
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 15 January 2025
Sec. Experimental Pharmacology and Drug Discovery
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1521188
Introduction: Resveratrol, a polyphenolic compound commonly found in natural plants and fruits, exhibits potential in preventing optic nerve damage in glaucoma, as indicated by several animal studies. However, there is presently a dearth of relevant evidence available for comprehensive summarization.
Methods: In this study, we conducted an extensive search across 7 electronic databases, encompassing all pertinent animal studies for a systematic review and meta-analysis. Methodological quality was evaluated using SYRCLE’s bias risk tool, with statistical analysis performed using Stata 17.0. The primary outcome measures included the survival of retinal ganglion cells and retinal thickness.
Results: The comprehensive analysis of the 30 included studies revealed that resveratrol can enhance the expression of Sirtuin 1(SIRT1) protein in retinal tissue (SMD: 3.00, 95% CI: 2.46, 3.53, P = 0.095), boost the survival rate of retinal ganglion cells (SMD: 4.33, 95% CI: 3.28, 5.38, P < 0.05), decelerate the thinning of retinal thickness (SMD: 4.26, 95% CI: 2.77, 5.75, P < 0.05), and enhance visual function. Its potential mechanism of action may involve the suppression of pro-inflammatory cytokine levels and cell apoptosis.
Discussion: Resveratrol emerges as a promising agent for mitigating glaucoma-related retinal damage. However, given that the animal research models utilized in the study may not fully reflect the intricate scenarios of multiple coexisting diseases in clinical settings, and the administration methods in animal models may differ from those in clinical practice, future studies should aim to provide higher levels of evidence to facilitate the clinical translation of these findings.
Systematic Review Registration:: identifier [CRD42024535673].
Glaucoma is an ocular condition characterized by the pathological elevation of intraocular pressure, leading to mechanical compression, optic nerve ischemia, and subsequent optic nerve damage along with visual field defects. With a blindness rate ranging from approximately 5%–20%, glaucoma stands as the second most prevalent cause of blindness in ophthalmology, following cataracts (Bourne et al., 2018). Research data indicates that a minimum of 80 million individuals globally are afflicted by glaucoma, and projections suggest that the number of patients is poised to surpass 110 million by the year 2040 (Liu et al., 2023; Tham et al., 2014). A healthcare cost research report revealed that glaucoma had a heavy disease burden (Prager et al., 2016). Glaucoma has imposed a substantial economic burden of $2.9 billion on the US economy (Thomas et al., 2015), highlighting its significance as a medical burden not only within the US but also globally (Barayev et al., 2021; Patel et al., 2021). Due to its high incidence rate, elevated blindness prevalence, and substantial economic impact, glaucoma has emerged as a noteworthy public health concern deserving attention.
The pathogenesis of glaucoma is intricate, with a prevailing belief that alterations in mechanical pressure and disruptions in blood flow regulation play significant roles (Flammer et al., 2002; Baudouin et al., 2021). Increased intraocular pressure or other vascular risk factors that potentially decrease ocular blood flow can result in inadequate blood delivery to the optic nerve. The cascade of ischemia and hypoxia can induce harm to retinal neurons, particularly axonal degeneration and diminished retinal ganglion cell (RGC) counts, culminating in irreversible impairment of visual function (Li et al., 2018; Liu et al., 2019). The activation of inflammatory responses and cell apoptosis within retinal tissue is intricately linked to RGC damage. In the compromised retina, the interplay of inflammatory cytokines such as iNOS (Liu and Neufeld, 2003; Shareef et al., 1999), COX-2 (Deng et al., 2020), IL-6 (Hu et al., 2021), and IL-1β (Chen et al., 2020; Coyle et al., 2021) instigates the immune-inflammatory response in the glaucomatous retina and triggers apoptosis of RGC cells. Studies indicate that in a mouse model of acute intraocular hypertension, retinal nerve cells undergo progressive apoptosis (Zhou et al., 2019), with aberrant expression levels of caspase-3, Bax, and Bcl-2 potentially serving as pivotal factors in RGC apoptosis (Quigley et al., 1995; Risner et al., 2022; Zalewska et al., 2004; Phatak et al., 2016). Among the various risk factors contributing to glaucoma damage, elevated intraocular pressure stands out as the modifiable factor (Fernandez-Albarral et al., 2024). As a result, the primary approach to treating glaucoma involves medication or surgical interventions aimed at lowering intraocular pressure. Nevertheless, the reduction of intraocular pressure alone may not entirely halt the progression of glaucoma or prevent damage to RGCs (Zeng et al., 2023). Retinal ischemia-reperfusion injury could be a crucial factor influencing treatment efficacy (Flammer et al., 2002; Goldblum and Mittag, 2002). Under the combined influence of various pathological mechanisms during ischemia-reperfusion, RGCs may experience cell death, morphological degeneration, and functional loss, ultimately resulting in vision impairment (Kim et al., 2013). Given the complexity of the underlying mechanisms, effective treatment methods are currently lacking. It is imperative to discover safe and efficient treatment strategies to enhance the survival rate of RGCs and safeguard the visual function of individuals with glaucoma.
Resveratrol (C14H12O3, Figure 1), a natural polyphenol abundant in diverse plants like grapes, possesses antioxidant, anti-inflammatory, and neuroprotective characteristics. It has found extensive application in various eye-related disorders (Lancon et al., 2016; Bryl et al., 2022; Delmas et al., 2021). One potential mechanism of resveratrol-mediated optic nerve protection entails the activation of silent information regulator factor 1 (SIRT1) (Pallas et al., 2009), a protein extensively distributed in the nucleus and cytoplasm of the retina. SIRT1 plays a pivotal role in modulating the activity of various transcription factors and co-factors, influencing the expression of downstream genes, and regulating associated physiological and pathological processes (Zhou et al., 2018). Studies have demonstrated that the upregulation of SIRT1 exerts a protective effect against various eye diseases (Zhou et al., 2018; Shindler et al., 2007), and resveratrol can offer retinal neuroprotection in acute glaucoma models through the activation of SIRT1 (Chen et al., 2013; Luo et al., 2020; Luna et al., 2009). While certain preclinical studies have indicated favorable outcomes of resveratrol in ameliorating retinal ischemic injury in animal models of glaucoma, there remains a shortage of high-quality evidence validating resveratrol’s ability to confer neuroprotective effects in such models. Further exploration is warranted to elucidate the protective mechanism of resveratrol on the optic nerve in glaucoma.
Preclinical animal experiments serve as a vital connection in scientific research, bridging findings from the molecular and cellular levels to clinical trial investigations. Regrettably, discrepancies in animal modeling and other facets of preclinical studies have hindered the optimistic clinical translation of their results. Previous preclinical inquiries into the neuroprotective impacts of resveratrol in glaucoma models have exhibited notable variations in reported outcomes stemming from differences in modeling techniques and assessment criteria. This study aims to gather pertinent animal research, conduct meta-analyses of animal experiments, systematically assess the protective efficacy of resveratrol in glaucoma animal models, and appraise the potential clinical translational applications of resveratrol.
This study adhered to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines and was registered with PROSPERO (CRD42024535673).
We conducted a search of electronic databases from their inception up to 30 April 2024, which included Web of Science, Embase, PubMed, China Biomedical Database (CBM), China National Knowledge Infrastructure (CNKI), Wanfang Database (WF), and China Science Journal Database (VIP). Additionally, we will explore potential eligible articles from the reference lists of retrieved papers to mitigate any potential gaps in the research literature. Search keywords comprised a combination of MeSH terms and free-text words (Supplementary Table 1).
According to the PICO principle, the studies included must meet the following criteria: (1) Population: Animal models of glaucoma-induced retinal injury, without restrictions on animal species, gender, age, or weight. (2) Intervention: Administration of resveratrol treatment, with no limitations on dosage, duration, or frequency. (3) Comparison: The control group should receive an equivalent carrier, physiological saline, or no treatment. (4) Outcome: Evaluation of the protective effects of resveratrol on the retina using outcome measures such as RGC survival rate, RGC apoptosis rate, visual function, retinal thickness, and retinal damage.
Exclusion criteria: (1) Clinical, in vitro, and computer simulation studies. (2) Studies where the control or treatment groups received a combination of resveratrol and other treatments. (3) Duplicate publications; in case of data duplication, the latest data will be retained. (4) Animal studies where experimental data cannot be retrieved.
The literature search was independently conducted by two researchers. Initially, irrelevant literature was excluded based on titles and abstracts. Subsequently, studies meeting the inclusion criteria were selected by reviewing the full text, and the following information was extracted: (1) Publication details including author(s) and publication year; (2) Details regarding animal species, gender, age, weight, and sample size; (3) Methods employed for establishing glaucoma models and administering anesthesia; (4) Information on the timing, dosage, route of administration, and control method of resveratrol; (5) Outcome measures. In cases where results are graphically presented, attempts were made to contact the corresponding authors of the research to obtain raw data. If unsuccessful, the data were processed using WebPlotDigitizer 4.5 (https://automeris.io/) to extract quantitative data from the graphs. When a study encompasses various doses and time points of administration, yielding multiple datasets for outcome indicators, the selection process for meta-analysis typically involves choosing the most efficacious dose or time point data. However, during dose-response analysis, data extraction entails utilizing information from distinct dose groups for comprehensive analysis. Any discrepancies in the data extraction procedure should be reconciled through consultation with a third researcher.
Two assessors independently assessed and graded the included studies using the SYRCLE tool for evaluating bias in animal studies (Hooijmans et al., 2014). The types of biases considered encompass selection bias, performance bias, detection bias, attrition bias, reporting bias, and other potential biases. Assessment outcomes were categorized as “yes” for low risk of bias, “no” for high risk of bias, and “uncertain” for unclear risk of bias.
Statistical analysis was performed using STATA software version 17.0. The outcome measures, represented as continuous variables, were compared by reporting the standard mean deviation (SMD) and 95% confidence interval (CI) for overall effect sizes. A significance level of p < 0.05 denoted statistical significance. Heterogeneity within the study was assessed using I2 values. A fixed-effects model was employed when I2 was 50% or less, while I2 values exceeding 50% indicated significant heterogeneity, warranting sensitivity, and subgroup analyses to investigate potential sources of heterogeneity. In cases where significant heterogeneity persisted unresolved, a random-effects model was utilized. Publication bias was evaluated using Egger’s linear regression analysis and Begg’s rank correlation analysis. If publication bias was detected, trimming and filling methods were employed to address it.
A total of 871 potentially relevant articles were retrieved from seven electronic databases: PubMed (112), Embase (378), Web of Science (167), CNKI (36), CBM (78), WF (43), and VIP (57). After removing duplicates, 549 articles remained. Subsequently, 446 irrelevant articles were excluded based on title and abstract reviews. Upon full-text assessment, 73 more articles were excluded, resulting in the inclusion of 30 articles. The research selection process is illustrated in Figure 2.
Out of 30 studies, 21 studies visually depicted their findings graphically, with 9 of these studies acquiring raw data directly from the corresponding author or first author. Furthermore, in 12 studies, two researchers used WebLotDigitizer 4.5 to extract data and took the average for meta-analysis. The 30 included studies involved a total of 559 animals, with 280 in the treatment group and 279 in the control group. Among these, 14 studies utilized 286 Sprague Dawley rats, 12 studies involved 211 C57BL/6J mice, 2 studies utilized 42 Wistar rats, 1 study employed 10 brown rats, and another study used 10 Agouti rats. Male animals were used in 24 studies, while 2 studies involved both male and female animals, and 4 studies did not specify the gender. The age of the animals was provided in 25 studies, and the weight was specified in 16 studies. Regarding the administration of resveratrol, 7 studies employed intravitreal injection, 17 studies utilized intraperitoneal injection, 4 studies employed oral or gavage administration, 1 study used eye drops, and 1 study did not specify the injection method. Concerning outcome measures, 23 studies reported on the survival or death of RGCs, 8 studies have reported the transcription factor Brn3 protein (Brn3a) that can be used to label and quantify RGCs (Nadal-Nicolas et al., 2009), 13 studies described changes in retinal thickness, 14 studies reported on apoptosis-related indicators of retinal tissue, 6 studies reported on A and B waves of electroretinography (ERG), 10 studies reported on inflammatory cytokine levels in local retinal tissue, and 11 studies reported on SIRT1 levels. Detailed characteristics of the included studies are presented in Table 1.
According to Figure 3, the quality assessment of the 30 studies was conducted using SYRCLE’s risk of bias tool, with 2 studies scoring 4 points (Xiong, 2021; Xia et al., 2020), 19 studies scored 5 points (Deng et al., 2020; Chronopoulos et al., 2023; Luo et al., 2018; Pang et al., 2020; Cao et al., 2020; Vin et al., 2013; Feng et al., 2024; Wu et al., 2020; Zhang et al., 2018; Ji et al., 2024; Chang et al., 2018; Liu, 2013; Chen, 2018; Li, 2012; Ji, 2022; Ji, 2016; He et al., 2021; Shamsher et al., 2022; Zhu et al., 2018),7 studies scored 6 points (Seong et al., 2017; Xie et al., 2023; Prasetya et al., 2023; Zhao et al., 2022; Seong et al., 2022; Luo, 2020; Academic Exchange Conference, 2016),2 studies scored 7 points (Pirhan et al., 2016; Guowu et al., 2020). Among the 30 included studies, only 4 studies mentioned random grouping, 25 studies detailed baseline characteristics, 11 studies outlined animal random housing, all random outcome assessments of the studies were rated as low-risk, but only 1 study documented the blinding of outcome assessors, and no study elucidated how blinding was implemented in allocation concealment and experimentalists. All studies were deemed low-risk in terms of incomplete outcome data, selective outcome reporting, and other sources of bias. The comprehensive evaluation results are presented in Supplementary Table 2.
In the included studies, a total of 23 investigations assessed the impact of resveratrol on RGCs in glaucoma models. Meta-analyses involving 19 studies indicated that resveratrol intervention significantly enhanced the survival rate of RGCs under elevated intraocular pressure [n = 231, SMD: 4.33 (95% CI: 3.28, 5.38), p < 0.05; heterogeneity: I2 = 76.5%, p < 0.05, Figure 4A]. Moreover, meta-analyses of 8 studies demonstrated that resveratrol could decrease the number of RGC deaths [n = 147, SMD: −3.86 (95% CI: −5.28, −2.44), p < 0.05; heterogeneity: I2 = 82.7%, p < 0.05, Figure 4B]. A meta-analysis of 7 studies revealed that resveratrol intervention led to an increase in Brn3a-labeled RGCs compared to the control group [n = 80, SMD: 3.57 (95% CI: 1.79, 5.36), p < 0.05; heterogeneity: I2 = 82.0%, p < 0.05, Figure 4C].
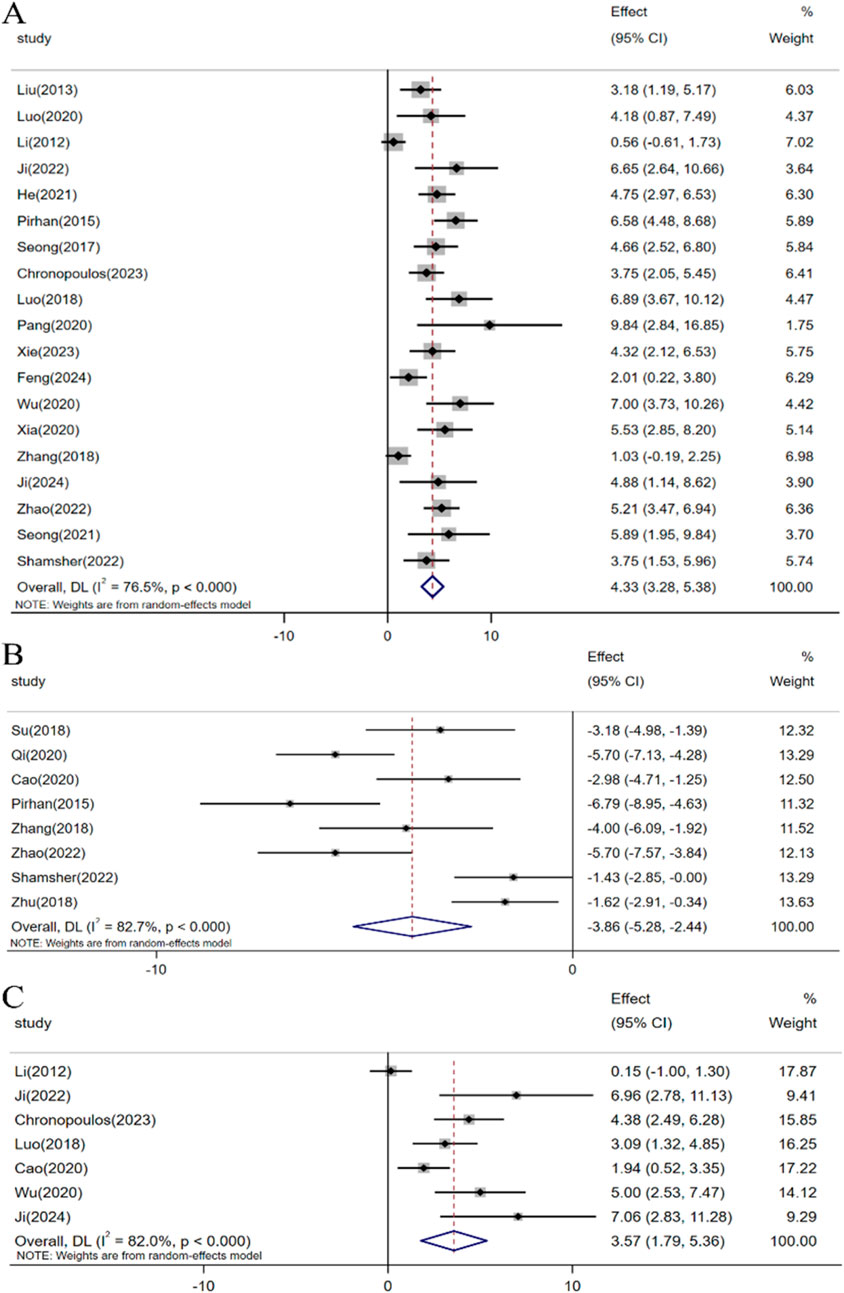
Figure 4. Forest plot: effect of resveratrol on (A) Survival status of RGC, (B) Death status of RGC, (C) Brn3a.
A meta-analysis of 12 studies revealed that resveratrol, when compared to the control group, can ameliorate retinal thickness thinning in high intraocular pressure conditions [n = 138, SMD: 4.26 (95% CI: 2.77, 5.75), p < 0.05; heterogeneity: I2 = 82.4%, p < 0.05, Figure 5].
Based on the dark adaptation flash ERG data included in the study, a meta-analysis of 4 studies indicated that resveratrol can increase the A-wave amplitude [n = 38, SMD: 3.98 (95% CI: 2.76, 5.20), p < 0.05; heterogeneity: I2 = 0.0%, p = 0.438, Figure 6A]. Similarly, a meta-analysis of 7 studies demonstrated that resveratrol can also elevate the B-wave amplitude [n = 76, SMD: 4.79 (95% CI: 2.84, 6.74), p < 0.05; heterogeneity: I2 = 76.3%, p < 0.05, Figure 6B]. Consequently, compared with the control group, resveratrol can increase the amplitude of “a” and “b” waves in ERG.
Incorporating data from 11 studies that assessed SIRT1 protein expression levels, the collective meta-analysis demonstrated that resveratrol can enhance the upregulation of SIRT1 protein expression [n = 136, SMD: 3.00 (95% CI: 2.46, 3.53), p < 0.05; heterogeneity: I2 = 38.1%, p = 0.095, Figure 7].
A meta-analysis of 4 studies indicated that resveratrol can decrease the level of the inflammatory cytokine iNOS [n = 36, SMD: −3.65 (95% CI: −4.84, −2.46), p < 0.05; Heterogeneity: I2 = 5.8%, p = 0.364, Figure 8A]. Another meta-analysis involving 3 studies demonstrated that resveratrol can lower the level of the inflammatory factor COX-2 [n = 26, SMD: −5.18 (95% CI: −8.33, −2.02), p < 0.05; Heterogeneity: I2 = 54%, p = 0.114, Figure 8B]. Similarly, a meta-analysis of 3 studies revealed that resveratrol can decrease the level of the inflammatory factor IL-6 [n = 38, SMD: −3.01 (95% CI: −4.01, −2.01), p < 0.05; Heterogeneity: I2 = 34.1%, p = 0.214, Figure 8C]. Lastly, an analysis of 3 studies found that resveratrol can reduce the level of the inflammatory factor IL-1β [n = 39, SMD: −2.24 (95% CI: −3.09, −1.40), p < 0.05; Heterogeneity: I2 = 0.0%, p = 0.488, Figure 8D].
In the evaluation of 6 studies, the combined meta-analysis revealed that resveratrol can induce a reduction in the expression level of Caspase-3 protein in rat retinal tissue [n = 68, SMD: −3.14 (95% CI: −3.91, −2.36), p < 0.05; Heterogeneity: I2 = 23.3%, p = 0.259, Figure 9A]. Furthermore, meta-analysis results from 7 studies illustrated that resveratrol can facilitate an increase in Bcl-2 protein expression levels in retinal tissue [n = 108, SMD: 3.58 (95% CI: 1.65, 5.51), p < 0.05; heterogeneity: I2 = 88.3%, p < 0.05, Figure 9B]. Concurrently, the meta-analysis findings from 7 studies indicated that resveratrol can impede the upregulation of Bax protein expression in retinal tissue [n = 106, SMD: −4.17 (95% CI: −6.18, −2.15), p < 0.05; heterogeneity: I2 = 86.3%, p < 0.05, Figure 9C].
In our analysis of the main outcome measures with significant heterogeneity, we systematically assessed the impact of each study’s exclusion on the combined effects related to RGC survival, RGC death, Brn3a, and retinal thickness. Notably, after excluding data from Pirhan (2015) and Zhang (2018), the range of combined effect sizes for RGC survival spanned from 4.17 (95% CI: 3.12, 5.21) to 4.55 (95% CI: 3.51, 5.58). Similarly, upon excluding data from Pirhan (2015) and Shamsher (2022), the combined effect sizes for RGC mortality varied between −3.47 (95% CI: −4.87, −2.08) and −4.22 (95% CI: −5.68, −2.76). Following the exclusion of Ji (2024) and Li (2012), the combined effect sizes for Brn3a ranged from 3.19 (95% CI: 1.40, 4.98) to 4.09 (95% CI: 2.60, 5.78). Moreover, after excluding data from Vin (2013) and Li (2012), the combined effect sizes for retinal thickness varied from 3.97 (95% CI: 2.49, 5.44) to 4.70 (95% CI: 3.15, 6.25).
Given the substantial heterogeneity observed between studies, we conducted subgroup analyses on RGC survival, RGC mortality, Brn3a, and retinal thickness, focusing on modeling methods, animal species, administration methods, and dosages. Our findings suggest that administration methods, dosages, and timing could potentially contribute to the observed heterogeneity in RGC survival. For RGC mortality, our analysis indicates that animal species, administration methods, and dosages may serve as possible sources of heterogeneity. Regarding Brn3a, the dosage and timing of administration are identified as potential sources of heterogeneity. Lastly, for retinal thickness, animal species and administration time are highlighted as potential sources of heterogeneity. The detailed results are presented in the attached Supplementary Table 3.
We utilized Egger’s test and Begg’s test to assess the potential publication bias in studies concerning RGC survival, RGC mortality, Brn3a, and retinal thickness. The findings revealed statistically significant publication bias for RGC survival, Brn3a, and retinal thickness, while no statistically significant publication bias was detected for RGC mortality (Supplementary Figure 1). Furthermore, employing pruning and filling methods, we conducted statistical analyses on studies that might have overlooked aspects related to RGC survival, Brn3a, and retinal thickness. The results suggest that the absence of certain research data does not significantly impact the overall consolidation effect, as depicted in Table 2 and Figure 10.
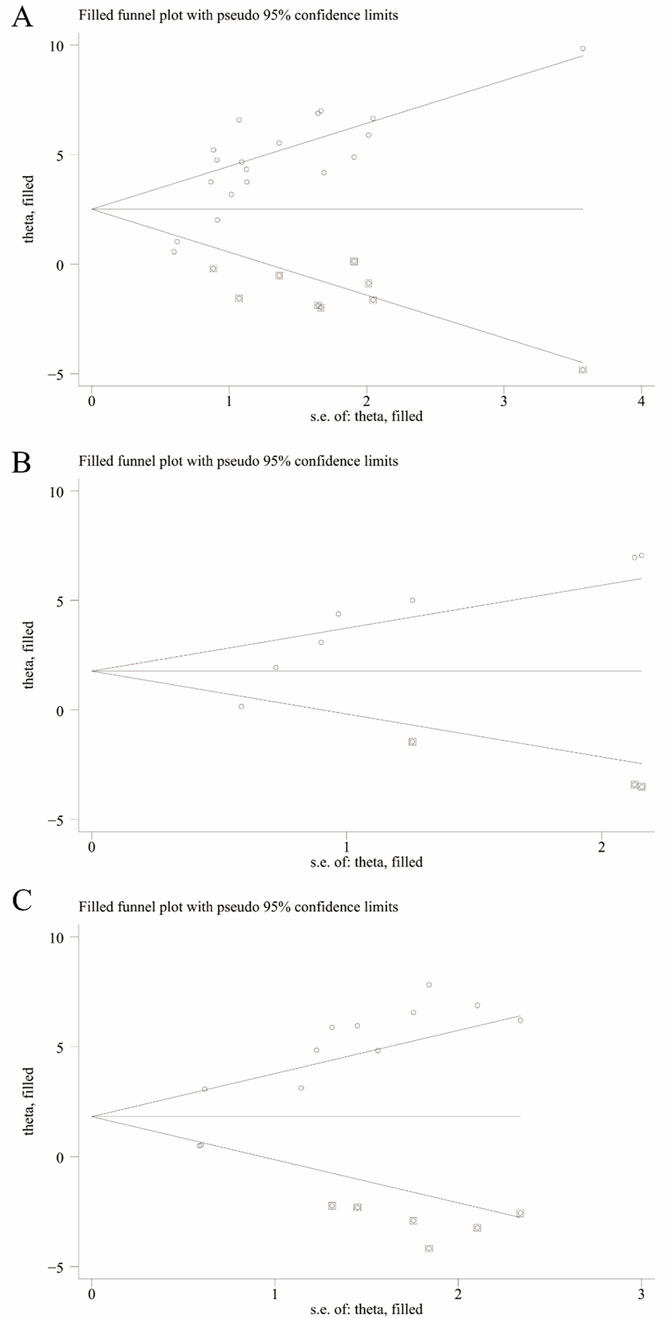
Figure 10. Trim-and-fill analysis for (A) Survival status of RGC, (B) Brn3a, and (C) Retinal thickness.
In our study, data from a total of 23 studies were analyzed concerning the dosage of resveratrol and its impact on RGC survival. Three studies were excluded from the analysis: one due to unclear dosing information and four due to the use of inconsistent dosage units. Among the remaining 18 studies, doses were administered in mg/kg, with 5 studies employing varying doses of resveratrol. Subsequently, the total dosage of resveratrol was categorized into 7 intervention methods. The research outcomes presented in Figure 11 suggest a non-linear correlation between resveratrol dosage and RGC survival. Notably, the maximum effect was observed when the total dose of resveratrol ranged between 160–240 mg/kg.
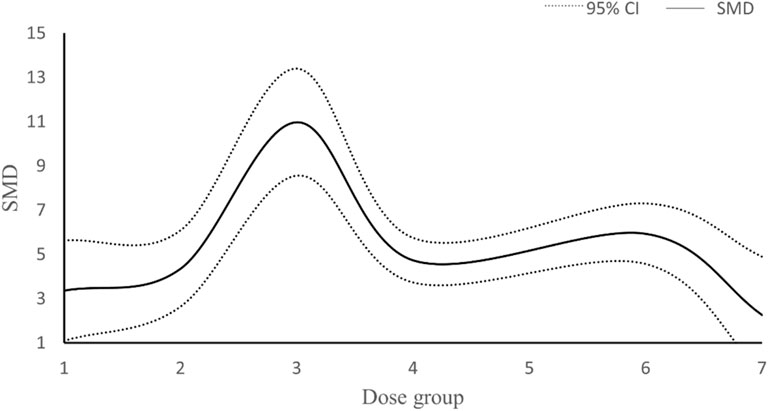
Figure 11. Dose-response curve of resveratrol in improving survival status of RGC. Dose group: (1) dose≤80 mg/kg, (2) 80<dose≤160 mg/kg, (3) 160<dose≤ 240 mg/kg, (4) 240<dose≤ 320 mg/kg, (5) 320<dose≤400 mg/kg, (6) 400<dose≤480 mg/kg, (7) >480dose mg/kg.
After synthesizing and evaluating data from 30 preclinical studies, our meta-analysis indicates that resveratrol exhibits promising therapeutic potential in the management of glaucoma-related retinal injuries. Notably, resveratrol demonstrates a protective effect by modulating key outcome measures in glaucoma retinal injury models. This includes enhancing the survival rate of RGCs, reducing the mortality rate of these cells, boosting Bra3a expression, and curbing retinal thinning. Furthermore, resveratrol shows efficacy in reversing retinal dysfunction induced by elevated intraocular pressure, as evidenced by increased A-wave and B-wave amplitudes. However, our meta-analysis uncovered significant heterogeneity in the primary indicators of RGC survival, RGC mortality, Bra3a expression, and retinal thickness. Despite conducting sensitivity analyses, the specific sources of this heterogeneity remained elusive. Subgroup analyses suggest that variations in animal species, administration methods, dosage, and timing of administration may contribute to this heterogeneity. To address potential publication biases in the assessment of RGC survival, Bra3a expression, and retinal thickness, we employed pruning and imputation techniques to estimate missing studies and data. The analysis indicates that publication bias does not significantly impact the robustness of our findings.
In glaucoma, optic nerve damage primarily manifests as structural alterations in the optic nerve head and progressive loss of RGC axons, resulting in distinct visual field deficits and impaired vision. A relatively recent discovery, the SIRT1 protein, is associated with ocular diseases, including those affecting the eyes (Wu et al., 2020). It plays a role in cellular stress response and cell survival (Porcu and Chiarugi, 2005). Upregulation of SIRT1 exhibits a protective effect against various ocular diseases (Zhou et al., 2018). Upon optic nerve damage, SIRT1 activation can enhance the survival of RGCs and mitigate inflammatory responses (Zuo et al., 2013; O'Neill et al., 2024). Its mechanism of action may involve the increased expression of the mitochondrial enzyme succinate dehydrogenase and the promotion of deacetylation of PGC-1α, a coenzyme crucial in mitochondrial function (Chen et al., 2013; O'Neill et al., 2024). Resveratrol, a natural polyphenolic compound known for boosting SIRT1 activity, can provide neuroprotection to RGCs in retinal IR injury, with this protective effect being attenuated by SIRT1 inhibitors (Luo et al., 2020). The meta-analysis comprising 11 studies revealed that resveratrol can enhance the expression of SIRT1 protein, thereby exerting a neuroprotective impact on the optic nerve.
The neuroprotective effects of resveratrol could also be associated with various other factors. Optic nerve damage in glaucoma involves multiple intertwined mechanisms such as inflammation and cellular apoptosis. Within the injured retina, pathogenic and reparative processes coexist during the inflammatory cascade. A controlled level of inflammatory response plays a pivotal role in preserving the retinal and neighboring environment’s homeostasis. However, an excessive inflammatory response can trigger a cascade of irreversible degenerative conditions including optic nerve damage and RGC demise (Baudouin et al., 2021). Key cytokines that regulate the inflammatory response could potentially mitigate optic nerve damage and prevent the loss of RGCs linked to glaucoma (Adornetto et al., 2019). According to our meta-analysis findings, resveratrol demonstrates the ability to diminish inflammatory cytokines like iNOS, COX-2, IL-6, and IL-1β in a model of glaucoma-related retinal injury. Inhibiting or decreasing the expression of associated inflammatory factors may attenuate RGC loss and confer a protective effect on the optic nerve in glaucoma (Neufeld, 2004; Brust et al., 2008; Song et al., 2018).
Apoptosis of RGC cells significantly contributes to the pathological alterations observed in glaucoma1924. By inhibiting cell apoptosis, it is possible to mitigate RGC loss and potentially salvage retinal nerve damage in glaucoma (Xia and Zhang, 2024; Chitranshi et al., 2023). Our meta-analysis findings indicate that resveratrol can increase the expression of the anti-apoptotic factor Bcl-2 while decreasing the expression of pro-apoptotic factors Bcl-2 and caspase-3. Prior research has illustrated that in models of retinal ischemia-reperfusion injury induced by elevated intraocular pressure, there is a rise in caspase-3 expression levels, leading to neuronal cell death in the retina via both exogenous and endogenous pathways (Katai and Yoshimura, 1999). Similarly, investigations have revealed a stronger expression of the pro-apoptotic Bax protein in the optic nerve axons of glaucoma patients in comparison to the anti-apoptotic Bcl-2 protein (Zalewska et al., 2004). In animal models, Bax can induce dendritic degeneration in RGCs (Risner et al., 2022) and plays a crucial role in RGC apoptosis (Libby et al., 2005). Upregulation of anti-apoptotic proteins such as Bcl-2 and downregulation of pro-apoptotic proteins such as Bax can exert a protective effect on the optic nerve (Phatak et al., 2016; Maes et al., 2017).
In conclusion, our study indicates that resveratrol may increase the expression of SIRT1 protein, decrease pro-inflammatory cytokine levels, enhance anti-apoptotic factors, and suppress pro-apoptotic factors. Nonetheless, additional research is warranted to elucidate whether resveratrol is involved in the comprehensive regulation of anti-inflammatory responses, cell death mechanisms, and other pathways.
Due to inherent methodological disparities, it is essential to exercise caution when extrapolating research findings from animal studies to human diseases. Although we strive to mitigate bias and enhance research accuracy by amalgamating data from multiple studies, it is important to recognize the unavoidable limitations that may impact the reliability of our results. Primarily, the variances in the animal models we incorporated possess discrepancies in replicating the extent of glaucoma-related retinal damage realistically. The absence of common clinical comorbidities like aging and diabetes could constrain the generalizability of our findings. Moreover, in instances where original research data were unattainable, a collaborative approach involving two researchers was adopted to extract data using graphic processing tools, subsequently calculating the average value for meta-analysis. However, it is important to acknowledge that this methodology may introduce inherent measurement biases. Additionally, the studies we integrated exhibited notable heterogeneity. Despite our efforts to explore potential sources of heterogeneity through sensitivity and subgroup analyses, differences in experimental design and research quality persist. Thus, for future investigations, the selection of animal models more reflective of clinical scenarios, stringent experimental protocols, and standardized methodologies are imperative to enhance research quality and facilitate clinical translation. Significantly, although we performed a basic dose-response analysis, more comprehensive pharmacological investigations are essential for the development of resveratrol as a viable drug, given the current gaps in pharmacokinetic and pharmacodynamic data.
Resveratrol has demonstrated protective effects on RGCs, retarding retinal thinning, and enhancing visual function in animal models of glaucoma and retinal injury. The protective mechanisms of resveratrol are likely linked to its activation of SIRT1, anti-inflammatory properties, and anti-apoptotic effects. Thus, resveratrol holds promise as a potential therapeutic agent for shielding against glaucoma-related retinal damage. However, further robust evidence is necessary in the future to facilitate its clinical translation and application.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
FZ: Conceptualization, Investigation, Methodology, Resources, Validation, Visualization, Writing–original draft, Writing–review and editing. TL: Conceptualization, Investigation, Methodology, Resources, Validation, Visualization, Writing–original draft, Writing–review and editing. JW: Investigation, Methodology, Validation, Writing–original draft. LW: Investigation, Methodology, Validation, Writing–original draft. WG: Investigation, Methodology, Validation, Writing–original draft. YH: Investigation, Methodology, Validation, Writing–review and editing. HW: Data curation, Formal Analysis, Methodology, Project administration, Software, Supervision, Visualization, Writing–review and editing. WB: Conceptualization, Project administration, Supervision, Writing–original draft, Writing–review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1521188/full#supplementary-material
RGC, retinal ganglion cell; SIRT1, silent information regulator factor 1; Brn3a, transcription factor Brn3 protein; ERG, electroretinography; IOP, intraocular pressure.
Academic Exchange Conference (2016). “Protective effect of the SIRT1 activator resveratrol on experimental glaucomatous retinas,” in The 13th Jiangxi Provincial Integrated Traditional Chinese and Western Medicine Ophthalmology Academic Exchange Conference and the 8th Academic Conference of the Ganzhou Medical Association Ophthalmology Society, Ganzhou, Jiangxi, China.
Adornetto, A., Russo, R., and Parisi, V. (2019). Neuroinflammation as a target for glaucoma therapy. Neural Regen. Res. 14 (3), 391–394. doi:10.4103/1673-5374.245465
Barayev, E., Geffen, N., Nahum, Y., and Gershoni, A. (2021). Changes in prices and eye-care providers prescribing patterns of glaucoma medications in the United States between 2013 and 2019. J. Glaucoma 30 (3), e83–e89. doi:10.1097/IJG.0000000000001728
Baudouin, C., Kolko, M., Melik-Parsadaniantz, S., and Messmer, E. M. (2021). Inflammation in Glaucoma: from the back to the front of the eye, and beyond. Prog. Retin Eye Res. 83, 100916. doi:10.1016/j.preteyeres.2020.100916
Bourne, R., Jonas, J. B., Bron, A. M., Cicinelli, M. V., Das, A., Flaxman, S. R., et al. (2018). Prevalence and causes of vision loss in high-income countries and in Eastern and Central Europe in 2015: magnitude, temporal trends and projections. Br. J. Ophthalmol. 102 (5), 575–585. doi:10.1136/bjophthalmol-2017-311258
Brust, A. K., Ulbrich, H. K., Seigel, G. M., Pfeiffer, N., and Grus, F. H. (2008). Effects of cyclooxygenase inhibitors on apoptotic neuroretinal cells. Biomark. Insights 3, 387–402. doi:10.4137/bmi.s692
Bryl, A., Falkowski, M., Zorena, K., and Mrugacz, M. (2022). The role of resveratrol in eye diseases-A review of the literature. Nutrients 14 (14), 2974. doi:10.3390/nu14142974
Cao, K., Ishida, T., Fang, Y., Shinohara, K., Li, X., Nagaoka, N., et al. (2020). Protection of the retinal ganglion cells: intravitreal injection of resveratrol in mouse model of ocular hypertension. Invest Ophthalmol. Vis. Sci. 61 (3), 13. doi:10.1167/iovs.61.3.13
Chang, S. U., Haisheng, Z., Shuling, G., Ruifeng, S., Xiaoxiao, F., Xiaobo, T., et al. (2018). Effects of resveratrol on expressions of Caspase-3 and Bcl-2 in retina tissue of rats with retinal ischemia-reperfusion injury. J. Jilin Univ. Med. Ed. 44 (2), 321–326. doi:10.13481/j.1671-587x.20180221
Chen, H., Deng, Y., Gan, X., Li, Y., Huang, W., Lu, L., et al. (2020). NLRP12 collaborates with NLRP3 and NLRC4 to promote pyroptosis inducing ganglion cell death of acute glaucoma. Mol. Neurodegener. 15 (1), 26. doi:10.1186/s13024-020-00372-w
Chen, S. (2018). Protective effects of resveratrol on retinal ischemia-reperfusion injury in rats. Nanchang University. Master’s thesis.
Chen, S., Fan, Q., Li, A., Liao, D., Ge, J., Laties, A. M., et al. (2013). Dynamic mobilization of PGC-1α mediates mitochondrial biogenesis for the protection of RGC-5 cells by resveratrol during serum deprivation. Apoptosis 18 (7), 786–799. doi:10.1007/s10495-013-0837-3
Chitranshi, N., Rajput, R., Godinez, A., Pushpitha, K., Mirzaei, M., Basavarajappa, D., et al. (2023). Neuroserpin gene therapy inhibits retinal ganglion cell apoptosis and promotes functional preservation in glaucoma. Mol. Ther. 31 (7), 2056–2076. doi:10.1016/j.ymthe.2023.03.008
Chronopoulos, P., Manicam, C., Zadeh, J. K., Laspas, P., Unkrig, J. C., Göbel, M. L., et al. (2023). Effects of resveratrol on vascular function in retinal ischemia-reperfusion injury. ANTIOXIDANTS 12 (4), 853. doi:10.3390/antiox12040853
Coyle, S., Khan, M. N., Chemaly, M., Callaghan, B., Doyle, C., Willoughby, C. E., et al. (2021). Targeting the NLRP3 inflammasome in glaucoma. Biomolecules 11 (8), 1239. doi:10.3390/biom11081239
Delmas, D., Cornebise, C., Courtaut, F., Xiao, J., and Aires, V. (2021). New Highlights of resveratrol: a review of properties against ocular diseases. Int. J. Mol. Sci. 22 (3), 1295. doi:10.3390/ijms22031295
Deng, C., Chen, S., Li, X., Luo, H., Zhang, Q., Hu, P., et al. (2020). Role of the PGE2 receptor in ischemia-reperfusion injury of the rat retina. Mol. Vis. 26, 36–47.
Feng, J. Z., Ji, K. B., Pan, Y. J., Huang, P., and Xing, Y. (2024). Resveratrol ameliorates retinal ischemia-reperfusion injury by modulating the NLRP3 inflammasome and keap1/Nrf2/HO-1 signaling pathway. Mol. Neurobiol. 61, 8454–8466. doi:10.1007/s12035-024-04105-8
Fernandez-Albarral, J. A., Ramirez, A. I., de Hoz, R., Matamoros, J. A., Salobrar-García, E., Elvira-Hurtado, L., et al. (2024). Glaucoma: from pathogenic mechanisms to retinal glial cell response to damage. Front. Cell Neurosci. 18, 1354569. doi:10.3389/fncel.2024.1354569
Flammer, J., Orgul, S., Costa, V. P., Orzalesi, N., Krieglstein, G. K., Serra, L. M., et al. (2002). The impact of ocular blood flow in glaucoma. Prog. Retin Eye Res. 21 (4), 359–393. doi:10.1016/s1350-9462(02)00008-3
Goldblum, D., and Mittag, T. (2002). Prospects for relevant glaucoma models with retinal ganglion cell damage in the rodent eye. Vis. Res. 42 (4), 471–478. doi:10.1016/s0042-6989(01)00194-8
Guowu, Q. I., Jingmei, H., Yiying, Y., Lin, Z., and Haiyan, W. (2020). Protective effect of resveratrol on optic nerve and its effect on the expression of Bcl-2, Bax and caspase-9 in rats with chronic glaucoma. China Hosp. Pharm. Eval. Analysis 20 (11), 1324–1328. doi:10.14009/j.issn.1672-2124.2020.11.011
He, L., Jiao, Y., Ma, G., Li, Y., and Li, X. (2021). Protective effect and mechanism of resveratrol on optic nerve injury in glaucoma rats. Int. J. Ophthalmol-Chi 21 (1), 27–31. doi:10.3980/j.issn.1672-5123.2021.1.05
Hooijmans, C. R., Rovers, M. M., de Vries, R. B., Leenaars, M., Ritskes-Hoitinga, M., and Langendam, M. W. (2014). SYRCLE's risk of bias tool for animal studies. Bmc Med. Res. Methodol. 14, 43. doi:10.1186/1471-2288-14-43
Hu, X., Zhao, G. L., Xu, M. X., Zhou, H., Li, F., Miao, Y., et al. (2021). Interplay between Muller cells and microglia aggravates retinal inflammatory response in experimental glaucoma. J. Neuroinflammation 18 (1), 303. doi:10.1186/s12974-021-02366-x
Ji, K. (2016). Changes in the expression of Sirtuins in the retina and optic nerve before and after acute retinal ischemia in rats, and the regulation of mitochondrial dynamics by resveratrol-mediated SIRT1 activation. Nanchang University. Doctoral dissertation.
Ji, K. (2022). Protective effects and mechanisms of resveratrol on retinal ganglion cells in murine retinal ischemic injury. Wuhan University. Doctoral dissertation.
Ji, K. B., Wan, W., Yang, Y., He, X. J., Xing, Y. Q., and Hu, Z. (2024). Ameliorative effect of resveratrol on acute ocular hypertension induced retinal injury through the SIRT1/NF-κB pathway. Neurosci. Lett. 826, 137712. doi:10.1016/j.neulet.2024.137712
Katai, N., and Yoshimura, N. (1999). Apoptotic retinal neuronal death by ischemia-reperfusion is executed by two distinct caspase family proteases. Invest Ophthalmol. Vis. Sci. 40 (11), 2697–2705.
Kim, B. J., Braun, T. A., Wordinger, R. J., and Clark, A. F. (2013). Progressive morphological changes and impaired retinal function associated with temporal regulation of gene expression after retinal ischemia/reperfusion injury in mice. Mol. Neurodegener. 8, 21. doi:10.1186/1750-1326-8-21
Lancon, A., Frazzi, R., and Latruffe, N. (2016). Anti-oxidant, anti-inflammatory and anti-angiogenic properties of resveratrol in ocular diseases. Molecules 21 (3), 304. doi:10.3390/molecules21030304
Li, C. (2012). The role of endoplasmic reticulum stress in retinal ischemia-reperfusion injury. Wuhan University. Doctoral dissertation.
Li, N., Wang, F., Zhang, Q., Jin, M., Lu, Y., Chen, S., et al. (2018). Rapamycin mediates mTOR signaling in reactive astrocytes and reduces retinal ganglion cell loss. Exp. Eye Res. 176, 10–19. doi:10.1016/j.exer.2018.06.014
Libby, R. T., Li, Y., Savinova, O. V., Barter, J., Smith, R. S., Nickells, R. W., et al. (2005). Susceptibility to neurodegeneration in a glaucoma is modified by Bax gene dosage. Plos Genet. 1 (1), 17–26. doi:10.1371/journal.pgen.0010004
Liu, B., and Neufeld, A. H. (2003). Activation of epidermal growth factor receptor signals induction of nitric oxide synthase-2 in human optic nerve head astrocytes in glaucomatous optic neuropathy. Neurobiol. Dis. 13 (2), 109–123. doi:10.1016/s0969-9961(03)00010-x
Liu, W., Guo, R., Huang, D., Ji, J., Gansevoort, R. T., Snieder, H., et al. (2023). Co-occurrence of chronic kidney disease and glaucoma: epidemiology and etiological mechanisms. Surv. Ophthalmol. 68 (1), 1–16. doi:10.1016/j.survophthal.2022.09.001
Liu, W., Xia, F., Ha, Y., Zhu, S., Li, Y., Folorunso, O., et al. (2019). Neuroprotective effects of HSF1 in retinal ischemia-reperfusion injury. Invest Ophthalmol. Vis. Sci. 60 (4), 965–977. doi:10.1167/iovs.18-26216
Liu, X. (2013). Neuroprotective effects and mechanisms of resveratrol on retinal ischemia-reperfusion injury in rats. Shandong University. Master’s thesis.
Luna, C., Li, G., Liton, P. B., Qiu, J., Epstein, D. L., Challa, P., et al. (2009). Resveratrol prevents the expression of glaucoma markers induced by chronic oxidative stress in trabecular meshwork cells. Food Chem. Toxicol. 47 (1), 198–204. doi:10.1016/j.fct.2008.10.029
Luo, H., Zhuang, J., Hu, P., Ye, W., Chen, S., Pang, Y., et al. (2018). Resveratrol delays retinal ganglion cell loss and attenuates gliosis-related inflammation from ischemia-reperfusion injury. Invest Ophthalmol. Vis. Sci. 59 (10), 3879–3888. doi:10.1167/iovs.18-23806
Luo, J. (2020). Study on the role and mechanism of resveratrol and SIRT1 in retinal ischemia-reperfusion injury in mice. Wuhan University. Doctoral dissertation.
Luo, J., He, T., Yang, J., Yang, N., Li, Z., and Xing, Y. (2020). SIRT1 is required for the neuroprotection of resveratrol on retinal ganglion cells after retinal ischemia-reperfusion injury in mice. Graefes Arch. Clin. Exp. Ophthalmol. 258 (2), 335–344. doi:10.1007/s00417-019-04580-z
Maes, M. E., Schlamp, C. L., and Nickells, R. W. (2017). BAX to basics: how the BCL2 gene family controls the death of retinal ganglion cells. Prog. Retin Eye Res. 57, 1–25. doi:10.1016/j.preteyeres.2017.01.002
Nadal-Nicolas, F. M., Jimenez-Lopez, M., Sobrado-Calvo, P., Nieto-López, L., Cánovas-Martínez, I., Salinas-Navarro, M., et al. (2009). Brn3a as a marker of retinal ganglion cells: qualitative and quantitative time course studies in naive and optic nerve-injured retinas. Invest Ophthalmol. Vis. Sci. 50 (8), 3860–3868. doi:10.1167/iovs.08-3267
Neufeld, A. H. (2004). Pharmacologic neuroprotection with an inhibitor of nitric oxide synthase for the treatment of glaucoma. Brain Res. Bull. 62 (6), 455–459. doi:10.1016/j.brainresbull.2003.07.005
O'Neill, N., Meng, M., Chaqour, B., Dine, K., Sarabu, N., Pham, J. C., et al. (2024). Comparison of SNCG and NEFH promoter-driven expression of human SIRT1 expression in a mouse model of glaucoma. Transl. Vis. Sci. Technol. 13 (8), 37. doi:10.1167/tvst.13.8.37
Pallas, M., Casadesus, G., Smith, M. A., Coto-Montes, A., Pelegri, C., Vilaplana, J., et al. (2009). Resveratrol and neurodegenerative diseases: activation of SIRT1 as the potential pathway towards neuroprotection. Curr. Neurovasc Res. 6 (1), 70–81. doi:10.2174/156720209787466019
Pang, Y., Qin, M., Hu, P., Ji, K., Xiao, R., Sun, N., et al. (2020). Resveratrol protects retinal ganglion cells against ischemia induced damage by increasing Opa1 expression. Int. J. Mol. Med. 46 (5), 1707–1720. doi:10.3892/ijmm.2020.4711
Patel, A. R., Schwartz, G. F., Campbell, J. H., Chen, C. C., McGuiness, C. B., Multani, J. K., et al. (2021). Economic and clinical burden associated with intensification of glaucoma topical therapy: a US claims-based analysis. J. Glaucoma 30 (3), 242–250. doi:10.1097/IJG.0000000000001730
Phatak, N. R., Stankowska, D. L., and Krishnamoorthy, R. R. (2016). Bcl-2, Bcl-xL, and p-AKT are involved in neuroprotective effects of transcription factor Brn3b in an ocular hypertension rat model of glaucoma. Mol. Vis. 22, 1048–1061.
Pirhan, D., Yüksel, N., Emre, E., Cengiz, A., and Kürşat, Y. D. (2016). Riluzole- and resveratrol-induced delay of retinal ganglion cell death in an experimental model of glaucoma. Curr. Eye Res. 41 (1), 59–69. doi:10.3109/02713683.2015.1004719
Porcu, M., and Chiarugi, A. (2005). The emerging therapeutic potential of sirtuin-interacting drugs: from cell death to lifespan extension. Trends Pharmacol. Sci. 26 (2), 94–103. doi:10.1016/j.tips.2004.12.009
Prager, A. J., Liebmann, J. M., Cioffi, G. A., and Blumberg, D. M. (2016). Self-reported function, health resource use, and total health care costs among medicare beneficiaries with glaucoma. Jama Ophthalmol. 134 (4), 357–365. doi:10.1001/jamaophthalmol.2015.5479
Prasetya, A. S., Komaratih, E., Sasono, W., Chrysanti, M., Niken Larasati, M. D., and Sudiana, I. K. (2023). Intravitreal resveratrol as anti apoptotic agent against retinal ganglion cell loss in ischemic reperfusion injury. Pharmacogn. J. 15 (6), 1207–1212. doi:10.5530/pj.2023.15.219
Quigley, H. A., Nickells, R. W., Kerrigan, L. A., Pease, M. E., Thibault, D. J., and Zack, D. J. (1995). Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest Ophthalmol. Vis. Sci. 36 (5), 774–786.
Risner, M. L., Pasini, S., McGrady, N. R., and Calkins, D. J. (2022). Bax contributes to retinal ganglion cell dendritic degeneration during glaucoma. Mol. Neurobiol. 59 (3), 1366–1380. doi:10.1007/s12035-021-02675-5
Seong, H., Jeong, J. Y., Ryu, J., Park, J., Han, Y. S., Cho, H. K., et al. (2022). Resveratrol prevents hypoxia-induced retinal ganglion cell death related with ErbB2. Int. J. Ophthalmol-Chi 15 (3), 394–400. doi:10.18240/ijo.2022.03.04
Seong, H., Ryu, J., Yoo, W. S., Kim, S. J., Han, Y. S., Park, J. M., et al. (2017). Resveratrol ameliorates retinal ischemia/reperfusion injury in C57BL/6J mice via downregulation of caspase-3. Curr. Eye Res. 42 (12), 1650–1658. doi:10.1080/02713683.2017.1344713
Shamsher, E., Guo, L., Davis, B. M., Luong, V., Ravindran, N., Somavarapu, S., et al. (2022). Resveratrol nanoparticles reduce retinal ganglion cell loss in glaucoma. Acta Ophthalmol. 100. doi:10.1111/j.1755-3768.2022.115
Shareef, S., Sawada, A., and Neufeld, A. H. (1999). Isoforms of nitric oxide synthase in the optic nerves of rat eyes with chronic moderately elevated intraocular pressure. Invest Ophthalmol. Vis. Sci. 40 (12), 2884–2891.
Shindler, K. S., Ventura, E., Rex, T. S., Elliott, P., and Rostami, A. (2007). SIRT1 activation confers neuroprotection in experimental optic neuritis. Invest Ophthalmol. Vis. Sci. 48 (8), 3602–3609. doi:10.1167/iovs.07-0131
Song, Y., Song, Q., Li, L., Xu, J., and Liu, X. (2018). Effect of ranibizumab on levels of IL-6 and VEGF in peripheral blood and aqueous humor of glaucoma rat model and association of IL-6 and VEGF with optic nerve damage. Exp. Ther. Med. 16 (3), 2506–2510. doi:10.3892/etm.2018.6441
Tham, Y. C., Li, X., Wong, T. Y., Quigley, H. A., Aung, T., and Cheng, C. Y. (2014). Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 121 (11), 2081–2090. doi:10.1016/j.ophtha.2014.05.013
Thomas, S., Hodge, W., and Malvankar-Mehta, M. (2015). The cost-effectiveness analysis of teleglaucoma screening device. Plos One 10 (9), e0137913. doi:10.1371/journal.pone.0137913
Vin, A. P., Hu, H., Zhai, Y., Von Zee, C. L., Logeman, A., Stubbs, E. B., et al. (2013). Neuroprotective effect of resveratrol prophylaxis on experimental retinal ischemic injury. Exp. Eye Res. 108, 72–75. doi:10.1016/j.exer.2012.11.022
Wu, Y., Pang, Y., Wei, W., Shao, A., Deng, C., Li, X., et al. (2020). Resveratrol protects retinal ganglion cell axons through regulation of the SIRT1-JNK pathway. Exp. Eye Res. 200, 108249. doi:10.1016/j.exer.2020.108249
Xia, J. Y., Yang, X. L., and Chen, W. A. (2020). Resveratrol protects the retina from I/R injury by inhibiting RGCS apoptosis, glial activation and expression of inflammatory factors. Trop. J. Pharm. Res. 19 (6), 1221–1226. doi:10.4314/tjpr.v19i6.16
Xia, Q., and Zhang, D. (2024). Apoptosis in glaucoma: a new direction for the treatment of glaucoma (Review). Mol. Med. Rep. 29 (5), 82. doi:10.3892/mmr.2024.13207
Xie, Z., Ying, Q., Luo, H., Qin, M., Pang, Y., Hu, H., et al. (2023). Resveratrol alleviates retinal ischemia-reperfusion injury by inhibiting the NLRP3/gasdermin D/Caspase-1/Interleukin-1β pyroptosis pathway. Invest Ophthalmol. Vis. Sci. 64 (15), 28. doi:10.1167/iovs.64.15.28
Xiong, Y. L. Q. (2021). Protective effects of resveratrol on the retina of glaucomatous rats based on the SIRT1 pathway. Northwest Pharm. J. 36 (04), 564–567. doi:10.3969/j.issn.1004-2407.2021.04.010
Zalewska, R., Reszec, J., Mariak, Z., and Sulkowski, S. (2004). The expression of Bcl-2, Bcl-xl, Bak and Bax proteins in axons of the optic nerve in closed-angle glaucoma. Klin. Ocz. 106 (1-2 Suppl. l), 155–157.
Zeng, Z., You, M., Fan, C., Rong, R., Li, H., and Xia, X. (2023). Pathologically high intraocular pressure induces mitochondrial dysfunction through Drp1 and leads to retinal ganglion cell PANoptosis in glaucoma. Redox Biol. 62, 102687. doi:10.1016/j.redox.2023.102687
Zhang, X., Feng, Y., Wang, Y., Wang, J., Xiang, D., Niu, W., et al. (2018). Resveratrol ameliorates disorders of mitochondrial biogenesis and dynamics in a rat chronic ocular hypertension model. Life Sci. 207, 234–245. doi:10.1016/j.lfs.2018.06.010
Zhao, Y. E., Yu, S. P., Huang, Z. Q., Chen, J., Zhang, X., and Qu, C. (2022). Therapeutic effects of sirtuin 1 activator on glaucoma mice and the regulation mechanism of mitogen-activated protein kinase pathway. J. Biomater. Tiss. Eng. 12 (1), 183–191. doi:10.1166/jbt.2022.2874
Zhou, L., Chen, W., Lin, D., Hu, W., and Tang, Z. (2019). Neuronal apoptosis, axon damage and synapse loss occur synchronously in acute ocular hypertension. Exp. Eye Res. 180, 77–85. doi:10.1016/j.exer.2018.12.006
Zhou, M., Liu, Z. K., Ji, K. B., Luo, H. D., Zhang, X., et al. (2001). “Protective effect of the SIRT1 activator resveratrol on experimental glaucomatous retinas,” in The 13th Jiangxi Provincial Integrated Traditional Chinese and Western Medicine Ophthalmology Academic Exchange Conference and the 8th Academic Conference of the Ganzhou Medical Association Ophthalmology Society. (Ganzhou, Jiangxi, China).
Zhou, M., Luo, J., and Zhang, H. (2018). Role of Sirtuin 1 in the pathogenesis of ocular disease (Review). Int. J. Mol. Med. 42 (1), 13–20. doi:10.3892/ijmm.2018.3623
Zhu, M., Choy, B. N. K., and Lai, J. S. M. (2018). Neuro-protective effect of resveratrol on ischemia/reperfusion-Induced Retinal Injury in mice. Investigative Ophthalmol. Vis. Sci. 59 (9).
Keywords: resveratrol, glaucoma, retina, preclinical studies, systematic review and meta-analysis
Citation: Zhang F, Li T, Wan J, Wang L, Guo W, Hu Y, Wang H and Bian W (2025) Protective effect of resveratrol on retinal damage in glaucoma: a systematic review and meta-analysis of preclinical studies. Front. Pharmacol. 15:1521188. doi: 10.3389/fphar.2024.1521188
Received: 01 November 2024; Accepted: 26 December 2024;
Published: 15 January 2025.
Edited by:
Mohammad S. Mubarak, The University of Jordan, JordanReviewed by:
Anand Giddabasappa, Pfizer, United StatesCopyright © 2025 Zhang, Li, Wan, Wang, Guo, Hu, Wang and Bian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Wang, d2FuZ2hhbzQyOTVAZm94bWFpbC5jb20=; Wei Bian, YmlhbndlaUBUbW11LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.