- 1Graduate School of Biotechnology and College of Life Science, Kyung Hee University, Yongin, Republic of Korea
- 2Department of Food and Nutrition, Chung Ang University, Anseong, Republic of Korea
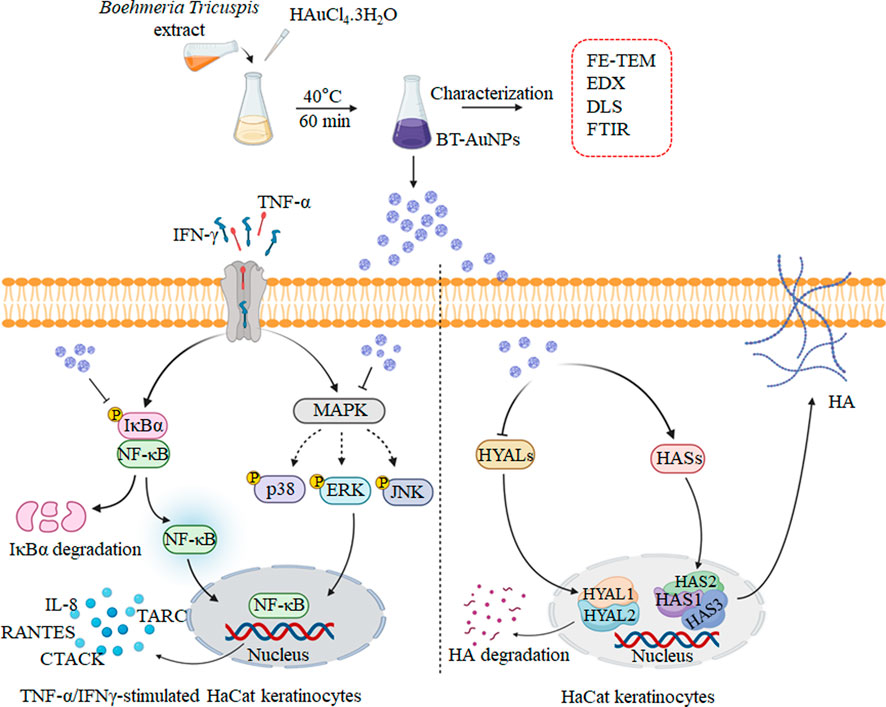
Introduction: Recently, nanotechnology has emerged as a potential technique for skin generation, which has several treatment advantages, such as decreased drug cytotoxicity and enhanced skin penetration. Boehmeria tricuspis (BT) belongs to the Urticaceae family and is rich in phenolic and flavonoid compounds. In this study, we biosynthesized gold nanoparticles (BT-AuNPs) using BT extract to explore their anti-inflammatory and skin-moisturizing properties in keratinocytes.
Methods: Field-emission transmission electron microscopy, energydispersive X-ray spectrometry, dynamic light scattering, and Fourier-transforminfrared spectroscopy were used to examine the synthesized BT-AuNPs. qRT-PCR, western blot, and ELISA were applied for investigating the effect of BT-AuNPs on anti-inflammation and moisturizing activity in HaCaT cells.
Results: At concentrations below 200 μg/mL, BT-AuNPs had no cytotoxic effect on keratinocytes. BT-AuNPs dramatically alleviated the expression and secretion of inflammatory chemokines/cytokine, such as IL-6, IL-8, TARC, CTACK, and RANTES in keratinocytes stimulated by tumor necrosis factor-α/interferon-γ (T + I). These anti-inflammatory properties of BT-AuNPs were regulated by inhibiting the NF-κB and MAPKs signaling pathways. Furthermore, BT-AuNPs greatly promoted hyaluronic acid (HA) production by enhancing the expression of hyaluronic acid synthase genes (HAS1, HAS2, and HAS3) and suppressing the expression of hyaluronidase genes (HYAL1 and HYAL2) in HaCaT cells.
Discussion: These results suggest that BT-AuNPs can be used as a promising therapeutic alternative for treating skin inflammation. Our findings provide a potential platform for the use of BT-AuNPs as candidates for treating inflammatory skin diseases and promoting skin health.
1 Introduction
The skin plays a critical role in the immune system, protecting against external stimuli while maintaining tissue homeostasis. However, an uncontrolled or abnormal immune response in the skin can lead to inflammatory skin diseases (ISDs), such as contact, atopic, seborrheic, and allergic dermatitis (Wang et al., 2022a). ISDs are associated with the overexpression of pro-inflammatory mediators, such as cytokines and chemokines (Wang et al., 2022a). In particular, keratinocytes might facilitate or enhance the inflammatory response by increasing the production of TNF-α and IFN-γ, leading to various skin diseases (Lin et al., 2018). In response to pro-inflammatory stimuli, keratinocytes naturally produce a distinct set of chemokines and cytokines including IL-6, IL-8, thymus and activation-regulated chemokine (TARC), and Regulated upon Activation, Normal T Cell Expressed and Presumably Secreted (RANTES) (Yang et al., 2015). These factors stimulate inflammatory cells and their infiltration into inflammatory skin lesions (Nedoszytko et al., 2014). Therefore, reducing inflammatory chemokine and cytokine secretion in keratinocytes could be an effective treatment strategy for ISDs (Kwon et al., 2012).
Recently, there has been a significant interest in nanotechnology due to the potential medical, pharmacological, and technological applications of metal nanoparticles, which have high biocompatibility (Moldovan et al., 2017). Gold nanoparticles (AuNPs) have been widely studied in biomedicine for their anti-inflammatory and antioxidant effects and their potential for drug delivery (Yamada et al., 2015). However, the conventional method for synthesizing AuNPs has several drawbacks including their hazardous natures and higher cost (Wallyn et al., 2019). To address these issues, researchers have utilized biological systems, such as microbial and plant-derived products for nanoparticles synthesis, which offer a safer and more eco-friendly approach (Mohanpuria et al., 2008). Recently, the green synthesis of nanoparticles (NPs), which involves the use of natural plant products, is becoming increasingly popular due to its nontoxic and eco-friendly approach for developing high-energy efficient materials (Hutchison, 2008). Therefore, utilizing natural plants to synthesis AuNPs may be a potential strategy to achieve results with enhanced safety and effectiveness.
Boehmeria tricuspis (BT) belongs to the Urticaceae family and is naturally grown in China, Japan, and Korea (Tang et al., 2016). The BT extract is rich in phenolic and flavoloids content which associated to significant anti-inflammatory and antioxidant activities (Chen et al., 2014; Akter et al., 2018). In addition, Chen et al. investigated the pharmaceutical application of BT leaves as potential anticancer agents (Chen et al., 2014). However, to our knowledge, the anti-inflammatory activity of AuNPs prepared with BT extract has not been investigated. Thus, this study aimed to biosynthesize AuNPs using BT extract to investigate the anti-inflammatory and moisturizing effects of BT-AuNPs on skin cells. Furthermore, we investigated the mechanisms underlying the anti-inflammatory activity of the AuNPs, particularly their involvement in NF-κB/MAPK pathway.
2 Material and methods
2.1 Materials
Dulbecco’s modified Eagle’s medium (DMEM), penicillin/streptomycin (P/S), and fetal bovine serum (FBS) were obtained from GenDEPOT (San Antonio, TX, United States). MTT (2,2-diphenyl-1-picrylhydrazyl) was provided by Thermo Fisher Scientific (Waltham, MA, United States). Primers for IL-6, IL-8, TARC, CTACK and RANTES were designed by Macrogen (Seoul, Republic of Korea) and their sequences are summarized in Supplementary Table S1. β-actin was purchased from Santa Cruz Biotechnology (Dallas, TX, United States) and other antibodies against p-IκBα, p-p38, P38, p-ERK 1/2, ERK 1/2, JNK, p-JNK, NF-κB, IκBα, and p-NF-κB were provided by Cell Signaling Technology (CST, MA, United States).
2.2 Methods
2.2.1 Preparation of B. tricuspis extract
The leaf tissue of BT was collected from northern Gyeonggi, South Korea, located near the demilitarized zone. The plant species were identified by Dr. J. K. Kim, a senior researcher at the Gyeonggido Business and Science Accelerator, Gyeonggi Biocenter (Suwon, South Korea), and the voucher specimen was GB-0011. The leaf tissues were then sun-dried. All dried tissues were extracted for 3 days in 70% ethanol at room temperature. The extract was filtered using a filtering cloth (20 nm; Hyundai Micro, Anseong, South Korea) and vacuum-evaporated (Buchi Korea Inc., Gwangmyeong, South Korea).
2.2.2 Biosynthesis and optimization of BT-AuNPs
BT-AuNPs were biologically synthesized and optimized according to a previous publication, with slight modifications (Tran et al., 2022). The following four parameters were considered during the biosynthesis optimization process: BT concentration (range: 0.25–2 mg), tetrachloroauric(III) acid trihydrate (HAuCl4⋅3H2O) concentration (range: 0.5–2 mM), reaction temperature (20°C–50°C), and incubation time (range: 20–50 min). To synthesize BT-AuNPs, a solution of 1 mL BT was mixed with a solution of HAuCl4⋅3H2O at the indicated concentrations. The reaction mixture was centrifuged at 13,500 rpm for 20 min to harvest the AuNPs. The resulting pellets were washed thrice with distilled water and dried at room temperature.
2.2.3 Characterization of BT-AuNPs
The BT-AuNPs were characterized using various techniques, according to a previous report (Liu et al., 2020). The absorbance spectra of the BT-AuNPs solution were measured using ultraviolet-visible (UV-Vis) spectroscopy, while the morphology and size of the particles were analyzed using FE-TEM and DLS (Shikha et al., 2020).
Specifically, the absorbance spectra of the purified nanoparticle suspension were verified using a UV-Vis spectrophotometer (Cary 60; Agilent, Santa Clara, CA, United States). The morphology, structure, purity, and elemental distribution of BT-AuNPs were evaluated using FE-TEM. The sample was prepared by placing droplets of purified nanoparticles dispersed in water on a carbon-coated copper grid and dried at 37°C before being transferred to the microscope. Additionally, the DLS (Otsuka Electronics, Shiga, Japan) were used to measure volume, number and intensity distribution the nanoparticles size. Spectrum™ One FTIR Spectrometer (PerkinElmer, Waltham, Massachusetts, United States) were used to acquire the FTIR spectra of dried BT-AuNPs and BT. FTIR analysis was employed to determine the functional groups of the plant extract which capped on the AuNPs surfaces.
2.2.4 Cell culture and cytotoxicity evaluation
HaCaT cells (Heidelberg, Germany) were cultured in DMEM supplemented with 10% FBS and 1% P/S at 37°C with 5% CO2/95% air. To assess the cytotoxic effects of BT-AuNPs and BT, cells were individually seeded into a 96-well plate (1 × 106 cells/mL; SPL, Pocheon, Republic of Korea) for 24 h. Thereafter, various doses of BT-AuNPs and BT samples dissolved in free DMEM were added to the cells. The cytotoxic effects of the BT-AuNPs and BT samples were measured using the MTT assay.
2.2.5 Live/dead fluorescent assay
Live/dead staining was performed to evaluate the cytotoxicity of BT-AuNPs and BT on HaCaT cells. Briefly, HaCaT cells were incubated with a live/dead assay kit (Thermo Fisher Scientific) for 30 min at 37°C in the dark. Cells were evaluated using a fluorescence microscope (Leica, Wetzlar, Germany).
2.2.6 Analysis of cytokine, chemokine and hyaluronic acid production
After treating the samples for 24 h, the supernatant was collected from the treatment media to measure the secretion of IL-6, IL-8, TARC/CCL17, and hyaluronic acid (HA) using ELISA, according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN, United States).
2.2.7 Quantitative reverse transcription-polymerase chain reaction
HaCaT cells (1 × 106 cells/mL) were seeded in a 35 × 10 mm cell culture dish for 24 h. The cells were then treated with various concentrations of BT-AuNPs for an additional 24 h. Total RNA was extracted and then cDNA was synthesized using a cDNA synthesis kit (NanoHelix). qRT-PCR was performed using the RealHelix™ Premier qPCR Kit (NanoHelix).
2.2.8 Western blotting
Similarly, HaCaT cell culture and sample treatment were performed for Western blot analysis. Western blotting was performed according to a previously reported method (Mi et al., 2021). The protein blots were exposed using ATTO LuminoGraph III Lite machine (Atto, Tokyo, Japan). The ImageJ software was used to determine the intensity of the visualized bands.
2.2.9 Statistical analysis
All experiments were performed in triplicate, and the data are displayed as the mean ± standard deviation. Student's t-test was used to statistically compare the two groups. Statistical significance was determined at p < 0.05, p < 0.01, and p < 0.001.
3 Results
3.1 Synthesis and optimization BT-AuNPs
The synthesis conditions were optimized according to a previous report describing the synthesis of AuNPs using plant extracts (Elbagory et al., 2019). The formation of AuNPs was verified using UV-Vis spectroscopy. Figure 1 illustrates the UV-Vis spectra used to confirm the formation of AuNPs under various conditions. Surface plasmon wavelengths of AuNPs were analyzed at various concentrations of BT-AuNPs (0.25, 0.5, 1, and 2 mg/mL) and 1 mM HAuCl4⋅3H2O reacted at room temperature (25°C), with a total reaction time of 30 min (Figure 1A). Optimal BT-AuNP production was observed at 0.5 and 1 mg/mL; however, 0.5 mg/mL (lower concentration) was chosen for further experiments since its use was more appropriate and economical in biological applications. Subsequently, 0.5 mg/mL BT and 1 mM HAuCl4⋅3H2O were incubated at various temperatures (20, 30, 40, and 50°C) for 30 min (Figure 1B), and the optimal temperature for the production of BT-AuNPs was found to be 40°C. Further, various concentrations of HAuCl4⋅3H2O (0.5, 1, 1.5, and 2 mM) were reacted with BT (0.5 mg/mL and 40°C) for 30 min (Figure 1C). The absorbance peak was more significant at the 1.5 mM concentration. Finally, BT (0.5 mg/mL) and HAuCl4⋅3H2O (1.5 mM) were used to synthesize BT-AuNPs for different durations (20, 30, 40, and 60 min) at 40°C. The major absorption peaks were more significant at 40 min (Figure 1D). In summary, the biosynthesis of BT-AuNPs was optimal when BT (0.5 mg/mL) reacted with HAuCl4⋅3H2O (1.5 mM) at 40°C for 40 min.
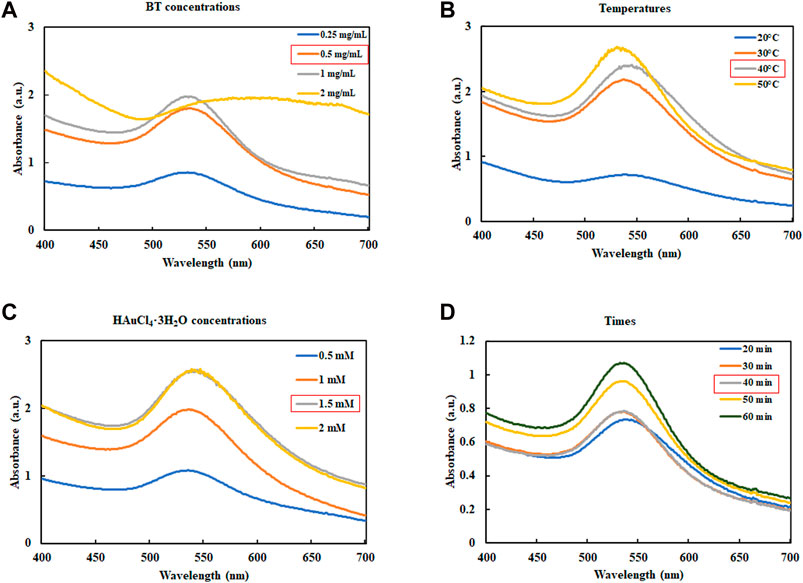
FIGURE 1. Optimization reaction condition of BT-AuNPs. (A) The concentrations of BT extract (BT) range from 0.25 to 2 mg/mL; (B) The consequence of reaction temperature (20°C–50°C); (C) The influence of HAuCl4⋅3H2O concentrations (0.5–2 mM); and (D) The consequence reaction time (20°C–50°C).
3.2 Characterization of BT-AuNPs
BT-AuNPs were evaluated using a UV-Vis spectrophotometer. BT-AuNPs revealed a strong peak at 538 nm, corresponding to the surface plasmon band of the synthesized AuNPs (Figure 2A). Additionally, the visible color changed from yellow to purple. These results confirmed the successful synthesis of BT-AuNPs (Liu et al., 2019). Figure 2B displays the FE-TEM image of BT-AuNPs, which reveals that the particles have a size range of 6–37 nm and exhibit spherical, triangular, and polygonal surface morphologies. Most surface morphologies consisted of circular nanohybrids with a few triangular and polygonal shapes. The elemental map in Figure 2C shows the distribution of Au (red) in the isolated particles. The Energy-dispersive X-ray (EDX) spectra showed multiple absorption peaks corresponding to the characteristic Au peaks (Figure 2D). Using DLS analysis, we determined the particle profiles of BT-AuNPs. Figure 2E shows the average values for the volume, number, and intensity of the BT-AuNPs, which were 43.3, 30.5, and 95.6 nm, respectively. Furthermore, BT-AuNPs had a hydrodynamic diameter of 62.7 nm and a polydispersity index of 0.29 (<0.3), indicating a narrow size distribution of AuNPs (Ricci-Júnior and Marchetti, 2006).
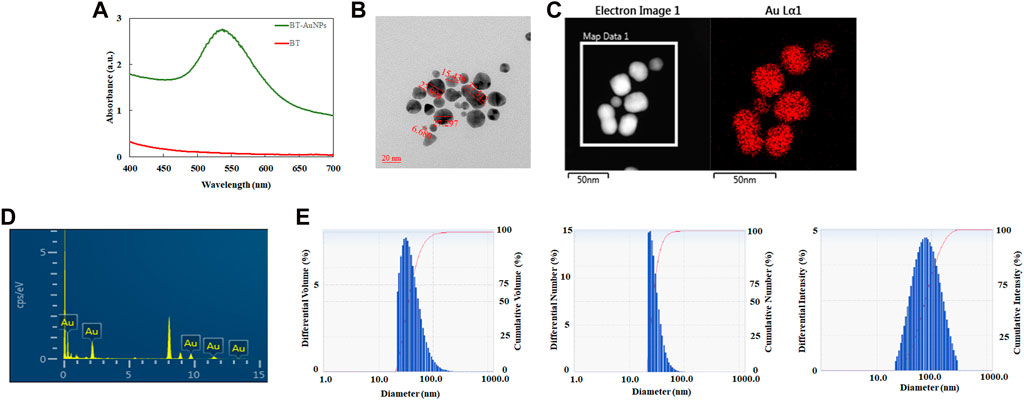
FIGURE 2. Characteration of BT-AuNPs. (A) UV−vis spectrum of BT-AuNPs and BT; (B) FE-TEM image was utilized to determine the morphology and size of BT-AuNPs. (C) Elemental mapping was used to confirm the gold distribution in nanoparticles; (D) EDX was analyzed to detect the Au peak of BT-AuNPs; (E) The volume, number, and intesity distributions of BT-AuNPs were determined using DLS spectrum.
The possible functional groups on the surfaces of the BT-AuNPs were identified using Fourier transform infrared (FT-IR) spectroscopy (Figures 3A, B), and the absorption peaks are shown in Figure 3C. The FT-IR spectra of BT-AuNPs and BT revealed bands at 3,405.09 cm−1 and 3,313.50 cm−1 corresponding to the O–H stretching; bands at 2,925.78–2,854.98 cm−1 and 2,929.88 cm−1 corresponding to the C-H stretching; and bands at 1,722.24–1,509.30 cm−1 and 1,698.47–1,520.76 cm−1 corresponding to the C=C and C=O stretching, respectively. The stretching of C-C-C and C-O double-bond functional groups was observed at 1,371.03–1,072.14 cm−1 and 1,388.56–1,053.86, respectively. The corresponding C-H band observed at 815.75–610.50 cm−1 was only present in BT. Therefore, we believe that HAuCl4⋅3H2O and BT combined individually to form BT-AuNPs (Mi et al., 2022a).

FIGURE 3. FT-IR analysis was utilized to confirm chemical linkage confirmation in BT-AuNPs. (A) BT-AuNPs; (B) BT; (C) The profile of a tabular view in the functional group.
3.3 Cytotoxicity of BT-AuNPs and BT
The MTT assay and live/dead cell staining were used to assess the cytotoxicity of BT-AuNPs and BT on HaCaT cells. The cells were incubated with different concentrations (25, 50, 100, and 200 μg/mL) of BT-AuNPs and BT for 24 h (Figure 4A). No significant cellular toxicity was observed on treatment with BT-AuNPs at concentrations ranging from 25 to 200 μg/mL. However, at concentrations of 50–100 g/mL, BT significantly decreased cell viability. Additionally, dead/live staining was used to determine the cytotoxic effects of BT-AuNPs and BT (Figure 4B). The data indicated that a large number of cells died in the BT-treated group, while the BT-AuNPs-treated group had relatively few dead cells. Therefore, BT-AuNPs at concentrations of 50, 100, and 200 μg/mL were chosen for further experiments.
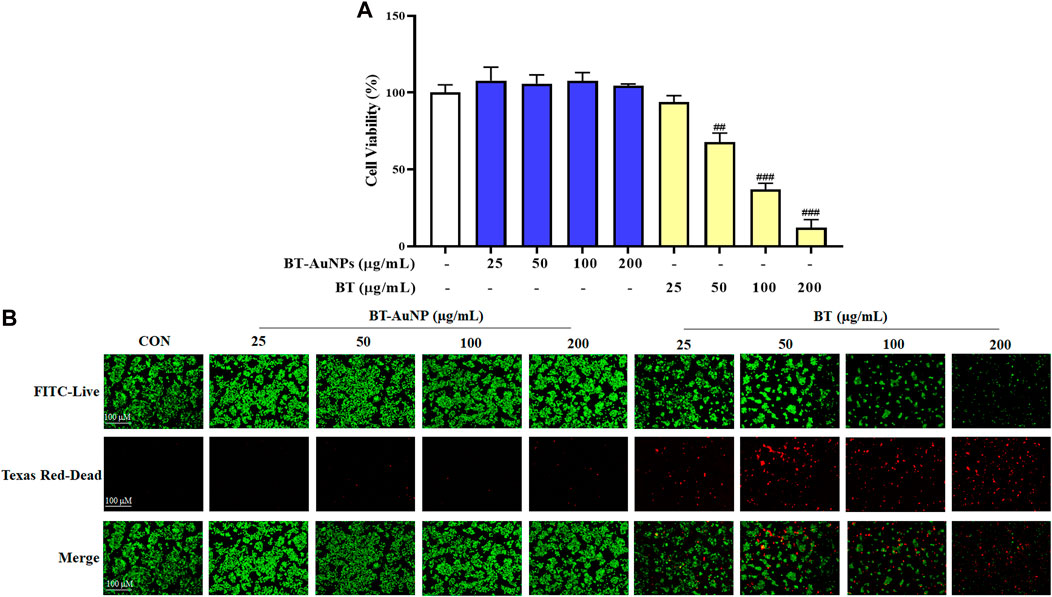
FIGURE 4. Cytotoxic of BT-AuNPs in keratinocytes. (A) Effects of BT-AuNPs and BT on viability in HaCaT cells were determined by MTT assay; (B) Live/Dead staining images were observed to confirm the cytotoxicity effect of BT-AuNPs and BT treated HaCaT cells. All values are expressed as mean ± S.D. ##p < 0.01, ###p < 0.001 vs. control group.
3.4 Effects of BT-AuNPs on TNF-α/IFN-γ-induced pro-inflammatory cytokines/chemokines
Chemokines and cytokines play critical roles in the immune and inflammatory responses. It is well-known that TNF-α/IFN-γ (T + I) can induce the aberrant expression of chemokines (such as IL-8, TARC, CTACK, and RANTES) and cytokines (IL-6), resulting in the infiltration of T cells or leukocytes into inflamed skin lesions (Homey et al., 2006; Nedoszytko et al., 2014). Therefore, we investigated the inhibitory effect of BT-AuNPs on pro-inflammatory chemokine and cytokine secretions induced by T + I in HaCaT cells using ELISA, with dexamethasone (Dex) as a positive control. T + I dramatically elevated the production of IL-6, IL-8, and TARC (Figures 5A–C) in keratinocytes. These increases were markedly reduced by BT-AuNPs treatment in a dose-dependent manner. Our results suggest that BT-AuNPs are promising therapeutic candidates for treating inflammatory skin disorders by inhibiting the secretion of pro-inflammatory chemokines and cytokines.
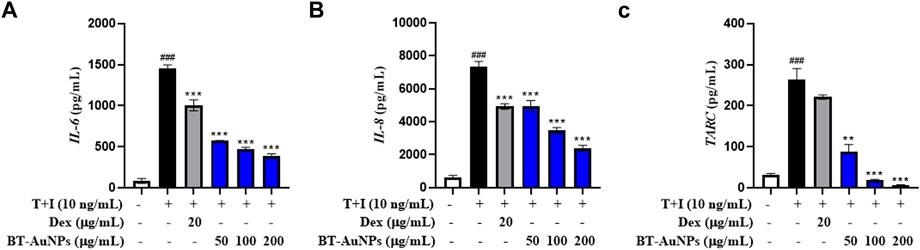
FIGURE 5. Effect of BT-AuNPs on cytokine and chemokines secretion in T + I-induced HaCaT cells were measured using ELISA assay. (A) IL-6; (B) IL-8; (C) TARC. All values are expressed as mean ± S.D. **p < 0.01, ***p < 0.001 vs. T + I group, ###p < 0.001 vs. control group.
Further, the mRNA expression of pro-inflammatory factors was investigated using qRT-PCR., The mRNA expression of IL-6, IL-8, CTACK, TARC, and RANTES (Figures 6A–E) significantly improved following T + I induction compared to those in the non-treated group. In contrast, Dex significantly suppressed the mRNA expression of pro-inflammatory chemokines and cytokines. In the group pretreated with BT-AuNPs, the levels of IL-6, IL-8, CTACK, TARC, and RANTES substantially attenuated. Additionally, the inhibitory effect of the BT-AuNPs was greater than that of the positive control at 200 g/mL. Overall, the ELISA and qRT-PCR results demonstrated that BT-AuNPs effectively inhibited the production and mRNA expression of pro-inflammatory chemokines and cytokines in T + I-induced HaCaT cells.
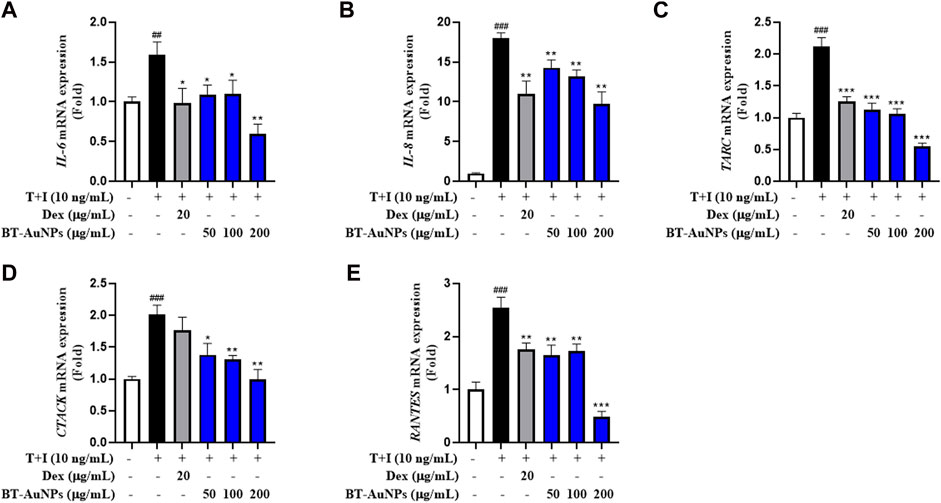
FIGURE 6. Effects of BT-AuNPs on mRNA expression in T + I-induced HaCaT cells were examined using qRT-PCR assay. (A) IL-6; (B) IL-8; (C) TARC; (D) CTACK; (E) RANTES. All values are expressed as mean ± S.D. *p < 0.05, **p < 0.01, ***p < 0.001 vs. T + I group, ###p < 0.001 vs. control group.
3.5 The anti-inflammatory mechanism of BT-AuNPs on TNF-α/IFN-γ-induced HaCaT cells
The MAPKs signaling pathway is crucial for various immune-mediated inflammatory responses. T + I stimulation activates the MAPKs signaling pathway, including p38, ERK, and JNK (Sikandan et al., 2018). In Figure 7A, the effects of BT-AuNPs on the activation of p38, ERK, and JNK were determined using Western blot. Immunoblotting results revealed that T + I significantly increased the protein levels of phosphorylated p38, ERK, and JNK; however, these increases were significantly attenuated by BT-AuNPs. Similarly, Dex markedly reduced the levels of phosphorylated MAPKs signaling pathway proteins. Interestingly, at the highest concentration of BT-AuNPs, the inhibitory effect on ERK and p38 phosphorylation was greater than that of the positive control. These findings imply that BT-AuNPs suppress the T + I-induced activation of the MAPKs signaling pathway.
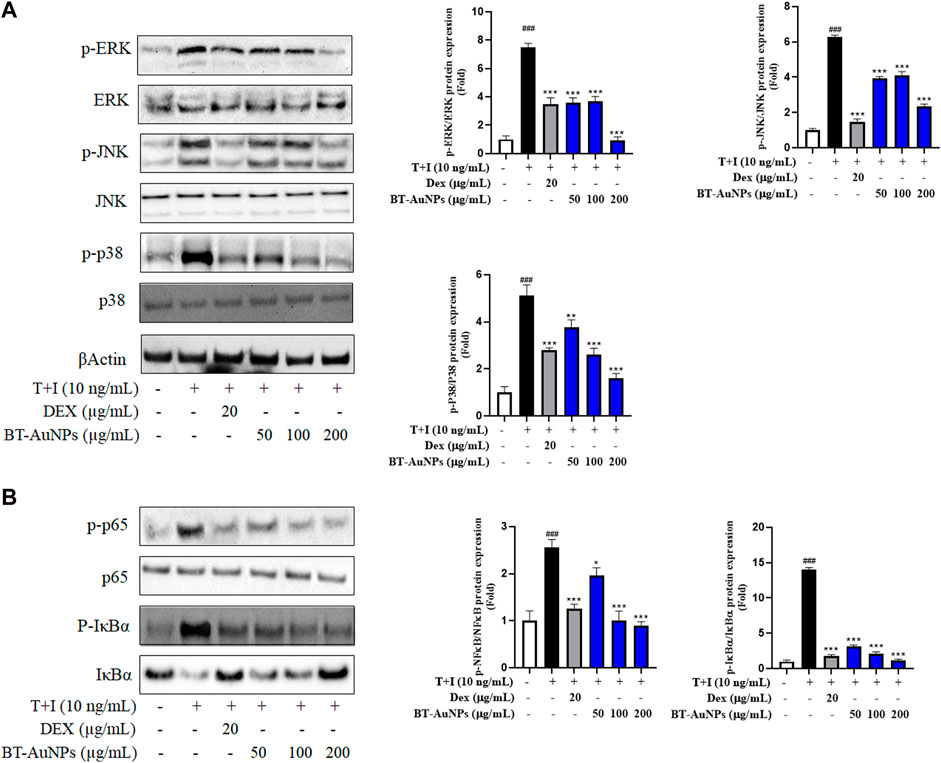
FIGURE 7. Effect of BT-AuNPs on (A) MAPK and (B) NF-κB signaling pathways in T + I-induced HaCaT cells. The protein expressions were presented as Western blotting pictures and these expressions were quantified according to the fold ratio of the phosphorylated/total form. All values are expressed as mean ± S.D. *p < 0.05, **p < 0.01, ***p < 0.001 vs. T + I group, ###p < 0.001 vs. control group.
Moreover, NF-κB can regulate the inflammatory response by producing chemokines and cytokines (Ju et al., 2009). To investigate the impact of BT-AuNPs on the NF-κB signaling pathways, we analyzed the levels of IκB-α degradation, phosphorylated IκB-α, and phosphorylated p65. As shown in Figure 7B, compared to the control, T + I treatment significantly degraded IκB-α and increased the levels of phosphorylated IκB-α and p65. However, compared to the T + I alone group, pretreatment with BT-AuNPs markedly reduced p-P65 and p-IκB-α levels. Furthermore, BT-AuNPs significantly restored IκB-α protein levels, which were degraded by T + I. Consequently, our results suggest that BT-AuNPs inhibit the phosphorylation of IκB-α and p65 and prevent T + I-stimulated IκB-α degradation.
3.6 Effect of BT-AuNPs on hyaluronic acid production and related gene and protein expression
Moisture maintenance is essential for normal skin function and is critical in regulating physiological processes in the skin, such as inflammation and wound recovery (Lee et al., 2019; Dias et al., 2021; Mi et al., 2022c). Thus, the effects of BT-AuNPs on skin moisture were evaluated in normal HaCaT cells using ELISA, Western blotting, and qRT-PCR. N-acetyl-D-glucosamine (NAD) was used as a positive control. HA production significantly improved in a dose-dependent manner in the BT-AuNP-treated group compared to that in the untreated group (Figure 8A). Notably, 200 μg/mL BT-AuNPs were more effectively produced HA than that by the NAD group. Moreover, BT-AuNPs enhanced the mRNA expression of HA synthesis (HAS) genes, such as HAS1, HAS2, and HAS3 (Figures 8B–D). Interestingly, HAS2 mRNA expression levels in the BT-AuNP-treated group at concentrations of 100 (6.1 fold) and 200 (7.4 fold) µg/mL were higher than that in the NAD (3.9 fold) group. Controlling HAS2 expression can preserve homeostasis and moisture in keratinocytes (Mi X. et al., 2022c). Thus, we further confirmed the moisturizing effect of BT-AuNPs on the HAS2 protein using Western blotting. The protein expression of HAS2 was dose-dependently enhanced by BT-AuNPs (Figure 8E) compared with that in the normal control. Additionally, the mRNA expression of hyaluronidases (HYALs, HYAL1, and HYAL2) was strongly suppressed after BT-AuNPs treatment (Figures 8F,G). These data indicate that BT-AuNPs may promote the production of HA by increasing the expression of HA-synthesizing enzymes and inhibiting HA-degrading enzymes.
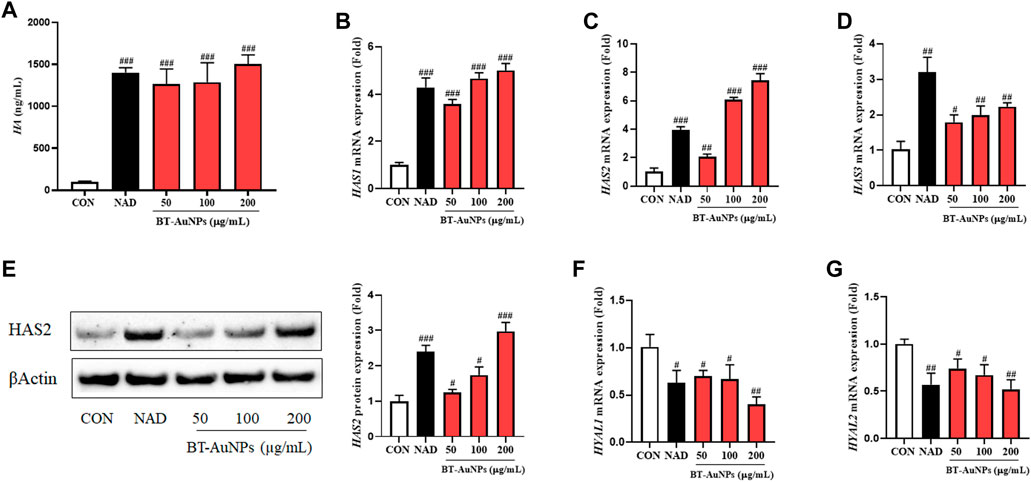
FIGURE 8. Effect of BT-AuNPs on HA production in normal keratinocytes. (A) HA secretion; gene expression of (B) HAS1, (C) HAS2, (D) HAS3; protein expression of (E) HAS2; gene expression of (F) HYAL1, (G) HYAL2. ELISA assay was conducted to measure HA secretion, and qRT-PCR was used to measure the expression of HA-related mRNA. All values are expressed as mean ± S.D. ##p < 0.05, ##p < 0.01, ###p < 0.001 vs. control group.
3.7 Identification of phytochemicals for BT-AuNPs synthesis
The major active compounds in BT were determined using a UPLC-MS/MS analysis. PDA (Photodiode array chromatogram) and BPC (base peak chromatogram) of BT are exhibited in Supplementary Figure S1. To calculate mass-to-charge ratio (m/z) and identify major peaks with mass accuracy less than ±5 ppm, mass spectrometry (MS) and tandem MS (MS2) were used. On the BPC, two peaks at retention periods of 6.49 and 6.70 min were verified to correspond to their source ions at 611.1581 and 465.1012, respectively, according to positive ionized mode [(M + H)] +) MS analysis. Thus, the results of MS and MS2 indicated that BT may contain 2 major phytochemicals, such as rutin and hyperoside (quercetin-3-O-galactoside).
4 Discussion
AuNPs are widely utilized in biomedical applications to treat various diseases, including inflammatory diseases, cancer, and immune system disorders (Xu et al., 2021; Mi et al., 2022b; Mi et al., 2022a). In this study, we synthesized BT-AuNPs using BT extract as a green reducing agent and investigated their bioactivity. Previous study showed that once the AuNPs are synthesized, the visual color of the reaction mixture changes to a deep purple or ruby red due to the surface plasmon resonance (Ahn et al., 2018). The color change of the reaction mixture from light yellow to dark purple, clearly suggesting that the BT extract is capable of reducing HAuCl4⋅3H2O to synthesize AuNPs. Next, analysis of the synthesis conditions of the nanoparticles indicated that BT-AuNPs were successfully synthesized under optimized conditions. The UV-vis absorption spectra of the reaction mixtures showed that there was a appearance of BT-AuNPs peak at λmax = 538 nm, which corresponds to the characteristic surface plasmon resonance phenomena of AuNPs. Further, the characteristics (crystalline nature, particle shape, and size) of the BT-AuNPs were determined using UV-Vis spectroscopy, FE-TEM, EDX, DLS, and FTIR analyses. FE-TEM exhibited particle sizes ranging from 6 to 37 nm and structures with almost spherical, a few triangular, and polygonal shapes, similar to those observed in a previous study (Salunke et al., 2014). The presence of typical metallic gold nanocrystal structure patterns was also confirmed using EDX analysis. Since DLS analysis showed that the size of the nanoparticles was dependent on the total size of the conjugates or their hydrodynamic size in colloids, the sizes obtained were normally considerably larger than those acquired using FE-TEM analysis (Colacio et al., 1996). AuNPs that are hydrodynamically smaller than 100 nm may be phagocytosed and internalized by caveolae and clathrin, indicating BT-AuNPs may have high biocompatibility (Colacio et al., 1996). The FT-IR spectra of the BT-AuNPs showed that the hydroxyl groups of phenolic compounds may contribute to capping and bioreduction during the synthesis of AuNPs (Ahn et al., 2018).
MTT assay and live/dead staining results suggested that BT-AuNPs were non-toxic to cells at concentrations ranging from 25 to 200 μg/mL, whereas BT was highly toxic to epidermal keratinocytes at concentrations 100 and 200 μg/mL. Similar to our results, a previous study has reported that Rosa rugosa-based Au nanoparticles are safer than the R. rugosa extract, indicating that the synthesis of BT-AuNPs may reduce the cytotoxicity of BT (Wang et al., 2022a). Further, we determined the anti-inflammatory properties of BT-AuNPs on T + I-stimulated HaCaT cells. Keratinocytes functionally constitute the epidermal barrier and play an important role in the pathophysiology of inflamed skin (Jiang et al., 2020). When keratinocytes are damaged by various stimuli, chemokines and cytokines are produced that stimulate inflammatory skin diseases (Tsai et al., 2022). Subsequently, immune cells, including neutrophils, macrophages, and T cells, are also stimulated and respond to inflammatory skin lesions (Wang et al., 2022b). The immune cells recruited to inflammatory skin lesions secrete chemokines such as IL-8, TARC, RANTES, and CTACK, as well as cytokines such as IL-4 and IL-6 (Si-Si et al., 2011; Mi et al., 2022c; Tsai et al., 2022). Accordingly, various studies have used T + I-stimulated HaCaT cell models to investigate their in vitro potential as skin anti-inflammatory agents (Albanesi and Pastore, 2010; Lim et al., 2015; Yang et al., 2018). Herein, T + I was used as an inflammation-inducing agent to investigate the anti-inflammatory properties of BT-AuNPs on keratinocytes. BT-AuNPs considerably decreased the mRNA expression of T + I-stimulated inflammatory genes, such as IL-6, IL-8, CTACK, TARC, and RANTES. Furthermore, the levels of IL-6, IL-8, and TARC decreased considerably after BT-AuNPs pretreatment. Similar to our results, AuNPs synthesized using R. rugosa extracts also inhibit inflammation in T + I-stimulated keratinocytes cells by suppressing chemokine/cytokine secretion and production (Wang et al., 2022a). These results suggested that BT-AuNPs could effectively attenuate inflammation in T + I-induced keratinocytes by inhibiting inflammatory cytokines and chemokines, such as IL-6, IL-8, CTACK, TARC, and RANTES.
Moreover, T + I can activate numerous intracellular signaling pathways, such as NF-κB and MAPK pathways, which are associated with inflammatory diseases like atopic dermatitis (Basu et al., 2019; Cho et al., 2020). The keratinocytes in patients with atopic dermatitis secrete chemokines and cytokines that stimulate the development of ISDs, which is triggered by MAPK signaling (Sung et al., 2012; Lim et al., 2016). The protein expression of phosphorylated ERK, JNK, and p38 were dramatically reduced in T + I-induced keratinocytes after BT-AuNPs pretreatment, implying that BT-AuNPs inhibit T + I-stimulated MAPK pathway activation. The MAPK signaling is important in immune responses and collaborates with NF-κB to modulate inflammatory pathways (Kim et al., 2010; Sung et al., 2012). Our results indicated that BT-AuNPs also markedly suppressed the protein expression of phosphorylated IκB-α and p65, suggesting that BT-AuNPs might inhibit T + I-stimulated NF-κB signaling activation which includes release and phosphorylation of IκB, NF-κB translocation into the nucleus, and activation of the inflammatory response (Mi et al., 2022c). Activation of MAPK and NF-κB pathways releases pro-inflammatory cytokines and chemokines, such as IL-6 and IL-8, potentially leading to an inflammatory response (Tang et al., 2017; Xiao et al., 2020). NF-κB and p38 are partially responsible in regulating the mechanism for TARC production in keratinocytes (Qi et al., 2009). Thus, BT-AuNPs inhibited the production of inflammation chemokines and cytokine via inhibiting the activation of NF-κB and MAPK pathways in keratinocytes.
HA is an important extracellular glycosaminoglycan component, which is associated with physiological processes of the skin like anti-aging, skin moisturizing, anti-inflammation, wound recovery, skin repair, and tissue regeneration (Bukhari et al., 2018; How et al., 2020). HA is a potential drug delivery agent in ISDs, such as psoriasis and atopic dermatitis (How et al., 2020; Marinho et al., 2021). Using HA or identifying substances that promote HA manufacturing are potential strategies to maintain skin health and cure a variety of skin-associated disorders (Qu et al., 2014; Mi et al., 2022c). HA is produced by the catalytic action of HAS, including HAS1, HAS2, ad HAS3 and conversely its depletion is promoted by hyaluronidase, including HYAL1 and HYAL2 (Oh et al., 2022). Therefore, the effect of BT-AuNPs on HA secretion was further examined via the analysis of related signaling molecules in HaCaT cells. ELISA and qRT-PCR results showed that BT-AuNPs remarkably increased HA secretion and HASs family (HAS1, HAS2, HAS3) mRNA expression. Notably, at a concentration 200 μg/mL BT-AuNPs, the gene expressions of HAS1 (5.0 fold) and HAS3 (2.2 fold) were lower than that of HAS2 (7.4 fold). Among the HAS family members, HAS2 is mainly expressed in organisms and plays a key role in HA production (Kim et al., 2021). Thus, the protein expression of HAS2 was investigated using Western blot analysis. As expected, BT-AuNPs increased the expression of the HAS2 protein in a concentration-dependent manner, implying that HAS2 might play a crucial role in HA secretion in keratinocytes following BT-AuNPs treatment. Moreover, BT-AuNPs significantly downregulated the expression of HYAL1 and HYAL2 in HaCaT cells. Consistent with our results, AuNPs synthesized using Diospyros kaki fruit extracts also stimulate the HAS genes and inhibit HYALs, leading to improved HA secretion (Dhandapani et al., 2023). In summary, the above results suggest that BT-AuNPs may stimulate HA production by promoting HA synthesis (HAS1, HAS2, and HAS3) and suppressing hyaluronidase (HYAL1 and HYAL2).
Finally, we determined the compounds that are critical to the anti-inflammatory and moisturizing effects of BT. Two major flavonoids, including rutin and hyperoside (quercetin-3-O-galactoside), were identified as the primary phytochemicals in BT by UPLC-MS/MS analysis. Similar with our findings, a previous study identified two flavonoids (rutin and isoquercetin) from B. tricuspis extract using HPLC analysis (Akter et al., 2018). Notably, both rutin and hyperoside were reported to have anti-inflammation properties on UVB irradiated keratinocytes (Gęgotek et al., 2019; Charachit et al., 2022). Kamel and Mostafa (2015) suggested that nanocream prepared from rutin exhibit moisture-enhancing properties for the skin. Therefore, these flavonoids may contribute to the an anti-inflammatory activity or HA production of BT-AuNPs.
5 Conclusion
In this study, we successfully synthesized uniformly shaped BT-AuNPs using a combination of the BT extract and Au salts. Our findings highlight the non-toxic effects of BT-AuNPs, whereas BT exhibits significant toxicity in HaCaT cells at the same concentration. This implies that the green-synthesized NPs can effectively reduce the toxicity of BT in HaCaT cells. Furthermore, BT-AuNPs greatly suppressed the inflammatory response via inhibiting NF-κB and MAPK pathways in T + I-stimulated keratinocytes. Additionally, we found that BT-AuNPs stimulated HA production by regulating the expression of HAS and HYAL biomarkers. Thus, our results provide valuable evidence of the anti-inflammatory and moisturizing properties of green-synthesized AuNPs. Therefore, BT-AuNPs can potentially be used as effective agents for treating inflammatory skin diseases and promoting skin health.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical approval was not required for the studies on humans in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used.
Author contributions
TT: Data curation, Investigation, Methodology, Writing–original draft. RW: Conceptualization, Writing–review and editing. HK: Methodology, Resources, Writing–review and editing. Y-JK: Conceptualization, Supervision, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the fund National Research Foundation of Korea (NRF, 2023R1A2C1007606) and the project (KHU-20202298, Kyung Hee University), Republic of Korea.
Conflict of interest
The authors declare that this study received funding from KDBIO Corp. The funder had the following involvement with the study: Supporting the data analysis of raw materials. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1258057/full#supplementary-material
References
Ahn, S., Singh, P., Jang, M., Kim, Y. J., Castro-Aceituno, V., Simu, S. Y., et al. (2018). Gold nanoflowers synthesized using Acanthopanacis cortex extract inhibit inflammatory mediators in LPS-induced RAW264.7 macrophages via NF-κB and AP-1 pathways. Colloids Surfaces B Biointerfaces 162, 398–404. doi:10.1016/j.colsurfb.2017.11.037
Akter, K. M., Kim, H. J., Khalil, A. A. K., Park, W. S., Lee, M. K., Park, J. H., et al. (2018). Inner morphological and chemical differentiation of Boehmeria species. J. Nat. Med. 72, 409–423. doi:10.1007/s11418-017-1164-8
Albanesi, C., and Pastore, S. (2010). Pathobiology of chronic inflammatory skin diseases: Interplay between keratino-cytes and immune cells as a target for anti-inflammatory drugs. Curr. Drug Metab. 11, 210–227. doi:10.2174/138920010791196328
Basu, C., Chatterjee, A., Bhattacharya, S., Dutta, N., and Sur, R. (2019). S-allyl cysteine inhibits TNF-α-induced inflammation in HaCaT keratinocytes by inhibition of NF- κB-dependent gene expression via sustained ERK activation. Exp. Dermatol. 28, 1328–1335. doi:10.1111/exd.14041
Bukhari, S. N. A., Roswandi, N. L., Waqas, M., Habib, H., Hussain, F., Khan, S., et al. (2018). Hyaluronic acid, a promising skin rejuvenating biomedicine: A review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int. J. Biol. Macromol. 120, 1682–1695. doi:10.1016/j.ijbiomac.2018.09.188
Charachit, N., Sukhamwang, A., Dejkriengkraikul, P., and Yodkeeree, S. (2022). Hyperoside and quercitrin in houttuynia cordata extract attenuate UVB-induced human keratinocyte cell damage and oxidative stress via modulation of MAPKs and akt signaling pathway. Antioxidants 11. doi:10.3390/antiox11020221
Chen, Y., Wang, G., Wang, H., Cheng, C., Zang, G., Guo, X., et al. (2014). Phytochemical profiles and antioxidant activities in six species of ramie leaves. PLoS One 9. doi:10.1371/journal.pone.0108140
Cho, S. H., Kim, H. S., Lee, W. W., Han, E. J., Kim, S. Y., Fernando, I. P. S., et al. (2020). Eckol from Ecklonia cava ameliorates TNF-α/IFN-γ-induced inflammatory responses via regulating MAPKs and NF-κB signaling pathway in HaCaT cells. Int. Immunopharmacol. 82, 106146. doi:10.1016/j.intimp.2019.106146
Colacio, E., Cuesta, R., Gutiérrez-Zorrilla, J. M., Luque, A., Román, P., Giraldi, T., et al. (1996). Gold(I)-Purine interactions: Synthesis and characterization of cyclic and open chain polynuclear gold(I) complexes containing xanthine derivatives and bis(phosphine) as bridging ligands. Crystal structures of [Au2(μ- HX)(μ-dmpe)]·3H2O and [Au2(μ-TT)(μ-dmp. Inorg. Chem. 35, 4232–4238. doi:10.1021/ic951591a
Dhandapani, S., Wang, R., Kim, H., and Kim, Y. (2023). Enhanced skin anti-inflammatory and moisturizing action of gold nanoparticles produced utilizing Diospyros kaki fruit extracts. Arab. J. Chem. 16, 104551. doi:10.1016/j.arabjc.2023.104551
Dias, M. K. H. M., Madusanka, D. M. D., Han, E. J., Kim, H. S., Jeon, Y. J., Jee, Y., et al. (2021). Sargassum horneri (Turner) C. Agardh ethanol extract attenuates fine dust-induced inflammatory responses and impaired skin barrier functions in HaCaT keratinocytes. J. Ethnopharmacol. 273, 114003. doi:10.1016/j.jep.2021.114003
Elbagory, A. M., Hussein, A. A., and Meyer, M. (2019). The in vitro immunomodulatory effects of gold nanoparticles synthesized from hypoxis hemerocallidea aqueous extract and hypoxoside on macrophage and natural killer cells. Int. J. Nanomedicine 14, 9007–9018. doi:10.2147/IJN.S216972
Gęgotek, A., Jarocka-Karpowicz, I., and Skrzydlewska, E. (2019). Synergistic cytoprotective effects of rutin and ascorbic acid on the proteomic profile of 3d-cultured keratinocytes exposed to uva or uvb radiation. Nutrients 11. doi:10.3390/nu11112672
Homey, B., Steinhoff, M., Ruzicka, T., and Leung, D. Y. M. (2006). Cytokines and chemokines orchestrate atopic skin inflammation. J. Allergy Clin. Immunol. 118, 178–189. doi:10.1016/j.jaci.2006.03.047
How, K. N., Yap, W. H., Lim, C. L. H., Goh, B. H., and Lai, Z. W. (2020). Hyaluronic acid-mediated drug delivery system targeting for inflammatory skin diseases: A mini review. Front. Pharmacol. 11, 1–8. doi:10.3389/fphar.2020.01105
Hutchison, J. E. (2008). Greener nanoscience: A proactive approach to advancing applications and reducing implications of nanotechnology. ACS Nano 2, 395–402. doi:10.1021/nn800131j
Jiang, Y., Tsoi, L. C., Billi, A. C., Ward, N. L., Harms, P. W., Zeng, C., et al. (2020). Cytokinocytes: The diverse contribution of keratinocytes to immune responses in skin. JCI Insight 5, 1–15. doi:10.1172/jci.insight.142067
Ju, S. M., Song, H. Y., Lee, S. J., Seo, W. Y., Sin, D. H., Goh, A. R., et al. (2009). Suppression of thymus- and activation-regulated chemokine (TARC/CCL17) production by 1,2,3,4,6-penta-O-galloyl-β-d-glucose via blockade of NF-κB and STAT1 activation in the HaCaT cells. Biochem. Biophys. Res. Commun. 387, 115–120. doi:10.1016/j.bbrc.2009.06.137
Kamel, R., and Mostafa, D. M. (2015). Rutin nanostructured lipid cosmeceutical preparation with sun protective potential. J. Photochem. Photobiol. B Biol. 153, 59–66. doi:10.1016/j.jphotobiol.2015.09.002
Kim, M. K., Chung, S. W., Kim, D. H., Kim, J. M., Lee, E. K., Kim, J. Y., et al. (2010). Modulation of age-related NF-κB activation by dietary zingerone via MAPK pathway. Exp. Gerontol. 45, 419–426. doi:10.1016/j.exger.2010.03.005
Kim, T. H., Kim, W. J., Park, S. Y., Kim, H., and Chung, D. K. (2021). In vitro anti-wrinkle and skin-moisturizing effects of evening primrose (Oenothera biennis) sprout and identification of its active components. Processes 9, 1–13. doi:10.3390/pr9010145
Kwon, D. J., Bae, Y. S., Ju, S. M., Goh, A. R., Youn, G. S., Choi, S. Y., et al. (2012). Casuarinin suppresses TARC/CCL17 and MDC/CCL22 production via blockade of NF-κB and STAT1 activation in HaCaT cells. Biochem. Biophys. Res. Commun. 417, 1254–1259. doi:10.1016/j.bbrc.2011.12.119
Lee, J. E., Kim, Y. A., Yu, S., Park, S. Y., Kim, K. H., and Kang, N. J. (2019). 3,6-Anhydro-L-galactose increases hyaluronic acid production via the EGFR and AMPKα signaling pathway in HaCaT keratinocytes. J. Dermatol. Sci. 96, 90–98. doi:10.1016/j.jdermsci.2019.10.005
Lim, H. S., Jin, S. E., Kim, O. S., Shin, H. K., and Jeong, S. J. (2015). Alantolactone from saussurea lappa exerts antiinflammatory effects by inhibiting chemokine production and STAT1 phosphorylation in TNF-α and IFN-γ-induced in HaCaT cells. Phyther. Res. 29, 1088–1096. doi:10.1002/ptr.5354
Lim, S. J., Kim, M., Randy, A., Nam, E. J., and Nho, C. W. (2016). Effects of Hovenia dulcis Thunb. extract and methyl vanillate on atopic dermatitis-like skin lesions and TNF-α/IFN-γ-induced chemokines production in HaCaT cells. J. Pharm. Pharmacol. 68, 1465–1479. doi:10.1111/jphp.12640
Lin, Z. M., Ma, M., Li, H., Qi, Q., Liu, Y. T., Yan, Y. X., et al. (2018). Topical administration of reversible SAHH inhibitor ameliorates imiquimod-induced psoriasis-like skin lesions in mice via suppression of TNF-α/IFN-γ-induced inflammatory response in keratinocytes and T cell-derived IL-17. Pharmacol. Res. 129, 443–452. doi:10.1016/j.phrs.2017.11.012
Liu, Y., Kim, S., Kim, Y. J., Perumalsamy, H., Lee, S., Hwang, E., et al. (2019). Green synthesis of gold nanoparticles using Euphrasia officinalis leaf extract to inhibit lipopolysaccharide-induced inflammation through NF-κB and JAK/STAT pathways in RAW 264.7 macrophages. Int. J. Nanomedicine 14, 2945–2959. doi:10.2147/IJN.S199781
Liu, Y., Perumalsamy, H., Kang, C. H., Kim, S. H., Hwang, J. S., Koh, S. C., et al. (2020). Intracellular synthesis of gold nanoparticles by Gluconacetobacter liquefaciens for delivery of peptide CopA3 and ginsenoside and anti-inflammatory effect on lipopolysaccharide-activated macrophages. Artif. Cells, Nanomedicine Biotechnol. 48, 777–788. doi:10.1080/21691401.2020.1748639
Marinho, A., Nunes, C., and Reis, S. (2021). Hyaluronic acid: A key ingredient in the therapy of inflammation. Biomolecules 11, 13–15. doi:10.3390/biom11101518
Mi, X., Hoa My Tran, T., Park, H.-R., Yue Xu, X., Subramaniyam, S., Sol Choi, H., et al. (2021). Immune-enhancing effects of postbiotic produced by Bacillus velezensis Kh2-2 isolated from Korea Foods. Food Res. Int. 152, 110911. doi:10.1016/j.foodres.2021.110911
Mi, X. jie, Choi, H. S., Perumalsamy, H., Shanmugam, R., Thangavelu, L., Balusamy, S. R., et al. (2022b). Biosynthesis and cytotoxic effect of silymarin-functionalized selenium nanoparticles induced autophagy mediated cellular apoptosis via downregulation of PI3K/Akt/mTOR pathway in gastric cancer. Phytomedicine 99, 154014. doi:10.1016/j.phymed.2022.154014
Mi, X. J., Xu, X. Y., Choi, H. S., Kim, H., Cho, I. H., Yi, T. H., et al. (2022a). The immune-enhancing properties of hwanglyeonhaedok-tang-mediated biosynthesized gold nanoparticles in macrophages and splenocytes. Int. J. Nanomedicine 17, 477–494. doi:10.2147/IJN.S338334
Mi, X., Kim, J., Lee, S., Moon, S., Kim, Y., and Kim, H. (2022c). In vitro assessment of the anti-inflammatory and skin-moisturizing effects of Filipendula palmata (Pall.) Maxim. On human keratinocytes and identification of its bioactive phytochemicals. J. Ethnopharmacol. 296, 115523. doi:10.1016/j.jep.2022.115523
Mohanpuria, P., Rana, N. K., and Yadav, S. K. (2008). Biosynthesis of nanoparticles: Technological concepts and future applications. J. Nanoparticle Res. 10, 507–517. doi:10.1007/s11051-007-9275-x
Moldovan, B., David, L., Vulcu, A., Olenic, L., Perde-Schrepler, M., Fischer-Fodor, E., et al. (2017). In vitro and in vivo anti-inflammatory properties of green synthesized silver nanoparticles using Viburnum opulus L. fruits extract. Mat. Sci. Eng. C 79, 720–727. doi:10.1016/j.msec.2017.05.122
Nedoszytko, B., Sokołowska-Wojdyło, M., Ruckemann-Dziurdzińska, K., Roszkiewicz, J., and Nowicki, R. J. (2014). Chemokines and cytokines network in the pathogenesis of the inflammatory skin diseases: Atopic dermatitis, psoriasis and skin mastocytosis. Postep. Dermatol. i Alergol. 31, 84–91. doi:10.5114/pdia.2014.40920
Oh, J. H., Hur, W., Li, N., and Jo, S. J. (2022). Effects of the epidermal growth factor receptor inhibitor, gefitinib, on lipid and hyaluronic acid synthesis in cultured HaCaT keratinocytes. Exp. Dermatol. 31, 918–927. doi:10.1111/exd.14538
Qi, X., Kim, D., Yoon, Y., Li, J., Song, S., Jin, D., et al. (2009). The adenylyl cyclase-cAMP system suppresses TARC/CCL17 and MDC/CCL22 production through p38 MAPK and NF- B in HaCaT keratinocytes. Mol. Immunol. 46, 1925–1934. doi:10.1016/j.molimm.2009.03.018
Qu, C., Rilla, K., Tammi, R., Tammi, M., Kröger, H., and Lammi, M. J. (2014). Extensive CD44-dependent hyaluronan coats on human bone marrow-derived mesenchymal stem cells produced by hyaluronan synthases HAS1, HAS2 and HAS3. Int. J. Biochem. Cell Biol. 48, 45–54. doi:10.1016/j.biocel.2013.12.016
Ricci-Júnior, E., and Marchetti, J. M. (2006). Zinc(II) phthalocyanine loaded PLGA nanoparticles for photodynamic therapy use. Int. J. Pharm. 310, 187–195. doi:10.1016/j.ijpharm.2005.10.048
Salunke, G. R., Ghosh, S., Santosh Kumar, R. J., Khade, S., Vashisth, P., Kale, T., et al. (2014). Rapid efficient synthesis and characterization of silver, gold, and bimetallic nanoparticles from the medicinal plant Plumbago zeylanica and their application in biofilm control. Int. J. Nanomedicine 9, 2635–2653. doi:10.2147/IJN.S59834
Shikha, S., Chaudhuri, S. R., and Bhattacharyya, M. S. (2020). Facile One pot greener synthesis of sophorolipid capped gold nanoparticles and its antimicrobial activity having special efficacy against gram negative Vibrio cholerae. Sci. Rep. 10, 1–13. doi:10.1038/s41598-019-57399-3
Si-Si, W., Liao, L., Ling, Z., and Yun-Xia, Y. (2011). Inhibition of TNF-α/IFN-γ induced RANTES expression in HaCaT cell by naringin. Pharm. Biol. 49, 810–814. doi:10.3109/13880209.2010.550054
Sikandan, A., Shinomiya, T., and Nagahara, Y. (2018). Ashwagandha root extract exerts anti-inflammatory effects in HaCaT cells by inhibiting the MAPK/NF-κB pathways and by regulating cytokines. Int. J. Mol. Med. 42, 425–434. doi:10.3892/ijmm.2018.3608
Sung, Y. Y., Kim, Y. S., and Kim, H. K. (2012). Illicium verum extract inhibits TNF-α- and IFN-γ-induced expression of chemokines and cytokines in human keratinocytes. J. Ethnopharmacol. 144, 182–189. doi:10.1016/j.jep.2012.08.049
Tang, Q., Chen, J. H., Zang, G. G., and Luan, M. B. (2016). Characterization of novel polymorphic genomic microsatellite markers of Boehmeria tricuspis (Hance) Makino. Genet. Mol. Res. 15. doi:10.4238/gmr.15027882
Tang, S. C., Liao, P. Y., Hung, S. J., Ge, J. S., Chen, S. M., Lai, J. C., et al. (2017). Topical application of glycolic acid suppresses the UVB induced IL-6, IL-8, MCP-1 and COX-2 inflammation by modulating NF-κB signaling pathway in keratinocytes and mice skin. J. Dermatol. Sci. 86, 238–248. doi:10.1016/j.jdermsci.2017.03.004
Tran, T. H. M., Puja, A. M., Kim, H., and Kim, Y.-J. (2022). Nanoemulsions prepared from mountain ginseng-mediated gold nanoparticles and silydianin increase the anti-inflammatory effects by regulating NF-κB and MAPK signaling pathways. Biomater. Adv., 212814. doi:10.1016/j.bioadv.2022.212814
Tsai, Y. C., Chang, H. H., Chou, S. C., Chu, T. W., Hsu, Y. J., Hsiao, C. Y., et al. (2022). Evaluation of the anti-atopic dermatitis effects of α-boswellic acid on tnf-α/ifn-γ-stimulated HaCat cells and DNCB-induced BALB/c mice. Int. J. Mol. Sci. 23. doi:10.3390/ijms23179863
Wallyn, J., Anton, N., and Vandamme, T. F. (2019). Synthesis, principles, and properties of magnetite nanoparticles for in vivo imaging applications—a review. Pharmaceutics 11, 1–29. doi:10.3390/pharmaceutics11110601
Wang, R., Moon, S. K., Kim, W. J., Dhandapani, S., Kim, H., and Kim, Y. J. (2022a). Biologically synthesized Rosa rugosa-based gold nanoparticles suppress skin inflammatory responses via MAPK and NF-κB signaling pathway in TNF-α/IFN-γ-Induced HaCaT keratinocytes. ACS Omega 7, 35951–35960. doi:10.1021/acsomega.2c04832
Wang, Z., Qi, F., Luo, H., Xu, G., and Wang, D. (2022b). Inflammatory microenvironment of skin wounds. Front. Immunol. 13, 1–17. doi:10.3389/fimmu.2022.789274
Xiao, K., Liu, C., Tu, Z., Xu, Q., Chen, S., Zhang, Y., et al. (2020). “Activation of the NF- κ B and MAPK signaling pathways contributes to the inflammatory responses, but not cell injury,” in IPEC-1 cells challenged with hydrogen peroxide. 2020.
Xu, X. Y., Tran, T. H. M., Perumalsamy, H., Sanjeevram, D., and Kim, Y. J. (2021). Biosynthetic gold nanoparticles of Hibiscus syriacus L. callus potentiates anti-inflammation efficacy via an autophagy-dependent mechanism. Mat. Sci. Eng. C 124, 112035. doi:10.1016/j.msec.2021.112035
Yamada, M., Foote, M., and Prow, T. W. (2015). Therapeutic gold, silver, and platinum nanoparticles. Wiley Interdiscip. Rev. Nanomedicine Nanobiotechnology 7, 428–445. doi:10.1002/wnan.1322
Yang, J. H., Hwang, Y. H., Gu, M. J., Cho, W. K., and Ma, J. Y. (2015). Ethanol extracts of Sanguisorba officinalis L. suppress TNF-α/IFN-γ-induced pro-inflammatory chemokine production in HaCaT cells. Phytomedicine 22, 1262–1268. doi:10.1016/j.phymed.2015.09.006
Keywords: Boehmeria tricuspis, gold nanoparticles, inflammatory skin diseases, hyaluronic acid, HaCaT keratinocytes
Citation: Tran THM, Wang R, Kim H and Kim Y-J (2023) The anti-inflammation and skin-moisturizing effects of Boehmeria tricuspis-mediated biosynthesized gold nanoparticles in human keratinocytes. Front. Pharmacol. 14:1258057. doi: 10.3389/fphar.2023.1258057
Received: 18 July 2023; Accepted: 12 September 2023;
Published: 06 October 2023.
Edited by:
Karl Tsim, Hong Kong University of Science and Technology, Hong Kong SAR, ChinaReviewed by:
Sun Young Park, Pusan National University, Republic of KoreaManel Ben Hammouda, Duke University, United States
Copyright © 2023 Tran, Wang, Kim and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hoon Kim, c2FwaGVhZDExMDZAaGFubWFpbC5uZXQ=; Yeon-Ju Kim, eWVvbmp1a2ltQGtodS5hYy5rcg==
 Thi Hoa My Tran1
Thi Hoa My Tran1 Hoon Kim
Hoon Kim Yeon-Ju Kim
Yeon-Ju Kim