- 1Department of Critical Care Medicine, The Dongguan Hospital of Guangzhou University of Chinese Medicine, Dongguan, China
- 2State Key Laboratory of Dampness Syndrome of Chinese Medicine, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
- 3Second Clinical Medical College, Guangzhou University of Chinese Medicine, Guangzhou, China
- 4Affiliated Zhuhai Hospital, Southern Medical University, Zhuhai Hospital of Integrated Traditional Chinese and Western Medicine, Zhuhai, China
- 5Department of Cardiovascular Medicine, Guangdong Provincial Hospital of Traditional Chinese Medicine, Guangzhou, China
- 6School of Biotechnology and Health Sciences, Wuyi University, Jiangmen, China
- 7State Key Laboratory of Quality Research in Chinese Medicine, Institute of Chinese Medical Sciences, University of Macau, Macau, China
- 8Guangdong-Hong Kong-Macau Joint Lab on Chinese Medicine and Immune Disease Research, Guangzhou, China
Psoriasis (PSO) is a common skin disease affecting approximately 1%–3% of the population, and the incidence rate is increasing yearly. PSO is associated with a dramatically increased risk of cardiovascular disease, the most common of which is atherosclerosis (AS). In the past, inflammation was considered to be the triggering factor of the two comorbidities, but in recent years, studies have found that lipid metabolism disorders increase the probability of atherosclerosis in patients with psoriasis. In this review, we discuss epidemiological studies, clinical treatment methods, risk factors, and lipid metabolism of psoriasis and atherosclerosis comorbidities.
1 Background
Psoriasis is a common disease, with approximately 150,000 new cases of psoriasis reported each year, with recent studies showing an upward trend over the past 3 years. The prevalence of psoriasis in the population is between 2% and 3% (Valacchi et al., 2010). The data indicated that the occurrence of psoriasis varied according to age and geographic region, being more frequent in countries more distant from the equator (Parisi et al., 2013). The prevalence of psoriasis is affected by genetic, viral, endocrine and psychological factors (Harden et al., 2015). In recent years, psoriasis has been identified as a systemic disease associated with multiorgan abnormalities and complications. In patients with psoriasis, an increased risk of cardiovascular disease with atherosclerosis has been noted (Masson et al., 2020). Most research experts believe that psoriasis is an autoimmune disease, and the main mechanism is mediated by T cells. The early pathogenesis of skin lesions is chronic infiltration dominated by CD4+T lymphocytes, and the pathogenesis of late stages is slightly different from that of the early stage, mainly infiltrated by CD8+T lymphocytes, but the specific pathogenesis is still unclear (Cabrijan et al., 2009).
Recent studies have found that abnormal fat metabolism is an important factor in the pathogenesis of psoriasis. Patients with psoriasis will have significantly abnormal blood lipids and an increased risk of cardiovascular atherosclerosis (Uyanik et al., 2002). Common clinical dyslipidemia, including various lipid metabolism abnormalities, including high-density lipoprotein (HDL) reduction, is a major risk factor for cardiovascular diseases. In fact, Apolipoprotein A-1 (ApoA-1) constitutes the principal protein fraction of HDL, which plays a protective role in atherosclerosis by reversing cholesterol transport and so on. However, some recent studies have suggested different perspectives (Vuilleumier et al., 2013). They found that during chronic systemic inflammation, HDL could lose some of its atheroprotective functions, become dysfunctional or even proinflammatory. It can be seen that in the context of systemic inflammation, the mechanisms driving ApoA-1 and HDL towards pro- or anti-inflammatory molecules still needs to be studied. (Vuilleumier et al., 2013). Dyslipidemia is a risk factor for cardiovascular disease and an important cause of chronic inflammation and tissue damage, mediating the occurrence of atherosclerosis (Pirillo et al., 2021). The infiltration and retention of lipoprotein-containing Apolipoprotein B (ApoB) in the arterial wall is a key initiating event that initiates the inflammatory response and promotes the development of atherosclerosis. Arterial injury leads to endothelial dysfunction, promoting modification of lipoprotein-containing ApoB and infiltration of monocytes into the subendothelial space (Sniderman et al., 2019). Internalization of lipoprotein-containing ApoB by macrophages promotes foam cell formation, a hallmark of the fatty streak phase of atherosclerosis. Macrophage inflammation leads to oxidative stress and enhanced cytokine/chemokine secretion, resulting in more low-density lipoprotein (LDL)/residual oxidation, endothelial cell activation, monocyte recruitment, and foam cell formation. HDL, ApoA-1 and endogenous Apolipoprotein E (ApoE) prevent inflammation and oxidative stress and promote cholesterol efflux to reduce lesion formation (Libby et al., 2019).
2 Epidemiological study of atherosclerosis and psoriasis
Epidemiological studies have also confirmed the association between psoriasis and atherosclerosis. In 1973, a study of 300 hospitalized patients demonstrated for the first time that compared with other skin disease control groups, the risk of adverse cardiovascular disease (CVD) outcomes in patients with psoriasis was 2.2 times that of theirs (Caiazzo et al., 2018). The study first found that psoriasis was associated with the linkage of CVD risks. The first systematic review of 40,000 psoriasis patients in 1995 found that psoriasis was associated not only with cardiac insufficiency but also with diabetes and obesity, which are traditionally thought to cause AS (Henseler and Christophers, 1995). Subsequently, although some cohort studies concluded that psoriasis was not significantly associated with an increased risk of CVD (Miller et al., 2013) or there was only a slight association in severe psoriasis (Horreau et al., 2013), most of the subsequent systematic reviews supported that psoriasis is significantly correlated with AS-induced diseases such as myocardial ischemia and stroke (Frieder and Ryan, 2016; Sajja et al., 2018). Psoriasis and CVD risk have also been proven to present a “dose effect”; that is, CVD risk is positively correlated with the severity of psoriasis, and the study also found that psoriasis may be an independent risk factor for coronary atherosclerosis (Boehncke, 2018). However, some studies in recent years have drawn different conclusions. After correcting for traditional risk factors, there was no correlation between psoriasis and the risk of CVD adverse events. That is, the CVD risk in patients with mild psoriasis is more dependent on traditional risk factors. Only in patients with severe psoriasis was the adjusted risk association of psoriasis and CVD adverse events highlighted (Osto et al., 2012). Evidence has also found that psoriasis may be positively correlated with other traditional CVD risk factors, such as obesity, hyperlipidemia, and hypertension, but various conclusions are still inconsistent (Katsiki et al., 2014).
2.1 Risk factors for comorbidities
Smoking, excessive drinking, obesity and other unhealthy lifestyles are risk factors for cardiovascular complications in patients with psoriasis. A retrospective study found that the probability of cardiovascular disease in smoking patients was 1.78 (95% CI, 1.52–2.06), which was higher than that in nonsmoking patients (Armstrong et al., 2014). Excessive drinking is more likely to cause cardiac complications, such as atrial fibrillation, cardiomyopathy and sudden death. In addition, psoriasis is clearly associated with central obesity and increased abdominal visceral fat, which are important risk factors for cardiovascular disease (Owczarczyk-Saczonek and Nowicki, 2015). Studies have proven that obesity not only leads to vascular inflammation but also exacerbates the development of the disease and the upregulation of C-reactive protein (CRP), leptin, resistin, etc., increasing the risk of atherosclerosis (Henning, 2021). Changing the lifestyle of patients with psoriasis is beneficial to reduce the prevalence of cardiovascular complications (Tablazon et al., 2013). Age is also associated with the comorbidity of psoriatic atherosclerosis. Many studies have divided psoriasis into early-onset and late-onset psoriasis with an age cut off of 40 years old. Early-onset psoriasis has the highest risk of atherosclerotic complications, which may be related to the degree of physical involvement and systemic inflammation (Cozzani et al., 2018). In one study, patients with early-onset psoriasis had significantly elevated serum tumor necrosis factor-α (TNF-α) levels, higher levels of systemic inflammation, elevated serum endothelin levels, and carotid intima-media thickness, suggesting more severe endothelial dysfunction and a higher risk of developing atherosclerosis (Elkamshoushi et al., 2019).
In summary, patients with psoriasis have a higher risk of developing coronary atherosclerosis. Early detection, avoidance of possible risk factors, and correct treatment are helpful for the positive development of the disease, but more research is still needed to find a more suitable diagnosis and treatment method.
2.2 The relationship between lipid metabolism and psoriasis and atherosclerosis
Abnormal fat metabolism is considered to be an important factor in the pathogenesis of psoriasis. Patients with psoriasis will have significantly abnormal blood lipids and an increased risk of cardiovascular atherosclerosis (Nowowiejska et al., 2021). Apolipoprotein ApoA-1 is the main protein component of HDL, which not only regulates cholesterol transport to prevent cardiovascular disease but also participates in the regulation of inflammation and the immune response. Lipid metabolism depends on the regulation of plasma apolipoprotein ApoA-1. Dyslipidemia is a risk factor for cardiovascular disease and a cause of chronic inflammatory tissue damage, which together mediate the occurrence of atherosclerosis (van der Vorst, 2020) (Figure 1).
2.2.1 Relationship between lipid metabolism and psoriasis
Metabolic syndrome characterized by lipid metabolism disorder is one of the important comorbidities in patients with psoriasis. This may be due to changes in serum cholesterol efflux capacity and macrophage cholesterol efflux mechanisms associated with atherosclerosis, mainly in blood lipid components. It is characterized by an increase or decrease in lipoproteins and changes in the ratio of other pure lipid components, such as triglycerides (Hao et al., 2021).
Metabolic syndrome is one of the important comorbidities of psoriasis. Approximately 20%–50% of psoriasis patients are affected by metabolic syndrome. It is a general term for metabolic disorders, and the diseases caused by them, including abdominal obesity, diabetes, hyperlipidemia, hypertension, and obesity-related nonalcoholic fatty liver disease, are closely related to lipid metabolism (Nowowiejska et al., 2021). A new single-center prospective study investigated the prevalence of metabolic syndrome in 60 children aged 3–10 years with psoriasis using the 2014 European Reference Standard for Metabolic Syndrome in Children. Compared with the incidence of metabolic syndrome in the general population, the risk of metabolic syndrome in children with psoriasis is significantly higher (30% vs. 5%) (Caroppo et al., 2021). This study complements the lack of epidemiological investigation of metabolic syndrome in young people and indirectly explains the high incidence of lipid metabolism disorders in psoriasis in all age groups.
Macrophages maintain the balance of normal lipid intake and excretion in lipid metabolism, and abnormal lipid metabolism can lead to abnormal deposition of lipids in macrophages (Yan and Horng, 2020). Flow cytometry and fluorescence analysis showed that bone marrow-derived macrophages (BMDMs) of diseased mice showed increased uptake of zinc oxide low-density lipoprotein and acetylated low-density lipoprotein, and the mechanism may be that the mRNA levels of modified LDL receptors were significantly increased, resulting in increased transcription (Baumer et al., 2018). Research demonstrated that targeted disruption of lipid transfer protein results in massive accumulation of both neutral lipids and phospholipids in macrophages within multiple tissues, following administration of a high-fat and -cholesterol diet (Kennedy et al., 2005). These gene changes can lead to reduced cholesterol export and increased storage. Stored cholesterol further leads to disordered lipid metabolism under the action of inflammatory factors in the inflammatory environment of psoriasis, such as interferon gamma and tumor necrosis factor alpha, in the mouse model by changing the pH of lysosomes and free cholesterol load. It accelerates the formation of cholesterol crystals in endothelial cells (Baumer et al., 2020).
The role of bioactive lipid mediators (LMS) in the development and regression of psoriasis is still not elucidated (Sorokin et al., 2018). Lysophosphatidic acid (LPA) is considered an inflammatory lipid whose elevated levels can contribute to psoriatic plaque inflammation or lead to complications (Zeng et al., 2017). In a study on the analysis of plasma glycerophospholipid metabolism, the results of an analysis of plasma glycerophospholipid metabolism by ultrahigh-performance liquid chromatography-tandem quadrupole mass spectrometry showed that LPA was hemolyzed. The levels of phosphatidylcholine and phosphatidic acidwere significantly increased, while the levels of phosphatidylcholine and phosphatidylinositol were significantly downregulated (Zeng et al., 2017). Using the same method, another study on phospholipid metabolism in monocytes found that free 4-hydroxynonenal-Hisadducts have a high level in psoriasis vulgaris patients and show increased endogenous cannabinoids in lipid enzyme metabolism. Cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2) activity are enhanced in patients with psoriasis vulgaris (Wójcik et al., 2019). There is currently a lack of research on the expression of COX in psoriasis using TNF-α inhibitors. However, research has shown that the down-stream effector PGE2 of COX-2 can inhibit the self-renewal of intestinal stem cells during tumor necrosis factor inhibitor (TNF-i) treatment, thereby compromising tissue remodeling and regeneration (Li et al., 2018). These lipid metabolites can enhance oxidative stress and lead to inflammation. The lesioned skin of psoriatic patients contained more arachidonic acid metabolites, such as 8-, 12-, and 15-hydroxyeicosatetraenoic acid, and more linoleic acid-derived LMS.
2.2.2 Relationship between lipid metabolism and atherosclerosis
Research on the relationship between lipid abnormalities and the pathogenesis of AS is still a hotspot. The previous concept believed that the increase in LDL level is the promotion factor of AS pathogenesis, while the increase in HDL level is the avoidance factor of AS. Therefore, LDL-lowering treatment is the first choice for AS treatment and is one of the core methods (Crismaru et al., 2020). Studies in recent years have further explored the necessity of lowering LDL levels; studies have been conducted on the remaining AS risk components after LDL reaches the treatment target, such as exploring serum triglyceride levels, medium-density lipoprotein cholesterol levels, and apolipoprotein B (Ling et al., 2017). Elevated risk association for AS. There are also new studies that have explored the deep association between LDL levels and AS, such as the correlation between “residual cholesterol” and AS. Residual cholesterol may be a more accurate measure of AS risk (Sandesara et al., 2019). The treatment of AS with elevated HDL has been proposed before, but the mortality rate of AS in people with high HDL levels is still high (Kingwell et al., 2014). Harmfully, improving the cholesterol reverse transport efficiency of HDL, that is, improving the cardiovascular protective function efficiency of HDL, has become a new research direction (Beazer et al., 2020).
The implementation of LDL-lowering therapy has achieved great benefits (Brandts and Ray, 2023), but whether it is safe and effective to apply it to older patients, use higher lipid-lowering intensity, and lower LDL target concentration has not been confirmed. A high-quality meta-analysis examining the benefits and harms of further lipid lowering at LDL levels of 1.8 mmol/L (70 mg/dL) showed that for every 1 mmol/L (38.7 mg/dL) reduction in LDL, the relative risk of major vascular events was 0.79 (95% CI, 0.71–0.87; p ≤ 0.001). Both statins alone and nonstatins combined had significant curative effects, and there were no obvious adverse events. Lipid is 1.2 mmol/L (Sabatine et al., 2018). A retrospective review of LDL therapy also confirmed that lowering LDL levels reduces the risk of AS-related stroke, regardless of initial LDL levels (Baigent et al., 2005). In the META analysis study of the sick population over 75 years old, the benefit of lowering LDL in the elderly population was affirmed. A 1 mmol/LDL level reduction can reduce the risk of AS by 26%, and this benefit is the same as that under the age of 75. Benefits were not significantly different across populations (Gencer et al., 2020).
In addition, a study found that regardless of low-density lipoprotein cholesterol levels (Raposeiras-Roubin et al., 2021), triglyceride (TG) levels ≥150 mg/dL were associated with subclinical noncoronary atherosclerosis, which was significantly associated with arterial inflammation. In terms of treatment research, in the case of already using statin drugs, a large randomized double-blind controlled multicenter study found that the use of ethyl eicosapentaenoic acid to further reduce the concentration of serum TGs in patients with various AS-induced CVD and stroke was associated with a significant reduction in risk, with a 20% reduction in cardiovascular mortality (Bhatt et al., 2019). This finding further confirms the feasibility of combination therapy with other therapeutic targets for treatment.
The ratio or concentration of cholesterol in lipoproteins is associated with AS (Burnett et al., 2020). The cholesterol component found in intermediate-density lipoprotein (IDL) and very-low-density lipoprotein (VLDL) particles has been termed “residual cholesterol” (Yang et al., 2023) or defined as that found in triglyceride-rich lipoprotein cholesterol content (Burnett et al., 2020). The study found that compared with patients with low levels of residual cholesterol, patients with residual cholesterol ≥30 mg/dL (0.78 mmol/L) had a higher risk of AS, which was independent of low-density lipoprotein cholesterol (LDL-C) levels (Masson et al., 2017). In other experiments, it was also found that the level of residual cholesterol has nothing to do with the level of apolipoprotein B, and the correlation between LDL-C and CVD decreased after controlling for residual cholesterol (Johannesen et al., 2021). Therefore, it is speculated that residual cholesterol may be one of the underlying reasons why LDL-C is associated with the risk of atherosclerotic cardiovascular disease, and it is beneficial for us to evaluate CVD risk more accurately (Hoogeveen and Ballantyne, 2021).
For the method of raising HDL to treat AS, the study used the cholesteryl ester transfer protein inhibitor evacetrapib. The HDL level of the test group was significantly increased, the LDL level was significantly decreased, and the cell cholesterol efflux capacity was increased, but the end-point CVD risk was still not statistically significant compared with the control group difference (Lincoff et al., 2017). The explanation for this may be that HDL is produced ineffectively, although various hypotheses are not supported. The latest scavenger receptor class B type 1mouse model study may explain this result; that is, higher levels of HDL may cause free cholesterol to flow from HDL to LDL and macrophages with poor cholesterol efflux ability. In the case of changes, the increase in this speed can increase the free cholesterol content of multiple tissue cells in the human body (Liu et al., 2021). In the experiment, the model mice lost HDL. The ability of cholesterol to be excreted into bile (Hoekstra and Van Eck, 2015) shows that the increase in HDL levels does not necessarily play a protective role in AS, and the function of HDL also needs to be re-examined.
In addition, genetic studies have found that AS disease populations are more susceptible to AS related to lipid metabolism genes than normal populations. Loss-of-function variants in the angiopoietin-like 3 gene are associated with reduced levels of blood triglycerides, LDL-C, and HDL cholesterol, and this case‒control study found that angiopoietin-like 3 has less loss of gene function variation (adjusted odds ratio is 0.59; 95% confidence interval is 0.41–0.85; p = 0.004), which may lead to the inability to maintain a low level of blood lipids in this population, thereby aggravating AS (Dewey et al., 2017).
2.3 The clinical method of treating psoriasis and AS comorbidity
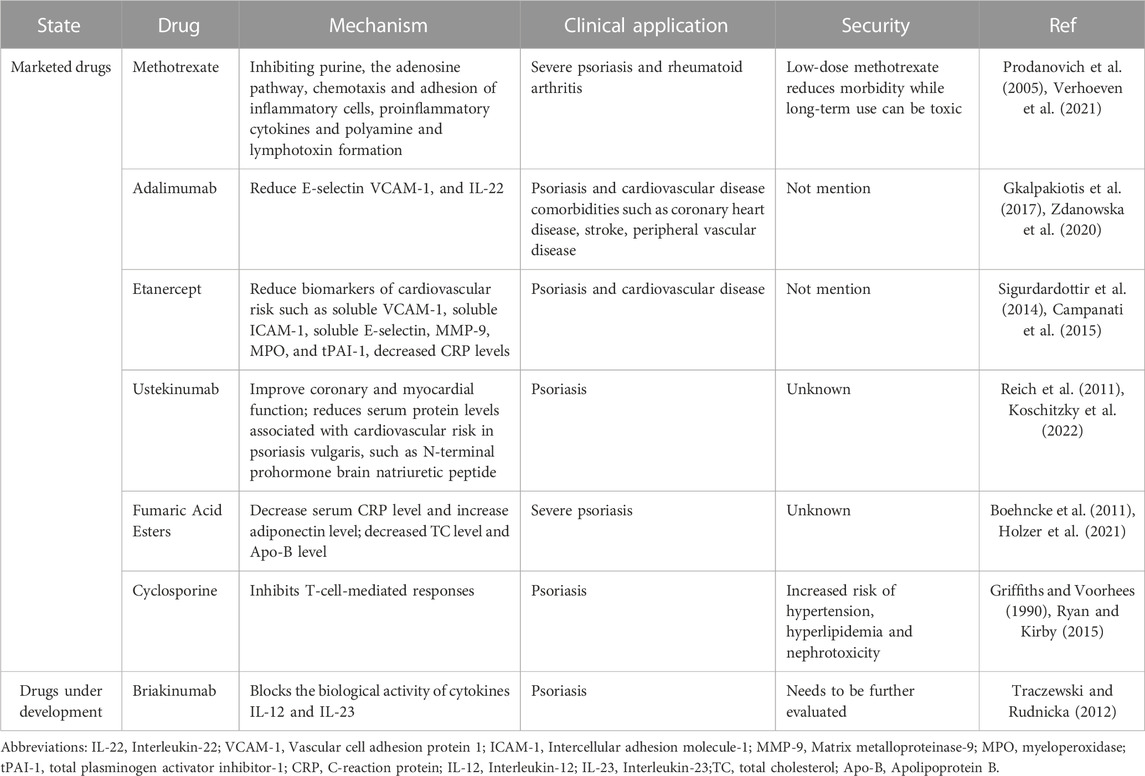
At present, the blood cholesterol management guidelines of the American Heart Association have clearly pointed out that chronic inflammatory diseases such as psoriasis are factors that enhance the risk of cardiovascular diseases such as atherosclerosis (Grundy et al., 2019). Studies have shown (Späh, 2008) that inflammation plays an important role in the link between psoriasis and coronary artery disease, so methotrexate (MTX), cyclosporin and TNF-i anti-inflammatory drugs play a key role in the treatment of comorbidities in psoriasis and AS (Shapiro et al., 2007) (Table 1). The guidelines of different countries are similar in the treatment of psoriasis complicated with coronary heart disease, but there are differences in the first choice of drugs (Shah et al., 2018). For example, the first-line system of France (Amatore et al., 2019) tends to prefer TNF as the preferred second-line systemic therapy over MTX. According to the United States (Elmets et al., 2019) combined care guidelines, patients with psoriasis treated with TNF is have a lower risk of major adverse cardiovascular events than those treated with methotrexate.
Regarding how to choose the first-line drug for the treatment of psoriasis and AS comorbidity, it has been pointed out (Yang et al., 2016) that compared with local treatment/phototherapy, methotrexate treatment with a TNF-α inhibitor was associated with a significantly lower risk of cardiovascular events compared with treatment with MTX. The risk of myocardial infarction was also reduced in psoriasis patients treated with TNF-α inhibitors compared with psoriasis patients treated with topical therapy and MTX (Shaaban and Al-Mutairi, 2018). In addition, long-term use of MTX leads to a risk of end-organ toxicity, whereas TNF-α inhibitors have a small risk of end-organ damage (Kaushik and LebwohlPsoriasis, 2019). Therefore, some experts believe that TNF-α inhibitors are the systemic drugs of choice for the treatment of psoriasis patients with cardiovascular risk factors. Wu and Poon reported (Wu and Poon, 2013) that the risk of myocardial infarction was substantially reduced in psoriasis patients treated with TNF-α inhibitors compared with untreated patients. At the same time, the abnormal effects of TNF-a antagonists on vascular function are also controversial (Dulai et al., 2012). A study found that the endothelial function and arterial elasticity of patients with moderate to severe psoriasis were improved after 6 months of adalimumab treatment (Pina et al., 2016). However, as there are few reports on how TNF-i affects COX, the specific mechanism of how adalimumab interferes via cyclooxygenase in preventing atherosclerosis remains to be explored. After 12 weeks of etanercept treatment in young patients with mild psoriasis, there was no significant change in endothelial function or vascular stiffness (Hayek et al., 2015). The reason for the difference. This suggests that more long-term data are needed on the therapeutic effect of TNF-α inhibitors.
With continuous in-depth research on psoriasis and cardiovascular diseases, new therapeutic targets have received more attention. Overall, epidemiological data suggest a positive or neutral impact on cardiovascular health for TNF, IL-17A and IL-12/23p40, but current evidence remains conflicting for anti-IL-23/p19 and JAK inhibitors (Weber et al., 2021). More research is needed to better assess the effect of biologic therapies on cardiovascular risk and to select more appropriate drugs for the treatment of psoriasis and AS comorbidities.
3 Summary
A large number of studies have explained the reasons for the comorbidity of psoriasis and coronary atherosclerosis from different angles, but there is still no clear mechanism. Psoriasis and AS have similar immune-mediated inflammatory responses, and it was previously believed that immune imbalance may be the common mechanism of both. However, increasing evidence shows that lipid metabolism disorders play an important role in the comorbidity of psoriasis and atherosclerosis. The association between psoriasis and AS may be the result of multifactorial interactions and is not limited to immune-inflammatory responses. In summary, literature reports and research results show that patients with psoriasis have an increased risk of developing central vascular diseases, lipid metabolism disorders may be the common pathogenesis of the two, and further research is needed.
Author contributions
LC: Conceptualization, Writing–original draft. HC: Software, Writing–original draft. SG: Conceptualization, Writing–original draft. ZC: Conceptualization, Writing–original draft. HY: Methodology, Writing–original draft. YL: Investigation, Writing–original draft. XaC: Investigation, Writing–original draft. XnC: Visualization, Writing–original draft. TD: Investigation, Writing–original draft. XL: Formal Analysis, Writing–original draft. JZ: Methodology, Writing–original draft. MG: Investigation, Writing–original draft. TL: Writing–review and editing, Visualization. DH: Writing–review and editing, Visualization. LW: Supervision, Writing–review and editing. JC: Supervision, Writing–review and editing. CL: Conceptualization, Validation, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82104495 and 82174161), Macao Youth Scholars Program (AM2021023), Guangdong Basic and Applied Basic Research Foundation (No. 2022A1515010395), Science and Technology Foundation of Guangzhou City (No. 202102010287), State Key Laboratory of Dampness Syndrome of Chinese Medicine Research Foundation (No. SZ2021ZZ21 and SZ2022QN02), TCM Research Fund of Guangdong Provincial Hospital of Chinese Medicine (No. YN2020MS13), 2020 Guangdong Provincial Science and Technology Innovation Strategy Special Fund (Guangdong-Hong Kong-Macau Joint Lab), (No. 2020B1212030006 and MY2022KF05), Dongguan Social Development Technology Project (20211800900022 and 20231800936882), and International Program for Postgraduates, Guangzhou University of Chinese Medicine.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AS, Atherosclerosis; HDL, High-density lipoprotein; ApoA-1, Apolipoprotein A-1; LDL, Low-density lipoprotein; ApoB, Apolipoprotein B; ApoE, Apolipoprotein E; CVD, Cardiovascular disease; CRP, C-reactive protein; TNF-α, Tumor necrosis factor-α; LMS, Lipid mediators; LPA, Lysophosphatidic acid; Cyclooxygenase, COX; COX-1, Cyclooxygenase-1; COX-2, Cyclooxygenase-2; TG, Triglyceride; IDL, Intermediate-density lipoprotein; VLDL, Very-low-density lipoprotein; LDL-C, Low-density lipoprotein cholesterol; MTX, Methotrexate; CSA, Cyclosporin.
References
Amatore, F., Villani, A. P., Tauber, M., Viguier, M., and Guillot, B.Psoriasis Research Group of the French Society of Dermatology Groupe de Recherche sur le Psoriasis de la Société Française de Dermatologie (2019). French guidelines on the use of systemic treatments for moderate-to-severe psoriasis in adults. J. Eur. Acad. Dermatol Venereol. 33, 464–483. doi:10.1111/jdv.15340
Armstrong, A. W., Harskamp, C. T., Dhillon, J. S., and Armstrong, E. J. (2014). Psoriasis and smoking: a systematic review and meta-analysis. Br. J. Dermatol 170, 304–314. doi:10.1111/bjd.12670
Baigent, C., Keech, A., Kearney, P. M., Blackwell, L., Buck, G., Pollicino, C., et al. (2005). Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 366, 1267–1278. doi:10.1016/s0140-6736(05)67394-1
Baumer, Y., Dey, A. K., Gutierrez-Huerta, C. A., Khalil, N. O., Sekine, Y., Sanda, G. E., et al. (2020). Hyperlipidaemia and IFNgamma/TNFalpha Synergism are associated with cholesterol crystal formation in Endothelial cells partly through modulation of Lysosomal pH and Cholesterol homeostasis. EBioMedicine 59, 102876. doi:10.1016/j.ebiom.2020.102876
Baumer, Y., Ng, Q., Sanda, G. E., Dey, A. K., Teague, H. L., Sorokin, A. V., et al. (2018). Chronic skin inflammation accelerates macrophage cholesterol crystal formation and atherosclerosis. JCI Insight 3, e97179. doi:10.1172/jci.insight.97179
Beazer, J. D., Patanapirunhakit, P., Gill, J. M. R., Graham, D., Karlsson, H., Ljunggren, S., et al. (2020). High-density lipoprotein's vascular protective functions in metabolic and cardiovascular disease - could extracellular vesicles be at play? Clin. Sci. (Lond) 134, 2977–2986. doi:10.1042/cs20200892
Bhatt, D. L., Steg, P. G., Miller, M., Brinton, E. A., Jacobson, T. A., Ketchum, S. B., et al. (2019). Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N. Engl. J. Med. 380, 11–22. doi:10.1056/NEJMoa1812792
Boehncke, S., Fichtlscherer, S., Salgo, R., Garbaraviciene, J., Beschmann, H., Diehl, S., et al. (2011). Systemic therapy of plaque-type psoriasis ameliorates endothelial cell function: results of a prospective longitudinal pilot trial. Arch. Dermatol Res. 303, 381–388. doi:10.1007/s00403-010-1108-6
Boehncke, W. H. (2018). Systemic inflammation and cardiovascular comorbidity in psoriasis patients: causes and consequences. Front. Immunol. 9, 579. doi:10.3389/fimmu.2018.00579
Brandts, J., and Ray, K. K. (2023). Novel and future lipid-modulating therapies for the prevention of cardiovascular disease. Nat. Rev. Cardiol. 20, 600–616. doi:10.1038/s41569-023-00860-8
Burnett, J. R., Hooper, A. J., and Hegele, R. A. (2020). Remnant cholesterol and atherosclerotic cardiovascular disease risk. J. Am. Coll. Cardiol. 76, 2736–2739. doi:10.1016/j.jacc.2020.10.029
Cabrijan, L., Lipozencić, J., Batinac, T., Peternel, S., and Pastar, Z. (2009). The role of CD4 and CD8 lymphocytes and macrophages in psoriasis vulgaris. Acta Dermatovenerol Croat. 17, 162–165.
Caiazzo, G., Fabbrocini, G., Di Caprio, R., Raimondo, A., Scala, E., Balato, N., et al. (2018). Psoriasis, cardiovascular events, and biologics: lights and shadows. Front. Immunol. 9, 1668. doi:10.3389/fimmu.2018.01668
Campanati, A., Ganzetti, G., Giuliodori, K., Marra, M., Bonfigli, A., Testa, R., et al. (2015). Serum levels of adipocytokines in psoriasis patients receiving tumor necrosis factor-α inhibitors: results of a retrospective analysis. Int. J. Dermatol 54, 839–845. doi:10.1111/ijd.12706
Caroppo, F., Galderisi, A., Ventura, L., and Belloni Fortina, A. (2021). Metabolic syndrome and insulin resistance in pre-pubertal children with psoriasis. Eur. J. Pediatr. 180, 1739–1745. doi:10.1007/s00431-020-03924-w
Cozzani, E., Rosa, G. M., Burlando, M., and Parodi, A. (2018). Psoriasis as a cardiovascular risk factor: updates and algorithmic approach. G. Ital. Dermatol Venereol. 153, 659–665. doi:10.23736/s0392-0488.18.06040-6
Crismaru, I., Pantea Stoian, A., Bratu, O. G., Gaman, M. A., Stanescu, A. M. A., Bacalbasa, N., et al. (2020). Low-density lipoprotein cholesterol lowering treatment: the current approach. Lipids Health Dis. 19, 85. doi:10.1186/s12944-020-01275-x
Dewey, F. E., Gusarova, V., Dunbar, R. L., O'Dushlaine, C., Schurmann, C., Gottesman, O., et al. (2017). Genetic and pharmacologic inactivation of ANGPTL3 and cardiovascular disease. N. Engl. J. Med. 377, 211–221. doi:10.1056/NEJMoa1612790
Dulai, R., Perry, M., Twycross-Lewis, R., Morrissey, D., Atzeni, F., and Greenwald, S. (2012). The effect of tumor necrosis factor-α antagonists on arterial stiffness in rheumatoid arthritis: a literature review. Semin. Arthritis Rheum. 42, 1–8. doi:10.1016/j.semarthrit.2012.02.002
Elkamshoushi, A. M., Omar, S. S., El Abd, A. M., Hassan, S. Z., Sultan, E. A., and Abd Elkawy, E. (2019). Subclinical atherosclerosis in psoriatic disease: relation to endocan, TNF-α, age of onset, and body fat. Int. J. Dermatol 58, 456–464. doi:10.1111/ijd.14290
Elmets, C. A., Leonardi, C. L., Davis, D. M. R., Gelfand, J. M., Lichten, J., Mehta, N. N., et al. (2019). Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J. Am. Acad. Dermatol 80, 1073–1113. doi:10.1016/j.jaad.2018.11.058
Frieder, J., and Ryan, C. (2016). Psoriasis and cardiovascular disorders. G. Ital. Dermatol Venereol. 151, 678–693.
Gencer, B., Marston, N. A., Im, K., Cannon, C. P., Sever, P., Keech, A., et al. (2020). Efficacy and safety of lowering LDL cholesterol in older patients: a systematic review and meta-analysis of randomised controlled trials. Lancet 396, 1637–1643. doi:10.1016/s0140-6736(20)32332-1
Gkalpakiotis, S., Arenbergerova, M., Gkalpakioti, P., Potockova, J., Arenberger, P., and Kraml, P. (2017). Impact of adalimumab treatment on cardiovascular risk biomarkers in psoriasis: results of a pilot study. J. Dermatol 44, 363–369. doi:10.1111/1346-8138.13661
Griffiths, C. E., and Voorhees, J. J. (1990). Cyclosporine A in the treatment of psoriasis: a clinical and mechanistic perspective. J. Invest. Dermatol 95, 53S–55s. doi:10.1111/1523-1747.ep12505786
Grundy, S. M., Stone, N. J., Bailey, A. L., Beam, C., Birtcher, K. K., Blumenthal, R. S., et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation 2019, 139, e1082-e1143. doi:10.1161/cir.0000000000000625
Hao, Y., Zhu, Y. J., Zou, S., Zhou, P., Hu, Y. W., Zhao, Q. X., et al. (2021). Metabolic syndrome and psoriasis: mechanisms and future directions. Front. Immunol. 12, 711060. doi:10.3389/fimmu.2021.711060
Harden, J. L., Krueger, J. G., and Bowcock, A. M. (2015). The immunogenetics of Psoriasis: a comprehensive review. J. Autoimmun. 64, 66–73. doi:10.1016/j.jaut.2015.07.008
Hayek, S. S., Neuman, R., Kavtaradze, N., Sher, S., Jones, D., Li, Q., et al. (2015). Tumor necrosis factor-alpha antagonism with etanercept improves endothelial progenitor cell counts in patients with psoriasis: etanercept, vascular function and endothelial progenitor cells in psoriasis. Int. J. Cardiol. 182, 387–389. doi:10.1016/j.ijcard.2014.12.093
Henning, R. J. (2021). Obesity and obesity-induced inflammatory disease contribute to atherosclerosis: a review of the pathophysiology and treatment of obesity. Am. J. Cardiovasc Dis. 11, 504–529.
Henseler, T., and Christophers, E. (1995). Disease concomitance in psoriasis. J. Am. Acad. Dermatol 32, 982–986. doi:10.1016/0190-9622(95)91336-x
Hoekstra, M., and Van Eck, M. (2015). Mouse models of disturbed HDL metabolism. Handb. Exp. Pharmacol. 224, 301–336. doi:10.1007/978-3-319-09665-0_9
Holzer, G., Hoke, M., Sabeti-Sandor, S., Perkmann, T., Rauscher, A., Strassegger, B., et al. (2021). Disparate effects of adalimumab and fumaric acid esters on cardiovascular risk factors in psoriasis patients: results from a prospective, randomized, observer-blinded head-to-head trial. J. Eur. Acad. Dermatol Venereol. 35, 441–449. doi:10.1111/jdv.16635
Hoogeveen, R. C., and Ballantyne, C. M. (2021). Residual cardiovascular risk at low LDL: remnants, lipoprotein(a), and inflammation. Clin. Chem. 67, 143–153. doi:10.1093/clinchem/hvaa252
Horreau, C., Pouplard, C., Brenaut, E., Barnetche, T., Misery, L., Cribier, B., et al. (2013). Cardiovascular morbidity and mortality in psoriasis and psoriatic arthritis: a systematic literature review. J. Eur. Acad. Dermatol Venereol. 27 (Suppl. 3), 12–29. doi:10.1111/jdv.12163
Johannesen, C. D. L., Mortensen, M. B., Langsted, A., and Nordestgaard, B. G. (2021). Apolipoprotein B and non-HDL cholesterol better reflect residual risk than LDL cholesterol in statin-treated patients. J. Am. Coll. Cardiol. 77, 1439–1450. doi:10.1016/j.jacc.2021.01.027
Katsiki, N., Anagnostis, P., Athyros, V. G., Karagiannis, A., and Mikhailidis, D. P. (2014). Psoriasis and vascular risk: an update. Curr. Pharm. Des. 20, 6114–6125. doi:10.2174/1381612820666140417105323
Kaushik, S. B., and LebwohlPsoriasis, M. G. (2019). Psoriasis: which therapy for which patient: psoriasis comorbidities and preferred systemic agents. J. Am. Acad. Dermatol 80, 27–40. doi:10.1016/j.jaad.2018.06.057
Kennedy, M. A., Barrera, G. C., Nakamura, K., Baldán, A., Tarr, P., Fishbein, M. C., et al. (2005). ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 1, 121–131. doi:10.1016/j.cmet.2005.01.002
Kingwell, B. A., Chapman, M. J., Kontush, A., and Miller, N. E. (2014). HDL-targeted therapies: progress, failures and future. Nat. Rev. Drug Discov. 13, 445–464. doi:10.1038/nrd4279
Koschitzky, M., Navrazhina, K., Garshick, M. S., Gonzalez, J., Han, J., Garcet, S., et al. (2022). Ustekinumab reduces serum protein levels associated with cardiovascular risk in psoriasis vulgaris. Exp. Dermatol 31, 1341–1351. doi:10.1111/exd.14582
Li, Y., Soendergaard, C., Bergenheim, F. H., Aronoff, D. M., Milne, G., Riis, L. B., et al. (2018). COX-2-PGE(2) signaling impairs intestinal epithelial regeneration and associates with TNF inhibitor responsiveness in ulcerative colitis. EBioMedicine 36, 497–507. doi:10.1016/j.ebiom.2018.08.040
Libby, P., Buring, J. E., Badimon, L., Hansson, G. K., Deanfield, J., Bittencourt, M. S., et al. (2019). Atheroscler. Nat. Rev. Dis. Prim. 5, 56. doi:10.1038/s41572-019-0106-z
Lincoff, A. M., Nicholls, S. J., Riesmeyer, J. S., Barter, P. J., Brewer, H. B., Fox, K. A. A., et al. (2017). Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N. Engl. J. Med. 376, 1933–1942. doi:10.1056/NEJMoa1609581
Ling, Y., Jiang, J., Wu, B., and Gao, X. (2017). Serum triglyceride, high-density lipoprotein cholesterol, apolipoprotein B, and coronary heart disease in a Chinese population undergoing coronary angiography. J. Clin. Lipidol. 11, 646–656. doi:10.1016/j.jacl.2017.02.017
Liu, J., Gillard, B. K., Yelamanchili, D., Gotto, A. M., Rosales, C., and Pownall, H. J. (2021). High free cholesterol bioavailability drives the tissue pathologies in Scarb1(-/-) mice. Arterioscler. Thromb. Vasc. Biol. 41, e453–e467. doi:10.1161/atvbaha.121.316535
Masson, W., Lobo, M., and Molinero, G. (2020). Psoriasis and cardiovascular risk: a comprehensive review. Adv. Ther. 37, 2017–2033. doi:10.1007/s12325-020-01346-6
Masson, W., Lobo, M., Molinero, G., and Siniawski, D. (2017). Discordant lipid pattern and carotid atherosclerotic plaque. Importance of remnant cholesterol. Arq. Bras. Cardiol. 108, 526–532. doi:10.5935/abc.20170069
Miller, I. M., Ellervik, C., Yazdanyar, S., and Jemec, G. B. (2013). Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. J. Am. Acad. Dermatol 69, 1014–1024. doi:10.1016/j.jaad.2013.06.053
Nowowiejska, J., Baran, A., and Flisiak, I. (2021). Aberrations in lipid expression and metabolism in psoriasis. Int. J. Mol. Sci. 22, 6561. doi:10.3390/ijms22126561
Osto, E., Piaserico, S., Maddalozzo, A., Forchetti, G., Montisci, R., Famoso, G., et al. (2012). Impaired coronary flow reserve in young patients affected by severe psoriasis. Atherosclerosis 221, 113–117. doi:10.1016/j.atherosclerosis.2011.12.015
Owczarczyk-Saczonek, A. B., and Nowicki, R. J. (2015). Prevalence of cardiovascular disease risk factors, and metabolic syndrome and its components in patients with psoriasis aged 30 to 49 years. Postepy Dermatol Alergol. 32, 290–295. doi:10.5114/pdia.2014.40966
Parisi, R., Symmons, D. P., Griffiths, C. E., and Ashcroft, D. M.Identification and Management of Psoriasis and Associated ComorbidiTy IMPACT project team (2013). Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J. Invest. Dermatol 133, 377–385. doi:10.1038/jid.2012.339
Pina, T., Corrales, A., Lopez-Mejias, R., Armesto, S., Gonzalez-Lopez, M. A., Gómez-Acebo, I., et al. (2016). Anti-tumor necrosis factor-alpha therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: a 6-month prospective study. J. Dermatol 43, 1267–1272. doi:10.1111/1346-8138.13398
Pirillo, A., Casula, M., Olmastroni, E., Norata, G. D., and Catapano, A. L. (2021). Global epidemiology of dyslipidaemias. Nat. Rev. Cardiol. 18, 689–700. doi:10.1038/s41569-021-00541-4
Prodanovich, S., Ma, F., Taylor, J. R., Pezon, C., Fasihi, T., Kirsner, R. S., et al. (2005). Methotrexate reduces incidence of vascular diseases in veterans with psoriasis or rheumatoid arthritis. J. Am. Acad. Dermatol 52, 262–267. doi:10.1016/j.jaad.2004.06.017
Raposeiras-Roubin, S., Rosselló, X., Oliva, B., Fernández-Friera, L., Mendiguren, J. M., Andrés, V., et al. (2021). Triglycerides and residual atherosclerotic risk. J. Am. Coll. Cardiol. 77, 3031–3041. doi:10.1016/j.jacc.2021.04.059
Reich, K., Langley, R. G., Lebwohl, M., Szapary, P., Guzzo, C., Yeilding, N., et al. (2011). Cardiovascular safety of ustekinumab in patients with moderate to severe psoriasis: results of integrated analyses of data from phase II and III clinical studies. Br. J. Dermatol 164, 862–872. doi:10.1111/j.1365-2133.2011.10257.x
Ryan, C., and Kirby, B. (2015). Psoriasis is a systemic disease with multiple cardiovascular and metabolic comorbidities. Dermatol Clin. 33, 41–55. doi:10.1016/j.det.2014.09.004
Sabatine, M. S., Wiviott, S. D., Im, K., Murphy, S. A., and Giugliano, R. P. (2018). Efficacy and safety of further lowering of low-density lipoprotein cholesterol in patients starting with very low levels: a meta-analysis. JAMA Cardiol. 3, 823–828. doi:10.1001/jamacardio.2018.2258
Sajja, A. P., Joshi, A. A., Teague, H. L., Dey, A. K., and Mehta, N. N. (2018). Potential immunological links between psoriasis and cardiovascular disease. Front. Immunol. 9, 1234. doi:10.3389/fimmu.2018.01234
Sandesara, P. B., Virani, S. S., Fazio, S., and Shapiro, M. D. (2019). The forgotten lipids: triglycerides, remnant cholesterol, and atherosclerotic cardiovascular disease risk. Endocr. Rev. 40, 537–557. doi:10.1210/er.2018-00184
Shaaban, D., and Al-Mutairi, N. (2018). The effect of tumor necrosis factor inhibitor therapy on the incidence of myocardial infarction in patients with psoriasis: a retrospective study. J. Dermatol. Treat. 29, 3–7. doi:10.1080/09546634.2016.1254145
Shah, V. V., Lee, E. B., Reddy, S., Lin, E. J., and Wu, J. J. (2018). Comparison of guidelines for the use of TNF inhibitors for psoriasis in the United States, Canada, Europe and the United Kingdom: a critical appraisal and comprehensive review. J. Dermatol. Treat. 29, 586–592. doi:10.1080/09546634.2018.1428723
Shapiro, J., Cohen, A. D., David, M., Hodak, E., Chodik, G., Viner, A., et al. (2007). The association between psoriasis, diabetes mellitus, and atherosclerosis in Israel: a case-control study. J. Am. Acad. Dermatol 56, 629–634. doi:10.1016/j.jaad.2006.09.017
Sigurdardottir, G., Ekman, A. K., Ståhle, M., Bivik, C., and Enerbäck, C. (2014). Systemic treatment and narrowband ultraviolet B differentially affect cardiovascular risk markers in psoriasis. J. Am. Acad. Dermatol 70, 1067–1075. doi:10.1016/j.jaad.2013.12.044
Sniderman, A. D., Thanassoulis, G., Glavinovic, T., Navar, A. M., Pencina, M., Catapano, A., et al. (2019). Apolipoprotein B particles and cardiovascular disease: a narrative review. JAMA Cardiol. 4, 1287–1295. doi:10.1001/jamacardio.2019.3780
Sorokin, A. V., Domenichiello, A. F., Dey, A. K., Yuan, Z. X., Goyal, A., Rose, S. M., et al. (2018). Bioactive lipid mediator profiles in human psoriasis skin and blood. J. Invest. Dermatol 138, 1518–1528. doi:10.1016/j.jid.2018.02.003
Späh, F. (2008). Inflammation in atherosclerosis and psoriasis: common pathogenic mechanisms and the potential for an integrated treatment approach. Br. J. Dermatol 159 (Suppl. 2), 10–17. doi:10.1111/j.1365-2133.2008.08780.x
Tablazon, I. L., Al-Dabagh, A., Davis, S. A., and Feldman, S. R. (2013). Risk of cardiovascular disorders in psoriasis patients: current and future. Am. J. Clin. Dermatol 14, 1–7. doi:10.1007/s40257-012-0005-5
Traczewski, P., and Rudnicka, L. (2012). Briakinumab for the treatment of plaque psoriasis. BioDrugs 26, 9–20. doi:10.2165/11595940-000000000-00000
Uyanik, B. S., Ari, Z., Onur, E., Gündüz, K., Tanülkü, S., and Durkan, K. (2002). Serum lipids and apolipoproteins in patients with psoriasis. Clin. Chem. Lab. Med. 40, 65–68. doi:10.1515/cclm.2002.013
Valacchi, G., De Luca, C., and Wertz, P. W. (2010). Lipid mediators in skin inflammation: updates and current views. Mediat. Inflamm. 2010, 398926. doi:10.1155/2010/398926
van der Vorst, E. P. C. (2020). High-density lipoproteins and apolipoprotein A1. Subcell. Biochem. 94, 399–420. doi:10.1007/978-3-030-41769-7_16
Verhoeven, F., Prati, C., Chouk, M., Demougeot, C., and Wendling, D. (2021). Methotrexate and cardiovascular risk in rheumatic diseases:A comprehensive review. Expert Rev. Clin. Pharmacol. 14, 1105–1112. doi:10.1080/17512433.2021.1932461
Vuilleumier, N., Dayer, J. M., von Eckardstein, A., and Roux-Lombard, P. (2013). Pro- or anti-inflammatory role of apolipoprotein A-1 in high-density lipoproteins? Swiss Med. Wkly. 143, w13781. doi:10.4414/smw.2013.13781
Weber, B., Merola, J. F., Husni, M. E., Di Carli, M., Berger, J. S., and Garshick, M. S. (2021). Psoriasis and cardiovascular disease: novel mechanisms and evolving therapeutics. Curr. Atheroscler. Rep. 23, 67. doi:10.1007/s11883-021-00963-y
Wójcik, P., Biernacki, M., Wroński, A., Łuczaj, W., Waeg, G., Žarković, N., et al. (2019). Altered lipid metabolism in blood mononuclear cells of psoriatic patients indicates differential changes in psoriasis vulgaris and psoriatic arthritis. Int. J. Mol. Sci. 20, 4249. doi:10.3390/ijms20174249
Wu, J. J., and Poon, K. Y. (2013). Association of gender, tumor necrosis factor inhibitor therapy, and myocardial infarction risk in patients with psoriasis. J. Am. Acad. Dermatol 69, 650–651. doi:10.1016/j.jaad.2013.04.035
Yan, J., and Horng, T. (2020). Lipid metabolism in regulation of macrophage functions. Trends Cell Biol. 30, 979–989. doi:10.1016/j.tcb.2020.09.006
Yang, L., Yue, Q., Fang, F., Zhang, Y., Liu, P., Zhang, Z., et al. (2023). Effect of dual residual risk of cholesterol and inflammation on all-cause mortality in patients with cardiovascular disease. Cardiovasc Diabetol. 22, 96. doi:10.1186/s12933-023-01826-3
Yang, Z. S., Lin, N. N., Li, L., and Li, Y. (2016). The effect of TNF inhibitors on cardiovascular events in psoriasis and psoriatic arthritis: an updated meta-analysis. Clin. Rev. Allergy Immunol. 51, 240–247. doi:10.1007/s12016-016-8560-9
Zdanowska, N., Owczarczyk-Saczonek, A., Czerwińska, J., Nowakowski, J. J., Kozera-Żywczyk, A., Owczarek, W., et al. (2020). Methotrexate and adalimumab decrease the serum levels of cardiovascular disease biomarkers (VCAM-1 and E-selectin) in plaque psoriasis. Med. Kaunas. 56, 473. doi:10.3390/medicina56090473
Keywords: comorbidity, lipid metabolism, psoriasis, atherosclerosis, inflammation
Citation: Chen L, Chen H, Guo S, Chen Z, Yang H, Liu Y, Chen X, Chen X, Du T, Long X, Zhao J, Guo M, Lao T, Huang D, Wang L, Chen J and Liu C (2023) Psoriasis comorbid with atherosclerosis meets in lipid metabolism. Front. Pharmacol. 14:1308965. doi: 10.3389/fphar.2023.1308965
Received: 07 October 2023; Accepted: 27 November 2023;
Published: 11 December 2023.
Edited by:
Gilberto De Nucci, State University of Campinas, BrazilReviewed by:
Renata Magalhaes, State University of Campinas, BrazilCopyright © 2023 Chen, Chen, Guo, Chen, Yang, Liu, Chen, Chen, Du, Long, Zhao, Guo, Lao, Huang, Wang, Chen and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunping Liu, Y2h1bnBpbmdsaXVAZ3p1Y20uZWR1LmNu; Jing Chen, d3l1Y2hlbWNqQDEyNi5jb20=; Lei Wang, ZHIud2FuZ2xlaUBnenVjbS5lZHUuY24=
†These authors have contributed equally to this work
 Liuping Chen
Liuping Chen Huiqi Chen
Huiqi Chen Sien Guo
Sien Guo Zhijun Chen2,3
Zhijun Chen2,3 Tingting Du
Tingting Du Xinyao Long
Xinyao Long Lei Wang
Lei Wang Jing Chen
Jing Chen Chunping Liu
Chunping Liu