
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Plant Sci. , 19 July 2012
Sec. Plant Genetics and Genomics
Volume 3 - 2012 | https://doi.org/10.3389/fpls.2012.00162
 Surya Kant1*
Surya Kant1* Saman Seneweera2
Saman Seneweera2 Joakim Rodin3
Joakim Rodin3 Michael Materne1
Michael Materne1 David Burch1
David Burch1 Steven J. Rothstein4
Steven J. Rothstein4 German Spangenberg3,5
German Spangenberg3,5Increasing crop productivity to meet burgeoning human food demand is challenging under changing environmental conditions. Since industrial revolution atmospheric CO2 levels have linearly increased. Developing crop varieties with increased utilization of CO2 for photosynthesis is an urgent requirement to cope with the irreversible rise of atmospheric CO2 and achieve higher food production. The primary effects of elevated CO2 levels in most crop plants, particularly C3 plants, include increased biomass accumulation, although initial stimulation of net photosynthesis rate is only temporal and plants fail to sustain the maximal stimulation, a phenomenon known as photosynthesis acclimation. Despite this acclimation, grain yield is known to marginally increase under elevated CO2. The yield potential of C3 crops is limited by their capacity to exploit sufficient carbon. The “C fertilization” through elevated CO2 levels could potentially be used for substantial yield increase. Rubisco is the rate-limiting enzyme in photosynthesis and its activity is largely affected by atmospheric CO2 and nitrogen availability. In addition, maintenance of the C/N ratio is pivotal for various growth and development processes in plants governing yield and seed quality. For maximizing the benefits of elevated CO2, raising plant nitrogen pools will be necessary as part of maintaining an optimal C/N balance. In this review, we discuss potential causes for the stagnation in yield increases under elevated CO2 levels and explore possibilities to overcome this limitation by improved photosynthetic capacity and enhanced nitrogen use efficiency. Opportunities of engineering nitrogen uptake, assimilatory, and responsive genes are also discussed that could ensure optimal nitrogen allocation toward expanding source and sink tissues. This might avert photosynthetic acclimation partially or completely and drive for improved crop production under elevated CO2 levels.
The human population has just crossed the mark of seven billion, and by the middle of this century it is expected to exceed nine billion (Godfray et al., 2010). To sufficiently feed such a large population, considerable stress will be imposed on increasing crop productivity due to a combination of factors, including shortage of arable land, resource constraints of water and nutrients, changing food habits, use of crop produce for biofuel, and rapid global environmental changes. Although, agronomic and breeding efforts in the past five decades have achieved a linear increase in food productivity, a further ability to increase or even sustain the crop yield and quality is uncertain in the face of rapid global environmental change (Rothstein, 2007; Tester and Langridge, 2010). The environmental changes are coincident with increasing biotic and abiotic threats such as heat and water stress, newer insect-pests as well as diseases and rising greenhouse gases including elevated CO2. Among these, atmospheric CO2 levels are increasing linearly over time; present atmospheric CO2 has increased from 280 to 390 μmol mol-1 since 1800, and is expected to double by the end of the twenty-first century (IPCC, 2007). Plants could adapt to these elevated levels through photosynthetic conversion of high CO2 into increased growth and productivity. However, the potential for different plant species to assimilate higher CO2 concentrations and their consequences is not yet fully understood.
Carbon (C) and nitrogen (N) are the key structural elements for plant growth and constitute ~45 and ~5% of plant dry matter, respectively (Ho, 1976; Marschner, 1995). The maintenance of optimum C and N balance (often referred as the C/N ratio) within plants as well as externally in soil or growth media is essential for optimal plant growth and development (Paul and Driscoll, 1997; Martin et al., 2002; Malamy, 2005; Wingler et al., 2006; Zhang et al., 2007). Simultaneous improvement of both C and N utilization efficiencies is of utmost importance, given the rising atmospheric CO2 levels and the necessity to lower input costs and reduce environmental pollution due to excessive use of nitrogenous fertilizers. Therefore, fine-tuning of genetic changes leading to metabolic adjustments of both C and N will be required in order to effectively harness excess C from elevated atmospheric CO2 and to simultaneously maintain an optimal C/N balance. The primary effects of elevated CO2 levels in most crop plants (especially C3 plants) include increased plant biomass accumulation, although initial stimulation of net photosynthesis rates for most C3 plants is only temporal, and they fail to sustain the maximum stimulation (though higher than ambient CO2 level) over longer exposure periods (months to years). This phenomenon is called CO2 or photosynthesis acclimation (Long et al., 2004; Reich et al., 2006; Bloom et al., 2010). This photosynthesis acclimation (initial stimulation followed by a partial reversal or stabilization at a lower rate) under elevated CO2 is accompanied by a decrease in carboxylation of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), a decrease in N concentration, a reduced stomatal conductance, and an increase of starch accumulation (Figure 1; Nakano et al., 1997; Geiger et al., 1999; Stitt and Krapp, 1999; Bloom et al., 2002, 2010; Long et al., 2004; Ainsworth and Long, 2005; Leakey et al., 2009).
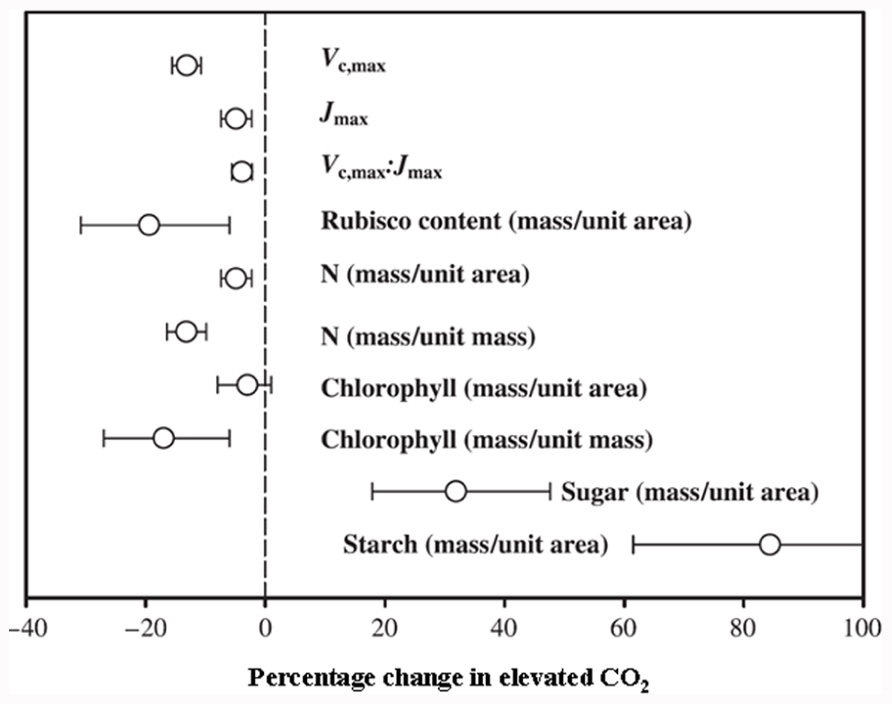
FIGURE 1. Mean response of maximum carboxylation rate (Vc,max), maximum rate of electron transport (Jmax), ratio of Vc,max:Jmax, and Rubisco, N, chlorophyll, sugar and starch contents. Rubisco, sugar and starch contents reported on area basis. N and chlorophyll contents reported on both area and mass basis at 95% confidence level. Number of species, FACE experiments and individual observations for each response are given in Ainsworth and Long (2005). LAI, leaf area index; DMP, above ground dry matter production (Reproduced with permission).
In C3 plants, Rubisco is the key chloroplast enzyme (comprising ~50% of total cellular proteins) involved in photosynthesis through catalyzing the carboxylation of ribulose-1,5-bisphosphate (RuBP) during capture and fixation of atmospheric CO2. This CO2 is later converted into sugars, the major building blocks for plants. In addition to the involvement of Rubisco in C metabolism, it is also a major storage protein for N (Makino and Osmond, 1991; Mae et al., 1993). This stored N is further utilized by the plants’ reproductive components when Rubisco degradation is initiated during leaf senescence. The consequences of elevated CO2 on plants are 2-fold; the decreased Rubisco level becomes a rate-limiting factor for photosynthetic efficiency compounded by a reduction in the available N pool. Genetically engineered plants producing increased levels of Rubisco protein could potentially improve CO2 fixation. However, plants under these conditions would require additional N for increased Rubisco production. Additionally, maintenance of an optimum C/N ratio within the plant is essential for efficient metabolism of C and N, optimal growth, and sustained quantitative and qualitative yield. High C status (specifically carbohydrates) due to increased CO2 levels would increase the C/N ratio with lower N levels resulting in lower protein content, thus reducing grain quality particularly in cereal crops.
There is mounting evidence that the yield potential of many crops is limited by their capacity to exploit sufficient C during their lifecycle, limiting grain size and quantity (Fischer et al., 1998). “C fertilization” through increased CO2 levels would be ideal for yield increase; however, photosynthetic acclimation restricts the plants’ ability to exploit elevated atmospheric CO2. In this review, we discuss the underlying causes of this stagnation in yield progress and explore the possibilities of improving the photosynthetic machinery in plants, combined with enhanced nitrogen use efficiency (NUE) under elevated CO2 conditions. Engineering N uptake, assimilatory and responsive genes would ensure optimal N allocation toward expanding source and sink tissues under elevated CO2 levels as well as improving grain yield and quality.
Photosynthesis is the process whereby light is harvested by the chloroplast thylakoids of the leaf and other photosynthetic structures. The resultant chemical energy (ATP and NADPH) is used to fix atmospheric CO2, either directly via Rubisco (C3 photosynthesis), or indirectly after primary fixation by phosphoenolpyruvate carboxylase (PEPC). C fixed through this mechanism is subsequently re-released into adjacent cells which are not in direct communication with atmospheric CO2 (C4 photosynthesis). The majority of crop species (rice, wheat, grain legumes, canola, and all root crops) and ~85% of terrestrial plants use C3 photosynthesis, while C4 crops are a minority, represented predominantly by maize, sorghum, and sugarcane among economically important crops (Ehleringer et al., 1991).
The Rubisco enzyme, which is fundamental to C fixation in both C3 and C4 plants, displays a high affinity to O2, and its inability to distinguish it from the CO2 molecule results in unnecessary O2 uptake, especially under hot and arid conditions. This oxygenation activity produces phosphoglycolate molecules, which are then broken down in a process referred to as photorespiration, an energy-consuming and wasteful process (Kajala et al., 2011). Photorespiration has been identified as the bottleneck preventing C3 plants from achieving full photosynthetic potential due to competition between CO2 and O2 at the C fixation site on the Rubisco enzyme. Whereas, C4 photosynthesis evolved to ameliorate photorespiration by utilizing two distinct cell types not involved in the C3 photosynthesis: mesophyll cells (MC) and bundle sheath cells (BSC), which are rarely more than one cell distant from each other, allowing ease of molecular transport between the two. The tissues within these cells are arranged concentrically relative to the surrounding vascular tissue, a structure characteristic of C4 plants known as Kranz anatomy (Muhaidat et al., 2007; Sage et al., 2012). When atmospheric CO2 is assimilated into the MC, carbonic anhydrase and PEPC hydrate and fix C molecules as oxaloacetate. This reaction has no affinity to O2 and is highly efficient (Sheen, 1999). The resulting C4 acid is decarboxylated within the BSC, delivering higher concentrations of CO2 directly to the Rubisco enzyme while minimizing the oxygenation of Rubisco. The increased concentration of CO2 at the site of Rubisco activity maximizes photosynthetic efficiency. These evolutionary adaptations in C4 plants provide an advantage over C3 photosynthesis while potentially improving water and nutrient use (Kajala et al., 2011). Zhu et al. (2008) reported a 60% increase in maximum photosynthetic efficiency in C4 plants compared to C3 plants. C4 plants can photosynthesize with ~50% greater water use efficiency, as C4 photosynthesis can assimilate an equivalent amount of CO2 with only half the stomatal conductance (Sage and Kubien, 2003; von Caemmerer and Furbank, 2003). Under N-limiting conditions, C4 plants also out-compete C3 plants, as they require less Rubisco to harness a similar amount of C due to increased photosynthetic efficiency (Sage and Kubien, 2003).
Since C4 plants are photosynthetically saturated at current CO2 conditions, predicted rises in atmospheric CO2 would have no major impact on their C fixation rate, biomass production, and yield (Figure 2; Cure and Acock, 1986; Ainsworth and Long, 2005). In contrast, as C3 plants are not photosynthetically saturated at present CO2 levels, photosynthesis, biomass, and subsequent yields should increase with elevated atmospheric CO2. The understanding of the biochemical and molecular nature of C3 and C4 photosynthesis provides a valuable tool for crop improvement in the twenty-first century, particularly with respect to improving C assimilation in C3 plants and reducing the impact of photosynthetic acclimation.
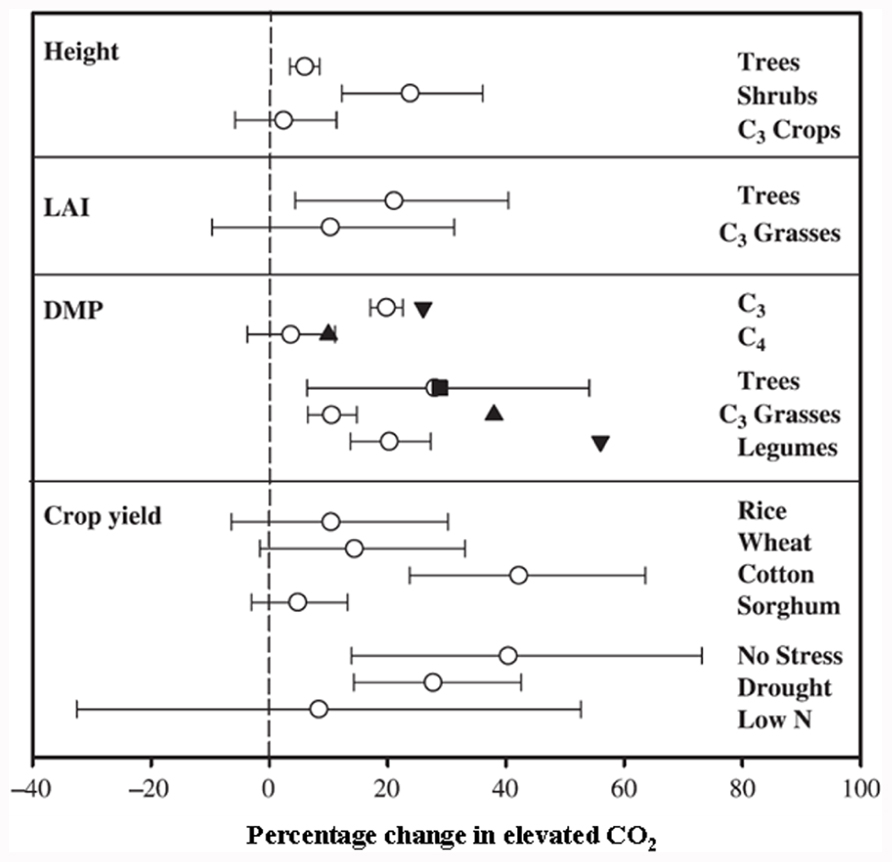
FIGURE 2. Responses to elevated CO2 of different plant species and experimental conditions on growth and yield variables. Results from: ◯, a meta-analysis of various species (Ainsworth and Long, 2005); ∎, a meta-analysis of tree species (Curtis and Wang, 1998); ▲, a meta-analysis of C4 plants and C3 grasses (Wand et al., 1999). ▼, a meta-analysis of C3 and legumes (Jablonski et al., 2002). Number of species, FACE experiments and individual observations for each response are given in Ainsworth and Long (2005; reproduced with permission).
The present atmospheric CO2 concentration of 390 μL CO2 L–1 limits the rate of photosynthesis in C3 plants (Farquhar et al., 1980; Farquhar and Sharkey, 1982), and presumably lower concentrations of CO2 in the recent past were even more limiting. Laboratory and field studies have shown that photosynthetic rates of C3 plants were approximately doubled when plants grown at about 380 μL CO2 L–1 were exposed to 700 μL CO2 L–1 (Ainsworth and Long, 2005). This increase in photosynthetic rate as atmospheric CO2 rises is primarily due to increase in Rubisco carboxylation capacity. Rubisco has an affinity for O2 as well as CO2 (Badger and Price, 2003); consequently, at 21% O2 and 390 μL CO2 L–1, a considerable amount of energy is wasted in the photorespiratory carbon oxidation cycle (PCO). This reduces photosynthetic rates by about 40% from the optimum level (Sharkey, 1985). Increasing the ambient CO2 concentration increases the ratio of CO2 to O2 at the site of fixation in the chloroplast, favoring PCR over PCO, and thus photosynthetic rates are increased in C3 plants. The limitation of photosynthesis imposed by Rubisco is referred to as the limitation due to supply and utilization of CO2 (Farquhar and Sharkey, 1982). Two other limitations were also identified: the supply and utilization of light and the utilization of triose phosphate. The former can be caused by low photon flux densities or inability to convert light energy to chemical energy. Triose phosphate is the end product of photosynthesis and can be formed into starches and sugars or utilized as a direct source of chemical energy. Limitation occurs when there are insufficient sinks for sucrose (Stitt and Schulze, 1994), thus reducing conversion to sugar and inhibiting photosynthesis. These three limitations to leaf photosynthesis were first identified in plants that were grown at a given CO2 concentration and then transferred to different CO2 concentration during measurement of photosynthesis (Drake et al., 1997). However, when plants are exposed to high CO2 for extended period, the photosynthetic rates slow down due to the so-called “acclimation” response (Long et al., 2004; Reich et al., 2006). This is thought to result from direct effects of sucrose on the transcription of genes encoding proteins involved in CO2 fixation and electron transport activity (Moore et al., 1999).
The effect of elevated CO2 on plant growth and yield has been studied in both controlled and field conditions, with the latter referred to as the Free Air Carbon dioxide Enrichment (FACE) system. The controlled conditions might produce larger artifacts, whereas FACE produces an environment similar to field conditions. The differential plant response under the two conditions has been reported. For example, Ainsworth et al. (2008) suggested a 14% yield increase in FACE and a 31% increase in controlled conditions in different plant species when CO2 was raised from ~373 to ~570 μmol moL–1. Generally, elevated CO2 increases photosynthesis, resulting in increased dry matter accumulation, leaf area, and plant height in trees and shrubs and to some extent in C3 plants (Figure 2; Ainsworth and Long, 2005). The yield increase in C3 crops under elevated CO2 is variable and dependent on other environmental factors such as water, temperature, and soil N (Ainsworth and Long, 2005). Irrespective of photosynthetic machinery, a yield increase requires a concomitant increase in sink capacity to match the source activity. The initial response of C3 plants to elevated CO2 is an increase in photosynthetic rate; however, due to the acclimation phenomenon this stimulation is not always maintained at the maximal level when plants are exposed to elevated CO2 for a longer period. This partial reversal of photosynthesis and settling at lower than maximal level could be ascribed to (i) reduced stomatal conductance resulting in depletion of intercellular CO2, leading to reduced CO2 supply to the photosynthetic machinery, and (ii) reduced rates of electron transport to Rubisco carboxylation (Figure 1 and also discussed in the following sub-section). Lower activation state and reduced concentration of Rubisco leads to changes in C assimilation and alters the whole plant N metabolism. Thereby, biochemical adjustments occur from the cellular to whole plant level in response to elevated CO2, accompanied by growth, development, and yield changes.
Rubisco is the rate-limiting enzyme in photosynthesis and its synthesis and degradation is affected by environmental factors such as temperature, light intensity, soil N, and atmospheric CO2. Prolonged exposure to elevated CO2 results in reduced Rubisco content and Rubisco activity (Moore et al., 1999; Aranjuelo et al., 2011; Seneweera et al., 2011). A concomitant reduction in the transcript level of genes encoding proteins involved in photosynthesis, including small subunit of Rubisco (RbcS), large subunit of Rubisco (RbcL), and Rubisco activase (Rca), has been observed in different plants (Nie et al., 1995; Cheng et al., 1998; Moore et al., 1998, 1999; Stitt and Krapp, 1999). In contrast, in expanding rice leaf blades there was no significant difference in RbcS transcript level between ambient and elevated CO2 levels (Aoki et al., 2003). This can be correlated with changes in Rubisco concentration during leaf development, with a rapid increase in Rubisco protein during leaf expansion, reaching a maximum when the leaf is fully expanded and a gradual decline with the onset of leaf senescence (Seneweera and Conroy, 2005; Imai et al., 2008). The decline in Rubisco and subsequent photosynthesis acclimation in plants under elevated CO2 could be attributed to two processes. It could be due to carbohydrate sink limitation since plants grown under CO2 enrichment initially assimilate more CO2 than they can incorporate in their sink tissues, and as a feedback response plants diminish CO2 assimilation by reducing levels of Rubisco and other proteins (Long et al., 2004). Previous reviews have reported that feedback repression of the RbcS and RbcL genes by soluble carbohydrates accumulation leads to a decline in Rubisco protein levels (Moore et al., 1999). Alternatively the C/N ratio usually increases under elevated CO2 (Geiger et al., 1999; Luo et al., 2004; Taub and Wang, 2008; Bloom et al., 2010), since N is a key constituent of Rubisco it becomes a rate-limiting factor for Rubisco synthesis (Nakano et al., 1997; Seneweera et al., 2011).
Elevated CO2 stimulates higher photosynthesis and an increased growth rate, which is required to match with an increased demand for nutrients. This may vary between plant species, nutrient availability, and the nutrient element in question. Among different nutrient elements, maintaining the C/N balance is important for optimal plant growth. For instance, under a higher C/N ratio in soil or growth media, there is a reduced uptake of N in plants, leading to reduced grain quality in cereals due to lower grain protein content. In cereals such as wheat, rice, and barley, a decrease of up to 15% grain protein was observed under elevated CO2, with an overall decrease in amino acid concentrations (Taub et al., 2008; Wieser et al., 2008; Högy et al., 2009). A decrease in cereal grain quality and a reduced protein composition may have serious health and economic implications. The spatial leaf N content has a strong correlation with Rubisco content in rice leaves, suggesting their inter-dependency with net photosynthetic rates (Seneweera, 2011). Leaf N allocation clearly declines under elevated CO2, accompanied by lower chlorophyll content, as both are closely linked (Figure 1; Conroy and Hocking, 1993; Nakano et al., 1997; Ainsworth and Long, 2005; Leakey et al., 2009), also discussed in a later section. Other macro- and micro-nutrient concentrations change under elevated CO2 conditions, though with lesser implications compared to N (Högy et al., 2009; Erbs et al., 2010). Potassium and phosphorous contents can increase or decrease depending upon growth conditions. Significantly lower levels of sodium, calcium, magnesium, sulfur, iron, zinc, manganese, and aluminum contents have been observed in wheat grain under elevated compared to ambient CO2 (Högy and Fangmeier, 2008).
Increase in net photosynthesis per unit leaf area is important for increasing crop production to meet the world food demand. To improve photosynthesis rates in C3 plants several approaches have been used, for example introducing C4 like characteristics into C3 cells (Kajala et al., 2011; Miyao et al., 2011; Peterhansel, 2011); introducing a CO2/HCO3 pump protein into chloroplast membranes from cyanobacteria (Price et al., 2008); introducing new catabolic pathways into plastids that bypass the photorespiratory recycling Rubisco oxygenation product, 2-phosphoglycolate, and concomitantly releasing CO2 into the stroma (Kebeish et al., 2007); and also improving the Rubisco kinetic characteristics. Some opportunities to improve photosynthetic efficiency in C3 plants are discussed here.
C4 photosynthesis has been identified to be evolving independently at least 66 times in 19 different families of angiosperms (Sage et al., 2011) with 21 of these lineages displaying the intermediate C3–C4 photosynthetic characteristics (Brown and Hattersley, 1989; Edwards et al., 2004). The evolution of C4 from C3 photosynthesis involves a number of intermediate steps, while the enzymes and structures present in C4 plants are also present in C3 plants in some form (Ehleringer et al., 1991). This is advantageous for plant biologists attempting to engineer C4 pathways in C3 plants, through the identification of genotypes expressing some degree of cellular similarities to C4 plants, such as high numbers of chloroplasts in the BSC, and separation of MC by only a single cell (Furbank et al., 2009). Partial C4 cycles genes have been introduced into rice, potato, and tobacco (Kajala et al., 2011) without incorporating the complete Kranz anatomy, which would require targeting multiple genes. The Kranz anatomy of C4 plants is generally considered to be too complex to engineer into C3 cells. However, two species of the family Chenopodiaceae were found to have a C4 photosynthesis system contained within a single chlorenchyma cell in the absence of Kranz anatomy. The cells performed the same role as Kranz anatomy by partitioning themselves into two cytoplasmic compartments (Edwards et al., 2004). Each of these cells performs a function analogous to the MC and BSC in Kranz anatomy, serving to concentrate C around Rubisco. This has provided hope that C4 like photosynthesis can be introduced into C3 plants in the absence of full Kranz anatomy. Specific genes have been suggested by Miyao (2003) in C3 plants very similar to those in C4; however their expression level is very low are thought to serve housekeeping functions (Kajala et al., 2011). Overexpression of native C3 genes or homologous C4 genes could possibly be useful for improving photosynthetic efficiency in C3 plants. Attempts have been made to engineer single-celled and two-celled photosynthetic pathways with expression of specific genes into rice (Kajala et al., 2011; Miyao et al., 2011). Four C4 genes, PEPC, malate dehydrogenase (MDH), NADP-malic enzyme (NADP-ME), and NADP-malate dehydrogenase (NADP-MDH) have been engineered in rice (Taniguchi et al., 2008; Miyao et al., 2011). However, a functional C4 cycle in leaves of C3 species has not yet achieved. In addition, the negative effects of engineering C4 genes in C3 plants have been reported, such as overexpression of maize NADP-ME in rice that led to enhanced photoinhibition of photosynthesis and pleiotropic effects (Tsuchida et al., 2001), stunted transgenic plants due to generation of futile cycles and improper circadian regulation of genes (Taniguchi et al., 2008; Kajala et al., 2011). To achieve C4 photosynthetic pathway operational in C3 plants is an enormous challenge and would require: careful selection and engineering of multiple genes encoding for both C4 photosynthetic genes and transporters of C4 metabolites, driving the optimal expression of genes, site specific expression of selected genes such as in MC or BSC, choice of coding sequence since C4 genes have acquired changes in coding regions during evolutionary process, and proper regulation of C4 enzymes (Kajala et al., 2011; Miyao et al., 2011; Peterhansel, 2011).
Cyanobacteria are a phylum of bacteria that obtain energy through photosynthesis and are the ancestors of chloroplasts in eukaryotic cells (Raven and Allen, 2003). Cyanobacteria are highly efficient for producing biomass from inorganic C (Nogales et al., 2012). As a photosynthetic organism, cyanobacteria have evolved a very efficient mechanism for converting CO2 into HCO3–, an important step in transporting C into the chloroplast stroma. The carbon concentrating mechanism (CCM) of cyanobacteria delivers several-fold higher CO2 to Rubisco sites compared to C3 photosynthesis. There have been a number of attempts made to introduce CCM into C3 plants, but so far limited progress has been made (Hibberd et al., 2008; Peterhansel et al., 2008). A cyanobacterial gene ictB has been linked to HCO3– accumulation within cyanobacteria, and when expressed in transgenic Arabidopsis and tobacco resulted in a significant increase in photosynthetic rates (Lieman-Hurwitz et al., 2003). The expression of such genes in crop plants could lead to significant yield increases by developing a C4 style C concentration mechanism within plants currently exhibiting C3 anatomy. Only a small subset of genes would need to be transferred to C3 crop species, and specialized anatomy and morphology may not be required; engineering such changes is within the scope of the current genomic technology.
As macronutrients, both C and N have pivotal roles in plant growth. Additionally, these nutrients can act as signaling molecules influencing several cellular processes through regulation of gene expression in plants. It has been suggested that about half of the Arabidopsis genome is regulated by C, N, or C/N interaction (Palenchar et al., 2004; Gutierrez et al., 2007). An optimum C/N ratio is required for smooth operation of several growth, developmental, and biochemical processes such as seedling development, root architecture, lateral root development, flowering time, senescence progression, photosynthesis, and regulation of C and N assimilation (Paul and Driscoll, 1997; Corbesier et al., 1998; Martin et al., 2002; Malamy, 2005; Wingler et al., 2006; Gutierrez et al., 2007; Zhang et al., 2007).
Photosynthetic acclimation and a decrease in Rubisco levels under elevated CO2 are pre-dominant in N-limited plants compared to sufficient N-supplied plants (Miglietta et al., 1996; Riviere-Rolland et al., 1996; Rogers et al., 1996; Geiger et al., 1999; Stitt and Krapp, 1999; Ainsworth et al., 2003; Reich et al., 2006). Photosynthetic acclimation is also associated with plants acquiring and assimilating insufficient N at elevated CO2, leading to N limitation in plant tissues and subsequently lower C acquisition. Under low N these response are much greater at elevated CO2 (Geiger et al., 1999; Luo et al., 2004; Taub and Wang, 2008; Bloom et al., 2010). Also, the exogenous supply of C as sucrose to N-limiting plants led to accumulation of higher carbohydrate and a decrease of Rubisco and chlorophyll content (Paul and Stitt, 1993; Paul and Driscoll, 1997). The decrease of N content in plant biomass at elevated CO2 compared to ambient CO2 is usually within the range of 10–15% (Figure 1; Jablonski et al., 2002; Kim et al., 2003; Ainsworth and Long, 2005; Seneweera, 2011; Seneweera et al., 2011). This decrease could be due to a range of factors. First, it could involve a dilution of N in plant tissues by an increased flux of photosynthate compounds from excess carbohydrate accumulation. Second, there could be a decreased transpirational driven flow of N due to reduced stomatal conductance. Third, there could be decreased N uptake due to both source effects (soil-root specific) and reduced demand (down-regulation of photosynthetic enzymes). Fourth, a decreased N assimilation capacity could be involved. Finally, there could be a reduced electron flow for nitrate assimilation. In particular, the photosynthetic C reduction cycle and nitrate assimilation compete for electrons from photosynthesis, and since CO2 assimilation is favored, this results in a reduced N influx (Bloom et al., 2002, 2010; McDonald et al., 2002; Luo et al., 2004; Taub and Wang, 2008).
The decrease in N content in plants grown under elevated CO2 aggravates N-limiting conditions in the following years, as plant leaves senescence and drops to the soil, thus increasing microbial immobilization of N. Due to a high C/N ratio, the availability of N to plants further declines as more N is fixed in soil microbes. Consequently, this leads to progressive N limitation in most agricultural cropping systems (Ball, 1997; Luo et al., 2004). This suggests that N supply needs to be matched with higher C assimilation under elevated CO2, requiring new N management strategies in agriculture. Legumes have the potential to respond maximally to higher CO2 due to their N-fixing capacity matching the excess C gain at elevated CO2. This allows maximum utilization of sink capacity (Rogers et al., 2006). This improved N assimilation has increased photosynthetic stimulation and higher productivity in legumes compared to non-legumes under elevated CO2 (Zanetti et al., 1996; Ainsworth et al., 2002; Rogers et al., 2006). Introducing N-fixation capabilities in non-leguminous plants has always been an attractive prospect, which could close the N acquisition gap, allowing efficient CO2 capture and maximizing yield gains under elevated atmospheric CO2. Otherwise, in non-leguminous plants additional N fertilization would be required to obtain similar yield benefits, which might not be economically and environmentally feasible. Maximization of plant growth under elevated CO2 conditions without additional N application would result in yield increases at no additional cost of fertilizer or risk to the environment. There is a large opportunity to efficiently utilize the existing N supply in most agricultural soils given that most cereal crops have a NUE of less than 40%. Therefore, over 60% of soil N is lost through a combination of leaching, surface runoff, de-nitrification, volatilization, and microbial consumption. It is estimated that a 1% increase in NUE could save ~$1.1 billion annually. Hence, developing crop varieties with a higher NUE would minimize the loss of N, reduce environmental pollution and decrease input cost (Kant et al., 2011). NUE has been defined in several ways (Good et al., 2004); the most simple and practical is yield per unit of N available in the soil. Coordinated efforts are required to increase N uptake, assimilation, and/or remobilization efficiency for improved NUE. During vegetative growth, N uptake is dedicated to storage and assimilation into amino acids where developing leaves and shoots act as sinks for N. During reproductive development, N assimilation and remobilization becomes more prominent, and leaves and shoots act as an N source, supplying amino acids for reproductive organs.
Attempts have been made to manipulate the expression of different nitrate and ammonium transporters and assimilatory genes, mostly in model plants and some crop plants (Good et al., 2004; Kant et al., 2011). Higher or lower N contents led by overexpression or mutation of genes for N transporters have been reported in several studies in Arabidopsis (Geelen et al., 2000; De Angeli et al., 2006; Chopin et al., 2007). However, evidence of improved yield or NUE in crop plants attributed to modification of N responsive genes is limited, with little improvement in some traits. For example, transgenic wheat plants displayed enhanced N assimilation capacity by overexpression of Glutamine Synthetase (GS1; Habash et al., 2001). Overexpression of the GS1.3 gene in maize resulted in an increase of 30% kernel number (Martin et al., 2006). The limited benefits of modifications of such genes could be ascribed to (i) lack of suitable combinations of promoters and regulatory elements for specific expression of the target genes that can match with a certain growth stage, plant tissue type, or environmental conditions, and (ii) limited sink capacity in crop plants to assimilate and utilize additional N for increased growth and yield. Additional C skeleton and C assimilation would be required to expand sink capacity where surplus N can be incorporated. Overexpression of genes for N uptake, transport, and assimilation would ensure increased availability of N content and amino acids utilized for photosynthetic machinery which mainly control plant growth and development. Simultaneously, elevated atmospheric CO2 would ensure adequate C supply for enhanced plant growth. Hence, orchestrating coordinated efforts for growing C3 plants with increased NUE and better C assimilation capacity under elevated CO2 would be an effective strategy for avoiding CO2 or photosynthesis acclimation, leading to higher growth rates, yield, and quality in cereals.
Plant growth is typically stimulated at elevated CO2, but often decreases with time, due to relaxation of photosynthesis to a lower rate under exposure to elevated CO2 over longer periods. The sustained and maximal stimulation of growth at elevated CO2 requires acquisition of additional N to maximize increased C assimilation. Coordinated efforts for increasing photosynthetic efficiency, enhancing sink capacity, and improving N uptake would potentially increase grain yield under rising atmospheric CO2. A marginal increase in crop growth and yield has been reported in several FACE experiments. Nevertheless, improving NUE and N uptake in crop plants could partially avert the limitations of both photosynthetic acclimation and reduced grain quality under elevated CO2 levels. The major private plant biotechnology companies are attempting to develop improved NUE transgenic lines in their research and development strategies. This would be an effective method and would reinforce their strategy for improved grain quality under commonly accepted climate change scenarios. In the past 15 years (1996–2010), the accumulated global land area for transgenic crops exceeded one billion hectares grown by over 15 million farmers (James, 2010). The use of genetically engineered crops has increased farmer profit, reduced herbicide and pesticide usage, and reduced chemical impact on the environment, which has mainly been achieved through single gene modifications. However, the traits of improving NUE and enhanced crop response to elevated CO2 are more complex and would require stacking of multiple modified genes. Efficient management of ammonium and nitrate application could also facilitate benefits of increased yield and sustained grain quality under forecasted atmospheric CO2 elevation. However, careful manipulations of N-responsive genes provide the greatest global advantages, since additional nitrogenous fertilizers pose undesirable economic and environmental threats.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank Carolyn Rothstein for critical reading of the draft manuscript.
Ainsworth, E. A., Davey, P. A., Bernacchi, C. J., Dermody, O. C., Heaton, E. A., Moore, D. J., Morgan, P. B., Naidu, S. L., Yoo Ra, H.-S., Zhu, X.-G., Curtis, P. S., and Long, S. P. (2002). A meta-analysis of elevated [CO2] effects on soybean (Glycine max) physiology, growth and yield. Glob. Change Biol. 8, 695–709.
Ainsworth, E. A., Davey, P. A., Hymus, G. J., Osborne, C. P., Rogers, A., Blum, H., NöSberger, J., and Long, S. P. (2003). Is stimulation of leaf photosynthesis by elevated carbon dioxide concentration maintained in the long term? A test with Lolium perenne grown for 10 years at two nitrogen fertilization levels under Free Air CO2 Enrichment (FACE). Plant Cell Environ. 26, 705–714.
Ainsworth, E. A., Leakey, A. D. B., Ort, D. R., and Long, S. P. (2008). FACE-ing the facts: inconsistencies and interdependence among field, chamber and modeling studies of elevated [CO2] impacts on crop yield and food supply. New Phytol. 179, 5–9.
Ainsworth, E. A., and Long, S. P. (2005). What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 165, 351–372.
Aoki, N., Ono, K., Sasaki, H., Seneweera, S. P., Sasaki, H., Kobatashi, K., and Ishimaru, K. (2003). Effects of elevated CO2 concentration on photosynthetic carbon metabolism in flag-leaf blades of rice before and after heading. Plant Prod. Sci. 6, 52–58.
Aranjuelo, I., Cabrera-Bosquet, L., Morcuende, R., Avice, J. C., Nogues, S., Araus, J. L., Martinez-Carrasco, R., and Perez, P. (2011). Does ear C sink strength contribute to overcoming photosynthetic acclimation of wheat plants exposed to elevated CO2? J. Exp. Bot. 62, 3957–3969.
Badger, M. R., and Price, G. D. (2003). CO2 concentrating mechanisms in cyanobacteria: molecular components, their diversity and evolution. J. Exp. Bot. 54, 609–622.
Ball, A. (1997). Microbial decomposition at elevated CO2 levels: effect of litter quality. Glob. Change Biol. 3, 379–386.
Bloom, A. J., Burger, M., Rubio Asensio, J. S., and Cousins, A. B. (2010). Carbon dioxide enrichment inhibits nitrate assimilation in wheat and Arabidopsis. Science 328, 899–903.
Bloom, A. J., Smart, D. R., Nguyen, D. T., and Searles, P. S. (2002). Nitrogen assimilation and growth of wheat under elevated carbon dioxide. Proc. Natl. Acad. Sci. U.S.A. 99, 1730–1735.
Brown, R. H., and Hattersley, P. W. (1989). Leaf anatomy of C3–C4 species as related to evolution of C4 photosynthesis. Plant Physiol. 91, 1543–1550.
Cheng, S. H., Moore, B., and Seemann, J. R. (1998). Effects of short- and long-term elevated CO2 on the expression of ribulose-1,5-bisphosphate carboxylase/oxygenase genes and carbohydrate accumulation in leaves of Arabidopsis thaliana (L.) Heynh. Plant Physiol. 116, 715–723.
Chopin, F., Orsel, M., Dorbe, M. F., Chardon, F., Truong, H. N., Miller, A. J., Krapp, A., and Daniel-Vedele, F. (2007). The Arabidopsis ATNRT2.7 nitrate transporter controls nitrate content in seeds. Plant Cell 19, 1590–1602.
Conroy, J., and Hocking, P. (1993). Nitrogen nutrition of C3 plants at elevated atmospheric CO2 concentrations. Physiol. Plant. 89, 570–576.
Corbesier, L., Lejeune, P., and Bernier, G. (1998). The role of carbohydrates in the induction of flowering in Arabidopsis thaliana: comparison between the wild type and a starchless mutant. Planta 206, 131–137.
Cure, J. D., and Acock, B. (1986). Crop responses to carbon dioxide doubling: a literature survey. Agric. For. Meteorol. 38, 127–145.
Curtis, P. S., and Wang, X. (1998). A meta-analysis of elevated CO2 effects on woody plant mass, form, and physiology. Oecologia 113, 299–313.
De Angeli, A., Monachello, D., Ephritikhine, G., Frachisse, J. M., Thomine, S., Gambale, F., and Barbier-Brygoo, H. (2006). The nitrate/proton antiporter AtCLCa mediates nitrate accumulation in plant vacuoles. Nature 442, 939–942.
Drake, B. G., Gonzalez-Meler, M. A., and Long, S. P. (1997). MORE EFFICIENT PLANTS: a consequence of rising atmospheric CO2? Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 609–639.
Edwards, G. E., Franceschi, V. R., and Voznesenskaya, E. V. (2004). Single-cell C4 photosynthesis versus the dual-cell (Kranz) paradigm. Annu. Rev. Plant Biol. 55, 173–196.
Ehleringer, J. R., Sage, R. F., Flanagan, L. B., and Pearcy, R. W. (1991). Climate change and the evolution of C4 photosynthesis. Trends Ecol. Evol. 6, 95–99.
Erbs, M., Manderscheid, R., Jansen, G., Seddig, S., Pacholski, A., and Weigel, H.-J. (2010). Effects of free-air CO2 enrichment and nitrogen supply on grain quality parameters and elemental composition of wheat and barley grown in a crop rotation. Agric. Ecosyst. Environ. 136, 59–68.
Farquhar, G. D., Caemmerer, S., and Berry, J. A. (1980). A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149, 78–90.
Farquhar, G. D., and Sharkey, T. D. (1982). Stomatal conductance and photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 33, 317–345.
Fischer, R. A., Rees, D., Sayre, K. D., Lu, Z. M., Condon, A. G., and Saavedra, A. L. (1998). Wheat yield progress associated with higher stomatal conductance and photosynthetic rate and cooler canopies. Crop Sci. 38, 1467–1475.
Furbank, R. T., von Caemmerer, S., Sheehy, J., and Edwards, G. (2009). C4 rice: a challenge for plant phenomics. Funct. Plant Biol. 36, 845–856.
Geelen, D., Lurin, C., Bouchez, D., Frachisse, J. M., Lelievre, F., Courtial, B., Barbier-Brygoo, H., and Maurel, C. (2000). Disruption of putative anion channel gene AtCLCa in Arabidopsis suggests a role in the regulation of nitrate content. Plant J. 21, 259–267.
Geiger, M., Haake, V., Ludewig, F., Sonnewald, U., and Stitt, M. (1999). The nitrate and ammonium nitrate supply have a major influence on the response of photosynthesis, carbon metabolism, nitrogen metabolism and growth to elevated carbon dioxide in tobacco. Plant Cell Environ. 22, 1177–1199.
Godfray, H. C. J., Beddington, J. R., Crute, I. R., Haddad, L., Lawrence, D., Muir, J. F., Pretty, J., Robinson, S., Thomas, S. M., and Toulmin, C. (2010). Food security: the challenge of feeding 9 billion people. Science 327, 812–818.
Good, A. G., Shrawat, A. K., and Muench, D. G. (2004). Can less yield more? Is reducing nutrient input into the environment compatible with maintaining crop production? Trends Plant Sci. 9, 597–605.
Gutierrez, R., Lejay, L., Dean, A., Chiaromonte, F., Shasha, D., and Coruzzi, G. (2007). Qualitative network models and genome-wide expression data define carbon/nitrogen-responsive molecular machines in Arabidopsis. Genome Biol. 8, R7.
Habash, D. Z., Massiah, A. J., Rong, H. L., Wallsgrove, R. M., and Leigh, R. A. (2001). The role of cytosolic glutamine synthetase in wheat. Ann. Appl. Biol. 138, 83–89.
Hibberd, J. M., Sheehy, J. E., and Langdale, J. A. (2008). Using C4 photosynthesis to increase the yield of rice-rationale and feasibility. Curr. Opin. Plant Biol. 11, 228–231.
Ho, L. C. (1976). Variation in the carbon/dry matter ratio in plant material. Ann. Bot. 40, 163–165.
Högy, P., and Fangmeier, A. (2008). Effects of elevated atmospheric CO2 on grain quality of wheat. J. Cereal Sci. 48, 580–591.
Högy, P., Wieser, H., Köhler, P., Schwadorf, K., Breuer, J., Franzaring, J., Muntifering, R., and Fangmeier, A. (2009). Effects of elevated CO2 on grain yield and quality of wheat: results from a 3-year free-air CO2 enrichment experiment. Plant Biol. 11, 60–69.
Imai, K., Suzuki, Y., Mae, T., and Makino, A. (2008). Changes in the synthesis of Rubisco in rice leaves in relation to senescence and N influx. Ann. Bot. 101, 135–144.
IPCC. (2007). “Summary for policymakers,” in Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, eds S. Solomon, D. Qin, M. Manning, Z. Chen, M. Marquis, K. B. Averyt, M. Tignor, and H. L. Miller (Cambridge: Cambridge University Press), 1–18.
Jablonski, L. M., Wang, X., and Curtis, P. S. (2002). Plant reproduction under elevated CO2 conditions: a meta-analysis of reports on 79 crop and wild species. New Phytol. 156, 9–26.
James, C. (2010). Global Status of Commercialized Biotech/GM Crops: 2010. ISAAA Brief No. 42-2010. Available at: http://www.isaaa.org/kc/
Kajala, K., Covshoff, S., Karki, S., Woodfield, H., Tolley, B. J., Dionora, M. J. A., Mogul, R. T., Mabilangan, A. E., Danila, F. R., Hibberd, J. M., and Quick, W. P. (2011). Strategies for engineering a two-celled C4 photosynthetic pathway into rice. J. Exp. Bot. 62, 3001–3010.
Kant, S., Bi, Y. M., and Rothstein, S. J. (2011). Understanding plant response to nitrogen limitation for the improvement of crop nitrogen use efficiency. J. Exp. Bot. 62, 1499–1509.
Kebeish, R., Niessen, M., Thiruveedhi, K., Bari, R., Hirsch, H.-J., Rosenkranz, R., Stabler, N., Schonfeld, B., Kreuzaler, F., and Peterhansel, C. (2007). Chloroplastic photorespiratory bypass increases photosynthesis and biomass production in Arabidopsis thaliana. Nat. Biotechnol. 25, 593–599.
Kim, H. Y., Lieffering, M., Kobayashi, K., Okada, M., Mitchell, M. W., and Gumpertz, M. (2003). Effects of free-air CO2 enrichment and nitrogen supply on the yield of temperate paddy rice crops. Field Crops Res. 83, 261–270.
Leakey, A. D. B., Ainsworth, E. A., Bernacchi, C. J., Rogers, A., Long, S. P., and Ort, D. R. (2009). Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important lessons from FACE. J. Exp. Bot. 60, 2859–2876.
Lieman-Hurwitz, J., Rachmilevitch, S., Mittler, R., Marcus, Y., and Kaplan, A. (2003). Enhanced photosynthesis and growth of transgenic plants that express ictB, a gene involved in HCO3- accumulation in cyanobacteria. Plant Biotechnol. J. 1, 43–50.
Long, S. P., Ainsworth, E. A., Rogers, A., and Ort, D. R. (2004). Rising atmospheric carbon dioxide: plants FACE the future. Annu. Rev. Plant Biol. 55, 591–628.
Luo, Y., Su, B. O., Currie, W. S., Dukes, J. S., Finzi, A., Hartwig, U., Hungate, B., Mc Murtrie, R. E., Oren, R. A. M., Parton, W. J., Pataki, D. E., Shaw, M. R., Zak, D. R., and Field, C. B. (2004). Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. Bioscience 54, 731–739.
Mae, T., Thomas, H., Gay, A. P., Makino, A., and Hidema, J. (1993). Leaf development in Lolium temulentum – photosynthesis and photosynthetic proteins in leaves senescing under different irradiances. Plant Cell Physiol. 34, 391–399.
Makino, A., and Osmond, B. (1991). Effects of nitrogen nutrition on nitrogen partitioning between chloroplasts and mitochondria in pea and wheat. Plant Physiol. 96, 355–362.
Malamy, J. E. (2005). Intrinsic and environmental response pathways that regulate root system architecture. Plant Cell Environ. 28, 67–77.
Martin, A., Lee, J., Kichey, T., Gerentes, D., Zivy, M., Tatout, C., Dubois, F., Balliau, T., Valot, B., Davanture, M., Terce-Laforgue, T., Quillere, I., Coque, M., Gallais, A., Gonzalez-Moro, M. B., Bethencourt, L., Habash, D. Z., Lea, P. J., Charcosset, A., Perez, P., Murigneux, A., Sakakibara, H., Edwards, K. J., and Hirel, B. (2006). Two cytosolic glutamine synthetase isoforms of maize are specifically involved in the control of grain production. Plant Cell 18, 3252–3274.
Martin, T., Oswald, O., and Graham, I. A. (2002). Arabidopsis seedling growth, storage lipid mobilization, and photosynthetic gene expression are regulated by carbon:nitrogen availability. Plant Physiol. 128, 472–481.
McDonald, E. P., Erickson, J. E., and Kruger, E. L. (2002). Can decreased transpiration limit plant nitrogen acquisition in elevated CO2? Funct. Plant Biol. 29, 1115–1120.
Miglietta, F., Giuntoli, A., and Bindi, M. (1996). The effect of free air carbon dioxide enrichment (FACE) and soil nitrogen availability on the photosynthetic capacity of wheat. Photosynth. Res. 47, 281–290.
Miyao, M. (2003). Molecular evolution and genetic engineering of C4 photosynthetic enzymes. J. Exp. Bot. 54, 179–189.
Miyao, M., Masumoto, C., Miyazawa, S.-I., and Fukayama, H. (2011). Lessons from engineering a single-cell C4 photosynthetic pathway into rice. J. Exp. Bot. 62, 3021–3029.
Moore, B. D., Cheng, S. H., Rice, J., and Seemann, J. R. (1998). Sucrose cycling, Rubisco expression, and prediction of photosynthetic acclimation to elevated atmospheric CO2. Plant Cell Environ. 21, 905–915.
Moore, B. D., Cheng, S. H., Sims, D., and Seemann, J. R. (1999). The biochemical and molecular basis for photosynthetic acclimation to elevated atmospheric CO2. Plant Cell Environ. 22, 567–582.
Muhaidat, R., Sage, R. F., and Dengler, N. G. (2007). Diversity of Kranz anatomy and biochemistry in C4 eudicots. Am. J. Bot. 94, 362–381.
Nakano, H., Makino, A., and Mae, T. (1997). The effect of elevated partial pressures of CO2 on the relationship between photosynthetic capacity and N content in rice leaves. Plant Physiol. 115, 191–198.
Nie, G., Hendrix, D. L., Webber, A. N., Kimball, B. A., and Long, S. P. (1995). Increased accumulation of carbohydrates and decreased photosynthetic gene transcript levels in wheat grown at an elevated CO2 concentration in the field. Plant Physiol. 108, 975–983.
Nogales, J., Gudmundsson, S., Knight, E. M., Palsson, B. O., and Thiele, I. (2012). Detailing the optimality of photosynthesis in cyanobacteria through systems biology analysis. Proc. Natl. Acad. Sci. U.S.A. 109, 2678–2683.
Palenchar, P., Kouranov, A., Lejay, L., and Coruzzi, G. (2004). Genome-wide patterns of carbon and nitrogen regulation of gene expression validate the combined carbon and nitrogen (CN)-signaling hypothesis in plants. Genome Biol. 5, R91.
Paul, M. J., and Driscoll, S. P. (1997). Sugar repression of photosynthesis: the role of carbohydrates in signalling nitrogen deficiency through source: sink imbalance. Plant Cell Environ. 20, 110–116.
Paul, M. J., and Stitt, M. (1993). Effects of nitrogen and phosphorus deficiencies on levels of carbohydrates, respiratory enzymes and metabolites in seedlings of tobacco and their response to exogenous sucrose. Plant Cell Environ. 16, 1047–1057.
Peterhansel, C. (2011). Best practice procedures for the establishment of a C4 cycle in transgenic C3 plants. J. Exp. Bot. 62, 3011–3019.
Peterhansel, C., Niessen, M., and Kebeish, R. M. (2008). Metabolic engineering towards the enhancement of photosynthesis. Photochem. Photobiol. 84, 1317–1323.
Price, G. D., Badger, M. R., Woodger, F. J., and Long, B. M. (2008). Advances in understanding the cyanobacterial CO2-concentrating-mechanism (CCM): functional components, Ci transporters, diversity, genetic regulation and prospects for engineering into plants. J. Exp. Bot. 59, 1441–1461.
Raven, J., and Allen, J. (2003). Genomics and chloroplast evolution: what did cyanobacteria do for plants? Genome Biol. 4, 209.
Reich, P. B., Hobbie, S. E., Lee, T., Ellsworth, D. S., West, J. B., Tilman, D., Knops, J. M., Naeem, S., and Trost, J. (2006). Nitrogen limitation constrains sustainability of ecosystem response to CO2. Nature 440, 922–925.
Riviere-Rolland, H., Contard, P., and Betsche, T. (1996). Adaptation of pea to elevated atmospheric CO2: Rubisco, phosphoenolpyruvate carboxylase and chloroplast phosphate translocator at different levels of nitrogen and phosphorus nutrition. Plant Cell Environ. 19, 109–117.
Rogers, A., Gibon, Y., Stitt, M., Morgan, P. B., Bernacchi, C. J., Ort, D. R., and Long, S. P. (2006). Increased C availability at elevated carbon dioxide concentration improves N assimilation in a legume. Plant Cell Environ. 29, 1651–1658.
Rogers, G., Milham, P., Gillings, M., and Conroy, J. (1996). Sink strength may be the key to growth and nitrogen responses in N-deficient wheat at elevated CO2. Funct. Plant Biol. 23, 253–264.
Rothstein, S. J. (2007). Returning to our roots: making plant biology research relevant to future challenges in agriculture. Plant Cell 19, 2695–2699.
Sage, R., and Kubien, D. (2003). Quo vadis C4? An ecophysiological perspective on global change and the future of C4 plants. Photosynth. Res. 77, 209–225.
Sage, R. F., Christin, P.-A., and Edwards, E. J. (2011). The C4 plant lineages of planet Earth. J. Exp. Bot. 62, 3155–3169.
Sage, R. F., Sage, T. L., and Kocacinar, F. (2012). Photorespiration and the evolution of C4 photosynthesis. Annu. Rev. Plant Biol. 63, 19–47.
Seneweera, S. (2011). Reduced nitrogen allocation to expanding leaf blades suppresses ribulose-1,5-bisphosphate carboxylase/oxygenase synthesis and leads to photosynthetic acclimation to elevated CO2 in rice. Photosynthetica 49, 145–148.
Seneweera, S., Makino, A., Hirotsu, N., Norton, R., and Suzuki, Y. (2011). New insight into photosynthetic acclimation to elevated CO2: The role of leaf nitrogen and ribulose-1,5-bisphosphate carboxylase/oxygenase content in rice leaves. Environ. Exp. Bot. 71, 128–136.
Seneweera, S. P., and Conroy, J. P. (2005). Enhanced leaf elongation rates of wheat at elevated CO2: is it related to carbon and nitrogen dynamics within the growing leaf blade? Environ. Exp. Bot. 54, 174–181.
Sharkey, T. D. (1985). Photosynthesis in intact leaves of C3 plants – physics, physiology and rate limitations. Bot. Rev. 51, 53–105.
Stitt, M., and Krapp, A. (1999). The interaction between elevated carbon dioxide and nitrogen nutrition: the physiological and molecular background. Plant Cell Environ. 22, 583–621.
Stitt, M., and Schulze, D. (1994). Does Rubisco control the rate of photosynthesis and plant growth? An exercise in molecular ecophysiology. Plant Cell Environ. 17, 465–487.
Taniguchi, Y., Ohkawa, H., Masumoto, C., Fukuda, T., Tamai, T., Lee, K., Sudoh, S., Tsuchida, H., Sasaki, H., Fukayama, H., and Miyao, M. (2008). Overproduction of C4 photosynthetic enzymes in transgenic rice plants: an approach to introduce the C4-like photosynthetic pathway into rice. J. Exp. Bot. 59, 1799–1809.
Taub, D. R., Miller, B., and Allen, H. (2008). Effects of elevated CO2 on the protein concentration of food crops: a meta-analysis. Glob. Change Biol. 14, 565–575.
Taub, D. R., and Wang, X. (2008). Why are nitrogen concentrations in plant tissues lower under elevated CO2? A critical examination of the hypotheses. J. Integr. Plant Biol. 50, 1365–1374.
Tester, M., and Langridge, P. (2010). Breeding technologies to increase crop production in a changing world. Science 327, 818–822.
Tsuchida, H., Tamai, T., Fukayama, H., Agarie, S., Nomura, M., Onodera, H., Ono, K., Nishizawa, Y., Lee, B.-H., Hirose, S., Toki, S., Ku, M. S. B., Matsuoka, M., and Miyao, M. (2001). High level expression of C4-specific NADP-malic enzyme in leaves and impairment of photoautotrophic growth in a C3 plant, rice. Plant Cell Physiol. 42, 138–145.
von Caemmerer, S., and Furbank, R. (2003). The C4 pathway: an efficient CO2 pump. Photosynth. Res. 77, 191–207.
Wand, S. J. E., Midgley, G. F., Jones, M. H., and Curtis, P. S. (1999). Responses of wild C4 and C3 grass (Poaceae) species to elevated atmospheric CO2 concentration: a meta-analytic test of current theories and perceptions. Glob. Change Biol. 5, 723–741.
Wieser, H., Manderscheid, R., Erbs, M., and Weigel, H. J. (2008). Effects of elevated atmospheric CO2 concentrations on the quantitative protein composition of wheat grain. J. Agric. Food Chem. 56, 6531–6535.
Wingler, A., Purdy, S., MacLean, J. A., and Pourtau, N. (2006). The role of sugars in integrating environmental signals during the regulation of leaf senescence. J. Exp. Bot. 57, 391–399.
Zanetti, S., Hartwig, U. A., Luscher, A., Hebeisen, T., Frehner, M., Fischer, B. U., Hendrey, G. R., Blum, H., and Nosberger, J. (1996). Stimulation of symbiotic N2 fixation in Trifolium repens L. under elevated atmospheric pCO2 in a grassland ecosystem. Plant Physiol. 112, 575–583.
Zhang, H. M., Rong, H. L., and Pilbeam, D. (2007). Signalling mechanisms underlying the morphological responses of the root system to nitrogen in Arabidopsis thaliana. J. Exp. Bot. 58, 2329–2338.
Keywords: photosynthesis, nitrogen use efficiency, Rubisco, carbon, nitrogen, elevated CO2, yield
Citation: Kant S, Seneweera S, Rodin J, Materne M, Burch D, Rothstein SJ and Spangenberg G (2012) Improving yield potential in crops under elevated CO2: integrating the photosynthetic and nitrogen utilization efficiencies. Front. Plant Sci. 3:162. doi: 10.3389/fpls.2012.00162
Received: 02 April 2012; Accepted: 30 June 2012;
Published online: 19 July 2012.
Edited by:
Takuji Sasaki, National Institute of Agrobiological Sciences, JapanReviewed by:
Steven B. Cannon, U.S. Department of Agriculture – Agricultural Research Service, USACopyright: © 2012 Kant, Seneweera, Rodin, Materne, Burch, Rothstein and Spangenberg. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and subject to any copyright notices concerning any third-party graphics etc.
*Correspondence: Surya Kant, Department of Primary Industries, Biosciences Research Division, Grains Innovation Park, 110 Natimuk Road, Horsham, VIC 3400, Australia. e-mail:c3VyeWEua2FudEBkcGkudmljLmdvdi5hdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.