- 1College of Agronomy and Biotechnology, Southwest University/Key Laboratory of Eco-environments in Three Gorges Reservoir Region, Ministry of Education/Engineering Research Center of South Upland Agriculture, Ministry of Education, Chongqing, China
- 2Jiangxi Agricultural University/Key Laboratory of Crop Physiology, Ecology and Genetic Breeding, Ministry of Education, Nanchang, China
- 3Department of Environmental Chemistry, University of Kassel, Witzenhausen, Germany
- 4College of Agriculture, Henan University of Science and Technology, Luoyang, China
- 5Key Laboratory of Vegetation Restoration and Management of Degraded Ecosystems, South China Botanical Garden, Chinese Academy of Sciences, Guangzhou, China
Introduction: Intercropping has a potential to reduce the CO2 emission from farmlands. Limited information is available on the underlying reasons.
Methods: This study investigated the effect of milk vetch (Astragalus sinicus L.) (MV), rapeseed (Brassica napus L.) monoculture (RS) and intercropping (Intercrop) on soil CO2 emissions, moisture and temperature in a bucket experiment during 210 days from October 2015 to May 2016 on Chongqing, China.
Results: The results showed that soil CO2 efflux of MV, RS and Intercrop was 1.44, 1.55 and 2.08 μmol·m-2·s-1 during seedling and stem elongation stages and 3.08, 1.59 and 1.95 μmol·m-2·s-1 during flowering and podding stages. At seeding and stem elongation stages Intercrop had 1.4 times higher soil CO2 efflux than the mean of MV and RS. In contrast, MVhad 1.6 times higher soil CO2 efflux than Intercrop thereafter, which shows it was inhibited if milk vetch presents as Intercrop only. Decreased sensitivity of soil respiration to temperature in 1.4 times and lower soil moisture by Intercrop were found compared to MV. Intercrop decreased soil moisture, especially at the seedling and stem elongation stages, compared to the monoculture. The fluctuation on soil respiration in RS and Intercrop was slight with changes in soil moisture.
Conclusion: Thus, milk vetch-rapeseed system has a potential to decrease CO2 emission from farmland, however soil moisture should be regulated properly.
1 Introduction
The CO2 production from agriculture accounts for 23% of anthropogenic greenhouse gas emissions (Smith et al., 2007). If intercropping with legumes or cereals is introduced (Hu et al., 2016; Cui et al., 2019), this emission can be substantially reduced (Gan et al., 2011), because such systems have a high potential to sequester soil organic carbon (SOC) by reducing soil respiration (Chai et al., 2014; Hu et al., 2015). Namely, pea - maize or wheat - maize intercropping reduces soil respiration from maize strips during the growing season (Li et al., 2001; Qin et al., 2013); pea - oat intercropping reduces CO2 emission during the period with higher precipitation; barley - pea intercropping also results in 10% higher soil C sequestration than barley monoculture (Chapagain and Riseman, 2014).
Agricultural ecosystems increase SOC sequestration up to 4% if intercropping with legumes or cereals is introduced compared to crop monoculture (Cong et al., 2015). This is associated with: i) regulation of crop growth by intercropping, and thus, reduction of root exudates and following CO2 efflux (Dyer et al., 2012; Qin et al., 2013); ii) changes in the composition of microbial community structure and decrease in biomass and functional diversity under one of the species (Zhou et al., 2019b) or stimulation of soil microbial biomass growth under intercropping (Latati et al., 2017), and iii) the regulation of soil CO2 efflux by plant species composition, which may be suppressed by reduce of net primary production because of water availability shortage (Zhou et al., 2019a). Therefore, various intercropping systems (e.g., rapeseed (pea) - maize, wheat-soybean (maize)) can have the potential to reduce the soil CO2 efflux from farmland (Li et al., 2001; Chai et al., 2014).
Soil hydrothermal factors play an important role in CO2 efflux from farmland (Hursh et al., 2017). Temperature is the most important factor affecting soil respiration, and there is a positive relationship between them (Hursh et al., 2017). Plant community composition affects soil respiration's temperature sensitivity. The temperature sensitivity of soil respiration is affected by plant community composition (Mauritz and Lipson, 2021). Higher temperatures are generally expected to enhance soil C losses due to increased soil decomposition (Crowther et al., 2016; Bond-Lamberty et al., 2018). The sensitivity of respiration to temperature changes with the soil water content, substrate availability, and species composition (Geist and Lambin, 2004). The combined factors of soil temperature and moisture would better predict soil respiration (Feng et al., 2018). Soil moisture also strongly affects the changes in SOM (Jassal et al., 2008; Wang et al., 2014), respiration (Bouma et al., 1997), and microbial activity (Hallett and Young, 1999; Drenovsky et al., 2004), and the synergistic relationship between soil respiration and moisture can greatly increase or decrease the decomposition rate of SOM, depending on the direction of moisture change (Sierra et al., 2015). However, it is not yet clear how hydrothermal factors will be changed in the intercropping of the legume with brassica and what the response of CO2 emission will be.
Milk vetch (Astragalus sinicus L.) intercropping with rapeseed (Brassica napus L.) can enhance farmland productivity (Zhou et al., 2018), change the microbial community structure and decrease microbial biomass and functional activity in the rapeseed rhizosphere (Zhou et al., 2019b). To verify the potential of intercropping to reduce the CO2 emission, the soil respiration from milk vetch - rapeseed system was monitored together with the temperature and moisture for the entire development of crops and compared to the monoculture. Considering that intercropping can improve water use efficiency (Ren et al., 2017), and can decrease soil temperature (Gong et al., 2019) because of the large soil surface cover, it was hypothesized that soil CO2 efflux would be lower compared to the monocultures. The objective of the experiment was to explore the effects of intercropping on soil respiration and to determine the relationship between soil respiration and hydrothermal factors.
2 Materials and methods
2.1 Experimental site
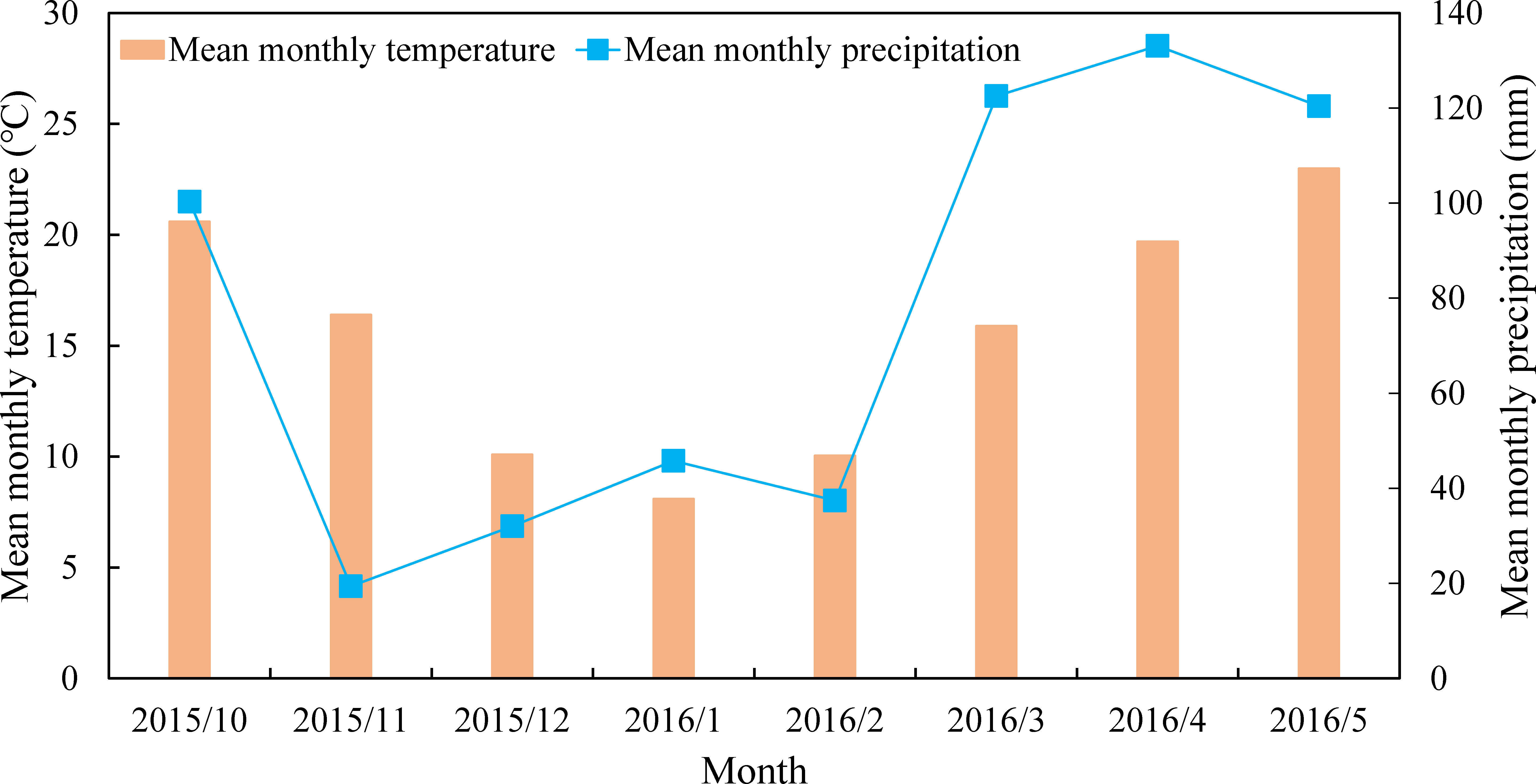
The study was conducted from October 2015 to May 2016 on Southwest University experimental farm (29°81′N, 106°41′E), Beibei, Chongqing, China, which belonged to humid subtropical monsoon climate zone (Figure 1). Soils (0-15 cm) were classified as dystric Regosols with a pH of 6.30, the total C content of 8.6 g kg-1 and total nitrogen content of 0.97 g kg-1.
2.2 Experimental design
The experiment was conducted in buckets (0.7 m in height, 0.4 m diameter at the bottom, and 0.57 m at the top. V = 0.12 m3), that were installed outside. Soil for the experiment was collected at 0-15 cm depth from the Southwest University experimental farm. All soil was well-mixed after being air-dried. Each pot contained 50 kg of dry soil in which the fertilizers (0.10 g N kg-1, 0.10 g P2O5 kg-1 and 0.10 g K2O kg-1 dry soil) was mixed before sowing. Make sure all soil was compacted into the buckets so that the density was equal to reduce the effect on soil respiration.
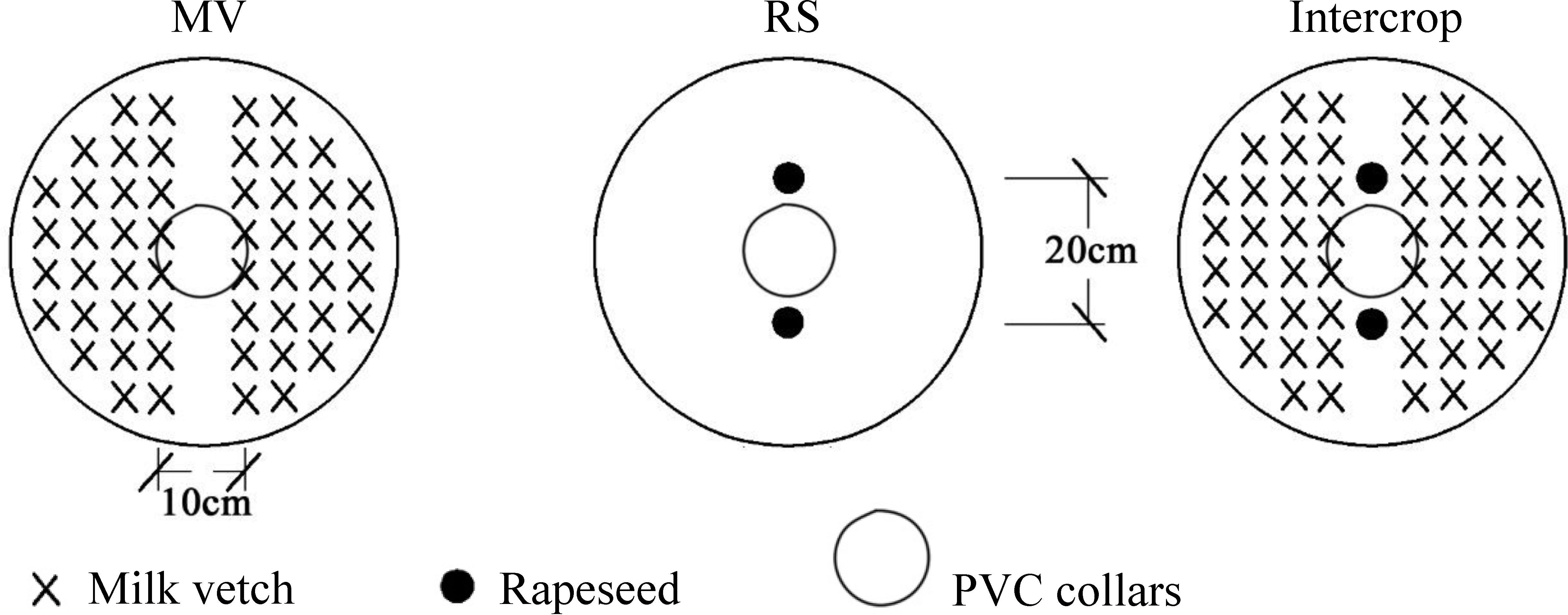
Three cropping systems were designed: a) monoculture milk vetch (MV): Leping variety sown by broadcasting with 1.0 g seeds in each bucket; b) monoculture rapeseed (RS): 94005 variety was sown in holes with 2 plants left after seedling emergence; c) milk vetch intercropping with rapeseed (Intercrop): rapeseed was sown in holes with 2 plants after seedling emergence and milk vetch was sown by broadcasting on both sides (0.5 g on each side) (Figure 2). Crops were sown in October 2015 and were harvested in May 2016. The experiment had a randomized complete block design with six replicates.
2.3 Soil respiration: Soil CO2 flux
Soil respiration was quantified using an infrared gas analyzer (Li-Cor 6400xt photosynthesis system installed a 6400-09 soil CO2 flux chamber, LI-COR Inc., Lincoln, USA). Cylindrical PVC collars (height, 0.05 m; diameter, 0.11 m) were placed at the core of buckets and inserted one day before measurement to reduce the disturbance of the soil. Each bucket had its own PVC collar. Soil respiration measurements were conducted once per 15 days from 1 November 2015 to 1 May 2016. Each treatment was measured in six replications, and 3 cycles were measured at every turn for each PVC collar. To minimize the influence of the diurnal variation on soil respiration, the measurements were carried out from 9:00 to 11:00 a.m.
2.4 Soil hydrothermal factors
Soil temperature (°C) outside the flux chamber at a depth of 5 cm was monitored simultaneously with soil respiration by the infrared gas analyzer. Soil moisture (m3 m-3) outside the flux chamber at a depth of 5 cm was measured with a handheld multifunction reader (ProCheck connected GS3 sensor, Decagon Inc., USA). The final soil moisture value of each experimental unit was the average of five values taken from the same unit. Each experimental unit's final soil moisture value was the average of five values taken from the same unit.
2.5 Statistical analysis
Statistical analysis of all experimental data was conducted using SPSS 17.0, Microsoft EXCEL 2010, and CANOCO 5. Soil respiration data were averaged for each growth stage and were evaluated with two-way ANOVA (two factors of crop system and growth stage). The residuals of the model were checked for normality and homogeneity by Shapiro and Leven’s tests, respectively. If conditions were met, the Tukey test was performed at P<0.05. Principal component analysis (PCA) was performed on the soil respiration of crop systems. Detrended correspondence analysis (DCA) and redundancy analysis (RDA) were performed on soil respiration and soil hydrothermal factors. The heterogeneity in soil respiration was tested with a DCA. Due to the gradient length<3.0, a RDA (linear method) was applied.
3 Results
3.1 Soil CO2 efflux
Soil CO2 efflux ranged between 0.53 and 4.15 μmol·m-2·s-1 during the growing period of crops (Figure 3). The turning point of respiration was observed at the lowest temperature during the stem elongation stage, and the pattern of respiration were also depended on the crop system. Soil CO2 efflux of MV, RS and Intercrop was 1.44, 1.55, and 2.08 μmol·m-2·s-1 during seedling and stem elongation stages and 3.08, 1.59, and 1.95 μmol·m-2·s-1 during flowering and podding stages (Figure 3). Soil CO2 efflux from Intercrop was 1.4 times higher than from mean of MV and RS at seedling and stem elongation stages, however soil CO2 efflux from MV was 1.6 times higher than Intercrop thereafter. The RS and Intercrop had similar CO2 efflux rates after the seedling stage, whereas maximum values were found under MV (Figure 3).
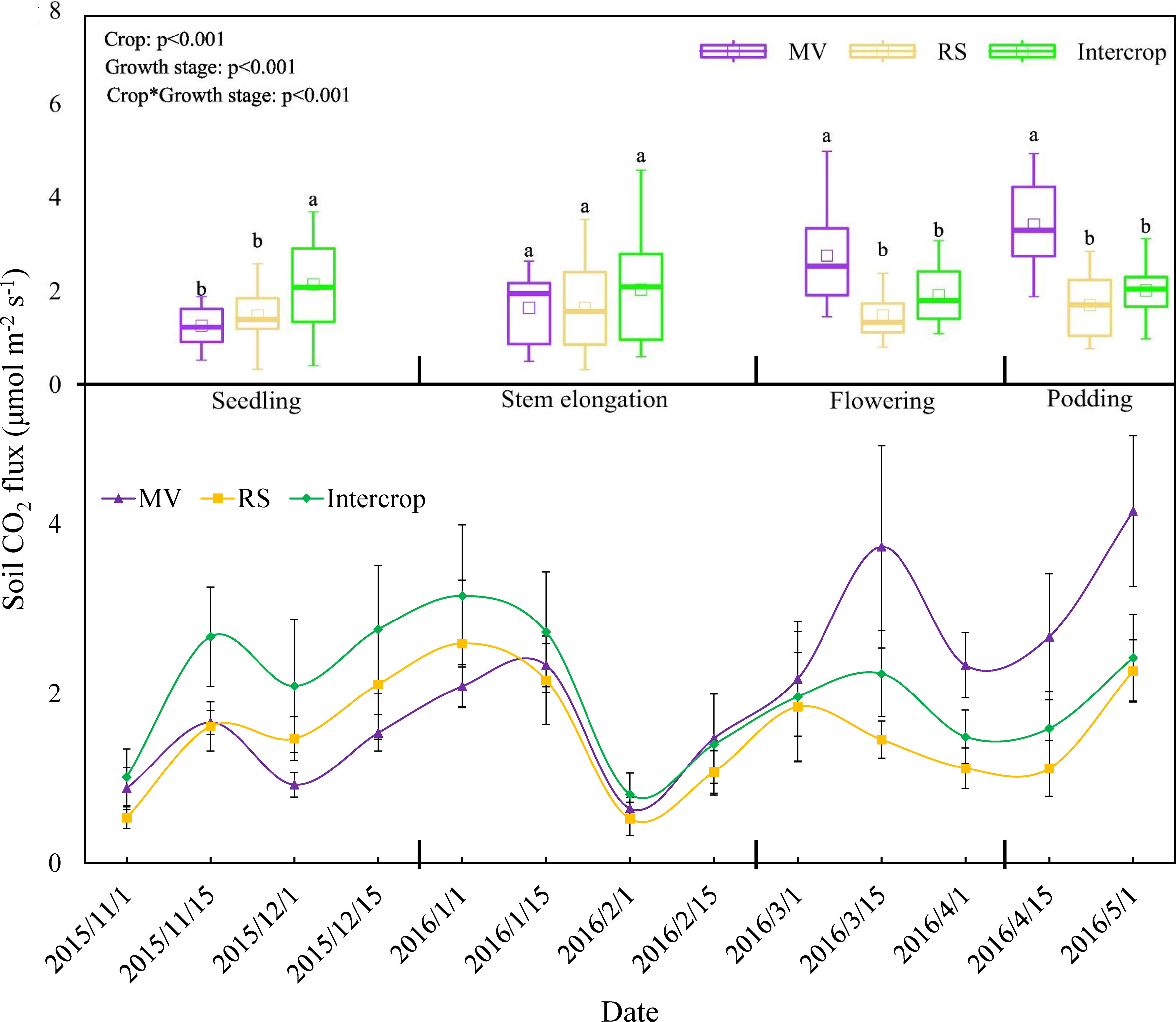
Figure 3 Effects of crop systems on soil respiration. Means are n=24 (seedling and stem elongation stages), n=18 (flowering stage) n=12 (podding stage) (these differences in replicates are because of the length of every growth stage was different) and standard deviations of each growth stage are show. Letters indicate significant differences among crop systems at P<0.05.
The first two PC explained together more than 80% of the soil respiration variation (Figure 4A). The MV was separated from Intercrop along PC1 and PC2 and only along PC1 from RS. In contrast, RS and Intercrop were only weakly separated along the PC2, and no separation along PC1 were found. Soil respiration in three crop systems could be classified into two types (Figure 4B). The separation along the PC1 was due to the CO2 efflux during the flowering to the podding stage, whereas separation along the PC2 was because CO2 efflux during the seedling and the stem elongation stages. Therefore, the difference in soil respiration between milk vetch and rapeseed was mainly determined by the later period of crop growth.
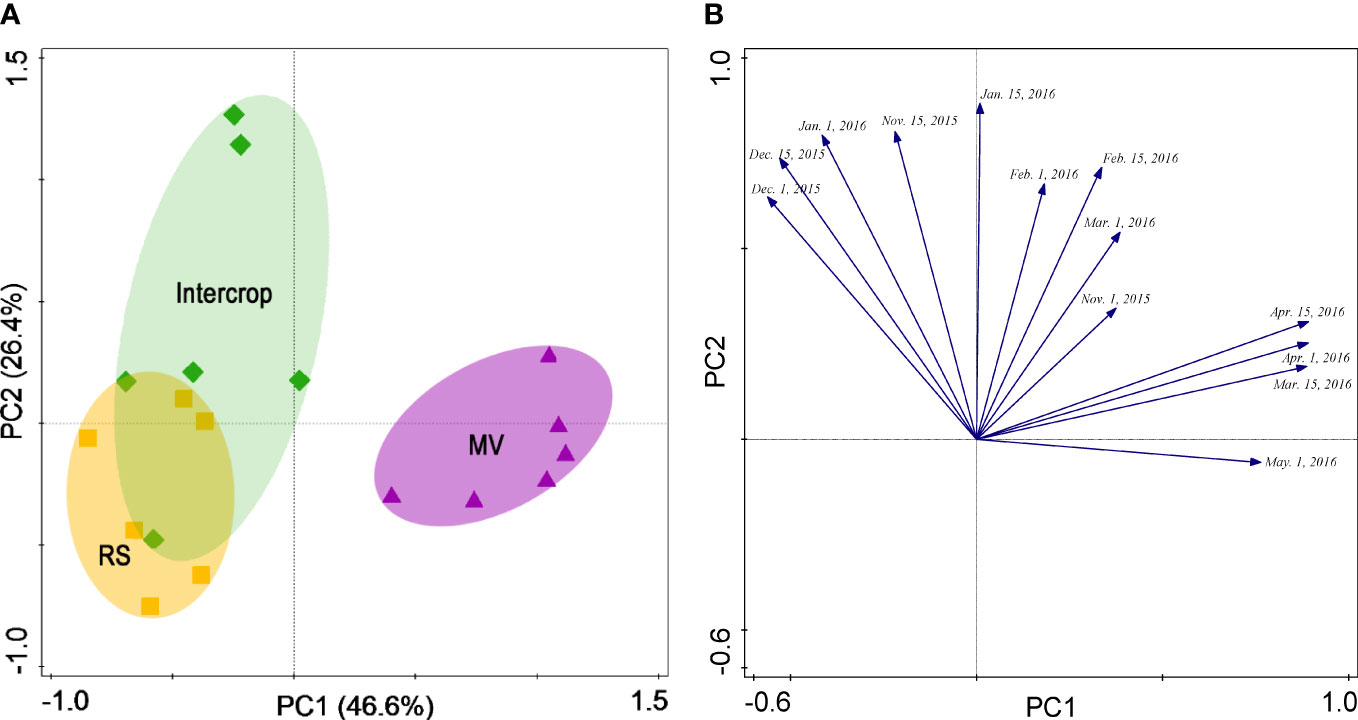
Figure 4 PCA scores (A) and corresponding loading values (B) for soil respiration (μmol·m-2·s-1) under various crop systems.
3.2 Soil respiration and hydrothermal factor
According to the DCA, soil respiration in MV, RS and Intercrop was found to be heterogeneous (gradient length<3.0). RDA showed that soil respiration was positively correlated with soil temperature and negatively with moisture (Figure 5). However, differences between crop systems were still observed. Firstly, MV had the closest correlation between soil respiration and hydrothermal factors, followed by Intercrop and RS. Secondly, the correlation between soil respiration and the temperature was more substantial than with soil moisture in the case of MV and RS; in the case of Intercrop, the correlation between soil respiration and moisture was stronger than that with temperature (Figure 5). This indicated that Intercrop changed the responses of soil respiration to hydrothermal factors.
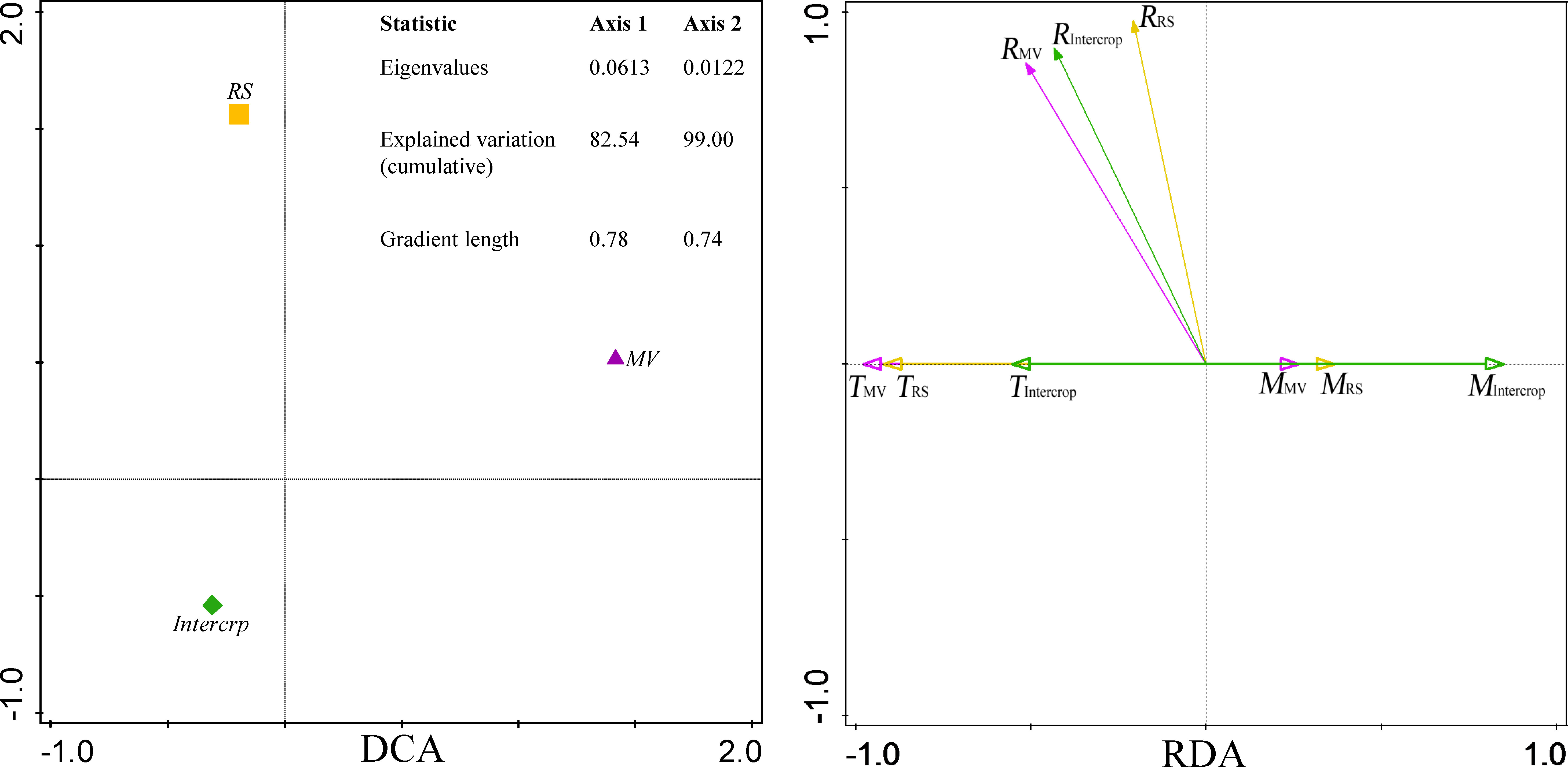
Figure 5 DCA and RDA of soil respiration and hydrothermal factors under various crop systems. DCA was perforemed on soil CO2 efflux (μmol m-2 s-1). RDA was performed on soil CO2 efflux (μmol m-2 s-1), soil temperature (°C), and soil moisture (m3 m-3). RMV, RRS, and RIntercrop indicate soil respiration under MV, RS and Intercrop, respectively; TMV, TRS, and TIntercrop indicate soil temperature; MMV, MRS and MIntercrop indicate soil moisture.
The Q10 values were 2.03, 1.39, and 1.45 in MV, RS, and Intercrop, respectively. Sensitivity of soil respiration to the temperature was lower in Intercrop than in MV and was independent on temperature changes, namely, although there was the same high temperature at the flowering and podding stages, the CO2 emission rates from Intercrop and RS were lower than from MV (Figure 6). Intercrop also decreased soil moisture, especially at the seedling and stem elongation stages, compared to the monoculture; soil CO2 efflux was smaller in RS and Intercrop than in MV when soil moisture was low. The CO2 efflux from RS and Intercrop was more constant under the moisture fluctuations compared with MV (Figure 6), which indicates that both RS and Intercrop reduced the response of soil respiration to moisture.
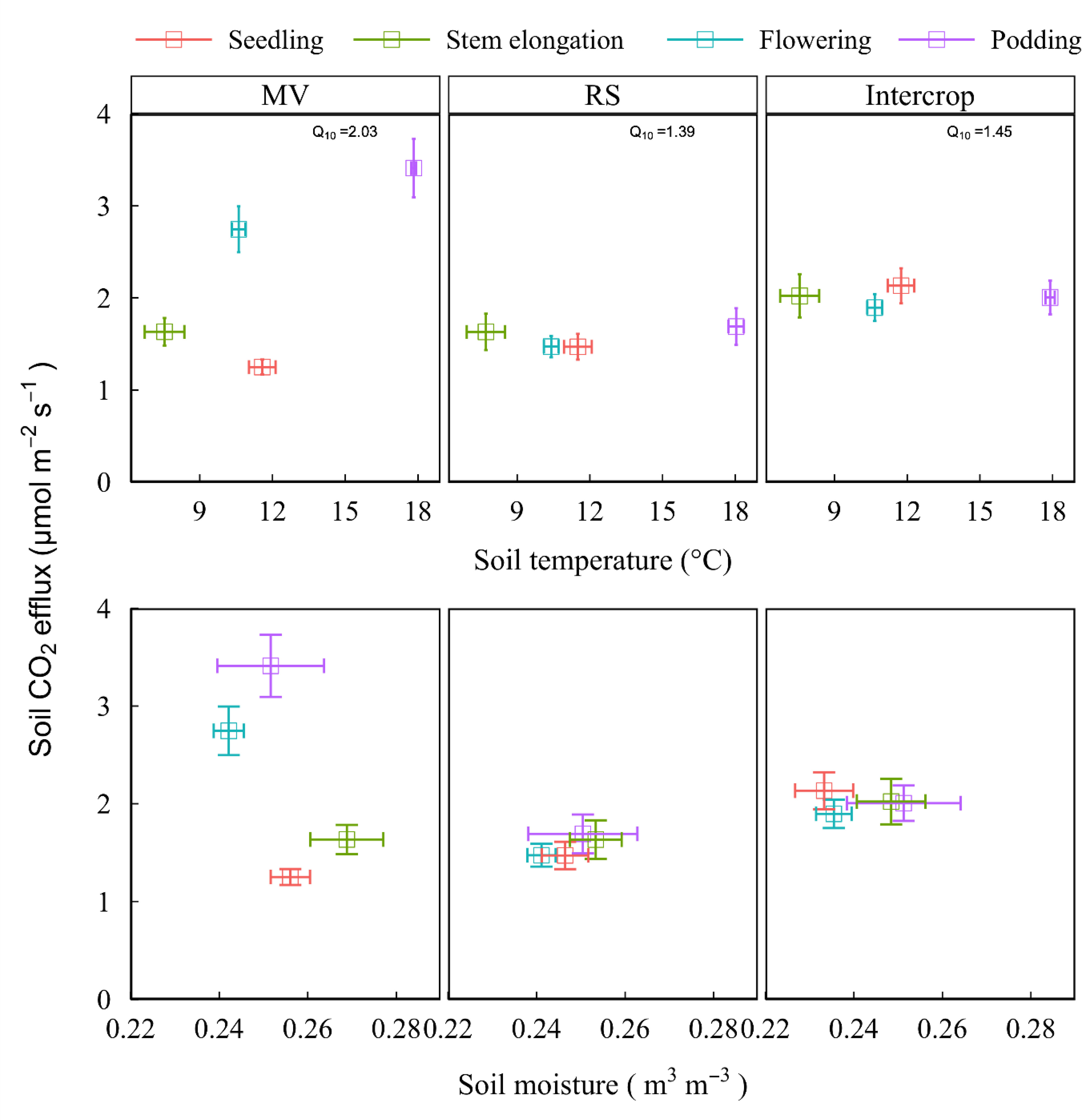
Figure 6 Soil respiration and hydrothermal factor under various crop systems and plant growth stages. Data shows means and standard errors. Q10 value is the sensitivity of soil respiration to temperature change, which was calculated as y=aebx, Q10 = e10b, where y is the soil respirartion, and x is temperature, a and b are fiited parameters.
4 Discussion
The high CO2 efflux from Intercrop at the early growth stages are explained by low competition for the soil resources between species (Mushagalusa et al., 2008), and intensive plant growth. Intercropping with RS may decrease the impact of MV on soil respiration at the later stage (Figure 3) i) due to inhibition of MV growth (Zhou et al., 2018), thus, decreasing the release of root exudates from MV into the soil (Liu et al., 2013) and ii) due to suppress microbial biomass and activity in the RS rhizosphere in the presence of MV (Zhou et al., 2019b), and thus, reducing rhizosphere respiration (Blaise et al., 2021; Yang et al., 2021). The RS and Intercrop had similar CO2 efflux rates after the seedling stage, whereas maximum values were found from MV (Figure 3). This is explained by higher root exudation under N2 fixing plant species (Zúñiga-Feest et al., 2018) and thus, higher rhizosphere respiration (Becker and Holz, 2021). This showed the positive effect of intercropping on the SOM accumulation because the CO2 efflux was reduced compared to N-fixing plant monoculture.
Seasonal and interannual variations of CO2 emission are related to the soil temperature and moisture (Suseela et al., 2012) because these parameters directly regulate microbial biomass and activity (Zhou et al., 2019b). Sensitivity of soil respiration to temperature was lower in Intercrop than in MV and was independent of temperature changes (Figure 6). Intercrop also decreased soil moisture, especially at the seedling and stem elongation stages, compared to the monoculture (Figure 6). This explains why in the Intercrop the growth of MV was suppressed (Zhou et al., 2018). High CO2 emission rate under MV observed at the flowering and podding stages can be explained more by the high soil moisture at the initial plant growth stages, then by the temperature. In contrast, the decreased CO2 efflux rate under Intercrop at later stages can be directly affected by variations in moisture or temperature, and probably other factors, such as changes in the composition of microbial communities (Zhou et al., 2019b), a decrease of rhizosphere C flux (Suseela and Dukes, 2013) and plant species-species interactions (Dijkstra et al., 2010).
The similar trends in CO2 efflux and temperature suggested that soil temperature was still the most important factor affecting soil respiration (Supplementary Figure 1). Soil respiration was positively correlated with soil temperature, and a negative correlation of soil respiration to soil moisture was also found, especially in Intercrop (Figure 5). It illustrated that the effect of soil moisture on soil respiration is more important in intercropping systems than in monoculture. Soil respiration responses to increases in temperature are constrained by soil moisture (Conant et al., 2004). If the soil moisture is often lower than the soil water holding capacity, the soil respiration cannot be high enough to reach the limiting point due to reduced oxygen diffusion into the soil and inhibited substrate decomposition (Tang et al., 2006; Hu et al., 2017). Furthermore, as microbial respiration is linearly related to soil water content and log-linearly related to water potential (Cook and Orchard, 2008), the decreased soil moisture will directly lead to the decrease of soil microbial biomass and functional activity (Zhou et al., 2019b). Thus, the variation in soil moisture can be the real reason for inhibited soil respiration by milk vetch intercropping with rapeseed.
5 Conclusions
Soil CO2 efflux from Intercrop was 1.4 times higher than from mean of MV and RS at seedling and stem elongation stages, however, soil CO2 efflux from MV was 1.6 times higher than Intercrop after that. Cultivation of legume in monoculture, although there is a positive contribution to soil N balance, can promote SOM losses compared to Brassica. In contrast, intercropping of Legume with Brassica is a beneficial agricultural practice to reduce the rate of CO2 efflux, which is related to the flowering and podding stages of plant growth. The sensitivity of soil respiration to temperature decreased in Intercrop, in which the variation of soil moisture was the primary factor to inhibit soil respiration. Therefore, milk vetch-rapeseed intercropping could be a potential approach to produce low CO2 emissions from farmland, however soil moisture should be adequately regulated so that agricultural intercropping systems can be well adaptable in the face of frequent global droughts.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by QZ, AG and JC. The first draft of the manuscript was written by QZ and all authors commented on previous versions of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was financially supported by the National Natural Science Foundation of China (31901476, 31871583, 31271673), the Jiangxi Provincial Natural Science Foundation (20202ACBL215002), the Special Fund for Agro-scientific Research in the Public Interest (201503127) and China Scholarship Council (CSC).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.1093507/full#supplementary-material
References
Becker, J. N., Holz, M. (2021). Hot or not? connecting rhizosphere hotspots to total soil respiration. Plant Soil 464, 489–499. doi: 10.1007/s11104-021-04963-4
Blaise, D., Velmourougane, K., Santosh, S., Manikandan, A. (2021). ). intercrop mulch affects soil biology and microbial diversity in rainfed transgenic bt cotton hybrids. Sci. Total Environ. 794, 148787. doi: 10.1016/j.scitotenv.2021.148787
Bond-Lamberty, B., Bailey, V. L., Chen, M., Gough, C. M., Vargas, R. (2018). Globally rising soil heterotrophic respiration over recent decades. Nature 560, 80–83. doi: 10.1038/s41586-018-0358-x
Bouma, T. J., Nielsen, K. L., Eissenstat, D. M., Lynch, J. P. (1997). Estimating respiration of roots in soil: Interactions with soil CO2, soil temperature and soil water content. Plant Soil 195, 221–232. doi: 10.1023/A:1004278421334
Chai, Q., Qin, A., Gan, Y., Yu, A. (2014). Higher yield and lower carbon emission by intercropping maize with rape, pea, and wheat in arid irrigation areas. Agron. Sustain. Dev. 34, 535–543. doi: 10.1007/s13593-013-0161-x
Chapagain, T., Riseman, A. (2014). Barley–pea intercropping: Effects on land productivity, carbon and nitrogen transformations. Field Crops Res. 166, 18–25. doi: 10.1016/j.fcr.2014.06.014
Conant, R. T., Dalla-Betta, P., Klopatek, C. C., Klopatek, J. M. (2004). Controls on soil respiration in semiarid soils. Soil Biol. Biochem. 36, 945–951. doi: 10.1016/j.soilbio.2004.02.013
Cong, W.-F., Hoffland, E., Li, L., Six, J., Sun, J.-H., Bao, X.-G., et al. (2015). Intercropping enhances soil carbon and nitrogen. Global Change Biol. 21, 1715–1726. doi: 10.1111/gcb.12738
Cook, F. J., Orchard, V. A. (2008). Relationships between soil respiration and soil moisture. Soil Biol. Biochem. 40, 1013–1018. doi: 10.1016/j.soilbio.2007.12.012
Crowther, T. W., Todd-Brown, K. E. O., Rowe, C. W., Wieder, W. R., Carey, J. C., Machmuller, M. B., et al. (2016). Quantifying global soil carbon losses in response to warming. Nature 540, 104–108. doi: 10.1038/nature20150
Cui, J., Sui, P., Wright, D. L., Wang, D., Sun, B., Ran, M., et al. (2019). Carbon emission of maize-based cropping systems in the north China plain. J. Cleaner Production 213, 300–308. doi: 10.1016/j.jclepro.2018.12.174
Dijkstra, F. A., Morgan, J. A., Blumenthal, D., Follett, R. F. (2010). Water limitation and plant inter-specific competition reduce rhizosphere-induced c decomposition and plant n uptake. Soil Biol. Biochem. 42, 1073–1082. doi: 10.1016/j.soilbio.2010.02.026
Drenovsky, R. E., Vo, D., Graham, K. J., Scow, K. M. (2004). Soil water content and organic carbon availability are major determinants of soil microbial community composition. Microb. Ecol. 48, 424–430. doi: 10.1007/s00248-003-1063-2
Dyer, L., Oelbermann, M., Echarte, L. (2012). Soil carbon dioxide and nitrous oxide emissions during the growing season from temperate maize-soybean intercrops. J. Plant Nutr. Soil Sci. 175, 394–400. doi: 10.1002/jpln.201100167
Feng, J., Wang, J., Song, Y., Zhu, B. (2018). Patterns of soil respiration and its temperature sensitivity in grassland ecosystems across China. Biogeosciences 15, 5329–5341. doi: 10.5194/bg-15-5329-2018
Gan, Y., Liang, C., Wang, X., McConkey, B. (2011). Lowering carbon footprint of durum wheat by diversifying cropping systems. Field Crops Res. 122, 199–206. doi: 10.1016/j.fcr.2011.03.020
Geist, H. J., Lambin, E. F. (2004). Dynamic causal patterns of desertification. BioScience 54, 817–829. doi: 10.1641/0006-3568(2004)054[0817:DCPOD]2.0.CO;2
Gong, X., Liu, C., Li, J., Luo, Y., Yang, Q., Zhang, W., et al. (2019). Responses of rhizosphere soil properties, enzyme activities and microbial diversity to intercropping patterns on the loess plateau of China. Soil Tillage Res. 195, 104355. doi: 10.1016/j.still.2019.104355
Hallett, P. D., Young, I. M. (1999). Changes to water repellence of soil aggregates caused by substrate-induced microbial activity. Eur. J. Soil Sci. 50, 35–40. doi: 10.1046/j.1365-2389.1999.00214.x
Hu, F., Chai, Q., Yu, A., Yin, W., Cui, H., Gan, Y. (2015). Less carbon emissions of wheat–maize intercropping under reduced tillage in arid areas. Agron. Sustain. Dev. 35, 701–711. doi: 10.1007/s13593-014-0257-y
Hu, F., Feng, F., Zhao, C., Chai, Q., Yu, A., Yin, W., et al. (2017). Integration of wheat-maize intercropping with conservation practices reduces CO2 emissions and enhances water use in dry areas. Soil Tillage Res. 169, 44–53. doi: 10.1016/j.still.2017.01.005
Hu, F., Gan, Y., Cui, H., Zhao, C., Feng, F., Yin, W., et al. (2016). Intercropping maize and wheat with conservation agriculture principles improves water harvesting and reduces carbon emissions in dry areas. Eur. J. Agron. 74, 9–17. doi: 10.1016/j.eja.2015.11.019
Hursh, A., Ballantyne, A., Cooper, L., Maneta, M., Kimball, J., Watts, J. (2017). The sensitivity of soil respiration to soil temperature, moisture, and carbon supply at the global scale. Global Change Biol. 23, 2090–2103. doi: 10.1111/gcb.13489
Jassal, R. S., Black, T. A., Novak, M. D., Gaumont-Guay, D., Nesic, Z. (2008). Effect of soil water stress on soil respiration and its temperature sensitivity in an 18-year-old temperate Douglas-fir stand. Global Change Biol. 14, 1305–1318. doi: 10.1111/j.1365-2486.2008.01573.x
Latati, M., Aouiche, A., Tellah, S., Laribi, A., Benlahrech, S., Kaci, G., et al. (2017). Intercropping maize and common bean enhances microbial carbon and nitrogen availability in low phosphorus soil under Mediterranean conditions. Eur. J. Soil Biol. 80, 9–18. doi: 10.1016/j.ejsobi.2017.03.003
Li, L., Sun, J., Zhang, F., Li, X., Rengel, Z., Yang, S. (2001). Wheat/maize or wheat/soybean strip intercropping: II. recovery or compensation of maize and soybean after wheat harvesting. Field Crops Res. 71, 173–181. doi: 10.1016/S0378-4290(01)00157-5
Liu, S., Chai, Q., Huang, G. (2013). Relationships among soil respiration, soil temperature and dry matter accumulation for wheat-maize intercropping in an arid environment. Can. J. Plant Sci. 93, 715–724. doi: 10.4141/cjps2012-274
Mauritz, M., Lipson, D. A. (2021). Plant community composition alters moisture and temperature sensitivity of soil respiration in semi-arid shrubland. Oecologia 197, 1003–1015. doi: 10.1007/s00442-021-04961-4
Mushagalusa, G. N., Ledent, J.-F., Draye, X. (2008). Shoot and root competition in potato/maize intercropping: Effects on growth and yield. Environ. Exp. Bot. 64, 180–188. doi: 10.1016/j.envexpbot.2008.05.008
Qin, A., Huang, G., Chai, Q., Yu, A., Huang, P. (2013). Grain yield and soil respiratory response to intercropping systems on arid land. Field Crops Res. 144, 1–10. doi: 10.1016/j.fcr.2012.12.005
Ren, Y. Y., Wang, X. L., Zhang, S. Q., Palta, J. A., Chen, Y. L. (2017). Influence of spatial arrangement in maize-soybean intercropping on root growth and water use efficiency. Plant Soil 415, 131–144. doi: 10.1007/s11104-016-3143-3
Sierra, C. A., Trumbore, S. E., Davidson, E. A., Vicca, S., Janssens, I. (2015). Sensitivity of decomposition rates of soil organic matter with respect to simultaneous changes in temperature and moisture. J. Adv. Modeling Earth Syst. 7, 335–356. doi: 10.1002/2014MS000358
Smith, P., Martino, D., Cai, Z., Gwary, D., Janzen, H., Kumar, P., et al. (2007). Greenhouse gas mitigation in agriculture. Philos. Trans. R. Soc. B: Biol. Sci. 363, 789–813. doi: 10.1098/rstb.2007.2184
Suseela, V., Conant, R. T., Wallenstein, M. D., Dukes, J. S. (2012). Effects of soil moisture on the temperature sensitivity of heterotrophic respiration vary seasonally in an old-field climate change experiment. Global Change Biol. 18, 336–348. doi: 10.1111/j.1365-2486.2011.02516.x
Suseela, V., Dukes, J. S. (2013). The responses of soil and rhizosphere respiration to simulated climatic changes vary by season. Ecology 94, 403–413. doi: 10.1890/12-0150.1
Tang, X.-L., Zhou, G.-Y., Liu, S.-G., Zhang, D.-Q., Liu, S.-Z., Li, J., et al. (2006). Dependence of soil respiration on soil temperature and soil moisture in successional forests in southern China. J. Integr. Plant Biol. 48, 654–663. doi: 10.1111/j.1744-7909.2006.00263.x
Wang, B., Zha, T. S., Jia, X., Wu, B., Zhang, Y. Q., Qin, S. G. (2014). ). soil moisture modifies the response of soil respiration to temperature in a desert shrub ecosystem. Biogeosciences 11, 259–268. doi: 10.5194/bg-11-259-2014
Yang, H., Hu, F., Yin, W., Chai, Q., Zhao, C., Yu, A., et al. (2021). Integration of tillage and planting density improves crop production and carbon mitigation of maize/pea intercropping in the oasis irrigation area of northwestern China. Field Crops Res. 272, 108281. doi: 10.1016/j.fcr.2021.108281
Zhou, C., Biederman, J. A., Zhang, H., Li, L., Cui, X., Kuzyakov, Y., et al. (2019a). Extreme-duration drought impacts on soil CO2 efflux are regulated by plant species composition. Plant Soil 439, 357–372. doi: 10.1007/s11104-019-04025-w
Zhou, Q., Chen, J., Xing, Y., Xie, X., Wang, L. (2019b). Influence of intercropping Chinese milk vetch on the soil microbial community in rhizosphere of rape. Plant Soil 440, 85–96. doi: 10.1007/s11104-019-04040-x
Zhou, Q., Wang, L., Ma, S., Zhang, X., Xing, Y., Zhang, S. (2018). Influences of rape intercropping with Chinese milk vetch and straw mulching on productive benefits in dryland of southwest China. Acta Agronomica Sin. 44, 431–441. doi: 10.3724/SP.J.1006.2018.00431
Zúñiga-Feest, A., Muñoz, G., Bustos-Salazar, A., Ramírez, F., Delgado, M., Valle, S., et al. (2018). The nitrogen fixing specie sophora cassioides (Fabaceae), is nutritionally favored and their rhizosphere bacteria modified when is co-cultivated with the cluster root forming embothrium coccineum (Proteaceae). J. Soil Sci. Plant Nutr. 18, 597–616. doi: 10.4067/S0718-95162018005001801
Keywords: legume-brassica intercrops, greenhouse gas emission, soil temperature, soil moisture, SOM balance
Citation: Zhou Q, Gunina A, Chen J, Xing Y, Xiong Y, Guo Z and Wang L (2023) Reduction in soil CO2 efflux through alteration of hydrothermal factor in milk vetch (Astragalus sinicus L.)-rapeseed (Brassica napus L.) intercropping system. Front. Plant Sci. 13:1093507. doi: 10.3389/fpls.2022.1093507
Received: 09 November 2022; Accepted: 15 December 2022;
Published: 10 January 2023.
Edited by:
Abraham J. Escobar-Gutiérrez, L’alimentation et l’environnement (INRAE), FranceReviewed by:
Honghai Luo, Shihezi University, ChinaJiban Shrestha, Nepal Agricultural Research Council, Nepal
Copyright © 2023 Zhou, Gunina, Chen, Xing, Xiong, Guo and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Longchang Wang, d2FuZ2xjMjAwM0AxNjMuY29t
 Quan Zhou
Quan Zhou Anna Gunina
Anna Gunina Jiao Chen1
Jiao Chen1 Yi Xing
Yi Xing