
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 03 January 2025
Sec. Cardiovascular and Smooth Muscle Pharmacology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1514293
This article is part of the Research TopicMendelian Randomization and Cardiovascular RemodelingView all 7 articles
 Qian Guo1†
Qian Guo1† Xinghua Xu2†
Xinghua Xu2† Xiaohui Li3†
Xiaohui Li3† Yang Mao4
Yang Mao4 Shengqiang Li5
Shengqiang Li5 Yuxin Yao3
Yuxin Yao3 Xiang Li3
Xiang Li3 Yaxing Li3
Yaxing Li3 Jiayue Feng6
Jiayue Feng6 Yan Shu6*
Yan Shu6* Xingli Xu3*
Xingli Xu3*Background: Abdominal aortic aneurysm (AAA) is one of the most dangerous types of vascular diseases worldwide. Metabolic disturbance affects disease risk and provide underlying therapeutic targets. Previous studies have reported an association between metabolic disorders and AAA. However, evidence of a causal relationship between blood metabolites and AAA is still lacking at present.
Methods: Using Mendelian randomization (MR), we assessed the causal association between 1,400 serum metabolites and AAA. The inverse variance weighted method (IVW), weighted median, MR-Egger regression, simple mode, as well as weighted mode methods were used for evaluating the causality between blood metabolites and AAA. Pleiotropy and heterogeneity tests were further conducted.
Results: Through strict screening, 17 known metabolites, 7 unknown metabolites and 5 metabolite ratios related to AAA were identified. Among all the metabolites, 24 were found to have negative associations, while 5 exhibited positive associations. The top five metabolites associated with an increased risk of AAA were Oleoyl-linoleoyl-glycerol (18:1/18:2) [2], Glycosyl-N-(2-hydroxynervonoyl)-sphingosine (d18:1/24:1(2OH)), Glycochenodeoxycholate 3-sulfate, X-21441 and X-24328. In contrast, the top five metabolites that were linked to a reduced risk of AAA included Uridine to pseudouridine ratio, Octadecanedioate, Phosphate to oleoyl-linoleoyl-glycerol (18:1 to 18:2) [2] ratio, 1-(1-enyl-palmitoyl)-GPE (p-16:0), and 1-stearoyl-GPG (18:0).
Conclusion: Among the 1,400 blood metabolites, we identified 17 known metabolites, 7 unknown metabolites, and 5 metabolite ratios associated with AAA. This MR study may provide a novel significant insight for the screening and prevention of AAA.
Abdominal aortic aneurysm (AAA) indicates as the maximal localized dilation of the abdominal aorta with the diameter ≥30 mm or 1.5 times greater than normal (Lu et al., 2022). The computed tomography (CT), magnetic resonance imaging (MRI) and ultrasound for aortic imaging are acceptable and reliable methods for early detection of AAA (Sakalihasan et al., 2018). AAA leads to about 200,000 deaths, including 9% in men over 65 years of age each year worldwide. The proven risk factors for AAA include male sex, age, smoking, hypercholesterolemia, hyperlipidemia, and hypertension with high heritability (Sakalihasan et al., 2018). Currently, surgical interventions including open aneurysm repair or endovascular aneurysm repair (EVAR) are limited options for patients with AAA larger than 5.5 cm in diameter (Vanmaele et al., 2024). Its high mortality is mainly due to the clinical lack of reliable and effective drugs treatment. The value of stains, β-blockers, antibiotics, or anti-platelet therapy in reducing the progression of AAA still needs further investigation (Zhang et al., 2024).
Metabolites are intermediates or end products of metabolic reactions. Their levels are affected by genetics, lifestyle, diet, gut microbiota and diseases. Besides, they can further influence disease conditions and being potential therapeutic targets. Recently, several studies have found that the plasma metabolites may be underlying biomarkers to explore the diagnosis and prognosis of AAA, as well as targets for alleviating the pathological progression of AAA (Tian et al., 2022; Benson et al., 2023; Li et al., 2024). Metabolic changes in patients with AAA are primarily related to carbohydrate and lipid metabolism, insulin resistance, energy metabolism, and alterations in amino acid (AA) metabolism (Li et al., 2024; Ling et al., 2022; Lieberg et al., 2021).
Due to hereditary variations in certain metabolite levels, human genetics can be utilized to assess the role of metabolites in disease outcomes. Mendelian randomization (MR) is a causal inference method that uses single nucleotide polymorphisms (SNPs) occurring randomly in the human genome as instrumental variables to test the impact of exposure, such as metabolites on disease outcomes (Wang et al., 2023; Chen et al., 2023). Currently, there is a lack of cohort-based causal studies linking metabolites to AAA. Therefore, this study elucidates changes in metabolite expression levels in AAA and their effects through comprehensive blood metabolomics data collection and MR analysis, providing new reliable targets for AAA diagnosis and treatment.
The complete dataset in this study is publicly accessible from published genome-wide association studies (GWAS) as reported on the database website. Written informed consent was individually obtained from all participants under approval from Institutional Review Boards’ ethics committees. No further ethical approval or informed consent is necessary. We conducted a comprehensive assessment using MR to investigate the relationships between metabolites as exposures and AAA as the outcome. A total of 1,400 metabolite, including 1,091 metabolites and 309 metabolite ratios was incorporated. The clinical diagnosis of AAA was determined as a localized dilatation of the abdominal aorta to a diameter ≥3.0 cm (outer wall to outer wall), as measured by imaging techniques such as using CT, MRI or ultrasound (Lu et al., 2022; Sakalihasan et al., 2018).
Three critical assumptions are necessary for fully consideration during the scientific MR studies. A), Genetic instruments should strongly correlate with the exposure under study. B), Genetic instrumental variables are not influenced by any known or unknown confounding factors and independent of the outcome. C), The instrumental variables should influence the outcome only through their impacts on the exposure of interests. Therefore, this dataset was used to investigate the role of 1,400 metabolites in patients diagnosed with AAA and healthy individuals without AAA using a MR analysis approach. The schematic illustration of this MR study is shown in Figure 1.
The Canadian Longitudinal Study on Aging (CLSA) is a large-scale and long-term research, designed to investigate and track the health status and life transitions in Canada over many years. It aims to collect various aspects of participants’ lives for evaluating the factors that contribute to healthy aging and the development of age-related diseases. Chen et al. performed the GWAS, involving metabolomic data of 1,091 metabolites and 309 metabolite ratios, from a cohort of 8,299 participants belonging to CLSA dataset. The results of this GWAS could be accessible including detailed human plasma metabolomic data from the website http://metabolomics.helmholtzmuenchen.de/gwas/.
The United Kingdom (UK) Biobank is a large-scale biomedical research resource, including around 500,000 participants aged 40–69 years between 2006 and 2010, established in the UK. It concludes detailed health information, lifestyle, donated biological samples, genome-wide genotyping, results of imaging and medical records over a period of decades. We defined AAA in the UK Biobank dataset based on the electronic health recodes (ICD-9/10 diagnosis and hospital procedure codes) from hospital episode statistics and death certificates. Age, sex, principal components and genotyping batch were all adjusted in the analysis. A total of 3,658 patients with AAA and 244,907 controls without AAA was included. The results of this statistics for AAA were obtained from Pan-UK Biobank service https://pan.ukbb.broadinstitute.org/.
In the MR analysis, the core assumptions refer to the fundamental principles that underpin the effectiveness of using genetic variants as proxies for modifiable exposures. Genetic variants as instrumental variables (IV) are associated with the environmental exposure as risk factors, independent of confounding factors common in traditional observational studies. Three core assumptions should be followed in the IV selection to avoid the biased estimates in MRandomization studies, including relevance (instrument-relevance assumption), independence (instrument-independence assumption) and exclusion restriction (no pleiotropy). We established a threshold of p < 5*10–8 of each metabolite for identifying SNPs that exhibit significant association on a genome-wide scale. Pairs of SNPs were considered to exhibit significant linkage disequilibrium if the squared correlation coefficient (r^2) was less than 0.1, and if the SNPs were located within a 500-kilobase (kb) genomic radius (Yang et al., 2020). Additionally, SNPs with an F-statistic below 10 were categorized as weak instruments and underwent rigorous scrutiny to minimize bias arising from weak instrumentality (Choi et al., 2019).
MR sensitivity analysis is a method performed to assess the robustness of causal inference, testing the potential violating assumptions or unmeasured confounding factors such as age, sex, and lifestyle variables. Researchers typically assess these assumptions rigorously through sensitivity analyses and by considering alternative explanations for their findings. Specifically, employing the Inverse Variance Weighted (IVW) method was used for assessing the causal relationship between metabolites and AAA, as the cornerstone of this analysis (Zhao and Liu, 2024). MR-Egger and the Weighted Median (WM) were further performed as the secondary methods of evaluation. First, Cochran’s Q test was conducted using both the IVW and MR-Egger methods to detect potential violations of assumptions due to heterogeneity in IV correlations. Second, the MR-Egger intercept was then utilized to assess pleiotropy, ensuring that genetic variants are independently associated with both metabolites and AAA. Third, we employed WM and Mode-based Estimation to enhance the reliability and stability of our hypothesis testing. Last, individual SNP analyses and leave-one-out (LOO) diagnostics were conducted to assess the robustness of observed associations for each SNP. The MR analysis assumes causality under the condition of genetic correlation between metabolites as exposure and AAA as outcome. To mitigate bias, SNPs associated with aneurysms were carefully selected, excluding those linked to other traits. Nonetheless, SNPs lacking known associations may still influence the incidence of AAA.
MR analyses were conducted using the ‘Two Sample MR’ package in R software (version 4.2.1). Odds ratios (ORs) were utilized to assess the magnitude and direction of the metabolic impact, accompanied by their respective 95% confidence intervals (CIs).
Through strict screening, 17 known metabolites, 7 unknown metabolites and 5 metabolite ratios related to AAA were identified. Among all metabolites, 24 were found to have negative associations, and 5 had positive associations. The top five metabolites that increased the risk of AAA were Oleoyl-linoleoyl-glycerol (18:1/18:2) [2], Glycosyl-N-(2-hydroxynervonoyl)-sphingosine (d18:1/24:1(2OH)), Glycochenodeoxycholate 3-sulfate, X-21441 and X-24328. Conversely, the top five metabolites that decreased the risk of AAA were Uridine to pseudouridine ratio, Octadecanedioate, Phosphate to oleoyl-linoleoyl-glycerol (18:1 to 18:2) [2] ratio, 1-(1-enyl-palmitoyl)-GPE (p-16:0), and 1-stearoyl-GPG (18:0).
Based on the threshold of p < 5*10–8 of each metabolite for identifying SNPs with significant associations on a genome-wide scale, a total of 29 plasma metabolites and metabolite ratios were selected. All computed F-statistics exceeded 10, indicating minimal susceptibility to weak instrument bias. All metabolic analyses utilized the IVW method as the primary approach, demonstrating uniformity and robust instrument strength. After screening for the primary outcomes and pleiotropy, 29 metabolites related to AAA were identified with IVW p < 0.05 and Pleiotropy p > 0.05, including 24 metabolites and 5 metabolite ratios.
Among the 24 metabolites, the chemical properties of 7 metabolites are unknown, while the remaining 17 known metabolites belong to multiple categories, such as lipid metabolism, bile acid metabolism, ketone body metabolism, and sphingolipid metabolism. Remarkably, lipids metabolism constitutes the most prevalent category, comprising approximately 60% of the identified substances. Within the group of 22 metabolites and metabolite ratios, only 3 known metabolites show a positive association with AAA, while the other 14 metabolites and 5 metabolite ratios are negative associated with the condition. The IVW forest plot depicting the association of 29 significantly associated metabolites and metabolite ratios is shown in Figure 2. The bubble plot is further performed to represent visually the relationships between the metabolites and metabolite rations and AAA as shown in Figure 3. Detailed results of alternative MR analyses, Q-tests, and sensitivity analyses for the 29 identified metabolites are provided in Table 1. All instrumental variables (IVs) passed rigorous sensitivity tests (p > 0.05).
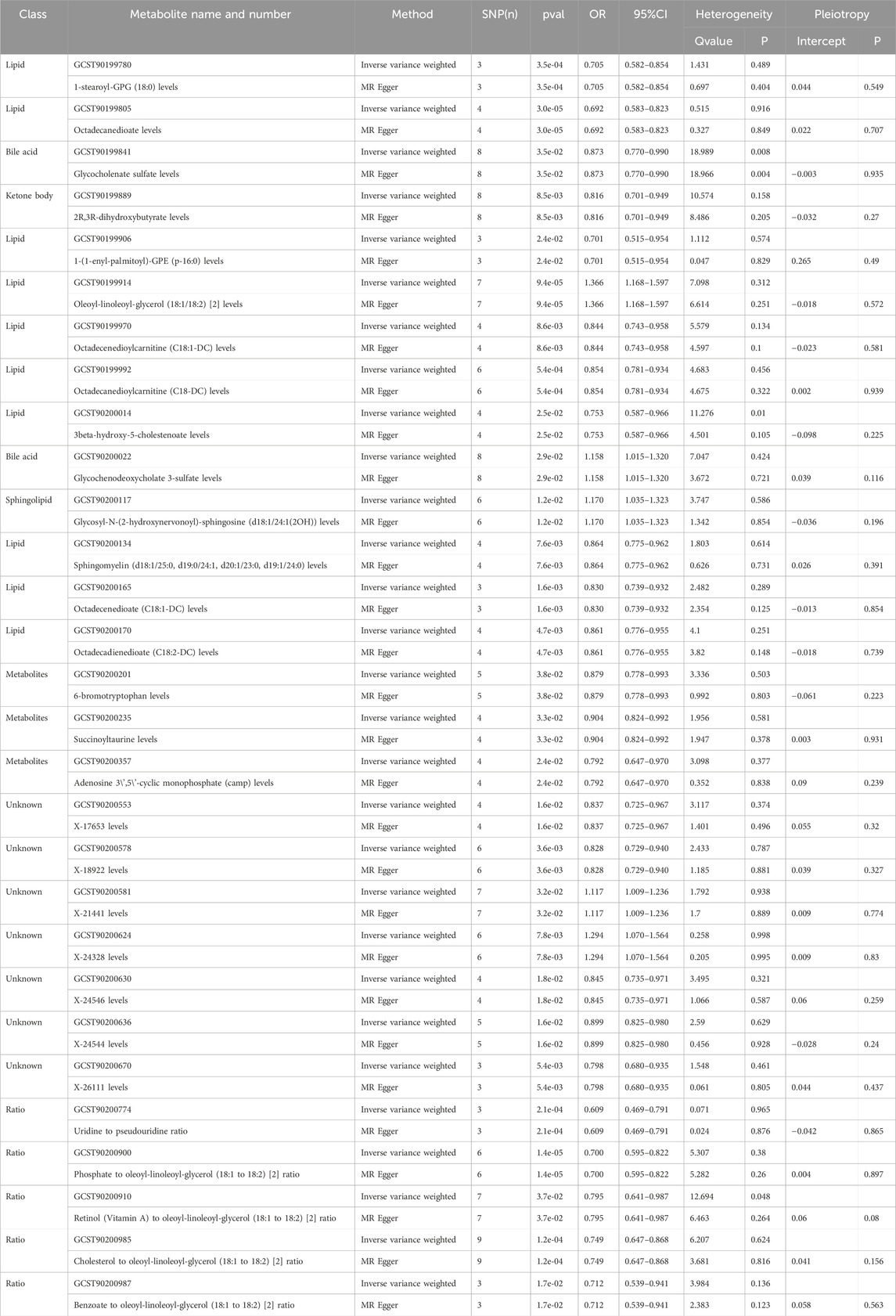
Table 1. Two MR models assessed causal relationships between 29 metabolites, their ratios, and AAA, examining heterogeneity and potential pleiotropy.
Among the 17 identified metabolites, we found that Octadecanedioate levels have the most significant negative correlation with AAA (IVW OR = 0.692, 95% CI = 0.583–0.823, p < 0.001), followed by 1-(1-enyl-palmitoyl)-GPE (p-16:0) levels (IVW OR = 0.701, 95% CI = 0.515–0.954, p = 0.024), 1-stearoyl-GPG (18:0) levels (IVW OR = 0.705, 95% CI = 0.582–0.854, p < 0.001), 2-linoleoylglycerol (18:2) levels (IVW OR = 0.96, 95% CI = 0.94–0.99, p = 0.003), 3beta-hydroxy-5-cholestenoate levels (IVW OR = 0.753, 95% CI = 0.587–0.966, p = 0.025), 2R,3R-dihydroxybutyrate levels (IVW OR = 0.816, 95% CI = 0.701–0.949, p < 0.001), Octadecenedioate (C18:1-DC) levels (IVW OR = 0.830, 95% CI = 0.739–0.932, p < 0.001), Octadecenedioylcarnitine (C18:1-DC) levels (IVW OR = 0.844, 95% CI = 0.743–0.958, p < 0.001), Octadecanedioylcarnitine (C18-DC) levels (IVW OR = 0.854, 95% CI = 0.781–0.934, p < 0.001), Octadecadienedioate (C18:2-DC) levels (IVW OR = 0.861, 95% CI = 0.776–0.955, p < 0.001), Sphingomyelin (d18:1/25:0, d19:0/24:1, d20:1/23:0, d19:1/24:0) levels (IVW OR = 0.864, 95% CI = 0.775–0.962, p < 0.001), Glycocholenate sulfate levels (IVW OR = 0.873, 95% CI = 0.770–0.990, p = 0.035), 6-bromotryptophan levels (IVW OR = 0.879, 95% CI = 0.778–0.993, p = 0.038), and Succinoyltaurine levels (IVW OR = 0.904, 95% CI = 0.824–0.992, p = 0.033).
The most significantly known positive correlation with AAA was observed in the Oleoyl-linoleoyl-glycerol (18:1/18:2) [2] levels (IVW OR = 1.366, 95% CI = 1.168–1.597, p < 0.001), Glycosyl-N-(2-hydroxynervonoyl)-sphingosine (d18:1/24:1(2OH)) levels (IVW OR = 1.170, 95% CI = 1.035–1.323; p = 0.012), and Glycochenodeoxycholate 3-sulfate levels (IVW OR = 1.158, 95% CI = 1.015–1.32, p = 0.029).
In relation to metabolite ratios, a collective of 5 ratios all exhibit a negative correlation with AAA. Among them, the most significant negative correlation with AAA was observed in the ratio of Uridine to pseudouridine (IVW OR = 0.609, 95% CI = 0.469–0.791, p < 0.001), followed by Phosphate to oleoyl-linoleoyl-glycerol (18:1 to 18:2) [2] ratio (IVW OR = 0.7, 95% CI = 0.595–0.822, p < 0.001), Benzoate to oleoyl-linoleoyl-glycerol (18:1 to 18:2) [2] ratio (IVW OR = 0.712, 95% CI = 0.539–0.941, p = 0.017), Cholesterol to oleoyl-linoleoyl-glycerol (18:1 to 18:2) [2] ratio (IVW OR = 0.749, 95% CI = 0.647–0.868, p < 0.001), Retinol (Vitamin A) to oleoyl-linoleoyl-glycerol (18:1 to 18:2) [2] ratio (IVW OR = 0.795, 95% CI = 0.641–0.987, p = 0.037).
In summary, MR estimates from IVW, WM, and MR-Egger regression models across 24 metabolites and 5 metabolite ratios consistently indicated both direction and magnitude, thereby bolstering the robustness of causal inference, with the exception of 3beta-hydroxy-5-cholestenoate levels (IVW OR = 0.753, 95% CI = 0.587–0.966, p = 0.025, heterogeneity Q value = 11.276; p = 0.01) and Retinol (Vitamin A) to oleoyl-linoleoyl-glycerol (18:1 to 18:2) [2] ratio (IVW OR = 0.795, 95% CI = 0.641–0.987, p = 0.037, heterogeneity Q value = 12.94, p = 0.048). No significant heterogeneity was observed in the p-values from the Cochran Q test across the remaining metabolites and metabolite ratios (Table 1). The MR-Egger intercept did not suggest the presence of pleiotropy (Table 1). Additionally, a LOO analysis did not reveal any highly influential SNPs that could bias the aggregated effect estimates (Supplementary Figures S1–S29). The funnel plot for the distribution of SNPs, scatter plot for the causal effect and forest plot of single SNP MR were also shown in (Supplementary Figures S1–S29. Consequently, these 24 metabolites and 5 metabolite ratios are identified as potential candidate markers in the metabolomic profile associated with the pathogenesis of AAA.
Our research findings substantiate a causal association between 24 metabolites and 5 metabolite ratios with AAA. The results indicate potential causal links between circulating metabolites and AAA. Specifically, increased levels of Oleoyl-linoleoyl-glycerol (18:1/18:2) [2], Glycosyl-N-(2-hydroxynervonoyl)-sphingosine (d18:1/24:1(2OH)), and Glycochenodeoxycholate 3-sulfate, exhibit a protective role in patients with AAA. Conversely, the elevation of 19 other metabolites and metabolite ratios including Octadecanedioate levels, 1-(1-enyl-palmitoyl)-GPE (p-16:0) levels, 1-stearoyl-GPG (18:0) levels, 2-linoleoylglycerol (18:2) levels, 3beta-hydroxy-5-cholestenoate levels, 2R,3R-dihydroxybutyrate levels, Octadecenedioate (C18:1-DC) levels, Octadecenedioylcarnitine (C18:1-DC) levels, Octadecanedioylcarnitine (C18-DC) levels, Octadecadienedioate (C18:2-DC) levels, Sphingomyelin (d18:1/25:0, d19:0/24:1, d20:1/23:0, d19:1/24:0) levels, Glycocholenate sulfate levels, 6-bromotryptophan levels, and Succinoyltaurine levels, Uridine to pseudouridine ratio, followed by Phosphate to oleoyl-linoleoyl-glycerol (18:1 to 18:2) [2] ratio (IVW OR = 0.7, 95% CI = 0.595–0.822, p = 0.000014), Benzoate to oleoyl-linoleoyl-glycerol (18:1 to 18:2) [2] ratio, Cholesterol to oleoyl-linoleoyl-glycerol (18:1 to 18:2) [2] ratio, as well as Retinol (Vitamin A) to oleoyl-linoleoyl-glycerol (18:1 to 18:2) [2] ratio, are associated with adverse effects on AAA.
AAA is a critical health concern that influences individuals throughout their entire lives (Lv et al., 2024a). It is characterized by pathological changes such as the loss of smooth muscle cells, alterations in extracellular matrix components, and significant inflammatory cell infiltration, which compromise arterial wall integrity (Xu et al., 2019). Additionally, metabolic homeostasis is disrupted, leading to altered serum concentrations of lipids, with elevated total cholesterol (TC), triglycerides and low-density lipoprotein cholesterol (LDL-C) as well as reduced high-density lipoprotein cholesterol (HDL-C) and phosphatidylcholines (Harrison et al., 2018). These changes lead to vascular dilation, increased wall stress, and an elevated risk of rupture, underscoring the importance of its early detection and monitoring (Golledge et al., 2023). Clinically, aneurysms frequently remain asymptomatic, but patients may report abdominal or back pain, a noticeable pulsatile mass, and fluctuations in blood pressure when symptoms do occur. Life-threatening complications, such as rupture, can result in severe internal bleeding, highlighting the importance of routine monitoring and imaging for those at higher risk (Golledge et al., 2023; Chen Z. et al., 2024). Patients with AAA are often complicated with atherosclerosis, hypertension, diabetes, and coronary artery disease, which can exacerbate its progression. Early identification and management of these comorbidities are further essential for reducing AAA risk and improving overall health outcomes.
The pathological process and progression of AAA are associated with lipid levels, particularly elevated LDL-C, which plays a significant role in atherosclerosis, a major contributor to AAA formation (Nana et al., 2021). Effective management of dyslipidemia may help mitigate AAA progression and enhance cardiovascular health, underscoring the necessity for routine lipid monitoring in at-risk individuals (Burillo et al., 2015). To our knowledge, this is the first investigation employing a MR approach to investigate the causal relationship between 1,400 blood metabolites and the risk of AAA.
After removing the unknown metabolites and metabolite ratios, we identified 19 metabolites and metabolite ratios that decreased the risk of AAA, including: Octadecanedioate, 1-(1-enyl-palmitoyl)-GPE (p-16:0), 1-stearoyl-GPG (18:0), 2-linoleoylglycerol (18:2), 3beta-hydroxy-5-cholestenoate, 2R,3R-dihydroxybutyrate, Octadecenedioate (C18:1-DC), Octadecenedioylcarnitine (C18:1-DC), Octadecanedioylcarnitine (C18-DC), Octadecadienedioate (C18:2-DC), Sphingomyelin (d18:1/25:0, d19:0/24:1, d20:1/23:0, d19:1/24:0), Glycocholenate sulfate, 6-bromotryptophan, and Succinoyltaurine, Uridine to pseudouridine ratio, followed by Phosphate to oleoyl-linoleoyl-glycerol (18:1 to 18:2) [2] ratio, Benzoate to oleoyl-linoleoyl-glycerol (18:1 to 18:2) [2] ratio, Cholesterol to oleoyl-linoleoyl-glycerol (18:1 to 18:2) [2] ratio, as well as Retinol (Vitamin A) to oleoyl-linoleoyl-glycerol (18:1 to 18:2) [2] ratio. Currently, no relevant studies have been found regarding 1-stearoyl-GPG (18:0), 3beta-hydroxy-5-cholestenoate, 2R,3R-dihydroxybutyrate, Octadecenedioylcarnitine (C18:1-DC), Sphingomyelin (d18:1/25:0, d19:0/24:1, d20:1/23:0, d19:1/24:0), Phosphate to oleoyl-linoleoyl-glycerol (18:1 to 18:2) [2] ratio, Benzoate to oleoyl-linoleoyl-glycerol (18:1 to 18:2) [2] ratio, and Retinol (Vitamin A) to oleoyl-linoleoyl-glycerol (18:1 to 18:2) [2] ratio. Lower levels of Octadecanedioate are related to the decreased odds of both preeclampsia and coronary heart disease (CHD) (Ross et al., 2019; Feofanova et al., 2020). Genes related to Octadecanedioate are significantly involved in the process of apoptosis (programmed cell death) based on the DAVID analysis (Feofanova et al., 2020). Different from the above two situations, exposure to Octadecanedioate downregulates the risk of AAA due to our MR results. High level of 1-(1-enyl-palmitoyl)-GPE (p-16:0) may be correlated with increased gastric cancer risk as a potential risk biomarker (Shu et al., 2021). 2-linoleoylglycerol (18:2) is one of reported circulating metabolome associated with colorectal cancer (CRC) (Gao et al., 2022). High level of Octadecenedioate (C18:1-DC) decreased susceptibility to CHD (Chen H. et al., 2024). Besides, its generation could be potentially decreased in patients with rosacea (Yao et al., 2024). Octadecanedioylcarnitine (C18-DC) mediates the genetic predictive effects on Alzheimer’s disease risk (Chen G. et al., 2024). Octadecadienedioate (C18:2-DC) is one of the plasma metabolites as significant mediators in the relationships between gut microbiota and type 2 diabetes (Zheng et al., 2024). Glycocholenate sulfate may be a new metabolite significantly associated with atrial fibrillation (AF), CHD, and CRC (Chen H. et al., 2024; Alonso et al., 2019; Alonso et al., 2015). Its concentration is negative associated with consumption of artificially sweetened beverages (Jia et al., 2024). 6-bromotryptophan may serve as a correlated metabolomics maker of kidney health, CKD progression, patients with cirrhosis, responder (Sekula et al., 2020; Tin et al., 2018; Sanchez et al., 2024). A MR study found a negative causal relationship with Succinoyltaurine and the risk of breast cancer (Ming et al., 2024). Uridine to pseudouridine ratio is related to decreased the risk of ischemia stroke (He et al., 2024). Sodium-glucose cotransporter 1 (SGLT1) and SGLT2 inhibitors play protective roles in small vessel disease (SVD) via Cholesterol to oleoyl-linoleoyl-glycerol (18:1 to 18:2) [2] ratio (Lv et al., 2024b). The upregulation of these metabolites and metabolite ratios may serve as good indicators for the occurrence and progression of AAA, while their upregulation could exert a protective effect. The metabolites are underlying diagnosis biomarkers and treatment targets for AAA. Given the correlation between these metabolites and AAA, it may be recommended to include clinical practice guidance to explore the changes in these above metabolites. Their downregulation may indicate early detection and development of AAA, and clinicians should pay attention to these metabolic markers for diagnosis and intervention.
After removing the unknown metabolites and metabolite ratios, we identified only 3 known metabolites that increased the risk of AAA, including: Oleoyl-linoleoyl-glycerol (18:1/18:2) [2], Glycosyl-N-(2-hydroxynervonoyl)-sphingosine (d18:1/24:1(2OH)), and Glycochenodeoxycholate 3-sulfate. Currently, no relevant studies have been found regarding Glycosyl-N-(2-hydroxynervonoyl)-sphingosine (d18:1/24:1(2OH)). Oleoyl-linoleoyl-glycerol (18:1/18:2) is a type of diacylglycerol that enhances the production and release of HDL-C and reduces the levels of TC and LDL-C by promoting the clearance of LDL-C and inhibiting cholesterol synthesis (Lv et al., 2024b). Glycochenodeoxycholate 3-sulfate, also named GCDCA-S, is produced from Glycochenodeoxycholic acid (GCDCA) in hepatocytes by sulfotransferase, reabsorbed in the distal small intestine, and taken up again by hepatocytes via Oatps, completing the enterohepatic circulation of bile acids (Li and Dawson, 2019). It helps regulate cholesterol metabolism by potentially reducing serum cholesterol levels and facilitates the fat digestion and fat-soluble vitamins absorption. GCDCA-S has been proved to be underlying diagnosis biomarkers for tuberculosis, severity of patients with acute hepatitis E infection (Deng et al., 2021; Wu et al., 2022), as well as the promising surrogate markers for quantitatively evaluating potential drug-drug interactions mediated by OATP1B (such as Rifampicin), OATP1B3 (such as micafungin), and Oatps-mediated hepatic uptake of atorvastatin (Takehara et al., 2018; Jin et al., 2022; Ma et al., 2021). The mediating effects of both oleoyl-linoleoyl-glycerol (18:1/18:2) and glycochenodeoxycholate 3-sulfate in the relationship between lipids and AAA further underscore the significance of lipid and its homeostasis in AAA. The upregulation of these three markers may serve as a risk signal for the occurrence and progression of AAA, while their downregulation could exert a protective effect. Treatment plans could consider modifying diet, exercise, or pharmacological interventions to address metabolic abnormalities associated with AAA, potentially slowing disease progression. Such recommendations would provide valuable insights for personalized treatment strategies.
This research presents numerous significant advantages. 1) By leveraging GWAS data, our MR analysis reveals novel potential causal mediators for 1,400 metabolites linked to AAAs. 2) The use of multiple cohorts derived from original GWAS data strengthens our ability to draw robust causal inferences across a large population, thereby increasing the statistical power of our findings. This makes it possible for developing potential effective drug targets and clinical trials. Nonetheless, our study has several limitations. 1) Our MR analysis is based on summary data from GWAS, while AAAs are influenced by a range of factors beyond genetic predisposition. Future research should prioritize investigating changes in relevant biomarkers to identify additional therapeutic targets for AAAs. 2) Our study predominantly involved individuals of European descent, which reduces population stratification bias but limits the generalizability of our findings to other ethnic groups. Further exploration in diverse populations is crucial to validate our results. 3) There may be overlap among participants in the GWAS cohorts, which could lead to weak instrument bias. While our F-statistics show that instrument bias is not present in the MR analysis, additional studies using independent cohorts without participant overlap are essential for deepening our understanding of the genetic factors involved in the development of AAAs.
Through MR and comprehensive circulating metabolomics, this study identified significant associations between metabolite expression and AAA. First, changes in the levels of specific metabolites may serve as indicators of AAA onset and progression, offering a potential strategy for the early detection of AAA. Second, certain metabolites could help predict a patient’s response to specific treatments, laying the groundwork for personalized therapy. Additionally, monitoring levels of metabolites may enable real-time assessment of treatment efficacy and guide necessary adjustments to the treatment, ultimately improving clinical outcomes and reducing side effects. By employing these strategies, personalized treatment not only enhances patient outcomes but also optimizes clinical management and overall prognosis. Therefore, the findings of this study may provide valuable insights for the clinical management of AAA and contribute to the advancement of precision medicine. These findings highlight promising avenues for the development of targeted diagnostic and therapeutic strategies for AAA, potentially improving patient outcomes and clinical management.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
QG: Data curation, Software, Validation, Writing–original draft, Visualization. XhX: Data curation, Investigation, Validation, Writing–original draft, Formal Analysis, Software. XhL: Data curation, Validation, Visualization, Writing–original draft, Conceptualization, Investigation. YM: Methodology, Resources, Software, Validation, Writing–original draft. SL: Investigation, Methodology, Software, Validation, Visualization, Writing–original draft. YY: Data curation, Investigation, Software, Validation, Writing–original draft. XnL: Data curation, Writing–original draft. YL: Data curation, Software, Writing–original draft. JF: Data curation, Software, Validation, Writing–original draft. YS: Formal Analysis, Resources, Visualization, Writing–original draft. XlX: Conceptualization, Data curation, Investigation, Software, Supervision, Visualization, Writing–review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82100439), China Postdoctoral Science Foundation (2024M751844), Shandong Provincial Natural Science Foundation (ZR2023MH267), Shandong Provincial Science and Technology Department Youth Science Fund Project (ZR2024QH242, ZR2022QH154), Key Laboratory of Medical Electrophysiology of Ministry of Education (Southwest Medical University) Open fund (KeyME-2021-05), and Natural Science Foundation of Sichuan Province (2022NSFSC0538).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1514293/full#supplementary-material
Alonso, A., Yu, B., Qureshi, W. T., Grams, M. E., Selvin, E., Soliman, E. Z., et al. (2015). Metabolomics and incidence of atrial fibrillation in african Americans: the atherosclerosis risk in communities (ARIC) study. PLoS One 10, e0142610. doi:10.1371/journal.pone.0142610
Alonso, A., Yu, B., Sun, Y. V., Chen, L. Y., Loehr, L. R., O'Neal, W. T., et al. (2019). Serum metabolomics and incidence of atrial fibrillation (from the atherosclerosis risk in communities study). Am. J. Cardiol. 123, 1955–1961. doi:10.1016/j.amjcard.2019.03.017
Benson, T. W., Conrad, K. A., Li, X. S., Wang, Z., Helsley, R. N., Schugar, R. C., et al. (2023). Gut microbiota-derived trimethylamine N-oxide contributes to abdominal aortic aneurysm through inflammatory and apoptotic mechanisms. Circulation 147, 1079–1096. doi:10.1161/CIRCULATIONAHA.122.060573
Burillo, E., Lindholt, J. S., Molina-Sanchez, P., Jorge, I., Martinez-Pinna, R., Blanco-Colio, L. M., et al. (2015). ApoA-I/HDL-C levels are inversely associated with abdominal aortic aneurysm progression. Thromb. Haemost. 113, 1335–1346. doi:10.1160/TH14-10-0874
Chen, G., Jin, Y., Chu, C., Zheng, Y., Chen, Y., and Zhu, X. (2024c). Genetic prediction of blood metabolites mediating the relationship between gut microbiota and Alzheimer's disease: a Mendelian randomization study. Front. Microbiol. 15, 1414977. doi:10.3389/fmicb.2024.1414977
Chen, H., Huang, Y., Wan, G., and Zou, X. (2024b). Circulating metabolites and coronary heart disease: a bidirectional Mendelian randomization. Front. Cardiovasc Med. 11, 1371805. doi:10.3389/fcvm.2024.1371805
Chen, Y., Lu, T., Pettersson-Kymmer, U., Stewart, I. D., Butler-Laporte, G., Nakanishi, T., et al. (2023). Genomic atlas of the plasma metabolome prioritizes metabolites implicated in human diseases. Nat. Genet. 55, 44–53. doi:10.1038/s41588-022-01270-1
Chen, Z., Gao, Q., Qiu, J., Ge, M., Wang, S., Liu, C., et al. (2024a). Genetic analysis reveals Key regulatory Axis in aortic dissection: CBL regulated by HOXB13 and microRNA-1321. CVIA 9 (1). doi:10.15212/cvia.2024.0034
Choi, K. W., Chen, C. Y., Stein, M. B., Klimentidis, Y. C., Wang, M. J., Koenen, K. C., et al. (2019). Assessment of bidirectional relationships between physical activity and depression among adults: a 2-sample mendelian randomization study. JAMA Psychiatry 76, 399–408. doi:10.1001/jamapsychiatry.2018.4175
Deng, J., Liu, L., Yang, Q., Wei, C., Zhang, H., Xin, H., et al. (2021). Urinary metabolomic analysis to identify potential markers for the diagnosis of tuberculosis and latent tuberculosis. Arch. Biochem. Biophys. 704, 108876. doi:10.1016/j.abb.2021.108876
Feofanova, E. V., Chen, H., Dai, Y., Jia, P., Grove, M. L., Morrison, A. C., et al. (2020). A genome-wide association study discovers 46 loci of the human metabolome in the hispanic community health study/study of latinos. Am. J. Hum. Genet. 107, 849–863. doi:10.1016/j.ajhg.2020.09.003
Gao, R., Wu, C., Zhu, Y., Kong, C., Zhu, Y., Gao, Y., et al. (2022). Integrated analysis of colorectal cancer reveals cross-cohort gut microbial signatures and associated serum metabolites. Gastroenterology 163, 1024–1037.e9. doi:10.1053/j.gastro.2022.06.069
Golledge, J., Thanigaimani, S., Powell, J. T., and Tsao, P. S. (2023). Pathogenesis and management of abdominal aortic aneurysm. Eur. Heart J. 44, 2682–2697. doi:10.1093/eurheartj/ehad386
Harrison, S. C., Holmes, M. V., Burgess, S., Asselbergs, F. W., Jones, G. T., Baas, A. F., et al. (2018). Genetic association of lipids and lipid drug targets with abdominal aortic aneurysm: a meta-analysis. JAMA Cardiol. 3, 26–33. doi:10.1001/jamacardio.2017.4293
He, M., Xu, C., Yang, R., Liu, L., Zhou, D., and Yan, S. (2024). Causal relationship between human blood metabolites and risk of ischemic stroke: a Mendelian randomization study. Front. Genet. 15, 1333454. doi:10.3389/fgene.2024.1333454
Jia, H., Bernard, L., Chen, J., Du, S., Steffen, L. M., Wong, K. E., et al. (2024). Serum metabolomic markers of artificially sweetened beverage consumption. J. Nutr. 154, 3266–3273. doi:10.1016/j.tjnut.2024.09.024
Jin, Y., Li, Y., Eisenmann, E. D., Figg, W. D., Baker, S. D., Sparreboom, A., et al. (2022). Determination of the endogenous OATP1B biomarkers glycochenodeoxycholate-3-sulfate and chenodeoxycholate-24-glucuronide in human and mouse plasma by a validated UHPLC-MS/MS method. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1210, 123437. doi:10.1016/j.jchromb.2022.123437
Li, C., Liu, Z., Yang, S., Li, W., Liang, B., Chen, H., et al. (2024). Causal relationship between gut microbiota, plasma metabolites, inflammatory cytokines and abdominal aortic aneurysm: a Mendelian randomization study. Clin. Exp. Hypertens. 46, 2390419. doi:10.1080/10641963.2024.2390419
Li, J., and Dawson, P. A. (2019). Animal models to study bile acid metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 1865, 895–911. doi:10.1016/j.bbadis.2018.05.011
Lieberg, J., Wanhainen, A., Ottas, A., Vähi, M., Zilmer, M., Soomets, U., et al. (2021). Metabolomic profile of abdominal aortic aneurysm. Metabolites 11, 555. doi:10.3390/metabo11080555
Ling, X., Jie, W., Qin, X., Zhang, S., Shi, K., Li, T., et al. (2022). Gut microbiome sheds light on the development and treatment of abdominal aortic aneurysm. Front. Cardiovasc Med. 9, 1063683. doi:10.3389/fcvm.2022.1063683
Lu, S., White, J. V., Nwaneshiudu, I., Nwaneshiudu, A., Monos, D. S., Solomides, C. C., et al. (2022). Human abdominal aortic aneurysm (AAA): evidence for an autoimmune antigen-driven disease. Autoimmun. Rev. 21, 103164. doi:10.1016/j.autrev.2022.103164
Lv, Y., Cheng, X., and Dong, Q. (2024b). SGLT1 and SGLT2 inhibition, circulating metabolites, and cerebral small vessel disease: a mediation Mendelian Randomization study. Cardiovasc Diabetol. 23, 157. doi:10.1186/s12933-024-02255-6
Lv, Y., Shen, D., Zhang, G., Wang, B., Wang, H., Zhang, J., et al. (2024a). Causal associations between the gut microbiome and aortic aneurysm: a mendelian randomization study. CVIA 9 (1). doi:10.15212/cvia.2024.0023
Ma, Y., Xin, M., Wen, Y., Wang, H., Zhang, G., Dai, J., et al. (2021). The utility of endogenous glycochenodeoxycholate-3-sulfate and 4β-hydroxycholesterol to evaluate the hepatic disposition of atorvastatin in rats. Asian J. Pharm. Sci. 16 (4), 519–529. doi:10.1016/j.ajps.2021.03.002
Ming, R., Wu, H., Liu, H., Zhan, F., Qiu, X., and Ji, M. (2024). Causal effects and metabolites mediators between immune cell and risk of breast cancer: a Mendelian randomization study. Front. Genet. 15, 1380249. doi:10.3389/fgene.2024.1380249
Nana, P., Dakis, K., Brodis, A., Spanos, K., and Kouvelos, G. (2021). Circulating biomarkers for the prediction of abdominal aortic aneurysm growth. J. Clin. Med. 10, 1718. doi:10.3390/jcm10081718
Ross, K. M., Baer, R. J., Ryckman, K., Feuer, S. K., Bandoli, G., Chambers, C., et al. (2019). Second trimester inflammatory and metabolic markers in women delivering preterm with and without preeclampsia. J. Perinatol. 39, 314–320. doi:10.1038/s41372-018-0275-8
Sakalihasan, N., Michel, J. B., Katsargyris, A., Kuivaniemi, H., Defraigne, J. O., Nchimi, A., et al. (2018). Abdominal aortic aneurysms. Nat. Rev. Dis. Prim. 4, 34. doi:10.1038/s41572-018-0030-7
Sanchez, J. I., Fontillas, A. C., Kwan, S. Y., Sanchez, C. I., Calderone, T. L., Lee, J. L., et al. (2024). Metabolomics biomarkers of hepatocellular carcinoma in a prospective cohort of patients with cirrhosis. JHEP Rep. 6, 101119. doi:10.1016/j.jhepr.2024.101119
Sekula, P., Tin, A., Schultheiss, U. T., Baid-Agrawal, S., Mohney, R. P., Steinbrenner, I., et al. (2020). Urine 6-bromotryptophan: associations with genetic variants and incident end-stage kidney disease. Sci. Rep. 10, 10018. doi:10.1038/s41598-020-66334-w
Shu, X., Cai, H., Lan, Q., Cai, Q., Ji, B. T., Zheng, W., et al. (2021). A prospective investigation of circulating metabolome identifies potential biomarkers for gastric cancer risk. Cancer Epidemiol. Biomarkers Prev. 30, 1634–1642. doi:10.1158/1055-9965.EPI-20-1633
Takehara, I., Yoshikado, T., Ishigame, K., Mori, D., Furihata, K. I., Watanabe, N., et al. (2018). Comparative study of the dose-dependence of OATP1B inhibition by Rifampicin using probe drugs and endogenous substrates in healthy volunteers. Pharm. Res. 35, 138. doi:10.1007/s11095-018-2416-3
Tian, Z., Zhang, Y., Zheng, Z., Zhang, M., Zhang, T., Jin, J., et al. (2022). Gut microbiome dysbiosis contributes to abdominal aortic aneurysm by promoting neutrophil extracellular trap formation. Cell Host Microbe 30, 1450–1463.e8. doi:10.1016/j.chom.2022.09.004
Tin, A., Nadkarni, G., Evans, A. M., Winkler, C. A., Bottinger, E., Rebholz, C. M., et al. (2018). Serum 6-bromotryptophan levels identified as a risk factor for CKD progression. J. Am. Soc. Nephrol. 29, 1939–1947. doi:10.1681/ASN.2017101064
Vanmaele, A., Bouwens, E., Hoeks, S. E., Kindt, A., Lamont, L., Fioole, B., et al. (2024). Targeted proteomics and metabolomics for biomarker discovery in abdominal aortic aneurysm and post-EVAR sac volume. Clin. Chim. Acta 554, 117786. doi:10.1016/j.cca.2024.117786
Wang, Q., Dai, H., Hou, T., Hou, Y., Wang, T., Lin, H., et al. (2023). Dissecting causal relationships between gut microbiota, blood metabolites, and stroke: a mendelian randomization study. J. Stroke 25, 350–360. doi:10.5853/jos.2023.00381
Wu, J., Xu, Y., Cui, Y., Bortolanza, M., Wang, M., Jiang, B., et al. (2022). Dynamic changes of serum metabolites associated with infection and severity of patients with acute hepatitis E infection. J. Med. Virol. 94, 2714–2726. doi:10.1002/jmv.27669
Xu, X., Zhang, F., Lu, Y., Yu, S., Sun, W., Sun, S., et al. (2019). Silencing of NONO inhibits abdominal aortic aneurysm in apolipoprotein E-knockout mice via collagen deposition and inflammatory inhibition. J. Cell Mol. Med. 23, 7449–7461. doi:10.1111/jcmm.14613
Yang, J., Yan, B., Zhao, B., Fan, Y., He, X., Yang, L., et al. (2020). Assessing the causal effects of human serum metabolites on 5 major psychiatric disorders. Schizophr. Bull. 46, 804–813. doi:10.1093/schbul/sbz138
Yao, H., Shen, S., Gao, X., Song, X., and Xiang, W. (2024). The causal relationship between blood metabolites and rosacea: a Mendelian randomization. Skin. Res. Technol. 30, e13796. doi:10.1111/srt.13796
Zhang, F., Li, K., Zhang, W., Zhao, Z., Chang, F., Du, J., et al. (2024). Ganglioside GM3 protects against abdominal aortic aneurysm by suppressing ferroptosis. Circulation 149, 843–859. doi:10.1161/CIRCULATIONAHA.123.066110
Zhao, X., and Liu, L. (2024). Mendelian randomization analyses for the causal relationship between early age at first sexual intercourse, early age at first live birth, and postpartum depression in pregnant women. Front. Psychiatry 15, 1287934. doi:10.3389/fpsyt.2024.1287934
Keywords: Mendelian randomization study, abdominal aortic aneurysm, metabolites, metabolite ratios, metabolomics
Citation: Guo Q, Xu X, Li X, Mao Y, Li S, Yao Y, Li X, Li Y, Feng J, Shu Y and Xu X (2025) Assessing the relationships of 1,400 blood metabolites with abdominal aortic aneurysm: a Mendelian randomization study. Front. Pharmacol. 15:1514293. doi: 10.3389/fphar.2024.1514293
Received: 20 October 2024; Accepted: 09 December 2024;
Published: 03 January 2025.
Edited by:
Aifeng Zhang, Boston Medical Center, United StatesReviewed by:
Chengming Fan, Central South University, ChinaCopyright © 2025 Guo, Xu, Li, Mao, Li, Yao, Li, Li, Feng, Shu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingli Xu, eHV4aW5nbGk2MjNAMTYzLmNvbQ==; Yan Shu, c2h1eWFuX3NjQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.