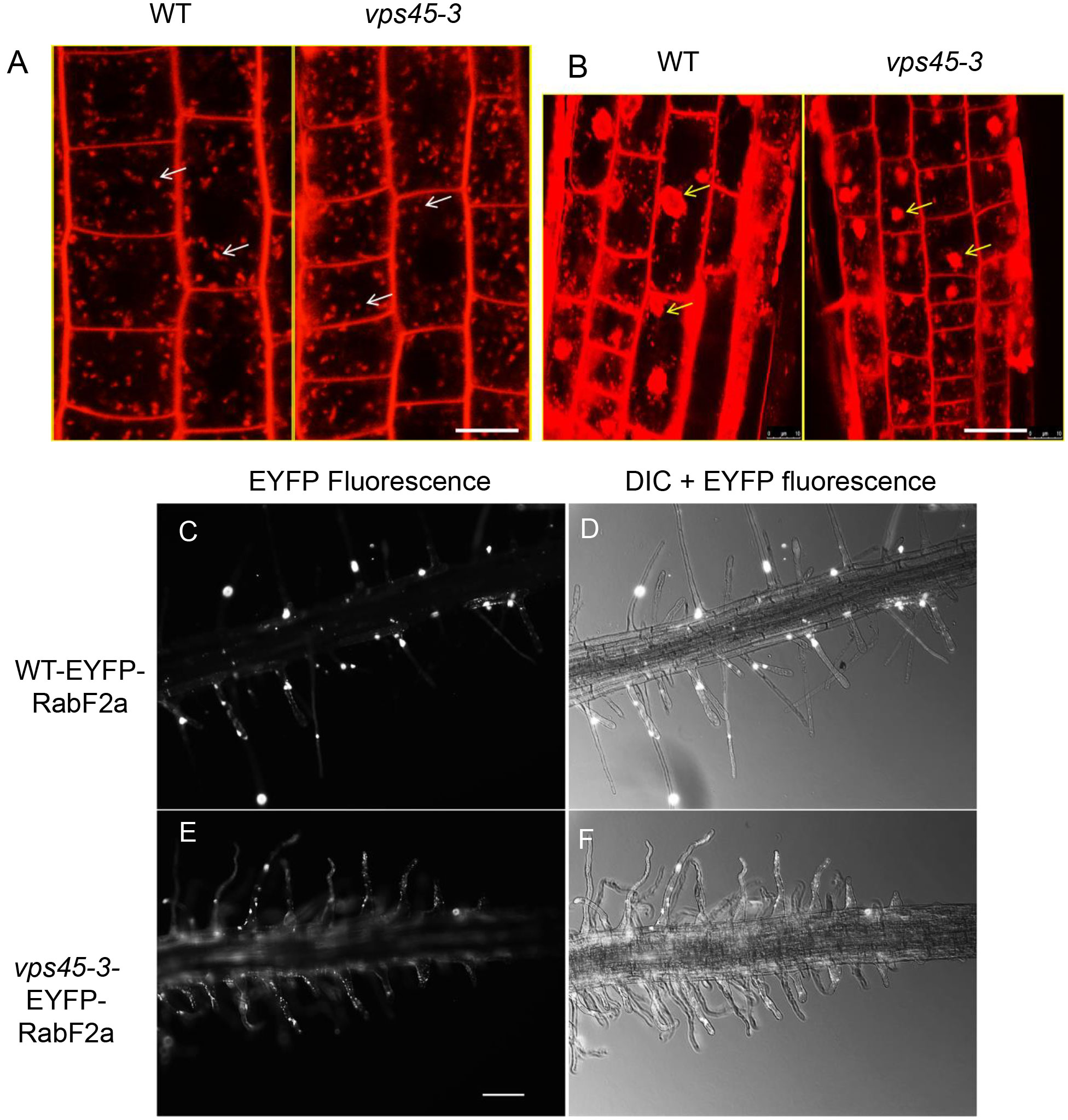
Figure 5 vps45-3 mutants have unaltered bulk endocytosis and transport of membrane cargo from the plasma membrane to BFA bodies. (A) 4-day old seedling roots were treated with 4 μM FM4-64 in liquid 0.5x MS medium and imaged by confocal microscopy after 2 min of treatment. White arrows indicate early endosomes/TGN. (B) Root cells were treated with 35 μm BFA for 1 h, followed by a 10 min incubation with 4 μM FM4-64, showing arrival of FM4-64 at BFA bodies. Yellow arrows indicate BFA bodies. Scale bar = 10 μm. (C–F) Root hairs of 5-day-old seedlings expressing the early endocytic compartment marker EYFP-RabF2a were imaged using a Zeiss Upright microscope with 40X objective lens either with transmitted light or with epifluorescence illumination and appropriate EYFP filters. Scale bar = 500 μm.
RabF2a is a Rab GTPase that localizes to early endocytic compartments in plants (Ueda et al., 2001; Preuss et al., 2004). We examined the distribution of RabF2a in root hairs of both WT and vps45-3. We transformed plants with an EYFP-RabF2a construct and observed three independently transformed lines by confocal microscopy. A similar distribution of EYFP-RabF2a was evident in both WT and vps45-3 root hairs, with small punctate structures spread along the length of the root hair as previously reported (Preuss et al., 2004) (Figures 5D–F). The organization of the endosomal system therefore appears to be intact in vps45-3. Taken together, these data suggest that endocytosis and membrane trafficking from the PM to the TGN are unaffected in vps45-3 mutants in both tip-growing and diffusely-growing cells.
3.6 vps45-3 root hairs have vacuolar defects
The root hair and pollen tube phenotypes suggest that the vps45-3 mutation might cause polarized tip growth defects. Polarized tip growth involves targeted deposition of cell wall and membrane material at the cell apex, and turgor pressure is a driving force for cell expansion via uptake of water into the vacuole (Cosgrove, 1993; Mendrinna and Persson, 2015). We reasoned that the root hair abnormality observed in vps45-3 could be due to vacuole defects that disrupt tip growth. To test this, we analyzed root hair vacuoles by staining root hairs of five day old seedlings with the tonoplast marker MDY-64 (Scheuring et al., 2015), and imaged them using confocal microscopy.
In elongating WT and vps45-3 COM root hairs, the vacuole was seen to occupy most of the cell, whereas in vps45-3, the root hair cell was filled with cytoplasm, with numerous small vacuoles visible (Figure 6; Movies M1, M2). Staining root hairs with fluorescein diacetate (FDA), which labels the cytoplasm, leaving the unstained vacuole visible, supported the idea that vps45-3 roots hairs have defects in vacuole morphology, as they appeared to have increased cytoplasmic staining (Figure S4). The changes in vacuolar morphology in root hairs therefore correlate with the cell expansion defects observed in vps45-3.
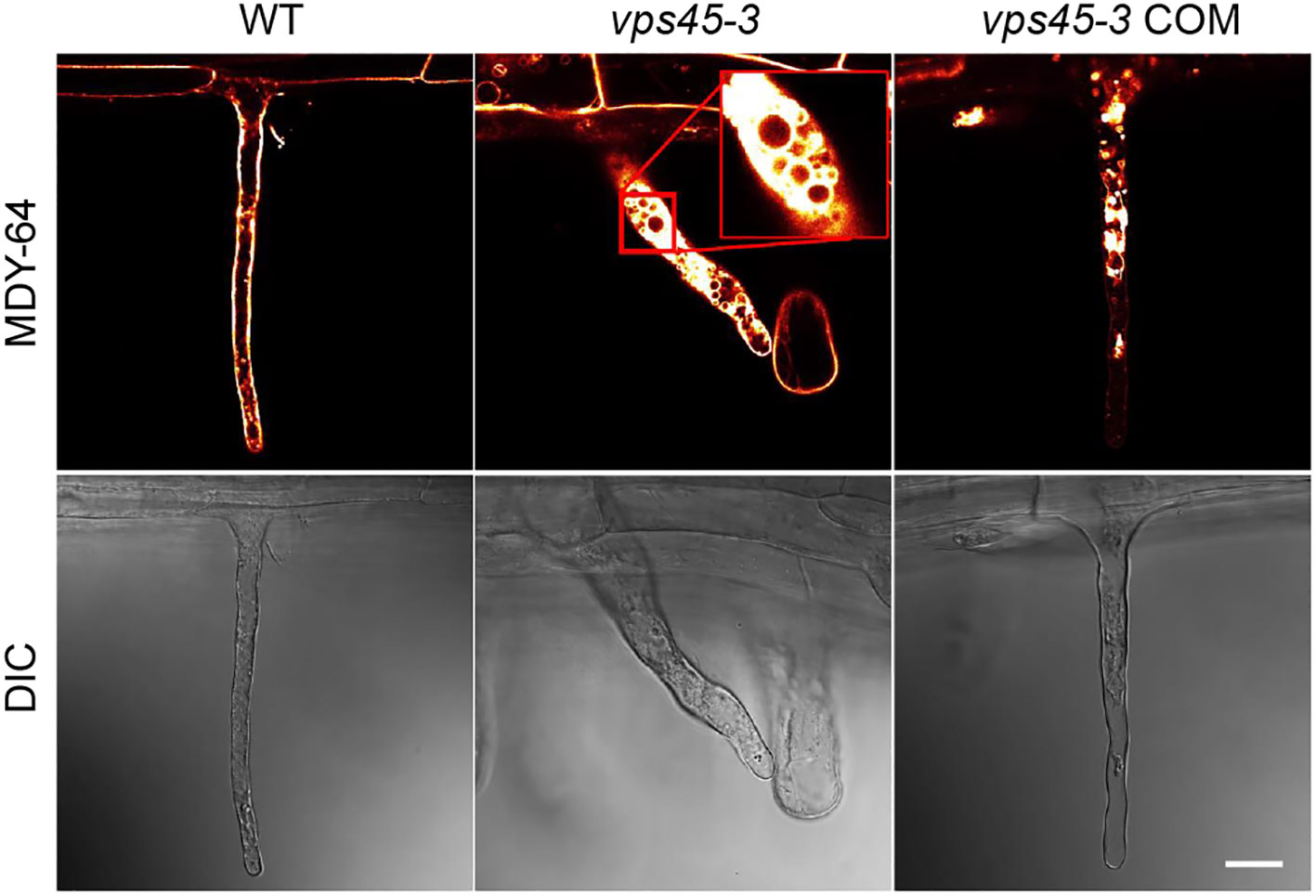
Figure 6 vps45-3 seedling root hairs show vacuole defects. Root hairs from 5-day-old seedlings were treated with the tonoplast membrane stain MDY-64 and imaged using confocal microscopy. Scale bar = 20 μm, DIC indicates differential interference contrast microscopy.
4 Discussion
We identified a new Arabidopsis vps45 mutant, vps45-3, which harbors a point mutation causing a serine-to-phenylalanine substitution at the 284th position of the VPS45 polypeptide chain. Previous studies revealed that a homozygous null allele of VPS45 is male gametophytic lethal, and RNAi lines with reduced VPS45 protein levels were used to study the function of VPS45 in plant growth (Zouhar et al., 2009). Similar to previously reported RNAi lines (Zouhar et al., 2009), vps45-3 showed a dwarf phenotype, with reduced organ size and cell expansion defects, thus confirming that this point mutation impacts the normal function of VPS45.
A fundamental step in vesicle trafficking is the interaction between the three t-SNAREs present on the target membrane and the v-SNARE present on the transport vesicle membrane (Parlati et al., 2000). SM proteins function as chaperones to enable SNARE complex assembly, typically by binding to the t-SNARE, which adopts an open conformation to expose the presumptive R-SNARE binding site (Eisemann et al., 2020; Zhang and Hughson, 2021). One possibility is that a mutation could change the conserved structure and thus affect the binding of VPS45-3 to its interaction partners. However, we showed that interaction between the mutant VPS45-3 protein and t-SNAREs SYP41 and SYP61 was unaffected, and therefore this is not the cause of the phenotypic defects. In yeast, Vps45 also interacts with the v-SNARE Snc2 (Shanks et al., 2012). The VPS45 cognate v-SNARE in Arabidopsis may be YKT6 (Chen et al., 2005), although this has not been shown in vivo. Further work is required to investigate whether the point mutation affects the interaction with the v-SNARE and the formation of the trans-SNARE complex.
Although our results show no change in interaction between VPS45-3 and cognate SNARE binding partners (SYP41 and SYP61), it is possible that the mutation might affect the interaction with other proteins. For example, the human VPS45 SM protein was shown to bind to the Rab5 effector Rabenosyn-5 (Nielsen et al., 2000). Further studies will be needed to ascertain any other interacting partners of VPS45 and the impact of the point mutation on such interactions.
VPS45 has been implicated in endocytic uptake of membrane cargo from the plasma membrane (Tanaka et al., 2013). Based on this, we hypothesized that the vps45-3 mutant may also have defects in endocytosis. Analysis of FM4-64 uptake in vps45-3 mutants suggested that bulk endocytosis is unaltered and studies with the fungal toxin Brefeldin A (Lippincott-Schwartz et al., 1991) suggested that membrane cargo arrival at the TGN-endosomal aggregates is also unaffected. The vps45-3 phenotypes seen are therefore likely to be a result of defects in biosynthetic trafficking to the vacuole.
We identified defects in polarized tip growth of both root hairs and pollen tubes in vps45-3. In root hairs, these tip growth defects correlated with vacuolar morphology defects, in which multiple small vacuoles were seen. VPS45 is important for localization of VSRs and for targeting of ctVSDs (Zouhar et al., 2009). Defects in VSR recycling and sorting of ctVSD-containing cargo may affect vacuolar morphology and subsequently cell expansion, and vacuole enlargement is critical during root hair expansion (Galway et al., 1997; Grierson and Schiefelbein, 2002). The SNARE VTI13 (belonging to the same family as VTI12) localizes to the vacuole and the TGN and is speculated to play roles in trafficking to the vacuole. Interestingly, a vti13 mutant has mislocalization of SYP41 and defective root hair growth (Larson et al., 2014). This supports a connection between vesicle fusion machineries at the TGN and vacuole and root hair growth. A recent report also demonstrated that an SM protein involved in secretion is required for pollen tube growth, further implicating SM proteins in tip growth (Beuder et al., 2022).
In summary, we identified a vps45-3 mutant which, unlike the previously described RNAi lines, maintained VPS45 protein levels and interaction with the t-SNARES SYP41 and SYP61 and their stability. Consistent with previous studies using knockdown lines (Zouhar et al., 2009), vps45-3 had reduced cell and plant size. We demonstrate a role for VPS45 in tip growth of root hair and pollen tubes and show that vps45-3 root hairs have fragmented vacuoles, compared to WT root hairs which have a single large vacuole occupying most of the root hair volume. We propose that reduced function of VPS45 leads to vacuole defects, which may affect vacuolar turgor pressure and consequently cause tip growth defects (Mendrinna and Persson, 2015).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
YM, RR and DB designed the experiments. YM and RR conducted the majority of the experiments. GS and YV performed pollen assays. WA performed cell size and root hair measurement. YM, RR and DB wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the US Department of Energy, grant # DE-SC0014038, and the US National Science Foundation, grant # MCB-2040582.
Acknowledgments
We thank Dr. Erik Nielsen for providing WTRabF2a seeds and a plasmid containing RabF2a. We thank Dr. Anton Sanderfoot for initial screening of the vps45-3 mutant.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2023.1120307/full#supplementary-material
Movie 1 | WT root hairs stained with MDY-64 and imaged by confocal microscopy.
Movie 2 | vps45-3 root hairs stained with MDY-64 and imaged by confocal microscopy.
References
Baker, R. W., Hughson, F. M. (2016). Chaperoning SNARE assembly and disassembly. Nat. Rev. Mol. Cell Biol. 17, 465–479. doi: 10.1038/nrm.2016.65
Bassham, D. C., Sanderfoot, A. A., Kovaleva, V., Zheng, H., Raikhel, N. V. (2000). AtVPS45 complex formation at the trans-golgi network. Mol. Biol. Cell 11, 2251–2265. doi: 10.1091/mbc.11.7.2251
Beuder, S., Lara-Mondragón, C., Dorchak, A., MacAlister, C. A. (2022). SEC1A is a major arabidopsis Sec1/Munc18 gene in vesicle trafficking during pollen tube tip growth. Plant J. 110, 1353–1369. doi: 10.1111/tpj.15742
Bonifacino, J. S., Glick, B. S. (2004). The mechanisms of vesicle budding and fusion. Cell 116, 153–166. doi: 10.1016/S0092-8674(03)01079-1
Bryant, N. J., James, D. E. (2001). Vps45p stabilizes the syntaxin homologue Tlg2p and positively regulates SNARE complex formation. EMBO J. 20, 3380–3388. doi: 10.1093/emboj/20.13.3380
Carr, C. M., Rizo, J. (2010). At The junction of SNARE and SM protein function. Curr. Opin. Cell Biol. 22, 488–495. doi: 10.1016/j.ceb.2010.04.006
Chen, Y., Shin, Y. K., Bassham, D. C. (2005). YKT6 is a core constituent of membrane fusion machineries at the arabidopsis trans-golgi network. J. Mol. Biol. 350, 92–101. doi: 10.1016/j.jmb.2005.04.061
Clough, S. J., Bent, A. F. (1998). Floral dip: A simplified method for agrobacterium-mediated transformation of arabidopsis thaliana. Plant J. 16, 735–743. doi: 10.1046/j.1365-313X.1998.00343.x
Colbert, T., Till, B. J., Tompa, R., Reynolds, S., Steine, M. N., Yeung, A. T., et al. (2001). High-throughput screening for induced point mutations. Plant Physiol. 126, 480–484. doi: 10.1104/pp.126.2.480
Cole, R. A., Fowler, J. E. (2006). Polarized growth: Maintaining focus on the tip. Curr. Opin. Plant Biol. 9, 579–588. doi: 10.1016/j.pbi.2006.09.014
Cosgrove, D. J. (1993). Water uptake by growing cells: An assessment of the controlling roles of wall relaxation, solute uptake, and hydraulic conductance. Int. J. Plant Sci. 154, 10–21. doi: 10.1086/297087
Dettmer, J., Hong-Hermesdorf, A., Stierhof, Y. D., Schumacher, K. (2006). Vacuolar h+-ATPase activity is required for endocytic and secretory trafficking in arabidopsis. Plant Cell 18, 715–730. doi: 10.1105/tpc.105.037978
de Vere, N., Rich, T. C. G., Trinder, S. A., Long, C. (2015). DNA Barcoding for plants. Methods Mol. Biol. 1245, 101–118. doi: 10.1007/978-1-4939-1966-6_8
Eisemann, T. J., Allen, F., Lau, K., Shimamura, G. R., Jeffrey, P. D., Hughson, F. M. (2020). The Sec1/Munc18 protein Vps45 holds the qa-SNARE Tlg2 in an open conformation. Elife 9, e60724. doi: 10.7554/ELIFE.60724
Furgason, M. L. M., MacDonald, C., Shanks, S. G., Ryder, S. P., Bryant, N. J., Munson, M. (2009). The n-terminal peptide of the syntaxin Tlg2p modulates binding of its closed conformation to Vps45p. Proc. Natl. Acad. Sci. U. S. A. 106, 14303–14308. doi: 10.1073/pnas.0902976106
Galway, M. E., Heckman, J. W., Schiefelbein, J. W. (1997). Growth and ultrastructure of arabidopsis root hairs: The rhd3 mutation alters vacuole enlargement and tip growth. Planta 201, 209–218. doi: 10.1007/BF01007706
Gendre, D., Jonsson, K., Boutté, Y., Bhalerao, R. P. (2015). Journey to the cell surface–the central role of the trans-golgi network in plants. Protoplasma 252, 385–398. doi: 10.1007/s00709-014-0693-1
Hong, W. J., Lev, S. (2014). Tethering the assembly of SNARE complexes. Trends Cell Biol. 24, 35–43. doi: 10.1016/j.tcb.2013.09.006
Jahn, R., Scheller, R. H. (2006). SNAREs - engines for membrane fusion. Nat. Rev. Mol. Cell Biol. 7, 631–643. doi: 10.1038/nrm2002
Kim, S. J., Brandizzi, F. (2012). News and views into the SNARE complexity in arabidopsis. Front. Plant Sci. 3. doi: 10.3389/fpls.2012.00028
Koumandou, V. L., Dacks, J. B., Coulson, R. M. R., Field, M. C. (2007). Control systems for membrane fusion in the ancestral eukaryote; evolution of tethering complexes and SM proteins. BMC Evol. Biol. 7, 29. doi: 10.1186/1471-2148-7-29
Larson, E. R., Domozych, D. S., Tierney, M. L. (2014). SNARE VTI13 plays a unique role in endosomal trafficking pathways associated with the vacuole and is essential for cell wall organization and root hair growth in arabidopsis. Ann. Bot. 114, 1147–1159. doi: 10.1093/aob/mcu041
Li, H., Lin, Y., Heath, R. M., Zhu, M. X., Yang, Z. (1999). Control of pollen tube tip growth by a rop GTPase-dependent pathway that leads to tip-localized calcium influx. Plant Cell 11, 1731–1742. doi: 10.2307/3871050
Lippincott-Schwartz, J., Yuan, L., Tipper, C., Amherdt, M., Orci, L., Klausner, R. D. (1991). Brefeldin a’s effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell 67, 601–616. doi: 10.1016/0092-8674(91)90534-6
Mathur, J., Hülskamp, M. (2001). Cell growth: How to grow and where to grow. Curr. Biol. 11, 402–404. doi: 10.1016/S0960-9822(01)00219-6
Mathur, J., Koncz, C. (1997). Method for preparation of epidermal imprints using agarose. Biotechniques 22, 280–282. doi: 10.2144/97222bm19
Mendrinna, A., Persson, S. (2015). Root hair growth: It’s a one way street. F1000Prime Rep. 7, 23. doi: 10.12703/P7-23
Nielsen, E., Christoforidis, S., Uttenweiler-Joseph, S., Miaczynska, M., Dewitte, F., Wilm, M., et al. (2000). Rabenosyn-5, a novel Rab5 effector, is complexed with hVPS45 and recruited to endosomes through a FYVE finger domain. J. Cell Biol. 151, 601–612. doi: 10.1083/jcb.151.3.601
Parlati, F., McNew, J. A., Fukuda, R., Miller, R., Söllner, T. H., Rothman, J. E. (2000). Topological restriction of SNARE-dependent membrane fusion. Nature 407, 194–198. doi: 10.1038/35025076
Preuss, M. L., Serna, J., Falbel, T. G., Bednarek, S. Y., Nielsen, E. (2004). The arabidopsis rab GTPase RabA4b localizes to the tips of growing root hair cells. Plant Cell 16, 1589–1603. doi: 10.1105/tpc.021634
Rizo, J., Südhof, T. C. (2012). The membrane fusion enigma: SNAREs, Sec1/Munc18 proteins, and their accomplices guilty as charged? Annu. Rev. Cell Dev. Biol. 28, 279–308. doi: 10.1146/annurev-cellbio-101011-155818
Rounds, C. M., Bezanilla, M. (2013). Growth mechanisms in tip-growing plant cells. Annu. Rev. Plant Biol. 64, 243–265. doi: 10.1146/annurev-arplant-050312-120150
Rout, M. P., Field, M. C. (2017). The evolution of organellar coat complexes and organization of the eukaryotic cell. Annu. Rev. Biochem. 86, 637–657. doi: 10.1146/annurev-biochem-061516-044643
Šamaj, J., Müller, J., Beck, M., Böhm, N., Menzel, D. (2006). Vesicular trafficking, cytoskeleton and signalling in root hairs and pollen tubes. Trends Plant Sci. 11, 594–600. doi: 10.1016/j.tplants.2006.10.002
Saedler, R., Jakoby, M., Marin, B., Galiana-Jaime, E., Hülskamp, M. (2009). The cell morphogenesis gene SPIRRIG in arabidopsis encodes a WD/BEACH domain protein. Plant J. 59, 612–621. doi: 10.1111/j.1365-313X.2009.03900.x
Scheuring, D., Schöller, M., Kleine-Vehn, J., Löfke, C. (2015). “Vacuolar staining methods in plant cells,” in Plant cell expansion (New York, NY: Humana Press), 83–92.
Schneider, C. A., Rasband, W. S., Eliceiri, K. W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. doi: 10.1038/nmeth.2089
Shanks, S. G., Carpp, L. N., Struthers, M. S., McCann, R. K., Bryant, N. J. (2012). The Sec1/Munc18 protein Vps45 regulates cellular levels of its SNARE binding partners Tlg2 and Snc2 in saccharomyces cerevisiae. PloS One 7, e49628. doi: 10.1371/journal.pone.0049628
Surpin, M., Zheng, H., Morita, M. T., Saito, C., Avila, E., Blakeslee, J. J., et al. (2003). The VTI family of SNARE proteins is necessary for plant viability and mediates different protein transport pathways. Plant Cell 15, 2885–2899. doi: 10.1105/tpc.016121
Tanaka, H., Kitakura, S., Rakusová, H., Uemura, T., Feraru, M. I., de Rycke, R., et al. (2013). Cell polarity and patterning by PIN trafficking through early endosomal compartments in arabidopsis thaliana. PloS Genet. 9, e1003540. doi: 10.1371/journal.pgen.1003540
Ueda, T., Yamaguchi, M., Uchimiya, H., Nakano, A. (2001). Ara6, a plant-unique novel type rab GTPase, functions in the endocytic pathway of arabidopsis thaliana. EMBO J. 20, 4730–4741. doi: 10.1093/emboj/20.17.4730
Uemura, T., Kim, H., Saito, C., Ebine, K., Ueda, T., Schulze-Lefert, P., et al. (2012). Qa-SNAREs localized to the trans-golgi network regulate multiple transport pathways and extracellular disease resistance in plants. Proc. Natl. Acad. Sci. U. S. A. 109, 1784–1789. doi: 10.1073/pnas.1115146109
Ungar, D., Hughson, F. M. (2003). SNARE protein structure and function. Annu. Rev. Cell Dev. Biol. 19, 493–517. doi: 10.1146/annurev.cellbio.19.110701.155609
Wang, N., Shi, L. (2015). “Screening of mutations by TILLING in plants,” in Plant genotyping (New York, NY: Humana Press), 193–203.
Wang, P., Hussey, P. J. (2015). Interactions between plant endomembrane systems and the actin cytoskeleton. Front. Plant Sci. 6. doi: 10.3389/fpls.2015.00422
Zhang, Y., Hughson, F. M. (2021). Chaperoning SNARE folding and assembly. Annu. Rev. Biochem. 90, 581–603. doi: 10.1146/annurev-biochem-081820-103615
Zhu, J., Gong, Z., Zhang, C., Song, C. P., Damsz, B., Inan, G., et al. (2002). OSM1/SYP61: A syntaxin protein in arabidopsis controls abscisic acid-mediated and non-abscisic acid-mediated responses to abiotic stress. Plant Cell 14, 3009–3028. doi: 10.1105/tpc.006981
Keywords: arabidopsis, endomembrane, tip growth, root hairs, SM protein, vacuole
Citation: Mugume Y, Roy R, Agbemafle W, Shepard GN, Vue Y and Bassham DC (2023) VPS45 is required for both diffuse and tip growth of Arabidopsis thaliana cells. Front. Plant Sci. 14:1120307. doi: 10.3389/fpls.2023.1120307
Received: 09 December 2022; Accepted: 07 February 2023;
Published: 27 February 2023.
Edited by:
Hao Wang, South China Agricultural University, ChinaReviewed by:
Yun Xiang, Lanzhou University, ChinaShutang Tan, University of Science and Technology of China, China
Copyright © 2023 Mugume, Roy, Agbemafle, Shepard, Vue and Bassham. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Diane C. Bassham, YmFzc2hhbUBpYXN0YXRlLmVkdQ==
†Present address: Rahul Roy, Department of Biology, Saint Catherine University, Saint Paul, MN, United States
 Yosia Mugume
Yosia Mugume Rahul Roy
Rahul Roy William Agbemafle
William Agbemafle Gabriella N. Shepard
Gabriella N. Shepard Yee Vue1
Yee Vue1 Diane C. Bassham
Diane C. Bassham