- 1Department of Pharmacy, Changzheng Hospital, Second Military Medical University, Shanghai, China
- 2Institute of Chinese Materia Madica, Shanghai University of Traditional Chinese Medicine, Shanghai, China
Background: Scutellaria baicalensis, the dry root of scutellaria baicalensis georgi, is a traditional Chinese medicine with long. In clinic, scutellaria baicalensis is commonly used in prescription for the treatment of depression. Additionally, numerous pre-clinical studies have shown that Scutellaria baicalensis and its active constituents are effective for depression. In this study, we aims to systematically review the roles of scutellaria baicalensis in depression and summarize the possible mechanism.
Methods: A systematic review and meta-analysis were conducted to analyze the existing studies on the effects of scutellaria baicalensis on depression in animal models. Briefly, we searched electronic databases including Pubmed and Embase for preclinical trial studies from inception to September 2023. The items in each study were evaluated by two independent reviewers, and meta-analyses were performed on scutellaria baicalensis-induced behavioral changes in the study. Finally, random effects model is used to collect data.
Results: A total of 49 studies were identified, and 13 studies were included in the final analysis. They all reported the different antidepressant effects of scutellaria baicalensis and the underlying biological mechanisms. Among the included 13 studies, the results of eight articles SPT[SMD = −2.80, 95%CI(-4.03, -1.57), p < 0.01], the results of the nine articles OFT[SMD = −2.38, 95%CI(-3.53, -1.23), p < 0.01], and the results of two articles NSFT[SMD = −2.98, 95%CI(-3.94, -2.02), p < 0.01] were significantly different from the control group. The risk of bias was moderate in all studies, however, there was a significant heterogeneity among studies.
Conclusion: These results preliminarily suggest that scutellaria baicalensis can alleviate depressive behaviors and modulate underlying mechanisms, which is expected to be a promising antidepressant.
1 Introduction
Depression (major depressive disorder), a common mental disease, is manifested by persistent feeling of sadness and loss of interest. This kind of disease can disturb person’s ability to work, study, sleep, eat, and enjoy once pleasurable activities. What’s more, people with depression possess cognitive behavioral, social dysfunction, and even suicide tendency in severe cases (Vos et al., 2017; Dwyer Jennifer et al., 2020). In recent years, its commonly defined that depression is global burden of disease with high mortality and morbidity, and high disability rate. In America, In 2017, the World Health Organization revealed that there were more than 300 million depression patients in the world, accounting for about 4.4% of the global population (World Health Organization WHO, 2017). Depression accounts for a large share of the global disease burden, with approximately 264 million people globally estimated to suffer from the condition (World Health Organization (WHO)). In China, a cross-sectional epidemiological study from 2013.07 to 2015.03 revealed that the economic burden of China is about 2.5 trillion US dollars, accounting for 10% of the total global disease burden (Jin et al., 2021). A cross-national comparison reported that lifetime prevalence estimates of major depressive disorders ranged from 1.0% (Czech Republic) to 16.9% (United States), with midpoints at 8.3% (Canada) and 9.0% (Chile), while the 12-month prevalence estimates ranged from 0.3% (Czech Republic) to 10% (United States), with midpoints at 4.5% (Mexico) and 5.2% (West Germany) (Kessler Ronald and Bromet Evelyn, 2013). Another cross-sectional survey analysis conducted in USA also pointed that individuals with depression diagnosis have substantial humanistic and economic burden (Jain et al., 2022). In 2015, The World Health Organization ranked depression as the single largest contributor to global disability, accounting for 7.5% of all years lived with disability (World Health Organization WHO, 2017). These epidemiological studies highlight that depressive disorder is a current issue for public health and will be a future challenge.
More and more studies on depression have shown that numerous factors, such as age, genetics, biology, and environment, influence depression morbidity and mortality (Hammen, 2018; Barrenetxea et al., 2022; Mars et al., 2022). Koh et al. reported that the incidence of population (70–80 years) was higher when compared with the population (60–69) (Barrenetxea et al., 2022). Bai et al. also revealed that in the last 3 decades, the incidence rate of depression among older individuals has increased though the age-standard incidence rate of depression has declined in China (Bai et al., 2022). In addition, the impact of other diseases such as cardiovascular diseases, obesity, and hypertension can not be ignored in recent years (Li et al., 2022). Indeed, there is a bidirectional association between cardiovascular diseases (Bobo et al., 2020), obesity (Luppino et al., 2010), hypertension (Jokela et al., 2014) and anxiety. In Korea, Park et al. found that depression increased the risk of ischemic heart disease by 38% and cerebrovascular disease by 46% among older adults through retrospective cohort study (Park et al., 2020). In addition, people with cardiovascular disease have a significantly increased risk of depression (Lesman-Leegte et al., 2009). An overview of a meta-analysis showed that Obese adults were 55 percent more likely to be depressed, and depressed adults were 58 percent more likely to be obese (Luppino et al., 2010). Lu et al. reported that incidence of depression in China were more high in women than that in men, unemployed people than employed, and those who were separated, widowed, or divorced than people who were married or cohabiting (Jin et al., 2021).
Presently, the clinical treatment strategy for depression contains first-line antidepressant drugs, cognitive-behavioral therapy, and physiotherapy (Kverno and Mangano, 2021). And the first-line antidepressant drugs includes selective serotonin reuptake inhibitor (SSRIs, fluoxetine, paroxetine, and sertraline), serotonin and noradrenaline reuptake inhibitor (SNRIs, venlafaxine, and duloxetine), noradrenergic and specific serotonergic antidepressants (NaSSA, mirtazapine), serotonin receptor antagonists and reuptake inhibitors (SARIs, trazodone), monoamine oxidase inhibitor (MAOI, moclobemide), and tricyclic antidepressants (TCA, imipramine) (Plenge et al., 2021). What’s more, ketamine (Nikayin et al., 2022) and nitrous oxide (Quach et al., 2022) are also used for the resistant depression. However, the cure rate of first-line antidepressants is low, and the adverse reactions of these drugs are obvious, and a response to conventional antidepressants requires several weeks of treatment and carries a non-negligible risk of suicide. Therefore, there is a major medical need for novel and improved antidepressant treatments. Acupuncture and herbal medicine were also used for the treatment of depression, and herbal medicine were shown to had superior efficacy and safety profiles (Chen and Shan, 2019).
Scutellaria baicalensis georgi is a herbal medicine frequently used in China, and its dry root (common name: Huang-Qin in Chinese) is widely used in prescription for the treatment of depression (Zhang et al., 2015; Lee et al., 2017). The beneficial effects of the root are due to different bioactive compounds in the brain, some of which are able to cross the blood-brain barrier (BBB). As far as it concerns scutellaria baicalensis, this corresponds to the two main flavonoids, namely, baicalin and baicalein, being purified from the plant’s dry roots (Wang et al., 2018; Zhao et al., 2019). Previous studies have shown that scutellaria baicalensis has a wide range of pharmacological effects including anti-inflammatory, anti-oxidative, neuroprotective, antibacterial, and anti-tumor activities (Zhao et al., 2016; Zhou et al., 2016; Zhao Yikai et al., 2018; Yoon et al., 2020). It has been found that Scutellaria baicalensis and its main components baicalin and baicalein have significant anti-depression effects and mechanism involves many aspects, such as improving the level of monoamine transmitter brain neurotrophic factor, regulating the HPA axis, anti-inflammation, anti-oxidation and promoting neurogenesis (Hai-Yang et al., 2016; Pazini Francis et al., 2017). In addition, as a traditional medicine, Scutellaria baicalensis has produced neuroprotective effects in various models of Parkinson’s disease (Mu X. et al., 2011), Alzheimer’s disease (Zhao J. et al., 2018)and so on. Recent studies have shown that baicalin and baicalein, in addition to protecting dopaminergic neurons from mitochondrial and oxidation-related toxicity, may also have a beneficial effect on DA-related brain diseases by increasing DA levels in the brain (Im H. I. et al., 2005; Zhao J. et al., 2018).
At present, accumulating evidence from the pharmacological effect indicated that scutellaria baicalensis may have great potential in treating depression. Nevertheless, up to now, the pre-clinical studies on scutellaria baicalensis for depression have not been systematically evaluated and summarized. In this study, we conducted a rigorous and comprehensive systematic review and meta-analysis of recent literature on the treatment of depression model animals by scutellaria baicalensis, and explored different behavioral changes and potential mechanisms, aiming to provide evidence and guidance for clinical practice.
2 Materials and methods
2.1 Search strategies
We searched relevant databases, including PubMed, Web of Science, Embase, and CNKI from inception to September 2023. The main search terms were composed by “Scutellaria baicalensis” or Radix Scutellariae [tiab] OR Scutellaria [tiab] OR baicalin [Mesh] OR baicalein [Mesh] AND (Depression [Mesh] OR “Depressive disorder” [Mesh] OR Depress [tiab] OR “emotional disorder” [tiab] OR “psychological disorder” [tiab] OR “psychological distress” [tiab] OR “emotional distress” [tiab] OR “emotional stress" [tiab]. Subsequently, the two researchers (Ying Ma and Xun Zhou) independently reviewed the title/abstract related to the topic. A full-text read was also performed to find the potential documents that met the eligibility criteria. Importantly, any disagreements between the two researchers were resolved through negotiation or thirdparty consensus.
2.2 Inclusion and exclusion criteria
Studies were be included when they meet the following criteria: (1) in vivo studies on animal subjects; (2) the animal disease model was depressive disorder model; (3) animals were treated with scutellaria baicalensis or its active components baicalin and baicalin; (4) the data included in the literature were represented by mean and standard (SD) or can be converted to mean and SD. Exclusion criteria are as follows: (1) other types of studies (in vitro studies, case reports, clinical trials, reviews, abstracts or comments), (2) combination with other compounds, (3) not depressive disorder model, (5) studies with insufficient data, (6) the sample size of control group and scutellaria treatment group was less than three animals. (7) plagiarism or duplicate publication of literature.
2.3 Data extraction
General data, intervention measures, efficacy indicators, test results and other data of patients were independently extracted by two researchers (Ying Ma and Xun Zhou) according to a unified table and cross-checked. The following information for each study include: (1) the year of publication of the first author’s name; (2) characteristics of the animal, including species, number, sex, weight, etc.; (3) the establishment of depression model and anesthesia used in the model; (4) Characteristics of intervention, including dose and route of administration; (5) main outcome indicators and differences between groups. If the main data were lost or displayed in a graphical manner, we would contact the publishers to obtain the original data. The values in the graph were measured by digital ruler software without receiving any reply from the author.
2.4 Quality assessment of included studies
The methodological quality of the included studies was evaluated by independently two investigators (Ying Ma and Xun Zhou) according to the Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies (CAMARADES)’s risk of bias tool (Macleod et al., 2004). The terms for quality assessment included 1) peer reviewed publication; 2) control of temperature; 3) random allocation to groups; 4) blinded induction of depression; 5) blinded assessment of behavioral outcome; 6) use of anesthetic without significant intrinsic neuroprotective activity; 7) calculation of the sample size necessary to achieve sufficient power; 8) appropriate animal model which uses animals without relevant comorbidities (aged, diabetic, or hypertensive); 9) compliance with animal welfare Regulations; 10) statement of potential conflict of interests. Any disagreements between the two researchers were resolved through negotiation or third party consensus.
2.5 Statistical analysis
Meta-analysis was performed using Review Manager (RevMan v5.3) software. Outcome measures were all expressed as continuous data and standardized mean difference (SMD) with 95% confidence interval (CI). If there was no statistical heterogeneity among studies (p ≥ 0.10, I2 ≤ 5%), fixed-effect model was used for analysis. Otherwise, random effect model is used to analyze. Probability value p < 0.05 was considered statistically significant.
3 Results
3.1 Study selection
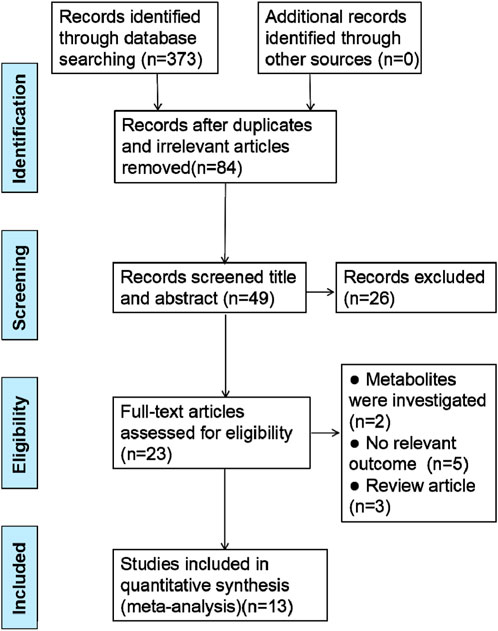
In the initial search of databases, 373 literature were retrieved. After eliminating redundant and irrelevant articles, 84 records remained. Subsequently, the investigators screened the titles and abstracts, and 26 studies were exclude. After reviewing the full-text articles carefully, 10 studies were excluded for at least one of the following reasons: (1) metabolites were studied; (2) no relevant outcome; (3) review article. Ultimately, 13 studies were included in this meta analysis. The search strategy built on this study using the PRISMA method (Moher et al., 2009) is described in Figure 1.
3.2 Characteristics of included studies
The basic characteristics of the 13 studies were shown in Table 1. The meta-analysis included 270 animals (136 in the model group and 134 in the Scutellaria baicalensis treatment group) in 13 studies.
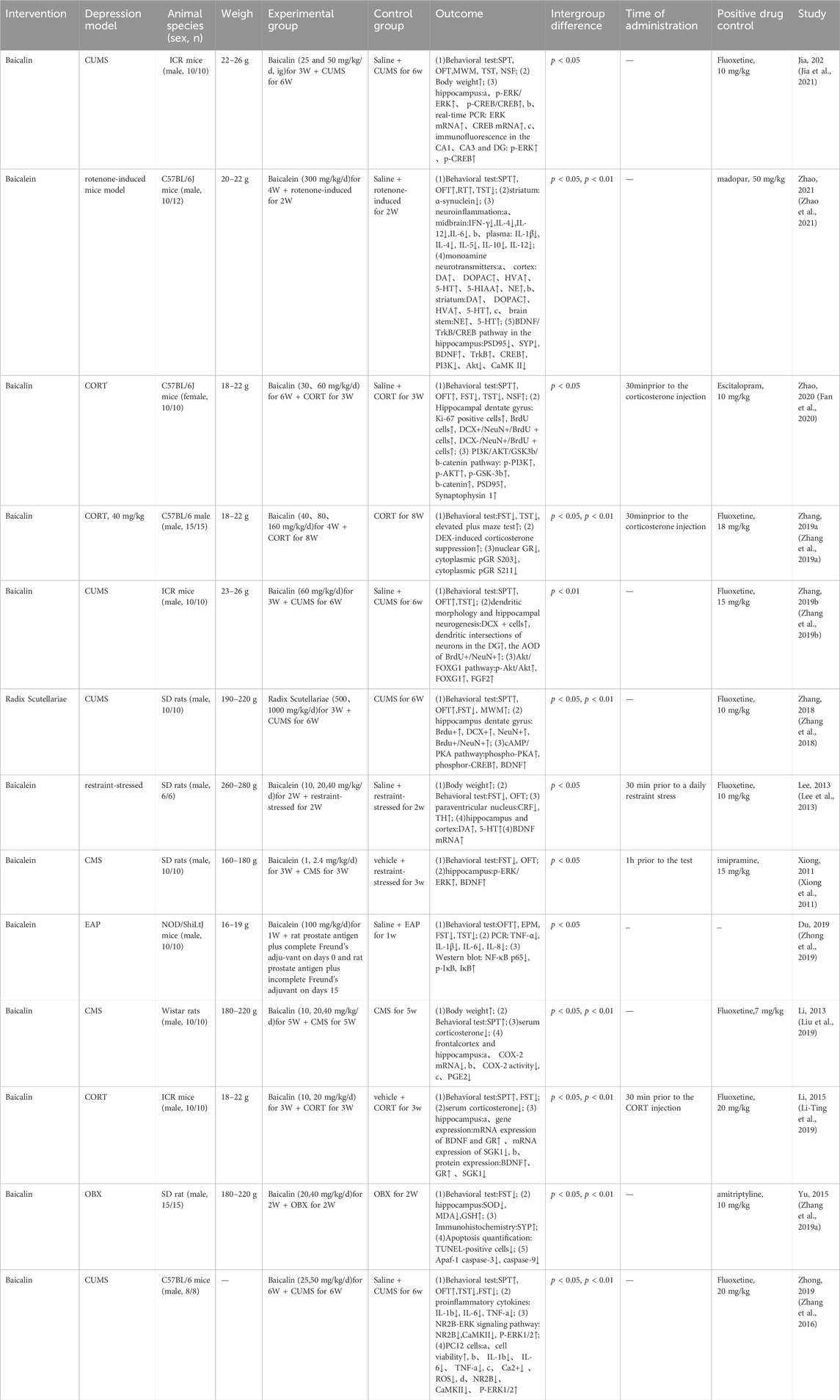
Table 1. Characteristics of studies included in systematic review of antidepressant effects of Scutellaria baicalensis.
3.2.1 Animals
C57BL/6 mice were used in 4 studies (Zhang et al., 2016; Zhang Kuo et al., 2019; Fan et al., 2020; Zhao et al., 2021), ICR mice were used in 3 studies (Zhang Ruyi et al., 2019; Li-Ting et al., 2019; Jia et al., 2021), and non-obese diabetic mice was used in only one study; Sprague Dawley rats were used in 4 studies (Xiong et al., 2011; Lee et al., 2013; Zhang et al., 2018; Zhang Kuo et al., 2019), and Wistar rats was used in one study (Liu et al., 2019). All but one of the studies involved males (Fan et al., 2020); The body weight of Sprague Dawley rats ranged from 160 g to 280 g, while the body weight of mice ranged from 18 g to 26 g. In the studies included in this meta-analysis, Chronic unpredictability mild stress (CUMS), corticosterone (CORT), rotenone, experimental autoimmune prostatitis (EAP), olfactory bulbectomy (OBX), and repeated restraint stress were used to construct the animal depression model. Currently, it is defined that chronic unpredictability mild stress (CUMS) is a valuable model to evaluate the etiology of depression. Among these studies, CUMS was adopted in six studies (Xiong et al., 2011; Zhang et al., 2016; Zhang et al., 2018; Zhang Ruyi et al., 2019; Liu et al., 2019; Jia et al., 2021). Long-term exposure to CORT was used in three studies (Zhang Kuo et al., 2019; Li-Ting et al., 2019; Fan et al., 2020). Rotenone (Zhao et al., 2021), EAP (Zhong et al., 2019), OBX (Zhang Kuo et al., 2019), and repeated restraint stress (Lee et al., 2013) were also applied in one studies, respectively.
3.2.2 Interventions
The animals in eight studies were treated with baicalin (Zhang et al., 2016; Zhang Kuo et al., 2019; Zhang Ruyi et al., 2019; Li-Ting et al., 2019; Liu et al., 2019; Fan et al., 2020; Jia et al., 2021), four with baicalein (Xiong et al., 2011; Lee et al., 2013; Zhong et al., 2019; Zhao et al., 2021), and one with Radix Scutellariae (Zhang et al., 2018). In the 13 studies, the duration of drug administration varied, with five studies lasting 3 weeks (Xiong et al., 2011; Zhang et al., 2018; Zhang Ruyi et al., 2019; Li-Ting et al., 2019; Jia et al., 2021), two studies lasting 4 weeks (Zhang Kuo et al., 2019; Zhao et al., 2021), two studies lasting 6 weeks (Zhang et al., 2016; Fan et al., 2020), two study lasting 2 weeks (Lee et al., 2013; Zhang Kuo et al., 2019), one study lasting 1 weeks (Zhong et al., 2019), and one study lasting 5 weeks (Zhang et al., 2016). All drugs were administered intragastrically and vehicles or saline were administered to the control group in all studies.
3.3 Methodological quality
The assessment of the quality of these studies included in these work was conducted from CAMARADES. As shown in table 2, the quality score ranged from 6 to 7, with median of 6.615. All of the studies have been peer-reviewed and reported. All studies have reported that animals were randomly divided into groups and blinded assessment of behavioral outcome. In addition, there were no study reported blinded induction of depression.
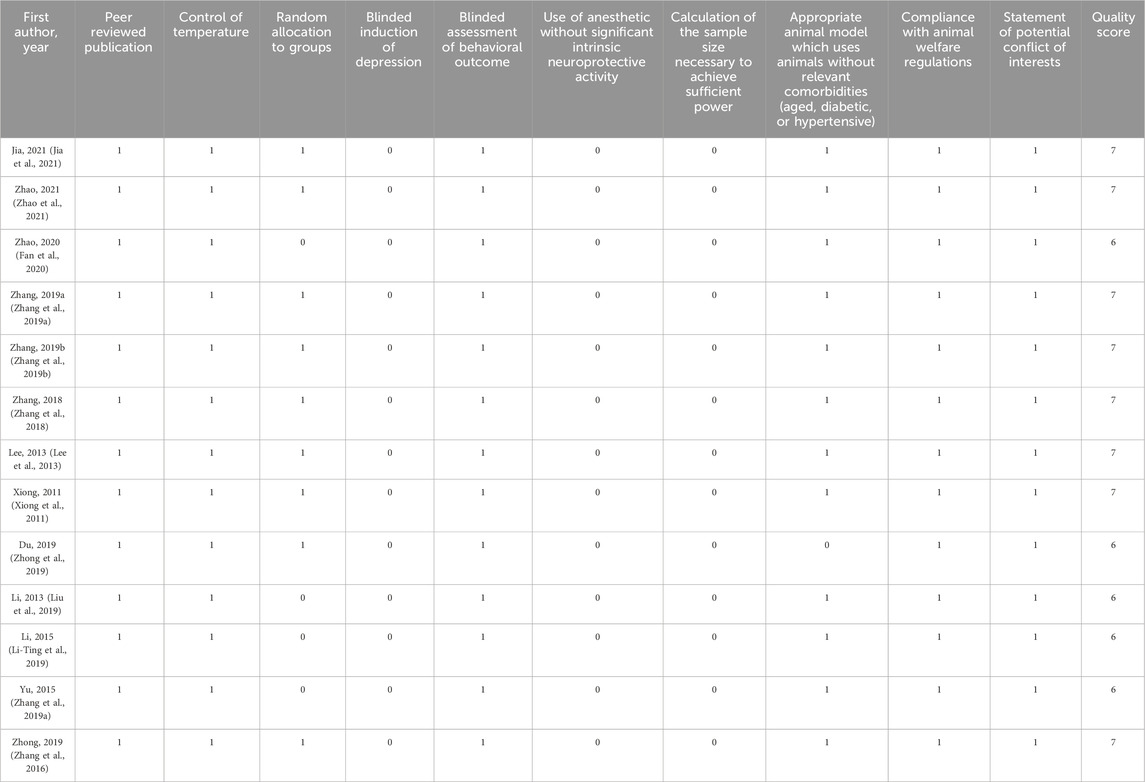
Table 2. Quality assessment of studies included in systematic review of antidepressant effects of Scutellaria Baicalensis following modified scale of CAMARADES.
3.4 Effects of scutellaria baicalensis on depression
3.4.1 Outcome measures
The outcome measures in the 13 studies included behavioral change, physiological change, and histological analysis. For the measurment of behavioral change, six behavioral tests were commonly used, namely, sucrose preference test (SPT) (Zhang et al., 2016; Zhang et al., 2018; Zhang Ruyi et al., 2019; Li-Ting et al., 2019; Liu et al., 2019; Fan et al., 2020; Jia et al., 2021; Zhao et al., 2021), open field test (OFT) (Xiong et al., 2011; Lee et al., 2013; Zhang et al., 2016; Zhang et al., 2018; Zhang Ruyi et al., 2019; Zhong et al., 2019; Fan et al., 2020; Jia et al., 2021; Zhao et al., 2021), morris water maze test (Zhang et al., 2018; Jia et al., 2021), tail suspension test (Zhang et al., 2016; Zhang Kuo et al., 2019; Zhang Ruyi et al., 2019; Zhong et al., 2019; Fan et al., 2020; Jia et al., 2021; Zhao et al., 2021), novelty suppressed feeding test (NSFT) (Fan et al., 2020; Jia et al., 2021), forced swimming test (Xiong et al., 2011; Lee et al., 2013; Zhang et al., 2016; Zhang Kuo et al., 2019; Li-Ting et al., 2019; Zhong et al., 2019; Fan et al., 2020). For the determination of physiological change, body weight, sucrose intake and sleep were assessed (Liu et al., 2019; Jia et al., 2021). For the detection of histological analysis, all of the studies have all focused on the hippocampus, and some of these studies also assessed a broader range of regions, such as striatum (Zhao et al., 2021), paraventrnucleus (Lee et al., 2013), cortex (Lee et al., 2013; Liu et al., 2019; Zhao et al., 2021), midbrain (Zhao et al., 2021) or brain stem (Zhao et al., 2021).
3.4.2 Effects of scutellaria baicalensis on depression by SPT analysis
A total of eight studies (Zhang et al., 2016; Zhang et al., 2018; Zhang Ruyi et al., 2019; Li-Ting et al., 2019; Liu et al., 2019; Fan et al., 2020; Jia et al., 2021; Zhao et al., 2021)compared the differences in SPT before and after treatment with 158 animals, including 78 in the experimental group and 80 in the control group. Due to significant heterogeneity between studies (p < 0.00001, I2 = 90%), the random-effects model was adopted. The difference between the two groups was statistically significant [SMD = −2.80, 95%CI(-4.03, -1.57), p < 0.01], suggesting that scutellaria baicalensis could significantly enhance the sucrose preference rate in depressed animals, as shown in Figure 2A.
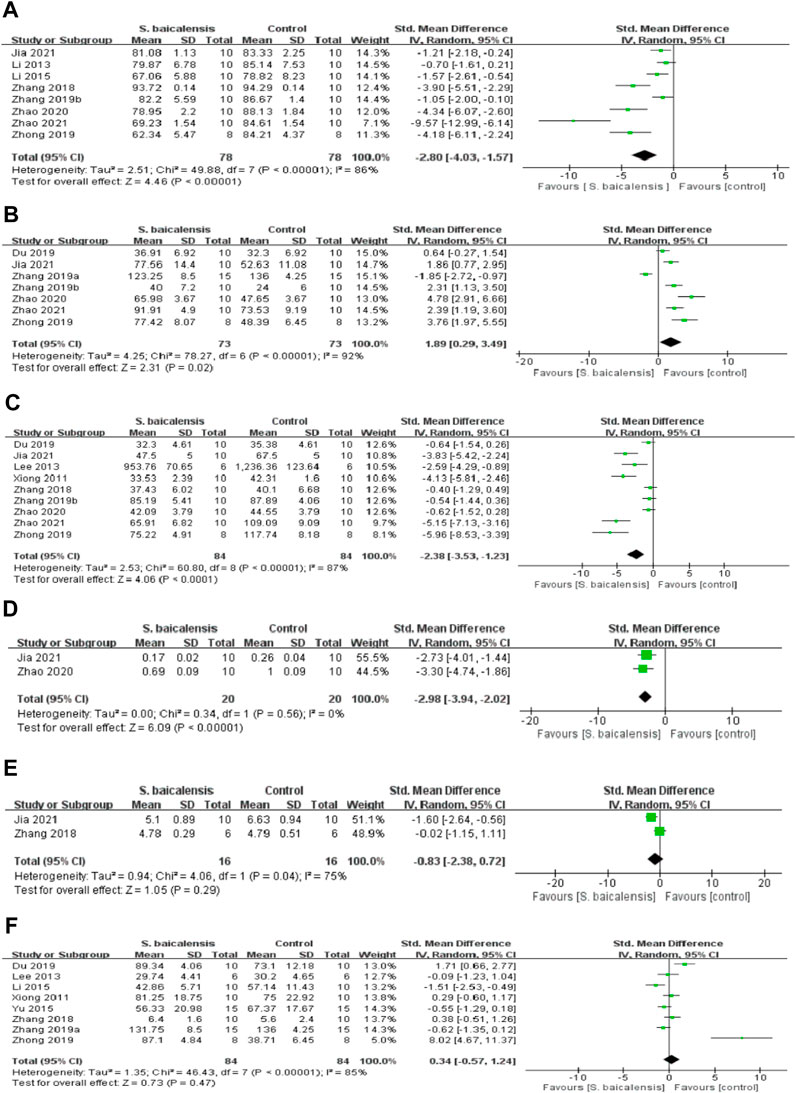
Figure 2. Forest plot of studies investigating the effect of Scutellaria baicalensis on animal behavior. The protective effects of Scutellaria baicalensis on animal behavior by (A) SPT, (B) TST, (C) OFT, (D) NSFT, (E) MWM, and (F) FST analysis.
3.4.3 Effects of scutellaria baicalensis on depression by TST analysis
The analysis of TST was applied in seven studies (Zhang et al., 2016; Zhang Kuo et al., 2019; Zhang Ruyi et al., 2019; Zhong et al., 2019; Fan et al., 2020; Jia et al., 2021; Zhao et al., 2021) that had a total sample size of 148, of which 73 animals received Scutellaria baicalensis and 75 received a vehicle or saline treatment, while the analysis of FST covered eight studies (Xiong et al., 2011; Lee et al., 2013; Zhang et al., 2016; Zhang Kuo et al., 2019; Li-Ting et al., 2019; Zhong et al., 2019; Fan et al., 2020)with 168 animals, 84 in the experimental group and 84 in the control group. There were significant heterogeneity among the studies (p < 0.00001, I2 = 92%; p < 0.00001, I2 = 85%), the random-effect model was adopted. Results as shown in Figures 2B, F, immobility time was dramatically reduced in the Scutellaria baicalensis group versus control group, the difference was no statistically significant ([SMD = 1.89, 95%CI(0.29, 3.49), p = 0.02]; [SMD = 0.34, 95%CI(-0.57, 1.24), p = 0.47].)
3.4.4 Effects of scutellaria baicalensis on depression by OFT analysis
In OFT, meta-analysis of nine studies (Xiong et al., 2011; Lee et al., 2013; Zhang et al., 2016; Zhang et al., 2018; Zhang Ruyi et al., 2019; Zhong et al., 2019; Fan et al., 2020; Jia et al., 2021; Zhao et al., 2021)had a total sample size of 170, of which 84 animals received Scutellaria baicalensis and 86 received a vehicle or saline treatment. There was Statistically significant between groups in the number of crossings and rearing, indicating that Scutellaria baicalensis treatment can ameliorate the frequency of crossing and rearing compared with the control group [SMD = −2.38, 95%CI(-3.53, -1.23), p < 0.01], as shown in Figure 2C.
3.4.5 Effects of scutellaria baicalensis on depression by NFST analysis
In the NSFT experiment (Fan et al., 2020; Jia et al., 2021), food consumption was increased [SMD = −2.98, 95%CI(-3.94, -2.02), p < 0.01 in Scutellaria baicalensis group compared to control group. In contrast, there was no statistically significant differences in the MWM (Zhang et al., 2018; Jia et al., 2021), implying that Scutellaria baicalensis administration did not affect the times of platform crossings and seconds spent in the target quarter [SMD = −0.83, 95%CI(-2.38,0.72), p = 0.29; Figure 2E].
3.5 Underlying mechanisms
Most of the included literature is based on studies of the neuroprotective effects of Scutellaria baicalensis on depression. Brain-derived neutrophic factor (BDNF) (Xiong et al., 2011; Lee et al., 2013; Zhang et al., 2018; Li-Ting et al., 2019; Jia et al., 2021; Zhao et al., 2021), extracellular-signal-regulated kinase (ERK) phosphorylation (Xiong et al., 2011; Zhang et al., 2016; Jia et al., 2021), cAMP response element-binding protein (CREB) phosphorylation (Zhang et al., 2018; Jia et al., 2021; Zhao et al., 2021), tropomyosin-related kinase B (TrkB) phosphorylation (Zhao et al., 2021), protein kinase A (PKA) phosphorylation (Zhang et al., 2018), phosphatidylinositol 3-kinasep (PI3K) phosphorylation (Fan et al., 2020), Glycogen synthase kinase-3 beta (GSK3b)phosphorylation (Fan et al., 2020), Protein Kinase B (AKT) phosphorylation (Zhang Kuo et al., 2019; Fan et al., 2020), NF-kB p65 phosphorylation (Zhong et al., 2019), inhibitor of kB (IkB) phosphorylation (Zhong et al., 2019), Fibroblast growth factor (FGF) (Zhang Ruyi et al., 2019), Forkhead box G1 (FOXG1) (Zhang Ruyi et al., 2019), Serum/Glucocorticoid Regulated Kinase 1(SGK-1) (Li-Ting et al., 2019), N-methyl-D-aspartate receptor 2B (NR2B) (Zhang et al., 2016), and CaMKII (Zhang et al., 2016) were studied. Mechanistically, by acting as partial, subtype-selective GABAA receptor ligands, scutellaria baicalensis and its bioactive ingredients (baicalin and baicalein) foster the interaction of GABAA receptors with TrkB to potentiate GABA-induced signaling. By increasing cAMP/pERK and PI3K/pAKT signaling, they promote the synthesis of neurotrophic factors (BDNF and NGF) as well as neurogenesis.
As far as monoamine neurotransmitters is concerned, dopamine (DA) (Lee et al., 2013; Zhao et al., 2021), 3,4-dihydroxyphenylacetic acid (DOPAC) (Lee et al., 2013; Zhao et al., 2021), homovanillic acid (HVA) (Lee et al., 2013; Zhao et al., 2021) serotonin (5-HT) (Lee et al., 2013; Zhao et al., 2021), 5-hydroxyindole-acetic acid (5-HIAA) (Zhao et al., 2021), and noradrenaline (NE) (Zhao et al., 2021) were assessed. In addition, serum Corticosterone (Zhang Kuo et al., 2019; Li-Ting et al., 2019; Liu et al., 2019), glucocorticoid receptor phosphorylation in hypothalamus (Zhang Kuo et al., 2019; Li-Ting et al., 2019), corticotrophin-releasing factor (CRF)in hypo-thalamic (Lee et al., 2013), and tyrosine hydroxylase (TH)in hypo-thalamic (Lee et al., 2013) were evaluated. BrdU+ (Zhang et al., 2018; Zhang Ruyi et al., 2019; Fan et al., 2020), NeuN+ (Zhang et al., 2018; Zhang Ruyi et al., 2019; Fan et al., 2020) and DCX+ (Zhang et al., 2018; Fan et al., 2020) in DG; SLC6A4 [473], IDO (Zhong et al., 2019) GFAP (for astrocytes) (Zhong et al., 2019), Iba1 (for microglia) (Zhong et al., 2019) in the CA1, CA3, andDG (Zhong et al., 2019); taurine (Tau)/totalcreatine (tCr,creatine + phosphocreatine), glutamate + glutamine (Glx)/tCr in the hippocampus (Zhong et al., 2019); and TUNEL-positive cells in the hippocampus (Zhang Kuo et al., 2019) were also examined. Additionally, α-synuclein (Zhao et al., 2021), PSD95 (Fan et al., 2020; Zhao et al., 2021), and SYP (Zhang Kuo et al., 2019; Fan et al., 2020; Zhao et al., 2021) were also studied. Actually, By acting as MAO A/B inhibitors, scutellaria baicalensis induce monoamine, and mostly DA release.
To determine the anti-inflammatory effects of Scutellaria baicalensis during depression, IFN-γ, TNF-α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12 in plasma (Zhao et al., 2021) and TNF-α, IL-1β, IL-6, IL-8, IL-18 in the hippocampus (Zhong et al., 2019) were studied respectively. Furthermore, inflammatory factors such as IL-1β, IL-6, and TNF-α were measured both in the serum and in the hippocampus (Zhang et al., 2016). In terms of chronic oxidative stress and apoptosis, COX-2 in the frontal cortex and hippocampus (Liu et al., 2019), PGE 2 in the frontalcortex and hippocampus (Liu et al., 2019), SOD (Zhang Kuo et al., 2019), MDA (Zhang Kuo et al., 2019), GSH (Zhang Kuo et al., 2019), Apaf-1 caspase-3 (Zhang Kuo et al., 2019), caspase-9 (Zhang Kuo et al., 2019)in the hippocampus, [Ca 2+ ] (Zhang et al., 2016) and ROS (Zhang et al., 2016) were mainly appraised.
4 Discussion
Depression is a common mental disease associated with high morbidity and huge social burden. Numerous preclinical studies have shown that Scutellaria baicalensis and its active constituents are effective for depression. The aim of this study is to systematically review the roles of scutellaria baicalensis in depression and summarize the possible mechanism. To our knowledge, it is a first systematic review and meta-analysis of preclinical studies on the efficacy of Scutellaria baicalensis and its main components in animal depression model. The results indicated that Scutellaria baicalensis can remarkably safeguard against depression evidenced by improved behavioral changes. And this activities were associated with the regulation of Scutellaria baicalensis on inflammatory responses, oxidative stress, apoptosis, and neurotransmitters production via modulating TrkB-BDNF, PI3K-AKT, MAPK and NF-κB pathways.
In this work, 13 studies were included to assess the efficacy of scutellaria baicalensis on depression. Chronic unpredictability mild stress, corticosterone, rotenone-induced depression model, experimental autoimmune prostatitis (EAP)-induced depression mice model, olfactory bulbectomy (OBX) depression mice model and repeated restraint stress-induced depression rat model were used to study the antidepressant effect of Scutellaria baicalensis, while sucrose preference test (SPT), open field test (OFT), Morris water maze test (MWM), tail suspension test (TST), novelty suppressed feeding test (NSFT), and forced swimming test (FST) were adopted to evaluate the efficacy.
So far, the anti-depressive actvities of scutellaria baicalensis are welly studied, and the commonly used animal models are CUMS (Xiong et al., 2011; Zhang et al., 2016; Zhang et al., 2018; Zhang Ruyi et al., 2019; Liu et al., 2019; Jia et al., 2021)and CORT (Zhang Kuo et al., 2019; Li-Ting et al., 2019; Fan et al., 2020), etc., and one of the criteria to evaluate the success of depression model and the efficacy of anti-depression is behavioral test. Sucrose preference test for evaluating degree of pleasure lack of mice, open field test reflect the spontaneous activity in mice and explore the behavior, forced swimming test and tail suspension test reflects the behavior of the mice desperation, reaction ability of learning and memory in mice water maze experiment, while novelty suppressed feeding test, the variation of the lack of animal euphoria (Lu et al., 2016). In a mouse model of chronic cort-induced depression, baicalin significantly ameliorates behavior change by reducing the time spent in the central area of the open field test and the time spent in the cross maze test with open arms, and increasing the immobile time in the tail suspension test and forced swimming test. (Zhang et al., 2016; Zhang Kuo et al., 2019). On the other hand, Scutellaria baicalensis alleviated depression-like behavior by increasing sucrose consumption and reducing the immobile time of tail suspension and forced swimming tests in mild stress chronic mouse model (CUMS) (Li-Ting et al., 2019; Liu et al., 2019; Zhong et al., 2019).
The anti-depressive mechanism of scutellaria baicalensis is still not fully understand. Scutellaria baicalensis and its active components have a wide range of antidepressive effects. This review shows that most studies have focused on neural protection by measuring BDNF, ERK, CREB, TrkB, PI3K, GSK3B, AKT, NF-KB P65, IkB and their phosphorylation (Xiong et al., 2011; Lee et al., 2013; Zhang et al., 2018; Zhang Kuo et al., 2019; Li-Ting et al., 2019; Zhong et al., 2019; Fan et al., 2020; Jia et al., 2021; Zhao et al., 2021). Neuroinflammation was concerned by detecting inflammatory cytokines IL-1β, IL-6 and TNF-α in serum and/or hippocampus (Zhang et al., 2016; Zhong et al., 2019; Zhao et al., 2021). Monoamine neurotransmitters including dopamine, 3, 4-dihydroxyphenylacetic acid, hypervanilic acid, serotonin (5-HT), 5-hydroxyindoleacetic acid, and norepinephrine were evaluated (Lee et al., 2013; Zhao et al., 2021). Oxidative stress and apoptosis were studied by measuring COX-2,SOD, MDA, GSH, APAF1 Caspase-3, Caspase-9, [Ca 2+]and ROS levels in frontal lobe and hippocampus (Zhang et al., 2016; Zhang Kuo et al., 2019; Liu et al., 2019). Moreover, protein expressions of the presynaptic marker (synaptophysin1) and the postsynaptic marker (PSD95) and so on were studied following baicalin treatment (Fan et al., 2020; Zhao et al., 2021). The included studies mostly confirmed that scutellaria baicalensis regulated monoamine neurotransmitter levels and inflammatory factors. In addition, scutellaria baicalensis and its active components can also regulate oxidative stress, apoptosis, and synaptic dysfunction.
These results resembled theoretical study. Studies have reported that scutellaria baicalensis may induce the release of monoamine (DA) and enhance GABA-induced signal transduction, thereby increasing cAMP/pERK and PI3K/pAKT signals, and promoting the synthesis and neurogenesis of neurotrophic factors (BDNF and NGF) (Im Heh-In et al., 2005; Mu Xin et al., 2011). Secondly, scutellaria baicalensis plays an anti-inflammatory role by decreasing the levels of inducible nitric oxide synthase, NF-κB and pro-inflammatory cytokines TNF-α, IL-6 and IL-1β (Ma et al., 2015). It is reported that scutellaria baicalensis can also improve mitochondrial membrane potential depolarization and ATP production, and increase AMPK to enhance mitochondrial autophagy and mitochondrial biogenesis, thus playing a protective role in mitochondria (Zhang et al., 2017). It also lowers levels of reactive oxygen species and nitrogen species, increases superoxide dismutase, glutathione, glutathione peroxidase and catalase activities, heat shock protein 70, heme oxygenase-1 and thioredoxin levels, and ultimately reduces lipid peroxidation. The content of malondialdehyde and lipoxygenase are decreased by Scutellaria baicalensis. It also inhibited p-P38, Bax/Bcl-2 ratio, caspase 3, caspase 6, caspase 9, and cytochrome C release, thereby inhibiting apoptosis (Liu et al., 2012; Wang et al., 2013; Li et al., 2019).
This study was screened strictly in accordance with the inclusion criteria, exclusion criteria and literature quality scoring criteria, but there may still be the following limitations that may affect the accuracy of the study: First, the databases searched in this review were all in English, so there may be some deviations; Second, the methodological quality of the included studies was moderate, with none of the studies reporting the blinded induction of depression and the sample size needed to calculate it to obtain sufficient power; Third, the lack of negative studies may lead to overestimation of the true role of Scutellaria baicalensis. Fourthly, in the included studies, there were significant differences in depression modeling method and time, dosage and treatment time of scutellaria baicalensis.
5 Conclusion
In this preclinical systematic review, scutellaria baicalum can improve the symptoms of anhedonia, reduce the degree of behavioral despair, improve the cognitive ability of mice and play an anti-depressive effect in experimental depression. The mechanism mainly includes antioxidant, anti-inflammatory, neurotransmitter regulation, and inhibition of apoptosis. Therefore, scutellaria baicalensis may be a candidate for further clinical trials of depression.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
YM: Data curation, Formal Analysis, Writing–original draft. XZ: Visualization, Writing–review and editing. FZ: Funding acquisition, Writing–review and editing. CH: Formal Analysis, Writing–review and editing. HY: Software, Writing–review and editing. WC: Writing–original draft. XT: Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by National Natural Science Foundation of China (Grant 81830109).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1313871/full#supplementary-material
References
Bai, R., Dong, W., Peng, Q., and Bai, Z. (2022). Trends in depression incidence in China, 1990-2019. J. Affect Disord. 296, 291–297. doi:10.1016/j.jad.2021.09.084
Barrenetxea, J., Pan, A., Feng, Q., and Koh, W. P. (2022). Factors associated with depression across age groups of older adults: the Singapore Chinese health study. Int. J. Geriatr. Psychiatry 37. undefined. doi:10.1002/gps.5666
Bobo, W. V., Ryu, E., Petterson, T. M., Lackore, K., Cheng, Y., Liu, H., et al. (2020). Bi-directional association between depression and HF: an electronic health records-based cohort study. J. Comorb 10, 2235042X20984059. doi:10.1177/2235042X20984059
Chen, C., and Shan, W. (2019). Pharmacological and non-pharmacological treatments for major depressive disorder in adults: a systematic review and network meta-analysis. Psychiatry Res. 281, 112595. doi:10.1016/j.psychres.2019.112595
Dwyer Jennifer, B., Awais, A., Rajiv, R., Widge, A., Rodriguez, C. I., Carpenter, L. L., et al. (2020). Hormonal treatments for major depressive disorder: state of the art. Am. J. Psychiatry 177, 686–705. doi:10.1176/appi.ajp.2020.19080848
Fan, Z., Tao, W., Shang, Z., Zhang, W., Ruan, J., Zhang, C., et al. (2020). Facilitating granule cell survival and maturation in dentate gyrus with baicalin for antidepressant therapeutics. Front. Pharmacol. 11, 556845. doi:10.3389/fphar.2020.556845
Hai-Yang, Y., Yin, Z.-J., Yang, S.-J., Ma, S. P., and Qu, R. (2016). Baicalin reverses depressive-like behaviours and regulates apoptotic signalling induced by olfactory bulbectomy. Phytotherapy Res. PTR 30 (3), 469–475. doi:10.1002/ptr.5550
Hammen, C. (2018). Risk factors for depression: an autobiographical review. Annu. Rev. Clin. Psychol. 14, 1–28. doi:10.1146/annurev-clinpsy-050817-084811
Im, H. I., Joo, W. S., Nam, E., Lee, E. S., Hwang, Y. J., and Kim, Y. S. (2005). Baicalein prevents 6-hydroxydopamine-induced dopaminergic dysfunction and lipid peroxidation in mice. Pharmacol 98, 185–189. doi:10.1254/jphs.sc0050014
Im, H.-I., Wan Seok, J., Nam, E., Lee, E. S., Hwang, Y. J., and Kim, Y. S. (2005). Baicalein prevents 6-hydroxydopamine-induced dopaminergic dysfunction and lipid peroxidation in mice. J. Pharmacol. Sci. 98, 185–189. doi:10.1254/jphs.sc0050014
Jain, S., Gupta, S., Li, V. W., Suthoff, E., and Arnaud, A. (2022). Humanistic and economic burden associated with depression in the United States: a cross-sectional survey analysis. BMC Psychiatry 22 (1), 542. doi:10.1186/s12888-022-04165-x
Jia, Z., Yang, J., Cao, Z., Zhao, J., Zhang, J., Lu, Y., et al. (2021). Baicalin ameliorates chronic unpredictable mild stress-induced depression through the BDNF/ERK/CREB signaling pathway. Behav. Brain Res. 414, 113463. undefined. doi:10.1016/j.bbr.2021.113463
Jin, L., Xu, X., Huang, Y., Li, T., Ma, C., Xu, G., et al. (2021). Prevalence of depressive disorders and treatment in China: a cross-sectional epidemiological study. Lancet Psychiatry 8, 981–990. doi:10.1016/S2215-0366(21)00251-0
Jokela, M., Hamer, M., Singh-Manoux, A., Batty, G. D., and Kivimäki, M. (2014). Association of metabolically healthy obesity with depressive symptoms: pooled analysis of eight studies. Mol. Psychiatry 19 (8), 910–914. doi:10.1038/mp.2013.162
Kessler Ronald, C., and Bromet Evelyn, J. (2013). The epidemiology of depression across cultures. Annu. Rev. Public Health 34, 119–138. doi:10.1146/annurev-publhealth-031912-114409
Kverno, K. S., and Mangano, E. (2021). Treatment-resistant depression: approaches to treatment. J. Psychosoc. Nurs. Ment. Health Serv. 59 (9), 7–11. doi:10.3928/02793695-20210816-01
Lee, B., Sur, B., Park, J., Kim, S. H., Kwon, S., Yeom, M., et al. (2013). Chronic administration of baicalein decreases depression-like behavior induced by repeated restraint stress in rats. Korean J. Physiol. Pharmacol. 17, 393–403. doi:10.4196/kjpp.2013.17.5.393
Lee, H. W., Ryu, H. W., Kang, M. G., Park, D., Lee, H., Shin, H. M., et al. (2017). Potent inhibition of monoamine oxidase A by decursin from Angelica gigas Nakai and by wogonin from Scutellaria baicalensis Georgi. Int. J. Biol. Macromol. 97, 598–605. doi:10.1016/j.ijbiomac.2017.01.080
Lesman-Leegte, I., van Veldhuisen, D. J., Hillege, H. L., Moser, D., Sanderman, R., and Jaarsma, T. (2009). Depressive symptoms and outcomes in patients with heart failure: data from the COACH study. Eur. J. Heart Fail 11 (12), 1202–1207. doi:10.1093/eurjhf/hfp155
Li, G. H., Cheung, C. L., Chung, A. K., Cheung, B. M. Y., Wong, I. C. K., Fok, M. L. Y., et al. (2022). Evaluation of bi-directional causal association between depression and cardiovascular diseases: a Mendelian randomization study. Psychol. Med. 52 (9), 1765–1776. doi:10.1017/S0033291720003566
Li, Q., Li, Q. Q., Jia, J. N., Sun, Q. Y., Zhou, H. H., Jin, W. L., et al. (2019). Baicalein exerts neuroprotective effects in FeCl3-induced posttraumatic epileptic seizures via suppressing ferroptosis. Front. Pharmacol. 10, 638. doi:10.3389/fphar.2019.00638
Li-Ting, G., Wang, S.-Q., Su, J., Xu, L. X., Ji, Z. Y., Zhang, R. Y., et al. (2019). Baicalin ameliorates neuroinflammation-induced depressive-like behavior through inhibition of toll-like receptor 4 expression via the PI3K/AKT/FoxO1 pathway. J. Neuroinflammation 16, 95. doi:10.1186/s12974-019-1474-8
Liu, L., Dong, Y., Xin, S., Xia, B., and Wang, H. (2019). Anti-depressive effectiveness of baicalin in vitro and in vivo. Molecules 24, 326. undefined. doi:10.3390/molecules24020326
Liu, Y.-F., Gao, F., Xiao-Wei, L., Jia, R. H., Meng, X. D., Zhao, R., et al. (2012). The anticonvulsant and neuroprotective effects of baicalin on pilocarpine-induced epileptic model in rats. Neurochem. Res. 37, 1670–1680. doi:10.1007/s11064-012-0771-8
Lu, Y., Hu, Q., Mak Marvin, S. H., Lou, J., Xu, S. L., Bi, C. W. C., et al. (2016). A Chinese herbal decoction, reformulated from Kai-Xin-San, relieves the depression-like symptoms in stressed rats and induces neurogenesis in cultured neurons. Sci. Rep. 6, 30014. doi:10.1038/srep30014
Luppino, F. S., de Wit, L. M., Bouvy, P. F., Stijnen, T., Cuijpers, P., Penninx, B. W. J. H., et al. (2010). Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch. Gen. Psychiatry 67 (3), 220–229. doi:10.1001/archgenpsychiatry.2010.2
Ma, P., Mao, X. Y., Li, X. L., Ma, Y., Qiao, Y. D., Liu, Z. Q., et al. (2015). Baicalin alleviates diabetes-associated cognitive deficits via modulation of mitogen-activated protein kinase signaling, brain-derived neurotrophic factor and apoptosis. Mol. Med. Rep. 12, 6377–6383. doi:10.3892/mmr.2015.4219
Macleod, M. R., O’Collins, T., Howells, D. W., and Donnan, G. A. (2004). Pooling of animal experimental data reveals influence of study design and publication bias. Stroke 35, 1203–1208. doi:10.1161/01.STR.0000125719.25853.20
Mars, N., Kerminen, S., Feng, Y. A., Kanai, M., Läll, K., Thomas, L. F., et al. (2022). Genome-wide risk prediction of common diseases across ancestries in one million people. Cell Genom 2 (4), 100118. None. doi:10.1016/j.xgen.2022.100118
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G.PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Clin. Epidemiol. 62, 1006–1012. doi:10.1016/j.jclinepi.2009.06.005
Mu, X., Guo-Rong, H., Yuan, X., Li, X. X., and Du, G. H. (2011b). Baicalein protects the brain against neuron impairments induced by MPTP in C57BL/6 mice. Pharmacol. Biochem. Behav. 98, 286–291. doi:10.1016/j.pbb.2011.01.011
Mu, X., He, G. R., Yuan, X., Li, X. X., and Du, G. H. (2011). Baicalein protects the brain against neuron impairments induced by MPTP in C57BL/6 mice. Pharmacol. Biochem. Behav. 98, 286–291. doi:10.1016/j.pbb.2011.01.011
Nikayin, S., Murphy, E., Krystal, J. H., and Wilkinson, S. T. (2022). Long-term safety of ketamine and esketamine in treatment of depression. Expert Opin. Drug Saf. 21 (6), 777–787. doi:10.1080/14740338.2022.2066651
Park, D. H., Cho, J. J., Yoon, J. L., Kim, M. Y., and Ju, Y. S. (2020). The impact of depression on cardiovascular disease: a nationwide population-based cohort study in Korean elderly. Korean J. Fam. Med. 41 (5), 299–305. doi:10.4082/kjfm.18.0134
Pazini Francis, L., Cunha Mauricio, P., Azevedo, D., Rosa, J. M., Colla, A., de Oliveira, J., et al. (2017). Creatine prevents corticosterone-induced reduction in hippocampal proliferation and differentiation: possible implication for its antidepressant effect. Mol. Neurobiol. 54 (8), 6245–6260. doi:10.1007/s12035-016-0148-0
Plenge, P., Yang, D., Salomon, K., Laursen, L., Kalenderoglou, I. E., Newman, A. H., et al. (2021). The antidepressant drug vilazodone is an allosteric inhibitor of the serotonin transporter. Nat. Commun. 12 (1), 5063. doi:10.1038/s41467-021-25363-3
Quach, D. F., de Leon, V. C., and Conway, C. R. (2022). Nitrous Oxide: an emerging novel treatment for treatment-resistant depression. J. Neurol. Sci. 434, 120092. doi:10.1016/j.jns.2021.120092
Vos, T., Abajobir, A. A., Abate, K. H., Abbafati, C., Abbas, K. M., Abd-Allah, F., et al. (2017). Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390, 1211–1259. GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. doi:10.1016/S0140-6736(17)32154-2
Wang, R., Shen, X., Xing, E., Guan, L., and Xin, L. (2013). Scutellaria baicalensis stem-leaf total flavonoid reduces neuronal apoptosis induced by amyloid beta-peptide (25–35). Neural Regen. Res. 8, 1081–1090. doi:10.3969/j.issn.1673-5374.2013.12.003
Wang, Z. L., Wang, S., Kuang, Y., Hu, Z. M., Qiao, X., and Ye, M. (2018). A comprehensive review on phytochemistry, pharmacology, and flavonoid biosynthesis of Scutellaria baicalensis. Pharm. Biol. 56, 465–484. doi:10.1080/13880209.2018.1492620
World Health Organization (Who), (2017). Depression and other common mental disorders global health estimates. Geneva, Switzerland: World Health Organization.
Xiong, Z., Jiang, B., Wu, P.-F., Tian, J., Shi, L. L., Gu, J., et al. (2011). Antidepressant effects of a plant-derived flavonoid baicalein involving extracellular signal-regulated kinases cascade. Biol. Pharm. Bull. 34, 253–259. doi:10.1248/bpb.34.253
Yoon, E. J., Lee, M. Y., Choi, B. I., Lim, K. J., Hong, S. Y., and Park, D. (2020). Pharmaceutical Advantages of GenoTX-407, A Combination of Extracts from Scutellaria baicalensis Root and Magnolia officinalis Bark. Antioxidants (Basel) 9 (11). doi:10.3390/antiox9111111
Zhang, K., He, M., Wang, F., Zhang, H., Li, Y., Yang, J., et al. (2019). Revealing antidepressant mechanisms of baicalin in hypothalamus through systems approaches in corticosterone- induced depressed mice. Front. Neurosci. 13, 834. doi:10.3389/fnins.2019.00834
Zhang, K., Pan, X., Wang, F., Ma, J., Su, G., Dong, Y., et al. (2016). Baicalin promotes hippocampal neurogenesis via SGK1- and FKBP5-mediated glucocorticoid receptor phosphorylation in a neuroendocrine mouse model of anxiety/depression. Sci. Rep. 6, 30951. doi:10.1038/srep30951
Zhang, K., Wang, F., Yang, J.-Y., Wang, L. J., Pang, H. H., Su, G. Y., et al. (2015). Analysis of main constituents and mechanisms underlying antidepressant-like effects of Xiaochaihutang in mice. J. Ethnopharmacol. 175, 48–57. doi:10.1016/j.jep.2015.08.031
Zhang, R., Guo, L., Zhouye, J., Li, X., Zhang, C., Ma, Z., et al. (2018). Radix Scutellariae attenuates CUMS-induced depressive-like behavior by promoting neurogenesis via cAMP/PKA pathway. Neurochem. Res. 43, 2111–2120. doi:10.1007/s11064-018-2635-3
Zhang, R., Ma, Z., Liu, K., Li, Y., Liu, D., Xu, L., et al. (2019). Baicalin exerts antidepressant effects through Akt/FOXG1 pathway promoting neuronal differentiation and survival. Life Sci. 221, 241–248. doi:10.1016/j.lfs.2019.02.033
Zhang, X., Du, L., Zhang, W., Yang, Y., and Zhou, Q. (2017). Therapeutic effects of baicalein on rotenone-induced Parkinson’s disease through protecting mitochondrial function and biogenesis. Sci. Rep. 7, 9968. doi:10.1038/s41598-017-07442-y
Zhao, J., Lu, S., Yu, H., Duan, S., and Zhao, J. (2018). Baicalin and ginsenoside Rb1 promote the proliferation and differentiation of neural stem cells in Alzheimer’s disease model rats. Brain Res. 1678, 187–194. doi:10.1016/j.brainres.2017.10.003
Zhao, Q., Chen, X.-Y., and Martin, C. (2016). Scutellaria baicalensis, the golden herb from the garden of Chinese medicinal plants. Sci. Bull. (Beijing) 61, 1391–1398. doi:10.1007/s11434-016-1136-5
Zhao, T., Tang, H., Xie, L., Zheng, Y., Ma, Z., Sun, Q., et al. (2019). Scutellaria baicalensis Georgi. (Lamiaceae): a review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Pharm. J. Pharmacol. 71, 1353–1369. doi:10.1111/jphp.13129
Zhao, X., Kong, D., Zhou, Q., Wei, G., Song, J., Liang, Y., et al. (2021). Baicalein alleviates depression-like behavior in rotenone-induced Parkinson's disease model in mice through activating the BDNF/TrkB/CREB pathway. Biomed. Pharmacother. 140, 111556. doi:10.1016/j.biopha.2021.111556
Zhao, Y., Zhang, L., Wu, Y., Dai, Q., Zhou, Y., et al. (2018). Selective anti-tumor activity of wogonin targeting the Warburg effect through stablizing p53. Pharmacol. Res. 135, 49–59. doi:10.1016/j.phrs.2018.07.011
Zhong, J., Li, G., Xu, H., Wang, Y., and Shi, M. (2019). Baicalin ameliorates chronic mild stress-induced depression-like behaviors in mice and attenuates inflammatory cytokines and oxidative stress. Braz J. Med. Biol. Res. 52, e8434. doi:10.1590/1414-431X20198434
Keywords: scutellaria baicalensis, depression, preclinical study, meta analysis, systematic review
Citation: Ma Y, Zhou X, Zhang F, Huang C, Yang H, Chen W and Tao X (2024) The effect of scutellaria baicalensis and its active ingredients on major depressive disorder: a systematic review and meta-analysis of literature in pre-clinical research. Front. Pharmacol. 15:1313871. doi: 10.3389/fphar.2024.1313871
Received: 27 October 2023; Accepted: 23 February 2024;
Published: 20 March 2024.
Edited by:
Pei Jiang, Jining First People’s Hospital, ChinaReviewed by:
Jia-Wen SHOU, The Chinese University of Hong Kong, ChinaGiovanna Rigillo, University of Modena and Reggio Emilia, Italy
Copyright © 2024 Ma, Zhou, Zhang, Huang, Yang, Chen and Tao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wansheng Chen, chenwansheng@smmu.edu.cn; Xia Tao, taoxia2003@126.com
†These authors share first authorship
 Ying Ma
Ying Ma