- 1The First School of Clinical Medicine, Zhejiang Chinese Medical University, Hangzhou, China
- 2Department of Acupuncture and Moxibustion, Zhejiang Provincial Hospital of Integrated Traditional Chinese and Western Medicine, Hangzhou, Zhenjiang, China
- 3Department of Digestive System, Jinhua Municipal Hospital of Traditional Chinese Medicine, Jinhua, China
- 4Department of General Practice, The First Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, China
Background: Inflammatory bowel disease (IBD) is a chronic condition that can be managed with treatment, but it is challenging to get IBD cured. Resveratrol, a non-flavonoid polyphenolic organic compound derived from various plants, has a potential effect on IBD. The current research was set out to investigate the therapeutic effects of resveratrol on animal models of IBD.
Methods: A comprehensive search of PubMed, Embase, Web of Science, and Chinese databases was performed. The literature search process was completed independently by two people and reviewed by a third person. The risk of bias in the included literature was assessed using the Collaborative Approach to Meta Analysis and Review of Animal Data from Experimental Stroke (CAMARADES) 10-point quality checklist. The meta-analysis utilized Review Manager 5.4 software to evaluate the efficacy of resveratrol, with histopathological index as the primary outcome measure. Subgroup analysis was conducted based on this indicator. Additionally, meta-analyses were carried out on different outcomes reported in the literature, including final disease activity index, final body weight change, colon length, splenic index, and inflammatory factors.
Results: After conducting a thorough literature search and selection process, a total of 28 studies were ultimately included in the analysis. It was found that over half of the selected studies had more than five items with low risk of bias in the bias risk assessment. Relevant datas from included literature indicated that the histopathological index of the resveratrol group was significantly lower than that of the control group (WMD = −2.58 [-3.29, −1.87]). Subgroup analysis revealed that higher doses of resveratrol (>80 mg/kg) had a better efficacy (WMD = −3.47 [-4.97, −1.98]). Furthermore, The data summary and quantitative analysis results of SI and colon length also showed that resveratrol was effective in alleviating intestinal mucosal pathological injury of IBD. In terms of biochemical indicators, the summary analysis revealed that resveratrol affected interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-8 (IL-8), interleukin-10 (IL-10), tumor necrosis factor-α (TNF-α), transforming growth factor-β (TGF-β), interferon-γ (IFN-γ), malondialdehyde (MDA), myeloperoxidase (MPO), superoxide dismutase (SOD), and prostaglandin E2 (PGE2) significantly. These effects may be attributed to the mechanism of resveratrol in regulating immune response and inhibiting oxidative stress.
Conclusion: This review suggests that resveratrol demonstrated a notable therapeutic impact in preclinical models of IBD, particularly at doses exceeding 80 mg/kg. This efficacy is attributed to the protective mechanisms targeting the intestinal mucosa involved in the pathogenesis of IBD through various pathways. As a result, resveratrol holds promising prospects for potential clinical use in the future.
1 Introduction
Inflammatory bowel disease (IBD) is a chronic, non-specific inflammatory condition that affects the bowel and mainly includes two types, namely, ulcerative colitis (UC) and Crohn’s disease (CD) (Lee and Eun, 2022). A systematic review of the global IBD epidemic reveals that Europe and North America have the highest incidence of IBD. In many countries in North America, Oceania, and Europe, the incidence of IBD exceeds 0.3% (Ng et al., 2017). On the other hand, newly industrialized countries in Africa, Asia, and South America have seen an increase in IBD incidence since 1990 (Ng et al., 2017; Mak et al., 2020; Agrawal and Jess, 2022; Aniwan et al., 2022). The IBD has a complex pathophysiology, and it has detrimental effects on the body. Its main clinical symptoms include abdominal pain, diarrhea, mucus, pus, and bloody stools. Initial prolonged damage to the intestinal lining can impair nutrient absorption (Seyedian et al., 2019). Repeated attacks of the disease may lead to the development of intestinal fistulas. As a result, there is a risk of leakage of digestive juices and feces, which can cause infection and pain, and this complication is particularly severe in individuals with CD (Seyedian et al., 2019). In addition, in severe cases, complications such as intestinal perforation, acute bleeding, and intestinal obstruction may arise, necessitating emergency surgery and contributing to a poor prognosis. Numerous studies have shown that compared with the general population, IBD patients have a higher risk of developing cancer, especially colorectal cancer (CRC), and additionally, the prognosis of IBD-related CRC is worse than that of sporadic CRC (Mattar et al., 2011). This can be attributed to the fact that the chronic inflammatory state caused by IBD promotes mucosal proliferation and does not follow the traditional adenoma-carcinoma sequence (Keller et al., 2019; Sato et al., 2023). In addition, IBD can impose a significant economic burden on patients, often affecting their treatment adherence.
During the process of IBD, the physical barrier of the intestinal mucosa can deteriorate, consequently affecting both innate and adaptive immunity (Beisner et al., 2010; Piechota-Polanczyk and Fichna, 2014). Activation of innate immune cells, such as macrophages, can result in the production of superoxide and nitric oxide by nitrogen oxide (NOX) and nitric oxide synthase (NOS), in addition to the generation of oxidant peroxynitrite (Mangerich et al., 2012; Piechota-Polanczyk and Fichna, 2014; Guan, 2019). Furthermore, other immune cells also contribute to the production of reactive oxygen species (ROS) during metabolic processes, leading to significant tissue damage (Piechota-Polanczyk and Fichna, 2014; Guan, 2019). Simultaneously, the imbalance between helper T cells (Th) and regulatory T cells are essential to the progression of IBD (Uniken et al., 2017; Guan, 2019; Qiuping et al., 2021; Korta et al., 2023). The CD is primarily mediated by Th1 cells, while UC inflammation is mainly caused by Th2 cells (Uniken et al., 2017). The interleukin 23/T helper cell 17 (IL-23/Th17) pathway also affects the progression of IBD (Toussirot, 2012; Guan, 2019; Qiuping et al., 2021; Korta et al., 2023). Additionally, the activation of nuclear factor κB (NF-κB) and other signaling pathways results in the uncontrolled release of inflammatory cytokines during the IBD process and contributes to the inflammatory cascade reaction, the proliferation of memory T cells, and alterations in the intestinal microenvironment, ultimately resulting in the persistent development of IBD (Piechota-Polanczyk and Fichna, 2014; Guan, 2019; Shen et al., 2020; Zhang Z. et al., 2022; Ni et al., 2022).
Currently, a combination of various methods is used in the treatment of IBD, including 5-aminosalicylate (5-ASA), thiopurines, anti-tumor necrosis factor drugs, probiotics, antibiotics, and surgery (Luo et al., 2022). However, these treatment options have certain limitations. Conventional treatment often involves the use of immunosuppressive and anti-inflammatory drugs, which can lead to serious side effects and complications. Biologic therapy, while effective, can vary greatly in its effectiveness among individuals and is often expensive, impacting patient compliance (Shivaji et al., 2020). Many patients demonstrate both primary and secondary drug resistance, and adjusting the biological treatment plan following the detection of resistance can lead to an increase in the patient’s side effects and medical costs (Zhou WP. et al., 2021). Furthermore, even if the initial treatment effectively manages the condition, a significant number of patients still encounter disease recurrence, which poses a challenge when it comes to adjusting treatment strategies.
Extracting effective compounds from plants and herbs for complementary and adjunctive treatment is a crucial avenue for future treatment of IBD. Resveratrol is a natural polyphenolic compound that can be derived from various sources such as cassia seed, mulberry bark, and tea (Zhang et al., 2021). Numerous studies have demonstrated the diverse biological functions of resveratrol, including metabolic regulation, anti-inflammatory effects, antioxidant properties, anti-cancer activity, anti-aging effects, and improvement of renal function in diabetic patients (Breuss et al., 2019; Huang et al., 2020; Zhou DD. et al., 2021; Ren et al., 2021; Yang et al., 2021). Based on the pathological characteristics of IBD, resveratrol may inhibit oxidative stress and inflammation to alleviate the severity of IBD (Yao et al., 2023). Currently, there are limited clinical RCT studies on the therapeutic effect of resveratrol on IBD. The present study aimed to address these shortcomings with a meta-analysis of all relevant studies, to provide more convincing and scientific evidence for the clinical application of resveratrol in the treatment of IBD.
2 Methods
2.1 Study selection
Animal experimental studies that investigate the effects of resveratrol in the treatment of IBD were systematically searched in PubMed, Embase, Web of Science, China Knowledge Infrastructure Network, and Wanfang database electronic database. To ensure comprehensive coverage of the literature, we conducted searches in OpenGrey, the National Technical Information Service, Health Canada, and CADTH-Canadian Agency for Drugs and Technologies. Despite extensive searching in large health databases, no studies on resveratrol for IBD treatment were identified. The time span for literature search was from inception to 23 September 2023, without any language restrictions. MeSH terms used in our research process included “inflammatory bowel disease,” “colitis, ulcerative,” “IBD,” “ulcerative colitis,” “colitis,” “anti-colitis,” “Crohn disease,” “Crohn’s disease,” “Crohns disease,” “Crohn’s enteritis,” “resveratrol,” “3,5,4-trihydroxystilbene,” “trihydroxystilbene,” “SRT 501,” “resveratrol-3-sulfate,” “cis-resveratrol,” “trans-resveratrol,” “trans-resveratrol-3-O-sulfate,” and “3,4,5-stilbenetriol.”
The literature was screened by two reviewers (Y.Y.G and Y.J.L) based on the abstracts and full texts of the research obtained. Any discrepancies were resolved through discussion with a third reviewer (Y.X.J).
2.2 Eligibility criteria
2.2.1 Types of studies
Animal studies investigating the therapeutic effects of resveratrol in rat or mouse models with IBD were included. Clinical case reports or in vitro studies were excluded.
2.2.2 Animal models
There were no strict restrictions on sex, age, or strain of rats or mice induced with IBD. Tritrobenzene sulfonic acid (TNBS), dextran sodium sulfate (DSS), and oxazolone (OXZ) were used to induce IBD in rats or mice. However, models created using radiation, allergic enteritis models induced by ovalbumin, and genetically deficient mouse models of spontaneous chronic colitis that lack the anti-inflammatory cytokine interleukin-10 (IL-10−/− mouse) were excluded. The three models exhibited significant heterogeneity in pathogenesis compared to the included IBD models. The genetic defect in the IL-10−/− model results in the loss of IL-10-mediated inhibition of macrophage and T cell function, preventing antigen-stimulated immune regulation (Kühn et al., 1993). As a result, it exhibits progressive development of chronic enteritis without intervention, contrasting with the chemical-induced IBD model in healthy animals. Additionally, the modeling cycle for this model is notably longer than the included model (Singh et al., 2012; Holcomb et al., 2023). OVA primarily induces allergic enteritis, predominantly mediated by type I hypersensitivity through IgE (Kato et al., 2023). Radiation-induced models mainly focus on radiation enteritis (Sun et al., 2020). Detailed characterizations of the relevant exclusion literatures were provided in the Supplementary Table S1.
2.2.3 Interventions
The treatment group received resveratrol with no limitations on the dose, dosage form, route, and time of administration. The control group either remained untreated or was treated with vehicles. Studies that combined resveratrol with other interventions for the treatment group were excluded. Studies without a control group were also excluded.
2.3 Outcome measures
2.3.1 Primary outcome
The histopathological index, extracted from animal experimental literature, serves as the primary outcome for quantitative and subgroup analysis. This index is a valuable tool for measuring intestinal mucosal damage in IBD.
2.3.2 Secondary outcomes
Secondary outcome measures extracted from the literature for meta-analysis included final DAI score, final body weight change, spleen index, colon length, inflammatory factor levels, oxidative stress-related biochemical indices, and enzyme metabolites.
2.4 Literature selection and data extraction
Two authors independently extracted data from the included studies. These data included title, first author, year of publication, animal strain, weight and sex, number of animals in each group, method used to induce IBD, resveratrol administration (including dose, method, and time), and measurable outcome measures. The mean and standard deviation were extracted to compare the values of each variable. Following the recommended intervention review in the Cochrane Systematic Manual, the experimental group was combined to create a single-pair comparison for study groups with multiple interventions. We used GetData graph digitizer 2.24 to interpret the graph data. Any differences were resolved through discussion with a third reviewer (Y.X.J).
2.5 Assessment of risk of bias in the included studies
The quality and design of all included studies were assessed using the ten-point quality checklist published by Collaborative Approach to Meta Analysis and Review of Animal Data from Experimental Studies (CAMARADES) (Sena et al., 2007). Ratings of “yes,” “no,” or “unclear” for the criteria items indicate low risk, high risk, or an inadequate assessment of risk of bias, respectively. The evaluation process was conducted independently by two individuals (Y.Y.G and Y.J.L), and in the event of disagreements, the opinion of a third person was sought (Y.X.J).
2.6 Statistical analysis
RevMan 5.4 software was used for summary analysis of data included in the study. Mean difference (MD) was used for the same unit, while standardized MD was used for different units. Cochrane I2 score was used to determine heterogeneity between groups. Heterogeneity was assumed if the p-value of the Chi-square test was less than 0.10. The I2 value greater than 50% indicated high heterogeneity. The fixed effects model was used for homogeneous clinical and statistical analysis, while the random effects model was used for heterogeneous clinical and statistical analysis. A subgroup meta-analysis was conducted by three dose levels of resveratrol: high-dose (>80 mg/kg), medium-dose (>40 mg/kg, ≤80 mg/kg), and low-dose (≤40 mg/kg). At the same time, subgroup analysis was performed based on the gender and model of the experimental animals, the concentration and duration of the modeling reagent, the mode of resveratrol administration. Line graphs were generated using GraphPad Prism 10 software to show trends in DAI values between the two groups.
3 Results
3.1 Description of the included studies
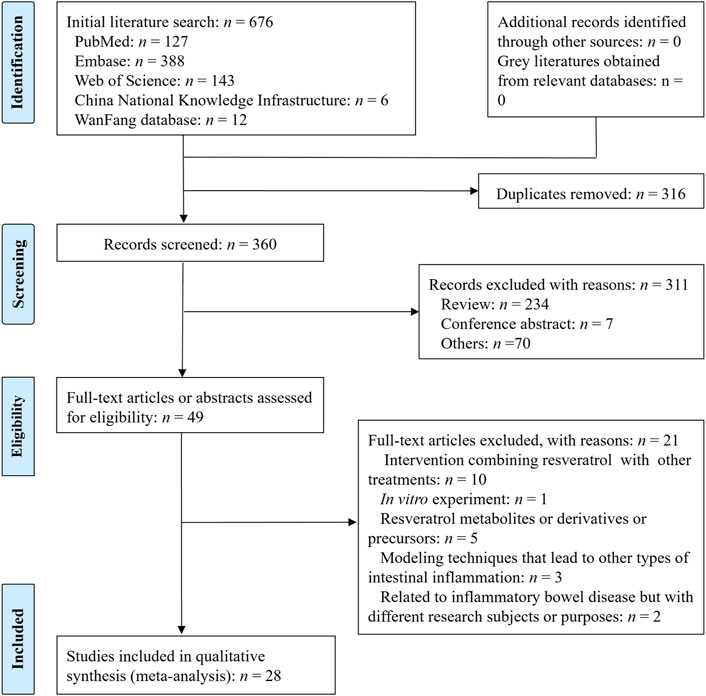
The process of literature screening is shown in Figure 1 (Liberati et al., 2009). A total of 676 articles were retrieved using specific search strategies. After excluding 316 duplicates, we further excluded 234 reviews, 7 conference abstracts, and 70 other records that were unrelated to the research topic. We obtained 49 articles for full-text review. Finally, 28 articles were included in this study. Among the excluded articles, 10 focused on the combination of resveratrol with other therapeutic methods as the intervention factor (Youn et al., 2009; Lozano-Pérez et al., 2014; Liu et al., 2018; Seoane-Viaño et al., 2019; Zhang et al., 2019; Gandhi et al., 2020; Lize et al., 2020; Naserifar et al., 2020; Pujara et al., 2021; Li et al., 2023), 1 involved an in vitro experiment (Sabzevary-Ghahfarokhi et al., 2020), 5 primarily studied resveratrol metabolites, derivatives, and precursors (Larrosa et al., 2010; Chen et al., 2017; Zhang B. et al., 2022; Fei et al., 2022; Zhang et al., 2023), 3 used modeling techniques to induce other types of intestinal inflammation (Singh et al., 2012; Sun et al., 2020; Bilotta et al., 2022), and 2 were related to IBD but had different research subjects or purposes (Wagnerova et al., 2017; Lu et al., 2019). Among the included studies, 19 were published in English and 9 were in Chinese (Jun et al., 2010a; Jun et al., 2010b; Singh et al., 2012; Wagnerova et al., 2017; Lu et al., 2019; Shenggao et al., 2019; Jianheng, 2021; Jianheng et al., 2021; Qiuping et al., 2021).
3.2 Characteristics of the included studies
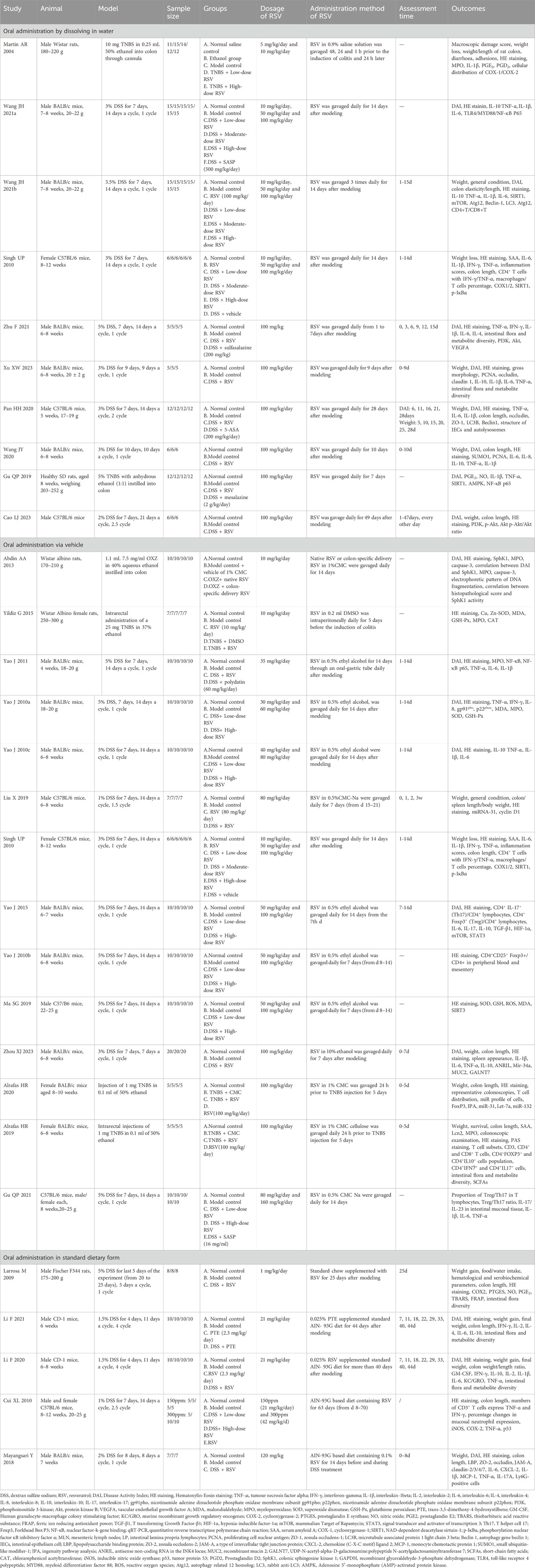
The features of the included research are summarized in Table 1 (Martín et al., 2004; Larrosa et al., 2009; Jun et al., 2010a; Jun et al., 2010b; Cui et al., 2010; Singh et al., 2010; Yao et al., 2010; Yao et al., 2011; Abdin, 2013; Yao et al., 2015; Yildiz et al., 2015; Mayangsari and Suzuki, 2018; Alrafas et al., 2019; Qiuping et al., 2019; Shenggao et al., 2019; Xin et al., 2019; Alrafas et al., 2020; Li et al., 2020; Pan et al., 2020; Wang et al., 2020; Jianheng, 2021; Jianheng et al., 2021; Li et al., 2021; Qiuping et al., 2021; Zhu et al., 2021; Liujing et al., 2023; Xu et al., 2023; Zhou et al., 2023). Among the studies included, 23 studies were conducted in mice, and 5 studies were conducted in rats. In terms of modeling techniques, 22 studies utilized oral DSS induction modeling, 5 studies employed TNBS intrarectal administration to induce IBD, and 1 study utilized the OXZ induction model. Among the studies using DSS molding reagents, the application time of reagents in a cycle varied from 4 to 10 days, with most studies applying them for 7 days, and 16 studies employed 1 cycle modeling, 3 studies employed 2 cycle modeling, 1 study employed 2.5 cycle modeling, while 2 studies employed 4 cycle modeling. Generally, the molding cycle was shorter. Among the studies using TNBS modeling reagent, 4 studies used TNBS mixed with ethanol, and 1 study used DMSO as the carrier. The sample sizes of all included studies ranged from 15 to 90.
In terms of the administration mode of resveratrol, 10 studies utilized resveratrol dissolved in water, 5 studies adopted resveratrol combined with a standard diet,. Another 13 studies administered resveratrol via vehicle routes.
3.3 Risk of bias assessment
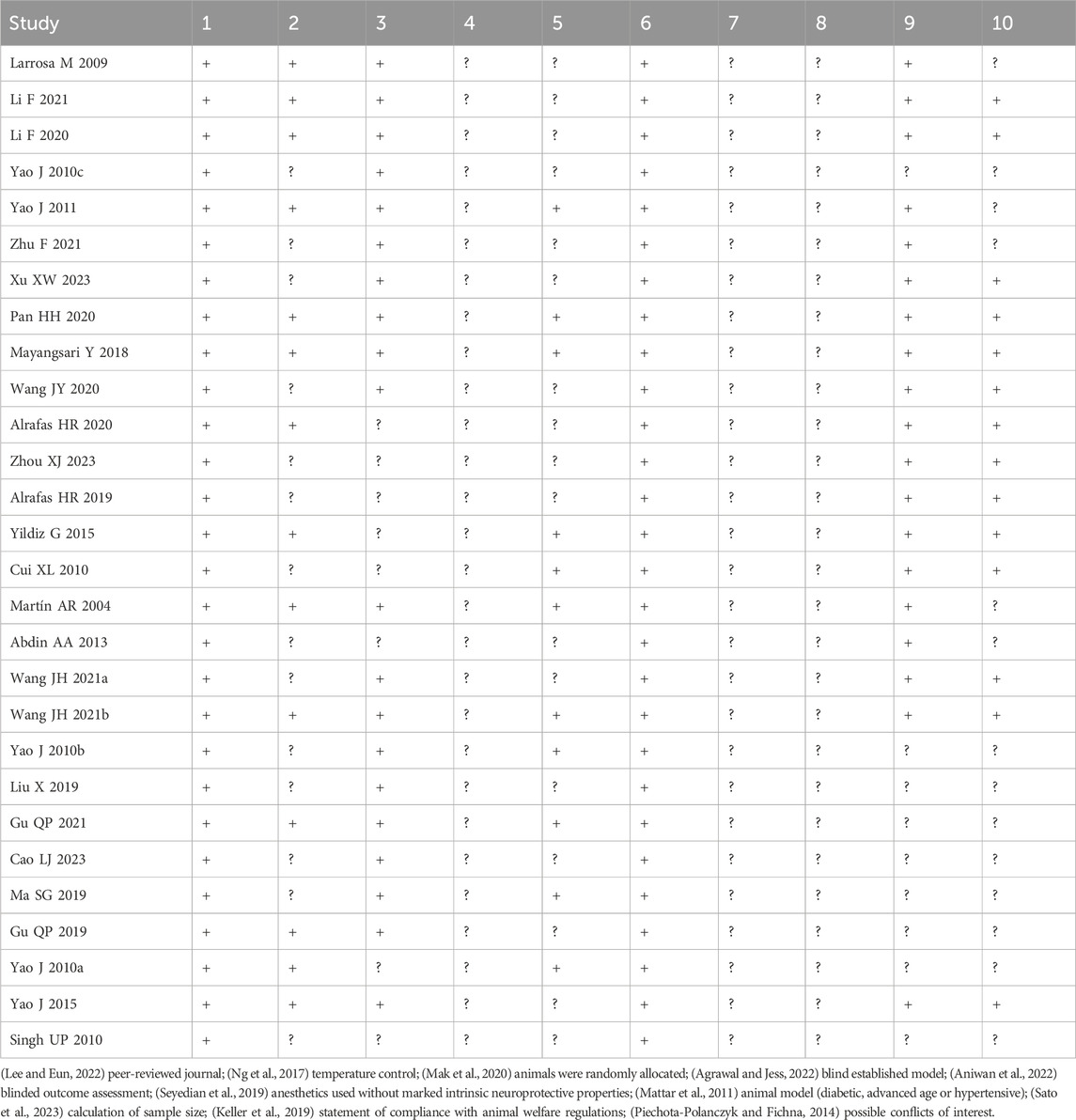
The results of the literature quality assessment are presented in Table 2. Overall, the quality of the included studies was acceptable, with more than half of the studies demonstrating low risk in five or more items. All the studies underwent peer review and showed a low risk in terms of the intrinsic neuroprotective properties of anesthetics. Specifically, temperature control was considered in 14 studies during animal breeding, the randomization of animals in the methodological design was clarified in 20 studies, blinding in the evaluation of outcome measures was adopted in 11 studies, adherence to animal welfare regulations was explicitly stated in 19 studies, and no possible conflicts of interest were declared in 14 studies. However, none of the studies reported the use of blinded modeling, representative animal-specific samples, or the calculation of sample size.
3.4 The role of resveratrol in the treatment of IBD
3.4.1 Histopathological index
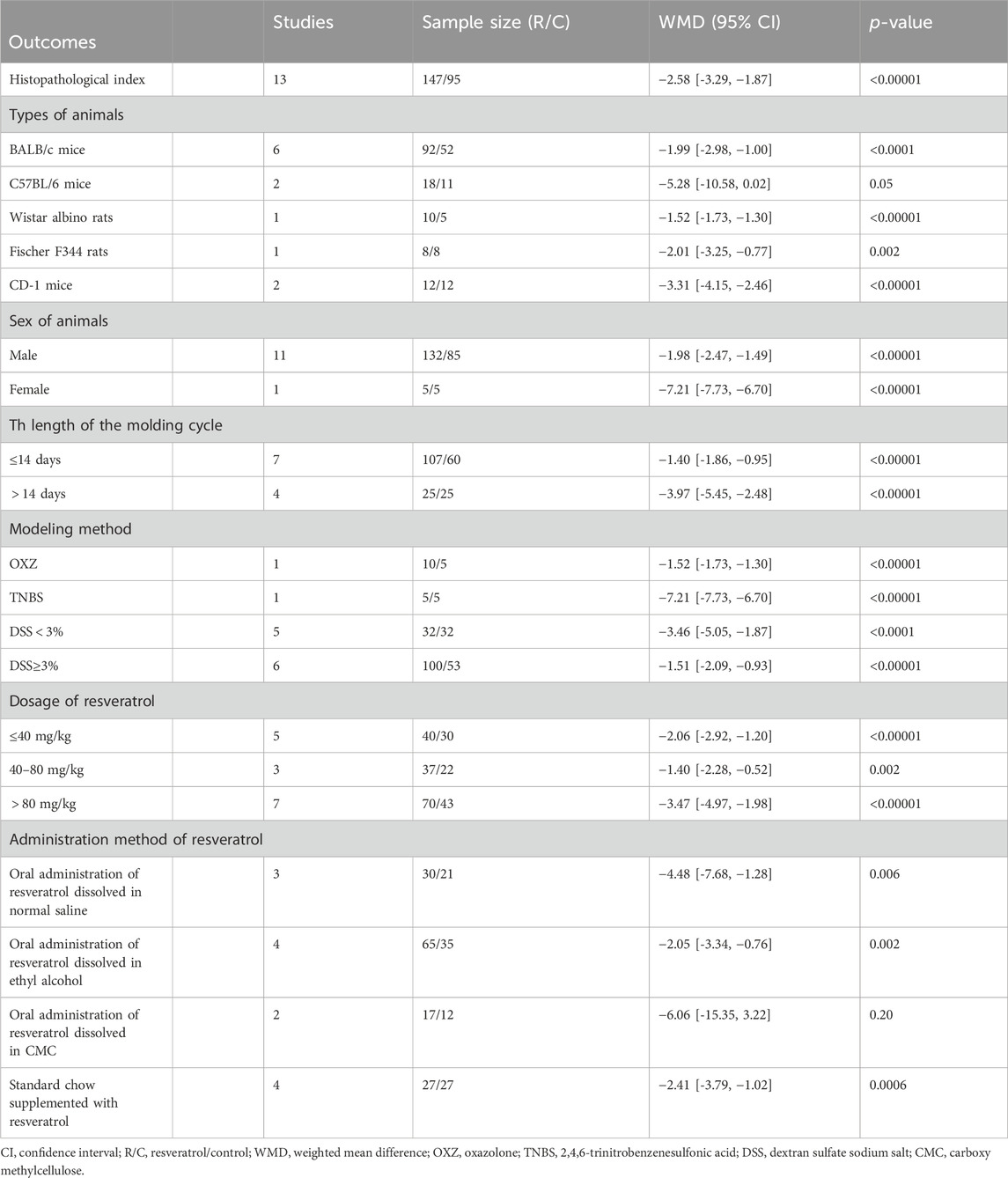
13 out of the 28 preclinical studies included in the analysis reported histopathological index as an outcome measure. Upon extraction and aggregation of these data, the results revealed that the histopathological index at the end of the experiment was effectively controlled and significantly lower in the resveratrol treatment group compared to the model group (n = 147/95, WMD = −2.58 [-3.29, −1.87], p < 0.00001; Table 3). Relevant forest plot was provided in the Supplementary Figure S1.
3.4.2 Subgroup analysis of histopathological index
A subgroup analysis was conducted on histopathological index, with six grouping criteria considered and all 13 studies that reported histopathological index included in the analysis. The findings revealed that high doses (>80 mg/kg) of resveratrol (7 studies, n = 70/43, WMD = −3.47 [-4.97, −1.98], p < 0.00001; Table 3) had the most significant control effect on histopathological index. Interestingly, incorporating resveratrol into the standard diet (4 studies, n = 27/27, WMD = −2.41 [-3.79, −1.02], p < 0.0006; Table 3) showed a low therapeutic effect similar to that of dissolved in alcohol (4 studies, n = 65/35, WMD = −2.05 [-3.34, −0.76], p < 0.002; Table 3) and significantly less than that of dissolved in water (3 studies, n = 30/21, WMD = -4.48 [-7.68, −1.28], p < 0.006) or in CMC (2 studies, n = 27/27, WMD = −2.41 [-3.79, −1.02], p < 0.0006; Table 3). However, due to the small number of studies included in each administration mode, it is difficult to make a judgment. Consequently, no definitive conclusions were reached regarding the impact of animal sex, animal species, or modeling method.
Notably, subgroup analyses of molding cycle revealed a more significant decline in histopathological index within the long-cycle group (4 studies, n = 25/25, WMD = −3.97 [-5.45, −2.48], p < 0.00001; Table 3). In terms of modeling methods, the subgroup using low-concentration DSS (6 studies, n = 100/53, WMD = −1.51 [-2.09, −0.93], p < 0.00001; Table 3) showed lower histopathological index compared to the high-concentration DSS group. Relevant forest plots were provided in the Supplementary Figure S2.
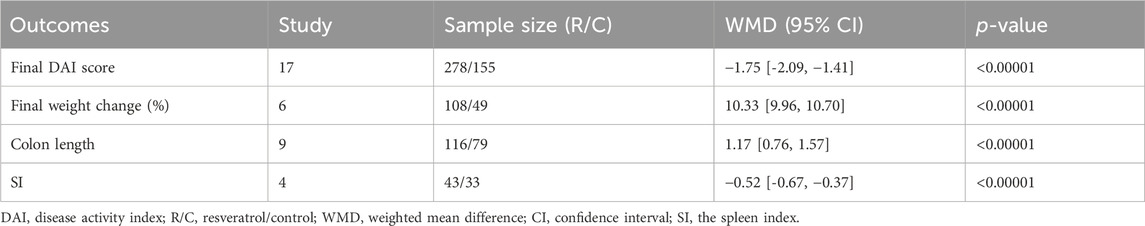
3.4.3 Final DAI score, final weight change and other histopathological indicators
The final DAI score was reported in 17 preclinical studies. The aggregate results indicated a significantly lower DAI score in the resveratrol group compared to the model group, suggesting that resveratrol effectively alleviates inflammation associated with IBD (n = 278/155, WMD = −1.75 [-2.09, −1.41], p < 0.00001; Table 4). 6 studies examined the final weight change, and our analysis showed resveratrol was effective in maintaining weight in IBD animals (n = 108/49, WMD = 10.33 [9.96, 10.70], p < 0.001; Table 4). In terms of pathological indicators other than histopathological index, we summarized SI data from 4 studies, and the results showed that resveratrol can reduce SI (n = 43/33, WMD = −0.52 [-0.67, −0.37], p < 0.00001; Table 4). Besides, 6 studies measured colon length, and analysis of the data manifested that the colon length of the resveratrol group was found to be significantly longer than that of the model group (n = 116/79, WMD = 1.17 [0.76, 1.57], p < 0.00001; Table 4). Relevant forest plots were provided in the Supplementary Figure S3.
3.4.4 Effects of resveratrol on inflammatory indicators
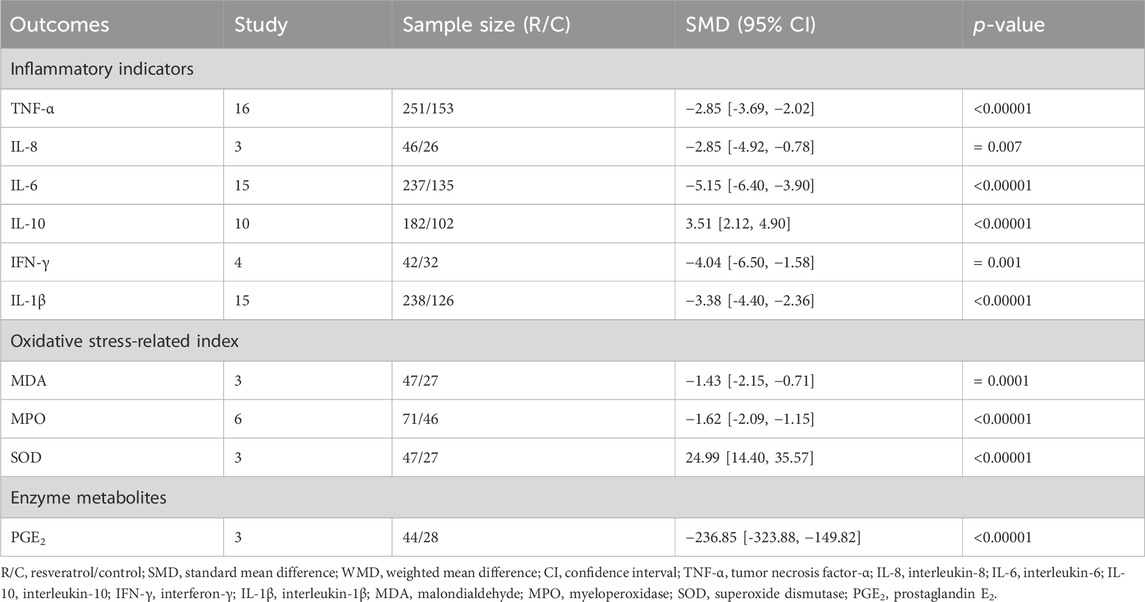
16 studies reported TNF-α, and combined data showed that resveratrol significantly reduced levels of this inflammatory factor in IBD animals (n = 251/153, SMD = −2.85 [-3.69, −2.02], p < 0.00001; Table 5). 15 studies selected IL-6 as an outcome indicator, and the summary results demonstrated a significant reduction in the resveratrol group compared to the model group (n = 237/175, SMD = −5.15 [-6.40, −3.90], p < 0.00001; Table 5). Similarly, 15 studies examined IL-1β, with results consistent with the previously mentioned factors (n = 238/126, SMD = −3.38 [-4.40, −2.36], p < 0.00001; Table 5). 3 studies investigated IL-8, and we found consistent results upon pooling the data (n = 46/26, SMD = −2.85 [-4.92, −0.78], p < 0.00001; Table 5). Additionally, 4 studies analyzed IFN-γ, and the result showed a decrease in this inflammatory factor (n = 42/32, SMD = −4.04 [-6.50, −1.58], p = 0.001; Table 5).
However, IL-10 was assessed in 10 studies, and the sammary of datas indicated that resveratrol increased level of this inflammatory factor in the treatment group (n = 182/102, SMD = 3.51 [2.12, 4.90], p < 0.00001; Table 5). Relevant forest plot was provided in the Supplementary Figure S4.
3.4.5 Effects of resveratrol on oxidative stress-related indicators and enzyme metabolites
3 studies reported PGE2, and the aggregate results showed that resveratrol had a downregulation effect on this index (n = 44/28, WMD = −236.85 [-323.88, −149.82], p < 0.00001; Table 5). 3 studies that evaluated MDA and summarized the extracted data also showed a decline (n = 47/27, SMD = −1.43 [-2.15, −0.71], p = 0.0001; Table 5). 6 studies used MPO as an outcome, and the results were the same when the data were combined (n = 71/46, SMD = −1.62 [-2.09, −1.15], p < 0.00001; Table 5).
Furthermore, in terms of the reduction of oxidative stress-related enzyme indexes mentioned above, summary statistics from the 3 studies measuring SOD indicated an upregulation effect (n = 47/27, SMD = 24.99 [14.40, 35.57], p < 0.00001; Table 5). Relevant forest plots were provided in the Supplementary Figure S5.
3.4.6 Publication bias
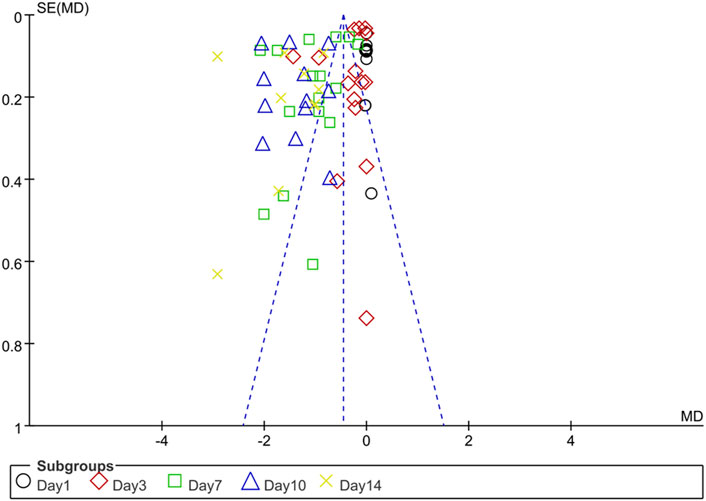
The funnel plot of publication bias for DAI scores over time after IBD showed significant asymmetry, suggesting that the included studies had a higher likelihood of publication bias (Figure 2).
4 Discussion
4.1 Summary of main results
In this study, we performed a quantitative meta-analysis to assess the effectiveness of resveratrol in animal models for Inflammatory Bowel Disease (IBD). Twenty-eight studies were included in the study, most of which demonstrated satisfactory results in risk of bias assessment. Analysis of the included preclinical studies revealed that resveratrol significantly decreased the severity of IBD compared to controls, particularly evident in controlling histopathological index. Subgroup analysis indicated that high-dose resveratrol (>80 mg/kg) had superior efficacy compared to medium-dose resveratrol (>40 mg/kg, ≤80 mg/kg) and low-dose resveratrol (≤40 mg/kg). Furthermore, resveratrol demonstrated effectiveness in alleviating weight loss in IBD animals and improving other pathological indicators like spleen index and colon length. Quantitative analysis of inflammatory factors and oxidative stress highlighted a significant correlation between the therapeutic benefits of resveratrol in IBD and its ability to reduce inflammation and oxidative stress.
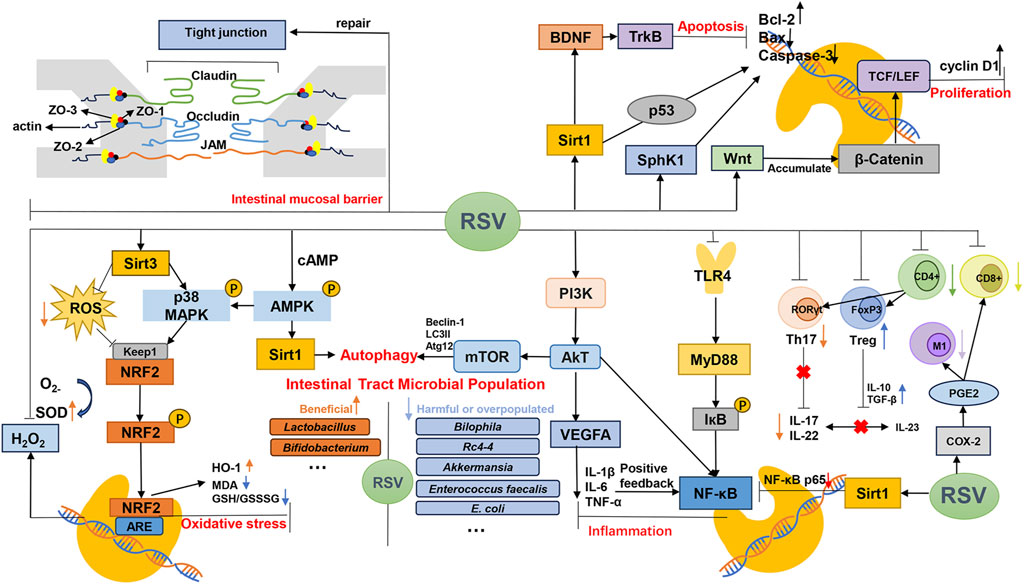
4.2 Possible mechanism of resveratrol in inflammatory factors
The IBD process triggers the release of inflammatory factors via various signaling pathways among which are the Toll-like receptor pathway, MyD88, mitogen-activated protein kinase (MAPK) pathway, and NF-κB pathway (Xiong et al., 2022; Zhu et al., 2023). Additionally, the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) pathway also contributes to this process (Shen et al., 2020; Zhang Z. et al., 2022).
TLR4 can stimulate the release of inflammatory mediators and activate the innate immune response (Feng et al., 2016). MyD88 is pivotal in relaying upstream signals (Zhou et al., 2018). The modeling reagent triggers TLR4 activation, which results in a marked increase in the phosphorylation of ERK (a member of the MAPK family), IκBα (an inhibitor of NF-κB), and mammalian target of rapamycin (mTOR) via MyD88-dependent signaling (Shen et al., 2020). Subsequently, the phosphorylation of IκBα promotes its degradation, thereby activating the transcription of genes involved in the NF-κB inflammatory cascade (Wang et al., 2022). PI3K engages and doubly phosphorylates the downstream signaling protein Akt through specific key metabolites (Vanhaesebroeck et al., 2010). mTOR, a downstream effector of Akt, modulates the pro-inflammatory activity of the NF-κB pathway and suppresses autophagy (Thorpe et al., 2015; Xianjuan et al., 2021). Moreover, activated Akt directly enhances the phosphorylation of NF-κB, leading to the transcription of inflammatory factors, such as TNF-α, which disturbs the cytokine balance and intensifies inflammatory responses (Arranz et al., 2012; Vergadi et al., 2017). The activation of TNF-α and IL-6 in the intestinal mucosa initiates a positive feedback loop in the NF-κB pathway. IL-6 facilitates this cycle by promoting the phosphorylation of signal transducer and activator of transcription 3 (STAT3) (Grivennikov et al., 2009).
We quantitatively analyzed the data related to inflammatory factors, and TNF-α, IL-6, IL-1β, IL-8, IFN-γ and IL-10 all showed positive results. Our findings suggest that resveratrol may exhibit anti-inflammatory effects in Inflammatory Bowel Disease (IBD) by increasing IL-10 levels and decreasing TNF-α, IL-6, IL-1β, and IL-8 levels.
4.3 Possible mechanism of resveratrol for alleviating oxidative stress response
The sirtuin (SIRT) family plays a protective role in cells by defending against oxidative stress damage. Specifically, SIRT3 boosts the activity of reactive oxygen species (ROS) scavenging enzymes, preserves mitochondrial functionality, and prevents ROS buildup within mitochondria, thereby exerting a potent antioxidant effect (Jianheng, 2021). Moreover, In the gastrointestinal tract, a diverse microbial community—encompassing both beneficial and detrimental bacteria—plays a critical role in modulating oxidative stress, which is a significant factor in the pathogenesis of IBD. Probiotic strains such as Lactobacillus and Bifidobacterium secrete cytoplasmic superoxide dismutase A (SodA), which mitigates oxidative stress and triggers the upregulation of nuclear factor erythroid 2-related factor 2 (Nrf2)-regulated antioxidant enzymes, thereby decelerating the inflammatory process in the colon (Larrosa et al., 2009; Yao et al., 2011; Hu et al., 2019). However, Enterococcus faecalis, found near the surface of the oxygenated colon, produces extracellular O2- at a higher rate, contributing to intestinal tissue damage (Larrosa et al., 2009; Yao et al., 2011; Hu et al., 2019).
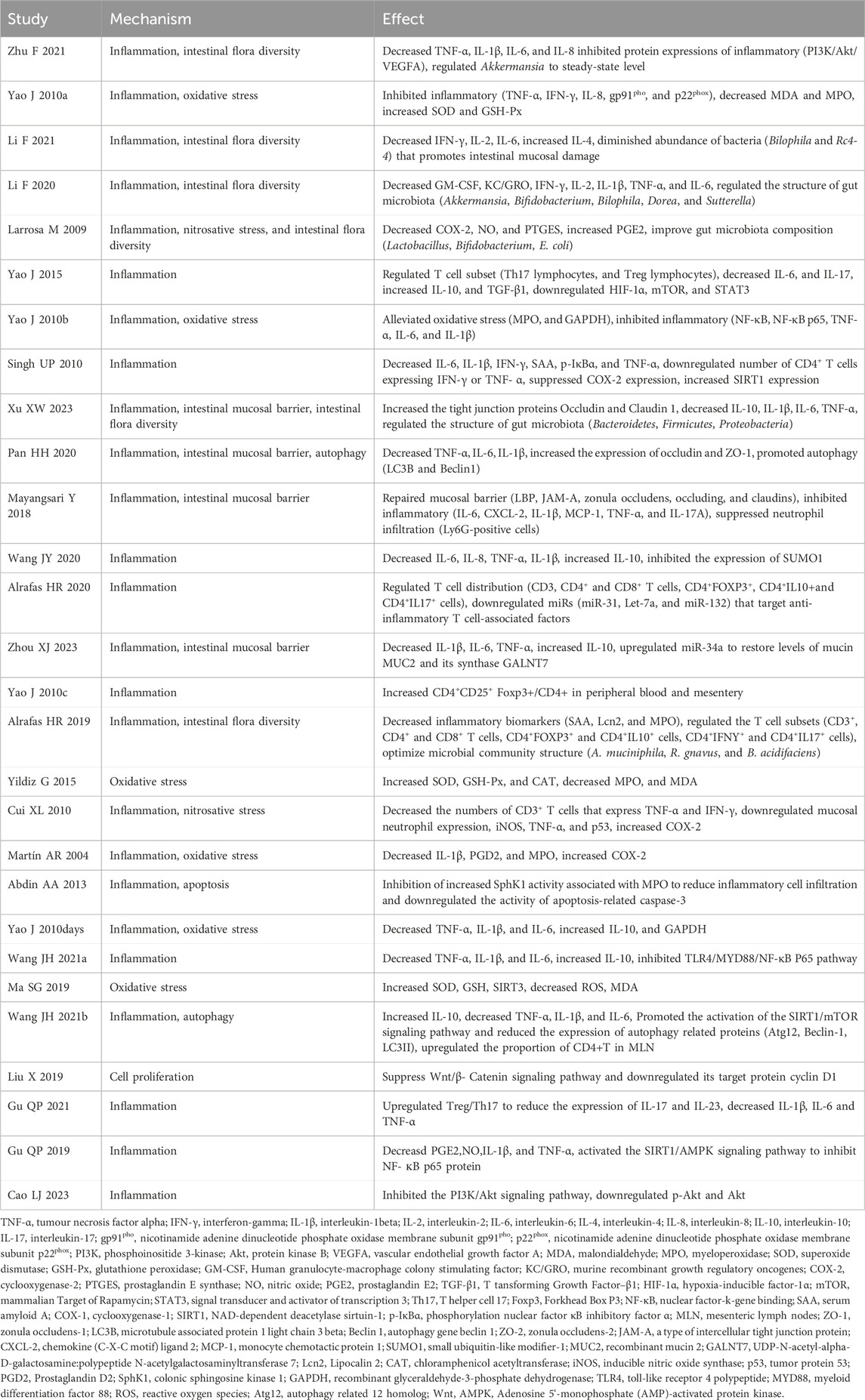
Our meta-analyses of MDA, MPO, ROS and SOD were all positive, suggesting that resveratrol may play an antioxidant stress role in IBD. The mechanisms of resveratrol in the treatment of IBD, as outlined in the included literature, have been comprehensively summarized (Table 6). Additionally, corresponding mechanism diagrams have been developed based on these mechanisms (Figure 3).
4.4 Dose and formulation of resveratrol for IBD
Subgroup analysis of effective resveratrol dosages for IBD treatment indicates that doses exceeding 80 mg/kg/day may enhance efficacy, particularly in controlling histopathological index. Furthermore, five of the reviewed studies utilized sulfasalazine as a positive control. High doses of resveratrol (>80 mg/kg/day) demonstrated comparable results to positive controls in terms of DAI control and reduction of inflammatory markers (Qiuping et al., 2019; Pan et al., 2020; Jianheng et al., 2021; Qiuping et al., 2021; Zhu et al., 2021). However, due to limited data on doses beyond 80 mg/kg/day, an optimal dosage range cannot be conclusively determined. While higher dosages may offer enhanced benefits, safety concerns may arise. Although resveratrol is generally considered to have low toxicity and good tolerance, the threshold for resveratrol toxicity remains unclear (Hebbar et al., 2005; Almeida et al., 2009; Williams et al., 2009; Gal et al., 2021; Zhang et al., 2021). Therefore, future research could explore a dose gradient with 80 mg as the middle or lower limit to identify the most effective dose without causing toxicity.
Subgroup analyses revealed that resveratrol dissolved in saline or CMC exhibited significantly greater efficacy compared to when incorporated in a standard diet or dissolved in alcohol. However, due to the limited number of included studies, definitive conclusions could not be made. One plausible explanation for this disparity is that incorporating resveratrol into the diet could potentially alter its distribution and absorption in the intestinal mucosa, consequently diminishing its bioavailability. Moreover, dissolving resveratrol in alcohol might lead to additional harm to the intestinal mucosa. It is noteworthy that despite the inclusion of only two studies, using CMC as a carrier notably enhanced the efficacy of resveratrol. This enhancement could be attributed to CMC’s ability to improve the stability of resveratrol suspension and facilitate its penetration in intestinal mucosal tissues. Therefore, the application of resveratrol using CMC as a carrier for IBD is worth further exploration. Additionally, there have been promising preclinical studies using nano-formulations of resveratrol (Lozano-Pérez et al., 2014; Gandhi et al., 2020; Naserifar et al., 2020; Pujara et al., 2021; Li et al., 2023). Although the number of such studies is currently limited, they show potential for addressing the issue of low bioavailability associated with resveratrol. In summary, resveratrol can be considered a highly effective nutritional additive and complementary drug, and its research as a candidate drug for treatment has important practical significance.
4.5 Effects of animal sex, types and modeling methods on the efficacy of resveratrol
In the subgroup analysis of sex, only 1 study utilized a female animal model (Alrafas et al., 2019). The result showed that resveratrol significantly reduced the histopathological index in female IBD animals compared to males. However, the evidence is not convincing and should be considered as reference only. As for the subgroup analysis of the types of experimental animals, C57BL/6 mice had the lowest histopathological index among the 4 types considered, although this subgroup only included 2 studies (Pan et al., 2020; Liujing et al., 2023). With a limited number of studies including subgroup analyses in rat models, it remains challenging to determine if the effectiveness of resveratrol differs between IBD rats and mice. When considering modeling methods, resveratrol demonstrated better control of histopathological index in low-concentration DSS animal models (DSS <3%) compared to high-concentration models (DSS ≥3%). While resveratrol showed promising results in animal models using TNBS as a modeling agent, definitive conclusions are hindered by the limited number of studies and other variables. Furthermore, in subgroup analysis based on the length of the modeling cycle, resveratrol exhibited a stronger protective effect on the pathological damage in IBD animals with longer modeling cycles (>14 days), suggesting a potential better efficacy in the chronic stage of intestinal inflammation compared to the acute stage. However, this conclusion is derived from preclinical studies with a limited sample size, warranting caution in interpretation.
4.6 Strengths and limitations of this review
To date, no quantitative studies have been conducted to analyze the therapeutic effects of resveratrol on animal models of IBD. This meta-analysis is a comprehensive and extensive inclusion of relevant studies. The inclusion criteria are not limited to the animal type and modeling method, ensuring more objective and representative results. In this review, histopathological index was chosen as the primary outcome indicator for quantitative analysis of the data in the included literature. This index is believed to provide a more precise and direct quantification of the extent and severity of intestinal mucosal pathological damage in IBD. Pathologists evaluate this index using a blind method, ensuring rigor and professionalism. Furthermore, this meta-analysis includes a subgroup analysis to evaluate the efficacy of various resveratrol doses in the treatment of IBD. Currently, there is a lack of in-depth clinical trials examining the dose-effect relationship of resveratrol in the treatment of IBD. Therefore, this study can provide certain dose evidence of IBD animal models for clinical trials. In subgroup analysis, we aimed to investigate the impact of resveratrol administration routes, IBD modeling methods, and modeling duration on histopathological index. By analyzing inflammatory factors and oxidative stress index, this study summarizes the specific mechanism of resveratrol in treating IBD with a larger sample size of animals.
However, this review has several limitations. Firstly, the small number of included studies may have overlooked unpublished or recently emerged animal studies, precluding the establishment of an upper limit for resveratrol administration based on the current data. Secondly, due to insufficient literature included, subgroup analysis of factors such as the sex of experimental animals, animal types, and modeling methods were limited. Thirdly, the presence of significant publication bias, as indicated by the funnel plot, should not be ignored. Last but not least, most of the animal studies we included used mice model. Although the mice model is the most established model for studying IBD, it does have clear limitations. Using mice model cannot dynamically track the pathological development of IBD. Mice have genetic and immune system differences compared to humans (Wen et al., 2024). Moreover, the longer pregnancy and growth cycle of mice increases the time and cost of experimental modeling (Kim et al., 2012). These limitations somewhat hinder the advancement of IBD research and the clinical translation of IBD treatments.
The current literature suggests that the zebrafish IBD model holds significant promise, particularly due to its high reproductive rate and short lifecycle, which can enhance experimental efficiency and reduce costs (Flores et al., 2020). Additionally, the transgenic strain exhibits fluorescence protein expression, facilitating the use of in vivo imaging technology to monitor intestinal tissue pathology in IBD (Marjoram and Bagnat, 2015; Hanyang et al., 2017). Zebrafish share genetic, digestive system, and immune system similarities with humans, making them a potential alternative to the conventional mouse model in future preclinical studies of IBD.
5 Conclusion
Our study demonstrated that resveratrol had a significant effect on reducing disease severity in animal models of IBD. This positive effect was observed through various indicators such as inflammation, general condition, histopathology, and oxidative stress. Resveratrol achieves improvements in these indicators by acting as an antioxidant, anti-inflammatory, and immunomodulatory agent. Notably, the effectiveness of high-dose resveratrol in promoting IBD disease remission was more pronounced compared with low-dose resveratrol. As a result, resveratrol shows promise as a potential candidate for future IBD clinical trials. However, it is important to interpret the results of this pre-clinical review with caution due to limitations in animal experimental methods and the quality of evidence.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
YG: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing–original draft, Writing–review and editing. YL: Data curation, Formal Analysis, Investigation, Methodology, Writing–original draft. ZZ: Data curation, Formal Analysis, Investigation, Methodology, Writing–original draft. XZ: Formal Analysis, Investigation, Methodology, Writing–original draft. XY: Data curation, Formal Analysis, Investigation, Writing–original draft. SW: Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Writing–original draft, Writing–review and editing. HL: Funding acquisition, Resources, Supervision, Writing–original draft, Writing–review and editing. YJ: Funding acquisition, Resources, Supervision, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China, NSFC Project, No. 81873268; Hangzhou Biomedicine and Health Industry Development Support Science and Technology Special Programme, No. 2022WJC205; Scientific Research Project 2022 of Zhejiang Chinese Medical University Affiliated Hospital (No. 892219A00332).
Acknowledgments
We sincerely thank my team members for their cooperation and support during the essay writing process.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1411566/full#supplementary-material
References
Abdin, A. A. (2013). Targeting sphingosine kinase 1 (SphK1) and apoptosis by colon-specific delivery formula of resveratrol in treatment of experimental ulcerative colitis in rats. Eur. J. Pharmacol. 718 (1-3), 145–153. doi:10.1016/j.ejphar.2013.08.040
Agrawal, M., and Jess, T. (2022). Implications of the changing epidemiology of inflammatory bowel disease in a changing world. United Eur. Gastroenterol. J. 10 (10), 1113–1120. doi:10.1002/ueg2.12317
Almeida, L., Vaz-da-Silva, M., Falcão, A., Soares, E., Costa, R., Loureiro, A. I., et al. (2009). Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol. Nutr. Food Res. 53 (Suppl. 1), S7–S15. doi:10.1002/mnfr.200800177
Alrafas, H. R., Busbee, P. B., Nagarkatti, M., and Nagarkatti, P. S. (2019). Resveratrol modulates the gut microbiota to prevent murine colitis development through induction of Tregs and suppression of Th17 cells. J. Leukoc. Biol. 106 (2), 467–480. doi:10.1002/JLB.3A1218-476RR
Alrafas, H. R., Busbee, P. B., Nagarkatti, M., and Nagarkatti, P. S. (2020). Resveratrol downregulates miR-31 to promote T regulatory cells during prevention of TNBS-induced colitis. Mol. Nutr. Food Res. 64 (1), e1900633. doi:10.1002/mnfr.201900633
Aniwan, S., Santiago, P., Loftus, E. V., and Park, S. H. (2022). The epidemiology of inflammatory bowel disease in Asia and Asian immigrants to Western countries. United Eur. Gastroenterol. J. 10 (10), 1063–1076. doi:10.1002/ueg2.12350
Arranz, A., Doxaki, C., Vergadi, E., Martinez de la Torre, Y., Vaporidi, K., Lagoudaki, E. D., et al. (2012). Akt1 and Akt2 protein kinases differentially contribute to macrophage polarization. Proc. Natl. Acad. Sci. U. S. A. 109 (24), 9517–9522. doi:10.1073/pnas.1119038109
Beisner, J., Stange, E. F., and Wehkamp, J. (2010). Innate antimicrobial immunity in inflammatory bowel diseases. Expert Rev. Clin. Immunol. 6 (5), 809–818. doi:10.1586/eci.10.56
Bilotta, S., Arbogast, J., Schart, N., Frei, M., and Lorentz, A. (2022). Resveratrol treatment prevents increase of mast cells in both murine OVA enteritis and IL-10(-/-) colitis. Int. J. Mol. Sci. 23 (3), 1213. doi:10.3390/ijms23031213
Breuss, J. M., Atanasov, A. G., and Uhrin, P. (2019). Resveratrol and its effects on the vascular system. Int. J. Mol. Sci. 20 (7), 1523. doi:10.3390/ijms20071523
Chen, Y., Park, J., Joe, Y., Park, H. J., Jekal, S. J., Sato, D., et al. (2017). Pterostilbene 4'-β-Glucoside protects against DSS-induced colitis via induction of tristetraprolin. Oxid. Med. Cell Longev. 2017, 9427583. doi:10.1155/2017/9427583
Cui, X., Jin, Y., Hofseth, A. B., Pena, E., Habiger, J., Chumanevich, A., et al. (2010). Resveratrol suppresses colitis and colon cancer associated with colitis. Cancer Prev. Res. (Phila) 3 (4), 549–559. doi:10.1158/1940-6207.CAPR-09-0117
Fei, Y., Zhang, S., Han, S., Qiu, B., Lu, Y., Huang, W., et al. (2022). The role of dihydroresveratrol in enhancing the synergistic effect of ligilactobacillus salivarius Li01 and resveratrol in ameliorating colitis in mice. Res. Wash D.C. 2022, 9863845. doi:10.34133/2022/9863845
Feng, Z., Wang, Z., Yang, M., Zhou, L., and Bao, Y. (2016). Polysaccharopeptide exerts immunoregulatory effects via MyD88-dependent signaling pathway. Int. J. Biol. Macromol. 82, 201–207. doi:10.1016/j.ijbiomac.2015.11.002
Flores, E. M., Nguyen, A. T., Odem, M. A., Eisenhoffer, G. T., and Krachler, A. M. (2020). The zebrafish as a model for gastrointestinal tract-microbe interactions. Cell Microbiol. 22 (3), e13152. doi:10.1111/cmi.13152
Gal, R., Deres, L., Toth, K., Halmosi, R., and Habon, T. (2021). The effect of resveratrol on the cardiovascular system from molecular mechanisms to clinical results. Int. J. Mol. Sci. 22 (18), 10152. doi:10.3390/ijms221810152
Gandhi, H., Rathore, C., Dua, K., Vihal, S., Tambuwala, M. M., and Negi, P. (2020). Efficacy of resveratrol encapsulated microsponges delivered by pectin based matrix tablets in rats with acetic acid-induced ulcerative colitis. Drug Dev. Ind. Pharm. 46 (3), 365–375. doi:10.1080/03639045.2020.1724127
Grivennikov, S., Karin, E., Terzic, J., Mucida, D., Yu, G. Y., Vallabhapurapu, S., et al. (2009). IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 15 (2), 103–113. doi:10.1016/j.ccr.2009.01.001
Guan, Q. (2019). A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J. Immunol. Res. 2019, 7247238. doi:10.1155/2019/7247238
Hanyang, L., Xuanzhe, L., Xuyang, C., Yujia, Q., Jiarong, F., Jun, S., et al. (2017). Application of zebrafish models in inflammatory bowel disease. Front. Immunol. 8, 501. doi:10.3389/fimmu.2017.00501
Hebbar, V., Shen, G., Hu, R., Kim, B. R., Chen, C., Korytko, P. J., et al. (2005). Toxicogenomics of resveratrol in rat liver. Life Sci. 76 (20), 2299–2314. doi:10.1016/j.lfs.2004.10.039
Holcomb, L., Holman, J. M., Hurd, M., Lavoie, B., Colucci, L., Hunt, B., et al. (2023). Early life exposure to broccoli sprouts confers stronger protection against enterocolitis development in an immunological mouse model of inflammatory bowel disease. Available at: https://www.biorxiv.org/content/10.1101/2023.01.27.525953v1.
Hu, Y., Chen, D., Zheng, P., Yu, J., He, J., Mao, X., et al. (2019). The bidirectional interactions between resveratrol and gut microbiota: an insight into oxidative stress and inflammatory bowel disease therapy. Biomed. Res. Int. 2019, 5403761. doi:10.1155/2019/5403761
Huang, D. D., Shi, G., Jiang, Y., Yao, C., and Zhu, C. (2020). A review on the potential of Resveratrol in prevention and therapy of diabetes and diabetic complications. Biomed. Pharmacother. 125, 109767. doi:10.1016/j.biopha.2019.109767
Jianheng, W. (2021) Resveratrol effects on the SIRT1/mTOR signaling pathway in colonic epithelium of mice with ulcerative colitis [MA thesis]. doi:10.27262/d.cnki.gqdau.2021.000447
Jianheng, W., Gang, Z., Lize, Z., Cuixia, Q., and Wenli, Y. (2021). Effect of resveratrol on colonic epithelium Toll-like receptor 4/myeloid differentiation factor 88/nuclear transcription factor-kappa B p65 signaling pathway in mice with ulcerative colitis. Chin. J. Exp. Surg. 38 (04), 662–664. doi:10.3760/cma.j.cn421213-20201213-00913
Jun, Y., Lisheng, W., Jianyao, W., Yingxue, L., Anying, X., Xiaoxia, W., et al. (2010b). Effect of resveratrol on cytokines expression in colonic mucosa of mice with ulcerative colitis. Traditional Chin. Drug Res. Clin. Pharmacol. 21 (03), 227–230.
Jun, Y., Lisheng, W., Yingxue, L., Jianyao, W., Liping, S., Jing, M., et al. (2010a). Resveratrol increases the percentages of CD4+CD25+Foxp3+ regulatory T cells in peripheral blood and mesenteric lymph nodes of mice with ulcerative colitis. World Chin. J. Dig. 18 (27), 2905–2908. doi:10.11569/wcjd.v18.i27.2905
Kato, S., Onishi, S., Sasai, M., Yasuda, H., Saeki, K., Matsumoto, K., et al. (2023). Deficiency of leukotriene B4 receptor type 1 ameliorates ovalbumin-induced allergic enteritis in mice. Clin. Exp. Pharmacol. Physiol. 50 (9), 766–775. doi:10.1111/1440-1681.13808
Keller, D. S., Windsor, A., Cohen, R., and Chand, M. (2019). Colorectal cancer in inflammatory bowel disease: review of the evidence. Tech. Coloproctol. 23 (1), 3–13. doi:10.1007/s10151-019-1926-2
Kim, J. J., Shajib, M. S., Manocha, M. M., and Khan, W. I. (2012). Investigating intestinal inflammation in DSS-induced model of IBD. J. Vis. Exp. (60), 3678. doi:10.3791/3678
Korta, A., Kula, J., and Gomułka, K. (2023). The role of IL-23 in the pathogenesis and therapy of inflammatory bowel disease. Int. J. Mol. Sci. 24 (12), 10172. doi:10.3390/ijms241210172
Kühn, R., Löhler, J., Rennick, D., Rajewsky, K., and Müller, W. (1993). Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75 (2), 263–274. doi:10.1016/0092-8674(93)80068-p
Larrosa, M., Tomé-Carneiro, J., Yáñez-Gascón, M. J., Alcántara, D., Selma, M. V., Beltrán, D., et al. (2010). Preventive oral treatment with resveratrol pro-prodrugs drastically reduce colon inflammation in rodents. J. Med. Chem. 53 (20), 7365–7376. doi:10.1021/jm1007006
Larrosa, M., Yañéz-Gascón, M. J., Selma, M. V., González-Sarrías, A., Toti, S., Cerón, J. J., et al. (2009). Effect of a low dose of dietary resveratrol on colon microbiota, inflammation and tissue damage in a DSS-induced colitis rat model. J. Agric. Food Chem. 57 (6), 2211–2220. doi:10.1021/jf803638d
Lee, J. W., and Eun, C. S. (2022). Inflammatory bowel disease in Korea: epidemiology and pathophysiology. Korean J. Intern Med. 37 (5), 885–894. doi:10.3904/kjim.2022.138
Li, F., Han, Y., Cai, X., Gu, M., Sun, J., Qi, C., et al. (2020). Dietary resveratrol attenuated colitis and modulated gut microbiota in dextran sulfate sodium-treated mice. Food Funct. 11 (1), 1063–1073. doi:10.1039/c9fo01519a
Li, F., Wang, Q., Han, Y., Song, M., Cai, X., Goulette, T., et al. (2021). Dietary pterostilbene inhibited colonic inflammation in dextran-sodium-sulfate-treated mice: a perspective of gut microbiota. Infect. Microbes Dis. 3 (1), 22–29. doi:10.1097/im9.0000000000000047
Li, W., Bi, D., Yi, J., Yao, L., Cao, J., Yang, P., et al. (2023). Soy protein isolate-polyguluronate nanoparticles loaded with resveratrol for effective treatment of colitis. Food Chem. 410, 135418. doi:10.1016/j.foodchem.2023.135418
Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gøtzsche, P. C., Ioannidis, J. P., et al. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Bmj 339, b2700. doi:10.1136/bmj.b2700
Liu, B., Li, S., Sui, X., Guo, L., Liu, X., Li, H., et al. (2018). Root extract of polygonum cuspidatum siebold and zucc. Ameliorates DSS-induced ulcerative colitis by affecting NF-kappaB signaling pathway in a mouse model via synergistic effects of polydatin, resveratrol, and emodin. Front. Pharmacol. 9, 347. doi:10.3389/fphar.2018.00347
Liujing, C., Yulun, W., Xinran, Z., Lihui, W., A-Ling, S., Jun, P., et al. (2023). Exploration on mechanism of resveratrol in treatment of ulcerative colitis based on network pharmacology and experimentation. Fujian J. Traditional Chin. Med. 54 (06), 52–58. doi:10.13260/j.cnki.jfjtcm.2023.06013
Lize, Z., Dandan, W., Cuixia, Q., Hairui, G., Wenli, Y., and Gang, Z. (2020). Effect and mechanism of curcumin and resveratrol on macrophages of mice with ulcerative colitis. Chin. J. Inflamm. Bowel Dis. 4 (2), 8. doi:10.3760/cma.j.cn101480-20190606-00083
Lozano-Pérez, A. A., Rodriguez-Nogales, A., Ortiz-Cullera, V., Algieri, F., Garrido-Mesa, J., Zorrilla, P., et al. (2014). Silk fibroin nanoparticles constitute a vector for controlled release of resveratrol in an experimental model of inflammatory bowel disease in rats. Int. J. Nanomedicine 9, 4507–4520. doi:10.2147/IJN.S68526
Lu, Y., Xu, H. M., Han, Y., and Zhang, Y. L. (2019). Analgesic effect of resveratrol on colitis-induced visceral pain via inhibition of TRAF6/NF-κB signaling pathway in the spinal cord. Brain Res. 1724, 146464. doi:10.1016/j.brainres.2019.146464
Luo, H., Cao, G., Luo, C., Tan, D., Vong, C. T., Xu, Y., et al. (2022). Emerging pharmacotherapy for inflammatory bowel diseases. Pharmacol. Res. 178, 106146. doi:10.1016/j.phrs.2022.106146
Mak, W. Y., Zhao, M., Ng, S. C., and Burisch, J. (2020). The epidemiology of inflammatory bowel disease: east meets west. J. Gastroenterol. Hepatol. 35 (3), 380–389. doi:10.1111/jgh.14872
Mangerich, A., Knutson, C. G., Parry, N. M., Muthupalani, S., Ye, W., Prestwich, E., et al. (2012). Infection-induced colitis in mice causes dynamic and tissue-specific changes in stress response and DNA damage leading to colon cancer. Proc. Natl. Acad. Sci. U. S. A. 109 (27), E1820–E1829. doi:10.1073/pnas.1207829109
Marjoram, L., and Bagnat, M. (2015). Infection, inflammation and healing in zebrafish: intestinal inflammation. Curr. Pathobiol. Rep. 3 (2), 147–153. doi:10.1007/s40139-015-0079-x
Martín, A. R., Villegas, I., La Casa, C., and de la Lastra, C. A. (2004). Resveratrol, a polyphenol found in grapes, suppresses oxidative damage and stimulates apoptosis during early colonic inflammation in rats. Biochem. Pharmacol. 67 (7), 1399–1410. doi:10.1016/j.bcp.2003.12.024
Mattar, M. C., Lough, D., Pishvaian, M. J., and Charabaty, A. (2011). Current management of inflammatory bowel disease and colorectal cancer. Gastrointest. Cancer Res. 4 (2), 53–61.
Mayangsari, Y., and Suzuki, T. (2018). Resveratrol ameliorates intestinal barrier defects and inflammation in colitic mice and intestinal cells. J. Agric. Food Chem. 66 (48), 12666–12674. doi:10.1021/acs.jafc.8b04138
Naserifar, M., Hosseinzadeh, H., Abnous, K., Mohammadi, M., Taghdisi, S. M., Ramezani, M., et al. (2020). Oral delivery of folate-targeted resveratrol-loaded nanoparticles for inflammatory bowel disease therapy in rats. Life Sci. 262, 118555. doi:10.1016/j.lfs.2020.118555
Ng, S. C., Shi, H. Y., Hamidi, N., Underwood, F. E., Tang, W., Benchimol, E. I., et al. (2017). Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 390 (10114), 2769–2778. doi:10.1016/S0140-6736(17)32448-0
Ni, L., Lu, Q., Tang, M., Tao, L., Zhao, H., Zhang, C., et al. (2022). Periplaneta americana extract ameliorates dextran sulfate sodium-induced ulcerative colitis via immunoregulatory and PI3K/AKT/NF-κB signaling pathways. Inflammopharmacology 30 (3), 907–918. doi:10.1007/s10787-022-00955-7
Pan, H. H., Zhou, X. X., Ma, Y. Y., Pan, W. S., Zhao, F., Yu, M. S., et al. (2020). Resveratrol alleviates intestinal mucosal barrier dysfunction in dextran sulfate sodium-induced colitis mice by enhancing autophagy. World J. Gastroenterol. 26 (33), 4945–4959. doi:10.3748/wjg.v26.i33.4945
Piechota-Polanczyk, A., and Fichna, J. (2014). Review article: the role of oxidative stress in pathogenesis and treatment of inflammatory bowel diseases. Naunyn Schmiedeb. Arch. Pharmacol. 387 (7), 605–620. doi:10.1007/s00210-014-0985-1
Pujara, N., Wong, K. Y., Qu, Z., Wang, R., Moniruzzaman, M., Rewatkar, P., et al. (2021). Oral delivery of β-lactoglobulin-nanosphere-encapsulated resveratrol alleviates inflammation in winnie mice with spontaneous ulcerative colitis. Mol. Pharm. 18 (2), 627–640. doi:10.1021/acs.molpharmaceut.0c00048
Qiuping, G., Fangqin, Z., Jinjin, L., Junfeng, X., and Jianhua, T. (2021). Experimental study on resveratrol regulating treg/Th17 to inhibit intestinal mucosal inflammation in ulcerative colitis. Drug Eval. 18 (10), 591–593. doi:10.19939/j.cnki.1672-2809.2021.10.05
Qiuping, G., Junfeng, X., Jinjin, L., Jianhua, T., and Xue, L. (2019). Experimental study on improvement effects of resveratrol in inflammatory bowel disease in rats by activating AMPK signaling pathway. Anti-Infection Pharm. 16 (4), 566–570. doi:10.13493/j.issn.1672-7878.2019.04-003
Ren, B., Kwah, M. X., Liu, C., Ma, Z., Shanmugam, M. K., Ding, L., et al. (2021). Resveratrol for cancer therapy: challenges and future perspectives. Cancer Lett. 515, 63–72. doi:10.1016/j.canlet.2021.05.001
Sabzevary-Ghahfarokhi, M., Soltani, A., Luzza, F., Larussa, T., Rahimian, G., Shirzad, H., et al. (2020). The protective effects of resveratrol on ulcerative colitis via changing the profile of Nrf2 and IL-1β protein. Mol. Biol. Rep. 47 (9), 6941–6947. doi:10.1007/s11033-020-05753-4
Sato, Y., Tsujinaka, S., Miura, T., Kitamura, Y., Suzuki, H., and Shibata, C. (2023). Inflammatory bowel disease and colorectal cancer: epidemiology, etiology, surveillance, and management. Cancers (Basel) 15 (16), 4154. doi:10.3390/cancers15164154
Sena, E., van der Worp, H. B., Howells, D., and Macleod, M. (2007). How can we improve the pre-clinical development of drugs for stroke? Trends Neurosci. 30 (9), 433–439. doi:10.1016/j.tins.2007.06.009
Seoane-Viaño, I., Gómez-Lado, N., Lázare-Iglesias, H., Rey-Bretal, D., Lamela-Gómez, I., Otero-Espinar, F. J., et al. (2019). Evaluation of the therapeutic activity of melatonin and resveratrol in Inflammatory Bowel Disease: a longitudinal PET/CT study in an animal model. Int. J. Pharm. 572, 118713. doi:10.1016/j.ijpharm.2019.118713
Seyedian, S. S., Nokhostin, F., and Malamir, M. D. (2019). A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J. Med. Life. 12 (2), 113–122. doi:10.25122/jml-2018-0075
Shen, N., Wang, Z., Wang, C., Zhang, J., and Liu, C. (2020). Methane alleviates inflammation and apoptosis of dextran sulfate sodium-induced inflammatory bowel diseases by inhibiting toll-like receptor 4 (TLR4)/Myeloid differentiation factor 88 (MyD88)/Nuclear translocation of nuclear factor-κb (NF-κB) and endoplasmic reticulum stress pathways in mice. Med. Sci. Monit. 26, e922248. doi:10.12659/MSM.922248
Shenggao, M., Qian, S., and Baohua, G. (2019). The effect of resveratrol on oxidative stress and SIRT3 expression in colonic mucosa of mice with ulcerative colitis. Anat. Res. 41 (01), 35–38+44.
Shivaji, U. N., Bazarova, A., Critchlow, T., Smith, S. C. L., Nardone, O. M., Love, M., et al. (2020). Clinical outcomes, predictors of prognosis and health economics consequences in IBD patients after discontinuation of the first biological therapy. Ther. Adv. Gastroenterol. 13, 1756284820981216. doi:10.1177/1756284820981216
Singh, U. P., Singh, N. P., Singh, B., Hofseth, L. J., Price, R. L., Nagarkatti, M., et al. (2010). Resveratrol (trans-3,5,4'-trihydroxystilbene) induces silent mating type information regulation-1 and down-regulates nuclear transcription factor-kappaB activation to abrogate dextran sulfate sodium-induced colitis. J. Pharmacol. Exp. Ther. 332 (3), 829–839. doi:10.1124/jpet.109.160838
Singh, U. P., Singh, N. P., Singh, B., Hofseth, L. J., Taub, D. D., Price, R. L., et al. (2012). Role of resveratrol-induced CD11b(+) Gr-1(+) myeloid derived suppressor cells (MDSCs) in the reduction of CXCR3(+) T cells and amelioration of chronic colitis in IL-10(-/-) mice. Brain Behav. Immun. 26 (1), 72–82. doi:10.1016/j.bbi.2011.07.236
Sun, H., Cai, H., Fu, Y., Wang, Q., Ji, K., Du, L., et al. (2020). The protection effect of resveratrol against radiation-induced inflammatory bowel disease via NLRP-3 inflammasome repression in mice. Dose Response 18 (2), 1559325820931292. doi:10.1177/1559325820931292
Thorpe, L. M., Yuzugullu, H., and Zhao, J. J. (2015). PI3K in cancer: divergent roles of isoforms, modes of activation and therapeutic targeting. Nat. Rev. Cancer 15 (1), 7–24. doi:10.1038/nrc3860
Toussirot, E. (2012). The IL23/Th17 pathway as a therapeutic target in chronic inflammatory diseases. Inflamm. Allergy Drug Targets 11 (2), 159–168. doi:10.2174/187152812800392805
Uniken, V. W. T., Voskuil, M. D., Dijkstra, G., Weersma, R. K., and Festen, E. A. (2017). The genetic background of inflammatory bowel disease: from correlation to causality. J. Pathol. 241 (2), 146–158. doi:10.1002/path.4817
Vanhaesebroeck, B., Guillermet-Guibert, J., Graupera, M., and Bilanges, B. (2010). The emerging mechanisms of isoform-specific PI3K signalling. Nat. Rev. Mol. Cell Biol. 11 (5), 329–341. doi:10.1038/nrm2882
Vergadi, E., Ieronymaki, E., Lyroni, K., Vaporidi, K., and Tsatsanis, C. (2017). Akt signaling pathway in macrophage activation and M1/M2 polarization. J. Immunol. 198 (3), 1006–1014. doi:10.4049/jimmunol.1601515
Wagnerova, A., Babickova, J., Liptak, R., Vlkova, B., Celec, P., and Gardlik, R. (2017). Sex differences in the effect of resveratrol on DSS-induced colitis in mice. Gastroenterol. Res. Pract. 2017, 8051870. doi:10.1155/2017/8051870
Wang, J., Zhang, Z., Fang, A., Wu, K., Chen, X., Wang, G., et al. (2020). Resveratrol attenuates inflammatory bowel disease in mice by regulating SUMO1. Biol. Pharm. Bull. 43 (3), 450–457. doi:10.1248/bpb.b19-00786
Wang, Z., Zhou, H., Cheng, F., Zhang, Z., and Long, S. (2022). miR-21 negatively regulates the PTEN-PI3K-Akt-mTOR signaling pathway in crohn's disease by altering immune tolerance and epithelial-mesenchymal transition. Discov. Med. 34 (171), 45–58.
Wen, C., Chen, D., Zhong, R., and Peng, X. (2024). Animal models of inflammatory bowel disease: category and evaluation indexes. Gastroenterol. Rep. (Oxf). 12, goae021. doi:10.1093/gastro/goae021
Williams, L. D., Burdock, G. A., Edwards, J. A., Beck, M., and Bausch, J. (2009). Safety studies conducted on high-purity trans-resveratrol in experimental animals. Food Chem. Toxicol. 47 (9), 2170–2182. doi:10.1016/j.fct.2009.06.002
Xianjuan, Y., Yin, F., Jiajun, W., Jian, W., Linxuan, X., Zhuo, X., et al. (2021). Effect of Coptidis Rhizoma-Magnoliae Officinalis Cortex on TNBS-induced ulcerative colitis in rats by inhibiting PI3K/Akt signaling pathway. Chin. Traditional Herb. Drugs 52 (15), 4587–4597. doi:10.7501/j.issn.0253-2670.2021.15.017
Xin, L., Yali, W., Kaili, L., Xiangli, C., Xinxin, D., and Wenqin, Z. (2019). Effects of resveratrol on ulcerative colitis in mice and its mechanism. Chin. J. Appl. Physiology 35 (05), 447–453. doi:10.12047/j.cjap.5826.2019.097
Xiong, T., Zheng, X., Zhang, K., Wu, H., Dong, Y., Zhou, F., et al. (2022). Ganluyin ameliorates DSS-induced ulcerative colitis by inhibiting the enteric-origin LPS/TLR4/NF-κB pathway. J. Ethnopharmacol. 289, 115001. doi:10.1016/j.jep.2022.115001
Xu, X., Ocansey, D. K. W., Pei, B., Zhang, Y., Wang, N., Wang, Z., et al. (2023). Resveratrol alleviates DSS-induced IBD in mice by regulating the intestinal microbiota-macrophage-arginine metabolism axis. Eur. J. Med. Res. 28 (1), 319. doi:10.1186/s40001-023-01257-6
Yang, A. J. T., Bagit, A., and MacPherson, R. E. K. (2021). Resveratrol, metabolic dysregulation, and alzheimer's disease: considerations for neurogenerative disease. Int. J. Mol. Sci. 22 (9), 4628. doi:10.3390/ijms22094628
Yao, J., Wang, J. Y., Liu, L., Li, Y. X., Xun, A. Y., Zeng, W. S., et al. (2010). Anti-oxidant effects of resveratrol on mice with DSS-induced ulcerative colitis. Arch. Med. Res. 41 (4), 288–294. doi:10.1016/j.arcmed.2010.05.002
Yao, J., Wang, J. Y., Liu, L., Zeng, W. S., Li, Y. X., Xun, A. Y., et al. (2011). Polydatin ameliorates DSS-induced colitis in mice through inhibition of nuclear factor-kappaB activation. Planta Med. 77 (5), 421–427. doi:10.1055/s-0030-1250462
Yao, J., Wei, C., Wang, J. Y., Zhang, R., Li, Y. X., and Wang, L. S. (2015). Effect of resveratrol on Treg/Th17 signaling and ulcerative colitis treatment in mice. World J. Gastroenterol. 21 (21), 6572–6581. doi:10.3748/wjg.v21.i21.6572
Yao, Y., Yuan, H., Chen, C., Liang, J., and Li, C. (2023). Study of the antioxidant capacity and oxidation products of resveratrol in soybean oil. Foods 13 (1), 29. doi:10.3390/foods13010029
Yildiz, G., Yildiz, Y., Ulutas, P. A., Yaylali, A., and Ural, M. (2015). Resveratrol pretreatment ameliorates TNBS colitis in rats. Recent Pat. Endocr. Metab. Immune Drug Discov. 9 (2), 134–140. doi:10.2174/1872214809666150806105737
Youn, J., Lee, J. S., Na, H. K., Kundu, J. K., and Surh, Y. J. (2009). Resveratrol and piceatannol inhibit iNOS expression and NF-kappaB activation in dextran sulfate sodium-induced mouse colitis. Nutr. Cancer 61 (6), 847–854. doi:10.1080/01635580903285072
Zhang, B., Zhang, Y., Liu, X., Yin, J., Li, X., Zhang, X., et al. (2022b). Differential protective effect of resveratrol and its microbial metabolites on intestinal barrier dysfunction is mediated by the AMPK pathway. J. Agric. Food Chem. 70 (36), 11301–11313. doi:10.1021/acs.jafc.2c04101
Zhang, B., Zhang, Y., Liu, X., Zhao, C., Yin, J., Li, X., et al. (2023). Distinctive anti-inflammatory effects of resveratrol, dihydroresveratrol, and 3-(4-hydroxyphenyl)-propionic acid on DSS-induced colitis in pseudo-germ-free mice. Food Chem. 400, 133904. doi:10.1016/j.foodchem.2022.133904
Zhang, L., Xue, H., Zhao, G., Qiao, C., Sun, X., Pang, C., et al. (2019). Curcumin and resveratrol suppress dextran sulfate sodium-induced colitis in mice. Mol. Med. Rep. 19 (4), 3053–3060. doi:10.3892/mmr.2019.9974
Zhang, L. X., Li, C. X., Kakar, M. U., Khan, M. S., Wu, P. F., Amir, R. M., et al. (2021). Resveratrol (RV): a pharmacological review and call for further research. Biomed. Pharmacother. 143, 112164. doi:10.1016/j.biopha.2021.112164
Zhang, Z., Chong, W., Xie, X., Liu, Y., Shang, L., and Li, L. (2022a). Hedysarum multijugum Maxim treats ulcerative colitis through the PI3K-AKT and TNF signaling pathway according to network pharmacology and molecular docking. Ann. Transl. Med. 10 (20), 1132. doi:10.21037/atm-22-4815
Zhou, D. D., Luo, M., Huang, S. Y., Saimaiti, A., Shang, A., Gan, R. Y., et al. (2021b). Effects and mechanisms of resveratrol on aging and age-related diseases. Oxid. Med. Cell Longev. 2021, 9932218. doi:10.1155/2021/9932218
Zhou, M., Xu, W., Wang, J., Yan, J., Shi, Y., Zhang, C., et al. (2018). Boosting mTOR-dependent autophagy via upstream TLR4-MyD88-MAPK signalling and downstream NF-κB pathway quenches intestinal inflammation and oxidative stress injury. EBioMedicine 35, 345–360. doi:10.1016/j.ebiom.2018.08.035
Zhou, W. P., Mu, N., Jian, W. Y., and Wang, H. H. (2021a). Economic burden and factors associated with Crohn's disease. Beijing Da Xue Xue Bao Yi Xue Ban. 53 (3), 555–559. doi:10.19723/j.issn.1671-167X.2021.03.019
Zhou, X., Zhang, Y., Hu, M., Ge, Z., and Zhou, G. (2023). Resveratrol enhances MUC2 synthesis via the ANRIL-miR-34a axis to mitigate IBD. Am. J. Transl. Res. 15 (1), 363–372.
Zhu, F., Zheng, J., Xu, F., Xi, Y., Chen, J., and Xu, X. (2021). Resveratrol alleviates dextran sulfate sodium-induced acute ulcerative colitis in mice by mediating PI3K/Akt/VEGFA pathway. Front. Pharmacol. 12, 693982. doi:10.3389/fphar.2021.693982
Keywords: resveratrol, inflammatory bowel disease, meta-analysis, systematic review, pharmacological mechanism
Citation: Gu Y, Lou Y, Zhou Z, Zhao X, Ye X, Wu S, Li H and Ji Y (2024) Resveratrol for inflammatory bowel disease in preclinical studies: a systematic review and meta-analysis. Front. Pharmacol. 15:1411566. doi: 10.3389/fphar.2024.1411566
Received: 03 April 2024; Accepted: 21 May 2024;
Published: 14 June 2024.
Edited by:
Sergio Fallone Andrade, Lusofona University, PortugalReviewed by:
Sikiru Olaitan Balogun, Federal University of Grande Dourados, BrazilEric Francelino Andrade, Universidade Federal de Lavras, Brazil
Copyright © 2024 Gu, Lou, Zhou, Zhao, Ye, Wu, Li and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunxi Ji, ODgyMDYzMzNAcXEuY29t; Haitao Li, YWF2dEAxNjMuY29t
 Yuting Gu
Yuting Gu Yijie Lou1
Yijie Lou1 Xuan Zhao
Xuan Zhao Shuwen Wu
Shuwen Wu