
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Pharmacol., 21 February 2023
Sec. Pharmacogenetics and Pharmacogenomics
Volume 14 - 2023 | https://doi.org/10.3389/fphar.2023.1080117
This article is part of the Research TopicThe Utilization of Bench-to-Bedside Approaches in PharmacogenomicsView all 5 articles
Pharmacogenetics has potential for optimizing use of psychotropics. CYP2D6 and CYP2C19 are two clinically relevant pharmacogenes in the prescribing of antidepressants. Using cases recruited from the Understanding Drug Reactions Using Genomic Sequencing (UDRUGS) study, we aimed to evaluate the clinical utility of genotyping CYP2D6 and CYP2C19 in antidepressant response. Genomic and clinical data for patients who were prescribed antidepressants for mental health disorders, and experienced adverse reactions (ADRs) or ineffectiveness, were extracted for analysis. Genotype-inferred phenotyping of CYP2D6 and CYP2C19 was carried out as per Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines. A total of 52 patients, predominantly New Zealand Europeans (85%) with a median age (range) of 36 years (15–73), were eligible for analysis. Thirty-one (60%) reported ADRs, 11 (21%) ineffectiveness, and 10 (19%) reported both. There were 19 CYP2C19 NMs, 15 IMs, 16 RMs, one PM and one UM. For CYP2D6, there were 22 NMs, 22 IMs, four PMs, three UMs, and one indeterminate. CPIC assigned a level to each gene-drug pair based on curated genotype-to-phenotype evidence. We analyzed a subgroup of 45 cases, inclusive of response type (ADRs/ineffectiveness). Seventy-nine (N = 37 for CYP2D6, N = 42 for CYP2C19) gene-drug/antidepressant-response pairs with CPIC evidence levels of A, A/B, or B were identified. Pairs were assigned as ‘actionable’ if the CYP phenotypes potentially contributed to the observed response. We observed actionability in 41% (15/37) of CYP2D6-antidepressant-response pairs and 36% (15/42) of CYP2C19-antidepressant-response pairs. In this cohort, CYP2D6 and CYP2C19 genotypes were actionable for a total of 38% pairs, consisting of 48% in relation to ADRs and 21% in relation to drug ineffectiveness.
Heterogeneity in drug response is a well-recognized challenge in mental health. In the management of depression, the average response rate reported for antidepressants ranged from 42% to 53% (Cipriani et al., 2018a; Cipriani et al., 2018b), while nearly 50% of patients experienced adverse reactions (ADRs) during treatment (Braund et al., 2021). ADRs and ineffectiveness are a major contributor towards poor adherence and discontinuation of antidepressants (Marasine and Sankhi, 2021).
Pharmacogenetics is a promising clinical tool in antidepressant prescribing, which aims to improve depression remission rates and minimize ADRs (Bousman et al., 2017). Systematic reviews and meta-analyses have supported the clinical utility of pharmacogenetic tests in depression management with significant improvement on patient outcomes observed for pharmacogenetics-guided groups (Arnone et al., 2023; Brown et al., 2020; Brown et al., 2022). Further, the recent Pre-emptive Pharmacogenomic Testing for Preventing Adverse Drug Reactions (PREPARE) randomised controlled trial, which included antidepressant-gene combinations, showed that genotype-guided prescribing reduced the incidence of clinically relevant ADRs by 30% (Swen et al., 2023).
Genetic variants of CYP2D6 and CYP2C19 are most clinically relevant to the pharmacokinetics of antidepressants. Literature investigating the association between CYP2D6/CYP2C19 genotypes and antidepressant response is extensive (Rosenblat et al., 2018; Bousman et al., 2019; Solomon et al., 2019), and its clinical implementation is advancing (van Westrhenen et al., 2020). The Clinical Pharmacogenetics Implementation Consortium (CPIC) has published CYP2D6 and CYP2C19 prescribing guidelines for two antidepressant classes (tricyclic antidepressants (TCAs) and selective serotonin reuptake inhibitors (SSRIs)) (Hicks et al., 2015; Hicks et al., 2017), with the updated guideline in review for publication. For each gene-drug pair, CPIC has reviewed the available evidence linking genotype to phenotype, and assigned a level of evidence (A, A/B, B, B/C, C, C/D, or D). Pharmacogenetics-guided treatments for antidepressants assigned with CPIC evidence levels A and B have been reported to be either cost-effective or cost-saving (Morris et al., 2022).
For gene-drug pairs with level A, the genetic information should be used to change prescribing of affected drug, while for B, the genetic information could be used to change prescribing of the affected drug because alternative therapies or dosing are extremely likely to be as effective and as safe as non-genetically based dosing. Currently, only gene-drug pairs with level A and B are sufficient for at least one prescribing action to be recommended. For level A/B, full evidence review is to be undertaken by CPIC, with preliminary review indicating a definitive CPIC level of either A or B (Clinical Pharmacogenetics Implementation Consortium, 2021b).
Understanding Adverse Drug Reactions using Genomic Sequencing (UDRUGS) is an ongoing project by our laboratory, which recruits patients who have experienced ADRs or drug ineffectiveness (Maggo et al., 2017). In addition to establishing a DNA bank linked to the clinical information of this cohort, UDRUGS also aims to study the role of genetic variations in known pharmacogenes that may contribute to the observed phenotypes. Recruited cases are mainly referred by healthcare practitioners, with established ADRs or ineffectiveness (treatment failure) phenotypes. In this case series report, we used cases recruited into UDRUGS to examine the explanatory role of CYP2D6 and CYP2C19 pharmacogenetics in responses associated with CPIC evidence levels A, A/B, or B-assigned gene-antidepressant pairs.
The Understanding Adverse Drug Reactions using Genomic Sequencing (UDRUGS) study (Maggo et al., 2017) received ethical approval from the New Zealand (NZ) Health and Disability Ethics Committees (HDEC URA/11/11/065). This retrospective case series report aims to evaluate the clinical utility of genotyping CYP2D6 and CYP2C19 in antidepressant response.
The recruitment and sampling of UDRUGS cases (Maggo et al., 2017), and deoxyribonucleic acid (DNA) extraction were as previously described (Miller et al., 1988; Maggo et al., 2019b). Screening was carried out on UDRUGS cases recruited during May 2019 - December 2021. The case inclusion criteria were patients who were prescribed antidepressants for mental health disorders, and experienced ADRs or ineffectiveness, and cases with complete CYP2D6 and CYP2C19 genotype results for analysis. The genomic and clinical information for these cases were extracted and analysed via two approaches as described in subsequent Section 2.3.
The genotypes of CYP2D6 and CYP2C19 were available for each of the identified UDRUGS cases, informed by previous screening of selected genetic variants. The genetic analysis for these pharmacogenes was carried out as previously reported (Maggo et al., 2019b; Kee et al., 2022). Briefly, gene regions of interest were amplified by polymerase chain reactions (PCR) and subsequently subjected to Sanger sequencing, run using BigDye® Terminator v3.1 on a 3130XL Genetic Analyser. Generated chromatograms were aligned with reference sequences for genotyping, via Geneious Prime Version 2020.1 (Biomatters Ltd. Auckland, NZ). *1 allele was assigned when no variations were observed. For CYP2C19, *2 (rs4244285), *3 (rs4986893), and *17 (rs12248560) alleles were genotyped. These are the first tier of CYP2C19 alleles recommended in clinical genotyping (Pratt et al., 2018). Unlike CYP2C19, the screening of CYP2D6 involved the whole 6.6 kb gene. This approach was preferred due to its polymorphic nature. To date, Pharmacogene Variation Consortium (PharmVar) has listed over 170 CYP2D6 star alleles (Gaedigk et al., 2018; Gaedigk et al., 2021). Alleles were assigned according to the PharmVar nomenclature system (Gaedigk et al., 2018). Based on the patient’s genotype, cases were assigned a phenotype according to CPIC guidelines (Caudle et al., 2020; Crews et al., 2021; Lima et al., 2021).
The methodology and study flow is as illustrated in Supplementary Figure S1.
This is defined as the number of cases reporting any negative response (ADRs or ineffectiveness) with each antidepressant. For example, a case which documented both ADRs and ineffectiveness with the use of citalopram is calculated as one response with the respective antidepressant.
The list of antidepressants for which CPIC has assigned an evidence level of A, A/B, or B for CYP2D6 or CYP2C19 is shown in Supplementary Table S1 (Clinical Pharmacogenetics Implementation Consortium, 2021a). From the eligible UDRUGS cases, gene-drug-response pairs were extracted for analysis. This observation also considered the type of negative response. For example, a case which documented both ADRs and ineffectiveness with the use of citalopram is calculated as two observation pairs. This is presented as ‘CYP2D6/CYP2C19-antidepressant-response pairs’.
Identified CYP2D6/CYP2C19-antidepressant-response pairs were categorized into either “actionable” or “non-actionable”. The proportion of actionable and non-actionable CYP2D6/CYP2C19-antidepressant-response pairs was assessed for 1) Antidepressant drug class, 2) Individual antidepressant, 3) CPIC evidence levels A, A/B, and B, 4) CYP2D6 and CYP2C19 pharmacogenes, and 5) Drug response phenotypes (ADRs and ineffectiveness).
The assignment was informed by critically assessing the available clinical and genetic data for the association between 1) genotype-inferred phenotypes, 2) drug exposure, and 3) drug response events.
Compared with normal metabolizer (NMs) phenotypes, a reduced metabolism rate is predicted in intermediate metabolizers (IMs) and poor metabolizers (PMs), while, an increased rate of metabolism is predicted in rapid metabolizers (RMs) and ultrarapid metabolizers (UMs). For drug exposure, it is predicted to be higher in IMs and PMs, and lower in RMs and UMs. In this cohort, two response phenotypes were analyzed: ADRs and ineffectiveness. High and low drug exposure levels are predicted to predispose to the risk of ADRs and ineffectiveness, respectively. “Ineffectiveness” included partial response, poor response, diminished response, or an “unusually” high dose requirement (as reported by the healthcare practitioner referring the case).
Pairs with a clear association between these three aspects are assigned as “actionable”. For example, for a medicine where the parent drug is active and its metabolites are inactive, the decreased or absent CYP2D6 and/or CYP2C19 metabolic capacity of a genetically-derived IM or PM could result in a higher drug exposure, increasing the risk of ADRs, or vice versa for RMs and UMs with the ineffectiveness phenotypes.
Binomial proportion confidence intervals, using the Wilson score interval method, were used to calculate all proportions and confidence intervals, using OpenEpi, a free and open source statistical software (Dean et al., 2013).
A total of 205 UDRUGS cases recruited between May 2019 to December 2021 were assessed. Of these, 52 eligible cases experienced ADRs or ineffectiveness with one or more antidepressants indicated for mental health conditions, and with complete CYP2D6 and CYP2C19 genotypes. The majority of cases (49) were referred by healthcare practitioners (including doctors and pharmacists), while three were self-referred.
A total of 111 antidepressant-response pairs were identified from 52 cases. Of the 111 antidepressant-response pairs observed, 58 (53%) involved SSRIs, 17 (15%) TCAs and 17 (15%) selective norepinephrine reuptake inhibitors (SNRIs). Others included 4 (4%) monoamine oxidase inhibitors (MAO-I), 13 (12%) atypical agents (bupropion and mirtazapine), and two (2%) were unspecified. The highest number of responses documented involved the use of fluoxetine (N = 22), followed by venlafaxine (N = 17) and sertraline (N = 15), while the recorded number of antidepressant-response pairs was comparable for nortriptyline (N = 12), escitalopram (N = 12) and mirtazapine (N = 11). Table 1 summarizes case demographic information and a breakdown of antidepressant-response pairs.
The documented ADRs covered a wide range of symptoms, mainly alteration of mental state (e.g., agitation, manic episode, paranoid), autonomic effects (e.g., diarrhoea, heart palpitation, diaphoresis), and neuromuscular effects (e.g., dystonia, stiffness). For efficacy, the majority of the cases either did not respond to or response diminished over time. These responses were documented across a range of doses and the time of event onset ranged from hours to months of being on treatment. The details of individual cases can be found in Supplementary Table S2.
Of the 52 cases, there were 22 (42%) CYP2D6 NMs, 22 (42%) IMs, four (8%) PMs, three (6%) UMs, and one (2%) indeterminate case. CYP2D6 duplications were detected in cases B35 (*1/*1xN), B38 (*1/*35xN or *1xN/*35), and B52 (*1/*27xN or *1xN/*27). Since *1, *35, and *27 are normal function alleles, the predicted phenotype was UM regardless of which allele is duplicated. The phenotype of sample B2 (*4/*32) was indeterminate due to the uncertain function of the *32 allele (Phasing was confirmed through long read Nanopore sequencing; protocols as described in (Hitchman et al., 2022)). With the presence of a non-functional *4 allele, B2 is expected to be either IM or PM. For CYP2C19, there was one (2%) UM, 16 (31%) RMs, 19 (37%) NMs, 15 (29%) IMs, and one (2%) PM.
The genotypes, inferred CYP2D6 and CYP2C19 phenotypes, and reported antidepressants and responses for each case can be found in Table 2.
Of the 52 cases, seven were excluded from this analysis for two reasons. First, the antidepressants documented have not been reviewed by CPIC, or have been assigned a lower level of evidence, specifically the drugs fluoxetine (CPIC evidence level C) and mirtazapine (CPIC evidence level B/C) (Clinical Pharmacogenetics Implementation Consortium, 2021a). Cases excluded for this reason were B3, B13, and B17. Second, specific antidepressants were not documented for B14, B45, B47, and B51.
Of the remaining 45 cases, 26 (57%) were CYP2D6 non-NMs and 28 (62%) were CYP2C19 non-NMs. A total of 79 gene-drug-response pairs were identified involving the antidepressant drug classes SSRIs, TCAs, and SNRIs. The specific drugs included nortriptyline, amitriptyline, clomipramine, paroxetine, sertraline, citalopram, escitalopram, and venlafaxine.
Table 3 lists the analyzed gene-drug-response pairs and their actionability based on CYP2D6 and CYP2C19 genotypes. The majority of the assignments were informed by the association between drug exposure and the metabolic rate predicted from genotype-inferred phenotypes, except for 1) Case B1 and B4 (CYP2C19-Amitriptyline-ADR pair), 2) Case B8 (CYP2C19-Clomipramine-ADR pair), and 3) Case B18 and B46 (CYP2D6-Venlafaxine-Ineffectiveness pair), where the associations were complex, mainly for two reasons. First, both the parent compound and metabolite are pharmacologically active (e.g., amitriptyline, clomipramine, and venlafaxine). Second, the role of CYP2D6 and CYP2C19 in the metabolic pathways is equally important, with similar CPIC evidence levels assigned (e.g., amitriptyline and clomipramine). These cases are described in further detail below.
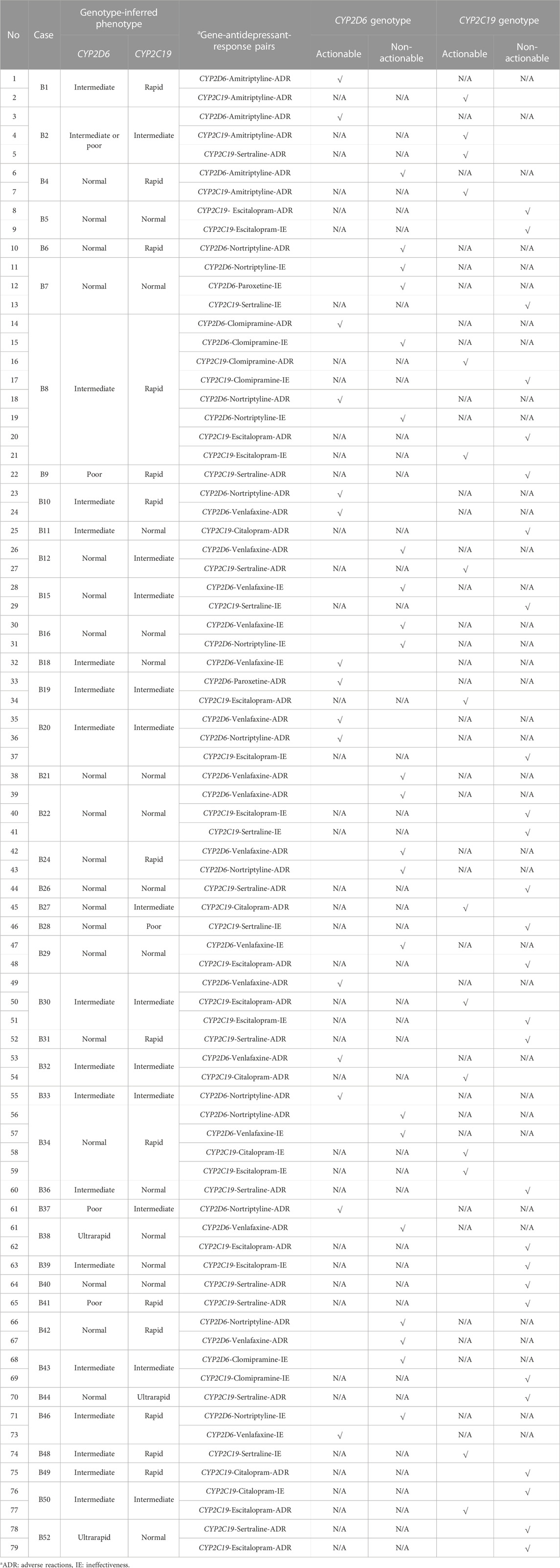
TABLE 3. Analysed gene-antidepressant-response pairs and actionability of CYP2D6 and CYP2C19 pharmacogenetics (N = 79).
B1, a CYP2D6 IM and CYP2C19 RM. B4, a CYP2D6 NM and CYP2C19 RM. The rapid metabolism of CYP2C19 is expected to increase the conversion of amitriptyline to nortriptyline, which itself is pharmacologically active. When compared with amitriptyline, nortriptyline was five times more potent in inhibiting noradrenaline with less affinity towards other post-synaptic receptors, thus less ADRs and better tolerability (Gillman, 2007). This may explain the reported mechanism-related reactions (nightmare and severe exhaustion) in B4, which are thought to be caused by a shift of neurotransmitter concentrations, rather than effects associated with the non-selective binding on other post-synaptic receptors.
B8, a CYP2D6 IM and CYP2C19 RM. The rapid metabolism of CYP2C19 is expected to increase the conversion of clomipramine to desmethyl-clomipramine, an active metabolite (Balant-Gorgia et al., 1991), which is further hydroxylated by CYP2D6. The association between CYP2D6 genotypes and the total clearance of clomipramine and hydroxylation indexes has been reported (Nielsen et al., 1994). Clinical case studies have also observed higher levels of desmethyl-clomipramine in CYP2D6 IMs and PMs (Stephan et al., 2006; Brown et al., 2017). Potentially, the CYP2D6 IM phenotype augmented the higher elevated concentration of desmethyl-clomipramine, produced from the rapid CYP2C19 enzymatic activity, leading to the reported ADRs.
B18, a CYP2D6 IM and CYP2C19 NM. B46, a CYP2D6 IM and CYP2C19 RM. Venlafaxine is mainly oxidized to O-desmethylvenlafaxine by CYP2D6 (Otton et al., 1996). Both venlafaxine and O-desmethylvenlafaxine are equipotent, but it has been suggested that the antidepressant effect of venlafaxine is largely accounted for by its metabolite (Lobello et al., 2010). The impaired CYP2D6 activity may reduce the formation of O-desmethylvenlaxine, thereby reducing the therapeutic effect.
Table 4 shows the proportion of actionable and non-actionable pairs for outcomes “antidepressant drug class,” “CPIC evidence level,” “CYP2D6 and CYP2C19 pharmacogenes,” and “drug response phenotype”.
Of the 79 CYP2D6/CYP2C19-antidepressant-response pairs, the pharmacogenetics of CYP2D6 and CYP2C19 was potentially able to explain a total of 30 (38%) pairs, making them actionable. A decreasing trend was observed for outcome ‘CPIC evidence levels’ from evidence level “A” (45%), “A/B” (38%) to “B” (24%). For “antidepressant drug class,” the proportion of actionable pairs across drug classes were 48% (TCAs), 38% (SNRIs), and 32% (SSRIs). Specifically, within TCAs-associated pairs, all CYP2C19-amitriptyline-response pairs (100%) were actionable, while the highest actionable proportion of SSRIs-associated pairs were CYP2D6-paroxetine-response pair (50%) and CYP2C19-citalopram-response pair (50%).
The actionability of CYP2D6 and CYP2C19 was comparable, up to 40% of the identified pairs were associated with the genotypes of the respective pharmacogene. Of the 50 CYP2D6/CYP2C19-antidepressant-ADRs pairs observed, up to 50% were actionable, while that of ineffectiveness pairs was lower, approximately 20%.
Among the 52 cases which experienced ADRs or ineffectiveness with the use of antidepressants, there were only eight cases (15%) with CYP2D6 and CYP2C19 NM phenotypes. This proportion is comparable to a recent study (∼13%) by Hahn and Roll (2022), who carried out a retrospective pharmacogenetic analysis in 108 European (German) adult depressive patients, where 51 of them were prescribed antidepressants or antipsychotics with CPIC and/or Dutch Pharmacogenetics Working Group (DPWG) guideline recommendations for CYP2D6 and CYP2C19 (Hahn and Roll, 2022). Hahn and Roll (2022) evaluated the clinical utility of CYP2D6 and CYP2C19 pharmacogenetics by comparing the proportion of actionable genotypes (genotypes with recommendations other than ‘initiate or treat with standard dose’) before and after pharmacogenetic testing service (Hahn and Roll, 2022). Separately, in a large Danish population-based case cohort of patients with mental disorders including depression (N = 51,464), 27% of the cases were reported with CYP2D6 and CYP2C19 NM phenotypes (Lunenburg et al., 2021). In addition to the larger sample size, the case cohort of this study also included patients with other mental disorders (e.g., bipolar disorder and schizophrenia), which may have explained the higher frequency observed. Despite the discordance, both the wider literature and our study showed that approximately 73%–85% of patients with mental disorders carry non-NM phenotypes in CYP2D6 and/or CYP2C19.
In the subgroup 45 cases used for CYP2D6/CYP2C19-antidepressant-response pair analysis, 57% were CYP2D6 non-NMs and 62% were CYP2C19 non-NMs. This is in accordance with Maggo et al. (2019a), who also reported comparably high proportions (42.7% CYP2D6 and 64% CYP2C19 non-NMs) in a cohort of Europeans and/or New Zealand Europeans with intolerance towards the use of SSRIs or SNRIs (Maggo et al., 2019a). However, the proportions of CYP2D6 and CYP2C19 actionable genotypes reported in Hahn and Roll (2022) were only 17% and 37%, respectively (Hahn and Roll, 2022). In addition to different study designs, the lower observation is likely due to the different definitions adopted for “actionable,”, which mainly concerns the “intermediate” metabolizer phenotype. For CPIC guidelines on antidepressants, not all IM phenotypes required dosing adjustments, the recommendation criterion which defined actionability in Hahn and Roll (2022). Taking amitriptyline as an example, CYP2D6 IMs are actionable, but not CYP2C19 IMs (Hicks et al., 2017). Unlike Hahn and Roll (2022), we consider all phenotypes in our cohort, apart from NMs, to potentially predispose to the risk of untoward drug responses. With external effects such as drug-drug interactions, IMs may be just as likely as PMs to experience ADRs, when compared with NMs. A recent systematic review suggested that IMs are more susceptible to phenoconversion associated with the concurrent administration of CYPs inhibitors, than other phenotypes (Klomp et al., 2020). With this assumption, the proportions reported by our study were consistent with other literature which observed approximately 20%–60% of depressed patients receiving psychotropic prescriptions potentially discordant with their pharmacogenetic profiles. However, these studies also included other pharmacogenes (e.g., CYP1A2, CYP2C9, and CYP3A4/5) (Hall-Flavin et al., 2012; Torrellas et al., 2017).
In this retrospective cohort reporting on ADRs or ineffectiveness with the use of antidepressants for mental health disorders, the majority of cases (∼60%) had actionable genotype-predicted CYP2D6 and/or CYP2C19 genotypes. When considering the type of responses, 48% of CYP2D6/CYP2C19-antidepressant-ADRs pairs and 21% of CYP2D6/CYP2C19-antidepressant-ineffectiveness pairs with CPIC evidence levels of A, A/B, or B had actionable pharmacogenetic information. This also means that for every 10 depressed patients presenting with ADRs and/or ineffectiveness, six patients would be expected to have a non-NM phenotype for either CYP2D6 or CYP2C19 or both. Furthermore, screening of these genotypes may potentially mitigate or prevent up to half of ADRs and one-fifth of ineffectiveness. This is a proportion considered to be of clinical significance for both prescriber and the patient.
Antidepressant response is a polygenic trait and has been studied using genome-wide association analyses (Tansey et al., 2013; Pain et al., 2021). While no clear association between antidepressant response and the pharmacogenetics of CYP2D6 and CYP2C19 were observed (Hicks et al., 2015; Hicks et al., 2017; Pain et al., 2021), the impact of CYP2D6 and CYP2C19 genetic variations on the variability in the metabolism of antidepressants is well-established with strong clinical implications (Carvalho Henriques et al., 2020). An early systematic review examined the pharmacokinetic influences of CYP2D6 and CYP2C19 genotype-inferred phenotypes on 20 antidepressants, expressed as percentages of dose adjustment (Kirchheiner et al., 2004). A good concordance between studies with respect to the dosing of TCAs was observed, which suggested halving the average TCAs doses in CYP2D6 PMs. This apparent association between the genetic variants of CYP2D6 and CYP2C19 and the pharmacokinetics of TCAs may have explained the high proportion of actionable CYP2D6/CYP2C19-TCAs-response pairs, as reported in our cohort.
Our findings from this real-world case series highlights the predictive value of CYP2D6 and CYP2C19 pharmacogenetics. This is supported by literature where the genotypes of either CYP2D6 and CYP2C19 were associated with the efficacy and tolerability profile of antidepressants (Shams et al., 2006; Penas-Lledo et al., 2013; He et al., 2017; Fabbri et al., 2018; Jukic et al., 2018; Zastrozhin et al., 2021; Campos et al., 2022; Jokovic et al., 2022; Thiele et al., 2022). However, there were also negative and mixed findings reported (Brandl et al., 2014; Hodgson et al., 2015; Taranu et al., 2017; Maggo et al., 2019a). Notwithstanding this, the predictive role of CYP2D6 and CYP2C19 pharmacogenetics in antidepressant response was substantiated in recent systematic reviews and meta-analyses (Arnone et al., 2023; Brown et al., 2022; Solomon et al., 2019).
By individualizing our analysis for each reported response, this case series showed that pharmacogenetics is associated with the efficacy profile (∼20%) of antidepressants to a lesser extent than their tolerability (∼50%). Mrazek et al. (2011) investigated the role of CYP2D6 and CYP2C19 genetic variants in citalopram response, in 1,074 White non-Hispanic subjects, previously enrolled in the STAR*D trial. They reported that the CYP2C19*2 allele was significantly associated with a lower tolerability (p = 0.02), but not remission rate (p = 0.95) (Mrazek et al., 2011). Pharmacodynamic aspects such as variation of genes involved in antidepressant response (Bahramali et al., 2016; Firouzabadi et al., 2017; van Westrhenen et al., 2020), and the functional selectivity and intrinsic efficacy of a drug (Berg and Clarke, 2018), may have contributed to the heterogenous ineffectiveness phenotypes. Furthermore, commonly prescribed antidepressants such as sertraline, citalopram, escitalopram and venlafaxine have a wide therapeutic window (Marken and Munro, 2000; Hansen et al., 2017), where the wide range between minimum effective and minimum toxic concentrations makes the efficacy profile of antidepressants less susceptible to a change in the drug exposure level induced by pharmacogenetics.
For ADRs, 48% of CYP2D6/CYP2C19-antidepressant-response pairs in our cohort were actionable. In comparison, the recent PREPARE trial that showed a reduction in ADRs stemming from genotype-guided prescribing, reported that of those specific antidepressants that were prescribed to at least 10 of a total of 6,944 enrolled patients, actionable CYP2D6/CYP2C19 variants were present in 25% (nortriptyline) to 47% (venlafaxine) (Swen et al., 2023). There are two possible reasons for the lower actionability observed in the PREPARE trial. First, the PREPARE participants were patients embarking on drug therapy, whereas our cohort included patients who had already started and experienced ADRs, which are therefore a more select group. Second, the PREPARE trial adopted DPWG guidelines for all phenotyping and actionability assignment, while for our study, we worked mainly on CPIC guidelines. However, this also means that almost half of the pairs in our study were not associated with the genotypes of CYP2D6 and/or CYP2C19, highlighting the role of external factors. Campos et al. (2021) applied a polygenic risk score approach to study common ADRs reported with the use of antidepressants (e.g., weight gain, suicidality, and sexual dysfunction). The significant associations observed were antidepressant- and ADR-dependent. For example, body mass index was strongly associated with weight gain across different antidepressant drug class, while headache showed significance with the use of sertraline only. However, this study also observed a high likelihood for a participant to report the same ADRs across different antidepressants, indicating the presence of an unknown common ‘element’ which may be a result of pharmacological and genetic factors (Campos et al., 2021). To add complexity, evidence suggesting pharmacogene-dependent ADRs is emerging. Eugene (2019) extracted a total of 5,000 post-marketing SSRIs-associated ADRs cases, and by determining the drugs as CYP2D6 or CYP2C19 substrates, clustered these cases into two groups. This study observed a differential nature of ADRs exhibited by CYP2D6 and CYP2C19 SSRIs substrates, with the latter mostly associated with the modulation of autonomic nervous system (Eugene, 2019).
Apart from a patient’s pharmacogenetic profile, other aspects such as drug-drug interactions and phenoconversion should be considered during regime optimization. In a cohort of 60 patients taking antidepressants, Gloor et al. (2022) compared the intrinsic (genotypic) and observed (phenotypic) activity of six cytochrome enzymes: CYP1A2, 2B6, 2C9, 2C19, 2D6, 3A4, and 3A5 (Gloor et al., 2022). They observed a consistently lower than predicted enzymatic activity for CYP2D6, CYP2C19, and CYP2C9 (p < 8 × 10−3 for all observations). The clinical impact of phenoconversion is increasingly recognized (Cicali and Wiisanen, 2022; Mostafa et al., 2022; Nahid and Johnson, 2022), with different approaches designed to incorporate this factor for efficient genotype-to-phenotype prediction (Cicali et al., 2021). While phenoconversion is multi-factorial, the proportion of patients taking antidepressants at risk of experiencing ADRs or ineffectiveness may be higher than is expected from their genotypes, highlighting the importance of pharmacogenetic screening.
There are several limitations in the current study. First, this is a small clinical cohort (N = 52). Second, the degree of association between the genotype-inferred phenotypes, drug exposure, and drug response events, used in determining the actionability for gene-antidepressant-response pairs, remains indefinite. In addition to having a polygenic complex trait, the presentation of drug response is heterogenous, as observed with the ineffectiveness phenotypes. In this study, separate analysis for these phenotypes was limited by the small sample size. Potentially, the observed response event may partly be accounted for by other factors, which may have an impact on the association, yet, were unknown at the time of recruitment.
A third limitation concerns the selective genotyping of CYP2D6 and CYP2C19, where screening for other CYPs, transporters or pharmacodynamic pharmacogenes relevant to antidepressant response may be useful. Specifically, for CYP2C19, the selective allelic screening may have missed novel or other clinically significant variants. Fourth, healthcare practitioners (referrer) or patient (self-referral) were asked to provide medical histories, which in part, were recall dependent. Thus, the risk of recall bias remains. Fifth, there were several cases with incomplete clinical information (e.g., the specific type, dosing, and frequency of antidepressant(s) associated with the reported ADRs or ineffectiveness, and event description), thus limiting analysis. Sixth, the lack of pharmacokinetic data. Collecting and measuring drug levels from appropriate serum or plasma samples will be useful in elucidating the association between genotypes and drug exposure.
There are several suggestions for future research. First, prioritizing referral cases from healthcare practitioners for pharmacogenetic analysis. Since antidepressant response is highly subjective, this approach may ensure that the reported responses are validated against clinical practice guidelines. Second, evaluating the clinical utility of CYP2D6 and CYP2C19 pharmacogenetics via further collaboration with clinical colleagues. For example, how did the pharmacogenetic results inform their subsequent prescribing and management of the patient? Third, encouraging more complete clinical information from the referrers. Fourth, stratifying and analysing cases according to drug class to minimize case heterogeneity. However, this would require a very large sample size. In regards to antidepressant responses, a combinatorial approach may be helpful in understanding the comprehensive impacts of pharmacogenetics. For example, the metabolism of sertraline involves CYP2D6, CYP2C19, CYP2C9, CYP2B6, and CYP3A4. However, apart from CYP2D6 and CYP2C19 to a lesser extent, the functional characterization of variants of other pharmacogenes remains uncertain, thus limiting the assignment of CPIC evidence levels.
In conclusion, this retrospective cohort describes 52 mental health cases who experienced ADRs or ineffectiveness or both with antidepressants. 79 CYP2D6/CYP2C19-antidepressant-response pairs with CPIC evidence level of A, A/B, or B were identified. Using CPIC guidelines, approximately 50% ADRs-associated pairs and 20% ineffectiveness-associated pairs were actionable with the pharmacogenetics of CYP2D6 and CYP2C19. With this, we provide an insight into the clinical utility of CYP2D6 and CYP2C19 in antidepressant prescribing. It is important to note that all pharmacogenetic data is valuable, regardless of its “actionability,” as it facilitates prescribers in initiating, maintaining, or adjusting antidepressant therapy.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by New Zealand (NZ) Health and Disability Ethics Committees (HDEC URA/11/11/065). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Conception and design: PC, SM, MK, and PK. Data acquisition: PC, SM, MK, and PK. Data analysis and interpretation: PC, SM, MK, and PK. Manuscript drafting: PC, SM, MK, and PK. All authors approved the authorship list and the final version of the article.
SM, PK, and this study were supported by the Jim and Mary Carney Charitable Trust (Whangarei, New Zealand). PK was supported by a doctoral scholarship from the University of Otago, and SM was supported by the Health Research Council of New Zealand.
The authors would like to thank all the participants who volunteered their time for this study; Allison Miller for her input in data interpretation; clinical colleagues for their collaboration and support; Carney Centre for Pharmacogenomics, Otago University, Christchurch; Department of Pathology and Biomedical Science, Otago University, Christchurch; and The Nicholls Research Centre, Otago University, Christchurch.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer AG declared a shared consortium, with the author MK to the handling Editor.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1080117/full#supplementary-material
ADRs, adverse reactions; CPIC, clinical pharmacogenetics implementation consortium; CYP, Cytochrome P450; DNA, deoxyribonucleic acid; DPWG, dutch pharmacogenetics working group; IM, intermediate metabolizer; MAO-I, monoamine oxidase inhibitors; NM, normal metabolizer; Pharmvar, pharmacogene variation consortium; PCR, polymerase chain reaction; PM, poor metabolizer; RM, rapid metabolizer; SNRIs, selective norepinephrine reuptake inhibitors; SSRIs, selective serotonin reuptake inhibitors; STAR*D, sequenced treatment alternatives to relieve depression; TCAs, tricyclic antidepressants; UDRUGS, understanding adverse drug reactions using genomic sequencing; UM, ultrarapid metabolizer.
Arnone, D., Omar, O., Arora, T., Ostlundh, L., Ramaraj, R., Javaid, S., et al. (2023). Effectiveness of pharmacogenomic tests including CYP2D6 and CYP2C19 genomic variants for guiding the treatment of depressive disorders: Systematic review and meta-analysis of randomised controlled trials. Neurosci. Biobehav. Rev. 144, 104965. doi:10.1016/j.neubiorev.2022.104965
Bahramali, E., Firouzabadi, N., Yavarian, I., Shayesteh, M. R., Erfani, N., Shoushtari, A. A., et al. (2016). Influence of ACE gene on differential response to sertraline versus fluoxetine in patients with major depression: A randomized controlled trial. Eur. J. Clin. Pharmacol. 72 (9), 1059–1064. doi:10.1007/s00228-016-2079-0
Balant-Gorgia, A. E., Gex-Fabry, M., and Balant, L. P. (1991). Clinical pharmacokinetics of clomipramine. Clin. Pharmacokinet. 20 (6), 447–462. doi:10.2165/00003088-199120060-00002
Berg, K. A., and Clarke, W. P. (2018). Making sense of pharmacology: Inverse agonism and functional selectivity. Int. J. Neuropsychopharmacol. 21 (10), 962–977. doi:10.1093/ijnp/pyy071
Bousman, C. A., Arandjelovic, K., Mancuso, S. G., Eyre, H. A., and Dunlop, B. W. (2019). Pharmacogenetic tests and depressive symptom remission: A meta-analysis of randomized controlled trials. Pharmacogenomics 20 (1), 37–47. doi:10.2217/pgs-2018-0142
Bousman, C. A., Forbes, M., Jayaram, M., Eyre, H., Reynolds, C. F., Berk, M., et al. (2017). Antidepressant prescribing in the precision medicine era: A prescriber's primer on pharmacogenetic tools. BMC Psychiatry 17 (1), 60. doi:10.1186/s12888-017-1230-5
Brandl, E. J., Tiwari, A. K., Zhou, X., Deluce, J., Kennedy, J. L., Muller, D. J., et al. (2014). Influence of CYP2D6 and CYP2C19 gene variants on antidepressant response in obsessive-compulsive disorder. Pharmacogenomics J. 14 (2), 176–181. doi:10.1038/tpj.2013.12
Braund, T. A., Tillman, G., Palmer, D. M., Gordon, E., Rush, A. J., and Harris, A. W. F. (2021). Antidepressant side effects and their impact on treatment outcome in people with major depressive disorder: An iSPOT-D report. Transl. Psychiatry 11 (1), 417. doi:10.1038/s41398-021-01533-1
Brown, J. T., Schneiderhan, M., Eum, S., and Bishop, J. R. (2017). Serum clomipramine and desmethylclomipramine levels in a CYP2C19 and CYP2D6 intermediate metabolizer. Pharmacogenomics 18 (7), 601–605. doi:10.2217/pgs-2017-0015
Brown, L. C., Stanton, J. D., Bharthi, K., Maruf, A., Muller, D. J., and Bousman, C. A. (2022). Pharmacogenomic testing and depressive symptom remission: A systematic review and meta-analysis of prospective, controlled clinical trials. Clin. Pharmacol. Ther. 112, 1303–1317. doi:10.1002/cpt.2748
Brown, L., Vranjkovic, O., Li, J., Yu, K., Al Habbab, T., Johnson, H., et al. (2020). The clinical utility of combinatorial pharmacogenomic testing for patients with depression: A meta-analysis. Pharmacogenomics 21 (8), 559–569. doi:10.2217/pgs-2019-0157
Campos, A. I., Byrne, E. M., Mitchell, B. L., Wray, N. R., Lind, P. A., Licinio, J., et al. (2022). Impact of CYP2C19 metaboliser status on SSRI response: A retrospective study of 9500 participants of the Australian genetics of depression study. Pharmacogenomics J. 22, 130–135. doi:10.1038/s41397-022-00267-7
Campos, A. I., Mulcahy, A., Thorp, J. G., Wray, N. R., Byrne, E. M., Lind, P. A., et al. (2021). Understanding genetic risk factors for common side effects of antidepressant medications. Commun. Med. 1 (1), 45. doi:10.1038/s43856-021-00046-8
Carvalho Henriques, B., Yang, E. H., Lapetina, D., Carr, M. S., Yavorskyy, V., Hague, J., et al. (2020). How can drug metabolism and transporter genetics inform psychotropic prescribing? Front. Genet. 11, 491895. doi:10.3389/fgene.2020.491895
Caudle, K. E., Sangkuhl, K., Whirl-Carrillo, M., Swen, J. J., Haidar, C. E., Klein, T. E., et al. (2020). Standardizing CYP2D6 genotype to phenotype translation: Consensus recommendations from the clinical pharmacogenetics implementation consortium and Dutch pharmacogenetics working group. Clin. Transl. Sci. 13 (1), 116–124. doi:10.1111/cts.12692
Cicali, E. J., Elchynski, A. L., Cook, K. J., Houder, J. T., Thomas, C. D., Smith, D. M., et al. (2021). How to integrate CYP2D6 phenoconversion into clinical pharmacogenetics: A tutorial. Clin. Pharmacol. Ther. 110 (3), 677–687. doi:10.1002/cpt.2354
Cicali, E. J., and Wiisanen, K. (2022). The importance of phenoconversion when using the CYP2D6 genotype in clinical practice. Pharmacogenomics 23 (14), 749–752. doi:10.2217/pgs-2022-0087
Cipriani, A., Furukawa, T. A., Salanti, G., Chaimani, A., Atkinson, L. Z., Ogawa, Y., et al. (2018a). Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. Lancet 391 (10128), 1357–1366. doi:10.1016/S0140-6736(17)32802-7
Cipriani, A., Salanti, G., Furukawa, T. A., Egger, M., Leucht, S., Ruhe, H. G., et al. (2018b). Antidepressants might work for people with major depression: Where do we go from here? Lancet Psychiatry 5 (6), 461–463. doi:10.1016/S2215-0366(18)30133-0
Clinical Pharmacogenetics Implementation Consortium (2021a). Genes-drugs. Retrieved from: https://cpicpgx.org/genes-drugs/.
Clinical Pharmacogenetics Implementation Consortium (2021b). Prioritization. Retrieved from: https://cpicpgx.org/prioritization/#flowchart.
Crews, K. R., Monte, A. A., Huddart, R., Caudle, K. E., Kharasch, E. D., Gaedigk, A., et al. (2021). Clinical pharmacogenetics implementation consortium guideline for CYP2D6, OPRM1, and COMT genotypes and select opioid therapy. Clin. Pharmacol. Ther. 110 (4), 888–896. doi:10.1002/cpt.2149
Dean, A. G., Sullivan, K. M., and Soe, M. M. (2013). OpenEpi: Open source epidemiologic statistics for public health. Retrieved from: https://www.openepi.com/Menu/OE_Menu.htm.
Eugene, A. R. (2019). Optimizing drug selection in psychopharmacology based on 40 significant CYP2C19- and CYP2D6-biased adverse drug reactions of selective serotonin reuptake inhibitors. PeerJ 7, e7860. doi:10.7717/peerj.7860
Fabbri, C., Tansey, K. E., Perlis, R. H., Hauser, J., Henigsberg, N., Maier, W., et al. (2018). Effect of cytochrome CYP2C19 metabolizing activity on antidepressant response and side effects: Meta-analysis of data from genome-wide association studies. Eur. Neuropsychopharmacol. 28 (8), 945–954. doi:10.1016/j.euroneuro.2018.05.009
Firouzabadi, N., Raeesi, R., Zomorrodian, K., Bahramali, E., and Yavarian, I. (2017). Beta adrenoceptor polymorphism and clinical response to sertraline in major depressive patients. J. Pharm. Pharm. Sci. 20, 1–7. doi:10.18433/J3W31F
Gaedigk, A., Casey, S. T., Whirl-Carrillo, M., Miller, N. A., and Klein, T. E. (2021). Pharmacogene variation consortium: A global resource and repository for pharmacogene variation. Clin. Pharmacol. Ther. 110 (3), 542–545. doi:10.1002/cpt.2321
Gaedigk, A., Ingelman-Sundberg, M., Miller, N. A., Leeder, J. S., Whirl-Carrillo, M., Klein, T. E., et al. (2018). The pharmacogene variation (PharmVar) consortium: Incorporation of the human cytochrome P450 (CYP) allele nomenclature database. Clin. Pharmacol. Ther. 103 (3), 399–401. doi:10.1002/cpt.910
Gillman, P. K. (2007). Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br. J. Pharmacol. 151 (6), 737–748. doi:10.1038/sj.bjp.0707253
Gloor, Y., Lloret-Linares, C., Bosilkovska, M., Perroud, N., Richard-Lepouriel, H., Aubry, J. M., et al. (2022). Drug metabolic enzyme genotype-phenotype discrepancy: High phenoconversion rate in patients treated with antidepressants. Biomed. Pharmacother. 152, 113202. doi:10.1016/j.biopha.2022.113202
Hahn, M., and Roll, S. C. (2022). A collaborative approach in pharmacogenetic testing: Actionable genotypes of antidepressants and their avoidance in a retrospective study. J. Explor. Res. Pharmacol. doi:10.14218/JERP.2022.00054
Hall-Flavin, D. K., Winner, J. G., Allen, J. D., Jordan, J. J., Nesheim, R. S., Snyder, K. A., et al. (2012). Using a pharmacogenomic algorithm to guide the treatment of depression. Transl. Psychiatry 2, e172. doi:10.1038/tp.2012.99
Hansen, M. R., Kuhlmann, I. B., Pottegard, A., and Damkier, P. (2017). Therapeutic drug monitoring of venlafaxine in an everyday clinical setting: Analysis of age, sex and dose concentration relationships. Basic Clin. Pharmacol. Toxicol. 121 (4), 298–302. doi:10.1111/bcpt.12796
He, Q., Yuan, Z., Liu, Y., Zhang, J., Yan, H., Shen, L., et al. (2017). Correlation between cytochrome P450 2C19 genetic polymorphism and treatment response to escitalopram in panic disorder. Pharmacogenetics Genomics 27 (8), 279–284. doi:10.1097/FPC.0000000000000290
Hicks, J. K., Bishop, J. R., Sangkuhl, K., Muller, D. J., Ji, Y., Leckband, S. G., et al. (2015). Clinical pharmacogenetics implementation, CClinical pharmacogenetics implementation consortium (CPIC) guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin. Pharmacol. Ther. 98 (2), 127–134. doi:10.1002/cpt.147
Hicks, J. K., Sangkuhl, K., Swen, J. J., Ellingrod, V. L., Muller, D. J., Shimoda, K., et al. (2017). Clinical pharmacogenetics implementation consortium guideline (CPIC) for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants: 2016 update. Clin. Pharmacol. Ther. 102 (1), 37–44. doi:10.1002/cpt.597
Hitchman, L. M., Faatoese, A., Merriman, T. R., Miller, A. L., Liau, Y., Graham, O. E. E., et al. (2022). Allelic diversity of the pharmacogene CYP2D6 in New Zealand Māori and Pacific peoples. Front. Genet. 13, 1016416. doi:10.3389/fgene.2022.1016416
Hodgson, K., Tansey, K. E., Uher, R., Dernovsek, M. Z., Mors, O., Hauser, J., et al. (2015). Exploring the role of drug-metabolising enzymes in antidepressant side effects. Psychopharmacol. Berl. 232 (14), 2609–2617. doi:10.1007/s00213-015-3898-x
Jokovic, D., Milosavljevic, F., Stojanovic, Z., Supic, G., Vojvodic, D., Uzelac, B., et al. (2022). CYP2C19 slow metabolizer phenotype is associated with lower antidepressant efficacy and tolerability. Psychiatry Res. 312, 114535. doi:10.1016/j.psychres.2022.114535
Jukic, M. M., Haslemo, T., Molden, E., and Ingelman-Sundberg, M. (2018). Impact of CYP2C19 genotype on escitalopram exposure and therapeutic failure: A retrospective study based on 2,087 patients. Am. J. Psychiatry 175 (5), 463–470. doi:10.1176/appi.ajp.2017.17050550
Kee, P. S., Maggo, S. D. S., Kennedy, M. A., Barclay, M. L., Miller, A. L., Lehnert, K., et al. (2022). Omeprazole treatment failure in gastroesophageal reflux disease and genetic variation at the CYP2C locus. Front. Genet. 13, 869160. doi:10.3389/fgene.2022.869160
Kirchheiner, J., Nickchen, K., Bauer, M., Wong, M. L., Licinio, J., Roots, I., et al. (2004). Pharmacogenetics of antidepressants and antipsychotics: The contribution of allelic variations to the phenotype of drug response. Mol. Psychiatry 9 (5), 442–473. doi:10.1038/sj.mp.4001494
Klomp, S. D., Manson, M. L., Guchelaar, H. J., and Swen, J. J. (2020). Phenoconversion of cytochrome P450 metabolism: A systematic review. J. Clin. Med. 9 (9), 2890. doi:10.3390/jcm9092890
Lima, J. J., Thomas, C. D., Barbarino, J., Desta, Z., Van Driest, S. L., El Rouby, N., et al. (2021). Clinical pharmacogenetics implementation consortium (CPIC) guideline for CYP2C19 and proton pump inhibitor dosing. Clin. Pharmacol. Ther. 109 (6), 1417–1423. doi:10.1002/cpt.2015
Lobello, K. W., Preskorn, S. H., Guico-Pabia, C. J., Jiang, Q., Paul, J., Nichols, A. I., et al. (2010). Cytochrome P450 2D6 phenotype predicts antidepressant efficacy of venlafaxine: A secondary analysis of 4 studies in major depressive disorder. J. Clin. Psychiatry 71 (11), 1482–1487. doi:10.4088/JCP.08m04773blu
Lunenburg, C., Thirstrup, J. P., Bybjerg-Grauholm, J., Baekvad-Hansen, M., Hougaard, D. M., Nordentoft, M., et al. (2021). Pharmacogenetic genotype and phenotype frequencies in a large Danish population-based case-cohort sample. Transl. Psychiatry 11 (1), 294. doi:10.1038/s41398-021-01417-4
Maggo, S. D., Chua, E. W., Chin, P., Cree, S., Pearson, J., Doogue, M., et al. (2017). A New Zealand platform to enable genetic investigation of adverse drug reactions. N. Z. Med. J. 130 (1466), 62–69.
Maggo, S., Kennedy, M. A., Barczyk, Z. A., Miller, A. L., Rucklidge, J. J., Mulder, R. T., et al. (2019a). Common CYP2D6, CYP2C9, and CYP2C19 gene variants, health anxiety, and neuroticism are not associated with self-reported antidepressant side effects. Front. Genet. 10, 1199. doi:10.3389/fgene.2019.01199
Maggo, S., Sycamore, K., Miller, A., and Kennedy, M. (2019b). The three ps: Psychiatry, pharmacy, and pharmacogenomics, a brief report from New Zealand. Front. psychiatry 10, 690. doi:10.3389/fpsyt.2019.00690
Marasine, N. R., and Sankhi, S. (2021). Factors associated with antidepressant medication non-adherence. Turk J. Pharm. Sci. 18 (2), 242–249. doi:10.4274/tjps.galenos.2020.49799
Marken, P. A., and Munro, J. S. (2000). Selecting a selective serotonin reuptake inhibitor: Clinically important distinguishing features. Prim. Care Companion J. Clin. Psychiatry 2 (6), 205–210. doi:10.4088/pcc.v02n0602
Miller, S. A., Dykes, D. D., and Polesky, H. F. (1988). A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic acids Res. 16 (3), 1215. doi:10.1093/nar/16.3.1215
Morris, S. A., Alsaidi, A. T., Verbyla, A., Cruz, A., Macfarlane, C., Bauer, J., et al. (2022). Cost effectiveness of pharmacogenetic testing for drugs with clinical pharmacogenetics implementation consortium (CPIC) guidelines: A systematic review. Clin. Pharmacol. Ther. 112, 1318–1328. doi:10.1002/cpt.2754
Mostafa, S., Polasek, T. M., Bousman, C. A., Mueller, D. J., Sheffield, L. J., Rembach, J., et al. (2022). Pharmacogenomics in psychiatry - the challenge of cytochrome P450 enzyme phenoconversion and solutions to assist precision dosing. Pharmacogenomics 23 (15), 857–867. doi:10.2217/pgs-2022-0104
Mrazek, D. A., Biernacka, J. M., O'Kane, D. J., Black, J. L., Cunningham, J. M., Drews, M. S., et al. (2011). CYP2C19 variation and citalopram response. Pharmacogenet Genomics 21 (1), 1–9. doi:10.1097/fpc.0b013e328340bc5a
Nahid, N. A., and Johnson, J. A. (2022). CYP2D6 pharmacogenetics and phenoconversion in personalized medicine. Expert Opin. Drug Metab. Toxicol. 18 (11), 769–785. doi:10.1080/17425255.2022.2160317
Nielsen, K. K., Brosen, K., Hansen, M. G., and Gram, L. F. (1994). Single-dose kinetics of clomipramine: Relationship to the sparteine and S-mephenytoin oxidation polymorphisms. Clin. Pharmacol. Ther. 55 (5), 518–527. doi:10.1038/clpt.1994.65
Otton, S. V., Ball, S. E., Cheung, S. W., Inaba, T., Rudolph, R. L., and Sellers, E. M. (1996). Venlafaxine oxidation in vitro is catalysed by CYP2D6. Br. J. Clin. Pharmacol. 41 (2), 149–156. doi:10.1111/j.1365-2125.1996.tb00173.x
Pain, O., Hodgson, K., Trubetskoy, V., Ripke, S., Marshe, V. S., Adams, M. J., et al. (2021). Identifying the common genetic basis of antidepressant response. Biol. Psychiatry 2, 115–126. doi:10.1016/j.bpsgos.2021.07.008
Penas-Lledo, E., Trejo, H., Dorado, P., Ortega, A., Jung, H., Alonso, E., et al. (2013). CYP2D6 ultrarapid metabolism and early dropout from fluoxetine or amitriptyline monotherapy treatment in major depressive patients. Mol. Psychiatry 18 (1), 8–9. doi:10.1038/mp.2012.91
Pratt, V. M., Del Tredici, A. L., Hachad, H., Ji, Y., Kalman, L. V., Scott, S. A., et al. (2018). Recommendations for clinical CYP2C19 genotyping allele selection: A report of the association for molecular Pathology. J. Mol. Diagn 20 (3), 269–276. doi:10.1016/j.jmoldx.2018.01.011
Rosenblat, J. D., Lee, Y., and McIntyre, R. S. (2018). The effect of pharmacogenomic testing on response and remission rates in the acute treatment of major depressive disorder: A meta-analysis. J. Affect Disord. 241, 484–491. doi:10.1016/j.jad.2018.08.056
Shams, M., Arneth, B., Hiemke, C., Dragicevic, A., Müller, M., Kaiser, R., et al. (2006). CYP2D6 polymorphism and clinical effect of the antidepressant venlafaxine. J. Clin. Pharm. Ther. 31 (5), 493–502. doi:10.1111/j.1365-2710.2006.00763.x
Solomon, H. V., Cates, K. W., and Li, K. J. (2019). Does obtaining CYP2D6 and CYP2C19 pharmacogenetic testing predict antidepressant response or adverse drug reactions? Psychiatry Res. 271, 604–613. doi:10.1016/j.psychres.2018.12.053
Stephan, P. L., Jaquenoud Sirot, E., Mueller, B., Eap, C. B., and Baumann, P. (2006). Adverse drug reactions following nonresponse in a depressed patient with CYP2D6 deficiency and low CYP 3A4/5 activity. Pharmacopsychiatry 39 (4), 150–152. doi:10.1055/s-2006-946705
Swen, J. J., van der Wouden, C. H., Manson, L. E., Abdullah-Koolmees, H., Blagec, K., Blagus, T., et al. (2023). A 12-gene pharmacogenetic panel to prevent adverse drug reactions: An open-label, multicentre, controlled, cluster-randomised crossover implementation study. Lancet 401 (10374), 347–356. doi:10.1016/S0140-6736(22)01841-4
Tansey, K. E., Guipponi, M., Hu, X., Domenici, E., Lewis, G., Malafosse, A., et al. (2013). Contribution of common genetic variants to antidepressant response. Biol. Psychiatry 73 (7), 679–682. doi:10.1016/j.biopsych.2012.10.030
Taranu, A., Colle, R., Gressier, F., El Asmar, K., Becquemont, L., Corruble, E., et al. (2017). Should a routine genotyping of CYP2D6 and CYP2C19 genetic polymorphisms be recommended to predict venlafaxine efficacy in depressed patients treated in psychiatric settings? Pharmacogenomics 18 (7), 639–650. doi:10.2217/pgs-2017-0003
Thiele, L. S., Ishtiak-Ahmed, K., Thirstrup, J. P., Agerbo, E., Lunenburg, C., Muller, D. J., et al. (2022). Clinical impact of functional CYP2C19 and CYP2D6 gene variants on treatment with antidepressants in young people with depression: A Danish cohort study. Pharm. (Basel) 15 (7), 870. doi:10.3390/ph15070870
Torrellas, C., Carril, J. C., and Cacabelos, R. (2017). Optimization of antidepressant use with pharmacogenetic strategies. Curr. Genomics 18 (5), 442–449. doi:10.2174/1389202918666170426164940
van Westrhenen, R., Aitchison, K. J., Ingelman-Sundberg, M., and Jukic, M. M. (2020). Pharmacogenomics of antidepressant and antipsychotic treatment: How far have we got and where are we going? Front. Psychiatry 11, 94. doi:10.3389/fpsyt.2020.00094
Zastrozhin, M. S., Skryabin, V. Y., Petukhov, A. E., Torrado, M. V., Pankratenko, E. P., Zastrozhina, A. K., et al. (2021). Effects of CYP2C19 genetic polymorphism on the steady-state concentration of citalopram in patients with major depressive disorder. Pharmacogenomics J. 21 (4), 435–439. doi:10.1038/s41397-021-00219-7
Keywords: antidepressant, Adverse drug reaction, drug response, CYP2D6, CYP2C19, Psychiatry, clinical utility, pharmacogenetics
Citation: Kee PS, Maggo SDS, Kennedy MA and Chin PKL (2023) The pharmacogenetics of CYP2D6 and CYP2C19 in a case series of antidepressant responses. Front. Pharmacol. 14:1080117. doi: 10.3389/fphar.2023.1080117
Received: 25 October 2022; Accepted: 08 February 2023;
Published: 21 February 2023.
Edited by:
Natalia Denisenko, Russian Medical Academy of Postgraduate Education, RussiaReviewed by:
Negar Firouzabadi, Shiraz University of Medical Sciences, IranCopyright © 2023 Kee, Maggo, Kennedy and Chin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paul K. L. Chin, cGF1bC5jaGluQG90YWdvLmFjLm56
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.