
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 19 June 2024
Sec. Renal Pharmacology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1380326
 Xiangmeng Li1,2,3
Xiangmeng Li1,2,3 Shimin Jiang1
Shimin Jiang1 Xia Gu1,2
Xia Gu1,2 Xiaojing Liu1,2
Xiaojing Liu1,2 Shunlai Shang1
Shunlai Shang1 Jiao Zhang4
Jiao Zhang4 Keying Pang5
Keying Pang5 Wenge Li1,2*
Wenge Li1,2*Objective: This study compares the cardiovascular risk in anemic chronic kidney disease patients treated with Roxadustat versus erythropoietin stimulating agents (ESAs). It also explores the cardiovascular impact of Roxadustat.
Methods: We searched PubMed, EMBASE, Cochrane, Scopus, and Web of Science databases up to 13 August 2023, using terms such as “ESA,” “Roxadustat,” “MACE,” “stroke,” “death,” “myocardial infarction,” and “heart failure.” Two researchers independently selected and extracted data based on predefined criteria. We assessed the risk of bias with the Cochrane tool and analyzed statistical heterogeneity using the Q and I2 tests. We conducted subgroup analyses by geographical region and performed data analysis with Stata 14.0 and RevMan 5.4 software. Data were sourced from the NCBI database by filtering for “Roxadustat” and “human,” and differentially expressed genes were identified using R software, setting the significance at p < 0.01 and a 2-fold logFC, followed by GO enrichment analysis, KEGG pathway analysis, and protein interaction network analysis.
Results: A total of 15 articles encompassing 1,43,065 patients were analyzed, including 1,38,739 patients treated with ESA and 4,326 patients treated with Roxadustat. In the overall population meta-analysis, the incidences of Major Adverse Cardiovascular Events (MACE), death, and heart failure (HF) were 13%, 8%, and 4% in the Roxadustat group, compared to 17%, 12%, and 6% in the ESA group, respectively, with P-values greater than 0.05. In the subgroup analysis, the incidences were 13%, 11%, and 4% for the Roxadustat group versus 17%, 15%, and 5% for the ESA group, also with p-values greater than 0.05. Bioinformatics analysis identified 59 differentially expressed genes, mainly involved in the inflammatory response. GO enrichment analysis revealed that these genes are primarily related to integrin binding. The main pathways identified were the TNF signaling pathway, NF-κB signaling pathway, and lipid metabolism related to atherosclerosis. The protein interaction network highlighted IL1B, CXCL8, ICAM1, CCL2, and CCL5 as the top five significantly different genes, all involved in the inflammatory response and downregulated by Roxadustat, suggesting a potential role in reducing inflammation.
Conclusion: The meta-analysis suggests that the use of Roxadustat and ESA in treating anemia associated with chronic kidney disease does not significantly alter the likelihood of cardiovascular events in the overall and American populations. However, Roxadustat exhibited a safer profile with respect to MACE, death, and heart failure. The bioinformatics findings suggest that Roxadustat may influence integrin adhesion and affect the TNF and NF-κB signaling pathways, along with lipid and atherosclerosis pathways, potentially reducing inflammation.
Anemia in patients with chronic kidney disease (CKD) often results from reduced erythropoietin (EPO) production, leading to lower levels of red blood cells and hemoglobin. This condition significantly impairs patient health and functionality, evident through symptoms such as fatigue, weakness, palpitations, and dizziness, which in turn increase mortality risk. Managing anemia in CKD patients is essential for enhancing their quality of life and prognosis. Erythropoiesis-stimulating agents (ESAs) are crucial for providing supplemental EPO through injections. However, many patients show poor compliance with ESA treatment, which is a major factor in anemia management failure. It is reported that non-compliance, defined as taking less than 90% of the prescribed dose, affects 35%–55% of the dialysis population (Johnson et al., 2007). The need for cold chain transportation for ESAs also adds significant economic burdens. Roxadustat, the first hypoxia-inducible factor prolyl hydroxylase inhibitor (HIF-PHI), has shown promise in improving anemia (Provenzano et al., 2021a; Provenzano et al., 2021b; Charytan et al., 2021; Fishbane et al., 2022) and is administered orally, which could enhance patient compliance. Nevertheless, its cardiovascular safety remains controversial. This study aims to assess the risk of cardiovascular events in CKD patients with anemia treated with Roxadustat through a two-part approach: a meta-analysis and bioinformatics analysis. The first part will conduct a meta-analysis of cardiovascular events in these patients to evaluate Roxadustat’s safety, registered under CRD42023453280 on the PROSPERO website. The second part will utilize GO enrichment analysis, pathway analysis, and protein interaction network analysis of RNAseq data from human cells treated with Roxadustat, available in the NCBI database, to investigate the drug’s regulatory mechanisms on cardiovascular events.
The PubMed, EMBASE, Cochrane, Scopus, and Web of Science databases were searched or studies using the search terms “ESA, Roxadustat, MACE, stroke, death, myocardial infarction, heart failure” up to 13 August 2023.
Inclusion criteria included (Johnson et al., 2007) randomized controlled trials; (Provenzano et al., 2021a); patients with chronic kidney disease (eGFR<90 mL/min/1.73 m2) including those on dialysis; (Provenzano et al., 2021b); anemia (Hb<10 g/L); (Charytan et al., 2021); participants aged over 18 years; (Fishbane et al., 2022); use of roxadustat or ESA for at least 6 months.
Exclusion criteria included (Johnson et al., 2007) anemia due to primary hematologic diseases; (Provenzano et al., 2021a); anemia secondary to tumors, bleeding, or severe infections; (Provenzano et al., 2021b); study populations with conditions other than chronic kidney disease, such as those with concurrent diabetes; (Charytan et al., 2021); incomplete data, even after contacting study authors, making certain indicators unavailable. Two authors independently screened the literature and extracted data to minimize selection bias.
Data were independently extracted by two authors from the selected literature. In case of discrepancies, a third author participated in the extraction process, and data were reported to the corresponding author of this study. Extracted data included the original geographical region of the study population (Asia, America, Europe), publication year, sample size, total follow-up time, eGFR levels and dialysis status, gender, average age, medical history, average hemoglobin levels, types and doses of medications, and cardiovascular events. Cardiovascular events comprised MACE and heart failure, with MACE including death, myocardial infarction, and stroke. The medical history primarily involved cardiac and cerebrovascular diseases. Cardiovascular events were the outcome events of this study. Differences in reporting led to treating death and all-cause mortality as equivalent, as well as acute myocardial infarction, myocardial infarction, and non-fatal myocardial infarction as equivalent, and similarly for stroke events and non-fatal stroke events.
This study applied the Cochrane risk of bias assessment tool, evaluating whether (Johnson et al., 2007) randomization methods introduced bias; (Provenzano et al., 2021a); allocation methods introduced bias; (Provenzano et al., 2021b); blinding was implemented; (Charytan et al., 2021); data results were complete; (Fishbane et al., 2022); study results introduced bias; (Chertow et al., 2021); other sources of bias were present. If all criteria were met, the risk of bias was considered low; if some were met, the risk was moderate; if none were met, the risk was high. The study excluded literature with a high risk of bias and conducted bias testing and sensitivity analysis on the results.
Johnson et al. (2007) Compare the risk of cardiovascular events between chronic kidney disease anemia patients treated with Roxadustat and those treated with ESA across the overall population (Provenzano et al., 2021a); Group the study population by geographic region in cohort studies to compare the risk of cardiovascular events post-treatment with Roxadustat and ESA. As cardiovascular events associated with Roxadustat were reported exclusively in the Americas, the analysis focused only on American patients (Provenzano et al., 2021b); Heterogeneity Test: Statistical heterogeneity of the included studies was analyzed using the Q test and the I2 test. An I2 value less than 50% indicates moderate heterogeneity, while values above 50% indicate high heterogeneity. A fixed-effect model is used when there is no statistical heterogeneity; otherwise, a random-effects model is employed (Charytan et al., 2021); Funnel plots were utilized to evaluate publication bias (Fishbane et al., 2022); Stata 14.0 software and RevMan 5.4 were employed for the meta-analysis.
The dataset was sourced using “Roxadustat” and “human (Homo sapiens)” as search terms in the NCBI database.
The selected dataset underwent preprocessing and analysis with R software, focusing on identifying differentially expressed genes based on a threshold of p < 0.01 and a 2-fold log fold change (logFC).
Initially, GO enrichment analysis was conducted to understand the biological processes, cellular components, and molecular functions of the differentially expressed genes using data from the GO database. This was followed by KEGG pathway enrichment analysis to explore the cellular signaling and biological metabolic pathways of the genes. All results from these analyses were required to meet a significance level of p < 0.05.
The protein-protein interaction network for differentially expressed genes was constructed using the STRING database and Cytoscape software to delineate interactions and identify potential key regulators influenced by roxadustat.
After removing duplicates, this study identified 633 potentially relevant articles (Figure 1). Exclusion of 567 articles based on title, abstract, and research methods left 66 articles for full-text evaluation. Of these, 48 were excluded for reasons including incomplete data, duplicate data, and medication duration not meeting the inclusion criteria. Additionally, three poster-format articles were excluded due to poor credibility and potential data duplication risks. Ultimately, 15 articles met the inclusion criteria (Fishbane et al., 2013; Macdougall et al., 2013; Malyszko et al., 2014; Saglimbene et al., 2017; Stirnadel-Farrant et al., 2018; Mc Causland et al., 2019; Stirnadel-Farrant et al., 2019; Cizman et al., 2020; Provenzano et al., 2021a; Provenzano et al., 2021b; Charytan et al., 2021; Chertow et al., 2021; Singh et al., 2021; Fishbane et al., 2022; Fujii et al., 2023). A keyword search for “Roxadustat” yielded three articles from phase 2 and phase 3 clinical trials in Chinese populations; however, three were removed for insufficient description of specific cardiovascular events (Chen et al., 2017; Chen et al., 2019a; Chen et al., 2019b). The analysis included 20 study cohorts with 1,43,065 patients, comprising 1,38,739 in the ESA treatment group and 4,326 in the Roxadustat group. All included articles were in English, and the quality assessment indicated no high-risk bias in the included literature (Figure 2).
The basic characteristics of the study cohorts are presented in Table 1. Of the 20 cohorts, 16 belonged to the ESA group—with 5 using Epoetin Alfa, 3 using Darbepoetin Alfa, 1 using CERA (Continuous Erythropoiesis Receptor Activator), 1 cohort using both Epoetin Alfa and Darbepoetin Alfa, and 6 unspecified. The Roxadustat group included four cohorts. Geographically, the ESA group had 2 cohorts from Asia, 5 from Europe, and 8 from the Americas, while all 4 Roxadustat cohorts were from the Americas. The studies reported consistent cardiovascular events in both the Roxadustat and ESA groups among the Chinese population with chronic kidney disease, though these were not analyzed in detail due to insufficient event descriptions (Chen et al., 2017; Chen et al., 2019a; Chen et al., 2019b). The cardiovascular event data for both treatment groups are shown in Table 2.
The ESA group consists of studies employing Epoetin Alfa, Darbepoetin Alfa, CERA, or unspecified ESA formulations, including 16 cohort studies involving 1,38,739 patients. The Roxadustat group comprises four cohort studies with 4,326 patients. Meta-analysis shows no significant difference in MACE events between the groups, with a lower incidence in the roxadustat group (13% vs. 17%). Subgroup analysis for MACE events was limited to populations from the Americas due to the absence of cohort studies on Asian and European populations. The ESA group includes data from eight cohort studies and 118,642 patients, whereas the Roxadustat group consists of four cohort studies encompassing 4,326 patients. The meta-analysis reveals no statistically significant difference in MACE (Major Adverse Cardiac Events) events between the ESA and roxadustat groups. However, there is a lower occurrence of MACE events in the roxadustat group compared to the ESA group (13% vs. 17%), as illustrated in Figure 3.
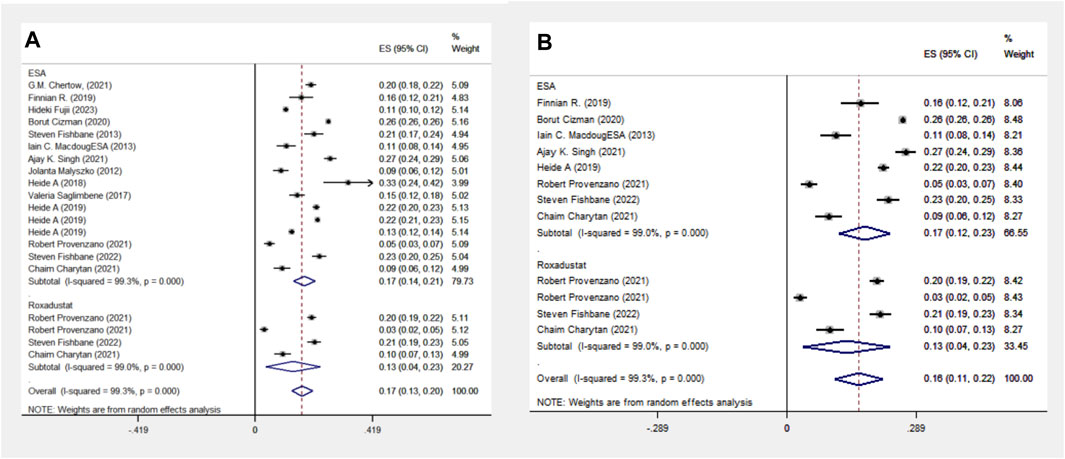
Figure 3. Overall group (A) and subgroup (B) forest plot of MACE events in the ESA and roxadustat groups.
The ESA group includes 11 cohort studies involving 1,16,454 patients, and the Roxadustat group includes four cohort studies with 4,326 patients. Meta-analysis reveals no significant difference in MI events between the two groups, each showing a 4% incidence rate. A subgroup analysis of the Americas confirmed this finding, with equal MI event probabilities in both groups, as indicated in Figure 4.
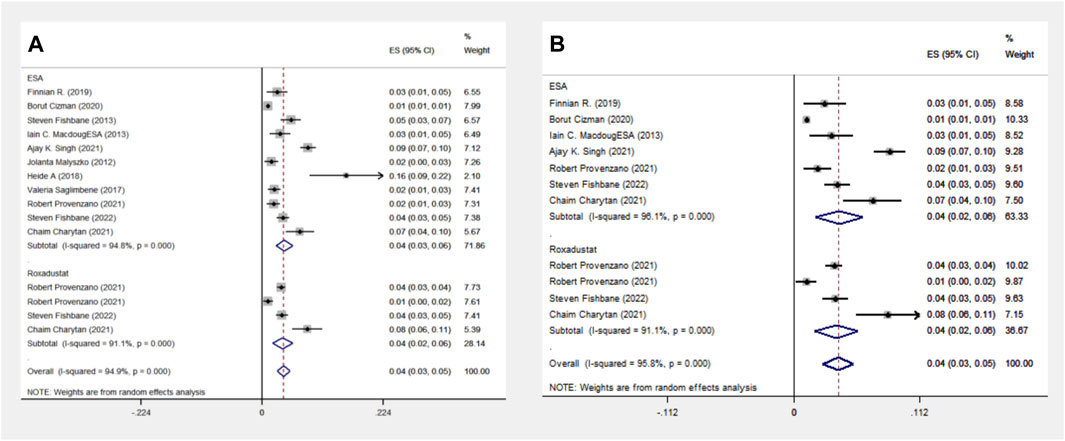
Figure 4. Overall group (A) and Subgroup (B) forest plot of MI events in the ESA and roxadustat groups. Abbreviations: MI, myocardial infarction.
The ESA group comprises 10 cohort studies with data from 115,798 patients, whereas the Roxadustat group includes four cohort studies with 4,326 patients. Meta-analysis indicates no significant difference in stroke events between the groups, both reporting a 2% incidence rate. However, subgroup analysis in the Americas shows a slightly higher stroke event rate in the Roxadustat group compared to the ESA group (2% vs. 1%), as presented in Figure 5.
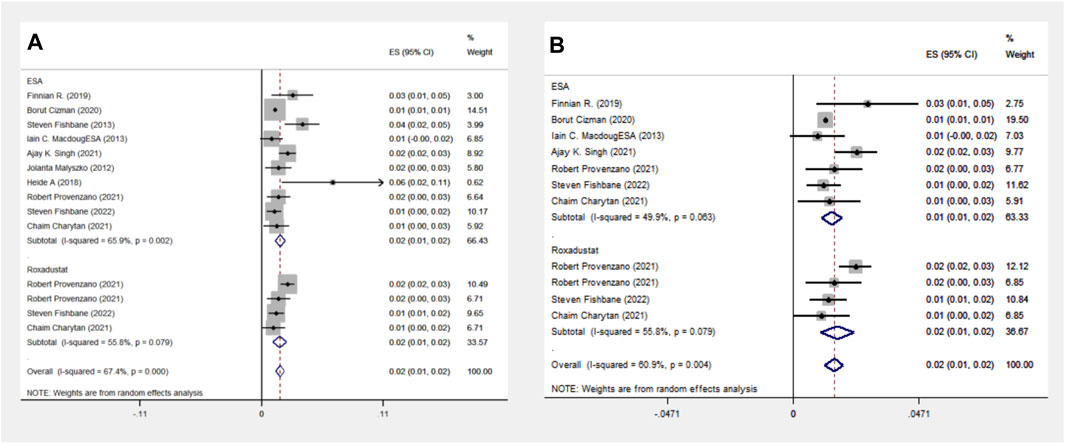
Figure 5. Overall group (A) and Subgroup (B) forest plot of stroke events in the ESA and roxadustat groups.
The ESA group consists of 16 cohort studies involving 1,38,739 patients, while the Roxadustat group comprises 4 cohort studies with 4,326 patients. The meta-analysis revealed no significant differences in death events between the two groups. However, the Roxadustat group showed a lower probability of death events compared to the ESA group (8% vs. 13%). A similar trend was observed in subgroup analysis for populations from the Americas, with the Roxadustat group also showing a lower incidence of death events than the ESA group (8% vs. 12%), as illustrated in Figure 6.
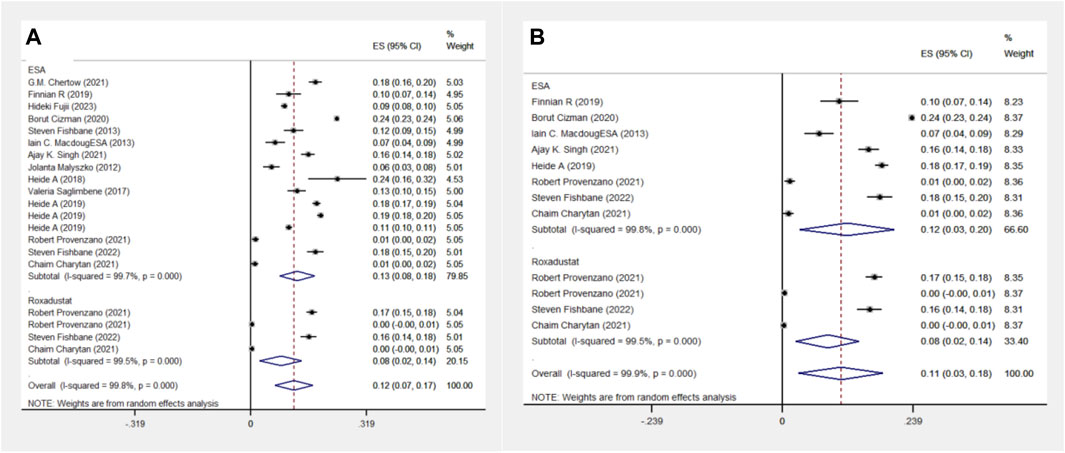
Figure 6. Overall group (A) and Subgroup (B) forest plot of death events in the ESA and roxadustat groups. Abbreviations: ESA, erythropoiesis-stimulating agent.
In another comparison, the ESA group encompassed 10 cohort studies with data from 119,393 patients, and the Roxadustat group included 4 cohort studies with 4,326 patients. The meta-analysis showed no significant differences in heart failure (HF) events between the groups. The Roxadustat group had a lower probability of HF events compared to the ESA group (4% vs. 6%). Subgroup analysis for populations from the Americas mirrored these findings, showing a reduced incidence of HF events in the Roxadustat group compared to the ESA group (4% vs. 5%), as depicted in Figure 7.
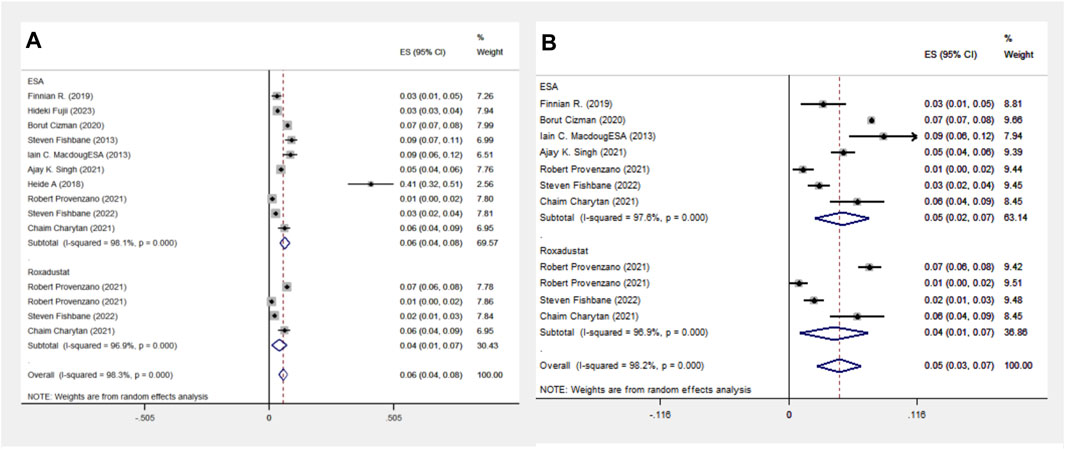
Figure 7. Overall group (A) and Subgroup (B) forest plots of HF events in the ESA and roxadustat groups.
Overall, 15 articles met the inclusion criteria, covering 20 study cohorts and 1,43,065 patients. Figure 8 displays the effect points of each study, indicating minimal publication bias among the cohorts.
Sensitivity analysis using the one-by-one exclusion method revealed no significant heterogeneity in the investigated indicators. This suggests the stability and reliability of the study conclusions, as illustrated in Figure 9.
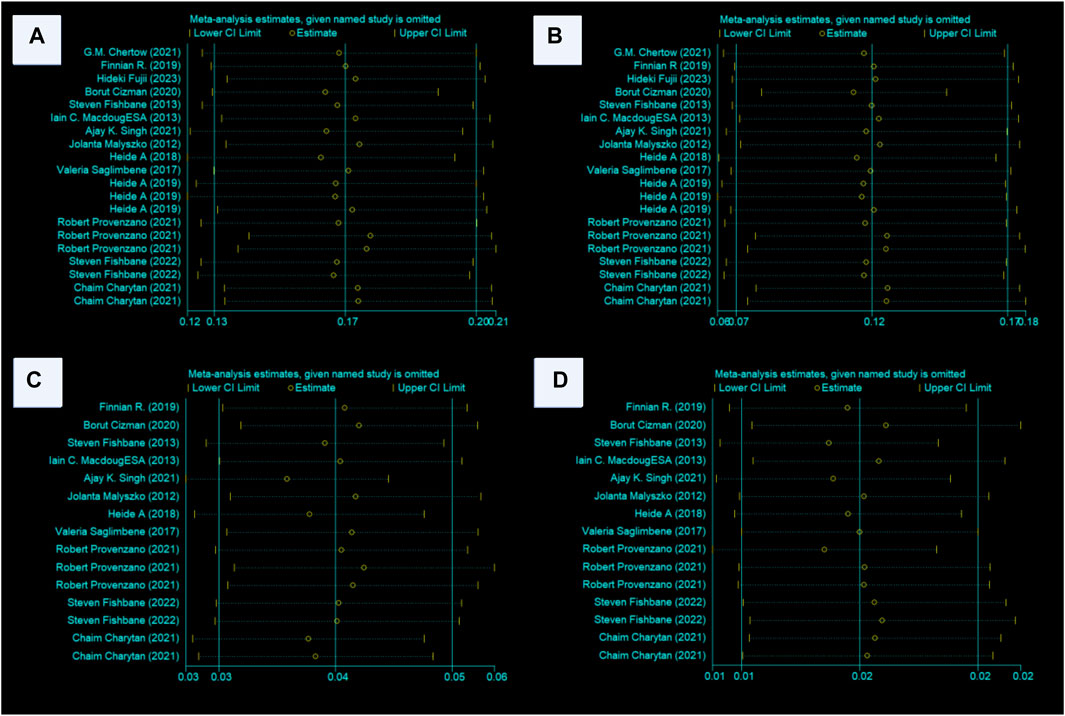
Figure 9. (A–D) Sensitivity analyses of the MACE, death, MI, and stroke conclusions. Abbreviations: MACE, major adverse cardiovascular events, MI, myocardial infarction, ESA, erythropoiesis-stimulating agent.
The dataset GSE189905 was retrieved from the NCBI database using “Roxadustat” as the search term and “human (H. sapiens)” as the sample source. The control group included samples GSM5709349, GSM5709350, and GSM5709351, while samples GSM5709355, GSM5709356, and GSM5709357 comprised the experimental group.
The dataset GSE189905 contains RNAseq data on the effects of Roxadustat in human neuroblastoma cell lines. Differentially expressed genes were identified using the criteria of “p < 0.01 and a 2-fold logFC,” resulting in a total of 59 differentially expressed genes.
GO analysis revealed the impact of Roxadustat on biological processes, molecular functions, and cellular components of the neuroblastoma cell line. Notably, Roxadustat influences inflammatory responses (GO:0006954, p-value 4.8310-12, FDR 2.8810-9), integrin binding (GO:0005178, p-value 1.8510-4, FDR 2.3010-2), and primarily affects the extracellular space (GO:0005615, p-Value 7.8210-6, FDR 7.0410-4). KEGGs pathway analysis identified significant pathways such as the TNF signaling pathway (hsa04668, p-Value 1.3610-9, FDR 1.5610-7), NF-κB signaling pathway (hsa04064, p-Value 6.5810-7, FDR 2.5210-5), and pathways related to lipid metabolism and atherosclerosis (hsa05417, p-Value 3.2510-6, FDR 9.3310-5) that are closely associated with inflammation [38][39][40]. Results were visualized using the “ggplot” package in R, as shown in Figure 10.
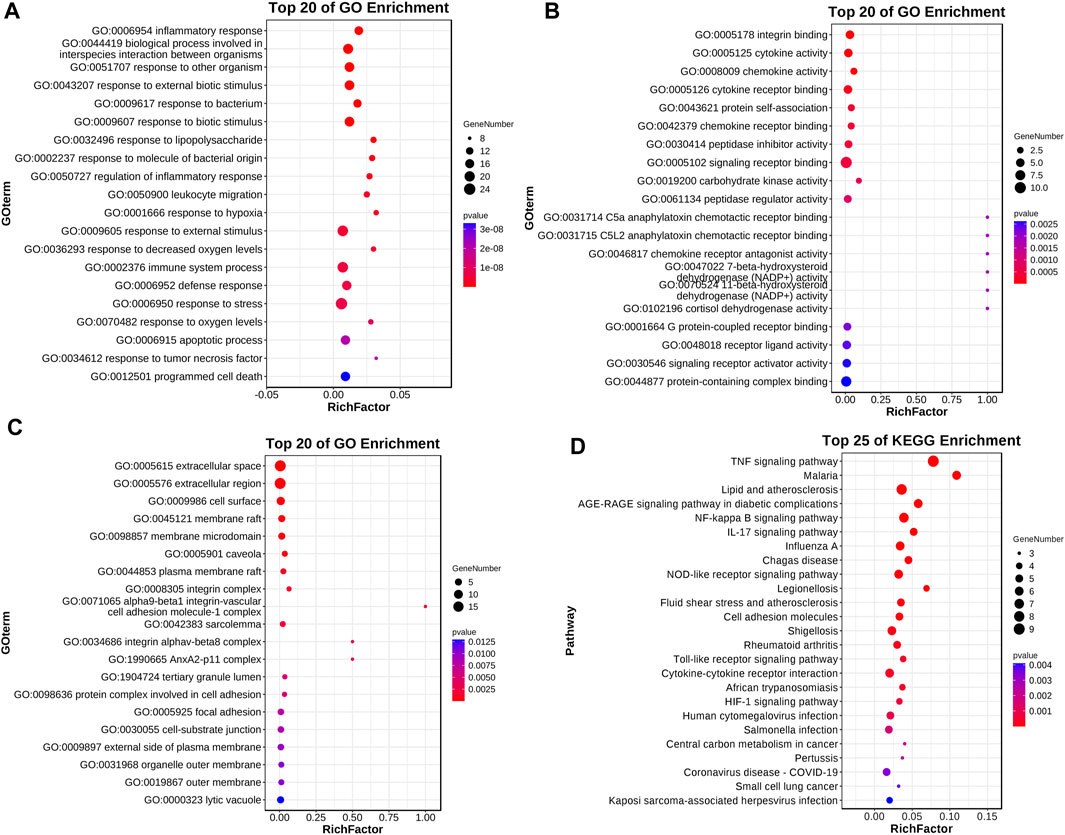
Figure 10. Roxadustat-related differential genes GO enrichment analysis and KEGG pathway analysis. Panel (A) displays the biological process enrichment, panel (B) shows the molecular function enrichment, panel (C) presents the cell component enrichment, and panel (D) illustrates the KEGG pathway analysis.
The network comprises 36 nodes and 130 edges, with an average node degree of 7.22. This complexity suggests a dense protein-protein interaction network with extensive interactions among the proteins. The observed number of edges significantly exceeds the expected number of 27, illustrating that the protein interactions surpass random connectivity. The PPI enrichment p-value is below 1.0E-16, emphasizing the significance and abundance of these interactions, which supports the reliability of the findings. Notably, proteins such as IL1B and CXCL8 have a degree of 20, indicating their potential as key nodes in the network. Similarly, ICAM1 has a degree of 18, and both CCL2 and CCL5 have degrees of 16, highlighting their important roles as depicted in Figure 11. These proteins, known for their role in inflammatory responses, are downregulated, suggesting that Roxadustat may help mitigate inflammation.
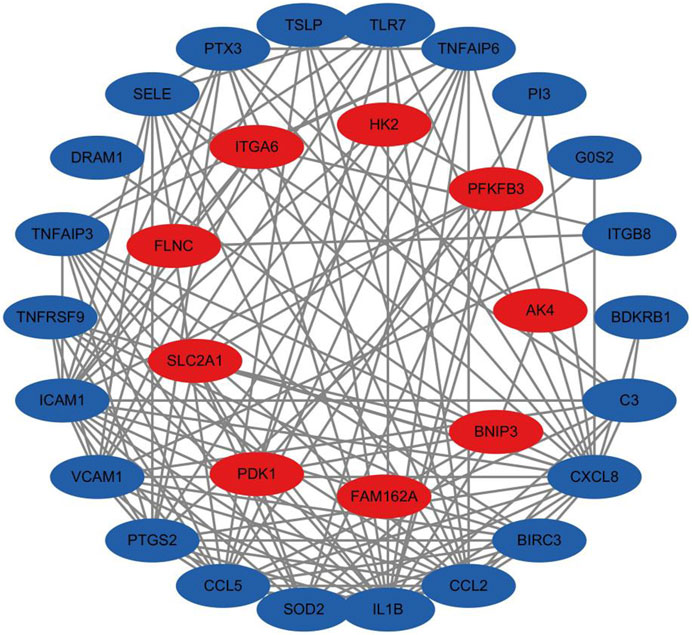
Figure 11. Protein interaction network analysis results, with red indicating genes of upregulated proteins and blue indicating genes of downregulated proteins.
Anemia is a frequent issue in chronic kidney disease, and the traditional treatment, erythropoiesis-stimulating agents (ESAs), can enhance life quality and reduce blood transfusion needs. However, high doses of ESAs may heighten the risk of cardiovascular problems and hasten kidney failure (Koulouridis et al., 2013). When ESAs show low efficacy, increasing the dose to meet the desired hemoglobin levels could also amplify these risks (Solomon et al., 2010). Under normal oxygen conditions, hypoxia-inducible factor prolyl hydroxylase inhibitors (HIF-PHIs) mimic low-oxygen effects by inhibiting prolyl hydroxylase domain (PHD) activity. This triggers a cascade of gene expressions related to hypoxia adaptation, boosts endogenous erythropoietin (EPO) production, elevates hemoglobin levels, and ameliorates anemia. Furthermore, HIF-PHIs can enhance iron metabolism by lowering hepcidin levels, thereby increasing iron absorption and mobilizing stored iron (Schwartz et al., 2019). Roxadustat, the first HIF-PHI introduced globally, enhances EPO production within normal ranges, improves iron metabolism, and effectively treats anemia in chronic kidney disease (Haase, 2017). Nonetheless, as HIF upregulation affects various metabolic pathways—including those involved in angiogenesis, lipid and glucose metabolism, glycolysis, mitochondrial function, cell growth, survival, vasodilation, and cell migration—the cardiovascular safety of Roxadustat is under scrutiny. Studies have shown that Roxadustat has a lower incidence of cardiovascular composite events compared to traditional ESA treatments (12% vs. 17%) (Provenzano et al., 2016). A meta-analysis focused on cardiovascular events in the treatment of anemia with ESA and Roxadustat revealed that Roxadustat appears safer concerning major adverse cardiac events (MACE), death, and heart failure. This safety profile may be linked to how Roxadustat maintains EPO production within a physiological range, unlike ESAs, which are administered in intermittent high doses, leading to peak EPO levels and a higher risk of cardiovascular events (Szczech et al., 2010). Additionally, the high doses of ESA needed to reach desired hemoglobin levels are associated with increased mortality rates (Koulouridis et al., 2013). Roxadustat, by producing endogenous EPO levels close to the physiological norm, minimizes the risk of adverse events (Gupta and Wish, 2017). Compared to ESAs, Roxadustat has less impact on blood pressure, can ameliorate cholesterol issues, lower blood glucose, enhance insulin sensitivity, and mitigate cardiovascular risk factors (Flamme et al., 2014; Provenzano et al., 2016; Riopel et al., 2020). Additionally, Roxadustat may improve myocardial ischemia and reduce the area of myocardial infarction (Deguchi et al., 2020). Animal studies indicate that HIF is crucial in myocardial ischemic preconditioning, enhancing heart muscle adaptability (Bishop and Ratcliffe, 2015).
In the absence of a database for Roxadustat in cardiovascular disease-related models, samples for this study were obtained from H. sapiens neuroblastoma cell lines, both with and without Roxadustat treatment. GO enrichment analysis revealed that Roxadustat primarily impacts the molecular function associated with integrin binding (GO:0005178), affecting genes such as ICAM1, VCAM1, IL1B, ITGB8, and ITGA6. These genes, which are linked to endothelial cells and inflammation, were identified using sources like The Human Gene Database (https://www.genecards.org/) and NCBI gene summaries. Roxadustat’s biological processes predominantly relate to the inflammatory response (GO:0006954). Integrins are crucial in endothelial and antigen-presenting cells within immune inflammation contexts (Takada et al., 2007), which are vital components of the vascular barrier and relate to cardiovascular events. Consequently, it is hypothesized that Roxadustat may influence endothelial cells and inflammatory responses, potentially impacting cardiovascular events, though further validation is needed. KEGG pathway analysis highlighted three significant pathways: the TNF signaling pathway, NF-κB signaling pathway, and the lipid and atherosclerosis pathway—all of which are closely associated with inflammation. The TNF signaling pathway is essential for regulating the inflammatory response (Yu et al., 2020). External stimuli cause cells to produce TNF-α, which activates several signaling pathways, including NF-κB, MAPK, and PI3K, leading to a cascade of biological effects. TNF-α specifically activates the NF-κB pathway, which promotes inflammatory factor expression in the nucleus (Hoesel and Schmid, 2013). Moreover, there is a strong link between lipid metabolism, atherosclerosis (Warnatsch et al., 2015; Libby, 2021), and inflammation. Protein interaction network analysis suggests that Roxadustat may help alleviate inflammation.
Hypoxia signaling is linked to calcification. Research using rodent models both in vivo and in vitro demonstrates that the hypoxia pathway may enhance vascular calcification (Mokas et al., 2016). HIF-1, a heterodimeric transcription factor, consists of an unstable α subunit (HIF-1α) and a stable β subunit (HIF-1β). In CKD mouse models, Roxadustat and Daprodustat have been shown to accelerate phosphate-induced calcification through activation of the HIF-1 pathway. However, extensive clinical studies are necessary to determine if these drugs heighten the risk of vascular calcification in CKD patients (Mokas et al., 2016; Tóth et al., 2022). Observational studies indicate that serum HIF-1α levels can predict vascular calcification in type 2 diabetes patients (Li et al., 2014). The 2020 guidelines from the Asia Pacific Society of Nephrology suggest that HIF-PHIs are linked with thrombosis and vascular calcification. Patients with a history of these conditions should exercise caution when using these drugs, and further studies are required to clarify the association between HIF-PHIs and these adverse outcomes (Yap et al., 2021).
Our study meticulously examined its limitations. The cohorts included, whether treated with ESA or Roxadustat, had varied drug dosages and study designs, potentially affecting the results due to inconsistency. The age, gender distribution, and comorbidities also varied across the cohorts, possibly influencing the outcomes due to heterogeneity. Moreover, no database exists for Roxadustat concerning cardiovascular disease in cell lines or animal models. We utilized biological samples from human neuroblastoma cell lines related to Roxadustat, which might have impacted data quality. Future in vivo or in vitro studies are encouraged to confirm the reliability of our findings. Despite these limitations, our sensitivity analysis did not reveal significant heterogeneity, and efforts were made to identify databases aligned with our research objectives. Overall, our study provides a deeper investigation into the cardiovascular safety of Roxadustat, aiming to aid in the development of improved clinical treatment strategies.
A meta-analysis of cohorts in the general and American populations showed no significant differences in cardiovascular event rates between patients treated with Roxadustat and ESA for anemia associated with chronic kidney disease. However, the Roxadustat group appears to exhibit a safer profile concerning major adverse cardiovascular events (MACE), death, and heart failure. Bioinformatics analysis indicates that Roxadustat may influence integrin adhesion and affect the TNF and NF-κB signaling pathways, along with lipid and atherosclerosis pathways, potentially reducing inflammation.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
XmL: Writing–original draft, Writing–review and editing. SJ: Data curation, Methodology, Writing–review and editing. XG: Data curation, Methodology, Writing–review and editing. XjL: Data curation, Methodology, Writing–review and editing. SS: Writing–review and editing. JZ: Data curation, Methodology, Writing–review and editing. KP: Data curation, Methodology, Writing–review and editing. WL: Data curation, Methodology, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was Supported by the China Postdoctoral Science Foundation under Grant Number 2023M733986 and 2023T160741.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Bishop, T., and Ratcliffe, P. J. (2015). HIF hydroxylase pathways in cardiovascular physiology and medicine. Circ. Res. 117 (1), 65–79. doi:10.1161/CIRCRESAHA.117.305109
Charytan, C., Manllo-Karim, R., Martin, E. R., Steer, D., Bernardo, M., Dua, S. L., et al. (2021). A randomized trial of roxadustat in anemia of kidney failure: SIERRAS study. Kidney Int. Rep. 6 (7), 1829–1839. doi:10.1016/j.ekir.2021.04.007
Chen, N., Hao, C., Liu, B. C., Lin, H., Wang, C., Xing, C., et al. (2019b). Roxadustat treatment for anemia in patients undergoing long-term dialysis. N. Engl. J. Med. 381 (11), 1011–1022. doi:10.1056/NEJMoa1901713
Chen, N., Hao, C., Peng, X., Lin, H., Yin, A., Hao, L., et al. (2019a). Roxadustat for anemia in patients with kidney disease not receiving dialysis. N. Engl. J. Med. 381 (11), 1001–1010. doi:10.1056/NEJMoa1813599
Chen, N., Qian, J., Chen, J., Yu, X., Mei, C., Hao, C., et al. (2017). Phase 2 studies of oral hypoxia-inducible factor prolyl hydroxylase inhibitor FG-4592 for treatment of anemia in China. Nephrol. Dial. Transpl. 32 (8), 1373–1386. doi:10.1093/ndt/gfx011
Chertow, G. M., Pergola, P. E., Farag, Y. M. K., Agarwal, R., Arnold, S., Bako, G., et al. (2021). Vadadustat in patients with anemia and non-dialysis-dependent CKD. N. Engl. J. Med. 384 (17), 1589–1600. doi:10.1056/NEJMoa2035938
Cizman, B., Smith, H. T., Camejo, R. R., Casillas, L., Dhillon, H., Mu, F., et al. (2020). Clinical and economic outcomes of erythropoiesis-stimulating agent hyporesponsiveness in the post-bundling era. Kidney Med. 2 (5), 589–599. doi:10.1016/j.xkme.2020.06.008
Deguchi, H., Ikeda, M., Ide, T., Tadokoro, T., Ikeda, S., Okabe, K., et al. (2020). Roxadustat markedly reduces myocardial ischemia reperfusion injury in mice. Circ. J. 84 (6), 1028–1033. doi:10.1253/circj.CJ-19-1039
Fishbane, S., Pollock, C. A., El-Shahawy, M., Escudero, E. T., Rastogi, A., Van, B. P., et al. (2022). Roxadustat versus epoetin Alfa for treating anemia in patients with chronic kidney disease on dialysis: results from the randomized phase 3 ROCKIES study. J. Am. Soc. Nephrol. 33 (4), 850–866. doi:10.1681/ASN.2020111638
Fishbane, S., Schiller, B., Locatelli, F., Covic, A. C., Provenzano, R., Wiecek, A., et al. (2013). Peginesatide in patients with anemia undergoing hemodialysis. N. Engl. J. Med. 368 (4), 307–319. doi:10.1056/NEJMoa1203165
Flamme, I., Oehme, F., Ellinghaus, P., Jeske, M., Keldenich, J., and Thuss, U. (2014). Mimicking hypoxia to treat anemia: HIF-stabilizer BAY 85-3934 (Molidustat) stimulates erythropoietin production without hypertensive effects. PLoS One 9 (11), e111838. doi:10.1371/journal.pone.0111838
Fujii, H., Hamano, T., Tsuchiya, K., Kuragano, T., Joki, N., Tsuruya, K., et al. (2023). Not baseline but time-dependent erythropoiesis-stimulating agent responsiveness predicts cardiovascular disease in hemodialysis patients receiving epoetin beta pegol: a multicenter prospective PARAMOUNT-HD Study. Int. J. Cardiol. 375, 110–118. doi:10.1016/j.ijcard.2022.12.051
Gupta, N., and Wish, J. B. (2017). Hypoxia-inducible factor prolyl hydroxylase inhibitors: a potential new treatment for anemia in patients with CKD. Am. J. Kidney Dis. 69 (6), 815–826. doi:10.1053/j.ajkd.2016.12.011
Haase, V. H. (2017). HIF-prolyl hydroxylases as therapeutic targets in erythropoiesis and iron metabolism. Hemodial. Int. 21 (Suppl. 1), S110–s24. doi:10.1111/hdi.12567
Hoesel, B., and Schmid, J. A. (2013). The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 12, 86. doi:10.1186/1476-4598-12-86
Johnson, D. W., Pollock, C. A., and Macdougall, I. C. (2007). Erythropoiesis-stimulating agent hyporesponsiveness. Nephrol. Carlt. 12 (4), 321–330. doi:10.1111/j.1440-1797.2007.00810.x
Koulouridis, I., Alfayez, M., Trikalinos, T. A., Balk, E. M., and Jaber, B. L. (2013). Dose of erythropoiesis-stimulating agents and adverse outcomes in CKD: a metaregression analysis. Am. J. Kidney Dis. 61 (1), 44–56. doi:10.1053/j.ajkd.2012.07.014
Li, G., Lu, W. H., Ai, R., Yang, J. H., Chen, F., and Tang, Z. Z. (2014). The relationship between serum hypoxia-inducible factor 1α and coronary artery calcification in asymptomatic type 2 diabetic patients. Cardiovasc Diabetol. 13, 52. doi:10.1186/1475-2840-13-52
Libby, P. (2021). Inflammation during the life cycle of the atherosclerotic plaque. Cardiovasc Res. 117 (13), 2525–2536. doi:10.1093/cvr/cvab303
Macdougall, I. C., Provenzano, R., Sharma, A., Spinowitz, B. S., Schmidt, R. J., Pergola, P. E., et al. (2013). Peginesatide for anemia in patients with chronic kidney disease not receiving dialysis. N. Engl. J. Med. 368 (4), 320–332. doi:10.1056/NEJMoa1203166
Malyszko, J., Drozdz, M., Zolkiewicz, A., and Rutkowski, B. (2014). Renal anaemia treatment in haemodialysis patients in the Central and Eastern European countries in everyday clinical practice follow-up. Int. Urol. Nephrol. 46 (1), 71–82. doi:10.1007/s11255-012-0303-0
Mc Causland, F. R., Claggett, B., Burdmann, E. A., Chertow, G. M., Cooper, M. E., Eckardt, K. U., et al. (2019). Treatment of anemia with Darbepoetin prior to dialysis initiation and clinical outcomes: analyses from the trial to reduce cardiovascular events with aranesp therapy (TREAT). Am. J. Kidney Dis. 73 (3), 309–315. doi:10.1053/j.ajkd.2018.10.006
Mokas, S., Larivière, R., Lamalice, L., Gobeil, S., Cornfield, D. N., Agharazii, M., et al. (2016). Hypoxia-inducible factor-1 plays a role in phosphate-induced vascular smooth muscle cell calcification. Kidney Int. 90 (3), 598–609. doi:10.1016/j.kint.2016.05.020
Provenzano, R., Besarab, A., Wright, S., Dua, S., Zeig, S., Nguyen, P., et al. (2016). Roxadustat (FG-4592) versus epoetin Alfa for anemia in patients receiving maintenance hemodialysis: a phase 2, randomized, 6- to 19-week, open-label, active-comparator, dose-ranging, safety and exploratory efficacy study. Am. J. Kidney Dis. 67 (6), 912–924. doi:10.1053/j.ajkd.2015.12.020
Provenzano, R., Shutov, E., Eremeeva, L., Korneyeva, S., Poole, L., Saha, G., et al. (2021b). Roxadustat for anemia in patients with end-stage renal disease incident to dialysis. Nephrol. Dial. Transpl. 36 (9), 1717–1730. doi:10.1093/ndt/gfab051
Provenzano, R., Szczech, L., Leong, R., Saikali, K. G., Zhong, M., Lee, T. T., et al. (2021a). Efficacy and cardiovascular safety of roxadustat for treatment of anemia in patients with non-dialysis-dependent CKD: pooled results of three randomized clinical trials. Clin. J. Am. Soc. Nephrol. 16 (8), 1190–1200. doi:10.2215/CJN.16191020
Riopel, M., Moon, J. S., Bandyopadhyay, G. K., You, S., Lam, K., Liu, X., et al. (2020). Inhibition of prolyl hydroxylases increases hepatic insulin and decreases glucagon sensitivity by an HIF-2α-dependent mechanism. Mol. Metab. 41, 101039. doi:10.1016/j.molmet.2020.101039
Saglimbene, V., Palmer, S. C., Craig, J. C., Ruospo, M., Nicolucci, A., Tonelli, M., et al. (2017). Low versus high dose erythropoiesis-stimulating agents in hemodialysis patients with anemia: a randomized clinical trial. PLoS One 12 (3), e0172735. doi:10.1371/journal.pone.0172735
Schwartz, A. J., Das, N. K., Ramakrishnan, S. K., Jain, C., Jurkovic, M. T., Wu, J., et al. (2019). Hepatic hepcidin/intestinal HIF-2α axis maintains iron absorption during iron deficiency and overload. J. Clin. Invest. 129 (1), 336–348. doi:10.1172/JCI122359
Singh, A. K., Carroll, K., Perkovic, V., Solomon, S., Jha, V., Johansen, K. L., et al. (2021). Daprodustat for the treatment of anemia in patients undergoing dialysis. N. Engl. J. Med. 385 (25), 2325–2335. doi:10.1056/NEJMoa2113379
Solomon, S. D., Uno, H., Lewis, E. F., Eckardt, K. U., Lin, J., Burdmann, E. A., et al. (2010). Erythropoietic response and outcomes in kidney disease and type 2 diabetes. N. Engl. J. Med. 363 (12), 1146–1155. doi:10.1056/NEJMoa1005109
Stirnadel-Farrant, H. A., Karaboyas, A., Cizman, B., Bieber, B. A., Kler, L., Jones, D., et al. (2019). Cardiovascular event rates among hemodialysis patients across geographical regions-A snapshot from the dialysis outcomes and practice patterns study (DOPPS). Kidney Int. Rep. 4 (6), 864–872. doi:10.1016/j.ekir.2019.03.016
Stirnadel-Farrant, H. A., Luo, J., Kler, L., Cizman, B., Jones, D., Brunelli, S. M., et al. (2018). Anemia and mortality in patients with nondialysis-dependent chronic kidney disease. BMC Nephrol. 19 (1), 135. doi:10.1186/s12882-018-0925-2
Szczech, L. A., Barnhart, H. X., Sapp, S., Felker, G. M., Hernandez, A., Reddan, D., et al. (2010). A secondary analysis of the CHOIR trial shows that comorbid conditions differentially affect outcomes during anemia treatment. Kidney Int. 77 (3), 239–246. doi:10.1038/ki.2009.415
Takada, Y., Ye, X., and Simon, S. (2007). The integrins. Genome Biol. 8 (5), 215. doi:10.1186/gb-2007-8-5-215
Tóth, A., Csiki, D. M., Nagy, B., Balogh, E., Lente, G., Ababneh, H., et al. (2022). Daprodustat accelerates high phosphate-induced calcification through the activation of HIF-1 signaling. Front. Pharmacol. 13, 798053. doi:10.3389/fphar.2022.798053
Warnatsch, A., Ioannou, M., Wang, Q., and Papayannopoulos, V. (2015). Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 349 (6245), 316–320. doi:10.1126/science.aaa8064
Yap, D. Y. H., McMahon, L. P., Hao, C. M., Hu, N., Okada, H., Suzuki, Y., et al. (2021). Recommendations by the Asian Pacific society of nephrology (APSN) on the appropriate use of HIF-PH inhibitors. Nephrol. Carlt. 26 (2), 105–118. doi:10.1111/nep.13835
Keywords: Roxadustat, erythropoietin stimulating agents, cardiovascular events, meta-analysis, inflammatory responses
Citation: Li X, Jiang S, Gu X, Liu X, Shang S, Zhang J, Pang K and Li W (2024) Assessment of the safety of Roxadustat for cardiovascular events in chronic kidney disease-related anemia using meta-analysis and bioinformatics. Front. Pharmacol. 15:1380326. doi: 10.3389/fphar.2024.1380326
Received: 01 February 2024; Accepted: 31 May 2024;
Published: 19 June 2024.
Edited by:
Wenhu Liu, Capital Medical University, ChinaReviewed by:
Ang Luo, Northwest A&F University, ChinaCopyright © 2024 Li, Jiang, Gu, Liu, Shang, Zhang, Pang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenge Li, d2VuZ2VfbGVlMjAwMkAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.