
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol. , 12 March 2025
Sec. Vascular Physiology
Volume 16 - 2025 | https://doi.org/10.3389/fphys.2025.1562582
This article is part of the Research Topic Physiological and Pathological Responses to Hypoxia and High Altitude, Volume III View all 8 articles
 Hannah R. Johnson1,2*
Hannah R. Johnson1,2* Max C. Wang1,2
Max C. Wang1,2 Rachael C. Stickland2
Rachael C. Stickland2 Yufen Chen3
Yufen Chen3 Todd B. Parrish1,3
Todd B. Parrish1,3 Farzaneh A. Sorond4
Farzaneh A. Sorond4 Molly G. Bright1,2
Molly G. Bright1,2Introduction: Cerebrovascular reactivity (CVR) to changes in blood carbon dioxide and oxygen levels is a robust indicator of vascular health. Although CVR is typically assessed with hypercapnia, the interplay between carbon dioxide and oxygen, and their ultimate roles in dictating vascular tone, can vary with pathology. Methods to characterize vasoreactivity to oxygen changes, particularly hypoxia, would provide important complementary information to established hypercapnia techniques. However, existing methods to study hypoxic CVR, typically with arterial spin labeling (ASL) MRI, demonstrate high variability and paradoxical responses.
Methods: To understand whether these responses are real or due to methodological confounds of ASL, we used phase-contrast MRI to quantify whole-brain blood flow in 21 participants during baseline, hypoxic, and hypercapnic respiratory states in three scan sessions.
Results: Hypoxic CVRreliability was poor-to-moderate (ICC = 0.42 for CVR relative to PETO2 changes, ICC = 0.56 relative to SpO2 changes) and was less reliable than hypercapnic CVR (ICC = 0.67).
Discussion: Without the uncertainty from ASL-related confounds, we still observed paradoxical responses at each timepoint. Concurrent changes in blood carbon dioxide levels did not account for paradoxical responses. Hypoxic CVR and hypercapnic CVR shared approximately 40% of variance across the dataset, indicating that the two effects may indeed reflect distinct, complementary elements of vascular regulation. The data included in this article were collected as part of a randomized cross-over clinical trial, but do not assess the outcomes of this trial: Improving Human Cerebrovascular Function Using Acute Intermittent Hypoxia (NCT05164705), https://clinicaltrials.gov/study/NCT05164705.
Cerebral blood flow (CBF) is regulated to ensure that sufficient oxygen and nutrients are delivered to brain tissue despite changes in metabolic demands, blood pressure, and blood gas levels. The ability of the brain vasculature to appropriately regulate blood flow can be assessed by measuring cerebrovascular reactivity (CVR), the change in CBF in response to a vasoactive stimulus like hypercapnia, hypoxia, or acetazolamide (Sleight et al., 2021). CVR is a useful metric of vascular health, changing with age (Peng et al., 2018) and decreasing with disease severity in Alzheimer’s Disease (Sur et al., 2020), Multiple Sclerosis (Marshall et al., 2014), Parkinson’s Disease (Ryman et al., 2023), and Obstructive Sleep Apnea (Ponsaing et al., 2018). CVR is also a promising predictive biomarker of stroke in carotid artery disease (Reinhard et al., 2014; Vernieri et al., 1999) and white matter hyperintensity development in white matter disease (Sam et al., 2016), increasing its clinical utility.
CVR is most commonly assessed using a hypercapnic stimulus, such as a breath-holding task or inhalation of gas mixtures with elevated CO2, as in the aforementioned studies. CVR to hypercapnic stimuli has been extensively characterized, with a large body of literature establishing normative values for multiple imaging modalities and evaluating the impact of a wide range of clinical conditions on CVR (Liu et al., 2019; Sleight et al., 2021). Although the cerebrovascular response to hypercapnia certainly provides valuable information about the health of the brain’s blood supply, the brain is also exquisitely sensitive to hypoxic conditions due to the physiological importance of oxygen (Steinback and Poulin, 2008). While oxygen and carbon dioxide levels are closely coupled through ventilation under healthy conditions, their relationship can change under pathology, particularly in respiratory disease and ischemia (Binks et al., 2008; Hoffman et al., 1999; West, 2011), and the two influence vascular tone through independent mechanisms (Beaudin et al., 2017; Carr et al., 2024; Cipolla, 2009). Characterization of the hypoxic vascular response, independent of carbon dioxide changes, could provide complementary information to the extensive body of literature on the hypercapnic cerebrovascular response and inform assessment of composite CVR in pathology.
There are technical challenges to studying CVR to hypoxia. Spontaneous breathing during hypoxic hypoxia (poikilocapnic conditions) results in decreased arterial CO2 concentrations, producing a vasoconstrictive effect that competes with and obscures the anticipated hypoxic vasodilatory response we seek to characterize (Friend et al., 2019; Shapiro et al., 1970). While transcranial doppler (TCD) provides unbiased measures of blood velocity changes in hypoxia, it is unable to account for arterial diameter changes and therefore cannot fully characterize CVR (Wilson et al., 2011). Hypoxia also introduces bias in typical MRI methods for assessing CVR: bulk changes in arterial oxygen confound characterization of CBF changes with blood oxygenation level-dependent (BOLD)-weighted functional MR imaging, and changes in blood velocity and arterial oxygenation can impact measurements of quantitative CBF with arterial spin labeling (ASL) MRI. Although the spatial distribution of CBF changes is lost, phase-contrast (PC) MRI offers a robust method for quantifying whole-brain CBF changes during hypoxia, accounting for modulations in both velocity and vessel area, and unbiased by BOLD-weighted contrast or ASL confounds related to labeling efficiency and T1 relaxation times.
Considering all imaging methods, the blood-flow response to severe hypoxia has been well-characterized, causing an invariable exponential increase in CBF with decreases in the partial pressure of oxygen (Harris et al., 2013; Poulin, 1998; Steinback and Poulin, 2008), but the response to mild-to-moderate hypoxia, particularly when assessed with non-invasive methods, remains ambiguous. While there is no current standardized classification system for defining hypoxia as mild, moderate, or severe, general thresholds exist (Damgaard et al., 2023). Mild hypoxia generally delivers a fraction of inspired oxygen (FiO2) of greater than 17% but less than room air, corresponding to inhaled partial pressures of oxygen (PO2) between 130 and 160 mmHg at sea level. Moderate hypoxia delivers FiO2 of between 10% and 16%, corresponding to PO2 of 76–130 mmHg. Severe hypoxia delivers air with less than 9% oxygen, equating to PO2 of less than 76 mmHg. As the typical PaO2/FiO2 ratio for healthy controls at sea level is between 400 and 500 mmHg (Kadkhodai et al., 2022), these thresholds approximately correspond to arterial PO2 (PaO2) levels above 75 mmHg and SpO2 levels above 94% for mild hypoxia, PaO2 between 40 and 75 mmHg and SpO2 between 75%–94% for moderate hypoxia, and PaO2 below 40 mmHg and SpO2 below 75% for severe hypoxia in healthy individuals (Madan, 2017).
At the (generally safe) levels of oxygen delivery used in mild-to-moderate hypoxia challenges, the blood flow response is comparatively small, limiting its detection, and has shown substantial variability between individuals. The typical response to mild-to-moderate hypoxia is an increase in blood flow. Kety and Schmidt first measured this response using arterial sampling, finding an average CBF increase of 37% (±23%) in response to 10% inhaled oxygen (Kety and Schmidt, 1948). Later, Ellingsen et al. found an invariable increase in blood velocity in the internal carotid artery using doppler ultrasound, averaging a 23% (range 12.5%–25%) increase in CBF, when inhaled oxygen was reduced by 35% to approximately 13.7% (Ellingsen et al., 1987). More recently, a 19% (±3%) increase in total CBF during an 11% inhaled oxygen challenge was measured using 4D-flow MRI (Kellawan et al., 2017). However, several published studies have shown that a subset of participants deviate from this expected response, exhibiting either no significant change or a paradoxical decrease in CBF during hypoxia administration. In an ASL MRI study, 30% of participants exhibited decreased CBF to moderate hypoxia, with an average decrease in blood flow of 0.68% (±0.31%) per % change in SaO2 (Nöth et al., 2008). In another study using positron emission tomography, Binks et al. observed that one of five participants had no change in CBF under moderate hypoxia (Binks et al., 2008). These studies demonstrate considerable deviation from the expected blood flow response to hypoxia, and such variable responsiveness could cause some individuals to have greater susceptibility to cerebral tissue deoxygenation. However, it is unclear whether this responsiveness to hypoxia is an artifact of methodological confounds, a result of day-to-day variability in physiology, or is a true representation of vascular reactivity in some individuals.
We therefore used phase-contrast MRI, which is free of the biases associated with other techniques, to quantify whole-brain blood flow under baseline, hypoxic, and hypercapnic respiratory conditions on 3 days spanning several months. We characterized inter-subject and inter-session variability in the cerebrovascular response to moderate hypoxic hypoxia, while accounting for important sources of physiological variability. We further compared hypoxic CVR with hypercapnic CVR measures in the same participants, evaluating the differences between the two metrics of vascular reactivity. Through this robust evaluation of CVR to mild-to-moderate hypoxia in healthy individuals, we hope to improve the utility of hypoxic CVR as a metric of cerebrovascular health and characterize sources of variability.
This study was approved by Northwestern University’s Institutional Review Board. All subjects provided written informed consent before undergoing any study procedures. 21 subjects (8 male, 12 female, 1 non-binary, 28 ± 7 years) were recruited. Subjects were free of known neurological, vascular, cardiac, or respiratory conditions, had no MRI contraindications, and were not pregnant. Participants were scanned twice, 21 ± 5 days apart, following an initial practice session outside of the scanner to ensure safety and comfort with gas challenges. Scans were scheduled at approximately the same time of day to minimize diurnal variations in blood flow and CVR, and subjects were asked to refrain from caffeine consumption for at least 2 h before scanning. 19 participants were scanned a third time, 124 ± 29 days (approximately 4 months) after the first MRI session using the same protocol. Note that scans were part of a larger randomized cross-over clinical trial with washout evaluating the effect of a respiratory intervention on CBF and CVR metrics; this study uses data from the sham intervention arm and from the first scan of the intervention arm but excludes data affected by the intervention.
Subjects were fit with an airtight face mask for scanning and connected to a computer-controlled gas blending system (RespirAct, Thornhill Medical, Toronto, Canada) used to prospectively target precise end-tidal partial pressures of oxygen (PETO2) and carbon dioxide (PETCO2) through manipulation of inhaled gas concentrations. The respiratory protocol consisted of 4 phases: (1) baseline, targeting participants’ resting PETO2 and PETCO2 levels, (2) hypoxia, targeting PETO2 of 55–70 mmHg and resting PETCO2, (3) recovery baseline, targeting resting PETO2 and PETCO2, and (4) hypercapnia, targeting resting PETO2 and PETCO2 of 6 mmHg above resting. The respiratory stimulus protocol is shown in Figure 1. Appropriate targets for PETO2 during hypoxia blocks, intended to maintain an oxygen saturation of at least 85%, were determined on a subject-specific basis during the practice session and ranged between 55 and 70 mmHg. Dynamic achieved PETO2 and PETCO2 were recorded continuously for the entire scan session through the gas blending system alongside respiratory rate and tidal volume. Participant SpO2 and heart rate were monitored continuously throughout scanning via a finger clip sensor, and values were recorded at the start of each PC scan. Blood pressure was taken before and after scanning.
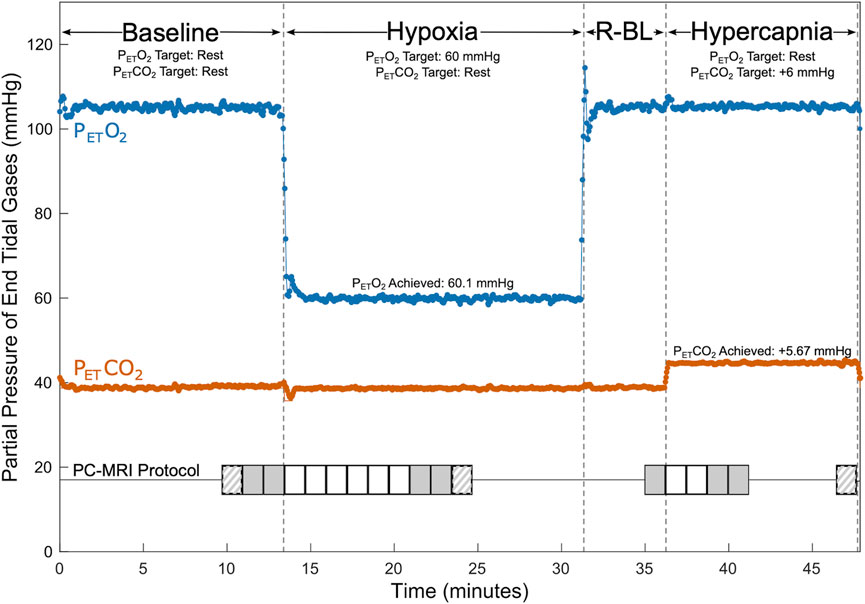
Figure 1. Respiratory and PC-MRI Acquisition Protocol. PETCO2 (shown in orange) and PETO2 (shown in blue) values are shown for an example participant during baseline, hypoxia, recovery baseline (R-BL), and hypercapnia. Baseline targeting lasted a minimum of 12 min (median 18 min), hypoxic targeting lasted at least 13 min (median 16 min), recovery baseline targeting lasted 5 min, and hypercapnic targeting lasted a minimum of 9 min (median 12 min). Timings of PC acquisitions are shown relative to respiratory state. Solid grey shading indicates placement 1 acquisitions used in calculations of CVR. Striped shading indicates placement 2 acquisitions that may have been used in calculations of CVR. Solid white shading indicates placement 1 acquisitions that were not used in CVR calculations. Additional scanning was completed between PC acquisitions.
Scanning was performed on a Siemens 3 T Prisma scanner; a 32-channel head coil was used to allow space for the face mask. A 3D time-of-flight angiogram (TR = 21.0 m, TE = 3.41 ms, voxel size = 0.8 × 0.8 × 1.2 mm3) was used to manually position imaging slices for subsequent PC measures. Slices were chosen perpendicular to the internal carotid and vertebral arteries, superior to the carotid bifurcation. Two placements were selected: placement 1 prioritized orthogonality of the imaging slice with the carotid arteries, and placement 2 prioritized orthogonality with any of the four vessels that were not considered orthogonal in placement 1, typically one or both vertebral arteries. These placements are shown for an example participant in Supplementary Figure S1. Slice positioning was replicated in subsequent scan sessions using screenshots from the first session. Consecutive retrospectively gated PC scans (TR = 47.52 ms, TE = 3.45 ms, voxel size = 0.5 × 0.5 × 5.0 mm3, encoding velocity = 100 cm/s, flip angle = 15°, eight frames per cardiac cycle) were acquired thrice (once at placement 2, twice at placement 1) during baseline gas delivery and repeatedly during the transition to steady-state, with three additional scans (two at placement 1, one at placement 2) collected at estimated steady-state hypoxia. Previous literature suggests that approximately 7.5 min are needed for blood flow to stabilize following the onset of hypoxia (Harris et al., 2013); based on this and pilot data, we allowed approximately 7.5 min before collecting steady-state scans. PC images were also collected once at the end of the recovery baseline, repeatedly during the transition to steady-state hypercapnia, and thrice at steady-state hypercapnia, allowing approximately 2.5 min for blood flow to stabilize. Images collected during the transitions to steady-state were all acquired at placement 1 (Figure 1). A T1-weighted MPRAGE scan (TR = 2,170.0 ms, TE = 1.69/3.55/5.41 ms, voxel size = 1.0 × 1.0 × 1.0 mm3, FOV read = 256 mm, flip angle = 7.0°) was acquired during baseline for calculation of brain volume.
The T1 image collected in session 1 was processed with FSL-BET and FAST for brain extraction and segmentation (Smith, 2002; Zhang et al., 2001). Grey matter and white matter volumes were summed to calculate total brain volume for each participant. Total brain mass was calculated from the total brain volume, assuming a tissue density of 1.06 g/mL (Aslan et al., 2010; Herscovitch and Raichle, 1985).
CBF was calculated for each PC-MRI acquisition using cvi42’s Flow 2D (Circle Cardiovascular Imaging, Calgary, Canada). Images were corrected for background phase errors. Regions of interest for internal carotid and vertebral arteries were manually selected for each cardiac bin in each acquisition. Mean flow, the product of average velocity and cross-sectional area, across the cardiac cycle for each vessel was calculated. The mean flows of the four vessels were summed to find total CBF in units of mL/min and further divided by the brain mass to find normalized CBF (nCBF), with units of mL/100 g/min.
Each reported measurement of CBF combines measurements from two or three acquisitions. Carotid artery blood flow measurements are taken as the average of the two steady-state PC acquisitions at placement 1. For vertebral arteries, the relationship between steady-state blood flow captured by placements 1 and 2 was modeled and used to identify outliers from this relationship, indicating that flow through the artery was not well-captured by placement 1. If the flow measured by placement 2 exceeded the flow measured by placement 1 by greater than 1.96 times the standard deviation of the sum-squared errors of the modeled group relationship at any point, the placement 2 flow values for that vertebral artery were used to calculate total CBF for all timepoints within each session rather than the average of the placement 1 flow values. Recall that placement 2 was generally chosen to better optimize orthogonality with one or both vertebral arteries and is therefore expected to give higher, and ostensibly more accurate, estimates of vertebral blood flow when placement 1 is inadequate.
End-tidal data were temporally aligned with scan acquisitions. A start marker was inserted in respiratory data synchronized with the start of the first baseline PC acquisition during data collection. The start time, relative to this first marked scan, and the length of each PC acquisition were calculated from header data and used to find the start and end time of each acquisition relative to the continuous respiratory data; the average PETCO2, PETO2, and minute ventilation across each PC-MRI acquisition was then calculated. For simplicity, average end-tidal and physiological data from placement 1 acquisitions were used for all comparisons and calculations, even when select vessel data were taken from placement 2 as described above. We make the assumption that this difference is small, given that measures are taken under identical physiological conditions and the time between measurements is on the order of 1–2 min.
CVR was calculated for hypoxic and hypercapnic conditions by finding the change in CBF as a function of the change in end-tidal partial pressure (Equations 1, 2) or as a function of the change in oxygen saturation (Equation 3). Data from the initial baseline, rather than recovery baseline, were used for all calculations.
The mean and standard deviation of end-tidal gases, SpO2, heart rate, minute ventilation, and nCBF were calculated for each experimental phase; we tested for significant differences with a mixed-effects model accounting for subjects and sessions as random effects and post hoc testing to compare stimuli to baseline. We verified that steady-state blood flow was sufficiently achieved through paired two-sided Wilcoxon tests of the differences in back-to-back CBF measurements at placement 1, considering repeated measures during baseline, hypoxic, and hypercapnic experimental phases. Furthermore, immediate test-retest repeatability from these back-to-back measures was assessed with the intra-class correlation coefficient (ICC).
Paired two-sided Wilcoxon tests were used to verify that the distributions of CBF changes due to stimuli were different than the distribution of error in CBF measurements during baseline. The reliability of CVR metrics across sessions was assessed using ICC and standard deviation (SD) estimates. ICCs were calculated in R Version 4.3.2 with software package ‘irr’ Version 0.84, with a one-way random effects model for absolute agreement between the averaged CVR metrics for each session and respiratory stimulus in complete datasets. Inter-subject and inter-session SDs were derived from a linear mixed-effects model, considering only complete datasets, with subject included as a random effect. Coefficients of determination were calculated for the relationships in hypoxic CVR and hypercapnic CVR between sessions, and between the two stimuli.
Nineteen subjects completed all three scan sessions; two subjects were unable to return for the third scan session. The hypercapnic portion of the scan was not completed in five otherwise complete scan sessions due to time constraints, and SpO2 data were not recorded in Sessions 1 or 2 for Subject 1. Details of participant data inclusion, as well as calculated CVR values, are summarized in Supplementary Table S1. Summary metrics of blood flow, gas levels, and physiological measures during each experimental phase are shown in Table 1, calculated from all available data. At the group level, during hypoxia, PETO2 and SpO2 decreased significantly compared to baseline, and heart rate and minute ventilation increased significantly. PETCO2 increased significantly during hypercapnia, as did minute ventilation. No significant differences were present between the two phases of baseline targeting.

Table 1. Achieved CBF, PETCO2, PETO2, and physiological parameters during each respiratory state. Group mean values across all scan sessions during steady-state PC acquisitions.
The immediate repeatability of CBF metrics was good, with back-to-back baseline measurements at placement 1 having an ICC of 0.88. The differences in CBF between back-to-back PC measurements during hypoxia or hypercapnia were not significantly different from differences in back-to-back PC measurements during baseline, indicating that steady-state blood flow was sufficiently reached (Supplementary Figure S2). Overall, back-to-back measurements showed a median absolute difference of 2 mL/100 g/min; this difference reflects the inherent influence of both measurement noise and natural physiological fluctuations on CBF measures. Consequently, in this study, we consider changes in CBF exceeding this threshold to be meaningful. The distributions of hypoxic nCBF changes (p < 0.001) and hypercapnic nCBF changes (p < 0.001) were significantly different from the null distribution of back-to-back changes in nCBF.
Mean total CBF during baseline was 779 mL/min, corresponding to a nCBF of 63 mL/100 g/min. CBF increased during hypoxia, with an average increase of 3 ± 4 mL/100 g/min or 5% ± 7%. CBF also increased during hypercapnia, with an average increase of 14 ± 7 mL/100 g/min or 22% ± 11%, shown in Figure 2. Percent changes are shown in Supplementary Figure S3. The average hypoxic CVR for the group was 0.10% ± 0.14%/-mmHg PETO2 and 0.58% ± 0.88%/-%SpO2. Average hypercapnic CVR was 4.33% ± 1.83%/mmHg PETCO2.
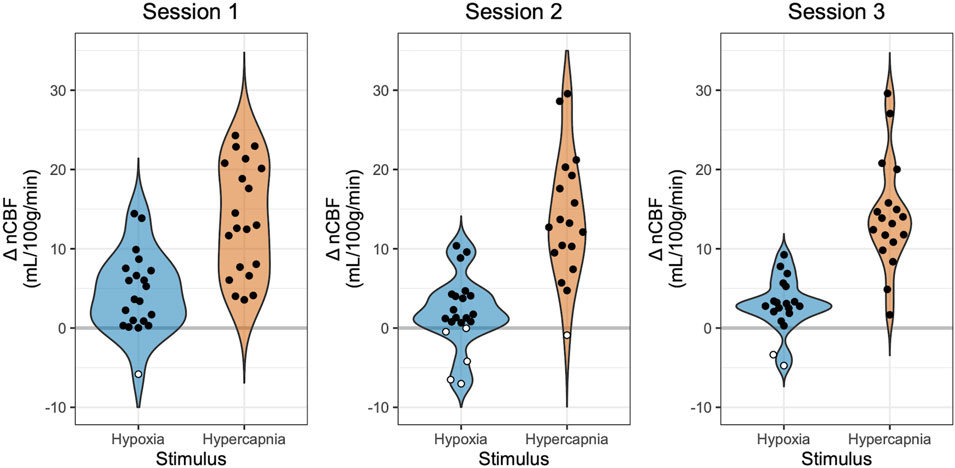
Figure 2. Changes in Blood Flow During Hypoxia and Hypercapnia. Changes in nCBF during hypoxia (blue) and hypercapnia (orange), relative to baseline, are shown for each session. Positive values, indicating an increase in CBF, are shown with black circles, while negative values, indicating a decrease in CBF, are shown with white circles. Note that sessions and gas stimuli have different numbers of subjects; all available data are shown.
The reliability of CVR measures is shown in Figure 3. Only subjects with complete datasets, Group A, were included in overall reliability metrics considering all three timepoints (see Supplementary Table S1 for individual CVR metrics and which subjects were included in groups). Hypoxic CVR showed relatively greater variability between subjects and between sessions than hypercapnic CVR, and inter-session variability dominated. Hypoxic CVR relative to changes in PETO2 showed an inter-subject standard deviation (SD) of 0.07%/-mmHg PETO2 and an inter-session SD of 0.14%/-mmHg PETO2; hypoxic CVR relative to changes in SpO2 showed slightly greater variability due to subject, with an inter-subject SD of 0.48%/-%SpO2 and an inter-session SD of 0.75%/-%SpO2. Hypoxic CVR was found to have overall ICCs of 0.42 and 0.56 relative to changes in PETO2 and SpO2, respectively. Hypercapnic CVR demonstrated substantially less variability relative to the magnitude, with an inter-subject SD of 1.13%/mmHg PETCO2, an inter-session SD of 1.37%/mmHg PETCO2, and an ICC of 0.67.
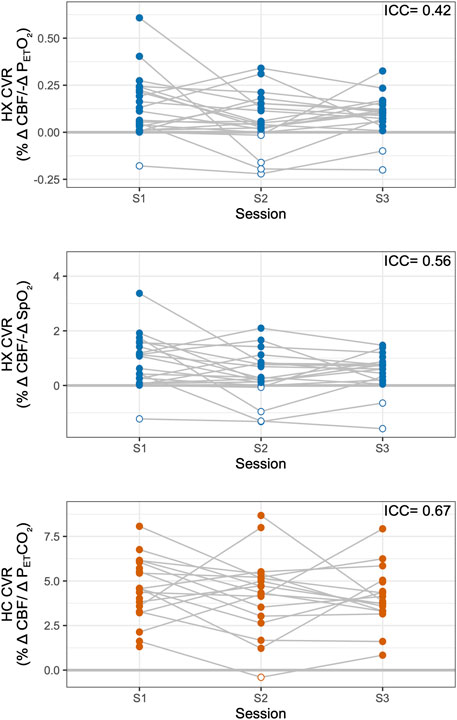
Figure 3. Reliability of Hypoxic and Hypercapnic CVR Measures. Hypoxic (HX) CVR metrics across sessions are shown, relative to PETO2 (top) and SpO2 (middle). Hypercapnic (HC) CVR is shown, relative to PETCO2, for comparison (bottom). Overall intraclass correlation coefficients (ICCs) are listed for subjects with complete datasets (Group A). Negative CVR values are indicated with open circles; all available data are shown.
ICCs considering only sessions 1 and 2, representing reliability over 3 weeks, were 0.33 for PETO2 hypoxic CVR, 0.50 for SpO2 hypoxic CVR, and 0.57 for hypercapnic CVR. Reliability for all CVR metrics decreased with time: ICCs considering only sessions 1 and 3, representing reliability over 3–5 months, were 0.20 for PETO2 hypoxic CVR, 0.23 for SpO2 hypoxic CVR, and 0.48 for hypercapnic CVR.
Hypoxia is expected to produce a positive change in CBF. However, previous literature in hypoxia reports observations of paradoxical negative changes in CBF, which may or may not reflect methodological confounds of the imaging modality used. In our study using PC-MRI, one individual (5% of participants) exhibited a decrease in CBF in session 1, five individuals (24%) exhibited a decrease in session 2, and two individuals (10%) exhibited a decrease in session 3, highlighted in white in Figure 2. One participant, Subject 7, showed a decrease in CBF in response to hypoxia in all three sessions, while all other participants with instances of negative hypoxic CBF changes only showed decreases in one or two sessions (Supplementary Table S1). Furthermore, many participants exhibited minimal CBF responses to hypoxia, with nineteen instances of nCBF changes of less than 2 mL/100 g/min (the median absolute change between back-to-back PC acquisitions in steady state, shown in Supplementary Figure S2). Only two instances of nCBF changes of less than 2 mL/100 g/min were recorded in hypercapnia, both in Subject 7 (who also showed a consistent paradoxical response to hypoxia, described above).
While resting levels of PETCO2 were targeted during the hypoxic experimental phase, PETCO2 was on average 0.4 ± 0.9 mmHg lower during hypoxia than during baseline. During data processing, it was noted that these unintentional concurrent changes in PETCO2, while minor, were moderately but significantly correlated (r = 0.4, p < 0.01) with hypoxic CBF changes (Figure 4). Based on this relationship, we implemented a novel correction strategy to account for such changes in PETCO2. The subject- and session-specific hypercapnic CVR measurement was used to estimate the CBF change attributable to the unintentional change in PETCO2 during hypoxia, and hypoxic CVR was re-calculated with this estimated CBF contribution removed.
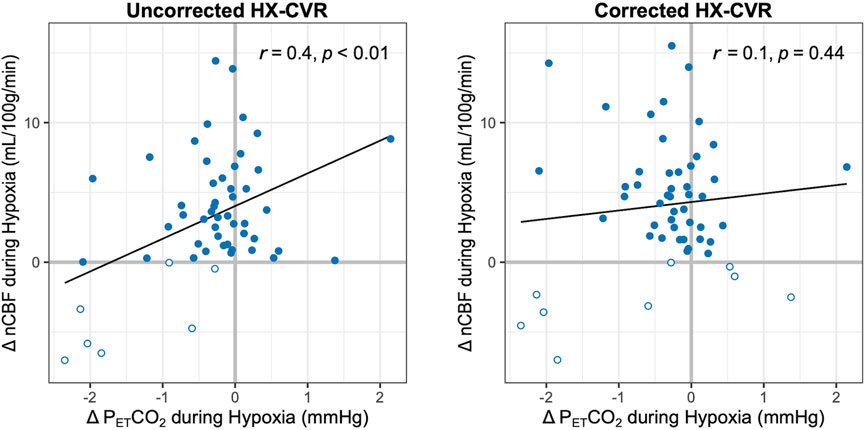
Figure 4. Relationship between Changes in PETCO2 and nCBF during Hypoxia. The correlations between nCBF changes in response to hypoxia and concurrent changes in PETCO2 are shown before (left) and after (right) correction for such PETCO2 changes. Pearson’s correlation coefficients and p-values are included. Decreases in blood flow are indicated with open circles; all available data are shown.
PETCO2 correction slightly increased the average nCBF change during hypoxia to 4 ± 5 mL/100 g/min or 6% ± 7%, and average hypoxic CVR increased to 0.12% ± 0.16%/-mmHg PETO2 and 0.82% ± 1.04%/-%SpO2. The correlation between concurrent PETCO2 changes and hypoxic CBF changes became weaker and non-significant (r = 0.1, p = 0.44), as shown in Figure 4. Removing potential outliers did not change this interpretation (Supplementary Figure S4). With correction for PETCO2 changes during hypoxia, three further instances of CBF decreases were identified in session 1; only one case of a negative CBF change became positive with this correction (Supplementary Figure S5). Inter-subject SDs for PETO2 and SpO2 hypoxic CVR changed to 0.07%/-mmHg PETO2 and 0.40%/-%SpO2, respectively, while inter-session SDs increased to 0.15%/-mmHg PETO2 and 1.02%/-%SpO2, respectively, indicating decreased inter-subject but increased inter-session variability. As such, ICCs ultimately decreased to 0.37 and 0.31 for PETO2 and SpO2 hypoxic CVR, respectively, as further instances of negative CVR were revealed. The impact of this correction on reproducibility is shown graphically in Supplementary Figure S6, and further details on the implementation of the correction method are included in the Supplemental Material.
Overall, hypercapnic CVR and hypoxic CVR demonstrated a positive but weak relationship, shown in Figure 5. Hypercapnic CVR and hypoxic PETO2 CVR demonstrate a coefficient of determination (R2) of 0.32, while hypercapnic CVR and hypoxic SpO2 CVR demonstrate an R2 of 0.42. PETCO2 correction of hypoxic CVR values increased this relationship slightly, with coefficients of determination increasing to 0.44 and 0.47 for PETO2 and SpO2 hypoxic CVR respectively. The positive relationships between hypercapnic and hypoxic CVR metrics indicate that differences in overall vascular reactivity contribute to the inter-subject variance observed as expected, but substantial variance in hypoxic CVR (>50%) cannot be explained by differences in overall reactivity.
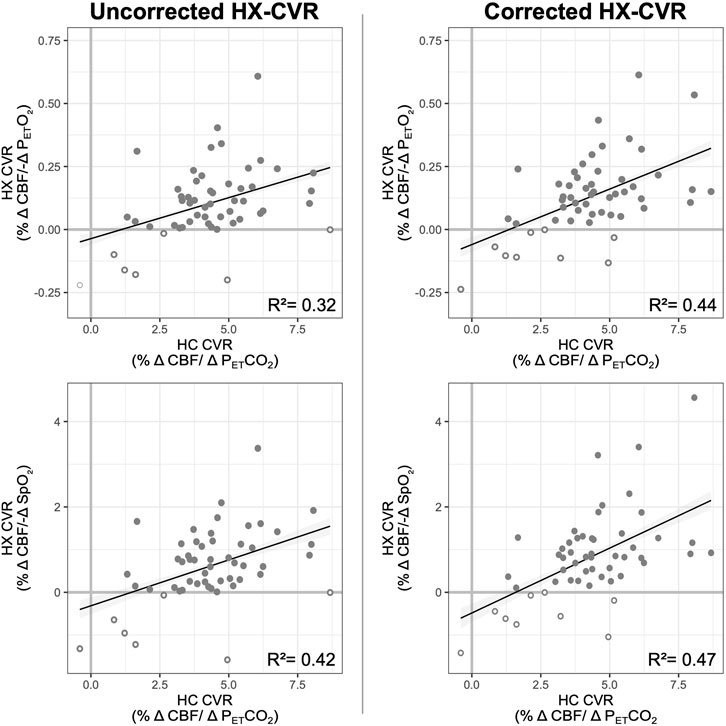
Figure 5. Relationship between Hypoxic and Hypercapnic CVR. The relationship between hypercapnic and uncorrected (left) and corrected (right) hypoxic CVR is shown, with hypoxic CVR relative to PETO2 on top and hypoxic CVR relative to SpO2 on the bottom. Coefficients of determination, calculated from linear mixed-effects models, are included; the relationship between hypercapnic and hypoxic CVR was significant (p less than a threshold 0.05/4 = 0.0125) for all four models. Negative CVR values (both hypoxic and hypercapnic) are indicated with open circles; data from all subjects and sessions where hypoxic and hypercapnic CVR were available are included.
This study quantified the CBF response to moderate hypoxia and mild hypercapnia using PC-MRI and assessed CVR to each stimulus. CVR was measured in three sessions, separated by approximately 3 weeks and 4 months. While previous studies have investigated the blood flow response to hypoxia (Binks et al., 2008; Ellingsen et al., 1987; Harris et al., 2013; Kellawan et al., 2017; Kety and Schmidt, 1948; Nöth et al., 2008; Steinback and Poulin, 2016), this is the first study to our knowledge to do so with PC-MRI and to assess variability in this metric. In particular, we aimed to investigate whether previously-observed inter-subject variability in hypoxic blood flow responses stems from methodological confounds, day-to-day changes, or characteristics inherent to certain individuals.
CBF is known to increase in response to hypercapnia and to mild-to-moderate hypoxia to maintain sufficient supply of nutrients and oxygen to cerebral tissue (Steinback and Poulin, 2016). Overall, we observed blood flow responses to both stimuli in agreement with this understanding, demonstrating a small increase during hypoxia and a more substantial increase during hypercapnia. CBF increased 5% ± 7% during hypoxia and 22% ± 11% during hypercapnia, corresponding to CVR metrics of 0.10% ± 0.14%/-mmHg PETO2 or 0.59% ± 0.88%/-%SpO2 for hypoxia and 4.33% ± 1.83%/mmHg PETCO2 for hypercapnia. Measured hypercapnic CVR in this study is consistent with previous literature, which reports blood flow increases of 3%–6% per mmHg PETCO2 when measured with ASL or PC-MRI (Liu et al., 2019). However, previous studies have generally reported higher CVR during hypoxia than we observe in this study: using ASL to measure perfusion changes in cerebral grey matter, Harris et al. observed an increase of 11.7 mL/100 g/min, corresponding to hypoxic CVRs of approximately 0.21%/-mmHg PETO2 and 1.02%/-%SpO2, while Nöth et al. saw approximately 0.11%/-mmHg PETO2 and 0.70%/-%SpO2 (Harris et al., 2013; Nöth et al., 2008). Measuring hypoxic CVR with H215O positron emission tomography, Binks et al. found an average increase of 6 mL/100 g/min, corresponding to CVRs of approximately 0.31%/-mmHg PETO2 and 0.89%/-%SpO2 (Binks et al., 2008). The greater magnitude of previously reported hypoxic CVR values, particularly with respect to PETO2, is potentially explained by their use of more severe hypoxic stimuli than the present study. Blood flow changes during hypoxia have a curvilinear relationship with PETO2 changes, and milder stimuli can produce smaller hypoxic CVR values (Steinback and Poulin, 2016). Furthermore, gray matter, as assessed by Harris et al. and Nöth et al., exhibits greater CVR than global metrics, as measured in the present study (Sleight et al., 2021). Further work could better characterize the dynamics of the nonlinear hypoxic whole-brain blood flow response by stepping through graded hypoxic stimuli (Poulin, 1998; Shapiro et al., 1970) [as done extensively with hypercapnic stimuli (Bhogal et al., 2016; Bhogal et al., 2014; Driver et al., 2010; Taylor et al., 2022; Tomoto et al., 2020)] and by employing both whole-brain and regionally-sensitive imaging metrics.
While no previous studies have assessed the reliability of hypoxic CVR, hypercapnic CVR has been determined to be a fairly stable metric of vascular health with good reproducibility (Goode et al., 2009; Spencer et al., 2015). CVR reflects the ability of the vasculature to dilate under stress, a characteristic that is not expected to change significantly over relatively short periods except in cases of pathology or in response to therapy (Sobczyk et al., 2016). In healthy individuals, hypercapnic CVR has been found to typically have ICCs of more than 0.7 between sessions and inter-session coefficients of variation (CV) of 16%–26% (Ances et al., 2011; Dengel et al., 2017; Goode et al., 2009; Spencer et al., 2015; Tancredi et al., 2015; Zhao et al., 2022). Our study produced an overall ICC of 0.67, with an inter-session SD of 1.37%/mmHg (CV = 34%). Importantly, many factors can affect the reproducibility of CVR metrics, including time of day, caffeine intake, time between scans, and imaging modality (Zhao et al., 2022). We minimized these effects by scanning at the approximate same time of day and asking participants to refrain from caffeine consumption. However, for 15 of the 53 scans for which these data were collected, participants had consumed caffeine on the day of the scan (average of 4.3 h before start of scan), which may have added to the variability observed (Chen and Parrish, 2009; Peng et al., 2022). Furthermore, our reliability is likely decreased by the longer period between scans relative to most literature [3–5 months compared with less than 1 week (Zhao et al., 2022)] and by the imaging modality, as blood flow responses have lower reproducibility than BOLD responses (Tancredi et al., 2015).
We predicted that hypoxic CVR would show similar reliability to hypercapnic CVR. However, we found that hypoxic CVR relative to PETO2 or SpO2 changes had higher variability relative to the magnitude of expected changes compared to hypercapnic CVR and overall ICCs of 0.42 and 0.56 relative to PETO2 and SpO2 changes, respectively, indicating poor-to-moderate reliability (Koo and Li, 2016). The magnitude of the CBF response varied greatly day-to-day, and while the directionality of most individuals’ responses was consistent between sessions, including one participant with a consistent decrease in CBF during hypoxia, several subjects exhibited negative hypoxic CVR in only one or two sessions.
Although blood flow is generally acknowledged to increase during hypoxia, in line with our overall average observations in this study, several previous studies of hypoxic CVR have identified unexpected variability between individuals in the cerebrovascular response to moderate hypoxic hypoxia. Nöth et al. found that 30% of participants had a decrease in global CBF, while Binks et al. reported that one of five subjects had no significant change in CBF (Binks et al., 2008; Nöth et al., 2008). The rate of minimal or paradoxical responses in this study was similar to previously published literature, with 1-5 participants, or 5%–24% of subjects, showing a decrease in CBF in response to hypoxia during a given scan session. Furthermore, 3-9 participants, or 16%–43% of subjects, did not show a meaningful change in CBF in response to hypoxia during a given scan session. The source of this variability is unclear. Previous work has relied on assumptions of unchanged labeling efficiency and T1 of arterial blood, both of which may change with hypoxia and confound CVR estimations (Nöth et al., 2008). However, using a method unbiased by these assumptions, we still observe paradoxical responses, indicating that these responses do not stem exclusively from measurement biases.
Paradoxical CBF responses to hypoxia could be caused by overall differences in the ability of the vasculature to react or by a truly atypical cerebrovascular response to hypoxia. In the lungs, arteries constrict in response to decreased oxygen concentrations in the alveoli (Dunham-Snary et al., 2017); similar vasoconstrictive mechanisms may be involved in regions of the brain in hypoxia, leading to competing vasoconstrictive and vasodilatory effects producing the variability seen in the global CBF response (Corfield and McKay, 2016). Lawley et al. verified that observed reductions in regional blood flow during hypoxia was caused by vasoconstriction, rather than an inability for the vasculature to dilate, as the regions in question displayed the typical vasodilatory response to a subsequent hypercapnic challenge (Lawley et al., 2017).
Prior studies have also not investigated the reliability of hypoxic CVR measures, leaving it ambiguous whether these minimal and negative responses are characteristic of certain individuals. If this response is a reliable trait of an individual, it could have substantial implications regarding the vulnerability of their cortical tissue to damage caused by moderate hypoxia. Of note, we observed one participant (S7, Supplementary Table S1) who demonstrated negative blood flow responses to hypoxia in all three sessions. No explanations for paradoxical responses in certain individuals were evident in the present study. Paradoxical responders were not set apart by any demographic characteristics (age, sex, ethnicity, or race), or by cardiovascular health, as indicated by blood pressure measurements. Paradoxical CBF responses to hypoxia agreed with the overall study trend in which hypoxic and hypercapnic CBF responses were positively and moderately correlated. Further work with larger sample sizes and further markers of cardiovascular health, including exercise habits, is needed to predict which individuals are likely to show a paradoxical CBF response to hypoxia.
Experimental methodology and general data quality likely still contribute to the observed variability in our PC-MRI measurements of CBF during different inhaled gas challenges. While not directly assessed in this study, motion artifacts could have impacted data quality and therefore flow quantification, particularly due to changes in ventilation induced by the respiratory stimuli. The requirement for a gas delivery face mask necessitated the use of a 32-channel coil as opposed to the 64-channel coil preferred for neck imaging, potentially resulting in a lower signal-to-noise ratio and thereby increasing flow measure variability. Subtle inconsistencies in vessel segmentation and slice placement across sessions, although minimized, may have further introduced variability in flow calculations. The implemented PC acquisition employed eight cardiac bins, resulting in a temporal resolution that may have been insufficient to fully capture the dynamics of pulsatile flow. Additionally, the fixed order of respiratory challenges may have contributed to the enhanced variability observed in hypoxic CVR compared to hypercapnic CVR. Although prioritizing hypoxic CVR ensured its collection even if participants became uncomfortable, acquiring these measurements before the hypercapnic phase may have resulted in greater physiological variability as participants were still acclimating to the MR and gas challenge environment. Future studies should counterbalance the two vasoactive stimuli to ensure that measures are not biased by this fixed order.
Variability in the measured CBF response to hypoxia could also result if CBF was not given enough time to stabilize under the stimulus. The blood flow response to hypoxia in gray matter is delayed by more than 3 minutes, with an additional 4.5 min required to reach steady state (Harris et al., 2013); we therefore allowed approximately 7.5 min before collecting steady-state CBF measures under hypoxia. We verified that blood flow had stabilized following the transition to hypoxia, as demonstrated by the differences in CBF measured in back-to-back repetitions of PC-MRI, indicating that instability of blood flow is not a dominant driver of the observed variability and select paradoxical responses.
Changes in PETCO2 levels substantially impact CBF, and concomitant changes in PETCO2 during hypoxia administration have been identified as a confound in isolating the hypoxic blood flow response and suggested as a potential source of variability (Binks et al., 2008; Nöth et al., 2008). As arterial carbon dioxide and oxygen concentrations are closely linked through respiration, changes in oxygen levels cause unintentional modulations of carbon dioxide concentrations, and hypoxia typically results in hypocapnia due to increased ventilation unless PETCO2 is externally controlled. This hypocapnia can result in a competing vasoconstrictive effect, potentially obscuring the true hypoxic blood flow response. We used a computer-controlled gas blending system to clamp PETCO2 at baseline levels. However, a slight decrease in PETCO2 during hypoxia was still observed, and individual changes in PETCO2 during hypoxia were found to be correlated with hypoxic nCBF changes (Figure 4). We therefore corrected CBF measurements during hypoxia for these effects, estimating the impact of the change in PETCO2 from subject- and session-specific hypercapnic CVR measures.
This correction removed the significant impact of confounding PETCO2 changes on measures of hypoxic CVR (Figure 4); however, it revealed further instances of decreased blood flow in response to hypoxia, and ICCs were lower following correction. This may mean that our correction approach makes us more sensitive to true physiologic variability in hypoxic CVR across sessions, although we cannot conclude this using our current study data. The reductions in reliability suggest, however, that the observed inter-session variability in hypoxic CBF responses likely does not stem directly from concurrent PETCO2 changes. While changes in ventilation, and therefore intrathoracic pressure, have been shown to influence cerebral perfusion (Winklewski et al., 2019), and were significant in hypoxia compared to baseline conditions (Table 1), we did not observe a meaningful relationship between changes in minute ventilation and CBF (r = 0.1, p = 0.53; Supplementary Figure S7). Although we are unable to directly assess the impact of intrathoracic pressure changes, the observed variability in hypoxic CVR is likely not substantially caused by ventilatory changes.
Variability in the hypoxic CBF response may instead result from variability in the degree of hypoxia achieved. Targets for PETO2 were selected for each subject in a practice session outside the scanner and were chosen to maintain participants at a SpO2 of 85%. However, many participants demonstrated smaller changes in oxygenation during MRI sessions despite identical PETO2 targets and substantial variability in the minimum SpO2 achieved between scan sessions (shown in Supplementary Figure S8), and the average SpO2 during hypoxia was only 90%. While previous literature indicates that there exists a linear relationship between arterial deoxygenation and CBF changes in hypoxia (Steinback and Poulin, 2016), some studies have suggested that the SpO2 threshold for a blood flow response to hypoxia is 90% (Gupta et al., 1997). Given this, we would expect participants who did not achieve SpO2 values below 90% to also not show meaningful increases in CBF (<2 mL/100 g/min) during hypoxia, and participants who achieve SpO2 values below this 90% threshold to demonstrate substantial CBF increases. This scenario is indicated by shading in Figure 6. Contrary to these literature predictions, many subjects in the present study demonstrated an increase in CBF at a SpO2 of greater than 90% or insubstantial changes in CBF with SpO2 levels well below this threshold, and still others displayed decreased CBF despite dramatic decreases in SpO2. While it is likely that we were unable to reach individual thresholds for vasoactive responses in some subjects, the lack of a strong relationship between CBF changes in hypoxia and SpO2 decreases (r = 0.21 uncorrected, r = 0.11 corrected for concurrent PETCO2 changes) suggests that the paradoxical or minimal responses we observed are not all attributable to insufficient decreases in oxygenation and may instead be due to other sources. Still, a greater degree of hypoxia may decrease inter- and intra-subject variability in hypoxic blood flow responses, and future studies should investigate whether a greater degree of hypoxia improves the reliability of hypoxic CVR measures. Furthermore, direct measures of arterial blood gases could provide further insight into the relationship between hypoxic CVR and arterial oxygenation. Although closely related, SpO2 values correspond to a range of partial pressures of arterial oxygen, rather than a single value; SpO2 may therefore not fully reflect variations in arterial oxygen, and consequently hypoxemia, that directly impact vascular tone (Carr et al., 2024; Röttgering et al., 2021).
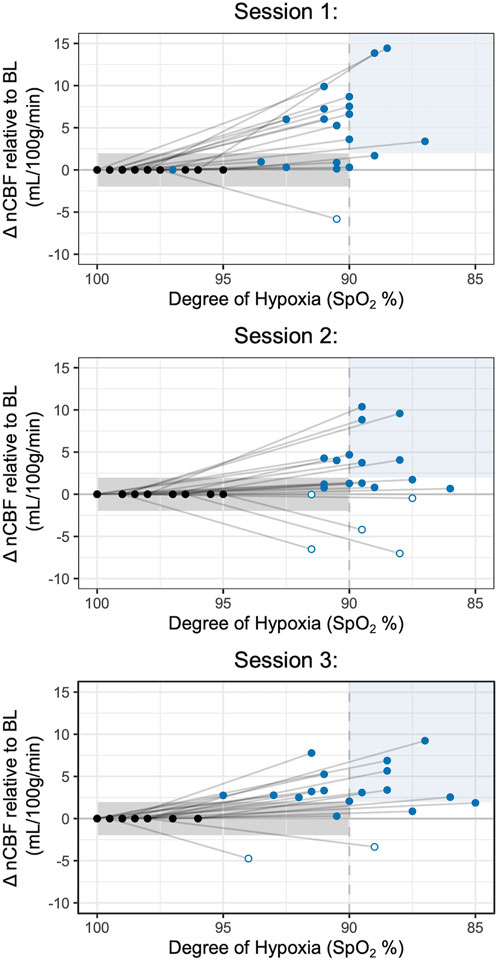
Figure 6. Comparisons of nCBF changes with SpO2 Decreases during Hypoxia. Changes in nCBF relative to baseline are shown during hypoxia (blue) and baseline (black) relative to the degree of hypoxia, measured by SpO2. Approximate ranges for expected changes in nCBF relative to SpO2, based on previous literature (Gupta et al., 1997), are shaded in light grey (greater than 90% SpO2) and light blue (less than 90% SpO2). Open circles indicate negative nCBF changes; all available data are included.
We compared hypercapnic and hypoxic measures of CVR to assess whether observed variability may be attributable to differences in overall cerebrovascular responsiveness. Across all sessions, less than half of variance is shared between hypercapnic and hypoxic reactivity, suggesting that additional sources of variability are present in the vascular response to hypoxia that are not present in the response to hypercapnia. The observed variance in hypoxic cerebral blood flow responses warrants further investigation, as hypoxic CVR may provide distinct information about vascular health not revealed by hypercapnic CVR. Notably, hypoxia and hypercapnia influence vascular tone through slightly different mechanisms, which may account for the differences observed between the two responses (Beaudin et al., 2011; Carr et al., 2024; Cummins et al., 2020; Prabhakar, 2016). Future work should explore the relationship between paradoxical CVR and additional measures of chemoreceptor sensitivity.
In conclusion, we investigated hypoxic CVR alongside hypercapnic CVR using PC-MRI and mild-to-moderate stimuli in three sessions. We observed great variability in hypoxia responses between subjects, including paradoxical decreases in CBF in some individuals, and hypoxic CVR metrics showed poor-to-moderate reliability in comparison with hypercapnic CVR metrics, which demonstrated moderate reliability (Koo and Li, 2016). Methodological confounds did not account for the high variability and paradoxical responses observed; PC-MRI is free of the assumptions that may bias CVR measures made with BOLD or ASL MRI, and neither concurrent changes in PETCO2 nor variability in deoxygenation achieved explained the noted variability in hypoxic CVR. While hypoxic and hypercapnic CVR demonstrated a positive relationship, less than half of their variance was shared, indicating that hypoxic CVR is subject to other sources of variability. We show that while paradoxical instances of hypoxic CVR likely reflect true inter-subject variations in the cerebrovascular response, further investigation of hypoxic CVR is warranted to understand the sources of this variability and its implications for cerebrovascular health.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: Improving Human Cerebrovascular Function Using Acute Intermittent Hypoxia: https://osf.io/j798q/.
The studies involving humans were approved by Northwestern University Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
HJ: Formal Analysis, Investigation, Project administration, Visualization, Writing–original draft, Writing–review and editing. MW: Investigation, Writing–review and editing. RS: Conceptualization, Investigation, Methodology, Writing–review and editing. YC: Conceptualization, Funding acquisition, Methodology, Writing–review and editing. TP: Conceptualization, Funding acquisition, Methodology, Writing–review and editing. FS: Conceptualization, Funding acquisition, Methodology, Writing–review and editing. MB: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Institute of Neurological Disorders and Stroke at the National Institutes of Health award R21NS121742. HJ was supported by the National Institute of Biomedical Imaging and Bioengineering at the National Institutes of Health award T32EB025766. Research reported in this publication was supported, in part, by the National Institutes of Health’s National Center for Advancing Translational Sciences, Grant Number UL1TR001422. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was supported by the Center for Translational Imaging at Northwestern University.
The authors would like to thank Andrew Vigotsky for his statistical guidance and Rachael Young for her assistance in data collection. This work was supported by the Center for Translational Imaging at Northwestern University. The contents of this manuscript have been posted to the preprint server bioRxiv (Johnson et al., 2024).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2025.1562582/full#supplementary-material
Ances B., Vaida F., Ellis R., Buxton R. (2011). Test-retest stability of calibrated BOLD-fMRI in HIV− and HIV+ subjects. Neuroimage 54, 2156–2162. doi:10.1016/j.neuroimage.2010.09.081
Aslan S., Xu F., Wang P. L., Uh J., Yezhuvath U. S., van Osch M., et al. (2010). Estimation of labeling efficiency in pseudo-continuous arterial spin labeling. Magn. Reson Med. 63, 765–771. doi:10.1002/mrm.22245
Beaudin A. E., Brugniaux J. V., Vöhringer M., Flewitt J., Green J. D., Friedrich M. G., et al. (2011). Cerebral and myocardial blood flow responses to hypercapnia and hypoxia in humans. Am. J. Physiology-Heart Circulatory Physiology 301, H1678–H1686. doi:10.1152/ajpheart.00281.2011
Beaudin A. E., Hartmann S. E., Pun M., Poulin M. J. (2017). Human cerebral blood flow control during hypoxia: focus on chronic pulmonary obstructive disease and obstructive sleep apnea. J. Appl. Physiology 123, 1350–1361. doi:10.1152/japplphysiol.00352.2017
Bhogal A. A., De Vis J. B., Siero J. C. W., Petersen E. T., Luijten P. R., Hendrikse J., et al. (2016). The BOLD cerebrovascular reactivity response to progressive hypercapnia in young and elderly. NeuroImage 139, 94–102. doi:10.1016/j.neuroimage.2016.06.010
Bhogal A. A., Siero J. C. W., Fisher J. A., Froeling M., Luijten P., Philippens M., et al. (2014). Investigating the non-linearity of the BOLD cerebrovascular reactivity response to targeted hypo/hypercapnia at 7 T. NeuroImage 98, 296–305. doi:10.1016/j.neuroimage.2014.05.006
Binks A. P., Cunningham V. J., Adams L., Banzett R. B. (2008). Gray matter blood flow change is unevenly distributed during moderate isocapnic hypoxia in humans. J. Appl. Physiol. (1985) 104, 212–217. doi:10.1152/japplphysiol.00069.2007
Carr J. M. J. R., Hoiland R. L., Fernandes I. A., Schrage W. G., Ainslie P. N. (2024). Recent insights into mechanisms of hypoxia-induced vasodilatation in the human brain. J. Physiol. 602, 5601–5618. doi:10.1113/JP284608
Chen Y., Parrish T. B. (2009). Caffeine’s effects on cerebrovascular reactivity and coupling between cerebral blood flow and oxygen metabolism. NeuroImage 44, 647–652. doi:10.1016/j.neuroimage.2008.09.057
Cipolla M. J. (2009). “Control of cerebral blood flow,” in The cerebral circulation (San Rafael, CA: Morgan and Claypool Life Sciences).
Corfield D. R., McKay L. C. (2016). “Regional cerebrovascular responses to hypercapnia and hypoxia,” in Hypoxia: translation in progress, advances in experimental medicine and biology. Editors R. C. Roach, P. H. Hackett, and P. D. Wagner (US, Boston, MA: Springer), 157–167. doi:10.1007/978-1-4899-7678-9_11
Cummins E. P., Strowitzki M. J., Taylor C. T. (2020). Mechanisms and consequences of oxygen and carbon dioxide sensing in mammals. Physiol. Rev. 100, 463–488. doi:10.1152/physrev.00003.2019
Damgaard V., Mariegaard J., Lindhardsen J. M., Ehrenreich H., Miskowiak K. W. (2023). Neuroprotective effects of moderate hypoxia: a systematic review. Brain Sci. 13, 1648. doi:10.3390/brainsci13121648
Dengel D. R., Evanoff N. G., Marlatt K. L., Geijer J. R., Mueller B. A., Lim K. O. (2017). Reproducibility of blood oxygen level-dependent signal changes with end-tidal carbon dioxide alterations. Clin. Physiology Funct. Imaging 37, 794–798. doi:10.1111/cpf.12358
Driver I., Blockley N., Fisher J., Francis S., Gowland P. (2010). The change in cerebrovascular reactivity between 3 T and 7 T measured using graded hypercapnia. NeuroImage 51, 274–279. doi:10.1016/j.neuroimage.2009.12.113
Dunham-Snary K. J., Wu D., Sykes E. A., Thakrar A., Parlow L. R. G., Mewburn J. D., et al. (2017). Hypoxic pulmonary vasoconstriction: from molecular mechanisms to medicine. Chest 151, 181–192. doi:10.1016/j.chest.2016.09.001
Ellingsen I., Hauge A., Nicolaysen G., Thoresen M., Walløe L. (1987). Changes in human cerebral blood flow due to step changes in PAO2 and PACO2. Acta Physiol. Scand. 129, 157–163. doi:10.1111/j.1748-1716.1987.tb08054.x
Friend A. T., Balanos G. M., Lucas S. J. E. (2019). Isolating the independent effects of hypoxia and hyperventilation-induced hypocapnia on cerebral haemodynamics and cognitive function. Exp. Physiol. 104, 1482–1493. doi:10.1113/EP087602
Goode S. D., Krishan S., Alexakis C., Mahajan R., Auer D. P. (2009). Precision of cerebrovascular reactivity assessment with use of different quantification methods for hypercapnia functional MR imaging. AJNR Am. J. Neuroradiol. 30, 972–977. doi:10.3174/ajnr.A1496
Gupta A. K., Menon D. K., Czosnyka M., Smielewski P., Jones J. G. (1997). Thresholds for hypoxic cerebral vasodilation in volunteers. Anesth. Analg. 85, 817–820. doi:10.1097/00000539-199710000-00018
Harris A. D., Murphy K., Diaz C. M., Saxena N., Hall J. E., Liu T. T., et al. (2013). Cerebral blood flow response to acute hypoxic hypoxia. NMR Biomed. 26, 1844–1852. doi:10.1002/nbm.3026
Herscovitch P., Raichle M. E. (1985). What is the correct value for the brain--blood partition coefficient for water? J. Cereb. Blood Flow. Metab. 5, 65–69. doi:10.1038/jcbfm.1985.9
Hoffman W. E., Charbel F. T., Gonzalez-Portillo G., Ausman J. I. (1999). Measurement of ischemia by changes in tissue oxygen, carbon dioxide, and pH. Surg. Neurol. 51, 654–658. doi:10.1016/S0090-3019(99)00011-7
Johnson H. R., Wang M. C., Stickland R. C., Chen Y., Parrish T. B., Sorond F. A., et al. (2024). Variable cerebral blood flow responsiveness to Acute hypoxic hypoxia. doi:10.1101/2024.11.14.623430
Kadkhodai L., Saghaei M., Habibzadeh M., Alikiaii B., Hashemi S. J. (2022). Estimating the best fraction of inspired oxygen for calculation of PaO2/FiO2 ratio in acute respiratory distress syndrome due to COVID-19 pneumonia. J. Res. Med. Sci. 27, 38. doi:10.4103/jrms.jrms_558_21
Kellawan J. M., Harrell J. W., Roldan-Alzate A., Wieben O., Schrage W. G. (2017). Regional hypoxic cerebral vasodilation facilitated by diameter changes primarily in anterior versus posterior circulation. J. Cereb. Blood Flow Metabolism 37, 2025–2034. doi:10.1177/0271678X16659497
Kety S. S., Schmidt C. F. (1948). The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal Young men. WWW Document. doi:10.1172/JCI101995
Koo T. K., Li M. Y. (2016). A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 15, 155–163. doi:10.1016/j.jcm.2016.02.012
Lawley J. S., Macdonald J. H., Oliver S. J., Mullins P. G. (2017). Unexpected reductions in regional cerebral perfusion during prolonged hypoxia. J. Physiol. 595, 935–947. doi:10.1113/JP272557
Liu P., De Vis J. B., Lu H. (2019). Cerebrovascular reactivity (CVR) MRI with CO2 challenge: a technical review. NeuroImage, Physiological Quantitative MRI 187, 104–115. doi:10.1016/j.neuroimage.2018.03.047
Madan A. (2017). Correlation between the levels of SpO2 and PaO2. Lung India 34, 307–308. doi:10.4103/lungindia.lungindia_106_17
Marshall O., Lu H., Brisset J.-C., Xu F., Liu P., Herbert J., et al. (2014). Impaired cerebrovascular reactivity in multiple Sclerosis. JAMA Neurol. 71, 1275–1281. doi:10.1001/jamaneurol.2014.1668
Nöth U., Kotajima F., Deichmann R., Turner R., Corfield D. R. (2008). Mapping of the cerebral vascular response to hypoxia and hypercapnia using quantitative perfusion MRI at 3 T. NMR Biomed. 21, 464–472. doi:10.1002/nbm.1210
Peng S.-L., Chen X., Li Y., Rodrigue K. M., Park D. C., Lu H. (2018). Age-related changes in cerebrovascular reactivity and their relationship to cognition: a four-year longitudinal study. NeuroImage 174, 257–262. doi:10.1016/j.neuroimage.2018.03.033
Peng S.-L., Chu L. W. L., Su F.-Y. (2022). Cerebral hemodynamic response to caffeine: effect of dietary caffeine consumption. NMR Biomed. 35, e4727. doi:10.1002/nbm.4727
Ponsaing L. B., Lindberg U., Rostrup E., Iversen H. K., Larsson H. B. W., Jennum P. (2018). Impaired cerebrovascular reactivity in obstructive sleep apnea: a case-control study. Sleep. Med. 43, 7–13. doi:10.1016/j.sleep.2017.10.010
Poulin M. J. (1998). Aspects of cerebral blood flow in humans. Oxford, UK: University of Oxford. Available online at: http://ora.ox.ac.uk.
Prabhakar N. R. (2016). “O2 and CO2 detection by the carotid and aortic bodies,” in Chemosensory transduction. Editors F. Zufall, and S. D. Munger (Academic Press), 321–338. doi:10.1016/B978-0-12-801694-7.00018-4
Reinhard M., Schwarzer G., Briel M., Altamura C., Palazzo P., King A., et al. (2014). Cerebrovascular reactivity predicts stroke in high-grade carotid artery disease. Neurology 83, 1424–1431. doi:10.1212/WNL.0000000000000888
Röttgering J. G., de Man A. M. E., Schuurs T. C., Wils E.-J., Daniels J. M., van den Aardweg J. G., et al. (2021). Determining a target SpO2 to maintain PaO2 within a physiological range. PLoS One 16, e0250740. doi:10.1371/journal.pone.0250740
Ryman S. G., Shaff N., Dodd A., Nitschke S., Wertz C., Julio K., et al. (2023). Reduced and delayed cerebrovascular reactivity in patients with Parkinson’s disease. Mov. Disord. 38, 1262–1272. doi:10.1002/mds.29429
Sam K., Conklin J., Holmes K. R., Sobczyk O., Poublanc J., Crawley A. P., et al. (2016). Impaired dynamic cerebrovascular response to hypercapnia predicts development of white matter hyperintensities. NeuroImage Clin. 11, 796–801. doi:10.1016/j.nicl.2016.05.008
Shapiro W., Wasserman A. J., Baker J. P., Patterson J. L. (1970). Cerebrovascular response to acute hypocapnic and eucapnic hypoxia in normal man. J. Clin. Invest. 49, 2362–2368. doi:10.1172/JCI106455
Sleight E., Stringer M. S., Marshall I., Wardlaw J. M., Thrippleton M. J. (2021). Cerebrovascular reactivity measurement using magnetic resonance imaging: a systematic review. Front. Physiology 12, 643468. doi:10.3389/fphys.2021.643468
Smith S. M. (2002). Fast robust automated brain extraction. Hum. Brain Mapp. 17, 143–155. doi:10.1002/hbm.10062
Sobczyk O., Crawley A. P., Poublanc J., Sam K., Mandell D. M., Mikulis D. J., et al. (2016). Identifying significant changes in cerebrovascular reactivity to carbon dioxide. Am. J. Neuroradiol. 37, 818–824. doi:10.3174/ajnr.A4679
Spencer M. D., Tyndall A. V., Davenport M. H., Argourd L., Anderson T. J., Eskes G. A., et al. (2015). Cerebrovascular responsiveness to hypercapnia is stable over six months in older adults. PLOS ONE 10, e0143059. doi:10.1371/journal.pone.0143059
Steinback C. D., Poulin M. J. (2008). Cardiovascular and cerebrovascular responses to acute isocapnic and poikilocapnic hypoxia in humans. J. Appl. Physiology 104, 482–489. doi:10.1152/japplphysiol.00553.2007
Steinback C. D., Poulin M. J. (2016). “Influence of hypoxia on cerebral blood flow regulation in humans,” in Hypoxia: translation in progress, advances in experimental medicine and biology. Editors R. C. Roach, P. H. Hackett, and P. D. Wagner (US, Boston, MA: Springer), 131–144. doi:10.1007/978-1-4899-7678-9_9
Sur S., Lin Z., Li Y., Yasar S., Rosenberg P., Moghekar A., et al. (2020). Association of cerebrovascular reactivity and Alzheimer pathologic markers with cognitive performance. Neurology 95, e962–e972. doi:10.1212/WNL.0000000000010133
Tancredi F. B., Lajoie I., Hoge R. D. (2015). Test-retest reliability of cerebral blood flow and blood oxygenation level-dependent responses to hypercapnia and hyperoxia using dual-echo pseudo-continuous arterial spin labeling and step changes in the fractional composition of inspired gases. J. Magn. Reson Imaging 42, 1144–1157. doi:10.1002/jmri.24878
Taylor J. L., Lavey J. A., Carlson A. R., Barnes J. N., Johnson B. D. (2022). A pilot study to investigate the effect of hypercapnia training on cerebrovascular reactivity in healthy adults. FASEB J. 36. doi:10.1096/fasebj.2022.36.S1.R4908
Tomoto T., Riley J., Turner M., Zhang R., Tarumi T. (2020). Cerebral vasomotor reactivity during hypo- and hypercapnia across the adult lifespan. J. Cereb. Blood Flow. Metab. 40, 600–610. doi:10.1177/0271678X19828327
Vernieri F., Pasqualetti P., Passarelli F., Rossini P. M., Silvestrini M. (1999). Outcome of carotid artery occlusion is predicted by cerebrovascular reactivity. Stroke 30, 593–598. doi:10.1161/01.STR.30.3.593
West J. B. (2011). Causes of and compensations for hypoxemia and hypercapnia. Compr. Physiol. 1, 1541–1553. doi:10.1002/cphy.c091007
Wilson M. H., Edsell M. E. G., Davagnanam I., Hirani S. P., Martin D. S., Levett D. Z. H., et al. (2011). Cerebral artery dilatation maintains cerebral oxygenation at extreme altitude and in acute hypoxia—an ultrasound and MRI study. J. Cereb. Blood Flow. Metab. 31, 2019–2029. doi:10.1038/jcbfm.2011.81
Winklewski P. J., Wolf J., Gruszecki M., Wszedybyl-Winklewska M., Narkiewicz K. (2019). Current understanding of the effects of inspiratory resistance on the interactions between systemic blood pressure, cerebral perfusion, intracranial pressure, and cerebrospinal fluid dynamics. J. Appl. Physiology 127, 1206–1214. doi:10.1152/japplphysiol.00058.2019
Zhang Y., Brady M., Smith S. (2001). Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging 20, 45–57. doi:10.1109/42.906424
Keywords: cerebral blood flow, cerebrovascular reactivity, hypoxia, phase contrast MRI, hypercapnia
Citation: Johnson HR, Wang MC, Stickland RC, Chen Y, Parrish TB, Sorond FA and Bright MG (2025) Variable cerebral blood flow responsiveness to acute hypoxic hypoxia. Front. Physiol. 16:1562582. doi: 10.3389/fphys.2025.1562582
Received: 17 January 2025; Accepted: 24 February 2025;
Published: 12 March 2025.
Edited by:
Rodrigo Iturriaga, Universidad Autonoma de Chile, ChileReviewed by:
Frank Pernett, Mid Sweden University, SwedenCopyright © 2025 Johnson, Wang, Stickland, Chen, Parrish, Sorond and Bright. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hannah R. Johnson, hannah.johnson3@northwestern.edu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.