
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Physiol., 10 March 2025
Sec. Exercise Physiology
Volume 16 - 2025 | https://doi.org/10.3389/fphys.2025.1571730
This article is part of the Research TopicPhysiological and Pathological Responses to Hypoxia and High Altitude, Volume IIIView all 8 articles
Objective: This study aims to assess the impact of hypoxia training on body composition and glycolipid metabolism in excess body weight or living with obese people through meta-analysis.
Methods: Randomized controlled trials investigating the effects of hypoxia training on body composition, glucose, and lipid metabolism in excess body weight or living with obese people were systematically searched from databases including CNKI, PubMed, and Web of Science. The meta-analysis was performed by using Stata 18 and RevMan 5.4 analytic tools. The risk of bias was assessed using the Cochrane evaluation tool, and the level of certainty of evidence was determined by the GRADE framework. Between-study heterogeneity was examined using the I2 test, and the publication bias was evaluated via the Egger test or funnel plot.
Results: A total of 32 RCTs with 1,011 participants were included. A meta-analysis of 25 RCTs was performed (499 men and 480 women, Age: 40.25 ± 15.69, BMI: 30.96 ± 3.65). In terms of body composition, the outcome indexes of body fat ratio (MD is −1.16, 95% CI -1.76 to −0.56, P = 0.00) in the hypoxia group were better than the normal oxygen group. There was no significant difference in body mass and BMI between the hypoxia group and the normal-oxygen group (P > 0.05). In terms of lipid and glucose metabolism, no significant changes were found between the hypoxia group and the normoxia group (P > 0.05). Subgroup analysis showed that training in hypoxic environment at altitude 2001–2,500 m could effectively improve body mass, TG and LDL-C (P < 0.05). The effective program to reduce body mass is to carry out moderate intensity training of 45–60 min for ≤8 weeks, ≥4 times a week (P < 0.05).
Conclusion: Hypoxic training is essential for reducing body fat ratio in excess body weight or obese people. It is recommended to carry out 45–60 min of moderate-intensity aerobic exercise for ≤8 weeks, ≥4 times a week, in a hypoxia environment of 2,001–2,500 m to lose body mass. The effects of hypoxia training and normoxia training on lipid and glucose metabolism in excess body weight or obese people are the same.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42024628550
In the clinical field, Excess adiposity has gradually become a global public health issue and a leading cause of death in most countries’ populations (Chen et al., 2022). The World Health Organization (WHO) defines excess body weight and excess adiposity by body mass index (BMI), individuals with a BMI exceeding 30 kg/m2 are typically classified as living with obesity, while those with a BMI ranging from 25 to 30 kg/m2 are considered excess body weight (Badimon et al., 2017). In recent years, alongside economic development and improved living standards, the prevalence of excess body weight and excess adiposity has continued to rise (Tee et al., 2023; Collaboration, 2016). The World Health Organization forecasts that by 2030, nearly 60% of the global population will be excess body weight or with obesity (Dragano et al., 2020). Excess body weight and excess adiposity can impair their exercise capacity and, coupled with excessive caloric intake, elevate the risk of cardiovascular ailments like diabetes mellitus and hypercholesterolemia (Chacaroun et al., 2020; Chen et al., 2022; Wang and Sun, 2019; Wang et al., 2012), consequently augmenting healthcare expenses and societal and economic burdens (Wang et al., 2021; Wang et al., 2011). Additionally, they may encounter discrimination from society, potentially impacting their mental wellbeing and reducing their quality of life (Heymsfield and Wadden, 2017; Han, 2020; Jin et al., 2023). Therefore, implementing appropriate interventions to manage people’s weight and enhance their glucose and lipid metabolism is imperative. Exercise for weight loss is globally important (Exercise for weight loss was no. One in China, Brazil, Mexico, and Spain and no. Four in Europe) (Kercher et al., 2021). Exercise aids in preserving lean body mass through weight loss efforts and helps consumers maintain long-term weight loss (Newsome et al., 2024). For excess body weight or obese individuals, although aerobic exercise can bring about effective weight loss, it usually takes a relatively long time; in contrast, interval training is an efficient and time - saving exercise option (Batrakoulis et al., 2021), and combined training has the most positive influence on cardiometabolic health indicators (Batrakoulis et al., 2022).
A hypoxia environment refers to an environment with reduced oxygen partial pressure compared to the normal sea-level oxygen environment, encompassing both natural high-altitude hypoxia environments and artificial hypoxia environments (Wang and Sun, 2019). Hypoxic exercise training is a method of exercise and fitness in a naturally occurring or artificially simulated plateau where the body is below normal oxygen conditions (Weng et al., 2006). Individuals accustomed to living at sea level may experience altitude sickness when first exposed to high altitudes, making altitude training impractical due to its associated challenges in distance and duration. Consequently, in recent years, artificial simulation of hypoxia environments, rather than natural high-altitude conditions, has been demonstrated to enhance athletes’ aerobic and anaerobic exercise capacities (Faulhaber et al., 2023; Feng et al., 2023; Mujika et al., 2019)and performance and is emerging as a potent non-pharmacological intervention against numerous diseases (Burtscher et al., 2023). This artificially simulated hypoxic environment is also progressively utilized to enhance the body composition and lipid metabolism of living with obese individuals. Research indicates that prolonged residence in high-altitude, hypoxia environments results in weight loss (Xie et al., 2017).
When the load is regulated by heart rate, hypoxic environments typically induce a slight increase in heart rate, potentially alleviating the body’s burden compared to normal oxygen conditions (Yang et al., 2014). Exercising in a hypoxic environment also creates greater metabolic strain and increases overall fatigue (Ruggiero et al., 2022). Furthermore, many training modalities in hypoxic environments involve stationary bicycles, which can mitigate the risk of bone, knee, and ankle injuries in excess body weight or living with obese individuals (Yan, 2020). At the same time, it is feasible, safe, and effective for excess body weight/with excess adiposity individuals (Batrakoulis and Fatouros, 2022). Previous studies have shown that training in the normobaric hypoxic environment does not produce better benefits (Chen et al., 2022). There are also no studies exploring the best hypoxic training prescriptions for people who are excess body weight or obese. Hence, this study aims to assess the impact of hypobaric/normobaric hypoxic training on enhancing body composition and glucose and lipid metabolism in excess body weight or living with obese people via meta-analysis, aiming to ascertain its efficacy compared to conventional oxygen training and explore the best hypoxic training program for excess body weight or obese people.
This paper follows the Systematic Review and Meta-Analysis Project (PRISMA) guidelines (Page et al., 2021). The PROSPERO registration number is CRD42024628550.
Inclusion criteria: Inclusion criteria followed the PICOS principles. (1) The participants were overweight or obese, and had no physical restrictions or health conditions that would preclude evaluation and exercise intervention; (2) Clinical randomized controlled trials; (3) In a hypobaric/normobaric hypoxic environment, oxygen concentration ≤17.4% or altitude ≥1,500 m; (4) The experimental group underwent hypoxia training hypoxic environme, while the control group received normal oxygen training; (5) Outcome: body mass, body fat ratio, body mass index, total cholesterol, triglycerides, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, fasting blood glucose, fasting blood insulin, and homeostatic assessment of insulin resistance, there is at least one outcome indicator in the literature.
Exclusion criteria: (1) Review Literature; (2) Case report studies; (3) Animal experimental literature; (4) Non-randomized controlled trials.
A literature search was performed on databases including China National Knowledge Infrastructure (CNKI), Pub Med, and Web of Science, covering the period from the inception of each database to 11 January 2024. The last literature search was conducted on 1 December 2024. We use the exact Boolean operator (AND, OR) to concatenate search terms. The following combination of terms was used: “hypoxia training” or “hypoxia exercise” or “Intermittent hypoxia” or “altitude training”. The Boolean operator “AND” was used to combine these descriptors with “overweight” or “obesity” or “obese”. References included in the study and meta-analysis were also manually checked to avoid possible missing studies.
The literature was retrieved from the databases using the established search strategy. Duplicate literature was removed using End Note X9 software, and irrelevant articles were excluded based on the examination of their titles and abstracts. Subsequently, articles that did not meet the inclusion and exclusion criteria were excluded.
Data extraction included retrieving information such as the first author’s name, publication year, sex distribution, sample size, age distribution, body mass index, intervention duration, intervention frequency, altitude, exercise modality, training duration, and outcome measures.
The Cochrane Collaboration RCT bias evaluation tool in Revman 5.4 software was employed to assess: (1) Random sequence generation; (2) Allocation concealment; (3) Blinding of participants and personnel; (4) Blinding of outcome assessment; (5) Incomplete outcome data; (6) Selective reporting; (7) Other bias. (This assessment was conducted independently by two researchers, with discrepancies resolved through discussion with a third researcher.)
The Grading of Recommendations Assessment, Developing and Evaluation (GRADE) method was employed to assess the quality of evidence. GRADE assesses the certainty of evidence to fall into the categories of very low, low, moderate, or high.
The outcome indicators extracted from the included literature were analyzed using Stata 18 software. Since the outcome indicators in the included studies are continuous variables, the unit of body composition was the same, so mean difference (MD) was used as the effect size. The units of lipid and sugar metabolism are different, and the effect sizes (Hedges’s d) were expressed as standardized mean difference (SMD) and 95% confidence intervals (CI). Heterogeneity among outcome indicators was assessed using I2 statistics. When I2 < 50%, indicating small heterogeneity, the fixed-effect model was employed for analysis. Conversely, if I2 suggested significant heterogeneity, exceeding 50%, the random-effects model was utilized. The source of atypia was identified by sensitivity analysis. The subgroup analysis method was used to explore the best hypoxic fat reduction training prescription. Publication bias was assessed by funnel plot or Egger’s regression test.
A total of 1,676 articles were retrieved from databases (Specific literature search strategies are shown in Supplementary Table 1). After excluding 353 replicated studies, the texts of the remaining articles were reviewed. Ultimately, 32 studies met the inclusion criteria and were included in the analysis. The flow chart illustrating the literature screening process is presented in Figure 1.
The basic information of the literature included in this study is presented in Supplementary Table 2. Of the 32 randomized controlled studies included (a meta-analysis of 25 studies (Park et al., 2024; Ghaith et al., 2022; Fu and Li, 2022; Hobbins et al., 2021; Ma, 2020; Jung et al., 2020; Gao et al., 2020; Chacaroun et al., 2020; Park et al., 2019; Zhang, 2019; Yang et al., 2018; Shin et al., 2018; Klug et al., 2018; Fernández Menéndez et al., 2018; Camacho-Cardenosa et al., 2018a; Park et al., 2017; Kong et al., 2017; Zhao and Shi, 2016; Nishiwaki et al., 2016; Gutwenger et al., 2015; Gatterer et al., 2015; Morishima et al., 2014; Kong et al., 2014; Li et al., 2014; Wiesner et al., 2010) was performed, 499 men and 480 women, Age: 40.25 ± 15.69, BMI: 30.96 ± 3.65; The seven studies (Han, 2020; Yan, 2020; Mai et al., 2020; Camacho-Cardenosa et al., 2019; De Groote et al., 2018; Camacho-Cardenosa et al., 2018b; Netzer et al., 2008) for which raw data could not be extracted have been analyzed descriptively in Supplementary Table 2), four specified allocation concealment. In terms of blind evaluation, 13 studies were single-blind and six studies were double-blind, and three of them informed the subjects of the entire trial process and risks. Eight of the studies had problems with participants dropping out of the trial during the intervention. The evaluation results are depicted in Figure 2.
The overall certainty of evidence underwent assessment with the application of the GRADE tool, and the findings are presented within Supplementary Table 3. The GRADE method demonstrated that the certainty levels for BM, BFR, TC and FBG were low, while those for BMI, TG, LDL - C, HDL - C, BFI and HOMA - IR were very low.
Twenty-two randomized controlled trials were utilized to assess the body mass (See Figure 3 for individual studies), there was no significant difference in the improvement of the body mass between the hypoxic group and the control group and no heterogeneity among the studies (MD -1.14, 95% CI -2.34 to −0.07; P = 0.07, I2 = 0%).
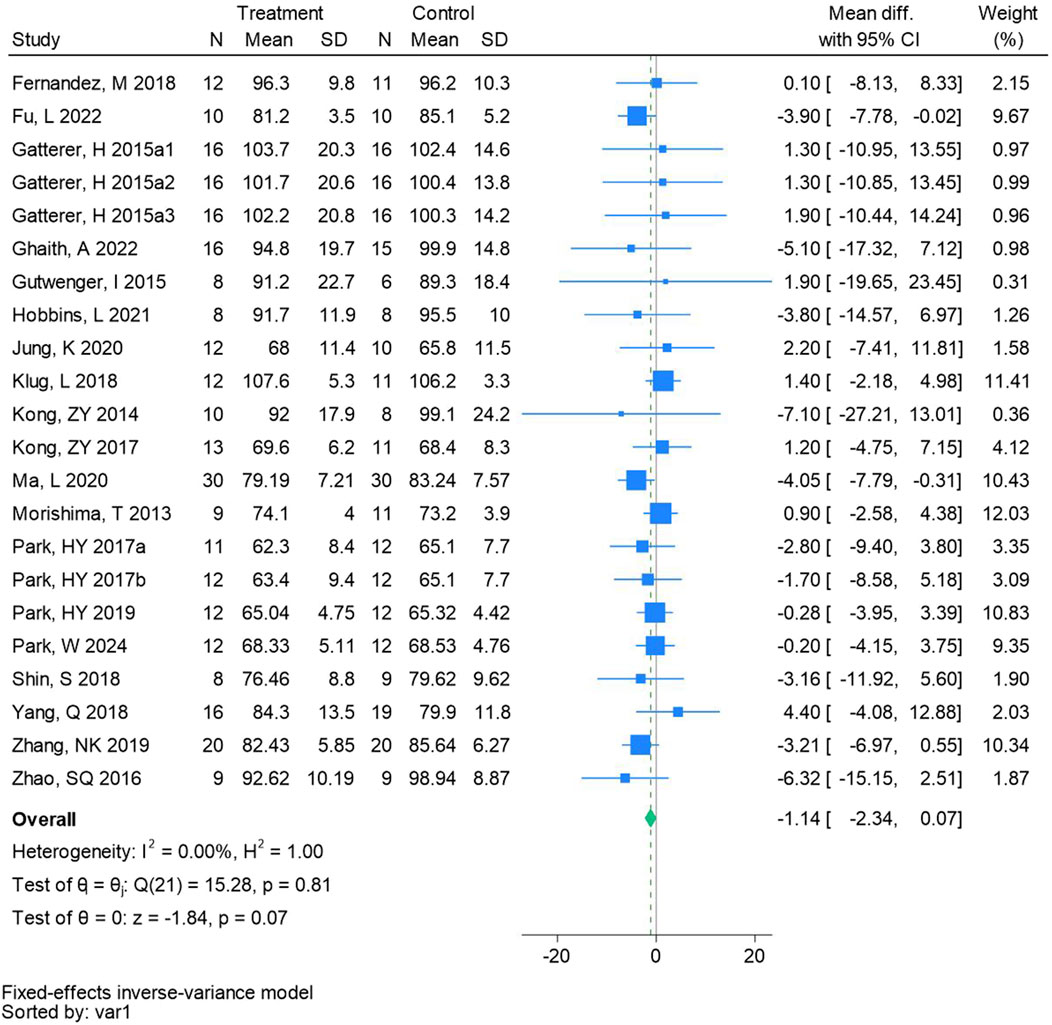
Figure 3. Forest plot of body mass meta-analysis. Gatterer, H 2015a1 indicated that the intervention period was 5 weeks, 2015a2 was 3 months, and 2015a3 was 8 months (Park et al., 2017). indicates training in a hypoxia environment at an altitude of 2,000 m, and 2017b indicates training in a hypoxia environment at an altitude of 3,000 m.
Sixteen randomized controlled trials were included in the analysis of body fat ratio (See Figure 4 for individual studies), there was a significant difference in the improvement of body fat ratio between the hypoxic group and the control group and no heterogeneity among the studies (MD -1.16, 95% CI -1.76 to −0.56; P = 0.0001, I2 = 42.92%).
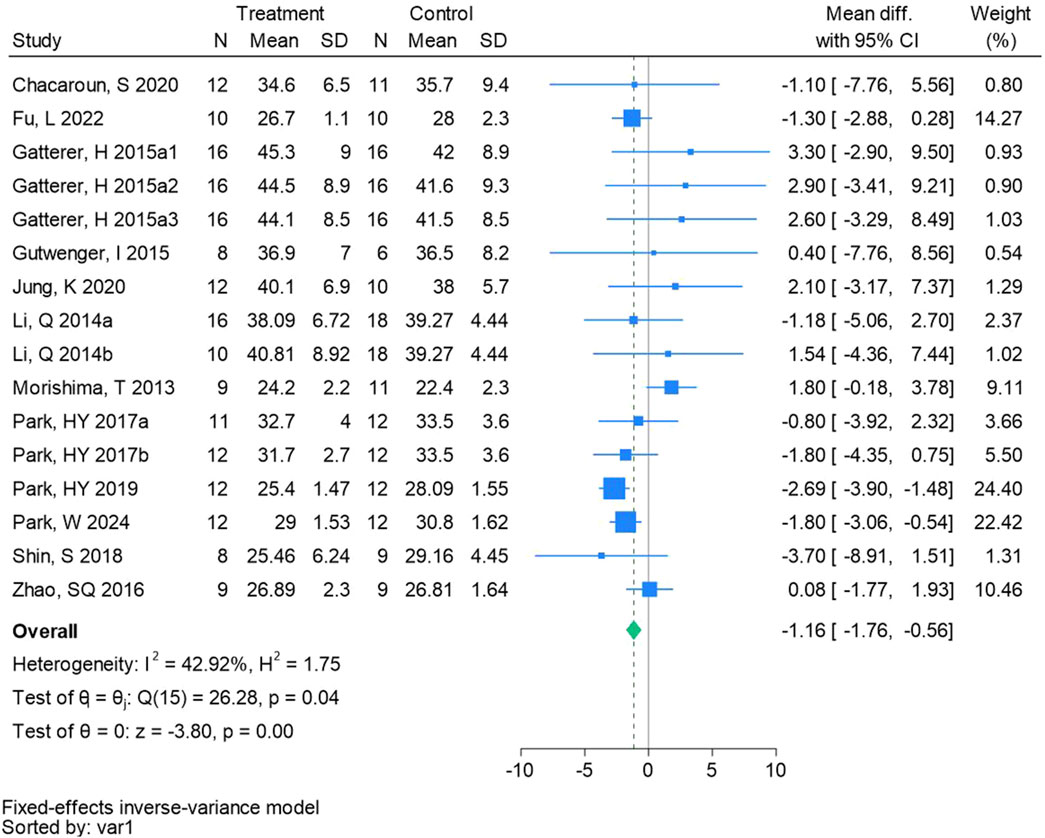
Figure 4. Forest plot of body fat ratio meta-analysis. Gatterer, H 2015a1 indicated that the intervention period was 5 weeks, 2015a2 was 3 months, and 2015a3 was 8 months (Park et al., 2017). indicates training in a hypoxia environment at an altitude of 2,000 m, and 2017b indicates training in a hypoxia environment at an altitude of 3,000 m.
Twenty-one randomized controlled trials were included in the analysis of BMI (See Figure 5 for individual studies), there was no significant difference in the improvement of BMI between the hypoxic group and the control group and heterogeneity among the studies (MD -0.42, 95% CI -1.23 to 0.39; P = 0.31, I2 = 69.55%).
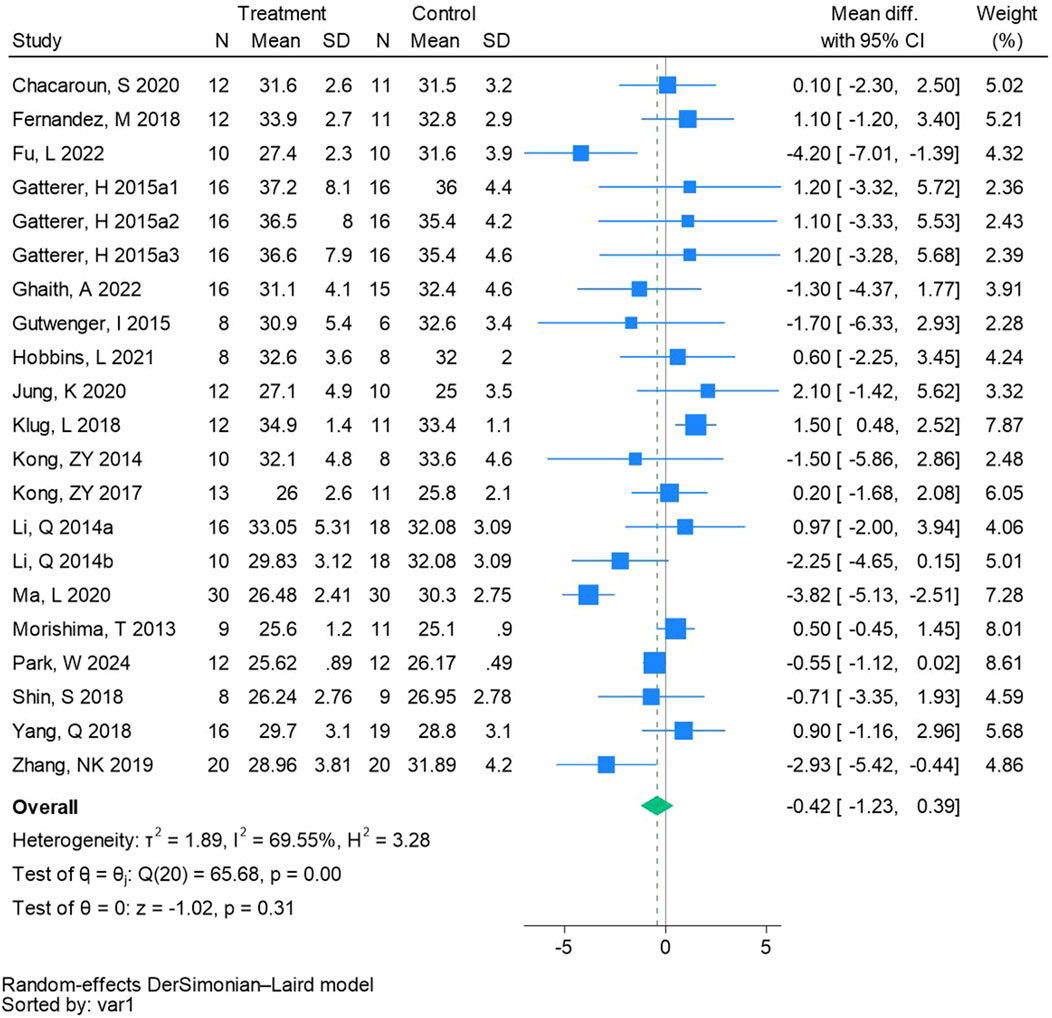
Figure 5. Forest plot of body mass index meta-analysis. Gatterer, H 2015a1 indicated that the intervention period was 5 weeks, 2015a2 was 3 months, and 2015a3 was 8 months (Li et al., 2014). represents the normobaric hypoxia environment, and 2014b represents the hypobaric hypoxia environment.
Twenty-two randomized controlled trials were utilized to analyze total cholesterol (See Figure 6 for individual studies), there was no significant difference in the improvement of total cholesterol between the hypoxic group and the control group and no heterogeneity among the studies (SMD -0.04, 95% CI -0.21 to 0.12; P = 0.59, I2 = 39.40%).
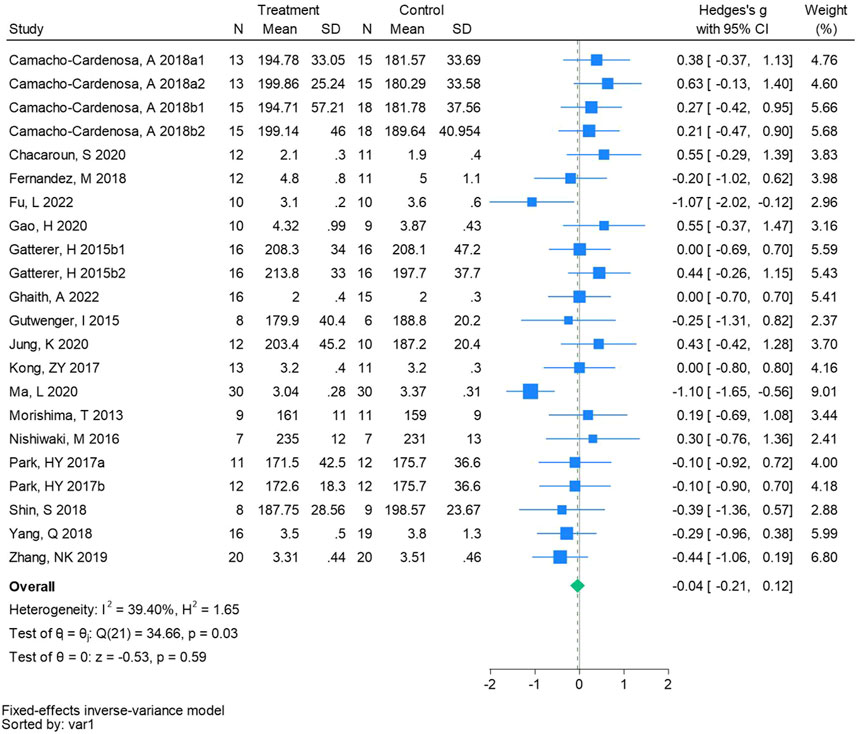
Figure 6. Forest plot of total cholesterol meta-analysis. Camacho-Cardenosa et al., 2018a stands for high-intensity interval training, 2018b stands for high intensity full sprint (1 means that the intervention period is 6 weeks, two means that the intervention period is 12 weeks). Gatterer, H 2015b1 indicated that the intervention period was 3 months, and 2015b2 indicated that the intervention period was 8 months (Park et al., 2017). indicates training in a hypoxia environment at an altitude of 2,000 m, and 2017b indicates training in a hypoxia environment at an altitude of 3,000 m.
Twenty-three randomized controlled trials were utilized to analyze triglycerides (See Figure 7 for individual studies), there was no significant difference in the improvement of triglycerides between the hypoxic group and the control group and heterogeneity among the studie (SMD 0.08, 95% CI -0.20 to 0.35; P = 0.59, I2 = 65.02%).
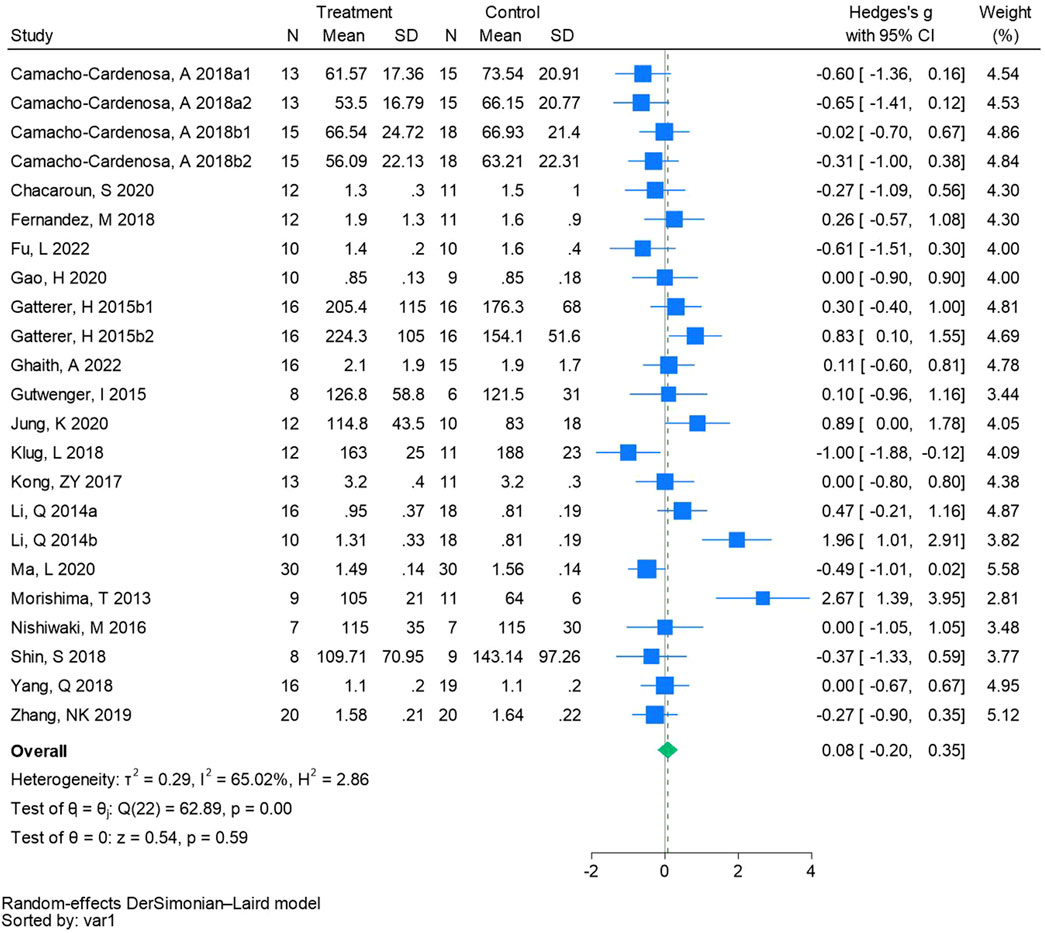
Figure 7. Forest plot of triglycerides meta-analysis. Camacho-Cardenosa et al., 2018a stands for high-intensity interval training, 2018b stands for high intensity full sprint (1 means that the intervention period is 6 weeks, two means that the intervention period is 12 weeks). Gatterer, H 2015b1 indicated that the intervention period was 3 months, and 2015b2 indicated that the intervention period was 8 months (Li et al., 2014). represents the normobaric hypoxia environment, and 2014b represents the hypobaric hypoxia environment.
Eighteen randomized controlled trials were utilized to analyze low-density lipoprotein cholesterol (See Figure 8 for individual studies), there was no significant difference in the improvement of low-density lipoprotein cholesterol between the hypoxic group and the control group and heterogeneity among the studies (SMD -0.23, 95% CI -0.65 to 0.19; P = 0.28, I2 = 78.59%).
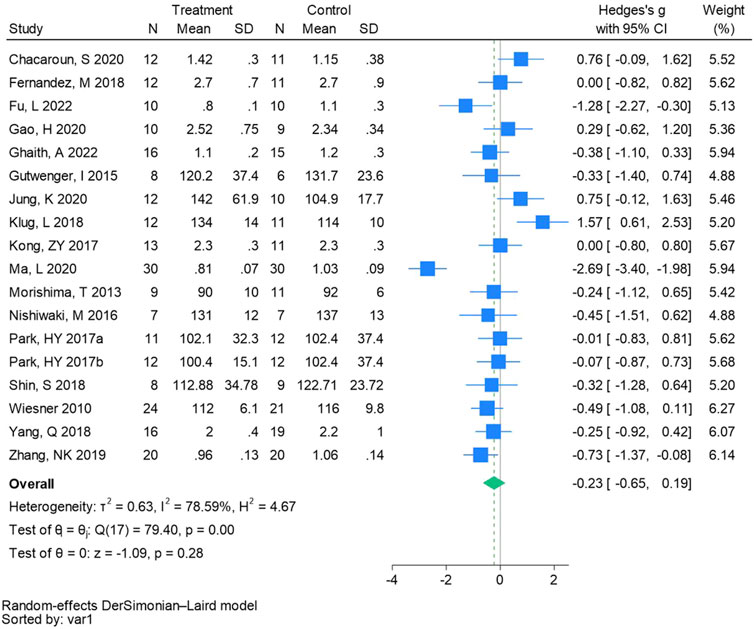
Figure 8. Forest plot of low-density lipoprotein cholesterol meta-analysis (Park et al., 2017). indicates training in a hypoxia environment at an altitude of 2,000 m, and 2017b indicates training in a hypoxia environment at an altitude of 3,000 m.
Nineteen randomized controlled trials were employed to analyze high-density lipoprotein cholesterol (See Figure 9 for individual studies), there was no significant difference in the improvement of high-density lipoprotein cholesterol between the hypoxic group and the control group and heterogeneity among the studies (SMD 0.17, 95% CI -0.26 to 0.59; P = 0.44, I2 = 80.28%).
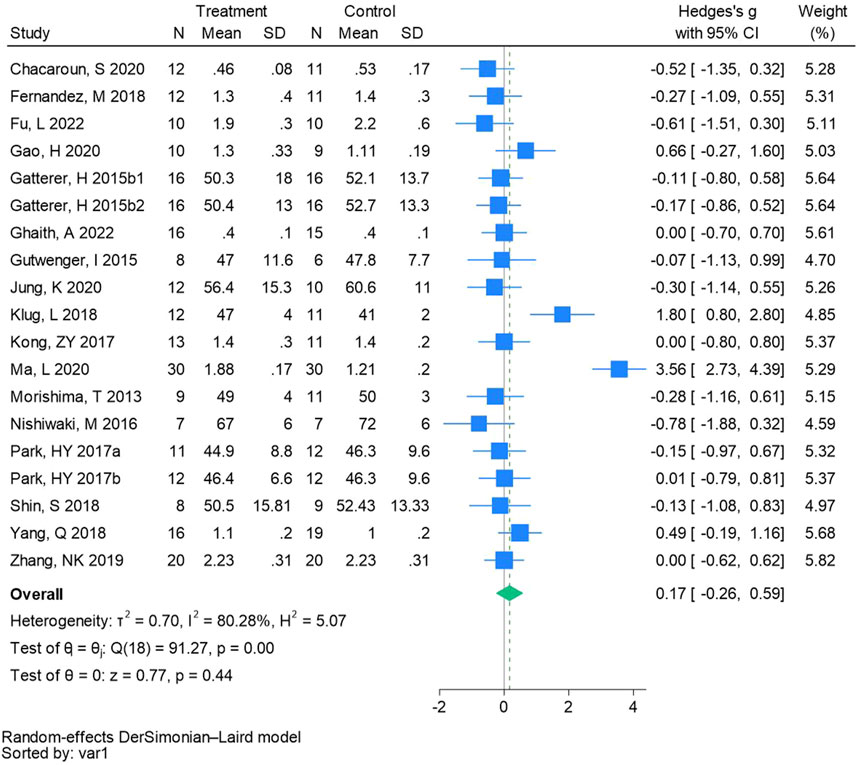
Figure 9. Forest plot of high-density lipoprotein cholesterol meta-analysis. Gatterer, H 2015b1 indicated that the intervention period was 3 months, and 2015b2 indicated that the intervention period was 8 months (Park et al., 2017). indicates training in a hypoxia environment at an altitude of 2,000 m, and 2017b indicates training in a hypoxia environment at an altitude of 3,000 m.
Eighteen randomized controlled trials were included in the analysis of glucose (See Figure 10 for individual studies), there was no significant difference in the improvement of glucose between the hypoxic group and the control group and no heterogeneity among the studies (SMD 0.01, 95% CI -0.16 to 0.19; P = 0.88, I2 = 29.22%).
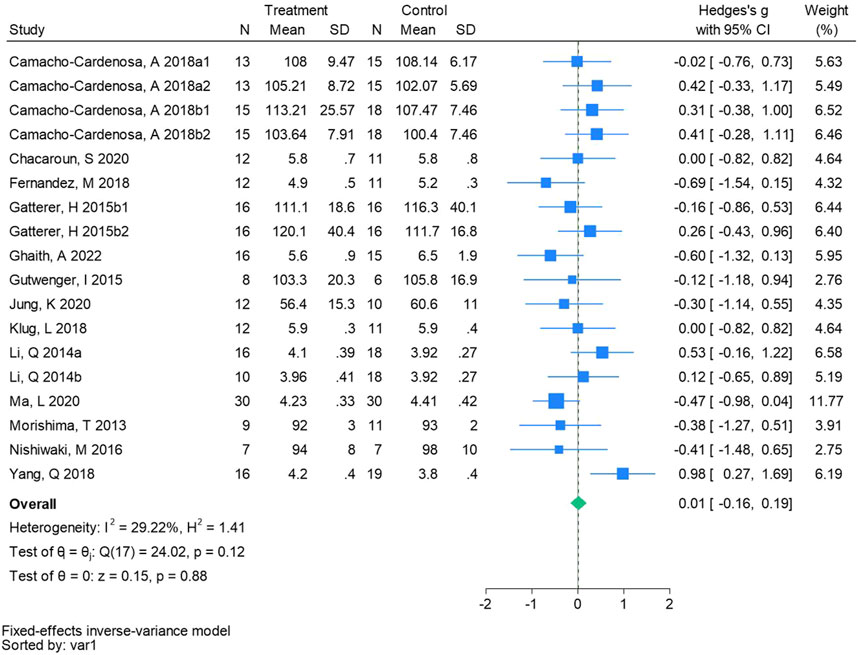
Figure 10. Forest plot of fasting blood glucose meta-analysis. Camacho-Cardenosa et al., 2018a stands for high-intensity interval training, 2018b stands for high intensity full sprint (1 means that the intervention period is 6 weeks, two means that the intervention period is 12 weeks). Gatterer, H 2015b1 indicated that the intervention period was 3 months, and 2015b2 indicated that the intervention period was 8 months (Li et al., 2014). represents the normobaric hypoxia environment, and 2014b represents the hypobaric hypoxia environment.
Twelve randomized controlled trials were included in the analysis of fasting blood insulin (See Figure 11 for individual studies), there was no significant difference in the improvement of fasting blood insulin between the hypoxic group and the control group and heterogeneity among the studies (SMD 0.24, 95% CI -0.30 to 0.79; P = 0.39, I2 = 80.62%).
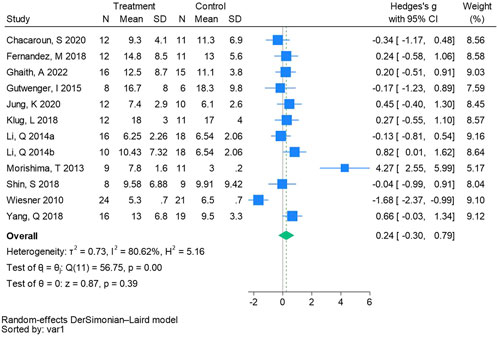
Figure 11. Forest plot of fasting blood insulin meta-analysis (Li et al., 2014). represents the normobaric hypoxia environment, and 2014b represents the hypobaric hypoxia environment.
Nine randomized controlled trials were included in the analysis of homeostatic assessment of insulin resistance (See Figure 12 for individual studies), there was no significant difference in the improvement of homeostatic assessment of insulin resistance between the hypoxic group and the control group and heterogeneity among the studies (SMD 0.04, 95% CI -0.44 to 0.52; P = 0.86, I2 = 71.48%).
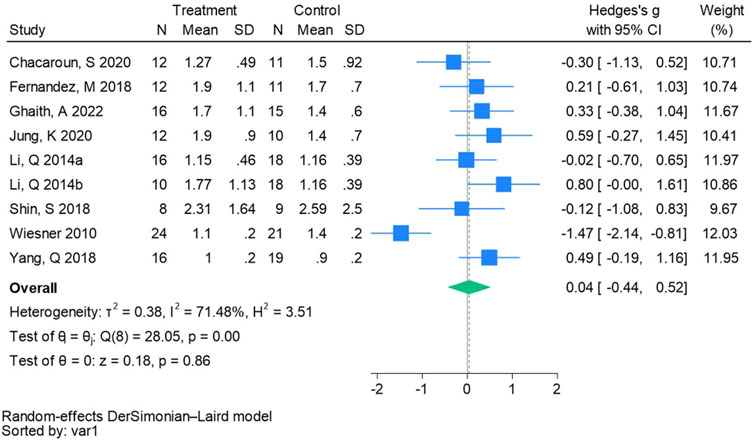
Figure 12. Forest plot of homeostatic assessment of insulin resistance meta-analysis (Li et al., 2014). represents the normobaric hypoxia environment, and 2014b represents the hypobaric hypoxia environment.
Sensitivity analysis of outcome indexes including BMI, TG, LDL - C, HDL - C, FBI, and HOMA - IR was performed. After deleting the literature with high heterogeneity, there were no significant differences in BMI (MD -0.06, 95% CI -0.43 to 0.31; P = 0.74, I2 = 48%), TG (SMD -0.09, 95% CI -0.25 to 0.08; P = 0.30, I2 = 30%), LDL - C (SMD -0.20, 95% CI -0.40 to 0.00; P = 0.06, I2 = 26%), HDL - C (SMD -0.02, 95% CI -0.21 to 0.17; P = 0.81, I2 = 27%), FBI (SMD 0.22, 95% CI -0.03 to 0.47; P = 0.09, I2 = 0%) and HOMA - IR (SMD 0.26, 95% CI -0.02 to 0.53; P = 0.07, I2 = 0%) between the hypoxia group and the normal-oxygen group (Supplementary Table 4).
The effects of hypoxic training on excess body weight or obese people may be influenced by duration, frequency, time, exercise intensity, intervention form, and altitude (Tables 1, 2).
(1) Compared with normoxic training, when the duration of hypoxic training ≤8 weeks, body mass (MD -1.51, 95% CI -2.89 to −0.13; P = 0.03, I2 = 0%) and LDL - C (SMD -0.25, 95% CI -0.46 to −0.04; P = 0.02, I2 = 9%) could be effectively improved.
(2) Compared with normoxic training, when the frequency of hypoxic training ≥4 days, body mass (MD -2.70, 95% CI -4.43 to −0.97; P = 0.002, I2 = 0%), TC (SMD -0.36, 95% CI -0.59 to −0.12; P = 0.003, I2 = 39%) and LDL - C (SMD -0.28, 95% CI -0.54 to −0.02; P = 0.04, I2 = 1%) can be effectively improved.
(3) Compared with normoxic training, when the time is 45–60 min of hypoxic training, body mass (MD -1.42, 95% CI -2.70 to −0.15; P = 0.03, I2 = 0%), TG (SMD -0.27, 95% CI -0.54 to −0.01; P = 0.04, I2 = 42%) and FBG (SMD -0.34, 95% CI -0.64 to −0.03; P = 0.03, I2 = 0%) can be effectively improved.
(4) Compared with normoxic training, when the hypoxic training is moderate intensity, body mass (MD -2.29, 95% CI -3.83 to −0.76; P = 0.003, I2 = 0%), BFR (MD -1.53, 95% CI -2.18 to −0.88; P < 0.00001, I2 = 31%) and BMI (MD -0.69, 95% CI -1.21 to −0.17; P = 0.009, I2 = 41%) can be effectively improved.
(5) Compared with normoxic training, when the intervention form of hypoxic training is a combination of aerobic training and resistance training, BFR (MD -2.26, 95% CI -3.14 to −1.39; P < 0.00001, I2 = 0%) can be effectively improved.
(6) Compared with normoxic training, when the altitude is between 2,001 m and 2,500 m, the body mass (MD -2.09, 95% CI -3.83 to −0.35; P = 0.02, I2 = 18%), TG (SMD -0.30, 95% CI -0.51 to −0.09; P = 0.005, I2 = 3%) and LDL - C (SMD -0.42, 95% CI -0.79 to −0.05; P = 0.03, I2 = 45%) can be effectively improved.
The funnel plot of body weight results showed reasonable symmetry, while the funnel plot analysis of other outcome measures showed slight asymmetry (Figure 13). In the meta-analysis, funnel plot analysis is not recommended for assessing publication bias when the included literature has fewer than 10 outcome indicators (Chang et al., 2015). Egger’s regression test for HOMA - IR did not reach statistical significance (P = 0.04727).
This study aims to assess the impact of hypoxic training on enhancing body composition and glucose and lipid metabolism in excess body weight and with obese people via meta-analysis, aiming to ascertain its efficacy compared to normoxic training. Explore the best hypoxic training program for excess body weight or obese people. The main finding of this meta-analysis was that hypoxic training significantly reduced body fat ratio in excess body weight or living with obese people, but there was no significant difference in glycolipid metabolism. Subgroup analysis showed that training in the hypoxic environment at an altitude of 2001–2500 m could effectively decrease body mass, TG, and LDL - C. The effective program to reduce body mass is to carry out moderate intensity training (MD -2.29, 95% CI -3.83 to −0.76; P = 0.003, I2 = 0%) of 45–60 min (MD -1.42, 95% CI -2.70 to −0.15; P = 0.03, I2 = 0%) for ≤8 weeks (MD -1.51, 95% CI -2.89 to −0.13; P = 0.03, I2 = 0%), ≥4 times a week (MD -2.70, 95% CI -4.43 to −0.97; P = 0.002, I2 = 0%).
Previous studies have not found that hypoxic training can significantly improve body composition, which may be affected by different training types and populations (Chen et al., 2022; Ramos-Campo et al., 2019; Guo et al., 2023). The results of this meta-analysis revealed a significant superiority of the hypoxia group over the normal-oxygen group in both body fat ratios (MD is −1.16, 95% CI -1.76 to −0.56, P = 0.00). It is suggested that hypoxia training can significantly reduce the body fat ratio of excess body weight and obese people, which is more significant in the combination of aerobic and resistance training intervention (MD is −2.26, 95% CI -3.14 to −1.39, P = 0.00). A recent meta - analysis has revealed that the integration of aerobic and resistance training leads to significant beneficial changes in multiple cardiometabolic parameters and mental health - associated markers among excess body weight or obese patients (Al-Mhanna et al., 2024). Furthermore, resistance training, when employed as an independent exercise intervention, confers numerous cardiometabolic advantages in the management and treatment of type 2 diabetes mellitus (T2DM) patients who are excess body weight or obese (Al-Mhanna et al., 2025). Despite the weak evidence for hypoxic training on body mass (MD is −1.14, 95% CI -2.34 to 0.07, P = 0.07) and BMI (MD is −0.42, 95% CI -1.23 to 0.39, P = 0.31) outcomes shown in our meta-analysis. A body of research indicates the efficacy of hypoxia training in improving the body composition of excess body weight and living with obese individuals (Tee et al., 2023; Kong et al., 2014). The combined effect of hypoxia and exercise triggers a stress response in the body, accelerating fat decomposition (Workman and Basset, 2012). Exposure to hypoxia enhanced the ability to transport oxygen to muscle and the improvement of fat oxidation (Park et al., 2016; Chen et al., 2013). Additionally, a hypoxia environment boosts metabolic rate, increases leptin and insulin-like growth factor levels, and stimulates the hematopoietic system, ultimately reducing body fat content (Yingzhong et al., 2006). Subgroup analysis showed that moderate-intensity aerobic exercise (≤8 weeks, ≥4 times/week, 45–60 min) at altitude 2001–2500 m in a hypoxia environment had the greatest improvement in body weight. Hypoxia can inhibit Ghrelin concentration (Bailey et al., 2015), and exercise in a hypoxia environment may lead to a substantial decrease in energy intake. The decrease in energy intake caused by low oxygen exposure and the increase in energy expenditure caused by an increase in basal metabolic rate may be the main causes of weight loss (Millet et al., 2016). The study found that exercise intensity is a crucial factor influencing fatty acids. When engaging in sustained exercise at 25% of the maximum intensity, 90% of the energy is sourced from fatty acid oxidation, 10% from liver glycogen, and the fatty acids primarily originate from the hydrolysis of triglycerides in adipose tissue (Romijn et al., 1993). Therefore, low or moderate exercise intensity is more conducive to fat mobilization. Vascular endothelial growth factor (VEGF) expression, skeletal muscle capillary density increase (Vogt et al., 2001), and myoglobin content significantly increased after hypoxia training, which is conducive to the transfer of more oxygen and fatty acids into skeletal muscle mitochondria to participate in oxidation and energy supply (Zoll et al., 2006), this may be a beneficial mechanism for weight loss. Therefore, for obese people who want to achieve a better weight loss effect in the short term, it is recommended to carry out moderate-intensity aerobic training (≥4 times a week, 45–60 min) in a hypoxia environment at an altitude of 2001–2,500 m, which can effectively reduce weight.
The results of this meta-analysis showed that there were no significant differences in TC (Hedges’s g is −0.04, 95% CI -0.21 to 0.12, P = 0.59), TG (Hedges’s g is 0.08, 95% CI -0.20 to 0.35, P = 0.59), LDL - C (Hedges’s g is −0.23, 95% CI -0.65 to 0.19, P = 0.28), and HDL - C (Hedges’s g is 0.17, 95% CI -0.26 to 0.59, P = 0.44) levels between the hypoxic group and the normal oxygen group. This is consistent with the research results of previous scholars (Chen et al., 2022; Guo et al., 2023; Ramos-Campo et al., 2019). Although there is no significant difference, there are many studies showing that hypoxic training is significantly better than normal oxygen training, and these studies are conducted in a hypoxic environment at an altitude of 2,500 m, five times a week, each time 60 min of aerobic exercise. Engaging in regular physical activity not only proves highly effective in boosting HDL - C levels but also brings about a significant reduction in TG, TC, and LDL - C levels, thus contributing comprehensively to better lipid metabolism and overall cardiovascular health (Badri Al-mhanna et al., 2024b). There is ongoing debate regarding the efficacy of hypoxic training in enhancing lipid metabolism among excess body weight and living with obese people. Research has demonstrated that a 4-week regimen of hypoxia training substantially enhances levels of TC, TG, LDL - C, and HDL - C in living with obese rats (Yan, 2020). Regular endurance exercise can induce favorable alterations in blood lipids and lipoproteins, particularly in hypoxia environments. This could be attributed to the combined effects of hypoxia and exercise, leading to an elevation in serum leptin levels, enhanced adipose tissue catabolism (Huan et al., 2008), improved insulin sensitivity, and reduced fat synthesis, ultimately contributing to the enhancement of blood lipid profiles (Wiesner et al., 2010). Elevated LDL - C levels and reduced HDL - C levels also elevate the risk of atherosclerosis (Marso et al., 2008). Subgroup analysis showed that exercise training for ≤8 weeks and ≥4 times per week at an altitude of 2,001–2,005 m was effective in improving LDL - C. Some suggest that the duration of training may impact the improvement of blood lipid levels (Chen et al., 2022). However, following 8 weeks of hypoxia training, there was a greater improvement in lipid metabolism levels among living with obese individuals (Netzer et al., 2017). In excess body weight and obese individuals, regular exercise is beneficial for enhancing HDL - C levels, yet the most effective strategy is a combination of moderate - to - high - intensity, long - term exercise performed at the aerobic threshold, accompanied by dietary changes (Badri Al-mhanna et al., 2024a). Furthermore, numerous studies suggest that exercise has a minimal impact on TG levels in living with obese individuals (Wiesner et al., 2010; Chacaroun et al., 2020; Wang et al., 2013), potentially attributable to TG primarily deriving from fat decomposition in food. A subgroup analysis then showed that 45–60 min of training in a hypoxia environment at an altitude of 2001–2,500 m had a significant effect on TG. Research has demonstrated that fat intake can be diminished by adhering to a low-fat diet (Chiu et al., 2015), eliminating lipid deposition in blood vessels, augmenting vascular formation (Tan et al., 2016), and mitigating the risk of atherosclerosis. However, the present study lacks rigorous dietary control for living with obese individuals, necessitating further investigation to ascertain whether strict dietary regulation could notably improve lipid metabolism. Therefore, we recommend that in a hypoxia environment at an altitude of 2001–2,500 m, develop good exercise habits (≥4 times a week, 45–60 min aerobic exercise) and strictly control diet, which is a better program to control lipid metabolism.
The results of this meta-analysis revealed no significant differences in FBG (Hedges’s g is 0.01, 95% CI -0.16 to 0.19, P = 0.88), FBI (Hedges’s g is 0.24, 95% CI -0.30 to 0.79, P = 0.39), and HOMA - IR (Hedges’s g is 0.04, 95% CI -0.44 to 0.52, P = 0.86) levels within the hypoxic group. This is consistent with the research results of previous scholars (Guo et al., 2023; Chen et al., 2022). Although there were no significant differences, two or three studies included in the analysis showed that both hypoxia and normoxia training boosted glucose metabolism levels. The exercise intervention in one study was 120 min of low-intensity exercise six times a week, and the exercise intervention in the other two studies was 60 min of moderate-intensity exercise 5/6 times a week. The majority of studies indicate that both hypoxia and normal oxygen training can enhance glucose metabolism in living with obese individuals (De Groote et al., 2018; Wiesner et al., 2010). Ounis et al. (2008) reported significant reductions in HOMA - IR among excess body weight and living with obese adolescents following 8 weeks of aerobic training. Excess adiposity severity correlates positively with diabetes incidence attributable to insulin resistance. Adequate physical activity enhances insulin sensitivity and promotes glucose utilization, thereby reducing blood sugar levels, improving glycemic control, and effectively preventing and managing diabetes. Presently, there is a paucity of randomized controlled trials (RCTs) investigating the combined effects of hypoxia and exercise intervention on glucose metabolism among excess body weight and living obese people. These trials are influenced by factors such as hypoxia mode, oxygen concentration, gender, load intensity, sample size, and diet. Research indicates that female youth in the hypoxic group exhibit greater reductions in HOMA - IR (Wang et al., 2013), possibly attributable to accelerated visceral fat decomposition in hypoxic environments, thereby ameliorating metabolic disorders and enhancing insulin sensitivity. Therefore, the effects of hypoxic training on glucose metabolism still need more high-quality literature to explore.
The limitations of this paper include the absence of allocation hiding and blind methods in some of the included literature, along with instances of subject loss, posing a significant risk of bias. Variability exists in the training protocols across the literature, including differences in hypoxia mode, oxygen concentration, and load intensity. In this paper, all age groups were included in the analysis, and the age span of subjects in some studies was too large to conduct a more in-depth analysis of age.
Hypoxic training is essential for reducing body fat ratio in excess body weight or obese people. It is recommended to carry out 45–60 min of moderate-intensity aerobic exercise for ≤8 weeks, ≥4 times a week, in a hypoxia environment of 2001–2,500 m to lose body mass. The effects of hypoxia training and normoxia training on lipid and glucose metabolism in excess body weight or obese people are the same.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
PL: Conceptualization, Data curation, Methodology, Software, Writing–original draft, Formal Analysis, Validation, Visualization, Writing–review and editing. HC: Data curation, Methodology, Software, Writing–review and editing, Supervision, Visualization. XJ: Conceptualization, Methodology, Supervision, Writing–review and editing. JD-C: Conceptualization, Methodology, Supervision, Writing–review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2025.1571730/full#supplementary-material
Al-Mhanna S. B., Batrakoulis A., Wan Ghazali W. S., Mohamed M., Aldayel A., Alhussain M. H., et al. (2024). Effects of combined aerobic and resistance training on glycemic control, blood pressure, inflammation, cardiorespiratory fitness and quality of life in patients with type 2 diabetes and overweight/obesity: a systematic review and meta-analysis. PeerJ 12, e17525. doi:10.7717/peerj.17525
Al-Mhanna S. B., Franklin B. A., Jakicic J. M., Stamatakis E., Pescatello L. S., Riebe D., et al. (2025). Impact of resistance training on cardiometabolic health-related indices in patients with type 2 diabetes and overweight/obesity: a systematic review and meta-analysis of randomised controlled trials. Br. J. Sports Med.–2024-108947. doi:10.1136/bjsports-2024-108947
Badimon L., Bugiardini R., Cenko E., Cubedo J., Dorobantu M., Duncker D. J., et al. (2017). Position paper of the European Society of Cardiology–working group of coronary pathophysiology and microcirculation: obesity and heart disease. Eur. heart J. 38, 1951–1958. doi:10.1093/eurheartj/ehx181
Badri Al-Mhanna S., Leão C., Wan Ghazali W. S., Mohamed M., Batrakoulis A., Abiola Afolabi H., et al. (2024a). Impact of exercise on high-density lipoprotein cholesterol in adults with overweight and obesity: a narrative review. Ann. Appl. Sport Sci. 12, 0. doi:10.61186/aassjournal.1300
Badri Al-Mhanna S., Wan Ghazali W. S., Batrakoulis A., Alkhamees N. H., Drenowatz C., Mohamed M., et al. (2024b). The impact of various types of exercise on lipid metabolism in patients with type 2 diabetes and concurrent overweight/obesity: a narrative review. Ann. Appl. Sport Sci. 12, 0. doi:10.61186/aassjournal.1324
Bailey D. P., Smith L. R., Chrismas B. C., Taylor L., Stensel D. J., Deighton K., et al. (2015). Appetite and gut hormone responses to moderate-intensity continuous exercise versus high-intensity interval exercise, in normoxic and hypoxic conditions. Appetite 89, 237–245. doi:10.1016/j.appet.2015.02.019
Batrakoulis A., Fatouros I. G. (2022). Psychological adaptations to high-intensity interval training in overweight and obese adults: a topical review. Sports (Basel) 10, 64. doi:10.3390/sports10050064
Batrakoulis A., Jamurtas A. Z., Fatouros I. G. (2021). High-intensity interval training in metabolic diseases: physiological adaptations. ACSM's Health and Fit. J. 25, 54–59. doi:10.1249/fit.0000000000000703
Batrakoulis A., Jamurtas A. Z., Metsios G. S., Perivoliotis K., Liguori G., Feito Y., et al. (2022). Comparative efficacy of 5 exercise types on cardiometabolic health in overweight and obese adults: a systematic review and network meta-analysis of 81 randomized controlled trials. Circ. Cardiovasc Qual. Outcomes 15, e008243. doi:10.1161/circoutcomes.121.008243
Burtscher J., Citherlet T., Camacho-Cardenosa A., Camacho-Cardenosa M., Raberin A., Krumm B., et al. (2023). Mechanisms underlying the health benefits of intermittent hypoxia conditioning. J. Physiol. 602, 5757–5783. doi:10.1113/jp285230
Camacho-Cardenosa A., Camacho-Cardenosa M., Brazo-Sayavera J., Burtscher M., Timón R., Olcina G. (2018a). Effects of high-intensity interval training under normobaric hypoxia on cardiometabolic risk markers in overweight/obese women. High. Alt. Med. Biol. 19, 356–366. doi:10.1089/ham.2018.0059
Camacho-Cardenosa A., Camacho-Cardenosa M., Burtscher M., Martínez-Guardado I., Timon R., Brazo-Sayavera J., et al. (2018b). High-intensity interval training in normobaric hypoxia leads to greater body fat loss in overweight/obese women than high-intensity interval training in normoxia. Front. Physiol. 9, 60. doi:10.3389/fphys.2018.00060
Camacho-Cardenosa A., Camacho-Cardenosa M., Olcina G., Timón R., Brazo-Sayavera J. (2019). Detraining effect on overweight/obese women after high-intensity interval training in hypoxia. Scand. J. Med. Sci. Sports 29, 535–543. doi:10.1111/sms.13380
Chacaroun S., Borowik A., Gonzalez V.-E. Y., Doutreleau S., Wuyam B., Belaidi E., et al. (2020). Hypoxic exercise training to improve exercise capacity in obese individuals. Med. Sci. Sports Exerc. 52, 1641–1649. doi:10.1249/MSS.0000000000002322
Chang Y. S., Chu H., Yang C. Y., Tsai J. C., Chung M. H., Liao Y. M., et al. (2015). The efficacy of music therapy for people with dementia: a meta-analysis of randomised controlled trials. J. Clin. Nurs. 24, 3425–3440. doi:10.1111/jocn.12976
Chen S., Su H., Liu X., Li Q., Yao Y., Cai J., et al. (2022). Effects of exercise training in hypoxia versus normoxia on fat-reducing in overweight and/or obese adults: a systematic review and meta-analysis of randomized clinical trials. Front. Physiology 13, 940749. doi:10.3389/fphys.2022.940749
Chen S. M., Lin H. Y., Kuo C. H. (2013). Altitude training improves glycemic control. Chin. J. Physiol. 56, 193–198. doi:10.4077/cjp.2013.Bab130
Chiu Y.-F., Hsu C.-C., Chiu T. H., Lee C.-Y., Liu T.-T., Tsao C. K., et al. (2015). Cross-sectional and longitudinal comparisons of metabolic profiles between vegetarian and non-vegetarian subjects: a matched cohort study. Br. J. Nutr. 114, 1313–1320. doi:10.1017/S0007114515002937
Collaboration N. R. F. (2016). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. lancet 387, 1377–1396. doi:10.1016/S0140-6736.(16).30054-X
De Groote E., Britto F. A., Bullock L., François M., De Buck C., Nielens H., et al. (2018). Hypoxic training improves normoxic glucose tolerance in adolescents with obesity. Med. Sci. Sports Exerc 50, 2200–2208. doi:10.1249/MSS.0000000000001694
Dragano N. R., Fernø J., Diéguez C., López M., Milbank E. (2020). Recent updates on obesity treatments: available drugs and future directions. Neuroscience 437, 215–239. doi:10.1016/j.neuroscience.2020.04.034
Faulhaber M., Schneider S., Rausch L. K., Dünnwald T., Menz V., Gatterer H., et al. (2023). Repeated short-term bouts of hyperoxia improve aerobic performance in acute hypoxia. J. Strength and Cond. Res. 37, 2016–2022. doi:10.1519/JSC.0000000000004502
Feng X., Zhao L., Chen Y., Wang Z., Lu H., Wang C. (2023). Optimal type and dose of hypoxic training for improving maximal aerobic capacity in athletes: a systematic review and Bayesian model-based network meta-analysis. Front. Physiology 14, 1223037. doi:10.3389/fphys.2023.1223037
Fernández Menéndez A., Saudan G., Sperisen L., Hans D., Saubade M., Millet G. P., et al. (2018). Effects of short-term normobaric hypoxic walking training on energetics and mechanics of gait in adults with obesity. Obes. (Silver Spring) 26, 819–827. doi:10.1002/oby.22131
Fu L., Li X. (2022). Effects of hypoxic training on body composition and aerobic endurance in obese population. Sci. and Technol. Station. and Sporting Goods 487, 92–94. doi:10.3969/j.issn.1006-8902.2022.06.033
Gao H., Xu J., Zhang L., Lu Y., Gao B., Feng L. (2020). Effects of living high-training low and high on body composition and metabolic risk markers in overweight and obese females. BioMed Res. Int. 2020, 3279710. doi:10.1155/2020/3279710
Gatterer H., Haacke S., Burtscher M., Faulhaber M., Melmer A., Ebenbichler C., et al. (2015). Normobaric intermittent hypoxia over 8 Months does not reduce body weight and metabolic risk factors--a randomized, single blind, placebo-controlled study in normobaric hypoxia and normobaric sham hypoxia. Obes. Facts 8, 200–209. doi:10.1159/000431157
Ghaith A., Chacaroun S., Borowik A., Chatel L., Doutreleau S., Wuyam B., et al. (2022). Hypoxic high-intensity interval training in individuals with overweight and obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 323, R700–r709. doi:10.1152/ajpregu.00049.2022
Guo H., Cheng L., Duolikun D., Yao Q. (2023). Aerobic exercise training under normobaric hypoxic conditions to improve glucose and lipid metabolism in overweight and obese individuals: a systematic review and meta-analysis. High. Alt. Med. Biol. 24, 312–320. doi:10.1089/ham.2022.0099
Gutwenger I., Hofer G., Gutwenger A. K., Sandri M., Wiedermann C. J. (2015). Pilot study on the effects of a 2-week hiking vacation at moderate versus low altitude on plasma parameters of carbohydrate and lipid metabolism in patients with metabolic syndrome. BMC Res. Notes 8, 103. doi:10.1186/s13104-015-1066-3
Han X. (2020). Hypoxia moderate-intensity exercise improves exercise capacity and anti-fatigue index in obese people. Genomics Appl. Biol. 39, 3847–3853. doi:10.13417/j.gab.039.003847
Heymsfield S. B., Wadden T. A. (2017). Mechanisms, pathophysiology, and management of obesity. N. Engl. J. Med. 376, 254–266. doi:10.1056/NEJMra1514009
Hobbins L., Hunter S., Gaoua N., Girard O. (2021). Short-term perceptually regulated interval-walk training in hypoxia and normoxia in overweight-to-obese adults. J. Sports Sci. Med. 20, 45–51. doi:10.52082/jssm.2021.45
Huan X., Feng L., Xu J., Lu Y. (2008). Changes of serum hormone related to body WeightRegulation during hypoxic training. CHINA SPORT Sci. 28, 39–46+72. doi:10.16469/j.css.2008.06.005
Jin X., Qiu T., Li L., Yu R., Chen X., Li C., et al. (2023). Pathophysiology of obesity and its associated diseases. Acta Pharm. Sin. B 13, 2403–2424. doi:10.1016/j.apsb.2023.01.012
Jung K., Kim J., Park H. Y., Jung W. S., Lim K. (2020). Hypoxic pilates intervention for obesity: a randomized controlled trial. Int. J. Environ. Res. Public Health 17, 7186. doi:10.3390/ijerph17197186
Kercher V. M., Kercher K., Bennion T., Yates B. A., Feito Y., Alexander C., et al. (2021). Fitness trends from around the globe. ACSM's Health and Fit. J. 25, 20–31. doi:10.1249/fit.0000000000000639
Klug L., Mähler A., Rakova N., Mai K., Schulz-Menger J., Rahn G., et al. (2018). Normobaric hypoxic conditioning in men with metabolic syndrome. Physiol. Rep. 6, e13949. doi:10.14814/phy2.13949
Kong Z., Shi Q., Nie J., Tong T. K., Song L., Yi L., et al. (2017). High-intensity interval training in normobaric hypoxia improves cardiorespiratory fitness in overweight Chinese young women. Front. physiology 8, 175. doi:10.3389/fphys.2017.00175
Kong Z., Zang Y., Hu Y. (2014). Normobaric hypoxia training causes more weight loss than normoxia training after a 4-week residential camp for obese young adults. Sleep Breath. 18, 591–597. doi:10.1007/s11325-013-0922-4
Li J., Feng L., Zhang L., Gao B., Xu J. (2014). Effects of altitude or hypoxia training on weight loss and blood glucose metabolism in obese adolescents. Chin. J. Sports Med. 33, 460–464. doi:10.16038/j.1000-6710.2014.05.005
Ma L. (2020). Strength training in hypoxia increases antioxidant capacity by upregulating HIF-1α. Genomics Appl. Biol. 39, 3425–3440. doi:10.13417/j.gab.039.000270
Mai K., Klug L., Rakova N., Piper S. K., Mähler A., Bobbert T., et al. (2020). Hypoxia and exercise interactions on skeletal muscle insulin sensitivity in obese subjects with metabolic syndrome: results of a randomized controlled trial. Int. J. Obes. (Lond) 44, 1119–1128. doi:10.1038/s41366-019-0504-z
Marso S. P., Mehta S. K., Frutkin A., House J. A., Mccrary J. R., Kulkarni K. R. (2008). Low adiponectin levels are associated with atherogenic dyslipidemia and lipid-rich plaque in nondiabetic coronary arteries. Diabetes Care 31, 989–994. doi:10.2337/dc07-2024
Millet G. P., Debevec T., Brocherie F., Malatesta D., Girard O. (2016). Therapeutic use of exercising in hypoxia: promises and limitations. Front. Physiol. 7, 224. doi:10.3389/fphys.2016.00224
Morishima T., Kurihara T., Hamaoka T., Goto K. (2014). Whole body, regional fat accumulation, and appetite-related hormonal response after hypoxic training. Clin. Physiol. Funct. Imaging 34, 90–97. doi:10.1111/cpf.12069
Mujika I., Sharma A. P., Stellingwerff T. (2019). Contemporary periodization of altitude training for elite endurance athletes: a narrative review. Sports Med. 49, 1651–1669. doi:10.1007/s40279-019-01165-y
Netzer N. C., Chytra R., Küpper T. (2008). Low intense physical exercise in normobaric hypoxia leads to more weight loss in obese people than low intense physical exercise in normobaric sham hypoxia. Sleep Breath. 12, 129–134. doi:10.1007/s11325-007-0149-3
Netzer N. C., Strohl K. P., Högel J., Gatterer H., Schilz R. (2017). Right ventricle dimensions and function in response to acute hypoxia in healthy human subjects. Acta physiol. 219, 478–485. doi:10.1111/apha.12740
Newsome A. N. M., Batrakoulis A., Camhi S. M., Mcavoy C. R., Sansone J., Reed R. (2024). 2025 ACSM worldwide fitness trends: future directions of the health and fitness industry. ACSM'S Health and Fit. J. doi:10.1249/FIT.0000000000001017
Nishiwaki M., Kawakami R., Saito K., Tamaki H., Ogita F. (2016). The effects of exercise training under mild hypoxic conditions on body composition and circulating adiponectin in postmenopausal women. Clin. Physiol. Funct. Imaging 36, 468–475. doi:10.1111/cpf.12252
Ounis O. B., Elloumi M., Amri M., Zbidi A., Tabka Z., Lac G. (2008). Impact of diet, exercise end diet combined with exercise programs on plasma lipoprotein and adiponectin levels in obese girls. J. sports Sci. and Med. 7, 437–445.
Page M. J., Mckenzie J. E., Bossuyt P. M., Boutron I., Hoffmann T. C., Mulrow C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj 372, n71. doi:10.1136/bmj.n71
Park H. Y., Hwang H., Park J., Lee S., Lim K. (2016). The effects of altitude/hypoxic training on oxygen delivery capacity of the blood and aerobic exercise capacity in elite athletes - a meta-analysis. J. Exerc Nutr. Biochem. 20, 15–22. doi:10.20463/jenb.2016.03.20.1.3
Park H. Y., Jung W. S., Kim J., Lim K. (2019). Twelve weeks of exercise modality in hypoxia enhances health-related function in obese older Korean men: a randomized controlled trial. Geriatr. Gerontol. Int. 19, 311–316. doi:10.1111/ggi.13625
Park H.-Y., Lim K. (2017). The effects of aerobic exercise at hypoxic condition during 6 Weeks on body composition, blood pressure, arterial stiffness, and blood lipid level in obese women. Int. J. Sports Sci. 1, 1–5.
Park W., Park H. Y., Kim S. W. (2024). Effects of 12 Weeks of combined exercise training in normobaric hypoxia on arterial stiffness, inflammatory biomarkers, and red blood cell hemorheological function in obese older women. Healthc. (Basel) 12, 1887. doi:10.3390/healthcare12181887
Ramos-Campo D. J., Girard O., Pérez A., Rubio-Arias J. (2019). Additive stress of normobaric hypoxic conditioning to improve body mass loss and cardiometabolic markers in individuals with overweight or obesity: a systematic review and meta-analysis. Physiol. Behav. 207, 28–40. doi:10.1016/j.physbeh.2019.04.027
Romijn J. A., Coyle E. F., Sidossis L. S., Gastaldelli A., Horowitz J. F., Endert E., et al. (1993). Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol. 265, E380–E391. doi:10.1152/ajpendo.1993.265.3.E380
Ruggiero L., Harrison S. W. D., Rice C. L., Mcneil C. J. (2022). Neuromuscular fatigability at high altitude: lowlanders with acute and chronic exposure, and native highlanders. Acta Physiol. (Oxf) 234, e13788. doi:10.1111/apha.13788
Shin S., Matsuoka T., So W.-Y. (2018). Influences of short-term normobaric hypoxic training on metabolic syndrome-related markers in overweight and normal-weight men. J. Men's Health 14, e44–e52. doi:10.22374/1875-6859.14.1.5
Tan J. T., Prosser H. C., Dunn L. L., Vanags L. Z., Ridiandries A., Tsatralis T., et al. (2016). High-density lipoproteins rescue diabetes-impaired angiogenesis via scavenger receptor class B type I. Diabetes 65, 3091–3103. doi:10.2337/db15-1668
Tee C. C. L., Cooke M. B., Chong M. C., Yeo W. K., Camera D. M. (2023). Mechanisms for combined hypoxic conditioning and divergent exercise modes to regulate inflammation, body composition, appetite, and blood glucose homeostasis in overweight and obese adults: a narrative review. Sports Med. 53, 327–348. doi:10.1007/s40279-022-01782-0
Vogt M., Puntschart A., Geiser J., Zuleger C., Billeter R., Hoppeler H. (2001). Molecular adaptations in human skeletal muscle to endurance training under simulated hypoxic conditions. J. Appl. Physiol. 91, 173–182. doi:10.1152/jappl.2001.91.1.173
Wang H., Sun Z. (2019). Effects of hypoxic training on body composition and blood lipid in obese people: a meta analysis. J. Environ. Occup. Med. 36, 157–163+69. doi:10.13213/j.cnki.jeom.2019.18435
Wang N., Hu Y., Guan Y., Zhao H. (2012). Effects of 4-week hypoxic exercise combined with diet on body weight, blood lipid and insulin resistance of obese adults. Chin. J. Sports Med. 31, 289–294. doi:10.16038/j.1000-6710.2012.04.013
Wang R., Wang H., Xu Y., Zhang Y., Maboundakounga P. R., Chen P. (2013). Effects of“Living high-training Low”on male/female obese adolescent's morphological indices and glucose/lipid metabolism. J. Beijing Sport Univ. 36, 81–87. doi:10.19582/j.cnki.11-3785/g8.2013.09.015
Wang Y., Zhao L., Gao L., Pan A., Xue H. (2021). Health policy and public health implications of obesity in China. lancet Diabetes and Endocrinol. 9, 446–461. doi:10.1016/S2213-8587(21)00118-2
Wang Y. C., Mcpherson K., Marsh T., Gortmaker S. L., Brown M. (2011). Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 378, 815–825. doi:10.1016/S0140-6736.(11).60814-3
Weng X., Lin W., Huang L., Xu G., Yu Q. (2006). Discussion on principle and application of hypoxic body exercise. China Sport Sci. Technolocy, 96–100. doi:10.16470/j.csst.2006.05.023
Wiesner S., Haufe S., Engeli S., Mutschler H., Haas U., Luft F. C., et al. (2010). Influences of normobaric hypoxia training on physical fitness and metabolic risk markers in overweight to obese subjects. Obesity 18, 116–120. doi:10.1038/oby.2009.193
Workman C., Basset F. A. (2012). Post-metabolic response to passive normobaric hypoxic exposure in sedendary overweight males: a pilot study. Nutr. and metabolism 9, 103–109. doi:10.1186/1743-7075-9-103
Xie C., Yagai T., Luo Y., Liang X., Chen T., Wang Q., et al. (2017). Activation of intestinal hypoxia-inducible factor 2α during obesity contributes to hepatic steatosis. Nat. Med. 23, 1298–1308. doi:10.1038/nm.4412
Yan P. (2020). Study on the molecular mechanism of hypoxic and medium-intensity training to improve body composition and lipid metabolism in obese. Genomics Appl. Biol. 39, 3777–3785. doi:10.13417/j.gab.039.003777
Yang Q., Huang G., Tian Q., Liu W., Sun X., Li N., et al. (2018). “Living High-Training Low” improved weight loss and glucagon-like peptide-1 level in a 4-week weight loss program in adolescents with obesity: a pilot study. Medicine 97, e9943. doi:10.1097/MD.0000000000009943
Yang X., He W., Shi D., He Y., Hu Y. (2014). The effects of hypoxic exercise on the energy intake, body composition and lipid metabolism in overweight and obese young men. Chin. J. Sports Med. 33, 638–645. doi:10.16038/j.1000-6710.2014.07.025
Yingzhong Y., Droma Y., Rili G., Kubo K. (2006). Regulation of body weight by leptin, with special reference to hypoxia-induced regulation. Intern Med. 45, 941–946. doi:10.2169/internalmedicine.45.1733
Zhang N. (2019). Effects of hypoxia and moderate intensity training therapy on body composition and blood lipid metabolism in obese subjects. Genomics Appl. Biol. 38, 5657–5663. doi:10.13417/j.gab.038.005657
Zhao S., Shi H. (2016). Effects of hypoxic training on body composition and endurance capacity of obese students. Chin. J. Sch. Health 37, 1637–1640. doi:10.16835/j.cnki.1000-9817.2016.11.012
Keywords: hypoxia training, obesity, body composition, metabolism, meta analysis
Citation: Liu P, Chen H, Jiang X and Diaz-Cidoncha Garcia J (2025) Impact of exercise training in a hypobaric/normobaric hypoxic environment on body composition and glycolipid metabolism in individuals with overweight or obesity: a systematic review and meta-analysis. Front. Physiol. 16:1571730. doi: 10.3389/fphys.2025.1571730
Received: 06 February 2025; Accepted: 17 February 2025;
Published: 10 March 2025.
Edited by:
Rodrigo Iturriaga, Universidad Autonoma de Chile, ChileReviewed by:
Alexios Batrakoulis, Democritus University of Thrace, GreeceCopyright © 2025 Liu, Chen, Jiang and Diaz-Cidoncha Garcia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Jiang, bmVudWVkdTIwMDZAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.