
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 04 December 2023
Sec. Drugs Outcomes Research and Policies
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1281235
This article is part of the Research Topic Rising Stars in Drugs Outcomes Research and Policies: 2023 View all 16 articles
 Jia-jia Huang1,2†
Jia-jia Huang1,2† Ji-zhen Cai3†
Ji-zhen Cai3† Zhi-peng Zhou1
Zhi-peng Zhou1 Yan Liu1,4
Yan Liu1,4 Zhen-jia Yang1
Zhen-jia Yang1 Da-zheng Li1
Da-zheng Li1 Yu-hua Chen1,5
Yu-hua Chen1,5 Ying-yi Luan6*
Ying-yi Luan6* Yong-ming Yao7*
Yong-ming Yao7* Ming Wu1,4*
Ming Wu1,4*Background: Previous studies documented that heparin can inhibit the invasion and metastasis of tumors, but its role on outcomes in patients with solid malignancy complicated sepsis remains unclear.
Methods: A retrospective cohort study was conducted in critically ill patients with solid malignancy associated sepsis from the Medical Information Mart for Intensive Care (MIMIC)-IV database. The primary endpoint was intensive care unit (ICU) mortality, secondary outcomes were thrombosis and hospital mortality. Propensity score matching (PSM), marginal structural Cox model (MSCM), cox proportional hazards model, stratification analysis and E-value were used to account for baseline differences, time-varying confounding and unmeasured variables.
Results: A total of 1,512 patients with solid malignancy complicated sepsis were enrolled, of which 683 in the heparin group with intensive care unit mortality, thrombosis rate and hospital mortality were 9.7%, 5.4%, 16.1%, and 829 in the non-heparin group with ICU mortality, thrombosis rate and hospital mortality were 14.6%, 12.5%, 22.6%. Similar results were observed on outcomes for patients with PSM (ICU mortality hazard ratio [HR] 0.61, 95% confidence interval [CI] 0.41–0.92), thrombosis rate (HR 0.42, 95% confidence interval 0.26–0.68); hospital mortality HR 0.70, 95% CI 0.50–0.99). marginal structural Cox model further reinforced the efficacy of heparin in reducing ICU mortality (HR 0.48, 95% CI 0.34–0.68). Logistic regression and Cox regression model showed heparin use also markedly reduced thrombosis (HR 0.42; 95% CI 0.26–0.68; p < 0.001) and hospital mortality (HR 0.70; 95% CI 0.50–0.99; p = 0.043). Stratification analysis with the MSCM showed an effect only those with digestive system cancer (HR 0.33, 95% CI 0.16–0.69).
Conclusion: Early heparin therapy improved outcomes in critically ill patients with solid malignancy complicated sepsis. These results are evident especially in those with digestive system cancer. A prospective randomized controlled study should be designed to further assess the relevant findings.
Over the past decades, although clinical research on tumor therapy has improved rapidly, but cancer is the leading cause of deaths in all over the world (Bray et al., 2021; Sung et al., 2021). Targeted antitumor therapy mainly affects tumor formation and regulation, the tumor microenvironment, specific tumor markers, immune modulators, and targeted tumor stem cells (Danai et al., 2006; Vincent et al., 2009; Schünemann et al., 2020; Khan et al., 2021). Antitumor therapy readily releases damage-associated molecules in patients with solid malignancies, exacerbating undesirable inflammatory responses (Yuan et al., 2020). Many tumor-targeting drugs may affect angiogenesis, damage endothelial cells, and lead to cancer-associated coagulopathy, which increases the risk of thrombosis. It has been demonstrated that patients with cancer are more likely to develop venous thromboembolism (VTE) than those without cancer (Schünemann et al., 2020).
Patients with cancer exhibit an increased risk of sepsis owing to immune dysfunction. It is reported that, among cancer cases, the sepsis incidence increases by approximately 10 times than that of non-cancer cases (Vincent et al., 2009). A recent study showed that malignant tumors can be detected in 1/6 of intensive care unit (ICUs) inpatients experiencing sepsis (Danai et al., 2006), and if solid tumors patients suffer sepsis shock, the 28-day mortality rate was 69.4% (Cuenca et al., 2022). With the development of social economy and the improvement of clinical treatment, patients with cancer are always admitted to the ICU because of secondary sepsis and sepsis-induced coagulopathy (SIC). SIC and cancer-associated coagulopathy intensify VTE incidence among patients with solid malignancies concomitant sepsis.
As an anticoagulation agent, heparin has been widely used for prophylaxis VTE for several decades. Previous studies documented that heparin inhibited the invasion and metastasis of tumors (Wei et al., 2023), but limited data exists on whether heparin administration provide a survival advantage in patients with solid malignancies concomitant sepsis. Therefore, we conducted a retrospective cohort study adopted data based on the Medical Information Mart for Intensive Care IV (MIMIC-IV) database to assess whether anticoagulation with heparin used in solid malignancy concomitant sepsis cases hospitalized in the ICU offered survival benefits, including advantages in ICU mortality, hospital mortality, and thrombosis, and estimated heparin application timing as well as dosing.
We performed a retrospective cohort study using data from MIMIC-IV (version 1.0). It included two in-hospital database systems, namely, ICU-specific clinical data and the custom hospital-wide electronic health record (EHR), which covered anonymous, integrated clinical information of cases at ICUs in Beth Israel Deaconess Medical Center in Boston, Massachusetts, from 2008 to 2019. Individuals finishing a collaborative institutional training initiative examination (certification number 38995627 for author Huang) were allowed to enter this database.
Altogether 382,278 individuals with 51,150 patients were admitted to the ICUs during our research. Patient selection criteria were as follows: 1) those aged over 18 years, 2) those meeting Sepsis 3.0 criteria (namely, a rapid elevation of Sequential Organ Failure Assessment (SOFA) score ≥2 in combination with suspicious infection (Singer et al., 2016), and 3) those diagnosed with malignant cancer.
Patients conforming to the following conditions were excluded: 1) those admitted to the ICU several times; 2) those aged <18 years or stayed in the ICU for <24 h; 3) those who used heparin in treatment or dialysis, but not in prophylactic application, or those who received warfarin or low-molecular-weight heparin (LMWH) in ICU stay; 4) pregnant women; 5) those with previous heparin-caused thrombocytopenia; 6) those with liver failure; 7) those with stage 5 chronic kidney disease (CKD); and 8) those with hematological malignancies.
The study collected information based on the MIMIC-IV database by Structured Query Language. Approaches described previously were adopted to search the above database (sepsis) and analyze the patient information collected (Zou et al., 2022). For patients who were hospitalized repeatedly, initial hospitalizations were collected. Basic features, together with laboratory findings on day one upon ICU admission, were extracted, which included age upon admission to the hospital, gender, weight, laboratory examinations (hemoglobin levels, white blood cell [WBC] count, platelet count, international normalized ratio [INR], prothrombin time [PT], partial thromboplastin time [PTT]), vital signs (heart rate, temperature, respiratory rate, mean arterial pressure [MAP], partial pressure of oxygen [PO2]), concurrent diseases (diabetes, hypertension, chronic lung disease, chronic heart disease (CHD)), urine output, mechanical ventilation, vasopressor use, acute kidney injury (AKI) stage, and renal replacement therapy (RRT). This study further collected the results of clinical severity scales, such as the SOFA score or Simplified Acute Physiology Score II (SAPS II). Typically, the SOFA score was determined in the initial 24-h after post-admission to the ICU. AKI diagnosis was based on the Kidney Disease: Improving Global Outcomes (KDIGO) standards (Ostermann et al., 2020). AKI stages were determined based on creatinine and urine output levels within the initial 24-h after post-admission to the ICU.
The laboratory variable activated partial thromboplastin time (APTT) was extracted throughout all ICU stay. In addition, the present work obtained the physiological values and measurement chart time based on a database. If the cases were measured repeatedly, only the highest daily APTT was included in the analyses. These screening variables had <15% missing values (Supplementary Table S1) and were later subjected to single imputation.
Participants were divided into two groups: a heparin group that enrolled patients receiving subcutaneous heparin 24 h after post-admission into the ICU at preventive doses and a control group that included patients not receiving heparin on day one. Our primary outcome was ICU mortality, which was deemed to be patient survival upon discharge from the ICU. The secondary outcomes included in-hospital mortality and thrombosis.
Categorical variables were presented in the form of numbers and percentages. They were compared between the heparin and nonheparin groups using Fisher’s exact test and chi-square test, whereas continuous variables were expressed as mean (standard deviation, SD) or median (interquartile range [IQR]) as appropriate.
The present study adopted propensity score matching (PSM) to interpret the basic differences in whether the patient received heparin treatment (Huang et al., 2023). During PSM analysis, prophylactic heparin was administered to the heparin group 24 h post-ICU admission. The treatment group was then matched to the control group through nearest-neighbor matching. Besides, this work also determined standardized mean difference (SMD) was calculated to examine whether PSM reduced the differences between two groups. Finally, a Cox proportional hazards model was used to adjust for residual imbalance by including parameters with p values < 0.05 and potential confounding factors judged by clinical expertise.
In this study, we determined heparin administration during the ICU stay to be the time-dependent variable for the marginal structural Cox model (MSCM). The possible basic confounders, including age, gender, mechanical ventilation, vasopressor, RRT, SOFA, and SAPS II scores, were acquired on the first day after ICM entry. The APTT in the entire ICU stay period was a time-varying confounder and was incorporated into the above model. In addition, MSCM parameters were predicted based on inverse probability weighting (IPW) (IPW) to correct selection bias or confounders, such as informative censoring (Shinozaki and Suzuki, 2020). IPW was adopted to weigh all cases, which helped to create two pseudopopulations close in terms of basic and time-dependent confounders, whereas they were distinct with regard to heparin exposure. IPWs were estimated using the IPW package (Grafféo et al., 2019).
To explore the differences in heparin use and ICU mortality among diverse subgroups stratified according to gender, race, AKI stage, vasopressor medication, mechanical ventilation use, and malignant cancer, a stratification analysis was performed. The Cox model, with adjustments for every variable in basic patient characteristics, was adopted for the subgroup analysis. E-values were determined to analyze the probability of non-measured confounders of heparin with ICU mortality (Haneuse et al., 2019). E-values could be used to quantify the necessary magnitude of the non-methylated confounder to negate the detected relationship of heparin with ICU mortality. Additionally, a pre-specified subgroup analysis was carried out using MSCM. p < 0.05 (two-tailed) stood for statistical difference. R software (version 4.1.1) was used for the analysis.
Upon a preliminary search, 382,278 ICU entries were found in the MIMIC-IV database. There were 32,404 cases satisfying the definition, while 1,512 septic patients had complicated malignant cancer 24 h after admission into the ICU. Of those study cases, 683 received heparin treatment within the initial 24 h post-admission to the ICU, whereas the remaining 829 did not administer heparin (Figure 1).
As shown in Table 1, temperature, hemoglobin levels, platelet levels, INR, PT, presence of diabetes, presence of chronic heart disease, AKI stage, ventilation, and SOFA score were significantly different between the two groups. Notably, the non-heparin group had a higher number of critical cases than the heparin group (SOFA score, 6 [4.8] vs. 5 [3.8], p < 0.001). Moreover, the heparin group displayed an increased requirement for mechanical ventilation (38.9% vs. 33.2%, p = 0.023) compared to the non-heparin group.
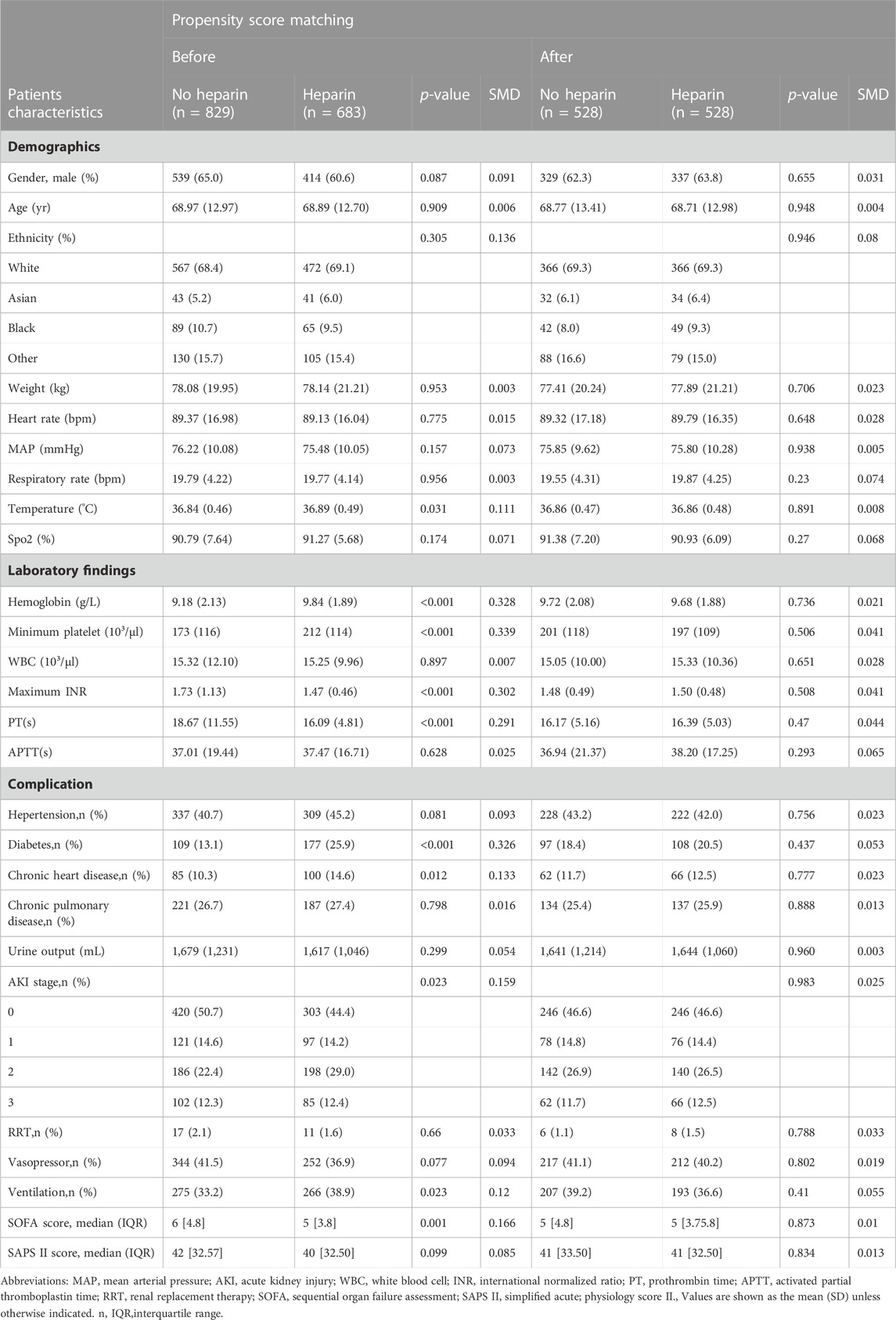
TABLE 1. Baseline characteristics of critically ill patients with solid malignancy associated sepsis before and after propensity score matching.
A total of 1,512 solid malignancy patients with sepsis were enrolled in the study, of which 683 in the heparin group had ICU mortality, thrombosis rate, and hospital mortality of 9.7%, 5.4%, 16.1%, and 829 in the non-heparin group, and ICU mortality, thrombosis rate, and hospital mortality were 14.6%, 12.5%, and 22.6%, respectively (Table 2). PSM was then conducted to match 528 cases receiving heparin with 528 patients not receiving heparin, which markedly decreased the imbalances between both groups (Supplementary Figure S1, Table 1). Owing to the presence of residual imbalances between both groups, this study utilized the Cox proportional hazards model. As a result, heparin use led to decreased mortality for the entire cohort (ICU mortality: hazard ratio [HR],0.61; 95% CI 0.41–0.92; p = 0.019). Secondary outcomes were similar (hospital mortality: HR, 0.70; 95% CI 0.50–0.99; p = 0.043, thrombosis: HR:0.42; 95% CI 0.26–0.68; p < 0.001) (Table 2).
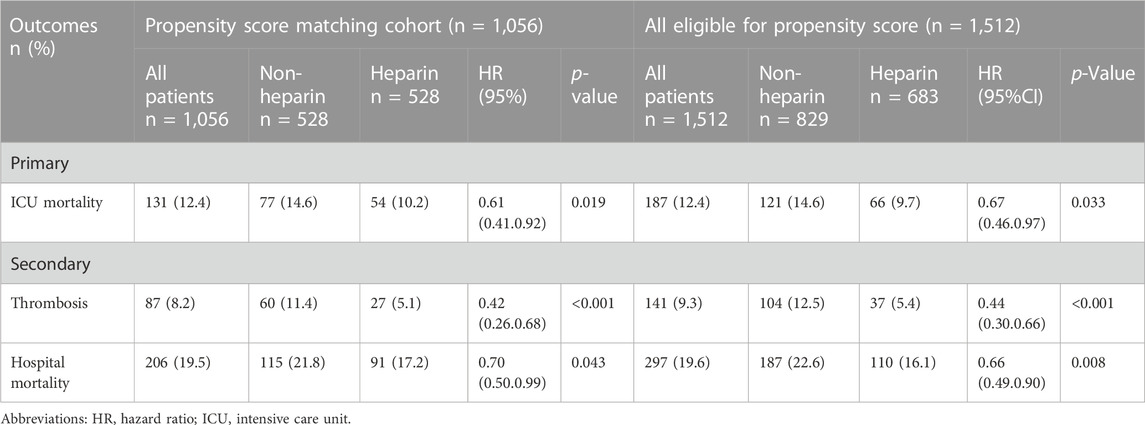
TABLE 2. Association between heparin use and clinic outcomes in critically ill patients with solid malignancy associated sepsis.
This study incorporated heparin use and time-varying confounders into MSCM. As a result, heparin use reduced ICU mortality (HR 0.48; 95% CI 0.34–0.68; p < 0.001) for the entire cohort. According to the stratification analysis results, heparin use decreased the incidence of ICU mortality among patients with solid malignancies concomitant sepsis among males (HR 0.30; 95% CI 0.18–0.51; p < 0.001) and those of white ethnicity (HR 0.36; 95% CI 0.22–0.59; p < 0.001), stage 3 AKI (HR 0.23; 95% CI 0.11–0.50; p < 0.001), and digestive system cancer (HR 0.33; 95% CI 0.16–0.69; p = 0.003) (Figure 2). It was observed that, in patients with solid malignancy concomitant sepsis, treatment with 7,500–12500 IU heparin daily was associated with a lower risk of ICU mortality than no heparin treatment (Table 3).
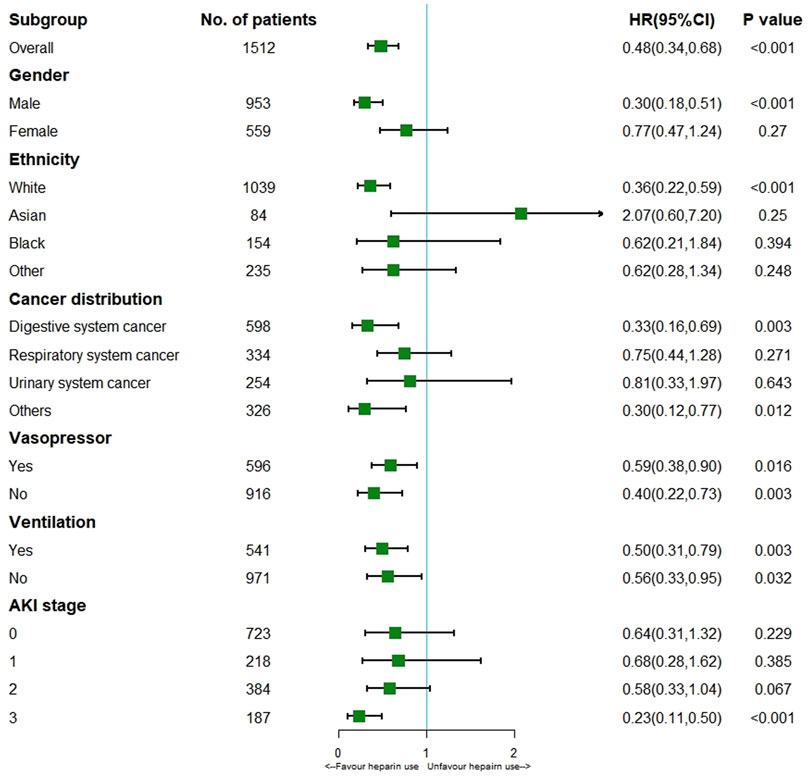
FIGURE 2. Results of ICU mortality in overall population with MSCM and stratification analysis Abbreviations: AKI, acute kidney injury; HR, hazard ratio.
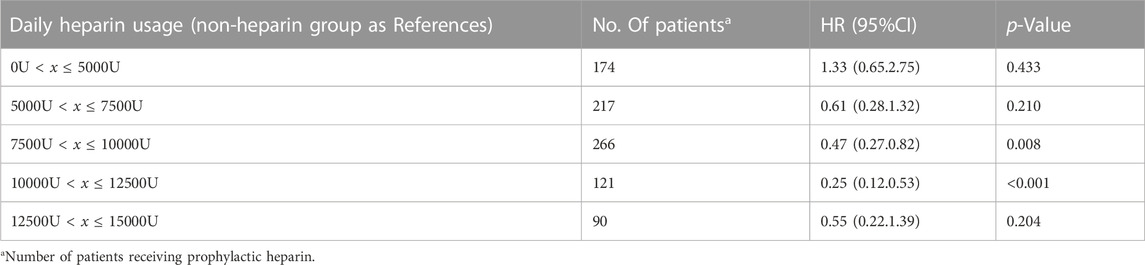
TABLE 3. Dose-response relationship between heparin and ICU mortality in critically ill patients with solid malignancy associated sepsis.
Logistic regression model showed heparin use markedly reduced thrombosis (HR 0.42; 95% CI 0.26–0.68; p < 0.001) for the entire cohort. According to the stratification analysis results, heparin use decreased the incidence of thrombosis in patients with solid malignancies concomitant sepsis among males (HR 0.35; 95% CI 0.18–0.68; p = 0.002) and those of white ethnicity (HR 0.40; 95% CI 0.22–0.71; p = 0.002) or other ethnicity (HR 0.36; 95% CI 0.14–0.91; p = 0.03), digestive system cancer (HR 0.27; 95% CI 0.12–0.63; p = 0.002), non-Vasopressor use (HR 0.31; 95% CI 0.15–0.64; p = 0.002), non-Ventilation (HR 0.29; 95% CI 0.15–0.56; p < 0.001) and AKI (HR 0.53; 95% CI 0.29–0.97; p = 0.039). (Figure 3).
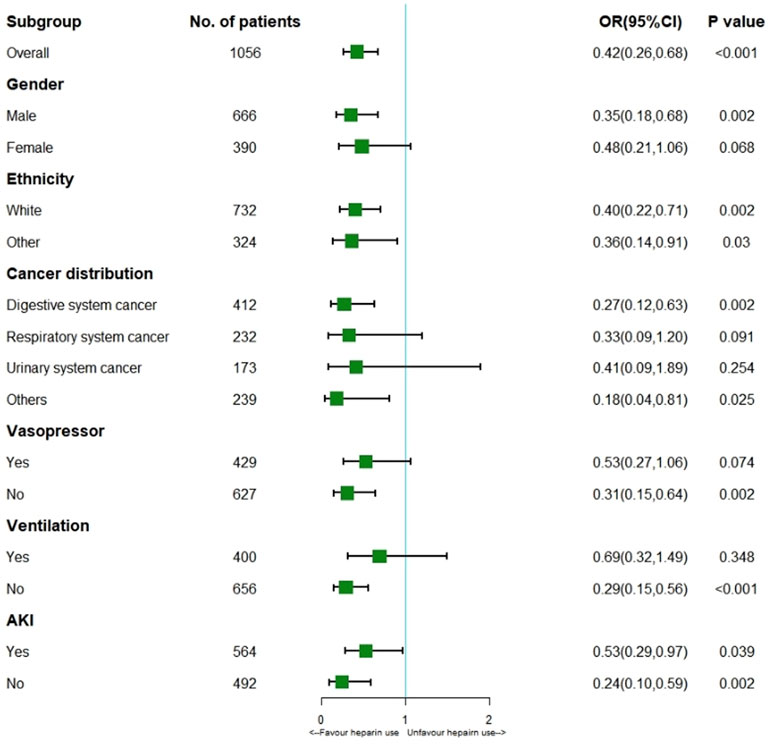
FIGURE 3. Results of thrombosis in overall population with logistic regression model and stratification analysis. Abbreviations: AKI, acute kidney injury; OR, odds ratio.
Cox regression model showed heparin use markedly reduced hospital mortality (HR 0.70; 95% CI 0.50–0.99; p = 0.043) for the all cohort. According to the stratification analysis results, heparin use decreased the incidence of hospital mortality in patients with solid malignancies concomitant sepsis among those of white ethnicity (HR 0.59; 95% CI 0.41–0.87; p = 0.007), digestive system cancer (HR 0.45; 95% CI 0.26–0.80; p = 0.007), Vasopressor use (HR 0.53; 95% CI 0.35–0.81; p = 0.004), Ventilation (HR 0.40; 95% CI 0.23–0.67; p < 0.001) and stage 3 AKI (HR 0.15; 95% CI 0.07–0.34; p < 0.001). (Figure 4).
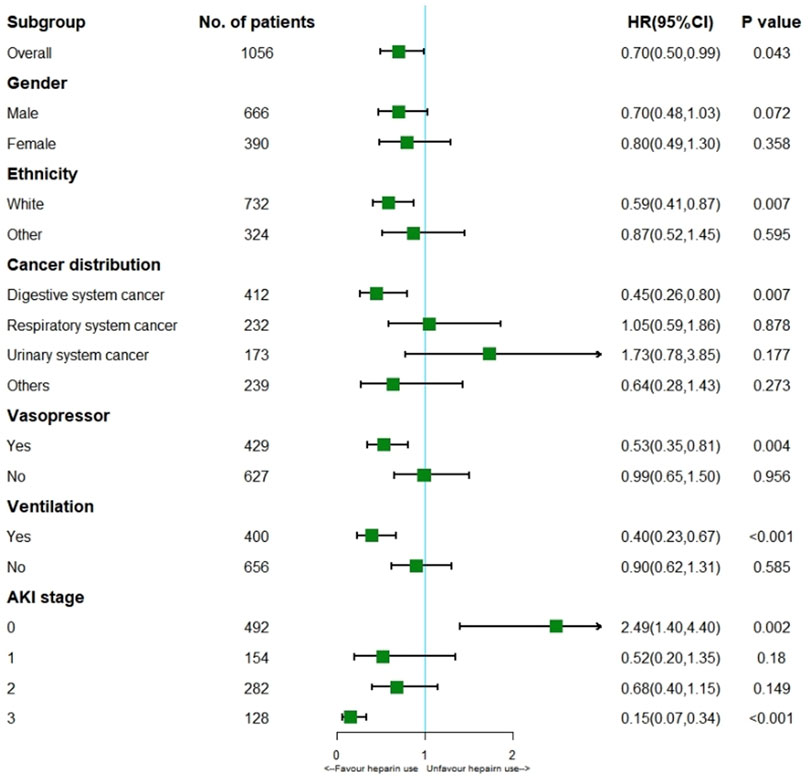
FIGURE 4. Results of hospital mortality in overall population with cox regression model and stratification analysis.
Distinct measured and known ICU mortality-related risk factors identified in the multivariable Cox proportional hazards model following PSM were SAPS II (HR, 1.02; 95% CI, 1.01–1.04), AKI 2 (HR, 1.91; 95% CI, 1.10–3.32), chronic pulmonary disease (HR, 2.27 (1.51.3.41); 95% CI, 1.51–3.41), and diabetes (HR, 1.74; 95% CI, 1.09–2.79) (Table 4).
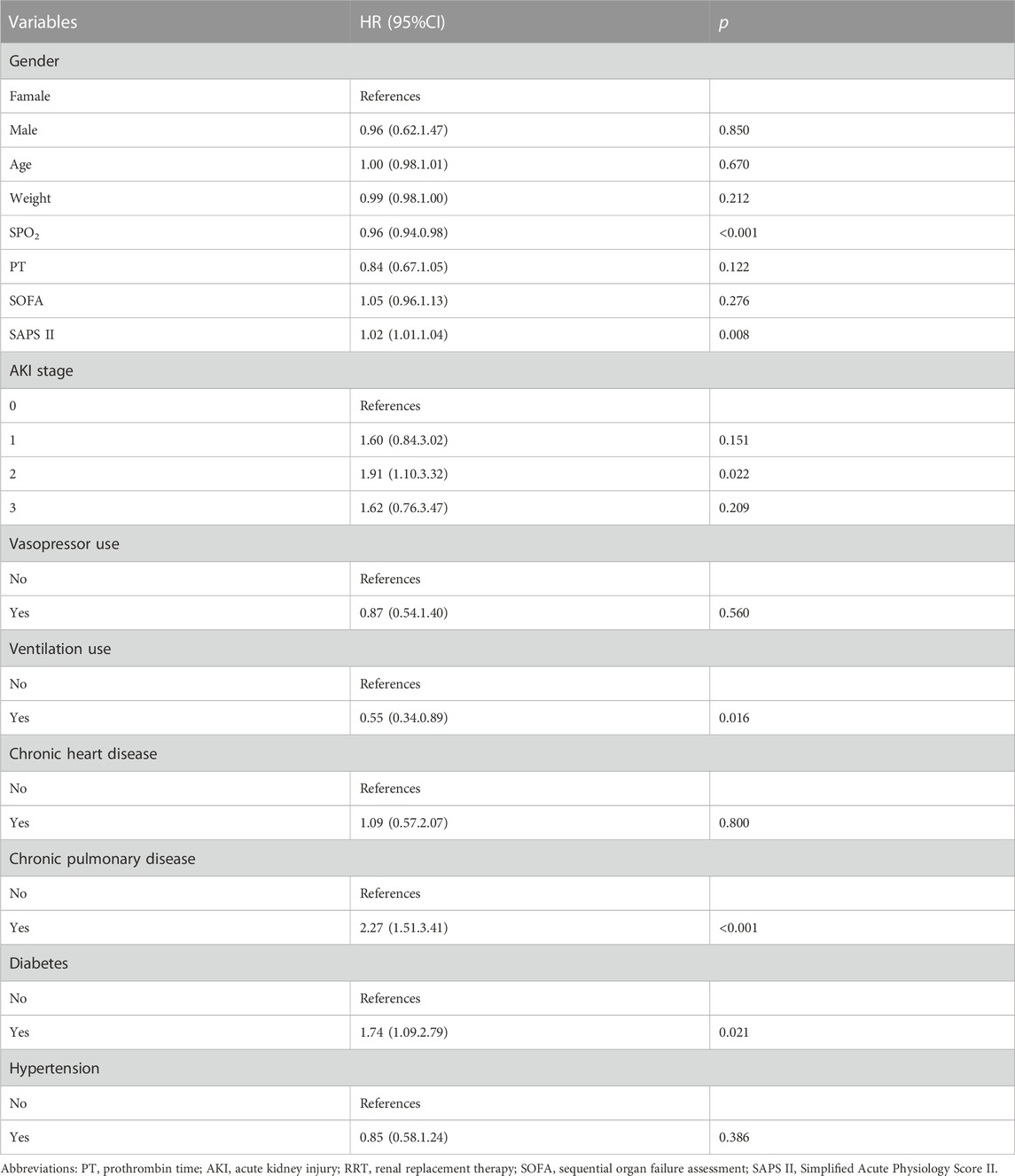
TABLE 4. Multivariable cox regression model after propensity score matching in critically ill patients with solid malignancy associated sepsis.
E-values were analyzed to assess the sensitivity to non-measured confounders (https://www.evalue-calculator.com/evalue/). Reliable preliminary results were obtained, only if non-measured confounders existed, the ICU mortality risk was relatively low and the HR was over 2.66 (upper limit of 4.05), suggesting the capability of residual confounders of explaining the detected connection when one non-measured covariate with the relative risk association with prophylactic heparin use and ICU mortality of >2.66 existed. Therefore, unknown and non-measured confounders might not significantly affect ICU mortality (relative risk >2.66) compared with known risk factors.
Due to the immunosuppression observed in cancer patients, sepsis may develop. Although animal studies have demonstrated that heparin can combine with lipopolysaccharide (LPS) to reduce the mortality resulting from Gram-negative bacterial infection (Tang et al., 2021; Yuan et al., 2021), and we found that early administration heparin provide a survival advantage in critically ill patients with sepsis (Zou et al., 2022), unfortunately, cancer patients were excluded from our previous studies. Thus, this conclusion may not be suitable for patients with cancer complicated with sepsis. Notably, minimal data exist on anticoagulation with heparin on outcomes in critically ill patients with solid malignancy associated sepsis as far. Whether administration heparin would provide a survival advantage in critically ill patients with solid malignancies concomitant sepsis remains unknown. Therefore, we designed another study on effectiveness of heparin therapy to patients with solid malignancies, and the results from the MIMIC-IV data demonstrate that heparin administration is associated with improved ICU mortality, thrombosis and hospital mortality in patients with solid malignancy concomitant sepsis.
As administration heparin is time-dependence variable, so we employed MSCM model to adjust additional time-dependent interventions (de Keyser et al., 2014; Karim et al., 2014; Huang et al., 2023). The MSCM further reinforces the efficacy of heparin in reducing ICU mortality. For the unmeasured confounding variables, we used risk factor analysis with a multivariable Cox proportional hazards model and E-value analysis to perform a combined analysis of the data. The result indicates that it is unlikely that an unmeasured confounder would have a substantially greater effect on ICU mortality than these known risk factors. E-value analysis suggested robustness to unmeasured confounding variables (Haneuse et al., 2019). Therefore, heparin therapy did demonstrate a benefit in patients not only in general populations but also in those with solid malignancies. Interestingly, stratification analysis with MSCM further indicated that administration heparin at 7,500–12500 IU a day decreased ICU mortality only among male patients and those with white ethnicity, stage 3 AKI, digestive system cancer and did not benefit those with respiratory system cancer. The underlying mechanism may involve the patient’s ethnicity and race, endocrine metabolism status, tumor type and targeted therapy. The findings presented in this report provide an insight role of heparin in patients with solid malignancies concomitant sepsis.
During targeted therapy for patients with solid malignancies, injury-related molecules are readily released and trigger inflammatory reactions and coagulation disorders (Wu et al., 2021), leading to an increased risk of thrombosis. Patients with cancer are significantly more likely to develop venous thromboembolism (VTE) than people without cancer (Heit, 2015; Schünemann et al., 2020). Cancer-associated thrombosis is the second leading cause of death in cancer patients after disease progression (Farge et al., 2022), furthermore, the incidence of cancer-associated thrombosis is increasing worldwide (Schünemann et al., 2020). The prevalence of cancer-associated thrombosis is increasing because of multiple factors, including longer patient survival, the use of anticancer therapies, increased detection of incidental VTE during surveillance imaging and wider use of central venous catheters (Mulder et al., 2021). The annual risk of a venous thromboembolic event in patients with solid cancer is 4%–5% overall, with wide variation between patients with different cancer types (Akl et al., 2017). A systematic review identified the greatest benefit from heparin treatment for VTE in patients with lung cancer (RR 0.59, 95% CI 0.42–0.81) (Cuenca et al., 2022). The result is similar to our results that heparin treatment has a benefit on reducing the risk of thrombosis (HR 0.42, 95% CI 0.26–0.68, p < 0.001). Therefore, the international clinical practice guidelines included the recommendation of LMWHs for the initial (first 10 days) treatment and maintenance treatment of cancer-associated thrombosis (Farge et al., 2022), but it does not improve survival (Cuenca et al., 2022).
Although previous studies have documented that heparin can inhibit tumor invasion and metastasis (Wei et al., 2023), heparin therapy has not been used as a conventional antitumor method in clinical practice. Recently, in the United States, an observational cohort study based on over 1 million sepsis hospitalizations showed that in-hospital mortality in cancer-related sepsis patients was 27.9% vs. 19.5% in patients without cancer-related sepsis, and cancer-related sepsis was associated with an adjusted absolute increase in in-hospital mortality that ranged from 2.2% to 15.2% of that associated with noncancer-related sepsis (Hensley et al., 2019). There are many plausible explanations for the differential outcomes between cancer-related and noncancer-related sepsis, including the cancer itself, cancer treatment and the resulting immune suppression, critical care provider bias and the differing goals of care (Hensley et al., 2019). Research shows that approximately 70% of cancer patients in the ICU have solid malignancies, and there is no recommendation in the international sepsis guidelines on whether they need anticoagulation treatment (Evans et al., 2021). The results of this study will provide a new therapeutic insight for the treatment of solid malignancies associated sepsis. The mechanism of effectiveness of heparin therapy to patients with solid malignancies concomitant sepsis, apart from anticoagulation effect, anti-inflammatory effects, anticomplement activity, and protease regulation on heparin (Li et al., 2013; Li et al., 2014), the underlying mechanism still needs further to investigate.
However, certain limitations of the present study should be noted. First, this study was carried out based on an EHR using clinically derived data. Therefore, the process adopted in cohort screening may not be identical to guideline-defined sepsis. However, sepsis cases were identified based on guidelines identical to the third definition of sepsis (such as infection combined with rapid alteration of total SOFA score ≥2 points). Second, confounders might have affected our results because of the retrospective nature of the present work. As a result, MSCM and PSM were adopted for balancing critical confounders, both of which verified our result creditability. Finally, certain patient variables were not obtained from the database, which might have led to bias or confounders. E-values were calculated in the sensitivity analysis to quantify possible results caused by those non-extracted confounders; according to our results, the non-extracted confounders might not interpret the whole therapeutic effect.
According to our results, early heparin application for patients with solid malignancy associated sepsis may improve survival, including ICU mortality, hospital mortality, and thrombosis, particularly for males and those with white ethnicity, stage 3 AKI, and digestive system cancer. In addition, patients with solid malignancy associated sepsis who received 7,500–12500 IU per day had lower ICU mortality than non-heparin cases. Consequently, more prospective randomized controlled trials are warranted to verify timing, dosage, and indications of heparin use for solid tumor cases.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by the Committee of Shenzhen Second People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because using data from MIMIC-IV (version 1.0).
JH: Writing–original draft, Data curation, Formal Analysis. Investigation. ZZ: Investigation. YL: Data curation, Formal ZY: Investigation. DL: Investigation, YC: Investigation. YL: original draft, Supervision. YY: Supervision, Conceptualization, Writing–review and editing. MW: Conceptualization, Supervision, Writing–review and editing, Funding acquisition.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the Sanming Project of Medicine in Shenzhen (SZSM20162011), Medical Science and Technology Research Foundation of Guangdong Province (No. A2023354), Science, Technology, and Innovation Commission of Shenzhen Municipality (JCYJ20190806163603504) and Shenzhen Second People’s Hospital Clinical Research Fund of Guangdong Province High-level Hospital Construction Projects (20203357014, 2023xgyj3357001,2023yjlcyj022).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1281235/full#supplementary-material
Akl, E. A., Kahale, L. A., Hakoum, M. B., Matar, C. F., Sperati, F., Barba, M., et al. (2017). Parenteral anticoagulation in ambulatory patients with cancer. Cochrane Database Syst. Rev. 9, Cd006652. doi:10.1002/14651858.CD006652.pub5
Bray, F., Laversanne, M., Weiderpass, E., and Soerjomataram, I. (2021). The everincreasing importance of cancer as a leading cause of premature death worldwide. Cancer 127, 3029–3030. doi:10.1002/cncr.33587
Cuenca, J. A., Manjappachar, N. K., Ramírez, C. M., Hernandez, M., Martin, P., Gutierrez, C., et al. (2022). Outcomes and predictors of 28-day mortality in patients with solid tumors and septic shock defined by third international consensus definitions for sepsis and septic shock criteria. Chest 162, 1063–1073. doi:10.1016/j.chest.2022.05.017
Danai, P. A., Moss, M., Mannino, D. M., and Martin, G. S. (2006). The epidemiology of sepsis in patients with malignancy. Chest 129, 1432–1440. doi:10.1378/chest.129.6.1432
de Keyser, C. E., Leening, M. J., Romio, S. A., Jukema, J. W., Hofman, A., Ikram, M. A., et al. (2014). Comparing a marginal structural model with a Cox proportional hazard model to estimate the effect of time-dependent drug use in observational studies: statin use for primary prevention of cardiovascular disease as an example from the Rotterdam Study. Eur. J. Epidemiol. 29 (11), 841–850. doi:10.1007/s10654-014-9951-y
Evans, L., Rhodes, A., Alhazzani, W., Antonelli, M., Coopersmith, C. M., French, C., et al. (2021). Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 47, 1181–1247. doi:10.1007/s00134-021-06506-y
Farge, D., Frere, C., Connors, J. M., Khorana, A. A., Kakkar, A., Ay, C., et al. (2022). 2022 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer, including patients with COVID-19. Lancet Oncol. 23, e334–e347. doi:10.1016/S1470-2045(22)00160-7
Grafféo, N., Latouche, A., Le Tourneau, C., and Chevret, S. (2019). ipcwswitch: an R package for inverse probability of censoring weighting with an application to switches in clinical trials. Comput. Biol. Med. 111, 103339. doi:10.1016/j.compbiomed.2019.103339
Haneuse, S., VanderWeele, T. J., and Arterburn, D. (2019). Using the E-value to assess the potential effect of unmeasured confounding in observational studies. Jama 321, 602–603. doi:10.1001/jama.2018.21554
Heit, J. A. (2015). Epidemiology of venous thromboembolism. Nat. Rev. Cardiol. 12, 464–474. doi:10.1038/nrcardio.2015.83
Hensley, M. K., Donnelly, J. P., Carlton, E. F., and Prescott, H. C. (2019). Epidemiology and outcomes of cancer-related versus non-cancer-related sepsis hospitalizations. Crit. Care Med. 47, 1310–1316. doi:10.1097/CCM.0000000000003896
Huang, J. J., Zou, Z. Y., Zhou, Z. P., Liu, Y., Yang, Z. J., Zhang, J. J., et al. (2023). Effectiveness of early heparin therapy on outcomes in critically ill patients with sepsis-induced coagulopathy. Front. Pharmacol. 14, 1173893. doi:10.3389/fphar.2023.1173893
Karim, M. E., Gustafson, P., Petkau, J., Zhao, Y., Shirani, A., Kingwell, E., et al. (2014). Marginal structural Cox models for estimating the association between β-interferon exposure and disease progression in a multiple sclerosis cohort. Am. J. Epidemiol. 180 (2), 160–171. doi:10.1093/aje/kwu125
Khan, F., Tritschler, T., Kahn, S. R., and Rodger, M. A. (2021). Venous thromboembolism. Lancet 398, 64–77. doi:10.1016/S0140-6736(20)32658-1
Li, X., Li, X., Zheng, Z., Liu, Y., and Ma, X. (2014). Unfractionated heparin suppresses lipopolysaccharide-induced monocyte chemoattractant protein-1 expression in human microvascular endothelial cells by blocking Krüppel-like factor 5 and nuclear factor-κB pathway. Immunobiology 219 (10), 778–785. doi:10.1016/j.imbio.2014.06.005
Li, X., Li, Z., Zheng, Z., Liu, Y., and Ma, X. (2013). Unfractionated heparin ameliorates lipopolysaccharide-induced lung inflammation by downregulating nuclear factor-κB signaling pathway. Inflammation 36 (6), 1201–1208. doi:10.1007/s10753-013-9656-5
Mulder, F. I., Horváth-Puhó, E., van Es, N., van Laarhoven, H. W. M., Pedersen, L., Moik, F., et al. (2021). Venous thromboembolism in cancer patients: a population-based cohort study. Blood 137, 1959–1969. doi:10.1182/blood.2020007338
Ostermann, M., Bellomo, R., Burdmann, E. A., Doi, K., Endre, Z. H., Goldstein, S. L., et al. (2020). Controversies in acute kidney injury: conclusions from a kidney disease: improving global outcomes (KDIGO) conference. Kidney Int. 98, 294–309. doi:10.1016/j.kint.2020.04.020
Schünemann, H. J., Ventresca, M., Crowther, M., Briel, M., Zhou, Q., Noble, S., et al. (2020). Evaluating prophylactic heparin in ambulatory patients with solid tumours: a systematic review and individual participant data meta-analysis. Lancet Haematol. 7, e746–e755. doi:10.1016/S2352-3026(20)30293-3
Shinozaki, T., and Suzuki, E. (2020). Understanding marginal structural models for time-varying exposures: pitfalls and tips. J. Epidemiol. 30, 377–389. doi:10.2188/jea.JE20200226
Singer, M., Deutschman, C. S., Seymour, C. W., Shankar-Hari, M., Annane, D., Bauer, M., et al. (2016). The third international consensus definitions for sepsis and septic shock (Sepsis-3). Jama 315, 801–810. doi:10.1001/jama.2016.0287
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. doi:10.3322/caac.21660
Tang, Y., Wang, X., Li, Z., He, Z., Yang, X., Cheng, X., et al. (2021). Heparin prevents caspase-11-dependent septic lethality independent of anticoagulant properties. Immunity 54, 454–467.e6. doi:10.1016/j.immuni.2021.01.007
Vincent, J. L., Rello, J., Marshall, J., Silva, E., Anzueto, A., Martin, C. D., et al. (2009). International study of the prevalence and outcomes of infection in intensive care units. Jama 302, 2323–2329. doi:10.1001/jama.2009.1754
Wei, F., Su, Y., Quan, Y., Li, X., Zou, Q., Zhang, L., et al. (2023). Anticoagulants enhance molecular and cellular immunotherapy of cancer by improving tumor microcirculation structure and function and redistributing tumor infiltrates. Clin. Cancer Res. 29, 2525–2539. doi:10.1158/1078-0432.CCR-22-2757
Wu, M., Shi, J., He, S., Wang, D., Zhang, N., Wang, Z., et al. (2021). cGAS promotes sepsis in radiotherapy of cancer by up-regulating caspase-11 signaling. Biochem. Biophys. Res. Commun. 551, 86–92. doi:10.1016/j.bbrc.2021.03.003
Yuan, C., Wang, D., Zhang, N., Wang, Z., Yang, F., He, J., et al. (2020). DNA damage/cGAS-triggered up-regulation of MALAT1 promotes undesirable inflammatory responses in radiotherapy of cancer. Biochem. Biophys. Res. Commun. 528, 746–752. doi:10.1016/j.bbrc.2020.05.064
Yuan, C., Wu, M., Xiao, Q., Zhao, W., Li, H., Zhong, Y., et al. (2021). Blocking Msr1 by berberine alkaloids inhibits caspase-11-dependent coagulation in bacterial sepsis. Signal Transduct. Target Ther. 6, 92. doi:10.1038/s41392-021-00483-w
Zou, Z. Y., Huang, J. J., Luan, Y. Y., Yang, Z. J., Zhou, Z. P., Zhang, J. J., et al. (2022). Early prophylactic anticoagulation with heparin alleviates mortality in critically ill patients with sepsis: a retrospective analysis from the MIMIC-IV database. Burns Trauma 10, tkac029. doi:10.1093/burnst/tkac029
Keywords: sepsis, heparin, mortality, thrombosis, solid malignancy
Citation: Huang J-j, Cai J-z, Zhou Z-p, Liu Y, Yang Z-j, Li D-z, Chen Y-h, Luan Y-y, Yao Y-m and Wu M (2023) Impact of early heparin therapy on outcomes in patients with solid malignancy associated sepsis: a marginal structural model causal analyse. Front. Pharmacol. 14:1281235. doi: 10.3389/fphar.2023.1281235
Received: 22 August 2023; Accepted: 21 November 2023;
Published: 04 December 2023.
Edited by:
Tanveer Ahmed Khan, National Institute of Health, PakistanReviewed by:
Fan Zhang, Qilu Hospital, Shandong University, ChinaCopyright © 2023 Huang, Cai, Zhou, Liu, Yang, Li, Chen, Luan, Yao and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying-yi Luan, luanyingyi@mail.ccmu.edu.cn; Yong-ming Yao, c_ff@sina.com; Ming Wu, boshiyy@126.com
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.