- Department of Transplantation Surgery, The Affiliated Hospital of Guizhou Medical University, Guiyang, Guizhou, China
Background: A systematic review and meta-analysis was performed to investigate the efficacy and safety of isoniazid (INH) prophylaxis to prevent tuberculosis (TB) infection in kidney transplant recipients (KTRs).
Methods: Web of Science, SCOPUS, and PubMed were searched to identify relevant studies that compared the effects among patients who received INH prophylaxis after transplantation.
Results: A total of 13 studies (involving 6,547 KTRs) were included in our analysis. We found that the risk of active TB infection (RR: 0.35, 95%CI 0.27–0.45, p < 0.01) for KTRs was lower in the INH treatment group than in those without prophylaxis. However, there was no significant difference between the two groups in mortality (RR: 0.93, 95%CI 0.67–1.28, p = 0.64), acute rejection (RR: 0.82, 95%CI 0.44–1.51, p = 0.52), and hepatotoxicity (RR: 1.25, 95%CI 0.94–1.65, p = 0.12).
Conclusion: Isoniazid prophylaxis is a safe and effective for KTRs on reactivation of latent TB infection.
Introduction
Kidney transplantation (KT) is the most important replacement therapy for patients with end-stage kidney disease (ESKD) (Lamb et al., 2011); it can improve the prognosis and life quality of ESKD patients (Park et al., 2020). With the wide use of immunosuppressive drugs, the survival rates of patients and grafts have improved remarkably (Singh et al., 2016; Hosohata et al., 2018), while opportunistic infections caused by excessive immunosuppression have also increased (Fang et al., 2021).
Tuberculosis (TB) has been a serious infectious disease in solid organ transplantation (SOT) (Roth et al., 2016; Clemente et al., 2018; Burguet et al., 2022). It is reported that the incidence of TB in SOT recipients ranges from 0.56% to 2.61% (Reis-Santos et al., 2013), which is 20 to 74 times higher than in the normal population (Subramanian and Dorman, 2009; Epstein and Subramanian, 2018). The mortality rate of TB infection after transplantation is 31% (Horne et al., 2013; Baker et al., 2017; Majeed et al., 2018). Due to atypical clinical symptoms, diagnosis may be delayed in some patients with active TB infection (Yi and Cheng, 2020). In addition, the interaction between anti-TB drugs and immunosuppressants makes diagnosis and treatment more difficult. Most active TB infections are considered to develop from the reactivation of latent tuberculosis infection (LTBI) after transplantation (Abad and Razonable, 2018; Sasi et al., 2020). Therefore, some experts have suggested that a TB prophylaxis strategy should be used in SOT recipients to reduce the incidence of post-transplant active TB infection (Naqvi et al., 2010; Adamu et al., 2014; Dodani et al., 2021). Some transplant centers use isoniazid (INH) prophylaxis in SOT recipients based on clinical experience, but this has been controversial, with side effects such as affected liver functioning and low medication compliance. Although some analogous meta-analyses have all been performed on clinical studies (Currie et al., 2010; Adamu et al., 2014), there is still a lack of comprehensive and accurate meta-analysis of INH prophylaxis among such patients. Thus, we conducted a systematic review and meta-analysis of relevant studies to evaluate the safety and efficacy of the INH prophylaxis strategy in KTRs.
Materials and methods
Our systematic review was conducted and reported according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines (Moher et al., 2009), presented in Supplementary Table S1.
Search strategy
Web of Science, SCOPUS, and PubMed were searched for all relevant studies up to 10 June 2022 (the latest search date) using logical combinations of relevant keywords: “kidney/renal transplant/allograft, tuberculosis”. The full text of the search terms for each database is presented in Supplementary Table S2. In addition, eligible references from relevant studies were also searched. All articles identified by this search strategy were evaluated by two independent reviewers (ZY and SC) according to their title, abstract, and full text to determine the final included studies. Studies about adult KTRs receiving INH prophylaxis for TB were included. When one study was reported many times, the study with a long follow-up period and complete case report was identified as the primary data source.
Outcome measures
The primary outcomes in this review were active TB infections after transplantation and all-cause mortality. The secondary outcomes were hepatotoxicity and acute rejection (AR).
Data extraction
Two investigators (ZY and CS) independently identified eligible studies by assessing the title, abstract, and full text of all studies. Data extraction was then independently performed by two reviewers (ZY and CS) according to the following items: study design, participant characteristics, interventions, and outcomes. Any data discrepancies were resolved by the whole team, and missing information was requested from study authors or sponsors.
Quality assessment and statistical analyses
We used Cochrane’s risk of bias to assess the methodological quality of all included studies (Higgins et al., 2011). The fixed effect model or random effect model was selected in a meta-analysis, depending on the value of heterogeneity. Heterogeneity across the studies was assessed using Cochrane’s Q (p < 0.1) and I2 statistics (I2 >50%). I2 values of 25%, 50%, and 75% corresponded to low, medium, and high levels of heterogeneity, respectively. If there was a value of more than 75% regarding heterogeneity (Higgins et al., 2003), a sensitivity analysis, subgroup analysis, or meta-regression analysis was performed to explore possible sources of heterogeneity. Data were pooled using the mean difference (MD) as the effect estimate, and the binary outcomes were presented as risk ratios (RRs) with 95% confidence intervals (CIs); p < 0.05 was considered statistically significant. All analyses were performed in R (version 4.1.1, R Project for Statistical Computing).
Results
Characteristics and quality assessments of included studies
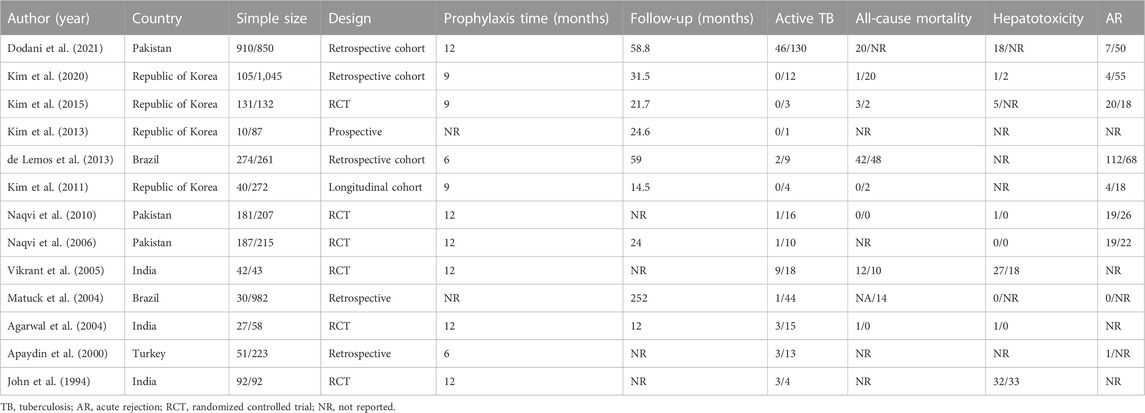
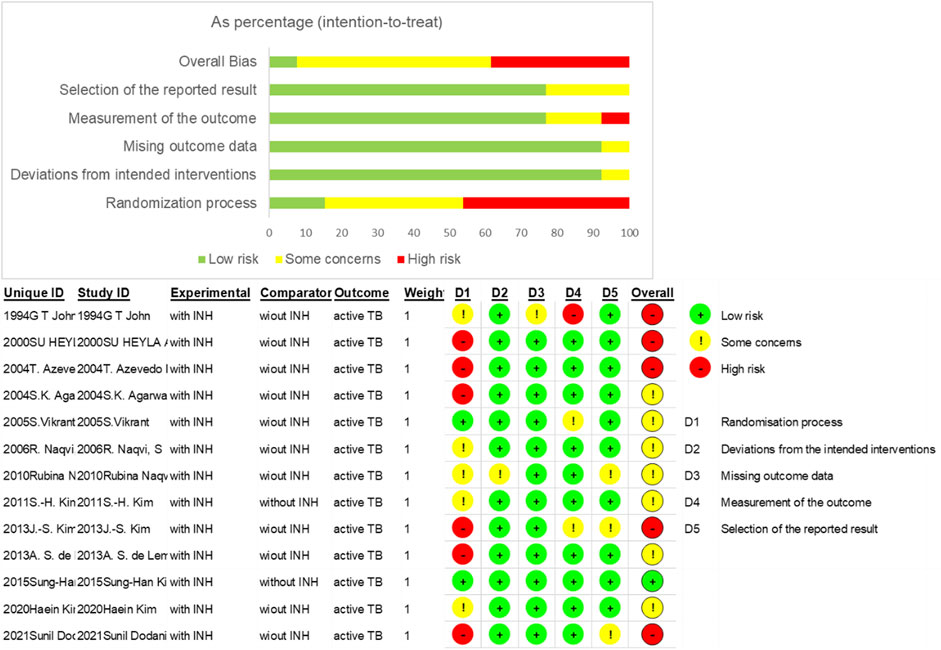
Some 1,818 potentially relevant articles were identified (Figure 1), of which 1,665 duplicates and irrelevant studies were removed. After screening titles and abstracts, 153 full-text articles were assessed. Finally, 13 studies (which included 6,547 patients) met the inclusion criteria (John et al., 1994; Apaydin et al., 2000; Agarwal et al., 2004; Matuck et al., 2004; Vikrant et al., 2005; Naqvi et al., 2006; Naqvi et al., 2010; Kim et al., 2011; de Lemos et al., 2013; Kim et al., 2013; Kim et al., 2015; Kim et al., 2020; Dodani et al., 2021). The basic characteristics of these 13 studies are shown in Table 1. Four studies were from the Republic of Korea (Kim et al., 2011; Kim et al., 2013; Kim et al., 2015; Kim et al., 2020), three each from India (John et al., 1994; Agarwal et al., 2004; Vikrant et al., 2005) and Pakistan (Naqvi et al., 2006; Naqvi et al., 2010; Dodani et al., 2021), two from Brazil (Matuck et al., 2004; de Lemos et al., 2013), and one study was from Turkey (Apaydin et al., 2000). The sample size of the studies ranged from 85 (Vikrant et al., 2005) to 1,760 (Dodani et al., 2021). Nine studies reported mean follow-up periods ranging from 12 to 252 months, while the other studies did not clarify the follow-up time. Of these nine studies, six were randomized controlled trials (RCTs) (John et al., 1994; Agarwal et al., 2004; Vikrant et al., 2005; Naqvi et al., 2006; Naqvi et al., 2010; Kim et al., 2015), four were cohort studies (Kim et al., 2011; de Lemos et al., 2013; Kim et al., 2020; Dodani et al., 2021), two were retrospective studies (Apaydin et al., 2000; Matuck et al., 2004), and one was a prospective study (Kim et al., 2013). Most of the included studies reported an INH prevention time of more than 9 months, the longest being 12 months. The quality of the 13 eligible studies assessed by Cochrane’s collaboration tool is shown in Figure 2. A total of six studies were RCTs, and only three studies detailed the process of random sequence generation. The eligible studies had a moderate risk of bias.
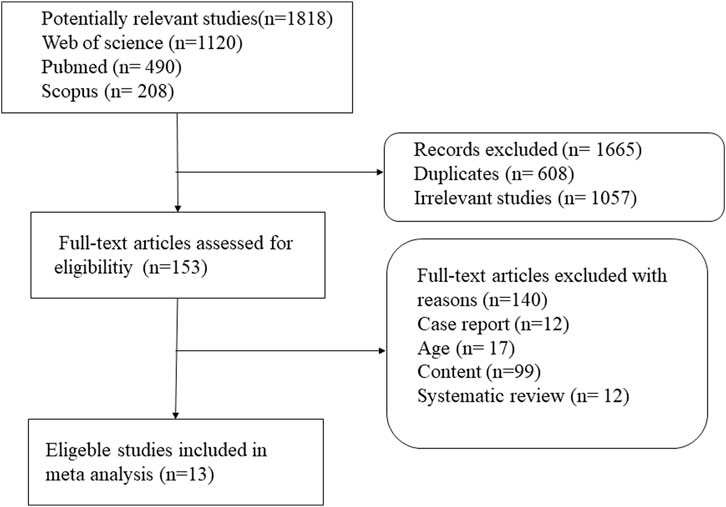
FIGURE 1. Procedure for search and selection of studies included in the systematic review and meta-analysis.
Primary outcomes
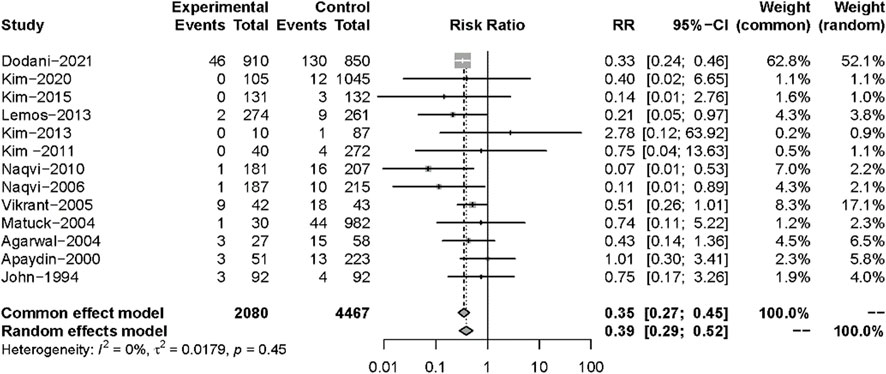
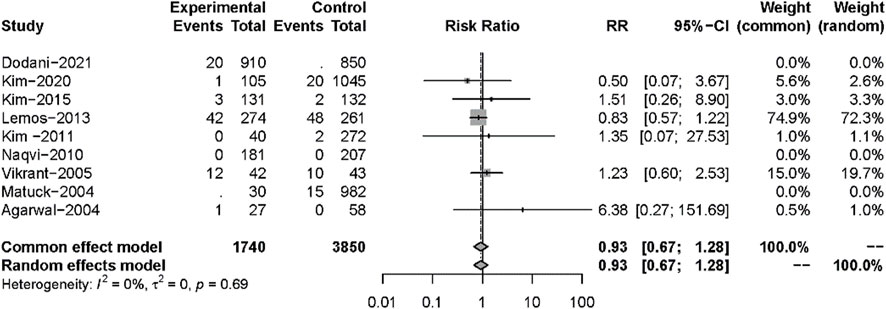
All 13 studies reported TB infections. The INH prophylaxis group had a lower risk of infection than the non-INH prophylaxis group (RR: 0.35, 95%CI 0.27–0.45, p < 0.01) (Figure 3), with no heterogeneity among these studies (I2 = 0%, p = 0.45). Nine studies reported the results of all-cause mortality (Figure 4), and no significant difference was found between the two groups (RR: 0.93, 95%CI 0.67–1.28, p = 0.64), with no heterogeneity among the nine studies (I2 = 0%, p = 0.69).
Secondary endpoints
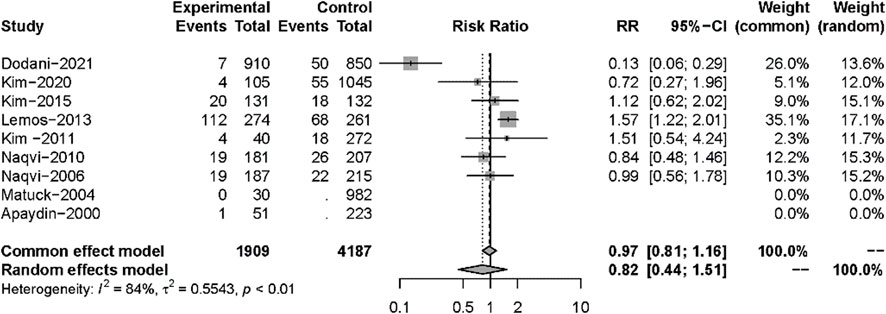
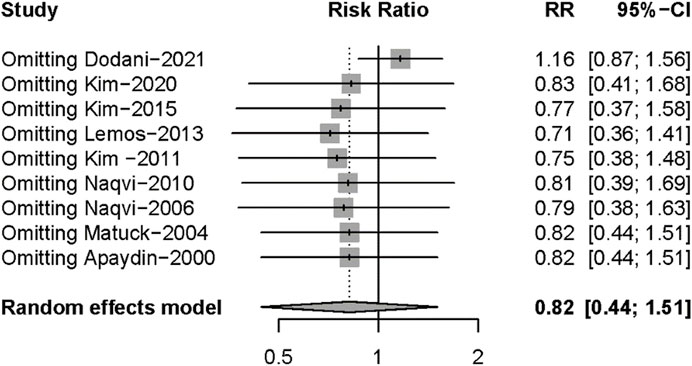
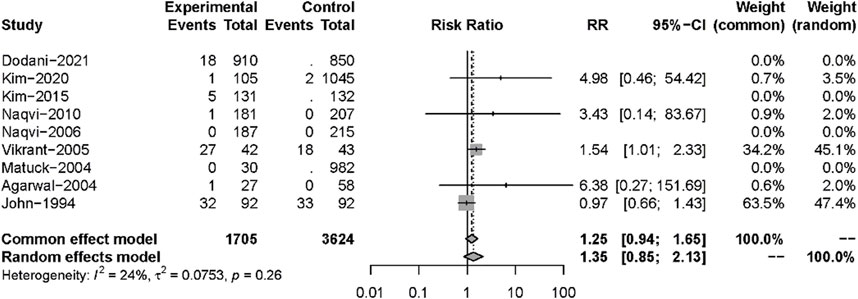
Nine studies described posttransplant ARs. Despite there being no significant difference in both groups (RR: 0.82, 95%CI 0.44–1.51, p = 0.52), significant heterogeneity was found in these studies (I2 = 84%; p < 0.01) (Figure 5). Additional sensitivity analyses to evaluate the variance found that the work of Dodani et al. (2021)might be responsible for the source of heterogeneity (Figure 6). We failed to find any difference in the subgroup analysis of ARs in Supplementary Figure S1, and we found no difference in these studies by subgroup analysis of intervention and observational studies (Supplementary Figure S2). There was also no difference in hepatotoxicity between the two groups (RR: 1.25, 95%CI 0.94–1.65, p = 0.12), and no significant heterogeneity was found among these studies (I2 = 24%, p = 0.26) (Figure 7).
Discussion
This meta-analysis (involving 6,547 KTRs) found that there was a significantly lower risk of active TB infection among KTRs who received INH prophylaxis. However, there were no significant differences between the groups on mortality and hepatotoxicity. Furthermore, ARs were not significantly different between the two groups, and the corresponding sensitive analysis showed no statistically significant difference. Thus, the application of INH is a safe and effective strategy for preventing TB infection after KT.
TB is one of the most common infections with negative impact post-transplantation (Karuthu and Blumberg, 2012). As KT is correlated with an immunosuppression status, the morbidity of active TB is obviously higher in KT recipients than in the general population (Torre-Cisneros et al., 2009; Kwon et al., 2021). It is reported that mortality can reach 60% (Lezaic et al., 2001), and graft rejection can reach 55.6% (el-Agroudy et al., 2003) in KT recipients with TB. Apaydin et al. (2000) found no statistical difference in the development of active TB after kidney transplantation in the INH treatment group compared with non-INH. In contrast, Dodani et al. (2021) conducted a study on primary INH prophylaxis in renal transplant recipients and found that the incidence of active TB decreased in the first two years. In addition, a systematic review of renal transplant recipients with TB suggested that INH prophylaxis was less likely to develop active TB compared with those who did not receive this treatment (Adamu et al., 2014). However, many transplant centers do not routinely use INH prophylaxis because of an increased risk of liver toxicity after INH treatment (Antony et al., 1997). Our study showed that INH prophylaxis can be beneficial in reducing the risk of TB infection and has no difference in side effects. Vikrant et al. (2005) explained that viral hepatitis was very common during dialysis, and that hepatotoxicity caused by viral hepatitis was sometimes difficult to distinguish from hepatotoxicity caused by INH. Meanwhile, the American Thoracic Society recommended that INH should be stopped only when liver enzyme levels increased three to five times in symptomatic patients (Van Stralen et al., 2013). Therefore, we still need to routinely monitor liver function based on these findings.
Our study had several limitations. First, there were differences in study design (six RCTs, four cohort studies, two retrospective studies, and one prospective study), leading to inherent bias. Second, some studies provided incomplete data, preventing a comprehensive review. Third, the diagnosis of latent TB was not the gold standard. Tuberculin skin test (TST) had limited sensitivity in renal failure patients, and the validity of interferon-gamma release assay was still uncertain in the immunocompromised population. However, INH prophylaxis therapy has been empirically initiated in some studies. Fourth, the outcomes of these studies may be influenced by many potential confounders. Fifth, the follow-up time in this study differed greatly from 12 months to 252 months. Finally, the prophylaxis period was not uniform, from 6 to 12 months, which may require new clinical studies for different prevention times to confirm its safety and effectiveness.
In conclusion, this meta-analysis revealed that INH prophylaxis could significantly reduce the risk of TB development in KTRs. Nevertheless, the available evidence is not robust and a large multicenter randomized trial is needed to evaluate the efficacy and safety of INH prophylaxis in KTRs (Bishai and Chaisson, 1997, Muñoz et al., 2005, Riella, 2018, Samavat et al., 2021, Aguado et al., 2009).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author contributions
ZY designed the study and wrote the manuscript. SC performed the meta-analysis. YX checked the results. YN designed the research strategy and revised the manuscript. All authors reviewed the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1022579/full#supplementary-material
Abbreviations
INH, isoniazid; KTRs, kidney transplant recipients; RR, risk ratios; AR, acute rejection; KT, kidney transplantation; ESKD, end-stage kidney disease; TB, tuberculosis; SOT, solid organ transplantation; LTBI, latent tuberculosis infection; MD, mean difference; CI, confidence interval; RCTs, randomized controlled trials; CYP3A4, cytochrome P450; TST, tuberculin skin test.
References
Abad, C. L. R., and Razonable, R. R. (2018). Mycobacterium tuberculosis after solid organ transplantation: A review of more than 2000 cases. Clin. Transpl. 32 (6), e13259. doi:10.1111/ctr.13259
Adamu, B., Abdu, A., Abba, A. A., Borodo, M. M., and Tleyjeh, I. M. (2014). Antibiotic prophylaxis for preventing post solid organ transplant tuberculosis. Cochrane Database Syst. Rev. 2014(3), Cd008597. doi:10.1002/14651858.CD008597.pub2
Agarwal, S. K., Gupta, S., Dash, S. C., Bhowmik, D., and Tiwari, S. C. (2004). Prospective randomised trial of isoniazid prophylaxis in renal transplant recipient. Int. Urol. Nephrol. 36 (3), 425–431. doi:10.1007/s11255-004-6251-6
Aguado, J. M., Torre-Cisneros, J., Fortún, J., Benito, N., Meije, Y., Doblas, A., et al. (2009). Tuberculosis in solid-organ transplant recipients: Consensus statement of the group for the study of infection in transplant recipients (GESITRA) of the Spanish society of infectious diseases and clinical microbiology. Clin. Infect. Dis. 48 (9), 1276–1284. doi:10.1086/597590
Antony, S. J., Ynares, C., and Dummer, J. S. (1997). Isoniazid hepatotoxicity in renal transplant recipients. Clin. Transpl. 11 (1), 34–37.
Apaydin, S., Altiparmak, M. R., Serdengeçti, K., Ataman, R., Oztürk, R., and Erek, E. (2000). Mycobacterium tuberculosis infections after renal transplantation. Scand. J. Infect. Dis. 32 (5), 501–505. doi:10.1080/003655400458776
Baker, R. J., Mark, P. B., Patel, R. K., Stevens, K. K., and Palmer, N. (2017). Renal association clinical practice guideline in post-operative care in the kidney transplant recipient. BMC Nephrol. 18(1), 174. doi:10.1186/s12882-017-0553-2
Bishai, W. R., and Chaisson, R. E. (1997). Short-course chemoprophylaxis for tuberculosis. Clin. Chest Med. 18 (1), 115–122. doi:10.1016/s0272-5231(05)70360-9
Burguet, L., Duvignaud, A., Nguyen, D., Receveur, M. C., Kaminski, H., Pellegrin, I., et al. (2022). Pulmonary tuberculosis and management of contact patients in a department of nephrology and kidney transplantation. Int. J. Infect. Dis. 117, 251–257. doi:10.1016/j.ijid.2021.05.045
Clemente, W. T., Pierrotti, L. C., Abdala, E., Morris, M. I., Azevedo, L. S., López-Vélez, R., et al. (2018). Recommendations for management of endemic diseases and travel medicine in solid-organ transplant recipients and donors: Latin America. Transplantation 102 (2), 193–208. doi:10.1097/tp.0000000000002027
Currie, A. C., Knight, S. R., and Morris, P. J. (2010). Tuberculosis in renal transplant recipients: The evidence for prophylaxis. Transplantation 90 (7), 695–704. doi:10.1097/TP.0b013e3181ecea8d
de Lemos, A. S., Vieira, M. A., Halpern, M., Quaresma, R. G., Borchardt, A. C., Santos, M. A., et al. (2013). Results of implementation of preventive recommendations for tuberculosis after renal transplantation in an endemic area. Am. J. Transpl. 13 (12), 3230–3235. doi:10.1111/ajt.12470
Dodani, S. K., Nasim, A., Aziz, T., and Naqvi, A. (2021). The efficacy of isoniazid prophylaxis in renal transplant recipients in a high tuberculosis burden country. Transpl. Infect. Dis. 23(5), e13709. doi:10.1111/tid.13709
el-Agroudy, A. E., and Refaie, A. F., (2003). Tuberculosis in Egyptian kidney transplant recipients: Study of clinical course and outcome. J. Nephrol. 16(3), 404–411.
Epstein, D. J., and Subramanian, A. K. (2018). Prevention and management of tuberculosis in solid organ transplant recipients. Infect. Dis. Clin. North Am. 32 (3), 703–718. doi:10.1016/j.idc.2018.05.002
Fang, G., Cheng, N. C., Huang, L. L., Xie, W. P., Hu, C. M., and Chen, W. (2021). The first report of co-existence of pulmonary tuberculosis and lung malignancy in a kidney transplant recipient: A case report and literature review. BMC Infect. Dis. 21 (1), 629. doi:10.1186/s12879-021-06350-x
Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Bmj 343, d5928. doi:10.1136/bmj.d5928
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. Bmj 327 (7414), 557–560. doi:10.1136/bmj.327.7414.557
Horne, D. J., Narita, M., Spitters, C. L., Parimi, S., Dodson, S., and Limaye, A. P. (2013). Challenging issues in tuberculosis in solid organ transplantation. Clin. Infect. Dis. 57 (10), 1473–1482. doi:10.1093/cid/cit488
Hosohata, K., Matsuoka, E., Inada, A., Oyama, S., Niinomi, I., Mori, Y., et al. (2018). Differential profiles of adverse events associated with mycophenolate mofetil between adult and pediatric renal transplant patients. J. Int. Med. Res. 46 (11), 4617–4623. doi:10.1177/0300060518786917
John, G. T., Thomas, P. P., Thomas, M., Jeyaseelan, L., Jacob, C. K., and Shastry, J. C. (1994). A double-blind randomized controlled trial of primary isoniazid prophylaxis in dialysis and transplant patients. Transplantation 57(11), 1683–1684. doi:10.1097/00007890-199457110-00029
Karuthu, S., and Blumberg, E. A. (2012). Common infections in kidney transplant recipients. Clin. J. Am. Soc. Nephrol. 7 (12), 2058–2070. doi:10.2215/cjn.04410512
Kim, H., Kim, S. H., Jung, J. H., Kim, M. J., Kim, H., Shin, S., et al. (2020). The usefulness of quantitative interferon-gamma releasing assay response for predicting active tuberculosis in kidney transplant recipients: A quasi-experimental study. J. Infect. 81 (3), 403–410. doi:10.1016/j.jinf.2020.06.070
Kim, J. S., Cho, J. H., Park, G. Y., Kang, Y. J., Kwon, O., Choi, J. Y., et al. (2013). Comparison of QuantiFERON-TB Gold with tuberculin skin test for detection of latent tuberculosis infection before kidney transplantation. Transpl. Proc. 45 (8), 2899–2902. doi:10.1016/j.transproceed.2013.08.059
Kim, S. H., Lee, S. O., Park, I. A., Kim, S. M., Park, S. J., Yun, S. C., et al. (2015). Isoniazid treatment to prevent TB in kidney and pancreas transplant recipients based on an interferon-γ-releasing assay: An exploratory randomized controlled trial. J. Antimicrob. Chemother. 70 (5), 1567–1572. doi:10.1093/jac/dku562
Kim, S. H., Lee, S. O., Park, J. B., Park, I. A., Park, S. J., Yun, S. C., et al. (2011). A prospective longitudinal study evaluating the usefulness of a T-cell-based assay for latent tuberculosis infection in kidney transplant recipients. Am. J. Transpl. 11 (9), 1927–1935. doi:10.1111/j.1600-6143.2011.03625.x
Kwon, D. E., Han, S. H., Han, K. D., La, Y., and Lee, K. H. (2021). Incidence rate of active tuberculosis in solid organ transplant recipients: Data from a nationwide population cohort in a high-endemic country. Transpl. Infect. Dis. 23(6), e13729. doi:10.1111/tid.13729
Lamb, K. E., Lodhi, S., and Meier-Kriesche, H. U. (2011). Long-term renal allograft survival in the United States: A critical reappraisal. Am. J. Transpl. 11(3), 450–462. doi:10.1111/j.1600-6143.2010.03283.x
Lezaic, V., Radivojevic, R., Radosavljevic, G., Blagojevic, R., Djukanovic, L., Simic, S., et al. (2001). Does tuberculosis after kidney transplantation follow the trend of tuberculosis in general population? Ren. Fail 23 (1), 97–106. doi:10.1081/jdi-100001289
Majeed, A., Beatty, N., Iftikhar, A., Mushtaq, A., Fisher, J., Gaynor, P., et al. (2018). A 20-year experience with nocardiosis in solid organ transplant (SOT) recipients in the southwestern United States: A single-center study. Transpl. Infect. Dis. 20 (4), e12904. doi:10.1111/tid.12904
Matuck, T. A., Brasil, P., Alvarenga Mde, F., Morgado, L., Rels, M. D., da Costa, A. C., et al. (2004). Tuberculosis in renal transplants in Rio de Janeiro. Transpl. Proc. 36 (4), 905–906. doi:10.1016/j.transproceed.2004.03.093
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., and PRISMA Group, (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 6 (7), e1000097. doi:10.1371/journal.pmed.1000097
Muñoz, P., Rodríguez, C., and Bouza, E. (2005). Mycobacterium tuberculosis infection in recipients of solid organ transplants. Clin. Infect. Dis. 40 (4), 581–587. doi:10.1086/427692
Naqvi, A., Rizvi, A., Hussain, Z., Hafeez, S., Hashmi, A., Akhtar, F., et al. (2001). Developing world perspective of posttransplant tuberculosis: Morbidity, mortality, and cost implications. Transpl. Proc. 33 (1-2), 1787–1788. doi:10.1016/s0041-1345(00)02680-4
Naqvi, R., Akhtar, S., Noor, H., Saeed, T., Bhatti, S., Sheikh, R., et al. (2006). Efficacy of isoniazid prophylaxis in renal allograft recipients. Transpl. Proc. 38 (7), 2057–2058. doi:10.1016/j.transproceed.2006.06.010
Naqvi, R., Naqvi, A., Akhtar, S., Ahmed, E., Noor, H., Saeed, T., et al. (2010). Use of isoniazid chemoprophylaxis in renal transplant recipients. Nephrol. Dial. Transpl. 25 (2), 634–637. doi:10.1093/ndt/gfp489
Park, S., Kim, M., Kim, J. E., Kim, K., Park, M., Kim, Y. C., et al. (2020). Characteristics of kidney transplantation recipients over time in South Korea. Korean J. Intern Med. 35 (6), 1457–1467. doi:10.3904/kjim.2019.292
Reis-Santos, B., Gomes, T., Horta, B. L., and Maciel, E. L. (2013). Tuberculosis prevalence in renal transplant recipients: Systematic review and meta-analysis. J. Bras. Nefrol. 35(3), 206–213. doi:10.5935/0101-2800.20130033
Riella, L. V. (2018). Understanding the causes of mortality post-transplantation - there is more than meets the eye. J. Bras. Nefrol. 40 (2), 102–104. doi:10.1590/2175-8239-jbn-2018-0002-0003
Roth, P. J., Grim, S. A., Gallitano, S., Adams, W., Clark, N. M., and Layden, J. E. (2016). Serial testing for latent tuberculosis infection in transplant candidates: A retrospective review. Transpl. Infect. Dis. 18 (1), 14–21. doi:10.1111/tid.12489
Samavat, S., Alahyari, S., Sangian, A., Nasiri, M., Nafar, M., Firoozan, A., et al. (2021). Agreement between the results of tuberculin skin test and Interferon-Gamma Release Assays in renal transplant candidates. J. Res. Med. Sci. 26, 88. doi:10.4103/jrms.JRMS_708_20
Sasi, S., Varghese, M. K., Nair, A. P., Hashim, S., and Al Maslamani, M. (2020). Tuberculosis in an allogeneic transplant kidney: A rare case report and review of literature. Cureus 12 (11), e11661. doi:10.7759/cureus.11661
Singh, P., Ng, Y. H., and Unruh, M. (2016). Kidney transplantation among the elderly: Challenges and opportunities to improve outcomes. Adv. Chronic Kidney Dis. 23 (1), 44–50. doi:10.1053/j.ackd.2015.11.002
Subramanian, A., Dorman, S., and AST Infectious Diseases Community of Practice, (2009). Mycobacterium tuberculosis in solid organ transplant recipients. Am. J. Transpl. 9 (4), S57–S62. doi:10.1111/j.1600-6143.2009.02894.x
Torre-Cisneros, J., Doblas, A., Aguado, J. M., San Juan, R., Blanes, M., Montejo, M., et al. (2009). Tuberculosis after solid-organ transplant: Incidence, risk factors, and clinical characteristics in the RESITRA (Spanish network of infection in transplantation) cohort. Clin. Infect. Dis. 48 (12), 1657–1665. doi:10.1086/599035
Van Stralen, K. J., Verrina, E., Belingheri, M., Dudley, J., Dusek, J., Grenda, R., et al. (2013). Impact of graft loss among kidney diseases with a high risk of post-transplant recurrence in the paediatric population. Nephrol. Dial. Transpl. 28 (4), 1031–1038. doi:10.1093/ndt/gfs549
Vikrant, S., Agarwal, S. K., Gupta, S., Bhowmik, D., Tiwari, S. C., Dash, S. C., et al. (2005). Prospective randomized control trial of isoniazid chemoprophylaxis during renal replacement therapy. Transpl. Infect. Dis. 7 (3-4), 99–108. doi:10.1111/j.1399-3062.2005.00103.x
Keywords: isoniazid, prophylaxis, tuberculosis, kidney transplant, meta, analysis
Citation: Yuan Z, Chao S, Xu Y and Niu Y (2023) Chemoprophylaxis for the prevention of tuberculosis in kidney transplant recipients: A systematic review and meta-analysis. Front. Pharmacol. 14:1022579. doi: 10.3389/fphar.2023.1022579
Received: 18 August 2022; Accepted: 23 February 2023;
Published: 16 March 2023.
Edited by:
Robert John Unwin, University College London, United KingdomReviewed by:
Yutaka Yoshii, The Jikei University School of Medicine, JapanBen Caplin, University College London, United Kingdom
Copyright © 2023 Yuan, Chao, Xu and Niu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yulin Niu, bm5pdXl1bGluQDE2My5jb20=
 Zhihui Yuan
Zhihui Yuan Sheng Chao
Sheng Chao